- 1Department of Pharmacy, The Second Xiangya Hospital, Central South University, Changsha, China
- 2International Research Center for Precision Medicine, Transformative Technology and Software Services, Changsha, China.
- 3Department of Microbiology and Infectious Diseases, St. Vincent’s Hospital, Sydney, NSW, Australia
- 4Unit of Clinical Pharmacology, ASST FBF Sacco University Hospital, Milan, Italy
- 5School of Pharmacy, Faculty of Medicine and Health, The University of Sydney, Sydney, NSW, Australia
- 6UNSW Sydney, St Vincent’s Clinical School, Sydney, Australia
- 7Department of Clinical Pharmacology and Toxicology, St Vincent’s Hospital Sydney, Sydney, NSW, Australia
- 8Westmead Hospital, Sydney, NSW, Australia
- 9Sydney Institute for Infectious Diseases, University of Sydney, Sydney, NSW, Australia
Objectives: The current practice of therapeutic drug monitoring (TDM) in Asia is poorly documented. Our aim was to capture and describe TDM services delivered in hospitals across Asia, including aspects such as assay availability, interpretation of results and clinical decision-making.
Methods: An online survey about anti-infective TDM practices, available in English and involving 50 questions, was promoted to people involved in TDM in Asia. The survey was open for responses from September to November 2021.
Results: Of 207 responses from participants working in 14 Asian countries, 150 responses from 10 countries could be included. TDM services are available for many anti-infectives, providing assays based on chromatographic assays (100.0%) or immunoassays (39.3%). Clinicians (82.6%) and pharmacists (86.8%) were responsible for ordering and interpreting TDM. Most services provided reference targets and dose recommendations. Interpretative support was available to a varying degree. Assay results were available and clinical decision-making could be completed within 24 h in most hospitals (87.9% and 88.9% respectively). As the turnaround time of assay results decreased, the proportion of clinical decision-making completed within 8 h increased. Barriers to implementation of TDM included lack of funding or equipment (71.1%), lack of clinician interest or cooperation (47.0%), and lack of expertise (42.3%). Lack of expertise was the primary barrier for using precision dosing software (50.5%).
Conclusion: There are significant differences and challenges in the development and practice of anti-infective TDM in Asian countries.
Introduction
Antibiotic resistance is increasing globally and few new antimicrobial agents are entering the market (The Review on Antimicrobial Resistance, 2016). Optimal, timely and appropriate anti-infective therapy is an important public health issue to minimise the development of antimicrobial resistance in available agents. Therapeutic drug monitoring (TDM) represents a strategy to personalize anti-infective therapy, leading to improved outcomes and avoiding resistance, particularly in the hospital setting where the development of resistance is of particular concern (Eliasson et al., 2013). TDM involves the measurement of the drug concentrations in blood or other validated specimen, interpretation of the result within the clinical context, and adjustment of the dose to achieve concentrations within the therapeutic range, thereby improving therapeutic efficacy and reducing the risk of concentration-dependent adverse effects (Abdul-Aziz et al., 2020; Vena et al., 2020).
TDM practices for anti-infectives are well described in Europe and North America (Jorgensen et al., 2021; Lanckohr et al., 2021), however, the pattern and extent of TDM implementation in Asian countries is poorly documented. TDM may be especially important in the Asia region given racial differences in key pharmacokinetic process. In a randomized 2-way crossover study, Koreans showed area under the concentration-time curve (AUC) for erythromycin that was 1.66 times higher than those of Caucasians (Yu et al., 2001). Numerous other examples of bioavailability and clearance that result in differential exposure in people of Asian descent have been described (Chen, 2006; Zhou et al., 2020; Zhou et al., 2021). Given vast differences in economic development and healthcare infrastructure across Asia, alongside cultural differences, it is anticipated that TDM practices may vary substantially compared to Europe and North America (Younger, 2016; Agustina et al., 2019; Haenssgen et al., 2019). Despite a growing body of literature regarding antimicrobial TDM, there is a paucity of reports concerning TDM practices in Asia. We therefore conducted a survey to evaluate current antimicrobial TDM practices in the region, including aspects such as assay availability, interpretation of results and clinical decision-making.
Materials and methods
Ethical approval
The survey involved procedural questions that were not of a sensitive nature. No information that could identify participants was collected. People were contacted by email and were free to choose whether they wished to participate. Responding to the survey was considered implied consent of the respondent. No compensation was offered to participants. Despite the nature of the questionnaire and the negligible-risk of the study, we sought ethical approval to the Ethics Committee of The Second XiangYa Hospital of Central South University with an approved [(2022) Ethical Review [C.R] No. (K049)] to conduct the survey.
Study design
A cross-sectional study was conducted by an online survey to assess TDM practices for anti-infective agents in Asia. A multidisciplinary group of medical and pharmacist anti-infectives specialists and TDM laboratory specialists developed a four-part survey with 50 questions and was based on previously published surveys (Tabah et al., 2015; Chen et al., 2018; He et al., 2020; Imani et al., 2020; Liu et al., 2021; Wicha et al., 2021). The survey was available in English and took about 10 min to complete (Supplementary material). Part one (12 questions) captured the demographic details of respondents and their healthcare institutions. Parts two and three captured information on availability of TDM for anti-infective agents, assay methods, and sampling (6 questions, respectively). The last part (26 questions) focused on institutional TDM practice. Clinical vignettes were used to evaluate institutional TDM practice in general and clinical decision-making for vancomycin and voriconazole, the most common anti-infectives managed by TDM. Specifically, this section focused on interpretation, clinical response and decision-making based on TDM results, including defining targets and critical values, the combination with MIC values and the individualized regimen of special populations or specific situation (for example, the next round of ordering TDM). To accurately capture differences in TDM practice, specific questions used branching logic, or remained open-ended with response options not mutually exclusive. The option to respond in free-text was also offered. The survey was pilot tested by nine experts in the field of TDM (seven Chinese, one Thai and one Australian) and was hosted on the Research Electronic Data Capture (REDCap) web application.
Study population
An email invitation to complete the survey was distributed to members of the International Association of Therapeutic Drug Monitoring & Clinical Toxicology (IATDMCT), registrants of the IATDMCT Asia-Pacific Regional Meeting and Division of Therapeutic Drug Monitoring and Chinese Pharmacological Society (China-TDM). The survey was also promoted by the authors within their professional networks.
Study procedure
The survey was conducted between September and November 2021. Following an initial invitation, three e-mail reminders were sent at 2, 3, 5 and 6 weeks. The survey was closed at 9 weeks as no further responses were captured. No identifying information was collected; a question to indicate the name of the institution where respondents work was voluntary and aimed to identify multiple responses from a single site. We did not measure distribution reach thus the response rate was not determined.
Statistical analysis
When the field “other” provided the option to enter free-text, answers were coded and sorted where possible. Only completed responses were analyzed, and the number of answers to each question were counted. For descriptive statistics used to summarize survey responses, categorical variables were expressed in number and/or percentage. For inferential statistics, nonparametric Spearman’s rank correlation coefficient and χ2 linear association test was used to study the relationship between two processes of TDM, as appropriate. Data analyses were performed using IBM SPSS Statistics version 25 (IBM, New York, 363NY). Figures were created using GraphPad Prism version 8 (San Diego, CA, United States) and OriginPro 2021b 9.8.5.212 (Learning Edition) (2012 OriginLab Corporation, Northampton, MA 01060, United States). Two-sided tests with a p-value of <0.05 was considered statistically significant.
Results
Respondent and institutional characteristics
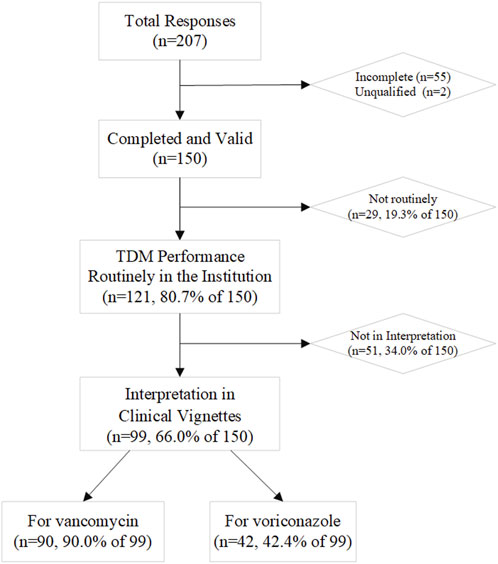
Two hundred and seven health professionals from 14 Asian countries responded; 150 respondents from 10 countries were included in description and statistical analysis: two were deemed ineligible and 55 were incomplete. TDM services were available at the institutions of 121 respondents (80.7%) (Figure 1), which has been increasing over time (Supplementary Figure S1).
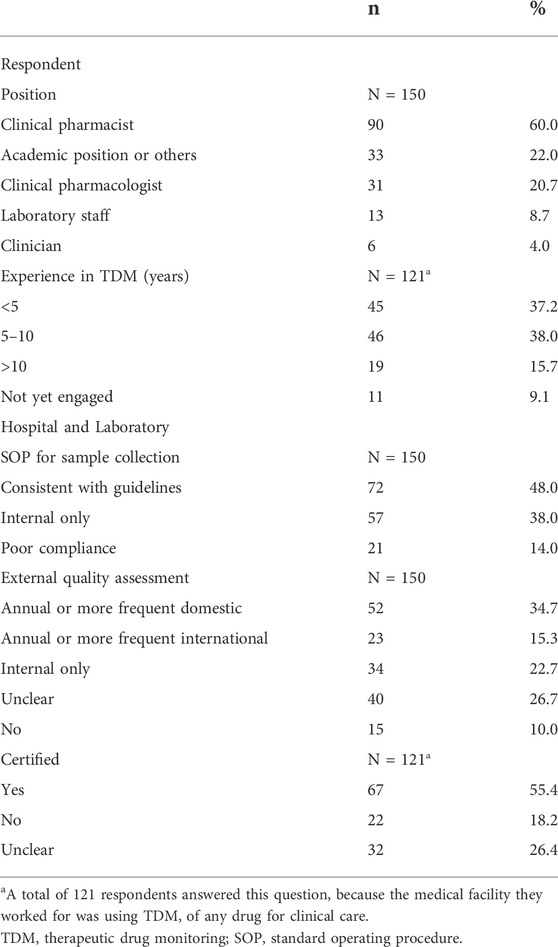
Respondents were predominantly from China, Malaysia and India (36.0%, 27.3% and 20.7% respectively, 84.0% in total; Table 1). Most respondents were pharmacists (60.0%), with 5–10 years (38.0%) or over 10 years (15.7%) experience in delivering TDM (Table 2). Most respondents indicated they had institutional guidelines in place for TDM, but 14.0% indicated poor guideline compliance. Most laboratories participate in annual or more frequent external quality assessment (55.4%), with either domestic (34.7%) or international (15.3%; Table 2) schemes (e.g., United Kingdom National External Quality Assessment Scheme, UKNEQAS).
Responsibilities and roles for TDM
Clinicians were predominantly responsible for ordering TDM for anti-infective agents (82.6%), followed by pharmacists (47.9%), laboratory staff (9.9%) or other professionals (5.0%; Figure 2 and Table 3). Interpretation of TDM results was predominantly performed by pharmacists (86.8%), followed by clinicians (50.4%), laboratory staff (18.2%), microbiologist and other health professionals (5.0%). Pharmacy laboratories were most commonly responsible for governance over TDM services (38.8%), followed by clinical pharmacology laboratories (28.1%) and analytical chemistry laboratories (24.0%; Table 3).
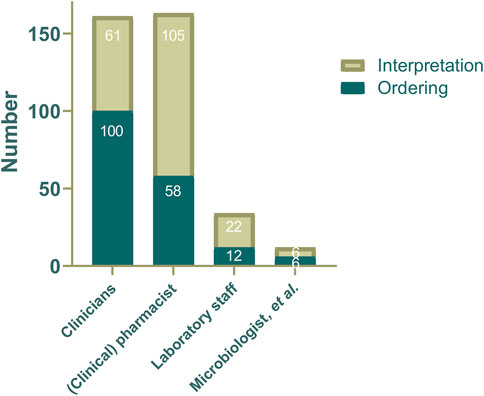
FIGURE 2. The distribution of professionals responsible for ordering or interpretation anti-infective agents TDM service at each institution (N = 121).
Current use of TDM
Regarding assay methods, 39.3% of respondents reported availability of immunoassays at their institution, while 100.0% reported chromatographic methods (Table 3). TDM was most commonly performed for antibacterial drugs (72.7% of respondents, Table 4), as are China, Malaysia, India (92.6% of 54, 97.6% of 41, 48.4% of 31, respectively, Supplementary Table S1) and the other countries (79.2% of 24). While 56.7% reported assay availability for anti-tuberculosis drugs, 33.3% indicated amikacin, and 24.0% reported others other than amikacin; 46.7% of respondents indicated availability of assays for antifungals. Most respondents reported availability of assays for glycopeptides (71.3%), 46.7% for aminoglycosides, 40.0% for voriconazole and 23.3% for β-lactams (Table 4). 15.3% respondents did not have any anti-infective assay available at their institutions, most of whom are clinical pharmacologist (7.3%) and academic professionals (4.7%). 3.7% of Chinese respondents and 48.4% of Indian respondents reported no implementation of anti-infectives TDM (n = 54, 31, respectively). Forty-one respondents in Malaysia reported that 97.6% practiced at least one anti-infectives TDM, involving four categories of antibacterial drugs and seven anti-tuberculosis drugs, but no antifungal drugs (Supplementary Table S1).
Clinical vignettes for interpretation
Two-thirds of respondents completed at least one of the clinical vignettes (n = 99, 66.0%; Figure 1 and Table 5). Most of those who did not participate in the interpretation were staff in academic or other positions (21, 41.2%) and clinical pharmacist or pharmacist (20, 39.2%). The first vignette concerned vancomycin for adult patients infected with methicillin-resistant Staphylococcus aureus (MRSA) (n = 90 responses). Trough level target ranges were 15–20 mg/L for 45.6% of respondents, 10–20 mg/L for 35.6% and 10–15 mg/L for 11.1%. Six respondents (6.7%) indicated that they targeted the AUC0-24 range of 400–600 mg·h/L rather than trough concentrations (Supplementary Table S2). The second vignette was for voriconazole in adults (n = 42 responses). Trough level target ranges were 1–5.5 mg/L for 54.8% of respondents, and 2–4 mg/L for 28.6% as the range in critically ill patients (Supplementary Table S2). 15–20 mg/L for vancomycin and 1.5–5 mg/L for voriconazole were the most often included by the target range (Figure 3). The target range of these two drugs mainly refers to the guidelines (61.1 and 73.8%, respectively). Respondents indicated established critical values were implemented for vancomycin and voriconazole as a hard upper limit (56.7% and 64.3%, respectively). TDM reports included a recommendation at the institutions of 72.7% of the respondents, with the same percentage reporting implementation of ‘active TDM’ in the last 2 weeks (Table 5). 41.4% reported that the number of interventions exceeded half of the total TDM services of the hospital.

TABLE 5. Clinical vignettes for interpretationa
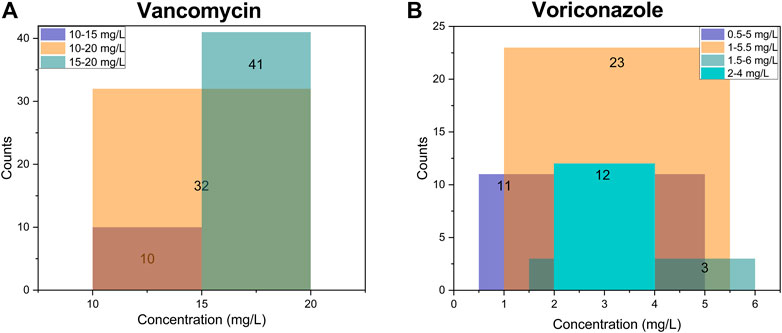
FIGURE 3. The top three target concentration ranges and their overlap in all institutions (N = 99). (A) of vancomycin for adult patients infected with MRSA (N = 90); (B) of voriconazole for adult patients (N = 42), and 12 respondents pursuing 2–4 mg/L as the target range considering clinical efficacy and safety in critically ill patients. The numbers on the column represent the respective counts. The color of the overlap will change when the target ranges cross, and the range with the most overlaps means the most common part of the target concentration.
The turnaround time of TDM services
The assay result and clinical decision-making can be completed within 24 h in most hospitals (87.9% and 88.9% respectively; Figure 4A). For assay results available within 4 h after sampling, clinical decisions can be made immediately (7 of 39, 17.9%) or within the next 8 h (27 of 39, 69.2%; Figure 4B). As the turnaround time for assay results increased, the proportion of respondents able to make a clinical decision within the next 8 h decreased. For results available after 24 h, two of 12 respondents (16.7%) were able to make a decision within the following 8 h, and six of 12 (50%) within 24–48 h (Figure 4B). In addition, there is a significantly moderate correlation between these two turnaround times, according to the result of nonparametric Spearman’s rank correlation coefficient (the value is 0.373, p < 0.01). Furtherly, there was significantly linear correlation between them (the value of χ2 is 16.082, p < 0.01).

FIGURE 4. The efficacy of TDM services in sample determination and clinical decision-making. (A) the cumulative percentage of these two processes completed over time (150 and 99 responses, respectively); (B) the distribution of the 99 responses. The sample determination means from sampling to TDM result; clinical decision-making means from TDM result to clinical response.
The barriers and challenges for TDM
‘Lack of funding or equipment’ was the primary barrier for implementing TDM (71.1% of 150), followed by ‘lack of interest or cooperation from clinicians’ (47.0%) and ‘lack of TDM expertise’ (42.3%; Suplementary Table S3). In contrast, patient cooperation was not considered a barrier (14.8%). Respondents that did not have a TDM service at their institution (18 of 29%, 62.1%; Supplementary Figure S2A) indicated ‘lack of experience’ as the second barrier. Lack of expertise was the primary barrier for using precision dosing software (50.5% of 99). For institutions that do not use dose optimisation software (78% of 99%, 78.8%), the primary barrier was insufficient experience (43 of 78%, 55.1%); followed by lack of availability of minimum inhibitory concentration (MIC) determination (30%, 38.5%) and drug concentration (24%, 30.8%; Supplementary Figure S2B). When dose prediction software was available, respondents expressed concerns with validity of results, insufficient experience with interpretation and software application, and lack of assays for drugs of interest (Supplementary Figure S2B).
Discussion
To our knowledge, this is the first report concerning TDM practices involving a range of anti-infectives in several Asian countries. Previous studies have been conducted in individual countries, have focused on specific anti-infective classes or have focused on applications in intensive care only (Ohnuma et al., 2018; Choi et al., 2019; Hamada et al., 2020; Liu et al., 2021; Zhang et al., 2021).
We found anti-infective TDM practices varied in terms of drugs involved, sample types, bioanalytical approaches and degree of clinical interpretation of results. TDM is common for drugs with available assays, including vancomycin and aminoglycosides, and TDM of β-lactam antibiotics is recommended in international guidelines (Richter et al., 2022), however there are emerging TDM candidates, including fluoroquinolones and linezolid (Roberts et al., 2012; Abdul-Aziz et al., 2020; Sandaradura et al., 2021).
Tuberculosis is of high incidence in the Asia region (World Health Organization, 2021). Efficacious, safe and economical medication treatment is always a priority, in which TDM plays an important role in personalise tuberculosis treatment and comparing with individual MICs to explore pharmacokinetics/pharmacodynamics indices. As the most important choices for multidrug-resistant tuberculosis, amikacin and linezolid are quantified by immunoassays and LC-MS, respectively (van Altena et al., 2017; Bolhuis et al., 2018).
In this sample, clinicians most frequently ordered TDM and interpretation of TDM was most commonly performed by pharmacists. Although anti-infective TDM was not performed at institutions for 15.3% of all respondents and for almost half of Indian respondents, as well as a limited variety of anti-infectives in Malaysia, their involvement in the survey, especially clinical pharmacologists and academic professionals, suggested significant interest.
In the section of clinical vignettes, we asked questions concerning vancomycin and voriconazole as common TDM candidate drugs with well-established ranges to reflect the anti-infectives TDM clinical interpretation and decision-making based on TDM results more specifically. We found most hospitals targeted range reference followed established guidelines, similar to the result in Australian hospitals (He et al., 2020; Imani et al., 2020). For countries and institutions that have not yet formulated the target, it could be preliminarily reference to 15–20 mg/L or AUC0-24 (400–600 mg·h/L) for vancomycin in adults infected with MRSA and 1–5.5 mg/L or 1.5–5 mg/L for voriconazole in adults. Individual differences in patients need to be noted. The target concentration range of voriconazole in critically ill patients may be reduced to 2–4 mg/L clinically, considering the clinical efficacy and safety. It confirms the clinical utility and rational application of TDM, although which is not clearly stated in the guidelines.
The critical values of drug concentration have been established in more than half of these hospitals. But so far, there was little reference of critical values even in the drug guidelines. Significantly, it was first published as early as 1982, which is a specific value of supratherapeutic concentration (e.g. 30 ml/L for vancomycin), to attract great attention to abnormally high exposure and encourage rapid clinical response to prevent the serious adverse drug events (Patton and Borshoff, 2018; Medical Laboratory Observer, 2022). So advocating attention to critical values is still of clinical significance.
We found the efficiency of TDM service can be ensure in most hospitals. The significantly linear correlation between the turnaround times of assay result and clinical decision-making suggested that the more efficient the sampling and assay are, the faster the TDM results can be applied to the follow-up adjustment by clinicians, which may be due to the surrounding concerns for TDM service and the daily schedule of clinicians in the real world. Whilst the role of many possible factors deserves further exploring to make TDM standard of care for anti-infective therapy, including Standard Operating Procedure compliance of sampling and determination, the time lag between serum sample and result, the development of point of care testing, and additional studies to standardize the approach for both reactive and proactive TDM (Strohbehn et al., 2018; Sandaradura et al., 2021). Optimization assay also plays a positive role in the efficient TDM practice and should been encouraged, which will promote clinicians to work based on the guidelines rather than the availability of technology and supplies, especially in most developing countries in Asia (Decosterd et al., 2020; Fuentes et al., 2021).
It was reassuring that the practice of TDM is expanding, accompanied by the emphasis on the innovation of instruments and methods (Dhaese et al., 2020). Pharmacokinetics and pharmacodynamics of anti-infectives based on TDM are increasingly acknowledged as a key component for optimal treatment, the prevention of a slow response to treatment, acquired drug resistance, and adverse drug effects. Therefore, further stimulation to initiate TDM is required (Sturkenboom et al., 2021). Lack of funding and equipment, lack of cooperation from clinicians, and lack of TDM expertise are considered the major barriers to TDM practice. Removing all identified barriers may begin with concerted efforts to refine TDM programmes at an institutional and national level, including establishing and implementation of standardized TDM interpretation scheme and guidelines, increasing the availability of standardized and accurate TDM assays, and carrying out professional knowledge training and exchange in ordering, sampling and interpretation for clinicians, pharmacists and nurses. TDM not only represents concentration measured, but also should be reflected in follow-up clinical decision-making. The clinical interpretation of TDM results by trained professionals plays a key role, which helps to tailor antimicrobial therapy, especially for special populations with different pathophysiology and pathogen’s susceptibility, including critically ill patients, the elderly, obesity, pediatric patients, and patients with renal failure (Gatti et al., 2022; Schmid et al., 2022). With the development of technology, more and more dose optimization software based on TDM was developed. Our survey revealed that only 21.2% institutions are using software to overcome some challenges, less than the Australian ones (51%) (Imani et al., 2020). And the challenges reported suggested that in addition to training in the use of dose optimisation software, developing and improving high-quality software is also important (Wicha et al., 2021).
There are some limitations to this work. Although every effort was made for widespread reach, respondents were primarily from China, Malaysia and India, meaning that results reflect essentially TDM practice of anti-infective agents in these three countries, and that in the other countries, TDM of anti-infective agents needs to be implemented, needing deeper and more widespread studies in further. As for any self-reported data, may or may not representing the wider practice in that institution or the local guidelines, there is also a risk of social desirability bias or reporting bias (Delannoy et al., 2019). It is therefore possible that our results present a more optimistic picture than in reality. Although clinical vignettes are widely used to evaluate clinical practices in the real-world (Das et al., 2015), responses may not reflect the complexities and nuances of clinical decision-making at the bedside. Although our study involved critically ill patients in the clinical vignettes, the lack of characteristics of the patient population managed by survey responders means that the results can not accurately reflect the different responses taken to different patient profiles, which is very meaningful for individual application of TDM service and needs further surveys. Despite the above limitations and preliminary understanding, we believe that these data are important to emphasize the initiation, efficiency, accuracy of intervention, and process improvement of anti-infective TDM practices.
Conclusion
The results point to TDM for some anti-infectives (vancomycin, aminoglycoside and voriconazole) is common across countries in Asia. Whereas TDM practices for other anti-infectives varies. TDM practice is being promoting and considerable in Asia, although it may not yet be widely available. A coordinated effort TDM procedure is needed to refine TDM programmes at an institutional and national level, including developing and implementation available and efficient assays, standardized interpretation scheme and guidelines, professional knowledge training and exchange in ordering and interpretation for clinicians and pharmacists, and the application of dose optimisation software. There is an urgency for well conducted research that will allow the understanding the phenomenon and seek the solution related to this topic more deeply and accurately.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of The Second XiangYa Hospital of Central South University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication. JH and MY wrote the main manuscript. All authors listed reviewed the manuscript. MY, JH, and YZ designed research. CX and JH designed the online questionnaire. DM, SS, and JS has reviewed the questionnaire. JH, MY, DM, DC, J-WA, SS, and JS forwarded the questionnaire. JH performed acquisition of data and the statistical analysis.
Funding
This work was supported by Health Commission of Hunan Provincial [202113012480].
Acknowledgments
The authors thank the Anti-infectives Committee of International Association of Therapeutic Drug Monitoring & Clinical Toxicology (IATDMCT) for initiating this survey. The authors thank all of our colleagues who participated in this survey. We would like to thank Prof. Hualin Cai, Dr. Mou-Ze Liu, Feng Chen, Jiao Xie, Xuemei Luo, Xuebin Wang, Yan Wang, Prof. Karunrat Tewthanom, Prof. Indy Sandaradura for pilot testing the questionnaire. The authors would like to acknowledge Shuang Xia, Jiakai Li, Thi Nguyen, Menino Cotta, Prof. Kaset Chimplee, Prof. Werawath Mahatthatrakul, Prof. Karunrat Tewthanom, Suzana Mustafa, Prof. Indy Sandaradura, Shamin Mohd Saffian, Ryuji kato, Yosuke Suzuki, Yuichi Ando, Prof. Yusuke Tanigawara, Prof. Liyan Miu and Prof. Xianglin Zhang for assistance in forwarding the questionnaire. The authors also thank the Division of Therapeutic Drug Monitoring, Chinese Pharmacological Society (CHINA-TDM) for supporting the forwarding the questionnaire.
This work was presented as an oral presentation at the 2021 Asia-Pacific Regional Meeting (29 October 2021).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.992354/full#supplementary-material
References
Abdul-Aziz, M. H., Alffenaar, J. C., Bassetti, M., Bracht, H., Dimopoulos, G., Marriott, D., et al. (2020). Antimicrobial therapeutic drug monitoring in critically ill adult patients: A position paper. Intensive Care Med. 46 (6), 1127–1153. doi:10.1007/s00134-020-06050-1
Agustina, R., Dartanto, T., Sitompul, R., Susiloretni, K. A., Suparmi, , , Achadi, E. L., et al. (2019). Universal health coverage in Indonesia: Concept, progress, and challenges. Lancet 393 (10166), 75–102. doi:10.1016/s0140-6736(18)31647-7
Bolhuis, M. S., Akkerman, O. W., Sturkenboom, M. G. G., Ghimire, S., Srivastava, S., Gumbo, T., et al. (2018). Linezolid-based regimens for multidrug-resistant tuberculosis (tb): A systematic review to establish or revise the current recommended dose for tb treatment. Clin. Infect. Dis. 67 (3), S327–s335. doi:10.1093/cid/ciy625
Chen, K., Zhang, X., Ke, X., Du, G., Yang, K., and Zhai, S. (2018). Individualized medication of voriconazole: A practice guideline of the division of therapeutic drug monitoring, Chinese pharmacological society. Ther. Drug Monit. 40 (6), 663–674. doi:10.1097/ftd.0000000000000561
Chen, M. L. (2006). Ethnic or racial differences revisited: Impact of dosage regimen and dosage form on pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet. 45 (10), 957–964. doi:10.2165/00003088-200645100-00001
Choi, R., Woo, H. I., Park, H. D., and Lee, S. Y. (2019). A nationwide utilization survey of therapeutic drug monitoring for five antibiotics in South Korea. Infect. Drug Resist. 12, 2163–2173. doi:10.2147/IDR.S208783
Das, J., Kwan, A., Daniels, B., Satyanarayana, S., Subbaraman, R., Bergkvist, S., et al. (2015). Use of standardised patients to assess quality of tuberculosis care: A pilot, cross-sectional study. Lancet. Infect. Dis. 15 (11), 1305–1313. doi:10.1016/s1473-3099(15)00077-8
Decosterd, L. A., Mercier, T., Ternon, B., Cruchon, S., Guignard, N., Lahrichi, S., et al. (2020). Validation and clinical application of a multiplex high performance liquid chromatography - tandem mass spectrometry assay for the monitoring of plasma concentrations of 12 antibiotics in patients with severe bacterial infections. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1157, 122160. doi:10.1016/j.jchromb.2020.122160
Delannoy, M., Agrinier, N., Charmillon, A., Degand, N., Dellamonica, J., Leone, M., et al. (2019). Implementation of antibiotic stewardship programmes in French ICUs in 2018: A nationwide cross-sectional survey. J. Antimicrob. Chemother. 74 (7), 2106–2114. doi:10.1093/jac/dkz113
Dhaese, S., Van Vooren, S., Boelens, J., and De Waele, J. (2020). Therapeutic drug monitoring of β-lactam antibiotics in the ICU. Expert Rev. anti. Infect. Ther. 18 (11), 1155–1164. doi:10.1080/14787210.2020.1788387
Eliasson, E., Lindh, J. D., Malmström, R. E., Beck, O., and Dahl, M.-L. (2013). Therapeutic drug monitoring for tomorrow. Eur. J. Clin. Pharmacol. 69 (1), 25–32. doi:10.1007/s00228-013-1504-x
Fuentes, Y. V., Blanco, J., Díaz-Quijano, D. M., Lechtig-Wasserman, S., Liebisch-Rey, H., Díaz-Pinilla, N., et al. (2021). Administration and therapeutic drug monitoring of β-lactams and vancomycin in critical care units in Colombia: The ANTIBIOCOL study. Pharmaceutics 13 (10), 1577. doi:10.3390/pharmaceutics13101577
Gatti, M., Cojutti, P. G., Bartoletti, M., Tonetti, T., Bianchini, A., Ramirez, S., et al. (2022). Expert clinical pharmacological advice may make an antimicrobial TDM program for emerging candidates more clinically useful in tailoring therapy of critically ill patients. Crit. Care 26 (1), 178. doi:10.1186/s13054-022-04050-9
Haenssgen, M. J., Charoenboon, N., Zanello, G., Mayxay, M., Reed-Tsochas, F., Lubell, Y., et al. (2019). Antibiotic knowledge, attitudes and practices: New insights from cross-sectional rural health behaviour surveys in low-income and middle-income south-east Asia. BMJ Open 9 (8), e028224. doi:10.1136/bmjopen-2018-028224
Hamada, Y., Ueda, T., Miyazaki, Y., Nakajima, K., Fukunaga, K., Miyazaki, T., et al. (2020). Effects of antifungal stewardship using therapeutic drug monitoring in voriconazole therapy on the prevention and control of hepatotoxicity and visual symptoms: A multicentre study conducted in Japan. Mycoses 63 (8), 779–786. doi:10.1111/myc.13129
He, N., Su, S., Ye, Z., Du, G., He, B., Li, D., et al. (2020). Evidence-based guideline for therapeutic drug monitoring of vancomycin: 2020 update by the division of therapeutic drug monitoring, Chinese pharmacological society. Clin. Infect. Dis. 71 (4), S363–S371. doi:10.1093/cid/ciaa1536
Imani, S., Alffenaar, J. W., Cotta, M. O., Daveson, K., van Hal, S., Lau, C., et al. (2020). Therapeutic drug monitoring of commonly used anti-infective agents: A nationwide cross-sectional survey of Australian hospital practices. Int. J. Antimicrob. Agents 56 (6), 106180. doi:10.1016/j.ijantimicag.2020.106180
Jorgensen, S. C. J., Spellberg, B., Shorr, A. F., and Wright, W. F. (2021). Should therapeutic drug monitoring based on the vancomycin area under the concentration-time curve Be standard for serious methicillin-resistant Staphylococcus aureus infections?-No. Clin. Infect. Dis. 72 (9), 1502–1506. doi:10.1093/cid/ciaa1743
Lanckohr, C., Boeing, C., De Waele, J. J., de Lange, D. W., Schouten, J., Prins, M., et al. (2021). Antimicrobial stewardship, therapeutic drug monitoring and infection management in the ICU: Results from the international A- TEAMICU survey. Ann. Intensive Care 11 (1), 131. doi:10.1186/s13613-021-00917-2
Liu, J., Zhang, S., Huang, S., Chen, Y., Zhang, L., Du, H., et al. (2021). Rationality of time-dependent antimicrobial use in intensive care units in China: A nationwide cross-sectional survey. Front. Med. 8, 584813. doi:10.3389/fmed.2021.584813
Medical Laboratory Observer (2022). CLR_2021-22_Critical_Values [Online]. Available: https://www.clr-online.com/CLR_2021-22_Critical_Values.pdf (Accessed June 21, 2022).
Ohnuma, T., Hayashi, Y., Yamashita, K., Marquess, J., Lefor, A. K., Sanui, M., et al. (2018). A nationwide survey of intravenous antimicrobial use in intensive care units in Japan. Int. J. Antimicrob. Agents 51 (4), 636–641. doi:10.1016/j.ijantimicag.2018.01.022
Patton, K., and Borshoff, D. C. (2018). Adverse drug reactions. Anaesthesia 73 (1), 76–84. doi:10.1111/anae.14143
Richter, D. C., Heininger, A., Chiriac, U., Frey, O. R., Rau, H., Fuchs, T., et al. (2022). Antibiotic stewardship and therapeutic drug monitoring of β-lactam antibiotics: Is there a link? An opinion paper. Ther. Drug Monit. 44 (1), 103–111. doi:10.1097/ftd.0000000000000949
Roberts, J. A., Norris, R., Paterson, D. L., and Martin, J. H. (2012). Therapeutic drug monitoring of antimicrobials. Br. J. Clin. Pharmacol. 73 (1), 27–36. doi:10.1111/j.1365-2125.2011.04080.x
Sandaradura, I., Alffenaar, J. W., Cotta, M. O., Daveson, K., Day, R. O., Van Hal, S., et al. (2021). Emerging therapeutic drug monitoring of anti-infective agents in Australian hospitals: Availability, performance and barriers to implementation. Br. J. Clin. Pharmacol. 88, 669–679. doi:10.1111/bcp.14995
Schmid, S., Schlosser, S., Gülow, K., Pavel, V., Müller, M., and Kratzer, A. (2022). Interprofessional collaboration between ICU physicians, staff nurses, and hospital pharmacists optimizes antimicrobial treatment and improves quality of care and economic outcome. Antibiot. (Basel) 11 (3), 381. doi:10.3390/antibiotics11030381
Strohbehn, G. W., Pan, W. W., Petrilli, C. M., Heidemann, L., Larson, S., Aaronson, K. D., et al. (2018). Large-scale variability of inpatient tacrolimus therapeutic drug monitoring at an academic transplant center: A retrospective study. Ther. Drug Monit. 40 (4), 394–400. doi:10.1097/ftd.0000000000000526
Sturkenboom, M. G. G., Märtson, A. G., Svensson, E. M., Sloan, D. J., Dooley, K. E., van den Elsen, S. H. J., et al. (2021). Population pharmacokinetics and bayesian dose adjustment to advance TDM of anti-TB drugs. Clin. Pharmacokinet. 60 (6), 685–710. doi:10.1007/s40262-021-00997-0
Tabah, A., De Waele, J., Lipman, J., Zahar, J. R., Cotta, M. O., Barton, G., et al. (2015). The ADMIN-ICU survey: A survey on antimicrobial dosing and monitoring in ICUs. J. Antimicrob. Chemother. 70 (9), 2671–2677. doi:10.1093/jac/dkv165
The Review on Antimicrobial Resistance (2016). The review on antimicrobial resistance 2016. Tackling drug-resistant infections globally: Final report and recommendations[Online]. Available: https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf (Accessed June 22, 2022).
van Altena, R., Dijkstra, J. A., van der Meer, M. E., Borjas Howard, J. F., Kosterink, J. G., van Soolingen, D., et al. (2017). Reduced chance of hearing loss associated with therapeutic drug monitoring of aminoglycosides in the treatment of multidrug-resistant tuberculosis. Antimicrob. Agents Chemother. 61 (3), e01400-e01416. doi:10.1128/aac.01400-16
Vena, A., Muñoz, P., Mateos, M., Guinea, J., Galar, A., Pea, F., et al. (2020). Therapeutic drug monitoring of antifungal drugs: Another tool to improve patient outcome? Infect. Dis. Ther. 9 (1), 137–149. doi:10.1007/s40121-020-00280-y
Wicha, S. G., Martson, A. G., Nielsen, E. I., Koch, B. C. P., Friberg, L. E., Alffenaar, J. W., et al. (2021). From therapeutic drug monitoring to model-informed precision dosing for antibiotics. Clin. Pharmacol. Ther. 109 (4), 928–941. doi:10.1002/cpt.2202
World Health Organization (2021). Global tuberculosis report 2021. [Online]. Available: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2021 (Accessed June 21, 2022).
Younger, D. S. (2016). Health care in China. Neurol. Clin. 34 (4), 1115–1125. doi:10.1016/j.ncl.2016.06.003
Yu, K. S., Cho, J. Y., Shon, J. H., Bae, K. S., Yi, S. Y., Lim, H. S., et al. (2001). Ethnic differences and relationships in the oral pharmacokinetics of nifedipine and erythromycin. Clin. Pharmacol. Ther. 70 (3), 228–236. doi:10.1067/mcp.2001.117703
Zhang, C., Lei, J., Liu, Y., Wang, Y., Huang, L., and Feng, Y. (2021). Therapeutic drug monitoring and pharmacogenetic testing in northern China. Front. Pharmacol. 12, 754380. doi:10.3389/fphar.2021.754380
Zhou, P. Y., Lim, T. P., Tang, S. L. S., Liew, Y., Chua, S. G. N., Lim, L. L. C., et al. (2020). The utility of voriconazole therapeutic drug monitoring in a multi-racial cohort in Southeast Asia. J. Glob. Antimicrob. Resist. 21, 427–433. doi:10.1016/j.jgar.2019.12.004
Keywords: therapeutic drug monitoring, anti-infective, interpretation, clinical practice, questionnaire
Citation: Hou J, Marriott D, Cattaneo D, Stocker S, Stojanova J, Alffenaar J-W, Xiao C, Zhao Y, Gong H and Yan M (2022) Therapeutic drug monitoring practices of anti-infectives: An Asia-wide cross-sectional survey. Front. Pharmacol. 13:992354. doi: 10.3389/fphar.2022.992354
Received: 12 July 2022; Accepted: 13 September 2022;
Published: 10 October 2022.
Edited by:
Erwin Dreesen, KU Leuven, BelgiumReviewed by:
Pier Giorgio Cojutti, Sant'Orsola-Malpighi Polyclinic, ItalyMehdi Oualha, Hôpital Necker-Enfants Malades, France
Copyright © 2022 Hou, Marriott, Cattaneo, Stocker, Stojanova, Alffenaar, Xiao, Zhao, Gong and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miao Yan, eWFubWlhb0Bjc3UuZWR1LmNu
 Jingjing Hou
Jingjing Hou Debbie Marriott3
Debbie Marriott3 Dario Cattaneo
Dario Cattaneo Yichang Zhao
Yichang Zhao Hui Gong
Hui Gong Miao Yan
Miao Yan