
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 07 October 2022
Sec. Neuropharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.988648
This article is part of the Research Topic The Pharmacotherapy of Depression - Searching for New Mechanisms and Drug Interactions. Basic and Clinical Research. View all 12 articles
Depression is a severe mental health problem that affects people regardless of social status or education, is associated with changes in mood and behavior, and can result in a suicide attempt. Therapy of depressive disorders is based mainly on drugs discovered in the 1960s and early 1970s. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are frontline pharmacological strategies for the medical treatment of depression. In addition, approved by FDA in 2019, esketamine [as nasal spray; N-methyl-D-aspartate (NMDA) receptors antagonist with additional effects on α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, hyperpolarization-activated cyclic nucleotide-gated (HCN) channels, L-type voltage-dependent calcium channel (L-VDCC), opioid receptors, and monoaminergic receptors] is an essential compound in suicide and drug-resistant depression. However, the treatment of depression is burdened with severe side effects, and in many cases, it is ineffective. An equally important issue is the choice of antidepressant therapy in people with comorbid somatic diseases, for example, due to possible interactions with the patient's other drugs. Therefore, there is a great need for new antidepressants with different mechanisms of action and the need to refine the search for new substances. The purpose of this review was to discuss new research directions and new trends that dominate laboratories worldwide. We have reviewed the literature to present new points on the pharmacological target of substances with antidepressant activity. In addition, we propose a new perspective on depressive therapies.
Following World Health Organization (WHO) data from the Institute of Health Metrics and Evaluation, the Global Health Data Exchange (GHDx) indicated that 280 million people in the world suffer from depression (Institute of Health Metrics and Evaluation Global Health Data Exchange, 2021). Depression is a very complex mental illness. Criteria for major depressive disorder (MDD) are one or more major depressive episodes (the lifetime absence of mania and hypermania) with five symptoms present during 2 weeks (DSM-5, 2013; Uher et al., 2014; Abdel-Bakky et al., 2021). The symptoms of depression can be divided into emotional and physical. The emotional symptoms of depression are stress, sadness, loss of interest, anxiety, hopelessness, difficulties with concentration, feeling of guilt, and suicidal thoughts (Abdel-Bakky et al., 2021). Physical symptoms include lack of energy, fatigue, pain, sleep disturbances, headaches, and psychomotor activity changes (Abdel-Bakky et al., 2021). The complexity of depression is evidenced by the classification of this illness proposed by the (American Psychiatric Association (APA), 2013) shown in Figure 1.
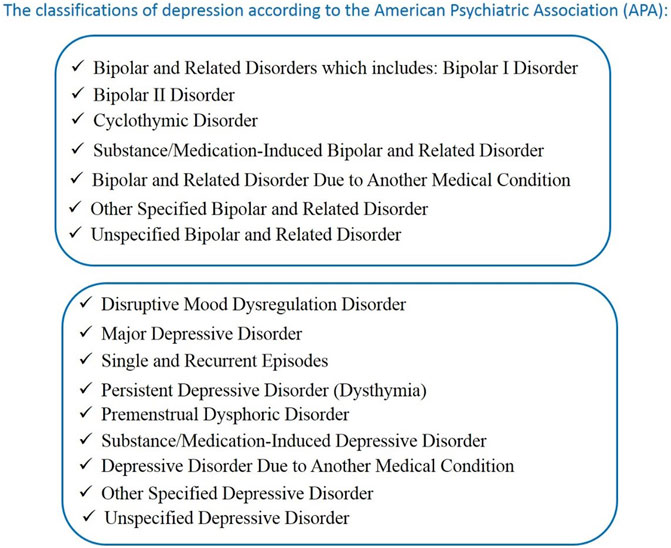
FIGURE 1. The classification of depression according to the American Psychiatric Association (APA). (DSM-5, 2013; Uher et al., 2014; Abdel-Bakky et al., 2021).
MDD can be divided into 14 subcategories, including that: “with anxious distress,” “with mixed features,” “with melancholic features,” “with mood-congruent psychotic features,” “with mood-incongruent psychotic features,” “with catatonia,” “with peripartum onset” categories (DSM-5, 2013).
As you can see, depression is a complex disease, which makes it challenging to diagnose unequivocally. According to statistics, women suffer from depression more often. It has been documented that depression in women occurs three times more often than in men. Hormonal aspects significantly impact the course of depression and treatment, which is particularly evident in postpartum depression (Kroska and Stowe, 2020). However, recent events related to the COVID-19 pandemic have shown increased incidence in all gender and age groups, including children (Bueno-Notivol et al., 2021; Hawes et al., 2021). Symptoms such as depression, anxiety, and cognitive impairment are considered to be the main symptoms of the post-acute COVID-19 syndrome (Mazza et al., 2022).
The etiology of depression has not yet been fully established and may involve genetic and environmental factors (Gaebel et al., 2017). Depression often coexists with other mental disorders (e.g., anxiety disorders and substance use disorders) and various somatic illnesses, including cardiovascular disease (e.g., hypertension, coronary artery disease), metabolic syndromes (e.g., diabetes), respiratory diseases (e.g., chronic obstructive pulmonary disease), various deficiencies (e.g., severe anemia), infections (e.g., tuberculosis, AIDS, influenza), collagen disorders, endocrine diseases (e.g., hypothyroidism, Cushing’s disease), and others (Dornquast et al., 2017; Abdel-Bakky et al., 2021). It's known that depression may induce somatic disorders and vice versa—the presence of chronic somatic conditions may lead to the development of mental diseases. Patients with somatic diseases have a higher risk of developing mental illness. On the other hand, in people with severe mental illness, the risk of developing somatic disorders is twice as high as in patients without psychiatric disorders. The data indicate that nearly 50% of patients experiencing mental disorders have clinically significant comorbid physical illnesses, which often go undiagnosed for extended periods (Dornquast et al., 2017; Steffen et al., 2020). Importantly, it is indicated that comorbidities are the most critical factor influencing the economic burden of depression. Hence, it is necessary to consider them in the treatment of depression (Steffen et al., 2020).
Between 2003 and 2005, in the European Union (EU), about 27% of adults were affected by a mental disorder (Wittchen et al., 2011). The following survey in 2011 detected 164.7 million people affected by mental health problems, which was about 38.2% of people (Wittchen et al., 2011). 2011 survey included 14 new disorders not included in the 2005 study (Wittchen et al., 2011) hence differences; but the number of affected people is enormous. Recent events related to the COVID-19 pandemic caused by the SARS-COV-2 virus have resulted in a significant increase in the incidence of depression in the population (Bueno-Notivol et al., 2021). A study by Bueno-Notivol et al. (2021) found a global estimated prevalence of depression in 2021 was seven times higher than in 2017, which is about a 25% increase. Authors searched for cross-sectional, community-based studies listed on PubMed or Web of Science from 1 January 2020, to 8 May 2020, that reported prevalence of depression (Bueno-Notivol et al., 2021). A random-effects model was used to estimate the pooled proportion of depression (Bueno-Notivol et al., 2021). Interestingly, the meta-regression observation showed that the prevalence of depression was independent of the percentage of women, mean age at baseline, response rate, or methodological quality (Bueno-Notivol et al., 2021), which suggests a severe global problem. Similar observations were documented in the United States after the pandemic of COVID-19 (Hawes et al., 2021). Another increase in depression can be expected in connection with the outbreak of the war in Ukraine in 2022 (Jain et al., 2022).
Today, the treatment of depression is primarily based on drugs discovered in the 1960s and 1970s. It should be mentioned here that Monoamine Oxidase Inhibitors (MAOIs) as selegiline, tranylcypromine, and phenelzine; Selective Serotonin Reuptake Inhibitors (SSRIs) as escitalopram, paroxetine, fluoxetine, fluvoxamine; Tricyclic Antidepressants (TADs) as amitriptyline, desipramine, iprindol; Serotonin-Norepinephrine Reuptake Inhibitors (S-NRIs) as venlafaxine, duloxetine, desvenlafaxine; Serotonin Antagonist and Reuptake Inhibitors (SAs and RIs) as nefazodone and trazodone; Norepinephrine Reuptake Inhibitors (NRIs) as reboxetine, viloxazine; Noradrenaline and Dopamine Reuptake Inhibitors (N/DRIs) as bupropion; and other drugs as esketamine, ansofaxine, vilazodone or quetiapine; see Figure 2. Moreover, just recently the FDA approved Zulresso (brexanolone) for postpartum depression (Powell et al., 2020).
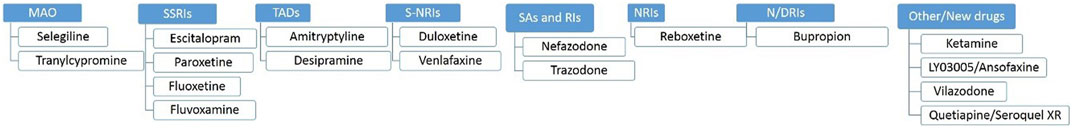
FIGURE 2. Selected examples of antidepressants by groups. MAO, Mono Amino Oxidase; SSRIs, Selective Serotonin Reuptake Inhibitors; TADs, Tricyclic Antidepressants; S-NRIs, Serotonin-Norepinephrine Reuptake Inhibitors; SAs and RIs, Serotonin Antagonist and Reuptake Inhibitors; NRIs, Norepinephrine Reuptake Inhibitors; N/DRIs, Noradrenaline and Dopamine Reuptake Inhibitors.
The number and variety of antidepressants available today seem pretty large, but their use causes many problems. First, these drugs require a long administration time (except for esketmine) to obtain a therapeutic effect (Abdel-Bakky et al., 2021; McCarron et al., 2021). In addition, their use is associated with many side effects, such as weight gain, sexual dysfunction, dizziness, headache, anxiety, psychosis, cognitive dysfunctions, etc. (Abdel-Bakky et al., 2021; McCarron et al., 2021). Considering SSRIs, prolonged bleeding time, and, when used during pregnancy, heart defects and pulmonary hypertension in newborns have been observed (Berard et al., 2017; Calvi et al., 2021). For newer medications, such as those approved by the FDA for treatment of MDD, ansofaxine (potential triple reuptake inhibitor of serotonin, norepinephrine, and dopamine, approved in 2019) or vilazodone (a selective serotonin reuptake inhibitor and serotonin 5-HT1A receptor partial agonist, approved in 2021), the side effects are very similar to traditional antidepressants. They are mild to moderate, with a slightly different frequency than, for example, SSRIs or NRIs. Interestingly, sexual dysfunctions typical for conventional antidepressants are not observed after using ansofaxine (Mi et al., 2022; Chauhan et al., 2022). Also, esketamine, recommended for patients with drug-resistant depression, may have many unpleasant consequences, including dissociation, anxiety, nausea, increased blood pressure, and headache. These side effects are mild, transient, and dose-related and will disappear with subsequent treatments. It is also indicated that the frequency of their occurrence is twice as high in patients receiving simultaneously nasal ketamine and orally another antidepressant than esketemine alone (Ceban et al., 2021). Many of the drugs mentioned have not been approved for pregnant women (Abdel-Bakky et al., 2021; McCarron et al., 2021).
On the other hand, in patients with comorbidities, there are severe contraindications to combining antidepressants with other drugs due to possible drug-drug interactions. The risk of their occurrence is exacerbated by complex polypharmacy regimens and extended treatment periods which result in tolerance problems to ineffectiveness and serious adverse events (Low et al., 2018; Woron et al., 2019). Pharmacokinetic interactions of antidepressants and cardiac drugs seem particularly dangerous because cytochrome 450 isoenzymes metabolize both in the liver. Significantly, both groups of medications can affect the activity of these enzymes. For example, fluoxetine and paroxetine are inhibitors of CYP2D6, and calcium channel blockers inhibit the activity of CYP3A4. Consequently, combined drugs from both groups may lead to hypotension or an increased risk of gastrointestinal bleeding (Woron et al., 2019). Hence, when using polypharmacy, careful selection of drugs with a low interaction potential is necessary. Unfortunately, this is not always possible due to the limited availability of antidepressants with a simple metabolic profile. Moreover, in many cases (especially patients with the severe clinical condition), the possibility of oral administration of antidepressants is severely limited. The exception is esketamine, which the FDA has approved for treating drug-resistant depression as a nasal spray; its action is fast, but also be careful when using it due to the potential risk of drug-drug interactions (Turner, 2019).
All of this necessitates the search for new, more effective treatments for depression and mental health. First, there is a need for drugs that will give a quick therapeutic response, few side effects, and a limited amount of interactions with other medications. Therefore, in the next chapter, we will consider new strategies for treating depression and identify potential pharmacological targets for new active substances designed to treat depression effectively.
When looking for a new way to treat depression, it is essential to define the main cellular/molecular targets for these findings. At present, the most important new targets for antidepressants seem to be glutamate receptors (GluRs) (Stachowicz 2021a; Stachowicz, 2021; Vasiliu 2022a) or gamma-aminobutyric acid (GABA)-ergic modulators; paying attention to both intra- and extra-synaptic GABA-A receptors (Brickley and Mody, 2012; Luo et al., 2013; Vasiliu, 2022). Although the use of GABA-ergic ligands seems to be effective in the treatment of depression, direct interference with the GABA pathway has side effects in the form of drowsiness or sedation, which may hinder daily activities (Vasiliu, 2022); for this reason, the weight appears to be tipping in favor of the GluRs ligands. These agents seem to be an auspicious new point in the pharmacological treatment of depression (Stachowicz 2021a; Stachowicz, 2021; Vasiliu 2022a), which is confirmed by the scale of both preclinical phase I and III clinical trials, where we can find as many as 27 active substances following the last finding (Stachowicz 2021a; Stachowicz, 2021; Vasiliu 2022a). Among GluRs ligands considered in clinical use were NMDA receptor (NMDAR) or its GluN2A/GluN2B subunits antagonists (e.g., NRX-101 or AZD6765, respectively), NMDAR positive allosteric modulators (e.g., AGN-241751), NMDAR-glycine site agonists (e.g., GLYX-13), AMPA receptor potentiators (e.g., TAK-653) and metabotropic glutamate receptors (mGluRs) antagonists (e.g., TS-161) (Stachowicz 2021a; Vasiliu 2022a). Among them, AZD6765, GLYX-13, and TAK-653 did not reach clinical use (Kadriu et al., 2020). However, compounds like GLYX-13 became the prototype for the synthesis of the next generation of compounds with similar mechanisms of action, including apimostinel (GATE-202, NRX-1074), a second-generation analog with increased potency, and zelquistinel (GATE-251, AGN-241751), a third-generation small molecule with increased power and high oral availability (Neurosciences Gate, 2022). The central hypothesis in using these compounds in depression is based on the idea that excessive excitatory transmission related to Glu and so-called neurotoxicity lead to impairment of brain functions, transmission, and plasticity, manifested by mental disorders such as depression (Skolnick, 1999; Stachowicz 2021a).
Other groups of active substances considered in the pharmacotherapy of depression are not apparent compounds acting as anti-cytokines and COX-2 inhibitors (Stachowicz 2021a; Vasiliu 2022a). This group of active substances is aimed at patients with immunological disorders coexisting with depression, where it achieves impressive results both as monotherapy and in combination with classical antidepressants (Stachowicz 2021a; Vasiliu 2022a). Importantly, these compounds could be effective in patients after COVID-19 infection because the psychopathological mechanisms underlying the symptoms of depression after COVID-19 are mainly related to inflammation caused by the peripheral immune response to viral infection and persistent psychological stress during and after an illness. Currently, eight active substances are in phase I to IV clinical trials (Vasiliu 2022). However, clinical reports are already showing promise with combination therapy for COX-2. Following Sethi et al. (2019), the favored use of celecoxib with reboxetine, fluoxetine, and sertraline was observed in depressed patients. In the latter, improvements in HRDS and HAM-D scales were observed if antidepressants were combined with celecoxib. Our preclinical studies are also optimistic, as positive antidepressant effects were observed in the animal model after co-administration of COX-2 inhibitor (NS398) with mGluR ligand and imipramine (Stachowicz 2021a). We have also started to decipher the mechanisms of the influence of antidepressants on fertility parameters in rodents, which may be crucial when looking for active substances without side effects (Solek et al., 2021; Tabecka-Lonczynska et al., 2021).
Quite a new group of antidepressants are orexin receptor antagonists, or compounds acting through microtubule-associated protein type-2 (MAP-2) via calcium channels or microglial mechanisms (for a more extensive review on the topic, please refer to Vasiliu 2022; Vasiliu 2022a). As far as orexins are concerned, ligands for type 1 and 2 receptors (OX1R and OX2R) can modulate feeding, sleep, motivated behavior, anxiety, and addiction; hence they have a vast potential to regulate many aspects of depression (Vasiliu 2022). For details described in this section, see Figure 3.
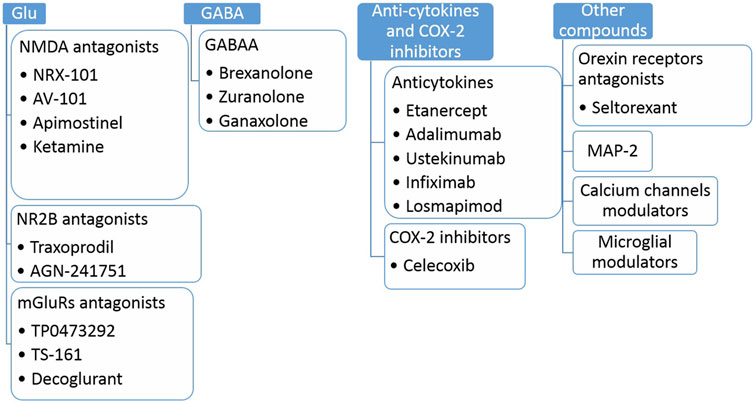
FIGURE 3. Selected examples of antidepressant compounds in clinical trials (Phase I-IV). Glu, glutamate; NMDA, N-methyl-D-aspartate; NR2B, NMDA receptor subunit 2B; mGluRs, metabotropic glutamate receptors, GABAA, gamma-aminobutyric acid receptors A; COX-2, cyclooxygenase 2; MAP-2, microtubule-associated protein 2.
Of course, we must not forget the substances that affect the 5-HT system. Intensive work is underway in this field to implement new compounds acting through new receptors such as 5-HT7 or safer compounds directed towards 5-HT1A or 5-HT2A (e.g., MIN-117 or psilocybin, respectively) (Vasiliu 2022); the topic will be expanded in the following subsections.
Because it was estimated that 15%–20% of depressed patients do not respond to the treatment (Rush et al., 2006; Kalmoe et al., 2020), there are ongoing attempts to use combination therapy to improve mental health (Figure 4).
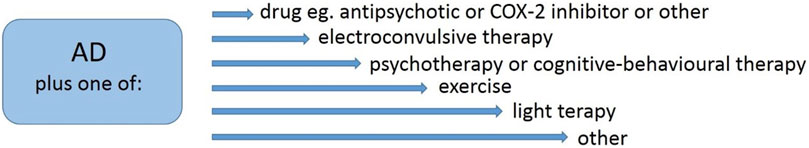
FIGURE 4. Examples of combined therapy in the treatment of depression. AD, antidepressant; COX-2, cyclooxygenase 2.
One way to improve the outcome of the treatment is a combination of known antidepressants (ADs) with the substance directed to a different target, e.g., combination with antipsychotics (Davis et al., 2021; Vázquez et al., 2021; Kishi et al., 2021) or COX-2 inhibitors (Muller et al., 2006; Muller, 2019; Sethi et al., 2019; Stachowicz, 2021). Second-generation antipsychotics (e.g., olanzapine, risperidone, or aripirazole) are effective in combination therapy; however, they still have not overcome the effectiveness of combination therapy with lithium (Vázquez et al., 2021; Kishi et al., 2021). Some hope is linked with combination therapy with COX-2 inhibitors, while much evidence is that this path is effective (Muller et al., 2006; Muller, 2019; Sethi et al., 2019).
A combination of electroconvulsive therapy with ADs is known, and the recent discovery regarding its use with esketamine seems very promising and demonstrates high efficacy in drug-resistant depression (Kavakbasi et al., 2021). Subsequent directions of combining treatments focus on ADs administration and application of psychotherapy (Guidi and Fava, 2021), cognitive-behavioral therapy with virtual reality (Stamou et al., 2021), exercise (Xie et al., 2021), or light therapy (Even et al., 2008) and others. These methods enrich the treatments that benefit and require specialists’ involvement and the patient’s engagement.
The last decade’s new finding is a microbiota-gut axis in depression (Cryan et al., 2019; Stachowicz, 2019; Simpson et al., 2021). Following Cryan et al. (2019), there is a new concept: “the concept of psychobiotics for treating various neurological and psychiatric disorders through targeting the gut microbiota.” The idea of enriching therapy of depression by psychobiotics directed research into entirely new paths; hence a vast amount of research in laboratories around the world is moving in this direction (Cryan et al., 2019; Stachowicz, 2019; Simpson et al., 2021). In connection with the above, mutual regulation between host microbiota and the effectiveness of ADs was recently described (Cryan et al., 2019; Stachowicz, 2019; Duan and Xie, 2020; Simpson et al., 2021); the topic will be expanded in the last subsection.
The latest findings of the COVID-19 pandemic and social isolation documented the positive effects of gardening and physical activity on mental health (Bu et al., 2021). The benefits of gentle exercises for mental health may be connected with reducing blood pressure, regulating neuroendocrine and neuroimmune systems, and giving psychological benefits (Bu et al., 2021).
When presenting new ideas for treating depression, one cannot ignore the idea of a “new look at old drugs.” Compounds that affect the 5-HT system are a large group of AD in the clinic, including 5-HT1A agonists, e.g., vilazodone and vortioxetine (Wróbel et al., 2019). Searching for new antidepressant compounds is focused on that directed to dual agonist 5-HT1A activity and SSRI (Herold et al., 2011; Wróbel et al., 2019) as a compound that both can accelerate desensitization and downregulation of autoreceptors and directly stimulate postsynaptically localized 5-HT neurons (Herold et al., 2011; Wróbel et al., 2019). The idea is that kind of stimulation has a faster antidepressant potential. Among dually acting compounds, molecular targets are 5-HT2A, 5-HT6, 5-HT7, and D2 (Wróbel et al., 2019). Analogs of gepirone, novel pyridol/pyrimidine derivatives, alkylnitroquipazines, and others are synthesized (Paluchowska et al., 2005; Herold et al., 2011; Wróbel et al., 2019; Ślifirski et al., 2019; Król et al., 2021).
Inhibitors of the serotonin transporter (SERT) have long been in clinical use. The “new look at old drugs” regarding SERT compounds is based on the physicochemical properties of SERT ligands. Understanding of physicochemical properties of interactions with targeted sites may be beneficial in designing new compounds with antidepressant properties. Does conformational rearrangement or ligand flexibility play a role in binding reaction and efficacy (Martin et al., 2008)? Martin et al. (2008) documented increased enthalpy with a polar surface area of S-citalopram, duloxetine, fluoxetine, indatraline, paroxetine, sertraline, venlafaxine, but not fluvoxamine; what suggests a SERT inhibitor binding site is polar and allows hydrated ligands to bind without imposing the enthalpic penalty expected from ligand dehydration (Martin et al., 2008). Furthermore, following Martin et al. (2008), entropy/enthalpy compensation in ligand-protein interactions is counteracted by the following regulation (conformational flexibility or hydrophobic properties are regulators of entropy H-bonds or van der Waals interactions in enthalpy). Martin's group found SERT inhibitors bind to 5-HT transporter in a competitive manner (Apparsundaram et al., 2008), further concluding that 5-HT pore may allow for more than one set of interactions with antidepressants (Apparsundaram et al., 2008).
Antipsychotics have attracted some attention in the last decade; the more it has been observed that discontinuation of antidepressants correlates with hypomania or mania (Kassm and Naja, 2018). Second-generation antipsychotics, e.g., aripiprazole, quetiapine, and olanzapine in combination with fluoxetine, have been approved to treat depression (Kato and Chang, 2013). The target audience is patients who failed to respond to monotherapy with ADs (Kato and Chang, 2013). However, Second-generation antipsychotics were more potent than placebo; esketamine or lithium use trumped the antipsychotic results (Vázquez et al., 2021). The research is ongoing, so there is no conclusion.
This subsection would not be presented with accepted and established trends, e.g., the antidepressant effects when targeting trophic factors like brain-derived neurotrophic factor (BDNF) or results of drugs directed to tropomyosin receptor kinase B (TrkB). Here, there will be many new ideas born in the depression field.
One fresh concept is described in Stachowicz (2018) and Wong et al. (2013). Rearrangement of the cytoskeleton of dendrites and spines and adhesion between spines as a predictor of mental health is an entirely new research direction. Following Wong et al. (2013), cytoskeletal abnormalities cause dendritic regression and decrease and are common in depression. In cytoskeletal rearrangement, actin filaments are engaged (F-actin, G-actin) and also actin-binding proteins (ABPs) and postsynaptic density (PSD) proteins, creating an interactive dendritic spine scaffold (Wong et al., 2013). The interplay of actin filaments with microtubules is responsible for organelles’ circulation in the dendritic spine and thus for the rotation of the receptor components and anchoring them in the cell membrane (Wong et al., 2013). Various types of receptors are present in the PSD. Still, the ionotropic glutamate receptors (iGluRs) seem particularly important in depressive disorders and the search for new anti-depressants. For a long time, it was thought that a sufficient explanation of the functional changes in the excitatory transmission is post-translational modifications (e.g., phosphorylation) located in the postsynaptic membrane receptors. However, the studies from the last 30 years have destabilized the static image of excitatory synapses and revealed its highly dynamic structure. It is known that glutamate receptors show lateral mobility along the cell surface between synaptic and extrasynaptic regions. They undergo constitutive trafficking to and from the cell surface with a surface half-life measured in 10 min (Nishimune et al., 1998). They are delivered to and removed from the synaptic membranes regulated by neural activity or the degree of electrical stimulation of the neuron. These mechanisms control the number of receptors and the receptors’ subunit composition, which determines the proper functioning of excitatory synapses. Regulation of the movement of receptors to synapses is multistep and includes transport from the endoplasmic reticulum, trafficking along dendrites, and local transport in a synaptic bulb. This process is controlled by numerous PSD proteins interacting with receptors via PDZ domains (e.g., PSD-95, Shank3/ProSAP2, SAP-97, PICK-1), and other scaffolding proteins (e.g., Homer, CaMKII) and the same receptors, which include many sites undergoing (not only) phosphorylation. Significantly, PSD proteins interact with many intracellular proteins (e.g., PSD-95 with synaptic Ras GTPase activating protein and guanylate kinase-related protein) (Dutta et al., 2021; Shaw and Koleske, 2021).
In addition to the above-discussed processes and molecules, adhesion is essential for synaptic formation by associating pre- and post-synaptic partners in a specific neuro space (Stachowicz, 2018). The importance of the problem was noted by Stachowicz (2018) in the review of the DSCAM protein. DSCAM is only an example, but the adhesive mechanisms involving other adhesive proteins are fundamental in synaptic plasticity and, thus, neural conduction and communication (Stachowicz, 2018; Jiang et al., 2021). Abnormal synaptic connection is associated with learning and memory disturbances and neuropsychiatric and neurodevelopmental disorders (Dutta et al., 2021).
An entirely new finding in the field of depression is the discovery of a new signal pathway involved in the disease—that is, engagement of the antioxidant pathway with nuclear factor erythroid-derived 2-like 2 (Nrf2) (Kansanen et al., 2013; Bouvier et al., 2017; Nakayama et al., 2020). Nrf2 was discovered in 1994 as a member of the human cap“n”collar (CNC) basic-region leucine zipper transcription factor family (Cuadrado et al., 2019). As a product of the NFE2L2 gene, Nrf2 forms heterodimers with other bZip proteins; and regulates the expression of about 250 human genes participating in inflammation, redox metabolism, or proteostasis (Robledinos-Anton et al., 2019). Activators of the Nrf2 pathway are under clinical investigation in Phase I-IV in multiple sclerosis, autism spectrum disorder, Alzheimer’s disease, major depression, and others (Robledinos-Anton et al., 2019). In a depression field, activation of Nrf2 translocation restores redox homeostasis and reverses vulnerability to depression (Bouvier et al., 2016). Furthermore, Nrf2-null mice show depressive-like behavior, and treatment with Nrf2 agonists possesses antidepressant-like potential (Nakayama et al., 2020).
In recent years another discovery in the field of depression is the already mentioned microbiota-gut axis (Cryan et al., 2019; Stachowicz, 2019; Simpson et al., 2021). Our results with the E. coli lipopolysaccharide (LPS) use suggest the engagement of this mechanism in synaptic plasticity with the involvement of excitatory amino acid transporters (EAATs)/COX-2/metabotropic glutamate receptors (mGluRs) (Stachowicz et al., 2021). However, more sophisticated research documented the gut microbiota may regulate the hypothalamic-pituitary-adrenal (HPA) axis, producing neurotransmitters (Sirisinha 2016). Probiotics containing appropriate species of bacteria can lower cortisol; microbiota transplanted from a healthy animal can change animal behavior (Sirisinha 2016). This search for mechanisms of depression, thus related to the immune system, also includes recent reports on the occurrence of depression after COVID-19. Following Mohammadkhanizadeh and Nikbakht (2021), central mechanisms involved in COVID-19-induced depression are inflammation, including uncontrolled activation of microglia, and following the release of inflammatory cytokines (TNF-alpha, IL-6, IL-1beta), nitric oxide, prostaglandin E2. Furthermore, damage to mitochondria directly by reaching them for transcription of the virus genome and indirectly by devasting properties of pro-inflammatory cytokines and ROS (Mohammadkhanizadeh and Nikbakht, 2021). Damage to the hippocampus observed after COVID-19 as a structure involved in depression has drawn the particular attention of researchers to the mechanisms linking respiratory viral infections and depression (Mohammadkhanizadeh and Nikbakht, 2021). Impairment of hippocampal synaptic plasticity and neurogenesis, followed by stress and dysregulation of the HPA axis, contributes to the progression of symptoms of depression (Mohammadkhanizadeh and Nikbakht, 2021). Next, vitamin D deficiency, Zinc, and magnesium are related to depression in COVID-19 (Mohammadkhanizadeh and Nikbakht, 2021).
Inflammatory processes in depression have long been a focus of research. Elevated levels of pro-inflammatory cytokines such as IL-1b, IL-6, and TNFa are observed in the serum of patients with depression (Iwata et al., 2013). The cytokines above are associated with somatic symptoms, described as illness behaviors, including fatigue and loss of appetite (Iwata et al., 2013), which overlap with typical symptoms of major depression. Subsequently, depression has been well described as a common complication of interferon treatment for malignant melanoma and chronic hepatitis (Musselman et al., 2001), and interferon-induced depression has been linked to a complex pathophysiological substrate involving serotonergic and dopaminergic neurotransmission as well as glucocorticoid and neurotrophic factors (Udina et al., 2016).
Summing up, as it can be seen based on this study, there is an intense search for new substances with an antidepressant profile. Researchers have focused on the hunt for compounds that directly interfere with the functioning of the Glu system through its various receptors, both NMDA, AMPA, and mGluRs. In addition, the next direction of research is still the 5-HT system. Still, in a new perspective, the game includes new receptors, such as 5-HT7, and multidirectional therapy, such as, e.g., triple reuptake inhibitors of 5-HT/norepinephrine/dopamine. These are, of course, the main directions of the search. Still, there are also attempts to search for active substances among compounds acting by orexin receptors, COX-2 inhibitors, incorporation of phagocytic, microglial, epigenetic mechanisms, or combination therapies. Personalized antidepressant treatment should also be considered in the future, considering gender differences or genetics, among other things. This problem was described in 2012 by Gvozdic et al. (2012) Undoubtedly, the development of new antidepressants based on new mechanisms of action is necessary due to the increasing number of patients, the unsatisfactory effectiveness of existing pharmacotherapy, or possible side effects and interactions with drugs used for other diseases.
Conceptualization: KS and MS-K; formal analysis: KS and MS-K; funding acquisition: KS and MS-K; investigation (literature review and collection): KS and MS-K; writing—original draft: KS; writing—review and editing: KS and MS-K Both authors have read and agreed to the published version of the manuscript.
The study was supported by grants from the National Science Centre (contracts UMO-2016/21/B/NZ7/01623 to MS-K) and the statutory fund of the Maj Institute of Pharmacology, PAS, Poland.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdel-Bakky, M. S., Amin, E., Faris, T. M., and Abdellatif, A. A. H. (2021). Mental depression: Relation to different disease status, newer treatments and its association with COVID-19 pandemic (review). Mol. Med. Rep. 24, 839. doi:10.3892/mmr.2021.12479
American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (DSM-5). Fifth edition. Washington, D.C, USA: American Psychiatric Association.
Apparsundaram, S., Stockdale, D. J., Henningsen, R. A., Milla, M. E., and Martin, R. S. (2008). Antidepressants targeting the serotonin reuptake transporter act via a competitive mechanism. J. Pharmacol. Exp. Ther. 327, 982–990. doi:10.1124/jpet.108.142315
Bérard, A., Sheehy, O., Zhao, J. P., Vinet, E., Bernatsky, S., and Abrahamowicz, M. (2017). SSRI and SNRI use during pregnancy and the risk of persistent pulmonary hypertension of the newborn. Br. J. Clin. Pharmacol. 83, 1126–1133. doi:10.1111/bcp.13194
Bouvier, E., Brouillard, F., Molet, J., Claverie, D., Cabungcal, J-H., Cresto, N., et al. (2017). Nrf2-dependent persistent oxidative stress results in stress-induced vulnerability to depression. Mol. Psychiatry 22, 1701–1713. doi:10.1038/mp.2016.144
Brickley, S., and Mody, I. (2012). Extrasynaptic GABAA receptors: Their function in the CNS and implications for disease. Neuron 73, 23–34. doi:10.1016/j.neuron.2011.12.012
Bu, F., Steptoe, A., Mak, H. W., and Fancourt, D. (2021). Time use and mental health in UK adults during an 11-week COVID-19 lockdown: A panel analysis. Br. J. Psychiatry. 219, 551–556. doi:10.1192/bjp.2021.44
Bueno-Notivol, J., Gracia-Garcia, P., Olaya, B., Lasheras, I., López-Antón, R., and Santabárbara, J. (2021). Prevalence of depression during the COVID-19 outbreak: A meta-analysis of community-based studies. Int. J. Clin. Health Psychol. 21, 100196. doi:10.1016/j.ijchp.2020.07.007
Calvi, A., Fischetti, I., Verzicco, I., Murri, B., Zanetidou, S., Volpi, R., et al. (2021). Antidepressant drugs effects on blood pressure. Front. Cardiovasc. Med. 8, 704281. doi:10.3389/fcvm.2021.704281
Chauhan, M., Parry, R., Bobo, M. R., Xu, X., Li, L., Tan, Q., Wang, G., et al. (2022). Vilazodone for Major Depression in Adults: Pharmacological Profile and an Updated Review for Clinical Practice. Neuropsychiatr. Dis. Treat . 14 (18), 1175–1193. doi:10.2147/NDT.S279342
Ceban, F., Rosenblat, J. D., Kratiuk, K., Lee, Y., Rodrigues, N. B., Gill, H., Subramaniapillai , M., et al. (2021). Prevention and Management of Common Adverse Effects of Ketamine and Esketamine in Patients with Mood Disorders. CNS Drugs 35 (20), 925–934. doi:10.1007/s40263-021-00846-5
Cryan, J. F., O’Riordan, K. J., Cowan, C. S. M., Sandhu, K. V., Bastiaanssen, T. F. S., Boehme, M., et al. (2019). The microbiota-gut-brain Axis. Physiol. Rev. 99, 1877–2013. doi:10.1152/physrev.00018.2018
Cuadrado, A., Rojo, A. I., Wells, G., Hayes, J. D., Cousin, S. P., Rumsey, W. L., et al. (2019). Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 18, 295–317. doi:10.1038/s41573-018-0008-x
Davis, A. K., Barett, F. S., May, D. G., Cosimano, M. P., Sepeda, N. D., Johnson, M. W., et al. (2021). Effects of psilocybin-assisted therapy on major depressive disorder a randomized clinical trial. JAMA Psychiatry 78, 481–489. doi:10.1001/jamapsychiatry.2020.3285
Dornquast, C., Tomzik, J., Reinhold, T., Walle, M., Mönter, N., and Berghöfer, A. (2017). To what extent are psychiatrists aware of the comorbid somatic illnesses of their patients with serious mental illnesses? - a cross-sectional secondary data analysis. BMC Health Serv. Res. 17, 162. doi:10.1186/s12913-017-2106-6
Duan, J., and Xie, P. (2020). The potential for metabolomics in the study and treatment of major depressive disorder and related conditions. Expert Rev. Proteomics 17, 309–322. doi:10.1080/14789450.2020.1772059
Dutta, P., Bharti, P., Kumar, J., and Maiti, S. (2021). Role of actin cytoskeleton in the organization and function of ionotropic glutamate receptors. Curr. Res. Struct. Biol. 3, 277–289. doi:10.1016/j.crstbi.2021.10.001
Even, C., Schroder, C. M., Friedman, S., and Rouillon, F. (2008). Efficacy of light therapy in nonseasonal depression: A systematic review. J. Affect. Disord. 108, 11–23. doi:10.1016/j.jad.2007.09.008
Gaebel, W., Zielasek, J., and Reed, G. M. (2017). Mental and behavioural disorders in the ICD-11: Concepts, methodologies, and current status. Psychiatr. Pol. 51, 169–195. doi:10.12740/PP/69660
Guidi, J., and Fava, G. A. (2021). Sequential combination of pharmacotherapy and psychotherapy in major depressive disorder: A systematic review and meta-analysis. JAMA Psychiatry 78, 261–269. doi:10.1001/jamapsychiatry.2020.3650
Gvozdic, K., Brandl, E. J., Taylor, D. L., and Muller, D. J. (2012). Genetics and personalized medicine in antidepressant treatment. Curr. Pharm. Des. 18, 5853–5878. doi:10.2174/138161212803523617
Hawes, M. T., Szenczy, A. K., Klein, D. N., Hajcak, G., and Nelson, B. D. (2021). Increases in depression and anxiety symptoms in adolescents and young adults during the COVID-19 pandemic. Psychol. Med. 13, 1–9. doi:10.1017/S0033291720005358
Herold, F., Chodkowski, A., Izbicki, Ł., Turło, J., Dawidowski, M., Kleps, J., et al. (2011). Novel 4-aryl-pyrido[1, 2-c]pyrimidines with dual SSRI and 5-HT1A activity. Part 3. Eur. J. Med. Chem. 46, 142–149. doi:10.1016/j.ejmech.2010.10.026
Institute of Health Metrics and Evaluation. Global Health Data Exchange (GHDx) (2019). “Institute of health Metrics and evaluation,” in Global health data Exchange (GHDx). Available at: http://ghdx.healthdata.org/gbd-results-tool?params=gbd-api-2019-permalink/d780dffbe8a381b25e1416884959e88b.
Iwata, M., Ota, K. T., and Duman, R. S. (2013). The inflammasome: Pathways linking psychological stress, depression, and systemic illnesses. Brain, Behav. Immun. 31, 105–114. doi:10.1016/j.bbi.2012.12.008
Jain, N., Prasad, S., Czárth, Z. C., Chodnekar, S. Y., Mohan, S., Savchenko, E., et al. (2022). War Psychiatry: Identifying and managing the neuropsychiatric consequences of armed conflicts. J. Prim. Care Community Health 13, 21501319221106625. doi:10.1177/21501319221106625
Jiang, X., Sando, R., and Sudhof, T. C. (2021). Multiple signaling pathways are essential for synapse formation induced by synaptic adhesion molecules. Proc. Natl. Acad. Sci. U. S. A. 118, e2000173118. doi:10.1073/pnas.2000173118
Kadriu, M. D. B., Deng, Z., Kraus, C., Henter, I. D., Lisanby, S. H., and Zarate, C. A. (2020). Not so fast. Recent successes and failures in treating depression. J. Clin. Psychiatry 81, 19ac13138. doi:10.4088/JCP.19ac13138
Kalmoe, M. C., Janski, A. M., Zorumski, C. F., Nagele, P., Palanca, B. J., and Conway, C. R. (2020). Ketamine and nitrous oxide: The evolution of NMDA receptor antagonists as antidepressant agents. J. Neurol. Sci. 412, 116778. doi:10.1016/j.jns.2020.116778
Kansanen, E., Kuosmanen, S. M., Leinonen, H., and Levonen, A. L. (2013). The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 1, 45–49. doi:10.1016/j.redox.2012.10.001
Kassm, S. A., and Naja, W. (2018). Looking for bipolarity in antidepressant discontinuation manic states: Update and diagnostic consideration of the phenomenon. J. Aff. Dis. 235, 551–556. doi:10.1016/j.jad.2018.04.080
Kato, M., and Chang, C. M. (2013). Augmentation treatments with second-generation antipsychotics to antidepressants in treatment-resistant depression. CNS Drugs 27, 811–S19. doi:10.1007/s40263-012-0029-7
Kavakbasi, E., Hassan, A., and Baune, B. T. (2021). Combination of electroconvulsive therapy alternating with intravenous esketamine can lead to rapid remission of treatment resistant depression. J. ECT. 37, e20–e21. doi:10.1097/YCT.0000000000000733
Kishi, T., Ikuta, T., Matsuda, Y., Sakuma , K., Okuya, M., Mishima, K., Iwata, N., et al. (2021). Mood stabilizers and/or antipsychotics for bipolar disorder in the maintenance phase: a systematic review and network meta-analysis of randomized controlled trials. Neuropsychiatr. Dis. Treat . 26 (8), 4146–4157. doi:10.1038/s41380-020-00946-6
Król, M., Ślifirski, G., Kleps, J., Ulenberg, Sz., Belka, M., Bączek, T., et al. (2021). Synthesis of novel pyrido[1, 2-c]pyrimidine derivatives with 6-Fluoro-3-(4-piperidynyl)-1, 2-benzisoxazole moiety as potential SSRI and 5-ht1a receptor ligands. Int. J. Mol. Sci. 22, 2329. doi:10.3390/ijms22052329
Kroska, E. B., and Stowe, Z. N. (2020). Postpartum depression identification and treatment in the clinic setting. Obstet. Gynecol. Clin. North Am. 47, 409–419. doi:10.1016/j.ogc.2020.05.001
Low, Y., Setia, S., and Lima, G. (2018). Drug-drug interactions involving antidepressants: Focus on desvenlafaxine. Neuropsychiatr. Dis. Treat. 14, 567–580. doi:10.2147/NDT.S157708
Luo, R., Partridge, J. G., and Vicini, S. (2013). Distinct roles of synaptic and extrasynaptic GABAAreceptors in striatal inhibition dynamics. Front. Neural Circuits 7, 186. doi:10.3389/fncir.2013.00186
Martin, R. S., Henningsen, R. A., Suen, A., Apparsundaram, S., Leung, B., Jia, Z., et al. (2008). Kinetic and thermodynamic assessment of binding of serotonin transporter inhibitors. J. Pharmacol. Exp. Ther. 327, 991–1000. doi:10.1124/jpet.108.142307
Mazza, M. G., Palladini, M., Poletti, S., and Benedetti, F. (2022). Post-COVID-19 depressive symptoms: Epidemiology, pathophysiology, and pharmacological treatment. CNS Drugs 21, 681–702. doi:10.1007/s40263-022-00931-3
McCarron, R. M., Shapiro, B., Rawles, J., and Luo, J. (2021). Depression. Ann. Intern. Med. 174, ITC65–ITC80. doi:10.7326/AITC202105180
Mi, W., Yang, F., Li, H., Xu, X., Li, L., Tan, Q., Wang, G., et al. (2022). Efficacy, Safety, and Tolerability of Ansofaxine (LY03005) Extended-Release Tablet for Major Depressive Disorder: A Randomized, Double-Blind, Placebo-Controlled, Dose-Finding, Phase 2 Clinical Trial. Int J Neuropsychopharmacol. 25 (3), 252–260. doi:10.1093/ijnp/pyab074
Mohammadkhanizadeh, A., and Nikbakht, F. (2021). Investigating the potential mechanisms of depression induced-by COVID-19 infection in patients. J. Clin. Neurosci. 91, 283–287. doi:10.1016/j.jocn.2021.07.023
Muller, N. (2019). COX-2 inhibitors, aspirin, and other potential anti-inflammatory treatments for psychiatric disorders. Front. Psychiatry 10, 375. doi:10.3389/fpsyt.2019.00375
Müller, N., Schwarz, M. J., Dehning, S., Douhe, A., Cerovecki, A., Goldstein-Müller, B., et al. (2006). The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: Results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol. Psychiatry 11, 680–684. doi:10.1038/sj.mp.4001805
Musselman, D. L., Lawson, D. H., Gumnick, J. F., Manatunga, A. K., Penna, S., Goodkin, S., et al. (2001). Paroxetine for the prevention of depression induced by high-dose interferon alfa. N. Engl. J. Med. 344, 961–966. doi:10.1056/NEJM200103293441303
Nakayama, T., Okimura, K., Shen, J., Yoshimura, T., Tamai, T. K., Shimada, A., et al. (2020). Seasonal changes in NRF2 antioxidant pathway regulates winter depression-like behavior. Proc. Natl. Acad. Sci. U. S. A. 117, 9594–9603. doi:10.1073/pnas.2000278117
Neurosciences Gate (2022). Neurosciences gate. Available at: https://www.gateneuro.com/Retrieved 2022-05-13.[Retrieved 2022 05 13].
Nishimune, A., Isaac, J. T., Molnar, E., Noel, J., Nash, S. R., Tagaya, M., et al. (1998). NSF binding to GluR2 regulates synaptic transmission. Neuron 21, 87–97. doi:10.1016/s0896-6273(00)80517-6
Paluchowska, M. H., Bugno, R., Bojarski, A. J., Charakchieva-Minol, S., Duszyńska, B., Tatarczyńska, E., et al. (2005). Novel, flexible, and conformationally defined analogs of gepirone: Synthesis and 5-ht1a, 5-ht2a, and D2 receptor activity. Bioorg. Med. Chem. 13, 1195–1200. doi:10.1016/j.bmc.2004.11.019
Powell, J. G., Garland, S., Preston, K., and Piszczatoski, C. (2020). Brexanolone (Zulresso): Finally, an FDA-approved treatment for postpartum depression. Ann. Pharmacother. 54, 157–163. doi:10.1177/1060028019873320
Robledinos-Anton, N., Fernandez-Gines, R., Manda, G., and Cuadrado, A. (2019). Activators and inhibitors of NRF2: A review of their potential for clinical development. Oxid. Med. Cell. Longev. 2019, 9372182. doi:10.1155/2019/9372182
Rush, A. J., Trivedi, M. H., Wisniewski, S. R., Nierenberg, A. A., Stewart, J. W., Warden, D., et al. (2006). Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am. J. Psychiatry 163, 1905–1917. doi:10.1176/ajp.2006.163.11.1905
Sethi, R., Gómez-Coronado, N., Walker, A. J., Robertson, O. D., Agustini, B., Berk, M., et al. (2019). Neurobiology and therapeutic potential of cyclooxygenase-2 (COX-2) inhibitors for inflammation in neuropsychiatric disorders. Front. Psychiatry 10, 605. doi:10.3389/fpsyt.2019.00605
Shaw, J. E., and Koleske, A. J. (2021). Functional interactions of ion channels with the actin cytoskeleton: Does coupling to dynamic actin regulate NMDA receptors? J. Physiol. 599, 431–441. doi:10.1113/JP278702
Simpson, C. A., Diaz-Arteche, C., Eliby, D., Schwartz, O. S., Simmons, J. G., and Cowan, C. S. M. (2021). The gut microbiota in anxiety and depression – a systemic review. Clin. Psych. Rev. 83, 101943. doi:10.1016/j.cpr.2020.101943
Sirisinha, S. (2016). The potential impact of gut microbiota on your health: Current status and future challenges. Asian pac. J. Allergy Immunol. 34, 249–264. doi:10.12932/AP0803
Skolnick, P. (1999). Antidepressants for the new millennium. Eur. J. Pharmacol. 375, 31–40. doi:10.1016/s0014-2999(99)00330-1
Ślifirski, G., Król, M., Kleps, J., Podsadni, P., Belka, M., Bączek, T., et al. (2019). Synthesis of new 5, 6, 7, 8-tetrahydropyrido[1, 2-c]pyrimidine derivatives with rigidized tryptamine moiety as potential SSRI and 5-HT1A receptor ligands. Eur. J. Med. Chem. 180, 383–397. doi:10.1016/j.ejmech.2019.07.027
Solek, P., Mytych, J., Sujkowska, E., Grzegorczyk, M., Jasiewicz, P., Sowa-Kucma, M., et al. (2021). Trade‐offs between male fertility reduction and selected growth factors or the klotho response in a lipopolysaccharide-dependent mouse model. Toxicol. Res. 38, 175–186. doi:10.1007/s43188-021-00098-x
Stachowicz, K. (2021). Application potential of modulation of cyclooxygenase-2 activity: A cognitive approach. Postepy Hig. i Med. Doswiadczalnej 75, 837–846. doi:10.2478/ahem-2021-0022
Stachowicz, K., Bobula, B., Kusek, M., Lenda, T., and Tokarski, K. (2021). Evidence for the interaction of COX-2 with mGluR5 in the regulation of EAAT1 and EAAT3 protein levels in the mouse hippocampus. The influence of oxidative stress mechanisms. Brain Res. 1771, 147660. doi:10.1016/j.brainres.2021.147660
Stachowicz, K. (2021a). Deciphering the mechanisms of regulation of an excitatory synapse via cyclooxygenase-2. A review. Biochem. Pharmacol. 192, 114729. doi:10.1016/j.bcp.2021.114729
Stachowicz, K. (2018). The role of DSCAM in the regulation of synaptic plasticity: Possible involvement in neuropsychiatric disorders. Acta Neurobiol. Exp. (Wars). 78, 210–219. doi:10.21307/ane-2018-019
Stamou, G., Garcia-Palacios, A., Woodford, B. J., Suso-Ribera, C., and Botella, C. (2021). The combination of cognitive-behavioural therapy with virtual reality for the treatment of postnatal depression in a brief intervention context: A single-case study trial. J. Healthc. Eng. 2021, 5514770. doi:10.1155/2021/5514770
Steffen, A., Nübel, J., Jacobi, F., Bätzing, J., and Holstiege, J. (2020). Mental and somatic comorbidity of depression: A comprehensive cross-sectional analysis of 202 diagnosis groups using German nationwide ambulatory claims data. BMC Psychiatry 20, 142. doi:10.1186/s12888-020-02546-8
Tabecka-Lonczynska, A., Mytych, J., Solek, P., Kulpa-Greszta, M., Jasiewicz, P., Sowa-Kucma, M., et al. (2021). IGF-1 as selected growth factor multi-response to antidepressant-like substances activity in C57BL/6J mouse testis model. Acta Histochem. 123, 151685. doi:10.1016/j.acthis.2021.151685
Turner, E. H. (2021). Esketamine for treatment-resistant depression: seven concerns about efficacy and FDA approval. Lancet Psychiatry 6 (12), 977–979. doi:10.1016/S2215-0366(19)30394-3
Udina, M., Navinés, R., Egmond, E., Oriolo, G., Langohr, K., Gimenez, D., et al. (2016). Glucocorticoid receptors, brain-derived neurotrophic factor, serotonin and dopamine neurotransmission are associated with interferon-induced depression. Oxf. Int. J. Neuropsychopharmacol. 19, pyv135–12. doi:10.1093/ijnp/pyv135
Uher, R., Payne, J. L., Pavlova, B., Pay, D. C., and Perlis, R. H. (2014). Major depressive disorder in DSM-5: Implications for clinical practice and research of changes from DSM-IV. Depress. Anxiety 31, 459–471. doi:10.1002/da.22217
Vasiliu, O. (2022). Investigational drugs for the treatment of depression (part 1): Monoaminergic, orexinergic, GABA-ergic, and antiinflammatory agents. Front. Pharmacol. 13, 884143. doi:10.3389/fphar.2022.884143
Vasiliu, O. (2022a). Investigational drugs for the treatment of depression (part 2): Glutamatergic, cholinergic, sestrin modulators and other agents. Front. Pharmacol. 13, 884155. doi:10.3389/fphar.2022.884155
Vázquez, G. H., Bahji, A., Undurraga, J., Tondo, L., and Baldessarini, R. J. (2021). Efficacy and tolerability of combination treatments for major depression: Antidepressants plus second-generation antipsychotics vs. esketamine vs. lithium. J. Psychopharmacol. 35, 890–900. doi:10.1177/02698811211013579
Wittchen, H. U., Jacob, F., Rehm, J., Gustavsson, A., Svensson, M., Jönsson, B., et al. (2011). The size and burden of mental disorders and other disorders of the brain in europe 2010. Eur. Neuropsychopharmacol. 21, 655–679. doi:10.1016/j.euroneuro.2011.07.018
Wong, G. T. H., Chang, R. C. C., and Law, A. C. K. (2013). A breach in the scaffold: The possible role of cytoskeleton dysfunction in the pathogenesis of major depression. Ageing Res. Rev. 12, 67–75. doi:10.1016/j.arr.2012.08.004
Woroń, J., Siwek, M., and Gorostowicz, A. (2019). Adverse effects of interactions between antidepressants and medications used in treatment of cardiovascular disorders. Psychiatr. Pol. 53, 977–995. doi:10.12740/PP/OnlineFirst/96286
Wróbel, M. Z., Chodkowski, A., Herold, F., Marciniak, M., Dawidowski, M., Siwek, A., et al. (2019). Synthesis and biological evaluation of new multi-target 3-(1H-indol-3-yl)pyrrolidine-2, 5-dione derivatives with potential antidepressant effect. Eur. J. Med. Chem. 183, 111736. doi:10.1016/j.ejmech.2019.111736
Keywords: depression, antidepressants, new target, glutamate receptors, DSCAM
Citation: Stachowicz K and Sowa-Kućma M (2022) The treatment of depression — searching for new ideas. Front. Pharmacol. 13:988648. doi: 10.3389/fphar.2022.988648
Received: 07 July 2022; Accepted: 20 September 2022;
Published: 07 October 2022.
Edited by:
Maria Grazia Morgese, University of Foggia, ItalyReviewed by:
Adrian Andrzej Chrobak, Jagiellonian University Medical College, PolandCopyright © 2022 Stachowicz and Sowa-Kućma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Magdalena Sowa-Kućma, bXNvd2FAdXIuZWR1LnBs
†ORCID: Magdalena Sowa-Kućma, orcid.org/0000-0001-5956-7229
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.