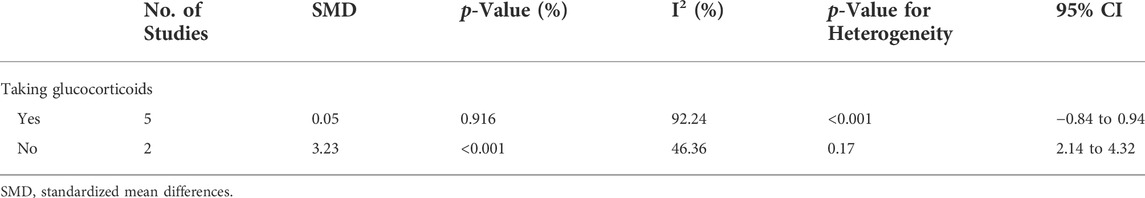- 1School of Basic Medical Sciences, Zhejiang Chinese Medical University, Hangzhou, China
- 2First Clinical School of Medicine, Nanjing Medical University, Nanjing, China
Objective: Th1 and Th2 cells and their associated cytokines function in the pathogenesis of systemic lupus erythematosus (SLE), but their exact roles are uncertain. We performed a meta-analysis to examine the relationship of these cells and cytokines with SLE.
Methods: Multiple databases were searched to identify publications that reported the percentages of Th1 and Th2 cells and their associated cytokines in SLE patients and healthy controls (HCs). Meta-analysis was performed using Stata MP version 16.
Results: SLE patients had a lower percentage of Th1 cells, a higher percentage of Th2 cells, and higher levels of Th1- and Th2-associated cytokines than HCs. SLE treatments normalized some but not all of these indicators. For studies in which the proportion of females was less than 94%, the percentage of Th2 cells and the level of IL-10 were higher in patients than HCs. SLE patients who had abnormal kidney function and were younger than 30 years old had a higher proportion of Th1 cells than HCs. SLE patients more than 30 years old had a higher level of IL-6 than HCs.
Conclusion: Medications appeared to restore the balance of Th1 cells and other disease indicators in patients with SLE. Gender and age affected the levels of Th1 and Th2 cells, and the abnormally elevated levels of Th2 cells appear to be more pronounced in older patients and males.
Systematic Review Registration: [https://www.crd.york.ac.uk/prospero/], identifier [CRD42022296540].
Highlights
1) Compared with healthy people, patients with systemic lupus erythematosus have lower levels of Th1 cells and higher levels of Th2 cells.
2) The main effect of conventional medications is to increase the level of Th1 cells in SLE patients.
3) Older patients and men appear to have more abnormally elevated Th2 cell levels.
Introduction
Systemic lupus erythematosus (SLE) is an inflammatory autoimmune disease characterized by the production of various autoantibodies and disruption of multiple organs and organ systems due to immunomodulatory dysfunction. The incidence of SLE is about 9 times higher in females than males (Yen and Singh, 2018). There is an incomplete understanding of the etiology of SLE, although evidence indicates that abnormal immune responses, B cell hyperactivation, and T cell abnormalities contribute to the pathogenesis (Catalina et al., 2020). For instance, a T cell autoimmune disorder leads to autoantibody production, and the production of several autoreactive T cells is an important precursor to B cell hyperactivation and the formation of immunoglobulins and immune complexes. Therefore, overactivation of T cells appears to have a major role in the pathogenesis of SLE (Katsuyama et al., 2018).
After antigen stimulation, immature CD4+T cells produce cytokines, T helper cells (Th1, Th2, and Th17) and regulatory T cells (Tregs) that have different immune functions (Zhu et al., 2010). The abnormal gene expression profiles of these cells are considered closely related to SLE (Wahren-Herlenius and Dörner, 2013; Talaat et al., 2015), and the balance of Th1/Th2 cells regulates the pathogenesis of SLE (Dolff et al., 2011). In particular, an imbalance of the pro-inflammatory effects of Th1 and the anti-inflammatory effects of Th2 is critical to immune dysfunction and infiltration of target organs by inflammatory cells (Moulton and Tsokos, 2011; Tsokos et al., 2016). Although many studies have examined the levels of Th1 and Th2 cells in patients with SLE, many of these results are discordant (Viallard et al., 1999; Amit et al., 2000; Li et al., 2002a; Oster et al., 2019). Thus, examination of changes in the levels of Th1 and Th2 cells during SLE is an active area of research (Matia-Garcia et al., 2021).
Different Th cells produce different cytokines. In particular, Th1 cells produce interferon (IFN)- γ, interleukin (IL)-2, and tumor necrosis factor (TNF)-α, whereas Th2 cells produce IL-4, IL-5, IL-6, and IL-10 (Muhammad Yusoff et al., 2020). Studies of the cytokine profiles of SLE patients indicated that dysregulated production of cytokines and soluble mediators were the primary factors responsible for increased inflammation (Lourenco and Cava, 2009). In particular, during SLE there is an increase in multiple cytokines, most of which have pro-inflammatory effects, although some of these cytokines have immunomodulatory and anti-inflammatory effects (Idborg and Oke, 2021). Because a variety of cytokines are associated with SLE disease activity, these cytokines were thought to be ideal biomarkers for defining SLE and as therapeutic targets for treatment of active SLE (La Cava, 2010; Rönnblom and Leonard, 2019; Aragón et al., 2020). Nevertheless, studies that examined changes of cytokines in the serum of SLE patients have reported contradictory results. For example, some studies reported increased levels of IL-4 (Davis et al., 2011), some studies reported no changes of IL-4 (Charles et al., 2010), and some studies reported reduced levels of IL-4 (Cavalcanti et al., 2017). Moreover, the effect of different factors on the regulation of cytokines during SLE is an active area of research. Although a few studies suggested that the effects of IL-4 may depend on genetic background, and that IL-2 function appears related to a sex-determined immune response, these conclusions remain uncertain (Moulton et al., 2012; Radmanesh et al., 2020).
In summary, there are many controversies in the research on the imbalance of Th1, Th2 and relevant cytokines in SLE patients. However, no evidence-based research has yet established the reasons for the inconsistent results of previous studies. Difference in the characteristics of patients (e.g., geographic region, gender, disease activity, medication use) may have contributed to the inconsistent results from previous studies. Therefore, this study aims to clarify the changes of Th1 cells, Th2 cells, and their related cytokines during SLE through meta-analysis, to better understand the pathogenesis of this disease, improve clinical diagnosis, and develop therapeutic strategies that have high efficacy and low toxicity.
Methods
This systematic review was registered under the number CRD42022296540 in the International Prospective Register of Systematic Reviews (PROSPERO).
Search strategy
Two researchers (M Zhang and J Zhang) independently searched the databases for publications from their dates of establishment to 30 September 2021. Search strategy was detailed in Supplementary file.
Study selection criteria
All included studies were case-control studies; examined a population as the study object; and assessed the percentages of Th1 cells and Th2 cells or the concentrations of their associated cytokines in peripheral blood. Studies were excluded if they were animal experiments; contained irrelevant research content, were literature reviews, or were conference abstracts not published in full; had missing data; examined cytokines secreted by non-CD4+ cells (such as IL-2, IL-17, and IL-6 secreted by Tregs); or did not measure the levels of cytokines in whole peripheral blood.
Data extraction and transformation
Two independent researchers (S Xiang and S Qian) extracted and cross-verified data necessary for analysis from eligible literature. Disagreements were resolved through discussion and consultation with a third researcher (Y Wang). The contents included: 1) the first author, the year of publication, the characteristics of subjects and country in which the study was performed; 2) The characteristics of included studies (number of patients, proportion of women, average age, average duration of disease, therapeutic drugs); 3) experimental methods (diagnostic criteria, determination methods); 4) Th1, Th2 and cytokine levels secreted by SLE patients and HCs.
All original data, including graphs and plots, were extracted for use in the meta-analysis. Because some publications only provided relevant data as graphs, GetData Graph Digitizer version 2.25 (http://getdata-graph-digitizer.com/) was used to extract these data (Liao et al., 2015; Tang et al., 2015; Shen et al., 2021). When studies provided medians and ranges (or interquartile ranges) instead of means and standard deviations, data conversion was performed using the method proposed by Xiang Wan (Wan et al., 2014). The Newcastle-Ottawa Quality (NOS) (Stang, 2010) was used to assess the quality of the included studies.
Statistical analysis
Stata version 16.0 was used to perform statistical analyses. When data were continuous variables and the measurement methods were similar, weighted mean differences (WMDs) were used as the effect scale. When the studies evaluated similar results using different measurement methods, or there was a large difference in the means or standard deviations, then standardized mean differences (SMDs) were used as the effect scale (Bakbergenuly et al., 2020). I2 was used to evaluate heterogeneity, and values of 25, 50, and 75% were used for classification of low, moderate, and high heterogeneity, respectively (Higgins et al., 2003). The meta-analysis was performed using a random-effects model that was weighted according to the sample size of studies (Wu et al., 2021). A difference with a p value below 0.05 was considered statistically significant. When the heterogeneity was high (I2 > 75%), subgroup analysis or sensitivity analysis (deleting one publication at a time) was used to assess the source of heterogeneity. Publication bias was evaluated using the Egger test (Hayashino et al., 2005), Begg test (Jiang et al., 2019), and a funnel plot.
Results
Study selection and characteristics
We retrieved 5,463 potentially eligible studies from the databases, and 4,601 studies remained after removal of duplicates. Screening of the titles and abstracts led to removal of 4,076 studies because the research content was considered irrelevant. Reading the full text of the remaining studies led to removal of 447 studies because they did not meet the eligibility criteria. Review of the remaining studies led to removal of 1 study that used different diagnostic criteria for SLE, 1 study that reported Th1 and Th2 cell levels in absolute numbers (cells/μL), and 38 studies that had research goals considered irrelevant to our meta-analysis. Further analysis of the 38 remaining studies indicated that 6 studies were ineligible because of insufficient quality and 6 studies were ineligible because of incomplete data. We finally included 26 studies in the meta-analysis (Supplementary Figure S1, Table 1). All 26 studies reported samples that were obtained from human peripheral blood, and used IFN-γ as a marker for Th1 cells and IL-4 as a marker for Th2 cells. The NOS score of each study was between 6 and 9 (Supplementary Table S1).
Changes in the percentage of Th1 and Th2 cells in SLE patients
Th1 cells
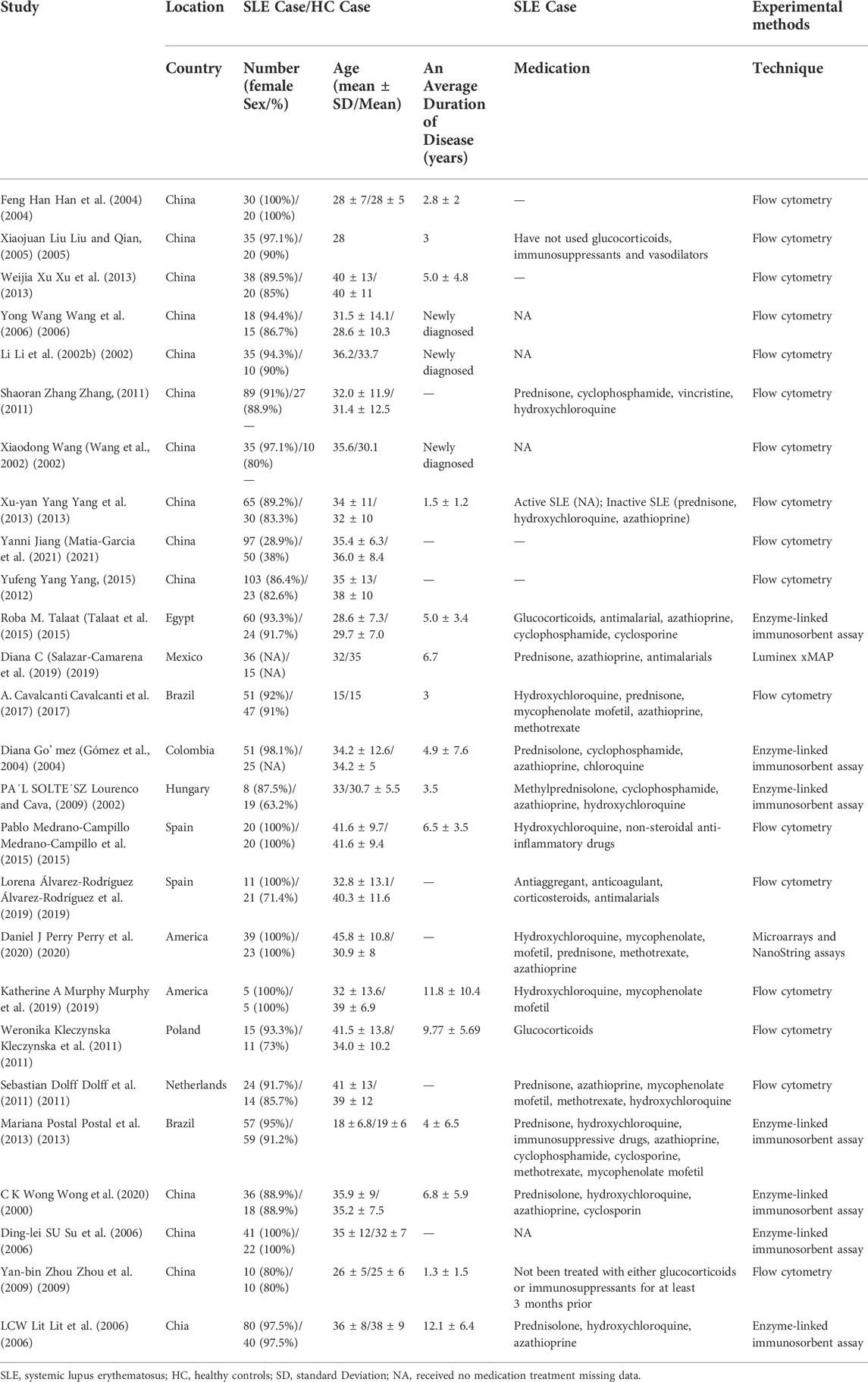
We found no significant difference in the percentage of Th1 cells in patients and HCs (WMD = −1.16; 95% CI = −4.06, 1.75; p = 0.43; n = 13; Figure 1), although there was high heterogeneity in these results (I2 = 97.67%).
FIGURE 1. Forest plot of the percentage change of Th1 cells in SLE patients compared with HCs. The overall results (bottom) show the meta-analysis of 13 studies, and the other four results show meta-analyses of different subgroups according to medication use. SLE, systemic lupus erythematosus; HCs, healthy controls.
We performed a subgroup analysis to assess the effect of medication use on Th1 cells. Studies that did not report medication use were categorized as an unrecorded medication group, and patients in other studies who were un-medicated or medicated were categorized into separate groups. The results showed that the percentage of Th1 cells was lower in unmedicated patients than HCs (WMD = −3.97; 95% CI = −4.76, −3.17; p < 0.001; n = 4; Figure 1). This implies that the use of medications by SLE patients affected the levels of their Th1 cells.
In total, 45 patients were unmedicated in the Xuyan Yang et al. study (Yang et al., 2013), and this accounted for 69% of their patients; their results showed that the percentage of Th1 cells was lower in patients than HCs (WMD = −1.38, 95% CI = −2.41, −0.35). A total of 41% of patients in the Yufeng Yang et al. study (Yang, 2015) were unmedicated; their results showed the level of Th1 cells was higher in patients than HCs (WMD = 1.55, 95% CI = 1.22, 1.88). Only 33% of patients in the Weronika Kleczynska et al. study (Kleczynska et al., 2011) were unmedicated; their results showed a trend for a higher level of Th1 cells in patients than HCs (WMD = 8.3, 95% CI = −0.75, 17.35). These results also suggest that SLE patients taking medications had a higher percentage of Th1 cells than HCs.
In addition, none of the patients in the Katherine A. Murphy et al. study (Murphy et al., 2019) used glucocorticoids (GCs), and their patients and HCs had similar percentages of Th1 cells. In contrast, all patients in the Sebastian Dolff et al. study (Dolff et al., 2011) used low-dose GCs, and their patients and HCs had very different percentages of Th1 cells. We speculate that use of low-dose GCs by SLE patients may alter the level of Th1 cells.
Th2 cells
The meta-analysis showed there was a higher percentage of Th2 cells in patients than HCs (SMD = 0.62; 95% CI = 0.03, 1.20; p = 0.04; n = 8; Figure 2). Sensitivity analysis that excluded the Yong Wang study (Wang et al., 2006) and the Yanni Jiang study (Matia-Garcia et al., 2021) reduced the heterogeneity of these results (I2 = 2.22%; Supplementary Figure S2), and the percentage of Th2 cells remained higher in patients than HCs (SMD = 0.29; 95% CI = 0.03, 0.56; p = 0.03; n = 6).
FIGURE 2. Forest plot of the percentage change of Th2 cells in SLE patients compared with HCs. The overall results (bottom) show the meta-analysis of 8 studies, and the other two results show meta-analyses of subgroups according to the percentage of females. SLE, systemic lupus erythematosus; HCs, healthy controls.
Because the incidence of SLE is higher in women than men, we performed a subgroup analysis to analyze the effect of the proportion of women in each study on the outcome. When the proportion of women was at least 94%, SLE patients had a greater change in the percentage of Th2 cells than HCs (p > 0.05, Figure 2); however, even when the proportion of women was less than 94%, SLE patients had a significantly greater level of Th2 cells than HCs (SMD = 1.00; 95% CI = 0.18, 1.82; p = 0.02; n = 2; Figure 2). Subgroup analysis that excluded the Yong Wang study (Wang et al., 2006) reduced the heterogeneity of the studies in which the proportion of women was greater than 94% (Supplementary Figure S3), but the overall results did not change. Thus, for studies in which the proportion of women was less than 94%, the significant difference in the proportions of men and women may explain the high heterogeneity of the results.
The two studies in which the proportion of women was less than 94% were the Yufeng Yang et al. study (Yang, 2015) (86.4% women) and the Yanni Jiang et al. study (Matia-Garcia et al., 2021) (28.9% women), and both of these studies reported a higher percentage of Th2 cells in patients than HCs (Figure 2). In addition, a subgroup analysis indicated that medicated patients had a higher percentage of Th2 cells than HCs (SMD = 0.39; 95%CI = 0.08, 0.71; p = 0.01; n = 4; Supplementary Figure S4).
Disease activity and levels of Th1 and Th2 cells
We then compared the percentage of Th1 and Th2 cells in patients with active disease and inactive disease. The meta-analysis indicated disease activity had no significant relationship with the levels of Th1 or Th2 cells (p > 0.05, Supplementary Figures S5, S6).
Abnormal kidney function and levels of Th1 and Th2 cells
The percentage of Th1 cells and Th2 cells were not obviously different in patients with abnormal kidney function (lupus nephritis or clinical presentation with proteinuria) and patients with normal kidney function (p > 0.05; Figure 3 and Supplementary Figure S7).
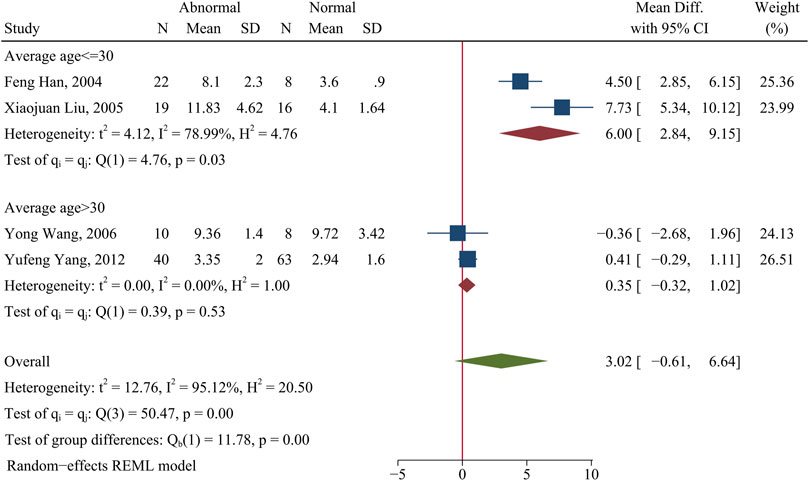
FIGURE 3. Forest plot of the percentage change of Th1 cells in SLE patients with abnormal kidney function compared with normal kidney function. The overall results (bottom) show the meta-analysis of 4 studies, and the other two results show meta-analyses of subgroups according to patient age. Abnormal: SLE patients with abnormal kidney function. Normal: SLE patients with normal kidney function.
However, subgroup analysis indicated that age significantly affected the percentage of Th1 cells in patients with abnormal kidney function. In particular, patients who were 30 years old or less had a higher level of Th1 cells than HCs (WMD = 6.00; 95%CI = 2.84, 9.15; p = 0.0002; n = 2; Figure 3), but there was no significant difference in patients more than 30 years old (p > 0.05; Figure 3).
Changes of the Th1/Th2 ratio in patients with SLE
There was no significant difference in the Th1/Th2 ratio of patients and HCs (p > 0.05; Figure 4). A subgroup analysis that assessed the effect of medication use showed that medicated patients had a higher Th1/Th2 ratio than HCs (WMD = 0.86; 95% CI = 0.42, 1.29; p < 0.001; n = 2; Figure 4), but there was no such relationship for unmedicated patients.
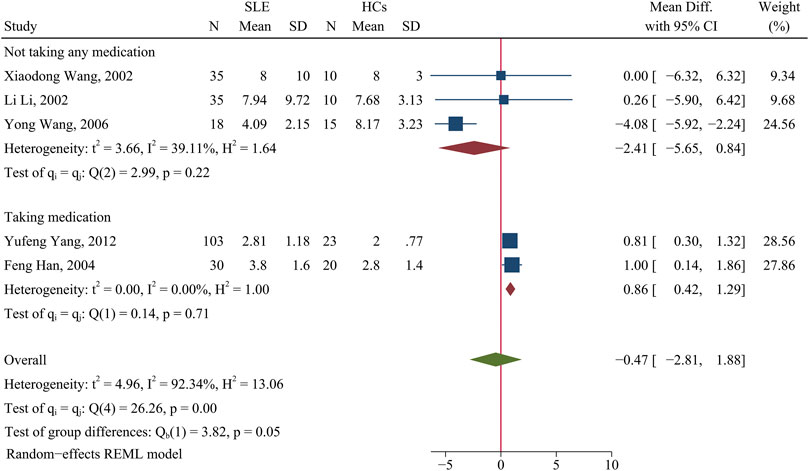
FIGURE 4. Forest plot of the ratio change of Th1/Th2 cells in SLE patients compared with HCs. The overall results (bottom) show the meta-analysis of 5 studies, and the two other results show meta-analyses of subgroups according to medication use. SLE, systemic lupus erythematosus; HCs, healthy controls.
Variation of Th1 and Th2 cytokines in patients with SLE
Th1 cytokines
Patients and HCs had no significant difference in the level of IFN-γ (SMD = 0.63; 95% CI = −1.81, 3.06; p = 0.61; n = 10; Figure 5). Subgroup analysis that examined the effect of medication use showed that unmedicated patients had a higher level of IFN-γ than HCs (SMD = 3.92; 95% CI = 1.01, 6.82; p = 0.008; n = 3; Figure 5), although there was no such difference in a comparison of medicated patients and HCs. This result suggests that unmedicated SLE patients have a higher level of IFN-γ than HCs, but medications normalize the level of IFN-γ in these patients.
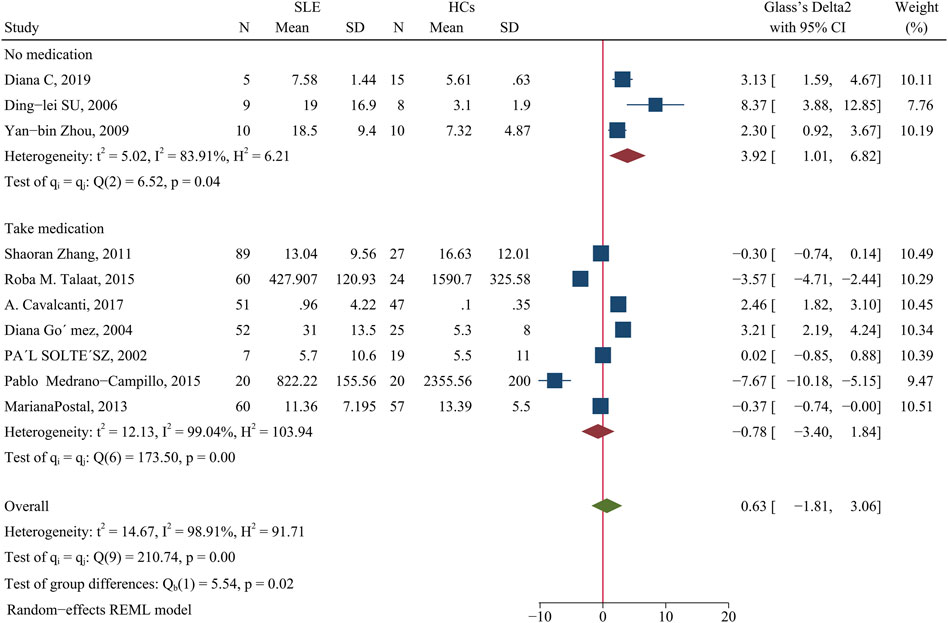
FIGURE 5. Forest plot of the concentration change of IFN-γ in SLE patients compared with HCs. The overall results (bottom) show the meta-analysis of 10 studies, and the other two results show meta-analyses of subgroups according to medication use. SLE, systemic lupus erythematosus; HCs, healthy controls.
The concentration of TNF-α was higher in patients than HCs (SMD = 0.32; 95% CI = 0.09, 0.56; P = 0.01; n = 4; Figure 6)
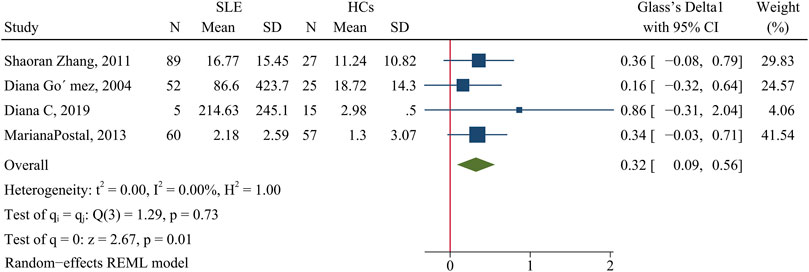
FIGURE 6. Forest plot of the concentration change of TNF-α in SLE patients compared with HCs. SLE, systemic lupus erythematosus; HCs, healthy controls.
A meta-analysis indicated no significant difference in the IL-2 concentration of patients and HCs (SMD = 0.47; 95% CI = −0.89, 1.84; p = 0.49; n = 4; Figure 7). However, subgroup analysis showed that the IL-2 level was lower in patients taking GCs than HCs (SMD = −0.53; 95% CI = −0.83, −0.23; p = 0.0005; n = 2; Figure 7), but higher in patients not using GCs than HCs (SMD = 1.63; 95% CI = 0.07, 3.19; p = 0.04; n = 2; Figure 7). Notably, patients in the Diana et al. study (Salazar-Camarena et al., 2019) did not use any medications, and those in the Pablo Medrano-Campillo et al. study (Medrano-Campillo et al., 2015) received hydroxychloroquine, diclofenac, ibuprofen, or other medications, and the heterogeneity of these studies could not be eliminated.
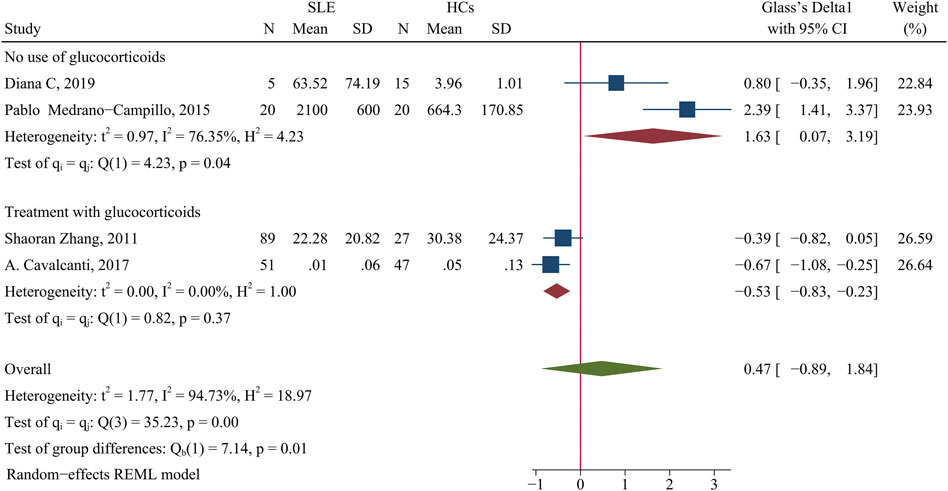
FIGURE 7. Forest plot of the concentration change of IL-2 in SLE patients compared with HCs. The overall results (bottom) show the meta-analysis of 4 studies, and the other two results show meta-analyses of two subgroups according to glucocorticoid use. SLE, systemic lupus erythematosus; HCs, healthy controls.
Th2 cytokines
Patients had a higher level of IL-10 than HCs (SMD = 4.2; 95% CI = 1.44, 6.96; p = 0.003; n = 10; Figure 8). Subgroup analysis showed that this difference was present in studies in which more than 50% of the patients had active disease (SMD = 2.99; 95% CI = 1.36, 4.63; p < 0.001; n = 7; Figure 8), but not in studies in which fewer than 50% of the patients had active disease.
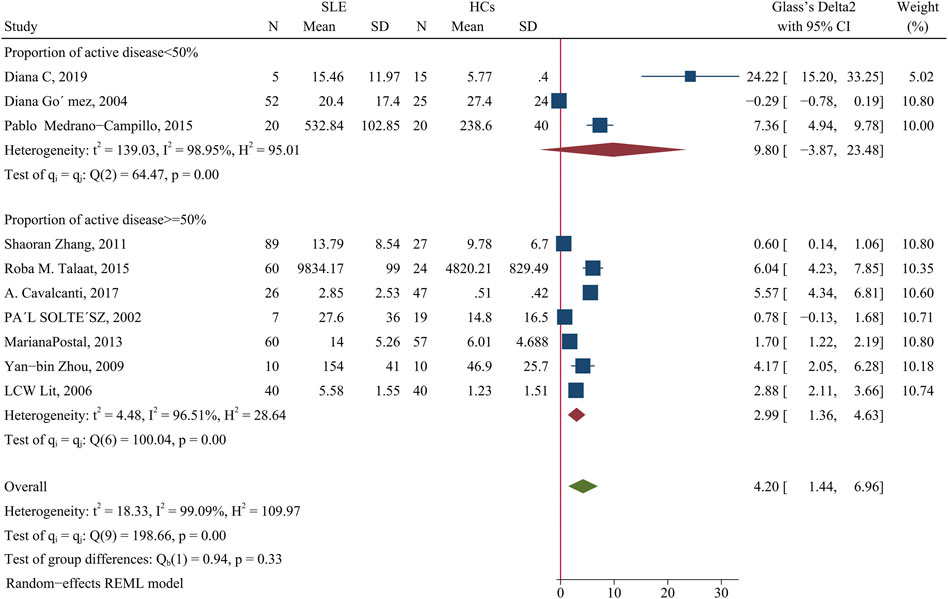
FIGURE 8. Forest plot of the concentration change of IL-10 in SLE patients compared with HCs. The overall results (bottom) show the meta-analysis of 10 studies, and the other two results show meta-analyses of subgroups according to the percentage of patients with active disease. SLE, systemic lupus erythematosus; HCs, healthy controls.
We performed another subgroup analysis to compare studies that had more than 94% women with those that had 94% or fewer women, with exclusion of the Diana C et al. study (Salazar-Camarena et al., 2019) because it did not provide the requisite data. The results showed that when the proportion of female patients was more than 94%, the IL-10 level was not significantly different in patients and HCs; however, this difference was significant when the proportion of females was less than 94% (SMD = 3.34; 95% CI = 1.01, 5.67; p = 0.005; Table 2).
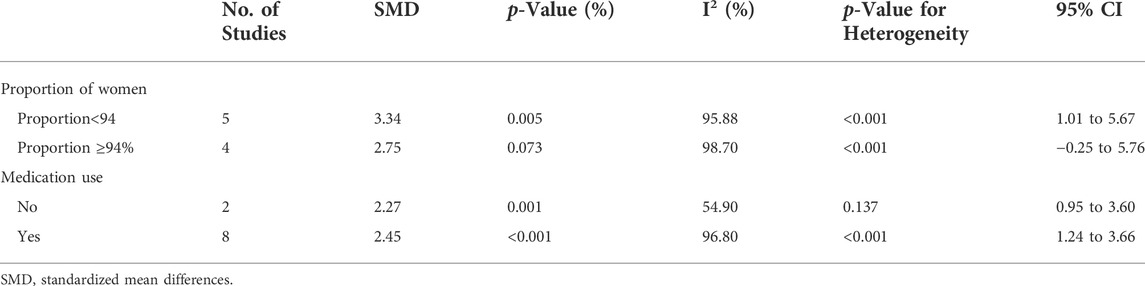
TABLE 2. Subgroup analysis in IL-10 according to the proportion of female SLE patients in total patients and the use of medication.
The level of IL-6 was higher in patients than HCs (SMD = 1.17; 95% CI = 0.32, 2.02; p = 0.007; n = 6; Figure 9). A subgroup analysis showed that when the average patient age was 30 years-old or more, the concentration of IL-6 was higher in patients than HCs (SMD = 1.32; 95% CI = 0.33, 2.30; p = 0.009; n = 3; Figure 9), but there was no significant difference when the average patient age was less than 30 years-old (p > 0.05).
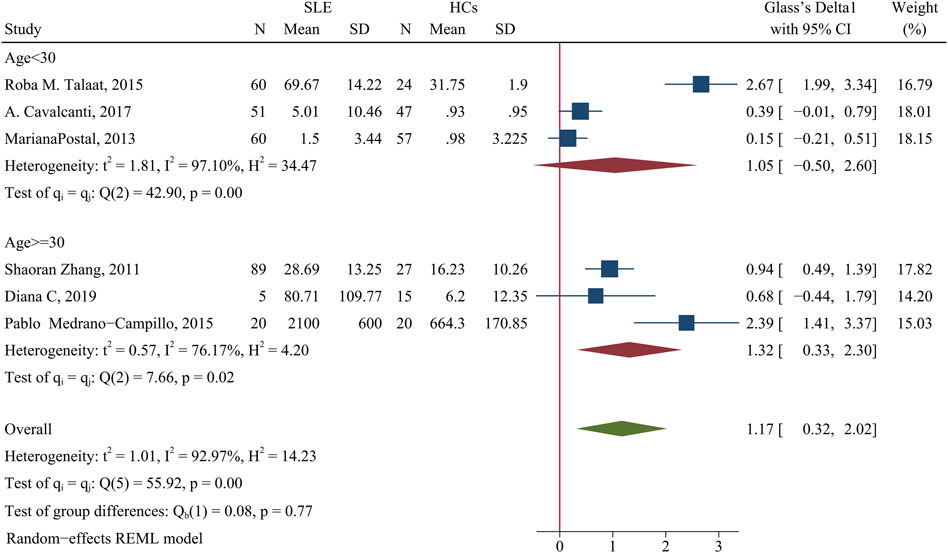
FIGURE 9. Forest plot of the concentration change of IL-6 in SLE patients compared with HCs. The overall results (bottom) show the meta-analysis of 6 studies, and the other two results show meta-analyses of subgroups according to patient age. SLE, systemic lupus erythematosus; HCs, healthy controls.
Sensitivity analysis of the effect of age indicated the heterogeneity between these two groups was eliminated after excluding Roba M. Talaat study (Talaat et al., 2015) and the Pablo Medrano-Campillo study (Medrano-Campillo et al., 2015), but the results did not change (Supplementary Figure S8). The use of GCs also affected the concentration of IL-6. When more than 90% of patients used GCs, the concentration of IL-6 cells was higher in patients than HCs (SMD = 1.78; 95% CI = 0.09, 3.48; p = 0.039; n = 2; Table 3). This suggests that GC use may increase the concentration of IL-6. In addition, the proportion of female patients in a study had no effect on IL-6 concentration (Table 3).
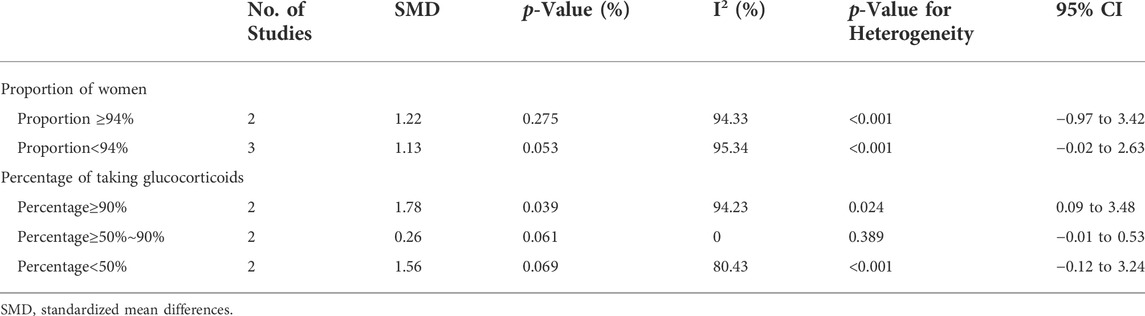
TABLE 3. Subgroup analysis in IL-6 according to the proportion of female SLE patients in total patients and the use of glucocorticoid.
Patients had a higher IL-4 concentration than HCs (SMD = 2.92; 95% CI = 0.37, 5.46; p = 0.02; n = 7; Figure 10). We performed a subgroup analysis of studies in which the proportion of females was 90% or more; more than 80% and less than 90%; and 80% or less. The results showed no difference of patients and HCs in studies that had 90% or more females (p > 0.05). However, when the proportion of women 80–90%, the concentration of IL-4 was higher in patients than HCs (SMD = 2.59; 95% CI = 0.01, 5.16; p = 0.049; Figure 10) and when the proportion of females was 80% or less, the concentration of IL-4 was even greater in patients than HCs (SMD = 8.03; 95% CI = 6.02, 10.05; p < 0. 001; Figure 10). In addition, a subgroup analysis showed no significant difference in the IL-4 level between patients using a GC and HCs (p > 0.05; Table 4).
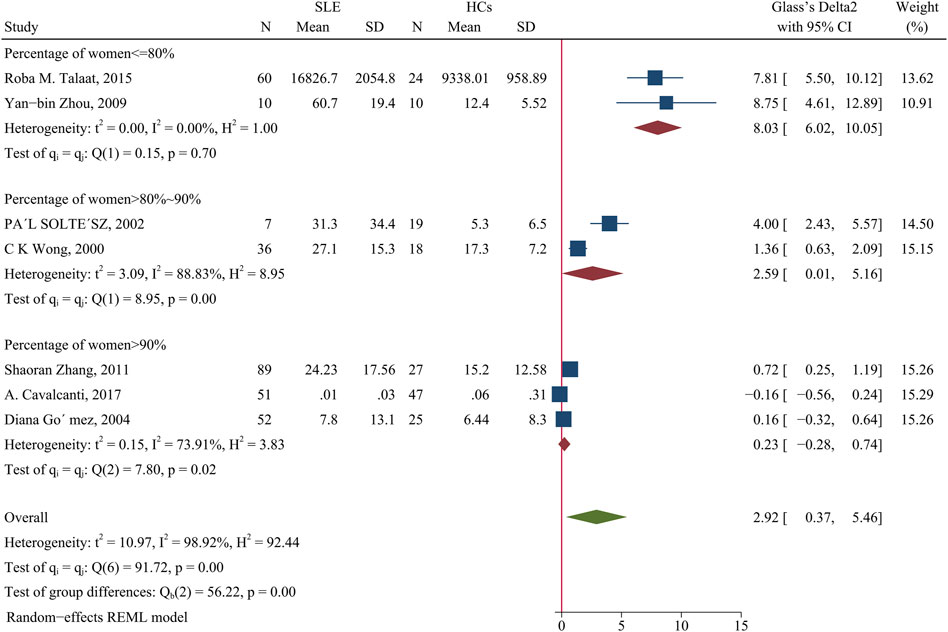
FIGURE 10. Forest plot of the concentration change of IL-4 in SLE patients compared with HCs. The overall results (bottom) show the meta-analysis of 7 studies, and the other three results show meta-analyses of subgroups according to the percentage of females. SLE, systemic lupus erythematosus; HCs, healthy controls.
Analysis of publication bias
We assessed the risk of publication bias using the Egger test, Begg test, and a funnel plot (Supplementary Figure S9 and Supplementary Table S2). The results showed no evidence of publication bias in most studies, suggesting that the conclusions of this meta-analysis were relatively robust. However, there was some evidence of publication bias regarding the results for IL-10 and IL-4.
Discussion
The results of the present meta-analysis showed that SLE patients had significantly lower percentage of Th1 cells, a higher percentage of Th2 cells and higher levels of Th1- and Th2-associated cytokines (IFN-γ, TNF-α, IL-2, IL-10, IL-6 and IL-4) than HCs. Moreover, disease activity appeared unrelated to the proportion of Th1 and Th2 cells.
SLE is characterized by polyclonal activation of B cells, which leads to the production of autoantibodies and pathological damage. Several cytokines secreted by Th2 cells promote the production of B cell antibodies (O'Brien et al., 2021). There is evidence that a negative feedback between Th1-type and Th2-type immune responses regulates normal immune balance. In SLE patients, an influx of Th2 cells inhibits the release of Th1 cells, thus supporting the hypothesis that the immune disorder of “Th2 dominance” is responsible for SLE (Ko et al., 2022).
However, we found a significantly higher proportion of Th1 cells in patients with abnormal kidney function. Previous studies showed that up-regulation of lipocalin-2 in SLE can promote the in vivo and in vitro differentiation of Th1 cells via the IL-12/STAT4 signalling pathway, thereby causing an immune imbalance and leading to lupus nephritis (LN) (Chen et al., 2020). Our study also showed that the level of Th2 cells was not greater in patients with abnormal kidney function, similar to previous studies (Uhm et al., 2003), and indicating an imbalanced Th1/Th2 ratio in LN.
Studies have shown that medications have significant effects on the level of Th1, Th2 and their associated cytokine levels in patients with systemic lupus erythematosus.
The different results regarding Th1 cells in previous studies of SLE patients may be attributed to whether or not these patients were receiving treatment, especially by GCs. Previous studies showed that GCs have a strong inhibitory effect on Th1 cells by suppressing the production and signal transduction mediated by IL-12 and IFN- γ and that GCs had a weaker effect on Th2 cells, and had little effect on IL-4-induced STAT6 phosphorylation (Franchimont et al., 2000; Taves and Ashwell, 2021). However, our meta-analysis results showed that untreated SLE patients had a lower proportion of Th1 cells relative to treated patients. This may be because Th1 cells mainly target exogenous antigens, such as infected and deteriorating cells (Hall, 2015), and cellular immunity mediated by Th1 cells does not play a major role in SLE because it is an antibody-mediated immune disease. We found that the percentage of Th1 cells was not significantly different in medicated patients and HCs. This may be because the medication reduced the autoimmune response of SLE by increasing the percentage of Th1 cells to achieve a relative balance in Th1 and Th2 levels.
Drug therapy can lead to the suppression of Th1-mediated production of IFN-γ. For example, a previous study showed that IFN-γ-responsive genes were significantly upregulated in SLE patients with untreated active disease relative to HCs, but there was no significant difference in a comparison of HCs with SLE patients who had stable disease due to treatment with GCs, hydroxychloroquine, and/or immunosuppressants (Dufour et al., 2018). However, the use of different dose of GC can lead to different results. For example, Talaat et al. (Talaat et al., 2015) studied 60 SLE patients, including 58 who received a GC dose higher than 10 mg per day, and found that only 32 had active disease. The 2019 European Rheumatic Alliance guidelines for the management of SLE recommend prednisone at a dose lower than 7.5 mg/day when SLE is in remission or low activity (Fanouriakis et al., 2019). Therefore, we hypothesize that the lower level of IFN-γ in medicated patients that were reported in some studies may be due to excessive use of GCs.
Moreover, our results showed the level of IL-2 was higher in SLE patients than HCs, and the use of GC was able to reduce the level of this cytokine. There is evidence that low-dose IL-2 therapy can increase the level of Tregs, and because the lack of Tregs may contribute to the pathogenesis of SLE, this may be an effective treatment for SLE (Tahvildari and Dana, 2019). However, even though IL-2 supplementation significantly increases Treg cells in SLE patients, it does not change the total CD4+ cells (Robinson and Thomas, 2021). This may be because SLE patients have Tregs that secrete less IL-2 and Th1 cells that secrete more IL-2. Although low-dose recombinant human IL-2 can selectively enhance Tregs (rather than Th1 cells) and significantly reduce disease activity (Tahvildari and Dana, 2019), it is unknown whether this treatment also reduces the secretion of IL-2 by Th1 cells. Our results showed that GC treatment of SLE patients effectively reduced the level of IL-2 secreted by Th1 cells. Thus, when considering the use of low-dose IL-2 treatment for SLE patients, it may be necessary to continue GC treatment.
Another interesting finding of this study is that imbalances in the levels of Th1, Th2 and their associated inflammatory cytokines may be associated with disease characteristics in male SLE patients.
The male SLE patients had a higher level of Th2 cells, IL-10 and IL-4 than female patients. The increased IL-10 level is associated with reduced kidney function and increased risk of cardiovascular disease (Yilmaz et al., 2014). IL-4 producing cells contribute to kidney pathology in SLE patients, and that animals lacking IL-4 had fewer pathological changes in the kidneys and lymph nodes (Reséndiz‐Mora et al., 2021). This may explain why men with SLE have more severe cardiovascular and kidney disease and are more likely to develop kidney failure (Parikh et al., 2020). However, the secretion of IL-10 is affected by many factors, including gender and disease activity, and this may have contributed to the high heterogeneity among the studies we analyzed.
Changes in the Th1/Th2 ratio corroborated the above results regarding the imbalance between Th1 and Th2 cells in SLE patients and the potential underlying causes. For example, medicated SLE patients had a higher Th1/Th2 ratio than HCs, possibly due to an increase in the proportion of Th1 cells. However, there was no significant difference in the Th1Th2 ratio between untreated patients and HCs. We suggest this result occurred because the sex ratio of a study affected the reported level of Th2 cells. The proportion of women was greater than 94% in the untreated subgroup. Here, we found no significant change in the proportion of Th2 cells, although the portion of Th1 cells decreased. Therefore, a change in Th1 cells may not significantly contribute to the imbalanced Th1/Th2 ratio in SLE patients. Interestingly, GCs appeared to primarily affect changes in the number of Th1 cells, not Th2 cells.
Furthermore, we found the effect of age on Th1 and IL-6 levels, which may be associated with the development of lupus nephropathy. The result showed that SLE patients had a higher level of IL-6 than in HCs, although this difference was mainly in studies in which the average patient age was more than 30 years old. Interestingly, Th1 cells were more abundant in patients with abnormal kidney function when the mean age was less than 30 years old, but this difference was not significant in patients when the mean age was greater than 30 years old. Recent research showed that Th1 cytokines functioned in the development of diffuse proliferative LN (Sigdel et al., 2016; Fava et al., 2020) and that Th2 cytokines functioned in the development of membranous LN (Masutani et al., 2004). However, analysis of the antigenic site of IL-6 in the glomerular mesangial cells of patients with LN (Malide et al., 1995) indicated that IL-6 may be closely related to the pathogenesis of membranous LN. Our subgroup analysis indicated that young patients with SLE were less likely to develop end-stage renal disease (ESRD). Another epidemiological investigation found that most children with LN had unchanged or normal kidney function, and that ESRD was rare (Vazzana et al., 2021). In addition, the probability of death from active SLE was much lower in children than adult men (Brunner et al., 2008). These results indicated that the Th1Th/Th2 balance was a critical determinant for the histopathology of LN (Miyake et al., 2011), and the type of LN was related to this balance.
Finally, we also found the contradiction that there was no significant difference in the level of Th1 cells between SLE patients and HCs, even though the patients had a significantly higher level of IFN-γ (Zhong et al., 2017). This may be because patients have increased activation of T cells relative to HCs (Araújo et al., 2016). When the concentration of Th2-associated cytokines was greater than that of Th1-associated cytokines, this may reflect a decreased percentage of Th1 cells and an increased percentage of Th2 cells.
This study has some limitations. All included studies had a retrospective case-control design, indicating the possibility of selection bias and publication bias. In particular, based on the Egger test and Begg test, we identified a statistically significant publication bias in studies that examined the levels of IL-10 and IL-4. Moreover, subgroup analysis cannot completely eliminate the heterogeneity among studies because many factors can affect the secretion of these cytokines. This topic therefore requires further study.
In summary, our results showed that Th2 cells play a crucial role in the pathogenesis of SLE. Notably, we confirmed that SLE patients have abnormal increases of multiple cytokines (IFN-γ, TNF-α, IL-2, IL-10, IL-6, and IL-4) that are associated with Th1 and Th2 cells. Medical therapies, especially GCs, seem to mainly affect Th1 cells. However, excessive use of GCs can apparently lead to an imbalance in the level of Th1 cells. Moreover, there was a higher proportion of Th2 cells in male than female SLE patients, and this abnormality may contribute to the more obvious clinical features, such as higher disease severity and worse long-term prognosis, in male patients. IL-10 and IL-4 may be closely associated with the higher incidence of kidney injury and poor prognosis in males. The higher levels of Th1 cells and IL-6 in different age groups may be associated with the development of LN.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
XD: Term, Conceptualization, Writing—Review and Editing, Supervision. SX: Methodology, Software, Formal analysis, Methodology, Data Curation, Writing—Original Draft. JZ: Methodology, Formal analysis, Investigation, Data Curation. MZ: Formal analysis, Investigation, Data Curation. SQ: Validation, Formal analysis, Visualization. RW: Investigation, Data Curation. YW: Writing—Original Draft. YX: Visualization.
Funding
This research was funded by the National Natural Science Foundation of China (Nos. 81973778 and 81774179). The role of the sponsor: In study design and the decision to submit the article for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.988512/full#supplementary-material
Abbreviations
ESRD, End-stage renal disease; GCs, Glucocorticoids; HCs, Healthy controls; INF, Interferon; IL, Interleukin; LN, Lupus nephritis; NOS, Newcastle-Ottawa scale; PROSPERO, Prospective Register of Systematic Reviews; SMDs, Standardized mean differences; SLE, Systemic lupus erythematosus; TNF, Tumor necrosis factor; WMDs, Weighted mean differences.
References
Álvarez-Rodríguez, L., Martínez-Taboada, V., Calvo-Alén, J., Beares, J., Villa, I., López-Hoyos, I., et al. (2019). Altered Th17/Treg ratio in peripheral blood of systemic lupus erythematosus but not primary antiphospholipid syndrome. Front. Immunol. 10, 391. doi:10.3389/fimmu.2019.00391
Amit, M., Mor, A., Weissgarten, J., Rosenberg, R., Ramot, Y., Wysenbeek, A. J., et al. (2000). Inactive systemic lupus erythematosus id associated with a normal stimulated Th(1)/Th(2) cytokine secretory pattern. Cytokine 12, 1405–1408. doi:10.1006/cyto.2000.0724
Aragón, C. C., Tafúr, R. A., Suárez-Avellaneda, A., Martínez, M. T., Salas, A. L., and Tobón, G. J. (2020). Urinary biomarkers in lupus nephritis. J. Transl. Autoimmun. 3, 100042. doi:10.1016/j.jtauto.2020.100042
Araújo, J. A., Mesquita, D., de Melo Cruvinel, W., Salmazi, K. I., Kallás, E. G., and Andrade, L. E. (2016). Th17 cells and CD4(+) multifunctional T cells in patients with systemic lupus erythematosus. Rev. Bras. Reumatol. Engl. Ed. 56, 28–36. doi:10.1016/j.rbre.2015.10.003
Bakbergenuly, I., Hoaglin, D. C., and Kulinskaya, E. (2020). Estimation in meta-analyses of mean difference and standardized mean difference. Stat. Med. 39, 171–191. doi:10.1002/sim.8422
Brunner, H. I., Gladman, D. D., Ibañez, D., Urowitz, M. D., and Silverman, E. D. (2008). Difference in disease features between childhood-onset and adult-onset systemic lupus erythematosus. Arthritis Rheum. 58, 556–562. doi:10.1002/art.23204
Catalina, M. D., Owen, K. A., Labonte, A. C., Grammer, A. C., and Lipsky, P. E. (2020). The pathogenesis of systemic lupus erythematosus: Harnessing big data to understand the molecular basis of lupus. J. Autoimmun. 110, 102359. doi:10.1016/j.jaut.2019.102359
Cavalcanti, A., Santos, R., Mesquita, Z., Duarte, A. L., and Lucena-Silva, N. (2017). Cytokine profile in childhood-onset systemic lupus erythematosus: A cross-sectional and longitudinal study. Braz J. Med. Biol. Res. 50, e5738. doi:10.1590/1414-431x20175738
Charles, N., Hardwick, D., Daugas, E., Illei, G. G., and Rivera, J. (2010). Basophils and the T helper 2 environment can promote the development of lupus nephritis. Nat. Med. 16, 701–707. doi:10.1038/nm.2159
Chen, W., Li, W., Zhang, Z., Tang, X., Wu, S., Yao, G., et al. (2020). Lipocalin-2 exacerbates lupus nephritis by promoting Th1 cell differentiation. J. Am. Soc. Nephrol. 31, 2263–2277. doi:10.1681/asn.2019090937
Davis, L. S., Hutcheson, J., and Mohan, C. (2011). The role of cytokines in the pathogenesis and treatment of systemic lupus erythematosus. J. Interferon Cytokine Res. 31, 781–789. doi:10.1089/jir.2011.0047
Dolff, S., Bijl, M., Huitema, M. G., Limburg, P. C., Kallenberg, C. G., and Abdulahad, W. H. (2011). Disturbed Th1, Th2, Th17 and T(reg) balance in patients with systemic lupus erythematosus. Clin. Immunol. 141, 197–204. doi:10.1016/j.clim.2011.08.005
Dufour, A., Bellac, C. L., Eckhard, U., Solis, N., Klein, T., Kappelhoff, R., et al. (2018). C-terminal truncation of IFN-γ inhibits proinflammatory macrophage responses and is deficient in autoimmune disease. Nat. Commun. 9, 2416. doi:10.1038/s41467-018-04717-4
Fanouriakis, A., Kostopoulou, M., Alunno, A., Aringer, M., Bajema, I., Boletis, J. N., et al. (2019). 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann. Rheum. Dis. 78, 736–745. doi:10.1136/annrheumdis-2019-215089
Fava, A., Buyon, J., Mohan, C., Zhang, T., Belmont, H. M., Izmirly, P., et al. (2020). Integrated urine proteomics and renal single-cell genomics identify an IFN-γ response gradient in lupus nephritis. JCI Insight 5, e138345. doi:10.1172/jci.insight.138345
Franchimont, D., Galon, J., Gadina, M., Visconti, R., Zhou, Y., Aringer, M., et al. (2000). Inhibition of Th1 immune response by glucocorticoids: Dexamethasone selectively inhibits IL-12-induced stat4 phosphorylation in T lymphocytes. J. Immunol. 164, 1768–1774. doi:10.4049/jimmunol.164.4.1768
Gómez, D., Correa, P. A., Gómez, L. M., Cadena, J., Molina, J. F., and Anaya, J. M. (2004). Th1/Th2 cytokines in patients with systemic lupus erythematosus: Is tumor necrosis factor α protective? Seminars Arthritis Rheumatism 33, 404–413. doi:10.1016/j.semarthrit.2003.11.002
Hall, B. M. (2015). T cells: Soldiers and spies--the surveillance and control of effector T cells by regulatory T cells. Clin. J. Am. Soc. Nephrol. 10, 2050–2064. doi:10.2215/cjn.06620714
Han, F., Qi, W., Tang, Z., and Wang, Y. (2004). Correlation between systemic lupus erythematosus and peripheral blood T cell subtypes. Chin Med Clin. 5, 385–387. doi:10.3969/j.issn.1671-2560.2004.05.025
Hayashino, Y., Noguchi, Y., and Fukui, T. (2005). Systematic evaluation and comparison of statistical tests for publication bias. J. Epidemiol. 15, 235–243. doi:10.2188/jea.15.235
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. Bmj 327, 557–560. doi:10.1136/bmj.327.7414.557
Idborg, H., and Oke, V. (2021). Cytokines as biomarkers in systemic lupus erythematosus: Value for diagnosis and drug therapy. Int. J. Mol. Sci. 22, 11327. doi:10.3390/ijms222111327
Jiang, D., Wang, L., Bai, C., and Chen, O. (2019). Association between abdominal obesity and asthma: A meta-analysis. Allergy Asthma Clin. Immunol. 15, 16. doi:10.1186/s13223-019-0333-6
Katsuyama, T., Tsokos, G. C., and Moulton, V. R. (2018). Aberrant T cell signaling and subsets in systemic lupus erythematosus. Front. Immunol. 9, 1088. doi:10.3389/fimmu.2018.01088
Kleczynska, W., Jakiela, B., Plutecka, H., Milewski, M., Sanak, M., and Musial, J. (2011). Imbalance between Th17 and regulatory T-cells in systemic lupus erythematosus. Folia Histochem Cytobiol. 49, 646–653. doi:10.5603/fhc.2011.0088
Ko, H., Kim, C. J., and Im, S. H. (2022). T Helper 2-associated immunity in the pathogenesis of systemic lupus erythematosus. Front. Immunol. 13, 866549. doi:10.3389/fimmu.2022.866549
La Cava, A. (2010). Anticytokine therapies in systemic lupus erythematosus. Immunotherapy 2, 575–582. doi:10.2217/imt.10.29
Li, L., Chen, S., Shen, N., Wang, X., Bao, C., and Gu, Y. (2002). Study on Th1/Th2 and its regulatory factor IL-10, IL-12 gene in patients with primary systemic lupus erythematosus. Chin J Rheumatol 1, 13–17. doi:10.3760/j:issn:1007-7480.2002.01.003
Li, L., Wang, X., Chen, S., Shen, N., Bao, C., Gu, Y., et al. (2002). Expression of Th1/Th2, IL-10 and IL-18 genes in patients with first-onset SLE. Shanghai J Immunol 03, 163–167. doi:10.3969/j.issn.1001-2478.2002.03.005
Liao, X., Li, G., Wang, A., Liu, T., Feng, S., Guo, Z., et al. (2015). Repetitive transcranial magnetic stimulation as an alternative therapy for cognitive impairment in alzheimer's disease: A meta-analysis. J. Alzheimers Dis. 48, 463–472. doi:10.3233/jad-150346
Lit, L. C., Wong, C. K., Tam, L. S., Li, E. K., and Lam, C. W. (2006). Raised plasma concentration and ex vivo production of inflammatory chemokines in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 65, 209–215. doi:10.1136/ard.2005.038315
Liu, X., and Qian, J. (2005). Study on the balance of Th1/Th2 cells in peripheral blood of patients with systemic lupus erythematosus. J Nant. Univ. (Med Ed. 4, 253–254. doi:10.3969/j.issn.1674-7887.2005.04.007
Lourenco, E. V., and Cava, A. (2009). Cytokines in systemic lupus erythematosus. Cmm 9, 242–254. doi:10.2174/156652409787847263
Malide, D., Russo, P., and Bendayan, M. (1995). Presence of tumor necrosis factor alpha and interleukin-6 in renal mesangial cells of lupus nephritis patients. Hum. Pathol. 26, 558–564. doi:10.1016/0046-8177(95)90253-8
Masutani, K., Taniguchi, M., Nakashima, H., Yotsueda, H., Kudoh, Y., Tsuruya, K., et al. (2004). Up-regulated interleukin-4 production by peripheral T-helper cells in idiopathic membranous nephropathy. Nephrol. Dial. Transpl. 19, 580–586. doi:10.1093/ndt/gfg572
Matia-Garcia, I., Vadillo, E., Pelayo, R., Muñoz-Valle, J. F., García-Chagollán, M., Loaeza-Loaeza, J., et al. (2021). Th1/Th2 balance in young subjects: Relationship with cytokine levels and metabolic profile. J. Inflamm. Res. 14, 6587–6600. doi:10.2147/JIR.S342545
Medrano-Campillo, P., Sarmiento-Soto, H., Álvarez-Sánchez, N., Álvarez-Ríos, A. I., Guerrero, J. M., Rodríguez-Prieto, I., et al. (2015). Evaluation of the immunomodulatory effect of melatonin on the T-cell response in peripheral blood from systemic lupus erythematosus patients. J. Pineal Res. 58, 219–226. doi:10.1111/jpi.12208
Miyake, K., Akahoshi, M., and Nakashima, H. (2011). Th subset balance in lupus nephritis. J. Biomed. Biotechnol. 2011, 980286. doi:10.1155/2011/980286
Moulton, V. R., Holcomb, D. R., Zajdel, M. C., and Tsokos, G. C. (2012). Estrogen upregulates cyclic AMP response element modulator α expression and downregulates interleukin-2 production by human T lymphocytes. Mol. Med. 18, 370–378. doi:10.2119/molmed.2011.00506
Moulton, V. R., and Tsokos, G. C. (2011). Abnormalities of T cell signaling in systemic lupus erythematosus. Arthritis Res. Ther. 13, 207. doi:10.1186/ar3251
Muhammad Yusoff, F., Wong, K. K., and Mohd Redzwan, N. (2020). Th1, Th2, and Th17 cytokines in systemic lupus erythematosus. Autoimmunity 53, 8–20. doi:10.1080/08916934.2019.1693545
Murphy, K. A., Bhamidipati, K., Rubin, S. J. S., Kipp, L., Robinson, W. H., and Lanz, T. V. (2019). Immunomodulatory receptors are differentially expressed in B and T cell subsets relevant to autoimmune disease. Clin. Immunol. 209, 108276. doi:10.1016/j.clim.2019.108276
O'Brien, S. A., Zhu, M., and Zhang, W. (2021). Spontaneous differentiation of T follicular helper cells in LATY136F mutant mice. Front. Immunol. 12, 656817. doi:10.3389/fimmu.2021.656817
Oster, C., Wilde, B., Specker, C., Sun, M., Kribben, A., Witzke, O., et al. (2019). BTLA expression on Th1, Th2 and Th17 effector T-cells of patients with systemic lupus erythematosus is associated with active disease. Int. J. Mol. Sci. 20, 4505. doi:10.3390/ijms20184505
Parikh, S. V., Almaani, S., Brodsky, S., and Rovin, B. H. (2020). Update on lupus nephritis: Core curriculum 2020. Am. J. Kidney Dis. 76, 265–281. doi:10.1053/j.ajkd.2019.10.017
Perry, D. J., Titov, A. A., Sobel, E. S., Brusko, L., and Morel, L. (2020). Immunophenotyping reveals distinct subgroups of lupus patients based on their activated T cell subsets. Clin. Immunol. 221, 108602. doi:10.1016/j.clim.2020.108602
Postal, M., Peliçari, K. O., Sinicato, N. A., Marini, R., Costallat, L. T., and Appenzeller, S. (2013). Th1/Th2 cytokine profile in childhood-onset systemic lupus erythematosus. Cytokine 61, 785–791. doi:10.1016/j.cyto.2012.11.023
Radmanesh, F., Mahmoudi, M., Yazdanpanah, V., Keyvani, N., Kia, A. R., Nikpoor, E., et al. (2020). The immunomodulatory effects of mesenchymal stromal cell-based therapy in human and animal models of systemic lupus erythematosus. IUBMB Life 72, 2366–2381. doi:10.1002/iub.2387
Reséndiz‐Mora, A., Wong‐Baeza, C., Nevárez‐Lechuga, I., Landa‐Saldívar, C., Molina‐Gómez, E., Hernández‐Pando, R., et al. (2021). Interleukin 4 deficiency limits the development of a lupus‐like disease in mice triggered by phospholipids in a non‐bilayer arrangement. Scand. J. Immunol. 93, e13002. doi:10.1111/sji.13002
Robinson, S., and Thomas, R. (2021). Potential for antigen-specific tolerizing immunotherapy in systematic lupus erythematosus. Front. Immunol. 12, 654701. doi:10.3389/fimmu.2021.654701
Rönnblom, L., and Leonard, D. (2019). Interferon pathway in SLE: One key to unlocking the mystery of the disease. Lupus Sci. Med. 6, e000270. doi:10.1136/lupus-2018-000270
Salazar-Camarena, D. C., Ortíz-Lazareno, P., Marín-Rosales, M., Cruz, A., Muñoz-Valle, F., Tapia-Llanos, R., et al. (2019). BAFF-R and TACI expression on CD3+ T cells: Interplay among BAFF, APRIL and T helper cytokines profile in systemic lupus erythematosus. Cytokine 114, 115–127. doi:10.1016/j.cyto.2018.11.008
Shen, T., Yue, Y., He, T., Huang, C., Qu, B., Lv, W., et al. (2021). The association between the gut microbiota and Parkinson's disease, a meta-analysis. Front. Aging Neurosci. 13, 636545. doi:10.3389/fnagi.2021.636545
Sigdel, K. R., Duan, L., Wang, Y., Hu, W., Wang, N., Sun, Q., et al. (2016). Serum cytokines Th1, Th2, and Th17 expression profiling in active lupus nephritis-IV: From a southern Chinese Han population. Mediat. Inflamm. 2016, 4927530. doi:10.1155/2016/4927530
Stang, A. (2010). Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605. doi:10.1007/s10654-010-9491-z
Su, D. L., Wang, H. J., Ji, X. H., Li, Y. Y., Xuan, H. B., Heng, C., et al. (2006). Mycophenolic acid inhibits SLE-associated cytokine expression and promotes apoptosis of peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Acta Pharmacol. Sin. 27, 1051–1057. doi:10.1111/j.1745-7254.2006.00352.x
Tahvildari, M., and Dana, R. (2019). Low-dose IL-2 therapy in transplantation, autoimmunity, and inflammatory diseases. J. Immunol. 203, 2749–2755. doi:10.4049/jimmunol.1900733
Talaat, R. M., Mohamed, S. F., Bassyouni, I. H., and Raouf, A. A. (2015). Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus erythematosus (SLE) patients: Correlation with disease activity. Cytokine 72, 146–153. doi:10.1016/j.cyto.2014.12.027
Tang, Q., Li, G., Liu, T., Wang, A., Feng, S., Liao, X., et al. (2015). Modulation of interhemispheric activation balance in motor-related areas of stroke patients with motor recovery: Systematic review and meta-analysis of fMRI studies. Neurosci. Biobehav Rev. 57, 392–400. doi:10.1016/j.neubiorev.2015.09.003
Taves, M. D., and Ashwell, J. D. (2021). Glucocorticoids in T cell development, differentiation and function. Nat. Rev. Immunol. 21, 233–243. doi:10.1038/s41577-020-00464-0
Tsokos, G. C., Lo, M. S., Costa Reis, P., and Sullivan, K. E. (2016). New insights into the immunopathogenesis of systemic lupus erythematosus. Nat. Rev. Rheumatol. 12, 716–730. doi:10.1038/nrrheum.2016.186
Uhm, W. S., Na, K., Song, G. W., Jung, S. S., Lee, T., Park, M. H., et al. (2003). Cytokine balance in kidney tissue from lupus nephritis patients. Rheumatol. Oxf. 42, 935–938. doi:10.1093/rheumatology/keg255
Vazzana, K. M., Daga, A., Goilav, B., Ogbu, E. A., Okamura, D. M., Park, C., et al. (2021). Principles of pediatric lupus nephritis in a prospective contemporary multi-center cohort. Lupus 30, 1660–1670. doi:10.1177/09612033211028658
Viallard, J. F., Pellegrin, J. L., Ranchin, V., Schaeverbeke, T., Dehais, J., Longy-Boursier, M., et al. (1999). Th1 (IL-2, interferon-gamma (IFN-gamma)) and Th2 (IL-10, IL-4) cytokine production by peripheral blood mononuclear cells (PBMC) from patients with systemic lupus erythematosus (SLE). Clin. Exp. Immunol. 115, 189–195. doi:10.1046/j.1365-2249.1999.00766.x
Wahren-Herlenius, M., and Dörner, T. (2013). Immunopathogenic mechanisms of systemic autoimmune disease. Lancet 382, 819–831. doi:10.1016/s0140-6736(13)60954-x
Wan, X., Wang, W., Liu, J., and Tong, T. (2014). Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 14, 135. doi:10.1186/1471-2288-14-135
Wang, X., Li, L., Shen, N., Hu, D., and Gu, Y. (2002). The study of Th1/Th2 imbalance in patients with primary systemic lupus erythematosus. Chin J Rheumatol 5, 316–319. doi:10.3760/j:issn:1007-7480.2002.05.003
Wang, Y., Lian, L., and Xu, J. (2006). Detection of Th0, Th1 and Th2 cell levels in patients with systemic lupus erythematosus. J Anhui Med Univ. 3, 321–323. doi:10.3969/j.issn.1000-1492.2006.03.028
Wong, C. K., Ho, C. Y., Li, E. K., and Lam, C. W. (2020). Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus 9, 589–593. doi:10.1191/096120300678828703
Wu, H., Zhang, S., Lin, X., He, J., Wang, S., and Zhou, P. (2021). Pregnancy-related complications and perinatal outcomes following progesterone supplementation before 20 weeks of pregnancy in spontaneously achieved singleton pregnancies: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 19, 165. doi:10.1186/s12958-021-00846-6
Xu, W., Li, Z., Yang, T., and Wang, B. (2013). Changes and clinical significance of Th17 and Th1 cells and their cytokine levels in patients with systemic lupus erythematosus. Lab. Med. 28, 396–399. doi:10.3969/j.issn.1673-8640.2013.05.012
Yang, X. Y., Wang, H. Y., Zhao, X. Y., Wang, L. J., Lv, Q. H., and Wang, Q. Q. (2013). Th22, but not th17 might be a good index to predict the tissue involvement of systemic lupus erythematosus. J. Clin. Immunol. 33, 767–774. doi:10.1007/s10875-013-9878-1
Yang, Y. (2015). Expression and clinical significance of T helper cell subsets in peripheral blood of systemic lupus erythematosus. Beijing: Chin People's Liberation Army Med College. [dissertation/master’s thesis].
Yen, E. Y., and Singh, R. R. (2018). Brief report: Lupus-an unrecognized leading cause of death in young females: A population-based study using nationwide death certificates, 2000-2015. Arthritis Rheumatol. 70, 1251–1255. doi:10.1002/art.40512
Yilmaz, M. I., Solak, Y., Saglam, M., Cayci, T., Acikel, C., Unal, H. U., et al. (2014). The relationship between IL-10 levels and cardiovascular events in patients with CKD. Clin. J. Am. Soc. Nephrol. 9, 1207–1216. doi:10.2215/CJN.08660813
Zhang, S. (2011). Study on the value of combined detection of T helper cell subsets in the evaluation of systemic lupus erythematosus. Shanxi: University of shanxi medicine. [dissertation/doctor’s thesis].
Zhong, W., Jiang, Y., Ma, H., Wu, J., Jiang, Z., and Zhao, L. (2017). Elevated levels of CCR6+ T helper 22 cells correlate with skin and renal impairment in systemic lupus erythematosus. Sci. Rep. 7, 12962. doi:10.1038/s41598-017-13344-w
Zhou, Y. B., Ye, R. G., Li, Y. J., and Xie, C. M. (2009). Targeting the CD134-CD134L interaction using anti-CD134 and/or rhCD134 fusion protein as a possible strategy to prevent lupus nephritis. Rheumatol. Int. 29, 417–425. doi:10.1007/s00296-008-0697-2
Keywords: systemic lupus erythematosus, helper T cell type 1, helper T cell type 2, cytokines, meta-analysis
Citation: Xiang S, Zhang J, Zhang M, Qian S, Wang R, Wang Y, Xiang Y and Ding X (2022) Imbalance of helper T cell type 1, helper T cell type 2 and associated cytokines in patients with systemic lupus erythematosus: A meta-analysis. Front. Pharmacol. 13:988512. doi: 10.3389/fphar.2022.988512
Received: 07 July 2022; Accepted: 05 September 2022;
Published: 29 September 2022.
Edited by:
Cecilia Beatrice Chighizola, University of Milan, ItalyReviewed by:
Ozgur Kasapcopur, Istanbul University-Cerrahpasa, TurkeyAleksandra Plavsic, University Clinical Centre of Serbia, Serbia
Copyright © 2022 Xiang, Zhang, Zhang, Qian, Wang, Wang, Xiang and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinghong Ding, ZHhoQHpjbXUuZWR1LmNu
†These authors share first authorship
 Shate Xiang
Shate Xiang Jingjing Zhang
Jingjing Zhang Mengge Zhang
Mengge Zhang Suhai Qian
Suhai Qian Rongyun Wang
Rongyun Wang Yao Wang
Yao Wang Yingshi Xiang
Yingshi Xiang Xinghong Ding
Xinghong Ding