
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 07 September 2022
Sec. Ethnopharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.988237
 Hongfei Zhu1,2†
Hongfei Zhu1,2† Mengting Li1,2†
Mengting Li1,2† Chen Tian1,2
Chen Tian1,2 Honghao Lai1,2
Honghao Lai1,2 Yuqing Zhang3,4,5,6
Yuqing Zhang3,4,5,6 Jiaheng Shi7,8
Jiaheng Shi7,8 Nannan Shi7
Nannan Shi7 Hui Zhao7
Hui Zhao7 Kehu Yang9,10,11
Kehu Yang9,10,11 Hongcai Shang12,13
Hongcai Shang12,13 Xin Sun14*
Xin Sun14* Jie Liu7,15*
Jie Liu7,15* Long Ge1,2,10,11*
Long Ge1,2,10,11* Luqi Huang7,16*
Luqi Huang7,16*Background: The coronavirus disease 2019 (COVID-19) is still a pandemic globally, about 80% of patients infected with COVID-19 were mild and moderate. Chinese herbal medicine (CHM) has played a positive role in the treatment of COVID-19, with a certain number of primary studies focused on CHM in managing COVID-19 published. This study aims to systematically review the currently published randomized controlled trials (RCTs) and observational studies (OBs), and summarize the effectiveness and safety of CHM in the treatment of mild/moderate COVID-19 patients.
Methods: We searched 9 databases up to 19 March 2022. Pairs of reviewers independently screened literature, extracted data and assessed risk of bias. For overall effect, we calculated the absolute risk difference (ARD) of weighted averages of different estimates, and certainty of evidence was assessed using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) system.
Results: We included 35 RCTs and 24 OBs enrolling 16,580 mild/moderate patients. The certainty of evidence was very low to low. Compared with usual supportive treatments, most effect estimates of CHM treatments were consistent in direction. CHMs presented significant benefits in reducing rate of conversion to severe cases (ARD = 99 less per 1000 patients in RCTs and 131 less per 1000 patients in OBs, baseline risk: 16.52%) and mortality (ARD = 3 less per 1000 patients in RCTs and OBs, baseline risk: 0.40%); shortening time to symptom resolution (3.35 days in RCTs and 2.94 days in OBs), length of hospital stay (2.36 days in RCTs and 2.12 days in OBs) and time to viral clearance (2.64 days in RCTs and 4.46 days in OBs); increasing rate of nucleic acid conversion (ARD = 73 more per 1000 patients in OBs, baseline risk: 16.30%). No serious adverse reactions were found and the differences between CHM and usual supportive care were insignificant.
Conclusion: Encouraging evidence showed that CHMs were beneficial in treating mild or moderate patients. CHMs have been proved to possess a safety profile that is comparable to that of usual supportive treatment alone. More rigorously designed clinical trials and mechanism studies are still warranted to further confirm the present findings.
Since December 2019, the number of Coronavirus disease 2019 (COVID-19) infections has increased rapidly and in March 2020, the World Health Organization (WHO) declared it to be a global pandemic (WHO, 2020). As of 17 April 2022, there have been more than 500 million confirmed cases and more than 6 million deaths globally (WHO, 2022a). About 80% of patients infected with COVID-19 were mild and moderate, so it was critical for their effective management (NHC, 2020). After evaluating 463 randomized controlled trials (RCTs) and 180 drugs on non-severe COVID patients, WHO guideline (Agarwal et al., 2020) only made strong recommendations for nirmatrelvir and ritonavir, and conditional recommendations for molnupiravir, sotrovimab, remdesivir, casirivimab and imdevimab.
Chinese herbal medicine (CHM), an important part of traditional medicine, has been spread to more than 170 countries (WHO, 2013) and played a huge role in the management of COVID-19. According to the WHO Expert Meeting on Evaluation of Traditional Chinese Medicine in the Treatment of COVID-19 released in March 2022, 12 selected RCTs demonstrated that Chinese patent medicine (CPM) as an additional intervention could shorten the time to viral clearance, resolution of clinical symptoms, and length of hospital stay compared with conventional treatment for mild-to-moderate patients (WHO, 2022b). However, in addition to this, there were many observational studies (OBs) on CPM, as well as RCTs or OBs on CHM. Therefore, it is crucial to timely summarize and evaluate all existed CHM evidence, including RCTs and OBs, to reflect the effectiveness and safety of CHM in the treatment of mild or moderate COVID-19 patients and further improve treatment measures and medical care worldwide.
We conducted and reported this systematic review and meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (Page et al., 2021).
An extremely sensitive search strategy that only included search terms related to disease (COVID-19) and study design were used to identify all relevant primary studies under the guidance of an experienced librarian, regardless of languages or types of publication. We conducted a systematic search from December 2019 to 19th March 2022 of the following 9 databases: PubMed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science, China national knowledge infrastructure (CNKI), WanFang Database, Chinese BioMedical Literature Database (CBM), China Science and Technology Journal Database (VIP), and the L-OVE COVID-19 Repository. We also tracked the references of relevant publications. The details of search strategies can be found in Supplementary Table S1.
Inclusion criteria: 1) Patients: confirm diagnosed mild or moderate COVID-19 according to the national or international recognized diagnosis standard; studies that included patients with both non-severe and severe or critical COVID-19 were eligible if more than 80% of patients were mild and/or moderate; studies included results of mild or moderate patients could be extracted from subgroup-analysis were also eligible. 2) Intervention: usual supportive treatment (e.g., bed rest, antibacterial treatment, antiviral treatment, immunotherapy, prone position treatment, etc.) any form of Chinese herbal medicine, such as granules, decoction and injections, were all considered to be included. Usual supportive treatment plus the combination of Chinese herbal medicines were also eligible. 3) Control: patients of the control group were given usual supportive treatment. 4) Outcomes: we decided the outcomes of interest according to a living network meta-analysis published in BMJ (Siemieniuk et al., 2020) and a core outcome set of COVID-19 (Jin X. et al., 2020); mainly including: a. clinical efficacy (e.g., mortality, viral clearance, length of hospital stay, rate of mechanical ventilation), b. clinical symptoms recovery (e.g., fever, cough, tiredness), c. adverse events (e.g., nausea and vomit, diarrhea, abnormal liver function). The study that reported at least one outcome listed above was considered eligible, 5) Study types: RCTs and OBs (e.g., cohort study and historical control study).
We excluded studies that enrolled 20% or more severe/critical patients, that did not report the outcome of interest, that were short reports or abstracts of which with key information missing, that did not report the approval information by the ethics committee or information about informed consent of patients, and that the study design was protocol, case report, case report series, cross-sectional study and controlled before-after study.
EndNote X8.0 was used to manage the initially searched records. After removing duplicate records, the remaining records were imported into an online reference management software Rayyan (Ouzzani et al., 2016). After receiving training and calibration exercises, four teams of 2 reviewers (ZHF and LMT, SMY and TC, LYF and ZWZ, RSM and JJY) independently screened the title and abstracts of each record, then further reviewed full texts of potentially eligible studies to determine the final eligibility. Any conflict was resolved through discussion or consultation with a third reviewer (GL).
A standardized, pilot tested data extraction form was used to extract information from each eligible trial. Teams of 2 reviewers (LYF and YQY, SMY and TC, CX and LHH), following training and calibration exercises, independently extracted data of interest, including 1) trial characteristics: first author, year of publication, trial registration number, published journal, language, study design and funding source; 2) baseline patient characteristics: geographic location and recruitment timeline of the study, age, gender, proportion of morbidities at baselines; 3) baseline clinical characteristics: type, dose and duration of care, details of CHM components, severity of COVID-19 symptoms; and 4) outcomes of interest: means or medians and measures of variability for continuous outcomes, and the number of patients analyzed and the number of patients who experienced relevant event for dichotomous outcomes. When eligible studies did not report interest data, we would contact authors to obtain data.
The risk of bias of eligible RCTs was independently assessed using a modified Cochrane risk of bias tool (RoB 2.0) (JAC et al., 2019) based on 6 domains: bias from the randomization process generated, bias due to deviations from the intended intervention, bias due to missing data, bias in measurement of the outcome, bias in selection of the reported results and bias due to other sources (e.g. consistency between the registration information and the final report, completeness of the report). For included OBs, a modified Risk of Bias in Non-randomized Studies-of Interventions (ROBINS-I) (Sterne et al., 2016) was used to assess the risk of bias according to the following 6 domains: bias due to confounding, bias in selection of participants into the study; bias from the interventions, bias due to missing data, bias due to measurement of the outcome, and bias in selection of the reported results.
Each item for included studies was categorized into four groups: low risk of bias, probably low risk of bias, probably high risk of bias, and high risk of bias. When information reported by primary studies was insufficient for reviewers to assess the risk of bias, we would contact authors for more adequate information. Discrepancies were solved by discussion and, when necessary, with adjudication by a third reviewer (GL). Detailed guidance for assessment of risk of bias was presented in Supplementary Table S2.
When there were two or more studies with the same study design, intervention and control treatments reporting on the same outcome measure, Review Manager software (RevMan, version 5.3, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) was used to perform meta-analysis. Where quantitative meta-analysis was unfeasible, qualitative systematic analysis was conducted to show the difference. The efficacy trend and distribution of point estimates of different Chinese medicines were visually presented with a forest plot. The direction and distribution of effect estimates of different CHM treatments across studies were compared to show the benefit and harm of CHM compared with usual supportive treatment. For dichotomous data, risk ratio (RR) with corresponding 95% confidence interval (CI) was calculated. For continuous data, we used the mean difference (MD) with 95% CI. When eligible study was unable to include in meta-analysis, if there existed OB that reported the results with adjustment of confounders, we directly used effect measures and effect estimates reported in OB. Due to the differences arising from the study design, we presented the analysis results of RCTs and OBs separately and did not merge the results.
In order to obtain the overall effect of CHM treatments, we also calculated weighted averages of different effect estimates using the inverse variance method, and presented the absolute risk difference (ARD) for weighted averages. We used a random model if there was considerable variation between studies, otherwise, we used a fixed model. To calculate ARD with corresponding 95% CI, wherever possible, we used the baseline risk from the WHO living guideline (Agarwal et al., 2020) for the corresponding outcome, otherwise, we calculated the median incidence rate in the usual supportive care group from studies with the same study design (More details of baseline risk were shown in Supplementary Table S3). Considering statistical power, we performed meta-regression, sensitivity analysis, and publication bias checks using Stata v.16.0 software (Stata Corporation LLC, College Station, United States) when there were10 or more studies included. The inter-study heterogeneity was examined by using standard Cochran’s Q test and I2 statistic. When there existed substantial heterogeneity, we conducted meta-regression analysis to explore the source of heterogeneity based on the following 2 factors: whether comorbidities are reported and the proportion of mild and moderate COVID-19 patients. Sensitivity analysis was undertaken by random effect models to observe the robustness of result. For the analysis that included 10 or more study, we evaluated the publication bias through Egger’s linear regression test (Egger et al., 1997) and funnel plot. p < 0.05 was considered as statistical significance.
Rader chart was used to show the rate of adverse reactions which were reported commonly in both RCTs and non-randomized trials, and each axis represented an adverse reaction. We would perform subgroup analyses by severity of patients, age, and comorbidities if the number of studies was sufficient.
For the weighted averages of different effect estimate, two reviewers (ZHF and LMT) independently assessed the certainty of evidence using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) system (Guyatt et al., 2008). The certainty of evidence was categorized as high, moderate, low or very low. At the beginning of the assessment, RCTs started as high certainty and could be downgraded due to five reasons: risk of bias, imprecision, inconsistency, indirectness, and publication bias; OBs started as low certainty, which could be downgraded due to five reasons same as RCTs, and upgraded due to three reasons: large magnitude of an effect, dose-response gradient, and effect of plausible residual confounding.
In total, 215,761 records were derived from electronic databases, of which 267 were potentially eligible and further underwent full-text screening. First, we obtained 231 primary studies involving drugs and non-drug treatments for the prevention, treatment, and rehabilitation stages. In the second step, 45 RCTs and 96 OBs focused on patients with mild or/and moderate COVID-19 patients were identified. In the third step, we included 35 RCTs and 24 OBs according to the eligibility criteria. The literature screening process was shown in Figure 1.
We included thirty-five RCTs enrolled 4,166 mild or moderate patients (male: 50.99%), with a mean age of 49.08 years. There were 22 studies published in Chinese (Ai X. Y. et al., 2020; Jin W. et al., 2020; Zhang C. T. et al., 2020; AI et al., 2020b; Yu P. et al., 2020; Wang Y. L. et al., 2020; Ding et al., 2020; Duan et al., 2020; Zhang Y. L. et al., 2020; Fu et al., 2020; Lin et al., 2020; Sun et al., 2020; Hu F. et al., 2021; Wang L. Q. et al., 2021; Wang Y. et al., 2021; He et al., 2021; Li et al., 2021; Ping et al., 2021; Qiu et al., 2021; Sun S et al., 2021; Wu et al., 2021; Yan et al., 2021) (2 registered prospectively (Jin W. et al., 2020; Sun Y et al., 2021)) and 13 published in English which were all registered (Xiao M. Z. et al., 2020; Wang J. B. et al., 2020; Xiong et al., 2020; Liu J. et al., 2021; An et al., 2021; Hu K. et al., 2021; Zhang X. Y. et al., 2021; Ma et al., 2021; Ni et al., 2021; Xu et al., 2021; Zeng et al., 2021; Zhao et al., 2021; Zhang et al., 2022). Twenty-four OBs(Chen et al., 2020a; Yang M. B. et al., 2020; Yu H. Y. et al., 2020; Chen et al., 2020b; Xiao Q. et al., 2020; Zhang C. Y. et al., 2020; Ai Z. Z. et al., 2020; Zhang N. et al., 2020; Lan et al., 2020; Li, 2020; Liu et al., 2020; Xin et al., 2020; Yao et al., 2020; Zeng et al., 2020; Chen J. et al., 2021; Zhang L. H. et al., 2021; Chen R. B. et al., 2021; Liu L. et al., 2021; Wang Q. L. et al., 2021; Shi et al., 2021; Sun S et al., 2021; Feng et al., 2022) (Tian et al., 2020; Zhang X. et al., 2021) enrolled 12,414 mild or moderate patients (male: 48.11%), with a mean age of 54.53 years. Fourteen OBs were published in Chinese (1 registered prospectively (Chen R. B. et al., 2021)) and 10 were published in English (4 registered prospectively (Tian et al., 2020; Xin et al., 2020; Zhang X. et al., 2021; Shi et al., 2021)).
All patients were recruited from China, of which 59.32% were in Hubei (Chen et al., 2020a; Xiao M. Z. et al., 2020; Yang M. B. et al., 2020; Yu H. Y. et al., 2020; Zhang C. T. et al., 2020; Chen et al., 2020b; Xiao Q. et al., 2020; Yu P. et al., 2020; Zhang C. Y. et al., 2020; Ai Z. Z. et al., 2020; Ding et al., 2020; Duan et al., 2020; Zhang Y. L. et al., 2020; Lan et al., 2020; Li, 2020; Tian et al., 2020; Xin et al., 2020; Xiong et al., 2020; Yao et al., 2020; Chen J. et al., 2021; Hu F. et al., 2021; Liu J. et al., 2021; An et al., 2021; Wang L. Q. et al., 2021; Zhang L. H. et al., 2021; Hu K. et al., 2021; Liu L. et al., 2021; Wang Y. et al., 2021; He et al., 2021; Ni et al., 2021; Shi et al., 2021; Sun S et al., 2021; Sun Y et al., 2021; Xu et al., 2021; Zhao et al., 2021). Except for 1 OB recruited from July to September 2020 (Wang Q. L. et al., 2021), the other studies were recruited at the beginning of the outbreak (December 2019 to May 2020). The most common comorbidities were diabetes, hypertension, cardiovascular disease or coronary heart disease, and respiratory conditions in eligible studies. In addition, there was one study on patients with COVID-19 and hepatitis B. Supplementary Table S4 presents the detailed study characteristics.
Fifty-nine studies involved 38 drugs, including Qingfei Paidu decoction (QFPD; 1 RCT and 7 OBs), Lianhua Qingwen granules/capsules (LHQW; 3 RCTs and 3 OBs), Jinyinhua oral liquid (JYH; 2 RCTs), Jinhua Qinggan granules (JHQG, 2 RCTs and 1 OB), Huashi Baidu granule (2 RCTs and 1 OB), Reduning injection (RDN, 2 RCTs), Shufeng Jiedu capsules (SFJD, 3 OBs), FeiyanYihao Chinese medicine granules (FY 1, 1 RCT and 1 OB), Lianhua Qingke granules (LHQK, 2 RCTs), Toujie Quwen granules (TJQW, 2 RCTs). In addition, there were CHMs only reported in one study: Buzhong YiQi decoction (BZYQ), Compound Yinchai Granules (FFCY) and Qingqiao Jiedu Granules (QQJD), Gegen Qinlian pill (GGQL), Ganlu Xiaodu decoction (GLXD), Hanshiyi Formula (HSY), Jiawei Dayuan decoction (JWDY), Keguan-1 decoction, Liushen pill (LS), Maxingshigan-Weijing decoction (MWD), Ma Xing Shigan decoction (MXSG), Maxin Xuanfei Jiedu decoction (MXXF), Qingfei Touxie Fuzheng decoction (QFTXFZ), Qingre Kangdu oral liquid (QRKD) and Lanxiang Jiedu oral liquid (LXJD), Qushi Paidu Fuzheng Recipe (QSPDFZ), Reyanning injection (RYN), Sanao Yulong mixture (SAYL), Self-formulated Compatible Decoction, Shuanghuanglian oral liquid (SHL), Tanreqing Capsule (TRQ), Xuebijing injection (XBJ), Xuanfei Baidu decoction (XFBD), Xuanfei Qingre decoction (XFQR), Xiyanping injection (XYP), Xueshuantong injection (XST), Yindan Jiedu granules (YDJD), and Yinghuang Qingfei capsules (YHQF).
The usual supportive treatment was performed mainly according to the Chinese treatment regimens recommended by the “Diagnosis and Treatment Protocol for COVID-19” (3rd to 7th Edition), including bed rest, monitoring life sign, oxygen therapy, prone position therapy, immunotherapy, antiviral, antibacterial, and anticoagulation therapy. The specific components of CHM, the measures of the control group and the intervention group were shown in Supplementary Table S5.
Figure 2 showed the risk of bias for RCTs (a) and OBs (b). Assessment of the risk of bias for single RCTs was presented in Supplementary Table S6. Only 3 RCTs (Wang J. B. et al., 2020; Zhang X. Y. et al., 2021; Sun Y et al., 2021) were assessed at low or probably low risk of bias in all domains. Other studies were assessed as high or probably high risk in at least one domain. The main biases were from random sequence generation and deviations from the intended intervention. Two RCTs (Xiao M. Z. et al., 2020; Xiong et al., 2020) also had risks with the missing data and subjective measurement of outcomes.
Assessment of the risk of bias for single OBs was presented in Supplementary Table S7. Four OBs (Chen et al., 2020a; Chen et al., 2020b; Tian et al., 2020; Zhang L. H. et al., 2021; Feng et al., 2022) were at low or probably low risk of bias in all domains. Other OBs were assessed as high risk of bias mainly because they did not adjust for confounding.
Twenty-three studies (16 RCTs, 7 OBs enrolled 4287mild/moderate patients) reported the rate of conversion to severe cases, involving 23 CHMs. We used forest plot to present the distribution of effects among included 24 studies (see Supplementary Figure S1.1 for RCTs, Supplementary Figure S1.2 for OBs). The point estimates of all studies showed consistent direction that CHMs were beneficial in reducing the rate of conversion of mild/moderate to severe cases and 9 CHMs showed statistical significance. Evidence from RCTs showed that compared with usual supportive care, LHQW (RR = 0.62, 95% CI: 0.40–0.94), JYH (RR = 0.08, 95% CI: 0.01, 0.64), JHQG (RR = 0.67, 95% CI: 0.15–2.98) and HSBD (RCT: RR = 0.31, 95% CI: 0.12–0.84) could significantly reduce the rate of conversion to severe cases. Evidence from OBs showed that QFPD (RR = 0.30, 95% CI: 0.14–0.63), LHQW (RR = 0.45, 95% CI: 0.25–0.80), JYBD (RR = 0.18, 95% CI: 0.04–0.70), QSPDFZ (RR = 0.23, 95% CI: 0.11–0.51), HSY (RR = 0.02, 95% CI: 0.00–0.29) and GLXD (RR = 0.03, 95% CI: 0.00–0.42) could significantly reduce the rate of conversion to severe cases.
The weighted averages of different effect estimates of RCTs and OBs showed that CHMs could significantly reduce the rate of conversion to severe cases by 58% (RR = 0.40, 95%CI: 0.29 to 0.57; ARD = 99 less per 1000 patients, 95%CI: 71 less to 117 less; low certainty) (see Supplementary Figure S1.1) and 80% (RR = 0.21, 95%CI: 0.15 to 0.30; ARD = 131 less per 1000 patients, 95%CI: 116 less to 140 less; very low certainty) (see Supplementary Figure S1.2) respectively. Table 1 showed the GRADE summary of finding table. Egger’s test indicated the possibility of publication bias (RCTs: p = 0.001; OBs: p = 0.002, Supplementary figure S10.1-2).
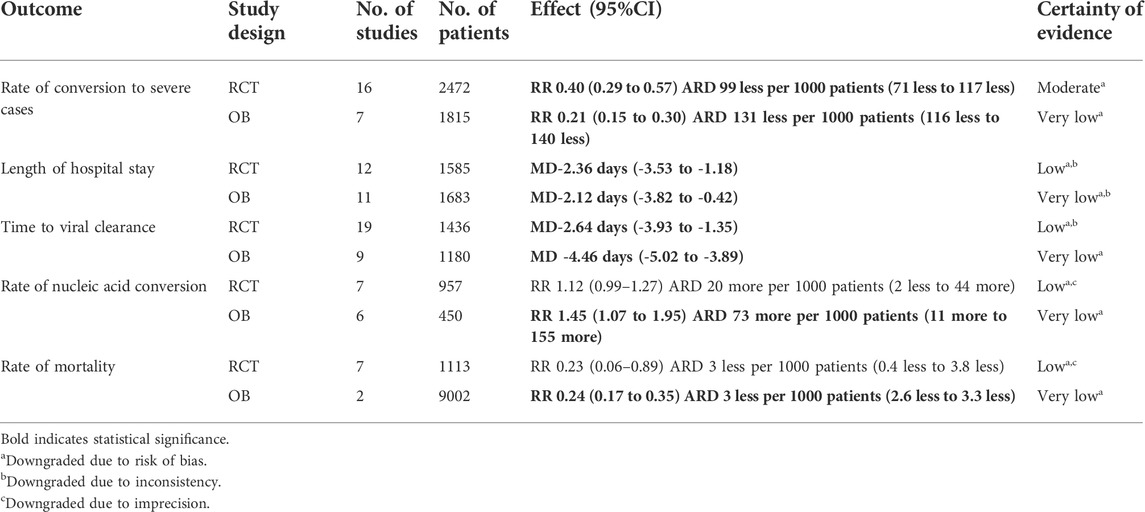
TABLE 1. GRADE summary of findings table showing certainty of evidence of weighted averages of different effect estimates on health outcomes in mild/moderate COVID-19 patients.
Twenty-two studies (12 RCTs and 10 OBs enrolled 1867 mild/moderate patients) reported the time to fever resolution, involving 18 CHMs. We used forest plot to present the distribution of effects among included 22 studies (see Supplementary Figure S2.1 for RCTs, Supplementary Figure S3.1 for OBs). The point estimates of most studies showed consistent direction that CHMs were beneficial in shortening the time to fever resolution and 12 CHMs showed statistical significance among them. Evidence from RCTs showed that compared with usual supportive care, LHQW (MD = −1.00 days, 95%CI: −1.25 to −0.75), YHQF (MD = −0.90 days, 95%CI: −1.00 to −0.80), LS (MD = −2.67 days, 95%CI: 4.59 to −0.75), Keguan-1 (MD = −1.75 days, 95%CI: −2.69 to −0.81), MWD (MD = −4.00 days, 95%CI: −6.34 to −1.66), SAYL (MD = −1.00 days, 95%CI: −1.69 to −0.31), MXXF (MD = −1.68 days, 95%CI: −2.38 to −0.98) and JWDY(MD = −2.52 days, 95%CI: −3.30 to −1.74) could significantly shorten the time to fever resolution. Evidence from OBs showed that SFJD (MD = −0.84 days, 95%CI: −1.09 to −0.59), GLXD (MD = −1.77 days, 95%CI: −2.42 to −1.12), TJQW (MD = −3.80 days, 95%CI: −4.47 to −3.13) and Self-formulated Compatible Decoction (MD = −1.08 days, 95%CI: −1.58 to −0.58) could significantly shorten the time to fever resolution.
The weighted averages of different effect estimates of RCTs showed that CHMs could significantly shorten the time to time to total symptom, fever, cough, tiredness and shortness of breath resolution. In addition, the weighted averages of different effect estimates of OBs indicated that CHMs could shorten the time to fever, cough, tiredness, expectoration and sore throat resolution (See Supplementary Figure S2.1–2.5 for RCTs, Supplementary Figure S3.1–3.6 for OBs). Table 2 shows the weighted averages of different effect estimates.
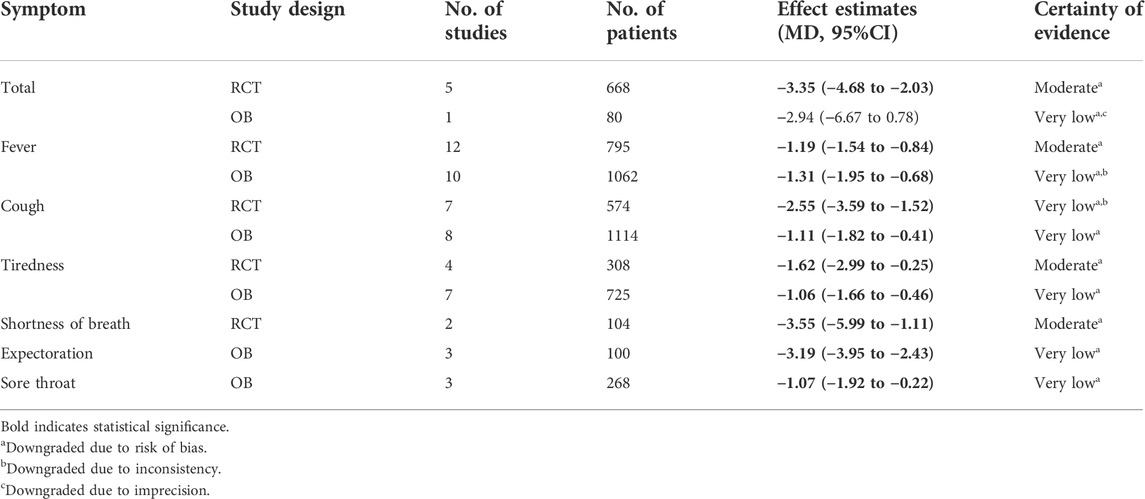
TABLE 2. GRADE summary of findings table showing certainty of evidence of weighted averages of different effect estimates on time to symptom resolution (days) in mild/moderate COVID-19 patients.
Twenty-three studies (12 RCTs and 11 OBs enrolled 3268 mild/moderate patients) reported the length of hospital stay, involving 17 CHMs. We used forest plot to present the distribution of effects among included 23 studies (see Supplementary Figure S4.1 for RCTs, Supplementary Figure S4.2 for OBs). The point estimates of most studies showed consistent direction that CHMs were beneficial in shortening the length of hospital stay and 10 CHMs showed statistical significance among them. Evidence from RCTs showed that compared with usual supportive care, QFPD (MD = −3.10 days, 95%CI: −3.72 to −2.48), JYH(MD = −6.16 days, 95%CI: −8.46 to −3.86), RDN (MD = −3.23 days, 95%CI: −4.92 to −1.55), JWYPF(MD = −8.17 days, 95%CI: −10.76 to −5.58), TJQW (MD = −2.70 days, 95%CI: −4.64 to −0.76), SAYL (MD = −1.00 days, 95%CI: −1.69 to −0.35) and XFQR (MD = −3.21 days, 95%CI: −6.08 to −0.34) could significantly shorten the length of hospital stay. Evidence from OBs showed that QFPD (MD = −2.74 days, 95%CI: −5.41 to −0.07), MXSG (MD = −2.85 days, 95%CI: −3.76 to −1.94), GLXD (MD = −1.10 days, 95%CI: −2.04 to −0.16) and Self-formulated Compatible Decoction (MD = −4.92 days, 95%CI: −5.83 to −4.01) could significantly shorten the length of hospital stay.
The weighted averages of different effect estimates of RCTs and OBs showed that CHMs could significantly shorten the length of hospital stay by −2.36 days (MD = −2.36 days, 95%CI: −3.53 to −1.18; very low certainty) (see Supplementary Figure S4.1) and 2.12 day (MD = −2.12 days, 95%CI: −3.82 to −0.42; very low certainty) (see Supplementary Figure S4.2). For RCTs, Egger’s test indicated the possibility of publication bias (RCTs: p = 0.039; OBs: p = 0.02, Supplementary Figure S10.3–4). The results of meta-regression demonstrated that comorbidities (p = 0.360), proportion of mild (p = 0.472) and moderate (p = 0.547) COVID-19 patients might not be the potential sources of heterogeneity (see Supplementary Figure S11.1). Sensitivity analysis revealed no outlier studies that might significantly alter the primary results (see Supplementary Figure S12.1-2).
Twenty-two studies (13 RCTs, 9 OBs enrolled 2616 mild/moderate patients) reported the time to viral clearance, involving 18 CHMs. We used forest plot to present the distribution of effects among included 22 studies (see Supplementary Figure S5.1 for RCTs, Supplementary Figure S5.2 for OBs). The point estimates of most studies showed consistent direction that CHMs were beneficial in shortening the time to viral clearance and 14 CHMs showed statistical significance among them. Evidence from RCTs showed that compared with usual supportive care, LHQW (MD = −1.34 days, 95%CI: −1.96 to −0.72), JYH (MD = −5.74 days, 95%CI: −7.77 to −3.71), RDN (MD = −3.75 days, 95%CI: −4.27 to −3.24), GGQL (MD = −2.17 days, 95%CI: −3.61 to −0.73), JWYPF(MD = −6.83 days, 95%CI: −9.10 to −4.56), XYP (MD = −3.53 days, 95%CI: −5.59 to −1.47), SAYL (MD = −2.00 days, 95%CI: −3.54 to −0.46) and XFQR (MD = −3.15 days, 95%CI: −5.44 to −0.86) could significantly shorten the time to viral clearance. Evidence from OBs showed that QFPD (MD = −4.04 days, 95%CI: −5.15 to −2.94), JHQG (MD = −3.00 days, 95%CI: −4.76 to −1.24), MSXG (MD = −3.90 days, 95%CI: −4.96 to −2.84), TJQW (MD = −5.66 days, 95%CI: −6.39 to −4.93), YDJD (MD = −5.02 days, 95%CI: −5.36 to −4.68) and TRQ (MD = −3.93 days, 95%CI: −7.50 to −0.36) could significantly shorten the time to viral clearance.
The weighted averages of different effect estimates of RCTs and OBs showed that CHMs could significantly shorten the time to viral clearance by 2.64 days (MD = −2.64 days, 95%CI: −3.93 to −1.35; low certainty) (see Supplementary Figure S5.1) and 4.46 days (MD = −4.46 days, 95%CI: −5.02 to −3.89; very low certainty) (see Supplementary Figure S5.2) respectively. For RCTs, no publication bias was detected (p = 0.701, see Supplementary Figure S10.5). The results of meta-regression demonstrated that comorbidities (p = 0.265), proportion of mild (p = 0.472) and moderate (p = 0.79) COVID-19 patients might not be the potential sources of heterogeneity (see Supplementary Figure S11.3). Sensitivity analysis of RCTs revealed no outlier studies that might significantly alter the primary results for RCTs (see Supplementary Figure S12.3).
Thirteen studies (7 RCTs, 6 OBs enrolled 1407 mild/moderate patients) reported the rate of nucleic acid conversion, involving 10 CHMs. We used forest plot to present the distribution of effects among included 13 studies (see Supplementary Figure S6.1 for OBs, Supplementary Figure S6.2 for RCTs). The point estimates of most studies showed consistent direction that CHMs were beneficial in increasing the rate of nucleic acid conversion and 5 CHMs showed statistical significance among them. Evidence from OBs showed that compared with usual supportive care, JHQG (RR = 2.05, 95%CI: 1.14–3.68), RYN (RR = 1.58, 95%CI: 1.13, 2.21), QSPDFZ (RR = 3.74, 95%CI: 1.70–8.26) and QFDYG (RCT: RR = 1.20, 95%CI: 1.01–1.43) could significantly increase the rate of nucleic acid conversion. Evidence from RCTs showed that BZYQ (RR = 2.19, 95%CI: 1.33–3.59) could significantly increase the rate of nucleic acid conversion.
The weighted average of different effect estimates of OBs showed that CHMs could significantly increase the rate of nucleic acid conversion by 50% (RR = 1.45, 95%CI: 1.07 to 1.95; ARD = 73 more per 1000 patients, 95%CI: 11 more to 155 more; very low certainty) (see Supplementary Figure S6.1), and RCTs showed CHMs could increase rate of nucleic acid conversion by 12% (RR = 1.12, 95%CI: 0.99 to 1.27; ARD = 20 more per 1000 patients, 95%CI: 2 less to 44 more; low certainty) (see Supplementary Figure S6.2).
Nine studies (7 RCTs, 2 OBs enrolled 10,115 mild/moderate patients) reported the rate of mortality, involving 7 CHMs. We used forest plot to present the distribution of effects among included 9 studies (see Supplementary Figure S7.1 for OBs, Supplementary Figure S7.2 for RCTs). The point estimates of all studies showed consistent direction that CHMs were beneficial in reducing the rate of mortality and 1 CHMs showed statistical significance among them. The weighted averages of different effect estimate of OBs and RCTs showed that QFPD could significantly reduce the rate of mortality by 76% (RR = 0.24, 95%CI: 0.17 to 0.35; ARD = 3 less per 1000 patients, 95%CI: 2.6 less to 3.3 less; very low certainty) (see Supplementary Figure S7.1) and 89% (RR = 0.23, 95%CI: 0.06 to 0.89; ARD = 3 less per 1000 patients, 95%CI: 0.4 less to 3.8 less; low certainty) (see Supplementary Figure S7.2).
The weighted averages of different effect estimates of RCTs showed that CHMs could significantly increase the rate of cough, tiredness, loss of appetite, shortness of breath resolution and CT improvement (see Supplementary Figures S8.1–S8.10). In addition, the weighted averages of different effect estimates of OBs indicated that CHMs could increase the rate of fever, tiredness, shortness of breath, chest tightness resolution and CT improvement (See Supplementary Figures S9.1–9.9). Table 3 shows the weighted averages of different effect estimates.
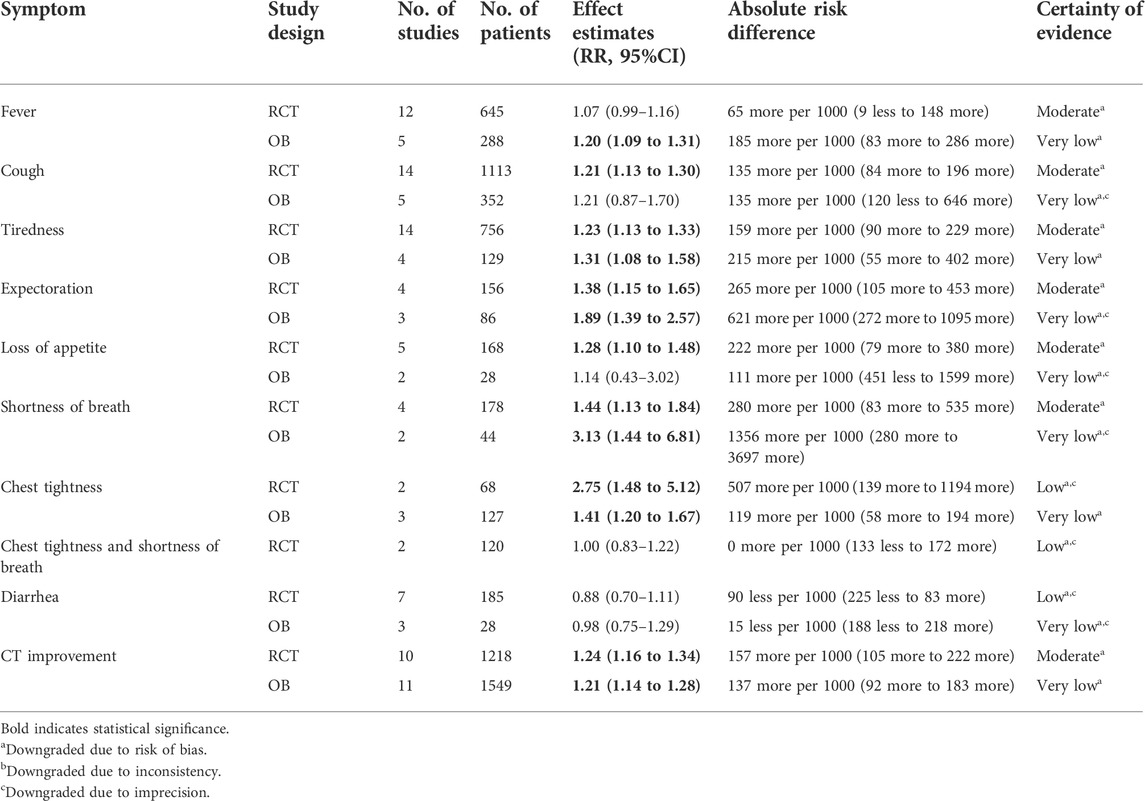
TABLE 3. GRADE summary of findings table showing certainty of evidence of weighted averages of different effect estimates on rate of symptom resolution in mild/moderate COVID-19 patients.
Thirty-seven studies (21 RCTs, 16 OBs) including 13,695 patients reported 61 types of adverse events (See Supplementary Table S8 for incidence and difference between groups of adverse reactions). Compared with the usual supportive treatment, the LHQW group showed significant difference in reducing the incidence of diarrhea (5.63% vs. 13.38%). However, JHQG group showed significant difference in increasing the incidence of diarrhea (32.93 vs. 0.00%). Commonly, patients in the CHM group had a lower incidence rate among the other adverse reactions. Adverse reactions reported in 4 or more studies included diarrhea, nausea, vomiting, loss of appetite, liver dysfunction and renal dysfunction (Figure 3), but no adverse reactions except diarrhea showed a statistical difference.
CHM applications in treating infectious diseases through the long history of China, its efficacies were also shown in treating other types of viral infections including those caused by SARS-CoV (severe acute respiratory syndrome coronavirus), MERS-CoV (Middle East respiratory syndrome coronavirus) as well as Ebola virus. During the COVID-19 pandemic, the unique advantages of CHM were completely utilized in treating pandemics and combined with western medicine to make great contributions to the control of the pandemic in China. This review provided a comprehensive overview of the evidence for CHM treatments in mild/moderate patients with COVID-19 as of 19th March 2022, including 59 studies (35 RCTs, 24 OBs) enrolling 16,580 patients, 38 kinds of CHM were involved. The certainty of evidence was very low to low, with all evidence downgrading primarily due to the risk of bias. Therefore, the interpretation of results was mainly based on the analysis of RCTs. The results indicated that, for mild/moderate patients, compared to usual supportive treatment, CHM presented significant effects in various patient-important outcomes, especially in reducing the rate of conversion to severe cases. Preventing exacerbation is key in moderate COVID-19 patients’ treatment. When the non-severe patient base is large, the rate of conversion to severe cases will directly influence the number of severe patients. Meanwhile, CHM also showed advantages in reducing rate of mortality; shortening time to symptom (fever, cough, tiredness, expectoration, shortness of breath and sore throat) resolution, length of hospital stay and time to viral clearance; increasing rate of nucleic acid conversion, rate of symptom (fever, cough, tiredness, expectoration, loss of appetite, shortness of breath and chest tightness) resolution and CT improvement. In terms of adverse reactions, we did not find serious adverse events related to CHMs in both mild and moderate patients, which indicated that CHM may be relatively safe for mild/moderate COVID-19 patients. In addition to diarrhea, no adverse reactions showed significant difference between CHM group and control group.
Because of the incomplete reporting of included studies, we failed to perform subgroup analyses. However, the univariate regression analysis result of a cohort study (Tian et al., 2020) revealed sex (male), age, fever, cough, and fatigue as risk factors for progression to severe disease, and HSY Formula could significantly reduce the rate of conversion to severe cases, which may effectively prevent and treat the COVID-19. Another RCT performed a multivariate logistic regression analysis, which indicated that age and patients’ source (centralized isolation site) were independent risk factors for worsening during treatment, the rate of conversion to severe cases also showed a significant difference between HSBD granule group and control group (Zhao et al., 2021). The performance of CHM in rate of conversion to severe cases is proof of its theory of “preventive treatment of disease” from a scientific perspective, as CHM could prevent the disease from becoming severe in the early stage, which also provided robust evidence for advancing the therapeutic window to the early stage of CHM in the treatment of COVID-19. The results of meta-regression analysis were similar to the original results. Besides, sensitivity analyses proved the robustness of our findings. Publication bias was detected in rate of conversion to severe cases and length of hospital stay, and we downgraded the certainty of evidence accordingly.
Regarding the adverse reactions, both evidence from RCTs and OBs showed a relatively high liver dysfunction rate, which is usually higher in control group than in CHM group without statistical difference. Chai et al. (Chai et al., 2020) pointed out that SARS-CoV-2 has been shown to result in an injury to the liver, and cholangiocyte dysfunction and other causes such as drug and systemic inflammatory response rather than hepatocyte damage may induce liver abnormalities. This may explain, to a certain extent, the higher incidence of liver dysfunction than other adverse reactions. For the incidence of diarrhea, as one of key components of LHQW, Pogostemon cablin has been shown to ameliorate diarrhea and improve the host-defense of the gastrointestinal tract (Zhou, 2018), which resulted in the significant advantage of LHQW in reducing the incidence rate of diarrhea (Hu K. et al., 2021). On the contrary, JHQG consist of the components such as Scutellaria baicalensis and Anemarrhena asphodeloides which were bitter in taste and could easily lead to diarrhea. JHQG was used in high doses in studies reporting adverse reactions of diarrhea, the above reasons may lead to diarrhea. In addition, 70.37% (19 cases/27 cases) of the patients with diarrhea in the CHM group had improved after 1–2 days without any special treatment (Duan et al., 2020).
CHM was effective against COVID-19 and could be verified by pharmacological mechanism research. Among the CHM treatments of COVID-19, LHQW, QFPD, JHQG, and XFQR were composed of MXSG and other prescriptions. MXSG could down-regulate the secretion level and protein expression level of Interferon α/β (IFN-α/β) in macrophages, inhibit the proliferation of virus (Shi et al., 2017; Zhang S. Y. et al., 2019), improve pulmonary interstitial edema caused by endotoxin (Han, 2020), and play an effective antiviral role. LHQW could block the binding of SARS-CoV-2 with the angiotensin converting enzyme (Niu M. et al., 2020), repress the action of the COVID-19 virus (Zhu et al., 2003), and lessen the content of the virus in cells (Jia et al., 2020; Li et al., 2020). JHQG, which is mainly composed of kaempferol, stigmasterol, has been proved to have antiviral, anti-inflammatory, and immunomodulatory effects based on a modern pharmacological study (Shen et al., 2020), and primarily through phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT), hypoxia-inducible factor-1 (HIF-1), tumor necrosis factor-α (TNF-α), mitogen-activated protein kinase (MAPK), and NF-kB signaling pathways for COVID-19 (Li et al., 2022). Network pharmacology studies have shown that QFPD had 51 potential targets for the treatment of COVID-19 and 26 core components (Zhou et al., 2020). QFPD could straightforwardly follow up on the 3CLpro, block its duplication and angiotensin converting enzyme II (ACE2) to diminish infection passage into cells (Xia et al., 2021). XFQR could remove inflammatory mediators and improve lung function (Zeng and Jin, 2019), and was effective in shortening the length of hospital stay. JYH had advantages in shortening length of hospital stay and accelerating nucleic acid negative conversion, mainly because Lonicera japonica Thunb. contained a large amount of microRNA (miRNA) components, which could effectively prevent or inhibit the virus from invading cells (Zhou et al., 2015). As the two phenolic acids with the highest content in JYH, honeysuckle glycoside and chlorogenic acid had a strong reducibility effect and could free radical scavenging (Du et al., 2019). The three components of RDN (Prunus armeniaca L. var.ansu Maxim., Lonicera japonica Thunb. and Carodira jasminoides Ellis) were proven antiviral properties that inhibit SARS-CoV-2 proliferation in vitro and shorten hospital stays (Romero et al., 2006; Efferth et al., 2008; Zhou et al., 2015; Haq et al., 2020). GGQL had a certain intervention effect on rotavirus adsorption to host cells (Yang et al., 2010); JWYPF had a two-way immunomodulatory effect (Li et al., 2011; Ren, 2018; Ma et al., 2020); SAYL was effective in relieving symptoms such as wheezing and coughing in mild patients, and had a favorable impact on inflammatory cytokines and lung function, these 3 CHMs could accelerate virus clearance (Wu et al., 2021).
Andrographolide, the active ingredient of XYP, possessed a high binding affinity to the main protease of SARS-CoV-2, which could activate T lymphocytes, recognize and kill virus-infected host cells or release antiviral cytokines to inhibit virus replication and accelerate time to virus clearance (Peng et al., 2002; Sheeja and Kuttan, 2007a; b). A network pharmacology study found that YDJD inhibited the phosphorylation of p65 by interacting strongly with kappa B (NF-κB) p65 residues to suppress the inflammatory response. QSPDFZ could inhibit the production of inflammatory factors, prevent the virus from attaching to cells, and mobilize the immune function to fight against COVID-19 (Zhong et al., 2018; Shao et al., 2020). RYN contained chemical components such as caffeic acid and flavonoids, which had anti-inflammatory, antiviral, and blood circulation promotion effects. The active ingredients in BZYQ could enhance the phagocytosis of bacteria and viruses by monocyte-macrophage and reticuloendothelial system, thereby enhancing human immune function (Zhan et al., 2017).
The most important thing for mild or moderate patients was to reduce their conversion to severe disease, and the severity of the disease is related to the inflammatory cytokine storm. CHM had an advantage in reducing the rate of severe cases. From the point of view of pharmacological mechanism, there were 246 targets in LHQW, which could act on interleukin 6 (IL-6), TNF-a and other signaling pathways to reduce the inflammatory response in patients (Peng W. et al., 2020). The main targets of HSBD in the treatment of COVID-19 were mitogen-activated protein kinase (MAPK) 3, MAPK 8, TNF, IL-6 and tumor protein p53 (TP53) (Tao et al., 2020), quercetin, ursolic acid and baicalein in HSBD could reduce IL-6 and angiotensin converting enzyme 2 (ACE2) (Niu W. H. et al., 2020; Niu et al., 2021). As the core prescription of QFPD, MXSG could exert an anti-platelet aggregation effect through ephedrine. Chemicals in QFPD could interfere with toll-like receptor 4 (TLR4), and regulate nuclear factor kappa light chain enhancer of activated B cells (NF-kβ) and MAPK signaling pathways to inhibit the release of inflammatory factors (Yang R. et al., 2020). There were 17 chemical components in JHQG, which mainly act on prostaglandin-endoperoxide synthase 2 (PTGS2), TNF-α, NF-κB, IL-6 and other multiple pathways through the Toll-like receptor signaling pathway, and play an anti-COVID-19 effect in a multi-target manner (Peng W. P. et al., 2020). In terms of the composition of QFTXFZ, Pogostemon cablin (Blanco)Benth. oil, Ephedra sinica Stapf, Glycine max (L.) Merr. and other ingredients had antibacterial and antiviral effects, which could inhibit virus replication and regulate inflammatory responses (Shao et al., 2020). Honokiol extracted in HSY could inhibit transmembrane glycoprotein cluster of differentiation 44 (CD44) and CD54, reduce IL-1β, IL-6 and TNF-α (Zhang X. W. et al., 2019), Amomum villosum Lour. could inhibit the binding of S-protein to human ACE2 and reduce virus replication (Niu M. et al., 2020). JYH contained iridoid components such as swertiamarine, which could inhibit the key PI3K/AKT inflammatory pathway and prevent the occurrence of inflammatory factor storm (Jin et al., 2006).
This study had several advantages. Firstly, the study systematically searched all available evidence to evaluate the efficacy and safety of CHM. Secondly, the back-to-back principle for literature screening, data extraction, and bias risk assessment was strictly followed, which ensured the methodological quality. In addition, this systematic review and meta-analysis were conducted and reported following internationally recognized standards to ensure both methodological and reporting quality, and improve research readability. Thirdly, we included all patient-important outcomes considered WHO “Therapeutics and COVID-19: living guideline” (Agarwal et al., 2020) and the “Core Outcome Set for Clinical Trials on Coronavirus Disease 2019”(Jin X. et al., 2020), which could help this review focus on more critical and important outcomes and prove the efficacy of Chinese herbal medicine from a widely recognized perspective. Fourthly, in order to present the efficacy of CHM taking into account the baseline risks, for the dichotomous outcomes, we also calculated the ARD (Agarwal et al., 2017). Moreover, we performed meta-regression analyses, sensitivity analyses and publication bias checks to explore the sources of heterogeneity and test the robustness of the results.
Meanwhile, this study also has some limitations. Firstly, the eligible studies only included general populations without comorbidities, which still lacked evidence for specific populations, such as people with tumors, obesity, chronic kidney disease, etc. Accordingly, the results may be indirect to inform care of patients with comorbidities in practice. Secondly, unlike western medicine, the clinical diagnosis and treatment of traditional Chinese medicine (TCM) are based on comprehensive information such as the patient’s symptoms, tongue image and pulse image, which is the syndrome differentiation, then treatments are conducted according to the identified TCM syndrome type. However, insufficient literature to support our interpretation of the results from the perspective of syndrome differentiation and treatment may limit the thorough assessment of the efficacy and advantages of CHM in the treatment of COVID-19. Thirdly, due to the complicated reality of the epidemic, the included studies are inadequate in study design and reporting, such as no allocation concealment during randomization, no blinding of researchers and patients and inadequate adjustment of confounders etc., these limitations may reduce the reliability of the results. Fourthly, due to the high risk of bias in the CHM primary studies, most results were assessed as low or very low certainty of evidence, indicating that the true effect might or probably be markedly different from the estimated effect.
CHM had an excellent performance in adapting to the specific symptoms of different mild or moderate patients, reducing the use of western medicine, and in the global promotion and acceptance. All patient recruitment was completed by October 2020 in eligible studies, when none of the patients had been vaccinated against COVID-19, nevertheless, Chinese patent medicines still showed good efficacy. Evidence-based evidence showed that CHM was beneficial in treating mild or moderate patients, but each CHM had specific symptoms to which it was adapted and should be differentiated in clinical use. Our discussion focused on CPM considering their internationally recognized advantages such as relatively mature production process and quality controllable pharmaceutical raw materials. According to the guidelines of traditional Chinese medicine (CAIM, 2020; Wang and Huang, 2020; NHC, 2022), LHQW, JHQG, SFJD and TRQ were used in mild or moderate patients with fever, chills, muscle aches, chest tightness, shortness of breath, sore throat and less phlegm, dry mouth and bitterness. XBJ was effective for patients with fever, palpitations, irritability, and for infection-induced systemic inflammatory syndrome and multiple organ dysfunction syndromes. RDN was suitable for patients with high fever, headache and body pain, cough, and yellow sputum. QFPD is applicable to patients of any type. Published clinical studies have shown that GGQL was suitable for patients with typical gastrointestinal symptoms, such as diarrhea and abdominal pain (Wang L. Q. et al., 2021); XYP may be more advantageous for mild or moderate patients with fever and respiratory symptoms or pulmonary impact characteristics (Zhang X. Y. et al., 2021); when patients were coughing and Expectoration, LHQK could be considered (Sun et al., 2020); RYN was more effective for the symptoms of dry throat, sore throat, cough, fatigue, fever, and chest tightness (Yang M. B. et al., 2020); XST could be used for patients who were chill, fever or no fever, dry cough, dry throat, fatigue, chest tightness and vomiting (Li et al., 2021).
Treated mild or moderate COVID-19 patients with CHM may reduce the use of western medicine. According to the condition of western medicine treatment reported by eligible studies, we found that the use rates of Lopinavir (Xin et al., 2020), Oseltamivir (Xiao M. Z. et al., 2020; Tian et al., 2020; An et al., 2021), Arbidol (Xiao M. Z. et al., 2020; Tian et al., 2020), Ribavirin (Tian et al., 2020), Anti-infective drugs (Xiao M. Z. et al., 2020; An et al., 2021) such as Macrolide (Xiao M. Z. et al., 2020), Antibiotics, Moxifloxacin, Clarithromycin (Tian et al., 2020) showed significant difference between CHM group and control group, and medication rates in CHM group was lower.
The CHM differentiation and treatment method have gradually spread throughout the world, and CHM also has great accessibility in the world. Chinese medicine has spread to more than 100 countries and has developed into an international industry (WHO, 2013). There are about 100,000 Chinese medicine clinics, 300,000 practitioners, and no less than 1,000 CHM education institutions worldwide. As the “Three Medicines and Three Prescriptions” recommended in the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia(NHC, 2022) issued by the National Health Commission (China), HSBD was recorded as an emergency registered drug in the United Arab Emirates; XFBD was approved and marketed by the Natural and non-Prescription Health Products Directorate in Canada; JHQG was the first Chinese patent medicine to complete a clinical trial oriented by drug registration overseas; LHQW has obtained marketing licenses in seven countries from 2012 to 2020, such as Canada. In terms of economic cost savings, CHM also possessed certain advantages. The results of a cost-benefit comparative analysis showed that as of 19 February 2020, 45,027 patients had been diagnosed in Wuhan, compared with western medicine using alone, CHM would save an average of 695.28 million dollars (Wang J. et al., 2020). In addition, a cross-sectional study also stated that CHM treatment was significantly negatively associated with non-pharmacologic treatment costs in total cases, moderate cases, and cases without comorbidities (Dong et al., 2021), which also indicated that CHM was beneficial for cost saving.
The study results showed that CHM had advantages in reducing rate of conversion to severe cases and mortality, shortening time to symptoms resolution, length of hospital stay and time to viral clearance, and increasing rate of nucleic acid conversion and rate of symptoms resolution for mild/moderate patients. No serious adverse events were observed for patients with the treatment of CHMs. However, due to the small sample size and high risk of bias in the randomization process generated and unadjusted confounders, in the context of the continuous variation of the virus, rigorously designed clinical trials and mechanism studies are still warranted to further confirm the effectiveness and safety of CHM in the treatment of COVID-19.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
LG, XS, JL, and LH designed the study. Search strategy has been developed by ML. HZ, ML, CT, and HL screened the references resulted from the literature search and extracted the required data. Data synthesis and paper writing by HZ, ML. LG, JL, YZ, and JS are responsible for the revision and proofreading of the manuscript. All authors reviewed and critically revised the protocol and the manuscript.
This work was supported by the Fundamental Research Funds for the Central Public Welfare Research Institutes (No. ZZ15-WT-05). The funding had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank Yajie Liu (China Academy of Chinese Medical Sciences), Wenzheng Zhang (China Academy of Chinese Medical Sciences), Jiayue Jin (China Academy of Chinese Medical Sciences), Simeng Ren (China Academy of Chinese Medical Sciences), and Sihong Yang (China Academy of Chinese Medical Sciences) for participating in study screening in the early of this study, Yafei Liu (Lanzhou University) for participating in search strategy developing and study screening, Yao Lu (Lanzhou University), Minyao Sun (Lanzhou University) and Bei Pan (Lanzhou University) for participating in study screening.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.988237/full#supplementary-material
Agarwal, A., Johnston, B. C., Vernooij, R. W. M., Carrasco-Labra, A., Brignardello-Petersen, R., Neumann, I., et al. (2017). Authors seldom report the most patient-important outcomes and absolute effect measures in systematic review abstracts. J. Clin. Epidemiol. 81, 3–12. doi:10.1016/j.jclinepi.2016.08.004
Agarwal, A., Rochwerg, B., Lamontagne, F., Siemieniuk, R. A., Agoritsas, T., Askie, L., et al. (2020). A living WHO guideline on drugs for covid-19. Bmj 370, m3379. doi:10.1136/bmj.m3379
Ai, X. Y., Lin, L. P., Xie, M., and Tan, X. H. (2020a). Effect of integrated traditional Chinese and Western medicine on T lymphocyte subsets of patients with normal type of. Guangdong Med. J. 41 (12), 1203–1206.
Ai, X. Y., Luo, C., Lin, L. P., Xie, M., Fan, H. M., and Tan, X. H. (2020b). Therapeutic effect of integrated traditional Chinese and western medicine on COVID-19 in Guangzhou. China Trop. Med. 20 (08), 746–750.
Ai, Z. Z., Zhou, S. S., Li, W. N., Wang, M. F., Wang, L. Q., Hu, G., et al. (2020c). Fei yan No. 1" as a combined treatment for COVID-19: An efficacy and potential mechanistic study. Front. Pharmacol. 11, 581277. doi:10.3389/fphar.2020.581277
An, X. D., Xu, X., Xiao, M. Z., Min, X. J., Lyu, Y., Tian, J., et al. (2021). Efficacy of Jinhua qinggan granules combined with western medicine in the treatment of confirmed and suspected COVID-19: A randomized controlled trial. Front. Med. 8, 728055. doi:10.3389/fmed.2021.728055
CAIM (2020). Expert consensus on the prevention and treatment of novel coronavirus pneumonia with integrated traditional Chinese and Western medicine. Chin J Integr Tradit West Med 40 (12), 1413–1423. doi:10.7661/j.cjim.20220126.034
Chai, X., Hu, L., Zhang, Y., Han, W., Lu, Z., Ke, A., et al. (2020). Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv 2020, 931766. doi:10.1101/2020.02.03.931766
Chen, J., Lin, S., Niu, C., and Xiao, Q. (2021a). Clinical evaluation of Shufeng Jiedu capsules combined with umifenovir (arbidol) in the treatment of common-type COVID-19: A retrospective study. Expert Rev. Respir. Med. 15 (2), 257–265. doi:10.1080/17476348.2020.1822741
Chen, L., Chen, Y. G., Chen, Z. G., Liu, F., Wu, J. H., Xia, Y., et al. (2020a). Retrospective analysis on clinical efficacy of Ganlu Xiaodu decoction combined with western medicine in treatment of common COVID-19 patients. Chin. J. Exp. Tradit. Med. Formulae 26 (19), 60–67. doi:10.13422/j.cnki.syfjx.20202011
Chen, L., Liu, F., Wu, J. H., Song, H. Y., Xia, J. S., Sheng, B., et al. (2020b). Clinical efficacy of Shufeng Jiedu capsule combined with western medicine in treatment of common COVID-19 patients by retrospective analysis. J. Emerg. Tradit. Chin. 24 (19), 14–20. doi:10.13422/j.cnki.syfjx.20201628
Chen, R. B., Shi, N. N., Li, H. Z., Jiao, L. W., Ma, Y., Liu, B., et al. (2021b). A multi-center retrospective study of Qingfei Paidu Decoction combined with conventional western medicine in the treatment of novel coronavirus pneumonia complicated with chronic hepatitis B. J. Tradit. Chin. Med. 62 (19), 1694–1701. doi:10.13288/j.11-2166/r.2021.19.008
Ding, X. J., Zhang, Y., He, D. C., Zhang, M. Y., Tan, Y. H., Yu, A. R., et al. (2020). Clinical effect and mechanism of Qingfei Touxie Fuzheng recipe in the treatment of novel coronavirus pneumonia. Her. Med. 39 (05), 640–644. doi:10.3870/j.issn.1004-0781.2020.05.012
Dong, M., Yang, Z., Chen, Y., Sun, J., Ma, W., Cheng, S., et al. (2021). Hospitalization costs of COVID-19 cases and their associated factors in guangdong, China: A cross-sectional study. Front. Med. 8, 655231. doi:10.3389/fmed.2021.655231
Du, Y. Q., Duan, Z. K., Dong, S. H., Zhao, P., and Song, S. J. (2019). Network pharmacology analysis of the anti-inflammatory pharmacological mechanisms of active ingredients from Lonicera japonica. Chin. J. Med. Chem. 29 (02), 002. doi:10.14142/j.cnki.cn21-1313/r.2019.02.002
Duan, C., Xia, W. G., Zhen, C. J., Sun, G. B., Li, Z. L., Li, Q. L., et al. (2020). Clinical observation of Jinhua Qinggan granule combined with conventional western medicine treatment regimen in the treatment of mild novel coronavirus pneumonia. J. Tradit. Chin. Med. 61 (17), 1473–1477. doi:10.13288/j.11-2166/r.2020.17.001
Efferth, T., Romero, M. R., Wolf, D. G., Stamminger, T., Marin, J. J. G., and Marschall, M. (2008). The antiviral activities of artemisinin and artesunate. Clin. Infect. Dis. 47 (6), 804–811. doi:10.1086/591195
Egger, M., Davey Smith, G., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. Bmj 315 (7109), 629–634. doi:10.1136/bmj.315.7109.629
Feng, Y., Zhu, B., Liu, Y., Liu, Y., Zhou, G., Yang, L., et al. (2022). Yindan Jiedu granules exhibit anti-inflammatory effect in patients with novel Coronavirus disease (COVID-19) by suppressing the NF-κB signaling pathway. Phytomedicine. 95, 153784. doi:10.1016/j.phymed.2021.153784
Fu, X. X., Lin, L. P., and Tan, X. H. (2020). Clinical observation on effect of Toujie quwen granules in treatment of COVID-19. Chin. J. Exp. Tradit. Med. Formulae 26 (12), 44–48. doi:10.13422/j.cnki.syfjx.20201314
Guyatt, G. H., Oxman, A. D., Vist, G. E., Kunz, R., Falck-Ytter, Y., Alonso-Coello, P., et al. (2008). Grade: An emerging consensus on rating quality of evidence and strength of recommendations. Bmj 336 (7650), 924–926. doi:10.1136/bmj.39489.470347.AD
Han, J. Y. (2020). The mechanisms underlying the beneficial effects of ma-xing-shi-Gan-tang on pulmonary interstitial edema and the beneficial effects of yiqifumai on shock. World Sci. Technol. Mod. Trad. Chin. Med. 22 (02), 248–256. doi:10.11842/wst.20200218002
Haq, F. U., Roman, M., Ahmad, K., Rahman, S. U., Shah, S. M. A., Suleman, N., et al. (2020). Artemisia annua: Trials are needed for COVID-19. Phytother. Res. 34 (10), 2423–2424. doi:10.1002/ptr.6733
He, Q., Zhang, Q. J., Gan, X. W., and Li, X. G. (2021). Analysis of clinical efficacy of Buzhong Yiqi decoction in the treatment of mild novel coronavirus pneumonia. J. Emerg. Tradit. Chin. 30 (03), 385–387. doi:10.3969/j.issn.1004-745X.2021.03.003
Hu, F., Guo, A. H., Huang, L., Yu, W. X., Liu, G. F., Gao, X. S., et al. (2021a). Multi-center clinical observation of Jinyinhua oral liquid combined with western medicine in the treatment of moderate type of novel coronavirus pneumonia. J. Tradit. Chin. Med. 62 (06), 510–515. doi:10.13288/j.11-2166/r.2021.06.011
Hu, K., Guan, W. J., Bi, Y., Zhang, W., Li, L. J., Zhang, B., et al. (2021b). Efficacy and safety of lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: A multicenter, prospective, randomized controlled trial [phytomedicine 85 (2021) 153242]. Phytomedicine. 93, 153775. doi:10.1016/j.phymed.2021.153775
Jac, S., J, S., and Mj, P. (2019). RoB 2: A revised tool for assessing risk of bias in randomized trials. BMJ 366, l4898. doi:10.1136/bmj.l4898
Jia, Z. H., Li, H. R., Chang, L. P., and Wei, C. (2020). Historical review and reflection on the response of traditional Chinese Medicine to epidemic Diseases. Chin. J. Exp. Tradit. Med. Formul. 26 (11), 1–7. doi:10.13422/j.cnki.syfjx.20201071
Jin, W., Lu, Y., Zhao, W., Tang, S. Y., Sang, X. Y., and Zhang, L. S. (2020a). The efficacy of recommended treatments with integrated Chinese and western medicine on coronavirus disease 2019 ( COVID-19) in sichuan: A clinical trial observation. Pharmacol. Clin. Chin. Mater Med. 36 (06), 6–10. doi:10.13412/j.cnki.zyyl.20201110.006
Jin, X. H., Ohgami, K., Shiratori, K., Suzuki, Y., Koyama, Y., Yoshida, K., et al. (2006). Effects of blue honeysuckle (Lonicera caerulea L.) extract on lipopolysaccharide-induced inflammation in vitro and in vivo. Exp. Eye Res. 82 (5), 860–867. doi:10.1016/j.exer.2005.10.024
Jin, X., Pang, B., Zhang, J., Liu, Q., Yang, Z., Feng, J., et al. (2020b). Core outcome set for clinical trials on coronavirus disease 2019 (COS-COVID). Eng. (Beijing) 6 (10), 1147–1152. doi:10.1016/j.eng.2020.03.002
Lan, J., Fan, G. C., and Meng, L. (2020). Clinical efficacy and mechanism of Qushi Paidu Fuzheng Recipe in treatment of corona virus disease 2019 with syndrome of cold dampness lung stagnation and epidemic toxin closing lung. Chin. J. Integr. Tradit. West Med. Intensive Crit. Care 27 (3), 262–266. doi:10.3969/j.issn.1008-9691.2020.03.002
Li, F. M., Chen, J. H., Sun, X. W., Liu, H. Z., and Liu, X. X. (2011). Effects of Jiawei Yupingfeng Powder on the immune function of T lymphocytes in mice irradiated with 60Coγ. Lishizhen Med. Mater Med. Res. 22 (05), 1179–1180. doi:10.3969/j.issn.1008-0805.2011.05.065
Li, H. L. (2020). Analysis of clinical effect of integrated traditional Chinese and western medicine in the treatment of patients with new coronary pneumonia. World Latest Med. Inf. 20 (91), 187–188. doi:10.3969/j.issn.1671-3141.2020.91.102
Li, M., Li, Y. P., Su, X. Y., Xing, S. L., and Xu, T. (2022). Analytical method of pharmacological mechanism of TCM based on Transcriptomics and its application in Jinhua Qinggan granules. Chin. J. Med. Guide 24 (02), 164–169. doi:10.3969/j.issn.1009-0959.2022.02.013
Li, R. F., Hou, Y. L., Huang, J. C., Pan, W. Q., Qinhai, M., Yongxia, S., et al. (2020). Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol. Res. 156, 104761. doi:10.1016/j.phrs.2020.104761
Li, Z. J., Cai, Q. X., Yuan, J., Tan, X. H., Wu, Q. K., Tang, S. H., et al. (2021). Clinical study on acupuncture point injection of xue shuan tong for coronavirus disease 2019. Shanghai J. Acu-mox 40 (10), 1182–1184. doi:10.13460/j.issn.1005-0957.2021.10.1182
Lin, F. F., Huang, J. P., Yang, J., Zhang, S. Z., Xie, S. R., and Zhou, F. (2020). Clinical study of Xuanfei Qingre recipe in adjuvant treatment of common novel coronavirus pneumonia. Zhejiang J. Integr. Tradit. 30 (12), 977–981. doi:10.3969/j.issn.1005-4561.2020.12.007
Liu, J., Yang, W., Liu, Y., Lu, C., Ruan, L., Zhao, C., et al. (2021a). Combination of hua Shi Bai du granule (Q-14) and standard care in the treatment of patients with coronavirus disease 2019 (COVID-19): A single-center, open-label, randomized controlled trial. Phytomedicine. 91, 153671. doi:10.1016/j.phymed.2021.153671
Liu, L., Shi, F., Tu, P., Chen, C., Zhang, M., Li, X., et al. (2021b). Arbidol combined with the Chinese medicine Lianhuaqingwen capsule versus arbidol alone in the treatment of COVID-19. Medicine 100 (4), e24475. doi:10.1097/MD.0000000000024475
Liu, Z., Li, X., Gou, C., Li, L., Luo, X., Zhang, C., et al. (2020). Effect of Jinhua Qinggan granules on novel coronavirus pneumonia in patients. J. Tradit. Chin. Med. 40 (3), 467–472. doi:10.19852/j.cnki.jtcm.2020.03.016
Ma, K., Liu, Y., Kang, S. G., Zhang, N. B., and Shi, X. L. (2020). Advances in pharmaceutical experiments and clinical application of Yupingfeng powder. Mod. J. Integr. Tradit. Chin. West Med. 29 (05), 565–570. doi:10.3969/j.issn.1008-8849.2020.05.028
Ma, Q., Xie, Y., Wang, Z., Lei, B., Chen, R., Liu, B., et al. (2021). Efficacy and safety of ReDuNing injection as a treatment for COVID-19 and its inhibitory effect against SARS-CoV-2. J. Ethnopharmacol. 279, 114367. doi:10.1016/j.jep.2021.114367
NHC (2020). China-WHO joint investigation report on novel coronavirus pneumonia (COVID-19). Available: http://www.nhc.gov.cn/jkj/s3578/202002/87fd92510d094e4b9bad597608f5cc2c/files/fa3ab9461d0540c294b9982ac22af64d.pdf.
NHC (2022). The ninth edition guideline for diagnosis and treatment COVID-19. The National Health Commission of the People’s Republic of China [Online]. Available: http://www.gov.cn/zhengce/zhengceku/2022-03/15/content_5679257.htm.
Ni, L., Wen, Z., Hu, X., Tang, W., Wang, H., Zhou, L., et al. (2021). Effects of shuanghuanglian oral liquids on patients with COVID-19: A randomized, open-label, parallel-controlled, multicenter clinical trial. Front. Med. 15 (5), 704–717. doi:10.1007/s11684-021-0853-6
Niu, M., Wang, R., and Wang, Z. (2020a). Rapid screening model and application of anti-new coronavirus TCM prescription based on clinical experience and molecular docking technology. China J. Chin. Mat. Med. 45 (6), 1213–1218. doi:10.19540/j.cnki.cjcmm.20200206.501
Niu, W. H., Wu, F., Cao, W. Y., Wu, Z.-G., Chao, Y.-C., Liang, C., et al. (2021). Network pharmacology for the identification of phytochemicals in traditional Chinese medicine for COVID-19 that may regulate interleukin-6. Biosci. Rep. 41 (1), BSR20202583. doi:10.1042/BSR20202583
Niu, W. H., Wu, F., Cui, H. M., Cao, W., Chao, Y., Wu, Z., et al. (2020b). Network pharmacology analysis to identify phytochemicals in traditional Chinese medicines that may regulate ACE2 for the treatment of COVID-19. Evid. Based. Complement. Altern. Med. 2020, 7493281. doi:10.1155/2020/7493281
Ouzzani, M., Hammady, H., Fedorowicz, Z., and Elmagarmid, A. (2016). Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 5 (1), 210. doi:10.1186/s13643-016-0384-4
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Bmj 372, n71. doi:10.1136/bmj.n71
Peng, G. Y., Zhou, F., Ding, R. N., Li, H. D., and Yao, K. (2002). Modulation of lianbizi injection (andrographolide) on some immune functions. China J. Chin. Mater Med.27 (02), 70–73. doi:10.3321/j.issn:1001-5302.2002.02.022
Peng, W., Han, D., Xu, Y., Feng, F., and He, H. (2020a). To explore the material basis and mechanism of Lianhua Qingwen Prescription against COVID-19 based on network pharmacology. Integr. Respir. Med. 1, 3. doi:10.1051/irm/2020004
Peng, W. P., Xu, Y., Han, D., Feng, F. C., Gu, C., and Wang, Z. C. (2020b). Mechanism of Jinhua qinggan granules on COVID-19 based on network pharmacology and molecular docking. Nat. Product. Res. Dev. 32 (12), 1992–2002.
Ping, X. H., Xu, H. L., Fu, D. F., Zhou, Y. F., liu, L., and Xu, H. X. (2021). Clinical observation of Jiawei Yupingfeng powder combined with western medicine in the treatment of the novel coronavirus pneumonia. Med Forum 25 (02), 149–151. doi:10.19435/j.1672-1721.2021.02.001
Qiu, M., Tin, L. Q., and Zhu, D. (2021). Efficacy observation of maxing Xuanfei Jiedu decoction on common type of NCP. J. Emerg. Tradit. Chin. Med. 29 (07), 1129–1130+1132. doi:10.3969/j.issn.1004-745X.2020.07.001
Ren, H. F. (2018). Analysis of the effect of Jiawei Yupingfeng Powder Decoction in the treatment of senile type 2 diabetic hyperhidrosis. Chin. J. Clin. Ration. Drug Use 11 (34), 76–77. doi:10.15887/j.cnki.13-1389/r.2018.34.040
Romero, M. R., Serrano, M. A., Vallejo, M., Efferth, T., Alvarez, M., and Marin, J. J. G. (2006). Antiviral effect of artemisinin from Artemisia annua against a model member of the Flaviviridae family, the bovine viral diarrhoea virus (BVDV). Planta Med. 72 (13), 1169–1174. doi:10.1055/s-2006-947198
Shao, Z. B., Zhu, Y. X., Liu, S., H., Jiang, J. K., Wu, Q., Shen, J. Y., et al. (2020). A review on the clinical application of high frequency traditional Chinese medicine in the treatment of new coronavirus pneumonia. Chin. Tradit. Herb. Drugs 51 (05), 1153–1158. doi:10.7501/j.issn.0253-2670.2020.05.009
Sheeja, K., and Kuttan, G. (2007a). Activation of cytotoxic T lymphocyte responses and attenuation of tumor growth in vivo by Andrographis paniculata extract and andrographolide. Immunopharmacol. Immunotoxicol. 29 (1), 81–93. doi:10.1080/08923970701282726
Sheeja, K., and Kuttan, G. (2007b). Modulation of natural killer cell activity, antibody-dependent cellular cytotoxicity, and antibody-dependent complement-mediated cytotoxicity by andrographolide in normal and Ehrlich ascites carcinoma-bearing mice. Integr. Cancer Ther. 6 (1), 66–73. doi:10.1177/1534735406298975
Shen, F., Zhongying, F., and Wang, Y. (2020). A study on the potential molecular mechanism of active compounds binding to specific target proteins of SARS-CoV-2 in Jinhua Qinggan Granules to interfere with COVID-19 on the basis of network pharmacology and high-throughput molecular docking. World Sci. Technol. Mod. Trad. Chin. Med. 21, 1–10. doi:10.11842/wst.20200317010
Shi, N. N., Guo, L. P., Liu, B., Bian, Y., Chen, R., Chen, S., et al. (2021). Efficacy and safety of Chinese herbal medicine versus lopinavir-ritonavir in adult patients with coronavirus disease 2019: A non-randomized controlled trial. Phytomedicine 81, 153367. doi:10.1016/j.phymed.2020.153367
Shi, P. H., Qu, X. P., Zhou, Z., and Wang, S. Q. (2017). Research progress on anti-influenza virus effects of TCM compound prescription. China J. Tradit. Chin. Med. Pharm. 32 (03), 1172–1175.
Siemieniuk, R. A., Bartoszko, J. J., Ge, L., Zeraatkar, D., Izcovich, A., Kum, E., et al. (2020). Drug treatments for covid-19: Living systematic review and network meta-analysis. BMJ 370, m2980. doi:10.1136/bmj.m2980
Sterne, J. A., Hernán, M. A., Reeves, B. C., Savović, J., Berkman, N. D., Viswanathan, M., et al. (2016). ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ Clin. Res. ed.) 355, i4919. doi:10.1136/bmj.i4919
Sun, H. M., Xu, F., Zhang, L., Wei, C., Chen, Y. J., Wang, Q. X., et al. (2020). Study on clinical efficacy of Lianhua Qingke granule in treatment of mild and ordinary COVID-19. Chin. J. Exp. Tradit. Med. Formulae 26 (14), 29–34. doi:10.13422/j.cnki.syfjx.20201438
Sun, S. Q., Chen, F. F., Yin, C. W., Wang, J., Cai, W. R., Guo, J., et al. (2021). Clinical effect of Liushen Pill combined with conventional treatment on patients with COVID-19. Chin. Tradit. Pat. Med. 43 (08), 2277–2280. doi:10.3969/j.issn.1001-1528.2021.08.058
Sun, Y. N., Lv, W. L., Li, H., Xiao, Y., Yang, H. J., et al. (2021). Qingfei Paidu decoction in the treatment of mild/ordinary novel coronavirus pneumonia 295 multicenter clinical studies. J. Tradit. Chin. Med. 62 (07), 599–603. doi:10.13288/j.11-2166/r.2021.07.010
Tao, Q. Y., Du, J. X., Li, X. T., Zeng, J. Y., Tan, B., Xu, J., et al. (2020). Network pharmacology and molecular docking analysis on molecular targets and mechanisms of Huashi Baidu formula in the treatment of COVID-19. Drug Dev. Ind. Pharm. 46 (8), 1345–1353. doi:10.1080/03639045.2020.1788070
Tian, J. X., Yan, S. Y., Wang, H., Zhang, Y., Zheng, Y. J., Wu, H., et al. (2020). Hanshiyi formula, a medicine for sars-CoV2 infection in China, reduced the proportion of mild and moderate COVID-19 patients turning to severe status: A cohort study. Pharmacol. Res. 161, 105127. doi:10.1016/j.phrs.2020.105127
Wang, J. B., Wang, Z. X., Jing, J., Zhao, P., Dong, J. H., Zhou, Y.-F., et al. (2020b). Exploring an integrative therapy for treating COVID-19: A randomized controlled trial. Chin. J. Integr. Med. 26 (9), 648–655. doi:10.1007/s11655-020-3426-7
Wang, J., Huang, M., Len, A., and Lv, M. (2020a). Cost-benefit comparative analysis of traditional Chinese medicine and Western medicine in the treatment of COVID-19 in Wuhan. J Integr Trad Chin West Med 12 (03), 204–207+211. doi:10.3969/j.issn.1674-4616.2020.03.018
Wang, L. Q., Li, W. N., Huang, W., Zhou, Z. M., Deng, Y. L., Hu, Y. L., et al. (2021a). Clinical study of Gegen Qinlian Pill in the treatment of novel coronavirus pneumonia. Mod. Tradit. Chin. Med. Mater Mater-World Sci. Technol. 22 (10), 3509–3514. doi:10.3969/j.issn.1674-4616.2020.03.018
Wang, Q. L., Sun, L. F., Zhao, M. F., Li, Y. Y., and Zhu, Y. L. (2021b). The application effect of Qingfei Paidu decoction in the treatment of patients with COVID-19 with the syndrome of deficiency of both qi and yin. Acta Chin. Med. 36 (05), 910–914. doi:10.16368/j.issn.1674-8999.2021.05.192
Wang, Y., Li, L., Zhen, L., Ku, B. Q., Yu, R., and Zhang, X. F. (2021c). Clinical effects of Qingfei Paidu Decoction combined with conventional treatment on patients with coronavirus disease 2019. Chin. Tradit. Pat. Med. 43 (02), 656–659. doi:10.3969/j.issn.1001-1528.2021.03.017
Wang, Y. L., Xue, J., Dai, E. H., Xu, Z. G., Fen, C. X., Liu, H. D., et al. (2020c). Clinical study on the treatment of patients with novel coronavirus pneumonia and asymptomatic infection with integrated traditional Chinese and western medicin. Hebei J. TCM 42 (05), 645–649. doi:10.3969/j.issn.1002-2619.2020.05.001
Wang, Y. Y., and Huang, L. Q. (2020). A clinical diagnosis & treatment rapid advice guideline for integrating Chinese and Western medicine of COVID-19. Chine Res. Hosp. 7 (02), 51–64. doi:10.19450/j.cnki.jcrh.2020.02.013
Who, (2020). Director-General's opening remarks at the media briefing on COVID-19. [Online]. Available: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11-march-2020 (Accessed March 11, 2020).
Who, (2022a). Weekly epidemiological update on COVID-19. Available: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---20-april-2022 (Accessed April 20, 2022).
Who, (2022b). WHO Expert meeting on evaluation of traditional Chinese medicine in the treatment of COVID-19. Available: https://www.who.int/publications/m/item/who-expert-meeting-on-evaluation-of-traditional-chinese-medicine-in-the-treatment-of-covid-19 (Accessed FebruaryMarch 282, 2022).
Who, (2013). WHO traditional medicine strategy 2014-2023. Available: https://apps.who.int/iris/handle/10665/312342.
Wu, R., Wang, Z. L., Jiao, C. X., Ban, W. M., and Zhao, W. X. (2021). Observation of the clinical efficacy of Sanao Yulong mixture in the treatment of new coronary pneumonia. Clinl J. Tradit. Chin. Med. 33 (07), 1368–1371. doi:10.16448/j.cjtcm.2021.0737
Xia, K. Y., Zhao, Z. Y., Shah, T., Wang, J. Y., and Baloch, Z. (2021). Composition, clinical efficiency, and mechanism of NHC-approved "three Chinese medicines and three Chinese recipes" for COVID-19 treatment. Front. Pharmacol. 12, 781090. doi:10.3389/fphar.2021.781090
Xiao, M. Z., Tian, J. X., Zhou, Y. N., Xu, X., Min, X. J., Lv, Y., et al. (2020a). Efficacy of huoxiang zhengqi dropping pills and Lianhua qingwen granules in treatment of COVID-19: A randomized controlled trial. Pharmacol. Res. 161, 105126. doi:10.1016/j.phrs.2020.105126
Xiao, Q., Jiang, Y. J., Wu, S. S., Wang, Y., An, J., Xu, W. P., et al. (2020b). Value analysis of traditional Chinese medicine Shufengjiedu capsule combined with Arbidol in the treatment of mild novel coronavirus pneumonia. J. Emerg. Tradit. Chin. 29 (05), 756–758. doi:10.3969/j.issn.1004-745X.2020.05.002
Xin, S. Y., Cheng, X., Q., Zhu, B., Liao, X. L., Yang, F., Song, L., et al. (2020). Clinical retrospective study on the efficacy of Qingfei Paidu decoction combined with Western medicine for COVID-19 treatment. Biomed. Pharmacother. 129, 110500. doi:10.1016/j.biopha.2020.110500
Xiong, W. Z., Wang, G., Du, J., and Ai, W. (2020). Efficacy of herbal medicine (Xuanfei Baidu decoction) combined with conventional drug in treating COVID-19:A pilot randomized clinical trial. Integr. Med. Res. 9 (3), 100489. doi:10.1016/j.imr.2020.100489
Xu, X., Zhang, J., Zheng, W., Yang, Z., Zhao, X., Wang, C., et al. (2021). Efficacy and safety of reduning injection in the treatment of COVID-19: A randomized, multicenter clinical study. Ann. Palliat. Med. 10 (5), 5146–5155. doi:10.21037/apm-20-2121
Yan, Y. S., Qu, J. Y., Wei, Y. R., Fan, F. Y., Tang, Y. L., and Gao, S. (2021). Effect of Yinghuang Qingfei capsule and lopinavir/ritonavir treatment in patients with mild COVID-19. Tradit. Chin. Med. 10 (2), 284–290. doi:10.12677/tcm.2021.102039
Yang, M. B., Dang, S. S., Huang, S., Li, Y. J., and Guo, Y. L. (2020a). Multi-center clinical observation of reyanning mixture in treatment of COVID-19. Chin. J. Exp. Tradit. Med. Formulae 26 (14), 7–12. doi:10.13422/j.cnki.syfjx.20201321
Yang, M. M., Zhang, Y., Chen, W., and Luo, J. B. (2010). Expermi ent of Gegenq inlian M icropellet. s Anti- rotavirus E ffect in V itro. Chin. Arch. Tradit. Chin. Med. 28 (09), 1981–1983.
Yang, R., Liu, H., Bai, C., Wang, Y., Zhang, X., Guo, R., et al. (2020b). Chemical composition and pharmacological mechanism of Qingfei Paidu decoction and Ma xing Shi Gan decoction against coronavirus disease 2019 (COVID-19): In silico and experimental study. Pharmacol. Res. 157, 104820. doi:10.1016/j.phrs.2020.104820
Yao, K. T., Liu, M. Y., Li, X., Huang, J. H., and Cai, H. B. (2020). Retrospective clinical analysis on treatment of coronavirus disease 2019 with traditional Chinese medicine Lianhua qingwen. J. Emerg. Tradit. Chin. 26 (11), 8–12. doi:10.13422/j.cnki.syfjx.20201099
Yu, H. Y., Ren, X. H., Qi, X. X., Zuo, Q., and Liu, D. (2020a). A retrospective study on the efficacy of arbidol, Qingfei Paidu decoction, Lianhua qingwen capsules and jinye Baidu granules on patients with mild/common COVID-19 in a fangcang shelter hospital. Pharmacol. Clin. Chin. Mater Med. 36 (06), 2–6. doi:10.13412/j.cnki.zyyl.20201110.005
Yu, P., Li, Y. Z., Wan, S. B., and Wang, Y. (2020b). Efficacy observation of Lianhua Qingwen granules combined with Arbidol in the treatment of mild novel coronavirus pneumonia. Chin. Pharm. J. 55 (12), 1042–1045. doi:10.11669/cpj.2020.12.014
Zeng, C., Yuan, Z., Zhu, J., Wang, Y., Xie, Y., Ye, R., et al. (2021). Therapeutic effects of traditional Chinese medicine (Maxingshigan-Weijing Decoction) on COVID-19: An open-label randomized controlled trial. Integr. Med. Res. 10, 100782. doi:10.1016/j.imr.2021.100782
Zeng, G. Y., and Jin, Y. S. (2019). Clinical observation on maxing shigan decoction combined with weijing decoction in treating senile pulmonary inhalation pneumonia with phlegm-heat stagnation lung. Guangming J. Chin. Med. 34 (04), 528–529+552. doi:10.3969/j.issn.1003-8914.2019.04.014
Zeng, X. H., Ma, W. H., and Wang, J. (2020). Effect of Qingfei Paidu on clinical eddicacy of COVID-19 pneumonia with Phlegm heat blocking lung. Med. J. West China 32 (12), 1799–1801. doi:10.3969/j.issn.1672-3511.2020.12.017
Zhan, T. J., Kou, J., and Shen, H. (2017). The effect of tonic traditional Chinese medicine Astragalus in promoting anti-tumor immune function. Int. J. Immunol. Res. 40 (02), 188–192. doi:10.3760/cma.j.issn.1673-4394.2017.02.016
Zhang, C. T., Yang, Y., You, F. M., Huang, Q. S., Gao, P. Y., Tang, J. Y., et al. (2020a). Clinical study on COVID-19 from the perspective of "yidujiashi" theory. Pharmacol. Clin. Chin. Mater Med. 36 (02), 43–45. doi:10.13412/j.cnki.zyyl.20200327.002
Zhang, C. Y., Li, Z. H., Zhang, S., Wang, W., and Jiang, X. Q. (2020b). Clinical observation of Xuebijing in the treatment of COVID-19. Chin. J. Hosp. Pharm. 40 (09), 964–967. doi:10.13286/j.1001-5213.2020.09.03
Zhang, L. H., Zheng, X., Bai, X. K., Wang, Q., Chen, B., Wang, H., et al. (2021a). Association between use of Qingfei Paidu tang and mortality in hospitalized patients with COVID-19: A national retrospective registry study. Phytomedicine. 85, 153531. doi:10.1016/j.phymed.2021.153531
Zhang, L., Wu, L., Xu, X. L., Yuan, Y. D., Jiang, R., Yan, X., et al. (2022). Efficacy and safety of Lianhua Qingke tablets in the treatment of mild and common-type COVID-19: A randomized, controlled, multicenter clinical study. Evid. Based. Complement. Altern. Med. 2022, 8733598. doi:10.1155/2022/8733598
Zhang, N., Qu, Y. F., Jin, Y. Z., et al. (2020c). Retrospective study of traditional Chinese medicine "Ma xing shigan decoction" combined with western medicine in the treatment of ordinary COVID-19. J. Front. Med. 10 (15), 31–34.
Zhang, S. Y., He, G. L., Lu, G. F., Li, L., Zhang, B., Dai, B., et al. (2019a). Mechanism research of anti influenza virus of ephedra decocted earlier Maxing Shigan Decoction from the expression level of IFN-α/β protein mediated by TLR7/8. China J. Tradit. Chin. Med. Pharm. 34 (03), 1188–1193.
Zhang, X. W., Fan, N., Guo, Y. D., Wang, X., J., and Chen, M. (2019b). Regulating effect of magnolol on lipid metabolism and immune response in high-fat diet-induced atherosclerotic rats. Chin. J. Clin. Pharmacol. 35 (07), 647–650. doi:10.13699/j.cnki.1001-6821.2019.07.014
Zhang, X., Xue, Y., Chen, X., Wu, J. M., Su, Z.-J., Sun, M., et al. (2021b). Effects of tanreqing capsule on the negative conversion time of nucleic acid in patients with COVID-19: A retrospective cohort study. J. Integr. Med. 19 (1), 36–41. doi:10.1016/j.joim.2020.10.002
Zhang, X. Y., Lv, L., Zhou, Y. L., Xie, L. D., Xu, Q., Zou, X. F., et al. (2021c). Efficacy and safety of xiyanping injection in the treatment of COVID-19: A multicenter, prospective, open-label and randomized controlled trial. Phytother. Res. 35 (8), 4401–4410. doi:10.1002/ptr.7141
Zhang, Y. L., Lei, L., Xu, Y., Wei, D. R., and Hu, F. (2020d). Clinical efficacy of Jinyinhua oral liquid in the treatment of 80 patients with coronavirus disease 2019. China Pharm. 20 (09), 23–26. doi:10.3969/j.issn.1006-4931.2020.09.006
Zhao, C., Li, L., Yang, W., Lv, W. L., Wang, J., Guo, J., et al. (2021). Chinese medicine formula huashibaidu granule early treatment for mild COVID-19 patients: An unblinded, cluster-randomized clinical trial. Front. Med. 8, 696976. doi:10.3389/fmed.2021.696976
Zhong, Y. H., Lin, C., and Zhong, Y. Q. (2018). Effect of huo Pu xia ling tang on NF- κB inflammatory signaling in HBZY- 1 cells and diabetic nephropathy rats. Tradit. Chin. Drug Res. Clin. Pharmacol. 29 (04), 381–386. doi:10.19378/j.issn.1003-9783.2018.04.001
Zhou, M. Q., Yang, L. P., Ma, H. J., Cheng, C. C., Zhang, Y. X., Zhang, J. K., et al. (2020). Network pharmacological study of Qingfei Paidu decoction intervening on cytojube storm mecganism of COVID-19. J. Hainan Med. Univ. 26 (10), 721–729. doi:10.13210/j.cnki.jhmu.20200507.003
Zhou, T. R. (2018). Study on the regulation mechanism of patchouli active components on intestinal smooth muscle nerve conduction in IBS-D rats. Guangzhou Univ. Chin. Med. 97.
Zhou, Z., Li, X., Liu, J. X., Dong, L., Chen, Q., Liu, J., et al. (2015). Honeysuckle-encoded atypical microRNA2911 directly targets influenza A viruses. Cell. Res. 25 (1), 39–49. doi:10.1038/cr.2014.130
Keywords: Chinese herbal medicine, COVID-19, Traditional Chinese Medicine, systematic review, meta-analysis
Citation: Zhu H, Li M, Tian C, Lai H, Zhang Y, Shi J, Shi N, Zhao H, Yang K, Shang H, Sun X, Liu J, Ge L and Huang L (2022) Efficacy and safety of chinese herbal medicine for treating mild or moderate COVID-19: A systematic review and meta-analysis of randomized controlled trials and observational studies. Front. Pharmacol. 13:988237. doi: 10.3389/fphar.2022.988237
Received: 07 July 2022; Accepted: 11 August 2022;
Published: 07 September 2022.
Edited by:
Anthony Booker, University of Westminster, United KingdomReviewed by:
Xuepeng Gong, Huazhong University of Science and Technology, ChinaCopyright © 2022 Zhu, Li, Tian, Lai, Zhang, Shi, Shi, Zhao, Yang, Shang, Sun, Liu, Ge and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Sun, c3VueGluQHdjaHNjdS5jbg==; Jie Liu, ZHIubGl1amllQDE2My5jb20=; Long Ge, Z2Vsb25nMjAwOUAxNjMuY29t; Luqi Huang, aHVhbmdsdXFpMDFAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.