
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Pharmacol. , 24 August 2022
Sec. Pharmacology of Ion Channels and Channelopathies
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.984299
This article is part of the Research Topic Women in Pharmacology of Ion Channels and Channelopathies: 2021 View all 4 articles
 Elena Zaklyazminskaya1,2*
Elena Zaklyazminskaya1,2* Anna Shestak1
Anna Shestak1 Dmitry Podolyak1
Dmitry Podolyak1 Vera Komoliatova3
Vera Komoliatova3 Leonid Makarov3
Leonid Makarov3 Anna Novitskaya4
Anna Novitskaya4 Amiran Revishvili5
Amiran Revishvili5Brugada syndrome (BrS) is an inherited cardiac arrhythmia characterized by ST-elevation, negative T-wave, and a high risk of sudden cardiac death (SCD) due to ventricular tachycardia. It is associated with mutations in over 20 genes but only SCN5A is recommended for routine genetic screening. This study was performed to estimate diagnostic yield and pathogenicity assessment of rare genetic variants in the genes encoding Nav1.5 channel in Russian patients with Brugada syndrome (BrS). Targeted genes panel sequencing of the five genes were screened using IonTorrent PGM with following Sanger confirmation. Detailed clinical evaluation of 75 unrelated BrS probands with a deep phenotyping of SCN5A (+) probands was performed. Twelve rare genetic variants (six missense, six truncating) were initially identified and classified as disease-causing. Reassessment of the clinical significance in the light of the current guidelines revealed: 2 Pathogenic (P) variants; 8 Likely Pathogenic (LP); two missense variants (p.G274S and p. S1778H) were re-classified later as a variant of uncertain significance (VUS). Unique VUS (p.Arg100Ser) was detected in the SCN4B gene. Lone Brugada-pattern was observed in 46% probands; 54% patients had concomitant arrhythmias. PR interval, the only electrocardiography parameter correlating with SCN5A-mutation, was longer (207 ± 24 ms) than normal in SCN5A (+) probands. SCD cases were registered in 31 families. Depression was the only recurring extra-cardiac complaint in SCN5A (+) probands; it was self-reported in five SCN5A (+) probands, and co-segregated with Brugada pattern in 2 families. After variants reassessment, the ratio of SCN5A (+) probands with Brugada syndrome accounts for 13% in Russian cohort.
Elevation of the ST segment in the right precordial leads in the absence of cardiac ischemia was noticed and described in 1953 as a rare variation of normal electrocardiograph (ECG) (Martini et al., 1989; Baranchuk, 2018). Forty years later, in 1992, P. and J. Brugada recognized this feature as a hallmark of a new inherited arrhythmic syndrome and complemented the description with pseudo-right bundle branch block (RBBB), T-wave inversion, and high risk of sudden cardiac death (SCD) due to polymorphic ventricular tachycardia (VT) (Brugada et al., 1998). Nowadays this disease named Brugada syndrome (BrS) counts as a cause of the 12% SCD events, and presumably underlies 20% of autopsy-negative cases (Juang and Huang, 2004). Its prevalence varies in different countries and ethnic groups with increasing gradient from Northwest to South East, with an intermediate rate between <0.5 per 1,000 individuals in United States and Western Europe and >3 per 1,000 individuals in Southeast Asia and Japan (Nademanee et al., 1997; Vutthikraivit et al., 2018). The incidence and prevalence of BrS in Russia are poorly understood. There was a single epidemiological study in the Central Volga (Samara Region) where ECG screening revealed 44 spontaneous Brugada-patterns in 47,000 inhabitants (Dupliakov et al., 2007). This corresponds to a prevalence of 0.9 per 1,000 individuals and it seems to be intermediate between Northern Europe and Asian populations.
The genetic heterogeneity of BrS is remarkable (Probst et al., 2009). By now mutations have been identified in two dozen other genes, except for SCN5A, encoding sodium, potassium, and calcium channels that are also known to be responsible for BrS. The mutation rate does not exceed 2–5% for most of them. Even screening of complete genes panel allows detection of pathogenic genetic variants in less than 50% of index cases. For routine screening of suspected BrS patients only the SCN5A gene is recommended without any ethnic specificity (Liu et al., 2010). This gene encodes the alpha subunit of the sodium channel Nav1.5 that plays a key role in the depolarization of cardiomyocytes. This channel also consists of four regulatory beta-subunits encoding by the SCN1B-SCN4B genes. A few publications describe mutations in these genes in BrS (Gottschalk et al., 2016; Dendramis et al., 2017; El-Battrawy et al., 2019).
Clinical manifestation of BrS also varies widely from syncope, ventricular and supraventricular arrhythmias, and cardiac arrest to asymptomatic longevity; therefore, risk assessment is still challenging. To date, implantable cardioverter-defibrillator (ICD) is considered the only proven method to reduce the risk of life-threatening events in these patients (Sacher et al., 2006; Benito et al., 2008). Some studies mention that extra-cardiac complications (i.e., gastroenterological complaints) may be a part of a more complex phenotype in patients with mutations in the SCN5A gene (Locke et al., 2006); but most published studies limit clinical description exclusively by arrhythmic presentation.
In this study, we present the results of molecular genetic screening of the genes encoding all subunits of the Nav1.5 channel (SCN5A, SCN1B, SCN2B, SCN3B, and SCN4B) in a pilot cohort of BrS probands of Russian origin. We also focused on deep phenotyping of the genotype-positive patients to better understand the phenotype-genotype correlation in Brugada Syndrome.
The head of the local Ethics Committee of Russian Scientific Center of Surgery signed a permit on 27/09/2019 to perform this retrospective study on Brugada syndrome. This study was performed in accordance with the 1964 Helsinki declaration. Written informed consent was obtained from all individual participants included in the study. For patients under 18, consent was taken from a parent and/or legal guardian.
Data obtained from each individual in the study included a personal and family medical history, general examination, 12-lead resting ECG, 24-h ECG Holter monitoring, transthoracic echocardiography (EchoCG), cardiac magnetic resonance imaging (MRI) with gadolinium enhancement, and pharmacological challenge test with class III anti-arrhythmic drugs (Sieira et al., 2015). The diagnosis of BrS was established based on current diagnostic criteria (Bayés de Luna et al., 2012).
Genetic screening of targeted genes panel encoding alpha- and beta-subunits of Nav1.5 (SCN5A, SCN1B, SCN2B, SCN3B, and SCN4B) was performed by semi-conductive sequencing using IonTorrent PGM. Confirmation of all variants detected by nest generation sequencing, resequencing of the low-coverage regions, and cascade familial screening were performed by bidirectional capillary Sanger sequencing. Sequencing data were analyzed using Torrent Suite (version 5.0.5), Ion Reporter annotation service (Thermo Scientific, Waltham, MA, United States), and the Integrative Genomic Viewer visualization tool. Analysis in silico of clinically relevant findings was performed using population databases (gnomAD, and 1,000 Genomes), missense variant effect predictors (SIFT, PolyPhen2, MutationTaster, etc.), splicing analysis tools (UMD HSF v3.1, NetGene2, SpliceAI), and data integrators (Varsome). Variants identified in 2009–2015 were assessed according to the traditional rules as “Mutation”, “Polymorphism” or “Rare variant”. Variants identified later were assessed based on American College of Medical Genetics and Genomics (ACMG) consensus recommendations (2015) (Richards et al., 2015). Variant reassessment found in BrS patients in 2009–2019 years was performed based on ACMG2015 guidelines and the latest refined recommendation (Richards et al., 2015; Walsh et al., 2021). Variants classified as “pathogenic”, “likely pathogenic”, and variants of unknown significance (Class III-V) were included into the final report for patients.
Seventy-five unrelated patients with Brugada syndrome (BrS) requested genetic counselling and DNA-diagnostics. Diagnosis of BrS was established in the 75 index patients based on current diagnostic criteria (2012, 2013) (Bayés de Luna et al., 2012; Priori et al., 2013). Spontaneous Brugada pattern type 1 was found in 29 out of 75 probands (38.6%). Their age ranged from 4 to 63 years at the time of initial diagnosis (30 ± 14 years). Male to female ratio was 6:1 (64 males and 11 females). Similar male gender predominance (3:1 in Europe and 9:1 in South Asia) was found in clinical observations worldwide (Nademanee et al., 1997). The follow-up was from 2 to 18 years but about 50% of patients fallen out from the follow up for different reasons.
Sporadic cases of BrS (single case in a family, no family history of SCD) were registered in 18 probands (24%). In 35% cases, familial data were incomplete or unavailable. A family history of SCD and/or Brugada pattern on ECG was reported by 31 probands (41%). Autopsy data from relatives died suddenly or their archive ECGs were only available in a minority of cases. For 10 adult probands and for the parents of a 5-year-old proband, information about SCD cases in the family became meaningful only in the context of genetic counseling. Fewer than half of the probands underwent systematic health screening within 1 year after the unexplained death of a relative. The relatives of the victims of SCD do not consider this a reason for clinical evaluation. A lack of information about the genetic background of SCD in society might be an important factor influencing the time of correct diagnosis and further compliance in cases of inherited arrhythmic syndromes.
Thirty-eight probands (51% of the whole cohort) including 3 children diagnosed before age 7 were asymptomatic and had no complaint. The reason for clinical and genetic evaluation in this subgroup was the occasional detection of the spontaneous Brugada pattern on resting ECG in otherwise healthy active people during professional, sport or preventive check-up, and pre-school examination.
Thirty-seven probands (49% of the whole group) underwent detailed clinical and genetic evaluation because of clinical symptoms. The most common complaints were syncope/pre-syncope (45%), palpitation (45%), dizziness (32%), and chest pain (30%) (Figure 1). Most individuals had more than one clinical symptom. Six probands (16% symptomatic patients, and 8% of the whole BrS cohort) survived cardiac arrest. Only three patients considered high-temperature conditions as a trigger for syncope (fever in one case and sauna in two cases), but many probands reported avoiding the sauna or other high-temperature conditions owing to “usual non-tolerance.” Five probands pointed out that moderate alcohol intake revealed chest pain, triggered syncope/cardiac arrest, and unmasked the Brugada pattern on ECG (none had reported alcohol addiction). This factor was previously described as a potentially underestimated trigger of BrS manifestation (Achaiah and Andrews, 2016).
Eight probands in symptomatic subgroup (11% of the whole BrS cohort) underwent at least one hospitalization in the intensive care department with a misdiagnosis of myocardial infarction and were discharged due to a lack of evidence for the ischemic origin of the phenotype and typical ECG dynamics. Transitory ST-segment and J-point elevation can mimic acute coronary episode, and differential diagnosis might be challenging (Abriel et al., 2000; Liu et al., 2010). It was shown that even expert cardiologists cannot always distinguish betwenn true Brugada syndrome and its phenocopies (Gottschalk et al., 2016). We assume that medical professionals, other than those with specific expertise, might be not sufficiently aware of BrS.
The Brugada pattern alone was observed in 46% probands. Most of patients (54%) had a wide spectrum of concomitant rhythm and conduction defects, including atrial fibrillation, premature ventricular contractions (PVC), non-sustained ventricular tachycardia (VT), ventricular fibrillation (VF), sick sinus syndrome (SSS), atrio-ventricular block (AVB) I-II degree, left bundle branch block (LBBB), and right bundle branch block (RBBB) (Figure 1).
Cardioverter-defibrillators were implanted in 8 probands (11%); 3 of them had appropriate shocks (1 male and 2 females). In one patient (NRF17, male, 38 y. o.) ICD was implanted in year 2011 because of spontaneous Brugada pattern and documented episode of non-sustained VT. After 6 years of episode-free follow-up and disappearance of the Brugada-pattern on ECG device, it was explanted by the agreement with patient. No QTc prolongation was ever registered. This proband is a carrier of heterozygous p. S1787N (rs199473316) variant initially described as a LQTS-causing mutation (Splawski et al., 2000). Cellular biophysical phenotype of this variant may vary significantly depending upon splice variant background and intracellular pH (Hu et al., 2015). Later it was shown that S1787N allele is associated with variable expressivity of BrS phenotype in SCN5A families, and can be considered as a risk factor (Wijeyeratne et al., 2020).
This observation enriches a list of pathogenic mutations affecting amino acid sequence of the Nav1.5 alpha subunit that may result in both, LQTS and Brugada syndrome (in-frame deletion ∆KPQ is the most known example) (Saber et al., 2015; Abdelsayed et al., 2017). Currently (2019) this rare genetic variant was reclassified as a variant of unknown significance (VUS) (Class III) (Glazer et al., 2020). The patient was invited for genetic counseling regarding variant reclassification.
Structural heart disease was found in six probands (8%). Three probands (2 females, one male) additionally met the diagnostic criteria (2010) for arrhythmogenic right ventricular cardiomyopathy (ARVC) (Marcus et al., 2010). Two out of 3 underwent radiofrequency ablation of the arrhythmogenic focus in the right ventricular outflow tract (RVOT). One male patient had dilated cardiomyopathy (DCM) with left ventricular enlargement and decreased ejection fraction up to 40%. Asymptomatic non-obstructive hypertrophic cardiomyopathy (HCM) was revealed by EchoCG and cardiac MRI in 2 male probands. One SCN5A (+) male proband was first diagnosed with unexplained cardiac hypertrophy in 2011 (38 y. o.) when IVS reached 14 mm (38 y. o.). During last 9 years of follow-up he remains asymptomatic but non-obstructive HCM is slowly progressing. Now IVS revealed 23 mm, LV posterior wall 13 mm, and RV wall 11 mm. Another male proband, SCN5A (-), had interventricular septum (IVS) up to 15 mm and no obstruction.
There are publications discussing phenotypic overlap between BrS and ARVC, and possible inter-relationship caused by a cross talk between desmosomal and sodium channel proteins (Corrado et al., 2016). Dilated cardiomyopathy as a part of phenotype in patients with both loss-of-function and gain-of-function mutations in the SCN5A gene, is also widely discussed (Wilde and Amin, 2018). But cardiac hypertrophy seems to be exclusively rare phenomenon in patients with channelopathies. We performed search through the current literature and found two clinical case reports of HCM patients: one with rare variant (p.Asp872Asn, VUS) in the SCN5A gene (no arrhythmic phenotype mentioned) (Cronin et al., 2018), and second patient with Brugada pattern on ECG and two rare variants in the MYH7 and MyBPC3 genes (Farnè et al., 2021). Whether BrS and HCM may have a common pathogenic mechanism remain unsolved. Mild cardiac hypertrophy might be an overlooked feature in patients with threatening diagnosis such as Brugada syndrome in these two observations.
We cannot exclude an occasional combination of two different genetic conditions (BrS and HCM; BrS and ARVC; BrS and DCM) in patients from our BrS cohort. It was known that multilocus variations can be found in more than 30% of patients with unusual phenotype (so called “phenotypic expantion”) (Karaca et al., 2018).
No mutation was found in SCN1B-4B, suggesting that these mutations are very rare in Russian patients. A unique heterozygous variant, p. R100S, in SCN4B was found in a 30-year-old proband of mid-Asian origin with the spontaneous Brugada type-1 pattern and 2 pre-syncopal episodes.
All in silico predictive tools (PolyPhen 2.0, SIFT, and MutationTaster) classify these variants as “disease causing” or “probably damaging.” In the absence of familial and functional data, further study is needed to evaluate the possible role of these variants in BrS pathogenesis.
Twelve rare variants (6 truncating and six missense) were found in SCN5A in 12 male probands (16%), and 11 out of 12 rare variants were considered related to the phenotype at the moment of detection (2009–2015) (Table 1). Eight variants were reported to patients as “mutations”, 3 variants (detected after 2015) were reported as Likely Pathogenic (LP), and one variant was considered as “Rare variant” (corresponds to VUS in current terminology).
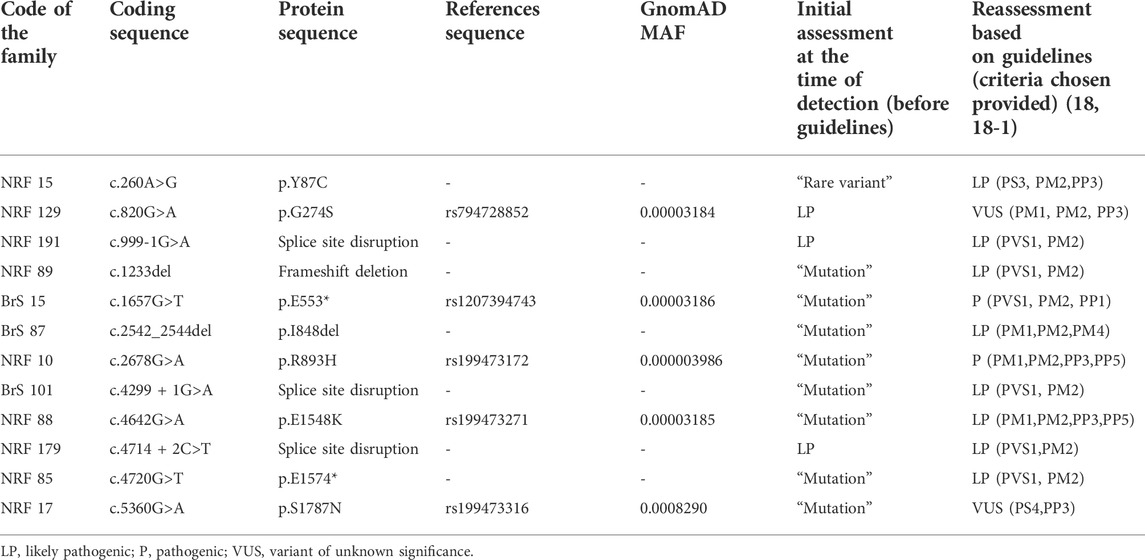
TABLE 1. Summary of SCN5A rare variants identified with Brugada syndrome. Classification of variant pathogenicity is according to the American College of Medical Genetics (ACMG) joint consensus standards and guidelines for the interpretation of sequence variants. All genetic variants were referred for a single transcript NM_198,056.2.
We reassessed all 12 rare variants in this cohort using ACMG (2015) criteria (Richards et al., 2015), Enhancing rare variants interpretation for inherited arrhythmias recommendations (Walsh et al., 2021), and additional published data available. All six truncating genetic variants (“mutations”) were reclassified as Pathogenic (P) (p.E533*) or Likely Pathogenic (LP) (c.999-1G>A, c.1233del, c.4299 + 1G>A, c.4714 + 2C>T, p. E1574*) (Table 1). Three out of six missense variants were reclassified based on ACMG criteria (2015, 2019). One missense variant, p. Y87C, was reclassified from “Rare variant” (=VUS, Class III) to Likely Pathogenic (P, Class V) because of the experimental evidence of deleterious effect on calmodulin binding with N-terminal domain of the Nav1.5 (Wang et al., 2020). Two missense variants, p. G274S and p. S1787N, were re-classified from “Mutations” (Class V/IV) to VUS (Variant of unknown significance, Class III) due to conflicting publications. All probands carrying these variants were invited for genetic counseling.
No mutation was found in a female proband; female gender of the proband is likely a negative predictive factor for the presence of SCN5A mutations.
Clinical characteristics of SCN5A (+) probands are summarized in Table 1. All truncating mutations (nonsense, frame-shift deletion, and splicing variants) in the SCN5A gene were of familial origin. Spontaneous Brugada pattern type 1 on the ECG in V1-V2 or V1’-V2’ was registered in all male carriers, including a young asymptomatic 4-year-old boy. Cases of SCD in families were reported for all probands harboring truncating mutations. The age at the time of the SCD or life-threatening VT in these families was 37–52 years. Four female family members carrying PTC mutations had Brugada pattern type 1 induced by pharmacological challenge test. Cascade familial screening revealed 12 additional family members (8 males, four females) carrying truncating variants, and all males had spontaneous Brugada pattern on ECG.
No significant differences in the cardiac manifestation of Brugada syndrome, such as the rate of syncope, SCD cases, age at manifestation, heart rate, QTc, spectrum of ventricular and supra-ventricular arrhythmias, and ICD implantation were found between genotype-positive and genotype-negative probands. The only parameter that differed significantly between these groups was the PR interval (207 ± 24 ms in SCN5A (+) probands vs. 172 ± 18 ms in SCN5A(−)probands, p < 0.01). These data are in accordance with the finding that in patients with BrS, a widening of the PQ interval (>210 ms) is predictive of the presence of SCN5A mutations (Smits et al., 2002).
Most of genes causing cardiac channelopathies have a wide expression profile and function in multiple tissue types. Thereby, extra-cardiac involvement is not unusual in cardiac channelopathies. We performed a survey of extra-cardiac complaints in patients with BrS. An increased prevalence of gut motility problems in SCN5A mutation carriers has been observed (Locke et al., 2006) but this symptom was not mentioned in the survey by any patient. No cases of proven epilepsy were found. Five probands (7%) self-reported at least one episode of depression requiring medication and/or hospitalization, and all these patients belonged to the SCN5A (+) group (Table 2). In family #BrS15, the recurrent depression co-segregated with BrS in 3 generations and the p.553* heterozygous mutation (Figure 2). In the family #NRF15 the co-inheritence of p. Y87C variant, Brugada Syndrome, and recurrent depression was found in 2 generation) (Wang et al., 2020).
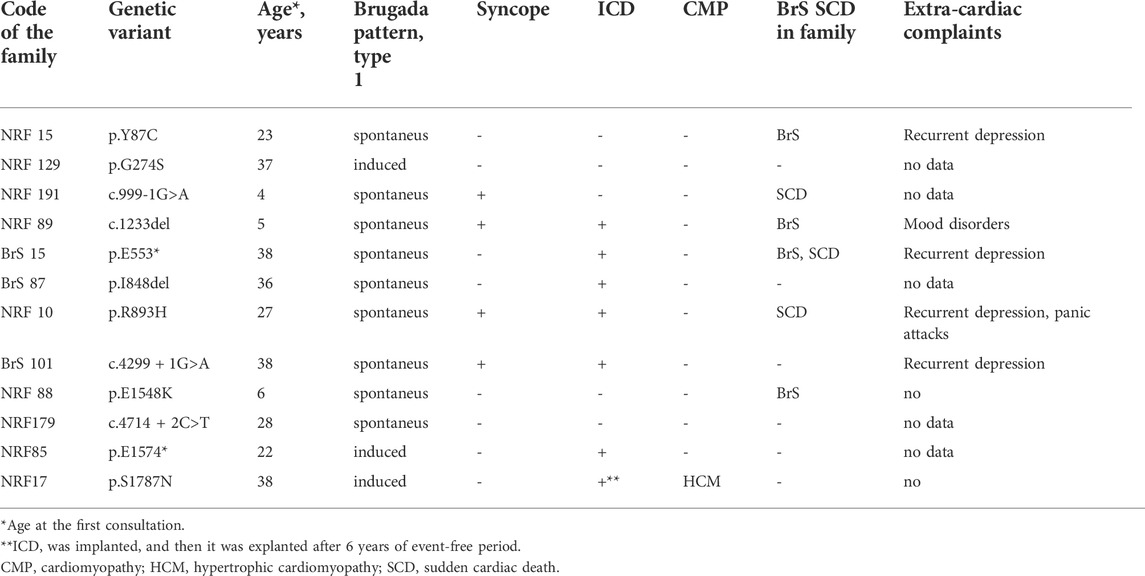
TABLE 2. Clinical characteristics of SCN5A (+) probands (all males) carrying heterozygous mutations in the SCN5A gene.
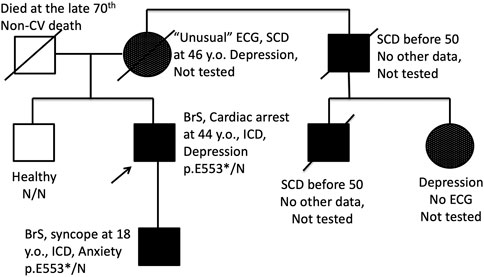
FIGURE 2. Pedigree of the family BrS15 with co-segregation of BrS pattern on ECG and endogenous depression. Proband and his son are carriers of heterozygous nonsense p. E553* mutation in the SCN5A gene. SCD, Sudden cardiac death; ↑, proband; □, male; ○, female.
Nav1.5 channels encoded by SCN5A are over-expressed in some non-excitable and excitable cells in the central nervous system and may contribute to diverse cellular functions (Catterall, 2014). We speculate that emotional disorders might be more common in SCN5A-related BrS than previously thought, but this observation requires confirmation in a larger study. The clinical cases of BrS reveled in patients with depression were published since 2014 (Chen and Sangha, 2014). This might have implications for the treatment of depression because a number of psychotropic agents are included in the “to avoid” and “to preferably avoid” lists for patients with BrS (Postema et al., 2009) and should be taken with caution.
After the rare variant reassessment, the prevalence of SCN5A-related BrS in Russian patients was 13%, consistent with the prevalence of SCN5A-associated BrS worldwide. Carriers of SCN5A mutations have a relatively longer PR interval. Female gender of proband is a negative predictive factor for mutations affecting Nav1.5 sodium channels. Genetic forms related to the beta-subunits are extremely rare in Russia, if any. Structural myocardial disorders were found in 8% of Russian BrS index cases. Further study is needed to evaluate if endogenous depression was an accidental finding of this group, or it really expands the clinical spectrum of SCN5A-related phenotype.
Risk stratification schemes and treatment strategies for patients with BrS are not currently based on genotypes but this could be a focus of future research. Preventive and therapeutic strategies will also benefit from increased knowledge of causal mutations and their consequences (Abriel et al., 2000). Current guidelines can be considered precursors to the development of further guidelines for genetic testing in cardiology and for a new approach for the integration of genetics and technology into clinical practice and personalized medicine.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author. The data presented are deposited in the repository ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/). Accession numbers for registered variants VCV000691966.6, VCV000201445.6, VCV001348900.1, VCV000067749.6, VCV000067913.1, VCV000067988.32, and VCV000691965.2.
This study was performed in accordance with the 1964 Helsinki declaration. The Head of local Ethics Committee of Russian Scientific Center of Surgery named after acad.B.V.Petrovsky signed a permit to perform this retrospective study on Brugada syndrome on 27/09/2019. Written informed consent to participate in this study was provided by the participants or participants’ legal guardian (if minor).
EZ—general management, drafting the manuscript, data analysis, genetic counseling, final approval of the manuscript AS—DNA diagnostic procedures, variant assessment, data analysis, drafting the manuscript DP and AR—patients evaluation and interventional treatment, data analysis VK and LM—patients ECG and EchCG evaluation and treatment, data analysis AN—neurological and psychiatric evaluation and treatment, data analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdelsayed, M., Baruteau, A. E., Gibbs, K., Sanatani, S., Krahn, A. D., Probst, V., et al. (2017). Differential calcium sensitivity in NaV 1.5 mixed syndrome mutants. J. Physiol. 595 (18), 6165–6186. doi:10.1113/JP274536
Abriel, H., Wehrens, X. H., Benhorin, J., Kerem, B., and Kass, R. S. (2000). Molecular pharmacology of the sodium channel mutation D1790G linked to the long-QT syndrome. Circulation 102 (8), 921–925. doi:10.1161/01.cir.102.8.921
Achaiah, A., and Andrews, N. (2016). Intoxication with alcohol: an underestimated trigger of Brugada syndrome? JRSM open 7 (5), 2054270416640153. doi:10.1177/2054270416640153
Baranchuk, A. (2018). Brugada Phenocopy: The art of recognizing the Brugada ECG pattern. Cambridge: Academic Press.
Bayés de Luna, A., Brugada, J., Baranchuk, A., Borggrefe, M., Breithardt, G., Goldwasser, D., et al. (2012). Current electrocardiographic criteria for diagnosis of brugada pattern: a consensus report. J. Electrocardiol. 45 (5), 433–442. doi:10.1016/j.jelectrocard.2012.06.004
Benito, B., Brugada, R., Brugada, J., and Brugada, P. (2008). Brugada syndrome. Prog. Cardiovasc. Dis. 51 (1), 1–22. doi:10.1016/j.pcad.2008.05.002
Brugada, J., Brugada, R., and Brugada, P. (1998). Right bundle-branch block and ST-segment elevation in leads V1 through V3: a marker for sudden death in patients without demonstrable structural heart disease. Circulation 97 (5), 457–460. doi:10.1161/01.cir.97.5.457
Catterall, W. A. (2014). Sodium channels, inherited epilepsy, and antiepileptic drugs. Annu. Rev. Pharmacol. Toxicol. 54, 317–338. doi:10.1146/annurev-pharmtox-011112-140232
Chen, J. J., and Sangha, R. S. (2014). Treatment of anxiety and depression in a patient with brugada syndrome. Case Rep. Psychiatry 2014, 478397. doi:10.1155/2014/478397
Corrado, D., Zorzi, A., Cerrone, M., Rigato, I., Mongillo, M., Bauce, B., et al. (2016). Relationship between arrhythmogenic right ventricular cardiomyopathy and brugada syndrome: New insights from molecular biology and clinical implications. Circ. Arrhythm. Electrophysiol. 9 (4), e003631. doi:10.1161/CIRCEP.115.003631
Cronin, H., Kerins, D., Fahy, G., and Vaughan, C. (2018). 20 the role of SCN5A mutations in hypertrophic cardiomyopathy. Heart 104, A15–A16. doi:10.1136/heartjnl-2018-ICS.20
Dendramis, G., Petrina, S. M., and Baranchuk, A. (2017). Not all ST-segment elevations are myocardial infarction: Hyperkalemia and Brugada phenocopy. Am. J. Emerg. Med. 35 (4), e1. doi:10.1016/j.ajem.2016.10.063
Dupliakov, D. V., Glukhova, V. L., Maksimova, S. V., Vozhdaeva, Z. I., Starostina, I. V., Vasil'eva, E. N., et al. (2007). Frequency of detection the of Brugada syndrome signs in the course of ECG registration. Kardiologiia 47 (11), 55–59.
El-Battrawy, I., Müller, J., Zhao, Z., Cyganek, L., Zhong, R., Zhang, F., et al. (2019). Studying brugada syndrome with an SCN1B variants in human-induced pluripotent stem cell-derived cardiomyocytes. Front. Cell Dev. Biol. 7, 261. doi:10.3389/fcell.2019.00261
Farnè, M., Balla, C., Margutti, A., Selvatici, R., De Raffele, M., Di Domenico, A., et al. (2021). Mutations in MYBPC3 and MYH7 in association with brugada type 1 ECG pattern: Overlap between brugada syndrome and hypertrophic cardiomyopathy? Cardiogenetics 11, 139–147. doi:10.3390/cardiogenetics11030016
Glazer, A. M., Wada, Y., Li, B., Muhammad, A., Kalash, O. R., O'Neill, M. J., et al. (2020). High-throughput reclassification of SCN5A variants. Am. J. Hum. Genet. 107 (1), 111–123. doi:10.1016/j.ajhg.2020.05.015
Gottschalk, B. H., Anselm, D. D., Brugada, J., Brugada, P., Wilde, A. A., Chiale, P. A., et al. (2016). Expert cardiologists cannot distinguish between brugada phenocopy and brugada syndrome electrocardiogram patterns. Europace : European pacing, arrhythmias, and cardiac electrophysiology. Europace 18 (7), 1095–1100. doi:10.1093/europace/euv278
Hu, R. M., Tan, B. H., Tester, D. J., Song, C., He, Y., Dovat, S., et al. (2015). Arrhythmogenic biophysical phenotype for SCN5A mutation S1787N depends upon splice variant background and intracellular acidosis. PloS one 10 (4), e0124921. doi:10.1371/journal.pone.0124921
Juang, J. M., and Huang, S. K. (2004). Brugada syndrome--an under-recognized electrical disease in patients with sudden cardiac death. Cardiology 101 (4), 157–169. doi:10.1159/000076693
Karaca, E., Posey, J. E., Coban Akdemir, Z., Pehlivan, D., Harel, T., Jhangiani, S. N., et al. (2018). Phenotypic expansion illuminates multilocus pathogenic variation. Genet. Med. 20 (12), 1528–1537. doi:10.1038/gim.2018.33
Liu, J. F., Moss, A. J., Jons, C., Benhorin, J., Schwartz, P. J., Spazzolini, C., et al. (2010). Mutation-specific risk in two genetic forms of type 3 long QT syndrome. Am. J. Cardiol. 105 (2), 210–213. doi:10.1016/j.amjcard.2009.08.676
Locke, G. R., Ackerman, M. J., Zinsmeister, A. R., Thapa, P., and Farrugia, G. (2006). Gastrointestinal symptoms in families of patients with an SCN5A-encoded cardiac channelopathy: evidence of an intestinal channelopathy. Am. J. Gastroenterol. 101 (6), 1299–1304. doi:10.1111/j.1572-0241.2006.00507.x
Marcus, F. I., McKenna, W. J., Sherrill, D., Basso, C., Bauce, B., Bluemke, D. A., et al. (2010). Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Eur. Heart J. 31 (7), 806–814. doi:10.1093/eurheartj/ehq025
Martini, B., Nava, A., Thiene, G., Buja, G. F., Canciani, B., Scognamiglio, R., et al. (1989). Ventricular fibrillation without apparent heart disease: description of six cases. Am. Heart J. 118 (6), 1203–1209. doi:10.1016/0002-8703(89)90011-2
Nademanee, K., Veerakul, G., Nimmannit, S., Chaowakul, V., Bhuripanyo, K., Likittanasombat, K., et al. (1997). Arrhythmogenic marker for the sudden unexplained death syndrome in Thai men. Circulation 96 (8), 2595–2600. doi:10.1161/01.cir.96.8.2595
Postema, P. G., Wolpert, C., Amin, A. S., Probst, V., Roden, D. M., Priori, S. G., et al. (2009). Drugs and Brugada syndrome patients: review of the literature, recommendations, and an up-to-date website (www.brugadadrugs.org). Heart R 6, 1335–1341. doi:10.1016/j.hrthm.2009.07.002
Priori, S. G., Wilde, A. A., Horie, M., Cho, Y., Behr, E. R., Berul, C., et al. (2013). Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm 10 (12), e85–e108. doi:10.1016/j.hrthm.2013.07.021
Probst, V., Wilde, A. A., Barc, J., Sacher, F., Babuty, D., Mabo, P., et al. (2009). SCN5A mutations and the role of genetic background in the pathophysiology of Brugada syndrome. Circ. Cardiovasc. Genet. 2 (6), 552–557. doi:10.1161/CIRCGENETICS.109.853374
Richards, S., Aziz, N., Bale, S., Bick, D., Das, S., Gastier-Foster, J., et al. (2015). Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of medical genetics and Genomics and the association for molecular pathology. Genet. Med. 17 (5), 405–424. doi:10.1038/gim.2015.30
Saber, S., Amarouch, M. Y., Fazelifar, A. F., Haghjoo, M., Emkanjoo, Z., Alizadeh, A., et al. (2015). Complex genetic background in a large family with Brugada syndrome. Physiol. Rep. 3 (1), e12256. doi:10.14814/phy2.12256
Sacher, F., Probst, V., Iesaka, Y., Jacon, P., Laborderie, J., Mizon-Gérard, F., et al. (2006). Outcome after implantation of a cardioverter-defibrillator in patients with brugada syndrome: a multicenter study. Circulation 114 (22), 2317–2324. doi:10.1161/CIRCULATIONAHA.106.628537
Sieira, J., Conte, G., Ciconte, G., de Asmundis, C., Chierchia, G. B., Baltogiannis, G., et al. (2015). Prognostic value of programmed electrical stimulation in brugada syndrome: 20 years experience. Circ. Arrhythm. Electrophysiol. 8 (4), 777–784. doi:10.1161/CIRCEP.114.002647
Smits, J. P., Eckardt, L., Probst, V., Bezzina, C. R., Schott, J. J., Remme, C. A., et al. (2002). Genotype-phenotype relationship in brugada syndrome: electrocardiographic features differentiate SCN5A-related patients from non-SCN5A-related patients. J. Am. Coll. Cardiol. 40 (2), 350–356. doi:10.1016/s0735-1097(02)01962-9
Splawski, I., Shen, J., Timothy, K. W., Lehmann, M. H., Priori, S., Robinson, J. L., et al. (2000). Spectrum of mutations in long-QT syndrome genes. KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation 102 (10), 1178–1185. doi:10.1161/01.cir.102.10.1178
Vutthikraivit, W., Rattanawong, P., Putthapiban, P., Sukhumthammarat, W., Vathesatogkit, P., Ngarmukos, T., et al. (2018). Worldwide prevalence of brugada syndrome: A systematic review and meta-analysis. Acta Cardiol. Sin. 34 (3), 267–277. doi:10.6515/ACS.201805_34(3).20180302B
Walsh, R., Lahrouchi, N., Tadros, R., Kyndt, F., Glinge, C., Postema, P. G., et al. (2021). Enhancing rare variant interpretation in inherited arrhythmias through quantitative analysis of consortium disease cohorts and population controls. Genet. Med. 23 (1), 47–58. doi:10.1038/s41436-020-00946-5
Wang, Z., Vermij, S. H., Sottas, V., Shestak, A., Ross-Kaschitza, D., Zaklyazminskaya, E. V., et al. (2020). Calmodulin binds to the N-terminal domain of the cardiac sodium channel Nav1.5. Channels (Austin, Tex.) 14 (1), 268–286. doi:10.1080/19336950.2020.1805999
Wijeyeratne, Y. D., Tanck, M. W., Mizusawa, Y., Batchvarov, V., Barc, J., Crotti, L., et al. (2020). SCN5A mutation type and a genetic risk score associate variably with brugada syndrome phenotype in SCN5A families. Circ. Genom. Precis. Med. 13 (6), e002911. doi:10.1161/CIRCGEN.120.002911
Keywords: SCN5A, Nav1.5 channel, Brugada syndrome comorbidity, Brugada syndrome, cardiac channelopathy
Citation: Zaklyazminskaya E, Shestak A, Podolyak D, Komoliatova V, Makarov L, Novitskaya A and Revishvili A (2022) Diagnostic yield and variant reassessment in the genes encoding Nav1.5 channel in Russian patients with Brugada syndrome. Front. Pharmacol. 13:984299. doi: 10.3389/fphar.2022.984299
Received: 01 July 2022; Accepted: 02 August 2022;
Published: 24 August 2022.
Edited by:
Barbara Niemeyer, Saarland University, GermanyReviewed by:
Pedro Brugada, Independent researcher, BelgiumCopyright © 2022 Zaklyazminskaya, Shestak, Podolyak, Komoliatova, Makarov, Novitskaya and Revishvili. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elena Zaklyazminskaya, aGVsZW5lemFrQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.