
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 19 October 2022
Sec. Pharmacology of Anti-Cancer Drugs
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.983092
Objective: Metastatic colon cancer (mCC) poses a great threat to the survival of patients suffering from it. In the past decade, many clinical trials have been carried out to improve the prognosis of patients with mCC. Numerous treatments have emerged, and satisfactory efficacy has been demonstrated in randomized phase III trials in highly selective patients with mCC. Our present study aims to investigate whether these therapeutic advances can be reflected to the broader mCC patients who performed cytoreductive colectomy.
Method: General and prognostic data for patients diagnosed with mCC who underwent cytoreductive colectomy between 2004–2018 were extracted from the Surveillance, Epidemiology, and End Results (SEER) database. Survival was analyzed using the Kaplan-Meier method and Cox proportional hazards model. The hazard ratio (HR) and its 95% confidence interval (CI) were used to evaluate the influence of risk factors on prognosis.
Results: A total of 26,301 patients diagnosed with mCC treated with cytoreductive colectomy were included in this study. The median overall survival was 19 months (range, 17–23). The good prognosis was associated with patients diagnosed at the most recent year, younger age, non-black race, female, married, without previous history of malignancy, no second malignancy onset, descending/sigmoid/splenic flexure colon tumor, normal CEA levels at diagnosis, low primary tumor burden, T1/T2 stage, N0 stage, single organ metastasis, underwent surgical resection of synchronous distant metastatic lymph nodes or organs, a high number of lymph-node examinations, low positive lymph-node ratio and received adjuvant chemotherapy. The proportion of patients surviving for ≥24 months increased from 37% in 2004 to 44.2% in 2016 (p < 0.001), especially in ≤49 years patients [46.8% in 2004 to 57.8% in 2016 (p < 0.001)]. The percentage of patients who died within 3 months decreased between 2004 and 2018 (from 19.6% to 15.7%; p < 0.001).
Conclusion: Over a span of 15 years, the long-term survival has improved in real-world mCC patients who were treated with cytoreductive colectomy, especially among younger patients. However, the median overall survival remains not substantial.
The incidence of colorectal cancer is rising in recent years, accounting for approximately 10% of all cancers. It is currently the third most common malignancy in the world, and the second most common cause of cancer-related deaths (Siegel et al., 2020; Biller and Schrag, 2021; Siegel et al., 2022). About 20% of patients with colorectal cancer are found to have distant metastases at the time of diagnosis, and up to 50% of patients progress to metastatic disease during subsequent follow-up (van der Geest et al., 2015). Metastatic colorectal cancer has a poor prognosis (Biller and Schrag, 2021; Modest et al., 2022). Since metastatic colorectal cancer encompasses a broad spectrum of clinical diseases, its clinical prognosis varies widely among patients. Unresectable metastatic disease has an extremely poor prognosis without systemic therapy and a mean OS of 6–8 months (Kawai et al., 2021). The chemotherapy regimen of 5-fluorouracil (5-FU) combined with oxaliplatin or irinotecan has been the cornerstone of the treatments of metastatic colorectal cancer since the 1960s (Douillard et al., 2000; Saltz et al., 2000; Goldberg et al., 2004; Lee et al., 2019; Modest et al., 2022). At the same time, by adding drugs that target vascular endothelial growth factor (VEGF) (such as bevacizumab, ramucirumab, and ziv-aflibercept), and drugs that inhibit the epidermal growth factor receptor (EGFR) signaling pathway (such as cetuximab and panitumumab) significantly improved median survival in patients with metastatic colorectal cancer (Mody and Bekaii-Saab, 2018). In addition, immune checkpoint inhibitors (such as pembrolizumab, and nivolumab) have made rapid progress in the field of colorectal cancer and brought new changes to the treatment of advanced colorectal cancer (Overman et al., 2017; Ganesh et al., 2019; André et al., 2020; Andre et al., 2021; Modest et al., 2022).
Through multidisciplinary comprehensive treatment strategies, the progression of colorectal cancer can be better controlled and alleviated, and the survival rate of most tumors can be improved. However, the progress made in the treatment of metastatic colorectal cancer is mostly reflected in the results of randomized controlled clinical studies, and these studies have a process of highly selective screening of cases for case inclusion. It is worth investigating whether the progress in the treatment of metastatic colorectal cancer is reflected in the real world. The purpose of this study was to investigate the short-term and long-term survival of patients with metastatic colon cancer (mCC), who underwent cytoreductive colectomy from 2004 to 2018. This will aid in determining whether the advances of metastatic colorectal cancer treatment from randomized controlled clinical studies are extended to the broader mCC population.
The analysis data in this study were obtained from Surveillance, Epidemiology, and End Results (SEER) database (https://seer.cancer.gov/) compiled by the National Cancer Institute of the United States. The study used 18 registrations of the SEER database, which accounted for about 28% of the total population of the United States. The basic characteristics of patients were similar to those of the general population, and they were representative. Demographic information, clinical features, the incidence of cancer, and treatment and survival of patients registered by each cancer registry were recorded in the SEER database. Since the SEER database is anonymous and has nothing to do with human studies, the ethics review committee of our hospital exempted ethical approval.
We identified patients with primary metastatic colon cancer, who underwent surgery from 2004 to 2018. The inclusion criteria were as follows: 1) All cases had pathological diagnosed, rather than an autopsy or death certificate. 2) Patients ≥ 18 years old. 3) Received recommended cytoreductive colectomy. 4) Complete information on causes of death and follow-up time. 5) Deleted the cases with duplicate IDs were excluded, and patients who underwent tumor reduction surgery but were not recommended by clinicians were removed.
Research variables included year of diagnosis (2004–2009 and 2010–2018), age of diagnosis (≤49, 50–59, 60–69, 70–79, and 80+), race (white, black, and others), gender (male and female), marital status (never married, married, and widowed/divorced/separated), history of malignant tumors (with or without), lifetime number of tumors (1, 2 and 3 and above), location of colon cancer (ascending colon, hepatic flexure, transverse colon, splenic flexure, descending colon, sigmoid colon, and large intestine NOS), tumor size (>5 cm and ≤5 cm), Carcino-embryonic antigen (CEA) level (normal, borderline, and elevated), histology (adenocarcinoma and non-adenocarcinoma), T stage (T1, T2, T3, and T4), N stage (N0, N1, and N2), M stage (M1a, M1b, M1c), metastasis site (bone, liver, lung, and brain), number of organ metastasis (1, 2, and 3+), peritoneal infiltration (yes and no), number of tumor deposits (none, 1-2, and 2+), number of lymph node examination (≤16 and >16; limited to patients with lymph node examination), number of positive lymph nodes (≤4 and >4; limited to patients with lymph node examination), lymph node positive rate (≤31% and >31%; limited to patients with lymph node examination, surgical methods (Subtotal colectomy hemicolectomy, Total colectomy/proctocolectomy, and Partial colectomy segmental/local excision), distant metastasis of lymph nodes and organs of surgery (yes and no), and radiotherapy and chemotherapy (yes and no).
All continuous variables in this study were described as mean ± standard deviation (SD) if they conformed to a normal distribution, and were compared by Student’s t-test. If the variables did not conform to the normal distribution, they were described as the median and interquartile range (IQR) and compared with Wilcoxon rank-sum test. Frequency (%) was used to represent the classification variables and the chi-square test for comparison. Kaplan-Meier method was used to calculate the survival rate of patients. Univariate and multivariate Cox proportional hazard model and Fine and Gray model were used to estimate the hazard ratios (HRs) and sub-distribution hazard ratios (sHRs) of total overall survival (OS) and cancer-specific survival (CSS), and their corresponding 95% confidence intervals, respectively. This was done to assess the impact of different covariates on OS and CSS, and to determine independent risk factors. Ordered logistic regression analysis was used to evaluate the impact of different covariates on early death risk, and the odds ratio (OR) of risk factors and its 95% CI were calculated. In addition, in order to evaluate the HR of age, tumor size, tumor deposits, number of lymph node examinations, number of positive lymph nodes, and rate of lymph node-positive on OS risk, the restricted cubic spline curve was used to display these correlations. The non-linear test was carried out through the likelihood ratio test, and the log-likelihood of the model with linear term and the model with cubic spline term was compared. All p-values were bilateral, and a p-value of <0.05 was considered to be statistically significant. R statistical package (v. 4.2.0) was used for evaluations.
A total of 26,301 mCC patients were identified between 2004–2018 through the SEER database. Of these, 12,068 (45.9%) were identified between 2004–2009 and 14,233 (54.1%) between 2010–2018. Detailed demographic information and clinical characteristics are shown in Table 1 and Supplementary Table S1. The median age at diagnosis for the total population was 65 years (IQR: 54–75). The diagnostic age of patients identified between 2010–2018 [66 years (IQR: 53–74)] was less than that of those identified between 2004–2009 [64 (IQR: 55–74)]. The percentage of patients in different age groups was different before and after 2010. The proportion of patients ≤49 years increased from 14.2% to 16.8%, while the proportion of patients ≥80 years decreased from 16.8% to 14.7% from 2004–2009 to 2010–2018. The proportion of tumors >5 cm increased from 40.3% to 46.4% compares 2004–2009 with 2010–2018, while the proportion of tumors <5 cm decreased from 53.6% to 49.4%. The proportion of T3 tumors decreased from 62.8% in 2004–2009 to 52.2% in 2010–2018, while the proportion of T4 tumors increased from 33.4% to 44.5%. It was found that the number of local lymph node examinations >16 increased from 37.0% in 2004–2009 to 52.0% in 2010–2018. In addition, patients receiving chemotherapy increased from 59.2% in 2004–2009 to 67.4% in 2010–2018.
Figure 1 shows that for the total population, the median OS is 19 months, and the median CSS is 21 months. For patients in different age groups, older patients had shorter median OS. For instance, patients ≤49 years had a median OS of 29 months, whereas for those >80 years was 7 months. The median OS of patients with mCC from 2004–2009 was 18 months, and for patients from 2010–2018 was 21 months.
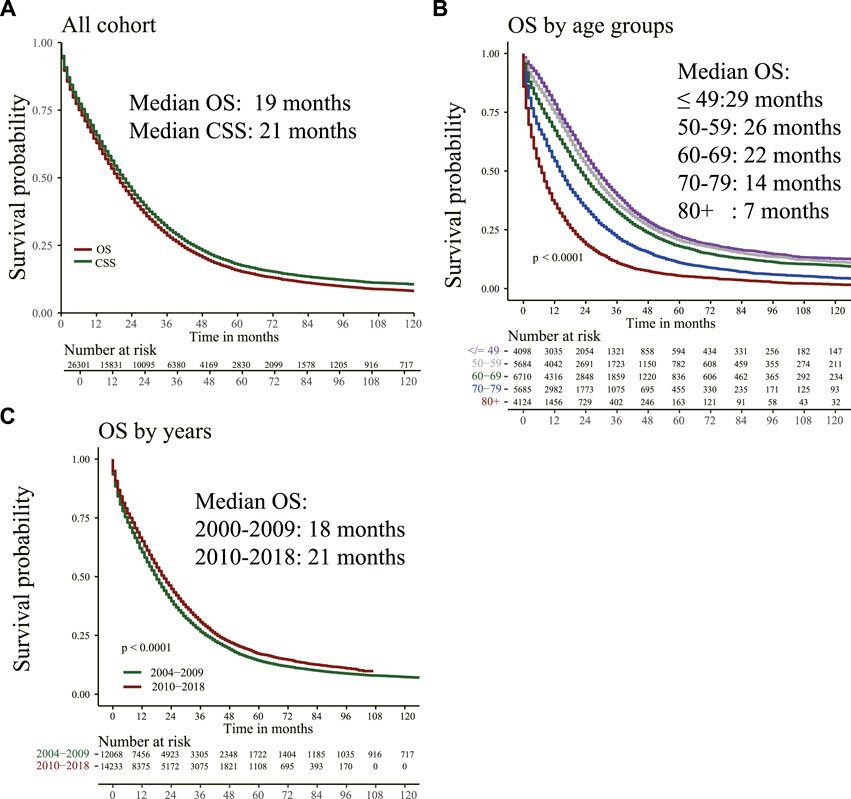
FIGURE 1. Overall survival (OS) and cancer-specific survival (CSS) were estimated by Kaplan–Meier analysis. (A) OS and CSS curve; (B) OS in different age groups; (C) OS of patients diagnosed at different time points.
The HR of death risk showed a consistent trend of statistically significant improvement after 2010. The HR of death risk of patients diagnosed at 2018 was 0.62 (95% CI, 0.50–0.77; p < 0.001) compared to patients diagnosed at 2004 (Table 2).
Figure 2 shows that age, tumor size, tumor deposits, number of regional lymph node tests, number of regional lymph node positives, and positive rate of regional lymph nodes at the time of diagnosis did not linearly correlate with the risk of all-cause deaths in patients with mCC (p for non-linear <0.05). These factors were then transformed into categorical variables and incorporated into the Cox model. After adjusting other covariates, they were all found to be independent risk factors for all-cause deaths (Table 3).
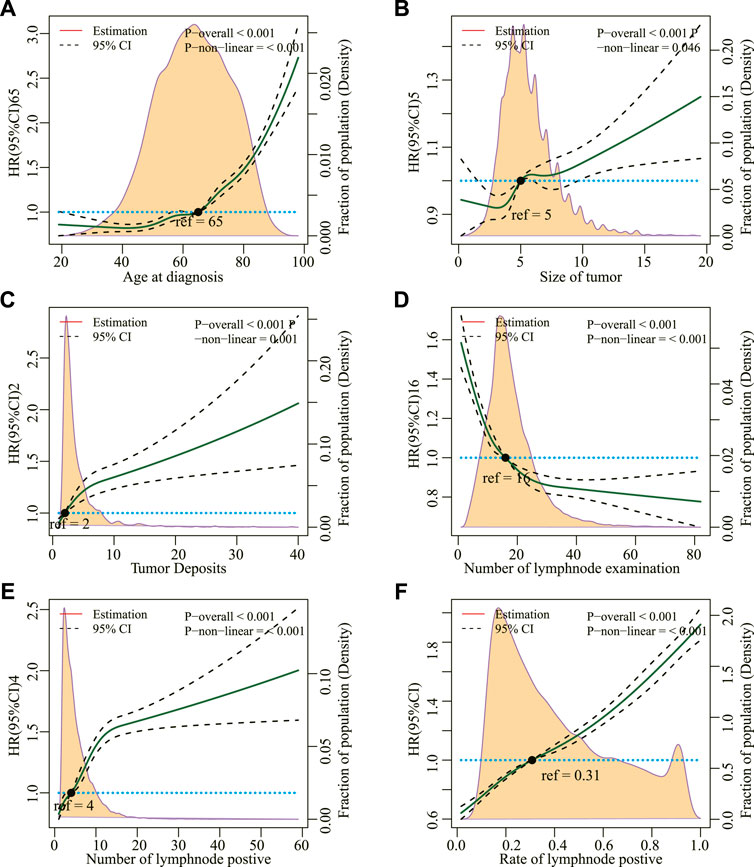
FIGURE 2. (A) Restricted cubic splines for the association between hazard risk of overall mortality and age at diagnosis, (B) Size of tumor, (C) Tumor Deposits, (D) Number of lymph node examination, (E) Number of lymph node positives, and (F) Rate of lymph node positives. Solid lines represent hazard ratio (HR); dashed lines represent 95% CIs. The hazard risk estimates were adjusted for all other covariables.
We define short-term survivors as patients who died within 3 months of initial diagnosis, and long-term survivors as patients who survived for at least 24 months. Figure 4 shows that the proportion of short-term survivors in the total population decreased from 19.6% in 2004 to 13.9% in 2015 (p < 0.001). The proportion of long-term survivors increased from 62.7% in 2004 to 55.8% in 2016 (p < 0.001). In addition, survivors who survived for at least 12 months increased from 58.4% in 2004 to 67.0% in 2015. It was found that the trend of survival improvement was more prominent in the younger population (Figures 3, 4).
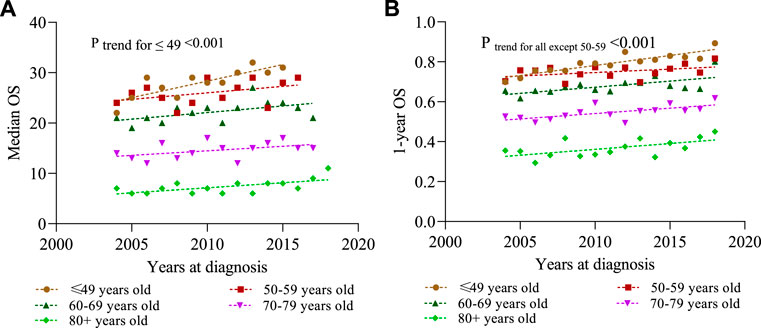
FIGURE 3. Overall survival (OS) is estimated by Kaplan–Meier method for different years at diagnosis stratified by different ages in the diagnosis cohort. (A) Median OS; (B) 1-year OS.
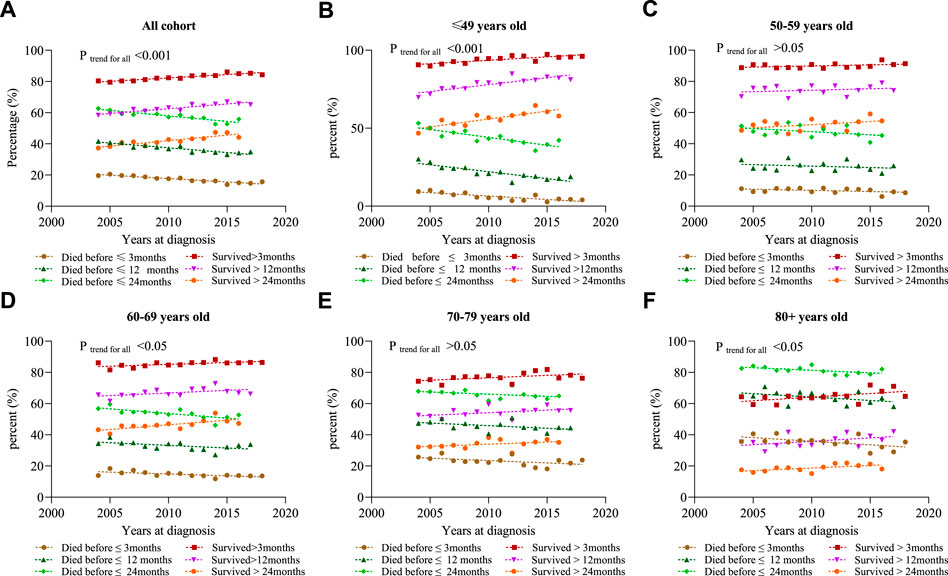
FIGURE 4. Percentage of death events in different years at diagnosis. (A) All cohort; (B) ≤ 49 years; (C) 50–59 years; (D) 60–69 years; (E) 70–79 years; and (F) 80 +years.
The good long-term survival was associated with patients diagnosed at the most recent year, younger age, non-black race, female, married, without previous history of malignancy, no second malignancy onset, descending/sigmoid/splenic flexure colon tumor, normal CEA levels at diagnosis, low primary tumor burden, T1/T2 stage, N0 stage, single organ metastasis, underwent surgical resection of synchronous distant metastatic lymph nodes or organs, a high number of lymph-node examinations, low positive lymph-node ratio and received adjuvant chemotherapy (Table 4).
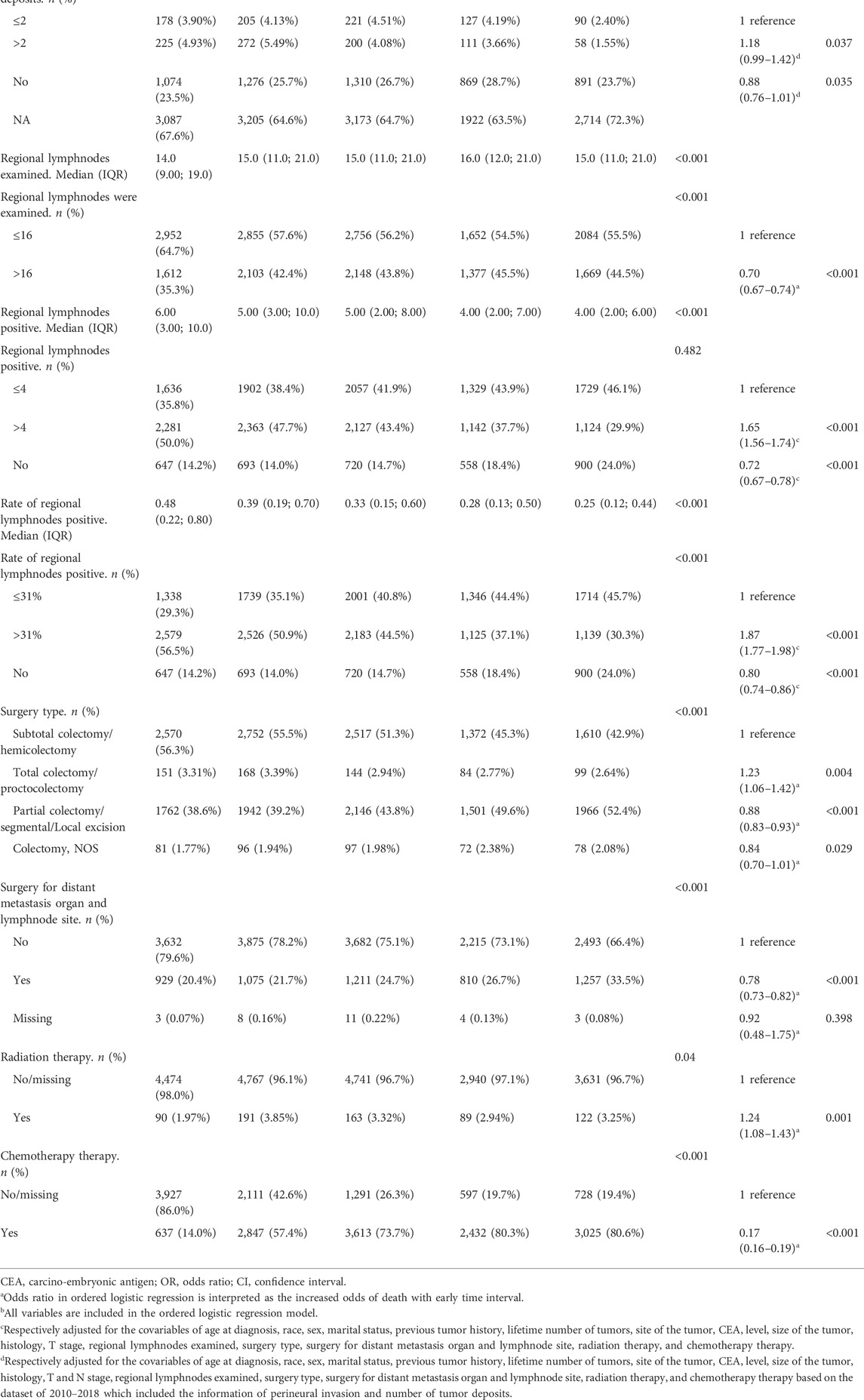
TABLE 4. Factors associated with early death in ordered logistic regressiona.
The study mainly found that the median OS of patients with mCC, who underwent cytoreductive surgery, remained largely stable and not substantial (17–21 months) over a duration of 15 years, however, the risk of mortality for mCC patients who underwent cytoreductive surgery has decreased in recent years compared to the past. Besides, we focusing on patients at both extremes of the survival spectrum and found that the percentage of patients who died within 3 months decreased and the percentage of patients who survival more than 24 months increased, which fully affirms the survival benefits of colon cancer patients underwent cytoreductive surgery in the era of rapid development of anticancer drugs.
Since the 1990s, 5-FU in combination with leucovorin has become the mainstay of treatment for metastatic colorectal cancer, and studies have reported a median survival of approximately 12 months (Poon et al., 1989). Subsequent studies have evaluated the effects of FOLFOX (a combination of 5-FU, leucovorin, and oxaliplatin) and FOLFIRI (a combination of 5-FU, leucovorin, and irinotecan) for metastatic colorectal cancer. These multidrug regimens resulted in a median OS of 12–20 months (de Gramont et al., 2000; Douillard et al., 2000; Saltz et al., 2000), suggesting that the multidrug combination may prolong patient survival. Therefore, FOLFOX and FOLFIRI regimens have become the standard first-line chemotherapy regimens for the treatment of metastatic colorectal cancer, and there is no significant difference in tumor benefit between the two regimens (Lee et al., 2019).
The emergence of molecularly targeted drugs has further improved the treatment of metastatic colorectal cancer. Bevacizumab, a monoclonal antibody targeting VEGF, inhibits tumor angiogenesis in combination with chemotherapy. Hurwitz et al. (2004) showed that compared with FOLFIRI alone, FOLFIRI combined with bevacizumab significantly prolonged the OS of patients (15.6 months vs. 20.3 months). The use of FOLFOXIRI combined with bevacizumab in patients with unresectable metastatic colorectal cancer was shown to have a significant overall objective response rate (69%), with a respectable conversion rate of 40% in selected patients (Tomasello et al., 2017). Other targeted angiogenesis drugs, such as ziv-aflibercept and ramucirumab, are recommended in combination with FOLFIRI for second-line treatment of metastatic colorectal cancer, and their median progression-free survival and objective response rate are superior to FOLFIRI therapy alone (Lee et al., 2019). Another important target for metastatic colorectal cancer therapy is EGFR, a tyrosine kinase closely related to HER2/neu that is overexpressed in many tumors. It is involved in signaling pathways, such as tumor proliferation, angiogenesis, and migration. Cetuximab and panitumumab are EGFR inhibitors and were found to have a significant survival advantage over supportive care in patients with chemotherapy-refractory tumors, and when combined with chemotherapy as first-line therapy, it significantly improved PFS and OS (Riedesser et al., 2022). Currently, anti-EGFR therapy is mainly used for treating metastatic colorectal cancer patients with wild-type RAS/BRAF, since RAS/BRAF mutant patients do not respond well to anti-EGFR therapy.
The RAS gene is often mutated in metastatic colorectal cancer, and the most mutated gene is the Kirsten Ras (KRAS) gene. More than 40% of metastatic colorectal cancers have KRAS mutations (Ros et al., 2021; Ciardiello et al., 2022). RAS mutations are often associated with poor prognosis and decreased response to antitumor therapy. Therefore, treatments targeting this RAS gene mutation are beneficial for the majority of patients. Currently, there is a lack of effective drugs for the treatment of RAS gene mutations. Sotorasib and adagrasib selectively inhibit KRASG12C mutation. Preliminary research results confirm their efficacy in KRASG12C mutation patients, and follow-up research is still in progress (Hong et al., 2020; Weiss et al., 2021). BRAF mutations are present in approximately 10–15% of patients with metastatic colorectal cancer. The V600E mutation is the most common mutation in the BRAF gene. BRAF mutations abnormally activate the MAPK signaling pathway, making tumors highly aggressive. Patients with BRAF V600E-mutated metastatic colorectal cancer are poorly responsive to chemotherapy and have an extremely poor prognosis with a median survival of only 12 months (Cohen et al., 2021). Some selective BRAF inhibitors have been developed, such as vemurafenib and dabrafenib. Unfortunately, these BRAF inhibitors negatively activate the MAPK signaling pathway, making their single-agent efficacy less than ideal. Combining multi-target blockade may help to obtain a more effective antitumor response. The BEACON study confirms that encorafenib plus cetuximab has significant advantages in objective response rate and OS in BRAF V600E-mutated metastatic colorectal cancer (Tabernero et al., 2021) In addition, studies combining encorafenib, cetuximab, and chemotherapy are also underway (Kopetz et al., 2021). Aberrant alterations in the HER2 gene are relatively uncommon in metastatic colorectal cancer, with HER2 gene amplification present in approximately 3% of metastatic colorectal cancers, mostly in RAS/BRAF wild-type patients (Ross et al., 2018). Retrospective studies have shown that HER2 gene amplification is associated with resistance to anti-EFGR therapy (Raghav et al., 2019). Currently, a series of studies on anti-HER2 therapy drugs in RAS/BRAF WT metastatic colorectal cancer patients is underway, and encouraging results have been obtained. This indicates that targeting HER2 therapy has a strong potential for treating metastatic colorectal cancer (Meric-Bernstam et al., 2019; Sartore-Bianchi et al., 2020; Siena et al., 2021).
Immunotherapy has yielded favorable outcomes in patients with a variety of solid tumors. However, in metastatic colorectal cancer, only a small proportion of patients may benefit. Microsatellite-instability-high (MSI-H)/mismatch repair-deficient (dMMR) is a biomarker for predicting the efficacy of immunotherapy in metastatic colorectal cancer, and it is present in approximately 5% of tumors in metastatic colorectal cancer (Arrichiello et al., 2021). Results of a multicenter randomized phase III clinical trial showed that compared with chemotherapy, pembrolizumab significantly prolonged median progression-free survival (PFS) in dMMR/MSI-H colorectal cancer patients, reaching 16.5 months. The majority of pembrolizumab monoclonal antibody-treated patients achieved objective responses over time (84% of patients lasting ≥2 years) (André et al., 2020). For microsatellite stable (MSS)/mismatch repair proficient (pMMR) patients with a high proportion of metastatic colorectal cancer, the efficacy of immunotherapy is poor, and whether it is suitable to receive immunotherapy is still under investigation. Studies have shown that cytotoxic drugs, anti-angiogenic drugs, molecularly targeted therapy, and radiotherapy can activate immunogenic cell death in tumor cells. Therefore, a series of studies are investigating the potential role of immune checkpoint inhibitor combination therapy (Ciardiello et al., 2022).
Reduction in the early death of cancer patients is an important indicator to evaluate the effect of comprehensive cancer treatment and nursing. McPhail et al. (2015) analyzed early mortality in patients with breast, colorectal, lung, prostate, and ovarian cancer in the United Kingdom, and found that age, tumor stage at diagnosis, income, and geographic location were significantly associated with early mortality in colorectal cancer. An analysis of colorectal cancer patients in the United Kingdom between 2006 and 2008 found that around 11.5% of colon cancer patients died within a month of being diagnosed, and about 33% died within a year of being diagnosed. Of these, old age, late tumor stage, poverty, and visits to the emergency department were associated with early mortality (Downing et al., 2013). Lieu et al. (2014) found that age was significantly associated with OS within 1 year of diagnosis in patients with metastatic colorectal cancer. The age effect was U-shaped, wherein both, young and old age, were unfavorable factors for early mortality in patients with metastatic colorectal cancer. Younger patients are generally healthier and have fewer underlying diseases than older patients, but their OS within 1 year of diagnosis is suboptimal, which may suggest different tumor biology. Renfro et al. (2016) found that low BMI was associated with an increased risk of metastatic colorectal cancer progression and death. Metastatic colorectal cancer patients are prone to cachexia, which leads to a significant decrease in BMI, affects the subsequent treatment of patients, and results in a significant increase in the risk of death.
A pooled analysis of 9 clinical trials examined the impact of performance status (PS) on chemotherapy in patients with metastatic colorectal cancer (Sargent et al., 2009). Although patients with PS grade 2 achieved similar treatment benefits as those with PS grades 0 and 1, they had significantly higher 60-day mortality (12.0% vs. 2.8%, p < 0.001). This suggests that performance status is an important risk factor for early mortality in patients with metastatic colorectal cancer. In a phase III randomized controlled trial of irinotecan in metastatic colorectal cancer, Giessen et al. (2011) assessed the clinicopathological factors associated with 60-day mortality in patients. The 60-day mortality rate in the study was 5.0% (24/479). Elevated LDH and WBC levels were considered independent predictors for early mortality, and fitness status showed a negative trend with an increased risk of early mortality in the study, but it was not statistically significant. However, in another study by Giessen et al. (2013). WBC count and performance status were identified as significant risk factors for early mortality, whereas LDH levels were not found to be associated with early mortality. In a randomized controlled study of primary tumor resection combined with systemic therapy (van der Kruijssen et al., 2021), patients with metastatic colorectal cancer who received systemic therapy after primary tumor resection had significantly higher 60-day mortality than patients who received systemic therapy alone. In the surgical group, factors such as serum lactate dehydrogenase, aspartate aminotransferase, alanine aminotransferase, and neutrophil count were associated with 60-day mortality.
In addition, the 60-day mortality appears to be significantly high in patients with multiple risk factors before surgery. There may be multiple reasons for how these factors affect early mortality in patients. 1) These risk factors are considered indicators of tumor mutational burden and malignancy (Kleespies et al., 2009; Ahn et al., 2014; Turner et al., 2015). Elevated levels of multiple indicators in patients suggest a large tumor burden, and therefore patients are at a risk of rapid progression after surgery. 2) These biochemical markers have been associated with poor prognosis in multiple studies (Ahn et al., 2014; Li et al., 2016). 3) Elevated levels of neutrophils in patients suggest a more pronounced systemic inflammatory response, which significantly increases the mortality of patients (Dell'Aquila et al., 2018). Early mortality was analyzed in a pooled analysis of 28 randomized clinical trials of metastatic colorectal cancer, which collected data from more than 22,000 patients with metastatic colorectal cancer from the ARCAD database. In this analysis, early mortality at 30 days, 60 days, and 90 days was 1.4%, 3.4%, and 5.5%, respectively. Older age, lower BMI, poor performance status, multiple metastatic sites, BRAF mutation status, and elevated laboratory markers (elevated bilirubin, WBC count, and neutrophil count) are associated with 90-day death. In contrast, KRAS mutation status, sex, single metastases, primary tumor site, and prior chemotherapy and treatment class (targeted versus non-targeted) were not associated with early mortality (Renfro et al., 2017). For patients with metastatic colorectal cancer with multiple risk factors, treatment options should be carefully evaluated to improve patient survival.
Our study has several limitations. First, the study was retrospective and the analysis relied on administrative claims data, with the potential for misclassification of cancer stage, vital status, and cause-specific survival. Second, the research object of this article is patients with mCC who underwent cytoreductive colectomy, it had clumped together a very heterogenous disease into a single category, which may be misleading. For example, the cohort will include patients with oligometastatic disease who underwent curative surgery and patients with bowel obstruction who underwent surgery, one has good outcomes, while the other is of poor prognosis. While, considering the different severity of metastatic colon cancer corresponding to different cytoreductive colectomy type and had a different survival outcome, we had classified the types of cytoreductive colectomy (Subtotal colectomy/hemicolectomy, Total colectomy/proctocolectomy, Partial colectomy/segmental/Local excision) and included into multivariate analysis. These type of cytoreductive colectomy are common in clinical practice for metastatic colon cancer patients. Third, there is no information on patient Karnofsky performance status, comorbidities, drug use of chemotherapy and targeted immunotherapy, or information on gene mutation, and imaging information (computerized tomography, magnetic resonance imaging, Positron Emission Tomography-Computed Tomography and so on) in the SEER database. However, the SEER database has done the following to ensure data accuracy: population-based case identification, detailed review of medical and pathological records, strict data collection and quality control standards, and high patient follow-up rates. This data has been thoroughly audited for accuracy and completeness. Therefore, it is reasonable to assume that our data on patient characteristics, tumor pathology and staging, treatment modalities, and survival status are reasonably accurate, and even with limited data, our conclusions remain reasonable. In addition, it should be noted that this study mainly included metastatic colon cancer patients who had undergone cytoreductive surgery and did not study rectal cancer, so the conclusions are not applicable to the metastatic rectal cancer population.
Over a span of 15 years, the OS and long-term survival of patients with mCC improved slightly, especially in the younger patient population. This demonstrates the progress in the comprehensive treatment for mCC over the past few decades. At the same time, we need to recognize that there is still a lot of room for improvement in the future.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
FC and GM conceived the research and wrote the manuscript. FC and SY analysed the data and prepared the figures and tables. All authors were involved in revising the manuscript and have approved its final version.
We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.983092/full#supplementary-material
Ahn, H. J., Oh, H. S., Ahn, Y., Lee, S. J., Kim, H. J., Kim, M. H., et al. (2014). Prognostic implications of primary tumor resection in stage IVB colorectal cancer in elderly patients. Ann. Coloproctol. 30 (4), 175–181. doi:10.3393/ac.2014.30.4.175
Andre, T., Shiu, K-K., Kim, T. W., Jensen, B. V., Jensen, L. H., Punt, C. J. A., et al. (2021). Final overall survival for the phase III KN177 study: Pembrolizumab versus chemotherapy in microsatellite instability-high/mismatch repair deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC). J. Clin. Oncol. 39 (15), 3500. doi:10.1200/jco.2021.39.15_suppl.3500
André, T., Shiu, K. K., Kim, T. W., Jensen, B. V., Jensen, L. H., Punt, C., et al. (2020). Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N. Engl. J. Med. 383 (23), 2207–2218. doi:10.1056/NEJMoa2017699
Arrichiello, G., Poliero, L., Borrelli, C., Paragliola, F., Nacca, V., Napolitano, S., et al. (2021). Immunotherapy in colorectal cancer: Is the long-awaited revolution finally happening? Cancer Treat. Res. Commun. 28, 100442. doi:10.1016/j.ctarc.2021.100442
Biller, L. H., and Schrag, D. (2021). Diagnosis and treatment of metastatic colorectal cancer: A review. JAMA 325 (7), 669–685. doi:10.1001/jama.2021.0106
Ciardiello, F., Ciardiello, D., Martini, G., Napolitano, S., Tabernero, J., and Cervantes, A. (2022). Clinical management of metastatic colorectal cancer in the era of precision medicine. Ca. Cancer J. Clin. 72, 372–401. doi:10.3322/caac.21728
Cohen, R., Liu, H., Fiskum, J., Adams, R., Chibaudel, B., Maughan, T. S., et al. (2021). BRAF V600E mutation in first-line metastatic colorectal cancer: An analysis of individual patient data from the ARCAD database. J. Natl. Cancer Inst. 113 (10), 1386–1395. doi:10.1093/jnci/djab042
de Gramont, A., Figer, A., Seymour, M., Homerin, M., Hmissi, A., Cassidy, J., et al. (2000). Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J. Clin. Oncol. 18 (16), 2938–2947. doi:10.1200/JCO.2000.18.16.2938
Dell'Aquila, E., Cremolini, C., Zeppola, T., Lonardi, S., Bergamo, F., Masi, G., et al. (2018). Prognostic and predictive role of neutrophil/lymphocytes ratio in metastatic colorectal cancer: A retrospective analysis of the TRIBE study by GONO. Ann. Oncol. 29 (4), 924–930. doi:10.1093/annonc/mdy004
Douillard, J. Y., Cunningham, D., Roth, A. D., Navarro, M., James, R. D., Karasek, P., et al. (2000). Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: A multicentre randomised trial. Lancet 355 (9209), 1041–1047. doi:10.1016/s0140-6736(00)02034-1
Downing, A., Aravani, A., Macleod, U., Oliver, S., Finan, P. J., Thomas, J. D., et al. (2013). Early mortality from colorectal cancer in england: A retrospective observational study of the factors associated with death in the first year after diagnosis. Br. J. Cancer 108 (3), 681–685. doi:10.1038/bjc.2012.585
Ganesh, K., Stadler, Z. K., Cercek, A., Mendelsohn, R. B., Shia, J., Segal, N. H., et al. (2019). Immunotherapy in colorectal cancer: Rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 16 (6), 361–375. doi:10.1038/s41575-019-0126-x
Giessen, C., Graeven, U., Laubender, R. P., Modest, D. P., Schulz, C., Porschen, R., et al. (2013). Prognostic factors for 60-day mortality in first-line treatment of metastatic colorectal cancer (mCRC): Individual patient analysis of four randomised, controlled trials by the AIO colorectal cancer study group. Ann. Oncol. 24 (12), 3051–3055. doi:10.1093/annonc/mdt402
Giessen, C., Stintzing, S., Laubender, R. P., Ankerst, D. P., Schulz, C., Moosmann, N., et al. (2011). Analysis for prognostic factors of 60-day mortality: Evaluation of an irinotecan-based phase III trial performed in the first-line treatment of metastatic colorectal cancer. Clin. Colorectal Cancer 10 (4), 317–324. doi:10.1016/j.clcc.2011.03.027
Goldberg, R. M., Sargent, D. J., Morton, R. F., Fuchs, C. S., Ramanathan, R. K., Williamson, S. K., et al. (2004). A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J. Clin. Oncol. 22 (1), 23–30. doi:10.1200/JCO.2004.09.046
Hong, D. S., Fakih, M. G., Strickler, J. H., Desai, J., Durm, G. A., Shapiro, G. I., et al. (2020). KRAS(G12C) inhibition with sotorasib in advanced solid tumors. N. Engl. J. Med. 383 (13), 1207–1217. doi:10.1056/NEJMoa1917239
Hurwitz, H., Fehrenbacher, L., Novotny, W., Cartwright, T., Hainsworth, J., Heim, W., et al. (2004). Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 350 (23), 2335–2342. doi:10.1056/NEJMoa032691
Kawai, S., Takeshima, N., Hayasaka, Y., Notsu, A., Yamazaki, M., Kawabata, T., et al. (2021). Comparison of irinotecan and oxaliplatin as the first-line therapies for metastatic colorectal cancer: A meta-analysis. BMC Cancer 21 (1), 116. doi:10.1186/s12885-021-07823-7
Kleespies, A., Füessl, K. E., Seeliger, H., Eichhorn, M. E., Müller, M. H., Rentsch, M., et al. (2009). Determinants of morbidity and survival after elective non-curative resection of stage IV colon and rectal cancer. Int. J. Colorectal Dis. 24 (9), 1097–1109. doi:10.1007/s00384-009-0734-y
Kopetz, S., Grothey, A., Yaeger, R., Ciardiello, F., Desai, J., Kim, T. W., et al. (2021). Breakwater: Randomized phase 3 study of encorafenib (enco) + cetuximab (cetux) ± chemotherapy for first-line (1L) treatment (tx) of BRAF V600E-mutant (BRAFV600E) metastatic colorectal cancer (mCRC). J. Clin. Oncol. 39 (15), TPS3619. doi:10.1200/jco.2021.39.15_suppl.tps3619
Lee, R. M., Cardona, K., and Russell, M. C. (2019). Historical perspective: Two decades of progress in treating metastatic colorectal cancer. J. Surg. Oncol. 119 (5), 549–563. doi:10.1002/jso.25431
Li, G., Wang, Z., Xu, J., Wu, H., Cai, S., and He, Y. (2016). The prognostic value of lactate dehydrogenase levels in colorectal cancer: A meta-analysis. BMC Cancer 16, 249. doi:10.1186/s12885-016-2276-3
Lieu, C. H., Renfro, L. A., de Gramont, A., Meyers, J. P., Maughan, T. S., Seymour, M. T., et al. (2014). Association of age with survival in patients with metastatic colorectal cancer: Analysis from the ARCAD clinical trials program. J. Clin. Oncol. 32 (27), 2975–2984. doi:10.1200/JCO.2013.54.9329
McPhail, S., Johnson, S., Greenberg, D., Peake, M., and Rous, B. (2015). Stage at diagnosis and early mortality from cancer in England. Br. J. Cancer 112, S108–S115. doi:10.1038/bjc.2015.49
Meric-Bernstam, F., Hurwitz, H., Raghav, K. P. S., McWilliams, R. R., Fakih, M., VanderWalde, A., et al. (2019). Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): An updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet. Oncol. 20 (4), 518–530. doi:10.1016/S1470-2045(18)30904-5
Modest, D. P., Karthaus, M., Fruehauf, S., Graeven, U., Muller, L., Konig, A. O., et al. (2022). Panitumumab plus fluorouracil and folinic acid versus fluorouracil and folinic acid alone as maintenance therapy in RAS wild-type metastatic colorectal cancer: The randomized Panama trial (AIO KRK 0212). J. Clin. Oncol. 40 (1), 72–82. doi:10.1200/JCO.21.01332
Mody, K., and Bekaii-Saab, T. (2018). Clinical trials and progress in metastatic colon cancer. Surg. Oncol. Clin. N. Am. 27 (2), 349–365. doi:10.1016/j.soc.2017.11.008
Overman, M. J., McDermott, R., Leach, J. L., Lonardi, S., Lenz, H. J., Morse, M. A., et al. (2017). Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet. Oncol. 18 (9), 1182–1191. doi:10.1016/S1470-2045(17)30422-9
Poon, M. A., O'Connell, M. J., Moertel, C. G., Wieand, H. S., Cullinan, S. A., Everson, L. K., et al. (1989). Biochemical modulation of fluorouracil: Evidence of significant improvement of survival and quality of life in patients with advanced colorectal carcinoma. J. Clin. Oncol. 7 (10), 1407–1418. doi:10.1200/JCO.1989.7.10.1407
Raghav, K., Loree, J. M., Morris, J. S., Overman, M. J., Yu, R., Meric-Bernstam, F., et al. (2019). Validation of HER2 amplification as a predictive biomarker for anti–epidermal growth factor receptor antibody therapy in metastatic colorectal cancer. JCO Precis. Oncol. 3 (3), 1–13. doi:10.1200/PO.18.00226
Renfro, L. A., Goldberg, R. M., Grothey, A., Sobrero, A., Adams, R., Seymour, M. T., et al. (2017). Clinical Calculator for Early Mortality in Metastatic Colorectal Cancer: An Analysis of Patients From 28 Clinical Trials in the Aide et Recherche en Cancérologie Digestive Database. J. Clin. Oncol. 35 (17), 1929–1937. doi:10.1200/JCO.2016.71.5771
Renfro, L. A., Loupakis, F., Adams, R. A., Seymour, M. T., Heinemann, V., Schmoll, H-J., et al. (2016). Body mass index is prognostic in metastatic colorectal cancer: Pooled analysis of patients from first-line clinical trials in the ARCAD database. J. Clin. Oncol. 34 (2), 144–150. doi:10.1200/JCO.2015.61.6441
Riedesser, J. E., Ebert, M. P., and Betge, J. (2022). Precision medicine for metastatic colorectal cancer in clinical practice. Ther. Adv. Med. Oncol. 14, 17588359211072703. doi:10.1177/17588359211072703
Ros, J., Baraibar, I., Martini, G., Salvà, F., Saoudi, N., Cuadra-Urteaga, J. L., et al. (2021). The evolving role of consensus molecular subtypes: A step beyond inpatient selection for treatment of colorectal cancer. Curr. Treat. Options Oncol. 22 (12), 113. doi:10.1007/s11864-021-00913-5
Ross, J. S., Fakih, M., Ali, S. M., Elvin, J. A., Schrock, A. B., Suh, J., et al. (2018). Targeting HER2 in colorectal cancer: The landscape of amplification and short variant mutations in ERBB2 and ERBB3. Cancer 124 (7), 1358–1373. doi:10.1002/cncr.31125
Saltz, L. B., Cox, J. V., Blanke, C., Rosen, L. S., Fehrenbacher, L., Moore, M. J., et al. (2000). Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N. Engl. J. Med. 343 (13), 905–914. doi:10.1056/NEJM200009283431302
Sargent, D. J., Köhne, C. H., Sanoff, H. K., Bot, B. M., Seymour, M. T., de Gramont, A., et al. (2009). Pooled safety and efficacy analysis examining the effect of performance status on outcomes in nine first-line treatment trials using individual data from patients with metastatic colorectal cancer. J. Clin. Oncol. 27 (12), 1948–1955. doi:10.1200/JCO.2008.20.2879
Sartore-Bianchi, A., Lonardi, S., Martino, C., Fenocchio, E., Tosi, F., Ghezzi, S., et al. (2020). Pertuzumab and trastuzumab emtansine in patients with HER2-amplified metastatic colorectal cancer: The phase II HERACLES-B trial. ESMO Open 5 (5), e000911. doi:10.1136/esmoopen-2020-000911
Siegel, R. L., Miller, K. D., Fuchs, H. E., and Jemal, A. (2022). Cancer statistics, 2016. Ca. Cancer J. Clin. 72 (1), 7–30. doi:10.3322/caac.21332
Siegel, R. L., Miller, K. D., Goding Sauer, A., Fedewa, S. A., Butterly, L. F., Anderson, J. C., et al. (2020). Colorectal cancer statistics, 2020. Ca. Cancer J. Clin. 70 (3), 145–164. doi:10.3322/caac.21601
Siena, S., Di Bartolomeo, M., Raghav, K., Masuishi, T., Loupakis, F., Kawakami, H., et al. (2021). Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): A multicentre, open-label, phase 2 trial. Lancet. Oncol. 22 (6), 779–789. doi:10.1016/S1470-2045(21)00086-3
Tabernero, J., Grothey, A., Van Cutsem, E., Yaeger, R., Wasan, H., Yoshino, T., et al. (2021). Encorafenib plus cetuximab as a new standard of care for previously treated BRAF V600e-mutant metastatic colorectal cancer: Updated survival results and subgroup Analyses from the BEACON study. J. Clin. Oncol. 39 (4), 273–284. doi:10.1200/JCO.20.02088
Tomasello, G., Petrelli, F., Ghidini, M., Russo, A., Passalacqua, R., and Barni, S. (2017). FOLFOXIRI plus bevacizumab as conversion therapy for patients with initially unresectable metastatic colorectal cancer: A systematic review and pooled analysis. JAMA Oncol. 3 (7), e170278. doi:10.1001/jamaoncol.2017.0278
Turner, N., Tran, B., Tran, P. V., Sinnathamby, M., Wong, H. L., Jones, I., et al. (2015). Primary tumor resection in patients with metastatic colorectal cancer is associated with reversal of systemic inflammation and improved survival. Clin. Colorectal Cancer 14 (3), 185–191. doi:10.1016/j.clcc.2015.02.004
van der Geest, L. G., Lam-Boer, J., Koopman, M., Verhoef, C., Elferink, M. A., and de Wilt, J. H. (2015). Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin. Exp. Metastasis 32 (5), 457–465. doi:10.1007/s10585-015-9719-0
van der Kruijssen, D. E. W., Elias, S. G., Vink, G. R., van Rooijen, K. L., ‘t Lam-Boer, J., Mol, L., et al. (2021). Sixty-day mortality of patients with metastatic colorectal cancer randomized to systemic treatment vs primary tumor resection followed by systemic treatment: The CAIRO4 phase 3 randomized clinical trial. JAMA Surg. 156 (12), 1093–1101. doi:10.1001/jamasurg.2021.4992
Weiss, J., Yaeger, R. D., Johnson, M. L., Spira, A., Klempner, S. J., Barve, M. A., et al. (2021). LBA6 KRYSTAL-1: Adagrasib (MRTX849) as monotherapy or combined with cetuximab (Cetux) in patients (Pts) with colorectal cancer (CRC) harboring a KRASG12C mutation. Ann. Oncol. 32, S1294. doi:10.1016/j.annonc.2021.08.2093
Keywords: survival outcomes, colon cancer, metastatic, cytoreductive colectomy, early mortality
Citation: Meng G, Yang S and Chen F (2022) Survival for patients with metastatic colon cancer underwent cytoreductive colectomy in the era of rapid development of anticancer drugs: A real-world analysis based on updated population dataset of 2004–2018. Front. Pharmacol. 13:983092. doi: 10.3389/fphar.2022.983092
Received: 01 July 2022; Accepted: 07 October 2022;
Published: 19 October 2022.
Edited by:
Fedor Moiseenko, N. N. Petrov National Medical Research Center of Oncology, RussiaReviewed by:
Hsueh Ju Lu, Chung Shan Medical University Hospital, TaiwanCopyright © 2022 Meng, Yang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangran Meng, MTM2MTg2MzQxODZAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.