
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 09 November 2022
Sec. Predictive Toxicology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.974376
This article is part of the Research Topic Toxicity Mechanism and Clinical features of PD-1/PD-L1 Inhibitors in Treatment of Cancer, Volume II View all 9 articles
Background & Aims: Immune checkpoint inhibitors (ICIs) have transformed the landscape of cancer treatment, and ICI-related toxicities (i.e., immune-related adverse events (irAEs) have been reported in many clinical studies. However, the toxicity data of real-world have not been fully assessed.
Methods: Patients with histologically confirmed solid tumors who had been treated with PD-1 inhibitors were included in the study. Patient data were collected from electronic medical records, including basic characteristics, data of irAEs, management and outcome. Incidences of irAEs were pooled and compared, and the risk of irAEs was also analyzed.
Results: A total of 362 solid tumor patients treated with sintilimab (n = 171), camrelizumab (n = 60), toripalimab (n = 72), and pembrolizumab (n = 59) were included. In total, any grade irAEs, grade 1–2 irAEs, and grade ≥3 irAEs accounted for 47.24%, 38.67% and 8.56% of cases, reapectively. Further, 29.24% of patients discontinued immunotherapy due to irAEs, with pneumonitis being the main reason for discontinuation. By comparing the toxicity profiles between different ICIs, we found that reactive capillary haemangiomas were camrelizumab-specific. Additionally, the frequency of irAEs was association with ICIs type, the pooled incidence (standardized rate) of irAEs related to sintilimab, camrelizumab, toripalimab and pembrolizumab were 55.56% (52.81%), 48.33% (55.55%), 33.33% (29.23%) and 38.98% (38.29%), respectively. Sintilimab and camrelizumab had higher incidences of any grade and grade 1–2 than toripalimab (55.56% vs. 33.33%, p = 0.002; 48.54% vs. 25.00%, p = 0.0001) and pembrolizumab (55.56% vs. 38.98%, p = 0.0028; 48.54% vs. 25.42%, p = 0.002), while the grade ≥3 irAEs of pembrolizumab (13.56%) were approximately 1.63- to 1.93-fold higher than other ICIs, and the standardized grade ≥3 of pembrolizumab was significantly higher than that of sintilimab (13.21% vs. 7.12%, p = 0.026), especially for grade ≥3 pneumonitis. Multivariate analysis found that cumulative cycles of ICI (OR = 1.081; 95% CI: 1.023–1.142; p = 0.006), and lung cancer (OR = 1.765; 95% CI: 1.105–2.820; p = 0.017) were independent risk factors for irAEs.
Conclusion: The frequency of irAEs is associated with ICI type. The pooled incidence of irAEs related to sintilimab and pneumonitis caused by pembrolizumab were higher. These data indicate the importance of having different monitoring priorities for different PD-1 inhibitors.
Over the past 5 years, immune therapy represented by immune checkpoint inhibitors (ICIs) targeting programmed death 1 (PD-1), programmed death ligand-1 (PD-L1) and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), have been important therapeutic drugs second only to chemotherapy, radiotherapy and targeted therapy, because ICIs have significantly improved clinical outcomes in multiple cancer types (Vaddepally et al., 2020). Different from conventional cytotoxic drugs, ICIs restore and promote antitumor immunity to kill cancer cells (Thompson et al., 2022). Currently, ICIs (e.g., nivolumab, pembrolizumab, atezolizumab, camrelizumab, toripalimab, tislelizumab and sintilimab) alone or combined with chemotherapy or anti-angiogenesis therapy have become the standard first- or second-line therapies for various malignancies. Moreover, domestic ICIs (camrelizumab, toripalimab, tislelizumab and sintilimab) are used more than abroad ICIs (pembrolizumab and nivolumab) because of drug costs.
With the widespread use of immunotherapy, the development of ICI-related toxicities (i.e., immune-related adverse events, irAEs) are concerning because many like myocarditis and pneumonitis are covert and fatal (Wang et al., 2018; Chen et al., 2021; Luo et al., 2021). Different from the adverse reactions mediated by traditional cytotoxic drugs, irAEs are associated with an immunologic mode of action, which can affect any organ system in the body. The most frequently organ include of skin, endocrine system, lungs, heart, gastrointestinal (GI) system, liver and other less common inflammatory events (Xu et al., 2018; Yang et al., 2019). Moreover, the characteristics of irAEs are not typical. The frequency of irAEs depends on the type of ICIs as well as the tumor type, the duration of exposure, the dose administered, and the specific characteristics of individual patients (e.g., such as intrinsic risk factors (Ma et al., 2018; Pillai et al., 2018; Tang et al., 2021). The results of a recent meta-analysis showed that GI toxicity and hypophysitis are more frequent with CTLA-4 inhibitors, whereas pneumonitis, hypothyroidism, arthralgia, and thyroid disorders are more prevalent with PD-1 inhibitors (Boutros et al., 2016; Choi and Lee, 2020). Patients receiving PD-1/PD-L1 inhibitors have a lower incidence of any-grade irAEs than those receiving CTLA-4 inhibitors, while patients receiving a combination of PD-1/PD-L1 inhibitors and CTLA-4 inhibitors have the highest incidence of irAEs. The incidence of grade ≥3 irAEs is 6%, 24% and 55% for patients receiving PD-1/PD-L1 inhibitors, CTLA-4 inhibitors, combination of the two immunotherapy, respectively (Haanen et al., 2017; Martins et al., 2019). Some systematic reviews have also shown that patients with non-small cell lung cancer (NSCLC) experience a higher frequency of pneumonitis and a lower frequency of GI and skin irAEs compared to melanoma (Khoja et al., 2017). Moreover, irAEs have very wide dispersion of onset times, with data from treatment process to 1 year after discontinuation (Zhai et al., 2019). These nonspecific features make it difficult to identify adverse reactions, and limit clinical applications.
Although many studies have reported on ICI-related toxicities, the toxicity data are mainly from clinical research which cannot fully substitute for real-world ICI-related toxicities due to certain limitations. Therefore, there is an urgent clinical need to obtain real-world safety data. Herein, we evaluate the real-world characteristics and potential risk of irAEs, with the aim of improving early recognition and management of irAEs and to also provide a reference for the use of ICIs.
We performed a retrospective observational study to investigate the characteristics of adverse drug reactions (ADRs) related to ICI, in a tertiary hospital (Chongqing, China) between January 2018 and July 2021. Informed consent was permitted obtained from all study participants. The inclusion criteria were as follows: (Vaddepally et al., 2020): patients were diagnosed as solid malignant tumors with pathologic diagnosis; (Thompson et al., 2022); patient with ≥2 cycle of ICI therapy (pembrolizumab, sintilimab, camrelizumab, tirelizumab, or toripalimab). The dosage of ICI was given referring to prescribing information and guidelines, such as pembrolizumab 2 mg/kg or 200 mg iv q3w, sintilimab 200 mg iv q3w, camrelizumab 200 mg iv q2w or q3w, tirelizumab 200 mg iv q3w, or toripalimab 3mg/kg iv q2w or 240 mg iv q3w. Patients with incomplete medical records were excluded. In our study, irAEs were defined according to the guidelines on the management of immunotherapy-related toxicities (Thompson et al., 2022) and finally diagnosed by medical specialists. The grade of irAEs was determined using the Common Terminology Criteria for Adverse Events version 5.0.
Available medical records in each case were reviewed for the patient’s medical history, including age, sex, history of disease, the type of tumor, duration of ICI therapy, and details of other chemotherapies and radiotherapy. The data of adverse reaction included onset time, symptoms, auxiliary examination, and the details of treatment approaches for ICI-related adverse reactions. Patient outcomes were also recorded.
The major endpoint of our study was to observe the characteristics of irAEs in real study, including irAE profiles, classification of adverse reactions, occurrence time, management and outcomes. We also sought to compare irAE profiles between different ICIs. Other assessed outcomes included an exploration of exploring risk factors associated with irAEs. Data are presented with descriptive statistics for each demographic. Association studies were conducted using Fisher’s exact test for categorical variables and the Mann-Whitney U test for continuous variables. Multivariate logistic regression analysis was conducted to identify potential risk factors for irAEs. The risk associations with a p-value < 0.05 were considered statistically significant, and all analyses were performed using SPSS. In addition, the incidence of different ICIs was standardized using the standardized equation (
From January 2018 to July 2021, 441 patients were treated with ICIs (Pembrolizumab, Sintilimab, Camrelizumab, Tirelizumab, or Toripalimab). A total of 79 patients were excluded because they received one treatment cycle, therefore, the study cohort consisted of 362 patients. The 362 patients received 1,256 treatment cycles. Of the 362 patients with ICI therapy, 275 (75.97%) were male, giving a male: female ratio of 3.16:1. The average age was 62.4 ± 11.4 years (range 11–86 years). Lung cancer was the most type of cancer (201 patients, 55.52%), followed by head and neck cancer (61 patients, 16.85%), and liver cancer (34 patients, 9.39%). The other details of the basic information are shown in Table 1, and the baseline information of people with different ICIs is also shown in Supplementary Table S1 of Supplementary Material. There were no significant differences among different ICI groups in terms of age, sex, smoking history, chronic obstructive pulmonary disease, medication information, targeted therapy history and radiation history; only the composition of tumor type was statistically significant (p = 0.001).
Of the 362 patients, 118 patients (32.6%) developed a total of 171 irAEs, among which 80 patients (67.80%) had one or two types of irAEs and nine patients (7.63%) suffered more than three kinds of irAEs. The common reported events (≥1%) by organ system were thyroid dysfunction (73, 20.17%), skin reaction (36, 9.94%), pneumonitis (19, 5.25%), infusion reaction (9, 2.49%), myocarditis (7, 1.93%), reactive capillary hemangiomas (7, 1.93%), colitis (6, 1.66%), and hepatitis (6, 1.66%). Moreover, rare irAEs (<1%) included fever (n = 2), thrombocytopenia (n = 1), nephritis (n = 1), arthritis (n = 1), diabetes (n = 1), adrenal (n = 1) and neurologic (n = 1), as listed in Supplementary Table S2 (Supplementary Material). Overall, the incidence of any grade irAEs, grade 1–2 irAEs, and grade ≥3 irAEs were 47.24%, 38.67%, and 8.56%, respectively. Further, irAEs were mostly mild, and there were significant differences between grade 1–2 and grade ≥3 (38.67% vs. 8.56%, p < 0.01) (Figure 1A, Supplementary Material, Supplementary Table S3). For grade ≥3 irAEs, the incidence of pneumonitis was the highest, reaching 4.7%, followed by hepatitis (1.1%), myocarditis (0.83%), skin reaction (0.55%), colitis (0.55%), thrombocytopenia (0.28%), diabetes (0.28%), and adrenal (0.28%). The toxicity data of different regimen were also revealed in Figure 1B, Figure 1, Figure 2, Figure 3 (Supplementary Material, Supplementary Table S3). These data showed that the ICI combination group had significantly higher rates of any grade irAEs (49.67% vs. 35%, p = 0.038) compared to the monotherapy group, while there were no significant differences between groups with grade 1–2 and grade ≥3 irAEs. Notably, the frequency of reactive capillary haemangiomas (RCCEP) were higher in the ICI monotherapy group than those in the ICI combination group (10% vs. 0.33%, p < 0.001).
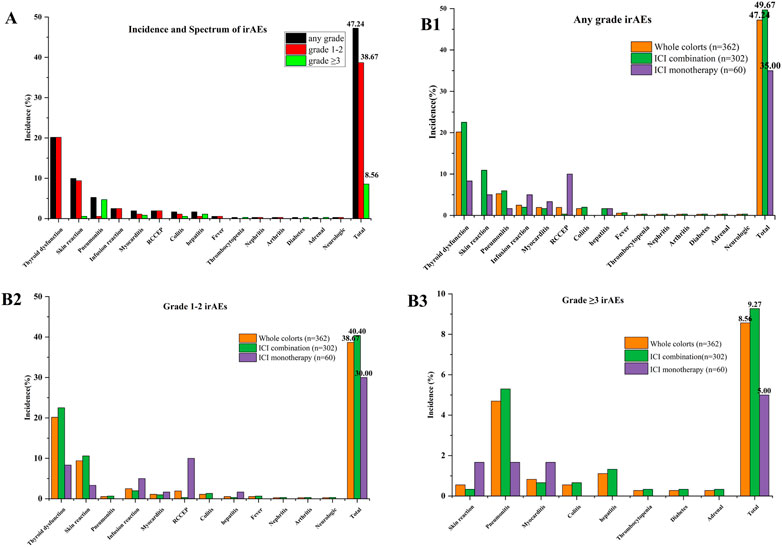
FIGURE 1. Incidence and spectrum of irAEs between immune checkpoint inhibitor monotherapy and combination: (A) Incidence and spectrum of irAEs; (B1–3) irAEs between immune checkpoint inhibitor monotherapy and combination. ICI combination: combination with chemotherapy, anti-vascular drug, anti-vascular and chemotherapy.
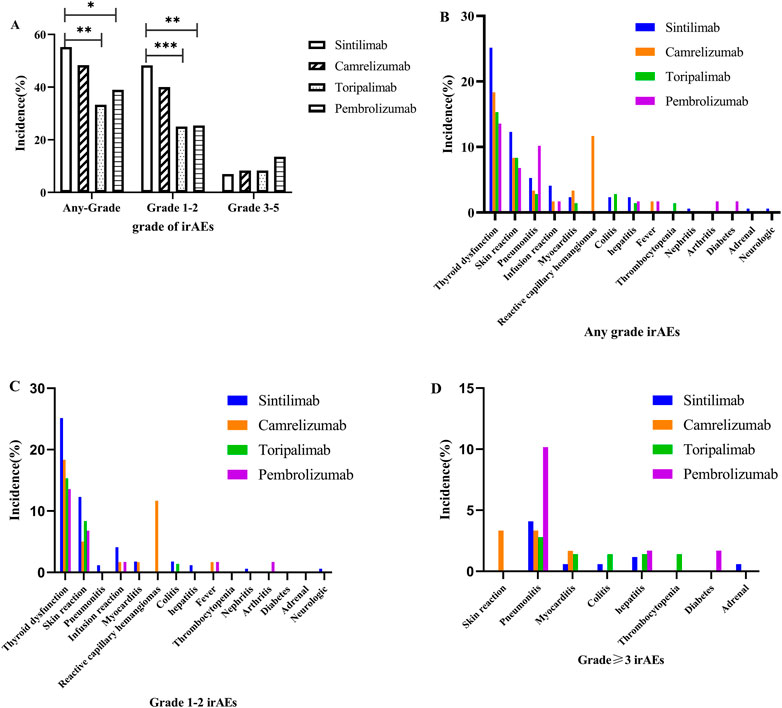
FIGURE 2. Immune-related adverse events between different immune checkpoint inhibitors: (A) any irAEs; (B) any grade; (C) grade 1–2; (D) grade ≥3.
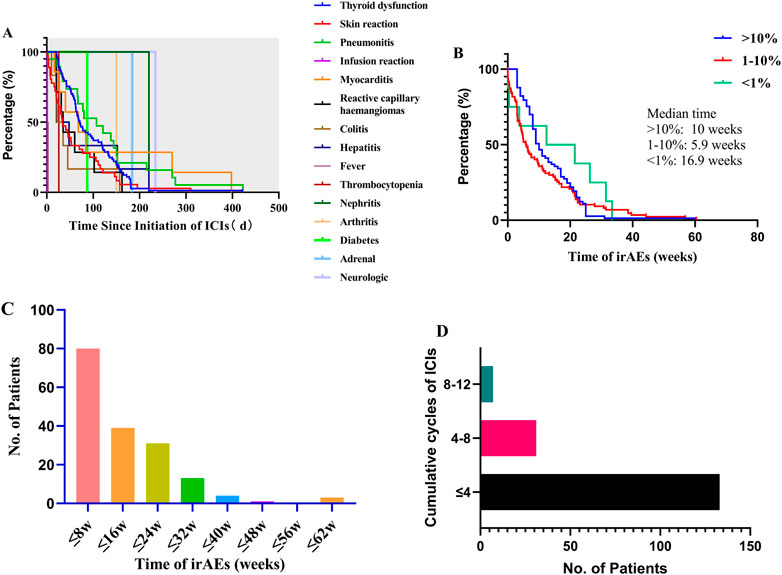
FIGURE 3. Onset time between different irAEs and frequency of all irAEs between onset time and treatment cycle: (A) onset time of any irAEs; (B) onset time between different frequency of irAEs; (C) frequency of all irAEs by onset time; (D) frequency of all irAEs by treatment cycle.
We further analyzed and compared the incidence to ICI-related adverse reactions associated with the ICI types. Figure 2 and Supplementary Table S4A–C of the Supplementary Material show that there were no significant differences among the spectrum of the four ICIs (sintilimab, camrelizumab, toripalimab, and pembrolizumab). Interestingly only camrelizumab caused reactive capillary haemangiomas, indicating that this AE should be camrelizumab-specifc. Moreover, the four ICIs had different incidence of any grade, and the pooled incidence of sintilimab, camrelizumab, toripalimab and pembrolizumab was 55.56%, 48.33%, 33.33% and 38.98%, respectively. Sintilimab had signifcantly higher incidences of any grade and grade 1–2 irAEs than toripalimab (55.56% vs. 33.33%, p = 0.002; 48.54% vs. 25%, p = 0.0001), and pembrolizumab (55.56% vs. 38.98%, p = 0.0028; 48.54% vs. 25.42%, p = 0.002). Considering the significant differences among groups in the composition of cancer type as shown in Supplementary Table S1(Supplementary Material), the incidence of irAEs with different ICI. The standardized incidence of all-grade irAEs with sintilimab, camrelizumab, toripalimab, and pembrolizumab was 52.81%, 55.55%, 29.23% and 38.29%, respectively, and grade 1–2 irAEs was 45.69%, 44.99%, 21.26%, and 25.08%, respectively. Sintilimab and camrelizumab had higher all-grade (52.81%/55.55% vs. 29.23%/38.29%, p < 0.001) and grade 1–2 irAEs (45.69%/44.99% vs. 21.26%/25.08%, p < 0.001) than toripalimab and pembrolizumab. For grade ≥3 irAEs, there were significant differences between sintilimab and pembrolizumab (7.12% vs. 13.21%, p = 0.026). Notably, the pooled incidences of grade ≥3 irAEs to pembrolizumab (13.56%) were approximately 1.93-, 1.63- and 1.63-fold higher than sintilimab (7.02%), camrelizumab (8.33%) and toripalimab (8.33%), which suggests pembrolizumab may have a bigger impact on severe irAEs. Additionally, pembrolizumab had a higher incidence of grade ≥3 pneumonitis (10.17% for pembrolizumab, 4.09% for sintilimab, 2.33% for camrelizumab and 2.78% for toripalimab), and the camrelizumab-induced grade ≥3 infusion reactions (3.33%) should not be ignored. The observed and standardized incidence of irAEs are shown in Table 2 and Supplementary Table S4A–C of the Supplementary Material.
Overall, the time to the start of any irAEs was variable (median: 62 d, range: 0–423 d), and there were significant differences between the onset time of different irAE (p < 0.001), such as infusion reaction (transfusion process), fever (median: 2 d, range: 1–3 d), thrombocytopenia (26 d), colitis (median: 28.5 d, range: 10–157 d), skin reaction (median: 33.5 d, range: 1–310 d), hepatitis (median: 34.5 d, range: 20–220 d), reactive capillary hemangiomas (median: 35 d, range: 21–162 d), myocarditis (median: 68 d, range: 16–398 d), diabetes (87 d), pneumonitis (median:107d, range: 3–423 d), arthritis (150 d), thyroid dysfunction (median:171 d, range: 21–421 d), nephritis (184 d), adrenal (220 d), and neurologic (234 d) as shown in Figure 3A. Interestingly, the onset time of irAEs was related to the incidence of irAEs and there were significant differences (p < 0.05) in the most common irAEs (>10%, median:10 weeks), common irAEs (1–10%, median:5.9 w), and rare irAEs (<1%, median:16.9 w) (Figure 3B). This suggested that we should play attention to rare irAEs for patients with using undergoing long-term ICI treatment. Moreover, we analyzed the incidence to ICI-related adverse reactions associated with the ICI using cycle. A total of 171 irAEs occurred in 80 patients, the majority of which were early in the treatment course (Figure 3C). In addition, 77.78% of these irAEs occurred in the first four cycles, 18.13% within four to eight cycles, and approximately 4.09% within 8–12 cycles (Figure 3D with increased medication time, the incidence of thyroid dysfunction, skin reaction, infusion reaction, and pneumonitis decreased, while the frequency of rare irAEs (e.g., nephritis, adrenal, and neurologic) increased.
The detailed clinical manifestations, management and outcomes of all irAEs are shown in Supplementary Table S5 of the Supplementary Material. These data demonstrated that most irAEs have no obvious clinical manifestations and patient outcomes were associated with the type and grade of irAEs. Overall, the mild or moderate irAEs of most patients were not associated with any apparent symptoms, and only showed abnormal laboratory results. Of the 171 irAEs, 159 had a good prognosis except for patients with thyroid dysfunction who required lifelong thyroid hormone replacement therapy. In addition, 1.75% (3 of 171) of patients were unchanged, and the health of 5.26% (9 of 171) patients worsened and some even died as shown in Figure 4A. We also found that 29.24% (50 of 171) discontinued immunotherapy because of their irAEs. In order of frequency, the irAEs of the nine patients whose health worsened and/or even died was pneumonitis (n = 5/9, 56%), myocarditis (n = 2/9, 22%), thrombocytopenia (n = 1/9, 11%) and colitis (n = 1/9, 11%) as shown in Figure 4B. Notably, 4 of 17 patients with grade ≥3 pneumonitis occurred rebound in the process of tapering off systemic steroids, while one patient died from pneumonitis; two of three patients with grade ≥3 myocarditis occurred rebound even died, and the patient with thrombocytopenia was neglected and died from delated treatment (Figure 4C). Moreover, ICI-related AEs leading to discontinuation were reported in 50 patients (29.24%), with the most common cause being pneumonitis (n = 19/50, 38%), reactive capillary hemangiomas (n = 7/50, 14%), colitis (n = 6/50, 12%), myocarditis (n = 5/10, 10%) and hepatitis (n = 4/10, 8%). Additionally, a few cases of discontinuation occurred following: skin reaction, infusion reaction, fever, thrombocytopenia, diabetes and adrenal. There were no instances of thyroid dysfunction.
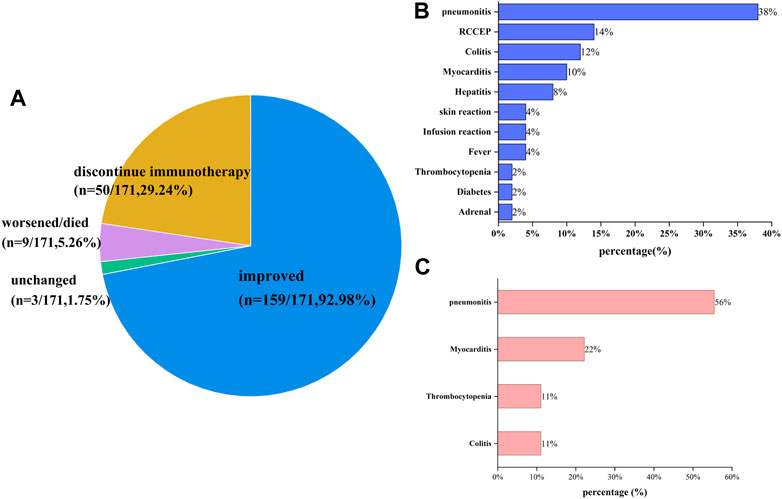
FIGURE 4. Outcomes of patients with irAEs: (A) the outcome distribution of patients with irAEs; (B) the reason for ending immunotherapy; (C) the cause of exacerbation or death for patients with irAEs. Overall, 92.98% of patients improved, and 1.75% of patients who experienced grade ≥3 RCCEP had unchanged. 5.26% of patients experienced worsened or died, with pneumonitis (n = 5/9, 55.56%), myocarditis (n = 2/9, 22.22%), colitis (n = 1/9, 11.11%) and thrombocytopenia (n = 1/9, 11.11%). 50 patients (29.24%) experienced discontinue immunotherapy for irAEs, with the common being pneumonitis (n = 19/50, 38%), reactive capillary hemangiomas (n = 7/50, 14%), colitis (n = 6/12, 12%), myocarditis (n = 5/50, 10%), and hepatitis (n = 4/50, 8%), and a few occurring in skin reaction (n = 2), infusion reaction (n = 2), fever (n = 2), thrombocytopenia (n = 1), diabetes (n = 1), and adrenal (n = 1).
In our study, we classified patients into two groups based on the prevalence of irAEs: those with irAEs (irAE group) and those without (non‐irAE group). Subsequently, we analyzed the susceptibility factors for irAEs using univariate and multivariate analyses. As shown in Table 3 and Table 4, univariate analysis revealed that there was a significant correlation between the incidences of irAEs and EGFR-TKI therapy history (p = 0.025), ICI cycles (p = 0.009), cancer types (p = 0.009) and Eos (p < 0.037). There were no significant differences between the incidence of irAEs and sex, age, treatment, line of therapy, or disease history. Using multivariate analysis, we found that cumulative cycles of ICI (OR = 1.081; 95% CI: 1.023–1.142; p = 0.006), and lung cancer (OR = 1.765; 95% CI: 1.105–2.820; p = 0.017) were independent risk factors for irAEs. Interestingly, the incidence of irAEs in patients, who had autoimmune diseases and had received radiotherapy in 90 d, did not markedly increase (p > 0.05) compared to the frequency of other patients.
With the expansion of the clinical indications for ICIs, their safety is a key factor for their broad clinical application. Due to the specific pharmacodynamics and pharmacokinetics of ICI immunotherapy, ICI-related toxicities are distinct from those observed with cytotoxic chemotherapy or targeted anti-cancer therapy. ICIs are capable of causing immune-related toxicity in almost all tissues. However, the prevalence and profile of ICI-related toxicity are not fully understanded and still study. The reported overall incidence of irAEs ranges widely from approximately 15–90% and is associated with ICI agent, tumor type, treatment lines of ICI initiation and derived neutrophil-to-lymphocyte ratio. These irAEs are most commonly found to affect the skin, GI tract, hepatitis, thyroid dysfunction, pneumonitis, and, rarely, myocarditis and the nervous system (Haanen et al., 2017). However, the data of ICI-related adverse reactions is mainly based on clinical trials and meta-analyses, which cannot substitute real-world treatment settings.
To our knowledge, there have been few retrospective observational studies to evaluate the real-world incidence, profile, management and risk factors of irAEs. Our analysis has revealed several important findings. We did not observe any novel irAEs but we did find that RCCEP was only reported in camrelizumab-treated patients, and that the incidence of RCCEP was lower in ICIs combination group (chemotherapy or anti-vascular drug). Further, we demonstrate that RCCEP is camrelizumab-specific as reported (Li et al., 2020). RCCEP is a highly specific off-target binding event, and the exact mechanisms of camrelizumab-induced RCCEP remain unknown. One possible explanation may be an imbalance in receptor/receptor-ligand interactions along with upregulation of vascular proliferative proteins (VEGF) (Teng et al., 2019). Camrelizumab, a potent agonist of human VEGFR-2, can drive hemangioma development via vascular endothelial cell activation and lead to high frequency of capillary hemangioma (Finlay et al., 2019). Anti-vascular drugs can block the VEGF-VEGFR signaling pathway, inhibit vascular cells, and lead to a decrease in the density of capillaries, which may explain why the incidence of RCCEP decreases for patients with camrelizumab and anti-vascular drugs (Li et al., 2019; Wang et al., 2021).
By indirectly comparing the toxicity data, we found that the prevalence of irAEs in different organs differed from those previously reported. Colitis and hepatitis were infrequent in our analysis, occurring in less than 2% of patients, and the incidence of skin toxicity was less common, whereas 13.6%, 29%, and 17–40% of patients experienced GI tract, hepatitis and skin toxicity, respectively, as previously reported (Wang et al., 2017; Suzman et al., 2018; Thompson et al., 2022). One underlying reason may be incomplete records in the electronic medical system and more patients receiving CTLA-4 inhibitor in clinical trials, as CTLA-4 inhibitors are associated with an increased risk of colitis and hepatitis compared to PD-1/PD-L1 inhibitors (Wang et al., 2017). This could lead to an underestimation of skin toxicity, colitis, and hepatitis in our analysis. In addition, patients included in this study usually received microecological agents, which may reduce the occurrence of colitis. Studies have shown that an increased baseline presence of Bacteroidetes species, such as Bacteroidetes, Bacteroidaceae, Rikenellaceae, and Barnesiellaceae, are linked to a reduced risk to colitis (Dubin et al., 2016; Aarnoutse et al., 2019; Som et al., 2019). Moreover, although the incidence of thyroid dysfunction was similar to those previously observed in a real-world setting, it was higher than that reported in a pooled analysis of many clinical trials (only with 5–10%) (Barroso-Sousa et al., 2018; Chang et al., 2019).
Importantly, we also found that there were certain differences between irAEs and ICI type. A resent meta-analysis looking at, the landscape and incidence of tr-AEs in a predominantly Chinese cohort, showed that the pooled incidence rates of all-grade TRAE (treatment-related adverse events) with sintilimab, camrelizumab, toripalimab, and pembrolizumab were 92.7%, 98%, 86.2%, and 74.1%, respectively. Moreover, the incidence of all-grade irAEs with sintilimab, camrelizumab, and toripalimab were 54.2%, 71.8% (RCCEP 58.6%), and 19.1%, respectively (Li et al., 2020). Additionally, a retrospective real-world study showed that the incidence rates of all-grade irAEs with sintilimab, camrelizumab, toripalimab, and pembrolizumab were 29.03%, 62.96% (RCCEP 22.22%), 9.52%, and 24.62%, respectively (Zheng et al., 2022). Furthermore, 147 articles and 23,761 cancer patients with 11 treated with different ICIs were included in a recent meta-analysis. Subgroup analysis showed that the frequency of all-grade and grade 3–5 irAEs with pembrolizumab were 67.25% and 16.58% (Song et al., 2020). Notably, our study found that sintilimab and camrelizumab had higher incidence of adverse reactions, and the irAEs of any grade and grade 1–2 irAEs were significantly different from toripalimab and pembrolizumab. However, standardized grade ≥3 irAEs of pembrolizumab was higher than those of sintilimab (13.21% vs. 7.12%, p = 0.026). Moreover, pembrolizumab had higher incidence of pneumonitis than other ICIs (sintilimab, toripalimab and camrelizumab), and the incidence was also higher than that previously reported in the literature (13.56% vs. 5%) (Nishino et al., 2016). This potential reason may be related to tumor type, for 55% of lung cancer and only <18% of melanoma in our study. As some studies have shown that many researchers support that there was higher incidence of pneumonitis but lower incidence of colitis in NSCLC and RCC compared with melanoma (Nishino et al., 2016; Wang et al., 2017). Further, pneumonitis was also the main reason for discontinuation in our study (38% of patients), where meta-analysis of pooled clinical trial data showed that 43% patients discontinued immunotherapy due to irAEs, with colitis being the most commonly reported reason for halting therapy (Kumar et al., 2017). this suggested that we should pay more attention or avoid using pembrolizumab for the people at high risk of developing pneumonia. In addition, in accordance with the literature, we found that the onset time of irAEs quite varied and our data highlight the correlation between incidence and onset time. We found that the median time of rare irAEs is later than that of other common irAEs (16.9 weeks vs. 5.9 weeks, p < 0.05), which suggests that we should be concerned about rare irAEs for patients on long-term treatment regimes.
Although early research shown that multiple factors were involved in the occurrence of irAEs, containing patient characteristics, medical history, medication history, ICIs type, therapeutic regimen and tumor type (Chennamadhavuni et al., 2022), there is still some debate about risk factors for irAEs. Some studies have reported that predictors for irAEs include concomitant chemotherapy, ICIs type, a higher body mass index, being female, C-reactive protein, neutrophil lymphocyte ratio, and smoking history (Suazo-Zepeda et al., 2021; Chennamadhavuni et al., 2022), whereas another study demonstrated no statistically significant irAE risk differences between the sexes (odds ratio [OR] = 1.19, 95% CI = 0.91–1.54, two-sided p = 0.21) (Jing et al., 2021). A real-world study also showed that serum albumin level ≥3.6 g/dl and history of Type I hypersensitivity reactions were risk factors for irAEs, but BIM, age and sex were not significant factors (Shimozaki et al., 2021). Although some studies showed that the incidence of irAEs was similar among different tumors, many researchers support that some irAEs was tumor-specific. As compared with melanoma, there was higher incidence of pneumonitis but lower incidence of colitis in NSCLC and RCC (Nishino et al., 2016; Wang et al., 2017). Additionally. Some studies indicated that pre-existing lung diseases increase the risk of irAE-pneumonitis (Shibaki et al., 2020), and patients with cardiovascular risk factors are more likely to occur cardiac-irAE (Pirozzi et al., 2021); whereas this is supported by conflicting evidence (Johns et al., 2020). In this study, we found that high eosinophil count, history of EGFR-TKI targeted therapy, cycles of ICI administration, and lung cancer were significantly correlated with the incidence of irAEs (p < 0.005), and cycles of ICI administration and lung cancer were independent risk factors for irAEs. In keeping with published clinical trial and meta-analysis data, these data suggest that we need to monitor the incidence of irAEs frequently for the people who have received EGFR-TKI therapy and are later receive ICIs treatment, especially the people with lung cancer. Interestingly, there were no significant difference between medical history, such as autoimmune condition, chronic pulmonary disease and history of thoracic radiation, and the development of irAEs; however, the relationship between preexisting autoimmune disorders and the development of irAEs remains controversial. With the increase in attention for irAEs, some studies showed that some baseline co-medications, such as antibiotics, PPI, diuretics and ACEI/ARD, could be associated with irAE. The underlying mechanisms was that altered anti-cancer activity to ICI were related to decreased incidence of irAEs, and gut microbial diversity was decreased by antibiotics (Kostine et al., 2021; Chennamadhavuni et al., 2022; Jing et al., 2022; Zhao et al., 2022). However, their clear relevance and the mechanism needs to be further studied in the future.
The correlation between irAEs and effective was not analyzed in our study due to confounding factors in the real world. However, based on the mechanism of action of ICIs, some published studies have shown that irAEs are associated with substantially improved ORR, DCR, PFS, and OS in patients who were treated with ICIs, and some experts consider irAEs to be a projection of the overall immune response to ICI therapy (Kobayashi et al., 2020; Kotwal et al., 2020; Shi et al., 2022). A prospective study showed that the development of vitiligo during melanoma treatment increases the likelihood of a positive clinical efficacy (Hua et al., 2016). Abu-Sbeih et al.,. performed a retrospectively studied the prognostic utility of the development of immune-mediated diarrhea and colitis in 173 patients with metastatic melanoma. The authors found that colitis was associated with improved OS and PFS, and higher grades of diarrhea were associated with even better patient’ OS rates. They also found that pancreatic adverse events were associated with worse PFS rates (Abu-Sbeih et al., 2019). A recent meta-analysis, which included 32 studies and 8,132 patients with NSCLC, melanoma, gastric cancer, renal cell carcinoma (RCC), urothelial carcinoma, and head and neck cancer (HNSCC), suggested that irAEs were, to some extent, a predictive factor for improved clinical outcomes and suggested irAEs as potential biomarkers for cancer patients undergoing ICI therapy (Park et al., 2021).
There are some limitations to the study, the main one being that it is a retrospective. All ICI-related ADRs were collected from the medical records, which could result in an over or underestimation of total irAEs. Further, there were some insufficient details in the description of clinical manifestations, and the detail duration of irAEs treatment was not available due to poor and incomplete medical records. In addition, the number of participants was relatively small and not symmetrical in different ICIs. Therefore, there were some bias may have been introduced based on the comparative analysis of spectrum and frequency of different ICI-induced toxicity, and the present findings should be interpreted with caution. Considering the complexity of participants, including, but not limited to, the medication, cancer type, and cumulative circles, etc. drug efficacy could not be evaluated objectively. Moreover, the analysis of risk factors could be not comprehensive. Thus, future studies are required to account for the bias and limitations included in this study.
Based on our retrospective observational study, we made the following conclusions: (Vaddepally et al., 2020): RCCEP is a specific ADR of camrelizumab, and the incidence of irAEs is associated with ICI type. The overall incidence of irAEs following sintilimab and camrelizumab was higher and the incidence of pembrolizumab-induced pneumonitis was the highest. Therefore, it indicates that pneumonitis should be closely monitored during treatment. (Thompson et al., 2022). The onset of irAEs ranges widely, and the median onset time of rare irAEs is longer than that of other common irAEs (p < 0.05). This suggests that uncommon irAEs should be a concern for patients with undergoing long-term treatment. (Chen et al., 2021). Patients with lung cancer and more cumulative cycles have a higher probability of developing irAEs, and other risks of irAEs need to be further studied in the future.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Third Affiliated Hospital of Chongqing Medical University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
GH and QD designed the experiments. QD and WL supervised the whole project. GH performed most experiments and wrote the paper. JD, XX, and RK provided technical support.
This research was supported by the Natural Science Foundation of Chongqing, China (No. cstc2020jcyj-msxmX0371), and the Scientific Research Program of Science and the Technology Commission of Yuzhong District of Chongqing (No. 20190113).
We thank all the patients whose details form the basis of this study. We extend our thanks to Rufu Xu, Ph. D. (Department of Pharmacy, The Second Affiliated Hospital of Army Medical College) for his statistical guidance. We also thank RK (Department of oncology, The Third Affiliated Hospital of Chongqing Medical University, Chongqing) for his help with pneumonitis image grading.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.974376/full#supplementary-material
Aarnoutse, R., Ziemons, J., Penders, J., Rensen, S. S., de Vos-Geelen, J., and Smidt, M. L. (2019). The clinical link between human intestinal microbiota and systemic cancer therapy. Int. J. Mol. Sci. 20 (17), 4145. doi:10.3390/ijms20174145
Abu-Sbeih, H., Ali, F. S., Qiao, W., Lu, Y., Patel, S., Diab, A., et al. (2019). Immune checkpoint inhibitor-induced colitis as a predictor of survival in metastatic melanoma. Cancer Immunol. Immunother. 68 (4), 553–561. doi:10.1007/s00262-019-02303-1
Barroso-Sousa, R., Barry, W. T., Garrido-Castro, A. C., Hodi, F. S., Min, L., Krop, I. E., et al. (2018). Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: A systematic review and meta-analysis. JAMA Oncol. 4 (2), 173–182. doi:10.1001/jamaoncol.2017.3064
Boutros, C., Tarhini, A., Routier, E., Lambotte, O., Ladurie, F. L., Carbonnel, F., et al. (2016). Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat. Rev. Clin. Oncol. 13 (8), 473–486. doi:10.1038/nrclinonc.2016.58
Chang, L., Barroso-Sousa, R., Tolaney, S., Hodi, F., Kaiser, U., and Min, L. (2019). Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr. Rev. 40 (1), 17–65. doi:10.1210/er.2018-00006
Chen, C., Wu, B., Zhang, C., and Xu, T. (2021). Immune-related adverse events associated with immune checkpoint inhibitors: An updated comprehensive disproportionality analysis of the FDA adverse event reporting system. Int. Immunopharmacol. 95, 107498. doi:10.1016/j.intimp.2021.107498
Chennamadhavuni, A., Abushahin, L., Jin, N., Presley, C. J., and Manne, A. (2022). Risk factors and biomarkers for immune-related adverse events: A practical guide to identifying high-risk patients and rechallenging immune checkpoint inhibitors. Front. Immunol. 13, 779691. doi:10.3389/fimmu.2022.779691
Choi, J., and Lee, S. Y. (2020). Clinical characteristics and treatment of immune-related adverse events of immune checkpoint inhibitors. Immune Netw. 20 (1), e9. doi:10.4110/in.2020.20.e9
Dubin, K., Callahan, M. K., Ren, B., Khanin, R., Viale, A., Ling, L., et al. (2016). Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 7, 10391. doi:10.1038/ncomms10391
Finlay, W. J. J., Coleman, J. E., Edwards, J. S., and Johnson, K. S. (2019). Anti-PD1 'SHR-1210' aberrantly targets pro-angiogenic receptors and this polyspecificity can be ablated by paratope refinement. MAbs 11 (1), 26–44. doi:10.1080/19420862.2018.1550321
Haanen, J., Carbonnel, F., Robert, C., Kerr, K. M., Peters, S., Larkin, J., et al. (2017). Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 28, iv119–iv142. doi:10.1093/annonc/mdx225
Hua, C., Boussemart, L., Mateus, C., Routier, E., Boutros, C., Cazenave, H., et al. (2016). Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 152 (1), 45–51. doi:10.1001/jamadermatol.2015.2707
Jing, Y., Chen, X., Li, K., Liu, Y., Zhang, Z., Chen, Y., et al. (2022). Association of antibiotic treatment with immune-related adverse events in patients with cancer receiving immunotherapy. J. Immunother. Cancer 10 (1), e003779. doi:10.1136/jitc-2021-003779
Jing, Y., Zhang, Y., Wang, J., Li, K., Chen, X., Heng, J., et al. (2021). Association between sex and immune-related adverse events during immune checkpoint inhibitor therapy. J. Natl. Cancer Inst. 113 (10), 1396–1404. doi:10.1093/jnci/djab035
Johns, A., Wei, L., Grogan, M., Patel, S., Li, M., Husain, M., et al. (2020). Association of medical comorbidities and cardiovascular disease with toxicity and survival in patients receiving checkpoint inhibitor immunotherapy. J. Clin. Oncol. 38, 7039–9. doi:10.1200/jco.2020.38.15_suppl.7039
Khoja, L., Day, D., Wei-Wu Chen, T., Siu, L. L., and Hansen, A. R. (2017). Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: A systematic review. Ann. Oncol. 28 (10), 2377–2385. doi:10.1093/annonc/mdx286
Kobayashi, T., Iwama, S., Yasuda, Y., Okada, N., Okuji, T., Ito, M., et al. (2020). Pituitary dysfunction induced by immune checkpoint inhibitors is associated with better overall survival in both malignant melanoma and non-small cell lung carcinoma: A prospective study. J. Immunother. Cancer 8 (2), e000779. doi:10.1136/jitc-2020-000779
Kostine, M., Mauric, E., Tison, A., Barnetche, T., Barre, A., Nikolski, M., et al. (2021). Baseline co-medications may alter the anti-tumoural effect of checkpoint inhibitors as well as the risk of immune-related adverse events. Eur. J. Cancer 157, 474–484. doi:10.1016/j.ejca.2021.08.036
Kotwal, A., Kottschade, L., and Ryder, M. (2020). PD-L1 inhibitor-induced thyroiditis is associated with better overall survival in cancer patients. Thyroid 30 (2), 177–184. doi:10.1089/thy.2019.0250
Kumar, V., Chaudhary, N., Garg, M., Floudas, C. S., Soni, P., and Chandra, A. B. (2017). Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front. Pharmacol. 8, 49. doi:10.3389/fphar.2017.00049
Li, L., Li, G., Rao, B., Dong, A. H., Liang, W., Zhu, J. X., et al. (2020). Landscape of immune checkpoint inhibitor-related adverse events in Chinese population. Sci. Rep. 10 (1), 15567. doi:10.1038/s41598-020-72649-5
Li, W., Wei, Z., Yang, X., Huang, G., Han, X., Ni, Y., et al. (2019). Salvage therapy of reactive capillary hemangiomas: Apatinib alleviates the unique adverse events induced by camrelizumab in non-small cell lung cancer. J. Cancer Res. Ther. 15 (7), 1624–1628. doi:10.4103/jcrt.JCRT_997_19
Luo, J., Beattie, J. A., Fuentes, P., Rizvi, H., Egger, J. V., Kern, J. A., et al. (2021). Beyond steroids: Immunosuppressants in steroid-refractory or resistant immune-related adverse events. J. Thorac. Oncol. 16 (10), 1759–1764. doi:10.1016/j.jtho.2021.06.024
Ma, K., Lu, Y., Jiang, S., Tang, J., Li, X., and Zhang, Y. (2018). The relative risk and incidence of immune checkpoint inhibitors related pneumonitis in patients with advanced cancer: A meta-analysis. Front. Pharmacol. 9, 1430. doi:10.3389/fphar.2018.01430
Martins, F., Sofiya, L., Sykiotis, G. P., Lamine, F., Maillard, M., Fraga, M., et al. (2019). Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 16 (9), 563–580. doi:10.1038/s41571-019-0218-0
Nishino, M., Giobbie-Hurder, A., Hatabu, H., Ramaiya, N. H., and Hodi, F. S. (2016). Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: A systematic review and meta-analysis. JAMA Oncol. 2 (12), 1607–1616. doi:10.1001/jamaoncol.2016.2453
Park, R., Lopes, L., and Saeed, A. (2021). Anti-PD-1/L1-associated immune-related adverse events as harbinger of favorable clinical outcome: Systematic review and meta-analysis. Clin. Transl. Oncol. 23 (1), 100–109. doi:10.1007/s12094-020-02397-5
Pillai, R. N., Behera, M., Owonikoko, T. K., Kamphorst, A. O., Pakkala, S., Belani, C. P., et al. (2018). Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer: A systematic analysis of the literature. Cancer 124 (2), 271–277. doi:10.1002/cncr.31043
Pirozzi, F., Poto, R., Aran, L., Cuomo, A., Galdiero, M. R., Spadaro, G., et al. (2021). Cardiovascular toxicity of immune checkpoint inhibitors: Clinical risk factors. Curr. Oncol. Rep. 23 (2), 13. doi:10.1007/s11912-020-01002-w
Shi, Y., Fang, J., Zhou, C., Liu, A., Wang, Y., Meng, Q., et al. (2022). Immune checkpoint inhibitor-related adverse events in lung cancer: Real-world incidence and management practices of 1905 patients in China. Thorac. Cancer 13 (3), 412–422. doi:10.1111/1759-7714.14274
Shibaki, R., Murakami, S., Matsumoto, Y., Yoshida, T., Goto, Y., Kanda, S., et al. (2020). Association of immune-related pneumonitis with the presence of preexisting interstitial lung disease in patients with non-small lung cancer receiving anti-programmed cell death 1 antibody. Cancer Immunol. Immunother. 69 (1), 15–22. doi:10.1007/s00262-019-02431-8
Shimozaki, K., Sukawa, Y., Sato, Y., Horie, S., Chida, A., Tsugaru, K., et al. (2021). Analysis of risk factors for immune-related adverse events in various solid tumors using real-world data. Future Oncol. 17 (20), 2593–2603. doi:10.2217/fon-2020-0861
Som, A., Mandaliya, R., Alsaadi, D., Farshidpour, M., Charabaty, A., Malhotra, N., et al. (2019). Immune checkpoint inhibitor-induced colitis: A comprehensive review. World J. Clin. Cases 7 (4), 405–418. doi:10.12998/wjcc.v7.i4.405
Song, P., Zhang, D., Cui, X., and Zhang, L. (2020). Meta-analysis of immune-related adverse events of immune checkpoint inhibitor therapy in cancer patients. Thorac. Cancer 11 (9), 2406–2430. doi:10.1111/1759-7714.13541
Suazo-Zepeda, E., Bokern, M., Vinke, P. C., Hiltermann, T. J. N., de Bock, G. H., and Sidorenkov, G. (2021). Risk factors for adverse events induced by immune checkpoint inhibitors in patients with non-small-cell lung cancer: A systematic review and meta-analysis. Cancer Immunol. Immunother. 70 (11), 3069–3080. doi:10.1007/s00262-021-02996-3
Suzman, D. L., Pelosof, L., Rosenberg, A., and Avigan, M. I. (2018). Hepatotoxicity of immune checkpoint inhibitors: An evolving picture of risk associated with a vital class of immunotherapy agents. Liver Int. 38 (6), 976–987. doi:10.1111/liv.13746
Tang, H., Zhou, J., and Bai, C. (2021). The efficacy and safety of immune checkpoint inhibitors in patients with cancer and preexisting autoimmune disease. Front. Oncol. 11, 625872. doi:10.3389/fonc.2021.625872
Teng, Y., Guo, R., Sun, J., Jiang, Y., and Liu, Y. (2019). Reactive capillary hemangiomas induced by camrelizumab (SHR-1210), an anti-PD-1 agent. Acta Oncol. 58 (3), 388–389. doi:10.1080/0284186X.2019.1567935
Thompson, J. A., Schneider, B. J., Brahmer, J., Achufusi, A., Armand, P., Berkenstock, M. K., et al. (2022). Management of immunotherapy-related toxicities, version 1.2022, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 20, 387–405. doi:10.6004/jnccn.2022.0020
Vaddepally, R. K., Kharel, P., Pandey, R., Garje, R., and Chandra, A. B. (2020). Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers 12 (3), 738. doi:10.3390/cancers12030738
Wang, D. Y., Salem, J. E., Cohen, J. V., Chandra, S., Menzer, C., Ye, F., et al. (2018). Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol. 4 (12), 1721–1728. doi:10.1001/jamaoncol.2018.3923
Wang, D. Y., Ye, F., Zhao, S., and Johnson, D. B. (2017). Incidence of immune checkpoint inhibitor-related colitis in solid tumor patients: A systematic review and meta-analysis. Oncoimmunology 6 (10), e1344805. doi:10.1080/2162402X.2017.1344805
Wang, J., Su, S., Li, J., and Li, Y. (2021). Efficacy and safety of camrelizumab monotherapy and combination therapy for cancers: A systematic review and meta-analysis. Front. Oncol. 11, 695512. doi:10.3389/fonc.2021.695512
Xu, C., Chen, Y. P., Du, X. J., Liu, J. Q., Huang, C. L., Chen, L., et al. (2018). Comparative safety of immune checkpoint inhibitors in cancer: Systematic review and network meta-analysis. BMJ 363, k4226. doi:10.1136/bmj.k4226
Yang, J., He, X., Lv, Q., Jing, J., and Shi, H. (2019). Management of adverse events in cancer patients treated with PD-1/PD-L1 blockade: Focus on asian populations. Front. Pharmacol. 10, 726. doi:10.3389/fphar.2019.00726
Zhai, Y., Ye, X., Hu, F., Xu, J., Guo, X., Zhuang, Y., et al. (2019). Endocrine toxicity of immune checkpoint inhibitors: A real-world study leveraging US food and drug administration adverse events reporting system. J. Immunother. Cancer 7 (1), 286. doi:10.1186/s40425-019-0754-2
Zhao, L., Li, Y., Jiang, N., Song, X., Xu, J., Zhu, X., et al. (2022). Association of blood biochemical mevents in patients with advanced cancers receiving PD-1 inhibitors. J. Immunother. 45 (4), 210–216. doi:10.1097/CJI.0000000000000415
Keywords: immune checkpoint inhibitor, PD-1 inhibitors, irAEs, real-world study, solid tumors
Citation: Huang G, Liu S, Dong J, Xi X, Kong R, Li W and Du Q (2022) PD-1 inhibitor-based adverse events in solid tumors: A retrospective real-world study. Front. Pharmacol. 13:974376. doi: 10.3389/fphar.2022.974376
Received: 21 June 2022; Accepted: 31 October 2022;
Published: 09 November 2022.
Edited by:
Lin Zhang, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaReviewed by:
Eleonora Lai, University Hospital and University of Cagliari, ItalyCopyright © 2022 Huang, Liu, Dong, Xi, Kong, Li and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenjun Li, bGl3ZW5qdW4wOTI4QG91dGxvb2suY29t; Qian Du, ZHVxaWFuQGhvc3BpdGFsLmNxbXUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.