- School of Medicine and Holistic Integrative Medicine, Nanjing University of Chinese Medicine, Nanjing, China
Realgar- and cinnabar-containing AnGongNiuHuang Pill (AGNHP) is widely used for treating encephalopathy syndrome. However, it raises great safety concerns due to the adverse effects reported by arsenic or mercury poisoning. Although AGNHP has been generally recognized, little is known about the metabolism of arsenic and mercury and their resulting potential health risk in vivo. Thus, comparative pharmacokinetics and urinary excretion of arsenic and mercury were conducted in rats after oral administration of realgar, cinnabar and AGNHP, respectively. The contents of arsenic and mercury in rat blood and urine were determined by hydride-generation atomic fluorescence spectrometry (HG-AFS) after wet digestion. AGNHP significantly reduced the absorption of arsenic in blood and promoted urinary arsenic excretion. Whereas, it increased the blood mercury absorption and reduced urinary mercury excretion. No significant toxicity was observed in the clinical dose range of AGNHP. However, excessive exposure to arsenic and mercury may still pose risks especially by long-term or excessive medication. The results are helpful for the rational clinical applications of realgar- and cinnabar-containing TCMs.
Introduction
Heavy metals are dense chemicals found naturally on a daily life basis. Heavy metals are classified as micronutrients or toxicants according to their toxicity (Kim et al., 2019). Arsenic and mercury are the most common toxic heavy metals in the environment, which exert toxic effects even at low concentrations. Ingestion, inhalation or dermal absorption of arsenic may result in gastrointestinal syndrome, neurodegenerative disorders, cardiovascular disease, diabetes, skin lesions, and cancers of the lung, kidney and bladder (Rehman et al., 2018). Mercury is a deadly neurotoxin substance, and its exposure is mainly through inhalation of mercury vapor and ingestion of organic mercury. Mercury toxicity is associated with neurological and immune dysfunction, pneumonitis, acute necrotizing bronchitis, tremor, gastrointestinal disturbance and brain damage (Rahman and Singh 2019; Tsoi et al., 2019). Since heavy metals can disturb the body’s metabolic functions in various ways, the WHO and the Food and Agriculture Organization (FAO) regulated the maximum weekly intake levels for arsenic and mercury (Sarma et al., 2011).
Interestingly, minerals containing large amounts of arsenic and mercury are intendedly added in numerous Traditional Chinese Medicine (TCM) formulas since ancient China, which has proved to have remarkable effects on various diseases. Realgar and cinnabar are the most extensively used arsenic- and mercury-containing mineral medical materials, respectively. Realgar (As2S2) has been widely used to treat carbuncles, malaria, psoriasis, convulsive epilepsy and parasitic infections (Wu et al., 2020). In recent years, realgar is used as an alternative to arsenic trioxide in treating acute promyelocytic leukemia (Lou et al., 2021). Cinnabar (HgS) has sedative and hypnotic effects, which is used to treat insomnia, dreaminess, epilepsy and infantile convulsions (Yang et al., 2020). In the 2020 edition of Chinese pharmacopeia, there are 38 (38/1607, 2.4%) and 74 (74/1607, 4.6%) types of TCMs containing realgar and cinnabar, respectively, among which 26 (26/1607, 1.6%) types contain both realgar and cinnabar. AnGongNiuHuang Pill (AGNHP) is the best-known realgar- and cinnabar-containing TCM preparation for treating stroke, encephalitis, meningitis, hematencephalon, convulsion, hyperpyrexia and coma (Zhang et al., 2021). It consists of realgar (10%), cinnabar (10%), Hyriopsis cumingii (Lea), Bovis Calculus, Powered Buffalo Horn Extract, natural or artificial Moschus, Coptis chinensis Franch., Dryobalanops aromatica C. F. Gaertn., Gardenia jasminoides J. Ellis, Curcuma aromatica Salisb. and Scutellaria baicalensis Georgi.
Because realgar and cinnabar are water-insoluble, their toxicities should not be deemed as toxic as the equivalent inorganic arsenic or mercury. Our previous studies had confirmed that realgar- or cinnabar-containing TCMs were relatively safe in the therapeutic dose range (Wu et al., 2018; Lu et al., 2020; Wu et al., 2020; Wu et al., 2022). However, poisoning cases caused by overdose or long-term use of realgar- or cinnabar-containing TCMs have been reported occasionally (Wu and Deng 2013; Wu et al., 2013; Chang et al., 2018). AGNHP is forbidden in foreign countries because it contains excessive levels of arsenic and mercury (Xia et al., 2018). Since realgar and cinnabar are the essentially active components of AGNHP (Xia et al., 2018; Tsoi et al., 2019), the safety of AGNHP has aroused great concerns among the public. Previous studies reported that AGNHP was protective against cinnabar- and realgar-induced hepatic and renal damage (Li et al., 2018; Wang et al., 2021). Whereas, the interaction between arsenic and mercury in AGNHP and their resulting potential health risk have not been well studied in vivo. Therefore, the metabolisms of arsenic and mercury after oral administration of AGNHP need to be urgently addressed in a biological system.
In this study, the safety of AGNHP was evaluated through the interaction of arsenic and mercury based on pharmacokinetics and urinary excretion. The metabolic differences of arsenic and mercury were compared in rats after single oral gavage of realgar, cinnabar and AGNHP, respectively. Arsenic and mercury in rat blood and urine were determined by hydride-generation atomic fluorescence spectrometry (HG-AFS) after wet digestion. The study aims to provide guidance for the safety and clinical usage of realgar- and cinnabar-containing TCMs.
Materials and methods
Chemicals and reagents
AGNHP (Batch No.19011426, 3 g/pill) was purchased from Beijing Tongrentang Technologies Co., Ltd. (Beijing, China). Water lapped realgar (Batch No.160319, purity of 92.86%) was obtained from Sanmenxia Yuhuangshan Pharmaceutical Co., Ltd. (Henan, China). Water lapped cinnabar (Batch No. 20181201, purity of 96.23%) was bought from Fenghuang Hongfei cinnabar Pharmaceutical Co., Ltd. (Hunan, China). Nitric acid, sulphuric acid, hydrochloric acid, perchloric acid, potassium borohydride, potassium dichromate, potassium hydroxide, potassium hydroxide, thiourea, ascorbic acid and sodium carboxymethyl cellulose (CMC-Na) were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Heparin sodium was supplied by Beijing Solarbio Science and Technology Co., Ltd. (Beijing, China). Arsenic and mercury standard solutions (1000 mg/L) were obtained from o2si smart solutions Co., Ltd. (Charleston, United States) and Guobiao Testing and Certification Co., Ltd. (Beijing, China), respectively. Deionized water was produced by a PCDX-F10 water purification system purchased from Pincheng Science and Technology Co., Ltd. (Chengdu, China).
Animals
Adult male Sprague-Dawley rats (180–200 g) were obtained from Xipuer-Bikai Laboratory Animal Co., Ltd (Shanghai, China). Rats were raised in the animal laboratory of Nanjing University of Chinese Medicine (Nanjing, China) at a temperature of (22 ± 3)°C and humidity of 40–60%. Rats were acclimatized for 1 week and fed sterile feed and water ad libitum. All animals were fasted overnight before the study.
The animal experiments were performed under animal use guidelines and approved by the Committee on Animal Research and Ethics of the Nanjing University of Chinese Medicine.
Quality control of TCMs
The quality of realgar, cinnabar and AGNHP was controlled following the 2020 edition of Chinese Pharmacopeia. The arsenic and mercury contents of TCMs were determined by the same procedure as previously reported (Lu Y. T. et al., 2017; Wu et al., 2022). Each AGNHP contained an average of 84 mg of mercury and 122 mg of arsenic. The average contents of arsenic and mercury were 677 mg/g and 860 mg/g in realgar and cinnabar, respectively.
Drug administration and sample collection
AGNHP was cut into pieces, and realgar and cinnabar were grounded into fine powders and suspended in 0.5% CMC-Na solution for administration. A total of 72 rats were randomly divided into 6 groups with 12 rats in each group. For each group, 7 rats were used for pharmacokinetic study, and the other 5 were placed individually in metabolic cages for urine excretion study. Rats were orally administrated with low and high doses of realgar, cinnabar and AGNHP at a single dose, respectively. The low and high doses of AGNHP were 308.5 and 1542.5 mg/kg for rats, equivalent to 1 and 5 times the human therapeutic dose, respectively. The corresponding low and high doses of realgar (18.0 and 90.0 mg/kg) and cinnabar (10.4 and 52.1 mg/kg) were calculated by equal amounts of realgar and cinnabar in AGNHP, respectively.
Blood samples of approximately 0.15 ml were collected from the rat angular vein into heparinized polythene tubes at 0, 0.25, 0.5, 0.75, 1, 2, 4, 8, 12, 24 and 48 h after dosing, respectively. Urine samples were collected at 0–4, 4–8, 8–12 and 12–24 h after dosing. The volumes of urine samples were measured and recorded. All the biological samples were stored at −80°C for further analysis.
Sample preparation and analytical methods
An aliquot of 0.1 ml of blood or urine samples were pretreated with a modified fast Kjeldahl digestion for arsenic determination as our previously reported (Wu et al., 2018; Wu et al., 2022). After digestion, thiourea and ascorbic acid were added to reduce As5+ to As3+ before HG-AFS analysis (Wu et al., 2018; Wu et al., 2022). For mercury determination in biological samples, 0.1 ml of blood or urine samples were placed in a Kjeldahl flask. After adding 4 ml of nitric acid and 1 ml of perchloric acid, the Kjeldahl flask was capped with a funnel and left overnight at room temperature. Then the mixture was digested on a hotplate until a clear and transparent solution was produced. The digested solution was cooled and transferred to a 10 ml volumetric flask with 5% HNO3 (v/v)-0.05% K2Cr2O7 (w/v) solution and diluted to volume. Samples beyond the linear ranges of arsenic and mercury were diluted to appropriate concentrations for analysis.
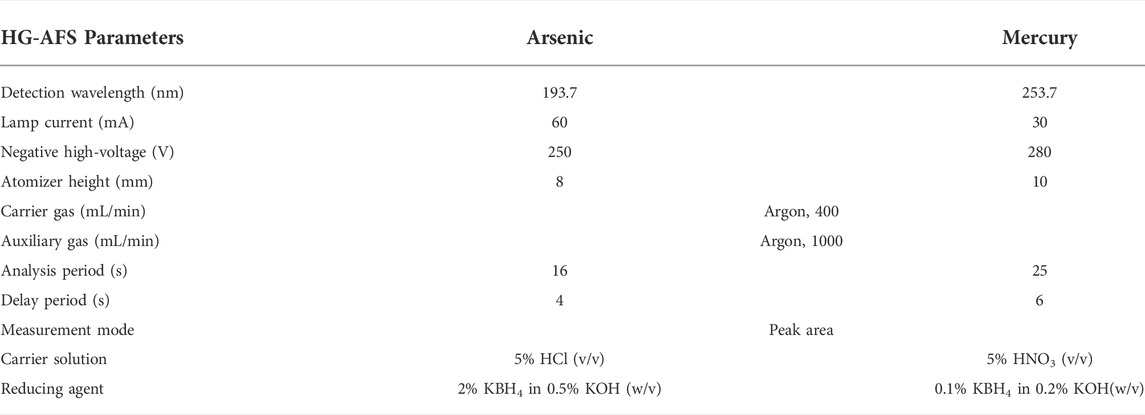
The calibration standards were prepared with an arsenic standard solution in the concentration range from 5 to 100 ng/ml in the same way as previously reported (Wu et al., 2018; Wu et al., 2022). Series of mercury standards were prepared by appropriate dilution of mercury stock solution with 5% HNO3 (v/v)-0.05% K2Cr2O7 (w/v) solution in the linear range from 0.2 to 1.0 ng/ml. Sensitive and simple methods were developed and validated for arsenic and mercury determination in biological samples. The concentrations of arsenic and mercury in rat blood and urine were determined by a 9750 HG-AFS system (Haiguang Instrument Co., Ltd., Beijing, China). The analytical parameters are displayed in Table 1.
Statistical analysis
The pharmacokinetics and urinary excretion data are presented as mean ± standard deviation (SD) and mean ± standard error of the mean (SEM), respectively. The pharmacokinetic parameters were calculated by non-compartmental analysis (NCA) using Phoenix WinNonLin 7.0 (Pharsight Corporation, California, United States). The independent t-test was used to compare the differences between the two groups. The statistical analysis was performed using GraphPad Prism 8.0 (GraphPad Software, Inc., San Diego, CA). Differences were considered statistically significant at p < 0.05.
Results and discussion
Method validation
The optimized methods were validated for the determination of arsenic and mercury in biological samples in terms of linearity, limit of detection (LOD), precision, accuracy and stability. Arsenic and mercury showed good linearity in the range of 5–100 ng/ml and 0.2–1.0 ng/ml, respectively, with the correlation coefficients greater than 0.999. LOD was calculated by the ratio of 3SD and the slope of the calibration curve after 11 blank injections. LODs of arsenic and mercury were 0.0019 and 0.017 ng/ml, respectively. The precision and accuracy were determined from five replicate determinations. The precisions were all within 10%, and the accuracies were in the range of 95–111%. Arsenic and mercury in biological samples were transformed into the oxidation forms of As5+ and Hg2+ after digestion, respectively, which was independent of the freeze–thaw cycles, storage temperature and time. Therefore, the stability of arsenic and mercury were evaluated by the digestion solutions at room temperature for 24 and 4 h, respectively. Arsenic and mercury were stable when stored at room temperature with RSDs less than 10%.
Pharmacokinetic study
The mean blood concentration-time curves of arsenic in rat blood after oral administration of realgar and AGNHP are shown in Figure 1. The pharmacokinetic parameters of Cmax (peak concentration), Tmax (peak time), AUC (area under the curve) and MRT (mean residence time) are displayed in Table 2. It was observed that blood arsenic showed a dose-dependent increase in realgar and AGNHP groups, respectively. As the dose increases, the increase of blood arsenic in AGNHP groups was not as obvious as that of realgar. The blood arsenic concentration profiles of rats after oral administration of AGNHP were decreased compared to the corresponding realgar groups, which displayed a significant difference at high doses. The Cmax of realgar and AGNHP was not significantly different at low doses due to the limited arsenic absorption in blood. Since the arsenic concentration did not decrease 48 h after dosing, T1/2 could not be calculated accurately. The prolonged Tmax and MRT showed a slow absorption of arsenic in blood after oral administration of realgar and AGNHP to rats. Slow absorption and elimination of arsenic were observed in rat blood after dosing. The AUC of arsenic in AGNHP group showed a decrease compared to the corresponding realgar groups, which was consistent with the trend of Cmax. Overall, AGNHP significantly influenced the pharmacokinetic behaviors of arsenic in realgar.
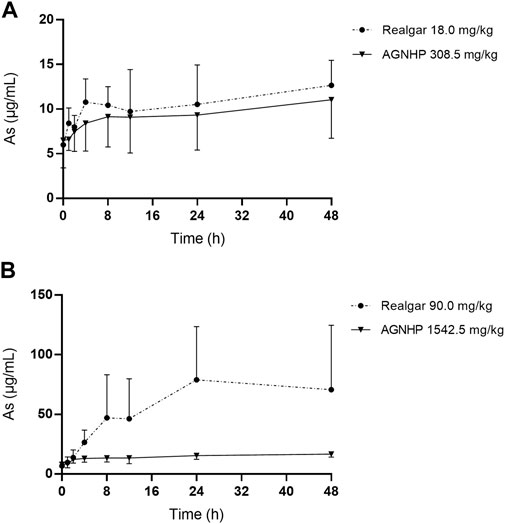
FIGURE 1. Blood concentration-time profiles of arsenic in rats after oral administration of realgar and AGNHP.
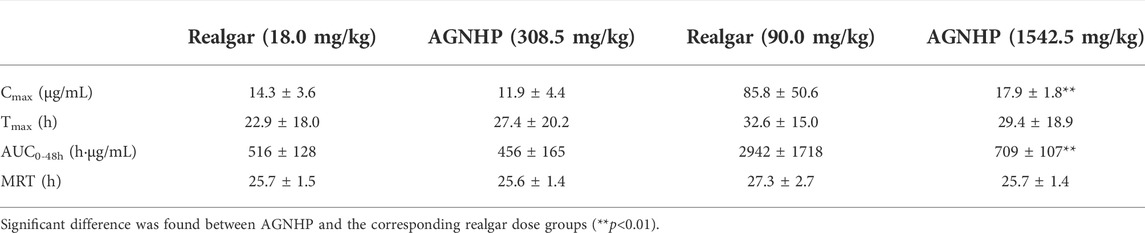
Table 2. Pharmacokinetic parameters of arsenic in rats after oral administration of realgar and AGNHP.
Previous studies had confirmed that realgar-containing TCMs significantly reduced the total blood arsenic exposure present in realgar, which might be attributed to the co-existing ingredients in realgar-containing TCMs (Wu et al., 2018; Wu et al., 2020). The reduced toxicity of realgar-containing TCMs might be related to the reduction of arsenic absorption by compatibility (Wang et al., 2021). Blood arsenic concentration reflected the absorption and was an important indicator of arsenic poisoning. As reported previously, a patient died of arsenic poisoning with a blood arsenic concentration of 21.1 μg/ml, and the fatal blood arsenic concentration was 0.16–41 μg/ml (Lech and Trela 2005; Lu et al., 2019). The blood arsenic concentration in poisoned rats was 144.00–166.11 μg/ml (Sayed et al., 2015). No significant toxicity occurred in rats after 30 days treatment of realgar, and the blood arsenic concentration was 68.62 μg/ml (Yi et al., 2019; Yi et al., 2020). Consequently, there is no risk of arsenic poisoning when taken AGNHP at clinical doses.
The blood concentration–time profiles of mercury in rats after oral administration of cinnabar and AGNHP are shown in Figure 2. The blood mercury absorption was much lower than arsenic after oral administration of cinnabar and AGNHP to rats. Blood mercury presented a dose-dependent increase in cinnabar and AGNHP groups, respectively. Interestingly, AGNHP significantly increased the blood mercury absorption compared to the corresponding cinnabar groups, which was in contrast to the results of arsenic aforementioned. The Cmax and AUC of mercury in AGNHP group showed a significant increase compared to the corresponding cinnabar groups (Table 3). The Tmax of mercury revealed that mercury was slowly absorbed in rat blood, and it was slightly shorter than arsenic. Compared with the AGNHP groups, the MRT of mercury was extended after cinnabar treatment, which presented a significant difference in high doses.
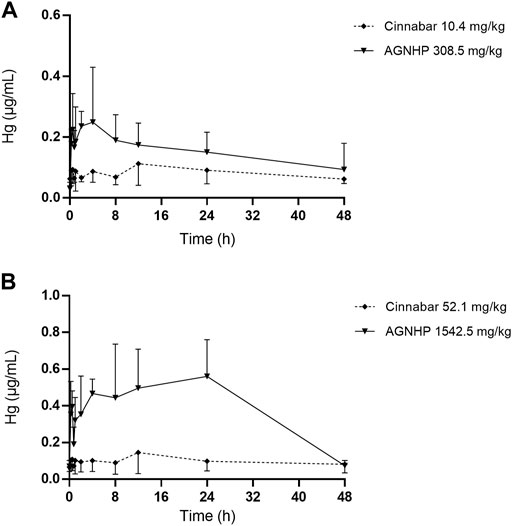
FIGURE 2. Blood concentration-time profiles of mercury in rats after oral administration of cinnabar and AGNHP.
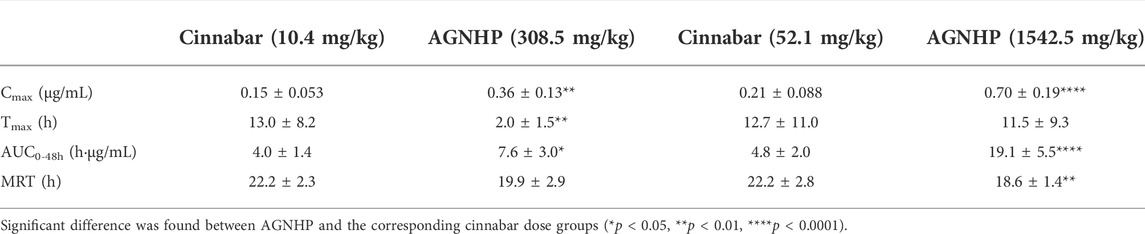
Table 3. Pharmacokinetic parameters of mercury in rats after oral administration of cinnabar and AGNHP.
Consistent with a previous study, cinnabar with herbal ingredients combination promoted the absorption of mercury and prolonged the elimination process (Lu et al., 2020). A three-year-old boy was diagnosed with mercury intoxication with high blood mercury levels detected in a private laboratory (Valido et al., 2019). The blood levels of mercury above 100 ng/ml were diagnosed with poisoning (Kamensky et al., 2019). After 28 days of exposure to methylmercury, the blood mercury concentration was 76.4 μg/ml, without apparent toxicity observed in rats (Pelletier et al., 2019). Although AGNHP significantly increased blood mercury exposure, it was proved safe by the limited blood mercury exposure at clinical doses. A California woman was diagnosed with mercury poisoning by using a skin-lightening cream on her face twice a day for 7 years, and the contents of mercury were 2,620 and 110 μg/L in her blood and urine samples, respectively (Kuehn 2020). Because mercury absorption through the skin can even lead to poisoning, long-term or excessive usage of cinnabar-containing TCMs should be paid more attention.
The pharmacokinetic profiles of arsenic and mercury in AGNHP were comprehensively elucidated at the first time. The blood exposure of arsenic and mercury were all within the safe range, and no noticeable toxic effects were observed at the experimental dose range of AGNHP. However, long-term or overdose of realgar- and cinnabar-containing TCMs may pose health risks of poisoning due to the slow absorption and elimination of arsenic and mercury.
Urinary excretion
The cumulative excretion of arsenic in rat urine after oral administration of realgar and AGNHP is shown in Figure 3. No significant differences were observed in urinary cumulative excretion of arsenic as the increase of dose in realgar and AGNHP groups, respectively. However, the cumulative urinary excretion of arsenic in AGNHP groups was much higher than the corresponding realgar groups. The compatibility of AGNHP promoted urinary arsenic excretion compared with realgar. As shown in Figure 4, the urinary arsenic concentration raised to the maximum at 8 h and then decreased within 24 h. The urinary arsenic concentration of AGNHP did not show noticeable differences between realgar at low doses due to the limited arsenic exposure. However, it was much higher than realgar at high doses. The urinary arsenic was not thoroughly eliminated within 24 h, which was in agreement with our previously reported (Wu et al., 2022). The total urinary cumulative excretion rates of arsenic in realgar and AGNHP low-dose groups were 0.57 and 1.0%, respectively. Meanwhile, those in high-dose groups were 0.12 and 0.18%, respectively. The extremely low urinary excretion rate of arsenic revealed that urine was not the dominant pathway of arsenic.
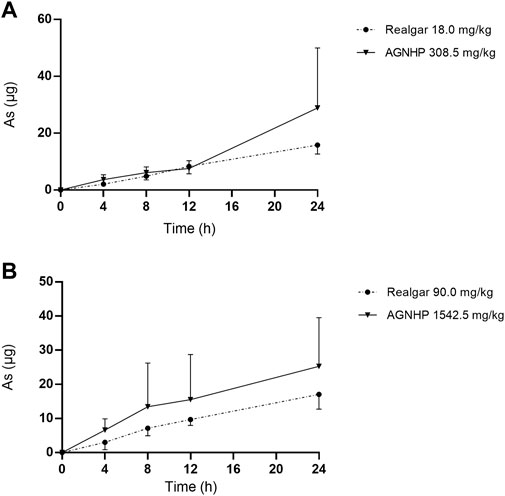
FIGURE 3. Cumulative excretion of arsenic in rat urine after oral administration of realgar and AGNHP.
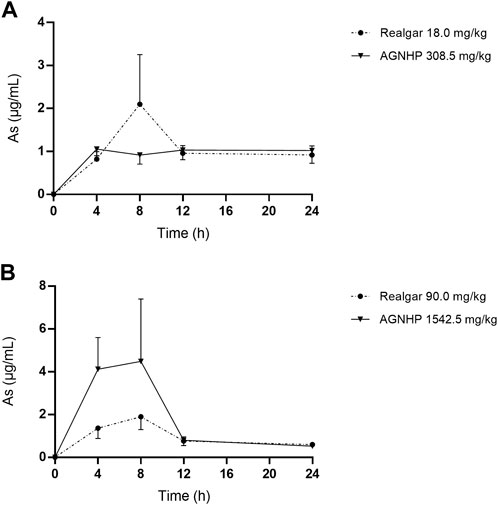
FIGURE 4. Concentration-time curves of arsenic in rat urine after oral administration of realgar and AGNHP.
The cumulative excretion of mercury in rat urine after oral administration of cinnabar and AGNHP is displayed in Figure 5. No significant differences were observed in urinary cumulative excretion of mercury as the increase of dose in cinnabar and AGNHP groups, respectively. Interestingly, the cumulative excretion of mercury in rat urine was opposite to that of arsenic after AGNHP compatibility. The cumulative urinary excretion of mercury in AGNHP was significantly reduced compared with the corresponding cinnabar groups. As displayed in Figure 6, the urinary concentration of mercury was much lower than arsenic. The urinary mercury concentration was gradually increased to 24 h after administration at low doses. However, it increased to the maximum at 12 h and then reduced within 24 h after 1542.5 mg/kg AGNHP dosing. Additionally, it was not decreased after treatment with 52.1 mg/kg of cinnabar. The trend of mercury urinary concentration was consistent with its cumulative excretion. Compared with cinnabar, urinary mercury was more difficult to excrete after taking AGNHP. The total urinary cumulative excretion rates of mercury in cinnabar and AGNHP low-dose groups were 0.91 and 0.40%, respectively. And those in high-dose groups were 0.25 and 0.03%, respectively. The results indicated that the elimination of mercury in urine was more difficult than arsenic after AGNHP administration. Urinary mercury was hardly excreted as the dose increases, which may pose a health risk of mercury accumulation by long-term or excessive medication.
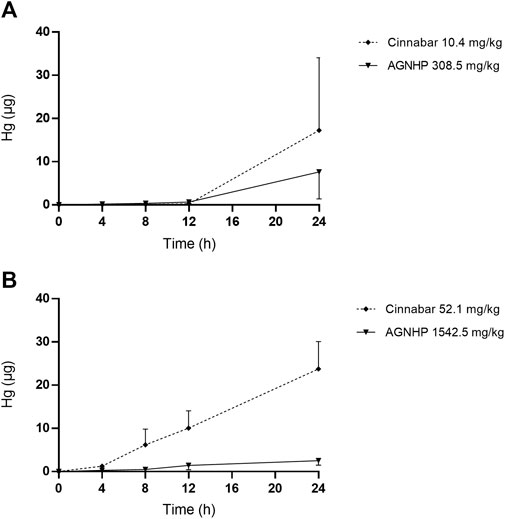
FIGURE 5. Cumulative excretion of mercury in rat urine after oral administration of cinnabar and AGNHP.
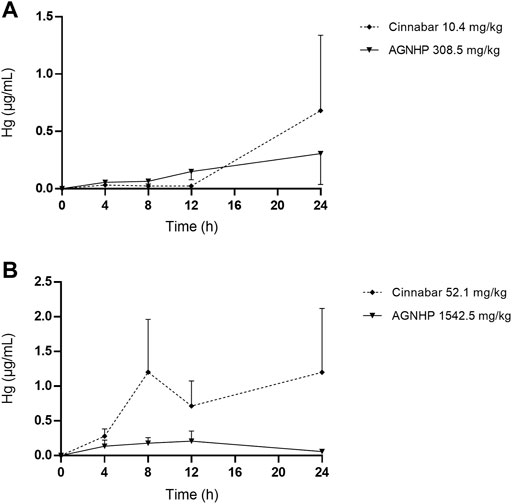
FIGURE 6. Concentration-time curves of mercury in rat urine after oral administration of cinnabar and AGNHP.
The results obtained in urine were in accordance with the findings of blood as we noted above. AGNHP formula compatibility significantly increased the excretion of arsenic in urine and reduced its exposure in blood. Conversely, AGNHP significantly reduced the excretion of mercury in urine and increased its exposure in blood. Urinary excretion was not the dominant excretion pathway of arsenic and mercury, and most of them excreted in feces in the form of As2S2 and HgS without metabolic transformation (Wu et al., 2022; Zhao et al., 2022). As reported, realgar-containing TCMs significantly inhibited the absorption of arsenic and promoted its excretion, which was responsible for the toxicity-reducing effect (Xu et al., 2022). More than 90.20 and 82.83% of mercury were excreted in rat feces within 24 h after treatment of cinnabar and AGNHP, respectively (Li et al., 2006). Others have also confirmed that cinnabar-containing TCMs significantly reduced the fecal mercury compared with cinnabar (Tian et al., 2015). These findings were highly consistent with our results. AGNHP significantly increased the excretion of arsenic, and conversely reduced the excretion of mercury. Although the fecal excretion profiles of arsenic and mercury have been generally recognized after treatment of realgar- or cinnabar-containing TCMs (Liu et al., 2008; Tinggi et al., 2016; Liu et al., 2018), little is known about the exposure of bioaccessible arsenic and mercury in vivo. Consequently, we focused on the bioaccessible arsenic and mercury in rat blood and urine to evaluate the safety of AGNHP in this study.
It has been demonstrated that the normal level of urinary arsenic was less than 0.02 μg/ml (Lech and Trela 2005; Spilchuk and Thompson 2019). Urinary mercury should not exceed 20 μg within 24 h, and more than 150 μg was potentially toxic (Nayfeh et al., 2018). The U.S. Federal Biological Exposure Index of mercury was 50 μg/L in urine (Kamensky et al., 2019). A clinical case reported patients with urinary mercury concentrations of 4,828 μg/L or 458 nmol/L were diagnosed with poisoning (Lu Q. Y. et al., 2017). No related toxicity occurred in 30 days realgar treated rats, and the urinary arsenic concentrations were 10.30 and 3.48 μg/ml at 1–12 and 12–24 h, respectively (Yi et al., 2019; Yi et al., 2020). Rats exposed to methylmercury for 9 weeks with a urinary mercury concentration of 3.20 μg/ml revealed no evidence of overt toxicity (Pingree et al., 2001). The urinary concentrations of arsenic and mercury were in the safe range after oral administration of AGNHP, and there were no significant toxic effects. Urine was not the main excretion route for arsenic and mercury (Wu et al., 2022; Zhao et al., 2022), and the limited excretion of arsenic and mercury could not accurately reflect the toxic exposure in vivo. Hence, the diagnosis of poisoning should be combined with the concentrations of arsenic and mercury in blood and urine.
Conclusion
In the present work, comparative pharmacokinetics and urinary excretion of arsenic and mercury were conducted in rats after oral administration of realgar, cinnabar and AGNHP, respectively. AGNHP significantly reduced the absorption of arsenic in blood and increased the excretion of arsenic in urine. Whereas, the trend of mercury in blood and urine was opposite to that of arsenic after AGNHP administration. AGNHP is safe at clinical doses by the limited arsenic and mercury exposure, but there is still a risk of toxicity by long-term or excessive medication. Therefore, more attentions must be paid to realgar- and cinnabar-containing TCMs due to the slow accumulation and excretion of arsenic and mercury.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by the Nanjing University of Chinese Medicine.
Author contributions
XW, ZZ, and YL conceived and designed the experiments. XW, ZZ, KL, XL, ZW, and ZL performed the experiments. XW and YL supervised the investigation and contributed to the writing of the paper.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82104523), National Natural Science Foundation of China by Nanjing University of Chinese Medicine (XPT82104523) and the Natural Science Foundation of Jiangsu Province (No. BK20200843).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Chang, R. S. K., William, L., Chun, Y., Cheung, T. T., and Chan, C. K. (2018). Arsenic-induced neuropathy by improper use of Chinese medicine: a case report. Am. J. Ther. 25, e392–e393. doi:10.1097/MJT.0000000000000576
Kamensky, O. L., Horton, D., Kingsley, D. P., and Bridges, C. C. (2019). A case of accidental mercury intoxication. J. Emerg. Med. 56, 275–278. doi:10.1016/j.jemermed.2018.12.039
Kim, J. J., Kim, Y. S., and Kumar, V. (2019). Heavy metal toxicity: an update of chelating therapeutic strategies. J. Trace Elem. Med. Biol. 54, 226–231. doi:10.1016/j.jtemb.2019.05.003
Kuehn, B. (2020). Mercury poisoning from skin cream. J. Am. Med. Assoc. 323, 500. doi:10.1001/jama.2020.0292
Lech, T., and Trela, F. (2005). Massive acute arsenic poisonings. Forensic Sci. Int. 151, 273–277. doi:10.1016/j.forsciint.2005.01.018
Li, A., Zhang, J. Y., Xiao, X., Wang, S. S., Wan, J. B., Chai, Y. S., et al. (2018). Hepatorenal protective effects of medicinal herbs in An-Gong-Niu-Huang Wan (AGNH) against cinnabar- and realgar-induced oxidative stress and inflammatory damage in mice. Food Chem. Toxicol. 119, 445–456. doi:10.1016/j.fct.2017.11.054
Li, J., Yuan, Z. B., Han, S. B., and Zhu, M. (2006). The existent form of mercury and arsenium in Angong Niuhuang Wan and the absorption and excreting in rats. J. Cap. Univ. Med. Sci. 27, 647–651. doi:10.3969/j.issn.1006-7795.2006.05.023
Liu, J., Shi, J. Z., Yu, L. M., Goyer, R. A., and Waalkes, M. P. (2008). Mercury in traditional medicines: is cinnabar toxicologically similar to common mercurials? Exp. Biol. Med. 233, 810–817. doi:10.3181/0712-MR-336
Liu, J., Wei, L. X., Wang, Q., Lu, Y. F., Zhang, F., Shi, J. Z., et al. (2018). A review of cinnabar (HgS) and/or realgar (As4S4)-containing traditional medicines. J. Ethnopharmacol. 210, 340–350. doi:10.1016/j.jep.2017.08.037
Lou, Y. J., Ma, Y. F., Jin, J., and Zhu, H. H. (2021). Oral realgar-Indigo naturalis formula plus retinoic acid for acute promyelocytic leukemia. Front. Oncol. 10, 597601. doi:10.3389/fonc.2020.597601
Lu, P., Ma, J. Q., Li, F., Xu, G. H., Guo, W., and Zhou, H. M. (2019). A fatal case of acute arsenic poisoning. J. Forensic Sci. 64, 1271–1273. doi:10.1111/1556-4029.14017
Lu, Q. Y., Liu, Z. L., and Chen, X. R. (2017a). Mercury poisoning through intravenous administration: two case reports with literature review. Medicine 96, e8643. doi:10.1097/MD.0000000000008643
Lu, Y. T., Qi, W. Z., Wang, S., Song, X. N., Yang, D. Y., Song, M., et al. (2020). Toxicity and risk assessment of mercury exposures from cinnabar and Baizi Yangxin Pills based on pharmacokinetic and tissue distribution studies. J. Ethnopharmacol. 250, 112489. doi:10.1016/j.jep.2019.112489
Lu, Y. T., Yang, D. Y., Song, X. N., Wang, S., Song, M., and Hang, T. J. (2017b). Bioaccessibility and health risk assessment of mercury in cinnabar containing Traditional Chinese Medicines. J. Trace Elem. Med. Biol. 44, 17–25. doi:10.1016/j.jtemb.2017.05.006
Nayfeh, A., Kassim, T., Addasi, N., Alghoula, F., Holewinski, C., and Depew, Z. (2018). Acute elemental mercury poisoning. Crit. Care Med. 46, 441. doi:10.1097/01.ccm.0000528923.44103.ea
Pelletier, G., Feng, Y. L., Leingartner, K., and Black, P. (2019). Co-administration of a Rhododendron tomentosum extract does not affect mercury tissue concentrations and excretion rate in methylmercury-treated adult male rats. BMC Res. Notes 12, 369. doi:10.1186/s13104-019-4409-7
Pingree, S. D., Simmonds, P. L., and Woods, J. S. (2001). Effects of 2, 3-dimercapto-1-propanesulfonic acid (DMPS) on tissue and urine mercury levels following prolonged methylmercury exposure in rats. Toxicol. Sci. 61, 224–233. doi:10.1093/toxsci/61.2.224
Rahman, Z., and Singh, V. P. (2019). The relative impact of toxic heavy metals (THMs) (arsenic (as), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: an overview. Environ. Monit. Assess. 191, 419. doi:10.1007/s10661-019-7528-7
Rehman, K., Fatima, F., Waheed, I., and Akas, M. S. H. (2018). Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 119, 157–184. doi:10.1002/jcb.26234
Sarma, H., Deka, S., Deka, H., and Saikia, R. R. (2011). Accumulation of heavy metals in selected medicinal plants. Rev. Environ. Contam. Toxicol. 214, 63–86. doi:10.1007/978-1-4614-0668-6_4
Sayed, M. A., Gofur, M. R., Khair, A., and Awal, M. A. (2015). Protective role of spirulina and vitamin E against arsenic toxicity in rats. Asian J. Anim. Sci. 9, 330–340. doi:10.3923/ajas.2015.330.340
Spilchuk, V., and Thompson, A. (2019). Chronic arsenic poisoning from traditional Chinese medicine. Can. Med. Assoc. J. 191, E424. doi:10.1503/cmaj.181176
Tian, X. Z., He, H. Y., Liu, J., Wu, Q., and Shi, J. Z. (2015). The absorption, distribution and excretion of mercury in cinnabar and cinnabar-containing remedy. Lishizhen Med. Mat. Medica Res. 26, 1106–1108. doi:10.3969/j.issn.1008-0805.2015.05.030
Tinggi, U., Sadler, R., Ng, J., Noller, B., and Seawright], A. (2016). Bioavailability study of arsenic and mercury in traditional Chinese medicines (TCM) using an animal model after a single dose exposure. Regul. Toxicol. Pharmacol. 76, 51–56. doi:10.1016/j.yrtph.2016.01.010
Tsoi, B., Wang, S. L., Gao, C., Luo, Y. H., Li, W. T., Yang, D., et al. (2019). Realgar and cinnabar are essential components contributing to neuroprotection of Angong Niuhuang Wan with no hepatorenal toxicity in transient ischemic brain injury. Toxicol. Appl. Pharmacol. 377, 114613. doi:10.1016/j.taap.2019.114613
Valido, H. M. C., Ferreira, J. P., Ibrahim, M. K., Argany, C., Ramos, T. D., Aguilar, G. G., et al. (2019). Mercury poisoning: A case report. Clin. Chim. Acta 493, S241–S242. doi:10.1016/j.cca.2019.03.499
Wang, S. S., Xiao, X., Li, A., and Li, P. (2021). The herbal constituents in An-Gong-Niu-Huang Wan (AGNH) protect against cinnabar- and realgar-induced hepatorenal toxicity and accumulations of mercury and arsenic in mice. Evid. Based. Complement. Altern. Med. 2021, 5566078. doi:10.1155/2021/5566078
Wu, M. L., Deng, J. F., Lin, K. P., and Tsai, W. J. (2013). Lead, mercury, and arsenic poisoning due to topical use of traditional chinese medicines. Am. J. Med. 126, 451–454. doi:10.1016/j.amjmed.2013.01.001
Wu, M. L., and Deng, J. F. (2013). Toxic epidermal necrolysis after extensive dermal use of realgar-containing (arsenic sulfide) herbal ointment. Clin. Toxicol. 51, 801–803. doi:10.3109/15563650.2013.831100
Wu, X., Guan, R., Liu, Y. X., Wu, S. H., Song, M., and Hang, T. J. (2020). Comparative health risk assessment of realgar and NiuHuangJieDu tablets based on tissue arsenic levels after multiple oral administration to rats. J. Ethnopharmacol. 249, 112370. doi:10.1016/j.jep.2019.112370
Wu, X., Wu, S. H., Liu, Y. X., Guan, R., Liang, F. M., Song, M., et al. (2018). Health risk assessment of arsenic in Realgar and NiuHuangJieDu tablets based on pharmacokinetic study. J. Trace Elem. Med. Biol. 48, 81–86. doi:10.1016/j.jtemb.2018.03.012
Wu, X., Yan, R. N., Guan, R., Du, Y., Liu, Y. X., Wu, S. H., et al. (2022). Arsenic-related health risk assessment of realgar-containing NiuHuangJieDu Tablets in healthy volunteers po administration. Front. Pharmacol. 12, 761801. doi:10.3389/fphar.2021.761801
Xia, F. B., Li, A., Chai, Y. S., Xiao, X., Wan, J. B., Li, P., et al. (2018). UPLC/Q-TOFMS-based metabolomics approach to reveal the protective role of other herbs in an-gong-niu-huang wan against the hepatorenal toxicity of cinnabar and realgar. Front. Pharmacol. 9, 618. doi:10.3389/fphar.2018.00618
Xu, W. F., Xu, S., Kuang, Y. M., He, X. R., and Jin, P. F. (2022). Compatibility of niuhuang jiedu tablets results in attenuated arsenic bioaccumulation and consequent protection against realgar-induced toxicity in mice. Evid. Based. Complement. Altern. Med. 2022, 7406694. doi:10.1155/2022/7406694
Yang, M. M., Wang, L. C., Zhang, T., Zhu, A., Sun, Y. Q., Zhao, J. W., et al. (2020). Different proteomic profiles of cinnabar upon therapeutic and toxic exposure reveal distinctive biological manifestations. J. Ethnopharmacol. 253, 112668. doi:10.1016/j.jep.2020.112668
Yi, Y., Gao, S. R., Xia, J., Li, C. Y., Zhao, Y., Zhang, Y. S., et al. (2019). Data on the sub-chronic toxicity in rats after 30 days of oral realgar administration and the accumulation and distribution of arsenic species. Data Brief. 23, 103572. doi:10.1016/j.dib.2018.12.011
Yi, Y., Gao, S. R., Xia, J., Li, C. Y., Zhao, Y., Zhang, Y. S., et al. (2020). Study of the accumulation and distribution of arsenic species and association with arsenic toxicity in rats after 30 days of oral realgar administration. J. Ethnopharmacol. 247, 111576. doi:10.1016/j.jep.2018.10.037
Zhang, S. Q., Jiang, X. L., Wang, Y., Lin, K. L., Zhang, Z., Zhang, Z., et al. (2021). Protective effect of An-Gong-Niu-Huang Wan pre-treatment against experimental cerebral ischemia injury via regulating GSK-3β/HO-1 pathway. Front. Pharmacol. 12, 640297. doi:10.3389/fphar.2021.640297
Keywords: realgar, cinnabar, AnGongNiuHuang pill, arsenic, mercury, pharmacokinetics, urinary excretion, hydride-generation atomic fluorescence spectrometry
Citation: Wu X, Zhong Z, Lin K, Liu X, Wu Z, Liu Z and Li Y (2022) Comparative pharmacokinetics and urinary excretion of arsenic and mercury after oral administration of realgar, cinnabar and AnGongNiuHuang Pill to rats. Front. Pharmacol. 13:967608. doi: 10.3389/fphar.2022.967608
Received: 13 June 2022; Accepted: 25 July 2022;
Published: 30 August 2022.
Edited by:
Rolf Teschke, Hospital Hanau, GermanyReviewed by:
Bun Tsoi, Hong Kong Polytechnic University, Hong Kong SAR, ChinaJan Kratzer, Institute of Analytical Chemistry (ASCR), Czechia
Copyright © 2022 Wu, Zhong, Lin, Liu, Wu, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao Wu, eHd1QG5qdWNtLmVkdS5jbg==; Yongming Li, bHltLTU2OUBuanVjbS5lZHUuY24=
 Xiao Wu
Xiao Wu Zeling Zhong
Zeling Zhong