- 1Senior Department of Hepatology, Fifth Medical Center of PLA General Hospital, Beijing, China
- 2Military Institute of Chinese Materia, Fifth Medical Center of PLA General Hospital, Beijing, China
- 3School of Traditional Chinese Medicine, Capital Medical University, Beijing, China
- 4The Third Affiliated Hospital of Zunyi Medical University (The First People’s Hospital of Zunyi), Guizhou, China
- 5Department of Pharmacy, The Fifth Medical Center of PLA General Hospital, Beijing, China
Inflammation is a key contributing factor in the pathogenesis of fatty liver diseases (FLD), such as nonalcoholic fatty liver disease (NAFLD) and alcohol-associated liver diseases (ALDs). The NLRP3 inflammasome is widely present in the hepatic parenchymal and non-parenchymal cells, which are assembled and activated by sensing intracellular and extracellular danger signals resulting in the matures of IL-1β/IL-18 and pyroptosis. Moreover, the aberrant activation of the NLRP3 inflammasome is considered the main factor to drives immune outbreaks in relation to hepatic injury, inflammation, steatosis, and fibrosis. Therefore, inhibition of NLRP3 inflammasome may be a promising therapeutic target for FLD. Currently, accumulating evidence has revealed that a number of traditional Chinese medicines (TCM) exert beneficial effects on liver injury via inhibiting the NLRP3 inflammasome activation. Here, we summarized the mechanism of NLRP3 inflammasomes in the progression of FLD, and TCM exerts beneficial effects on FLD via positive modulation of inflammation. We describe that TCM is a promising valuable resource for the prevention and treatment agents against FLD and has the potential to be developed into clinical drugs.
Introduction
Liver diseases have been a global health concern and ranked as one of the major causes of morbidity and mortality worldwide (Asrani et al., 2019). Among the various forms of liver disease, FLD has become the most common liver disease globally, which is associated with fibrosis and the risk of hepatocellular carcinoma and has been classified into NAFLD and ALD (The global, 2020). The vast majority of Europe and Asia encounter a huge burden of fatty liver pathologies, and about 25% of the European population is affected by NAFLD (Younossi et al., 2018). Moreover, approximately 300 million Chinese people suffer from liver disease. Notably, in contrast to the number of newly HBV-infected patients, the burden of NAFLD and ALD in China continued to grow, paralleling the increase in obesity (Wang et al., 2014; Pimpin et al., 2018; Younossi et al., 2018).
The liver is anatomically and physiologically connected to the gut, leading to the liver being constantly exposed to the gut-derived pathogen-associated molecular patterns (PAMPs), such as microbial and toxins, which trigger immune responses (Zhang et al., 2021a). In addition to PAMPs, hepatic inflammation is also activated by intracellular damage signals, which are released by damaged or dying hepatocytes (called damage-associated molecular patterns, DAMPs) (Kubes and Mehal, 2012). PAMPs and DAMPs can be recognized by pattern recognition receptors (PPRs) to induce PAMPs- and DAMPs-triggered immunity and test studies of PPRs include NOD-like receptors (NLR), Toll-like receptors (TLR), and AIM2-like receptors (AIM2) (Takeuchi and Akira, 2010). NLRP3, as the best characterized NLRs, is activated by PAMPs or DAMPs and promotes the expression of inflammatory cytokines to amplify the inflammatory response. The aberrant activation of NLRP3 inflammasome is considered the main driving force behind excessive immune outbreaks. Increasing studies have indicated that the aberration of the NLRP3 inflammasome is implicated in liver diseases, including drug-induced liver injury (DILI), hepatocellular carcinoma (HCC), cholestatic liver injury (CLI), and autoimmune hepatitis (AIH) (Neumann et al., 2018). For example, previous studies demonstrated that traditional Chinese medicines (TCMs), such as Epimedii Folium [Berberidaceae; Epimedium brevicornu Maxim.], Psoraleae Fructus [Leguminosae; Psoralea corylifolia L.], and Sophora flavescens [Leguminosae; Sophorae flavescentis Radix], as well as some chemical drugs carbamazepine, isoniazid, and nevirapine, promote NLRP3 inflammasome activation and result in liver injury (Wang et al., 2019a; Gao et al., 2021; Qin et al., 2021; and Lin et al., 2022). A clinical study showed that NLRP3 inflammasome activation exhibits a protective effect on the development of HCC, but other experimental data indicated that NLRP3 deficiency in HCC cells enhance surveillance of NK cells to delay the tumor development in the xenograft mice model (Wei et al., 2014; Lee et al., 2021). Some research studies showed a high level of NLPR3 expression in cholestatic liver injury via the S1P/S1PR2 pathway (Hou et al., 2021). Over-activation of the NLRP3 inflammasome has also been found in trichloroethene- and ConA-induced autoimmune hepatitis mice models, indicating that the inflammasome activation-dependent IL-1β and pyroptosis contributed to exacerbating the liver injury (Luan et al., 2018; Wang et al., 2019b). Emerging evidence revealed that NRLP3 inflammasome activation is a driver of the pathological process of FLD (including NAFLD and ALD), which contributes to hepatic steatosis, liver tissue damage, and necrotic cell death (Del Campo et al., 2018). In both NASH and ALD patients, the level of IL-1β was increased and contributed to the progression of the disease (Tilg et al., 1992; Henao-Mejia et al., 2012). In accordance with human NAFLD and ALD patients, the expressions of NLRP3 and IL-1β were significantly increased in NAFLD and ALD mouse models (Knorr et al., 2020). Hence, NLRP3 inflammasomes might be a novel target for the treatment of liver disease, especially in FLD.
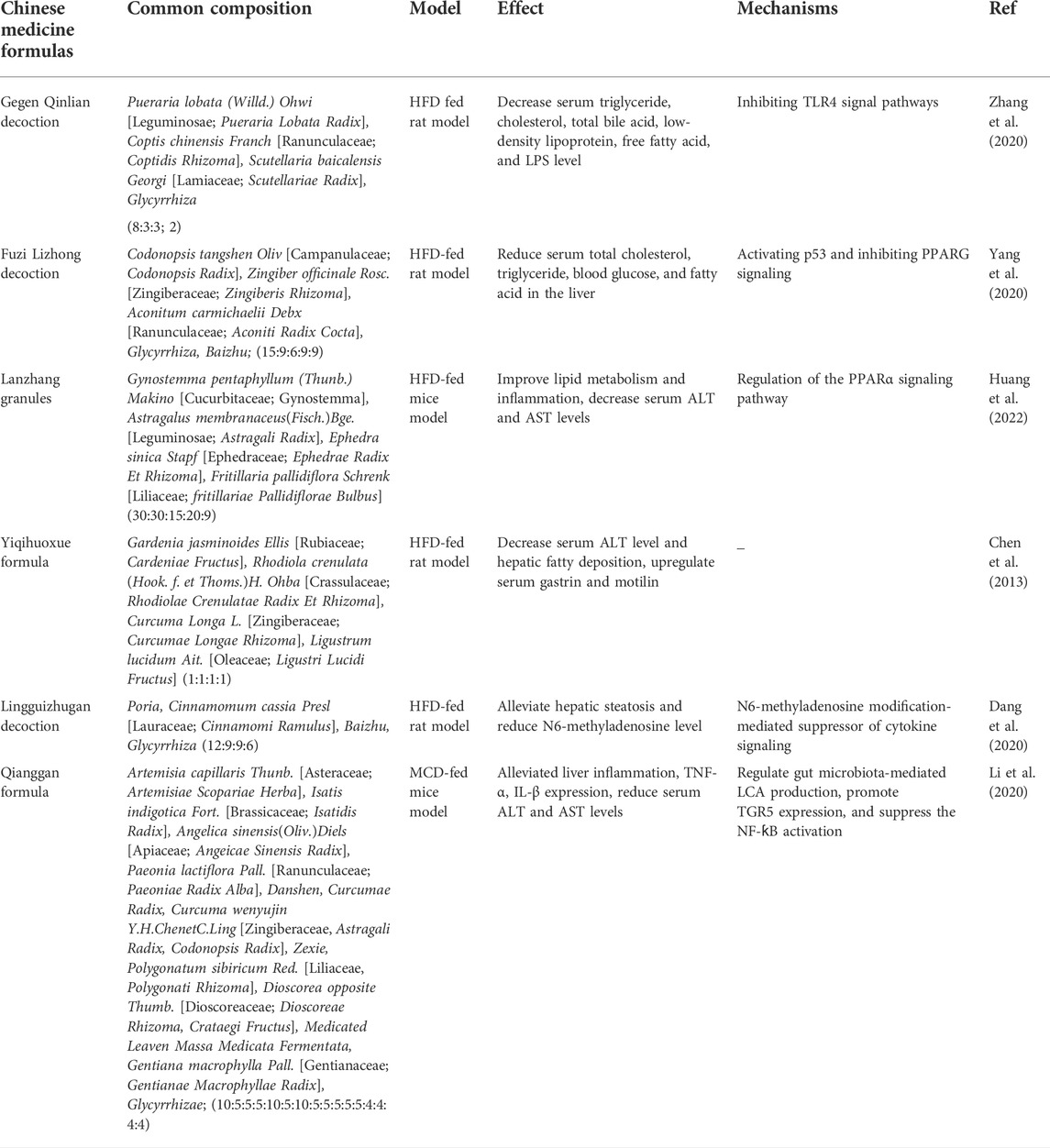
Currently, there is still no availability of approved pharmacological agents approved for the management of ALD and lifestyle modification, such as weight loss and alcohol abstinence, is considered the best therapeutic strategy (Pessione et al., 2003; Ferro et al., 2020). Several market-available drugs have been evaluated in ALDs, such as vitamin E, metformin, and statins, but most of them only provide limited success (Sanyal et al., 2010; Ford et al., 2015; Tziomalos et al., 2015). Thus, there is an urgent need to identify a high efficacy and minimal side effects treatment for ALD. Traditional Chinese medicine (TCM) has a long history of complementary and therapy applications in many countries (Zhang et al., 2021b), and the efficacy and safety for many diseases have been widely verified via long-term empirical trials (Li et al., 2017; Yang et al., 2019). Recently, TCM has gained much attention as a potential application in the prevention and treatment of FLD due to the characteristic of multi-targets, multi-pathway, and less toxic side effects. ALDs are referred to as “Gan-Pi” (NAFLD) or “Jiu-Pi” (ALD), respectively, due to the different etiology in Chinese medicine, and “internal retention of phlegm and dampness”, “liver qi stagnation”, “blood stasis”, and “a deficiency of spleen or kidney” is considered as its pathogenesis (Zhu et al., 2021a; Dong et al., 2012; 张欢 et al., 2022). Thus, the main principle of Chinese medicine in the treatment of ALD involves evacuating phlegm and dampness from the body, relieving qi stagnancy in the liver, removing blood stasis, and strengthening the function of the spleen and kidney (Dong et al., 2012; 张欢 et al., 2022). According to the therapeutics in Chinese medicine, numerous Chinese herbal formulations have been proposed and used for FLD (Tables 1, 2).
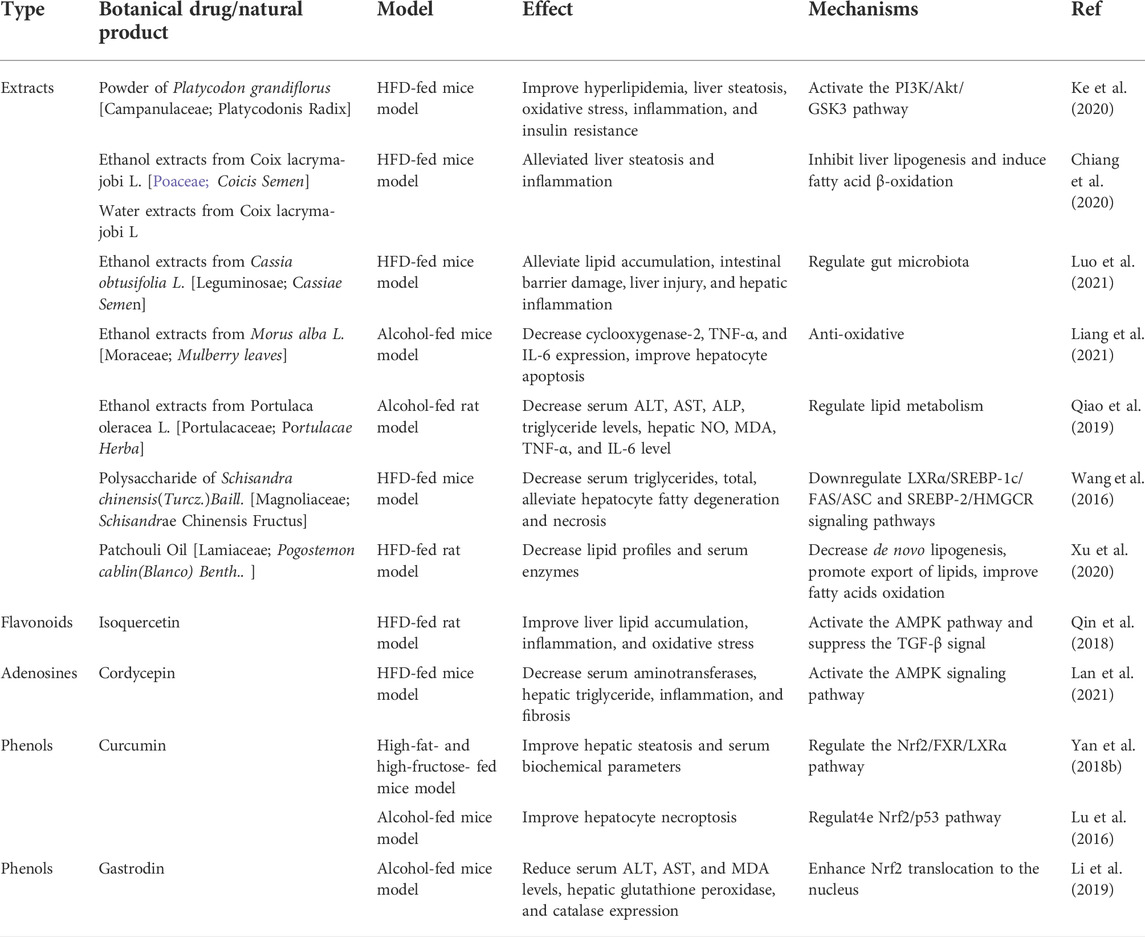
Emerging immunological studies also show that NLRP3 inflammasomes play an important role in the development of FLD and might be a promising therapeutic target for the treatment of FLD. Moreover, a variety of Chinese herbal formulations, TCM extracts, and natural products exert a wide range of anti-inflammatory effects by inhibiting the activation of the NLRP3 inflammasome and showing a potent and effective effect in various FLD (Fan et al., 2020; Wang et al., 2022). In this review, we systematically summarized the role and mechanisms of NLRP3 inflammasome activation in FLD and how the TCM targets and regulates NLRP3 inflammasome to improve the development of FLD.
The activation of NLRP3 inflammasomes and potential modulating factors
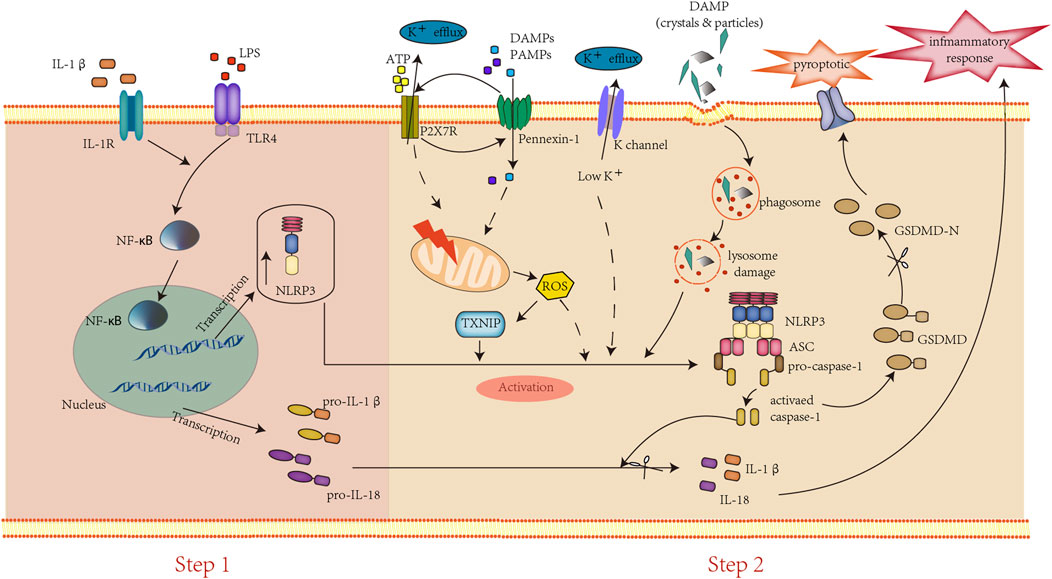
NLRP3 inflammasomes are well known as cytosolic multiprotein complexes consisting of the innate immune sensor protein NLRP3 (also called Cryopyrin), adaptor speck-like protein (ASC), and the caspase-1 protease (Deng et al., 2019). Some studies indicated that NLRP3 may act as a sensor of the homeostatic intracellular process that is activated by sensing the intracellular and extracellular PAMPs and DAMPs (Masters et al., 2010). Typically, the NLRP3 inflammasome activation requires a two-step process, including priming and activating (Figure 1). First, the priming step is usually induced by lipopolysaccharide (LPS), activating the transcription factor nuclear factor-kappa B (NF-ƙB) to upregulate the transcription of inflammasome proteins and pro-cytokines (pro-IL 1β, pro-IL-18). Second, the activating step is provided by a diverse group of DAMPs and PAMPs, such as ATP, cholesterol, reactive oxygen species (ROS), etc., that assemble and activate the NLRP3 inflammasome through three main pathways. 1) Extracellular ATP binds to the ionotropic P2X purinoceptor7 (P2X7) and activates the NLRP3 inflammasome by inducing intracellular K+ efflux (Carta et al., 2006). Moreover, the persistently activated P2X7 recruit membrane pore protein pannexin-1 and presumably formed, the “P2X7-PANX1 pore complex”, which allows a variety of PAMPs and DAMPs into the cytosol to trigger NLPR3 inflammasome activation (Kanneganti et al., 2007). 2) The endocytosis of crystals or large particles (amyloid, silica, cholesterol, etc.) induced lysosomal damage, leading to their components and lysosomal proteases release to induce NLRP3 inflammasome activation (Halle et al., 2008; Hornung et al., 2008; Broz and Dixit, 2016). 3) The increase of ROS leads to thioredoxin-interacting protein (TXNIP) translocating from the nucleus to the cytoplasm and bound to thioredoxin to associated with NLRP3 inflammasome activation (Brocker et al., 2020). Subsequently, the activated NLRP3/ASC/pro-caspase-1 complex converts pro-caspase-1 to caspase-1, which in turn processes the mature pro-IL-1β/pro-IL-18 into their secretory bio-active (IL-1β/IL-18) forms, triggering the inflammatory cascade and gasdermin D (GSDMD) cleavage (Basiorka et al., 2016). In addition to the above three main pathways, numerous NLRP3-interacting proteins, including mitosis A-related kinase-7 (NEK7), heat shock protein 90 (HSP90), etc., have been proved to promote the activation of NLRP3 inflammasomes (Duan et al., 2020).
Historically, inflammasomes are central to regulating liver diseases, which has been attributed to their ability to induce hepatic inflammation by up-regulating the expression of IL-1β/IL-18. Increasing clinical and experimental studies have demonstrated that inflammasome activation-dependent IL-1β is a major cause and contributes to liver disease progression (Iracheta-Vellve et al., 2017). The secreted active IL-1β synergistic action with TLR signaling amplifies inflammation by increasing the expression of pro- IL-1β, TNF, CCL2, etc. (Granowitz et al., 1992; Mandrekar et al., 2011). Moreover, IL-1β promotes hepatic stellate cells (HSCs) activation, resulting in liver fibrosis, as well as enhances the accumulation of triglyceride and hepatocyte injury contributing to liver steatosis (Miura et al., 2010; Petrasek et al., 2011). Compared to IL-1β, IL-18 aggravates NASH severity via altering the gut microbiota, and it has been proved in the MCD diet-induced NASH model that IL-18-deficient mice progressed to severe NASH more than the control group (Henao-Mejia et al., 2012). However, the role of IL-1β/IL-18 in NAFLD and ALD remains to be elucidated, and the underlying mechanisms require deep investigation.
The activation of the NLRP3 inflammasome requires two steps: first, DAMPs, PAMPs, or cytokines bind to their receptors, leading to the NF-κB signaling pathway activation, resulting in the increased expression of pro-IL-1β/IL-18 and inflammasome components. Second, several DAMPs and PAMPs induce the activation of the NLRP3 inflammasome to trigger caspase-1 cleavage and IL-1β/IL-18 mature. Subsequently, activated caspase-1 cleaves GSDMD to GSDMD-N, resulting in pyroptosis.
TCM for the treatment of NAFLD by inhibiting the NLRP3 inflammasome
NAFLD is the most frequent type of FLD, affecting more than 20% of people worldwide, and is highly correlated with obesity and metabolic syndrome (Lee et al., 2020). The clinical spectrum of NAFLD is spammed from noninflammatory isolated hepatic steatosis, NASH, progressive to cirrhosis, or even carcinoma (HCC) (Arab et al., 2018). NASH is a severe liver condition that is characterized by hepatocellular damage, steatosis, inflammation, and fibrogenesis. Approximately 10–30% of patients with NAFLD will develop NASH (Liang et al., 2018a). A “two-hit” hypothesis that explains the progression of NAFLD into NASH. The “first hit” involves an abnormal accumulation of lipid and insulin resistance that leads to hepatic steatosis, thereby resulting in the liver being susceptible to “second hits” including dysfunction of mitochondria, endotoxins, inflammation, and oxidative stress. Emerging evidence has indicated that NLRP3 inflammasome activation is implicated in metabolic syndrome, obesity, and NAFLD (Szabo and Csak, 2012; Lee et al., 2013; Esser et al., 2014). Recently, increasing clinical and experimental studies have shown that the expression of NLRP3 inflammasome components (NLRP3, caspase-1, and ASC) was remarkably increased in the patients with NAFLD and in the mice model (Wree et al., 2014; Mitsuyoshi et al., 2017; Gaul et al., 2021). Moreover, both NLRP3 inflammasome components, deficient or treated with NLRP3 inhibitors, attenuated the inflammation, liver fibrosis, and liver cell death in a mouse model, which further demonstrated the role of the NLRP3 inflammasome in NAFLD (Dixon et al., 2013; Li et al., 2022). In animal models, feeding rodents a diet deficient in methionine and choline (MCD) is a classic method of inducing NASH, as well as a prolonged high-fat diet (HFD) and high-fat/high-cholesterol/high-sugar diet (HF-HC-HSD). It is noteworthy that the short period of HFD or HF-HC-HSD feeding causes hepatic steatosis but not NASH (Ganz et al., 2015; Farrell et al., 2019).
Most types of TCMs, including TCM formulas, extracts, and its natural products, have been used in treating NAFLD and exhibit a promising treatment efficacy via modulating a variety of risk signals in the process of NAFLD, such as oxidized lipids, DAMPs, and ROS, resulting result in NLRP3 expression in liver tissue through TLR4 (Farrell et al., 2018; Wang et al., 2020; Zhang et al., 2020). Many of them showed a potent effect in modulating NLRP3 inflammasome activation by regulating inflammasome activation-associated signaling pathways, such as the release of ROS, LPS, NF-κB, toll-like, etc. Dansheng Zexie decoction is the water extract of three Chinese medicines, including Salvia miltiorrhiza Bunge (Danshen, 15 g) [ Lamiaceae; Salviae miltiorrhizae radix and rhizoma], Alisma plantago-aquatica Linn. (Zexie, 30 g) [Alismataceae; Alismatis Rhizoma], and Atractylodes macrocephala Koidz. (Baizhu, 12 g) [Asteraceae, Atractylodis Macrocephalae Rhizoma]. Both single compound and formulae of the Dansheng Zexie decoction have the effect of modulating cholesterol metabolism to decrease the level of lipid, showing a significant ability for treatment for NAFLD (Ding et al., 2019; Wu et al., 2021; Cao et al., 2022). Recently, experimental research revealed the mechanism underlying Dansheng Zexie decoction in treating NAFLD, which reduces lipid accumulation and alleviates hepatic steatosis, oxidative stress, and inflammation via inhibiting the ROS/NLRP3/IL-1β signaling pathway (Biao et al., 2022). Shenling Baizhu powder is composed of Dolichos lablab L. (4 g) [Leguminosae; Lablab Semen Album], Poria cocos (Schw.) Wolf (5 g) [Polyporaceae; Poria], Glycyrrhiza (3 g) [Leguminosae, Glycyrrhizae Radix Et Rhizoma], Platycodon grandiflorus (2 g) [Campanulaceae; Platycodonis Radix], Nelumbo nucifera (3 g) [Nymphaeaceae; Nelumbinis Semen], Panax ginseng C. A. Mey. (PG, 5 g) [Araliaceae, Ginseng Radix Et Rhizoma], Amomum villosum Lour. (2 g) [Zingiberaceae; Amomi Fructus], Dioscorea opposita Thunb. (5 g) [Dioscoreaceae; Dioscoreae Rhizoma], Coix lacryma-jobi L. var.ma-yuen (Roman.) Stapf (3 g) [Poaceae; Coicis Semen], and Baizhu (5 g), which has been proven to reduce body weight, serum-free fatty acid, and ameliorated liver microcirculation and ultrastructural abnormalities via inhibiting the TLR4/NLRP3 signaling pathway in the HFD-fed rat model (Pan et al., 2021). Chaihu–Shugan–San (CSS) decoction is composed of seven kinds of botanical drugs, including Citrus reticulata Blanco (6 g) [Rutaceae; Citri Reticulatae Pericarpium], Bupleurum chinense DC. (6 g) [Apiaceae; Bupleuri Radix], Ligusticum chuanxiong Hort. (5 g) [Apiaceae, Chuangxiong Rhizoma], Cyperus rotundus (5 g) [Cyperaceae; Cyperi Rhizoma], Citrus aurantium (5 g) [Rutaceae; Aurantii Fructus], Paeonia lactiflora (5 g) [Ranunculaceae; Paeoniae Radix Alba], and Glycyrrhiza (3 g), and has been frequently used for the prevention and treatment of chronic diseases (NAFLD and gastroenteropathy), significantly improving lipid peroxidation, inflammation, and liver fibrosis (Yang et al., 2014). The CSS contributes to the reducing serum LPS level, NLRP3 expression, liver steatosis, and reconstruction of the intestinal microflora in the HFD-fed rat model, all processes associated with the NLRP3 inflammasome pathway, suggesting that the inhibition of NLRP3 inflammasome activation is responsible for the treatment of NAFLD with CSS (Liang et al., 2018b).
Antrodia cinnamomea (AC) [Polyporaceae; Antrodia camphorata], a fungus of the Fomitopsidaceae family, has been used for treating many kinds of diseases and showed an effect in reducing hepatic triglycerides and total cholesterol concentrations in the HFD hamster model. Recent studies demonstrated that the AC ethanol extract attenuated steatohepatitis, oxidative stress, hepatic inflammation, ameliorating the MCD-diet-induced NAFLD by inhibiting NLRP3 inflammasome activation (Yen et al., 2020). Honey, a natural substance produced by bees from nectar, is a classical medicinal and edible TCM that has been investigated and used for various diseases, such as chemical-induced liver injury, hepatic cancer, and diabetes (Erejuwa et al., 2010; El‐kott et al., 2012; Al-Yahya et al., 2013). In recent studies, honey has been used for treating NAFLD and showed a potent effect in improving hepatic histology, lipid metabolism, oxidative stress, and hepatic inflammation via inhibiting the TXNIP-NLRP3 pathway in the HFD-fed rat model (Xiao et al., 2016). Rheum palmatun L. (RP) [Polygonaceae; Rhei Radix Et Rhizoma] is one of the most used TCM for “pursing fire and detoxification” and “promoting blood circulation for removing blood stasis” in clinical Chinese medicine, which is frequently prescribed for treating a set of metabolic disorders, and the RP aqueous extract has been reported to ameliorate NAFLD (Yang et al., 2016). Recent studies demonstrated that the RP aqueous extract improved the MCD diet-induced serum inflammation and liver function by inhibiting the activation of NLRP3 inflammasome in vivo (Wu et al., 2022).
In addition, numerous natural products isolated from TCMs have been shown to address the treatment potentials of NAFLD through modulating the NLRP3 inflammasome. Increasing studies showed that many natural products of GL exhibit a potent effect in the treatment of NAFLD. Glycyrrhiza uralensis Fisch. (GL) is one of the most popular TCM in clinical Chinese medicine shows a wide range of biological activities and common therapies for multisystem inflammatory diseases, such as NAFLD (El-Saber Batiha et al., 2020). A randomized double-blind clinical trial of treating NAFLD demonstrated that licorice, the powder from the root of GL, supplementation contributes to a reduction of ALT levels and liver steatosis in patients with lifestyle modification, suggesting that licorice supplementation can improve the effectiveness of lifestyle modification alone in treating NAFLD (Rostamizadeh et al., 2022). Our group’s previous studies showed that licochalcone B (flavonoids from GL.) inhibits NLRP3 inflammasome activation by preventing NEK7 from binding to NLRP3, and echinatin (flavonoids from GL) showed a negative effect on NLRP3 inflammasome activation by binding to HSP90. Furthermore, both licochalcone B and echinatin attenuate the MCD-induced increase of alanine transaminase (ALT) and aminotransferase (AST), liver inflammation changes, hepatic steatosis, and fibrosis (Xu et al., 2021; Li et al., 2022). In addition, another study also found that both glycyrrhizin and glycyrrhetinic acid (terpenoids from GL) alleviated the degree of inflammation infiltration and lipid disruption in MCD-diet mice (Yan et al., 2018a). Baicalin (a flavonoid glycoside from Scutellaria baicalensis Georgi [Lamiaceae; Scutellariae Radix] significantly reduced NLRP3, gasdermin D (GSDMD), IL-1β expression, and protected hepatocytes from free fatty acids-induced morphological damage and death, protecting hepatocytes from apoptosis by blocking NLRP3-GSDMD signaling in vitro (Shi et al., 2020a). Berberine is an isoquinoline alkaloid isolated from numerous herbal plants, which significantly ameliorated lipid accumulation, reducing TNF-α expression and phosphorylation of NF-κB, and inhibiting NLRP3 inflammasome activation by modulating the ROS/TXNIP axis in MCD-diet mice model (Mai et al., 2020). Emodin, rhein, diacerein, aloe-emodin, and 1,8-dihydroxyanthraquinone are free anthraquinones from Rheum palmatum L., and all of them remarkably decreased serum ALT, AST, IL-1β, and TNF-α levels, improved hepatic inflammation, and fibrosis by blocking the activation of the NLRP3 inflammasome and the underlying mechanism of this role is related to inhibited ASC oligomerization.
TCM for the treatment of ALD by inhibiting the NLRP3 inflammasome
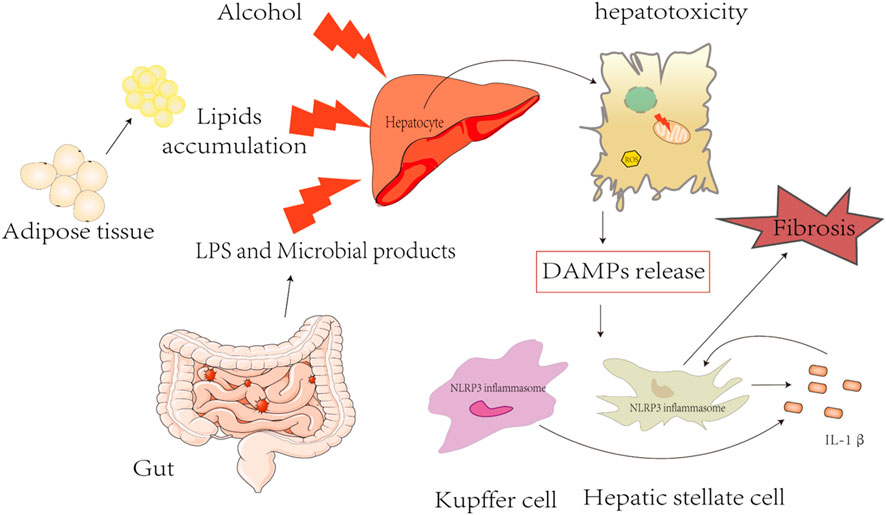
A major cause of ALD is due to the intake of excessive alcohol and is similar to NAFLD in pathology, ranging from hepatic steatohepatitis to fibrosis and cirrhosis (Silva et al., 2017). The early stage of ALD can be reversible with limited alcohol intake, but the advanced stages (including cirrhosis and severe alcoholic hepatitis) are irreversible, with fatal outcomes mediated by liver failure (Szabo et al., 2006). Activation of NLRP3 inflammation plays an important role in the progression of ALD, and the increased IL-1β and neutrophilia are characteristic features of sterile inflammation. The expression of IL-1β is significantly increased in patients with ALD, which is more than 10 times higher than in healthy controls (McClain et al., 1986). Moreover, the level of NLRP3 inflammasome components and IL-1β are increased in mice fed with excess ethanol (Petrasek et al., 2012). Similarly, caspase-1 or ASC deficient and IL-1 receptor knockout mice showed decreased ethanol-induced hepatic injury and steatosis (Petrasek et al., 2012). Moreover, liver macrophages (Kupffer cells) were important in mediating inflammasome activation in the progression of ALD (Adachi et al., 1994). The activation of the inflammasome is triggered by a variety of potential molecules in ALD, including DAMPs and PAMPs. Alcohol-induced intestinal barrier, leading to gut permeability, increased along with the leakage of gut microbiota product lipopolysaccharide (LPS) (DeSantis et al., 2013). Subsequently, the LPS binds to the TLR4 on Kupffer cells, which acts as the priming signal to induce the inflammasome component’s gene expression, to activate the NLRP3 inflammasome (Ganz et al., 2011). Additionally, alcohol-induced hepatocyte damage results in the release of DAMPs (ATP and uric acid) and mediated inflammasome activation (Tilg et al., 2016). It was verified by clinical and experimental studies that the increase in ATP and uric acid have been found in alcohol-fed healthy humans (Petrasek et al., 2015) and mice that were fed the ethanol diet (Iracheta-Vellve et al., 2015). Both P2X7-deficient and uric acid-inhibited mice lack inflammasome activation in alcohol-fed groups (Petrasek et al., 2015).
Many kinds of TCMs that play an important role in the development of ALD and have been used for the treatment of ALD. Lycium barbarum L. (LB) [Solanaceae; Lycii Fructus], a traditional TCM has a wide range of pharmacological effects, such as anti-inflammation, antioxidation, and hepatoprotective, and is usually used for “nourishing the liver” in clinical Chinese medicine (Chang and So, 2008; Tang et al., 2017). Recently, the LB polysaccharides (LBPs), the liquid fraction extracted from LB, showed an effect on ameliorating the progression of ALD, in vitro experiments confirm that LBP could reduce ethanol-induced oxidative stress, apoptosis, and the underlying mechanism of this role is proved by inhibiting NLRP3 inflammasome activation (Cheng and Kong, 2011; Xiao et al., 2014a). Moreover, zeaxanthin dipalmitate (ZD), one of the carotenoids of LB, showed an inhibiting effect on the NLRP3 inflammasome via modulating P2X7 and adipoR1, and drastically reduced inflammation infiltration and accumulation of fatty droplets in the ALD model rat (Gao et al., 2019).
In addition, many other natural products from TCM are also able to improve the development of ALD, such as gentiopicroside and active terpenoids of Gentiana Manchuria Kitag. [Gentianaceae; Gentianae Radix Et Rhizoma] regulated P2X7-NLRP3 to decrease the accumulation of aminotransferases and triglycerides in serum and reduced liver lipogenesis (Li et al., 2018).
Quercetin, a flavonoid from many TCMs, and ginsenoside Rg1, a natural terpenoid derived from PG, both showed a marked decrease effect on serum AST and ALT production, ameliorating the liver histology by inhibiting the activation of NRLP3 inflammasome via blocking oxidant stress in alcohol-fed mice and rats (Liu et al., 2018; Yang et al., 2021).
TCM for the treatment of liver fibrosis by inhibiting the NLRP3 inflammasome
Liver fibrosis is a result of chronic liver inflammation that is majorly regulated by the inflammasome, and the advanced form of fibrosis is responsible for liver failure (Bataller and Brenner, 2005). Numerous studies have indicated that the activation of the NLRP3 inflammasome is a critical contributor to the development of liver fibrosis. The inflammasome activator (uric acid crystals) increase the expression of transforming growth factor (TGF)-β1 to activate the hepatic stellate cells (HSCs), triggering collagen production and deposition in humans and mouse, but does not occur in ASC-deficient situation (Watanabe et al., 2009). Kupffer cell activation-dependent IL-1 also is an indirect factor in the progression of fibrosis, which activates HSC by binding to IL-1β receptors (Weiskirchen and Tacke, 2014). Furthermore, some experimental studies showed that NLRP3 or ASC deficiency protects mice from carbon tetrachloride (CCl4)-induced increase of hepatic TFG-β1 and collagen-1α1 expression. NLRP3 knock-out reduced liver fibrosis and inflammation in NASH model mice (Gaul et al., 2021).
25-0CH3-PDD (PDD), one of the ginsenosides derived from PG, showed an activation effect on LXRs to inhibit P2X7-mediated NLRP3 inflammasome activation, decreasing serum ALT/AST expression and ameliorating liver injury and fibrosis in thioacetamide (TAA)-induced mouse model (Han et al., 2018). The liver X receptors (LXRs) are considered a critical regulator of energy metabolism, which had been reported to downregulate inflammatory gene expression, including il-1β, il-6, P2X7, etc., inhibiting inflammation (Zhu et al., 2012). Recently, a study showed that ursolic acid, a natural terpenoid isolated from a variety of herbal medicine, decreased collagen deposition and fibrosis-related factors expression and inhibited the level of NADPH oxidase 4 (NOX4) and NLRP3 in the CCl4-induced liver fibrosis model. NOX4 activates liver fibrosis via regulating ROS to trigger apoptosis and HSC activation (Crosas-Molist and Fabregat, 2015). NLRP3 and NOX4 deficiency ameliorates the progression of ALD (Nie et al., 2021). In addition, alpinetin, a flavonoid isolated from Alpinia katsumadai [Zingiberaceae; Alpiniae Katsumadai Semen], also affects ameliorated liver injury and fibrosis via inhibiting NLRP3 inflammasome activation (Zhu et al., 2021b).
Discussion
NLRP3 inflammasomes play a pivotal role in FLD, especially in the progression of chronic types, including NAFLD, ALD, and liver fibrosis (Figure 2). However, the current knowledge of the mechanism of NLRP3 inflammasome activation is still very limited, and there is a lack of efficient clinical drugs for targeting NLRP3 inflammasome. Currently, therapeutic strategies are aimed at inhibiting the NRLP3 inflammasome signaling pathway by using NLRP3, IL-1β, TNF-α, and caspase inhibitors. MCC950 is well known as an NLRP3-specific inhibitor, showing a promising therapeutic effect in a variety of NLRP3-dependent immunopathological mouse models, including colitis, steatohepatitis, etc., but it was withdrawn from phase II clinical trial for the treatment of rheumatoid arthritis due to hepatotoxicity (Mangan et al., 2018). In addition, antibodies or antagonists (canakinumab) are used as inhibitors for IL-1β, which have been evaluated in humans (Kuemmerle-Deschner et al., 2011); however, multiple pro-inflammatory cytokines are induced by NLRP3 inflammasome activation and the treatment strategies to block IL-1β still need further study. Pentoxifylline has been known as a selective inhibitor of TNF-α and has been used in treating patients with severe alcoholic hepatitis in a randomized study, but it did not improve outcomes (Thursz et al., 2015). GS-9450 is an effective caspase inhibitor for caspases 1,8, and 9 and has been explored for NASH in a randomized, double-blind, placebo-controlled study, which demonstrated the potent effect in decreasing ALT levels safely and with tolerance. However, episodes of GS-9450-induced DILI occurred in a 6-month study in hepatitis C subjects, and the safety and efficacy of long-term caspase inhibitor in NASH still need to be further investigated (Ratziu et al., 2012).
TCM has been extensively applied for the prevention and treatment of various liver diseases, particularly FLD. Many Chinese herbal formulations, TCM extracts, and natural products exhibit beneficial effects on the progression of FLD via modulating the NLRP3 inflammasome pathway (Table 3, 4). Carnosol is one of the phenols isolated from Rosmarinus officinalis [Lamiaceae; Rosmarinus officinalis L.]; cryptotanshinone is a quinones components in Salvia miltiorrhiza Bunge and serves as therapeutics against NLRP3-drive disease, including LPS-induced mortality and MCD-fed induced NASH mouse model via inhibiting the activation of NLRP3 inflammasomes (Shi et al., 2020b; Liu et al., 2021). Therefore, TCM has shown promising therapeutic anti-inflammatory, antioxidant, and anti-fibrosis t that might take beneficial effects on curtailing the progression of ALD.
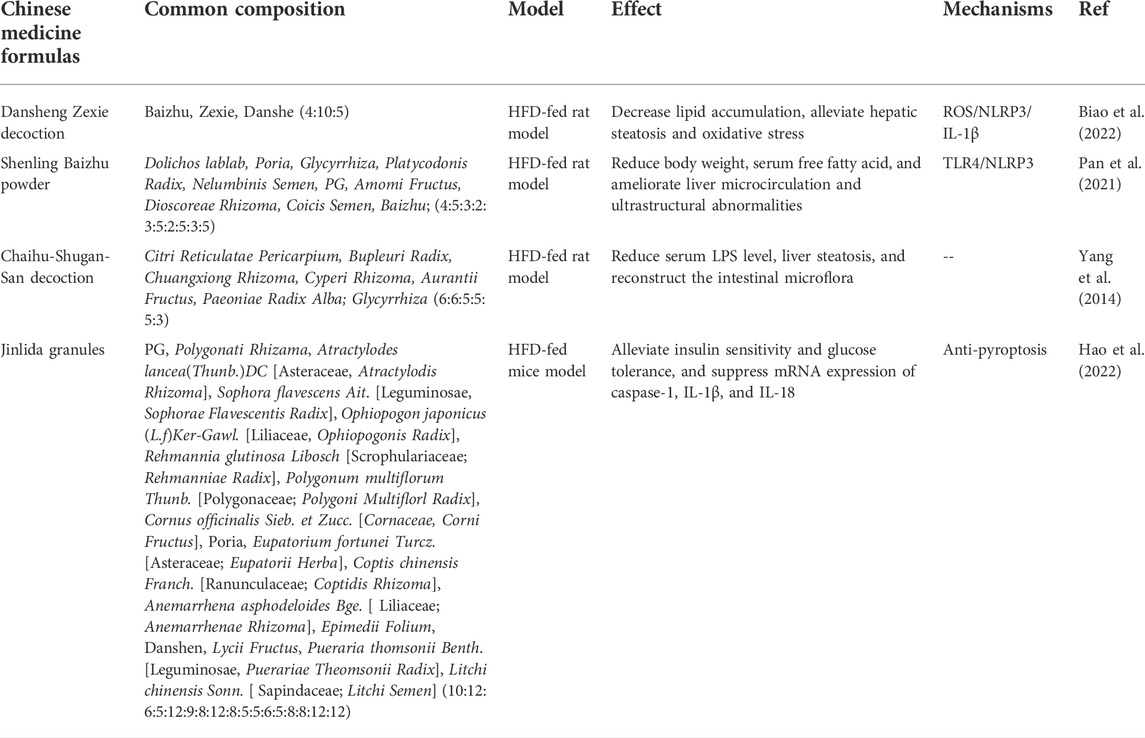
TABLE 3. Traditional Chinese medicine formulas for the treatment of FLD by inhibiting NLRP3 inflammasome activation.
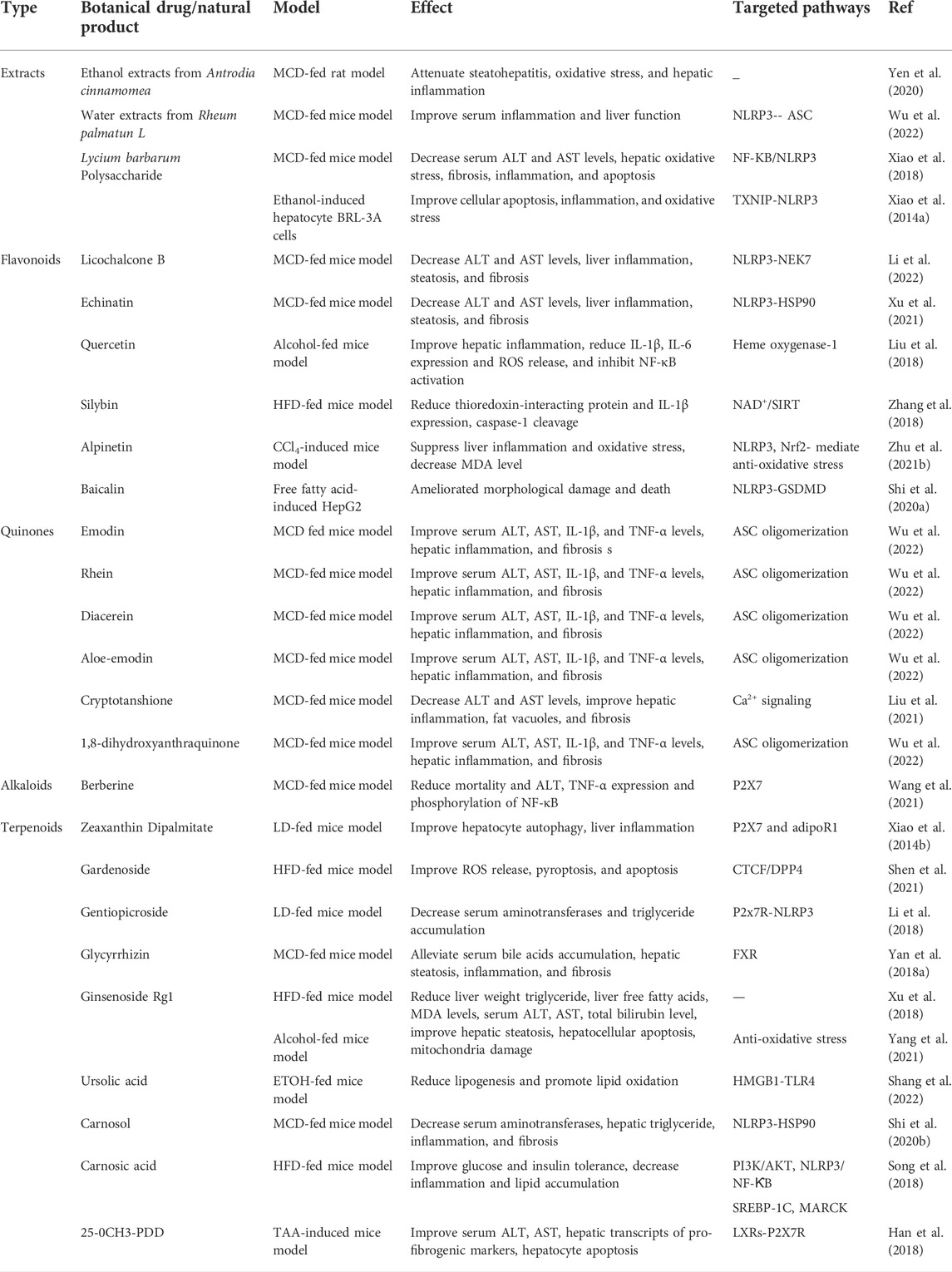
TABLE 4. Botanical drugs and natural products for the treatment of FLD by inhibiting NLRP3 inflammasome activation.
In this review, we summarized and discussed recent research on Chinese herbal formulations, TCM extracts, and natural products that improve the status of ALD via inhibiting the NLRP3 inflammasome.
Most of them, such as Glycyrrhiza, and Salvia miltiorrhiza Bunge, are commonly used in clinical practice, and their formulations and natural products can achieve an anti-inflammatory effect inhibiting NLRP3 inflammasome activation, showing advantages in reducing side effects, improving prognosis during FLD treatment, and improving the survival rate of patients. Moreover, the natural products, such as licochalcone B and cryptotanshinone, have the potential to be developed as inhibitors for treating FLD due to their specifically inhibiting NLRP3 inflammasome activation. However, the mechanisms underlying TCM inhibiting NLRP3 inflammasome activation have not yet been systematically studied and remain to be further investigated. The bioactive components of TCM are complex and many of them exhibit an anti-inflammation effect, but it is unknown whether there is synergistic or antagonistic interaction between these components. Moreover, most research based on animals and cells is urgently required to undergo clinical safety and efficacy studies. Collectively, even though further studies are required to disentangle the mechanism of TCM, we believe that TCM and its natural products are promising therapeutic applications for the treatment of FLD.
Author contributions
BZ, XG, XX, and ZY, supervised the project and acquired funding for the study; LT and LL collected the relevant literature, XG, LL, and LT designed the pictures and tables; LT wrote the manuscript; BZ, ZY, LTT, LLX, and XG revised the manuscript.
Funding
National Natural Science Foundation of China (81874368, 81903891), Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (No: ZYYCXTD-C-202005), Youth Foundation of Chinese PLA General Hospital (QNF19065), China Postdoctoral Science Foundation Funded Project (2020M673676).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adachi, Y., Bradford, B. U., Gao, W., Bojes, H. K., and Thurman, R. G. (1994). Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology 20, 453–460. doi:10.1002/hep.1840200227
Al-Yahya, M., Mothana, R., Al-Said, M., Al-Dosari, M., Al-Musayeib, N., Al-Sohaibani, M., et al. (2013). Attenuation of CCl4-induced oxidative stress and hepatonephrotoxicity by Saudi sidr honey in rats. Evid. Based. Complement. Altern. Med. 2013, 569037. doi:10.1155/2013/569037
Arab, J. P., Arrese, M., and Trauner, M. (2018). Recent insights into the pathogenesis of nonalcoholic fatty liver disease. Annu. Rev. Pathol. 13, 321–350. doi:10.1146/annurev-pathol-020117-043617
Asrani, S. K., Devarbhavi, H., Eaton, J., and Kamath, P. S. (2019). Burden of liver diseases in the world. J. Hepatol. 70, 151–171. doi:10.1016/j.jhep.2018.09.014
Basiorka, A. A., McGraw, K. L., Eksioglu, E. A., Chen, X., Johnson, J., Zhang, L., et al. (2016). The NLRP3 inflammasome functions as a driver of the myelodysplastic syndrome phenotype. Blood 128, 2960–2975. doi:10.1182/blood-2016-07-730556
Bataller, R., and Brenner, D. A. (2005). Liver fibrosis. J. Clin. Investig. 115, 209–218. doi:10.1172/JCI24282
Biao, Y., Chen, J., Liu, C., Wang, R., Han, X., Li, L., et al. (2022). Protective effect of danshen Zexie decoction against non-alcoholic fatty liver disease through inhibition of ROS/NLRP3/IL-1β pathway by Nrf2 signaling activation. Front. Pharmacol. 13, 877924. doi:10.3389/fphar.2022.877924
Brocker, C. N., Kim, D., Melia, T., Karri, K., Velenosi, T. J., Takahashi, S., et al. (2020). Long non-coding RNA Gm15441 attenuates hepatic inflammasome activation in response to PPARA agonism and fasting. Nat. Commun. 11, 5847. doi:10.1038/s41467-020-19554-7
Broz, P., and Dixit, V. M. (2016). Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 16, 407–420. doi:10.1038/nri.2016.58
Cao, Y., Shi, J., Song, L., Xu, J., Lu, H., Sun, J., et al. (2022). Multi-omics integration analysis identifies lipid disorder of a non-alcoholic fatty liver disease (NAFLD) mouse model improved by zexie-baizhu decoction. Front. Pharmacol. 13, 858795. doi:10.3389/fphar.2022.858795
Carta, S., Tassi, S., Semino, C., Fossati, G., Mascagni, P., Dinarello, C. A., et al. (2006). Histone deacetylase inhibitors prevent exocytosis of interleukin-1beta-containing secretory lysosomes: Role of microtubules. Blood 108, 1618–1626. doi:10.1182/blood-2006-03-014126
Chang, R. C., and So, K. F. (2008). Use of anti-aging herbal medicine, Lycium barbarum, against aging-associated diseases. What do we know so far? Cell. Mol. Neurobiol. 28, 643–652. doi:10.1007/s10571-007-9181-x
Chen, S., Zhou, H., Lin, M., Mi, R., and Li, L. (2013). Decoction vs extracts-mixed solution: Effect of yiqihuoxue formula on non-alcoholic fatty liver disease in rats. J. Tradit. Chin. Med. 33, 513–517. doi:10.1016/s0254-6272(13)60157-0
Cheng, D., and Kong, H. (2011). The effect of Lycium barbarum polysaccharide on alcohol-induced oxidative stress in rats. Molecules 16, 2542–2550. doi:10.3390/molecules16032542
Chiang, H., Lu, H. F., Chen, J. C., Chen, Y. H., Sun, H. T., Huang, H. C., et al. (2020). Adlay seed (Coix lacryma-jobi L.) extracts exhibit a prophylactic effect on diet-induced metabolic dysfunction and nonalcoholic fatty liver disease in mice. Evid. Based. Complement. Altern. Med. 2020, 9519625. doi:10.1155/2020/9519625
Kuemmerle-Deschner, J. B., Ramos, E., Blank, N., Roesler, F., Felix, S. D., Jung, T., et al. (2011). Canakinumab (ACZ885, a fully human IgG1 anti-IL-1β mAb) induces sustained remission in pediatric patients with cryopyrin-associated periodic syndrome (CAPS) Arthritis. Res. Ther. 13 (1), R34. doi:10.1186/ar3266
Crosas-Molist, E., and Fabregat, I. (2015). Role of NADPH oxidases in the redox biology of liver fibrosis. Redox Biol. 6, 106–111. doi:10.1016/j.redox.2015.07.005
Dang, Y., Xu, J., Yang, Y., Li, C., Zhang, Q., Zhou, W., et al. (2020). Ling-gui-zhu-gan decoction alleviates hepatic steatosis through SOCS2 modification by N6-methyladenosine. Biomed. Pharmacother. 127, 109976. doi:10.1016/j.biopha.2020.109976
Del Campo, J. A., Gallego, P., and Grande, L. (2018). Role of inflammatory response in liver diseases: Therapeutic strategies. World J. Hepatol. 10, 1–7. doi:10.4254/wjh.v10.i1.1
Deng, M., Guo, H., Tam, J. W., Johnson, B. M., Brickey, W. J., New, J. S., et al. (2019). Platelet-activating factor (PAF) mediates NLRP3-NEK7 inflammasome induction independently of PAFR. J. Exp. Med. 216, 2838–2853. doi:10.1084/jem.20190111
DeSantis, D. A., Ko, C. W., Liu, Y., Liu, X., Hise, A. G., Nunez, G., et al. (2013). Alcohol-induced liver injury is modulated by Nlrp3 and Nlrc4 inflammasomes in mice. Mediat. Inflamm. 2013, 751374. doi:10.1155/2013/751374
Ding, J., Zhang, B., Wang, P. J., He, G. N., Wei, D. M., Ding, J. L., et al. (2019). Analysis on mechanisms and medication rules of herbal prescriptions for nonalcoholic fatty liver disease based on methods of data mining and biological information. Zhongguo Zhong Yao Za Zhi 44, 1689–1695. doi:10.19540/j.cnki.cjcmm.20190110.001
Dixon, L. J., Flask, C. A., Papouchado, B. G., Feldstein, A. E., and Nagy, L. E. (2013). Caspase-1 as a central regulator of high fat diet-induced non-alcoholic steatohepatitis. PLoS One 8, e56100. doi:10.1371/journal.pone.0056100
Dong, H., Lu, F. E., and Zhao, L. (2012). Chinese herbal medicine in the treatment of nonalcoholic fatty liver disease. Chin. J. Integr. Med. 18, 152–160. doi:10.1007/s11655-012-0993-2
Duan, Y., Zhang, L., Angosto-Bazarra, D., Pelegrín, P., Núñez, G., and He, Y. (2020). RACK1 mediates NLRP3 inflammasome activation by promoting NLRP3 active conformation and inflammasome assembly. Cell. Rep. 33, 108405. doi:10.1016/j.celrep.2020.108405
El-Saber Batiha, G., Magdy Beshbishy, A., El-Mleeh, A., Abdel-Daim, M. M., and Prasad Devkota, H. (2020). Traditional uses, bioactive chemical constituents, and pharmacological and toxicological activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules 10, E352. doi:10.3390/biom10030352
El‐kott, A. F., Kandeel, A. A., El-Az, S. F. A., and Ribea, H. M. (2012). Anti-tumor effects of bee honey on PCNA and P53 expression in the rat hepatocarcinogenesis. Int. J. Cancer Res. 8, 130–139. doi:10.3923/ijcr.2012.130.139
Erejuwa, O. O., Gurtu, S., Sulaiman, S. A., Ab Wahab, M. S., Sirajudeen, K. N., Salleh, M. S., et al. (2010). Hypoglycemic and antioxidant effects of honey supplementation in streptozotocin-induced diabetic rats. Int. J. Vitam. Nutr. Res. 80, 74–82. doi:10.1024/0300-9831/a000008
Esser, N., Legrand-Poels, S., Piette, J., Scheen, A. J., and Paquot, N. (2014). Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 105, 141–150. doi:10.1016/j.diabres.2014.04.006
Fan, X., Lin, L., Cui, B., Zhao, T., Mao, L., Song, Y., et al. (2020). Therapeutic potential of genipin in various acute liver injury, fulminant hepatitis, NAFLD and other non-cancer liver diseases: More friend than foe. Pharmacol. Res. 159, 104945. doi:10.1016/j.phrs.2020.104945
Farrell, G. C., Haczeyni, F., and Chitturi, S. (2018). Pathogenesis of NASH: How metabolic complications of overnutrition favour lipotoxicity and pro-inflammatory fatty liver disease. Adv. Exp. Med. Biol. 1061, 19–44. doi:10.1007/978-981-10-8684-7_3
Farrell, G., Schattenberg, J. M., Leclercq, I., Yeh, M. M., Goldin, R., Teoh, N., et al. (2019). Mouse models of nonalcoholic steatohepatitis: Toward optimization of their relevance to human nonalcoholic steatohepatitis. Hepatology 69, 2241–2257. doi:10.1002/hep.30333
Ferro, Y., Montalcini, T., Mazza, E., Foti, D., Angotti, E., Gliozzi, M., et al. (2020). Randomized clinical trial: Bergamot Citrus and wild cardoon reduce liver steatosis and body weight in non-diabetic individuals aged over 50 years. Front. Endocrinol. 11, 494. doi:10.3389/fendo.2020.00494
Ford, R. J., Fullerton, M. D., Pinkosky, S. L., Day, E. A., Scott, J. W., Oakhill, J. S., et al. (2015). Metformin and salicylate synergistically activate liver AMPK, inhibit lipogenesis and improve insulin sensitivity. Biochem. J. 468, 125–132. doi:10.1042/BJ20150125
Ganz, M., Bukong, T. N., Csak, T., Saha, B., Park, J. K., Ambade, A., et al. (2015). Progression of non-alcoholic steatosis to steatohepatitis and fibrosis parallels cumulative accumulation of danger signals that promote inflammation and liver tumors in a high fat-cholesterol-sugar diet model in mice. J. Transl. Med. 13, 193. doi:10.1186/s12967-015-0552-7
Ganz, M., Csak, T., Nath, B., and Szabo, G. (2011). Lipopolysaccharide induces and activates the Nalp3 inflammasome in the liver. World J. Gastroenterol. 17, 4772–4778. doi:10.3748/wjg.v17.i43.4772
Gao, H., Lv, Y., Liu, Y., Li, J., Wang, X., Zhou, Z., et al. (2019). Wolfberry-derived zeaxanthin dipalmitate attenuates ethanol-induced hepatic damage. Mol. Nutr. Food Res. 63, e1801339. doi:10.1002/mnfr.201801339
Gao, Y., Xu, G., Ma, L., Shi, W., Wang, Z., Zhan, X., et al. (2021). Icariside I specifically facilitates ATP or nigericin-induced NLRP3 inflammasome activation and causes idiosyncratic hepatotoxicity. Cell. Commun. Signal. 19, 13. doi:10.1186/s12964-020-00647-1
Gaul, S., Leszczynska, A., Alegre, F., Kaufmann, B., Johnson, C. D., Adams, L. A., et al. (2021). Hepatocyte pyroptosis and release of inflammasome particles induce stellate cell activation and liver fibrosis. J. Hepatol. 74, 156–167. doi:10.1016/j.jhep.2020.07.041
Granowitz, E. V., Vannier, E., Poutsiaka, D. D., and Dinarello, C. A. (1992). Effect of interleukin-1 (IL-1) blockade on cytokine synthesis: II. IL-1 receptor antagonist inhibits lipopolysaccharide-induced cytokine synthesis by human monocytes. Blood 79, 2364–2369. doi:10.1182/blood.v79.9.2364.bloodjournal7992364
Halle, A., Hornung, V., Petzold, G. C., Stewart, C. R., Monks, B. G., Reinheckel, T., et al. (2008). The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 9, 857–865. doi:10.1038/ni.1636
Han, X., Song, J., Lian, L. H., Yao, Y. L., Shao, D. Y., Fan, Y., et al. (2018). Ginsenoside 25-OCH(3)-PPD promotes activity of LXRs to ameliorate P2x7r-mediated NLRP3 inflammasome in the development of hepatic fibrosis. J. Agric. Food Chem. 66, 7023–7035. doi:10.1021/acs.jafc.8b01982
Hao, Y. Y., Cui, W. W., Gao, H. L., Wang, M. Y., Liu, Y., Li, C. R., et al. (2022). Jinlida granules ameliorate the high-fat-diet induced liver injury in mice by antagonising hepatocytes pyroptosis. Pharm. Biol. 60, 274–281. doi:10.1080/13880209.2022.2029501
Henao-Mejia, J., Elinav, E., Jin, C., Hao, L., Mehal, W. Z., Strowig, T., et al. (2012). Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482, 179–185. doi:10.1038/nature10809
Hornung, V., Bauernfeind, F., Halle, A., Samstad, E. O., Kono, H., Rock, K. L., et al. (2008). Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9, 847–856. doi:10.1038/ni.1631
Hou, L., Zhang, Z., Yang, L., Chang, N., Zhao, X., Zhou, X., et al. (2021). NLRP3 inflammasome priming and activation in cholestatic liver injury via the sphingosine 1-phosphate/S1P receptor 2/Gα((12/13))/MAPK signaling pathway. J. Mol. Med. 99, 273–288. doi:10.1007/s00109-020-02032-4
Huang, P., Yang, L., Liu, Y., Jiang, Y., Li, Y., Chen, Z., et al. (2022). Lanzhang granules ameliorate nonalcoholic fatty liver disease by regulating the PPARα signaling pathway. Evidence-Based Complementary Altern. Med. 2022, 1124901–1124915. doi:10.1155/2022/1124901
Iracheta-Vellve, A., Petrasek, J., Gyogyosi, B., Bala, S., Csak, T., Kodys, K., et al. (2017). Interleukin-1 inhibition facilitates recovery from liver injury and promotes regeneration of hepatocytes in alcoholic hepatitis in mice. Liver Int. 37, 968–973. doi:10.1111/liv.13430
Iracheta-Vellve, A., Petrasek, J., Satishchandran, A., Gyongyosi, B., Saha, B., Kodys, K., et al. (2015). Inhibition of sterile danger signals, uric acid and ATP, prevents inflammasome activation and protects from alcoholic steatohepatitis in mice. J. Hepatol. 63, 1147–1155. doi:10.1016/j.jhep.2015.06.013
Kanneganti, T. D., Lamkanfi, M., Kim, Y. G., Chen, G., Park, J. H., Franchi, L., et al. (2007). Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity 26, 433–443. doi:10.1016/j.immuni.2007.03.008
Ke, W., Wang, P., Wang, X., Zhou, X., Hu, X., and Chen, F. (2020). Dietary Platycodon grandiflorus attenuates hepatic insulin resistance and oxidative stress in high-fat-diet induced non-alcoholic fatty liver disease. Nutrients 12, E480. doi:10.3390/nu12020480
Knorr, J., Wree, A., Tacke, F., and Feldstein, A. E. (2020). The NLRP3 inflammasome in alcoholic and nonalcoholic steatohepatitis. Semin. Liver Dis. 40, 298–306. doi:10.1055/s-0040-1708540
Kubes, P., and Mehal, W. Z. (2012). Sterile inflammation in the liver. Gastroenterology 143, 1158–1172. doi:10.1053/j.gastro.2012.09.008
Lan, T., Yu, Y., Zhang, J., Li, H., Weng, Q., Jiang, S., et al. (2021). Cordycepin ameliorates nonalcoholic steatohepatitis by activation of the AMP-activated protein kinase signaling pathway. Hepatology 74, 686–703. doi:10.1002/hep.31749
Lee, G., You, H. J., Bajaj, J. S., Joo, S. K., Yu, J., Park, S., et al. (2020). Distinct signatures of gut microbiome and metabolites associated with significant fibrosis in non-obese NAFLD. Nat. Commun. 11, 4982. doi:10.1038/s41467-020-18754-5
Lee, H. H., Kim, D., Jung, J., Kang, H., and Cho, H. (2021). NLRP3 deficiency in hepatocellular carcinoma enhances surveillance of NK-92 through a modulation of MICA/B. Int. J. Mol. Sci. 22, 9285. doi:10.3390/ijms22179285
Lee, H. M., Kim, J. J., Kim, H. J., Shong, M., Ku, B. J., and Jo, E. K. (2013). Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes 62, 194–204. doi:10.2337/db12-0420
Li, J. Y., Cao, H. Y., Sun, L., Sun, R. F., Wu, C., Bian, Y. Q., et al. (2017). Therapeutic mechanism of Yīn-Chén-Hāo decoction in hepatic diseases. World J. Gastroenterol. 23, 1125–1138. doi:10.3748/wjg.v23.i7.1125
Li, Q., Feng, H., Wang, H., Wang, Y., Mou, W., Xu, G., et al. (2022). Licochalcone B specifically inhibits the NLRP3 inflammasome by disrupting NEK7-NLRP3 interaction. EMBO Rep. 23, e53499. doi:10.15252/embr.202153499
Li, Q., Li, M., Li, F., Zhou, W., Dang, Y., Zhang, L., et al. (2020). Qiang-Gan formula extract improves non-alcoholic steatohepatitis via regulating bile acid metabolism and gut microbiota in mice. J. Ethnopharmacol. 258, 112896. doi:10.1016/j.jep.2020.112896
Li, X. X., Jiang, Z. H., Zhou, B., Chen, C., and Zhang, X. Y. (2019). Hepatoprotective effect of gastrodin against alcohol-induced liver injury in mice. J. Physiol. Biochem. 75, 29–37. doi:10.1007/s13105-018-0647-8
Li, X., Zhang, Y., Jin, Q., Xia, K. L., Jiang, M., Cui, B. W., et al. (2018). Liver kinase B1/AMP-activated protein kinase-mediated regulation by gentiopicroside ameliorates P2X7 receptor-dependent alcoholic hepatosteatosis. Br. J. Pharmacol. 175, 1451–1470. doi:10.1111/bph.14145
Liang, H. W., Yang, T. Y., Teng, C. S., Lee, Y. J., Yu, M. H., Lee, H. J., et al. (2021). Mulberry leaves extract ameliorates alcohol-induced liver damages through reduction of acetaldehyde toxicity and inhibition of apoptosis caused by oxidative stress signals. Int. J. Med. Sci. 18, 53–64. doi:10.7150/ijms.50174
Liang, J. Q., Teoh, N., Xu, L., Pok, S., Li, X., Chu, E. S. H., et al. (2018). Dietary cholesterol promotes steatohepatitis related hepatocellular carcinoma through dysregulated metabolism and calcium signaling. Nat. Commun. 9, 4490. doi:10.1038/s41467-018-06931-6
Liang, Y., Zhang, Y., Deng, Y., Liang, S., He, Y., Chen, Y., et al. (2018). Chaihu-shugan-san decoction modulates intestinal microbe dysbiosis and alleviates chronic metabolic inflammation in NAFLD rats via the NLRP3 inflammasome pathway. Evid. Based. Complement. Altern. Med. 2018, 9390786. doi:10.1155/2018/9390786
Lin, L., Chen, Y., Li, Q., Xu, G., Ding, K., Ren, L., et al. (2022). Isoxanthohumol, a component of Sophora flavescens, promotes the activation of the NLRP3 inflammasome and induces idiosyncratic hepatotoxicity. J. Ethnopharmacol. 285, 114796. doi:10.1016/j.jep.2021.114796
Liu, H., Zhan, X., Xu, G., Wang, Z., Li, R., Wang, Y., et al. (2021). Cryptotanshinone specifically suppresses NLRP3 inflammasome activation and protects against inflammasome-mediated diseases. Pharmacol. Res. 164, 105384. doi:10.1016/j.phrs.2020.105384
Liu, S., Tian, L., Chai, G., Wen, B., and Wang, B. (2018). Targeting heme oxygenase-1 by quercetin ameliorates alcohol-induced acute liver injury via inhibiting NLRP3 inflammasome activation. Food Funct. 9, 4184–4193. doi:10.1039/c8fo00650d
Lu, C., Xu, W., Zhang, F., Shao, J., and Zheng, S. (2016). Nrf2 knockdown disrupts the protective effect of curcumin on alcohol-induced hepatocyte necroptosis. Mol. Pharm. 13, 4043–4053. doi:10.1021/acs.molpharmaceut.6b00562
Luan, J., Zhang, X., Wang, S., Li, Y., Fan, J., Chen, W., et al. (2018). NOD-like receptor protein 3 inflammasome-dependent IL-1β accelerated ConA-induced hepatitis. Front. Immunol. 9, 758. doi:10.3389/fimmu.2018.00758
Luo, H., Wu, H., Wang, L., Xiao, S., Lu, Y., Liu, C., et al. (2021). Hepatoprotective effects of Cassiae Semen on mice with non-alcoholic fatty liver disease based on gut microbiota. Commun. Biol. 4, 1357. doi:10.1038/s42003-021-02883-8
Mai, W., Xu, Y., Xu, J., Zhao, D., Ye, L., Yu, G., et al. (2020). Berberine inhibits nod-like receptor family pyrin domain containing 3 inflammasome activation and pyroptosis in nonalcoholic steatohepatitis via the ROS/TXNIP Axis. Front. Pharmacol. 11, 185. doi:10.3389/fphar.2020.00185
Mandrekar, P., Ambade, A., Lim, A., Szabo, G., and Catalano, D. (2011). An essential role for monocyte chemoattractant protein-1 in alcoholic liver injury: Regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology 54, 2185–2197. doi:10.1002/hep.24599
Mangan, M. S. J., Olhava, E. J., Roush, W. R., Seidel, H. M., Glick, G. D., and Latz, E. (2018). Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 17, 688. doi:10.1038/nrd.2018.149
Masters, S. L., Dunne, A., Subramanian, S. L., Hull, R. L., Tannahill, G. M., Sharp, F. A., et al. (2010). Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat. Immunol. 11, 897–904. doi:10.1038/ni.1935
McClain, C., Cohen, D., Dinarello, C., Cannon, J., Shedlofsky, S., and Kaplan, A. (1986). Serum interleukin-1 (IL-1) activity in alcoholic hepatitis. Life Sci. 39, 1479–1485. doi:10.1016/0024-3205(86)90554-0
Mitsuyoshi, H., Yasui, K., Hara, T., Taketani, H., Ishiba, H., Okajima, A., et al. (2017). Hepatic nucleotide binding oligomerization domain-like receptors pyrin domain-containing 3 inflammasomes are associated with the histologic severity of non-alcoholic fatty liver disease. Hepatol. Res. 47, 1459–1468. doi:10.1111/hepr.12883
Miura, K., Kodama, Y., Inokuchi, S., Schnabl, B., Aoyama, T., Ohnishi, H., et al. (2010). Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology 139, 323–334. doi:10.1053/j.gastro.2010.03.052
Neumann, K., Schiller, B., and Tiegs, G. (2018). NLRP3 inflammasome and IL-33: Novel players in sterile liver inflammation. Int. J. Mol. Sci. 19, E2732. doi:10.3390/ijms19092732
Nie, Y., Liu, Q., Zhang, W., Wan, Y., Huang, C., and Zhu, X. (2021). Ursolic acid reverses liver fibrosis by inhibiting NOX4/NLRP3 inflammasome pathways and bacterial dysbiosis. Gut Microbes 13, 1972746. doi:10.1080/19490976.2021.1972746
Pan, M. X., Zheng, C. Y., Deng, Y. J., Tang, K. R., Nie, H., Xie, J. Q., et al. (2021). Hepatic protective effects of Shenling Baizhu powder, a herbal compound, against inflammatory damage via TLR4/NLRP3 signalling pathway in rats with nonalcoholic fatty liver disease. J. Integr. Med. 19, 428–438. doi:10.1016/j.joim.2021.07.004
Pessione, F., Ramond, M. J., Peters, L., Pham, B. N., Batel, P., Rueff, B., et al. (2003). Five-year survival predictive factors in patients with excessive alcohol intake and cirrhosis. Effect of alcoholic hepatitis, smoking and abstinence. Liver Int. 23, 45–53. doi:10.1034/j.1600-0676.2003.01804.x
Petrasek, J., Bala, S., Csak, T., Lippai, D., Kodys, K., Menashy, V., et al. (2012). IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J. Clin. Investig. 122, 3476–3489. doi:10.1172/JCI60777
Petrasek, J., Dolganiuc, A., Csak, T., Kurt-Jones, E. A., and Szabo, G. (2011). Type I interferons protect from Toll-like receptor 9-associated liver injury and regulate IL-1 receptor antagonist in mice. Gastroenterology 140, 697–708. doi:10.1053/j.gastro.2010.08.020
Petrasek, J., Iracheta-Vellve, A., Saha, B., Satishchandran, A., Kodys, K., Fitzgerald, K. A., et al. (2015). Metabolic danger signals, uric acid and ATP, mediate inflammatory cross-talk between hepatocytes and immune cells in alcoholic liver disease. J. Leukoc. Biol. 98, 249–256. doi:10.1189/jlb.3AB1214-590R
Pimpin, L., Cortez-Pinto, H., Negro, F., Corbould, E., Lazarus, J. V., Webber, L., et al. (2018). Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. J. Hepatol. 69, 718–735. doi:10.1016/j.jhep.2018.05.011
Qiao, J. Y., Li, H. W., Liu, F. G., Li, Y. C., Tian, S., Cao, L. H., et al. (2019). Effects of Portulaca oleracea extract on acute alcoholic liver injury of rats. Molecules 24, E2887. doi:10.3390/molecules24162887
Qin, G., Ma, J., Huang, Q., Yin, H., Han, J., Li, M., et al. (2018). Isoquercetin improves hepatic lipid accumulation by activating AMPK pathway and suppressing TGF-β signaling on an HFD-induced nonalcoholic fatty liver disease rat model. Int. J. Mol. Sci. 19, E4126. doi:10.3390/ijms19124126
Qin, N., Xu, G., Wang, Y., Zhan, X., Gao, Y., Wang, Z., et al. (2021). Bavachin enhances NLRP3 inflammasome activation induced by ATP or nigericin and causes idiosyncratic hepatotoxicity. Front. Med. 15, 594–607. doi:10.1007/s11684-020-0809-2
Ratziu, V., Sheikh, M. Y., Sanyal, A. J., Lim, J. K., Conjeevaram, H., Chalasani, N., et al. (2012). A phase 2, randomized, double-blind, placebo-controlled study of GS-9450 in subjects with nonalcoholic steatohepatitis. Hepatology 55, 419–428. doi:10.1002/hep.24747
Rostamizadeh, P., Asl, S., Far, Z. G., Ahmadijoo, P., Mahmudiono, T., Bokov, D. O., et al. (2022). Effects of licorice root supplementation on liver enzymes, hepatic steatosis, metabolic and oxidative stress parameters in women with nonalcoholic fatty liver disease: A randomized double-blind clinical trial. Phytotherapy Res. doi:10.1002/ptr.7543
Sanyal, A. J., Chalasani, N., Kowdley, K. V., McCullough, A., Diehl, A. M., Bass, N. M., et al. (2010). Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 362, 1675–1685. doi:10.1056/NEJMoa0907929
Shang, Y., Jiang, M., Chen, N., Jiang, X. L., Zhan, Z. Y., Zhang, Z. H., et al. (2022). Inhibition of HMGB1/TLR4 signaling pathway by digitoflavone: A potential therapeutic role in alcohol-associated liver disease. J. Agric. Food Chem. 70, 2968–2983. doi:10.1021/acs.jafc.2c00195
Shen, T., Lei, T., Chen, L., Zhu, B. B., Xu, B. L., Zhang, C. P., et al. (2021). Gardenoside hinders caspase-1-mediated hepatocyte pyroptosis through the CTCF/DPP4 signaling pathway. Front. Physiol. 12, 669202. doi:10.3389/fphys.2021.669202
Shi, H., Zhang, Y., Xing, J., Liu, L., Qiao, F., Li, J., et al. (2020). Baicalin attenuates hepatic injury in non-alcoholic steatohepatitis cell model by suppressing inflammasome-dependent GSDMD-mediated cell pyroptosis. Int. Immunopharmacol. 81, 106195. doi:10.1016/j.intimp.2020.106195
Shi, W., Xu, G., Zhan, X., Gao, Y., Wang, Z., Fu, S., et al. (2020). Carnosol inhibits inflammasome activation by directly targeting HSP90 to treat inflammasome-mediated diseases. Cell. Death Dis. 11, 252. doi:10.1038/s41419-020-2460-x
Silva, I., Rausch, V., Seitz, H. K., and Mueller, S. (2017). Does hypoxia cause carcinogenic iron accumulation in alcoholic liver disease (ALD)? Cancers (Basel) 9, E145. doi:10.3390/cancers9110145
Song, H. M., Li, X., Liu, Y. Y., Lu, W. P., Cui, Z. H., Zhou, L., et al. (2018). Carnosic acid protects mice from high-fat diet-induced NAFLD by regulating MARCKS. Int. J. Mol. Med. 42, 193–207. doi:10.3892/ijmm.2018.3593
Szabo, G., and Csak, T. (2012). Inflammasomes in liver diseases. J. Hepatol. 57, 642–654. doi:10.1016/j.jhep.2012.03.035
Szabo, G., Dolganiuc, A., and Mandrekar, P. (2006). Pattern recognition receptors: A contemporary view on liver diseases. Hepatology 44, 287–298. doi:10.1002/hep.21308
Takeuchi, O., and Akira, S. (2010). Pattern recognition receptors and inflammation. Cell. 140, 805–820. doi:10.1016/j.cell.2010.01.022
Tang, X., Olatunji, O. J., Zhou, Y., and Hou, X. (2017). Allium tuberosum: Antidiabetic and hepatoprotective activities. Food Res. Int. 102, 681–689. doi:10.1016/j.foodres.2017.08.034
The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: A systematic analysis for the global burden of disease study 2017. Lancet. Gastroenterol. Hepatol. 5 (2020) 245–266. doi:10.1016/S2468-1253(19)30349-8
Thursz, M. R., Richardson, P., Allison, M., Austin, A., Bowers, M., Day, C. P., et al. (2015). Prednisolone or pentoxifylline for alcoholic hepatitis. N. Engl. J. Med. 372, 1619–1628. doi:10.1056/NEJMoa1412278
Tilg, H., Moschen, A. R., and Szabo, G. (2016). Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology 64, 955–965. doi:10.1002/hep.28456
Tilg, H., Wilmer, A., Vogel, W., Herold, M., Nölchen, B., Judmaier, G., et al. (1992). Serum levels of cytokines in chronic liver diseases. Gastroenterology 103, 264–274. doi:10.1016/0016-5085(92)91122-k
Tziomalos, K., Athyros, V. G., Paschos, P., and Karagiannis, A. (2015). Nonalcoholic fatty liver disease and statins. Metabolism. 64, 1215–1223. doi:10.1016/j.metabol.2015.07.003
Wang, C. M., Yuan, R. S., Zhuang, W. Y., Sun, J. H., Wu, J. Y., Li, H., et al. (2016). Schisandra polysaccharide inhibits hepatic lipid accumulation by downregulating expression of SREBPs in NAFLD mice. Lipids Health Dis. 15, 195. doi:10.1186/s12944-016-0358-5
Wang, F., Park, J. S., Ma, Y., Ma, H., Lee, Y. J., Lee, G. R., et al. (2021). Ginseng saponin enriched in Rh1 and Rg2 ameliorates nonalcoholic fatty liver disease by inhibiting inflammasome activation. Nutrients 13, 856. doi:10.3390/nu13030856
Wang, F. S., Fan, J. G., Zhang, Z., Gao, B., and Wang, H. Y. (2014). The global burden of liver disease: The major impact of China. Hepatology 60, 2099–2108. doi:10.1002/hep.27406
Wang, H., Wang, G., Liang, Y., Du, X., Boor, P. J., Sun, J., et al. (2019). Redox regulation of hepatic NLRP3 inflammasome activation and immune dysregulation in trichloroethene-mediated autoimmunity. Free Radic. Biol. Med. 143, 223–231. doi:10.1016/j.freeradbiomed.2019.08.014
Wang, L., Jia, Z., Wang, B., and Zhang, B. (2020). Berberine inhibits liver damage in rats with non-alcoholic fatty liver disease by regulating TLR4/MyD88/NF-κB pathway. Turk. J. Gastroenterol. 31, 902–909. doi:10.5152/tjg.2020.19568
Wang, Z., Xu, G., Li, Z., Xiao, X., Tang, J., and Bai, Z. (2022). NLRP3 inflammasome pharmacological inhibitors in Glycyrrhiza for NLRP3-driven diseases treatment: Extinguishing the fire of inflammation. J. Inflamm. Res. 15, 409–422. doi:10.2147/JIR.S344071
Wang, Z., Xu, G., Zhan, X., Liu, Y., Gao, Y., Chen, N., et al. (2019). Carbamazepine promotes specific stimuli-induced NLRP3 inflammasome activation and causes idiosyncratic liver injury in mice. Arch. Toxicol. 93, 3585–3599. doi:10.1007/s00204-019-02606-3
Watanabe, A., Sohail, M. A., Gomes, D. A., Hashmi, A., Nagata, J., Sutterwala, F. S., et al. (2009). Inflammasome-mediated regulation of hepatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G1248–G1257. doi:10.1152/ajpgi.90223.2008
Wei, Q., Mu, K., Li, T., Zhang, Y., Yang, Z., Jia, X., et al. (2014). Deregulation of the NLRP3 inflammasome in hepatic parenchymal cells during liver cancer progression. Lab. Investig. 94, 52–62. doi:10.1038/labinvest.2013.126
Weiskirchen, R., and Tacke, F. (2014). Cellular and molecular functions of hepatic stellate cells in inflammatory responses and liver immunology. Hepatobiliary Surg. Nutr. 3, 344–363. doi:10.3978/j.issn.2304-3881.2014.11.03
Wree, A., McGeough, M. D., Peña, C. A., Schlattjan, M., Li, H., Inzaugarat, M. E., et al. (2014). NLRP3 inflammasome activation is required for fibrosis development in NAFLD. J. Mol. Med. 92, 1069–1082. doi:10.1007/s00109-014-1170-1
Wu, C., Bian, Y., Lu, B., Wang, D., Azami, N. L. B., Wei, G., et al. (2022). Rhubarb free anthraquinones improved mice nonalcoholic fatty liver disease by inhibiting NLRP3 inflammasome. J. Transl. Med. 20, 294. doi:10.1186/s12967-022-03495-4
Wu, J., Zhang, F., Ruan, H., Chang, X., Wang, J., Li, Z., et al. (2021). Integrating network Pharmacology and RT-qPCR analysis to investigate the mechanisms underlying ZeXie decoction-mediated treatment of non-alcoholic fatty liver disease. Front. Pharmacol. 12, 722016. doi:10.3389/fphar.2021.722016
Xiao, J., Liu, Y., Xing, F., Leung, T. M., Liong, E. C., and Tipoe, G. L. (2016). Bee's honey attenuates non-alcoholic steatohepatitis-induced hepatic injury through the regulation of thioredoxin-interacting protein-NLRP3 inflammasome pathway. Eur. J. Nutr. 55, 1465–1477. doi:10.1007/s00394-015-0964-4
Xiao, J., Wang, F., Liong, E. C., So, K. F., and Tipoe, G. L. (2018). Lycium barbarum polysaccharides improve hepatic injury through NFkappa-B and NLRP3/6 pathways in a methionine choline deficient diet steatohepatitis mouse model. Int. J. Biol. Macromol. 120, 1480–1489. doi:10.1016/j.ijbiomac.2018.09.151
Xiao, J., Wang, J., Xing, F., Han, T., Jiao, R., Liong, E. C., et al. (2014). Zeaxanthin dipalmitate therapeutically improves hepatic functions in an alcoholic fatty liver disease model through modulating MAPK pathway. PLoS One 9, e95214. doi:10.1371/journal.pone.0095214
Xiao, J., Zhu, Y., Liu, Y., Tipoe, G. L., Xing, F., and So, K. F. (2014). Lycium barbarum polysaccharide attenuates alcoholic cellular injury through TXNIP-NLRP3 inflammasome pathway. Int. J. Biol. Macromol. 69, 73–78. doi:10.1016/j.ijbiomac.2014.05.034
Xu, G., Fu, S., Zhan, X., Wang, Z., Zhang, P., Shi, W., et al. (2021). Echinatin effectively protects against NLRP3 inflammasome-driven diseases by targeting HSP90. JCI Insight 6, 134601. doi:10.1172/jci.insight.134601
Xu, N., Wu, X., Luo, H. J., Xu, F. F., Huang, Q. H., Wu, J. Z., et al. (2020). Patchouli Oil attenuates high fat diet-induced non-alcoholic hepatic steatosis. Planta Med. 86, 255–266. doi:10.1055/a-1087-7405
Xu, Y., Yang, C., Zhang, S., Li, J., Xiao, Q., and Huang, W. (2018). Ginsenoside Rg1 protects against non-alcoholic fatty liver disease by ameliorating lipid peroxidation, endoplasmic reticulum stress, and inflammasome activation. Biol. Pharm. Bull. 41, 1638–1644. doi:10.1248/bpb.b18-00132
Yan, C., Zhang, Y., Zhang, X., Aa, J., Wang, G., and Xie, Y. (2018). Curcumin regulates endogenous and exogenous metabolism via Nrf2-FXR-LXR pathway in NAFLD mice. Biomed. Pharmacother. 105, 274–281. doi:10.1016/j.biopha.2018.05.135
Yan, T., Wang, H., Cao, L., Wang, Q., Takahashi, S., Yagai, T., et al. (2018). Glycyrrhizin alleviates nonalcoholic steatohepatitis via modulating bile acids and meta-inflammation. Drug Metab. Dispos. 46, 1310–1319. doi:10.1124/dmd.118.082008
Yang, C., He, X., Zhao, J., and Huang, W. (2021). Hepatoprotection by Ginsenoside Rg1 in alcoholic liver disease. Int. Immunopharmacol. 92, 107327. doi:10.1016/j.intimp.2020.107327
Yang, J., Ma, W., Mei, Q., Song, J., Shu, L., Zhang, S., et al. (2020). Protective effect of fuzi Lizhong decoction against non-alcoholic fatty liver disease via anti-inflammatory response through regulating p53 and PPARG signaling. Biol. Pharm. Bull. 43, 1626–1633. doi:10.1248/bpb.b20-00053
Yang, J. M., Sun, Y., Wang, M., Zhang, X. L., Zhang, S. J., Gao, Y. S., et al. (2019). Regulatory effect of a Chinese herbal medicine formula on non-alcoholic fatty liver disease. World J. Gastroenterol. 25, 5105–5119. doi:10.3748/wjg.v25.i34.5105
Yang, M., Li, X., Zeng, X., Ou, Z., Xue, M., Gao, D., et al. (2016). Rheum palmatum L. Attenuates high fat diet-induced hepatosteatosis by activating AMP-activated protein kinase. Am. J. Chin. Med. 44, 551–564. doi:10.1142/S0192415X16500300
Yang, Q. H., Xu, Y. J., Liu, Y. Z., Liang, Y. J., Feng, G. F., Zhang, Y. P., et al. (2014). Effects of chaihu-shugan-san and shen-ling-Bai-Zhu-San on p38 MAPK pathway in kupffer cells of nonalcoholic steatohepatitis. Evid. Based. Complement. Altern. Med. 2014, 671013. doi:10.1155/2014/671013
Yen, I. C., Lin, J. C., Chen, Y., Tu, Q. W., and Lee, S. Y. (2020). Antrodia cinnamomea attenuates non-alcoholic steatohepatitis by suppressing NLRP3 inflammasome activation in vitro and in vivo. Am. J. Chin. Med. 48, 1859–1874. doi:10.1142/S0192415X20500937
Younossi, Z., Anstee, Q. M., Marietti, M., Hardy, T., Henry, L., Eslam, M., et al. (2018). Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 15, 11–20. doi:10.1038/nrgastro.2017.109
Zhang, B., Xu, D., She, L., Wang, Z., Yang, N., Sun, R., et al. (2018). Silybin inhibits NLRP3 inflammasome assembly through the NAD(+)/SIRT2 pathway in mice with nonalcoholic fatty liver disease. Faseb J. 32, 757–767. doi:10.1096/fj.201700602R
Zhang, C. H., Xiao, Q., Sheng, J. Q., Liu, T. T., Cao, Y. Q., Xue, Y. N., et al. (2020). Gegen Qinlian Decoction abates nonalcoholic steatohepatitis associated liver injuries via anti-oxidative stress and anti-inflammatory response involved inhibition of toll-like receptor 4 signaling pathways. Biomed. Pharmacother. 126, 110076. doi:10.1016/j.biopha.2020.110076
Zhang, H. Y., Tian, J. X., Lian, F. M., Li, M., Liu, W. K., Zhen, Z., et al. (2021). Therapeutic mechanisms of traditional Chinese medicine to improve metabolic diseases via the gut microbiota. Biomed. Pharmacother. 133, 110857. doi:10.1016/j.biopha.2020.110857
Zhang, Q., Ma, C., Duan, Y., Heinrich, B., Rosato, U., Diggs, L. P., et al. (2021). Gut microbiome directs hepatocytes to recruit MDSCs and promote cholangiocarcinoma. Cancer Discov. 11, 1248–1267. doi:10.1158/2159-8290.CD-20-0304
Zhu, K., Guo, Y., Zhao, C., Kang, S., Li, J., Wang, J., et al. (2021). Etiology exploration of non-alcoholic fatty liver disease from traditional Chinese medicine constitution perspective: A cross-sectional study. Front. Public Health 9, 635818. doi:10.3389/fpubh.2021.635818
Zhu, R., Ou, Z., Ruan, X., and Gong, J. (2012). Role of liver X receptors in cholesterol efflux and inflammatory signaling (review). Mol. Med. Rep. 5, 895–900. doi:10.3892/mmr.2012.758
Zhu, Z., Hu, R., Li, J., Xing, X., Chen, J., Zhou, Q., et al. (2021). Alpinetin exerts anti-inflammatory, anti-oxidative and anti-angiogenic effects through activating the Nrf2 pathway and inhibiting NLRP3 pathway in carbon tetrachloride-induced liver fibrosis. Int. Immunopharmacol. 96, 107660. doi:10.1016/j.intimp.2021.107660
Keywords: Chinese medicine, NLRP3 inflammasome, inhibitor, NAFLD, ALD, liver fibrosis
Citation: Liu T, Xu G, Liang L, Xiao X, Zhao Y and Bai Z (2022) Pharmacological effects of Chinese medicine modulating NLRP3 inflammasomes in fatty liver treatment. Front. Pharmacol. 13:967594. doi: 10.3389/fphar.2022.967594
Received: 13 June 2022; Accepted: 11 August 2022;
Published: 08 September 2022.
Edited by:
Fu Li, Chengdu Institute of Biology (CAS), ChinaReviewed by:
Chao-Zhan Lin, Guangzhou University of Chinese Medicine, ChinaXu-Dong Zhou, Hunan University of Chinese Medicine, China
Copyright © 2022 Liu, Xu, Liang, Xiao, Zhao and Bai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaofang Bai, YmFpemYyMDA4QGhvdG1haWwuY29t; Guang Xu, Z3VhbmdfeHVAY2NtdS5lZHUuY24=; Yanling Zhao, emhhb3lsMjg1NUAxMjYuY29t
†These authors have contributed equally to this work
 Tingting Liu
Tingting Liu Guang Xu2*†
Guang Xu2*† Longxin Liang
Longxin Liang Xiaohe Xiao
Xiaohe Xiao Yanling Zhao
Yanling Zhao Zhaofang Bai
Zhaofang Bai