
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Pharmacol. , 04 August 2022
Sec. Gastrointestinal and Hepatic Pharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.962671
 Zhiyao Chen1†
Zhiyao Chen1† Kun Jiang1†
Kun Jiang1† Fei Liu2†
Fei Liu2† Ping Zhu1
Ping Zhu1 Fei Cai1
Fei Cai1 Yanqiu He1
Yanqiu He1 Tao Jin1
Tao Jin1 Ziqi Lin1
Ziqi Lin1 Qian Li2
Qian Li2 Cheng Hu1
Cheng Hu1 Qingyuan Tan1
Qingyuan Tan1 Xiaonan Yang1
Xiaonan Yang1 Jia Guo1
Jia Guo1 Wei Huang1*
Wei Huang1* Lihui Deng1*
Lihui Deng1* Qing Xia1*
Qing Xia1*Background: Hydromorphone patient-controlled analgesia (PCA) provides satisfactory postoperative pain therapy, but its effect has not been assessed in acute pancreatitis (AP).
Aim: To assess the safety and efficacy of intravenous hydromorphone PCA for pain relief in AP.
Methods: This open-label trial included AP patients admitted within 72 h of symptom onset, aged 18–70 years old, and with Visual Analog Scale (VAS) for pain intensity ≥5. They were randomized to receive intravenous hydromorphone PCA (0.05 mg/h with 0.2 mg on-demand) or intramuscular pethidine (50 mg as required) for three consecutive days. Intramuscular dezocine (5 mg on demand) was the rescue analgesia. The primary outcome was the change of VAS score recorded every 4 h for 3 days. Interim analysis was conducted by an Independent Data and Safety Monitoring Committee (IDSMC).
Results: From 26 July 2019 to 15 January 2020, 77 patients were eligible for the intention-to-treat analysis in the interim analysis (39 in the hydromorphone group and 38 in the pethidine group). Baseline parameters were comparable between groups. No difference in VAS between the two groups was found. Hydromorphone PCA was associated with higher moderately severe to severe cases (82.1% vs. 55.3%, p = 0.011), acute peripancreatic fluid collections (53.9% vs. 28.9%, p = 0.027), more cumulative opioid consumption (median 46.7 vs. 5 mg, p < 0.001), higher analgesia costs (median 85.5 vs. 0.5 $, p < 0.001) and hospitalization costs (median 3,778 vs. 2,273 $, p = 0.007), and more adverse events (20.5% vs. 2.6%, p = 0.087). The per-protocol analysis did not change the results. Although a sample size of 122 patients was planned, the IDSMC halted further recruitment as disease worsening or worse clinical outcomes between the groups in the interim analysis.
Conclusion: Hydromorphone PCA was not superior to pethidine in relieving pain in AP patients and might have worse clinical outcomes. Therefore, its use is not recommended.
Clinical Trial Registration: Chictr.org.cn. ChiCTR1900025971
Acute pancreatitis (AP) is a critical digestive disease, with severe pain as one of its cardinal symptoms, often necessitating analgesia (Drewes et al., 2020). Pain is included as the fifth vital sign and one of the diagnostic criteria for AP (Banks et al., 2013; Morone and Weiner, 2013). Although no solid evidence shows that the intensity of pain correlates with disease severity (Kapoor et al., 2013), the importance of abdominal pain has been considered in the Pancreatitis Activity Scoring System (PASS) in 2017 (Wu et al., 2017), which was correlated with AP clinical outcomes (Buxbaum et al., 2018; Thiruvengadam et al., 2021). Therefore, analgesia is a clinical priority for AP management.
Parenteral analgesics, such as opioids, nonsteroidal anti-inflammatory drugs (NSAIDs), and other adjuvant analgesics, are suggested for pain relief of AP (Basurto Ona et al., 2013; Moggia et al., 2017). Opioids, strong pain killers used in 93% of AP patients in North America (Matta et al., 2020), have been evaluated in over 70% of AP randomized controlled trials (RCTs) about analgesics for pain relief (Blamey et al., 1984; Ebbehøj et al., 1985; Jakobs et al., 2000; Stevens et al., 2002; Kahl et al., 2004; Peiró et al., 2008; Layer et al., 2011; Sadowski et al., 2015; Gülen et al., 2016; Mahapatra et al., 2019; Huang et al., 2020; Kumar et al., 2020). The first RCT on opioid use, with 32 AP participants, showed no difference between intramuscular buprenorphine and intramuscular pethidine for pain relief (Blamey et al., 1984). Another trial showed that epidural analgesia of a combination of bupivacaine and fentanyl increased arterial perfusion of the pancreas to a higher degree than that in fentanyl in patient-controlled analgesia (PCA) (Sadowski et al., 2015), and was the only study reporting the use of PCA in AP patients. One recent RCT showed that the opioid pentazocine had better efficacy than the NSAID diclofenac in AP (Mahapatra et al., 2019). In contrast, another trial concluded that diclofenac and tramadol were equally effective in AP pain management (Kumar et al., 2020). However, these studies had relatively small sample sizes, included participants with mild acute pancreatitis (MAP), and adopted different diagnostic criteria for AP. In our recent systematic review and meta-analysis, NSAIDs and opioids are equally effective for analgesia in MAP, but the optimal analgesic strategy for moderately severe acute pancreatitis (MSAP) and severe acute pancreatitis (SAP) patients remains unclear (Cai et al., 2021).
The use of opioids should be individualized, following a gradual addition of small doses, and an ordinary intramuscular injection or intravenous infusion cannot achieve this purpose. Patients frequently request the use of painkillers due to unbearable pain, which increases the workload of the medical staff and reduces the efficiency of their work. PCA achieves satisfactory analgesia by allowing patients to control their medication doses. A systematic review showed that opioid PCA provided better pain control in postoperative patients (McNicol et al., 2015). Hydromorphone, a semi-synthetic opioid agonist clinically applied since 1926 (Murray and Hagen, 2005), plays its analgesic role by stimulating the central nervous system μ-opioid receptors and is widely used for acute, chronic and cancerous pains (Quigley and Wiffen, 2003; Bao et al., 2016). A meta-analysis of eight studies showed that hydromorphone had better analgesic effects than morphine (Felden et al., 2011). However, this notion has never been tested in the AP setting. Although the 2019 World Society of Emergency Surgery (WSES) recommended the use of PCA and hydromorphone in AP due to their superiority to morphine (Leppäniemi et al., 2019), most AP guidelines lack a recommendation regarding optimal pain medications and analgesic approaches (Cai et al., 2021).
In this study, we aimed to evaluate the safety and efficacy of intravenous hydromorphone PCA for pain relief in AP. Based on better analgesic results provided by previous studies comparing PCA with conventional intramuscular pethidine (Searle et al., 1994; Wilson et al., 2018), we hypothesized that intravenous hydromorphone PCA would achieve a better effect of pain relief than intramuscular pethidine in AP patients.
We carried out an open-label RCT in the Department of Integrated Traditional Chinese and Western Medicine, West China Hospital of Sichuan University. Consolidated Standards of Reporting Trials (CONSORT) guidelines were used to design this trial (Schulz et al., 2010). Before trial implementation, we obtained approval from the Ethics Committee on Biomedical Research, West China Hospital of Sichuan University (Number 2019511) and completed the clinical trial registration on the Chinese Clinical Trial Registry website (Number ChiCTR1900025971).
All patients were considered eligible if they met the following inclusion criteria: 1) a definite diagnosis of AP by the revised Atlanta classification (Banks et al., 2013); 2) aged from 18 to 70 years; 3) admission within 72 h from abdominal pain onset; and 4) the Visual Analog Scale (VAS) score at admission was greater than or equal to five scores (Jensen et al., 1986). Ineligible patients were excluded if they met the exclusion criteria: 1) known pregnant or lactating at admission; 2) patients with acute onset of chronic pancreatitis, acute traumatic pancreatitis and recurrent acute pancreatitis; 3) patients with severe chronic diseases such as coronary heart disease, chronic obstructive pulmonary disease, liver and kidney dysfunction, anemia, mental illness and malignant tumors; 4) patients suffering from contraindications to hydromorphone or pethidine such as severe pulmonary insufficiency, paralytic ileus, supraventricular tachycardia, traumatic brain injury, and intracranial space-occupying lesions; 5) patients who were allergic to hydromorphone or pethidine; 6) patients unwilling to sign the informed consent form.
Interim analysis was conducted by an Independent Data and Safety Monitoring Committee (IDSMC), which also determined whether the trial should be halted. The termination criteria were as follows: 1) patients who suffered severe adverse events (SAEs) related to the intervention drugs; 2) the planned interim analysis achieved the expected outcome differences; and 3) interim analysis showed disease worsening or worse clinical outcomes between the groups.
The definition of a drop-out case was any patient who was enrolled in the trial but quit the study for any reason. If a patient dropped out, a researcher completed a case report form, outlining their reasons. For patients who dropped out due to adverse events (AEs), researchers closely followed up on their conditions until AEs disappeared. All the drop-out cases could not be replaced and were included in the intention-to-treat analysis.
After the inclusion and exclusion criteria were screened, eligible patients were randomized in a 1:1 ratio to receive intravenous hydromorphone PCA or intramuscular pethidine. An independent researcher generated random numbers using SPSS (Version 21, IBM, Armonk, New York, United States ) before the first subject was recruited. Random numbers were kept in a sealed envelope in the order of their selection. An independent hospital staff member who was available 24 h, 7 days a week by telephone kept the sealed envelopes to ensure the concealment of the allocation sequence. Due to the different patterns of the two drug administrations, the study participants and researchers were not blinded to the study group assignment.
All participants received the same treatment (Tenner et al., 2013; Crockett et al., 2018), including fluid resuscitation, nutrition support, organ function support, antibiotics with indications, and surgical intervention, if necessary.
In the hydromorphone group, 10 mg of hydromorphone (2 ml: 2 mg) was mixed with 0.9% saline to a volume dose of 200 ml. The PCA pump (Rehn MedTech Co. Ltd. Nantong, Jiangsu, China) was programmed by a specialized anesthetist using background infusion at 0.05 mg every hour, a demand dose of 0.2 mg each time. Patients were trained to press the button on the PCA pump when they felt pain. To avoid hydromorphone overdosage, the pump was automatically stopped from transfusing hydromorphone with a lockout period of 10 min, and a 1-h maximum dose of 1.2 mg. A PCA pump was used for three consecutive days in the hydromorphone group. In the pethidine group, patients were given intramuscular pethidine (50 mg) on demand for three consecutive days.
If patients still complained of insufferable pain after PCA or pethidine administration after 3 days, intramuscular dezocine (5 mg) was used as the rescue analgesic.
During hospitalization, the routine use of the following therapeutics was not allowed: 1) acupuncture; 2) ultrasonic analgesic therapy; and 3) other analgesics, such as NSAIDs and opioid analgesics.
The primary outcome was a change in the VAS score, which was recorded every 4 h for 3 days. During the time points corresponding to nighttime, when patients were sleeping, VAS was recorded as SLEEP, and the scores were recorded as zero (Wu et al., 2017). Secondary outcomes included: 1) daily evaluation of clinical scores in the first 3 days after admission, including the Modified Marshall score (Marshall et al., 1995), Sequential Organ Failure Assessment (SOFA) score (Ferreira et al., 2001), Bedside index of severity in acute pancreatitis (BISAP) score (Wu et al., 2008), Acute physiology and chronic health evaluation (APACHE II) (Larvin and McMahon, 1989), and PASS; 2) serum C-reactive protein (CRP), tumor necrosis factor (TNF)-α, procalcitonin (PCT), interleukin (IL)-6, on admission and on the 4th day after admission; 3) organ failure (OF) occurrence; 4) local complications, such as acute peripancreatic fluid collection (APFC) and acute necrotic collection (ANC) as per revised Atlanta criteria, which were evaluated by contrast-enhanced computed tomography (CECT); 5) in-hospital mortality; 6) opioid consumption was calculated based on equivalent morphine doses (Mcpherson, 2018); 7) length of hospital stay; and 8) costs of analgesics and hospitalization.
AEs and SAEs were defined in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events v4.0. Common side effects of the study medication were closely monitored and recorded (Els et al., 2017; Verberkt et al., 2017). The time window for AEs was defined as those occurring within 72 h after PCA or within 2 h after pethidine injection.
In a previous study (Blamey et al., 1984), the linear analog scale of pain after AP patients received intramuscular pethidine was 3.6. In this study, we anticipated that the patient’s VAS score after receiving hydromorphone would be 2. The standard deviation (SD) in the hydromorphone and pethidine groups was 3. Assuming a significance level of 0.05 and a study power of 0.8 with a 10% drop-out rate, 122 patients were required for this trial, with 61 patients in each group. The sample size was calculated by Power Analysis and Sample Size (Version 11.0.7, NCSS).
Statistical analyses were performed using SPSS 21. Continuous data were expressed as medians and interquartile ranges. Categorical data were presented as numbers and percentages. Continuous variables were compared using the independent-sample t test or the Mann–Whitney U test (for non-normal distributions). Categorical variables were compared with the Chi-squared test or Fisher’s exact test (for 2 × 2 tables with cells under 5). Two-sided p-values less than 0.05 indicated statistically significant differences. Baseline characteristics and clinical outcomes were described based on the intention-to-treat population, which included participants who had at least one treatment and one primary outcome measure (n = 77). Per-protocol analysis was performed to test the efficacy of treatment measures, which included participants completing the treatment plan as per the protocol (n = 72). Figures were performed using GraphPad Prism (Version 8, San Diego, California, United States ).
After the interim analysis of the inclusion of 77 participants from 26 July 2019 to 15 January 2020, the IDSMC suggested the termination of participant recruitment, as increased MSAP to SAP and a higher incidence of APFC occurred in the hydromorphone group versus the pethidine group, greatly threatening participant health.
In the initial screening of 744 AP patients, 77 participants were randomized (39 in the hydromorphone group and 38 in the pethidine group) and included in the intention-to-treat population. Five patients (three in the hydromorphone group and two in the pethidine group) dropped out. Among them, two refused to use PCA halfway, one had their PCA pump taken away by mistake within the first 72 h after enrollment, one patient received sedation treatment in the intensive care unit (ICU) and could not control the pump, and one patient abandoned treatment and had to be discharged. The patient selection process is shown in Figure 1.
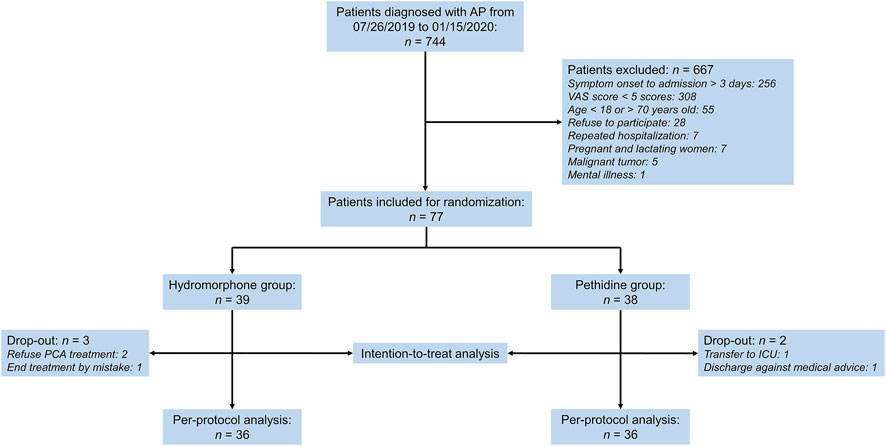
FIGURE 1. Patient selection flowchart. AP, acute pancreatitis, VAS, visual analog scale score, PCA, patient-controlled analgesia, ICU, intensive care unit.
In total of 77 patients, there were 54 males and 23 females, and the mean age was 44.9 years. Hypertriglyceridemia (37.7%) and biliary (31.2%) were the main etiologies, consistent with our previous studies (Zhang et al., 2019; Li et al., 2020; Shi et al., 2020). Of the 29 cases of hypertriglyceridemia-induced AP, only one patient had a history of alcohol abuse in the hydromorphone group. The baseline characteristics of the included patients are provided in Table 1. The gender distribution, age, etiology, intervals from onset to admission, Charlson comorbidity index, and clinical severity scores of participants were similar (p > 0.05). The respiratory rate in the hydromorphone group was higher than that in the pethidine group (22, IQR 20–26 versus 20, IQR 20–22, p = 0.036). There were no significant differences in the serum levels of triglycerides, amylase, lipase, urea and creatinine between the two groups (p > 0.05).
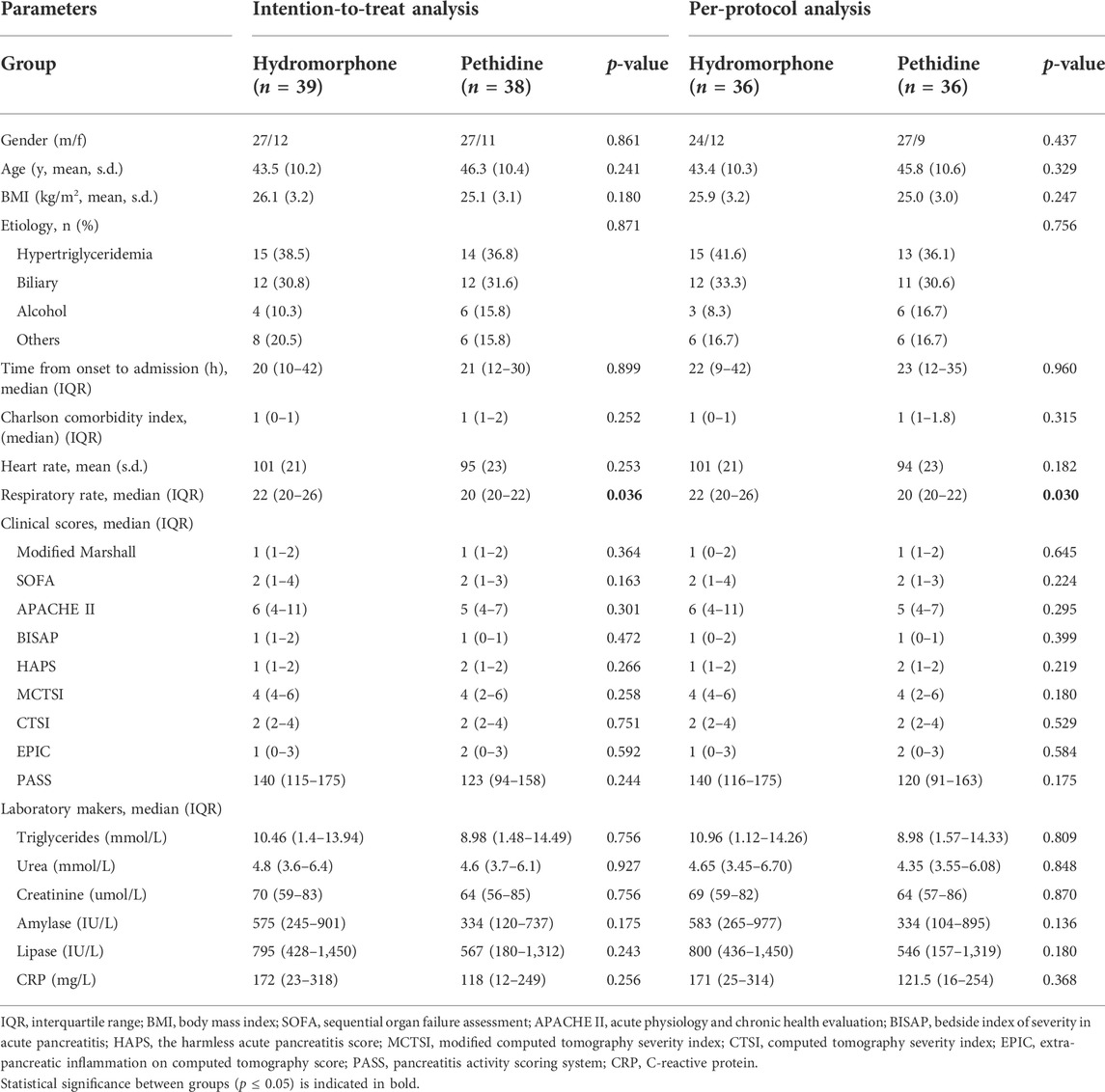
TABLE 1. Baseline characteristics of patients based on intention-to-treat and per-protocol analyses.
On admission, the median VAS scores of both groups were similar (6, IQR 6–7 in the hydromorphone group versus 6, IQR 5–6 in the pethidine group, p = 0.261) (Figure 2). Thereafter, the VAS scores of both groups declined. The lowest VAS score in the hydromorphone group was 0 and was reported during the 36th hour, while that in the pethidine group was 0.5 and was detected during the 64th hour. There were no significant differences at any timepoints between the groups (p > 0.05) (Table 2). Moreover, the cumulative VAS scores for the first 24 h, 24–48 h, and 48–72 h showed no significant differences (p > 0.05) (Supplementary Table S1).
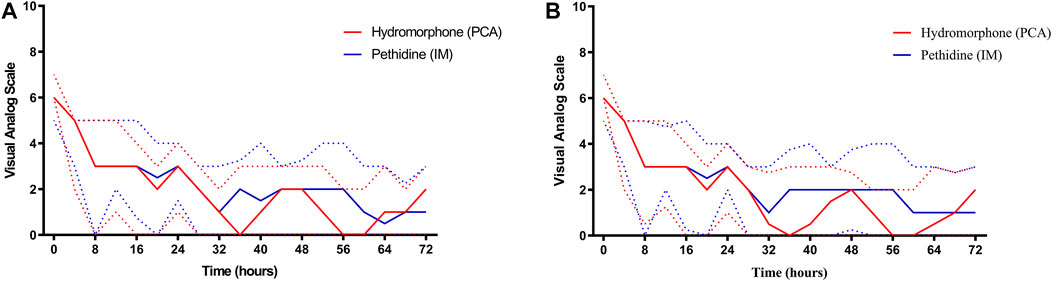
FIGURE 2. Trends of VAS scores in the two groups. Solid red and solid blue lines represent changes in VAS score over 72 h in the hydromorphone and pethidine groups, respectively. Dotted red and dotted blue lines represent the interquartile range of VAS scores in the hydromorphone and pethidine groups, respectively. (A) VAS score based on intention-to-treat analysis. (B) VAS score based on per-protocol analysis. PCA, patient-controlled analgesia, IM, intramuscular, VAS, visual analog scale score.
The incidence of MSAP to SAP (82.1% versus 55.3%, p = 0.011) and APFC (53.9% versus 28.9%, p = 0.027) was higher in the hydromorphone group than those in the pethidine group (Table 3). The two groups had no significant difference in organ failure, persistent organ failure, ICU admission, length of hospitalization, and mortality (p > 0.05).
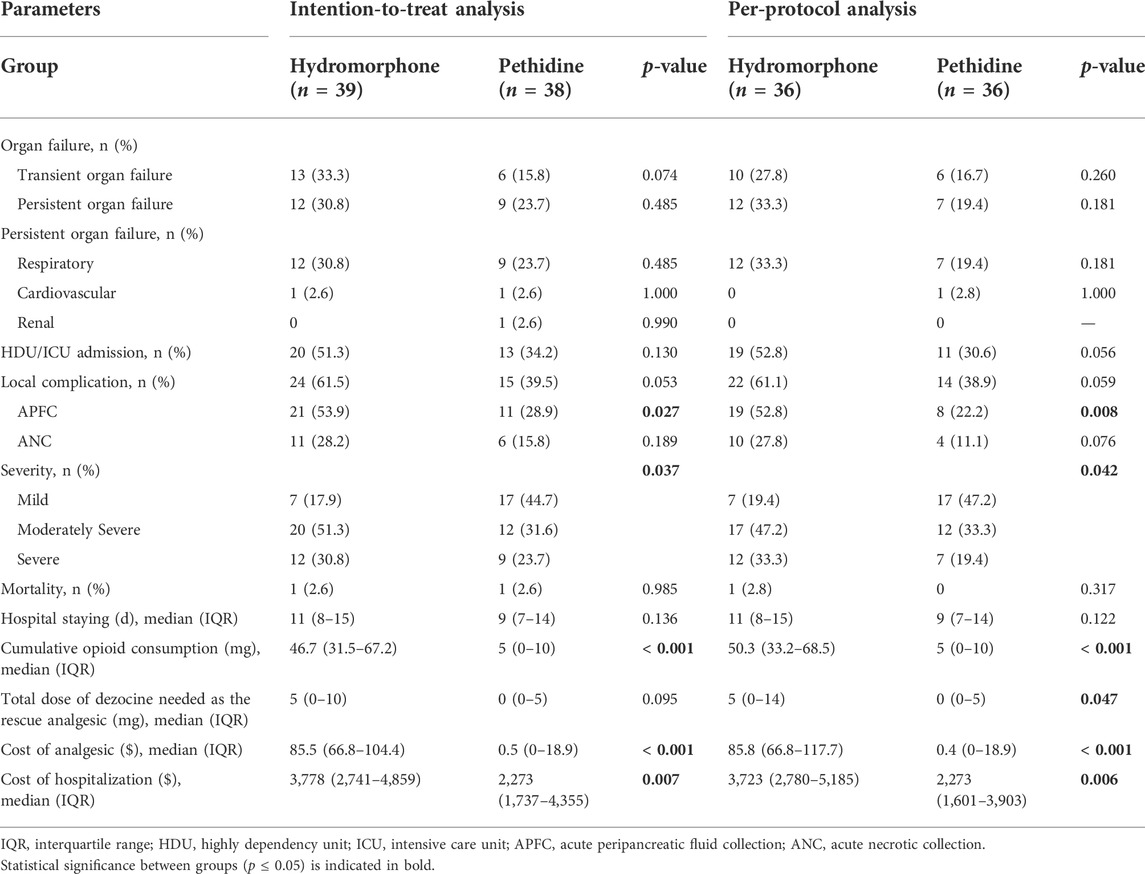
TABLE 3. Secondary outcomes of the two groups based on intention-to-treat and per-protocol analyses.
The cumulative dosage of opioid consumption in the hydromorphone group (46.7 mg, IQR 31.5–67.2 mg) was higher than that in the pethidine group (5 mg, IQR 0–10 mg, p < 0.001) (Supplementary Table S2). The daily opioid consumption in both groups can be found in Supplementary Tables S3, 4. The dosage of dezocine as the rescue analgesia was similar and had no significant difference between the groups (p > 0.05). The cost of analgesics and hospitalization in the hydromorphone group were higher than those in the pethidine group (p < 0.05).
There were no significant differences in the daily Modified Marshall score and APACHE II score between the two groups (p > 0.05). The SOFA score on 48 h after admission in the hydromorphone group was higher than that in the pethidine group (p = 0.011). The BISAP scores on 48 and 72 h after admission in the hydromorphone group were higher than those in the pethidine group (0, IQR 0–1, p < 0.05), based on per-protocol analysis. The PASS scores in the hydromorphone group from day 1 to day 3 were significantly higher than those in the pethidine group (p < 0.05). Daily clinical scores are shown in Supplementary Table S5.
After treatment for three consecutive days, the serum levels of CRP, TNF-α, PCT, IL-6, IL-8 and IL-10 between the groups were not significantly different (p > 0.05) (Supplementary Table S6).
A total of nine patients showed AEs, eight in the hydromorphone group and one in the pethidine group (p = 0.087) (Table 4). Specifically, five patients in the hydromorphone group and one in the pethidine group felt nausea and experienced vomiting during the use of medication. One male patient in the hydromorphone group showed urine retention on the first day after admission and dropped out (Parker et al., 1997; Goodarzi, 1999). After placing the urine catheter and discontinuing hydromorphone, urine retention disappeared. One male patient had a numbness sensation on his face and tongue within 24 h of hydromorphone PCA, which was relieved soon after the administration of intravenous 2 g calcium gluconate. One female suffered from 50 ml of bloody stool in the first hour after hydromorphone PCA, but the bleeding stopped after giving 2 units of hemocoagulase atrox.
To the best of our knowledge, this is the first RCT evaluating the safety and efficacy of intravenous hydromorphone PCA in AP patients. The strengths of this study include the following: 1) our treatment design the novel analgesic technology PCA versus the traditional analgesia in AP; 2) according to our inclusion criteria, 70% of patients were MSAP to SAP, compared to a high proportion of MAP cases in the existing 12 RCTs (Cai et al., 2021); 3) the onset of symptoms of patients in the two groups was limited within 72 h, which maintained homogeneity between the groups; and 4) a VAS score for pain intensity greater than five on admission was one of the inclusion criteria to more accurately evaluate the analgesic effects of the drugs, which was not clearly defined in most of the previous RCTs.
For the primary outcome, we did not find that intravenous hydromorphone PCA was superior to intramuscular pethidine in terms of analgesic effects, which was consistent with some previous studies comparing the two opioid drugs (Blamey et al., 1984; Stevens et al., 2002). In another study, epidural versus PCA opioids only had better analgesic effects on day 10 (Sadowski et al., 2015). Based on this result and the possibility that multiple factors likely interfere with analgesic effects in the late period of AP, we only evaluated analgesic effects based on VAS within the first 72 h in the two groups.
The interim analysis also found that hydromorphone PCA is associated with severity aggravation. Saluja and others (Barlass et al., 2018) have shown that morphine worsens AP severity and delays regeneration. Similar results were obtained in another experiment, whereby hydromorphone could aggravate the severity of AP models (Cheema et al., 2018). The results from basic experiments might explain, at least partly, our trial findings. A cohort study found in 1.14 million patients that the risk of opioid-related AEs increased with increasing opioid dosages (Herzig et al., 2014). However, the mean daily dosage of opioid consumption in our study was lower than 68 mg of oral morphine equivalents in that study. The cost of hydromorphone and PCA pump is more expensive than that of pethidine, indicating that hydromorphone PCA has a relatively poor health-economic value in AP pain management.
Hydromorphone PCA is also associated with a higher incidence of local complications. A recent study using AP models found that fentanyl pre-treatment exacerbated pancreatic necrosis and buprenorphine pre-treatment increased pancreatic edema (Bálint et al., 2022). These results were similar to our findings.
A potentially dangerous opioid-related AE is respiratory depression. However, we did not detect the occurrence of respiratory depression from patient self-reports, which might result from the low medication dose used in this study. However, over 20% of participants reported AEs during hydromorphone administration, which represents a higher incidence than that of the pethidine group. Nausea, vomiting, and urine retention—common side effects previously reported for opioid use (Benyamin et al., 2008) —occurred more frequently in the hydromorphone group. Gastrointestinal bleeding occurred in one patient in the hydromorphone group, and is a common side effect of NSAIDs but had not been reported during hydromorphone use (Lanas, 2012).
Our study has several limitations. First, researcher and participant blinding could not be performed, given the different administration of PCA and intramuscular injection. Bias during clinical observations and data collection by participants and researchers could not be absolutely eliminated, although outcome assessments and data analyses were conducted by an independent researcher blinded to the groups. Second, we used a relatively small sample size (77 participants). However, ours has been one of the largest sample size trials in AP pain management. Third, the control administration was designed as intramuscular pethidine based on the fact that there is no standard AP pain relief strategy and based on a recently published trial (Wilson et al., 2018).
In conclusion, intravenous hydromorphone PCA did not have superior analgesic effects or sufficient safety in comparison with intramuscular pethidine in AP pain management. We do not strongly recommend the application of hydromorphone PCA in the initial period of AP. Future multicenter randomized controlled trials with a large sample size should be conducted to validate the efficacy and safety of hydromorphone in patients with AP.
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Ethics Committee on Biomedical Research, West China Hospital of Sichuan University. The patients/participants provided their written informed consent to participate in this study.
QX, LD, WH, KJ, and FL contributed to the conception and design of the study. ZC, KJ, LD, and WH drafted the manuscript. ZC, KJ, FL, FC, and YH participated in clinical observation and data collection. JG, XY, LD, TJ, and ZL contributed to clinical treatment of the patients. FL and QL managed the analgesic treatment. CH and QT contributed to data qualification. PZ performed the statistical analysis.
This study was supported by the Project of Sichuan Provincial Administration of Traditional Chinese Medicine (2020ZD004) and Health Commision of Sichuan province (19PJ092).
We thank all the members from the pancreas multidisciplinary team in West China Hospital of Sichuan University for their continuous support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.962671/full#supplementary-material
Bálint, E. R., Fűr, G., Kui, B., Balla, Z., Kormányos, E. S., Orján, E. M., et al. (2022). Fentanyl but not morphine or buprenorphine improves the severity of necrotizing acute pancreatitis in rats. Int. J. Mol. Sci. 23 (3), 1192. doi:10.3390/ijms23031192
Banks, P. A., Bollen, T. L., Dervenis, C., Gooszen, H. G., Johnson, C. D., Sarr, M. G., et al. (2013). Classification of acute pancreatitis--2012: Revision of the Atlanta classification and definitions by international consensus. Gut 62 (1), 102–111. doi:10.1136/gutjnl-2012-302779
Bao, Y. J., Hou, W., Kong, X. Y., Yang, L., Xia, J., Hua, B. J., et al. (2016). Hydromorphone for cancer pain. Cochrane Database Syst. Rev. 10 (10), Cd011108. doi:10.1002/14651858.CD011108.pub2
Barlass, U., Dutta, R., Cheema, H., George, J., Sareen, A., Dixit, A., et al. (2018). Morphine worsens the severity and prevents pancreatic regeneration in mouse models of acute pancreatitis. Gut 67 (4), 600–602. doi:10.1136/gutjnl-2017-313717
Basurto Ona, X., Rigau Comas, D., and Urrútia, G. (2013). Opioids for acute pancreatitis pain. Cochrane Database Syst. Rev. 7, Cd009179. doi:10.1002/14651858.CD009179.pub2
Benyamin, R., Trescot, A. M., Datta, S., Buenaventura, R., Adlaka, R., Sehgal, N., et al. (2008). Opioid complications and side effects. Pain Physician 11 (2 Suppl. l), S105–S120. doi:10.36076/ppj.2008/11/s105
Blamey, S. L., Finlay, I. G., Carter, D. C., and Imrie, C. W. (1984). Analgesia in acute pancreatitis: Comparison of buprenorphine and pethidine. Br. Med. J. 288 (6429), 1494–1495. doi:10.1136/bmj.288.6429.1494-a
Buxbaum, J., Quezada, M., Chong, B., Gupta, N., Yu, C. Y., Lane, C., et al. (2018). The pancreatitis activity scoring system predicts clinical outcomes in acute pancreatitis: Findings from a prospective cohort study. Am. J. Gastroenterol. 113 (5), 755–764. doi:10.1038/s41395-018-0048-1
Cai, W., Liu, F., Wen, Y., Han, C., Prasad, M., Xia, Q., et al. (2021). Pain management in acute pancreatitis: A systematic review and meta-analysis of randomised controlled trials. Front. Med. 8, 782151. doi:10.3389/fmed.2021.782151
Cheema, H., Tarique, M., Hooda, U., Kashyap, M., Bava, E. P., Dudeja, V., et al. (2018). Tu1371 - hydromorphone worsens the severity of acute pancreatitis in animal model of the disease. Gastroenterology 154 (6), 946. doi:10.1016/S0016-5085(18)33187-1
Crockett, S. D., Wani, S., Gardner, T. B., Falck-Ytter, Y., and Barkun, A. N. (2018). American gastroenterological association Institute guideline on initial management of acute pancreatitis. Gastroenterology 154 (4), 1096–1101. doi:10.1053/j.gastro.2018.01.032
Drewes, A. M., Olesen, A. E., Farmer, A. D., Szigethy, E., Rebours, V., Olesen, S. S., et al. (2020). Gastrointestinal pain. Nat. Rev. Dis. Prim. 6 (1), 1. doi:10.1038/s41572-019-0135-7
Ebbehøj, N., Friis, J., Svendsen, L. B., Bülow, S., and Madsen, P. (1985). Indomethacin treatment of acute pancreatitis. A controlled double-blind trial. Scand. J. Gastroenterol. 20 (7), 798–800. doi:10.3109/00365528509088825
Els, C., Jackson, T. D., Kunyk, D., Lappi, V. G., Sonnenberg, B., Hagtvedt, R., et al. (2017). Adverse events associated with medium- and long-term use of opioids for chronic non-cancer pain: An overview of cochrane reviews. Cochrane Database Syst. Rev. 10 (10), Cd012509. doi:10.1002/14651858.CD012509.pub2
Felden, L., Walter, C., Harder, S., Treede, R. D., Kayser, H., Drover, D., et al. (2011). Comparative clinical effects of hydromorphone and morphine: A meta-analysis. Br. J. Anaesth. 107 (3), 319–328. doi:10.1093/bja/aer232
Ferreira, F. L., Bota, D. P., Bross, A., Mélot, C., and Vincent, J. L. (2001). Serial evaluation of the SOFA score to predict outcome in critically ill patients. Jama 286 (14), 1754–1758. doi:10.1001/jama.286.14.1754
Goodarzi, M. (1999). Comparison of epidural morphine, hydromorphone and fentanyl for postoperative pain control in children undergoing orthopaedic surgery. Paediatr. Anaesth. 9 (5), 419–422. doi:10.1046/j.1460-9592.1999.00370.x
Gülen, B., Dur, A., Serinken, M., Karcıoğlu, Ö., and Sönmez, E. (2016). Pain treatment in patients with acute pancreatitis: A randomized controlled trial. Turk. J. Gastroenterol. 27 (2), 192–196. doi:10.5152/tjg.2015.150398
Herzig, S. J., Rothberg, M. B., Cheung, M., Ngo, L. H., and Marcantonio, E. R. (2014). Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J. Hosp. Med. 9 (2), 73–81. doi:10.1002/jhm.2102
Huang, Z., Ma, X., Jia, X., Wang, R., Liu, L., Zhang, M., et al. (2020). Prevention of severe acute pancreatitis with cyclooxygenase-2 inhibitors: A randomized controlled clinical trial. Am. J. Gastroenterol. 115 (3), 473–480. doi:10.14309/ajg.0000000000000529
Jakobs, R., Adamek, M. U., von Bubnoff, A. C., and Riemann, J. F. (2000). Buprenorphine or procaine for pain relief in acute pancreatitis. A prospective randomized study. Scand. J. Gastroenterol. 35 (12), 1319–1323. doi:10.1080/003655200453692
Jensen, M. P., Karoly, P., and Braver, S. (1986). The measurement of clinical pain intensity: A comparison of six methods. Pain 27 (1), 117–126. doi:10.1016/0304-3959(86)90228-9
Kahl, S., Zimmermann, S., Pross, M., Schulz, H. U., Schmidt, U., Malfertheiner, P., et al. (2004). Procaine hydrochloride fails to relieve pain in patients with acute pancreatitis. Digestion 69 (1), 5–9. doi:10.1159/000076541
Kapoor, K., Repas, K., Singh, V. K., Conwell, D. L., Mortele, K. J., Wu, B. U., et al. (2013). Does the duration of abdominal pain prior to admission influence the severity of acute pancreatitis? Jop 14 (2), 171–175. doi:10.6092/1590-8577/1283
Kumar, N. S., Muktesh, G., Samra, T., Sarma, P., Samanta, J., Sinha, S. K., et al. (2020). Comparison of efficacy of diclofenac and tramadol in relieving pain in patients of acute pancreatitis: A randomized parallel group double blind active controlled pilot study. Eur. J. Pain 24 (3), 639–648. doi:10.1002/ejp.1515
Lanas, A. (2012). Gastrointestinal bleeding associated with NSAIDs, antiplatelet therapy and anticoagulant agent. Gastroenterol. Hepatol. 35 (Suppl. 1), 35–42. doi:10.1016/s0210-5705(12)70032-8
Larvin, M., and McMahon, M. J. (1989). Apache-II score for assessment and monitoring of acute pancreatitis. Lancet 2 (8656), 201–205. doi:10.1016/s0140-6736(89)90381-4
Layer, P., Bronisch, H. J., Henniges, U. M., Koop, I., Kahl, M., Dignass, A., et al. (2011). Effects of systemic administration of a local anesthetic on pain in acute pancreatitis: A randomized clinical trial. Pancreas 40 (5), 673–679. doi:10.1097/MPA.0b013e318215ad38
Leppäniemi, A., Tolonen, M., Tarasconi, A., Segovia-Lohse, H., Gamberini, E., Kirkpatrick, A. W., et al. (2019). 2019 WSES guidelines for the management of severe acute pancreatitis. World J. Emerg. Surg. 14, 27. doi:10.1186/s13017-019-0247-0
Li, L., Jin, T., Wen, S., Shi, N., Zhang, R., Zhu, P., et al. (2020). Early rapid fluid therapy is associated with increased rate of noninvasive positive-pressure ventilation in hemoconcentrated patients with severe acute pancreatitis. Dig. Dis. Sci. 65 (9), 2700–2711. doi:10.1007/s10620-019-05985-w
Mahapatra, S. J., Jain, S., Bopanna, S., Gupta, S., Singh, P., Trikha, A., et al. (2019). Pentazocine, a kappa-opioid agonist, is better than diclofenac for analgesia in acute pancreatitis: A randomized controlled trial. Am. J. Gastroenterol. 114 (5), 813–821. doi:10.14309/ajg.0000000000000224
Marshall, J. C., Cook, D. J., Christou, N. V., Bernard, G. R., Sprung, C. L., Sibbald, W. J., et al. (1995). Multiple organ dysfunction score: A reliable descriptor of a complex clinical outcome. Crit. Care Med. 23 (10), 1638–1652. doi:10.1097/00003246-199510000-00007
Matta, B., Gougol, A., Gao, X., Reddy, N., Talukdar, R., Kochhar, R., et al. (2020). Worldwide variations in demographics, management, and outcomes of acute pancreatitis. Clin. Gastroenterol. Hepatol. 18 (7), 1567–1575. e1562. doi:10.1016/j.cgh.2019.11.017
McNicol, E. D., Ferguson, M. C., and Hudcova, J. (2015). Patient controlled opioid analgesia versus non-patient controlled opioid analgesia for postoperative pain. Cochrane Database Syst. Rev. 2015 (6), Cd003348. doi:10.1002/14651858.CD003348.pub3
Mcpherson, M. (2018). Demystifying opioid conversion calculations: A guide for effective dosing. Maryland: American Society of Health-Systems Pharmacists.
Moggia, E., Koti, R., Belgaumkar, A. P., Fazio, F., Pereira, S. P., Davidson, B. R., et al. (2017). Pharmacological interventions for acute pancreatitis. Cochrane Database Syst. Rev. 4 (4), Cd011384. doi:10.1002/14651858.CD011384.pub2
Morone, N. E., and Weiner, D. K. (2013). Pain as the fifth vital sign: Exposing the vital need for pain education. Clin. Ther. 35 (11), 1728–1732. doi:10.1016/j.clinthera.2013.10.001
Murray, A., and Hagen, N. A. (2005). Hydromorphone. J. Pain Symptom Manage. 29 (5 Suppl. l), S57–S66. doi:10.1016/j.jpainsymman.2005.01.007
Parker, R. K., Holtmann, B., and White, P. F. (1997). Patient-controlled epidural analgesia: Interactions between nalbuphine and hydromorphone. Anesth. Analg. 84 (4), 757–763. doi:10.1097/00000539-199704000-00011
Peiró, A. M., Martínez, J., Martínez, E., de Madaria, E., Llorens, P., Horga, J. F., et al. (2008). Efficacy and tolerance of metamizole versus morphine for acute pancreatitis pain. Pancreatology 8 (1), 25–29. doi:10.1159/000114852
Quigley, C., and Wiffen, P. (2003). A systematic review of hydromorphone in acute and chronic pain. J. Pain Symptom Manage. 25 (2), 169–178. doi:10.1016/s0885-3924(02)00643-7
Sadowski, S. M., Andres, A., Morel, P., Schiffer, E., Frossard, J. L., Platon, A., et al. (2015). Epidural anesthesia improves pancreatic perfusion and decreases the severity of acute pancreatitis. World J. Gastroenterol. 21 (43), 12448–12456. doi:10.3748/wjg.v21.i43.12448
Schulz, K. F., Altman, D. G., and Moher, D. (2010). CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. Bmj 340, c332. doi:10.1136/bmj.c332
Searle, N. R., Roy, M., Bergeron, G., Perrault, J., Roof, J., Heermans, C., et al. (1994). Hydromorphone patient-controlled analgesia (PCA) after coronary artery bypass surgery. Can. J. Anaesth. 41 (3), 198–205. doi:10.1007/bf03009831
Shi, N., Liu, T., de la Iglesia-Garcia, D., Deng, L., Jin, T., Lan, L., et al. (2020). Duration of organ failure impacts mortality in acute pancreatitis. Gut 69 (3), 604–605. doi:10.1136/gutjnl-2019-318241
Stevens, M., Esler, R., and Asher, G. (2002). Transdermal fentanyl for the management of acute pancreatitis pain. Appl. Nurs. Res. 15 (2), 102–110. doi:10.1053/apnr.2002.29532
Tenner, S., Baillie, J., DeWitt, J., and Vege, S. S. (2013). American college of gastroenterology guideline: Management of acute pancreatitis. Am. J. Gastroenterol. 108 (9), 1400–1415. doi:10.1038/ajg.2013.218
Thiruvengadam, N. R., Miranda, J., Kim, C., Behr, S., and Arain, M. A. (2021). The pancreatitis activity scoring system predicts clinical outcomes in patients with infected pancreatic necrosis. Pancreas 50 (6), 859–866. doi:10.1097/mpa.0000000000001838
Verberkt, C. A., van den Beuken-van Everdingen, M. H. J., Schols, J., Datla, S., Dirksen, C. D., Johnson, M. J., et al. (2017). Respiratory adverse effects of opioids for breathlessness: A systematic review and meta-analysis. Eur. Respir. J. 50 (5), 1701153. doi:10.1183/13993003.01153-2017
Wilson, M. J. A., MacArthur, C., Hewitt, C. A., Handley, K., Gao, F., Beeson, L., et al. (2018). Intravenous remifentanil patient-controlled analgesia versus intramuscular pethidine for pain relief in labour (RESPITE): An open-label, multicentre, randomised controlled trial. Lancet 392 (10148), 662–672. doi:10.1016/s0140-6736(18)31613-1
Wu, B. U., Batech, M., Quezada, M., Lew, D., Fujikawa, K., Kung, J., et al. (2017). Dynamic measurement of disease activity in acute pancreatitis: The pancreatitis activity scoring system. Am. J. Gastroenterol. 112 (7), 1144–1152. doi:10.1038/ajg.2017.114
Wu, B. U., Johannes, R. S., Sun, X., Tabak, Y., Conwell, D. L., Banks, P. A., et al. (2008). The early prediction of mortality in acute pancreatitis: A large population-based study. Gut 57 (12), 1698–1703. doi:10.1136/gut.2008.152702
Keywords: acute pancreatitis, hydromorphone, pethidine, patient-controlled analgesia, randomized controlled trial
Citation: Chen Z, Jiang K, Liu F, Zhu P, Cai F, He Y, Jin T, Lin Z, Li Q, Hu C, Tan Q, Yang X, Guo J, Huang W, Deng L and Xia Q (2022) Safety and efficacy of intravenous hydromorphone patient-controlled analgesia versus intramuscular pethidine in acute pancreatitis: An open-label, randomized controlled trial. Front. Pharmacol. 13:962671. doi: 10.3389/fphar.2022.962671
Received: 06 June 2022; Accepted: 04 July 2022;
Published: 04 August 2022.
Edited by:
Raffaele Capasso, University of Naples Federico II, ItalyReviewed by:
Ravi Kumar Sharma, Chandigarh University, IndiaCopyright © 2022 Chen, Jiang, Liu, Zhu, Cai, He, Jin, Lin, Li, Hu, Tan, Yang, Guo, Huang, Deng and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Huang, d2VpLmh1YW5nLnNjdUB2aXAuMTYzLmNvbQ==; Lihui Deng, ZGVuZ2xpaHVpQHNjdS5lZHUuY24=; Qing Xia, eGlhcWluZ0BtZWRtYWlsLmNvbS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.