
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 08 September 2022
Sec. Renal Pharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.961590
This article is part of the Research Topic Methods and Application in Renal Pharmacology: 2022 View all 4 articles
Background: Hemodialysis (HD) patients are at risk of malnutrition, cardiovascular complications, and all-cause mortality due to oxidative stress and inflammation. Some studies have demonstrated that rutin attenuates oxidative stress and inflammation in CKD rats, but its effects in HD patients are unknown to date.
Aim: The aim of this study was to evaluate the effect of rutin and vitamin C versus vitamin C alone on oxidative stress and inflammation in HD patients.
Methods: A prospective randomized, open-label, controlled trial enrolled on hundred and five HD patients divided into three groups as follows: patients in group 1 were given a rutin/vitamin C combination (Ruta C group as the combination trade name is known as Ruta C 60 tablets), patients in group 2 were given vitamin C (1 g) (vitamin C group), and group 3 was the control group; the study period was 16 weeks. The following were assessed at baseline and at the end of the study: serum malondialdehyde (MDA), glutathione peroxidase (GPx), high-sensitivity C-reactive protein (hs-CRP), tumor necrosis factor-α (TNF-α), lipid profile levels, and erythrocyte sedimentation rate.
Results: It was found that vitamin C significantly increased serum GPx in group 2 (p = 0.001) compared to a non-significant result in both group 1 and 3; in addition, serum MDA and TNF-α values had decreased significantly in the three groups compared to their baselines; however, a non-significant difference was seen among the studied groups at the end of the study. On the other hand, MDA levels were reduced by 50% in interventional groups compared to 28% in the control group, while the Ruta C group showed an 80% reduction in the level of TNF α compared to the 78% reduction observed in the vitamin C group, and finally, the interventional drugs showed a significant improvement in the lipid profile.
Conclusion: Vitamin C supplementation alone for 16 weeks had a potential effect on the antioxidant’s GPx activity. Moreover, it was reported that both vitamin C alone or the rutin/vitamin C combination (Ruta C) showed a protective role against lipid peroxidation, evidenced by the reduced levels of MDA. Finally, rutin had a favorable synergistic effect with vitamin C in reducing TG and TNF-α levels and increasing HDL-C level.
Chronic kidney disease (CKD) is a widespread universal problem (Levey et al., 2007). Several biological/clinical functions are affected with the progressive deterioration of the kidney’s function, such as the increase in the production of inflammatory/oxidative stress mediators (Russa et al., 2019). The level of pro-oxidants is increased in CKD because the kidney is an important source of antioxidant enzymes, including glutathione peroxidase (Kelly and Rothwell, 2020). Therefore, dialysis or transplantation as kidney replacement treatments can improve both the outcome and the prognosis of end-stage renal disease (Eckardt et al., 2018).
The oxidative stress (OS) is a global challenge in life which might be exaggerated as a result of the hemodialysis (HD) procedure, where the related factors responsible for this are as follows: first, activation of leukocytes by the dialysis membrane and dialysate which in turn induces inflammation and triggers reactive oxygen species production and accumulation of oxidative products; second, the filters of HD trap the low- or the very-low-molecular-weight antioxidants; and third, dietary restrictions imposed on HD patients which aggravate the deficiency of exogenous antioxidants which in turn decreases their antioxidant function, and OS is highly related with chronic inflammation (Liakopoulos et al., 2017).
On the other hand, dietary restrictions on vegetables and fruits in hemodialysis (HD) patients may potentiate the depletion of the antioxidant defense system resembled in decreased levels of vitamin E and vitamin C, in addition to reduced levels of selenium, and reduced function of glutathione (Canaud et al., 1999; Ross, 1999). In addition, several factors induce pro-oxidant activity in HD patients, including chronic inflammation, uremic status, hypertension, obesity, dyslipidemia, diabetes, advanced age, enhanced vascular calcification, and other dialysis-related factors (Locatelli et al., 2003).
It is worthy to mention that reactive oxygen species (ROS) can be inactivated by enzyme systems and scavenged by antioxidants, on the contrary, when ROS are produced in excessive amounts, they cannot be neutralized causing cellular structure’s alterations as DNA damage, oxidation of lipids and proteins, impairment of cellular activity, and retarded enzymatic activity (Rapa et al., 2019).
It has been demonstrated that significantly enhanced oxidative stress and damage in peripheral and mononuclear leukocytes and increased plasma levels of xanthine oxidase, oxidized glutathione (GSSG), and malondialdehyde; decreased superoxide dismutase, catalase, glutathione peroxidase, and glutathione (GSH); and altered GSSG/GSH balance were found in non-dialysis-dependent-CKD, and hemodialysis patients compared to healthy controls (Vida et al., 2021).
Regarding chronic inflammation, it is considered a comorbid factor in CKD, and especially patients on hemodialysis (HD) (Akchurin and Kaskel, 2015), evidenced by the presence of cardiovascular disease in greater than 50% of patients undergoing dialysis, which is the major cause of their death (Cozzolino et al., 2018). In addition, increased inflammation and lipid peroxidation are characteristics of HD patients (Bayés et al., 2001). Handelman et al. (2001) reported significantly higher levels of F2-isoprostanes in HD patients compared to controls with normal kidney function. Also, a strong association between F2-isoprostanes and C-reactive protein levels has been found in the HD group, suggesting a tight relationship between inflammation and oxidative stress in patients with HD.
Due to the reported deficiency of vitamin C in hemodialysis patients (HD), thus, an improvement in their health may be achieved with greater intake of this vitamin (Raimann et al., 2013), which competitively reacts with reactive oxygen species scavenging and in turn is oxidized, resulting in protection of the structure of lipid membranes, proteins, and DNA from being damaged (Berretta et al., 2020). Moreover, plasma and intracellular vitamin C, being a potent antioxidant, are readily oxidized to dehydroascorbic acid, unfortunately, plasma levels of vitamin C are low in HD, while, urinary vitamin C loss is increased due to the administration of diuretics by dialysis population, where about 200 mg of vitamin C is lost in the dialysate/week (Deicher and Hörl, 2003).
Morena et al. (2002) recorded the loss of vitamin C during a hemodialysis session, leading to a deficiency of vitamin C, which in turn was associated with a high level of malondialdhyde (MDA) and reduced activity of glutathione peroxidase. Besides, it was reported that oral supplementation of vitamin C decreased oxidative stress by decreasing MDA and lipoperoxide levels while causing an increase in the antioxidant capacity of hemodialysis and CKD stage 3 and 4 patients (Ghiadoni et al., 2004). The previous studies were diverse in terms of population, doses used, follow-up length, and the markers assessed (Liakopoulos et al., 2019), thus, targeting chronic oxidative stress and inflammation is a necessary proposal to test the influence of vitamin C and put an end to their complications in HD patients (Chaghouri et al., 2021).
Concerning rutin (vitamin P) which belongs to the flavonoid group and is present in tea leaves, apples, and many more contain rutin as active component and these days has been investigated for its nutraceutical action (Ganeshpurkar and Saluja, 2017). Rutin has both anti-oxidative and anti-inflammatory effects that make it extensively utilized in pharmacological approaches (Perk et al., 2014; Al-Dhabi et al., 2015), not only that, but it also has anticancer (Webster et al., 1996), anti-diabetic (Ahmed et al., 2010), and antimicrobial (Panasiak et al., 1989) actions. It has been confirmed that rutin attenuates schizophrenia-like behavior induced by ketamine in mice through reducing nicotinamide adenine dinucleotide phosphate oxidase-2 (Nox-2) expression, oxidative/nitrergic stresses, acetylcholinesterase activity, and preventing the decrease of glutamic-acid decarboxylase-67 (GAD67) expression (Oshodi et al., 2021).
Interestingly, there is growing evidence that rutin has the ability to influence the cellular metabolism by affecting the inflammatory process, where, there is preclinical evidence that rutin can attenuate the inflammation by decreasing the pro-inflammatory markers levels via its strong antioxidant activity such as TNF-α, interleukin-1 (IL-1β), interleukin (IL)-6, and cyclooxygenase-2 (Muvhulawa et al., 2022). Quercetin (a metabolite of Rutin) displays a broad spectrum of antiviral activities. A recent study suggested the administration of quercetin and vitamin C combination for the prophylaxis and early treatment of infections of the respiratory tract, particularly coronavirus patients (Colunga Biancatelli et al., 2020).
The combination of rutin and vitamin C is often used in oral dosage form for the treatment of colds and flu. In addition, the use of this formulation is recommended for vascular disorders as rutin decreases the permeability of capillaries, increases their resistance, and prevents edema (Gabor, 1972).
Up to the present, the interaction of orally supplemented rutin and vitamin C has been studied and investigated with regard to their anti-inflammatory effect and vascular sealing action (Milde et al., 2004; Al-Rejaie et al., 2012), also, it has been reported that rutin enhances the reducing power of vitamin C (Afanas’ev et al., 1989). Moreover, on undergoing an in vitro investigation by combining rutin with vitamin C, a synergistic effect and cytoprotective ability from UVA and UVB radiation were reported, which was interpreted to occur through the inhibition of inflammatory processes approved by deceased Nuclear Factor Kappa B (NFκB) expression (Gęgotek et al., 2019).
Despite the antioxidant and anti-inflammatory effects of each component (vitamin C and rutin) individually, up to date, there has been no prospective clinical trial performed to evaluate the antioxidant and anti-inflammatory effects of the supplementation of hemodialysis (HD) patients with the vitamin C/rutin combination. This randomized clinical trial aimed to evaluate the combined effect of rutin and vitamin C supplementation compared to control and vitamin C supplementation alone on the oxidative stress and inflammatory markers in HD patients.
The current study design was a prospective, interventional, open-label, randomized controlled clinical trial conducted on adult patients with end-stage renal disease on regular hemodialysis at the hemodialysis unit in Ain Shams University hospital, Cairo, Egypt, from the start of August (2021) to the end of November (2021).
All patients were assessed for eligibility criteria and were included in the study only when they had met the following inclusion criteria: age greater than 18 years old, hemodialysis in the last 3 months or longer, and hemodialysis frequency of three times per week or more. On the other hand, the exclusion criteria included treatment with antioxidant agents such as vitamin C and E during the two preceding months prior to the study, active liver disease, and pregnant patients or patients planning pregnancy.
A total of 156 patients were assessed for eligibility; 51 patients were excluded (49 patients had not met the inclusion criteria, and two patients refused to participate in the study). One hundred and five patients were randomly allocated and distributed to the three studied groups as follows; 35 patients in the RUTA C group (group1), 35 patients to the vitamin C group (group2), and 35 patients to the control group (group3). Eleven patients all-over the three groups were withdrawn and could not complete the study due to different reasons as described in Figure 1.
Eligible hemodialysis patients were randomized into three groups. The first group (group1) included 35 patients who received a dose of two tablets of rutin 60 mg in combination with vitamin C 160 mg manufactured by KAHIRA PHARM. and CHEM.IND.CO.CAIRO-EGYPT under the trade name of RUTA C 60®, three times daily for a period of 4 months in addition to their routine therapy.
The second group (group2) was constituted of 35 patients who received a dose of one capsule of vitamin C 500 mg manufactured by Hikma pharma S.A.E-Egypt under the trade name of C-Retard®, two times daily for a period of 4 months in addition to their routine therapy.
The dose of vitamin C in our study was selected based on the Ramos et al. study in which a dose of 1 g/day of vitamin C was supplemented to HD patients (Ramos and Martínez-Castelao, 2008).
Finally, the control group (group 3) was constituted of 35 patients who received their routine therapy.
The routine therapy was calcium-based phosphate binder (calcium acetate 700 mg = calcium 180 mg), calcimimetic agent (cinacalcet 30 mg), epoetin alpha injection, alfacalcidol (0.25 µg each other day), low dose aspirin (75 mg/QD), and antihypertensive medications: B-Blockers, Angiotensin converting enzyme inhibitor (captopril), calcium channel blockers (amlodipine), diuretics (hydrochlorothiazide), and methyldopa.
Relevant information for all groups, such as the age, sex, weight, cause of end-stage renal disease, duration of end-stage renal disease and HD comorbidities were collected, routine biochemical data corresponding to collection date, such as hemoglobin, white blood cells, platelet counts, serum phosphorus, potassium, calcium, urea, and albumin were also obtained from routine medical records, body mass index (BMI), lipid profile including; total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C), and liver enzymes; alanine transaminase (ALT), and aspartate transaminase (AST) were determined.
In the current study, both oxidative stress and inflammation were determined through evaluation of levels of malondialdehyde (MDA), glutathione peroxidase (GPx), high sensitivity C-reactive protein (hs-CRP), tumor necrosis factor-alpha (TNF-α), and erythrocyte sedimentation rate (ESR), for all groups before and after intervention.
Follow-up of patients throughout the 16 weeks of the study was performed, where, patients were interviewed during their regular visits at the dialysis unit, which was three times weekly, and were educated regarding: medication side effects and medication adherence, the patients were asked to return the empty strips of the interventional medications to assess their adherence, and were asked about the incidence of any side and/or adverse effects to assess tolerability.
Blood samples were withdrawn and collected in Vacutainer® SST™ tubes (serum separator tubes) at baseline and at the end of the study from all groups just before the start of the hemodialysis session. Then, it was centrifuged at 3,000 rpm, for 10 min at 4°C, to separate sera and stored at -80°C until analysis for biochemical evaluation of MDA, GPx, hs-CRP, and TNF-alpha, lipid profile (TC, TG, HDL-c, and LDL-c), ALT, and AST. Besides, blood samples were also withdrawn at baseline and at the end of the study from all groups just before the start of the hemodialysis session for the measurement of erythrocyte sedimentation rate (ESR).
Study outcomes included the improvement of the following oxidative stress and inflammatory markers: MDA, GPx, hs-CRP, TNF-α, and ESR. The Tumor Necrosis Factor-alpha (TNF-α), MDA, and GPx were determined by enzyme-linked immunosorbent assay (ELISA) by using the human tumor necrosis factor-alpha ELISA Kit, Cat. No E0082Hu, MDA by using the human malondialdehyde ELISA KIT, Cat. No E1371Hu., and GPx by using the human glutathione peroxidase ELISA KIT, Cat. No. E3696Hu. All ELISA kits are manufactured by Bioassay Technology Laboratory BT LAB, China.
Concerning the high sensitivity C-reactive protein (hs-CRP), it was measured using an Elisa kit manufactured by Monocent, Inc. USA (Yudkin et al., 1999). All ELISA procedures were performed according to the manufacturer’s instructions, using a Thermo Scientific Multiskan FC Microplate Reader by Thermo Fisher Scientific (Skanlt Software 4.1).
Regarding the lipid profile, including: TC, LDL-C, HDL-C, and TG were determined using enzymatic-colorimetric tests manufactured by Human Gesellschaft fur Biochemica und Diagnostica mbH-Germany. All methods were performed according to the instructions of the manufacturer. The total cholesterol was assayed using an enzymatic-colorimetric method (CHOD-PAP-Method) (Schettler and Nussei, 1975). The high-density lipid (HDL) cholesterol was assayed using precipitant and standard, for use with the human cholesterol liquicolor Test Kit (Gordon et al., 1977). Triglyceride (TG) was measured using an enzymatic-colorimetric method (GPO-PAP-method) (Schettler and Nussei, 1975), while the concentration of the low-density lipid (LDL) cholesterol was calculated from the total cholesterol concentration and concentrations of both HDL-C and TG were determined according to Friedewald et al., (Friedewald et al., 1972).
Finally, Alanine transaminase (ALT) and aspartate transaminase (AST) were determined by using the International Federation of Clinical Chemistry (IFCC) kinetic methods (Bergmeyer et al., 1976; Bergmeyer, 1980), the liquiUV Test kits used for ALT and AST assays are manufactured by Human Gesellschaft fur Biochemica und Diagnostica mbH-Germany.
The study was conducted in accordance with good clinical practice guidelines and the ethical principles in the Declaration of Helsinki (as revised in 2013). This clinical trial followed the CONSORT guidelines and ICMJE recommendations. The protocol was approved by the ethics committee of faculty of the pharmacy, Ain Shams University, Cairo, Egypt, which is registered at the Egyptain Ministry of Health (MOH) under registration number (26), moreover, the study has been registered on ClinicalTrials.gov: NCT04955145. The participants were informed about the study protocol and a written informed consent was obtained from all the patients prior to their participation in the study without any obligation to withdraw from the study if they wanted to.
The sample size was calculated by GPower v.3.1.9.4 (Erdfelder et al., 1996). According to Connelly (2008), the literature suggests that a pilot study sample should be 10% of the sample projected for the larger parent study (Connelly, 2008). Therefore, for a full-scale study, expecting a small effect size of 0.10, to reach a power of 80% to reject the null hypothesis, with three equally sized groups and an alpha of 0.05, the sample size for our pilot study would be expected to be 96.6 (approximated to 97) participants. To account for dropouts and equality of group sizes, we decided to recruit a total of 105 participants, equally divided among the three groups (35 participants in each group).
The statistical analysis was performed in R software (version 4.1.1). Two-sided p-values of less than 0.05 were considered to represent a statistically significant result. Shapiro-Wilk’s test was performed to test the normality of data across all treatment groups, and non-parametric tests were chosen given the violation of the normality assumption in at least one of the comparator groups in most instances. Electrolyte levels (Ca, K, and PO4), albumin levels, total leucocytic count, hemoglobin, platelet count, pre and post-dialysis urea, inflammatory and oxidative stress markers (MDA, GPx, hs-CRP, TNF-α, and ESR), cholesterol (LDL, HDL, TC, and TG), and hepatic transaminases were compared using the Kruskal–Wallis test before and after treatment across groups and were compared before and after treatment within each group using Wilcoxon’s signed-rank test.
A total of 94 patients on maintenance HD completed the study and were analyzed. Eleven patients were lost to follow-up for the reasons described in Figure 1.
At baseline, demographic and clinical characteristics were assessed, and there were no significant differences between the three study groups (Table 1) Statistical analysis of the baseline laboratory measurements presented in Table 2 showed a non-significant difference between the three studied groups, except for platelet count and hs C-reactive protein, with a p-value = 0.05 for both of them.
The data represented in Table 4 showed that at the end of the study, evaluation of the antioxidant effect through measurement of GPx showed a significant increase in the vitamin C group (p = 0.001) compared to its baseline results. However, a non-significant difference was reported in the other groups when compared to the baseline values (Table 3). In addition, no significant differences were found among the three groups. Concerning the serum level of MDA, a significant decrease in all groups compared to the baseline values, with a non-significant difference among the three groups at the end of the study was reported (Table 4).
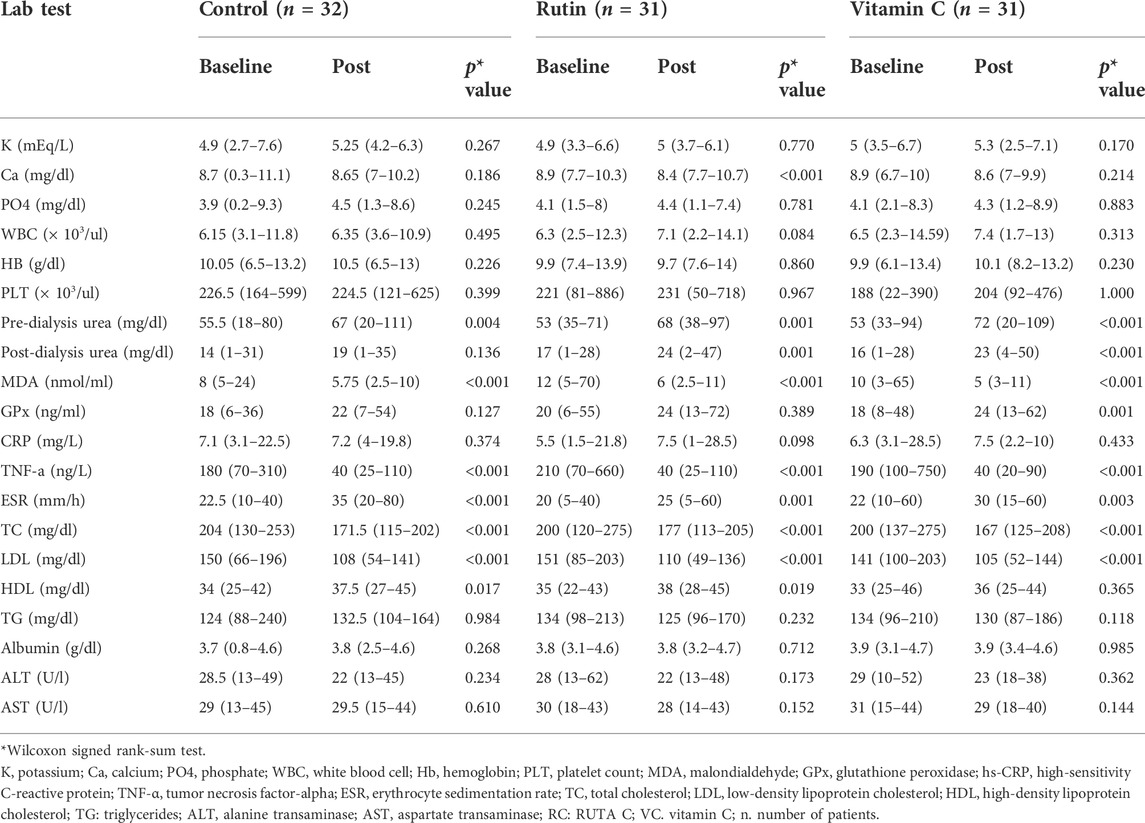
TABLE 3. Values of laboratory parameters and markers of oxidative stress before and at the end of the study period among the studied groups.
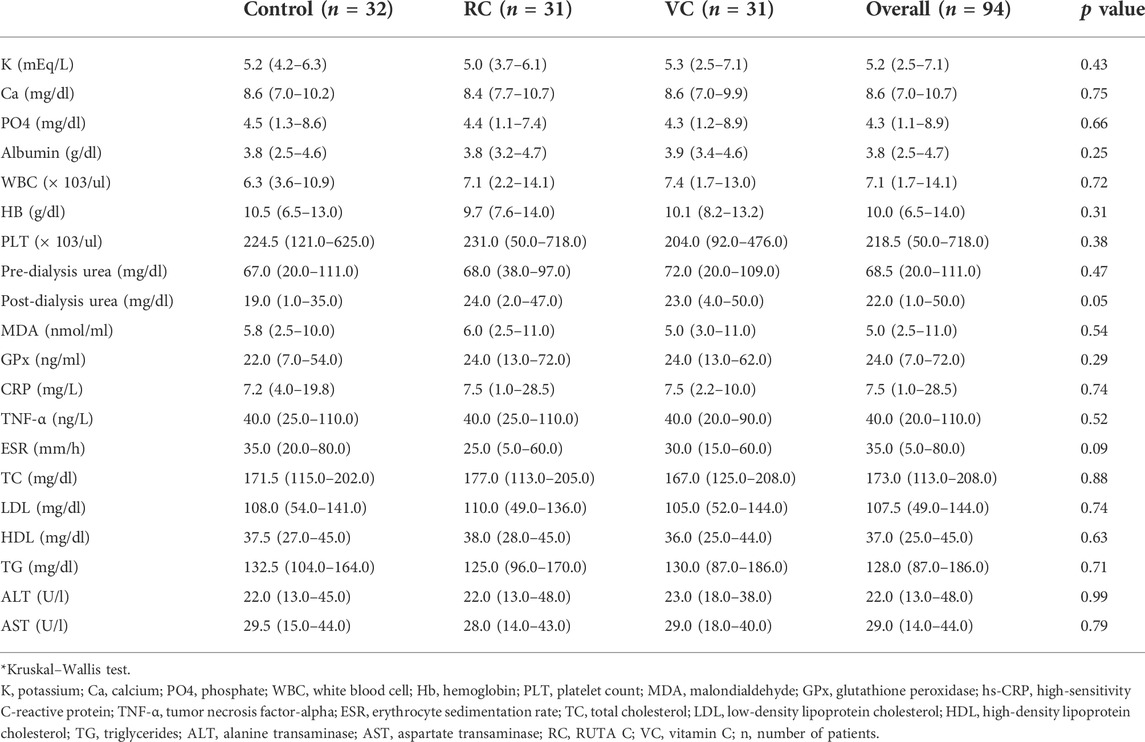
TABLE 4. Values of laboratory parameters and markers of oxidative stress at the end of the study period among the studied groups.
Concerning the evaluation of the anti-inflammatory effect of the interventional drugs, it was clear from the results presented in (Tables 3, 4) that the serum hs-CRP has increased in all groups with a non-significant difference, although there was a smaller increase in the vitamin C group, from 6.3 (3.1–28.5) mg/L to 7.5 (2.2–10) mg/L (p = 0.433) compared to a higher increase in the Ruta C group, from 5.5 (1.5–21.8) mg/L to 7.5 (1–28.5) mg/L (p = 0.098).
Moreover, the values of serum TNF-α had decreased significantly (p < 0.001) among the three groups in comparison to their baseline values (Table 3), on the other hand there was no significant difference shown on comparing the three groups with each other (p = 0.52).
Additionally, the results presented in (Table 4) showed a significant increase in the ESR values among the three groups compared to baseline values (Table 3),where, a higher increase in values was observed in the control group from 22.5 (10–40) mm/hr to 35 (20–80) mm/hr, p < 0.001 compared to the interventional groups, which showed a lower increase from 22 (10–60) mm/hr to 30 (15–60) mm/hr, p = 0.003, and from 20 (5–40) mm/hr to 25 (5–60) mm/hr, p = 0.001, in vitamin C and RUTA C, respectively. However, there was a non-significant difference among the three groups, as shown in (Table 4).
Regarding the results of the lipid profile, it was apparent from (Tables 3, 4) that there was a non-significant difference among the three groups, with an increase in HDL-C levels in all of them, this increase was significant only in the RUTA C and control groups [from 35 (22–43) mg/dl to 38 (28–45) mg/dl, p = 0.019, and from 34 (25–42) mg/dl to 37.5 (27–45) mg/dl, p = 0.017, respectively]. On the other hand, there was a non-significant increase in the vitamin C group, [from 33 (25–46) mg/dl to 36 (25–44) mg/dl, p = 0.365].Besides, the statistical analysis result of the LDL-C levels showed a significant decrease from 141 (100–203) mg/dl to 105 (52–144) mg/dl, p < 0.001, from 151 (85–203) mg/dl to 110 (49–136) mg/dl, p < 0.001, and from 150 (66–196) mg/dl to 108 (54–141) mg/dl, p < 0.001 for the vitamin C, RUTA C, and control groups, respectively, before and at the end of the study, moreover, a non-significant decrease was obtained among the three studied groups.
Concerning the total cholesterol (TC) levels, there was a non-significant difference among the studied groups before and at the end of the study, while there was a significant decrease from 200 (137–275) mg/dl to 167 (125–208) mg/dl, p < 0.001, from 204 (130–253) mg/L to 171.5 (115–202) mg/dl, p < 0.001, and from 200 (120–275) mg/dl to 177 (113–205) mg/dl, p < 0.001 for vitamin C, control, and RUTA C groups, respectively.
Triglycerides results showed a non-significant difference among the three groups a non-significant decrease in both the vitamin C and RUTA C groups, and a non-significant increase in the control group were observed before and at the end of the study.
Statistical analysis of the obtained results showed a non-significant difference among the studied groups with regard to the values of, ALT, AST, Albumin, Hb, K, Ca, PO4, WBC, and PLT count at the end of the study Tables 3, 4.
Regarding safety and tolerability of the interventional drugs, it was reported that one patient suffered from gastrointestinal tract (GIT) upset and one patient recorded a decrease in blood pressure on administration of RUTA C. While with the vitamin C supplement alone, only one patient suffered from GIT upset and vomiting from the beginning of its administration.
Malondialdehyde (MDA) is the end product of lipid peroxidation and is used as a great oxidative stress marker (Chen et al., 2011), MDA-LDL/LDL-C ratio has been shown to have a significant elevation in HD patients and could be considered as a potential risk factor for cardiovascular events in those patients (Asamiya et al., 2013). Also, a decrease in the levels of GSH or GPx has been seen in CKD patients (Duni et al., 2017). Vera et al. (2018) suggested that the potentiation of the GPx pathway could be future promising method to reduce the endothelial dysfunction induced in CKD patients, as GPx is known to minimize lipid peroxidation of the cell membranes accompanying OS.
In the current study, the administration of oral vitamin C alone (group 2) showed a significant increase in the serum levels of glutathione peroxidase GPx from 18 (8–48) ng/ml at baseline to 24 (13–62) ng/ml (p = 0.001) at the end of the 16-week study period compared to a non-significant difference in both groups 1 and 3. To the best of our knowledge, this observation was the first one to be documented compared to the other previously performed studies, which lacked such a newly detected elevation in HD patients supplemented with vitamin C.
Wen et al., demonstrated that glutathione (GSH) values were increased by 28% after 4 weeks of supplementation with 1000 mg/day of vitamin C compared to the control (p > 0.001) (Wen et al., 1997), which was in accordance with our results.
On the other hand, Fumeron et al. (2005) reported a non-significant change in GSH levels upon the administration of 250 mg of vitamin C for 8 weeks compared to controls.
In addition, Martins et al. (2021) reported a non-significant decrease in GPx levels when patients were given 0.250 g of vitamin C three times/week for 8 weeks. The discrepancy between the previously mentioned studies’ results and the current study ones might be due to their shorter length of study intervention (8 weeks) compared to ours (16 weeks), about double the study period.
In the present study, after 16 weeks of supplementation, the data showed a significant reduction in MDA levels at the end of the study in the three studied groups compared to their baseline levels (p > 0.001). Interestingly, the percentage change in MDA levels was reduced by 50% in interventional groups and by only 28% in the control group. On considering the vitamin C effect, our findings were consistent with those obtained by Sarandol et al. (2022), who reported that the supplementation of vitamin C showed a significant decrease in MDA.
Also, Abdollahzad et al. (2009) study results showed a decrease in the level of serum MDA in the vitamin C group after 12 weeks of supplementation of 0.25 g three times/week and a significant difference in mean MDA changes between the vitamin C group and placebo group. In addition, Candan et al. (2002) reported that MDA levels significantly decreased in vitamin C supplemented patients when compared with those receiving placebo at the end of 3 months.
In the present study, there was a non-significant difference in hs-CRP levels among the three groups at the end of the study. Interestingly, when we compared the interquartile range values at baselines 6.3 (3.1–28.5) with that at end of the study 7.5 (2.2–10) of the vitamin C group, we found 75% of patients had hs-CRP levels ≤10 mg/L after treatment compared to ≤28.5 mg/L before treatment, this result may reflects the tendency of vitamin C to decrease hs-CRP levels in the majority of patients, and it may be due to the differences in their duration of dialysis, wide range of the age and comorbidities of the patients that the rest of patients resembling 25% did not show such a large reduction in their hs-CRP’ levels.
In agreement with our results, Fumeron et al. (2005) reported no effect on plasma hs-CRP after oral supplementation of vitamin C every second day for a period of 2 months, although the level of vitamin C was increased. However, in contrast with Biniaz et al. (2014) who demonstrated a significant fall in median CRP level in a group of patients on hemodialysis after 2 months of supplementation with vitamin C 250 mg three times/week.
In the current study, although the three studied groups did not show any significant difference regarding TNF-α levels at the end of our study, all groups showed a significant decrease in the TNF-α levels compared to their baseline values.
When we compared median TNF-α values of the three groups at end of the study with their baseline values, we found that administration of rutin/vitamin C combination (Ruta C) causes a reduction of TNF-α levels in 50% of the patients from 210 ng/L to 40 ng/L indicating the ability of rutin to alleviate the inflammatory response and manifested itself to produce a small synergistic effect with vitamin C, when given together as a combination, and also as a promise anti-inflammatory treatment attenuating inflammation and oxidative stress in HD patients.
On the other side, we could not ignore the effect of vitamin C alone (vitamin C group) in decreasing the TNF-α level in 25% of patients to ≤20 at the end of the study compared to ≤100 at baseline in accordance with those reported by Khosroshahi et al. (2011) that TNF-α levels reduced significantly in HD patients after intravenous administration of Vitamin C with 500 mg two times/week after each dialysis session for 8 weeks.
In fact, there were limited data in the literature which focused on the anti-inflammatory effect of vitamin C in HD patients. The anti-inflammatory mechanism of vitamin C was clarified by its inhibitory effect on Nuclear Factor Kappa B (NF-kB) which is responsible for the activation of a number of pro-inflammatory cytokines such as TNF-α (Carr and Maggini, 2017).
Concerning the non-significant increase in ESR values in the three studied groups which were observed at the end of our study period, with a higher increase in values observed in the control group compared to the interventional groups revealed that although vitamin C failed to decrease ESR significantly, it succeeded in avoiding its increase above the highest baseline value (60 mm/h). Moreover, it was suggested that the longer duration of patients’ intake of vitamin C might have induced favorable actions supported by those reported by Khosroshahi et al. (2011) for the reduction in ESR level in their study group supplemented with vitamin C.
Considering the effect of vitamin C on lipid profile levels, our results were in accordance with a meta-analysis of 13 randomized controlled trials, which concluded that the administration of vitamin C at least 0.5 g/d for a minimum of 4 weeks can result in a significant decrease in serum LDL-C and reported a non-significant elevation of serum HDL-C with a significant fall in serum triglycerides (McRae, 2008). In addition, our results of the vitamin C group were supported by those found by El Mashad et al. (2016) where, a significant decrease in the levels of cholesterol and LDL after 12 weeks of supplementation with vitamin C failed to agree with their TG results.
From the results obtained in this study, it can be concluded that oral administration of 1 g/day of vitamin C for a period of 16 weeks resulted in the reduction of serum levels of malondialdehyde (MDA) and elevation of glutathione peroxidase (GPx) levels among hemodialysis patients (HD). Moreover, rutin had a favorable synergistic effect with vitamin C in reducing TG and TNF-α levels, and increasing HDL-C level. Rutin might provide a positive future result associated with promise as an anti-inflammatory supplementation, attenuating inflammation and oxidative stress in HD patients when combined with vitamin C.
A multi-center randomized controlled study with a relatively longer duration is required to confirm the role of vitamin C in improving the inflammatory status of HD patients. Moreover, a long-term trials and larger doses as well are recommended to explore the impact of rutin supplementation on the oxidative stress and inflammatory markers in ESRD population. Finally, future studies are to include detection of cytokine levels as well as broader investigations of the anti-inflammatory effect of vitamin C and rutin in HD patients.
The raw data supporting the conclusion of this article will be made available by the authors without undue reservation.
The studies involving human participants were reviewed and approved by the ethics committee of the faculty of pharmacy, Ain Shams University, Cairo, Egypt. The patients/participants provided their written informed consent to participate in this study.
All authors contributed to the study’s conception and design. Material presentation, data collection, and analysis were performed by SO, RE, and NS. The practical part of the clinical trial was carried out under the supervision of TE. The first draft of the manuscript was written by SO, and all authors commented on versions of the manuscript. All authors read and approved the final manuscript.
The authors would like to thank the nephrology department at Ain Shams University Hospital for help and collaboration.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdollahzad, H., Eghtesadi, S., Nourmohammadi, I., Khadem-Ansari, M., Nejad-Gashti, H., and Esmaillzadeh, A. (2009). Effect of vitamin C supplementation on oxidative stress and lipid profiles in hemodialysis patients. Int. J. Vitam. Nutr. Res. 79 (56), 281–287. doi:10.1024/0300-9831.79.56.281
Afanas' ev, I. B., Dcrozhko, A. I., Brodskii, A. V., Kostyuk, V. A., and Potapovitch, A. I. (1989). Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem. Pharmacol. 38 (11), 1763–1769. doi:10.1016/0006-2952(89)90410-3
Ahmed, O. M., Moneim, A. A., Yazid, I. A., and Mahmoud, A. M. (2010). Antihyperglycemic, antihyperlipidemic and antioxidant effects and the probable mechanisms of action of Ruta graveolens infusion and rutin in nicotinamide-streptozotocin-induced diabetic rats. Diabetol. Croat. 39 (1), 564–70.
Akchurin, M., and Kaskel, F. (2015). Update on inflammation in chronic kidney disease. Blood Purif. 39 (1-3), 84–92. doi:10.1159/000368940
Al-Dhabi, N. A., Arasu, M. V., Park, C. H., and Park, S. U. (2015). An up-to-date review of rutin and its biological and pharmacological activities. EXCLI J. 14, 59–63. doi:10.17179/excli2014-663
Al-Rejaie, S. S., Abuohashish, H. M., Alkhamees, O. A., Aleisa, A. M., and Alroujayee, A. S. (2012). Gender difference following high cholesterol diet induced renal injury and the protective role of rutin and ascorbic acid combination in Wistar albino rats. Lipids Health Dis. 11 (1), 41–10. doi:10.1186/1476-511X-11-41
Asamiya, Y., Yajima, A., Tsuruta, Y., Otsubo, S., and Nitta, K. (2013). Oxidised LDL/LDL-cholesterol ratio and coronary artery calcification in haemodialysis patients. Nutr. Metab. Cardiovasc. Dis. 23 (7), 619–627. doi:10.1016/j.numecd.2012.02.001
Bayés, B., Pastor, M. C., Bonal, J., Junca, J., and Romero, R. (2001). Homocysteine and lipid peroxidation in haemodialysis: Role of folinic acid and vitamin E. Nephrol. Dial. Transpl. 16 (11), 2172–2175. doi:10.1093/ndt/16.11.2172
Bergmeyer, H. U., Bowes, G. N., Hörder, M., and Moss, D. W. (1976). Provisional recommendations on IFCC methods for the measurement of catalytic concentrations of enzymes Part 2. IFCC method for aspartate aminotransferase. Clin. Chim. Acta 70 (2), F19–F42. doi:10.1016/0009-8981(76)90437-x
Bergmeyer, H. U. (1980). IFCC methods for the measurement of catalytic concentrations of enzymes: Part 3. IFCC method for alanine aminotransferase (L-alanine: 2-oxoglutarate aminotransferase, EC 2.6. 1.2). Clin. Chim. acta 105 (1), 147–154. doi:10.1016/0009-8981(80)90105-9
Berretta, M., Quagliariello, V., Maurea, N., Di Francia, R., Sharifi, S., Facchini, G., et al. (2020). Multiple effects of ascorbic acid against chronic diseases: Updated evidence from preclinical and clinical studies. Antioxidants 9 (12), 1182. doi:10.3390/antiox9121182
Biniaz, V., Shermeh, M. S., Ebadi, A., Tayebi, A., and Einollahi, B. (2014). Effect of vitamin C supplementation on C-reactive protein levels in patients undergoing hemodialysis: A randomized, double blind, placebo-controlled study. Nephrourol. Mon. 6 (1), e13351. doi:10.5812/numonthly.13351
Canaud, B., Cristol, J. P., Morena, M., Leray-Moragues, H., Bosc, J. Y., and Vaussenat, F. (1999). Imbalance of oxidants and antioxidants in haemodialysis patients. Blood Purif. 17 (2-3), 99–106. doi:10.1159/000014381
Candan, F., Gültekin, F., and Candan, F. (2002). Effect of vitamin C and zinc on osmotic fragility and lipid peroxidation in zinc‐deficient haemodialysis patients. Cell biochem. Funct. 20 (2), 95–98. doi:10.1002/cbf.947
Carr, A. C., and Maggini, S. (2017). Vitamin C and immune function. Nutrients 9 (11), 1211. doi:10.3390/nu9111211
Chaghouri, P., Maalouf, N., Peters, S. L., Nowak, P. J., Peczek, K., Zasowska-Nowak, A., et al. (2021). Two faces of vitamin C in hemodialysis patients: Relation to oxidative stress and inflammation. Nutrients 13 (3), 791. doi:10.3390/nu13030791
Chen, J. L., Huang, Y. J., Pan, C. H., Hu, C. W., and Chao, M. R. (2011). Determination of urinary malondialdehyde by isotope dilution LC-MS/MS with automated solid-phase extraction: A cautionary note on derivatization optimization. Free Radic. Biol. Med. 51 (9), 1823–1829. doi:10.1016/j.freeradbiomed.2011.08.012
Colunga Biancatelli, R. M., Berrill, M., Catravas, J. D., and Marik, P. E. (2020). Quercetin and vitamin C: An experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19). Front. Immunol. 11, 1451. doi:10.3389/fimmu.2020.01451
Cozzolino, M., Mangano, M., Stucchi, A., Ciceri, P., Conte, F., and Galassi, A. (2018). Cardiovascular disease in dialysis patients. Nephrol. Dial. Transpl. 33 (3), iii28–34. doi:10.1093/ndt/gfy174
Deicher, R., and Hörl, W. H. (2003). Vitamin C in chronic kidney disease and hemodialysis patients. Kidney Blood Press. Res. 26 (2), 100–106. doi:10.1159/000070991
Duni, A., Liakopoulos, V., Rapsomanikis, K. P., and Dounousi, E. (2017). Chronic kidney disease and disproportionally increased cardiovascular damage: Does oxidative stress explain the burden? Oxidative Med. Cell. Longev. 2017, 9036450. doi:10.1155/2017/9036450
Eckardt, K. U., Bansal, N., Coresh, J., Evans, M., Grams, M. E., Herzog, C. A., et al. (2018). Improving the prognosis of patients with severely decreased glomerular filtration rate (CKD G4+): Conclusions from a kidney disease: Improving global outcomes (KDIGO) controversies conference. Kidney Int. 93 (6), 1281–1292. doi:10.1016/j.kint.2018.02.006
El Mashad, G. M., ElSayed, H. M., and Nosair, N. A. (2016). Effect of vitamin C supplementation on lipid profile, serum uric acid, and ascorbic acid in children on hemodialysis. Saudi J. Kidney Dis. Transpl. 27 (6), 1148–1154. doi:10.4103/1319-2442.194602
Erdfelder, E., Faul, F., and Buchner, A. (1996). Gpower: A general power analysis program. Behav. Res. Methods Instrum. Comput. 28 (1), 1–11. doi:10.3758/bf03203630
Friedewald, W. T., Levy, R. I., and Fredrickson, D. S. (1972). Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18 (6), 499–502. doi:10.1093/clinchem/18.6.499
Fumeron, C., Nguyen-Khoa, T., Saltiel, C., Kebede, M., Buisson, C., Drüeke, T. B., et al. (2005). Effects of oral vitamin C supplementation on oxidative stress and inflammation status in haemodialysis patients. Nephrol. Dial. Transpl. 20 (9), 1874–1879. doi:10.1093/ndt/gfh928
Gabor, M. (1972). Pharmacologic effects of flavonoids on blood vessels. Angiologica 9 (3-6), 355–374. doi:10.1159/000157944
Ganeshpurkar, A., and Saluja, A. K. (2017). The pharmacological potential of rutin. Saudi Pharm. J. 25 (2), 149–164. doi:10.1016/j.jsps.2016.04.025
Gęgotek, A., Ambrożewicz, E., Jastrząb, A., Jarocka-Karpowicz, I., and Skrzydlewska, E. (2019). Rutin and ascorbic acid cooperation in antioxidant and antiapoptotic effect on human skin keratinocytes and fibroblasts exposed to UVA and UVB radiation. Arch. Dermatol. Res. 311 (3), 203–219. doi:10.1007/s00403-019-01898-w
Ghiadoni, L., Cupisti, A., Huang, Y., Mattei, P., Cardinal, H., Favilla, S., et al. (2004). Endothelial dysfunction and oxidative stress in chronic renal failure. J. Nephrol. 17 (4), 512–519.
Gordon, T., Castelli, W. P., Hjortland, M. C., Kannel, W. B., and Dawber, T. R. (1977). High density lipoprotein as a protective factor against coronary heart disease: The framingham study. Am. J. Med. 62 (5), 707–714. doi:10.1016/0002-9343(77)90874-9
Handelman, G. J., Walter, M. F., Adhikarla, R., Gross, J., Dallal, G. E., Levin, N. W., et al. (2001). Elevated plasma F2-isoprostanes in patients on long-term hemodialysis. Kidney Int. 59 (5), 1960–1966. doi:10.1046/j.1523-1755.2001.0590051960.x
Kelly, D., and Rothwell, P. M. (2020). Disentangling the multiple links between renal dysfunction and cerebrovascular disease. J. Neurol. Neurosurg. Psychiatry 91 (1), 88–97. doi:10.1136/jnnp-2019-320526
Khosroshahi, H. T., Talebi, Y., Ahmadzadeh, S., Habibzadeh, A., Mousavi, S. E., and Khalilzadeh, M. (2011). The effects of intravenous vitamin C administration on hs-CRP and tumor necrosis factor-α levels in haemodialysis patients. Funct. Foods Health Dis. 1 (8), 255–261. doi:10.31989/ffhd.v1i8.125
Levey, A. S., Andreoli, S. P., DuBose, T., Provenzano, R., and Collins, A. J. (2007). Ckd: Common, harmful, and treatable—world kidney day 2007. Am. J. Kidney Dis. 49 (2), 175–179. doi:10.1053/j.ajkd.2006.12.013
Liakopoulos, V., Roumeliotis, S., Gorny, X., Dounousi, E., and Mertens, P. R. (2017). Oxidative stress in hemodialysis patients: A review of the literature. Oxidative Med. Cell. Longev. 2017, 3081856. doi:10.1155/2017/3081856
Liakopoulos, V., Roumeliotis, S., Zarogiannis, S., Eleftheriadis, T., and Mertens, P. R. (2019). Oxidative stress in hemodialysis: Causative mechanisms, clinical implications, and possible therapeutic interventions. Semin. Dial. 32 (1), 58–71. doi:10.1111/sdi.12745
Locatelli, F., Canaud, B., Eckardt, K. U., Stenvinkel, P., Wanner, C., and Zoccali, C. (2003). Oxidative stress in end‐stage renal disease: An emerging threat to patient outcome. Nephrol. Dial. Transpl. 18 (7), 1272–1280. doi:10.1093/ndt/gfg074
Martins, M. L., da Silva, A. T., Machado, R. P., Ramos, H. P., Martinelli, C., Silveira, T. T., et al. (2021). Vitamin C decreases reduced glutathione in chronic haemodialysis patients: A pilot, randomised, double-blind trial. Int. Urol. Nephrol. 53 (8), 1695–1704. doi:10.1007/s11255-021-02797-8
McRae, M. P. (2008). Vitamin C supplementation lowers serum low-density lipoprotein cholesterol and triglycerides: A meta-analysis of 13 randomized controlled trials. J. Chiropr. Med. 7 (2), 48–58. doi:10.1016/j.jcme.2008.01.002
Milde, J., Elstner, E. F., and Grassmann, J. (2004). Synergistic inhibition of low-density lipoprotein oxidation by rutin, γ-terpinene, and ascorbic acid. Phytomedicine 11 (2-3), 105–113. doi:10.1078/0944-7113-00380
Morena, M., Cristol, J. P., Bosc, J. Y., Tetta, C., Forret, G., Leger, C. L., et al. (2002). Convective and diffusive losses of vitamin C during haemodiafiltration session: A contributive factor to oxidative stress in haemodialysis patients. Nephrol. Dial. Transpl. 17 (3), 422–427. doi:10.1093/ndt/17.3.422
Muvhulawa, N., Dludla, P. V., Ziqubu, K., Mthembu, S. X., Mthiyane, F., Nkambule, B. B., et al. (2022). Rutin ameliorates inflammation and improves metabolic function: A comprehensive analysis of scientific literature. Pharmacol. Res. 178, 106163. doi:10.1016/j.phrs.2022.106163
Oshodi, T. O., Ben-Azu, B., Ishola, I. O., Ajayi, A. M., Emokpae, O., and Umukoro, S. (2021). Molecular mechanisms involved in the prevention and reversal of ketamine-induced schizophrenia-like behavior by rutin: The role of glutamic acid decarboxylase isoform-67, cholinergic, nox-2-oxidative stress pathways in mice. Mol. Biol. Rep. 48 (3), 2335–2350. doi:10.1007/s11033-021-06264-6
Panasiak, W., Wleklik, M., Oraczewska, A., and Luczak, M. (1989). Influence of flavonoids on combined experimental infections with EMC virus and Staphylococcus aureus in mice. Acta Microbiol. Pol. 38 (2), 185–188.
Perk, A. A., Shatynska-Mytsyk, I., Gerçek, Y. C., Boztaş, K., Yazgan, M., Fayyaz, S., et al. (2014). Rutin mediated targeting of signaling machinery in cancer cells. Cancer Cell Int. 14 (1), 124–125. doi:10.1186/s12935-014-0124-6
Raimann, J. G., Levin, N. W., Craig, R. G., Sirover, W., Kotanko, P., and Handelman, G. (2013). Is vitamin C intake too low in dialysis patients? InSeminars dialysis 26 (1), 1–5. doi:10.1111/sdi.12030
Ramos, R., and Martínez-Castelao, A. (2008). Lipoperoxidation and hemodialysis. Metabolism. 57 (10), 1369–1374. doi:10.1016/j.metabol.2008.05.004
Rapa, S. F., Di Iorio, B. R., Campiglia, P., Heidland, A., and Marzocco, S. (2019). Inflammation and oxidative stress in chronic kidney disease—Potential therapeutic role of minerals, vitamins and plant-derived metabolites. Int. J. Mol. Sci. 21 (1), 263. doi:10.3390/ijms21010263
Ross, R. (1999). Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 340 (2), 115–126. doi:10.1056/NEJM199901143400207
Russa, D. L., Pellegrino, D., Montesanto, A., Gigliotti, P., Perri, A., Russa, A. L., et al. (2019). Oxidative balance and inflammation in hemodialysis patients: Biomarkers of cardiovascular risk? Oxid. Med. Cell. Longev. 2019, 8567275. doi:10.1155/2019/8567275
Sarandol, E., Erdinc, S., Senol, E., Ersoy, A., and Surmen-Gur, E. (2022). Effects of vitamin C supplementation on oxidative stress and serum paraoxonase/arylesterase activities in patients on long-term hemodialysis. Nefrologia. doi:10.1016/j.nefro.2021.11.003
Schettler, G., and Nussei, E. (1975). Massnahmen zur Prävention der Arteriosklerose [in German]. Arb. Med. Soz. Med. Prav. Med. 10, 25.
Vera, M., Torramade-Moix, S., Martin-Rodriguez, S., Cases, A., Cruzado, J. M., Rivera, J., et al. (2018). Antioxidant and anti-inflammatory strategies based on the potentiation of glutathione peroxidase activity prevent endothelial dysfunction in chronic kidney disease. Cell. Physiol. biochem. 51 (3), 1287–1300. doi:10.1159/000495540
Vida, C., Oliva, C., Yuste, C., Ceprián, N., Caro, P. J., Valera, G., et al. (2021). Oxidative stress in patients with advanced ckd and renal replacement therapy: The key role of peripheral blood leukocytes. Antioxidants 10 (7), 1155. doi:10.3390/antiox10071155
Webster, R. P., Gawde, M. D., and Bhattacharya, R. K. (1996). Protective effect of rutin, a flavonol glycoside, on the carcinogen-induced DNA damage and repair enzymes in rats. Cancer Lett. 109 (1-2), 185–191. doi:10.1016/s0304-3835(96)04443-6
Wen, Y., Cooke, T., and Feely, J. (1997). The effect of pharmacological supplementation with vitamin C on low‐density lipoprotein oxidation. Br. J. Clin. Pharmacol. 44 (1), 94–97. doi:10.1046/j.1365-2125.1997.00623.x
Yudkin, J. S., Stehouwer, C., Emeis, J., and Coppack, S. (1999). C-Reactive protein in healthy subjects: Associations with obesity, insulin resistance, and endothelial dysfunction: A potential role for cytokines originating from adipose tissue? Arterioscler. Thromb. Vasc. Biol. 19 (4), 972–978. doi:10.1161/01.atv.19.4.972
Keywords: rutin (PubChem CID), vitamin C, inflammation markers, oxidative stress, hemodialysis
Citation: Omar S, El Borolossy RM, Elsaid T and Sabri NA (2022) Evaluation of the combination effect of rutin and vitamin C supplementation on the oxidative stress and inflammation in hemodialysis patients. Front. Pharmacol. 13:961590. doi: 10.3389/fphar.2022.961590
Received: 04 June 2022; Accepted: 15 August 2022;
Published: 08 September 2022.
Edited by:
Marika Cordaro, University of Messina, ItalyReviewed by:
Phiwayinkosi V. Dludla, South African Medical Research Council, South AfricaCopyright © 2022 Omar, El Borolossy, Elsaid and Sabri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samia Omar, c2FtaWEub21hcjE4QHBoYXJtYS5hc3UuZWR1LmVn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.