- 1Graduate School, Beijing University of Chinese Medicine, Beijing, China
- 2The 2nd Department of Pulmonary Disease in Traditional Chinese Medicine (TCM), China-Japan Friendship Hospital, Beijing, China
- 3Department of Respiratory, Dongfang Hospital, Beijing University of Chinese Medicine, Beijing, China
Purpose: To investigate the impact of different baseline characteristics on the efficacy of immune checkpoint inhibitors (ICIs) for advanced lung cancer.
Methods: In order to identify eligible randomized controlled trials (RCTs), a systematic search was conducted in PubMed, Embase, Cochrane Library, Web of Science, and Scopus databases. The primary outcomes were hazard ratios (HRs) and 95% confidence intervals (CIs) for overall survival (OS). To explore the potential interaction during the administration of ICI, patients were stratified by baseline characteristics.
Results: The meta-analysis included 24 RCTs. ① Compared with non-ICI therapy, patients with lung cancer benefitted more from immunotherapy (HR, 0.78; p < 0.0001). ② Patients without liver metastases could get more survival benefits than those with liver metastases (HR, 1.20; p = 0.0139). Similar outcomes were also observed in the following subgroups: small-cell lung cancer (HR, 1.20; p = 0.0433), subsequent line (HR, 1.40; p = 0.0147), and ICI monotherapy (HR, 1.40; p = 0.0147). ③ Subgroup analysis showed that tumor type affected the efficacy of immunotherapy in patients with brain metastases (HR, 0.72 vs. 1.41; interaction, p < 0.01). Among patients with smoking history (HR, 0.87 vs. 1.23; interaction, p = 0.05) and brain metastases (HR, 0.69 vs. 1.21; interaction, p = 0.05), the type of therapy (i.e., monotherapy or combination therapy) had potential influences on the efficacy of immunotherapy.
Conclusion: Some critical baseline characteristics could indicate the efficacy of ICI therapy. Liver metastasis status could predict the efficacy of ICI therapy for lung cancer. Compared with small-cell lung cancer, patients with brain metastases might have durable OS in non-small-cell lung cancer. The smoking history or brain metastasis status of patients could indicate the potential clinical benefits of monotherapy or combination therapy.
1 Introduction
Lung cancer is a major threat to people’s health. It is the main cause of cancer death (Siegel et al., 2022). In the past decades, chemotherapy was the main therapy for advanced cancer. However, patients who underwent chemotherapy had a poor prognosis (Sibiya et al., 2019). Although new molecular targeted therapy has improved the treatment of lung cancer, only patients with corresponding genetic mutations can benefit from this therapy (Lee et al., 2018), and drug resistance during the therapeutic process constitutes a pending challenge (Song et al., 2019).
The emergence of immunotherapy improves the treatment mode for lung cancer (Xu et al., 2021). Immune checkpoint inhibitors (ICIs) can block the pathway of cytotoxic T lymphocyte-associated protein-4 (CTLA-4) or programmed death-1 (PD-1). It can enhance T-cell immune responses to prevent the immune escape of tumor cells (Pardoll, 2012). Previous studies have proved a promising survival benefit of immunotherapy for patients with lung cancer (Brahmer et al., 2015; Fehrenbacher et al., 2016; Sugawara et al., 2021). However, only a minority of patients get durable survival from immunotherapy (Hegde and Chen, 2020). Meanwhile, due to the high cost of immunotherapy (Yong et al., 2022) and immune-related adverse reactions (Yu et al., 2021), it is necessary to standardize the application of ICIs. Appropriate baseline characteristics contribute to implementing rational therapeutic strategies. Furthermore, it is conducive to identifying patients suitable for immunotherapy and achieving precise treatment for lung cancer.
In some randomized control trials (RCTs) with prespecified subgroups, the efficacy of immunotherapy varied among patients with different baseline characteristics such as age, gender, and smoking status. For example, in the CheckMate 227 trial (Hellmann et al., 2019), nivolumab combined with ipilimumab significantly improved survival in smoking patients and patients without liver metastases but not in non-smokers and patients with liver metastases. In the CheckMate 057 trial (Borghaei et al., 2015), nivolumab prolonged patients’ overall survival (OS) in second-line therapy and negative epidermal growth factor receptor (EGFR) mutation status. The STIMULI trial (Peters et al., 2022) discovered that no survival benefit of ICIs was observed in male patients, current smoking patients, and 0-point patients in the performance status (PS) of the Eastern Cooperative Oncology Group (ECOG). Therefore, in order to screen the predictors of efficacy of ICIs for lung cancer, it is necessary to conduct pooled analysis of relative RCTs. Recent research (Yan et al., 2020; Wang et al., 2021; Xue et al., 2021) has reported the association between unique baseline characteristics and the efficacy of ICIs for lung cancer. To further investigate the potential influence of baseline characteristics on efficacy, we conducted a comprehensive study in this field.
In this meta-analysis, the efficacy of immunotherapy and non-ICI therapy in different baseline characteristics was systematically assessed. Meanwhile, we also calculated the interaction of baseline characteristics that might influence the efficacy. Moreover, we summarized the immune-related adverse events (irAEs) of immunotherapy for lung cancer and illustrated them with a table in the Results section. This study involved a total of 11 systems and contained the incidence and common disease severity. Then, we expounded the importance of standardized and precise medication of ICIs.
2 Materials and methods
2.1 Protocol and registration
This review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (Moher et al., 2009) (Supplementary Table S1). The research protocol has been submitted to the PROSPERO platform (https://www.crd.york.ac.uk/prospero/) (Registration No.: CRD42022326099).
2.2 Search strategy
PubMed, Embase, Cochrane Library, Web of Science, and Scopus databases were searched for phase 2 or 3 RCTs up to 10 February 2022. Conference proceedings of medical societies, such as the European Society of Medical Oncology and the American Society of Clinical Oncology, were also reviewed. Two authors (Xiao and Yu) independently completed the retrieval of the literature. The search terms used are as follows: “Nivolumab,” “Pembrolizumab,” “Cemiplimab,” “Atezolizumab,” “Durvalumab,” “Avelumab,” “Ipilimumab,” “Tremelimumab,” “Programmed Cell Death 1 Receptor (PD-1),” “Programmed Cell Death 1 Ligand 1 Protein (PD-L1),” “Cytotoxic T-Lymphocyte Associated Antigen 4 (CTLA-4),” “immune checkpoint inhibitor,” “Lung Cancer,” and “randomized controlled trial.” The details of the search strategy are provided in Supplementary Table S2.
2.3 Inclusion and exclusion criteria
The studies were included in this meta-analysis when they met the following inclusion criteria: 1) Population: patients diagnosed as lung cancer. 2) Intervention: ICI monotherapy or combined with other therapies (i.e., another immunotherapy, chemotherapy, target therapy, or radiotherapy). 3) Comparison: chemotherapy or placebo. 4) Outcome: hazard ratios (HRs) and 95% confidence intervals (CIs) for OS. 5) Study design: phase 2 or 3 RCTs.
The exclusion criteria of this study were as follows: 1) observational studies, 2) single-arm trials, 3) studies with duplicated data in the same population, 4) conference articles for which the full text is not available, and 5) articles in a language other than English.
2.4 Data extraction and quality assessment
Data extraction was carried out by two reviewers (Shuai and Yao) according to the predetermined list of information. All of the disagreements were resolved through consultation with all investigators. The basic information of the study was extracted, including first author, publication year, trial name, types of lung cancer, study phase, line of therapy, PD-L1 expression, therapeutic drug, number of patients, overall HRs, and HRs for each baseline characteristic.
The risk of bias was evaluated according to seven aspects (Higgins et al., 2011): random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias.
2.5 Statistical analysis
The clinical heterogeneity of the included studies was assessed by the characteristics of populations, interventions, control group, outcomes, and study design. All statistical analyses were conducted by the “meta” package in R version 4.1.3 (Balduzzi et al., 2019). The HRs and 95% CIs for OS in patients stratified by each baseline characteristic were collected from original studies. We used the standard Cochran’s Q test and I2 statistics to evaluate the heterogeneity of included studies (Higgins et al., 2003). I2 > 40% or p < 0.1 represented significant heterogeneity, and a random-effects model was chosen. Otherwise, a fixed-effects model was used (Sethi et al., 2019). In order to assess the difference in immunotherapy efficacy among patients stratified by baseline characteristics, we used the following methods: first, the inverse variance method was applied to calculate pooled HRs for OS in patients. Then, we calculated interaction HRs for baseline characteristics (Altman and Bland, 2003) and integrated these data. Subgroup analysis was also performed in this review. The subgroups include types of lung cancer, lines of treatment, and types of therapy. The publication bias was evaluated using the funnel plot, Egger’s test, and Begg’s test.
3 Results
3.1 Study selection
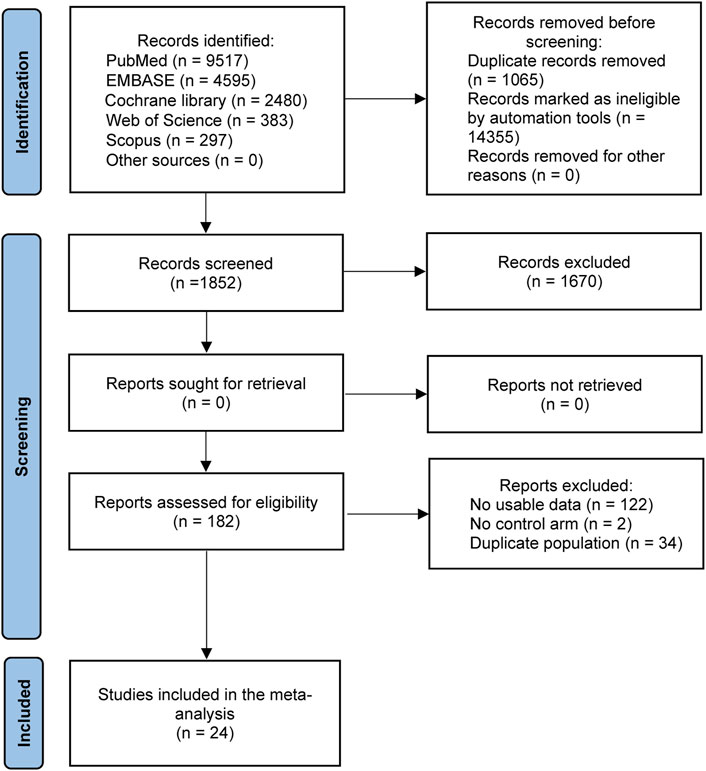
The flow chart of literature selection is shown in Figure 1. In total, 17,272 literature works were identified through searching PubMed, Embase, Cochrane library, Web of Science, and Scopus databases and conference proceedings up to 10 February 2022. After removing duplicate studies, filtering titles and abstracts, and reviewing the full texts, 24 studies with 15,628 patients were ultimately included in this meta-analysis (Borghaei et al., 2015; Reck et al., 2016; Carbone et al., 2017; Govindan et al., 2017; Rittmeyer et al., 2017; Paz-Ares et al., 2018; Hellmann et al., 2019; Reck et al., 2019; West et al., 2019; Rudin et al., 2020; Goldman et al., 2021; Herbst et al., 2021; Liu et al., 2021; Nishio et al., 2021; Owonikoko et al., 2021; Park et al., 2021; Reck et al., 2021; Rodriguez-Abreu et al., 2021; Sezer et al., 2021; Socinski et al., 2021; Spigel et al., 2021; Wu et al., 2021; Peters et al., 2022; Spigel et al., 2022).
3.2 Study characteristics
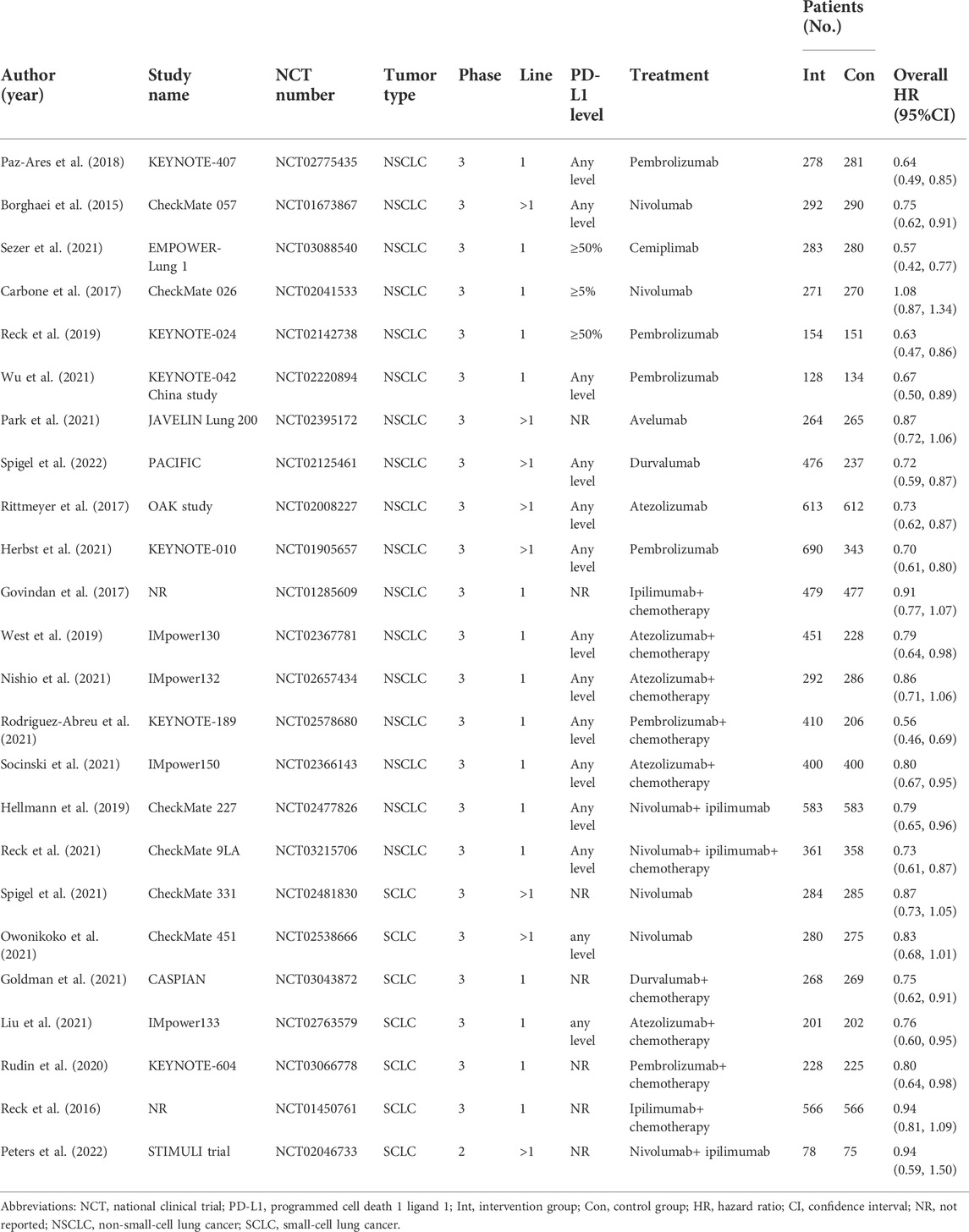
The study characteristics of 24 RCTs are summarized in Table 1. All eligible studies were published between 2015 and 2022. Among the 24 studies, a total of 17 studies researched on non-small-cell lung cancer (NSCLC) and seven studies researched on small-cell lung cancer (SCLC). In all the included trials, there were 16 trials that focused on first-line therapy of patients with lung cancer and 8 trials focused on subsequent-line therapy. All of the included studies were comparisons between ICI-based therapy and non-ICI therapy. A total of 12 trials used ICI monotherapy, and nine trials used ICI combined with chemotherapy, and only three trials used ICI combined with ICI. Overall HRs were collected from each study.
3.3 Risk of bias assessment
The risk of bias assessment is provided in Supplementary Table S3. In all trials, the risk of bias was low in terms of random sequence generation, completeness of outcome data, selective reporting, and other biases. Because of the open-label design, some trials may increase the risk of bias. In general, the quality of these trials was satisfactory.
3.4 Pooled hazard ratios of patients with lung cancer
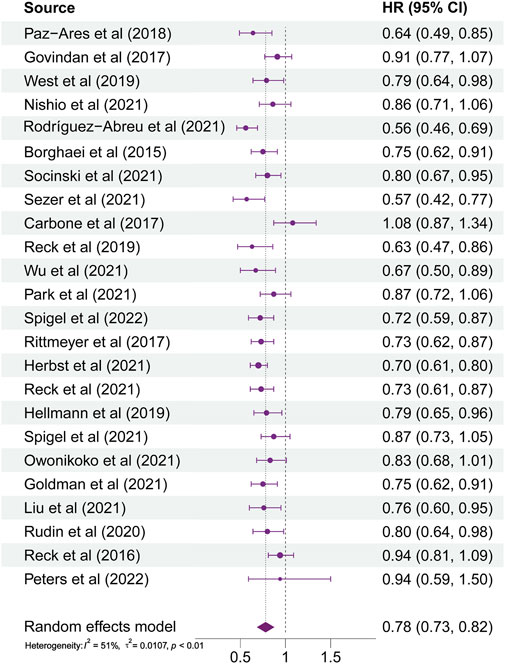
As illustrated in Figure 2, HRs of almost all studies were on the left side of the axis in the forest plots. It indicated that ICI therapy had more benefits than non-ICI therapy. However, the HR reported by Carbone et al. (2017) trended to the right side of the axis. It represented that patients administered with ICI therapy did not gain a more lasting survival than those in the chemotherapy group. The range of 95% CI reported by Peters et al. (2022) illustrated that the efficacy of ICI therapy for different patients varies greatly in this study. In general, patients with lung cancer using ICI therapy received more significant benefits than those using non-ICI therapy (HR, 0.78; 95% CI, 0.73–0.82; p < 0.0001). Subgroup analyses were also conducted according to patients’ different baseline characteristics. The results showed that the source of heterogeneity was liver metastases status and PD-L1 expression level.
3.5 Pooled hazard ratios and interaction hazard ratios of patients stratified by baseline characteristics
The pooled HRs and potential interactions are shown in Figure 3. The left forest plot represented pooled HRs (ICIs vs. non-ICIs) for each baseline characteristic. In the group of patients with brain metastases, there was no significant difference between ICI therapy and non-ICI therapy (HR, 0.76; 95% CI, 0.49–1.17; p = 0.2142). It indicated that patients with brain metastases may receive relatively rare clinical benefits from ICI-based therapy.
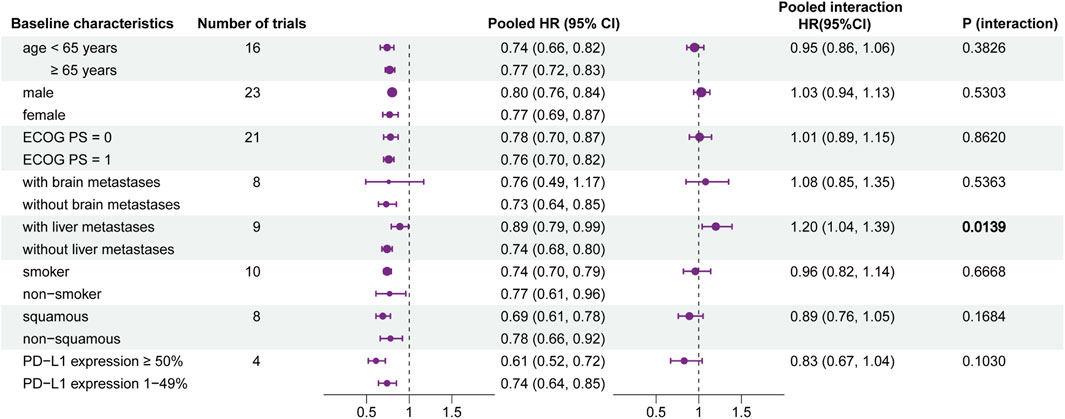
FIGURE 3. Forest plot of hazard ratios for overall survival according to baseline characteristics. Left forest plot: hazard ratios of overall survival for patients assigned to the intervention group, compared with those assigned to the control group, stratified by each baseline characteristic. Right forest plot: interaction between immunotherapy efficacy and each baseline characteristic.
The interaction is shown in the right part of Figure 3. It represented the interaction between immunotherapy and each baseline characteristic. The pooled interaction HR between patients with liver metastases and without liver metastases was 1.20 (95% CI, 1.04–1.39; p = 0.0139). This illustrated that patients without liver metastases may gain more benefits from ICI therapy. Patients stratified by other baseline characteristics did not show a significant difference in efficacy.
3.6 Subgroup analysis
3.6.1 Type of lung cancer
The pooled interaction in patients with brain metastases or without brain metastases was 0.72 (95% CI, 0.50–1.03) in NSCLC and 1.41 (95% CI, 1.05–1.89) in SCLC. By conducting a heterogeneity test of interaction between subgroups, the results showed that there was significant heterogeneity between NSCLC and SCLC (HR, 0.72 vs. 1.41; interaction, p < 0.01) (Figure 4). In other baseline characteristics, no significant difference was observed (Supplementary Figure S1).
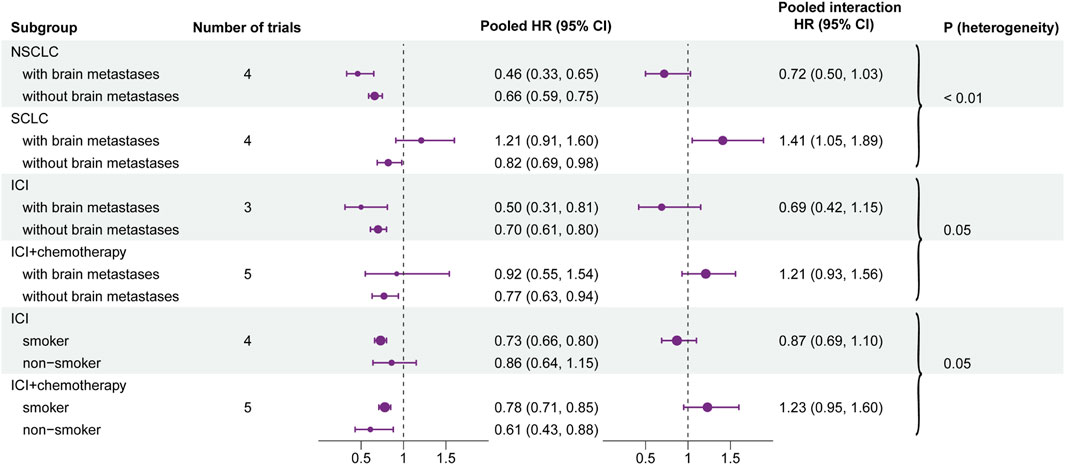
FIGURE 4. Forest plot of hazard ratios for overall survival according to the positive results of each subgroup. Left forest plot: hazard ratios of overall survival for subgroups and baseline characteristics. Right forest plot: interaction between immunotherapy efficacy and baseline characteristics in different subgroups (i.e., type of therapy and type of cancer).
3.6.2 Line of treatment
Details on the subgroup of line of treatment are shown in Supplementary Figure S2. In the following baseline characteristics, no difference in interaction in the lines of therapy was observed: age, gender, ECOG PS, liver metastasis status, smoking status, and histological type.
3.6.3 Type of therapy
The pooled interaction in patients with brain metastases or without brain metastases was 0.69 (95% CI, 0.42–1.15) in the group of ICI monotherapy and 1.21 (95% CI, 0.93–1.56) in the group of ICI combined with chemotherapy. Further analyses illustrated that potential heterogeneity existed between ICI monotherapy and combination therapy (HR, 0.69 vs. 1.21; interaction, p = 0.05). Similar results were also observed in the smoking status. The pooled interaction between smoking patients and non-smokers was 0.87 (95% CI, 0.69–1.10) in ICI monotherapy and 1.23 (95% CI, 0.95–1.60) in combination therapy. Also, the heterogeneity test demonstrated that there was a significant difference between the two groups (HR, 0.87 vs. 1.23; interaction, p = 0.05) (Figure 4). In other baseline characteristics, no obvious heterogeneity was observed (Supplementary Figure S3).
3.7 Immune-related adverse events
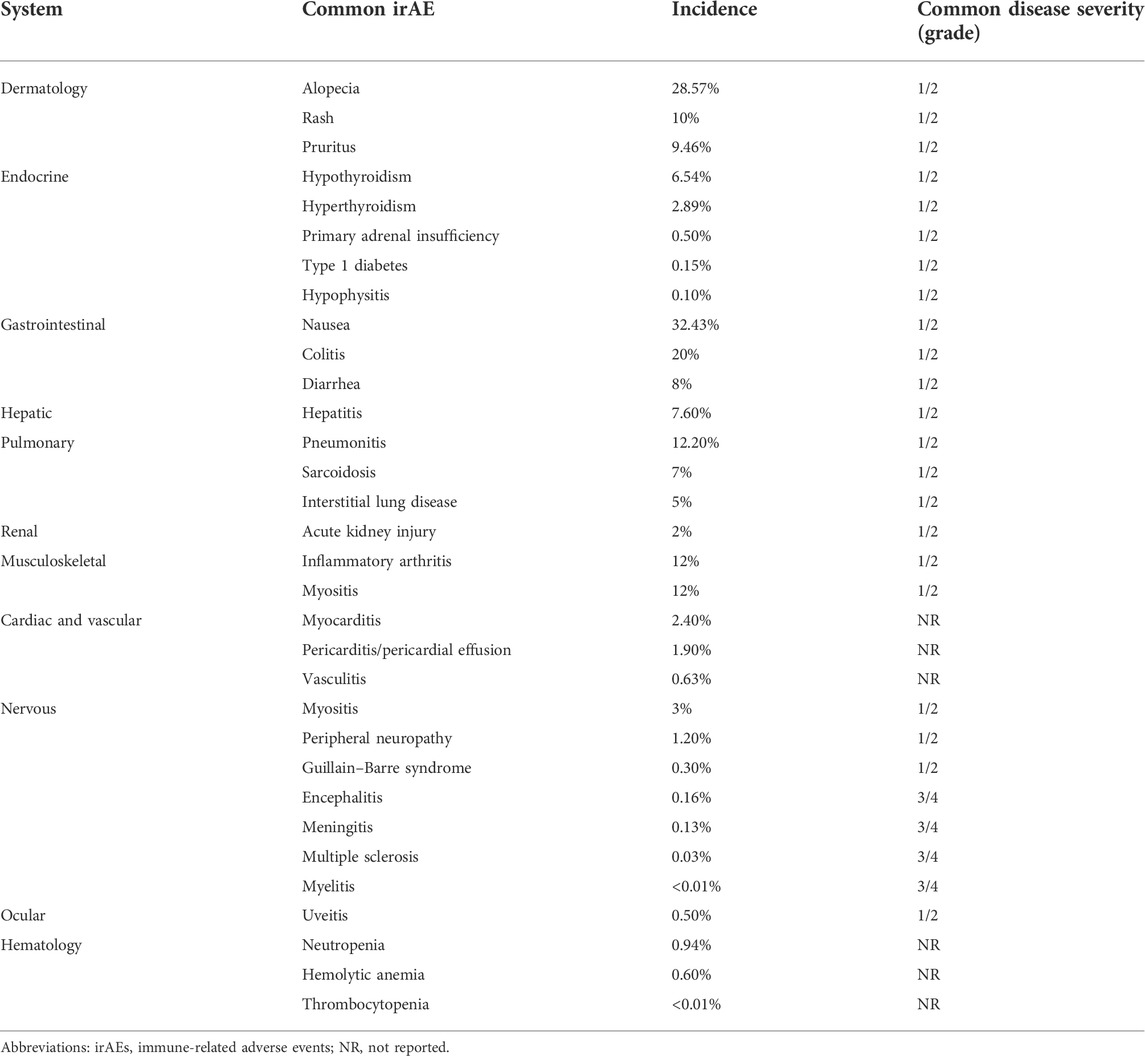
Common irAEs of immunotherapy for lung cancer are shown in Table 2. A total of 11 systems were involved in this study, and it contained the incidence and common disease severity of irAEs. The prevalent severity of most adverse events was graded 1 and 2, while encephalitis, meningitis, multiple sclerosis, and myelitis were graded 3 and 4. Due to the rare incidence of some irAEs, there is currently no report on their disease severity.
3.8 Sensitivity analysis and publication bias
In order to evaluate the influence of each study on overall outcomes, we conducted the sensitivity analysis with both the fixed effects model and random effects model. Similar results were obtained from two models. The absence of each study cannot significantly change the overall values, and it verified the stability of the results in our meta-analysis (Supplementary Figure S4).
We used two methods to assess the publication bias. The p-value of Egger’s test was 0.3003 and that of Begg’s test was 0.2145. Evidence of publication bias was not detected by the funnel plot (Supplementary Figure S5).
4 Discussion
Despite considerable breakthroughs in immunotherapy in the treatment of lung cancer, only few patients benefit from ICIs. Therefore, it makes sense to explore appropriate predictors for suitable patients. Previous studies (Yan et al., 2020; Wang et al., 2021; Xue et al., 2021) found that baseline characteristics (i.e., age, gender, and brain metastases, etc.) could influence the efficacy of ICIs on lung cancer. In practical clinical applications, the interaction among these single characteristics might influence the final responses of the immunotherapy. From a whole perspective, the interaction should be given more importance. Therefore, in order to provide references for clinical decisions, this review analyzed the impact of common baseline characteristics on efficacy. As far as we know, this is the first meta-analysis to investigate the effect of comprehensive baseline characteristics on the efficacy of ICIs for lung cancer.
In general, patients who received ICI-based therapy acquired better survival benefits than those who received non-ICI therapy. However, the HR reported by Carbone et al. (2017). trended to the right side of the axis. The reason was unbalanced baseline characteristics between the two groups. Disease characteristics associated with better prognosis are favored by the chemotherapy group. Because of the discontinuation of some patients, the range of 95% CI reported by Peters et al. (2022) illustrated that the efficacy of ICI therapy for different patients varies greatly in this study.
The statistical heterogeneity of all patients’ HRs for OS among included studies was detected (I2 = 51%; p < 0.01), and we performed this meta-analysis via the random effects model. Statistical heterogeneity was the result of the synergy of clinical and methodological diversity among studies. Due to differences in terms of the populations, interventions, control group, outcome indicators, and study design, there may be clinical heterogeneity among studies. We carefully assessed the differences in these aspects and conducted subgroup analyses to explore the source of heterogeneity. The results showed that the source of heterogeneity was liver metastasis status and PD-L1 expression level. Differences in the study design may lead to methodological heterogeneity, which included, randomization, application of blinding, allocation concealment, completeness of outcome reporting, and rigorousness of statistical analysis. In this meta-analysis, the study design and research quality of the included trials did not show obvious differences.
Although current genetic tests and immunohistochemical tests can direct the application of ICIs in most cases, some baseline characteristics may have potentially directive functions in the choice of ICIs. Liver metastases were potentially related to the poor prognosis of immunotherapy on lung cancer according to the results of our meta-analysis. Immunotherapy in patients without liver metastases might result in a more lasting OS than those with liver metastases. Similar results were also observed in other subgroups (i.e., pathological type, subsequent line, and ICI monotherapy). It was consistent with other reports (Tumeh et al., 2017; Tournoy et al., 2018). The incidence of liver metastases of lung cancer is rare in clinical real-world settings. In the included studies of the liver metastases group, the sample size of patients with or without liver metastases was 1,346 and 3,807, respectively. The incidence of liver metastases in patients with lung cancer was 26.12%. Since we included patients with advanced lung cancer, the sample size of patients with liver metastases would be relatively large. This value did not represent the incidence in the general population. Due to the limitation of sample size, we tentatively drew this conclusion. It is necessary to increase the sample size to further explore the influence of liver metastasis status on immunotherapy for lung cancer.
The liver induces immunotolerance by multiple mechanisms, including poor activation of CD4+ T cells (Wang and Livingstone, 2003), incomplete activation of CD8+ T cells (Limmer et al., 2000), and the apoptosis of activated CD8+ T cells (Crispe, 2003). The tolerable hepatic microenvironment may interfere with the reaction of ICIs in patients with liver metastasis. Meanwhile, CD8+ T cells are associated with the reaction of PD-1 inhibitors. CD8+ T cells are depleted in patients with liver metastases. Therefore, liver metastasis status has an impact on the response of ICIs (Tumeh et al., 2017). Recently, Wu et al. (2022) found that immunosuppressive cells, such as MRC1 CCL18 M2-like macrophages, showed an upward trend in the liver metastasis sites. MRC1 CCL18 M2-like macrophages displayed enhanced metabolic activity in all myeloid cells, resulting in a suppressed immune state and reduced efficacy of immunotherapy. The neoadjuvant chemotherapy mentioned in this study may offer new treatment ideas for patients with liver metastases. The study by Liu et al. (2022) bridged immune phenotypes of primary and metastatic tumors. They found that for myeloid cells, M-type SPP1 macrophages were predominant in liver metastasis, leading to the immunosuppressive state.
In addition, we conducted heterogeneous tests for the interaction of each baseline characteristic in every subgroup. The result showed that the efficacy of patients with brain metastases exhibited significant heterogeneity between NSCLC and SCLC (p < 0.01). It illustrated that tumor types may influence the efficacy of immunotherapy on patients with brain metastases. This phenomenon may be related to the PD-L1 expression level. A higher PD-L1 expression level has been proved to be associated with enhanced efficacy of immunotherapy in NSCLC (Mansour et al., 2022). In our review, patients with brain metastases who received ICI therapy got less benefits than patients who received non-ICI therapy in SCLC. For asymptomatic patients with brain metastases in extensive-stage SCLC, National Comprehensive Cancer Network (NCCN) guidelines (Ganti et al., 2021) recommended the systemic therapy of atezolizumab or durvalumab combined with chemotherapy. The ICI drugs used in two of four included studies were beyond the recommendation of the NCCN guidelines, and corresponding research was still under investigation. The efficacy of ICIs for SCLC still needs further exploration.
In the subgroup of type of treatment, the efficacy of patients with brain metastases between ICI monotherapy and combination therapy had potential heterogeneity. The same research results were also observed in smoking status. It indicated that the type of treatment may affect the efficacy of immunotherapy for patients with brain metastases and smoking status. In our meta-analysis, compared with ICI monotherapy, patients with brain metastases who received combination therapy showed less clinical benefits. In our included articles, three of four studies in NSCLC applied ICI monotherapy, and all four studies in SCLC applied ICI combined with chemotherapy. In the aforementioned discussion, we discussed the reason for the poor efficacy of ICIs for SCLC in this review. So patients with brain metastases who received combination therapy gained less benefits from ICI therapy.
Moreover, our meta-analysis discovered that patients with smoking history benefitted more from ICI monotherapy, while non-smokers obtained more benefits from combination therapy. On the one hand, the study by Yang et al. (2021) pointed out that smoking may increase tumor mutation burden (TMB) and microsatellite instability (MSI). This mechanism may improve the reaction of immunotherapy. On the other hand, from the point of pathological type, patients with smoking history took higher risks on suffering from squamous cell carcinoma (Park et al., 2010), while non-smokers may have higher genetic susceptibility to adenocarcinoma (Fu et al., 2022). The research by Tian et al. (2021) indicated that patients with squamous cell carcinoma may obtain more lasting OS from ICIs than those with adenocarcinoma. More functional tumor-infiltrating lymphocytes and chemokines in the tumor microenvironment are present in squamous NSCLC.
Our study found that gender is not associated with the efficacy of immunotherapy for lung cancer. This is different from the results of previous studies (Jang et al., 2021; Wu et al., 2018b). We found that the reason for the different results among the studies may be due to different patient populations. The study by Jang et al. (2021) included patients with melanoma; Wu et al. (2018b) included patients with various tumor types, while our study just included patients with lung cancer. The efficacy of immunotherapy varies by tumor types.
In terms of the comparison between PD-1 and PD-L1 agents, no statistically significant difference was found in the efficacy between PD-1 and PD-L1 agents for patients with lung cancer (HR, 0.73 vs. 0.78; p = 0.34). This result is different from the previous literature (Duan et al., 2020; Wu et al., 2018a). The possible reason may be due to the different patient populations. The study by Duan et al. (2020) included patients with solid tumors; Wu et al. (2018a) included patients with NSCLC, while our study included patients with lung cancer. Immunotherapy responds differently in different tumor types.
A previous study indicated that about 40% of patients using immunotherapy may experience irAEs (D'Souza et al., 2021). The study by Garrett et al. (2020) also reported the incidence and disease severity of dermatological toxicities and provided a rational strategy for the management of irAEs. Our study summarized the frequent irAEs of immunotherapy for lung cancer. We found that irAEs are difficult to avoid in the process of immunotherapy. Individualized precision medicine may have the potential effects on balancing the efficacy of immunotherapy and the occurrence of irAEs.
Empirical targeted therapy is less dependent on emerging technologies. However, only a minority of patients with cancer could get durable survival from it. It is necessary to determine the most suitable treatment plan for different patients. Precision medicine fully considers the individual heterogeneity of patients. In this way, the treatment plan will take into account the best treatment effect and the best medical cost-effectiveness ratio. Therefore, precision medicine is becoming the trend in cancer treatment.
However, there are several potential limitations to this study. First, HRs for OS in patients with various baseline characteristics were provided by different clinical trials and various institutions. It may lead to inaccurate data collection. Second, owing to the fact that we just focussed on RCTs, the data of patients who did not meet the inclusion criteria were missing. Third, although our review covered the effect of common baseline characteristics on the efficacy of ICIs for lung cancer, due to insufficient data on other baseline characteristics, there may exist other confounding factors which might affect the efficacy, such as region and disease severity.
5 Conclusion
Current data indicated that ICI-based therapy improved the OS of most patients stratified by different baseline characteristics compared with non-ICI therapy. Liver metastasis status would influence the final efficacy of ICIs, and other baseline characteristics might be independent of it. Through subgroup analysis, compared with small-cell lung cancer, patients with brain metastases might have a more durable OS in non-small-cell lung cancer. The smoking history or brain metastasis status of patients could indicate the potential clinical benefits of monotherapy or combination therapy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author contributions
Work design and task assignment: QX and YZ. Data collection and analysis: QX, XYu, ZS, and TY. Manuscript writing: QX and XYu. Manuscript revision: TY, XYa, and YZ. Manuscript approval: all authors.
Funding
The present study was funded by the National Natural Science Foundation of China (Grant No.: 81603577) and the National Key Clinical Specialty of the National Health and Family Planning Commission (pulmonary disease) (Grant No.: 040203001001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.956788/full#supplementary-material
References
Altman, D. G., and Bland, J. M. (2003). Interaction revisited: the difference between two estimates. BMJ 326 (7382), 219. doi:10.1136/bmj.326.7382.219
Balduzzi, S., Rucker, G., and Schwarzer, G. (2019). How to perform a meta-analysis with R: a practical tutorial. Evid. Based. Ment. Health 22 (4), 153–160. doi:10.1136/ebmental-2019-300117
Borghaei, H., Paz-Ares, L., Horn, L., Spigel, D. R., Steins, M., Ready, N. E., et al. (2015). Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 373 (17), 1627–1639. doi:10.1056/NEJMoa1507643
Brahmer, J., Reckamp, K. L., Baas, P., Crino, L., Eberhardt, W. E., Poddubskaya, E., et al. (2015). Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 373 (2), 123–135. doi:10.1056/NEJMoa1504627
Carbone, D. P., Reck, M., Paz-Ares, L., Creelan, B., Horn, L., Steins, M., et al. (2017). First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N. Engl. J. Med. 376 (25), 2415–2426. doi:10.1056/NEJMoa1613493
Crispe, I. N. (2003). Hepatic T cells and liver tolerance. Nat. Rev. Immunol. 3 (1), 51–62. doi:10.1038/nri981
D'Souza, M., Nielsen, D., Svane, I. M., Iversen, K., Rasmussen, P. V., Madelaire, C., et al. (2021). The risk of cardiac events in patients receiving immune checkpoint inhibitors: a nationwide Danish study. Eur. Heart J. 42 (16), 1621–1631. doi:10.1093/eurheartj/ehaa884
Duan, J., Cui, L., Zhao, X., Bai, H., Cai, S., Wang, G., et al. (2020). Use of immunotherapy with programmed cell death 1 vs programmed cell death ligand 1 inhibitors in patients with cancer: A systematic review and meta-analysis. JAMA Oncol. 6 (3), 375–384. doi:10.1001/jamaoncol.2019.5367
Fehrenbacher, L., Spira, A., Ballinger, M., Kowanetz, M., Vansteenkiste, J., Mazieres, J., et al. (2016). Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 387 (10030), 1837–1846. doi:10.1016/s0140-6736(16)00587-0
Fu, R., Zhang, J. T., Chen, R. R., Li, H., Tai, Z. X., Lin, H. X., et al. (2022). Identification of heritable rare variants associated with early-stage lung adenocarcinoma risk. Transl. Lung Cancer Res. 11 (4), 509–522. doi:10.21037/tlcr-21-789
Ganti, A. K. P., Loo, B. W., Bassetti, M., Blakely, C., Chiang, A., D'Amico, T. A., et al. (2021). Small cell lung cancer, version 2.2022, NCCN clinical practice guidelines in Oncology. J. Natl. Compr. Canc. Netw. 19 (12), 1441–1464. doi:10.6004/jnccn.2021.0058
Garrett, N., da Costa, A. C. C., Damiani, G., and Vasques, C. I. (2020). Patients with lung cancer undergoing immune checkpoint inhibitors: A meta-analysis of dermatological toxicities. Crit. Rev. Oncol. Hematol. 152, 102983. doi:10.1016/j.critrevonc.2020.102983
Goldman, J. W., Dvorkin, M., Chen, Y., Reinmuth, N., Hotta, K., Trukhin, D., et al. (2021). Durvalumab, with or without tremelimumab, plus platinum–etoposide versus platinum–etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet. Oncol. 22 (1), 51–65. doi:10.1016/s1470-2045(20)30539-8
Govindan, R., Szczesna, A., Ahn, M. J., Schneider, C. P., Gonzalez Mella, P. F., Barlesi, F., et al. (2017). Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung cancer. J. Clin. Oncol. 35 (30), 3449–3457. doi:10.1200/JCO.2016.71.7629
Hegde, P. S., and Chen, D. S. (2020). Top 10 challenges in cancer immunotherapy. Immunity 52 (1), 17–35. doi:10.1016/j.immuni.2019.12.011
Hellmann, M. D., Paz-Ares, L., Bernabe Caro, R., Zurawski, B., Kim, S. W., Carcereny Costa, E., et al. (2019). Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N. Engl. J. Med. 381 (21), 2020–2031. doi:10.1056/NEJMoa1910231
Herbst, R. S., Garon, E. B., Kim, D. W., Cho, B. C., Gervais, R., Perez-Gracia, J. L., et al. (2021). Five year survival update from KEYNOTE-010: Pembrolizumab versus docetaxel for previously treated, programmed death-ligand 1-positive advanced NSCLC. J. Thorac. Oncol. 16 (10), 1718–1732. doi:10.1016/j.jtho.2021.05.001
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Higgins, J. P., Altman, D. G., Gotzsche, P. C., Juni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Jang, S. R., Nikita, N., Banks, J., Keith, S. W., Johnson, J. M., Wilson, M., et al. (2021). Association between sex and immune checkpoint inhibitor outcomes for patients with melanoma. JAMA Netw. Open 4 (12), e2136823. doi:10.1001/jamanetworkopen.2021.36823
Lee, Y. T., Tan, Y. J., and Oon, C. E. (2018). Molecular targeted therapy: treating cancer with specificity. Eur. J. Pharmacol. 834, 188–196. doi:10.1016/j.ejphar.2018.07.034
Limmer, A., Ohl, J., Kurts, C., Ljunggren, H. G., Reiss, Y., Groettrup, M., et al. (2000). Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat. Med. 6 (12), 1348–1354. doi:10.1038/82161
Liu, S. V., Reck, M., Mansfield, A. S., Mok, T., Scherpereel, A., Reinmuth, N., et al. (2021). Updated overall survival and PD-L1 subgroup Analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J. Clin. Oncol. 39 (6), 619–630. doi:10.1200/JCO.20.01055
Liu, Y., Zhang, Q., Xing, B., Luo, N., Gao, R., Yu, K., et al. (2022). Immune phenotypic linkage between colorectal cancer and liver metastasis. Cancer Cell 40 (4), 424–437.e5. doi:10.1016/j.ccell.2022.02.013
Mansour, M. S. I., Malmros, K., Mager, U., Ericson Lindquist, K., Hejny, K., Holmgren, B., et al. (2022). PD-L1 expression in non-small cell lung cancer specimens: Association with clinicopathological factors and molecular alterations. Int. J. Mol. Sci. 23 (9), 4517. doi:10.3390/ijms23094517
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and Group, P. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J. Clin. Epidemiol. 62 (10), 1006–1012. doi:10.1016/j.jclinepi.2009.06.005
Nishio, M., Barlesi, F., West, H., Ball, S., Bordoni, R., Cobo, M., et al. (2021). Atezolizumab plus chemotherapy for first-line treatment of nonsquamous NSCLC: Results from the randomized phase 3 IMpower132 trial. J. Thorac. Oncol. 16 (4), 653–664. doi:10.1016/j.jtho.2020.11.025
Owonikoko, T. K., Park, K., Govindan, R., Ready, N., Reck, M., Peters, S., et al. (2021). Nivolumab and ipilimumab as maintenance therapy in extensive-disease small-cell lung cancer: CheckMate 451. J. Clin. Oncol. 39 (12), 1349–1359. doi:10.1200/JCO.20.02212
Pardoll, D. M. (2012). The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12 (4), 252–264. doi:10.1038/nrc3239
Park, S. K., Cho, L. Y., Yang, J. J., Park, B., Chang, S. H., Lee, K. S., et al. (2010). Lung cancer risk and cigarette smoking, lung tuberculosis according to histologic type and gender in a population based case-control study. Lung Cancer 68 (1), 20–26. doi:10.1016/j.lungcan.2009.05.017
Park, K., Ozguroglu, M., Vansteenkiste, J., Spigel, D., Yang, J. C. H., Ishii, H., et al. (2021). Avelumab versus docetaxel in patients with platinum-treated advanced NSCLC: 2-Year follow-up from the JAVELIN lung 200 phase 3 trial. J. Thorac. Oncol. 16 (8), 1369–1378. doi:10.1016/j.jtho.2021.03.009
Paz-Ares, L., Luft, A., Vicente, D., Tafreshi, A., Gumus, M., Mazieres, J., et al. (2018). Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N. Engl. J. Med. 379 (21), 2040–2051. doi:10.1056/NEJMoa1810865
Peters, S., Pujol, J. L., Dafni, U., Domine, M., Popat, S., Reck, M., et al. (2022). Consolidation nivolumab and ipilimumab versus observation in limited-disease small-cell lung cancer after chemo-radiotherapy - results from the randomised phase II ETOP/IFCT 4-12 STIMULI trial. Ann. Oncol. 33 (1), 67–79. doi:10.1016/j.annonc.2021.09.011
Reck, M., Luft, A., Szczesna, A., Havel, L., Kim, S. W., Akerley, W., et al. (2016). Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J. Clin. Oncol. 34 (31), 3740–3748. doi:10.1200/JCO.2016.67.6601
Reck, M., Rodriguez-Abreu, D., Robinson, A. G., Hui, R., Csoszi, T., Fulop, A., et al. (2019). Updated analysis of KEYNOTE-024: Pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J. Clin. Oncol. 37 (7), 537–546. doi:10.1200/JCO.18.00149
Reck, M., Ciuleanu, T. E., Cobo, M., Schenker, M., Zurawski, B., Menezes, J., et al. (2021). First-line nivolumab plus ipilimumab with two cycles of chemotherapy versus chemotherapy alone (four cycles) in advanced non-small-cell lung cancer: CheckMate 9LA 2-year update. ESMO Open 6 (5), 100273. doi:10.1016/j.esmoop.2021.100273
Rittmeyer, A., Barlesi, F., Waterkamp, D., Park, K., Ciardiello, F., von Pawel, J., et al. (2017). Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389 (10066), 255–265. doi:10.1016/s0140-6736(16)32517-x
Rodriguez-Abreu, D., Powell, S. F., Hochmair, M. J., Gadgeel, S., Esteban, E., Felip, E., et al. (2021). Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: Protocol-specified final analysis from KEYNOTE-189. Ann. Oncol. 32 (7), 881–895. doi:10.1016/j.annonc.2021.04.008
Rudin, C. M., Awad, M. M., Navarro, A., Gottfried, M., Peters, S., Csoszi, T., et al. (2020). Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: Randomized, double-blind, phase III KEYNOTE-604 study. J. Clin. Oncol. 38 (21), 2369–2379. doi:10.1200/JCO.20.00793
Sethi, J., Ali, M. S., Mohananey, D., Nanchal, R., Maldonado, F., and Musani, A. (2019). Are transbronchial cryobiopsies ready for prime time?: A systematic review and meta-analysis. J. Bronchology Interv. Pulmonol. 26 (1), 22–32. doi:10.1097/LBR.0000000000000519
Sezer, A., Kilickap, S., Gümüş, M., Bondarenko, I., Özgüroğlu, M., Gogishvili, M., et al. (2021). Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet 397 (10274), 592–604. doi:10.1016/s0140-6736(21)00228-2
Sibiya, M. A., Raphoko, L., Mangokoana, D., Makola, R., Nxumalo, W., and Matsebatlela, T. M. (2019). Induction of cell death in human A549 cells using 3-(Quinoxaline-3-yl) prop-2-ynyl methanosulphonate and 3-(Quinoxaline-3-yl) prop-2-yn-1-ol. Molecules 24 (3), E407. doi:10.3390/molecules24030407
Siegel, R. L., Miller, K. D., Fuchs, H. E., and Jemal, A. (2022). Cancer statistics, 2022. CA. Cancer J. Clin. 72 (1), 7–33. doi:10.3322/caac.21708
Socinski, M. A., Nishio, M., Jotte, R. M., Cappuzzo, F., Orlandi, F., Stroyakovskiy, D., et al. (2021). IMpower150 final overall survival analyses for atezolizumab plus bevacizumab and chemotherapy in first-line metastatic nonsquamous NSCLC. J. Thorac. Oncol. 16 (11), 1909–1924. doi:10.1016/j.jtho.2021.07.009
Song, P., Zhang, J., Shang, C., and Zhang, L. (2019). Real-world evidenceand clinical observations of the treatment of advanced non-small cell lung cancer with PD-1/PD-L1 inhibitors. Sci. Rep. 9 (1), 4278. doi:10.1038/s41598-019-40748-7
Spigel, D. R., Vicente, D., Ciuleanu, T. E., Gettinger, S., Peters, S., Horn, L., et al. (2021). Second-line nivolumab in relapsed small-cell lung cancer: CheckMate 331. Ann. Oncol. 32 (5), 631–641. doi:10.1016/j.annonc.2021.01.071
Spigel, D. R., Faivre-Finn, C., Gray, J. E., Vicente, D., Planchard, D., Paz-Ares, L., et al. (2022). Five-year survival outcomes from the PACIFIC trial: Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J. Clin. Oncol. 40 (12), 1301–1311. doi:10.1200/JCO.21.01308
Sugawara, S., Lee, J. S., Kang, J. H., Kim, H. R., Inui, N., Hida, T., et al. (2021). Nivolumab with carboplatin, paclitaxel, and bevacizumab for first-line treatment of advanced nonsquamous non-small-cell lung cancer. Ann. Oncol. 32 (9), 1137–1147. doi:10.1016/j.annonc.2021.06.004
Tian, Y., Zhai, X., Yan, W., Zhu, H., and Yu, J. (2021). Clinical outcomes of immune checkpoint blockades and the underlying immune escape mechanisms in squamous and adenocarcinoma NSCLC. Cancer Med. 10 (1), 3–14. doi:10.1002/cam4.3590
Tournoy, K. G., Thomeer, M., Germonpre, P., Derijcke, S., De Pauw, R., Galdermans, D., et al. (2018). Does nivolumab for progressed metastatic lung cancer fulfill its promises? An efficacy and safety analysis in 20 general hospitals. Lung Cancer 115, 49–55. doi:10.1016/j.lungcan.2017.11.008
Tumeh, P. C., Hellmann, M. D., Hamid, O., Tsai, K. K., Loo, K. L., Gubens, M. A., et al. (2017). Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol. Res. 5 (5), 417–424. doi:10.1158/2326-6066.CIR-16-0325
Wang, J. C., and Livingstone, A. M. (2003). Cutting edge: CD4+ T cell help can be essential for primary CD8+ T cell responses in vivo. J. Immunol. 171 (12), 6339–6343. doi:10.4049/jimmunol.171.12.6339
Wang, Y., Zhang, Q., Chen, C., Hu, Y., Miao, L., and Zhou, Y. (2021). Association of brain metastases with immune checkpoint inhibitors efficacy in advanced lung cancer: A systematic review and meta-analysis. Front. Oncol. 11, 721760. doi:10.3389/fonc.2021.721760
West, H., McCleod, M., Hussein, M., Morabito, A., Rittmeyer, A., Conter, H. J., et al. (2019). Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet. Oncol. 20 (7), 924–937. doi:10.1016/s1470-2045(19)30167-6
Wu, Y., Lin, L., Shen, Y., and Wu, H. (2018a). Comparison between PD-1/PD-L1 inhibitors (nivolumab, pembrolizumab, and atezolizumab) in pretreated NSCLC patients: Evidence from a Bayesian network model. Int. J. Cancer 143 (11), 3038–3040. doi:10.1002/ijc.31733
Wu, Y., Ju, Q., Jia, K., Yu, J., Shi, H., Wu, H., et al. (2018b). Correlation between sex and efficacy of immune checkpoint inhibitors (PD-1 and CTLA-4 inhibitors). Int. J. Cancer 143 (1), 45–51. doi:10.1002/ijc.31301
Wu, Y. L., Zhang, L., Fan, Y., Zhou, J., Zhang, L., Zhou, Q., et al. (2021). Randomized clinical trial of pembrolizumab vs chemotherapy for previously untreated Chinese patients with PD-L1-positive locally advanced or metastatic non-small-cell lung cancer: KEYNOTE-042 China study. Int. J. Cancer 148 (9), 2313–2320. doi:10.1002/ijc.33399
Wu, Y., Yang, S., Ma, J., Chen, Z., Song, G., Rao, D., et al. (2022). Spatiotemporal immune landscape of colorectal cancer liver metastasis at single-cell level. Cancer Discov. 12 (1), 134–153. doi:10.1158/2159-8290.CD-21-0316
Xu, Y., Wang, Q., Xie, J., Chen, M., Liu, H., Zhan, P., et al. (2021). The predictive value of clinical and molecular characteristics or immunotherapy in non-small cell lung cancer: A meta-analysis of randomized controlled trials. Front. Oncol. 11, 732214. doi:10.3389/fonc.2021.732214
Xue, C., Zheng, S., Dong, H., Lu, X., Zhang, X., Zhang, J., et al. (2021). Association between efficacy of immune checkpoint inhibitors and sex: An updated meta-analysis on 21 trials and 12, 675 non-small cell lung cancer patients. Front. Oncol. 11, 627016. doi:10.3389/fonc.2021.627016
Yan, X., Tian, X., Wu, Z., and Han, W. (2020). Impact of age on the efficacy of immune checkpoint inhibitor-based combination therapy for non-small-cell lung cancer: A systematic review and meta-analysis. Front. Oncol. 10, 1671. doi:10.3389/fonc.2020.01671
Yang, H., Ma, W., Sun, B., Fan, L., Xu, K., Hall, S. R. R., et al. (2021). Smoking signature is superior to programmed death-ligand 1 expression in predicting pathological response to neoadjuvant immunotherapy in lung cancer patients. Transl. Lung Cancer Res. 10 (9), 3807–3822. doi:10.21037/tlcr-21-734
Yong, T., Chang, K. K., Wang, Y. S., and Ma, C. (2022). Active humoral response reverts tumorigenicity through disruption of key signaling pathway. Vaccines (Basel) 10 (2), 163. doi:10.3390/vaccines10020163
Keywords: baseline characteristic, immune checkpoint inhibitor, lung cancer, efficacy, meta-analysis
Citation: Xiao Q, Yu X, Shuai Z, Yao T, Yang X and Zhang Y (2022) The influence of baseline characteristics on the efficacy of immune checkpoint inhibitors for advanced lung cancer: A systematic review and meta-analysis. Front. Pharmacol. 13:956788. doi: 10.3389/fphar.2022.956788
Received: 30 May 2022; Accepted: 19 August 2022;
Published: 09 September 2022.
Edited by:
Giovanni Damiani, University of Milan, ItalyCopyright © 2022 Xiao, Yu, Shuai, Yao, Yang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanxia Zhang, emhhbmd5eDkyOUBidWNtLmVkdS5jbg==
 Qionghua Xiao
Qionghua Xiao Xiaolin Yu
Xiaolin Yu Zhihao Shuai1
Zhihao Shuai1 Ting Yao
Ting Yao Yanxia Zhang
Yanxia Zhang