- Department of Orthopedics and Spine Surgery, The First Affiliated Hospital of Anhui Medical University, Hefei, China
Low back pain is a major cause of disability worldwide that declines the quality of life; it poses a substantial economic burden for the patient and society. Intervertebral disc (IVD) degeneration (IDD) is the main cause of low back pain, and it is also the pathological basis of several spinal degenerative diseases, such as intervertebral disc herniation and spinal stenosis. The current clinical drug treatment of IDD focuses on the symptoms and not their pathogenesis, which results in frequent recurrence and gradual aggravation. Moreover, the side effects associated with the long-term use of these drugs further limit their use. The pathological mechanism of IDD is complex, and oxidative stress and inflammation play an important role in promoting IDD. They induce the destruction of the extracellular matrix in IVD and reduce the number of living cells and functional cells, thereby destroying the function of IVD and promoting the occurrence and development of IDD. Phytochemicals from fruits, vegetables, grains, and other herbs play a protective role in the treatment of IDD as they have anti-inflammatory and antioxidant properties. This article reviews the protective effects of phytochemicals on IDD and their regulatory effects on different molecular pathways related to the pathogenesis of IDD. Moreover, the therapeutic limitations and future prospects of IDD treatment have also been reviewed. Phytochemicals are promising candidates for further development and research on IDD treatment.
Introduction
Low back pain (LBP) is one of the most prevalent musculoskeletal disorders in the world, and it is estimated that nearly 80% of the population suffers from LBP during their lifetime. The occurrence of LBP in a patient induces a severe burden on their families and society. Intervertebral disc (IVD) degeneration (IDD) is currently believed to be an essential cause of LBP, and it forms the pathophysiological basis of several spinal degenerative diseases, such as intervertebral disc herniation and spinal stenosis (Wang et al., 2014; Vo et al., 2016; Oichi et al., 2020). The conservative clinical treatment for the early stages of IDD primarily includes nonsteroidal anti-inflammatory drugs, physical therapy, and rest. However, such treatment is limited to reducing or controlling pain and does not reverse the process of IDD. Moreover, the long-term use of nonsteroidal anti-inflammatory drugs has apparent side effects (Conaghan, 2012; Bindu et al., 2020). If IDD develops to an advanced stage, surgical treatment, including discectomy and interbody fusion, is required. Although surgical treatment is recommended to be effective for IDD, it is expensive and is associated with several complications, such as adjacent segment disease (Lau et al., 2021), decreased spinal mobility, and limited function. Hence, it is imperative to explore new methods for the treatment of IDD.
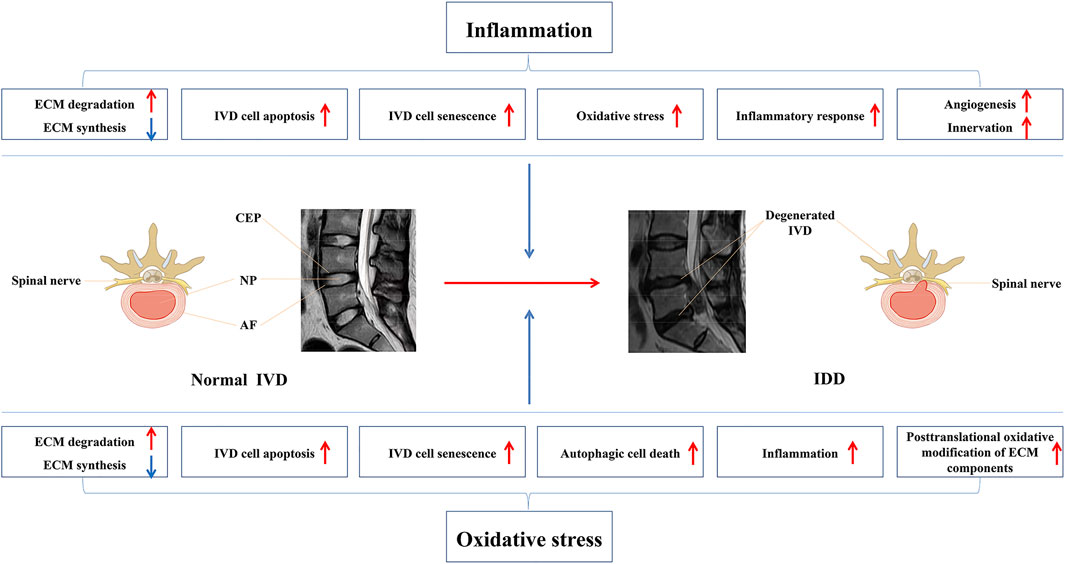
The IVD, which is the largest avascular structure, is an important component of the load-bearing capacity of the spine. It comprises three distinct regions: the centrally located nucleus pulposus (NP), peripheral annulus fibrosus (AF) surrounding NP, and cartilage endplate (CEP) located above and below (Adams and Roughley, 2006). The NP is composed mainly of water and a rich extracellular matrix (ECM), which provides resistance to IVD against axial pressures that are transmitted down the spine. The AF consists of concentrically arranged fibrous layers (15–25 layers) that resist the lateral expansion of the IVD during weight-bearing activities. The CEP not only seals the IVD, but also attaches it to the vertebral body. Most importantly, CEP furnishes a permeable barrier between the IVD and the vertebral body to provide nutrition for the IVD cells, but its ability to provide nutrition is limited. Therefore, it is difficult for IVD to repair itself in case of injury (Freemont, 2009).
The presence of three distinct anatomical regions generates the structural complexity of the disc. Healthy discs transmit and absorb stresses in the spine and maintain the multi-axial flexibility of the spine. In contrast, degenerating discs show structural damage that is characterized by high disc collapse, AF rupture, NP tissue loss, decreased water content, and CEP calcification (Ding et al., 2013). IDD is a multifactorial disease with etiologies including infection, genetic susceptibility, aging, trauma, smoking, and diabetes. Many signaling pathways and effector molecules have been implicated in the IDD process, and elucidation of the pathological mechanisms of IDD will facilitate the improvement of its treatment options. Recently, a growing number of studies have revealed a close relationship between inflammation, oxidative stress, and the incidence of IDD (Dowdell et al., 2017). Therefore, antioxidant and anti-inflammatory treatments have been proposed as promising strategies for the treatment of IDD. The following section details the roles of inflammation and oxidative stress in the pathogenesis of IDD.
Inflammation in Intervertebral Disc Degeneration
Increasing evidence has shown that inflammation is implicated in the occurrence and development of IDD. Several pro-inflammatory cytokines, such as IL-6, IL-17, IL-1α, TNF-α, IL-1β, and IL-8, significantly contribute to an increase in degenerative IVD. These cytokines are closely associated with several key pathophysiological processes of IDD (Johnson et al., 2015; Wang Y. et al., 2020). IL-1β stimulation has been reported to significantly enhance the expression of IL-6, IL-8, and IL-17 in human IVD cells, resulting in an inflammatory cascade. Simultaneously, a feedback loop is formed between these pro-inflammatory cytokines, which forms a persistent local inflammatory microenvironment (Jimbo et al., 2005; Jia et al., 2020). Furthermore, the balance between catabolism and anabolism of ECM is essential for maintaining the structural and functional integrity of IVD, i.e., if the ECM catabolic activity is higher than anabolic activity, it can result in the occurrence of IDD. The primary enzymes that cleave ECM components include matrix metalloproteinases (MMPs) and a disintegrin and metalloprotease with thrombospondin motifs (ADAMTS) (Le Maitre et al., 2007; Vo et al., 2013). Some ECM-degrading enzymes, such as MMP-1/3/7/9 and ADAMTS-1/4/5/9 are significantly increased in degenerative IVD (Le Maitre et al., 2007). Furthermore, pro-inflammatory cytokines can contribute to ECM degradation and the subsequent destruction of the IVD structure by promoting the expression of ECM-degrading enzymes. Apoptosis refers to the spontaneous and orderly death of cells regulated by genes in order to maintain tissue homeostasis. Under physiological conditions, apoptosis plays an important role in maintaining tissue homeostasis. However, in the pathological state, excessive apoptosis stimulated by risk factors will lead to a significant reduction in the number of IVD cells, resulting in the destruction of the structure and function of IVD. Notably, several studies have shown that pro-inflammatory cytokines can induce apoptosis of IVD cells, and then lead to the occurrence and development of IDD. Cell senescence is an irreversible cell cycle arrest caused by a number of factors, such as oxidative stress, pro-inflammatory cytokines, DNA damage. Senescent cells are active and exhibit pro-inflammatory and catabolic phenotypes. Pro-inflammatory cytokines can also accelerate the senescence of IVD cells. Senescent cells can produce more matrix-degrading enzymes and pro-inflammatory cytokines, resulting in further deterioration of the IVD microenvironment (Zhang et al., 2020). Recently, the relationship between inflammation and oxidative stress has also attracted much attention. Pro-inflammatory cytokines have been proved to promote the excessive production of ROS in IVD cells, and then induce oxidative injury of IVD cells (Wang Y. et al., 2020). Vascular endothelial growth factor (VEGF) is an essential member of the pro-angiogenic factor. The expression of VEGF in degenerative IVD is significantly upregulated. Studies have revealed that pro-inflammatory cytokines can upregulate the expression of VEGF in IVD (Kwon et al., 2017). Most importantly, these pro-inflammatory factors can induce stimulation of sinus vertebral nerve endings (Ohtori et al., 2012a; Ohtori et al., 2012b). These nerve endings grow into the IVD and cause nerve root pain (Freemont et al., 2002; Orita et al., 2010), which is the main cause of chronic LBP (Johnson et al., 2001). These findings highlight that inflammation has a central role in the pathogenesis of IDD (Francisco et al., 2022) and suggest that anti-inflammatory strategies can be promising for the treatment of IDD.
Oxidative Stress in Intervertebral Disc Degeneration
Reactive oxygen species (ROS) are unstable and highly reactive molecules (Feng et al., 2017). They include superoxide anions (O2−), hydrogen peroxide (H2O2), hydroxyl radicals (OH−), and hypochlorite ions (OCL−) (Cao et al., 2022). They are the by-products of oxidative metabolism (Reuter et al., 2010; Kim and Yim, 2015). Although the nutrition supply of IVD is low, the NP, AF, and CEP cells are not anaerobic (Bartels et al., 1998; Lee et al., 2007). During IDD, neovascularization increases the blood supply in IVD cells, thereby increasing the nutrient supply, which promotes ROS production from the original nutrient-deficient disc cells and results in oxidative stress (Gille and Nohl, 2001; Turrens, 2003). Oxidative stress is attributed to an imbalance between ROS production and the protective mechanism of antioxidants, resulting in molecular oxidative damage and cell destruction, which has adverse implications on the body. Studies have shown that ROS are widely involved in signal transduction, metabolic regulation, apoptosis, cell senescence, and the phenotypic transformation of cells in IDD (Feng et al., 2017; Cao et al., 2022). Excessive ROS activates the NF-κB and MAPK pathways, resulting in an imbalance between degradation and synthesis of ECM in disc cells and an increase in the secretion of pro-inflammatory factors. These changes eventually lead to the loss of disc cells and the persistence of the inflammatory microenvironment, which further leads to the destruction of IVD and the production of ROS (Zhou et al., 2010; Zhu et al., 2019). Autophagy is a protective process in which cells degrade metabolic waste and further reuse it; however continuous oxidative stress can induce excessive autophagy and lead to cell death (Chen et al., 2015; Filomeni et al., 2015; Cao et al., 2022). In addition, oxidative stress can destroy mitochondria and release pro-apoptotic molecules from the mitochondria into the cytoplasm, causing cascade reactions and cell apoptosis (Chen et al., 2014; Yang et al., 2015). Excessive ROS production can also promote IVD cell senescence, thus promoting the secretion of pro-inflammatory factors, leading to adjacent IVD cell senescence, apoptosis, and ECM degradation (Dimozi et al., 2015). Moreover, the increase of ROS production leads to the triggering of glycosylation reaction which further results in the increase of endogenous active by-products and the generation of advanced glycation end products (AGEs) (Vistoli et al., 2013). AGEs have been proved to be closely related to the pathogenesis of IDD. They can promote the inflammation, apoptosis, and ECM degradation of IVD cells, resulting in the destruction of IVD structure and function (Song et al., 2017; Song et al., 2018). These findings indicate that antioxidation is a new and effective treatment for IDD. Figure 1 provides an overview of the mechanisms by which inflammation and oxidative stress participate in the occurrence and development of IDD.
Phytochemicals for the Treatment of IDD by Targeting Inflammation and Oxidative Stress
In recent years, several phytochemicals from traditional medicinal plants have been studied owing to their low cost, wide availability, and diverse biological activities. Owing to their anti-inflammatory and antioxidant properties, phytochemicals have been used to treat several diseases, such as myocardial ischemia, traumatic brain injury, osteoarthritis, and cancer (Cai et al., 2021; Deng et al., 2021; Tian et al., 2021; Chen C. et al., 2022). Several in vivo and in vitro experiments (Zhu et al., 2020; Chen et al., 2021) have shown that phytochemicals can play a protective role against IDD by targeting inflammation and oxidative stress. The effects and mechanisms of various phytochemicals against IDD are described in the following sections (Table 1).
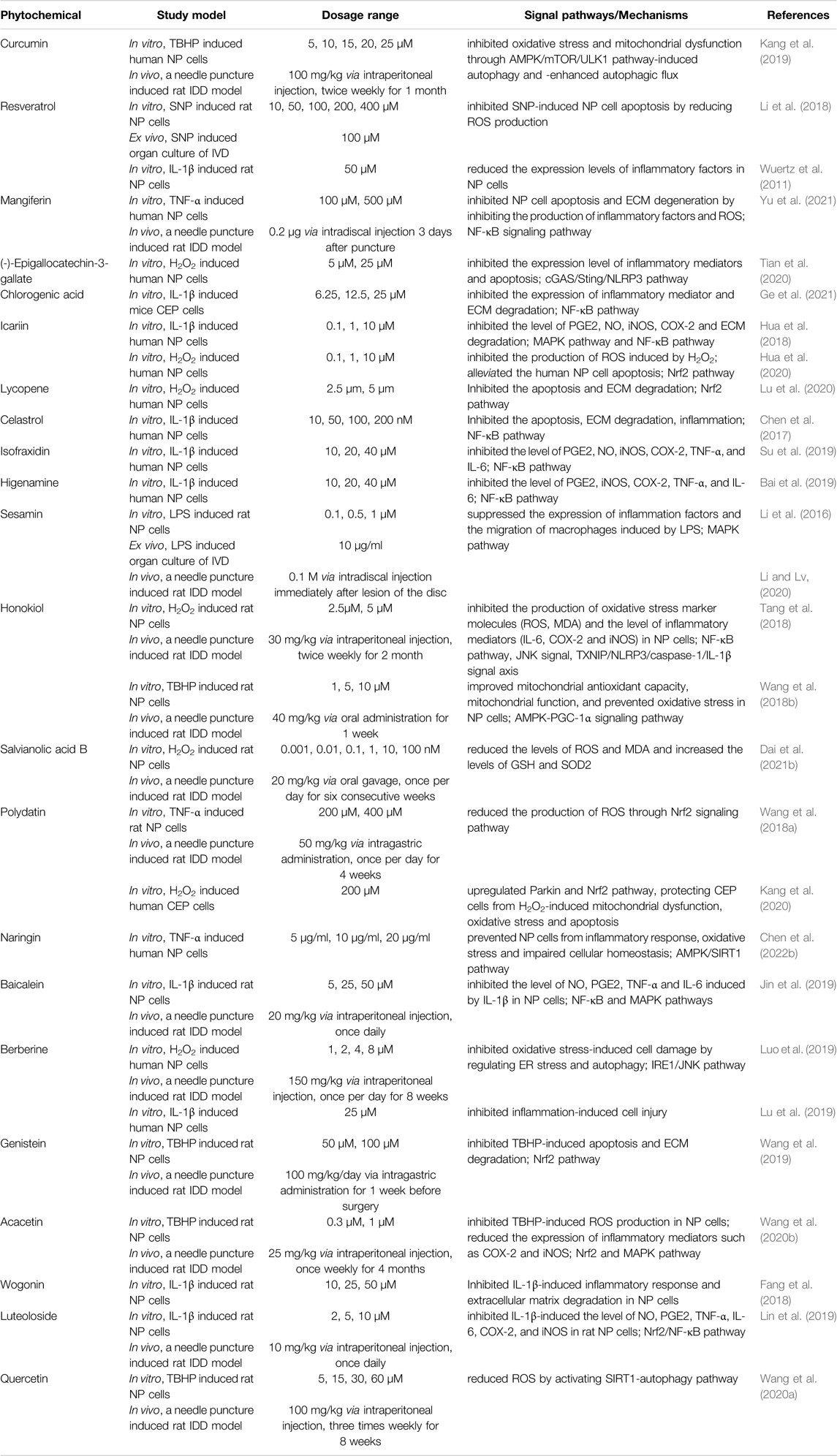
TABLE 1. Phytochemicals possess multiple pharmacological effects via the anti-inflammatory and antioxidant mechanism in various in vitro and in vivo models of IDD.
Curcumin
Curcumin (CUR)—an active polyphenol extracted from the dried rhizomes of Curcuma longa—has been traditionally used for dietary and medical purposes worldwide (Li KX. et al., 2022; Jin et al., 2022; Ojo et al., 2022). CUR shows a wide range of pharmacological activities, including anti-inflammatory and antioxidant activities, in various disease models (Yang et al., 2020; Uddin et al., 2021; Fan and Lei, 2022). Kang et al. confirmed the protective effect of CUR against IDD using in vivo and in vitro experiments (Kang et al., 2019). CUR induces autophagy and enhanced autophagic flux via the AMPK pathway (Kang et al., 2019), thereby inhibiting oxidative stress and mitochondrial dysfunction. Moreover, the apoptosis, ECM degradation, and senescence were reversed by CUR in NP cells that were treated with tert-butyl hydroperoxide (TBHP). This study provides sufficient evidence suggesting that CUR delays IDD development by inhibiting oxidative stress.
Resveratrol
Resveratrol (RES) is a polyphenolic compound present in many plants, such as berries and peanuts (Li KX. et al., 2021; Izzo et al., 2021). It has strong protective effects against many diseases, such as osteoarthritis and cancer (Mobasheri et al., 2012; Nguyen et al., 2017; Xiao et al., 2018; Deng et al., 2019; Cháirez-Ramírez et al., 2021). Li et al. found that RES protects rat NP cells from apoptosis induced by sodium nitroprusside by scavenging ROS in vitro experiments (Li et al., 2018). Moreover, ex vivo experiments showed that RES could reduce the development of experimental IDD. The secretion of pro-inflammatory cytokines by IVD cells appears to be the key mediator in the development of pain. Wuertz et al. found that RES significantly reduces the expression of IL-6 and IL-8 in NP cells (Wuertz et al., 2011). Therefore, RES may inhibit the progression of IDD through its dual effects of anti-inflammatory and antioxidant activities. Hence, it can be explored as a new method for IDD treatment.
Mangiferin
Mangiferinis mainly extracted from Mangifera persiciformis, Anemarrhena asphodeloides, and Mangifera indica (Akter et al., 2022; Wang et al., 2022). It plays an important role in the progression of kidney disorders (Lum et al., 2022), diabetes (Aswal et al., 2020), cancer (Morozkina et al., 2021), and osteoarthritis (Qu et al., 2017; Li et al., 2019). It has crucial anti-inflammatory (Wang R. et al., 2021; Li N. et al., 2021) and antioxidant functions (Huang et al., 2020; Samadarsi and Dutta, 2020; Ismail et al., 2021); its application has also been reported in the treatment of IDD. Yu et al. found that mangiferin treatment can inhibit the loss of ECM by inhibiting TNF-α-induced inflammatory cytokines such as iNOS and COX-2 (Yu et al., 2021). In addition, it can alleviate mitochondrial damage and apoptosis indicators, such as cleaved-caspase-3 and Bax, by reducing ROS production. Moreover, in vivo experiments have confirmed the protective effect of mangiferin against IDD (Yu et al., 2021). These results suggest that mangiferin can provide a potential treatment for IDD.
(-)-Epigallocatechin-3-Gallate
(-)-Epigallocatechin-3-gallate (EGCG) is a polyphenol that is abundantly found in tea (Butt et al., 2015). EGCG shows a variety of functions in many diseases, such as antiarteriosclerosis (Liu S. et al., 2017), antioxidant (Park et al., 2021), antibacterial (Noor Mohammadi et al., 2019), anti-inflammatory (Ma et al., 2021), and anti-tumor activities (Farabegoli and Pinheiro, 2022). The flow cytometry results of the study (Tian et al., 2020) conducted by Tian et al. showed that EGCG could inhibit the apoptosis and cell cycle arrest induced by H2O2. Western blot results showed that EGCG upregulates the anti-apoptotic proteins expression and downregulates the pro-apoptotic protein expression in H2O2-treated cells. Tian et al. analyzed the expression of IL-6,IL-1β, IL-10, and TNF-α in NP cells to explore the effect of EGCG on the NP cell’s inflammatory response. It was found that H2O2 could promote their expression, whereas EGCG could reverse the changes induced by H2O2. These results suggest that EGCG may be an alternative treatment for IDD.
Chlorogenic Acid
Chlorogenic acid (CGA) is a natural biologically active compound that is abundantly present in coffee, fruits, and vegetables (Nwafor et al., 2022). It is also the main active ingredient in Chinese herbal medicines, such as Honeysuckle and Eucommia. Its biological functions in disease treatment, such as antioxidant, anti-inflammatory, and immune protective functions, have attracted considerable attention (Liang and Kitts, 2015; Bagdas et al., 2020; Kzhyshkowska, 2022). Ge et al. showed that CGA could reverse the downregulation of aggrecan, the main protein involved in the extracellular matrix anabolism of CEP cells induced by IL-1β, and inhibit the upregulation of MMP-13, the main protein involved in extracellular matrix catabolism (Ge et al., 2021). It can also inhibit the expression of inflammatory factors. Exploration of the molecular mechanisms revealed that the NF-κB signaling is the anti-degenerative effector molecule of CGA. Considering the important role of the NF-κB in the pathogenesis of IDD, these results suggest that CGA can reverse IDD development by regulating this pathway.
Icariin
Icariin (ICA) is a flavonoid compound extracted from the widely known Chinese herbal medicine Epimedium (Wang M. et al., 2020), which is also recognized as “Yin Yang Huo”. ICA has been revealed by many studies to play a crucial role in antioxidation (Liu XJ. et al., 2021; Zheng et al., 2021) and anti-inflammatory (Guangtao et al., 2021; Zhang et al., 2022). Hua et al. found that IL-1β induces significant expression of COX-2 and iNOS and stimulates the production of PGE2 and nitric oxide in human NP cells (Hua et al., 2018). ICA usage can significantly reduce the levels of these inflammatory mediators. In addition, ICA reduces the expression levels of MMP-3/9/13 and ADAMTS-4/5 induced byIL-1β and increases the expression levels of type II collagen and aggrecan (Hua et al., 2018). A molecular mechanism study showed that MAPK and NF-κB are closely related to ICA. The research group also conducted relevant studies on oxidative stress. They found that ROS production increased in H2O2-treated human NP cells; however, this increase was inhibited by ICA in a dose-dependent manner (Hua et al., 2020). ICA can inhibit the mitochondrial cytochrome c translocation to cytoplasm, decrease Bax and caspase-3 levels, and increase Bcl-2 in H2O2-treated NP cells (Hua et al., 2020). The Nrf2 signaling pathway is an important member of the anti-oxidative stress system in cells. It has been shown to be involved in the antioxidant effects of ICA. Therefore, the study of ICA against inflammation and oxidative stress to maintain IVD cell homeostasis could prove the significance of ICA in the treatment of IDD.
Lycopene
Lycopene is a naturally occurring, effective antioxidant found in reddish pink-colored fruits and vegetables, such as tomatoes (Mozos et al., 2018). The human body cannot synthesize lycopene and must be ingested through the diet. The powerful antioxidant effects of lycopene have attracted considerable attention and have been verified in many disease models (Müller et al., 2016; Chen et al., 2019; Imran et al., 2020). The upregulation of Bax and downregulation of Bcl-2 in H2O2-treated human NP cells were attenuated by lycopene (Lu et al., 2020). Flow cytometry also showed that lycopene inhibits NP cell apoptosis. In addition, lycopene can promote the expression of type II collagen, aggrecan, and Sox9, in NP cells. The molecular mechanism of lycopene involves Nrf2, which is a powerful antioxidant transcription factor closely related to the role of lycopene in H2O2-treated human NP cells. Therefore, lycopene has the potential to mediate antioxidation and treat IDD.
Celastrol
Celastrolis a natural triterpenoid found in Tripterygium wilfordii that has been used to treat a variety of common diseases because of its strong anti-inflammatory activity (Jing et al., 2021; Zhu et al., 2021; Li M. et al., 2022). Celastrol can inhibit the upregulation of IL-6andTNF-α expression in NP cells induced by IL-1β (Chen et al., 2017). IL-1β can promote the MMP-3/9/13 and ADAMTS-4/5 expression. Celastrol inhibits the upregulation of these ECM-degrading enzymes. Moreover, since NF-κB acts as a crucial factor in promoting the inflammatory responses during IDD, celastrol can inhibit the activation of the NF-κB pathway (Chen et al., 2017). Therefore, celastrol has the potential for the treatment of IDD.
Isofraxidin
Isofraxidin is a coumarin compound found in traditional Chinese herbs (Majnooni et al., 2020) and has been clearly shown by previous studies to have strong anti-inflammatory activity (Lin et al., 2018; Chen et al., 2020). Su et al. found that isofraxidin alleviated the IL-1β-induced upregulation of inflammatory mediators and cytokines (Su et al., 2019). In NP ECM metabolism, it can inhibit the expression of the ECM-degrading enzymes and promote the expression of type II collagen and aggrecan. In terms of the molecular mechanism, isofraxidin can inhibit the nuclear translocation and phosphorylation of p65, indicating that the NF-κB pathway is involved in the anti-inflammatory effect of isofraxidin. These studies show that isofraxidin can be used for treating IDD.
Higenamine
Higenamine was extracted initially from the traditional Chinese herb aconite root in 1976 and later was identified as the main active component of many Chinese herbs (Ha et al., 2012). Higenamine has been found to have many biological activities (Bai et al., 2019; Zhang et al., 2019; Romeo et al., 2020; Yang et al., 2021), such as anti-inflammatory and antioxidant. Bai et al. found that the IL-1β-induced iNOS, PGE2, COX-2, TNF-α, and IL-6 levels, were attenuated by higenamine in NP cells (Bai et al., 2019). Moreover, Bai et al. have also found that higenamine suppressed the IL-1β-induced activation of the NF-κB signaling pathway.
Sesamin
Sesamum indicum (sesame) is often used as a source of spices and edible oil. Sesamin is a type of sesame lignans that can be extracted from sesame oil (Dalibalta et al., 2020). Many studies have shown that sesamin has potential anti-inflammatory, antioxidant, and anti-tumor effects in different tissues (Majdalawieh et al., 2017; Dalibalta et al., 2020). The role of sesamin in IDD development has also been confirmed. The anti-inflammatory effects of sesamin on rat IVD have been examined by Li et al. The expression of inflammation factors and the migration of macrophages can be suppressed by sesamin treatment (Li et al., 2016). Subsequently, the inhibition of MAPK pathway activation was involved in its anti-inflammatory effect. In addition, Li et al. proved the inhibitory effects of sesamin on the occurrence and development of IDD through in vivo experiments (Li and Lv, 2020). These results suggest that sesamin can reverse the process of IDD by inhibiting inflammation.
Honokiol
Honokiol (HKL) is a natural compound extracted from the roots and bark of Magnolia trees (Prasad and Katiyar, 2016). Previous studies have shown that it has apparent antagonistic effects on oxidative stress, inflammation, and tumor, and hence has been reported to be used in disease treatment (Chiu et al., 2021; Liu Y. et al., 2021; Lu et al., 2022). Tang et al. demonstrated that HKL inhibited the expression of NP cell apoptosis-related proteins induced by H2O2, the production of oxidative stress marker molecules, the level of inflammatory mediators, the expression of major extracellular matrix-degrading proteases, and then enhanced the expression of ECM anabolic proteins (Tang et al., 2018). The molecular mechanism of the action of HKL involves the inhibition of NF-κB/JNK signaling and TXNIP/NLRP3/caspase-1/IL-1β activation. SIRT3 is an important deacetylation modifying enzyme in mitochondria and is important for mitochondrial health. Wang et al. reported that HKL induced the upregulation of SIRT3 through the AMPK-PGC-1α axis, which improves the activity of antioxidant enzymes in mitochondria, and further prevents oxidative damage toNP cells (Wang et al., 2018b). In vivo experiments also confirmed the results of in vitro experiments. Therefore, HKL has the potential to treat IDD.
Salvianolic Acid B
Salvia miltiorrhiza Bunge, also called Danshen, is atraditional Chinese herb. It has been used in Chinafor centuries (Wu et al., 2020; Xiao et al., 2020). Salvianolic acid B (SAB) is an abundant active ingredient from Danshen. SAB has been proved to have antioxidant (Zhao et al., 2019; Xiao et al., 2020) and anti-inflammatory activities (Ho and Hong, 2011). Dai et al. found that SAB slowed down the process of IDD and reconstructed the structure of IVD through in vivo experimental study. Subsequently, it was also confirmed that the levels of GSH and SOD2 in the degenerative IVD were reduced, and SAB treatment significantly reversed this change (Dai S. et al., 2021). In additional in vitro experiments, SAB was found to reduce the levels of ROS and MDA and increase the levels of GSH and SOD2. JAK2/STAT3 signaling pathway is suggested to be related to the antioxidant effect of SAB.
Polydatin
Polydatin is the abundant form of resveratrol found in nature, and its average concentration in Polygonum cuspidatumandred wine is about 10times that of resveratrol (Liu W. et al., 2017). Polydatin, like resveratrol, has anti-inflammatory and antioxidant activities. It is worth noting that, unlike resveratrol, polydatin can enter cells through an active mechanism using glucose carriers and has a stronger anti-enzymatic oxidation ability than resveratrol. These properties allow polydatin to exhibit a higher absorption and better bioavailability relative to resveratrol (Tang, 2021). Wang et al. found that polydatin reduced the production of ROS through theNrf2 signaling pathway and protected rat NP cells from TNF-α-induced mitochondrial dysfunction and ECM degradation (Wang et al., 2018a). Moreover, Kang et al. found that polydatin upregulated Parkin and Nrf2 pathways, protecting CEP cells from H2O2-induced mitochondrial dysfunction, oxidative stress, and apoptosis, thereby inhibiting the development of IDD (Kang et al., 2020).
Naringin
Naringin is a bioflavonoid found in the tangerine peel (Lavrador et al., 2018). It reportedly has a wide range of pharmacological activities, including antioxidant (Long et al., 2020; Bao et al., 2022) and anti-inflammatory effects (Zhao et al., 2020; Wu et al., 2021). Naringin has been confirmed to increase autophagy flux by activating the AMPK/SIRT1 pathway, thereby protecting NP cells from inflammatory response, oxidative stress, and impaired cellular homeostasis (Chen R. et al., 2022). Naringin can be developed into an effective drug to treat IDD.
Baicalein
Baicalein is a flavonoid compound naturally found in the Chinese herb Scutellaria baicalensis. Baicalein possesses several pharmacological activities, including alleviation of inflammation (Wang X. et al., 2021; Jiang et al., 2022) and oxidative stress (Dai C. et al., 2021; Liu BY. et al., 2021). Baicalein helps treat IDD mainly via countering inflammation. It can inhibit NO, PGE2, TNF-α, and IL-6 induced by IL-1β (Jin et al., 2019). At the same time, it can reduce the expression of degrading enzymes MMP-13 and ADAMTS5 and upregulate the expression of aggrecan and type II collagen. A study examining its mechanism found that baicalein inhibited NF-κB and MAPK pathways. In vivo experiments also showed that baicalein could inhibit the process that led to IDD.
Berberine
Berberine (BBR) is an isoquinoline alkaloid found in the long-used traditional Chinese herbs Rhizomacoptidis, Cortex Phellodendri, and Mahonia bealei. BBR reportedly has anti-inflammatory (Li Y. et al., 2022; Dai et al., 2022) and antioxidant properties (Cao et al., 2021; Seth et al., 2021). BBR has therapeutic effects on various diseases, including osteoarthritis. Its therapeutic potential in IDD has also been tested. Luo et al. demonstrated that BBR could inhibit oxidative stress-induced cell damage by regulating ER stress and autophagy (Luo et al., 2019). In vivo studies have also yielded similar evidence, suggesting that BBR treatment can delay the IVD destruction process induced by puncture in the rat model. Lu et al. found that BBR can inhibit the upregulation of NP extracellular matrix-degrading enzymes and the downregulation of the key components of the matrix, and excessive apoptosis induced by IL-1β (Lu et al., 2019).
Genistein
Genistein is an isoflavone primarily identified in Glycine max (soybean) extract, among many other sources, such as peanuts, green peas, and legumes. Genistein reportedly prevents various diseases, such as anti-inflammatory, reducing osteoporosis, improving obesity, and anti-tumor (Fuloria et al., 2022; Goh et al., 2022; Ji et al., 2022). Wang et al. found that genistein can activate the Nrf2-mediated antioxidant defense system in NP cells (Wang et al., 2019) and subsequently inhibit TBHP-induced apoptosis and ECM degradation.
Acacetin
Acacetin is a flavonoid compound from Saussurea involucrata plant and Damiana. Acacetin has been found to have anti-inflammatory (Ren et al., 2020; Singh et al., 2020), antioxidant (Song et al., 2022; Wu et al., 2022), anti-cancer (Wang S. et al., 2020; Yun et al., 2021), anti-osteoporosis (Jin et al., 2021; Lin et al., 2022), anti-diabetic (Han et al., 2020; Song et al., 2022) and other properties. Acacetin reportedly inhibits TBHP-induced ROS production by upregulating the expression of antioxidant proteins such as HO1, NQO1, and SOD in NP cells (Wang H. et al., 2020). Acacetin can also reduce the expression of inflammatory mediators such as COX-2 and iNOS induced by TBHP and also inhibits the degradation of extracellular matrix in NP cells. The activation of the Nrf2 pathway and the inhibition of the MAPK pathway are the specific mechanisms of the biological action of Acacetin. In vivo experiments also confirmed that Acacetin can reduce the process of IDD induced by puncture.
Wogonin
Wogonin is an important flavonoid, isolated from the root of Scutellaria baicalensis Georgi (Chirumbolo, 2013). It has many pharmacological effects, including antioxidant and anti-inflammatory (Zheng et al., 2020; Feng et al., 2022; Shao et al., 2022). Fang et al. found that wogonin can inhibit IL-1β-induced inflammatory response and extracellular matrix degradation in NP cells (Fang et al., 2018). Further studies revealed that wogonin playsa key role by activating Nrf2/HO-1 pathway and inhibiting MAPK signaling pathway.
Luteoloside
Luteoloside, a type of flavonoid glycoside, can be isolated from the plant Lonicera japonica (Xiong et al., 2013; Lin et al., 2019; Shi et al., 2020). It has recently been reported to possess anti-inflammatory and antibacterial properties. Lin et al. found that luteoloside inhibited IL-1β-induced the level of TNF-α, iNOS, NO, IL-6, COX-2, and PGE2 in rat NP cells (Lin et al., 2019). The level of apoptosis and ECM degradation were also improved by luteoloside (Lin et al., 2019). In addition, luteoloside has been proved to play a role by activating the Nrf2 pathway and then inhibiting the NF-κB pathway.
Quercetin
Quercetin is a type of natural flavonoid isolated from various fruits and vegetables (Mirazimi et al., 2022). Previous studies have shown that quercetin possesses many functions, including anti-cancer, anti-oxidative, and anti-inflammatory properties (Pinheiro et al., 2021; Yin et al., 2021; Sun et al., 2022). Wang et al. found that quercetin can activate the autophagy pathway through SIRT1 so as to reduce the level of ROS and alleviate apoptosis and extracellular matrix degeneration (Wang D. et al., 2020). These findings hint at a new and effective treatment for IDD.
Summary and Prospect
Oxidative stress and inflammation play an important role in the progression of IDD. Elevated levels of ROS and increased production of inflammatory cytokines in the degenerative discs can activate multiple signaling pathways that cause damage to IVD cells, resulting in structural damage and dysfunction of IVD, which in turn leads to the development of IDD (Johnson et al., 2015; Feng et al., 2017; Cao et al., 2022). Therefore, using oxidative stress and inflammation as therapeutic targets for IDD has wide-ranging prospects. In recent years, the research and application of phytochemicals have attracted significant attention. This review revealed that various phytochemicals could exert inhibitory effects on IDD based on the data from in vitro and in vivo experiments, mainly by inhibiting oxidative stress and inflammation. Also, given the cost-effectiveness and the availability of phytochemicals and their therapeutic role in IDD, research on the use of phytochemicals to improve IDD is rapidly increasing. Phytochemicals have emerged as an important source for developing therapeutic agents for IDD. Future research needs to consider several key points. The first is that phytochemicals can often have multiple targets; therefore, the synergistic effects between multiple signaling pathways and the broad range of cellular functions involved must be analyzed in an integrated manner when designing therapeutic agents for IDD to minimize side effects and expand therapeutic effects. Secondly, most of the research on the role of phytochemicals in IDD treatment is to deliver drugs to animals through IVD injection and other methods. However, very few studies have focused on the metabolism and distribution of phytochemicals in animals. Due to the extensive distribution of phytochemicals in animals, side effects may be encountered in other organs apart from the therapeutic effect on IDD. Exploring the panorama of the metabolic processes and organ distribution of phytochemicals in animals can help select appropriate phytochemicals and delivery methodsso as to improve the therapeutic efficacy of phytochemicals on IDD. Additionally, the dose of phytochemicals also needs to be focused upon in future studies. Currently, many in vitro experiments are focusing on the therapeutic effect and toxicity of different doses of phytochemicals on IDD models. In contrast, little attention is paid to the therapeutic effect of different doses and long-term toxicity to animals in vivo experiments. Therefore, in future studies, it may be necessary to perform multiple-dose in vivo experiments to verify its therapeutic effect so as to evaluate the effect of phytochemicals more comprehensively. Finally, although many studies have uncovered the favorable effects of phytochemicals for treating IDD, a large number of clinical trial studies are needed to further confirm their effects when applying them to the treatment of patients. Researchers and experts need to work together to develop a systematic experimental design and experimental analysis and establish an effective evaluation system to evaluate phytochemicals more safely and rationally and make these phytochemicals more effective in benefiting patients with IDD.
Author Contributions
LK, RZ, and CS conceptualized the review. LK, HZ, CJ drafted the manuscript. LK, HZ, CJ, RZ, and CS revised and supplemented the manuscript. All the authors participated in writing and giving feedback on the manuscript. All authors have read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81772408).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
LBP, low back pain; IVD, intervertebral disc; IDD, intervertebral disc degeneration; NP, nucleus pulposus; AF, annulus fibrosus; CEP, cartilage endplate; ECM, extracellular matrix; MMPs, matrix metalloproteinases; ADAMTS, a disintegrin and metalloprotease with thrombospondin motifs; VEGF, vascular endothelial growth factor; ROS, reactive oxygen species; AGEs, advanced glycation end products; CUR, curcumin; TBHP, Tert-butyl hydroperoxide; RES, resveratrol; EGCG, (-)-Epigallocatechin-3-gallate; CGA, chlorogenic acid; ICA, icariin; HKL, honokiol; SAB, salvianolic acid B; BBR, berberine.
References
Adams, M. A., and Roughley, P. J. (2006). What Is Intervertebral Disc Degeneration, and what Causes it? Spine (Phila Pa 1976) 31 (18), 2151–2161. doi:10.1097/01.brs.0000231761.73859.2c
Akter, S., Moni, A., Faisal, G. M., Uddin, M. R., Jahan, N., Hannan, M. A., et al. (2022). Renoprotective Effects of Mangiferin: Pharmacological Advances and Future Perspectives. Ijerph 19 (3), 1864. doi:10.3390/ijerph19031864
Aswal, S., Kumar, A., Chauhan, A., Semwal, R. B., Kumar, A., and Semwal, D. K. (2020). A Molecular Approach on the Protective Effects of Mangiferin against Diabetes and Diabetes-Related Complications. Curr. Diabetes Rev. 16 (7), 690–698. doi:10.2174/1573399815666191004112023
Bagdas, D., Gul, Z., Meade, J. A., Cam, B., Cinkilic, N., and Gurun, M. S. (2020). Pharmacologic Overview of Chlorogenic Acid and its Metabolites in Chronic Pain and Inflammation. Curr. Neuropharmacol. 18 (3), 216–228. doi:10.2174/1570159x17666191021111809
Bai, X., Ding, W., Yang, S., and Guo, X. (2019). Higenamine Inhibits IL-1β-induced Inflammation in Human Nucleus Pulposus Cells. Biosci. Rep. 39 (6). doi:10.1042/bsr20190857
Bao, T., Yao, J., Zhou, S., Ma, Y., Dong, J., Zhang, C., et al. (2022). Naringin Prevents Follicular Atresia by Inhibiting Oxidative Stress in the Aging Chicken. Poult. Sci. 101 (7), 101891. doi:10.1016/j.psj.2022.101891
Bartels, E. M., Fairbank, J. C., Winlove, C. P., and Urban, J. P. (1998). Oxygen and Lactate Concentrations Measured In Vivo in the Intervertebral Discs of Patients with Scoliosis and Back Pain. Spine (Phila Pa 1976) 23 (1), 1–8. ; discussion 8. doi:10.1097/00007632-199801010-00001
Bindu, S., Mazumder, S., and Bandyopadhyay, U. (2020). Non-steroidal Anti-inflammatory Drugs (NSAIDs) and Organ Damage: A Current Perspective. Biochem. Pharmacol. 180, 114147. doi:10.1016/j.bcp.2020.114147
Butt, M. S., Ahmad, R. S., Sultan, M. T., Qayyum, M. M., and Naz, A. (2015). Green Tea and Anticancer Perspectives: Updates from Last Decade. Crit. Rev. Food Sci. Nutr. 55 (6), 792–805. doi:10.1080/10408398.2012.680205
Cai, X., Yuan, S., Zeng, Y., Wang, C., Yu, N., and Ding, C. (2021). New Trends in Pharmacological Treatments for Osteoarthritis. Front. Pharmacol. 12. doi:10.3389/fphar.2021.645842
Cao, G., Yang, S., Cao, J., Tan, Z., Wu, L., Dong, F., et al. (2022). The Role of Oxidative Stress in Intervertebral Disc Degeneration. Oxid. Med. Cell Longev. 2022, 2166817. doi:10.1155/2022/2166817
Cao, R. Y., Zhang, Y., Feng, Z., Liu, S., Liu, Y., Zheng, H., et al. (2021). The Effective Role of Natural Product Berberine in Modulating Oxidative Stress and Inflammation Related Atherosclerosis: Novel Insights into the Gut-Heart Axis Evidenced by Genetic Sequencing Analysis. Front. Pharmacol. 12, 764994. doi:10.3389/fphar.2021.764994
Cháirez-Ramírez, M. H., de la Cruz-López, K. G., and García-Carrancá, A. (2021). Polyphenols as Antitumor Agents Targeting Key Players in Cancer-Driving Signaling Pathways. Front. Pharmacol. 12, 710304. doi:10.3389/fphar.2021.710304
Chen, C., Yu, L.-T., Cheng, B.-R., Xu, J.-L., Cai, Y., Jin, J.-L., et al. (2022a). Promising Therapeutic Candidate for Myocardial Ischemia/Reperfusion Injury: What Are the Possible Mechanisms and Roles of Phytochemicals? Front. Cardiovasc. Med. 8. doi:10.3389/fcvm.2021.792592
Chen, D., Huang, C., and Chen, Z. (2019). A Review for the Pharmacological Effect of Lycopene in Central Nervous System Disorders. Biomed. Pharmacother. 111, 791–801. doi:10.1016/j.biopha.2018.12.151
Chen, G., Song, X., Lin, D., and Xu, P. (2020). Isofraxidin Alleviates Myocardial Infarction through NLRP3 Inflammasome Inhibition. Inflammation 43 (2), 712–721. doi:10.1007/s10753-019-01158-z
Chen, H. W., Zhang, G. Z., Liu, M. Q., Zhang, L. J., Kang, J. H., Wang, Z. H., et al. (2021). Natural Products of Pharmacology and Mechanisms in Nucleus Pulposus Cells and Intervertebral Disc Degeneration. Evid. Based Complement. Altern. Med. 2021, 9963677. doi:10.1155/2021/9963677
Chen, J., Xuan, J., Gu, Y. T., Shi, K. S., Xie, J. J., Chen, J. X., et al. (2017). Celastrol Reduces IL-1β Induced Matrix Catabolism, Oxidative Stress and Inflammation in Human Nucleus Pulposus Cells and Attenuates Rat Intervertebral Disc Degeneration In Vivo. Biomed. Pharmacother. 91, 208–219. doi:10.1016/j.biopha.2017.04.093
Chen, J. W., Ni, B. B., Li, B., Yang, Y. H., Jiang, S. D., and Jiang, L. S. (2014). The Responses of Autophagy and Apoptosis to Oxidative Stress in Nucleus Pulposus Cells: Implications for Disc Degeneration. Cell Physiol. Biochem. 34 (4), 1175–1189. doi:10.1159/000366330
Chen, J. W., Ni, B. B., Zheng, X. F., Li, B., Jiang, S. D., and Jiang, L. S. (2015). Hypoxia Facilitates the Survival of Nucleus Pulposus Cells in Serum Deprivation by Down-Regulating Excessive Autophagy through Restricting ROS Generation. Int. J. Biochem. Cell Biol. 59, 1–10. doi:10.1016/j.biocel.2014.11.009
Chen, R., Gao, S., Guan, H., Zhang, X., Gao, Y., Su, Y., et al. (2022b). Naringin Protects Human Nucleus Pulposus Cells against TNF-α-Induced Inflammation, Oxidative Stress, and Loss of Cellular Homeostasis by Enhancing Autophagic Flux via AMPK/SIRT1 Activation. Oxidative Med. Cell. Longev. 2022, 7655142. doi:10.1155/2022/7655142
Chirumbolo, S. (2013). Anticancer Properties of the Flavone Wogonin. Toxicology 314 (1), 60–64. doi:10.1016/j.tox.2013.08.016
Chiu, K. C., Shih, Y. H., Wang, T. H., Lan, W. C., Li, P. J., Jhuang, H. S., et al. (2021). In Vitro antimicrobial and Antipro-Inflammation Potential of Honokiol and Magnolol against Oral Pathogens and Macrophages. J. Formos. Med. Assoc. 120 (2), 827–837. doi:10.1016/j.jfma.2020.09.002
Conaghan, P. G. (2012). A Turbulent Decade for NSAIDs: Update on Current Concepts of Classification, Epidemiology, Comparative Efficacy, and Toxicity. Rheumatol. Int. 32 (6), 1491–1502. doi:10.1007/s00296-011-2263-6
Dai, C., Li, H., Wang, Y., Tang, S., Velkov, T., and Shen, J. (2021a). Inhibition of Oxidative Stress and ALOX12 and NF-Κb Pathways Contribute to the Protective Effect of Baicalein on Carbon Tetrachloride-Induced Acute Liver Injury. Antioxidants (Basel) 10 (6). doi:10.3390/antiox10060976
Dai, L., Zhu, L., Ma, S., Liu, J., Zhang, M., Li, J., et al. (2022). Berberine Alleviates NLRP3 Inflammasome Induced Endothelial Junction Dysfunction through Ca2+ Signalling in Inflammatory Vascular Injury. Phytomedicine 101, 154131. doi:10.1016/j.phymed.2022.154131
Dai, S., Liang, T., Shi, X., Luo, Z., and Yang, H. (2021b). Salvianolic Acid B Protects Intervertebral Discs from Oxidative Stress-Induced Degeneration via Activation of the JAK2/STAT3 Signaling Pathway. Oxidative Med. Cell. Longev. 2021, 6672978. doi:10.1155/2021/6672978
Dalibalta, S., Majdalawieh, A. F., and Manjikian, H. (2020). Health Benefits of Sesamin on Cardiovascular Disease and its Associated Risk Factors. Saudi Pharm. J. 28 (10), 1276–1289. doi:10.1016/j.jsps.2020.08.018
Deng, J., Zong, Z., Su, Z., Chen, H., Huang, J., Niu, Y., et al. (2021). Recent Advances in Pharmacological Intervention of Osteoarthritis: A Biological Aspect. Front. Pharmacol. 12. doi:10.3389/fphar.2021.772678
Deng, Z., Li, Y., Liu, H., Xiao, S., Li, L., Tian, J., et al. (2019). The Role of Sirtuin 1 and its Activator, Resveratrol in Osteoarthritis. Biosci. Rep. 39 (5). doi:10.1042/bsr20190189
Dimozi, A., Mavrogonatou, E., Sklirou, A., and Kletsas, D. (2015). Oxidative Stress Inhibits the Proliferation, Induces Premature Senescence and Promotes a Catabolic Phenotype in Human Nucleus Pulposus Intervertebral Disc Cells. Eur. Cell Mater 30, 89–103. discussion 103. doi:10.22203/ecm.v030a07
Ding, F., Shao, Z. W., and Xiong, L. M. (2013). Cell Death in Intervertebral Disc Degeneration. Apoptosis 18 (7), 777–785. doi:10.1007/s10495-013-0839-1
Dowdell, J., Erwin, M., Choma, T., Vaccaro, A., Iatridis, J., and Cho, S. K. (2017). Intervertebral Disk Degeneration and Repair. Neurosurgery 80 (3s), S46–s54. doi:10.1093/neuros/nyw078
Fan, F., and Lei, M. (2022). Mechanisms Underlying Curcumin-Induced Neuroprotection in Cerebral Ischemia. Front. Pharmacol. 13, 893118. doi:10.3389/fphar.2022.893118
Fang, W., Zhou, X., Wang, J., Xu, L., Zhou, L., Yu, W., et al. (2018). Wogonin Mitigates Intervertebral Disc Degeneration through the Nrf2/ARE and MAPK Signaling Pathways. Int. Immunopharmacol. 65, 539–549. doi:10.1016/j.intimp.2018.10.024
Farabegoli, F., and Pinheiro, M. (2022). Epigallocatechin-3-Gallate Delivery in Lipid-Based Nanoparticles: Potentiality and Perspectives for Future Applications in Cancer Chemoprevention and Therapy. Front. Pharmacol. 13, 809706. doi:10.3389/fphar.2022.809706
Feng, C., Yang, M., Lan, M., Liu, C., Zhang, Y., Huang, B., et al. (2017). ROS: Crucial Intermediators in the Pathogenesis of Intervertebral Disc Degeneration. Oxid. Med. Cell Longev. 2017, 5601593. doi:10.1155/2017/5601593
Feng, Y., Ju, Y., Yan, Z., Ji, M., Yang, M., Wu, Q., et al. (2022). Protective Role of Wogonin Following Traumatic Brain Injury by Reducing Oxidative Stress and Apoptosis via the PI3K/Nrf2/HO-1 P-athway. Int. J. Mol. Med. 49 (4). doi:10.3892/ijmm.2022.5109
Filomeni, G., De Zio, D., and Cecconi, F. (2015). Oxidative Stress and Autophagy: the Clash between Damage and Metabolic Needs. Cell Death Differ. 22 (3), 377–388. doi:10.1038/cdd.2014.150
Francisco, V., Pino, J., González-Gay, M. Á., Lago, F., Karppinen, J., Tervonen, O., et al. (2022). A New Immunometabolic Perspective of Intervertebral Disc Degeneration. Nat. Rev. Rheumatol. 18 (1), 47–60. doi:10.1038/s41584-021-00713-z
Freemont, A. J. (2009). The Cellular Pathobiology of the Degenerate Intervertebral Disc and Discogenic Back Pain. Rheumatol. Oxf. 48 (1), 5–10. doi:10.1093/rheumatology/ken396
Freemont, A. J., Watkins, A., Le Maitre, C., Baird, P., Jeziorska, M., Knight, M. T., et al. (2002). Nerve Growth Factor Expression and Innervation of the Painful Intervertebral Disc. J. Pathol. 197 (3), 286–292. doi:10.1002/path.1108
Fuloria, S., Yusri, M. A. A., Sekar, M., Gan, S. H., Rani, N. N. I. M., Lum, P. T., et al. (2022). Genistein: A Potential Natural Lead Molecule for New Drug Design and Development for Treating Memory Impairment. Molecules 27 (1), 265. doi:10.3390/molecules27010265
Ge, Q., Ying, J., Shi, Z., Mao, Q., Jin, H., Wang, P. E., et al. (2021). Chlorogenic Acid Retards Cartilaginous Endplate Degeneration and Ameliorates Intervertebral Disc Degeneration via Suppressing NF-Κb Signaling. Life Sci. 274, 119324. doi:10.1016/j.lfs.2021.119324
Gille, L., and Nohl, H. (2001). The Ubiquinol/bc1 Redox Couple Regulates Mitochondrial Oxygen Radical Formation. Arch. Biochem. Biophys. 388 (1), 34–38. doi:10.1006/abbi.2000.2257
Goh, Y. X., Jalil, J., Lam, K. W., Husain, K., and Premakumar, C. M. (2022). Genistein: A Review on its Anti-inflammatory Properties. Front. Pharmacol. 13, 820969. doi:10.3389/fphar.2022.820969
Guangtao, F., Zhenkang, W., Zhantao, D., Mengyuan, L., Qingtian, L., Yuanchen, M., et al. (2021). Icariin Alleviates Wear Particle-Induced Periprosthetic Osteolysis via Down-Regulation of the Estrogen Receptor α-mediated NF-Κb Signaling Pathway in Macrophages. Front. Pharmacol. 12, 746391. doi:10.3389/fphar.2021.746391
Ha, Y. M., Kim, M. Y., Park, M. K., Lee, Y. S., Kim, Y. M., Kim, H. J., et al. (2012). Higenamine Reduces HMGB1 during Hypoxia-Induced Brain Injury by Induction of Heme Oxygenase-1 through PI3K/Akt/Nrf-2 Signal Pathways. Apoptosis 17 (5), 463–474. doi:10.1007/s10495-011-0688-8
Han, W. M., Chen, X. C., Li, G. R., and Wang, Y. (2020). Acacetin Protects against High Glucose-Induced Endothelial Cells Injury by Preserving Mitochondrial Function via Activating Sirt1/Sirt3/AMPK Signals. Front. Pharmacol. 11, 607796. doi:10.3389/fphar.2020.607796
Ho, J. H., and Hong, C. Y. (2011). Salvianolic Acids: Small Compounds with Multiple Mechanisms for Cardiovascular Protection. J. Biomed. Sci. 18 (1), 30. doi:10.1186/1423-0127-18-30
Hua, W., Li, S., Luo, R., Wu, X., Zhang, Y., Liao, Z., et al. (2020). Icariin Protects Human Nucleus Pulposus Cells from Hydrogen Peroxide-Induced Mitochondria-Mediated Apoptosis by Activating Nuclear Factor Erythroid 2-related Factor 2. Biochim. Biophys. Acta Mol. Basis Dis. 1866 (1), 165575. doi:10.1016/j.bbadis.2019.165575
Hua, W., Zhang, Y., Wu, X., Kang, L., Tu, J., Zhao, K., et al. (2018). Icariin Attenuates Interleukin-1β-Induced Inflammatory Response in Human Nucleus Pulposus Cells. Curr. Pharm. Des. 23 (39), 6071–6078. doi:10.2174/1381612823666170615112158
Huang, J., Zheng, L., Wang, F., Su, Y., Kong, H., and Xin, H. (2020). Mangiferin Ameliorates Placental Oxidative Stress and Activates PI3K/Akt/mTOR Pathway in Mouse Model of Preeclampsia. Arch. Pharm. Res. 43 (2), 233–241. doi:10.1007/s12272-020-01220-7
Imran, M., Ghorat, F., Ul-Haq, I., Ur-Rehman, H., Aslam, F., Heydari, M., et al. (2020). Lycopene as a Natural Antioxidant Used to Prevent Human Health Disorders. Antioxidants (Basel) 9 (8). doi:10.3390/antiox9080706
Ismail, M. B., Rajendran, P., AbuZahra, H. M., and Veeraraghavan, V. P. (2021). Mangiferin Inhibits Apoptosis in Doxorubicin-Induced Vascular Endothelial Cells via the Nrf2 Signaling Pathway. Ijms 22 (8), 4259. doi:10.3390/ijms22084259
Izzo, C., Annunziata, M., Melara, G., Sciorio, R., Dallio, M., Masarone, M., et al. (2021). The Role of Resveratrol in Liver Disease: A Comprehensive Review from In Vitro to Clinical Trials. Nutrients 13 (3). doi:10.3390/nu13030933
Ji, X., Liu, K., Li, Q., Shen, Q., Han, F., Ye, Q., et al. (2022). A Mini-Review of Flavone Isomers Apigenin and Genistein in Prostate Cancer Treatment. Front. Pharmacol. 13, 851589. doi:10.3389/fphar.2022.851589
Jia, J., Nie, L., and Liu, Y. (2020). Butyrate Alleviates Inflammatory Response and NF-Κb Activation in Human Degenerated Intervertebral Disc Tissues. Int. Immunopharmacol. 78, 106004. doi:10.1016/j.intimp.2019.106004
Jiang, C., Zhang, J., Xie, H., Guan, H., Li, R., Chen, C., et al. (2022). Baicalein Suppresses Lipopolysaccharide-Induced Acute Lung Injury by Regulating Drp1-dependent Mitochondrial Fission of Macrophages. Biomed. Pharmacother. 145, 112408. doi:10.1016/j.biopha.2021.112408
Jimbo, K., Park, J. S., Yokosuka, K., Sato, K., and Nagata, K. (2005). Positive Feedback Loop of Interleukin-1beta Upregulating Production of Inflammatory Mediators in Human Intervertebral Disc Cells In Vitro. J. Neurosurg. Spine 2 (5), 589–595. doi:10.3171/spi.2005.2.5.0589
Jin, H., Wang, Q., Wu, J., Han, X., Qian, T., Zhang, Z., et al. (2019). Baicalein Inhibits the IL-1β-Induced Inflammatory Response in Nucleus Pulposus Cells and Attenuates Disc Degeneration In Vivo. Inflammation 42 (3), 1032–1044. doi:10.1007/s10753-019-00965-8
Jin, M., Nie, J., Zhu, J., Li, J., Fang, T., Xu, J., et al. (2021). Acacetin Inhibits RANKL-Induced Osteoclastogenesis and LPS-Induced Bone Loss by Modulating NFATc1 Transcription. Biochem. Biophys. Res. Commun. 583, 146–153. doi:10.1016/j.bbrc.2021.10.066
Jin, T., Zhang, Y., Botchway, B. O. A., Zhang, J., Fan, R., Zhang, Y., et al. (2022). Curcumin Can Improve Parkinson's Disease via Activating BDNF/PI3k/Akt Signaling Pathways. Food Chem. Toxicol. 164, 113091. doi:10.1016/j.fct.2022.113091
Jing, M., Yang, J., Zhang, L., Liu, J., Xu, S., Wang, M., et al. (2021). Celastrol Inhibits Rheumatoid Arthritis through the ROS-NF-Κb-NLRP3 Inflammasome axis. Int. Immunopharmacol. 98, 107879. doi:10.1016/j.intimp.2021.107879
Johnson, W. E., Evans, H., Menage, J., Eisenstein, S. M., El Haj, A., and Roberts, S. (2001). Immunohistochemical Detection of Schwann Cells in Innervated and Vascularized Human Intervertebral Discs. Spine (Phila Pa 1976) 26 (23), 2550–2557. doi:10.1097/00007632-200112010-00007
Johnson, Z. I., Schoepflin, Z. R., Choi, H., Shapiro, I. M., and Risbud, M. V. (2015). Disc in Flames: Roles of TNF-α and IL-1β in Intervertebral Disc Degeneration. Eur. Cell Mater 30, 104–107. ; discussion 116-107. doi:10.22203/ecm.v030a08
Kang, L., Liu, S., Li, J., Tian, Y., Xue, Y., and Liu, X. (2020). Parkin and Nrf2 Prevent Oxidative Stress-Induced Apoptosis in Intervertebral Endplate Chondrocytes via Inducing Mitophagy and Anti-oxidant Defenses. Life Sci. 243, 117244. doi:10.1016/j.lfs.2019.117244
Kang, L., Xiang, Q., Zhan, S., Song, Y., Wang, K., Zhao, K., et al. (2019). Restoration of Autophagic Flux Rescues Oxidative Damage and Mitochondrial Dysfunction to Protect against Intervertebral Disc Degeneration. Oxid. Med. Cell Longev. 2019, 7810320. doi:10.1155/2019/7810320
Kim, K. A., and Yim, J. E. (2015). Antioxidative Activity of Onion Peel Extract in Obese Women: A Randomized, Double-Blind, Placebo Controlled Study. J. Cancer Prev. 20 (3), 202–207. doi:10.15430/jcp.2015.20.3.202
Kwon, W. K., Moon, H. J., Kwon, T. H., Park, Y. K., and Kim, J. H. (2017). The Role of Hypoxia in Angiogenesis and Extracellular Matrix Regulation of Intervertebral Disc Cells during Inflammatory Reactions. Neurosurgery 81 (5), 867–875. doi:10.1093/neuros/nyx149
Kzhyshkowska, J. (2022). Stabilizing the Immune System by Chlorogenic Acid. J. Leukoc. Bio. doi:10.1002/jlb.3ce0821-427rr
Lau, K. K. L., Samartzis, D., To, N. S. C., Harada, G. K., An, H. S., and Wong, A. Y. L. (2021). Demographic, Surgical, and Radiographic Risk Factors for Symptomatic Adjacent Segment Disease after Lumbar Fusion: A Systematic Review and Meta-Analysis. J. Bone Jt. Surg. 103 (15). doi:10.2106/jbjs.20.00408
Lavrador, P., Gaspar, V. M., and Mano, J. F. (2018). Bioinspired Bone Therapies Using Naringin: Applications and Advances. Drug Discov. Today 23 (6), 1293–1304. doi:10.1016/j.drudis.2018.05.012
Le Maitre, C. L., Pockert, A., Buttle, D. J., Freemont, A. J., and Hoyland, J. A. (2007). Matrix Synthesis and Degradation in Human Intervertebral Disc Degeneration. Biochem. Soc. Trans. 35 (Pt 4), 652–655. doi:10.1042/bst0350652
Lee, D. C., Adams, C. S., Albert, T. J., Shapiro, I. M., Evans, S. M., and Koch, C. J. (2007). In Situ oxygen Utilization in the Rat Intervertebral Disc. J. Anat. 210 (3), 294–303. doi:10.1111/j.1469-7580.2007.00692.x
Li, K., Li, Y., Mi, J., Mao, L., Han, X., and Zhao, J. (2018). Resveratrol Protects against Sodium Nitroprusside Induced Nucleus Pulposus Cell Apoptosis by Scavenging ROS. Int. J. Mol. Med. 41 (5), 2485–2492. doi:10.3892/ijmm.2018.3461
Li, K., Li, Y., Xu, B., Mao, L., and Zhao, J. (2016). Sesamin Inhibits Lipopolysaccharide-Induced Inflammation and Extracellular Matrix Catabolism in Rat Intervertebral Disc. Connect. Tissue Res. 57 (5), 347–359. doi:10.1080/03008207.2016.1182998
Li, K., and Lv, C. (2020). Intradiscal Injection of Sesamin Protects from Lesion-Induced Degeneration. Connect. Tissue Res. 61 (6), 594–603. doi:10.1080/03008207.2019.1651847
Li, K. X., Ji, M. J., and Sun, H. J. (2021a). An Updated Pharmacological Insight of Resveratrol in the Treatment of Diabetic Nephropathy. Gene 780, 145532. doi:10.1016/j.gene.2021.145532
Li, K. X., Wang, Z. C., Machuki, J. O., Li, M. Z., Wu, Y. J., Niu, M. K., et al. (2022a). Benefits of Curcumin in the Vasculature: A Therapeutic Candidate for Vascular Remodeling in Arterial Hypertension and Pulmonary Arterial Hypertension? Front. Physiol. 13, 848867. doi:10.3389/fphys.2022.848867
Li, M., Xie, F., Wang, L., Zhu, G., Qi, L.-W., and Jiang, S. (2022b). Celastrol: An Update on its Hepatoprotective Properties and the Linked Molecular Mechanisms. Front. Pharmacol. 13, 857956. doi:10.3389/fphar.2022.857956
Li, N., Xiong, R., He, R., Liu, B., Wang, B., and Geng, Q. (2021b). Mangiferin Mitigates Lipopolysaccharide-Induced Lung Injury by Inhibiting NLRP3 Inflammasome Activation. J. Inflamm. Res. 14, 2289–2300. doi:10.2147/jir.s304492
Li, Y., Wu, Y., Jiang, K., Han, W., Zhang, J., Xie, L., et al. (2019). Mangiferin Prevents TBHP-Induced Apoptosis and ECM Degradation in Mouse Osteoarthritic Chondrocytes via Restoring Autophagy and Ameliorates Murine Osteoarthritis. Oxid. Med. Cell Longev. 2019, 8783197. doi:10.1155/2019/8783197
Li, Y., Chen, X., Chen, Y., Yu, D., Jiang, R., Kou, X., et al. (2022c). Berberine Improves TNF-α-Induced Hepatic Insulin Resistance by Targeting MEKK1/MEK Pathway. Inflammation. doi:10.1007/s10753-022-01671-8
Liang, N., and Kitts, D. D. (2015). Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 8 (1). doi:10.3390/nu8010016
Lin, J., Chen, J., Zhang, Z., Xu, T., Shao, Z., Wang, X., et al. (2019). Luteoloside Inhibits IL-1β-Induced Apoptosis and Catabolism in Nucleus Pulposus Cells and Ameliorates Intervertebral Disk Degeneration. Front. Pharmacol. 10, 868. doi:10.3389/fphar.2019.00868
Lin, J., Li, X., Qi, W., Yan, Y., Chen, K., Xue, X., et al. (2018). Isofraxidin Inhibits Interleukin-1β Induced Inflammatory Response in Human Osteoarthritis Chondrocytes. Int. Immunopharmacol. 64, 238–245. doi:10.1016/j.intimp.2018.09.003
Lin, X., Xu, F., Zhang, K.-W., Qiu, W.-X., Zhang, H., Hao, Q., et al. (2022). Acacetin Prevents Bone Loss by Disrupting Osteoclast Formation and Promoting Type H Vessel Formation in Ovariectomy-Induced Osteoporosis. Front. Cell Dev. Biol. 10, 796227. doi:10.3389/fcell.2022.796227
Liu, B. Y., Li, L., Liu, G. L., Ding, W., Chang, W. G., Xu, T., et al. (2021a). Baicalein Attenuates Cardiac Hypertrophy in Mice via Suppressing Oxidative Stress and Activating Autophagy in Cardiomyocytes. Acta Pharmacol. Sin. 42 (5), 701–714. doi:10.1038/s41401-020-0496-1
Liu, S., Sun, Z., Chu, P., Li, H., Ahsan, A., Zhou, Z., et al. (2017a). EGCG Protects against Homocysteine-Induced Human Umbilical Vein Endothelial Cells Apoptosis by Modulating Mitochondrial-dependent Apoptotic Signaling and PI3K/Akt/eNOS Signaling Pathways. Apoptosis 22 (5), 672–680. doi:10.1007/s10495-017-1360-8
Liu, W., Chen, P., Deng, J., Lv, J., and Liu, J. (2017b). Resveratrol and Polydatin as Modulators of Ca2+ Mobilization in the Cardiovascular System. Ann. N. Y. Acad. Sci. 1403 (1), 82–91. doi:10.1111/nyas.13386
Liu, X. J., Lv, Y. F., Cui, W. Z., Li, Y., Liu, Y., Xue, Y. T., et al. (2021b). Icariin Inhibits Hypoxia/reoxygenation-Induced Ferroptosis of Cardiomyocytes via Regulation of the Nrf2/HO-1 Signaling Pathway. FEBS open bio 11 (11), 2966–2976. doi:10.1002/2211-5463.13276
Liu, Y., Zhou, J., Luo, Y., Li, J., Shang, L., Zhou, F., et al. (2021c). Honokiol Alleviates LPS-Induced Acute Lung Injury by Inhibiting NLRP3 Inflammasome-Mediated Pyroptosis via Nrf2 Activation In Vitro and In Vivo. Chin. Med. 16 (1), 127. doi:10.1186/s13020-021-00541-z
Long, J. Y., Chen, J. M., Liao, Y. J., Zhou, Y. J., Liang, B. Y., and Zhou, Y. (2020). Naringin Provides Neuroprotection in CCL2-Induced Cognition Impairment by Attenuating Neuronal Apoptosis in the hippocampus. Behav. Brain Funct. 16 (1), 4. doi:10.1186/s12993-020-00166-6
Lu, L., Hu, J., Wu, Q., An, Y., Cui, W., Wang, J., et al. (2019). Berberine Prevents Human Nucleus Pulposus Cells from IL-1β-induced E-xtracellular M-atrix D-egradation and A-poptosis by I-nhibiting the NF-κB P-athway. Int. J. Mol. Med. 43 (4), 1679–1686. doi:10.3892/ijmm.2019.4105
Lu, X., Lu, X., Yang, P., Zhang, Z., and Lv, H. (2022). Honokiol Nanosuspensions Loaded Thermosensitive Hydrogels as the Local Delivery System in Combination with Systemic Paclitaxel for Synergistic Therapy of Breast Cancer. Eur. J. Pharm. Sci. 175, 106212. doi:10.1016/j.ejps.2022.106212
Lu, Y., Zhou, L., He, S., Ren, H. L., Zhou, N., and Hu, Z. M. (2020). Lycopene Alleviates Disc Degeneration under Oxidative Stress through the Nrf2 Signaling Pathway. Mol. Cell Probes 51, 101559. doi:10.1016/j.mcp.2020.101559
Lum, P. T., Sekar, M., Gan, S. H., Jeyabalan, S., Bonam, S. R., Rani, N. N. I. M., et al. (2022). Therapeutic Potential of Mangiferin against Kidney Disorders and its Mechanism of Action: A Review. Saudi J. Biol. Sci. 29 (3), 1530–1542. doi:10.1016/j.sjbs.2021.11.016
Luo, R., Liao, Z., Song, Y., Yin, H., Zhan, S., Li, G., et al. (2019). Berberine Ameliorates Oxidative Stress-Induced Apoptosis by Modulating ER Stress and Autophagy in Human Nucleus Pulposus Cells. Life Sci. 228, 85–97. doi:10.1016/j.lfs.2019.04.064
Ma, Y., Liu, G., Tang, M., Fang, J., and Jiang, H. (2021). Epigallocatechin Gallate Can Protect Mice from Acute Stress Induced by LPS while Stabilizing Gut Microbes and Serum Metabolites Levels. Front. Immunol. 12, 640305. doi:10.3389/fimmu.2021.640305
Majdalawieh, A. F., Massri, M., and Nasrallah, G. K. (2017). A Comprehensive Review on the Anti-cancer Properties and Mechanisms of Action of Sesamin, a Lignan in Sesame Seeds (Sesamum indicum). Eur. J. Pharmacol. 815, 512–521. doi:10.1016/j.ejphar.2017.10.020
Majnooni, M. B., Fakhri, S., Shokoohinia, Y., Mojarrab, M., Kazemi-Afrakoti, S., and Farzaei, M. H. (2020). Isofraxidin: Synthesis, Biosynthesis, Isolation, Pharmacokinetic and Pharmacological Properties. Molecules 25 (9). doi:10.3390/molecules25092040
Mirazimi, S. M. A., Dashti, F., Tobeiha, M., Shahini, A., Jafari, R., Khoddami, M., et al. (2022). Application of Quercetin in the Treatment of Gastrointestinal Cancers. Front. Pharmacol. 13, 860209. doi:10.3389/fphar.2022.860209
Mobasheri, A., Henrotin, Y., Biesalski, H. K., and Shakibaei, M. (2012). Scientific Evidence and Rationale for the Development of Curcumin and Resveratrol as Nutraceutricals for Joint Health. Int. J. Mol. Sci. 13 (4), 4202–4232. doi:10.3390/ijms13044202
Morozkina, S. N., Nhung Vu, T. H., Generalova, Y. E., Snetkov, P. P., and Uspenskaya, M. V. (2021). Mangiferin as New Potential Anti-cancer Agent and Mangiferin-Integrated Polymer Systems-A Novel Research Direction. Biomolecules 11 (1). doi:10.3390/biom11010079
Mozos, I., Stoian, D., Caraba, A., Malainer, C., Horbańczuk, J. O., and Atanasov, A. G. (2018). Lycopene and Vascular Health. Front. Pharmacol. 9, 521. doi:10.3389/fphar.2018.00521
Müller, L., Caris-Veyrat, C., Lowe, G., and Böhm, V. (2016). Lycopene and its Antioxidant Role in the Prevention of Cardiovascular Diseases-A Critical Review. Crit. Rev. Food Sci. Nutr. 56 (11), 1868–1879. doi:10.1080/10408398.2013.801827
Nguyen, C., Savouret, J. F., Widerak, M., Corvol, M. T., and Rannou, F. (2017). Resveratrol, Potential Therapeutic Interest in Joint Disorders: A Critical Narrative Review. Nutrients 9 (1). doi:10.3390/nu9010045
Noor Mohammadi, T., Maung, A. T., Sato, J., Sonoda, T., Masuda, Y., Honjoh, K., et al. (2019). Mechanism for Antibacterial Action of Epigallocatechin Gallate and Theaflavin-3,3'-Digallate on Clostridium perfringens. J. Appl. Microbiol. 126 (2), 633–640. doi:10.1111/jam.14134
Nwafor, E. O., Lu, P., Zhang, Y., Liu, R., Peng, H., Xing, B., et al. (2022). Chlorogenic Acid: Potential Source of Natural Drugs for the Therapeutics of Fibrosis and Cancer. Transl. Oncol. 15 (1), 101294. doi:10.1016/j.tranon.2021.101294
Ohtori, S., Miyagi, M., Eguchi, Y., Inoue, G., Orita, S., Ochiai, N., et al. (2012a). Efficacy of Epidural Administration of Anti-interleukin-6 Receptor Antibody onto Spinal Nerve for Treatment of Sciatica. Eur. Spine J. 21 (10), 2079–2084. official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. doi:10.1007/s00586-012-2183-5
Ohtori, S., Miyagi, M., Eguchi, Y., Inoue, G., Orita, S., Ochiai, N., et al. (2012b). Epidural Administration of Spinal Nerves with the Tumor Necrosis Factor-Alpha Inhibitor, Etanercept, Compared with Dexamethasone for Treatment of Sciatica in Patients with Lumbar Spinal Stenosis: a Prospective Randomized Study. Spine (Phila Pa 1976) 37 (6), 439–444. doi:10.1097/BRS.0b013e318238af83
Oichi, T., Taniguchi, Y., Oshima, Y., Tanaka, S., and Saito, T. (2020). Pathomechanism of Intervertebral Disc Degeneration. JOR Spine 3 (1), e1076. doi:10.1002/jsp2.1076
Ojo, O. A., Adeyemo, T. R., Rotimi, D., Batiha, G. E., Mostafa-Hedeab, G., Iyobhebhe, M. E., et al. (2022). Anticancer Properties of Curcumin against Colorectal Cancer: A Review. Front. Oncol. 12, 881641. doi:10.3389/fonc.2022.881641
Orita, S., Ohtori, S., Nagata, M., Horii, M., Yamashita, M., Yamauchi, K., et al. (2010). Inhibiting Nerve Growth Factor or its Receptors Downregulates Calcitonin Gene-Related Peptide Expression in Rat Lumbar Dorsal Root Ganglia Innervating Injured Intervertebral Discs. J. Orthop. Res. 28 (12), 1614–1620. doi:10.1002/jor.21170
Park, D. H., Park, J. Y., Kang, K. S., and Hwang, G. S. (2021). Neuroprotective Effect of Gallocatechin Gallate on Glutamate-Induced Oxidative Stress in Hippocampal HT22 Cells. Molecules 26 (5), 1387. doi:10.3390/molecules26051387
Pinheiro, R. G. R., Pinheiro, M., and Neves, A. R. (2021). Nanotechnology Innovations to Enhance the Therapeutic Efficacy of Quercetin. Nanomaterials 11 (10), 2658. doi:10.3390/nano11102658
Prasad, R., and Katiyar, S. K. (2016). Honokiol, an Active Compound of Magnolia Plant, Inhibits Growth, and Progression of Cancers of Different Organs. Adv. Exp. Med. Biol. 928, 245–265. doi:10.1007/978-3-319-41334-1_11
Qu, Y., Zhou, L., and Wang, C. (2017). Mangiferin Inhibits IL-1β-Induced Inflammatory Response by Activating PPAR-γ in Human Osteoarthritis Chondrocytes. Inflammation 40 (1), 52–57. doi:10.1007/s10753-016-0451-y
Ren, J., Yue, B., Wang, H., Zhang, B., Luo, X., Yu, Z., et al. (2020). Acacetin Ameliorates Experimental Colitis in Mice via Inhibiting Macrophage Inflammatory Response and Regulating the Composition of Gut Microbiota. Front. Physiol. 11, 577237. doi:10.3389/fphys.2020.577237
Reuter, S., Gupta, S. C., Chaturvedi, M. M., and Aggarwal, B. B. (2010). Oxidative Stress, Inflammation, and Cancer: How Are They Linked? Free Radic. Biol. Med. 49 (11), 1603–1616. doi:10.1016/j.freeradbiomed.2010.09.006
Romeo, I., Parise, A., Galano, A., Russo, N., Alvarez-Idaboy, J. R., and Marino, T. (2020). The Antioxidant Capability of Higenamine: Insights from Theory. Antioxidants (Basel) 9 (5). doi:10.3390/antiox9050358
Samadarsi, R., and Dutta, D. (2020). Anti-oxidative Effect of Mangiferin-Chitosan Nanoparticles on Oxidative Stress-Induced Renal Cells. Int. J. Biol. Macromol. 151, 36–46. doi:10.1016/j.ijbiomac.2020.02.112
Seth, E., Ahsan, A. U., Kaushal, S., Mehra, S., and Chopra, M. (2021). Berberine Affords Protection against Oxidative Stress and Apoptotic Damage in F1 Generation of Wistar Rats Following Lactational Exposure to Chlorpyrifos. Pestic. Biochem. Physiol. 179, 104977. doi:10.1016/j.pestbp.2021.104977
Shao, W., Zhang, C., Li, K., Lu, Z., Zhao, Z., Gao, K., et al. (2022). Wogonin Inhibits Inflammation and Apoptosis through STAT3 Signal Pathway to Promote the Recovery of Spinal Cord Injury. Brain Res. 1782, 147843. doi:10.1016/j.brainres.2022.147843
Shi, C. Y., He, X. B., Zhao, C., and Wang, H. J. (2020). Luteoloside Exerts Analgesic Effect in a Complete Freund's Adjuvant-Induced Inflammatory Model via Inhibiting Interleukin-1β Expression and Macrophage/Microglia Activation. Front. Pharmacol. 11, 1158. doi:10.3389/fphar.2020.01158
Singh, S., Gupta, P., Meena, A., and Luqman, S. (2020). Acacetin, a Flavone with Diverse Therapeutic Potential in Cancer, Inflammation, Infections and Other Metabolic Disorders. Food Chem. Toxicol. 145, 111708. doi:10.1016/j.fct.2020.111708
Song, F., Mao, Y. J., Hu, Y., Zhao, S. S., Wang, R., Wu, W. Y., et al. (2022). Acacetin Attenuates Diabetes-Induced Cardiomyopathy by Inhibiting Oxidative Stress and Energy Metabolism via PPAR-α/AMPK Pathway. Eur. J. Pharmacol. 922, 174916. doi:10.1016/j.ejphar.2022.174916
Song, Y., Li, S., Geng, W., Luo, R., Liu, W., Tu, J., et al. (2018). Sirtuin 3-dependent Mitochondrial Redox Homeostasis Protects against AGEs-Induced Intervertebral Disc Degeneration. Redox Biol. 19, 339–353. doi:10.1016/j.redox.2018.09.006
Song, Y., Wang, Y., Zhang, Y., Geng, W., Liu, W., Gao, Y., et al. (2017). Advanced Glycation End Products Regulate Anabolic and Catabolic Activities via NLRP3-Inflammasome Activation in Human Nucleus Pulposus Cells. J. Cell Mol. Med. 21 (7), 1373–1387. doi:10.1111/jcmm.13067
Su, X., Liu, B., Gong, F., Yin, J., Sun, Q., Gao, Y., et al. (2019). Isofraxidin Attenuates IL-1β-induced Inflammatory Response in Human Nucleus Pulposus Cells. J. Cell Biochem. 120 (8), 13302–13309. doi:10.1002/jcb.28604
Sun, L., Guo, L., Xu, G., Li, Z., Appiah, M. O., Yang, L., et al. (2022). Quercetin Reduces Inflammation and Protects Gut Microbiota in Broilers. Molecules 27 (10), 3269. doi:10.3390/molecules27103269
Tang, K. S. (2020). Protective Effects of Polydatin against Dementia-Related Disorders. Curr. Neuropharmacol. 19 (2), 127–135. doi:10.2174/1570159x18666200611144825
Tang, P., Gu, J. M., Xie, Z. A., Gu, Y., Jie, Z. W., Huang, K. M., et al. (2018). Honokiol Alleviates the Degeneration of Intervertebral Disc via Suppressing the Activation of TXNIP-NLRP3 Inflammasome Signal Pathway. Free Radic. Biol. Med. 120, 368–379. doi:10.1016/j.freeradbiomed.2018.04.008
Tian, Y., Bao, Z., Ji, Y., Mei, X., and Yang, H. (2020). Epigallocatechin-3-Gallate Protects H2O2-Induced Nucleus Pulposus Cell Apoptosis and Inflammation by Inhibiting cGAS/Sting/NLRP3 Activation. Drug Des. Devel Ther. 14, 2113–2122. doi:10.2147/dddt.s251623
Tian, Z., Zhang, X., and Sun, M. (2021). Phytochemicals Mediate Autophagy against Osteoarthritis by Maintaining Cartilage Homeostasis. Front. Pharmacol. 12, 795058. doi:10.3389/fphar.2021.795058
Turrens, J. F. (2003). Mitochondrial Formation of Reactive Oxygen Species. J. Physiol. 552 (Pt 2), 335–344. doi:10.1113/jphysiol.2003.049478
Uddin, S. J., Hasan, M. F., Afroz, M., Sarker, D. K., Rouf, R., Islam, M. T., et al. (2021). Curcumin and its Multi-Target Function against Pain and Inflammation: An Update of Pre-clinical Data. Curr. Drug Targets 22 (6), 656–671. doi:10.2174/1389450121666200925150022
Vistoli, G., De Maddis, D., Cipak, A., Zarkovic, N., Carini, M., and Aldini, G. (2013). Advanced Glycoxidation and Lipoxidation End Products (AGEs and ALEs): an Overview of Their Mechanisms of Formation. Free Radic. Res. 47 (Suppl. 1), 3–27. doi:10.3109/10715762.2013.815348
Vo, N. V., Hartman, R. A., Patil, P. R., Risbud, M. V., Kletsas, D., Iatridis, J. C., et al. (2016). Molecular Mechanisms of Biological Aging in Intervertebral Discs. J. Orthop. Res. 34 (8), 1289–1306. doi:10.1002/jor.23195
Vo, N. V., Hartman, R. A., Yurube, T., Jacobs, L. J., Sowa, G. A., and Kang, J. D. (2013). Expression and Regulation of Metalloproteinases and Their Inhibitors in Intervertebral Disc Aging and Degeneration. Spine J. 13 (3), 331–341. doi:10.1016/j.spinee.2012.02.027
Wang, D., He, X., Wang, D., Peng, P., Xu, X., Gao, B., et al. (2020a). Quercetin Suppresses Apoptosis and Attenuates Intervertebral Disc Degeneration via the SIRT1-Autophagy Pathway. Front. Cell Dev. Biol. 8, 613006. doi:10.3389/fcell.2020.613006
Wang, H., Jiang, Z., Pang, Z., Zhou, T., and Gu, Y. (2020b). Acacetin Alleviates Inflammation and Matrix Degradation in Nucleus Pulposus Cells and Ameliorates Intervertebral Disc Degeneration In Vivo. Drug Des. Devel Ther. 14, 4801–4813. doi:10.2147/dddt.s274812
Wang, J., Huang, C., Lin, Z., Pan, X., Chen, J., Zheng, G., et al. (2018a). Polydatin Suppresses Nucleus Pulposus Cell Senescence, Promotes Matrix Homeostasis and Attenuates Intervertebral Disc Degeneration in Rats. J. Cell Mol. Med. 22 (11), 5720–5731. doi:10.1111/jcmm.13848
Wang, J., Nisar, M., Huang, C., Pan, X., Lin, D., Zheng, G., et al. (2018b). Small Molecule Natural Compound Agonist of SIRT3 as a Therapeutic Target for the Treatment of Intervertebral Disc Degeneration. Exp. Mol. Med. 50 (11), 1–14. doi:10.1038/s12276-018-0173-3
Wang, K., Hu, S., Wang, B., Wang, J., Wang, X., and Xu, C. (2019). Genistein Protects Intervertebral Discs from Degeneration via Nrf2-Mediated Antioxidant Defense System: An In Vitro and In Vivo Study. J. Cell Physiol. 234 (9), 16348–16356. doi:10.1002/jcp.28301
Wang, M., Gao, H., Li, W., and Wu, B. (2020c). Icariin and its Metabolites Regulate Lipid Metabolism: From Effects to Molecular Mechanisms. Biomed. Pharmacother. 131, 110675. doi:10.1016/j.biopha.2020.110675
Wang, M., Liang, Y., Chen, K., Wang, M., Long, X., Liu, H., et al. (2022). The Management of Diabetes Mellitus by Mangiferin: Advances and Prospects. Nanoscale 14 (6), 2119–2135. doi:10.1039/d1nr06690k
Wang, R., Liu, J., Wang, Z., Wu, X., Guo, H., Jiao, X., et al. (2021a). Mangiferin Exert Protective Effects on Joints of Adjuvant-Induced Arthritis Rats by Regulating the MAPKs/NF-Κb Pathway of Fibroblast-like Synoviocytes. Int. Immunopharmacol. 101, 108352. doi:10.1016/j.intimp.2021.108352
Wang, S., Lin, B., Liu, W., Wei, G., Li, Z., Yu, N., et al. (2020d). Acacetin Induces Apoptosis in Human Osteosarcoma Cells by Modulation of ROS/JNK Activation. Drug Des. Devel Ther. 14, 5077–5085. doi:10.2147/dddt.s275148
Wang, S. Z., Rui, Y. F., Lu, J., and Wang, C. (2014). Cell and Molecular Biology of Intervertebral Disc Degeneration: Current Understanding and Implications for Potential Therapeutic Strategies. Cell Prolif. 47 (5), 381–390. doi:10.1111/cpr.12121
Wang, X., Cai, H., Chen, Z., Zhang, Y., Wu, M., Xu, X., et al. (2021b). Baicalein Alleviates Pyroptosis and Inflammation in Hyperlipidemic Pancreatitis by Inhibiting NLRP3/Caspase-1 Pathway through the miR-192-5p/TXNIP axis. Int. Immunopharmacol. 101 (Pt B), 108315. doi:10.1016/j.intimp.2021.108315
Wang, Y., Che, M., Xin, J., Zheng, Z., Li, J., and Zhang, S. (2020e). The Role of IL-1β and TNF-α in Intervertebral Disc Degeneration. Biomed. Pharmacother. 131, 110660. doi:10.1016/j.biopha.2020.110660
Wu, C., Chen, R. L., Wang, Y., Wu, W. Y., and Li, G. (2022). Acacetin Alleviates Myocardial Ischaemia/reperfusion Injury by Inhibiting Oxidative Stress and Apoptosis via the Nrf-2/HO-1 Pathway. Pharm. Biol. 60 (1), 553–561. doi:10.1080/13880209.2022.2041675
Wu, Y., Xu, S., and Tian, X. Y. (2020). The Effect of Salvianolic Acid on Vascular Protection and Possible Mechanisms. Oxid. Med. Cell Longev. 2020, 5472096. doi:10.1155/2020/5472096
Wu, Y., Cai, C., Xiang, Y., Zhao, H., Lv, L., and Zeng, C. (2021). Naringin Ameliorates Monocrotaline-Induced Pulmonary Arterial Hypertension through Endothelial-To-Mesenchymal Transition Inhibition. Front. Pharmacol. 12, 696135. doi:10.3389/fphar.2021.696135
Wuertz, K., Quero, L., Sekiguchi, M., Klawitter, M., Nerlich, A., Konno, S., et al. (2011). The Red Wine Polyphenol Resveratrol Shows Promising Potential for the Treatment of Nucleus Pulposus-Mediated Pain In Vitro and In Vivo. Spine (Phila Pa 1976) 36 (21), E1373–E1384. doi:10.1097/BRS.0b013e318221e655
Xiao, Q., Zhu, W., Feng, W., Lee, S. S., Leung, A. W., Shen, J., et al. (2018). A Review of Resveratrol as a Potent Chemoprotective and Synergistic Agent in Cancer Chemotherapy. Front. Pharmacol. 9, 1534. doi:10.3389/fphar.2018.01534
Xiao, Z., Liu, W., Mu, Y. P., Zhang, H., Wang, X. N., Zhao, C. Q., et al. (2020). Pharmacological Effects of Salvianolic Acid B against Oxidative Damage. Front. Pharmacol. 11, 572373. doi:10.3389/fphar.2020.572373
Xiong, J., Li, S., Wang, W., Hong, Y., Tang, K., and Luo, Q. (2013). Screening and Identification of the Antibacterial Bioactive Compounds from Lonicera japonica Thunb. Leaves. Food Chem. 138 (1), 327–333. doi:10.1016/j.foodchem.2012.10.127
Yang, B., Ma, S., Zhang, C., Sun, J., Zhang, D., Chang, S., et al. (2021). Higenamine Attenuates Neuropathic Pain by Inhibition of NOX2/ROS/TRP/P38 Mitogen-Activated Protein Kinase/NF-ĸb Signaling Pathway. Front. Pharmacol. 12, 716684. doi:10.3389/fphar.2021.716684
Yang, L., Rong, Z., Zeng, M., Cao, Y., Gong, X., Lin, L., et al. (2015). Pyrroloquinoline Quinone Protects Nucleus Pulposus Cells from Hydrogen Peroxide-Induced Apoptosis by Inhibiting the Mitochondria-Mediated Pathway. Eur. Spine J. 24 (8), 1702–1710. doi:10.1007/s00586-014-3630-2
Yang, L., Chen, Y., Liu, Y., Xing, Y., Miao, C., Zhao, Y., et al. (2020). The Role of Oxidative Stress and Natural Antioxidants in Ovarian Aging. Front. Pharmacol. 11, 617843. doi:10.3389/fphar.2020.617843
Yin, M., Liu, Y., and Chen, Y. (2021). Iron Metabolism: an Emerging Therapeutic Target Underlying the Anti-cancer Effect of Quercetin. Free Radic. Res. 55 (3), 296–303. doi:10.1080/10715762.2021.1898604
Yu, H., Hou, G., Cao, J., Yin, Y., Zhao, Y., and Cheng, L. (2021). Mangiferin Alleviates Mitochondrial ROS in Nucleus Pulposus Cells and Protects against Intervertebral Disc Degeneration via Suppression of NF-Κb Signaling Pathway. Oxidative Med. Cell. Longev. 2021, 6632786. doi:10.1155/2021/6632786
Yun, S., Lee, Y.-J., Choi, J., Kim, N. D., Han, D. C., and Kwon, B.-M. (2021). Acacetin Inhibits the Growth of STAT3-Activated DU145 Prostate Cancer Cells by Directly Binding to Signal Transducer and Activator of Transcription 3 (STAT3). Molecules 26 (20), 6204. doi:10.3390/molecules26206204
Zhang, J., Fan, F., Liu, A., Zhang, C., Li, Q., Zhang, C., et al. (2022). Icariin: A Potential Molecule for Treatment of Knee Osteoarthritis. Front. Pharmacol. 13, 811808. doi:10.3389/fphar.2022.811808
Zhang, Y., Yang, B., Wang, J., Cheng, F., Shi, K., Ying, L., et al. (2020). Cell Senescence: A Nonnegligible Cell State under Survival Stress in Pathology of Intervertebral Disc Degeneration. Oxid. Med. Cell Longev. 2020, 9503562. doi:10.1155/2020/9503562
Zhang, Y., Zhang, J., Wu, C., Guo, S., Su, J., Zhao, W., et al. (2019). Higenamine Protects Neuronal Cells from Oxygen-Glucose Deprivation/reoxygenation-Induced Injury. J. Cell Biochem. 120 (3), 3757–3764. doi:10.1002/jcb.27656
Zhao, H., Liu, M., Liu, H., Suo, R., and Lu, C. (2020). Naringin Protects Endothelial Cells from Apoptosis and Inflammation by Regulating the Hippo-YAP Pathway. Biosci. Rep. 40 (3). doi:10.1042/bsr20193431
Zhao, R., Liu, X., Zhang, L., Yang, H., and Zhang, Q. (2019). Current Progress of Research on Neurodegenerative Diseases of Salvianolic Acid B. Oxidative Med. Cell. Longev. 2019–9. doi:10.1155/2019/3281260
Zheng, X. X., Li, Y. C., Yang, K. L., He, Z. X., Wang, Z. L., Wang, X., et al. (2021). Icariin Reduces Glu-Induced Excitatory Neurotoxicity via Antioxidative and Antiapoptotic Pathways in SH-Sy5y Cells. Phytother. Res. 35 (6), 3377–3389. doi:10.1002/ptr.7057
Zheng, Z. C., Zhu, W., Lei, L., Liu, X. Q., and Wu, Y. G. (2020). Wogonin Ameliorates Renal Inflammation and Fibrosis by Inhibiting NF-Κb and TGF-β1/Smad3 Signaling Pathways in Diabetic Nephropathy. Drug Des. Devel Ther. 14, 4135–4148. doi:10.2147/dddt.s274256
Zhou, R., Tardivel, A., Thorens, B., Choi, I., and Tschopp, J. (2010). Thioredoxin-interacting Protein Links Oxidative Stress to Inflammasome Activation. Nat. Immunol. 11 (2), 136–140. doi:10.1038/ni.1831
Zhu, H., Sun, B., and Shen, Q. (2019). TNF-Α Induces Apoptosis of Human Nucleus Pulposus Cells via Activating the TRIM14/NF-Κb Signalling Pathway. Artif. Cells Nanomed Biotechnol. 47 (1), 3004–3012. doi:10.1080/21691401.2019.1643733
Zhu, L., Yu, C., Zhang, X., Yu, Z., Zhan, F., Yu, X., et al. (2020). The Treatment of Intervertebral Disc Degeneration Using Traditional Chinese Medicine. J. Ethnopharmacol. 263, 113117. doi:10.1016/j.jep.2020.113117
Zhu, Y., Wan, N., Shan, X., Deng, G., Xu, Q., Ye, H., et al. (2021). Celastrol Targets Adenylyl Cyclase-Associated Protein 1 to Reduce Macrophages-Mediated Inflammation and Ameliorates High Fat Diet-Induced Metabolic Syndrome in Mice. Acta Pharm. Sin. B 11 (5), 1200–1212. doi:10.1016/j.apsb.2020.12.008
Keywords: intervertebral disc degeneration, oxidative stress, inflammation, phytochemicals, therapeutic implication
Citation: Kang L, Zhang H, Jia C, Zhang R and Shen C (2022) Targeting Oxidative Stress and Inflammation in Intervertebral Disc Degeneration: Therapeutic Perspectives of Phytochemicals. Front. Pharmacol. 13:956355. doi: 10.3389/fphar.2022.956355
Received: 30 May 2022; Accepted: 20 June 2022;
Published: 12 July 2022.
Edited by:
Cheng Chen, Hefei Institutes of Physical Science (CAS), ChinaCopyright © 2022 Kang, Zhang, Jia, Zhang and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Renjie Zhang, emhhbmdyZW5qaWUxMDg5QDEyNi5jb20=; Cailiang Shen, c2hlbmNhaWxpYW5nQGFobXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Liang Kang
Liang Kang Huaqing Zhang†
Huaqing Zhang† Cailiang Shen
Cailiang Shen