- 1Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 3Department of Neurology, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, China
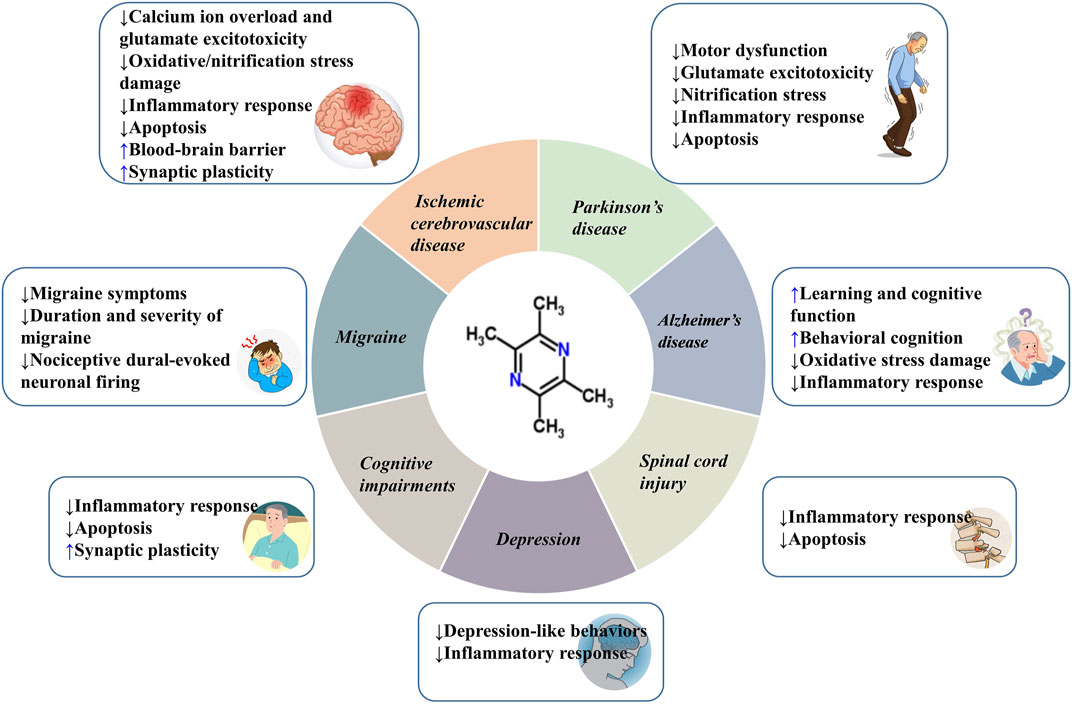
Central nervous system (CNS) diseases can lead to motor, sensory, speech, cognitive dysfunction, and sometimes even death. These diseases are recognized to cause a substantial socio-economic impact on a global scale. Tetramethylpyrazine (TMP) is one of the main active ingredients extracted from the Chinese herbal medicine Ligusticum striatum DC. (Chuan Xiong). Many in vivo and in vitro studies have demonstrated that TMP has a certain role in the treatment of CNS diseases through inhibiting calcium ion overload and glutamate excitotoxicity, anti-oxidative/nitrification stress, mitigating inflammatory response, anti-apoptosis, protecting the integrity of the blood-brain barrier (BBB) and facilitating synaptic plasticity. In this review, we summarize the roles and mechanisms of action of TMP on ischemic cerebrovascular disease, spinal cord injury, Parkinson’s disease, Alzheimer’s disease, cognitive impairments, migraine, and depression. Our review will provide new insights into the clinical applications of TMP and the development of novel therapeutics.
1 Introduction
Central nervous system (CNS) disorders are a group of neurological disorders that affect the structure or function of the brain or spinal cord and are characterized by motor, sensory, speech, and cognitive impairment (Naval et al., 2011; Kundap et al., 2017). The increasing number of people affected by CNS diseases in recent years has undoubtedly caused a significant socio-economic burden on a global scale (Alavijeh et al., 2005). However, only a few efficient drugs are available to treat CNS diseases (Alavijeh et al., 2005; Kant, 2014). Several reasons contribute to this pitfall, such as the complex pathogenesis of the disease, the permeability of the blood-brain barrier (BBB), the side effects of CNS drugs, etc. Hence, there is a pressing need to develop novel therapeutic drugs effective against CNS diseases. Recent research has indicated that Chinese herbal medicines and their active ingredients can be promising in treating CNS diseases due to their beneficial role (Zhou et al., 2012; Liu et al., 2018; Fan et al., 2020).
Tetramethylpyrazine (ligustrazine, TMP) is an alkaloid monomer extracted from the dried rhizome of the Chinese herbal medicine Ligusticum striatum DC. (Chuan Xiong) [Figure 1 (Li and Tetramethylpyrazine, 2022)]. Chuan Xiong is a vital ingredient in Buyanghuanwu decoction, Houshiheisan, and Naoxintong capsules. All of them are classic prescriptions of traditional Chinese medicine (TCM) and have remarkable efficacy in treating cardiovascular and CNS diseases (Wang et al., 2014; Han et al., 2019; Gao et al., 2021). TMP is the more functional and structural representative of Chuan Xiong (Chang et al., 2007). Several studies have shown that TMP can exhibit multiple biopharmacological activities such as inhibition of platelet aggregation (Li et al., 2018), oxidative stress (Su et al., 2019), inflammation (Fan et al., 2011), apoptosis (Michel et al., 2016), and so forth. However, to date, the neuroprotective effects of TMP in CNS diseases have not been systematically reviewed. Our work tries to provide an overview of the pharmacological properties and therapeutic mechanisms of TMP associated with treating CNS diseases to facilitate its clinical application and provide a basis and direction for developing new drugs.
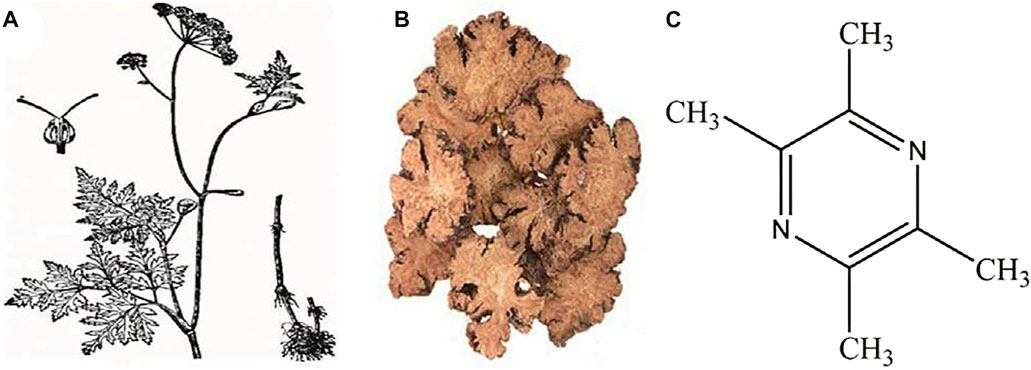
FIGURE 1. (A) Ligusticum striatum DC. (Chuan Xiong) plant; (B) Decoction pieces; (C) Chemical structure of tetramethylpyrazine [cited from Li J, Gong X. Front Pharmacol. 2022 Li and Tetramethylpyrazine (2022)].
2 Pharmacokinetics of tetramethylpyrazine
Through various detection methods, the absorption, distribution, metabolism, and excretion of TMP in vivo have been extensively studied over the past few years. Studies showed that TMP was quickly absorbed in the human body with an absorption half-life of 0.15 h and the time required to reach its peak concentration in the blood was 0.51 h (Cai et al., 1989). Subsequently, TMP was rapidly and widely distributed in vivo, and the plasma protein binding rate of TMP was 64.64% (Ye et al., 2010). The blood pharmacokinetic parameters of unbound TMP conformed to the first-order rate and two-chamber open model (Tsai and Liang, 2001; Wang et al., 2012). In rats, different concentrations of TMP were detected in the kidneys, liver, fat, heart, spleen, muscle, and lungs (Wang et al., 2019). TMP penetrated the BBB and was distributed in various regions of the brain (cerebral cortex, brainstem, striatum, hippocampus, cerebellum, and midbrain) (Tsai and Liang, 2013), suggesting TMP may be effective in the treatment of cerebrovascular diseases (Liang et al., 1999).
In addition, the elimination of TMP was also quick. Following the intravenous injection, the pharmacokinetic profiles of TMP in rats were linear, and the half-life was about 35 min (Wang et al., 2019). It was also reported that the elimination half-life of TMP in rat blood and brain was 82.1 and 184.6 min, respectively (Tsai and Liang, 2013). 2-hydroxymethyl-3,5,6-trimethylpyrazine (HTMP) is the main active metabolite of TMP (Jiang, 1993). After administration of tetramethylpyrazine phosphate (TMPP) injection, high concentrations of HTMP were observed in the liver, lung, brain, kidney, heart, and spleen in rats, indicating that TMP is widely metabolized in vivo (Cao et al., 2013). Cytochrome P450 (CYP450) is the principal enzyme responsible for drug metabolism in the body and is distributed mainly in the liver microsomes (Schelstraete et al., 2018). About 15 enzymes belonging to the CYP1, CYP2, and CYP3 families are involved in the metabolism of foreign chemicals (Korobkova, 2015). A study further revealed that CYP3A4, 2C9, and 1A2 are the main CYP isoenzymes involved in the oxidative metabolism of TMP in human and rat liver microsomes (Tan et al., 2014). In summary, TMP has the pharmacokinetic characteristics of rapid absorption, wide distribution, and rapid elimination.
3 Drug delivery system of tetramethylpyrazine
The characteristic of short half-life makes TMP present a defect of low bioavailability, which limits its clinical application. Various methods have been used to enhance the TMP bioavailability, feasibility, and targeting. The drug delivery methods mainly include three crucial aspects.
3.1 Tetramethylpyrazine administration methods
A study showed that the bioavailability obtained by intranasal administration was significantly higher than that of intragastric administration (86.33% vs. 50.39%) (Meng et al., 2014a). TMP was absorbed more quickly through the nasal cavity, entered into the systemic circulation, and penetrated the BBB to target the brain (Jian et al., 2009; Meng et al., 2014b). Another clinical study compared the pharmacokinetics of transdermal patches and oral tablets of TMP in humans, indicating that the patch achieved the same therapeutic effect as oral administration (Shen et al., 2013). Also, it could prolong plasma TMP levels and lower drug fluctuations (Shen et al., 2013).
3.2 Combination of tetramethylpyrazine with other drugs
Borneol (BO), a crystalline isomer extracted from Blumea balsamifera (L.) DC. or Cinnamomum camphora (L.) J. Presl., effectively improves the bioavailability and blood concentration of co-administered drugs. It also increases the permeability of the BBB and promotes the drug to enter the brain, which is essential for its therapeutic role (Chen et al., 2019). BO can act as an effective adjuvant for delivering TMP to the brain. Studies have shown that the co-administration of BO could significantly increase oral absorption, areas under the concentration-time curve (AUC), and the concentration of TMP in the brain tissue (Yan-Yu et al., 2007; Liao et al., 2018; Wu et al., 2018).
3.3 Use of carrier systems for delivering drugs
Microemulsion (ME) is a new drug carrier system comprising a mixture of water, oil, and surfactants, and shows thermodynamic and kinetic stability (Karasulu, 2008). In mice, TMP-loaded microemulsion (TMP ME) presented a prolonged residence of time of the drug and markedly improved its overall targeting efficiency in the mice brain. A higher concentration of TMP was detected in mice’s brain, spleen, and lungs with the administration of TMP ME (Ma et al., 2013). In addition, ME-based transdermal delivery of TMP enhanced the TMP distribution rate to the brain and decreased its clearance rate from the brain (Zhao et al., 2011; Hu et al., 2019). The XBC microemulsion, a refined compound mixture of Rhizoma ligustric Chuanxiong extracts, BO, and TMP, enhances the bioavailability by increasing the half-life of TMP and prolonging the TMP residence time (Wang et al., 2012). The mean residence time of TMP in the brain also increased upon XBC microemulsion administration (Wang et al., 2012). Moreover, the lipid emulsion-based drug delivery system could significantly increase the mean residence time and half-life and decrease the clearance of TMP (Wei et al., 2012). Nanodrug delivery systems have received much attention recently because of their advantages, such as sustained, controlled release, and targeting (Altinoglu and Adali, 2019). According to another study, TMP-loaded nanoparticles modified with HIV-1 transcription factor (TAT) can boost the TMP half-life and membrane penetration rate, resulting in prolonged drug action and effective spinal cord targeting (Li et al., 2021a).
4 Effects of tetramethylpyrazine on central nervous system diseases
4.1 Ischemic cerebrovascular diseases
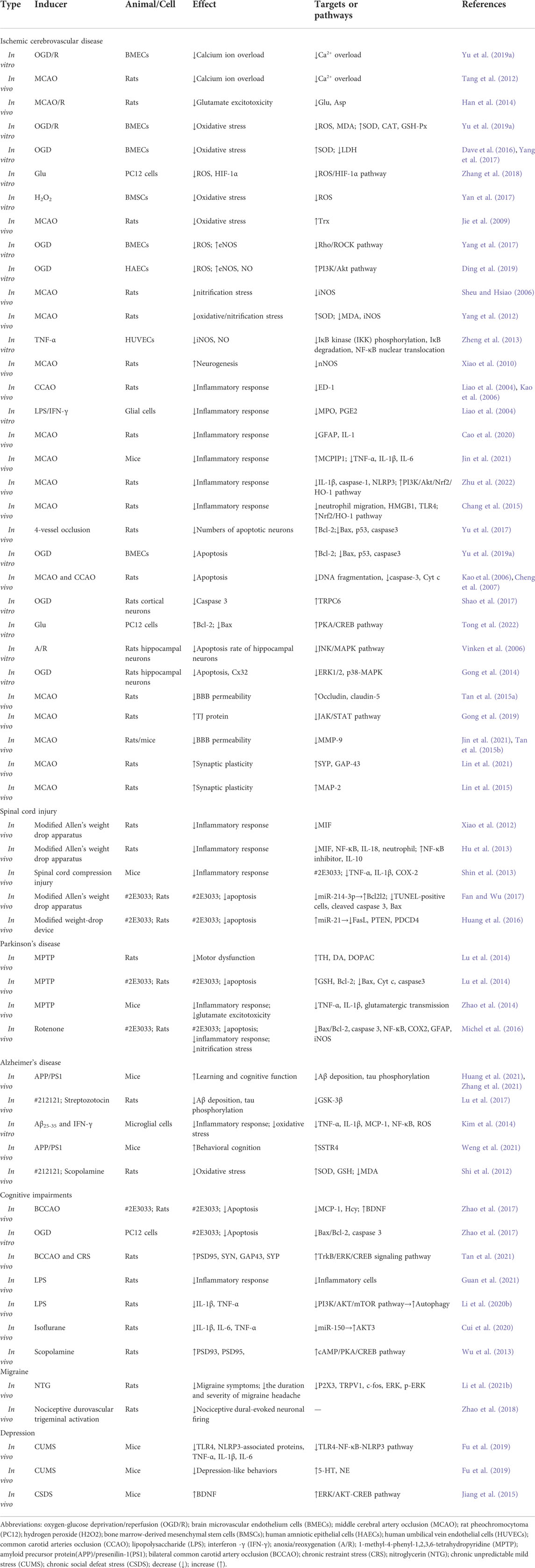
Ischemic cerebrovascular diseases (ICVDs) are a major public health concern and affect human health worldwide. Among the ICVDs, ischemic stroke is the most common and one of the leading causes of high morbidity and mortality (Li et al., 2020a). Evidence suggests that thrombolytic therapy effectively treats ischemic stroke and decreases morbidity and mortality when timely provided (Man et al., 2020). Ischemia and reperfusion result in several cellular and biochemical consequences, including intracellular Ca2+ overload, excitatory amino acid poisoning, inflammatory response, oxidative damage, apoptosis, and so forth (Lo et al., 2003; Khatri et al., 2012; Radak et al., 2017; Lian et al., 2020; Lim et al., 2021). Novel treatments are urgently needed to prevent and treat the cerebral ischemia-reperfusion (I/R) injury. Prior reports indicate that TMP protects against the above-mentioned pathological mechanisms in vivo and in vitro (Figure 2).
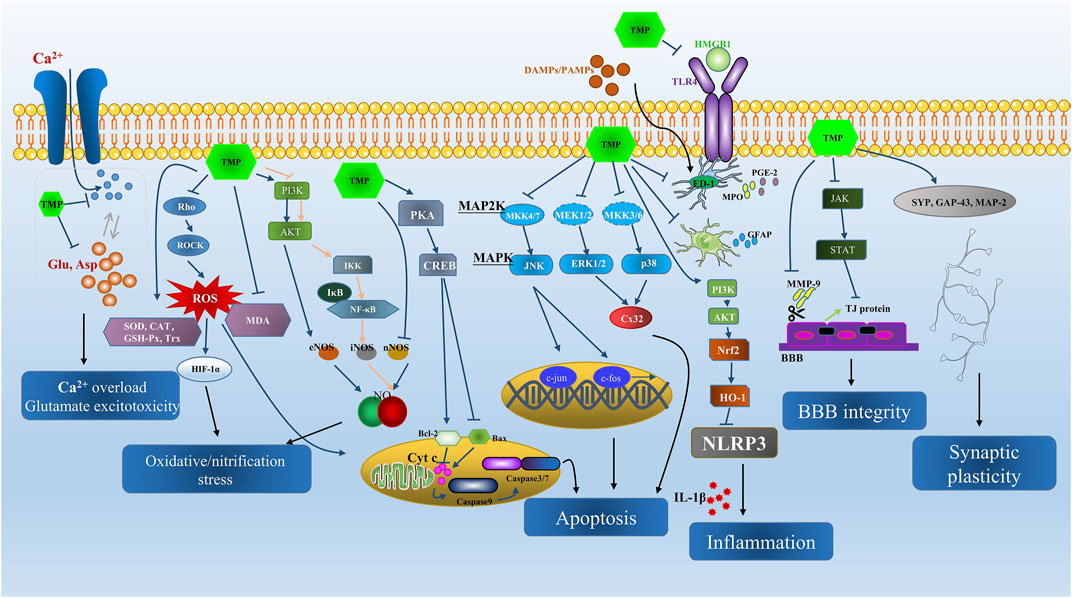
FIGURE 2. The summarization of neuroprotective effects and main signal pathways regulated by TMP in ICVD from both in vivo and in vitro studies.
4.1.1 Inhibiting calcium ion overload and glutamate excitotoxicity
During ischemia and reperfusion, energy expenditure leads to the depletion of ATP and the imbalance of calcium homeostasis, causing several excitatory neurotransmitters (mainly glutamic acid) to be released into the synaptic space (Belov Kirdajova et al., 2020). Further, energy-dependent presynapses fail to reuptake the glutamate (Glu), resulting in its accumulation, a permanent influx of calcium ions, and neuronal death through excitotoxicity (Lipton, 1999; Hurtado et al., 2005; Chamorro et al., 2016; Mahmoud et al., 2019). As evident above, there is a synergistic effect between Ca2+ overload and the toxic effects of excessive excitatory amino acids.
TMP has been reported to suppress calcium mobilization from extracellular medium and intracellular stores (Liu and Sylvester, 1990). One study showed TMP as a calcium antagonist that blocked the influx of extracellular calcium through calcium channels and inhibited the release of intracellularly stored calcium in vascular smooth muscle cells (Pang et al., 1996). In vitro, using oxygen-glucose deprivation/reperfusion (OGD/R)-induced brain microvascular endothelium cells (BMECs) injury model, TMP treatment was found to significantly attenuate the Ca2+ overload in BMECs (Yu et al., 2019a). Also, in the permanent middle cerebral artery occlusion (MCAO)-induced cerebral ischemic model, TMP was shown to decrease the concentration of intercellular free [Ca2+]ions (Tang et al., 2012). The regulation of the level of excitatory amino acids (EAAs) during cerebral ischemia has always been the focus of research on the prevention and treatment drugs of cerebral ischemia. The concentrations of two EAAs, Glu and aspartic acid (Asp), were evaluated in MCAO/reperfusion (MCAO/R) rat models. The results indicated that ischemia increased the striatal concentrations of Glu and Asp, while TMP treatment notably abrogated the aberration (Han et al., 2014). Hence, it is apparent that the inhibition of Ca2+ overload and glutamate excitotoxicity is the key effect of TMP on cerebral ischemia and reperfusion, which could provide new insights into treating this disease.
4.1.2 Anti-oxidative/nitrification stress damage
Free radicals include reactive oxygen species (ROS) and reactive nitrogen species (RNS), and oxidative/nitrification stress occurs when the production of ROS/RNS exceeds the clearance capacity of the antioxidant defense system (Diaz De Barboza et al., 2017). During ischemic stroke and reperfusion, ROS and RNS are generated in the brain tissue, activating mitochondria, death receptors, and endoplasmic reticulum stress pathways, ultimately mediating neuronal apoptosis (Stoll et al., 1998; Niizuma et al., 2009; Yuan et al., 2021). Thus, investigation of anti-oxidant/nitrification strategies becomes indispensable for treating cerebral ischemia and reperfusion.
In an OGD/R-induced BMECs injury model, TMP treatment relieved the oxidative stress by decreasing the concentrations of ROS and malondialdehyde (MDA) and upregulating the activities of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) (Yu et al., 2019a). Another study reported that TMP treatment enhanced the SOD levels and decreased the expression of lactate dehydrogenase (LDH), the earliest blood biochemical marker recognized during oxidative stress (Dave et al., 2016; Yang et al., 2017).
The transcriptional regulator hypoxia-inducible factor (HIF)-1α is a vital checkpoint of oxygen homeostasis, the expression of which rises rapidly after ischemia/hypoxia (He et al., 2021), and it plays a bidirectional role in ischemic stroke. On the one hand, it facilitates the adaptive response of cells in a hypoxic environment and modulates the expression of genes related to neurogenesis, angiogenesis, and cell proliferation (He et al., 2021). While on the other hand, it increases infarction volume and worsens nerve function by activating apoptosis and neuroinflammation, thus demonstrating a harmful effect (Chen et al., 2009; Cheng et al., 2014). Some researchers believe that low levels of HIF-1α can protect nerves, whereas the high expression of HIF-1α can cause cell death (Arumugam et al., 2018). Inhibiting the expression of HIF-1α in the early stages of ischemia is conducive to reducing cerebral edema and the number of apoptotic cells (Yeh et al., 2010). ROS plays a crucial role in promoting the stabilization of HIF-1α by inhibiting its ubiquitin degradation (Semenza, 2012; Zepeda et al., 2013). A study reported that ultrasound (US) exposure paired with TMP decreased intracellular ROS levels and inhibited the overexpression of HIF-1α in rat pheochromocytoma (PC12) cells. Thus, TMP exerted a neuroprotective effect by inhibiting the ROS/HIF-1α signaling pathway (Zhang et al., 2018).
Bone marrow-derived mesenchymal stem cells (BMSCs) transplantation has become a promising strategy for treating ischemic stroke (Li et al., 2007). However, transplanted BMSCs face the threat of oxidative stress in the ischemic microenvironment (Sun et al., 2012). TMP preconditioning could decrease intracellular ROS generation in hydrogen peroxide (H2O2)-induced BMSCs and is beneficial for cell survival in BMSCs transplantation therapy (Yan et al., 2017). Compelling evidence indicates that thioredoxin (Trx) plays an important role in the antioxidant defense mechanism (Rhee et al., 2001; Andoh et al., 2002; Kasuno et al., 2003). In vivo administration of TMP significantly decreased the volume of cerebral infarction and brain injury in MACO rats within 4 h post-reperfusion. This was likely achieved by upregulating the transcription of Trx (Jie et al., 2009).
Nitric oxide (NO), produced by inducible nitric oxide synthase (iNOS) and neuronal nitric oxide synthase (nNOS), is a typical representative of RNS. It can quickly react with superoxide (O2−) to produce a significant amount of peroxynitrite (ONOO-), resulting in nitrification stress (Pryor and Squadrito, 1995; Radi, 2004). On the contrary, NO produced by endothelial nitric oxide synthase (eNOS) exerts neuroprotective effects (Huang, 2004). Polymorphonuclear leukocytes (PMN) respiratory eruption induced by n-formylmethionyl-leucine-phenylalanine (fMLP) is often used to decipher the endogenous ROS and NO pathophysiology. Previous studies showed that TMP could scavenge the endogenous O2− and reduce the generation of NO in a dose-dependent fashion in fMLP-stimulated PMN (Zhang et al., 2003). The results indicated that TMP could be used as a NOS activity regulator and ROS scavenger.
In vitro, TMP treatment could attenuate ROS overproduction and improve the expression level of eNOS (Yang et al., 2017). This effect is achieved through inhibition of the Rho/Rho-kinase (Rho-associated kinases, ROCK) signaling pathway, which plays a vital role in the pathogenesis of stroke (Koumura et al., 2011). Likewise, the increased levels of eNOS and NO with protective properties were noted after TMP treatment in OGD-induced human amniotic epithelial cells (HAECs) which may be related to the activation of phosphoinositide 3-kinase (PI3K) and serine/threonine kinase (Akt) signaling pathways (Ding et al., 2019). Furthermore, TMP therapy reduced infarct size in an MCAO-induced cerebral I/R rat model, with the underlying mechanism related primarily to reducing iNOS expression and preventing free radical generation (Sheu and Hsiao, 2006). Another study reported that TMP combined with astragaloside IV treatment modulated SOD activity upregulation and downregulated MDA content and iNOS activity (Yang et al., 2012). According to Zheng et al. (2013), TMP decreased the NO production and had toxic effects in vitro mediated by repressing the iNOS expression. The harmful effects were attributed to inhibition of the PI3K/Akt pathway and further suppressing IκB kinase (IKK) phosphorylation, IκB degradation, and nuclear factor κB (NF-κB) translocation, all of which are required for NO transcription (Zheng et al., 2013). It is worth noting that activation and inhibition of the PI3K/Akt pathway are beneficial to nitrification stress resistance (Zheng et al., 2013; Ding et al., 2019). The former targets to increase eNOS expression, while the latter targets to decrease the iNOS expression. However, in-depth research is needed to confirm the dual role of the PI3K/Akt pathway and the effects of TMP intervention.
It is well established that neurogenesis is necessary to repair the damaged brain (Rahman et al., 2021). Significant levels of NO are required to inhibit the proliferation of neural stem cells, and as nNOS decreases, cell proliferation and migration increase (Zhang et al., 2006; Zhang et al., 2007; Carreira et al., 2014). After TMP treatment in the MCAO-induced I/R rat model, Xiao et al. (2010) found that TMP could reduce infarction volume, neuronal loss, and water content by decreasing the expression of nNOS and further promoting cell proliferation and differentiation. These findings suggest that TMP can control antioxidant/nitrification stress damage during cerebral ischemia and reperfusion treatment. However, more detailed studies are required to confirm this theory.
4.1.3 Mitigation of inflammatory response
Inflammation response involves in the pathogenesis of ischemic stroke and reperfusion. During ischemic stroke, oxygen deprivation and energy exhaustion lead to the accumulation of toxic metabolites in brain tissue, causing neuronal necrosis and apoptosis (Shah et al., 2019). Dead neurons release damage-associated molecular patterns (DAMPs) into the extracellular environment and produce large numbers of pro-inflammatory factors and chemokines, which rapidly activate microglia-mediated innate immune response (Xu et al., 2020). At the same time, reactive astrocytes promote the expression of pro-inflammatory factors such as interleukin- 6 (IL-6), tumor necrosis factor (TNF)-a, and IL-1β, and increase free radical ROS and NO levels (Liu and Chopp, 2016; Xu et al., 2020). Neutrophils are thought to be the primary source of free radicals during reperfusion (Funk et al., 2013; Jayaraj et al., 2019; Chen et al., 2021), and their counts are highest between 24 and 72 h after stroke. The activation of these immune cells can exacerbate the permeability of the BBB and destroy its integrity, aggravating nerve function damage (Huang et al., 2020).
Increasing evidence suggests that TMP exhibits anti-inflammatory effects against cerebral I/R. In vivo, TMP was proven to remarkably inhibit the immunoreactivity of ED-1, a microglia/macrophage marker protein (Liao et al., 2004; Kao et al., 2006). In the meantime, the activity of myeloperoxidase (MPO) and the production of prostaglandin E2 (PGE2) was significantly suppressed in the rat ischemic hemisphere, demonstrating the suppressive effect of TMP on the inflammatory cell activation and recruitment (Liao et al., 2004). Activation of astrocytes is characterized by increased glial fibrillary acidic protein (GFAP) in the blood (Dvorak et al., 2009). TMP combined with umbilical cord mesenchymal stem cells (ucMSCs) was found to rapidly downregulate the expression levels of GFAP and IL-1 (Cao et al., 2020).
The monocyte chemotactic protein-induced protein 1 (MCPIP1) is a suppressor of inflammation (Liang et al., 2010). Results from the cerebral I/R mice model induced by MCAO indicated that the TMP treatment effectively upregulates the expression of MCPIP1 and decreases the levels of inflammatory cytokines TNF-α, IL-1β, and IL-6 (Jin et al., 2021). Nuclear factor E2 correlation factor 2 (Nrf2) can regulate the oxidative stress and inflammation response by promoting the expression of the downstream heme oxygenase-1 (HO-1) gene (He et al., 2020). The PI3K/Akt pathway activation can promote Nrf2 nuclear translocation (Martin et al., 2004; Zhai et al., 2018). TMP sodium chloride injection was reported to enhance the expression of HO-1 by activating the PI3K/Akt/Nrf2 signal pathway (Zhu et al., 2022). At the same time, the expression levels of IL-1β, caspase-1, and NOD-like receptors family pyrin domain containing 3 (NLRP3) were significantly decreased by the treatment of TMP sodium chloride injection (Zhu et al., 2022).
High mobility group box 1 (HMGB1) is a highly conserved protein rapidly released extracellularly by the stimulation of pathogenic products (Andersson and Tracey, 2011). HMGB1 can bind to toll-like receptor-4 (TLR4), initiating the release of pro-inflammatory factors and causing tissue damage (Park et al., 2004; Fan et al., 2012). A clinical study showed that the serum HMGB1 levels in patients with cerebral ischemia were higher than in healthy volunteers (Goldstein et al., 2006). In the MCAO-induced cerebral ischemia rat model, TMP administration significantly inhibited neutrophil migration and suppressed the activities of HMGB1 and TLR4 (Chang et al., 2015). Interestingly, the inhibitory action of TMP on neutrophils was accompanied by increased expression levels of Nrf2 and HO-1 (Kao et al., 2013; Chang et al., 2015). Thus, according to the above findings, it is apparent that TMP could exert a strong anti-inflammatory response through the regulation of key targets or pathways in the pathogenesis of cerebral ischemia and reperfusion.
4.1.4 Anti-apoptosis
Apoptosis is an essential form of programmed cell death (Wood and Bristow, 2010). A series of biochemical reactions after an ischemic stroke can cause apoptosis and necrosis. Mitochondria-mediated endogenous apoptosis plays a vital part in neurological impairment. Cytochrome c (Cyt c), a key regulator of apoptosis, activates the caspase enzyme during its release from the mitochondria to the cytoplasm, initiating the caspase cascade and apoptosis (Jemmerson et al., 2005; Yadav et al., 2020). B-cell lymphoma-2 (Bcl-2) and Bcl-2-associated X (Bax) are two crucial regulatory proteins of the mitochondria-mediated apoptotic pathway (Gross et al., 1999). A critical tumor suppressor gene, p53, regulates the expression of Bax to trigger apoptosis (Miyashita et al., 1995). TMP exerts anti-apoptosis effects mainly by inhibiting the pro-apoptotic protein Bax expression, promoting anti-apoptotic protein Bcl-2 expression, and blocking the caspase cascade.
In the cerebral ischemia-reperfusion rat model, administration of TMP combined with BO improved neuronal ultrastructure, reduced the numbers of apoptotic neurons by increasing the level of Bcl-2, decreased the expression of Bax, and inhibited the mRNA levels of p53 and caspase 3 (Yu et al., 2017). Yu et al. (2019a) also pointed out that TMP and BO work synergistically in regulating the expressions of Bcl-2, Bax, p53, and caspase 3 enzyme. In addition, in vivo and in vitro, TMP treatment suppressed DNA fragmentation, caspase-3 activation, and Cyt c release (Kao et al., 2006; Cheng et al., 2007). TMP can exert the above-mentioned regulatory effects in a variety of ways. The transient receptor potential ion channel subfamily C member 6 (TRPC6) is a Ca2+ permeable, non-selective ion channel widely distributed in neurons (Lin et al., 2013). Inhibiting the proteolysis of TRPC6 is favorable for combating neuronal death in cerebral ischemia (Johansson et al., 2015; Guo et al., 2017). TMP may protect neurons from OGD-induced apoptosis and inhibit the expression of caspase-3 by blocking the TRPC6 degradation (Shao et al., 2017). The cAMP-dependent protein kinase (PKA)/cAMP response element binding protein (CREB) signaling pathway plays a crucial regulatory role in apoptosis by promoting the Bcl-2 expression (Jiang et al., 2016). Cell experiments showed that TMP administration regulated the expression of Bcl-2 and Bax by activating the PKA/CREB signaling pathway (Tong et al., 2022). Further, pre-administration of TMP lowered the apoptosis rate of hippocampal neurons by inhibiting the JNK/MAPK signal pathway (Zhong et al., 2016). Connexins (Cx) are a family of membrane proteins that act as key players in cell death (Vinken et al., 2006). In OGD-induced hippocampal neurons, apoptosis and the expression of Cx32 were significantly increased (Gong et al., 2014). It was reported that TMP could reverse these changes by inhibiting the phosphorylation levels of extracellular signal-related kinases 1/2 (ERK1/2) and p38-MAPK (Gong et al., 2014).
4.1.5 Protecting the blood-brain barrier
The BBB maintains a stable environment within the brain tissue. The tight junction (TJ) builds the structure and foundation of BBB. Cerebral ischemia and reperfusion increase BBB permeability, resulting in an enlarged infarct volume (Fan et al., 2018a). In the MCAO-induced cerebral ischemia/reperfusion rat model, TMP plays a neuroprotective role by reducing the infarct volume and BBB permeability, decreasing nerve score, and alleviating brain edema. The neuroprotective effect of TMP is related to attenuating the loss of TJ proteins, occludin and claudin-5 (Tan et al., 2015a). Further, the effects of TMP on enhancing the expression of TJ proteins are suggested through the inhibition of the activation of the JAK/STAT signaling pathway (Gong et al., 2019). Matrix metalloproteinases-9 (MMP-9) is an enzyme responsible for degrading the extracellular matrix and tight junctions (Candelario-Jalil et al., 2011). TMP treatment was also shown to preserve the BBB integrity by decreasing the levels of MMP-9 (Tan et al., 2015b; Jin et al., 2021).
4.1.6 Enhancing synaptic plasticity
After ischemic stroke, the disruption of normal neuronal function leads to motor, memory, and cognition dysfunction. Evidence shows synaptic plasticity is involved in restoring neurological function after stroke (Yu et al., 2019b; Yepes, 2020). Synaptic morphology is the structural basis of synaptic function and plasticity (Harris and Weinberg, 2012). Synaptophysin (SYP) and growth-associated binding protein 43 (GAP-43) are considered molecular markers of synaptogenesis, and the increase in their expression levels is indicative of the synaptic number and function recovery (Hami et al., 2017; Merino et al., 2019). In vivo, TMP treatment improved the synaptic ultrastructure significantly, as observed by transmission electron microscopy. This was reflected in the modified main curvature of the synaptic interface, postsynaptic density thickness, and synaptic cleft width (Lin et al., 2021). Further, the expression levels of SYP and GAP-43 were upregulated upon TMP treatment (Lin et al., 2021). The microtubule-associated protein 2 (MAP-2) is a postsynaptic protein and a sensitive marker of ischemic stroke, the loss of which often indicates neuronal dysfunction (Li et al., 1998). In another in vivo study, TMP treatment markedly improved the expression levels of MAP-2 in peri-infarct area after stroke and alleviated the neurological deficits (Lin et al., 2015). The above studies show that TMP has a remarkable potential to enhance synaptic plasticity.
4.2 Spinal cord injury
Spinal cord injury (SCI), a central nervous system disorder defined by motor, sensory, or autonomic dysfunction, is caused by direct or indirect external factors that damage the spinal cord entirely or partially. SCI often predisposes the patients to limited mobility and various complications (Mcdonald and Sadowsky, 2002). Current treatments of SCI include early surgical decompression and fixation, mean arterial blood pressure augmentation, and corticosteroids (Karsy and Hawryluk, 2019). However, these strategies can only improve symptoms and reduce complications, and have a limited effect on nerve regeneration and functional restoration. Disorders of the spinal cord microenvironment after injury can lead to a series of pathophysiological changes (Fan et al., 2018b). Achieving rebalancing of the spinal cord microenvironment is a key strategy for repairing nerves (Fan et al., 2022). In vivo and in vitro studies have demonstrated that TMP has a regulatory effect on the spinal cord microenvironmen.
Macrophages and microglia are the vital effector cells of the inflammatory response that follows SCI (David and Kroner, 2011; Brockie et al., 2021). In acute spinal cord injury rat model, TMP has been found to decrease the expression of migration inhibitory factor (MIF), which may repair the injured spinal cord tissue (Xiao et al., 2012). The experiment designed by Hu suggested that TMP treatment could inhibit the expression of MIF, NF-кB, pro-inflammatory cytokine IL-18 and neutrophil infiltration and increase the level of NF-κB inhibitor and anti-inflammation cytokine IL-10 (Hu et al., 2013). Cyclooxygenase-2 (COX-2) and iNOS are downstream signaling molecules of the NF-κB signaling pathway, which induce the production of NO and PGE2, thus promoting cellular inflammatory response (Liou et al., 2014). In vivo, the TNF-α, IL-1β expression, and mRNA levels of COX-2 were upregulated in SCI-mice model. However, the TMP treatment could reverse the above mentioned changes, attenuating microglial activation and neutrophil infiltration (Shin et al., 2013).
The long-term neurological deficits after SCI are thought to be caused by extensive apoptosis of neurons and oligodendrocytes (Shi et al., 2021). In vivo, TMP visibly reduced the number of TUNEL-positive cells, downregulated the protein expression of cleaved caspase 3 and Bax, and upregulated the protein levels of B-cell CLL/lymphoma 2 like 2 (Bcl2l2) (Fan and Wu, 2017). Further, the microRNA-214-3p (miR-214-3p) expression levels decreased following the TMP treatment. The luciferase reporter gene assay and cell experiments showed that the miR-214-3p targets the 3′-UTR of the Bcl2l2 gene (Fan and Wu, 2017). Hence, it is reasonable to speculate that TMP promotes the expression of the anti-apoptotic gene Bcl2l2 by regulating the miR-214-3p levels, thereby alleviating neuronal cell apoptosis in SCI rats. TMP has also been found to reduce the expression of Fas ligand gene (FasL), phosphatase and tensin homolog (PTEN), and programmed cell death 4 (PDCD4), all of which are the contributors to apoptosis (Huang et al., 2016). The mechanisms may be related to enhancing the miR-21 expression levels (Huang et al., 2016). The above results showed that TMP is a promising candidate for SCI treatment.
4.3 Parkinson’s disease
Parkinson’s disease (PD) is a common CNS disease characterized by tremors and bradykinesia. Currently, the drugs for PD treatment mainly involve levodopa, dopamine agonists, monoamine oxidase B inhibitors, catechol-O-methyl transferase inhibitors, anticholinergics, and amantadine (Reich and Savitt, 2019). To further improve the clinical efficacy of the therapy and reduce the incidence of complications, combination strategies are intended for the prevention and treatment of PD.
The underlying pathological change in PD is the denaturation and necrosis of dopaminergic neurons in substantia nigra compacta (Xu et al., 2002), leading to a serious loss of tyrosine hydroxylase (TH) and dopamine (DA) (Kawahata and Fukunaga, 2020; Miller and O’callaghan, 2015). TMP has been proven to attenuate motor dysfunction, inhibit the decrease of TH expression, and prevent the reduction of DA and its metabolite- DOPAC (Lu et al., 2014). The regulatory effects of TMP in PD primarily involve inhibition of apoptosis, nitrification stress, inflammation, and glutamate excitotoxicity. Neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) causes PD-like behavioral symptoms. Thus, the MPTP model has been used for experimental studies of PD. In an MPTP-induced PD rat model, TMP prevented the decrease of GSH, downregulated Bax expression, upregulated Bcl-2 expression, and inhibited the release of Cyt c and the lysis of caspase 3 (Lu et al., 2014). In addition, TMP could suppress the upregulation of TNF-α and IL-1β and modulate the glutamate levels in vivo (Zhao et al., 2014). In the rotenone-induced PD rat model, TMP attenuated the ratio of Bax/Bcl-2, inhibited the activation of caspase 3, and downregulated the expression levels of NF-кB, iNOS, COX-2, and GFAP (Michel et al., 2016). The findings reported in these studies demonstrate that TMP has a favorable effect on the treatment of PD (Figure 3).
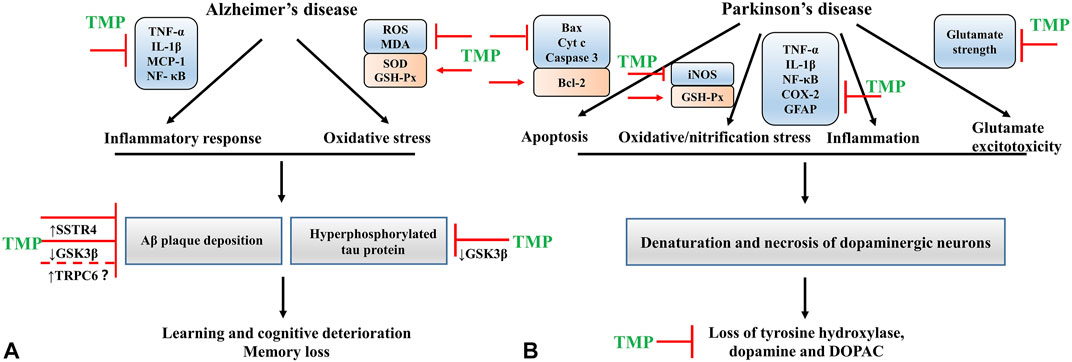
FIGURE 3. (A) The mechanisms of TMP intervention in AD; (B) The mechanisms of TMP intervention in PD.
4.4 Alzheimer’s disease
Alzheimer’s disease (AD) is a progressive neurodegenerative disease characterized by cognitive deterioration and memory loss, threatening human health and public health systems. Despite the serious public health problems the disease poses, only five drugs have been approved to treat AD (Briggs et al., 2016). Among them, donepezil, rivastigmine, galantamine and memantine are the most commonly used drugs that effectively relieve AD symptoms. Though they have side effects such as gastrointestinal, fatigue, dizziness and muscle cramps (Briggs et al., 2016). Given the rising prevalence of AD, there is an urgent need to develop new therapeutic drugs.
Evidence shows that β amyloid (Aβ) plaque deposition and hyperphosphorylated tau protein are the typical pathological changes observed in AD (Lane et al., 2018). In vivo studies, TMP has been proven to improve learning and cognitive function in AD models by lowering the Aβ deposition and tau phosphorylation levels (Huang et al., 2021; Zhang et al., 2021). The sequential cleavage of amyloid-beta precursor protein (APP) by β and γ-secretases is critical in Aβ production (Xiaojie and Weihong, 2013). One study demonstrated that TRPC6 could reduce the generation of Aβ by inhibiting the cleavage by γ-secretase (Wang et al., 2015). As mentioned before, TMP has a regulatory effect on TRPC6 (Shao et al., 2017). However, this effect is unknown in AD diseases and needs to be further addressed. The glycogen synthase kinase-3β (GSK-3β) is a multi-potential serine/threonine kinase that plays a crucial role in several cellular processes, including cell proliferation, differentiation, transformation, and apoptosis (Demuro et al., 2021). The activation of GSK3β contributes to memory loss, tau hyper-phosphorylation, increased Aβ deposition, and local plaque-associated microglial-mediated inflammatory responses (Hooper et al., 2008). In the AD rat model, TMP treatment inhibited the activity of GSK-3β and restored the function of cholinergic neurons. This neuroprotection provided by TMP may be mediated by the activation of Akt (Lu et al., 2017).
Neuroinflammation is a pathological feature of AD. Aβ can activate the microglial cells to release proinflammatory and cytotoxic factors, thus aggravating the inflammatory response of the CNS (Lue et al., 2010). In cultured microglial cells stimulated with Aβ25-35 and interferon (IFN)-γ, TMP could suppress the production of microglial proinflammatory mediators TNF-α, IL-1β and monocyte chemotactic protein 1 (MCP-1), and inhibit the activity of NF-κB (Kim et al., 2014). TMP pretreatment also decreased the ROS production in microglia (Kim et al., 2014). Somatostatin (SST) is a circular polypeptide that binds to somatostatin receptors (SSTR) on the cell membrane and is widely distributed in the CNS and peripheral tissues. It plays a vital role in memory, cognition, and emotional function (Arimura et al., 1975; Patel and Reichlin, 1978; Finley et al., 1981; Epelbaum et al., 2009). SST enhances the activity of neprilysin, an enzyme that degrades Aβ (Rofo et al., 2021). A study showed that the SST and SSTR protein levels in the cerebral cortex of Alzheimer’s patients were significantly lower than that in healthy individuals. Thus these proteins could serve as markers of the disease (Kumar, 2005). In vivo, TMP treatment improved the learning and memory function, attenuating AD mice’s cognitive impairment. The potential mechanism might be related to reducing the ubiquitination of SSTR4, thus increasing its levels in the system (Weng et al., 2021). Apart from these, researchers have designed the ligustrazine phosphate (LP) transdermal ethosomal system, and evaluated the effect of LP on AD in scopolamine-induced amnesia rats. The results showed that the LP transdermal ethosomal system could markedly increase SOD and GSH-Px activities, decrease MDA levels and reverse these activities/levels to the similar status of the normal rats (Shi et al., 2012) (Figure 3).
4.5 Cognitive impairment
Vascular dementia (VD) is the second leading type of dementia after Alzheimer’s disease. The clinical manifestations of VD include memory loss, emotional and behavioral changes, cognitive impairment, and executive dysfunction. Chronic cerebral ischemia, hypoxia, and hypoperfusion are the important causes of VD (Iadecola, 2013). Studies have demonstrated that TMP has a protective effect against VD, primarily through apoptosis inhibition and synaptic plasticity facilitation. In the bilateral common carotid artery occlusion (BCCAO) surgery-induced VD rats, TMP therapy modulated the pro-and anti-apoptotic indicators, inhibited the elevated MCP-1 and homocysteine (Hcy) levels, and suppressed the lowered levels of brain-derived neurotrophic factor (BDNF) (Zhao et al., 2017). TMP also significantly reduced the ratio of Bax/Bcl-2 protein and cleaved caspase-3 expression in OGD PC12 cells (Zhao et al., 2017). In the BCCAO stimulated rats, chronic restraint stress (CRS) is measured by the novel object recognition test, social interaction test, and Barnes maze paradigm. It was discovered in these rats that TMP treatment improved cognition, sociability, and learning/memory impairments, by increasing the expressions of synapse-related proteins PSD95, SYN, GAP43, and SYP by activating the TrkB/ERK/CREB signaling pathway (Tan et al., 2021).
TMP has been effective in alleviating learning and cognitive impairment through various mechanisms. In LPS-induced cognitive impairment model, TMP reduced the inflammatory cells in the brain tissue, suggesting its role in neuroinflammation alleviation and cognitive impairment improvement (Guan et al., 2021). Li et al. (2020b) reported that TMP could activate autophagy by inhibiting the PI3K/Akt/mTOR pathway and further reduce the expression levels of IL-1β and TNF-α, thus mitigating learning/memory function and neurocognitive impairments. MiR-150 is a key anti-inflammatory miRNA that regulates neuroinflammation by targeting Akt3 pathway (Ji et al., 2018; Cai et al., 2019). TMP promoted the modulation of miR-150/Akt pathway in vivo, reducing neuroinflammation and improving learning and memory function (Cui et al., 2020). The cAMP/PKA/CREB pathway is critical for memory formation because it promotes synaptic plasticity (Kandel, 2012). Evidence indicates that TMP treatment preserved the expression levels of PSD93 and PSD95 by restoring the normal function of cAMP/PKA/CREB pathway (Wu et al., 2013). Cumulatively these studies indicate that TMP might attenuate CI by suppressing the inflammatory response and apoptosis and promote synaptic plasticity (Figure 4).
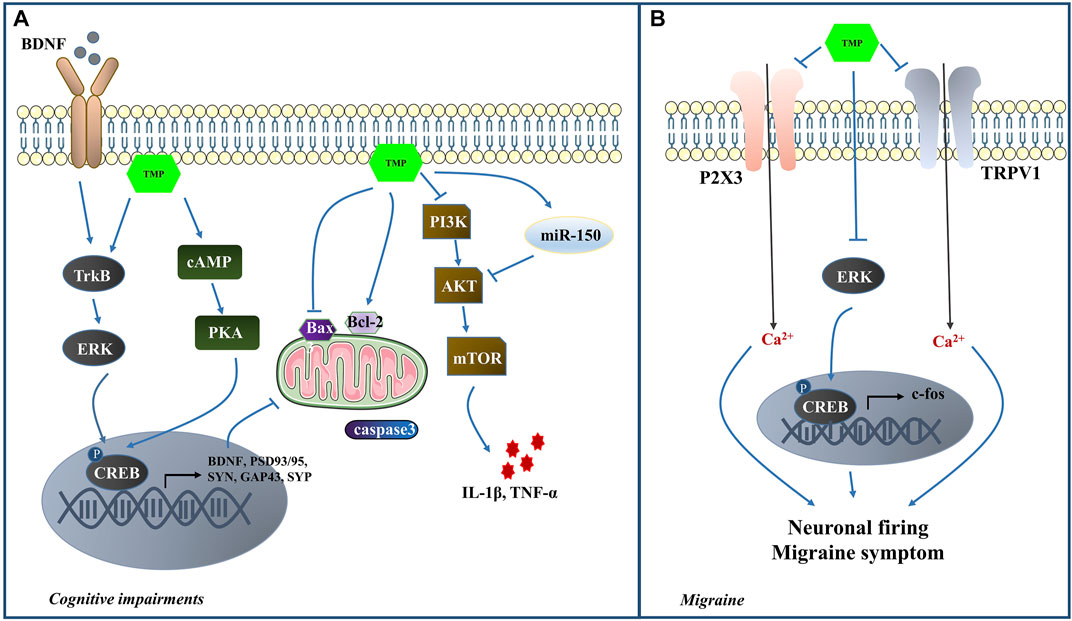
FIGURE 4. (A) The mechanisms of TMP intervention in CI; (B) The mechanisms of TMP intervention in MI.
4.6 Migraine
Migraine (MI), classified clinically as a primary headache, ranks second among disabling medical illnesses worldwide, according to the 2017 Global Burden of Disease Survey (Global, 2019). The mainstay of MI treatment involves ergot alkaloids and triptans. However, they can cause severe vasoconstriction (Raut et al., 2020). Further research into new drugs is warranted for the treatment of MI.
Previous studies have demonstrated that the activation of the trigeminovascular system plays a crucial role in MI (Chen and Wu, 2020). The interaction of Purinergic (P2X3) receptors and transient receptor potential vanilloid subtype 1 (TRPV1) in trigeminal sensory neurons contributes to mechanical hyperalgesia (Saloman et al., 2013). In addition, ERK1/2 has an essential role in the occurrence and development of neuropathic pain (Chen et al., 2016). p-ERK is translocated from the cytoplasm into the nucleus, where it exerts its regulatory effects on the target gene expression by phosphorylation of CREB. Finally, the target gene c-fos participates in the course of MI (Tang et al., 2020). Nitroglycerin (NTG) was used in a study to establish the MI rat model and elucidate TMP’s effects on analgesia. The results indicated that TMP could relieve MI symptoms and reduce the duration and severity of MI headaches. The TMP mechanism may be associated with the inhibition of P2X3, TRPV1, c-fos, ERK and p-ERK expression in the NTG-induced MI rats (Li et al., 2021b). In the nociceptive durovascular trigeminal activation induced rat MI model, TMP could inhibit nociceptive dural-evoked neuronal firing in the trigeminocervical complex (Zhao et al., 2018). Thus it is worth exploring the potential of TMP-mediated MI protection (Figure 4).
4.7 Depression
Depression is a mood disorder and serious medical illness and it is a significant contributor to the global burden of diseases affecting millions of people worldwide. As reported by World Health Organization that depression will become the second most devastating disease only to cardiovascular disease by year 2030 (Uphoff et al., 2020).
The pathogenesis of depression has not been fully elucidated to date. However, the immune-inflammatory response may have some involvement in the disease progression (Haapakoski et al., 2016). NLRP3 is known to mediate the activation of microglia and the release of inflammatory factors, playing an essential role in the development of depression (Alcocer-Gómez and Cordero, 2014; Wong et al., 2016; Kaufmann et al., 2017). TLR4 on cell membranes recognizes pathogen-associated molecular patterns (PAMPs) or DAMPs and activates NF-κB, which upregulates the mRNA transcription levels of NLRP3, pro-IL-1β and pro-IL-18, thus mediating the inflammatory responses (He et al., 2016). In a chronic unpredictable mild stress (CUMS) -induced mice model, TMP exerted strong anti-inflammatory effects by inhibiting the TLR4 and NLRP3-associated proteins and suppressing the levels of inflammatory cytokines TNF-α, IL-1β, and IL-6 (Fu et al., 2019). Additionally, TMP also remarkably improved the levels of serotonin (5-HT) and norepinephrine (NE) (Fu et al., 2019). It is well established that the inhibition of the ERK pathway contributes to the onset of depression (Wang and Mao, 2019). The reduction in the BDNF and CREB levels, which are respectively the upstream and downstream molecules involved in the ERK pathway, is crucial in the pathogenesis of depression (Wang and Mao, 2019). In vivo, TMP was reported to reverse the decrease of BDNF protein and enhance the phosphorylation levels of ERK1/2 and CREB (Jiang et al., 2015). Although there is a lack of suffcient data to support TMP’s involvement in ameliorating depression, current evidence indicates that TMP usage may be a novel strategy in treating depression.
5 Conclusion
In this paper, we summarize the neuroprotective effects of TMP on various CNS diseases, including ICVD, SCI, PD, AD, CI, MI, and depression. Although the pathological manifestations and mechanisms are different, the protective effects of TMP against these CNS diseases are based on the following aspects: inhibiting the calcium ion overload and glutamate excitotoxicity, oxidative/nitrification stress, inflammatory response, apoptosis, and protecting the integrity of BBB and facilitating synaptic plasticity (Figure 5; Table 1). To improve the pharmacodynamics activities and pharmacokinetic properties, and given the short half-life and low bioavailability of TMP, multiple drug delivery systems and TMP derivatives have been developed to date (Zou et al., 2018). This greatly improves the drugability and clinical application value of TMP. Meta-analysis showed that TMP preparation had high safety, but there are also some adverse reactions, such as dry mouth, vomiting, and dizziness (Yu et al., 2016). Thus, TMP should be used reasonably under the guidance of instructions.
Currently, the injection of the traditional Chinese patent medicine ligustrazine hydrochloride has been widely used to treat ischemic cerebrovascular disease in China. However, the acceptance and usage of TMP in other countries are still limited. The evidence reviewed in this paper provides possibilities for the widespread clinical application of TMP as a promising therapeutic agent for CNS diseases.
Though TMP has shown promising potential in treating CNS diseases, some lacunae need to be addressed in future studies. First, it should be noted that the evidence mentioned in this paper is mainly obtained from cell and animal experiments, which is not enough to support the efficacy of TMP in clinical practice. Hence, conducting elaborate high-quality, large-scale, multicenter, and randomized controlled clinical trials to explore TMP’s effectiveness and safety is essential. Second, the use of modern detection technologies to explore the pharmacokinetic characteristics of TMP in the CNS is necessary. Also, various drug delivery systems that provide TMP with good solubility, high bioavailability, stable metabolism, and significant biological activity must be assessed. Third, modern molecular biology technologies are developing rapidly, so future research can further study the specific underlying mechanisms of TMP from the perspectives of multiomics and epigenetic modification. In conclusion, more detailed evidence is expected to support the benefical effects of TMP, in order to promote its clinical application worldwide.
Author contributions
YL and GY conceived and designed this study. YL retrieved the literature and drafted the manuscript. XL, WC and GY revised the manuscript. XL and YZ directed the research. All authors contributed to the article and approved the final manuscript.
Funding
This research was supported by the China Academy of Chinese Medical Sciences Innovation Fund (Nos. CI2021A01310, CI2021A00911), the Fundamental Research Funds for the Central public welfare research institutes (No. 2020YJSZX-3), the Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (No. ZYYCXTD-C-202007) and the Scientific and Technological Innovation Project of China Academy of Chinese Medical Sciences (No. CI2021B006).
Acknowledgments
We would like to express our appreciation to all authors of this review. We would like to thank Professor Xiangning Jiang of the University of California, San Francisco for revising this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alavijeh, M. S., Chishty, M., Qaiser, M. Z., and Palmer, A. M. (2005). Drug metabolism and pharmacokinetics, the blood-brain barrier, and central nervous system drug discovery. NeuroRX 2 (4), 554–571. doi:10.1602/neurorx.2.4.554
Alcocer-Gómez, E., and Cordero, M. D. (2014). NLRP3 inflammasome: A new target in major depressive disorder. CNS Neurosci. Ther. 20 (3), 294–295. doi:10.1111/cns.12230
Altinoglu, G., and Adali, T. (2019). Alzheimer's disease targeted nano-based drug delivery systems[J]. Curr. Drug Targets 20 (7).
Andersson, U., and Tracey, K. J. (2011). HMGB1 is a therapeutic target for sterile inflammation and infection. Annu. Rev. Immunol. 29 (1), 139–162. doi:10.1146/annurev-immunol-030409-101323
Andoh, T., Chock, P. B., and Chiueh, C. C. (2002). The roles of thioredoxin in protection against oxidative stress-induced apoptosis in SH-SY5Y cells. J. Biol. Chem. 277 (12), 9655–9660. doi:10.1074/jbc.M110701200
Arimura, A., Sato, H., Dupont, A., Nishi, N., and Schally, A. V. (1975). Somatostatin: Abundance of immunoreactive hormone in rat stomach and pancreas. Science 189 (4207), 1007–1009. doi:10.1126/science.56779
Arumugam, T. V., Baik, S. H., Balaganapathy, P., Sobey, C. G., Mattson, M. P., Jo, D. G., et al. (2018). Notch signaling and neuronal death in stroke[J]. Prog. Neurobiol. 165-167, 103–116. doi:10.1016/j.pneurobio.2018.03.002
Belov Kirdajova, D., Kriska, J., Tureckova, J., and Anderova, M. (2020). Ischemia-triggered glutamate excitotoxicity from the perspective of glial cells. Front. Cell. Neurosci. 14, 51. doi:10.3389/fncel.2020.00051
Briggs, R., Kennelly, S. P., and O'neill, D. (2016). Drug treatments in Alzheimer's disease. Clin. Med. 16 (3), 247–253. doi:10.7861/clinmedicine.16-3-247
Brockie, S., Hong, J., and Fehlings, M. G. (2021). The role of microglia in modulating neuroinflammation after spinal cord injury. Int. J. Mol. Sci. 22 (18), 9706. doi:10.3390/ijms22189706
Cai, W., Dong, S. N., and Lou, Y. (1989). [HPLC determination of tetramethylpyrazine in human serum and its pharmacokinetic parameters]. Acta Pharm. Sin. 24 (12), 881–886.
Candelario-Jalil, E., Thompson, J., Taheri, S., Grossetete, M., Adair, J. C., Edmonds, E., et al. (2011). Matrix metalloproteinases are associated with increased blood-brain barrier opening in vascular cognitive impairment. Stroke. 42 (5), 1345–1350. doi:10.1161/STROKEAHA.110.600825
Cai, W., Zhang, Y., Liu, Y., Liu, H., Zhang, Z., and Su, Z. (2019). Effects of miR-150 on neuropathic pain process via targeting AKT3. Biochem. Biophys. Res. Commun. 517 (3), 532–537. doi:10.1016/j.bbrc.2019.07.061
Cao, H., Cheng, Y., Zhang, J., Xu, M., and Ge, L. (2020). The effect of umbilical cord mesenchymal stem cells combined with tetramethylpyrazine therapy on ischemic brain injury: A histological study. J. Stroke Cerebrovasc. Dis. 29 (12), 105298. doi:10.1016/j.jstrokecerebrovasdis.2020.105298
Cao, R., Li, Q., Li, H., Chu, T., Jin, H., and Mao, S. J. (2013). Development of 2-hydroxymethyl-3, 5, 6-trimethylpyrazine palmitate-loaded lipid emulsion: Formulation, optimization, characterization, pharmacokinetics, biodistribution and pharmacodynamics. J. Drug Target. 21 (4), 341–353. doi:10.3109/1061186X.2012.751536
Carreira, B. P., Morte, M. I., Santos, A. I., Lourenco, A. S., Ambrosio, A. F., Carvalho, C. M., et al. (2014). Nitric oxide from inflammatory origin impairs neural stem cell proliferation by inhibiting epidermal growth factor receptor signaling. Front. Cell. Neurosci. 8, 343. doi:10.3389/fncel.2014.00343
Chamorro, Á., Dirnagl, U., Urra, X., and Planas, A. M. (2016). Neuroprotection in acute stroke: Targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet. Neurol. 15 (8), 869–881. doi:10.1016/S1474-4422(16)00114-9
Chang, C. Y., Kao, T. K., Chen, W. Y., Ou, Y. C., Li, J. R., Liao, S. L., et al. (2015). Tetramethylpyrazine inhibits neutrophil activation following permanent cerebral ischemia in rats. Biochem. Biophys. Res. Commun. 463 (3), 421–427. doi:10.1016/j.bbrc.2015.05.088
Chang, X. L., Ma, Y. B., Zhang, X. M., Jiang, Z. y., and Chen, J. j. (2007). [Studies on chemical constituents of rhizomes of Ligusticum chuanxiong]. Zhongguo Zhong Yao Za Zhi 32 (15), 1533–1536.
Chen, C., Huang, T., Zhai, X., Ma, Y., Xie, L., Lu, B., et al. (2021). Targeting neutrophils as a novel therapeutic strategy after stroke. J. Cereb. Blood Flow. Metab. 41 (9), 2150–2161. doi:10.1177/0271678X211000137
Chen, C., Qin, H., Yan, J., Yang, X., Shi, X., Lei, J., et al. (2009). Early inhibition of HIF-1alpha with small interfering RNA reduces ischemic-reperfused brain injury in rats. Neurobiol. Dis. 33 (3), 509–517. doi:10.1016/j.nbd.2008.12.010
Chen, N. F., Chen, W. F., Sung, C. S., Lu, C. H., Chen, C. L., Hung, H. C., et al. (2016). Contributions of p38 and ERK to the antinociceptive effects of TGF-β1 in chronic constriction injury-induced neuropathic rats. J. Headache Pain 17 (1), 72. doi:10.1186/s10194-016-0665-2
Chen, S. T., and Wu, J. W. (2020). A new era for migraine: The role of calcitonin gene-related peptide in the trigeminovascular system. Prog. Brain Res. 255, 123–142. doi:10.1016/bs.pbr.2020.05.012
Chen, Z. X., Xu, Q. Q., Shan, C. S., Shi, Y. h., Wang, Y., Chang, R. C. C., et al. (2019). Borneol for regulating the permeability of the blood-brain barrier in experimental ischemic stroke: Preclinical evidence and possible mechanism. Oxid. Med. Cell. Longev. 2019, 1–15. doi:10.1155/2019/2936737
Cheng, X. R., Zhang, L., Hu, J. J., Sun, L., and Du, G. H. (2007). Neuroprotective effects of tetramethylpyrazine on hydrogen peroxide-induced apoptosis in PC12 cells. Cell Biol. Int. 31 (5), 438–443. doi:10.1016/j.cellbi.2006.10.001
Cheng, Y. L., Park, J. S., Manzanero, S., Choi, Y., Baik, S. H., Okun, E., et al. (2014). Evidence that collaboration between HIF-1α and Notch-1 promotes neuronal cell death in ischemic stroke. Neurobiol. Dis. 62, 286–295. doi:10.1016/j.nbd.2013.10.009
Cui, H., Xu, Z., and Qu, C. (2020). Tetramethylpyrazine ameliorates isofluraneinduced cognitive dysfunction by inhibiting neuroinflammation via miR150 in rats[J]. Exp. Ther. Med. 20 (4), 3878–3887. doi:10.3892/etm.2020.9110
Dave, A., Maru, L., and Jain, A. (2016). LDH (lactate dehydrogenase): A biochemical marker for the prediction of adverse outcomes in pre-eclampsia and eclampsia. J. Obstet. Gynaecol. India 66 (1), 23–29. doi:10.1007/s13224-014-0645-x
David, S., and Kroner, A. (2011). Repertoire of microglial and macrophage responses after spinal cord injury. Nat. Rev. Neurosci. 12 (7), 388–399. doi:10.1038/nrn3053
Demuro, S., Di Martino, R. M. C., Ortega, J. A., and Cavalli, A. (2021). GSK-3β, FYN, and DYRK1A: Master regulators in neurodegenerative pathways. Int. J. Mol. Sci. 22 (16), 9098. doi:10.3390/ijms22169098
Diaz De Barboza, G., Guizzardi, S., Moine, L., and Tolosa de Talamoni, N. (2017). Oxidative stress, antioxidants and intestinal calcium absorption. World J. Gastroenterol. 23 (16), 2841–2853. doi:10.3748/wjg.v23.i16.2841
Ding, Y., Du, J., Cui, F., Chen, L., and Li, K. (2019). The protective effect of ligustrazine on rats with cerebral ischemia-reperfusion injury via activating PI3K/Akt pathway. Hum. Exp. Toxicol. 38 (10), 1168–1177. doi:10.1177/0960327119851260
Dvorak, F., Haberer, I., Sitzer, M., and Foerch, C. (2009). Characterisation of the diagnostic window of serum glial fibrillary acidic protein for the differentiation of intracerebral haemorrhage and ischaemic stroke. Cerebrovasc. Dis. 27 (1), 37–41. doi:10.1159/000172632
Epelbaum, J., Guillou, J. L., Gastambide, F., Hoyer, D., Duron, E., and Viollet, C. (2009). Somatostatin, Alzheimer's disease and cognition: An old story coming of age?[J]. Prog. Neurobiol. 89 (2), 153–161. doi:10.1016/j.pneurobio.2009.07.002
Fan, B., Wei, Z., and Feng, S. (2022). Progression in translational research on spinal cord injury based on microenvironment imbalance. Bone Res. 10 (1), 35. doi:10.1038/s41413-022-00199-9
Fan, B., Wei, Z., Yao, X., Shi, G., Cheng, X., Zhou, X., et al. (2018). Microenvironment imbalance of spinal cord injury. Cell Transpl. 27 (6), 853–866. doi:10.1177/0963689718755778
Fan, F., Yang, J., Xu, Y., and Guan, S. (2018). MiR-539 targets MMP-9 to regulate the permeability of blood-brain barrier in ischemia/reperfusion injury of brain. Neurochem. Res. 43 (12), 2260–2267. doi:10.1007/s11064-018-2646-0
Fan, F., Yang, L., Li, R., Zou, X., Li, N., Meng, X., et al. (2020). Salidroside as a potential neuroprotective agent for ischemic stroke: A review of sources, pharmacokinetics, mechanism and safety. Biomed. Pharmacother. 129, 110458. doi:10.1016/j.biopha.2020.110458
Fan, J., Li, Y., Levy, R. M., Fan, J. J., Hackam, D. J., Vodovotz, Y., et al. (2012). Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: Role of HMGB1-TLR4 signaling. J. Immunol. 178 (10), 6573–6580. doi:10.4049/jimmunol.178.10.6573
Fan, L., Wang, K., Shi, Z., Die, J., Wang, C., and Dang, X. (2011). Tetramethylpyrazine protects spinal cord and reduces inflammation in a rat model of spinal cord ischemia-reperfusion injury. J. Vasc. Surg. 54 (1), 192–200. doi:10.1016/j.jvs.2010.12.030
Fan, Y., and Wu, Y. (2017). Tetramethylpyrazine alleviates neural apoptosis in injured spinal cord via the downregulation of miR-214-3p. Biomed. Pharmacother. 94, 827–833. doi:10.1016/j.biopha.2017.07.162
Feng, J., Li, F., Zhao, Y., Feng, Y., and Abe, Y. (2009). Brain pharmacokinetics of tetramethylpyrazine after intranasal and intravenous administration in awake rats. Int. J. Pharm. 375 (1-2), 55–60. doi:10.1016/j.ijpharm.2009.03.034
Finley, J. C., Maderdrut, J. L., Roger, L. J., and Petrusz, P. (1981). The immunocytochemical localization of somatostatin-containing neurons in the rat central nervous system. Neuroscience 6 (11), 2173–2192. doi:10.1016/0306-4522(81)90006-3
Fu, S., Wang, J., Hao, C., Dang, H., and Jiang, S. (2019). Tetramethylpyrazine ameliorates depression by inhibiting TLR4-NLRP3 inflammasome signal pathway in mice. Psychopharmacol. Berl. 236 (7), 2173–2185. doi:10.1007/s00213-019-05210-6
Funk, J. L., Frye, J. B., Davis-Gorman, G., Spera, A. L., Bernas, M. J., Witte, M. H., et al. (2013). Curcuminoids limit neutrophil-mediated reperfusion injury in experimental stroke by targeting the endothelium. Microcirculation 20 (6), 544–554. doi:10.1111/micc.12054
Gao, L., Xiao, Z., Jia, C., and Wang, W. (2021). Effect of buyang huanwu decoction for the rehabilitation of ischemic stroke patients: A meta-analysis of randomized controlled trials. Health Qual. Life Outcomes 19 (1), 79. doi:10.1186/s12955-021-01728-6
Global, regional (2019). , and national burden of neurological disorders, 1990-2016: A systematic analysis for the global burden of disease study 2016[J]. Lancet Neurol. 18 (5), 459–480.
Goldstein, R. S., Gallowitsch-Puerta, M., Yang, L. H., Rosas-Ballina, M., Huston, J. M., Czura, C. J., et al. (2006). Elevated high-mobility group box 1 levels in patients with cerebral and myocardial ischemia. Shock 25 (6), 571–574. doi:10.1097/01.shk.0000209540.99176.72
Gong, G., Yuan, L., Cai, L., Ran, M., Zhang, Y., Gong, H., et al. (2014). Tetramethylpyrazine suppresses transient oxygen-glucose deprivation-induced connexin32 expression and cell apoptosis via the ERK1/2 and p38 MAPK pathway in cultured hippocampal neurons. PLoS One 9 (9), e105944. doi:10.1371/journal.pone.0105944
Gong, P., Zhang, Z., Zou, Y., Tian, Q., Han, S., Xu, Z., et al. (2019). Tetramethylpyrazine attenuates blood-brain barrier disruption in ischemia/reperfusion injury through the JAK/STAT signaling pathway[J]. Eur. J. Pharmacol. 854, 289–297. doi:10.1016/j.ejphar.2019.04.028
Gross, A., Mcdonnell, J. M., and Korsmeyer, S. J. (1999). BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 13 (15), 1899–1911. doi:10.1101/gad.13.15.1899
Guan, X., Liu, S., Liang, M., Li, G., Dong, J., Zhou, Q., et al. (2021). Diffusion kurtosis imaging to evaluate the effect and mechanism of tetramethylpyrazine on cognitive impairment induced by lipopolysaccharide in rats[J]. Brain Imaging Behav. 15 (5), 2492–2501. doi:10.1007/s11682-021-00449-0
Guo, C., Ma, Y., Ma, S., Mu, F., Deng, J., Duan, J., et al. (2017). The role of TRPC6 in the neuroprotection of calycosin against cerebral ischemic injury. Sci. Rep. 7 (1), 3039. doi:10.1038/s41598-017-03404-6
Haapakoski, R., Ebmeier, K. P., Alenius, H., and Kivimaki, M. (2016). Innate and adaptive immunity in the development of depression: An update on current knowledge and technological advances. Prog. Neuropsychopharmacol. Biol. Psychiatry 66, 63–72. doi:10.1016/j.pnpbp.2015.11.012
Hami, J., Vafaei-Nezhad, S., Sadeghi, A., Ghaemi, K., Taheri, M. M. H., Fereidouni, M., et al. (2017). Synaptogenesis in the cerebellum of offspring born to diabetic mothers. J. Pediatr. Neurosci. 12 (3), 215–221. doi:10.4103/jpn.JPN_144_16
Han, J., Tan, H., Duan, Y., Chen, Y., Zhu, Y., Zhao, B., et al. (2019). The cardioprotective properties and the involved mechanisms of NaoXinTong Capsule. Pharmacol. Res. 141, 409–417. doi:10.1016/j.phrs.2019.01.024
Han, J., Wan, H. T., Yang, J. H., Zhang, Y. Y., Ge, L. J., and Bie, X. D. (2014). Effect of ligustrazine on levels of amino acid neurotransmitters in rat striatum after cerebral ischemia-reperfusion injury. J. Asian Nat. Prod. Res. 16 (11), 1060–1067. doi:10.1080/10286020.2014.935347
Harris, K. M., and Weinberg, R. J. (2012). Ultrastructure of synapses in the mammalian brain[J]. Cold Spring Harb. Perspect. Biol. 4 (5), 570–581.
He, F., Ru, X., and Wen, T. (2020). NRF2, a transcription factor for stress response and beyond. Int. J. Mol. Sci. 21 (13), 4777. doi:10.3390/ijms21134777
He, Q., Ma, Y., Liu, J., Zhang, D., Ren, J., Zhao, R., et al. (2021). Biological functions and regulatory mechanisms of hypoxia-inducible factor-1α in ischemic stroke. Front. Immunol. 12, 801985. doi:10.3389/fimmu.2021.801985
He, Y., Hara, H., and Núñez, G. (2016). Mechanism and regulation of NLRP3 inflammasome activation. Trends biochem. Sci. 41 (12), 1012–1021. doi:10.1016/j.tibs.2016.09.002
Hooper, C., Killick, R., and Lovestone, S. (2008). The GSK3 hypothesis of Alzheimer's disease. J. Neurochem. 104 (6), 1433–1439. doi:10.1111/j.1471-4159.2007.05194.x
Hu, J. Z., Huang, J. H., Xiao, Z. M., Li, J. H., Li, X. M., and Lu, H. B. (2013). Tetramethylpyrazine accelerates the function recovery of traumatic spinal cord in rat model by attenuating inflammation. J. Neurol. Sci. 324 (1-2), 94–99. doi:10.1016/j.jns.2012.10.009
Hu, X., Cheng, N., Zhao, J., Piao, X., Yan, Y., Zhang, Q., et al. (2019). Percutaneous absorption and brain distribution facilitation of borneol on tetramethylpyrazine in a microemulsion-based transdermal therapeutic system. Asian J. Pharm. Sci. 14 (3), 305–312. doi:10.1016/j.ajps.2018.06.003
Huang, J. H., Cao, Y., Zeng, L., Wang, G., Cao, M., Lu, H. B., et al. (2016). Tetramethylpyrazine enhances functional recovery after contusion spinal cord injury by modulation of MicroRNA-21, FasL, PDCD4 and PTEN expression. Brain Res. 1648 (1), 35–45. doi:10.1016/j.brainres.2016.07.023
Huang, P. L. (2004). Nitric oxide and cerebral ischemic preconditioning. Cell Calcium 36 (3-4), 323–329. doi:10.1016/j.ceca.2004.02.007
Huang, X., Yang, J., Huang, X., Zhang, Z., Liu, J., Zou, L., et al. (2021). Tetramethylpyrazine improves cognitive impairment and modifies the hippocampal proteome in two mouse models of Alzheimer's disease. Front. Cell Dev. Biol. 9, 632843. doi:10.3389/fcell.2021.632843
Huang, Y., Chen, S., Luo, Y., and Han, Z. (2020). Crosstalk between inflammation and the BBB in stroke. Curr. Neuropharmacol. 18 (12), 1227–1236. doi:10.2174/1570159X18666200620230321
Hurtado, O., Moro, M. A., Cárdenas, A., Sanchez, V., Fernandez-Tome, P., Leza, J. C., et al. (2005). Neuroprotection afforded by prior citicoline administration in experimental brain ischemia: Effects on glutamate transport. Neurobiol. Dis. 18 (2), 336–345. doi:10.1016/j.nbd.2004.10.006
Iadecola, C. (2013). The pathobiology of vascular dementia[J]. Neuron 80 (4), 844–866. doi:10.1016/j.neuron.2013.10.008
Jayaraj, R. L., Azimullah, S., Beiram, R., Jalal, F. Y., and Rosenberg, G. A. (2019). Neuroinflammation: Friend and foe for ischemic stroke. J. Neuroinflammation 16 (1), 142. doi:10.1186/s12974-019-1516-2
Jemmerson, R., Dubinsky, J. M., and Brustovetsky, N. (2005). Cytochrome C release from CNS mitochondria and potential for clinical intervention in apoptosis-mediated CNS diseases. Antioxid. Redox Signal. 7 (9-10), 1158–1172. doi:10.1089/ars.2005.7.1158
Ji, L. J., Lu, J. M., and Huang, Q. M. (2018). MiR‐150 alleviates neuropathic pain via inhibiting toll‐like receptor 5[J]. J. Cell Biochem. 119 (1), 1017–1026. doi:10.1002/jcb.26269
Jiang, B., Huang, C., Chen, X. F., Tong, L. J., and Zhang, W. (2015). Tetramethylpyrazine produces antidepressant-like effects in mice through promotion of BDNF signaling pathway. Int. J. Neuropsychopharmacol. 18 (8), pyv010. doi:10.1093/ijnp/pyv010
Jiang, J. (1993). [The metabolism of TMPz in vivo]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 15 (2), 79–82.
Jiang, Y., Yang, S., Tao, J., Lin, Z., Ye, X., You, Y., et al. (2016). Opposing needling promotes behavior recovery and exerts neuroprotection via the cAMP/PKA/CREB signal transduction pathway in transient MCAO rats[J]. Mol. Med. Rep. 13 (3), 2060–2070. doi:10.3892/mmr.2016.4773
Jie, J., Xi, Z., Hu, Y. S., Wu, Y., Wang, Q. Z., Li, N. N., et al. (2009). Protective effect of tetraethyl pyrazine against focal cerebral ischemia/reperfusion injury in rats: Therapeutic time window and its mechanism[J]. Thromb. Res. 123 (5), 727–730. doi:10.1016/j.thromres.2008.11.004
Jin, Z., Liang, J., and Kolattukudy, P. E. (2021). Tetramethylpyrazine preserves the integrity of blood-brain barrier associated with upregulation of MCPIP1 in a murine model of focal ischemic stroke. Front. Pharmacol. 12, 710358. doi:10.3389/fphar.2021.710358
Johansson, S. E., Andersen, X. E., Hansen, R. H., Povlsen, G. K., and Edvinsson, L. (2015). Cerebrovascular endothelin-1 hyper-reactivity is associated with transient receptor potential canonical channels 1 and 6 activation and delayed cerebral hypoperfusion after forebrain ischaemia in rats. Acta Physiol. 214 (3), 376–389. doi:10.1111/apha.12519
Kandel, E. R. (2012). The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol. Brain 5 (1), 14. doi:10.1186/1756-6606-5-14
Kant, U. R. (2014). Drug delivery systems, CNS protection, and the blood brain barrier[J]. Biomed. Res. Int. 2014, 37.
Kao, T. K., Ou, Y. C., Kuo, J. S., Chen, W. Y., Liao, S. L., Wu, C. W., et al. (2006). Neuroprotection by tetramethylpyrazine against ischemic brain injury in rats. Neurochem. Int. 48 (3), 166–176. doi:10.1016/j.neuint.2005.10.008
Kao, T-K., Chang, C-Y., Ou, Y-C., Chen, W. Y., Kuan, Y. H., Pan, H. C., et al. (2013). Tetramethylpyrazine reduces cellular inflammatory response following permanent focal cerebral ischemia in rats[J]. Exp. Neurol. 247, 188–201. doi:10.1016/j.expneurol.2013.04.010
Karasulu, H. Y. (2008). Microemulsions as novel drug carriers: The formation, stability, applications and toxicity. Expert Opin. Drug Deliv. 5 (1), 119–135. doi:10.1517/17425247.5.1.119
Karsy, M., and Hawryluk, G. (2019). Modern medical management of spinal cord injury. Curr. Neurol. Neurosci. Rep. 19 (9), 65. doi:10.1007/s11910-019-0984-1
Kasuno, K., Nakamura, H., Ono, T., Muso, E., and Yodoi, J. (2003). Protective roles of thioredoxin, a redox-regulating protein, in renal ischemia/reperfusion injury. Kidney Int. 64 (4), 1273–1282. doi:10.1046/j.1523-1755.2003.00224.x
Kaufmann, F. N., Costa, A. P., Ghisleni, G., Diaz, A. P., Rodrigues, A. L. S., Peluffo, H., et al. (2017). NLRP3 inflammasome-driven pathways in depression: Clinical and preclinical findings. Brain Behav. Immun. 64, 367–383. doi:10.1016/j.bbi.2017.03.002
Kawahata, I., and Fukunaga, K. (2020). Degradation of tyrosine hydroxylase by the ubiquitin-proteasome system in the pathogenesis of Parkinson's disease and dopa-responsive dystonia. Int. J. Mol. Sci. 21 (11), E3779. doi:10.3390/ijms21113779
Khatri, R., Mckinney, A. M., Swenson, B., and Janardhan, V. (2012). Blood-brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology 79 (13), S52–S57. doi:10.1212/WNL.0b013e3182697e70
Kim, M., Kim, S. O., Lee, M., Lee, J. H., Jung, W. S., Moon, S. K., et al. (2014). Tetramethylpyrazine, a natural alkaloid, attenuates pro-inflammatory mediators induced by amyloid β and interferon-γ in rat brain microglia[J]. Eur. J. Pharmacol. 740, 504–511. doi:10.1016/j.ejphar.2014.06.037
Korobkova, E. A. (2015). Effect of natural polyphenols on CYP metabolism: Implications for diseases. Chem. Res. Toxicol. 28 (7), 1359–1390. doi:10.1021/acs.chemrestox.5b00121
Koumura, A., Hamanaka, J., Kawasaki, K., Tsuruma, K., Shimazawa, M., Hozumi, I., et al. (2011). Fasudil and ozagrel in combination show neuroprotective effects on cerebral infarction after murine middle cerebral artery occlusion. J. Pharmacol. Exp. Ther. 338 (1), 337–344. doi:10.1124/jpet.110.177675
Kumar, U. (2005). Expression of somatostatin receptor subtypes (SSTR1-5) in Alzheimer's disease brain: An immunohistochemical analysis. Neuroscience 134 (2), 525–538. doi:10.1016/j.neuroscience.2005.04.001
Kundap, U. P., Saatheeyavaane, B., Yatinesh, K., Othman, I., and Shaikh, M. F. (2017). Plant derived phytocompound, embelin in CNS disorders: A systematic review. Front. Pharmacol. 8, 76. doi:10.3389/fphar.2017.00076
Lane, C. A., Hardy, J., and Schott, J. M. (2018). Alzheimer's disease. Eur. J. Neurol. 25 (1), 59–70. doi:10.1111/ene.13439
Li, G., Liu, S., Wang, H., Pan, R., Tang, H., Yan, X., et al. (2020). Ligustrazine ameliorates lipopolysaccharideinduced neurocognitive impairment by activating autophagy via the PI3K/AKT/mTOR pathway[J]. Int. J. Mol. Med. 45 (6), 1711–1720. doi:10.3892/ijmm.2020.4548
Li, H., Bai, F., Cong, C., Chen, B., Xie, W., Li, S., et al. (2021). Effects of ligustrazine on the expression of neurotransmitters in the trigeminal ganglion of a rat migraine model. Ann. Transl. Med. 9 (16), 1318. doi:10.21037/atm-21-3423
Li, J., and Tetramethylpyrazine, Gong X. (2022). Tetramethylpyrazine: An active ingredient of Chinese herbal medicine with therapeutic potential in acute kidney injury and renal fibrosis. Front. Pharmacol. 13, 820071. doi:10.3389/fphar.2022.820071
Li, J., Wei, J., Wan, Y., Du, X., Bai, X., Li, C., et al. (2021). TAT-modified tetramethylpyrazine-loaded nanoparticles for targeted treatment of spinal cord injury[J]. J. Control. Release 335, 103–116. doi:10.1016/j.jconrel.2021.05.016
Li, J., Zhang, Q., Wang, W., Lin, F., Wang, S., Zhao, J., et al. (2020). Mesenchymal stem cell therapy for ischemic stroke: A look into treatment mechanism and therapeutic potential[J]. J. Neurology 268, 1–13. doi:10.1007/s00415-020-10138-5
Li, L., Chen, H., Shen, A., Li, Q., Chen, Y., Chu, J., et al. (2018). Ligustrazine inhibits platelet activation via suppression of the Akt pathway[J]. Int. J. Mol. Med. 43 (1), 575–582. doi:10.3892/ijmm.2018.3970
Li, Y., Chopp, M., Chen, J., Wang, L., Gautam, S. C., Xu, Y. X., et al. (2007). Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J. Cereb. Blood Flow. Metab. 20 (9), 1311–1319. doi:10.1097/00004647-200009000-00006
Li, Y., Jiang, N., Powers, C., and ChoppM., (1998). Neuronal damage and plasticity identified by microtubule-associated protein 2, growth-associated protein 43, and cyclin D1 immunoreactivity after focal cerebral ischemia in rats. Stroke 29 (9), 1972–1980. doi:10.1161/01.str.29.9.1972
Lian, L., Zhang, Y., Liu, L., Yang, L., Cai, Y., Zhang, J., et al. (2020). Neuroinflammation in ischemic stroke: Focus on MicroRNA-mediated polarization of microglia. Front. Mol. Neurosci. 13, 612439. doi:10.3389/fnmol.2020.612439
Liang, C. C., Hong, C. Y., Chen, C. F., and Tsai, T. H. (1999). Measurement and pharmacokinetic study of tetramethylpyrazine in rat blood and its regional brain tissue by high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 724 (2), 303–309. doi:10.1016/s0378-4347(99)00010-9
Liang, J., Saad, Y., Lei, T., Wang, J., Qi, D., Yang, Q., et al. (2010). MCP-induced protein 1 deubiquitinates TRAF proteins and negatively regulates JNK and NF-κB signaling. J. Cell Biol. 191 (6), i14. doi:10.1083/jcb1916oia14
Liao, S. L., Kao, T. K., Chen, W. Y., Lin, Y. S., Chen, S. Y., Raung, S. L., et al. (2004). Tetramethylpyrazine reduces ischemic brain injury in rats. Neurosci. Lett. 372 (1-2), 40–45. doi:10.1016/j.neulet.2004.09.013
Liao, W., Huang, X., Yin, Y., Liu, B., and Zhu, R. (2018). In vivo microdialysis with ultra performance liquid chromatography-mass spectrometry for analysis of tetramethylpyrazine and its interaction with borneol in rat brain and blood. Biomed. Chromatogr. 32 (6), e4210. doi:10.1002/bmc.4210
Lim, S., Kim, T. J., Kim, Y. J., Kim, C., Ko, S. B., and Kim, B. S. (2021). Senolytic therapy for cerebral ischemia-reperfusion injury. Int. J. Mol. Sci. 22 (21), 11967. doi:10.3390/ijms222111967
Lin, J., Hao, C., Gong, Y., Zhang, Y., Li, Y., Feng, Z., et al. (2021). Effect of tetramethylpyrazine on neuroplasticity after transient focal cerebral ischemia reperfusion in rats. Evid. Based. Complement. Altern. Med. 2021, 1587241. doi:10.1155/2021/1587241
Lin, J. B., Zheng, C. J., Zhang, X., Chen, J., Liao, W. J., Wan, Q., et al. (2015). Effects of tetramethylpyrazine on functional recovery and neuronal dendritic plasticity after experimental stroke[J]. Evidence-Based Complementray Altern. Med. 2015, 394926. doi:10.1155/2015/394926
Lin, Y., Pan, W., Ning, S., Song, X., Jin, Z., and Lv, S. (2013). Prevalence and management of hypertension in patients with acute coronary syndrome vary with gender: Observations from the Chinese registry of acute coronary events (CRACE). Mol. Med. Rep. 8 (2), 173–177. doi:10.3892/mmr.2013.1461
Liou, C. J., Len, W. B., Wu, S. J., Lin, C. F., Wu, X. L., and Huang, W. C. (2014). Casticin inhibits COX-2 and iNOS expression via suppression of NF-κB and MAPK signaling in lipopolysaccharide-stimulated mouse macrophages. J. Ethnopharmacol. 158, 310–316. doi:10.1016/j.jep.2014.10.046
Lipton, P. (1999). Ischemic cell death in brain neurons. Physiol. Rev. 79 (4), 1431–1568. doi:10.1152/physrev.1999.79.4.1431
Liu, S. Y., and Sylvester, D. M. (1990). Antithrombotic/antiplatelet activity of tetramethylpyrazine. Thromb. Res. 58 (2), 129–140. doi:10.1016/0049-3848(90)90170-h
Liu, Y., Gao, J., Peng, M., Meng, H., Ma, H., Cai, P., et al. (2018). A review on central nervous system effects of gastrodin. Front. Pharmacol. 9, 24. doi:10.3389/fphar.2018.00024
Liu, Z., and Chopp, M. (2016). Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Prog. Neurobiol. 144, 103–120. doi:10.1016/j.pneurobio.2015.09.008
Lo, E. H., Dalkara, T., and Moskowitz, M. A. (2003). Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci. 4 (5), 399–415. doi:10.1038/nrn1106
Lu, C., Zhang, J., Shi, X., Miao, S., Bi, L., Zhang, S., et al. (2014). Neuroprotective effects of tetramethylpyrazine against dopaminergic neuron injury in a rat model of Parkinson's disease induced by MPTP. Int. J. Biol. Sci. 10 (4), 350–357. doi:10.7150/ijbs.8366
Lu, F., Li, X., Li, W., Wei, K., Yao, Y., Zhang, Q., et al. (2017). Tetramethylpyrazine reverses intracerebroventricular streptozotocin-induced memory deficits by inhibiting GSK-3β. Acta Biochim. Biophys. Sin. 49 (8), 722–728. doi:10.1093/abbs/gmx059
Lue, L. F., Kuo, Y. M., Beach, T., and Walker, D. G. (2010). Microglia activation and anti-inflammatory regulation in Alzheimer's disease. Mol. Neurobiol. 41 (2-3), 115–128. doi:10.1007/s12035-010-8106-8
Ma, L., Fan, Y., Wu, H., Na, S., Wang, L., and Lu, C. (2013). Tissue distribution and targeting evaluation of TMP after oral administration of TMP-loaded microemulsion to mice. Drug Dev. Ind. Pharm. 39 (12), 1951–1958. doi:10.3109/03639045.2012.725733
Mahmoud, S., Gharagozloo, M., Simard, C., and Gris, D. (2019). Astrocytes maintain glutamate homeostasis in the CNS by controlling the balance between glutamate uptake and release. Cells 8 (2), E184. doi:10.3390/cells8020184
Man, S., Xian, Y., Holmes, D. N., Matsouaka, R. A., Saver, J. L., Smith, E. E., et al. (2020). Target: Stroke was associated with faster intravenous thrombolysis and improved one-year outcomes for acute ischemic stroke in medicare beneficiaries. Circ. Cardiovasc. Qual. Outcomes 13 (12), e007150. doi:10.1161/CIRCOUTCOMES.120.007150
Martin, D., Rojo, A. I., Salinas, M., Diaz, R., Gallardo, G., Alam, J., et al. (2004). Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J. Biol. Chem. 279 (10), 8919–8929. doi:10.1074/jbc.M309660200
Mcdonald, J. W., and Sadowsky, C. (2002). Spinal-cord injury. Lancet 359 (9304), 417–425. doi:10.1016/S0140-6736(02)07603-1
Meng, D., Lu, H., Huang, S., Wei, M., Ding, P., Xiao, X., et al. (2014). Comparative pharmacokinetics of tetramethylpyrazine phosphate in rat plasma and extracellular fluid of brain after intranasal, intragastric and intravenous administration[J]. Acta Pharm. Sin. B 4 (1), 74–78. doi:10.1016/j.apsb.2013.12.009
Meng, D., Lu, H., Huang, S., Wei, M., Ding, P., Xiao, X., et al. (2014). Comparative pharmacokinetics of tetramethylpyrazine phosphate in rat plasma and extracellular fluid of brain after intranasal, intragastric and intravenous administration. Acta Pharm. Sin. B 4 (1), 74–78. doi:10.1016/j.apsb.2013.12.009
Merino, P., Diaz, A., Torre, E. R., and Yepes, M. (2019). Urokinase-type plasminogen activator (uPA) regulates the expression and function of the growth- associated protein-43 (GAP-43) in the synapse[J]. J. Biol. Chem. 295 (2), 619–630. doi:10.1074/jbc.RA119.010644
Michel, H. E., Tadros, M. G., Esmat, A., Khalifa, A. E., and Abdel-Tawab, A. M. (2016). Tetramethylpyrazine ameliorates rotenone-induced Parkinson's disease in rats: Involvement of its anti-inflammatory and anti-apoptotic actions[J]. Mol. Neurobiol. 54 (7), 4866–4878. doi:10.1007/s12035-016-0028-7
Miller, D. B., and O'callaghan, J. P. (2015). Biomarkers of Parkinson's disease: Present and future. Metabolism. 64 (3), S40–S46. doi:10.1016/j.metabol.2014.10.030
Miyashita, T., Reed, J. C., and Miyashita, T. (1995). Reed JCTumour suppressor p53 is a direct transcriptional activator of human BAX gene. Cell 80: 293-299[J]. Cell 80 (2), 293–299. doi:10.1016/0092-8674(95)90412-3
Naval, N., Chandolu, S., and Mirski, M. (2011). Organ failure: Central nervous system. Semin. Respir. Crit. Care Med. 32 (05), 587–597. doi:10.1055/s-0031-1287867
Niizuma, K., Endo, H., and Chan, P. H. (2009). Oxidative stress and mitochondrial dysfunction as determinants of ischemic neuronal death and survival. J. Neurochem. 109 (1), 133–138. doi:10.1111/j.1471-4159.2009.05897.x
Pang, P. K., Shan, J. J., and Chiu, K. W. (1996). Tetramethylpyrazine, a calcium antagonist. Planta Med. 62 (5), 431–435. doi:10.1055/s-2006-957933
Park, J. S., Svetkauskaite, D., He, Q., Kim, J. Y., Strassheim, D., Ishizaka, A., et al. (2004). Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem. 279 (9), 7370–7377. doi:10.1074/jbc.M306793200
Patel, Y. C., and Reichlin, S. (1978). Somatostatin in hypothalamus, extrahypothalamic brain, and peripheral tissues of the rat. Endocrinology 102 (2), 523–530. doi:10.1210/endo-102-2-523
Pryor, W. A., and Squadrito, G. L. (1995). The chemistry of peroxynitrite: A product from the reaction of nitric oxide with superoxide. Am. J. Physiol. 268 (5), L699–L722. doi:10.1152/ajplung.1995.268.5.L699
Radak, D., Katsiki, N., Resanovic, I., Jovanovic, A., Sudar-Milovanovic, E., Zafirovic, S., et al. (2017). Apoptosis and acute brain ischemia in ischemic stroke. Curr. Vasc. Pharmacol. 15 (2), 115–122. doi:10.2174/1570161115666161104095522
Radi, R. (2004). Nitric oxide, oxidants, and protein tyrosine nitration. Proc. Natl. Acad. Sci. U. S. A. 101 (12), 4003–4008. doi:10.1073/pnas.0307446101
Rahman, A. A., Amruta, N., Pinteaux, E., and Bix, G. J. (2021). Neurogenesis after stroke: A therapeutic perspective. Transl. Stroke Res. 12 (1)–14. doi:10.1007/s12975-020-00841-w
Raut, S., Singh, U., Sarmah, D., Datta, A., Baidya, F., Shah, B., et al. (2020). Migraine and ischemic stroke: Deciphering the bidirectional pathway. ACS Chem. Neurosci. 11 (11), 1525–1538. doi:10.1021/acschemneuro.0c00137
Reich, S. G., and Savitt, J. M. (2019). Parkinson's disease. Med. Clin. North Am. 103 (2), 337–350. doi:10.1016/j.mcna.2018.10.014
Rhee, S. G., Kang, S. W., Chang, T. S., Jeong, W., and Kim, K. (2001). Peroxiredoxin, a novel family of peroxidases. IUBMB Life 52 (1-2), 35–41. doi:10.1080/15216540252774748
Rofo, F., Ugur Yilmaz, C., Metzendorf, N., Gustavsson, T., Beretta, C., Erlandsson, A., et al. (2021). Enhanced neprilysin-mediated degradation of hippocampal Aβ42 with a somatostatin peptide that enters the brain. Theranostics 11 (2), 789–804. doi:10.7150/thno.50263
Saloman, J. L., Chung, M. K., and Ro, J. Y. (2013). P2X3 and TRPV1 functionally interact and mediate sensitization of trigeminal sensory neurons. Neuroscience 232, 226–238. doi:10.1016/j.neuroscience.2012.11.015
Schelstraete, W., Devreese, M., and Croubels, S. (2018). Storage stability study of porcine hepatic and intestinal cytochrome P450 isoenzymes by use of a newly developed and fully validated highly sensitive HPLC-MS/MS method. Anal. Bioanal. Chem. 410 (6), 1833–1843. doi:10.1007/s00216-017-0839-z
Semenza, G. (2012). Hypoxia-inducible factors in physiology and medicine. Cell 148 (3), 399–408. doi:10.1016/j.cell.2012.01.021
Shah, F. A., Kury, L. A., Li, T., Zeb, A., Koh, P. O., Liu, F., et al. (2019). Polydatin attenuates neuronal loss via reducing neuroinflammation and oxidative stress in rat MCAO models. Front. Pharmacol. 10, 663. doi:10.3389/fphar.2019.00663
Shao, Z., Wang, L., Liu, S., and Wang, X. (2017). Tetramethylpyrazine protects neurons from oxygen-glucose deprivation-induced death. Med. Sci. Monit. 23, 5277–5282. doi:10.12659/msm.904554
Shen, T., Xu, H., Weng, W., and Zhang, J. (2013). Single- and multiple-dose pharmacokinetics of a novel tetramethylpyrazine reservoir-type transdermal patch versus tetramethylpyrazine phosphate oral tablets in healthy normal volunteers, and in vitro/in vivo correlation. Biol. Pharm. Bull. 36 (6), 931–937. doi:10.1248/bpb.b12-00909
Sheu, J. R., and Hsiao, G. (2006). Inhibitory mechanisms of tetramethylpyrazine in middle cerebral artery occlusion (MCAO)-induced focal cerebral ischemia in rats[J]. Planta. Med. 72 (5), 411–417. doi:10.1055/s-2005-917242
Shi, J., Wang, Y., and Luo, G. (2012). Ligustrazine phosphate ethosomes for treatment of Alzheimer's disease, in vitro and in animal model studies. AAPS PharmSciTech 13 (2), 485–492. doi:10.1208/s12249-012-9767-6
Shi, Z., Yuan, S., Shi, L., Li, J., Ning, G., Kong, X., et al. (2021). Programmed cell death in spinal cord injury pathogenesis and therapy. Cell Prolif. 54 (9), e12992. doi:10.1111/cpr.12992
Shin, J. W., Moon, J. Y., Seong, J. W., Song, S. H., Cheong, Y. J., Kang, C., et al. (2013). Effects of tetramethylpyrazine on microglia activation in spinal cord compression injury of mice. Am. J. Chin. Med. 41 (06), 1361–1376. doi:10.1142/S0192415X13500912
Stoll, G., Jander, S., and Schroeter, M. (1998). Inflammation and glial responses in ischemic brain lesions. Prog. Neurobiol. 56 (2), 149–171. doi:10.1016/s0301-0082(98)00034-3
Su, Q., Lv, X., and Ye, Z. (2019). Ligustrazine Attenuates Myocardial injury induced by Coronary Microembolization in rats by activating the PI3K/Akt pathway. Oxid. Med. Cell. Longev. 2019 (2), 6791457. doi:10.1155/2019/6791457
Sun, B., Feng, M., Tian, X., Lu, X., Zhang, Y., Ke, X., et al. (2012). DL-3-n-Butylphthalide protects rat bone marrow stem cells against hydrogen peroxide-induced cell death through antioxidation and activation of PI3K-Akt pathway. Neurosci. Lett. 516 (2), 247–252. doi:10.1016/j.neulet.2012.04.003
Tan, F., Fu, W., Cheng, N., Meng, D., and Gu, Y. (2015). Ligustrazine reduces blood-brain barrier permeability in a rat model of focal cerebral ischemia and reperfusion[J]. Exp. Ther. Med. 9 (5), 1757–1762. doi:10.3892/etm.2015.2365
Tan, F., Fu, W., Cheng, N., Meng, D. I., and Gu, Y. (2015). Ligustrazine reduces blood-brain barrier permeability in a rat model of focal cerebral ischemia and reperfusion. Exp. Ther. Med. 9 (5), 1757–1762. doi:10.3892/etm.2015.2365
Tan, Y., Zhuang, X. M., Shen, G. L., Li, H., and Gao, Y. (2014). [Investigation of metabolic kinetics and reaction phenotyping of ligustrazin by using liver microsomes and recombinant human enzymes]. Acta Pharm. Sin. 49 (3), 374–379.
Tan, Z., Qiu, J., Zhang, Y., Yang, Q., Yin, X., Li, J., et al. (2021). Tetramethylpyrazine alleviates behavioral and psychological symptoms of dementia through facilitating hippocampal synaptic plasticity in rats with chronic cerebral hypoperfusion. Front. Neurosci. 15, 646537. doi:10.3389/fnins.2021.646537
Tang, C., Unekawa, M., Shibata, M., Tomita, Y., Izawa, Y., Sugimoto, H., et al. (2020). Characteristics of cortical spreading depression and c-Fos expression in transgenic mice having a mutation associated with familial hemiplegic migraine 2. Cephalalgia 40 (11), 1177–1190. doi:10.1177/0333102420929028
Tang, Q., Han, R., Xiao, H., Shen, J., Luo, Q., and Li, J. (2012). Neuroprotective effects of tanshinone IIA and/or tetramethylpyrazine in cerebral ischemic injury in vivo and in vitro. Brain Res. 1488, 81–91. doi:10.1016/j.brainres.2012.09.034
Tong, H., Wang, K., Wang, X., and Lu, T. (2022). Molecular mechanism of tetramethylpyrazine ameliorating neuroexcitotoxicity through activating the PKA/CREB signaling pathway. Biomed. Res. Int. 2022, 2812839. doi:10.1155/2022/2812839
Tsai, T. H., and Liang, C. C. (2001). Pharmacokinetics of tetramethylpyrazine in rat blood and brain using microdialysis. Int. J. Pharm. 216 (1-2), 61–66. doi:10.1016/s0378-5173(01)00572-5
Tsai, T. H., and Liang, C. (2013). Pharmacokinetics of tetramethylpyrazine in rat blood and brain using microdialysis[J]. Int. J. Pharm. 216 (1-2), 61–66. doi:10.1016/s0378-5173(01)00572-5
Uphoff, E., Pires, M., Barbui, C., Barua, D., Churchill, R., Cristofalo, D., et al. (2020). Behavioural activation therapy for depression in adults with non-communicable diseases. Cochrane Database Syst. Rev. 8 (8), Cd013461. doi:10.1002/14651858.CD013461.pub2
Vinken, M., Vanhaecke, T., Papeleu, P., Snykers, S., Henkens, T., Rogiers, V., et al. (2006). Connexins and their channels in cell growth and cell death[J]. Cell. Signal. 18 (5), 592–600. doi:10.1016/j.cellsig.2005.08.012
Wang, H., Wang, L., Zhang, N., Zhang, Q., Zhao, H., and Zhang, Q. (2014). Houshiheisan compound prescription protects neurovascular units after cerebral ischemia. Neural Regen. Res. 9 (7), 741–748. doi:10.4103/1673-5374.131580
Wang, J., Lu, R., Yang, J., Li, H., He, Z., Jing, N., et al. (2015). TRPC6 specifically interacts with APP to inhibit its cleavage by γ-secretase and reduce Aβ production. Nat. Commun. 6, 397–399. doi:10.1038/ncomms9876
Wang, J. Q., and Mao, L. (2019). The ERK pathway: Molecular mechanisms and treatment of depression. Mol. Neurobiol. 56 (9), 6197–6205. doi:10.1007/s12035-019-1524-3
Wang, L. S., Shi, Z. F., Zhang, Y. F., Guo, Q., Huang, Y. W., and Zhou, L. L. (2012). Effect of Xiongbing compound on the pharmacokinetics and brain targeting of tetramethylpyrazine. J. Pharm. Pharmacol. 64, 1688–1694. doi:10.1111/j.2042-7158.2012.01546.x
Wang, Q., Sun, H., Yu, L., Ma, X., Jiang, B., Bi, C., et al. (2019). Pharmacokinetic behaviors of ligustrazine after single- and multiple-dose intravenous Shenxiong glucose injection in rats by high-performance liquid chromatography[J]. Naunyn Schmiedebergs Archives Pharmacol. 392 (5), 565–572. doi:10.1007/s00210-018-01608-9
Wei, L., Marasini, N., Li, G., Yong, C. S., Kim, J. O., and Quan, Q. (2012). Development of ligustrazine-loaded lipid emulsion: Formulation optimization, characterization and biodistribution. Int. J. Pharm. 437 (1-2), 203–212. doi:10.1016/j.ijpharm.2012.08.027
Weng, G., Zhou, B., Liu, T., Huang, Z., and Huang, S. (2021). Tetramethylpyrazine improves cognitive function of Alzheimer's disease mice by regulating SSTR4 ubiquitination. Drug Des. devel. Ther. 15, 2385–2399. doi:10.2147/DDDT.S290030
Wong, M. L., Inserra, A., Lewis, M. D., Mastronardi, C. A., Leong, L., Choo, J., et al. (2016). Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol. Psychiatry. 21 (6), 797–805. doi:10.1038/mp.2016.46
Wood, A. M., and Bristow, D. R. (2010). N-methyl-D-aspartate receptor desensitisation is neuroprotective by inhibiting glutamate-induced apoptotic-like death. J. Neurochem. 70 (2), 677–687. doi:10.1046/j.1471-4159.1998.70020677.x
Wu, J. Y., Li, Y. J., Yang, L., Hu, Y. Y., Hu, X. B., Tang, T. T., et al. (2018). Borneol and Α-asarone as adjuvant agents for improving blood-brain barrier permeability of puerarin and tetramethylpyrazine by activating adenosine receptors. Drug Deliv. 25 (1), 1858–1864. doi:10.1080/10717544.2018.1516005
Wu, W., Yu, X., Luo, X. P., Yang, S. H., and Zheng, D. (2013). Tetramethylpyrazine protects against scopolamine-induced memory impairments in rats by reversing the cAMP/PKA/CREB pathway. Behav. Brain Res. 253, 212–216. doi:10.1016/j.bbr.2013.07.052
Xiao, X., Liu, Y., Qi, C., Qiu, F., Chen, X., Zhang, J., et al. (2010). Neuroprotection and enhanced neurogenesis by tetramethylpyrazine in adult rat brain after focal ischemia. Neurol. Res. 32 (5), 547–555. doi:10.1179/174313209X414533
Xiao, Z., Hu, J., Lu, H., Zhuo, X., Xu, D., Wang, S., et al. (2012). [Effect of tetramethylpyrazine on the expression of macrophage migration inhibitory factor in acute spinal cord injury in rats]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 37 (10), 1031–1036. doi:10.3969/j.issn.1672-7347.2012.10.011
Xiaojie, Z., and Weihong, S. (2013). The role of APP and BACE1 trafficking in APP processing and amyloid-β generation[J]. Alzheimer's Res. Ther. 5 (5), 46. doi:10.1186/alzrt211
Xu, J., Kao, S. Y., Lee, F. J., Song, W., Jin, L. W., Yankner, B. A., et al. (2002). Dopamine-dependent neurotoxicity of α-synuclein: A mechanism for selective neurodegeneration in Parkinson disease[J]. Nat. Med. 8 (6), 600–606. doi:10.1038/nm0602-600
Xu, S., Lu, J., Shao, A., Zhang, J. H., and Zhang, J. (2020). Glial cells: Role of the immune response in ischemic stroke. Front. Immunol. 11, 294. doi:10.3389/fimmu.2020.00294
Yadav, N., Gogada, R., O’Malley, J., Gundampati, R. K., Jayanthi, S., Hashmi, S., et al. (2020). Molecular insights on cytochrome c and nucleotide regulation of apoptosome function and its implication in cancer. Biochim. Biophys. Acta Mol. Cell Res. 1867 (1), 118573. doi:10.1016/j.bbamcr.2019.118573
Yan, F., Chu, L., Li, L., Wang, J., Yang, Y., Gu, J., et al. (2017). Tetramethylpyrazine protects bone marrow-derived mesenchymal stem cells against hydrogen peroxide-induced apoptosis through PI3K/Akt and ERK1/2 pathways[J]. Biol. Pharm. Bull. 40 (12), 2146–2152. doi:10.1248/bpb.b17-00524
Yan-Yu, X., Qi-Neng, P., and Zhi-Peng, C. (2007). The enhancing effect of synthetical borneol on the absorption of tetramethylpyrazine phosphate in mouse. Int. J. Pharm. 337 (1-2), 74–79. doi:10.1016/j.ijpharm.2006.12.034
Yang, G., Qian, C., Wang, N., Lin, C., Wang, Y., Wang, G., et al. (2017). Tetramethylpyrazine protects against oxygen-glucose deprivation-induced brain microvascular endothelial cells injury via rho/rho-kinase signaling pathway. Cell. Mol. Neurobiol. 37 (4), 619–633. doi:10.1007/s10571-016-0398-4
Yang, J., Li, J., Jing, L., Zhang, Y., Zhu, Z., and Wan, H. (2012). Synergistic protective effect of astragaloside IV-tetramethylpyrazine against cerebral ischemic-reperfusion injury induced by transient focal ischemia. J. Ethnopharmacol. 140 (1), 64–72. doi:10.1016/j.jep.2011.12.023
Ye, Y., Zhou, L. L., Yan, Y. L., and Huang, Q. j. (2010). [Determination of plasma protein binding rate of tetramethylpyrazine phosphate by ultrafiltration]. Zhong Yao Cai 33 (8), 1282–1285.
Yeh, S. H., Ou, L. C., Gean, P. W., Hung, J. J., and Chang, W. C. (2010). Selective inhibition of early-but not late-expressed HIF-1α is neuroprotective in rats after focal ischemic brain damage. Brain Pathol. 21 (3), 249–262. doi:10.1111/j.1750-3639.2010.00443.x
Yepes, M. (2020). Urokinase-type plasminogen activator is a modulator of synaptic plasticity in the central nervous system: Implications for neurorepair in the ischemic brain. Neural Regen. Res. 15 (4), 620–624. doi:10.4103/1673-5374.266904
Yu, B., Ruan, M., Liang, T., Huang, S. W., Liu, S. J., Cheng, H. B., et al. (2017). Tetramethylpyrazine phosphate and borneol combination therapy synergistically attenuated ischemia-reperfusion injury of the hypothalamus and striatum via regulation of apoptosis and autophagy in a rat model. Am. J. Transl. Res. 9 (11), 4807–4820.
Yu, B., Zhong, F. M., Yao, Y., Deng, S. Q., Xu, H. Q., Lu, J. F., et al. (2019). Synergistic protection of tetramethylpyrazine phosphate and borneol on brain microvascular endothelium cells injured by hypoxia. Am. J. Transl. Res. 11 (4), 2168–2180.
Yu, D., Cheng, Z., Ali, A. I., Wang, J., Le, K., Chibaatar, E., et al. (2019). Chronic unexpected mild stress destroys synaptic plasticity of neurons through a glutamate transporter, GLT-1, of astrocytes in the ischemic stroke rat. Neural Plast. 2019, 1615925. doi:10.1155/2019/1615925
Yu, T., Guo, X., Zhang, Z., Liu, R., Zou, L., Fu, J., et al. (2016). Meta-analysis of the clinical effectiveness and safety of ligustrazine in cerebral infarction. Evid. Based. Complement. Altern. Med. 2016, 3595946. doi:10.1155/2016/3595946
Yuan, Q., Yuan, Y., Zheng, Y., Sheng, R., Liu, L., Xie, F., et al. (2021). Anti-cerebral ischemia reperfusion injury of polysaccharides: A review of the mechanisms. Biomed. Pharmacother. 137, 111303. doi:10.1016/j.biopha.2021.111303
Zepeda, Ab, Castillo, R. L., Figueroa, C. A., Pulgar, V. M., and Farias, J. G. (2013). Cellular and molecular mechanisms in the hypoxic tissue: Role of HIF-1 and ROS. Cell biochem. Funct. 31 (6), 451–459. doi:10.1002/cbf.2985
Zhai, Y., Meng, X., Luo, Y., Wu, Y., Ye, T., Zhou, P., et al. (2018). Notoginsenoside R1 ameliorates diabetic encephalopathy by activating the Nrf2 pathway and inhibiting NLRP3 inflammasome activation. Oncotarget 9 (10), 9344–9363. doi:10.18632/oncotarget.24295
Zhang, C., Shen, M., Teng, F., Li, P., Gao, F., Tu, J., et al. (2018). Ultrasound-enhanced protective effect of tetramethylpyrazine via the ROS/HIF-1A signaling pathway in an in vitro cerebral ischemia/reperfusion injury model. Ultrasound Med. Biol. 44, 1786–1798. doi:10.1016/j.ultrasmedbio.2018.04.005
Zhang, P., Liu, Y., Li, J., Kang, Q., Tian, Y., Chen, X., et al. (2006). Cell proliferation in ependymal/subventricular zone and nNOS expression following focal cerebral ischemia in adult rats. Neurol. Res. 28 (1), 91–96. doi:10.1179/016164106X91942
Zhang, P., Liu, Y., Li, J., Kang, Q., Tian, Y., Chen, X., et al. (2007). Decreased neuronal nitric oxide synthase expression and cell migration in the peri-infarction after focal cerebral ischemia in rats. Neuropathology 27 (4), 347–354. doi:10.1111/j.1440-1789.2007.00791.x
Zhang, Q., Wang, J., Zhu, L., Shi-jie, J., Juan, L., Lin-xiao, W., et al. (2021). Ligustrazine attenuates hyperhomocysteinemia-induced alzheimer-like pathologies in rats[J]. Springer.
Zhang, Z., Wei, T., Hou, J., Li, G., Yu, S., and Xin, W. (2003). Tetramethylpyrazine scavenges superoxide anion and decreases nitric oxide production in human polymorphonuclear leukocytes. Life Sci. 72 (22), 2465–2472. doi:10.1016/s0024-3205(03)00139-5
Zhao, H., Xu, M. L., Zhang, Q., Guo, Z. H., Peng, Y., Qu, Z. Y., et al. (2014). Tetramethylpyrazine alleviated cytokine synthesis and dopamine deficit and improved motor dysfunction in the mice model of Parkinson's disease. Neurol. Sci. 35 (12), 1963–1967. doi:10.1007/s10072-014-1871-9
Zhao, J. H., Ji, L., Wang, H., Chen, Z. Q., Zhang, Y. T., Liu, Y., et al. (2011). Microemulsion-based novel transdermal delivery system of tetramethylpyrazine: Preparation and evaluation in vitro and in vivo. Int. J. Nanomedicine 6, 1611–1619. doi:10.2147/IJN.S23597
Zhao, T., Fu, Y., Hao, S., and Liu, X. (2017). Ligustrazine suppresses neuron apoptosis via the Bax/Bcl‐2 and caspase‐3 pathway in PC12 cells and in rats with vascular dementia[J]. IUBMB Life 70, 60–70. doi:10.1002/iub.1704
Zhao, Y., Martins-Oliveira, M., Akerman, S., and Goadsby, P. J. (2018). Comparative effects of traditional Chinese and Western migraine medicines in an animal model of nociceptive trigeminovascular activation. Cephalalgia 38 (7), 1215–1224. doi:10.1177/0333102417728245
Zheng, Z., Li, Z., Chen, S., Pan, J., and Ma, X. (2013). Tetramethylpyrazine attenuates TNF-α-induced iNOS expression in human endothelial cells: Involvement of Syk-mediated activation of PI3K-IKK-IκB signaling pathways. Exp. Cell Res. 319 (14), 2145–2151. doi:10.1016/j.yexcr.2013.05.018
Zhong, M., Ma, W., Zhang, X., Wang, Y., and Gao, X. (2016). Tetramethyl pyrazine protects hippocampal neurons against anoxia/reoxygenation injury through inhibiting apoptosis mediated by JNK/MARK signal pathway. Med. Sci. Monit. 22, 5082–5090. doi:10.12659/msm.898921
Zhou, J., Chan, L., and Zhou, S. (2012). Trigonelline: A plant alkaloid with therapeutic potential for diabetes and central nervous system disease. Curr. Med. Chem. 19 (21), 3523–3531. doi:10.2174/092986712801323171
Zhu, T., Fang, B. Y., Meng, X. B., Zhang, S. X., Wang, H., Gao, G., et al. (2022). Folium Ginkgo extract and tetramethylpyrazine sodium chloride injection (Xingxiong injection) protects against focal cerebral ischaemia/reperfusion injury via activating the Akt/Nrf2 pathway and inhibiting NLRP3 inflammasome activation. Pharm. Biol. 60 (1), 195–205. doi:10.1080/13880209.2021.2014895
Keywords: tetramethylpyrazine, central nervous system diseases, pharmacokinetics, protective mechanisms, ischemic stroke and reperfusion
Citation: Liu Y, Yang G, Cui W, Zhang Y and Liang X (2022) Regulatory mechanisms of tetramethylpyrazine on central nervous system diseases: A review. Front. Pharmacol. 13:948600. doi: 10.3389/fphar.2022.948600
Received: 20 May 2022; Accepted: 05 August 2022;
Published: 05 September 2022.
Edited by:
Shuai Ji, Xuzhou Medical University, ChinaReviewed by:
Jaspreet Kaur, University of Copenhagen, DenmarkYijun Pan, The University of Melbourne, Australia
Shengquan Hu, Shenzhen Second People’s Hospital, China
Copyright © 2022 Liu, Yang, Cui, Zhang and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunling Zhang, eXVubGluZ3poYW5nMjAwNEAxMjYuY29t; Xiao Liang, bGlhbmd4aWFvMDIyQDEyNi5jb20=
 Yue Liu
Yue Liu Guang Yang
Guang Yang Wenqiang Cui
Wenqiang Cui Yunling Zhang
Yunling Zhang Xiao Liang
Xiao Liang