
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Pharmacol. , 14 October 2022
Sec. Neuropharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.948412
This article is part of the Research Topic Experimental Models of Epilepsy and Related Comorbidities, Volume II View all 7 articles
The main strategy for the treatment of epilepsy is the use of pharmacological agents known as antiseizure medication (ASM). These drugs control the seizure onset and improves the life expectancy and quality of life of patients. Several ASMs are contraindicated during pregnancy, due to a potential teratogen risk. For this reason, the pharmacological treatments of the pregnant Women with Epilepsy (WWE) need comprehensive analyses to reduce fetal risk during the first trimester of pregnancy. The mechanisms by which ASM are teratogens are still under study and scientists in the field, propose different hypotheses. One of them, which will be addressed in this review, corresponds to the potential alteration of ASM on ion channels and proteins involved in relevant signaling and cellular responses (i.e., migration, differentiation) during embryonic development. The actual information related to the action of ASM and its possible targets it is poorly understood. In this review, we will focus on describing the eventual presence of some ion channels and synaptic proteins of the neurotransmitter signaling pathways present during early neural development, which could potentially interacting as targets of ASM. This information leads to elucidate whether these drugs would have the ability to affect critical signaling during periods of neural development that in turn could explain the fetal malformations observed by the use of ASM during pregnancy.
Epilepsy is a chronic pathology that affects near 50 million people globally. Its causes include genetic, structural and metabolic aspects, while in a half of reported cases have an undetermined etiology (Pan American Health Organization, 2018). According to the International League Against Epilepsy (ILAE), this disease is defined as a brain disorder characterized by at least one of three conditions. 1) epileptic syndrome diagnostic, 2) exhibit at least two non-induced seizures in a 24-h range, and 3) present at least a 60% probability of generating a new non-induced seizures during the 10 years after the first two seizures (Fisher et al., 2014).
Treatment for epilepsy tries to contain seizures through pharmacologic management, using a set of drugs called antiseizure medication (ASM). Only 70% of affected people respond effectively to ASM, mostly using monotherapy, but it has been documented that between 20%–30% of all those patients do not respond to pharmacological treatments (Pan American Health Organization, 2018; Fattorusso et al., 2021).
Food and Drug Administration (FDA) and European Medicines Agency (EMA) provide a list containing the drugs approved for use, while the choice and concentration of these ASMs vary for each patient based on factors such as type of epilepsy (syndrome), lifestyle, age, seizure frequency and others. Some of the most frequently ASM used are Phenobarbital (PB), Phenytoin (PHT), Carbamazepine (CBZ) and Valproic acid (VPA), from the first generation drugs. Lamotrigine (LTG), Topiramate (TPM), Levetiracetam (LEV) and Gabapentin (GBP) corresponding to second generation and Lacosamide (LCM), Rufinamide (RUF), Cannabidiol (CBD) between others from third generation (Hakami, 2021).
The malformations rates decreases with the use of third generation ASM associating a more safe profile to the newer drugs (Tomson et al., 2019). Regarding MCM rate, ASM can be classified as low: ≤3% (OXC, GBP, LTG, LEV); intermediate: 3.1%–6% (TPM, CBZ, PHT); high 6.1%–9% (PB); very high > 9% (VPA) (Abou-Khalil, 2022). Despite this, the use of first generation ASM is still broadly use, not only against epilepsy, but is also use for migraine, mood stabilizer and even pain. In addition, the use of ASM can lead to psycho-behavioral side effects and physical dysfunction, such as irritability, sedation, nausea and others (Johnson, 2019).
Fetal malformations include heart defects, cleft palate and failures related to development of the nervous system, such as neural tube defects (NTDs), all of them classified as major congenital Malformations (MCMs) (Källén et al., 1989; Werler et al., 2011; Wallingford et al., 2013). The relationship between MCM and the use of ASM comes mainly from the three registries: NAAPR, UK and Ireland and EURAP. Since these antecedents, the teratogenicity in children of pregnant WWE has been associated especially at the use of ASM in high doses (Pennell, 2016). VPA, have cut-offs for higher risks ranging from 500–650 mg/day. A dose-dependent effect was also identified for LTG, CBZ and PB, while the lowest risk was associated with LTG at ≤325 mg/day (Tomson et al., 2019b). In general, the data shows that elevated MCM rates are associated with the use of high concentrations of VPA and CBZ in comparison with other ASM like LEV (Tomson et al., 2019a). For more detailed information associated with dosage, change in serum levels and bioavailability during pregnancy related with MCM refer to Hakami (2021) and Nucera et al. (2022). In relation with polytherapy, it has been usually considered that multidrug treatments correlate with greater MCMs (Veroniki et al., 2017), nevertheless, more recent studies identify that the specific ASM used is more significant than the number. Once again, the inclusion of VPA was associated with higher prevalence of MCMs (Holmes et al., 2011).
Analyses of teratogenicity in the Central Nervous System (CNS) has been evaluated using frog embryos (Xenopus laevis), showing that exposure in early stages of development, such as neurulation, interferes processes related to cell migration and proliferation generating alterations in glutamate signaling (Sequerra et al., 2018). In addition, autism spectrum disorders (ASD) and intellectual disabilities has been associated with the prenatal exposure of ASM (Bjørk et al., 2022).
Although the mechanisms of ASM to control epilepsy, through ion channels or receptors have been extensively studied, the pathways underlying the teratogenicity during intrauterine development are far for complete. Therefore, it is necessary to investigate the possible association between ASM, ion channels, synaptic proteins and teratogenicity during embryonic development.
There is large evidence describing teratogenicity in pregnant WWE with ASM treatment during her first trimester of pregnancy (Kilic et al., 2014; Vossler, 2019). Focused on nervous system development, normally its begins with neurulation process in the first month of pregnancy, followed with a series of complex cellular and tissue modifications such as segmentation, migration, differentiation, axonal guidance, synaptogenesis, among others (Knuesel et al., 2014). Based on this information, we could hypothesize that the generation of some neural teratogen alterations would occur due to the disturbance of the ASM with active signaling pathways required for aforementioned biological processes.
Neurulation is one of the first step for the development of the nervous system in chordates (Colas & Schoenwolf, 2001; Knuesel et al., 2014). This event is preceded by neural induction (described by Spemann & Mangold on 1924), where a layer of ectodermal cells differentiates and forms the neural plate. This flat layer stretches cephalo-caudally and divides symmetrically while the lateral edges elevate to converge medially and merging to create an internal cavity known as the neural tube. Neurulation takes place in humans during the third week of gestation but its temporary window is specific for each chordates. It is a previous event to synaptogenesis, which occurs around the 20th week of gestation in humans (Knuesel et al., 2014). There is evidence that electrical activity and neurotransmitter signaling is present during neurulation (Root et al., 2008), participating in the regulation of neural plate cell proliferation and migration necessary for the formation of the neural tube (Sequerra et al., 2018; Benavides-Rivas et al., 2020).
Failures in neurulation process leads to NTDs, being anencephaly (erroneous closure of the cranial region) and spina bifida (failure of closure in the caudal zone) (Hughes et al., 2018) the most common malformations. The etiology of NTDs is diverse, involving genetic and environmental factors (Padmanabhan, 2006). Associate to genetic causes, folate deficiency deregulates critical cell remodeling, necessary for this period, like apical constriction (Balashova et al., 2017), while and important environmental factor is the use of ASM during pregnancy (Pippenger, 2003).
Some hypotheses suggests that there is an increase in apoptosis of neural cells results from the interaction of ASM with neurotrophins, NGF and BDNF, interfering with their neuroprotective action (Huang & Reichardt, 2001; Roullet et al., 2010). It is also postulated that the deleterious action of excessive free radicals present in ASM-treated women during pregnancy could be the cause for birth defects (Pippenger, 2003). Other studies argue that the teratogenicity of ASM like valproic acid is related to its known inhibitory action on histone-deacetylase (HDAC), which leads to indirect changes in DNA methylation and gene expression (Eyal et al., 2004; Smith et al., 2012). Here, we will discus the possible teratogen mechanism of ASM through their principal targets.
ASMs are drugs used to control epilepsy by reducing the frequency or intensity of seizures. It should be noted that these drugs do not modify disease properties, and instead they intended to stabilize its manifestations controlling epileptic seizures. In fact, currently the term use is ASM in replacement of antiepileptic like before. The principal mechanism of action of ASMs is based on controlling the over-excitability of nervous system by modulating ion channels associated with this function, like voltage-gate channels, selectively permeable to Na+, K+, and Ca 2+ and excitatory (glutamate) and inhibitory (GABA) receptors and signaling (Figure 1). In this review, we will focus on describing those proteins that are the principal targets of ASMs to suggest possible interactions between these drugs and embryonic proteins and signaling.
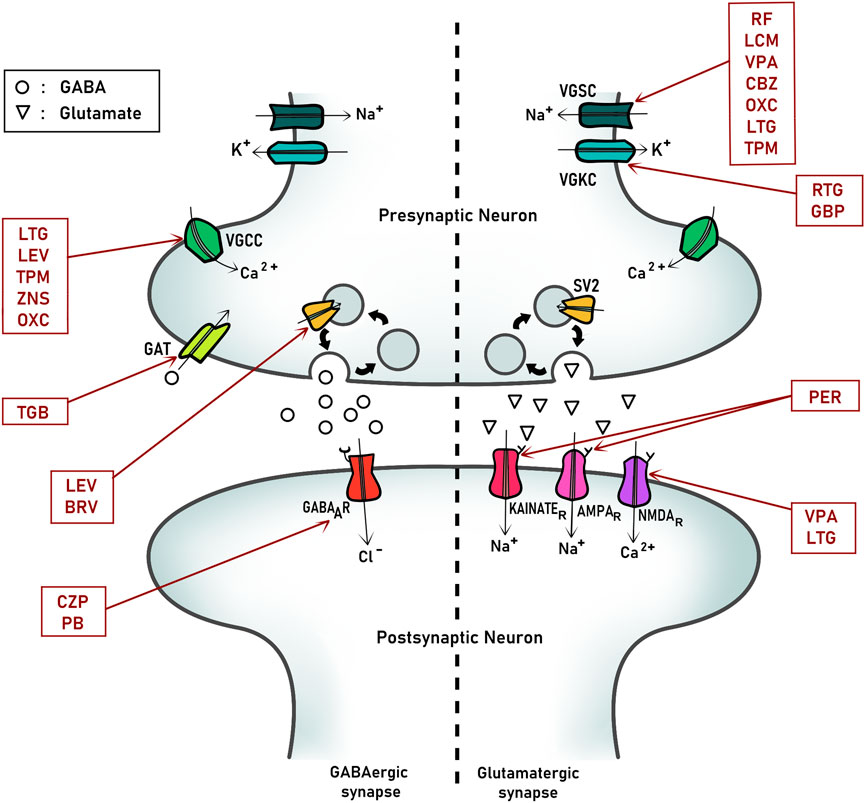
FIGURE 1. Principal pharmacological targets of antiseizure medication (ASMs) at synapses. A representative scheme of an inhibitory (GABAergic, left) and excitatory (Glutamatergic, right) synapse from a mature nervous system. The main proteins and ion channels targets of relevant antiseizure medications are indicated. VCCC, Voltage-gated calcium channel; VGSC, Voltage-gated sodium channel; VGKC, Voltage-gated potassium channel; SV2, Synaptic-vesicle protein 2. LEV, Levetiracetam; TPM, Topiramate; TGB, Tiagabine; BRV, Brivaracetam; CZP, Clonazepam; PB, Phenobarbital; RF, Rufinamide; LCM, Lacosamide; RTG, Retigabine; GBP, Gabapentin; PER, Perampanel; VPA, Valproate; CBZ, carbazepine; LTG, Lamotrigine; OXC, oxcarbazepine.
Ion channels allow the passage of Na+, K+, Ca 2 + or Cl-ions, modulating the action potential. These channels can be classified into three broad types depending on the stimulus they need to open or close: 1) mechanosensitive channels, 2) ligand-activated channels, and 3) voltage-dependent channels. The voltage-dependent channels are the target of several ASMs listed below.
Voltage-gated sodium channels (VGSCs) are composed by one α subunit, with genes encoding the proteins Nav1.1 through Nav1.9. They may also have one or two β subunits encoding the Navβ1 to Navβ4 proteins. In the adult mammalian central nervous system, four of these α subunits are present: Nav1.1, Nav1.2, Nav1.3, and Nav1.6 (Goldin, 2001; Whitaker et al., 2001).
During rat development, Nav1.1 transcripts are first detected before birth on embryonic day 18 (E18) and their levels increase towards adulthood. Nav1.2 begins to be expressed a little earlier at stage E15 with greater levels detected in the spinal cord peaking at postnatal day 7 (P7) and increasing further in other regions. Nav1.3 is robustly expressed at E12 and decreases thereafter reaching a plateau during P7-P15 (Beckh et al., 1989). Relative expression of Nav1.6 transcripts is quite low in embryonic periods in rats, but it increases early after birth (P1) with development (Schaller & Caldwell, 2000).
Studies show that mutations in the Nav1.1, Nav1.2, Nav1.3, Nav 1.6, and Navβ1 subunits correlate with epilepsy (Guo et al., 2008; Larsen et al., 2015; Wolff et al., 2017) and ASMs are aimed to restore normal ion channel activity altered by these mutant subunits.
A depolarization of plasma membrane generates an Action Potential that in turn is transmitted by axonal VGSCs to further spread the seizure activities. Because of this VGSC are the main targets of several ASM like PHT, CBZ, VPA, LMT, OXC, TPM, ESL, RUF, and LCM. Rufinamide (RF) for example, has reported to have a higher affinity for the Nav1.1 and Nav1.6 subunit proteins (Gilchrist et al., 2014), and Lacosamide (LCM) exerts inhibitory effects on Nav1.3 and Nav1.7 (Sheets et al., 2008). Here, a valid question is if the VGSC signaling is active and participate in early development. Preliminary, the expression levels of VGSC in early stages of neural development (neurulation) would be weak, then, why some ASM with a sodium channel blocker (SCB) action mechanism displays a teratogen risk. Analyses shows that one possible explanation could be its unspecific action. VPA for example, induce ROS formation and apoptosis and inhibit histone deacetylase (HDAC) (Tomson et al., 2016). CBZ, is a potent enzyme inducer acting through 1A2, 2B6, 2C9, 2C19, and 3A4/5 CYP targets acting directly on endogenous metabolic pathways and also has been documented that enhances adipogenesis inhibiting Wnt/β-Catenin expression (Brodie et al., 2013; Lawthom, 2020). PHT, inhibit non-NMDA glutamate receptors with greater affinity to the Ca2+-impermeable AMPA receptors (Dron et al., 2021) and additionally inhibit the cardiac calcium release channels ryanodine receptor 2 (RyR2; Ashna et al., 2020). TPM is an antagonist of AMPA and Kainate receptors, increases GABA(R) responses, inhibits carbonic anhydrase isoenzymes, affects voltage-activated Ca2+ channels and interact with protein kinase phosphorylation sites (Bai et al., 2022). LMT inhibits postsynaptic AMPA receptors, N- and P/Q-type calcium channels on presynaptic nerve terminals and glutamate release (Dron et al., 2021). Altogether, shows that probably secondary activities of the SCB could contribute significantly to the teratogen risk.
Voltage-gated calcium channels (VGCCs) are composed of an α1 subunit that detects the potential change, forms the pore, and other auxiliary subunits such as α2δ (encoded by four genes: CACNA2D1-4), β (encoded by four genes: CACNB1-4), and γ (encoded by eight known genes CACNGG1-8) (Catterall, 2000). VGCCs are classified according to the activation of its α1 subunit, channels of high conductance (type L, P/Q, N and R) and low conductance (type T). The L-type include the Cav1.1 to Cav1.4 proteins, the P/Q, N, and R types have only one member each, Cav2.1, Cav2.2, and Cav2.3, respectively, while the T-Type contains the Cav3.1 to Cav3.3 subunits.
Associated to nervous system development, Cav2.1 and Cav2.2 are already functional in St.5-6 In Xenopus laevis embryos (Motin et al., 2007; Cohen-Kutner et al., 2010), and Cav1.2, Cav2.1, Cav2.2 and Cav3.2 channels are present at St.14 in neural cell cultures. It is important to mention that expression of Cav1.2 disappear at St.18 while Cav1.3 show up only from St.22 (Lewis et al., 2014).
In patients with epilepsy, have been detected alterations in several genes encoded by Cav2.1 (Chioza et al., 2001; Bomben et al., 2016), Cav2.3 (Weiergraber et al., 2006), Cav3.1 (B. Singh et al., 2007), Cav3.2 (Chen et al., 2003; Eckle et al., 2014), and α2δ subunits encoded by the CACNA2D1 and CACNA2D2 genes (Edvardson et al., 2013; Hino-Fukuyo et al., 2015; Vergult et al., 2015).
Levetiracetam (LEV) and Lamotrigine (LTG) have a higher affinity for Cav2.2 (N-) channels (Wang et al., 1996; Lukyanetz et al., 2002). Topiramate (TPM) exerts part of its function on Cav2.2, Cav2.3 channels and L-type channels (Zhang et al., 2000; Kuzmiski et al., 2005), while Zonisamide (ZNS) inhibits T-type channels (Suzuki et al., 1992). ASM have also been reported as therapeutic targets of VGCC complementary subunits, for example, Pregabalin and Gabapentin bind to α2δ helper subunits encoded by the CACNA2D1 and CACNA2D2 genes (Gee et al., 1996; Hendrich et al., 2008).
One study show that use of 200 μM nifedipine a broadly VGCC blocker generates NTDs inhibiting apical constriction of neural plate cells (Suzuki et al., 2017). Other investigation report that neural tube closure signaling pathway require T-type calcium channels (TTCCs) that controls EphrinA expression and loss of TTCCs produces a failure to seal the anterior neural folds, generating NTDs (Abdul-Wajid et al., 2015). These investigations shows that Ca2+i is active and relevant during neurulation through VGCC and that alterations on this signaling lead NTDs, like spine bifida.
Glutamate is the main excitatory neurotransmitter of the central nervous system. An aberrant enhancement of glutamatergic neurotransmission can result in epileptic activity. In the nervous system, glutamate receptors are divided into metabotropic (mGluR) whit eight receptors (mGluR1-R8), and ionotropic (iGluR), which are subdivided into three groups: NMDA (containing the GluN1, GluN2A-2D and GluN3A-3B subunits), AMPA (GluA1-GluA4 subunits), and KAINATE (GluK1 to GluK5 subunits). Several receptors have been associated with epilepsy such us: GluA1, GluA2, GluN1, GluN2A, GluN2B, GluK2 and GluK5 (Smolders et al., 2002; Li et al., 2010; Peret et al., 2014; Egbenya et al., 2018; Zubareva et al., 2018).
Several ASMs target glutamatergic-signaling components. Perampanel (PER) is an AMPA receptor antagonist that decreases the affinity of GluA1/2 and GluA2/3 subunit combinations for glutamate (Augustin et al., 2018; Lange et al., 2019). Lamotrigine also inhibits AMPA channels in a dose-dependent manner (Lee et al., 2008) and topiramate inhibits AMPA and KAINATE receptors (Ängehagen et al., 2004).
A study showed that in the neural plate stage of Xenopus laevis (St.13) there is glutamate signaling that regulates Ca2+ transients through the GluN1 subunit of NMDARs, which it will be a target of the VPA (Sequerra et al., 2018). In addition, the presence of GluA1 receptor transcripts was described in the same development stages, as GluA2 transcripts begin to be expressed in rats at E18 (Qiu et al., 2012) and GluK1 and GluK2 transcripts are present in E17 rats (Joseph et al., 2011). As mentioned early, Ca2+ signaling is relevant during neurilation even before and several glutamate-mediated Ca2+ receptors like NMDAR and AMPAR will be active at these stages of nervous system development.
The main inhibitory neurotransmitter in the brain is γ-aminobutyric acid (GABA), synthesized from glutamate by the enzymes GAD65/67. GABA receptors can be divided into metabotropic [GABA(B)R] coupled to Gαi protein, which are composed of the B1 and B2 subunits; and ionotropic [GABA(A)R], which allow the selective passage of Cl−, composed by varied heteropentameric subunits (α1-6, β1-4, γ1-3, δ, and ρ) (Bettler & Fakler, 2017). A third type of GABA receptor called GABA(A)ρ [also known as GABA(C)R], is a sub-class of the ionotropic GABA(A)R receptor that presents the ρ subunit, and is expressed principally in the retina (Polenzani et al., 1991). In epilepsy, animal models suggest that alterations in GABA(A)R which contain α1, α5 (Friedman et al., 1994; Hernandez et al., 2019), δ (Dibbens et al., 2004; H.-J. Feng et al., 2006), γ2 (Baulac et al., 2001; Eugène et al., 2007), β1 and β3 (Homanics et al., 1997; Brooks-Kayal et al., 1998; Janve et al., 2016) subunits correlate with seizure states.
Before year 2000, studies showed that the α4, β1, γ1 subunits and the GAD65 and GAD67 enzymes were already present in mice at embryonic E17 (Ma & Barker, 1998). Kaeser and colleagues (Kaeser et al., 2011) identified, in stage 8 Xenopus laevis, transcripts of the α2 and ρ2 subunits, and transcripts of the ρ1 subunit that decrease by St.16. Levels of transcripts for α1, α3 and β1 increase from St.12-16 and the β2 subunit is detected later at around St.28. Another study in Lhx6-eGFP transgenic mice described the presence of transcripts for GABA(A) subunits α1-5, β1-3, γ1-3 at E14.5, (Cuzon Carlson and Yeh, 2011).
Benzodiazepine and barbiturates also has been use as ASM. Clonazepam (CZP) is a positive allosteric modulators to the α-γ2 site of the GABA(A) channel, increasing its opening, (Löscher & Rogawski, 2012; Kubová et al., 2020). Similarly, pentobarbital (PB) are also a positive allosteric modulators of GABA(A)R through their binding to the β3 subunit, binding site different from that of benzodiazepines (Serafini et al., 2000; Zeller et al., 2007; Löscher & Rogawski, 2012). Additionally, PB is considered a high inductor of MCM and has been shows that additional mechanism of action is related to inhibit more selectively the Ca2+-impermeable AMPA receptors and N- and L-voltage-activated Ca2+ currents (Sills and Rogawski, 2020). A study in pregnant rats where GABA agonists and antagonists were administered, showed that GABA(A)R or GABA(B)R agonists or GABA(B)R antagonist lead to NTDs suggesting a role for GABAergic signaling during neural tube formation (Briner, 2001).
Synaptic Vesicle Protein 2 (SV2) family are proteins with vesicular localization that participate in neurotransmitter release. In vertebrates, there are three isoforms (SV2A, SV2B, and SV2C) (Bajjalieh et al., 1994; Abdellah et al., 2004; Gregory et al., 2006; Zody et al., 2006). SV2A is the most ubiquitously expressed in the brain, while SV2B has a more restricted expression pattern and SV2C is poorly expressed in the brain, because is highly present in the basal ganglia (Bajjalieh et al., 1994; Janz & Südhof, 1999; Dardou et al., 2011; Crèvecœur et al., 2013; Edvinsson et al., 2015; Steinberg et al., 2016). It has been seen that all these isoforms are closely related to the protein Synaptotagmin, a Ca2+ sensor belonging to the SNARE complex, in a binding site inhibited by Ca2+, in addition, the SV2A and SV2C isoforms present an additional site of interaction (Schivell et al., 2005).
SV2A knockout mice exhibit a high number of seizures and die by third week of their life, while SV2B knockout animals are viable and do not present severe phenotypic characteristics (Crowder et al., 1999; Janz et al., 1999; Venkatesan et al., 2012). In addition, SV2B levels are decreased in epileptic models and SV2A can be decreased or increased in some epileptic patients (Contreras-García et al., 2018, Contreras-García et al., 2021, Crèvecœur et al., 2014; Feng et al., 2009; Hanaya et al., 2012; Ohno et al., 2009; Shi et al., 2015) which challenges the understanding of the role of SV2 in epilepsy.
The mechanisms by which ASMs might alter the levels or function of these proteins are still under study. The drug Levetiracetam (LEV) exerts its mechanism of action specifically on SV2A proteins (Lynch et al., 2004; Nowack et al., 2011), and a recently developed drug Brivaracetam (BRV), also shows affinity for SV2A, decreasing synaptic frequency and vesicular recycling, presenting a greater affinity for the protein through binding to a distinct site (Yang et al., 2015; Wood & Gillard, 2017).
LEV show a low rate on MCM and the expression of its target proteins during embryogenesis have not been extensively studied, but SV2A is detected as early as in E14 mouse brain (Crèvecœur et al., 2013). Additional mechanism of action of LEV is related with a reduction the amplitude of kainate induced current in cortical neurons (Carunchio et al., 2007) which has not been analyzed during early nervous system development.
ASMs are the principal treatment for controlling seizures on epileptic patients, but several of these drugs have a secondary effect related with increase the risk of generating MCMs, such as spine bifida (NTDs), which correspond to the first birth defects associate with CNS. ASM target specific channels and receptors related with the control of neuronal excitability (glutamatergic/GABAergic) and then seizure initiation and propagation. Because its association with MCMs, it becomes relevant, describe which of the principal ASM targets proteins are expressed and controls active signaling in early developmental stages of the nervous system. The presence ASM, could interfere with their physiological role and generate birth malformations.
Our analyses identified several investigations, that found the presence of a number of transcripts of voltage-gated channels and receptors in embryonic periods, like Nav1.3 Cav2.1, Cav2.2, GABA(A)α2, and GluA1 which can be target for several ASM like LCM, GBP, RTG, LEV, LTG, TPM, CZP, and PER. One investigation that propose an interaction mechanism between an ASM with an a receptor during neurulation, show that VPA induces NTDs blocking NMDAR and altering excitatory glutamatergic signaling in embryos necessary for generates Ca2+i transients and in turn, regulate oriented migration of neural plate cells, fundamental for the normal Neural tube closure (Sequerra et al., 2018). Similarly, the direct blockade of active VGCC decreasing Ca2+i signaling with nifedipine (Suzuki et al., 2017) or inhibition of T-Type Ca2+ Channel directly generates NTDs, because alter active and necessary signaling for these periods.
The interaction between ASM and its principal target, interfering with an active signaling important for early development, correspond to first possibility of generate malformations of the nervous system. An additional hypothesis is related with the action of ASM and secondary targets. Almost all ASM, interact with additional proteins different to the principal targets (Table 1) and the possibility to interfere with signals different to Na+ channels, Ca2+ channels, glutamate and GABA receptors and synaptic vesicles proteins (SV2) increases significantly. For example, VPA present at least four targets besides Na+ channel, including HDAC, ROS generation, TCA enzymes and GABAergic system. Similarly, TPM affects Na+ channels, GABA augmentation, AMPAR and KaiRs. Then, exist a good association between drugs development and safety profile, whose older drugs (first generation) are more unsafe that new (third generation). In correlation, first ASM have more targets possible versus new drugs, restricting the alterations of multiple signaling.
Despite the development of new ASM, investigations of third generation ASM can generate nervous system malformations in vitro. LCM and its metabolites may have teratogenic effects on the developing mice embryos, reflected in embryonic lethality and malformations, as well as behavioral and histological alterations (López-escobar et al., 2020). Then, LCM generates growth retardation and major malformations increased in a dose-dependent manner and observed mostly in the supratherapeutic group (Mete et al., 2016). These preclinical data will need to be corroborated with new investigations and clinical studies, which should confirm the potential risk of using LCM and third generation ASMs.
In summary, the expression of diverse channels and receptors in early stages of development should be associate with a functional role during embryogenesis. The comprehensive knowledge of the function of these components as possible targets of ASMs will help to evaluate possible interactions during intrauterine gestation in pregnant WWE. More studies are needed to determine if these interactions occurs in vivo, in order to contribute to the understanding the teratogenic effect of old and new ASM during pregnancy. Finally, in relation with the pathology of epilepsy and seizure onset, it has been shown that the expression of several receptors and ion channels changes with epileptic seizures (Bender et al., 2003) and could be a relevant strategy and target for future analyses of ASM.
IP-B and PC wrote the manuscript. IP-B, JF, GM-C, GY, and PC read, contributed and comment to the manuscript and approved the final version of the manuscript.
This work was funded by FONDECYT 11160562 (PAC), VRID 220.033.112-4 (PAC), Fondecyt 1211095 (GM-C), Fondecyt 1200908 (JF) and the Millennium Nucleus for the Study of Pain (MiNuSPain) (GEY). MiNuSPain is a Millennium Nucleus supported by the Millennium Science Initiative NCN19_038 of the Ministry of Science, Technology, Knowledge and Innovation, Chile.
The authors thank Laura Borodinsky for comments of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdellah, Z., Ahmadi, A., Ahmed, S., Aimable, M., Ainscough, R., Almeida, J., et al. (2004). Finishing the euchromatic sequence of the human genome. Nature 431 (7011), 931–945. doi:10.1038/nature03001
Abdul-Wajid, S., Morales-Diaz, H., Khairallah, S. M., and Smith, W. C. (2015). T-Type calcium channel regulation of neural tube closure and EphrinA/EPHA expression. Cell Rep. 13 (4), 829–839. doi:10.1016/j.celrep.2015.09.035
Abou-Khalil, B. W. (2022). Update on antiseizure medications 2022. Contin. (Minneap Minn) 28 (2), 500–535. doi:10.1212/CON.0000000000001104
Ängehagen, M., Ben-Menachem, E., Shank, R., Rönnbäck, L., and Hansson, E. (2004). Topiramate modulation of kainate-induced calcium currents is inversely related to channel phosphorylation level. J. Neurochem. 88 (2), 320–325. doi:10.1046/j.1471-4159.2003.02186.x
Ashna, A., van Helden, D. F., Dos Remedios, C., Molenaar, P., and Laver, D. R. (2020). Phenytoin reduces activity of cardiac ryanodine receptor 2; A potential mechanism for its cardioprotective action. Mol. Pharmacol. 97 (4), 250–258. doi:10.1124/mol.119.117721
Augustin, K., Williams, S., Cunningham, M., Devlin, A. M., Friedrich, M., Jayasekera, A., et al. (2018). Perampanel and decanoic acid show synergistic action against AMPA receptors and seizures. Epilepsia 59 (11), e172–e178. doi:10.1111/epi.14578
Bai, Y. F., Zeng, C., Jia, M., and Xiao, B. (2022). Molecular mechanisms of topiramate and its clinical value in epilepsy. Seizure 98, 51–56. doi:10.1016/j.seizure.2022.03.024
Bajjalieh, S., Frantz, G., Weimann, J., McConnell, S., and Scheller, R. (1994). Differential expression of synaptic vesicle protein 2 (SV2) isoforms. J. Neurosci. 14 (9), 5223–5235. doi:10.1523/JNEUROSCI.14-09-05223.1994
Balashova, O. A., Visina, O., and Borodinsky, L. N. (2017). Folate receptor 1 is necessary for neural plate cell apical constriction during Xenopus neural tube formation. Development 144 (8), 1518–1530. doi:10.1242/dev.137315
Baulac, S., Huberfeld, G., Gourfinkel-An, I., Mitropoulou, G., Beranger, A., Prud’homme, J. F., et al. (2001). First genetic evidence of GABA(A) receptor dysfunction in epilepsy: A mutation in the gamma2-subunit gene. Nat. Genet. 28 (1), 46–48. doi:10.1038/ng0501-46
Beckh, S., Noda, M., Lübbert, H., and Numa, S. (1989). Differential regulation of three sodium channel messenger RNAs in the rat central nervous system during development. EMBO J. 8 (12), 3611–3616. doi:10.1002/j.1460-2075.1989.tb08534.x
Benavides-Rivas, C., Tovar, L. M., Zúñiga, N., Pinto-Borguero, I., Retamal, C., Yévenes, G. E., et al. (2020). Altered glutaminase 1 activity during neurulation and its potential implications in neural tube defects. Front. Pharmacol. 11, 900–908. doi:10.3389/fphar.2020.00900
Bender, R. A., Soleymani, S. V., Brewster, A. L., Nguyen, S. T., Beck, H., Mathern, G. W., et al. (2003). Enhanced expression of a specific hyperpolarization-activated cyclic nucleotide-gated cation channel (HCN) in surviving dentate gyrus granule cells of human and experimental epileptic hippocampus. J. Neurosci. 23 (17), 6826–6836. doi:10.1523/JNEUROSCI.23-17-06826.2003
Bettler, B., and Fakler, B. (2017). Ionotropic AMPA-type glutamate and metabotropic GABAB receptors: Determining cellular physiology by proteomes. Curr. Opin. Neurobiol. 45, 16–23. doi:10.1016/j.conb.2017.02.011
Bjørk, M. H., Zoega, H., Leinonen, M. K., Cohen, J. M., Dreier, J. W., Furu, K., et al. (2022). Association of prenatal exposure to antiseizure medication with risk of autism and intellectual disability. JAMA Neurol. 79, 672–681. doi:10.1001/jamaneurol.2022.1269
Bomben, V. C., Aiba, I., Qian, J., Mark, M. D., Herlitze, S., and Noebels, J. L. (2016). Isolated P/Q calcium channel deletion in layer VI corticothalamic neurons generates absence epilepsy. J. Neurosci. 36 (2), 405–418. doi:10.1523/JNEUROSCI.2555-15.2016
Briner, W. (2001). The effect of GABA receptor ligands in experimental spina bifida occulta. BMC Pharmacol. 1, 2. doi:10.1186/1471-2210-1-2
Brodie, M. J., Mintzer, S., Pack, A. M., Gidal, B. E., Vecht, C. J., and Schmidt, D. (2013). Enzyme induction with antiepileptic drugs: Cause for concern? Epilepsia 54 (1), 11–27. doi:10.1111/j.1528-1167.2012.03671.x
Brooks-Kayal, A. R., Shumate, M. D., Jin, H., Rikhter, T. Y., and Coulter, D. A. (1998). Selective changes in single cell GABA(A) receptor subunit expression and function in temporal lobe epilepsy. Nat. Med. 4 (10), 1166–1172. doi:10.1038/2661
Carunchio, I., Pieri, M., Ciotti, M. T., Albo, F., and Zona, C. (2007). Modulation of AMPA receptors in cultured cortical neurons induced by the antiepileptic drug levetiracetam. Epilepsia 48, 654–662. doi:10.1111/j.1528-1167.2006.00973.x
Catterall, W. (2000). Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 16, 521–555. doi:10.1146/annurev.cellbio.16.1.521
Chen, Y., Lu, J., Pan, H., Zhang, Y., Wu, H., Xu, K., et al. (2003). Association between genetic variation of CACNA1H and childhood absence epilepsy. Ann. Neurol. 54 (2), 239–243. doi:10.1002/ana.10607
Chioza, B., Wilkie, H., Nashef, L., Blower, J., McCormick, D., Sham, P., et al. (2001). Association between the alpha(1a) calcium channel gene CACNA1A and idiopathic generalized epilepsy. Neurology 56 (9), 1245–1246. doi:10.1212/WNL.56.9.1245
Cohen-Kutner, M., Nachmanni, D., and Atlas, D. (2010). CaV2.1 (P/Q channel) interaction with synaptic proteins is essential for depolarization-evoked release. Channels 4 (4), 266–277. doi:10.4161/chan.4.4.12130
Colas, J.-F., and Schoenwolf, G. C. (2001). Towards a cellular and molecular understanding of neurulation. Dev. Dyn. 221 (2), 117–145. doi:10.1002/dvdy.1144
Contreras-García, I. J., Gómez-Lira, G., Phillips-Farfán, B. V., Pichardo-Macías, L. A., García-Cruz, M. E., Chávez-Pacheco, J. L., et al. (2021). Synaptic vesicle protein 2A expression in glutamatergic terminals is associated with the response to levetiracetam treatment. Brain Sci. 11 (5), 531. doi:10.3390/brainsci11050531
Contreras-García, I. J., Pichardo-Macías, L. A., Santana-Gómez, C. E., Sánchez-Huerta, K., Ramírez-Hernández, R., Gómez-González, B., et al. (2018). Differential expression of synaptic vesicle protein 2A after status epilepticus and during epilepsy in a lithium-pilocarpine model. Epilepsy Behav. 88, 283–294. doi:10.1016/j.yebeh.2018.08.023
Crèvecœur, J., Foerch, P., Doupagne, M., Thielen, C., Vandenplas, C., Moonen, G., et al. (2013). Expression of SV2 isoforms during rodent brain development. BMC Neurosci. 14 (1), 87. doi:10.1186/1471-2202-14-87
Crèvecœur, J., Kaminski, R. M., Rogister, B., Foerch, P., Vandenplas, C., Neveux, M., et al. (2014). Expression pattern of synaptic vesicle protein 2 (SV2) isoforms in patients with temporal lobe epilepsy and hippocampal sclerosis. Neuropathol. Appl. Neurobiol. 40 (2), 191–204. doi:10.1111/nan.12054
Crowder, K. M., Gunther, J. M., Jones, T. A., Hale, B. D., Zhang, H. Z., Peterson, M. R., et al. (1999). Abnormal neurotransmission in mice lacking synaptic vesicle protein 2A (SV2A). Proc. Natl. Acad. Sci. U. S. A. 96 (26), 15268–15273. doi:10.1073/pnas.96.26.15268
Cuzon Carlson, V. C., and Yeh, H. H. (2011). GABAA receptor subunit profiles of tangentially migrating neurons derived from the medial ganglionic eminence. Cereb. Cortex 21 (8), 1792–1802. doi:10.1093/cercor/bhq247
Dardou, D., Dassesse, D., Cuvelier, L., Deprez, T., De Ryck, M., and Schiffmann, S. N. (2011). Distribution of SV2C mRNA and protein expression in the mouse brain with a particular emphasis on the basal ganglia system. Brain Res. 1367, 130–145. doi:10.1016/j.brainres.2010.09.063
Dibbens, L. M., Feng, H.-J., Richards, M. C., Harkin, L. A., Hodgson, B. L., Scott, D., et al. (2004). GABRD encoding a protein for extra- or peri-synaptic GABAA receptors is a susceptibility locus for generalized epilepsies. Hum. Mol. Genet. 13 (13), 1315–1319. doi:10.1093/hmg/ddh146
Dron, M. Y., Zhigulin, A. S., Tikhonov, D. B., and Barygin, O. I. (2021). Screening for activity against AMPA receptors among anticonvulsants-focus on Phenytoin. Front. Pharmacol. 12, 775040. doi:10.3389/fphar.2021.775040
Eckle, V.-S., Shcheglovitov, A., Vitko, I., Dey, D., Yap, C. C., Winckler, B., et al. (2014). Mechanisms by which a CACNA1H mutation in epilepsy patients increases seizure susceptibility. J. Physiol. 592 (4), 795–809. doi:10.1113/jphysiol.2013.264176
Edvardson, S., Oz, S., Abulhijaa, F. A., Taher, F. B., Shaag, A., Zenvirt, S., et al. (2013). Early infantile epileptic encephalopathy associated with a high voltage gated calcium channelopathy. J. Med. Genet. 50 (2), 118–123. doi:10.1136/jmedgenet-2012-101223
Edvinsson, J., Warfvinge, K., and Edvinsson, L. (2015). Modulation of inflammatory mediators in the trigeminal ganglion by botulinum neurotoxin type A: An organ culture study. J. Headache Pain 16 (1), 555. doi:10.1186/s10194-015-0555-z
Egbenya, D. L., Hussain, S., Lai, Y.-C., Xia, J., Anderson, A. E., and Davanger, S. (2018). Changes in synaptic AMPA receptor concentration and composition in chronic temporal lobe epilepsy. Mol. Cell. Neurosci. 92, 93–103. doi:10.1016/j.mcn.2018.07.004
Eugène, E., Depienne, C., Baulac, S., Baulac, M., Fritschy, J. M., Le Guern, E., et al. (2007). GABA(A) receptor gamma 2 subunit mutations linked to human epileptic syndromes differentially affect phasic and tonic inhibition. J. Neurosci. 27 (51), 14108–14116. doi:10.1523/JNEUROSCI.2618-07.2007
Eyal, S., Yagen, B., Sobol, E., Altschuler, Y., Shmuel, M., and Bialer, M. (2004). The activity of antiepileptic drugs as histone deacetylase inhibitors. Epilepsia 45 (7), 737–744. doi:10.1111/j.0013-9580.2004.00104.x
Fattorusso, A., Matricardi, S., Mencaroni, E., Dell’Isola, G. B., Di Cara, G., Striano, P., et al. (2021). The pharmacoresistant epilepsy: An overview on existant and new emerging therapies. Front. Neurol. 12, 674483. doi:10.3389/fneur.2021.674483
Feng, G., Xiao, F., Lu, Y., Huang, Z., Yuan, J., Xiao, Z., et al. (2009). Down-regulation synaptic vesicle protein 2A in the anterior temporal neocortex of patients with intractable epilepsy. J. Mol. Neurosci. 39 (3), 354–359. doi:10.1007/s12031-009-9288-2
Feng, H.-J., Kang, J.-Q., Song, L., Dibbens, L., Mulley, J., and Macdonald, R. L. (2006). Delta subunit susceptibility variants E177A and R220H associated with complex epilepsy alter channel gating and surface expression of alpha4beta2delta GABAA receptors. J. Neurosci. 26 (5), 1499–1506. doi:10.1523/JNEUROSCI.2913-05.2006
Fisher, R. S., Acevedo, C., Arzimanoglou, A., Bogacz, A., Cross, J. H., Elger, C. E., et al. (2014). ILAE official report: A practical clinical definition of epilepsy. Epilepsia 55 (4), 475–482. doi:10.1111/epi.12550
Friedman, L., Pellegrini-Giampietro, D., Sperber, E., Bennett, M., Moshe, S., and Zukin, R. (1994). Kainate-induced status epilepticus alters glutamate and GABAA receptor gene expression in adult rat hippocampus: An in situ hybridization study. J. Neurosci. 14 (5), 2697–2707. doi:10.1523/JNEUROSCI.14-05-02697.1994
Gee, N. S., Brown, J. P., Dissanayake, V. U. K., Offord, J., Thurlow, R., and Woodruff, G. N. (1996). The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J. Biol. Chem. 271 (10), 5768–5776. doi:10.1074/jbc.271.10.5768
Gilchrist, J., Dutton, S., Diaz-Bustamante, M., McPherson, A., Olivares, N., Kalia, J., et al. (2014). Na v 1.1 modulation by a novel triazole compound attenuates epileptic seizures in rodents. ACS Chem. Biol. 9 (5), 1204–1212. doi:10.1021/cb500108p
Goldin, A. L. (2001). Resurgence of sodium channel research. Annu. Rev. Physiol. 63 (1), 871–894. doi:10.1146/annurev.physiol.63.1.871
Gregory, S. G., Barlow, K. F., McLay, K. E., Kaul, R., Swarbreck, D., Dunham, A., et al. (2006). The DNA sequence and biological annotation of human chromosome 1. Nature 441 (7091), 315–321. doi:10.1038/nature04727
Guo, F., Yu, N., Cai, J.-Q., Quinn, T., Zong, Z.-H., Zeng, Y.-J., et al. (2008). Voltage-gated sodium channel Nav1.1, Nav1.3 and beta1 subunit were up-regulated in the hippocampus of spontaneously epileptic rat. Brain Res. Bull. 75 (1), 179–187. doi:10.1016/j.brainresbull.2007.10.005
Hakami, T. (2021). Neuropharmacology of antiseizure drugs. Neuropsychopharmacol. Rep. 41 (3), 336–351. doi:10.1002/npr2.12196
Hanaya, R., Hosoyama, H., Sugata, S., Tokudome, M., Hirano, H., Tokimura, H., et al. (2012). Low distribution of synaptic vesicle protein 2A and synaptotagimin-1 in the cerebral cortex and hippocampus of spontaneously epileptic rats exhibiting both tonic convulsion and absence seizure. Neuroscience 221, 12–20. doi:10.1016/j.neuroscience.2012.06.058
Hendrich, J., Van Minh, A. T., Heblich, F., Nieto-Rostro, M., Watschinger, K., Striessnig, J., et al. (2008). Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proc. Natl. Acad. Sci. U. S. A. 105 (9), 3628–3633. doi:10.1073/pnas.0708930105
Hernandez, C. C., Xiangwei, W., Hu, N., Shen, D., Shen, W., Lagrange, A. H., et al. (2019). Altered inhibitory synapses in de novo GABRA5 and GABRA1 mutations associated with early onset epileptic encephalopathies. Brain 142 (7), 1938–1954. doi:10.1093/brain/awz123
Hino-Fukuyo, N., Kikuchi, A., Arai-Ichinoi, N., Niihori, T., Sato, R., Suzuki, T., et al. (2015). Genomic analysis identifies candidate pathogenic variants in 9 of 18 patients with unexplained West syndrome. Hum. Genet. 134 (6), 649–658. doi:10.1007/s00439-015-1553-6
Holmes, L. B., Mittendorf, R., Shen, A., Smith, C. R., and Hernandez-Diaz, S. (2011). Fetal effects of anticonvulsant polytherapies: Different risks from different drug combinations. Arch. Neurol. 68 (10), 1275–1281. doi:10.1001/archneurol.2011.133
Homanics, G. E., DeLorey, T. M., Firestone, L. L., Quinlan, J. J., Handforth, A., Harrison, N. L., et al. (1997). Mice devoid of gamma-aminobutyrate type A receptor beta3 subunit have epilepsy, cleft palate, and hypersensitive behavior. Proc. Natl. Acad. Sci. U. S. A. 94 (8), 4143–4148. doi:10.1073/pnas.94.8.4143
Huang, E. J., and Reichardt, L. F. (2001). Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 24 (1), 677–736. doi:10.1146/annurev.neuro.24.1.677
Hughes, A., Greene, N. D. E., Copp, A. J., and Galea, G. L. (2018). Valproic acid disrupts the biomechanics of late spinal neural tube closure in mouse embryos. Mech. Dev. 149, 20–26. doi:10.1016/j.mod.2017.12.001
Janve, V. S., Hernandez, C. C., Verdier, K. M., Hu, N., and Macdonald, R. L. (2016). Epileptic encephalopathy de novo GABRB mutations impair γ-aminobutyric acid type A receptor function. Ann. Neurol. 79 (5), 806–825. doi:10.1002/ana.24631
Janz, R., Goda, Y., Geppert, M., Missler, M., and Südhof, T. C. (1999). SV2A and SV2B function as redundant Ca2+ regulators in neurotransmitter release. Neuron 24 (4), 1003–1016. doi:10.1016/S0896-6273(00)81046-6
Janz, R., and Südhof, T. (1999). SV2C is a synaptic vesicle protein with an unusually restricted localization: Anatomy of a synaptic vesicle protein family. Neuroscience 94 (4), 1279–1290. doi:10.1016/S0306-4522(99)00370-X
Johnson, E. L. (2019). Seizures and epilepsy. Med. Clin. North Am. 103 (2), 309–324. doi:10.1016/j.mcna.2018.10.002
Joseph, D. J., Williams, D. J., and MacDermott, A. B. (2011). Modulation of neurite outgrowth by activation of calcium-permeable kainate receptors expressed by rat nociceptive-like dorsal root ganglion neurons. Dev. Neurobiol. 71 (10), 818–835. doi:10.1002/dneu.20906
Kaeser, G. E., Rabe, B. A., and Saha, M. S. (2011). Cloning and characterization of GABAA α subunits and GABAB subunits in Xenopus laevis during development. Dev. Dyn. 240 (4), 862–873. doi:10.1002/dvdy.22580
Källén, B., Robert, E., Mastroiacovo, P., Martínez-Frías, M. L., Castilla, E. E., and Cocchi, G. (1989). Anticonvulsant drugs and malformations is there a drug specificity? Eur. J. Epidemiol. 5 (1), 31–36. doi:10.1007/BF00145041
Kilic, D., Pedersen, H., Kjaersgaard, M. I. S., Parner, E. T., Vestergaard, M., Sørensen, M. J., et al. (2014). Birth outcomes after prenatal exposure to antiepileptic drugs-A population-based study. Epilepsia 55 (11), 1714–1721. doi:10.1111/epi.12758
Knuesel, I., Chicha, L., Britschgi, M., Schobel, S. A., Bodmer, M., Hellings, J. A., et al. (2014). Maternal immune activation and abnormal brain development across CNS disorders. Nat. Rev. Neurol. 10 (11), 643–660. doi:10.1038/nrneurol.2014.187
Kubová, H., Bendová, Z., Moravcová, S., Pačesová, D., Rocha, L., and Mareš, P. (2020). Neonatal Clonazepam administration induced long-lasting changes in GABAA and GABAB receptors. Int. J. Mol. Sci. 21 (9), 3184. doi:10.3390/ijms21093184
Kuzmiski, J. B., Barr, W., Zamponi, G. W., and MacVicar, B. A. (2005). Topiramate inhibits the initiation of plateau potentials in CA1 neurons by depressing R-type calcium channels. Epilepsia 46 (4), 481–489. doi:10.1111/j.0013-9580.2005.35304.x
Lange, F., Weßlau, K., Porath, K., Hörnschemeyer, J., Bergner, C., Krause, B. J., et al. (2019). AMPA receptor antagonist perampanel affects glioblastoma cell growth and glutamate release in vitro. PLOS ONE 14 (2), e0211644. doi:10.1371/journal.pone.0211644
Larsen, J., Carvill, G. L., Gardella, E., Kluger, G., Schmiedel, G., Barisic, N., et al. (2015). The phenotypic spectrum of SCN8A encephalopathy. Neurology 84 (5), 480–489. doi:10.1212/WNL.0000000000001211
Lawthom, C. (2020). Carbamazepine: Out with the old, in with the new? Seizure 83, 246–248. doi:10.1016/j.seizure.2020.10.026
Lee, C. Y., Fu, W. M., Chen, C. C., Su, M. J., and Liou, H. H. (2008). Lamotrigine inhibits postsynaptic AMPA receptor and glutamate release in the dentate gyrus. Epilepsia 49 (5), 888–897. doi:10.1111/j.1528-1167.2007.01526.x
Lewis, B. B., Miller, L. E., Herbst, W. A., and Saha, M. S. (2014). The role of voltage-gated calcium channels in neurotransmitter phenotype specification: Coexpression and functional analysis in Xenopus laevis. J. Comp. Neurol. 522 (11), 2518–2531. doi:10.1002/cne.23547
Li, J.-M., Zeng, Y.-J., Peng, F., Li, L., Yang, T.-H., Hong, Z., et al. (2010). Aberrant glutamate receptor 5 expression in temporal lobe epilepsy lesions. Brain Res. 1311, 166–174. doi:10.1016/j.brainres.2009.11.024
López-Escobar, B., Fernández-Torres, R., Vargas-López, V., Villar-Navarro, M., Rybkina, T., Rivas-Infante, E., et al. (2020). Lacosamide intake during pregnancy increases the incidence of foetal malformations and symptoms associated with schizophrenia in the offspring of mice. Sci. Rep. 10 (1), 7615. doi:10.1038/s41598-020-64626-9
Löscher, W., and Rogawski, M. A. (2012). How theories evolved concerning the mechanism of action of barbiturates. Epilepsia 53 (8), 12–25. doi:10.1111/epi.12025
Lukyanetz, E. A., Shkryl, V. M., and Kostyuk, P. G. (2002). Selective blockade of N-type calcium channels by levetiracetam. Epilepsia 43 (1), 9–18. doi:10.1046/j.1528-1157.2002.24501.x
Lynch, B. A., Lambeng, N., Nocka, K., Kensel-Hammes, P., Bajjalieh, S. M., Matagne, A., et al. (2004). The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc. Natl. Acad. Sci. U. S. A. 101 (26), 9861–9866. doi:10.1073/pnas.0308208101
Ma, W., and Barker, J. L. (1998). GABA, GAD, and GABAA receptor α4, β1, and γ1 subunits are expressed in the late embryonic and early postnatal neocortical germinal matrix and coincide with gliogenesis. Microsc. Res. Tech. 40 (5), 398–407. doi:10.1002/(SICI)1097-0029(19980301)40:5<398:AID-JEMT6>3.0.CO;2-N
Mete, M., Gurcu, B., Collu, F., Unsal, U. U., Duransoy, Y. K., Tuglu, M. I., et al. (2016). Effects of lacosamide "a novel antiepileptic drug" in the early stages of chicken embryo development. Childs Nerv. Syst. 32 (9), 1715–1719. doi:10.1007/s00381-016-3181-4
Motin, L., Yasuda, T., Schroeder, C. I., Lewis, R. J., and Adams, D. J. (2007). Omega-conotoxin CVIB differentially inhibits native and recombinant N- and P/Q-type calcium channels. Eur. J. Neurosci. 25 (2), 435–444. doi:10.1111/j.1460-9568.2006.05299.x
Nowack, A., Malarkey, E. B., Yao, J., Bleckert, A., Hill, J., and Bajjalieh, S. M. (2011). Levetiracetam reverses synaptic deficits produced by overexpression of SV2A. PLoS ONE 6 (12), e29560. doi:10.1371/journal.pone.0029560
Nucera, B., Brigo, F., Trinka, E., and Kalss, G. (2022). Treatment and care of women with epilepsy before, during, and after pregnancy: A practical guide. Ther. Adv. Neurol. Disord. 15, 17562864221101687. doi:10.1177/17562864221101687
Ohno, Y., Ishihara, S., Terada, R., Kikuta, M., Sofue, N., Kawai, Y., et al. (2009). Preferential increase in the hippocampal synaptic vesicle protein 2A (SV2A) by pentylenetetrazole kindling. Biochem. Biophys. Res. Commun. 390 (3), 415–420. doi:10.1016/j.bbrc.2009.09.035
Padmanabhan, R. (2006). Etiology, pathogenesis and prevention of neural tube defects. Congenit. Anom. 46 (2), 55–67. doi:10.1111/j.1741-4520.2006.00104.x
Pan American Health Organization (2018). The management of epilepsy in the public Health sector, 2018. Washington, DC: PAHO. doi:10.37774/9789275120279
Pennell, P. B. (2016). Use of antiepileptic drugs during pregnancy: Evolving concepts. Neurotherapeutics 13 (4), 811–820. doi:10.1007/s13311-016-0464-0
Peret, A., Christie, L. A., Ouedraogo, D. W., Gorlewicz, A., Epsztein, J., Mulle, C., et al. (2014). Contribution of aberrant GluK2-containing kainate receptors to chronic seizures in temporal lobe epilepsy. Cell Rep. 8 (2), 347–354. doi:10.1016/j.celrep.2014.06.032
Pippenger, C. E. (2003). Pharmacology of neural tube defects. Epilepsia 44, 24–32. doi:10.1046/j.1528-1157.44.s3.3.x
Polenzani, L., Woodward, R. M., and Miledi, R. (1991). Expression of mammalian gamma-aminobutyric acid receptors with distinct pharmacology in Xenopus oocytes. Proc. Natl. Acad. Sci. U. S. A. 88 (10), 4318–4322. doi:10.1073/pnas.88.10.4318
Qiu, Z., Sylwestrak, E. L., Lieberman, D. N., Zhang, Y., Liu, X.-Y., and Ghosh, A. (2012). The rett syndrome protein MeCP2 regulates synaptic scaling. J. Neurosci. 32 (3), 989–994. doi:10.1523/JNEUROSCI.0175-11.2012
Root, C. M., Velázquez-Ulloa, N. A., Monsalve, G. C., Minakova, E., and Spitzer, N. C. (2008). Embryonically expressed GABA and glutamate drive electrical activity regulating neurotransmitter specification. J. Neurosci. 28 (18), 4777–4784. doi:10.1523/JNEUROSCI.4873-07.2008
Roullet, F. I., Wollaston, L., Decatanzaro, D., and Foster, J. A. (2010). Behavioral and molecular changes in the mouse in response to prenatal exposure to the anti-epileptic drug valproic acid. Neuroscience 170 (2), 514–522. doi:10.1016/j.neuroscience.2010.06.069
Schaller, K. L., and Caldwell, J. H. (2000). Developmental and regional expression of sodium channel isoform NaCh6 in the rat central nervous system. J. Comp. Neurol. 420 (1), 84–97. doi:10.1002/(sici)1096-9861(20000424)420:1<84:aid-cne6>3.0.co;2-9
Schivell, A. E., Mochida, S., Kensel-Hammes, P., Custer, K. L., and Bajjalieh, S. M. (2005). SV2A and SV2C contain a unique synaptotagmin-binding site. Mol. Cell. Neurosci. 29 (1), 56–64. doi:10.1016/j.mcn.2004.12.011
Sequerra, E. B., Goyal, R., Castro, P. A., Levin, J. B., and Borodinsky, L. N. (2018). NMDA receptor signaling is important for neural tube formation and for preventing antiepileptic drug-induced neural tube defects. J. Neurosci. 38 (20), 4762–4773. doi:10.1523/JNEUROSCI.2634-17.2018
Serafini, R., Bracamontes, J., and Steinbach, J. H. (2000). Structural domains of the human GABA A receptor β3 subunit involved in the actions of pentobarbital. J. Physiol. 524 (3), 649–676. doi:10.1111/j.1469-7793.2000.00649.x
Sheets, P. L., Heers, C., Stoehr, T., and Cummins, T. R. (2008). Differential block of sensory neuronal voltage-gated sodium channels by lacosamide [(2R)-2-(acetylamino)-N-benzyl-3-methoxypropanamide], lidocaine, and carbamazepine. J. Pharmacol. Exp. Ther. 326 (1), 89–99. doi:10.1124/jpet.107.133413
Shi, J., Zhou, F., Wang, L., and Wu, G. (2015). Synaptic vesicle protein2A decreases in amygdaloid-kindling pharmcoresistant epileptic rats. J. Huazhong Univ. Sci. Technol. Med. Sci. 35 (5), 716–722. doi:10.1007/s11596-015-1496-0
Sills, G. J., and Rogawski, M. A. (2020). Mechanisms of action of currently used antiseizure drugs. Neuropharmacology 168, 107966. doi:10.1016/j.neuropharm.2020.107966
Singh, B., Monteil, A., Bidaud, I., Sugimoto, Y., Suzuki, T., Hamano, S., et al. (2007). Mutational analysis of CACNA1G in idiopathic generalized epilepsy. Mutation in brief #962. Online. Hum. Mutat. 28 (5), 524–525. doi:10.1002/humu.9491
Smith, A. K., Conneely, K. N., Newport, D. J., Kilaru, V., Schroeder, J. W., Pennell, P. B., et al. (2012). Prenatal antiepileptic exposure associates with neonatal DNA methylation differences. Epigenetics 7 (5), 458–463. doi:10.4161/epi.19617
Smolders, I., Bortolotto, Z. A., Clarke, V. R. J., Warre, R., Khan, G. M., O’Neill, M. J., et al. (2002). Antagonists of GLUK5-containing kainate receptors prevent pilocarpine-induced limbic seizures. Nat. Neurosci. 5 (8), 796–804. doi:10.1038/nn880
Stefani, A., Pisani, A., De Murtas, M., Mercuri, N. B., Marciani, M. G., and Calabresi, P. (1995). Action of GP 47779, the active metabolite of oxcarbazepine, on the corticostriatal system. II. Modulation of high-voltage-activated calcium currents. Epilepsia 36 (10), 997–1002. doi:10.1111/j.1528-1157.1995.tb00958.x
Steinberg, A., Frederiksen, S. D., Blixt, F. W., Warfvinge, K., and Edvinsson, L. (2016). Expression of messenger molecules and receptors in rat and human sphenopalatine ganglion indicating therapeutic targets. J. Headache Pain 17 (1), 78. doi:10.1186/s10194-016-0664-3
Suzuki, M., Sato, M., Koyama, H., Hara, Y., Hayashi, K., Yasue, N., et al. (2017). Distinct intracellular Ca2+ dynamics regulate apical constriction and differentially contribute to neural tube closure. Development 144 (7), 1307–1316. doi:10.1242/dev.141952
Suzuki, S., Kawakami, K., Nishimura, S., Watanabe, Y., Yagi, K., Scino, M., et al. (1992). Zonisamide blocks T-type calcium channel in cultured neurons of rat cerebral cortex. Epilepsy Res. 12 (1), 21–27. doi:10.1016/0920-1211(92)90087-A
Tomson, T., Battino, D., Bonizzoni, E., Craig, J., Lindhout, D., Perucca, E., et al. EURAP Study Group (2019a). Declining malformation rates with changed antiepileptic drug prescribing: An observational study. Neurology 93 (9), e831–e840. doi:10.1212/WNL.0000000000008001
Tomson, T., Battino, D., Bromley, R., Kochen, S., Meador, K., Pennell, P., et al. (2019b). Management of epilepsy in pregnancy: A report from the international League against epilepsy task force on women and pregnancy. Epileptic Disord. 21 (6), 497–517. doi:10.1684/epd.2019.1105
Tomson, T., Battino, D., and Perucca, E. (2016). Valproic acid after five decades of use in epilepsy: Time to reconsider the indications of a time-honoured drug. Lancet. Neurol. 15 (2), 210–218. doi:10.1016/S1474-4422(15)00314-2
Venkatesan, K., Alix, P., Marquet, A., Doupagne, M., Niespodziany, I., Rogister, B., et al. (2012). Altered balance between excitatory and inhibitory inputs onto CA1 pyramidal neurons from SV2A-deficient but not SV2B-deficient mice. J. Neurosci. Res. 90 (12), 2317–2327. doi:10.1002/jnr.23111
Vergult, S., Dheedene, A., Meurs, A., Faes, F., Isidor, B., Janssens, S., et al. (2015). Genomic aberrations of the CACNA2D1 gene in three patients with epilepsy and intellectual disability. Eur. J. Hum. Genet. 23 (5), 628–632. doi:10.1038/ejhg.2014.141
Veroniki, A. A., Cogo, E., Rios, P., Straus, S. E., Finkelstein, Y., Kealey, R., et al. (2017). Comparative safety of antiepileptic drugs during pregnancy: A systematic review and network meta-analysis of congenital malformations and prenatal outcomes. BMC Med. 15 (1), 95. doi:10.1186/s12916-017-0845-1
Vossler, D. G. (2019). Comparative risk of major congenital malformations with 8 different antiepileptic drugs: A prospective cohort study of the eurap registry. Epilepsy Curr. 19 (2), 83–85. doi:10.1177/1535759719835353
Wallingford, J. B., Niswander, L. A., Shaw, G. M., and Finnell, R. H. (2013). The continuing challenge of understanding, preventing, and treating neural tube defects. Science 339 (6123), 1222002. doi:10.1126/science.1222002
Wang, S.-J., Huang, C.-C., Hsu, K.-S., Tsai, J.-J., and Gean, P.-W. (1996). Inhibition of N-type calcium currents by lamotrigine in rat amygdalar neurones. NeuroReport 7 (18), 3037–3040. doi:10.1097/00001756-199611250-00048
Weiergraber, M., Henry, M., Krieger, A., Kamp, M., Radhakrishnan, K., Hescheler, J., et al. (2006). Altered seizure susceptibility in mice lacking the Cav2.3 E-type Ca2+ channel. Epilepsia 47 (5), 839–850. doi:10.1111/j.1528-1167.2006.00541.x
Werler, M. M., Ahrens, K. A., Bosco, J. L. F., Mitchell, A. A., Anderka, M. T., Gilboa, S. M., et al. (2011). Use of antiepileptic medications in pregnancy in relation to risks of birth defects. Ann. Epidemiol. 21 (11), 842–850. doi:10.1016/j.annepidem.2011.08.002
Whitaker, W. R. J., Faull, R. L. M., Waldvogel, H. J., Plumpton, C. J., Emson, P. C., and Clare, J. J. (2001). Comparative distribution of voltage-gated sodium channel proteins in human brain. Brain Res. Mol. Brain Res. 88 (1–2), 37–53. doi:10.1016/S0169-328X(00)00289-8
Wolff, M., Johannesen, K. M., Hedrich, U. B. S., Masnada, S., Rubboli, G., Gardella, E., et al. (2017). Genetic and phenotypic heterogeneity suggest therapeutic implications in SCN2A-related disorders. Brain 140 (5), 1316–1336. doi:10.1093/brain/awx054
Wood, M. D., and Gillard, M. (2017). Evidence for a differential interaction of brivaracetam and levetiracetam with the synaptic vesicle 2A protein. Epilepsia 58 (2), 255–262. doi:10.1111/epi.13638
Yang, X., Bognar, J., He, T., Mohammed, M., Niespodziany, I., Wolff, C., et al. (2015). Brivaracetam augments short-term depression and slows vesicle recycling. Epilepsia 56 (12), 1899–1909. doi:10.1111/epi.13223
Zeller, A., Arras, M., Jurd, R., and Rudolph, U. (2007). Identification of a molecular target mediating the general anesthetic actions of pentobarbital. Mol. Pharmacol. 71 (3), 852–859. doi:10.1124/mol.106.030049
Zhang, X., Velumian, A. A., Jones, O. T., and Carlen, P. L. (2000). Modulation of high-voltage-activated calcium channels in dentate granule cells by topiramate. Epilepsia 41 (1), 52–60. doi:10.1111/j.1528-1157.2000.tb02173.x
Zody, M. C., Garber, M., Sharpe, T., Young, S. K., Rowen, L., O’Neill, K., et al. (2006). Analysis of the DNA sequence and duplication history of human chromosome 15. Nature 440 (7084), 671–675. doi:10.1038/nature04601
Zubareva, O. E., Kovalenko, A. A., Kalemenev, S. V., Schwarz, A. P., Karyakin, V. B., and Zaitsev, A. V. (2018). Alterations in mRNA expression of glutamate receptor subunits and excitatory amino acid transporters following pilocarpine-induced seizures in rats. Neurosci. Lett. 686, 94–100. doi:10.1016/j.neulet.2018.08.047
Keywords: pregnancy, teratogenicity, neural development, epilepsy, antiseizure medication (ASM)
Citation: Castro PA, Pinto-Borguero I, Yévenes GE, Moraga-Cid G and Fuentealba J (2022) Antiseizure medication in early nervous system development. Ion channels and synaptic proteins as principal targets. Front. Pharmacol. 13:948412. doi: 10.3389/fphar.2022.948412
Received: 19 May 2022; Accepted: 05 September 2022;
Published: 14 October 2022.
Edited by:
Francisco Lopez-Munoz, Camilo José Cela University, SpainReviewed by:
Wladyslaw-Lason, Maj Institute of Pharmacology, Polish Academy of Sciences (IF PAS), PolandCopyright © 2022 Castro, Pinto-Borguero, Yévenes, Moraga-Cid and Fuentealba. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patricio A. Castro, cGFjYXN0cm9AdWRlYy5jbA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.