- 1Experiment Center of Teaching and Learning, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 2School of Pharmacy, Shanghai University of Traditional Chinese Medicine, Shanghai, China
Cinobufacini capsule and injection are two different formulations from the same source, obtained from the extraction of the skin of Bufo bufo gargarizans Cantor, which have been approved by the Chinese State Food and Drug Administration (CFDA) for the treatment of various cancers. Our previous study has found that the cinobufacini capsule and injection exhibited different anticancer effects, but their different pharmacokinetic behaviors, which could give a cause of that, have never been reported. So a sensitive and selective method for the simultaneous quantitation of 13 compounds in the rat plasma, including bufothionine, hellebrigenin, bufalin, gamabufotalin, telocinobufagin, cinobufagin, arenobufagin, cinobufotalin, desacetylcinobufotalin, bufotalin, pseudobufarenogin, resibufogenin, and desacetylcinobufagin, was established by using the Agilent 6460 mass spectrometer equipped with an ESI ion source in a multiple-reaction monitoring (MRM) mode. Chromatographic analysis was accomplished in 6 min by using an Agilent SB-C18 column and a mobile phase consisting of 0.1% formic acid in water and acetonitrile in an optimized gradient program at a flow rate of 0.3 ml/min. The correlation coefficients (r) of all analytes ranged from 0.9967 to 0.9996, while their lower limits of quantification ranged from 0.20 to 4.84 ng/ml. The method has been fully verified and applied for the pharmacokinetic difference study of the Cinobufacini capsule and injection in rats. The results showed that nine components could be quantitated in rat plasma samples after the administration of the cinobufacini capsule, while only bufothionine, bufalin, arenobufagin, and pseudobufarenogin could be detected in the cinobufacini injection group. Their pharmacokinetic studies indicated telocinobufagin, bufalin, desacetylcinobufagin, and arenobufagin were predicted as the potential active substances of the Cinobufacini capsule, while bufothionine was considered as a major ingredient in the cinobufacini injection due to its relatively high blood drug exposure. Also, the AUC of the nine components in cinobufacini capsule groups with three different doses showed a similar trend with significant differences, and the exposure increased with the increase of the dose. The pharmacokinetic characteristics of all major ingredients in cinobufacini capsules and injection were of wide variation, which could be used to explain differences in the efficacy of the cinobufacini capsule and injection and infer the pharmacodynamic ingredients of various cinobufacini preparations.
1 Introduction
Cancer is considered as the critical public health problem and the second leading cause of morbidity and mortality worldwide, according to GLOBOCAN 2020 official statistics from the World Health Organization (WHO) in 2020. New cases of cancer have reached 19.3 million, and the number of cancer deaths is up to 10 million in 2020 (Sung et al., 2021). Although various preventative and curative interventions including surgery operation, chemotherapy, and radiation treatment have been used as critical oncological therapeutic strategies, they could not ameliorate their high mortality rates over the last few decades due to the numerous shortcomings of current treatments (Zhong et al., 2021). The chemotherapy combined with surgical operation, radiotherapy, and biotherapy was chosen as the major medical practice for cancer treatment. However, chemotherapy was mainly used to kill tumor cells and inhibit tumor growth and metastasis but sometimes failed to discriminate cancerous cells from normal cells, causing serious toxicity and side effects. Most common chemotherapy drugs, such as sorafenib, regorafenib, and lenvatinib, have been reported to induce side effects, including diarrhea, weight loss, and hair loss (Mansouri et al., 2021). Moreover, low response rate and drug resistance were other challenges for chemotherapy, so it is urgent to develop new effective anti-cancer drugs with low toxicity and making the patients “survival with cancer” for a long time.
Traditional Chinese medicine (TCM) has been widely used to treat cancers in China and other East Asian countries, as adjunctive or complementary therapy. The empirical applications have indicated that TCM not only enhances life quality and progression-free survival in patients with cancer through multi-pathways but also diminishes adverse reactions of chemotherapy, radiotherapy, or targeted therapy (Huang et al., 2020). Some famous herbal formulae have been developed into the Chinese patent medicine and approved by the China Food and Drug Administration (CFDA) for cancer treatment, such as cinobufacini capsule, cinobufacini injection (Xu et al., 2019; Peng et al., 2021), Aidi injection (Yang et al., 2022), Shenyi capsule, Xiaoaiping injection (Huang et al., 2013), and Kanglaite injection (Fu et al., 2014). Cinobufacini is a well-known Chinese medicine, from the boiling water extraction of the skin of Bufo bufo gargarizans Cantor, also called Huachansu in Chinese. It has been used clinically for various cancers, including hepatocellular carcinoma (Qi et al., 2018a), colon cancer (Wang et al., 2020), and gastric cancer (Shen et al., 2018). The anticancer mechanisms of cinobufacini mainly included the effects of inducing cancer cell apoptosis, inhibiting cancer cell proliferation, and metastasis (Nakata et al., 2015; Yang et al., 2015; Chen et al., 2018). It has been approved by the FDA in two preparation forms of the cinobufacini capsule and injection for the clinical treatment of cancer. Although the methods for the simultaneous determination of major components including eight bufadienolides and other compounds in Cinobufacini injection have been developed by LC-MS/MS and HPLC-PAD (Wu et al., 2012; Zhao et al., 2013), the chemical composition of cinobufacini capsule and injection has never been compared. Moreover, we have found that the anti-tumor effect of cinobufacini capsule and injection was quite different, which also has not been distinguished in their clinical application. It is also inferred that the in vivo effective substance of cinobufacini capsule and injection would be different. Unfortunately, the comparative pharmacokinetics (PK) study of cinobufacini capsule and injection has never been reported. As bufadienolides are major components in cinobufacini and toad venom preparations, previous studies have focused on the pharmacokinetic behaviors of the major bufadienolides such as resibufogenin, bufalin, gamabufotalin, arenobufagin, and bufotalin after the administration of Shexiang Baoxin pill (Huang et al., 2015). In addition, the difference in pharmacokinetics of cinobufotalin between normal and diethylnitrosamine-injured rats has also been studied, which indicated that the pharmacokinetic behaviors of cinobufotalin will be altered in rats with HCC (Zhang et al., 2019). All of these references would provide some clues for the pharmacokinetics study of cinobufacini capsule and injection.
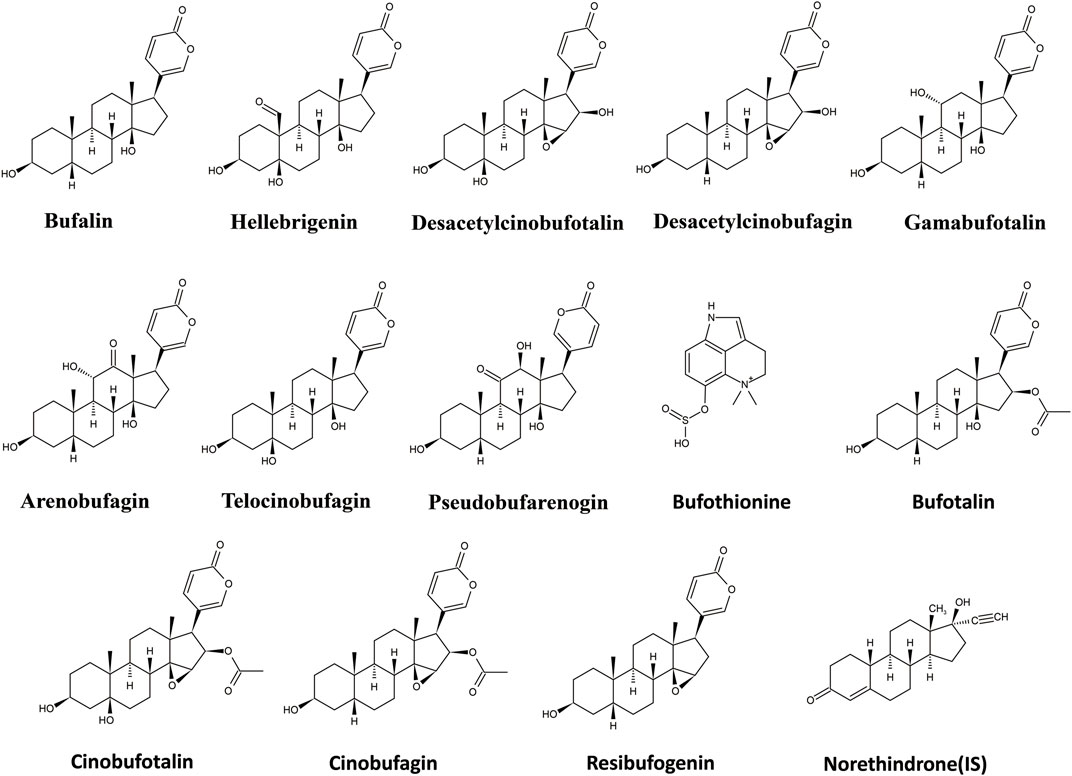
UPLC-MS/MS is the best tool for quantitative analysis for targeted analytes due to high selectivity and sensitivity (Abdelhameed et al., 2019; Al-Shakliah et al., 2020; Attwa et al., 2020). Furthermore, extraction methodology is also a crucial step to remove matrix constituent interferences and target compound extraction. In the present study, a rapid, selective, and efficient UPLC-MS/MS method for the simultaneous determination of bufothionine, hellebrigenin, bufalin, gamabufotalin, telocinobufagin, cinobufagin, arenobufagin, cinobufotalin, desacetylcinobufotalin, bufotalin, pseudobufarenogin, resibufogenin, and desacetylcinobufagin in the rat plasma was developed and fully validated to evaluate the comparative pharmacokinetics of cinobufacini capsule and injection. The chemical structures of the 13 analytes are shown in Figure 1. The pharmacokinetic parameters of nine compounds following the oral administration of cinobufacini capsule (0.9, 1.8, 3.6 g/kg) in rats have been calculated, while only bufothionine, telocinobufagin, bufalin, and arenobufagin could be detected in the rat plasma cinobufacini injection group. The present study for the first time revealed the significant difference in pharmacokinetic behaviors between cinobufacini capsule and injection, which would provide valuable information to discover the potential pharmacodynamic ingredients of cinobufacini capsule and injection.
2 Materials and Methods
2.1 Chemicals and Reagents
Cinobufacini injection (200504-1) was purchased from Anhui China Resources Jinchan Pharmaceutical Co., Ltd., and cinobufacini (0K01) capsules were purchased from Shaanxi Dongtai Pharmaceutical Co., Ltd. The standards (purity ≥ 98%) bufothionine (BTI), hellebrigenin (HBG), bufalin (BF), gamabufotalin (GBL), telocinobufagin (TCG), cinobufagin (CBG), arenobufagin (ABG), cinobufotalin (CBT), desacetylcinobufotalin (DCT), bufotalin (BL), pseudobufarenogin (PBG), resibufogenin (RBG), desacetylcinobufagin (DCG), and norethisterone (IS) were all purchased from Hongyong Biotechnology Co., Ltd. (Shanghai, China). Formic acid (98% purity), acetonitrile, and methanol of MS grade were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) and Merck (Darmstadt, Germany).
2.2 Animal Handing
Male Sprague–Dawley rats (weighing 200–220 g) of SPF grade were offered by Sippr/BK laboratory Animal, Corp., Ltd. (Shanghai, China) and fed in the Laboratory Animal Center of the Shanghai University of Traditional Chinese Medicine. All the rats were kept under environmentally controlled conditions with constant temperature (22–24°C) and humidity (60–65%). The animal experiment program in the present research was approved by the Animal Committee of the Shanghai University of Traditional Chinese Medicine (PZSHUTCM210618012).
2.3 Comparative Pharmacokinetic Studies of Cinobufacini Capsule and Injection
2.3.1 Instrumentation and Chromatographic Conditions
2.3.1.1 Liquid Chromatography
An Agilent 1290 liquid chromatographic system includes a G4220A quaternary pump, a G1326C column incubator, a G4226A automatic sampler, and a G1330B degasser. The column temperature was set to 35°C. A measure of 5 µL of samples were separated in an Agilent SB-C18 column (2.1 mm ×50 mm, 1.8 μm) and eluted at 0.3 ml/min, followed by gradient elution with water (0.1% V/V formic acid) (A) and acetonitrile (B) (0–1 min, 20%–20% B; 1–3.5 min, 20%–80% B; 4–4.1 min, 80%-20% B; 4.1–6 min, 20% B). The total run time was 6 minutes.
2.3.1.2 Mass Spectrometric Conditions
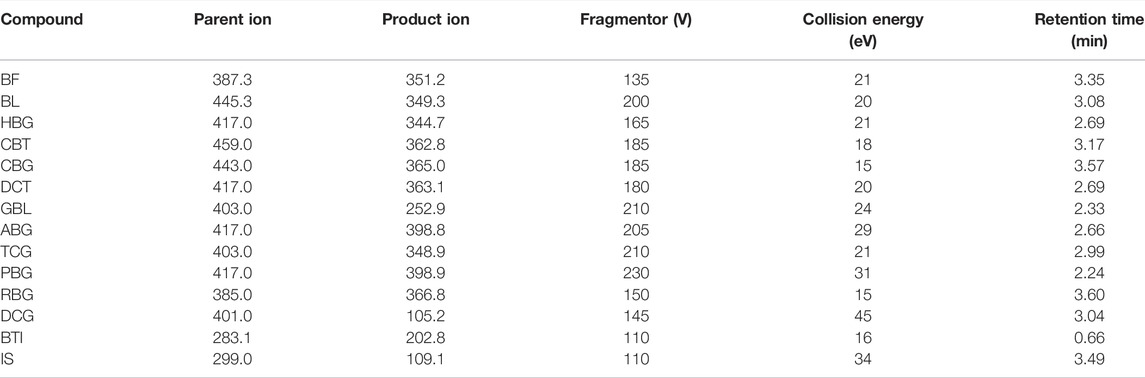
An Agilent 1290 Infinity series UPLC system was combined with an Agilent 6460 series triple quadrupole mass spectrometer (Agilent Technologies, Santa Clara, CA, United States) to quantify all 13 analytes in an ESI-positive ionization mode. Quantitative analysis was performed in an MRM mode. The mass spectrum conditions were as follows: capillary voltage, 4000V; drying gas (N2), gas flow rate: 10 L/min; sprayer, 30 psi; gas temperature 350°C; The delta EMV (+) 200 V; collision gas (N2); dwell time, 25 ms. The optimized parent ion, product ion, fragmentor, and collision energy of all analytes are shown in Table 1. Agilent’s MassHunter Workstation software was used for data collection, peak area calculation, and quantitative analysis.
2.3.2 Preparation of the Standard Solutions and Quality Control Samples
Standard substances of 13 compounds were accurately weighed in appropriate amounts and dissolved with methanol in a 10-ml volumetric flask to prepare the stock solutions. Then, 1 ml of each stock solution of 13 compounds was mixed to prepare the standard working solution and then diluted with methanol at eight concentration levels. Norethisterone was chosen as the internal standard (IS) to prepare its stock solution at 1 mg/ml. Then, it was diluted into an internal standard solution with a concentration of 345 ng/ml. All solutions were stored at 4°C before use. The calibration standard solution was prepared by spiking appropriate amounts of the standard working solutions into blank rat plasma, and the range of their final concentrations is shown in Table 2. The quality control (QC) samples at three different levels were also prepared following the same procedures, according to that of the calibration standard solution.
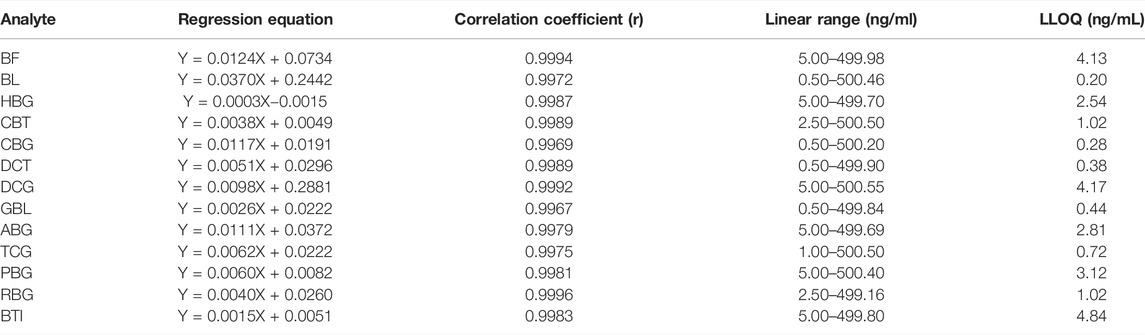
TABLE 2. Regression equations, linear range, and lower limit of quantification (LLOQ) of analytes in the rat plasma.
2.3.3 Biosample Preparation
A measure of 50 µL of the plasma sample was mixed with 10 µL of the internal standard solution (345 ng/ml) in a 1.5-ml centrifuge tube and vortexed for 5 min. The samples were extracted by 500 µL of ethyl acetate for 5 min and centrifuged at 4°C for 10 min (18000 rpm). All the supernatants were collected into another 1.5-ml centrifuge tube, dried under a gentle stream of nitrogen at 37°C, and then added with 50 µL of 80% methanol for resolution. The resolution samples were centrifuged at 4°C for 10 min (18000 rpm), and 5 µL supernatant was extracted and injected for analysis.
2.3.4 Method Validation
Validation parameters of the method included selectivity, linearity, precision and accuracy, recovery, matrix effect, and stability. All tests were performed in accordance with the U. S. Food and Drug Administration guidelines for the validation of bioanalytical methods (The Food And Drug Administration., 2018).
2.3.4.1 Selectivity
The specificity of the present method was evaluated by chromatograms of six different batches of blank rat plasma samples, blank samples spiked with all 13 analytes and IS, and rat plasma samples after drug administration of 0.5 h to check for endogenous interference.
2.3.4.2 Linearity and Lower Limit of Quantification
The calibration curve was generated by measuring standard plasma samples at eight concentration levels, and the linear relationship between the peak area ratio of the 13 analytes to the internal standard and the analyte concentrations in the calibrated plasma samples was constructed. The lower limit of quantification (LLOQ) is defined as the lowest quantifiable calibration concentration with a signal-to-noise ratio (S/N) of 10, with an acceptable accuracy within ±20%.
2.3.4.3 Precision and Accuracy
The intra- and inter-day precision and accuracy were evaluated by determining QC samples at three different concentrations (six replicates for each concentration level) in one day and over three consecutive days. The concentrations were determined using calibration curves obtained daily. The intra-day and inter-day precisions were evaluated as a variability with a relative standard deviation percentage (RSD) of <15%, and accuracy was expressed as a relative error percentage (RE, %) within ±15%.
2.3.4.4 Recovery Efficiency and Matrix Effect
The extraction recoveries were determined at three QC levels by comparing the peak area of the plasma samples spiked with all 13 analytes added before extraction to that of plasma samples spiked with analytes after extraction. Matrix effects were measured at three QC levels by comparing the peak area of the extracted blank plasma samples (from six different batches of rat plasma) with that of the corresponding pure standard solution. The extraction recoveries and matrix effects were acceptable when their RSD <15%.
2.3.4.5 Stability
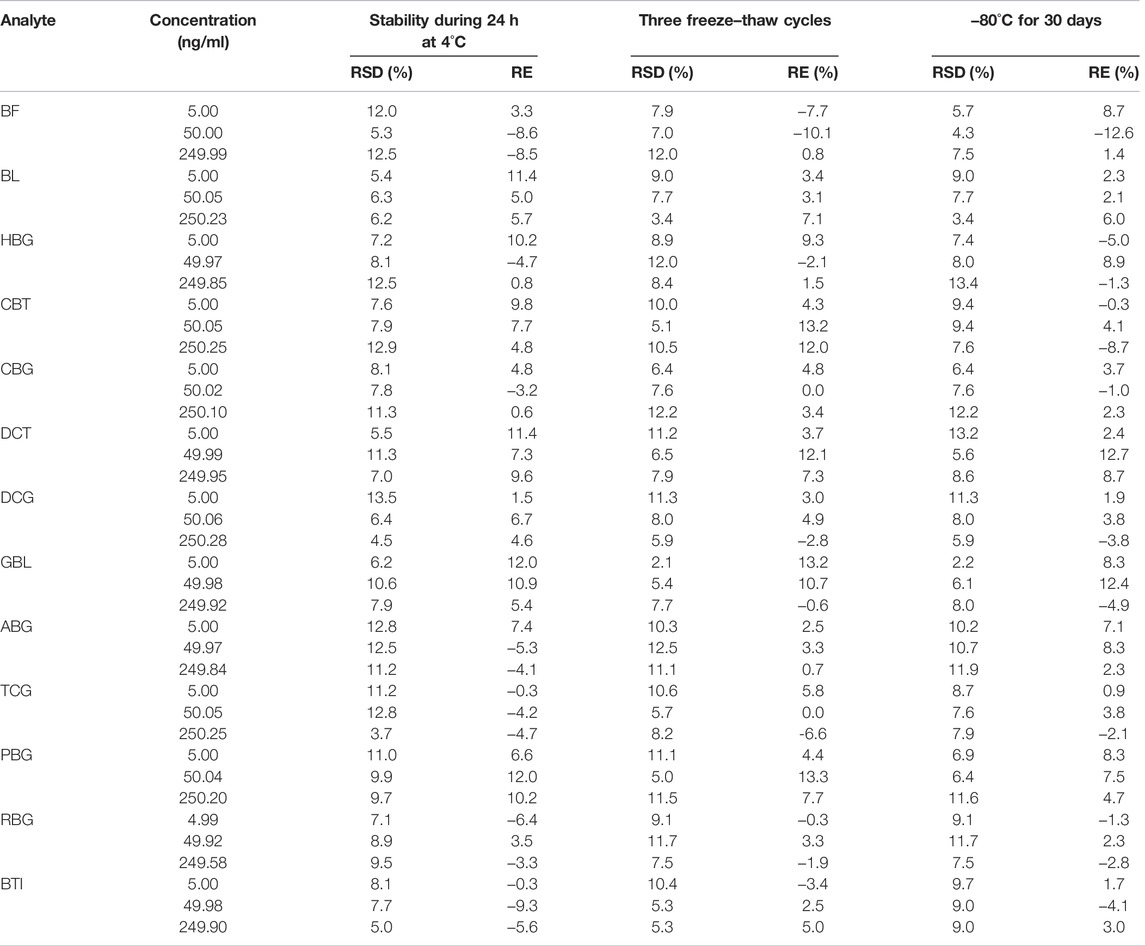
The stability of QC samples in six replicates at three concentrations (low, medium, and high levels) was analyzed under different storage conditions: the post-treatment stability was analyzed after storage in an autosampler (4°C) for 24 h. Freeze–thaw stability was investigated after three freeze–thaw cycles (−80°C to room temperature as one cycle). Long-term stability was assessed by analyzing QC samples kept at −80°C for 30 days. The concentrations of the analytes in the plasma samples and the QC samples were calculated by the calibration standard curve prepared each day. The stabilities of analytes were acceptable when the accuracy (RE, %) and precision (RSD, %) of all QC samples in all storage conditions were within ±15% compared to nominal concentrations.
2.3.5 Application to Pharmacokinetic Analysis
Twenty-four male Sprague–Dawley rats (weigh 200–220 g) were divided into four groups with six rats in each group randomly, including three capsule groups at three different doses and one injection group. In the instructions for cinobufacini capsule and injection, the clinical dosage for adults is 2 g per 70 kg orally and 20 ml per 70 kg intravenously, converting to rat doses of 180 mg/kg and 1.8 ml/kg, respectively.
In the pre-experiments, we compared cinobufacini capsule of 180 mg/kg (one-time clinical dose) and 900 mg/kg (five times of clinical dose) and Cinobufacini injection of 1.8 ml/kg, but concentrations of some compounds in plasma were too low and difficult to be quantified. Therefore, 0.9, 1.8, 3.6 g/kg (5, 10, and 20 times of clinical dose), and 9 ml/kg (five times of clinical dose) were chosen as administered doses of cinobufacini capsule and injection, which could detect more active components in the rat plasma and could demonstrate the pharmacokinetic characteristics of all compounds sufficiently. The experimental rats were led to fast the night and were free to get access to water for 12 h before the pharmacokinetic study. After administration, blood samples (approximately 150 μL) of rats were collected via retro-orbital sinus into heparinized centrifuge tubes at 0.05, 0.083, 0.25, 0.5, 0.75, 1, 2, 4, 6, 8, and 10 h. After centrifugation at 5,000 rpm for 10 min, the plasma was transferred to 1.5-ml polypropylene tubes and stored at −80°C until UPLC-MS/MS analysis.
2.4 Data Processing and Statistical Analysis
GraphPad Prism software (version 8.0.2) was used to draw the mean plasma concentration–time curves of all analytes. The pharmacokinetic parameters were calculated by DAS 3.2.8 software. Meanwhile, the pharmacokinetic data on rats were presented as mean ± standard deviation (SD). Differences of pharmacokinetic parameters between groups were analyzed by the t-test using SPSS software (version 26.0). Differences were considered significant statistically when the p-values were <0.05 and very significant statistically when the p-values were <0.01.
3 Results
3.1 Optimization of UPLC-MS/MS Conditions
The standard solution of each compound (500 ng/ml) was analyzed by the mass spectrometer with spectra from m/z 50 to 1000 in order to optimize the mass spectrometry conditions. In the positive ion mode, all 13 analytes could produce a strong mass spectral response. The mass spectrum parameters such as capillary voltage and collision energy were optimized in the multiple reaction monitoring (MRM) model, and the parent ions, product ions, fragmentors (V), and collision energies (CE) of all analytes are revealed in Table 1. The mobile phase for gradient elution was optimized, while acetonitrile could obtain good separation efficiency for the majority of all analytes in 6 min by using an Agilent SB C18 column (2.1 mm× 50 mm, 1.8 μm). Moreover, we evaluated the effects of different proportions of formic acid in mobile phases to maximize the mass spectrum response of the compounds, indicating that acetonitrile–0.1% formic acid solution could be used as a gradient elution condition to obtain a better peak shape.
3.2 Optimization of Extraction Conditions
The protein precipitation (PPT) method using methanol, acetonitrile, or their mixture and liquid–liquid extraction (LLE) with ethyl acetate has been used to extract 13 compounds and IS from rat plasma simultaneously. The protein precipitation (PPT) method by using methanol, acetonitrile, and their mixture was not suitable, while the extraction recoveries of all analytes were low. Also, the LLE method with ethyl acetate could provide higher extraction recoveries than 50% for all analytes and also offer better selectivity. Hence, LLE with ethyl acetate was used for sample pretreatment in this study.
3.2.1 Method Validation
3.2.1.1 Selectivity
No endogenous interference was found in the retention time of analytes and internal standard in blank plasma of six different rats, which proved the specificity of this method. Typical chromatograms of blank plasma, blank plasma with nine analytes added, and plasma samples of normal rats 0.5 h after oral are shown in Figure 2 to describe that there were no endogenous interferences for their quantification. The MRM mass spectra with the corresponding fragmentation for all analytes are shown in Supplementary Figure S1 of the supplementary material. Another four analytes including BL, CBT, CBG, and RBG could not be detected in the rat plasma in both cinobufacini capsules and injection groups, and typical chromatograms of blank plasma and blank plasma with four analytes added were also compared and are shown in Supplementary Figure S2 of the supplementary material. Blank plasma added with standard samples was freshly prepared according to the QC samples in a low level, with a concentration of 5.00 ng/ml for BF; 5.00 ng/ml for BL; 5.00 ng/ml for HBG; 5.00 ng/ml for CBT; 5.00 ng/ml for CBG; 5.00 ng/ml for DCT; 5.00 ng/ml for DCG; 5.00 ng/ml for GBL; 5.00 ng/ml for ABG; 5.00 ng/ml for TCG; 5.00 ng/ml for PBG; 4.99 ng/ml for RBG, and 5.00 ng/ml for BTI.
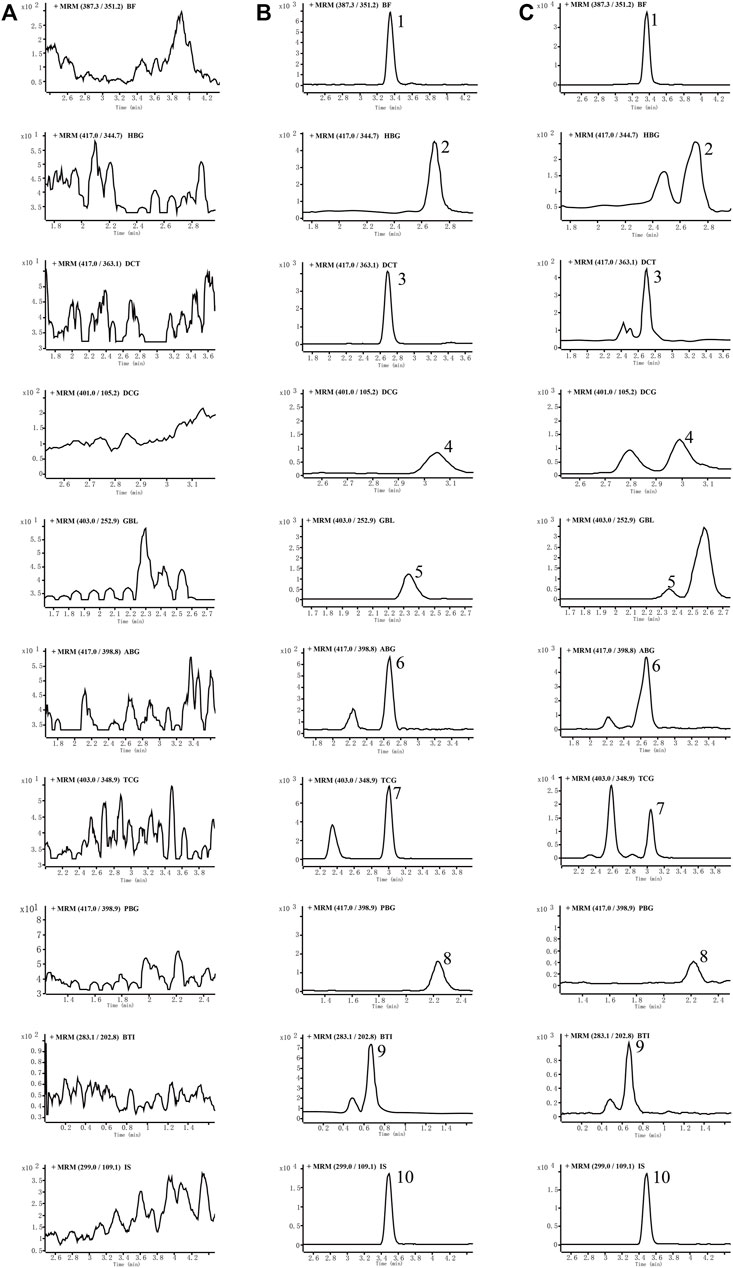
FIGURE 2. Representative MRM chromatograms of nine analytes and internal standards (IS), in rat plasma: blank rat plasma (A), blank rat plasma spiked with the analytes and IS (B), and rat plasma samples at 0.5 h after drug administration (C). Peak 1: bufalin, 2: hellebrigenin, 3: desacetylcinobufotalin, 4: desacetylcinobufagin, 5: gamabufotalin, 6: arenobufagin, 7: telocinobufagin, 8: pseudobufarenogin, 9: bufothionine, and 10: norethisterone (IS).
3.2.1.2 Linear and Quantitative Lower Bound
By weighted (1/x2) least-squares linear regression, the relationship curve between the peak area ratio (y) of each analyte to IS and the corresponding nominal concentration (x) of the analyte was plotted. The correlation coefficient for all calibration curves above 0.9967 showed good linearity for quantitation of all analytes in rat plasma. The established method was verified to meet the requirements of the quantitative determination for pharmacokinetic studies, and results of the calibration curves, linear ranges, and correlation coefficients are shown in Table 2.
3.2.1.3 Precision and Accuracy
Intra-day and daytime accuracy (relative standard deviation, RSD) is less than 14.3%, and accuracy (relative error, RE) is less than 13.0%, as summarized in Table 3. All results were within acceptable standards, according to the guidelines for bioassay methods.
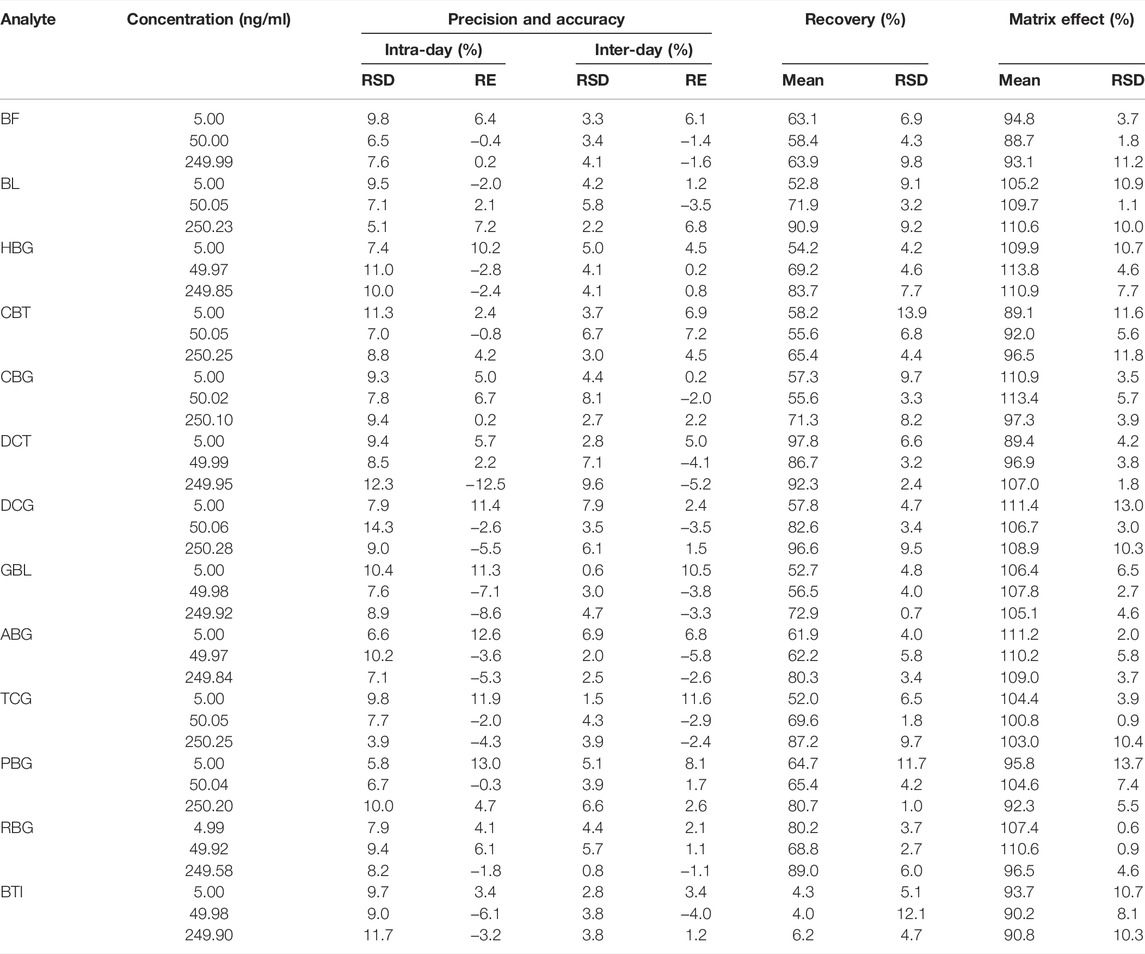
TABLE 3. Summary of accuracy, precision, recovery, and matrix effect of the analytes in rat plasma (n = 6).
3.2.1.4 Recovery and Matrix Effect
The average extraction recoveries and matrix effects of 13 analytes at three quality control levels are summarized in Table 3, while the recoveries of 12 analytes except BTI at three concentration levels in rat plasma samples ranged from 52.0 % to 97.8%, and the matrix effect ranged from 88.7% to 113.8%. But the extraction recovery of BTI ranged from 4.0% to 6.2%, which was out of requirement of method validation. Most sample pretreatment methods have been used to increase its extraction recovery rate, but it does not work. The pharmacokinetics of BTI has never been reported in previous studies. Fortunately, the concentration of BTI in rat plasma is high enough for quantitation. We hope it would be solved in further research.
3.2.1.5 Stability
The stability of 13 analytes at three quality control levels is summarized in Table 4. The accuracy (RE, %) of all QC samples in the stability test ranged from −12.6% to 13.3% with precision (RSD, %) in the range of 2.1%–13.5%. It indicated that analytes in rat plasma remained stable in plasma samples under the following conditions: post-treatment storage in an autosampler (4°C) for 24 h, three freeze–thaw cycles, and at −80°C for 30 days.
4 Discussion
The established UPLC-MS/MS method has been successfully applied for the pharmacokinetic study of cinobufacini capsule and injection in rats. The concentrations of BF, HBG, DCT, DCG, GBL, ABG, TCG, BTI, and PBG in rat plasma after the oral administration of cinobufacini capsule could be determined. The concentrations of BTI, BF, ABG, and PBG in rat plasma after the intravenous administration of cinobufacini injection through the tail vein could be determined. The mean plasma concentration–time curves are presented in Figure 3. The pharmacokinetic parameters including the maximum plasma concentration (C max), area under the concentration–time curve (AUC) and mean residence time (MRT), the half-life life (t 1/2z), and clearance (CL/F) are summarized in Tables 5, 6. The pharmacokinetic parameters in the cinobufacini capsule group were calculated by a two-compartmental model by DAS 3.2 data analysis software.
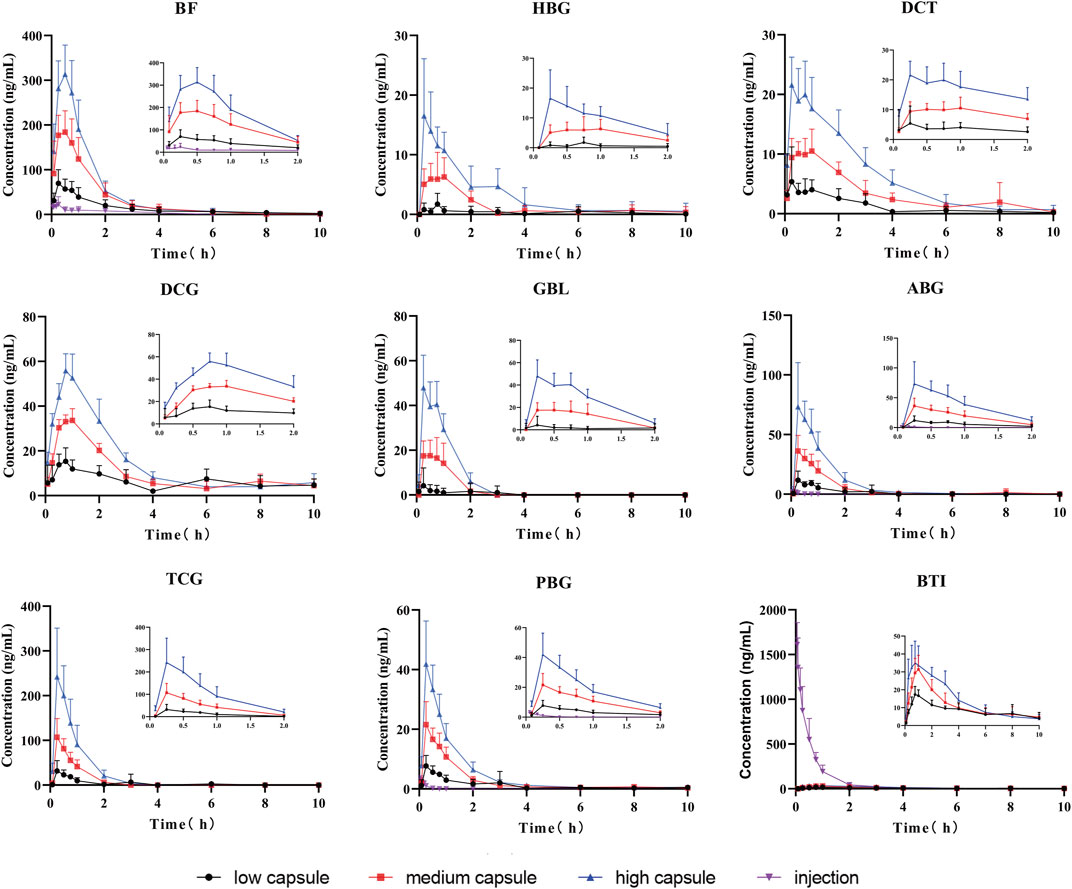
FIGURE 3. Mean concentration–time curves of nine compounds in rat plasma after the oral administration of cinobufacini capsule (0.9, 1.8, and 3.6 g/kg) and intravenous administration of cinobufacini injection at a dose of 9 ml/kg to male SD rats (mean ± SD, n = 6).
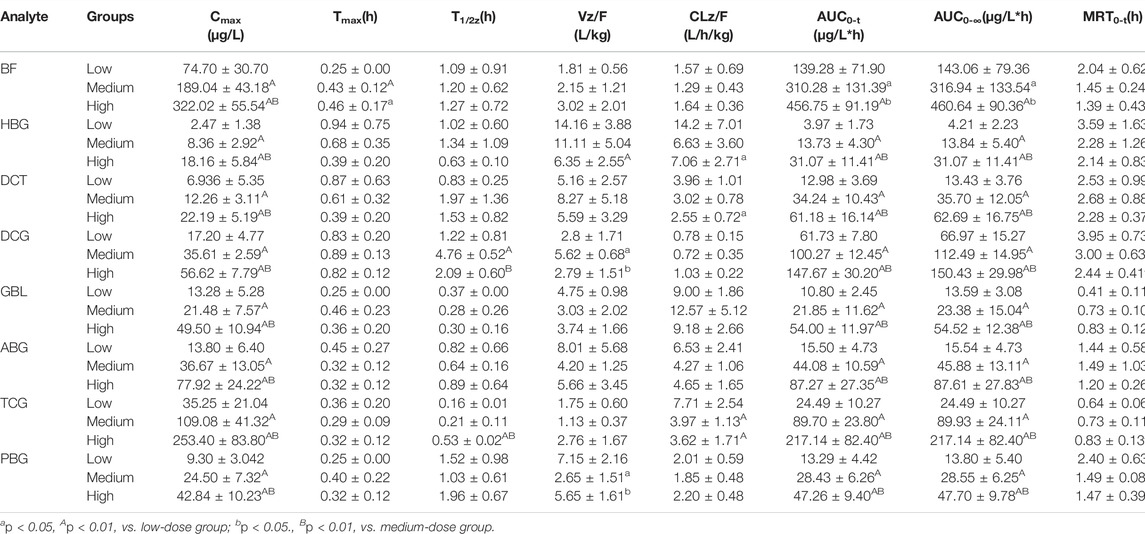
TABLE 5. Pharmacokinetic parameters of the target analytes after the oral administration of cinobufacini capsule (0.9, 1.8, and 3.6 g/kg) and intravenous administration of cinobufacini injection at a dose of 9 ml/kg to male SD rats (mean ± SD, n = 6).
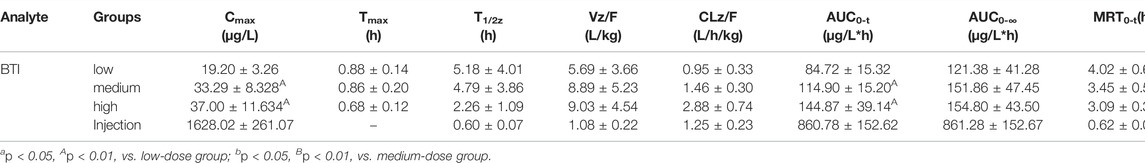
TABLE 6. Pharmacokinetic parameters of bufothionine after the oral administration of cinobufacini capsule (0.9, 1.8, and 3.6 g/kg) and intravenous administration of cinobufacini injection at a dose of 9 ml/kg to male SD rats (mean ± SD, n = 6).
After gavage administration, HBG, DCT, GBL, ABG, TCG, and PBG reached maximum plasma concentrations (Cmax) within 30 min, and BF, BTI, and DCG reached maximum plasma concentrations within 60 min. It suggested that these nine analytes were immediately absorbed and distributed into blood. Moreover, T1/2, MRT0-∞, and MRT0-t of the nine analytes ranged from 0.16 to 4.86 h, indicating that the elimination rates of all analytes were metabolized and eliminated fast in vivo. The T1/2z (h), MRT0-t (h), and CLz/F (μg/L*h) values of the compounds were between 0.16 ± 0.01 and 4.76 ± 0.52, between 0.41 ± 0.11 and 3.95 ± 0.73, and between 0.95 ± 0.33 and 14.16 ± 3.88, respectively. The CLz/F values of GBL and HBG were higher than other compounds, indicating that the elimination rates of most of the analytes in rat plasma were rapid, and the elimination rates of GBL and HBG were the fastest in nine compounds. Cmax, AUC0-t, and AUC0-∞ of nine compounds were dose-dependently increased and showed significant difference among three dosage groups (p < 0.05; p < 0.01). BF had the highest Cmax value and the highest AUC0-t value compared with the other compounds, indicating that BF had a high level exposure in rat plasma. Cmax, AUC0-t, and AUC0-∞ values of TBG, BF, DCG, and ABG were relatively higher than other compounds, suggesting that these four compounds could be the major effective components in cinobufacini capsule.
Furthermore, the comparative pharmacokinetic behaviors of cinobufacini capsule and injection have been compared at the same dose, which indicated that TBG, BF, DCG, and ABG were the principal active compounds in cinobufacini capsule, and only BTI was the major active compound in cinobufacini injection. Meanwhile, BF, ABG, and PBG could be detected in rat plasma after the intravenous administration of cinobufacini injection. But their concentrations in most rat plasma samples were far below the LLOQs so that their pharmacokinetic parameters could not be calculated. The mean plasma concentration–time curve of BTI could be drawn, and its pharmacokinetic parameters could be calculated (Figure 3). The Cmax (1628.02 ± 261.07 ng/ml) and AUC0-t (860.78 ± 152.62 μg/L*h) of BTI in the cinobufacini injection group were higher than those in the cinobufacini capsule group. It could be speculated that BTI is the main component in cinobufacini injection, while bufadienolides including BTI, BF, HBG, DCT, DCG, GBL, ABG, TCG, and PBG were the major components in cinobufacini capsule. Despite a few reports on the main components in Cinobufacini capsules or injection, some previous studies about the pharmacokinetic characteristics of bufadienolides in rats after the administration of a single bufadienolide or the single medicinal material Venenum Bufonis, which is the dry secretion of Bufo bufo gargarizans Cantor, have been reported. Moreover, five bufadienolides, including RBG, BF, ABG, GBL, and BL, after administration of Shexiang Baoxin pill, were also determined in rat plasma, simultaneously (Huang et al., 2015). Interestingly, the pharmacokinetic parameters of BF and ABG including Tmax, t1/2, and MRT were similar in both Huang’s and our studies. Meanwhile, even the pharmacokinetic parameters of BL have been calculated in Huang’s studies, but the concentration of BL is lower than 4.4 ng/ml. In our present study, the concentration of BL in plasma is too low to calculate the pharmacokinetics parameters accurately. CBG, another indicative component in Venenum Bufonis, was not detected in both Huang’s and our studies. However, CBG was detected in Wang’s studies with Cmax at 4.49 ng/ml when the administration dose was 1.11 mg/kg (Wang et al., 2014). In our present study, the administration dose was calculated at 0.36 mg/kg for CBG, which was lower than the dose in Wang’s studies. So CBG could not be detected in the present study. Moreover, it has been reported that CBG could be metabolized into forms of desacetylcinobufagin and 3-epidesacetylcinobufagin, or in other forms through hydroxylation (Toma et al., 1987; He et al., 2012), which can also cause the decrease in CBG concentration in rat plasma. So the metabolism process of bufadienolides in cinobufacini preparations should be studied in further research.
To the best of our knowledge, bufadienolides and indolealkylamines are the two major ingredients of active compounds in cinobufacini, as BF, HBG, DCT, DCG, GBL, ABG, TCG, and PBG belong to bufadienolides, and BTI belongs to indolealkylamines. The different preparation processes between cinobufacini capsule and injection cause a huge difference in their components, especially the preparation process of the boiling water extraction into water-soluble injection, leading to most of the bufadienolides being removed. So the present comparative pharmacokinetic studies inferred that bufadienolides including BF, HBG, DCT, DCG, GBL, ABG, TCG, and PBG were the main bioactive components in cinobufacini capsule, while BTI is the main bioactive component in cinobufacini injection. Moreover, the antitumor effect of BTI was much less than most of bufadienolides, such as CBG and RBG (Xie et al., 2012; Qi et al., 2018). So their antitumor effects have been proven to be widely different from our other studies. So the present research would provide a more valuable information and scientific basis for further discovery of potential pharmacodynamic ingredients, clinical application, and quality control of cinobufacini capsule and injection.
5 Conclusion
A rapid, selective, and sensitive UPLC-MS/MS method was established and validated for the simultaneous determination of BTI, HBG, BF, GBL, TCG, CBG, ABG, CBT, DCT, BL, RBG, PBG, and DCG in rat plasma. It was fully validated via the linearity, precision, extraction recovery, matrix effect, and stability test. The method was successfully applied for the pharmacokinetic study of cinobufacini capsule and injection in rats, which indicated that the pharmacokinetic characteristics of cinobufacini capsules and injection were significantly different for the first time. It indicated that bufadienolides and BTI were the main bioactive components in cinobufacini capsule and injection, respectively, which would give comprehensive information for understanding the difference of pharmacodynamics between cinobufacini capsules and injection. This could facilitate further research on the action mechanism of cinobufacini capsules and injection.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Ethics Statement
The animal study was reviewed and approved by the Animal Committee of Shanghai University of Traditional Chinese Medicine.
Author Contributions
TZ and YD formulated the study concept and design and guided the critical revision of this manuscript. ML performed the research, acquired and analyzed the data, and drafted the manuscript. ZL and JL helped in executing part of research. YQ and YD provide important intellectual content and improved the manuscript. All the authors have reviewed the manuscript and agreed to all the contents and the submission.
Funding
This work was supported by programs of the National Natural Science Foundation of China (grant number 81872981); “Shuguang Program” supported by Shanghai Education Development Foundation and Shanghai Municipal Education Commission (grant numbers 20SG43); Shanghai Sailing Program from the Shanghai Committee of Science and Technology (grant number 19YF1449000); Shanghai Science and technology innovation project (grant number 20S21902500); Program of Shanghai Leading Talents (grant number 2019100); The Innovation activity plan for College Students of SHUTCM (grant number 2020SHUTCM131); Program from the Shanghai Committee of Science and Technology (grant number 21010504200).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.944041/full#supplementary-material
References
Abdelhameed, A. S., Kadi, A. A., Attwa, M. W., and Alrabiah, H. (2019). Validated LC-MS/MS Assay for Quantification of the Newly Approved Tyrosine Kinase Inhibitor, Dacomitinib, and Application to Investigating its Metabolic Stability. PLoS One 14 (4), e0214598. doi:10.1371/journal.pone.0214598
Al-Shakliah, N. S., Attwa, M. W., Kadi, A. A., and Alrabiah, H. (2020). Identification and Characterization of In Silico, In Vivo, In Vitro, and Reactive Metabolites of Infigratinib Using LC-ITMS: Bioactivation Pathway Elucidation and In Silico Toxicity Studies of its Metabolites. RSC Adv. 10 (28), 16231–16244. doi:10.1039/c9ra10871h
Attwa, M. W., Kadi, A. A., Abdelhameed, A. S., and Alhazmi, H. A. (2020). Metabolic Stability Assessment of New PARP Inhibitor Talazoparib Using Validated LC-MS/MS Methodology: In Silico Metabolic Vulnerability and Toxicity Studies. Drug Des. Devel Ther. 14, 783–793. doi:10.2147/DDDT.S239458
Chen, D., Chen, J., Guo, Y., and Li, Y. (2018). Cinobufacini Promotes Apoptosis of Bladder Cancer Cells by Influencing the Expression of Autophagy-Related Genes. Oncol. Lett. 15 (5), 7104–7110. doi:10.3892/ol.2018.8206
Food and Drug Administration (2018). Bioanalytical Method Validation Guidance for Industry. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry (accessed 28 04, 2022).
Fu, F., Wan, Y., MulatiWu, T., and Wu, T. (2014). Kanglaite Injection Combined with Hepatic Arterial Intervention for Unresectable Hepatocellular Carcinoma: a Meta-Analysis. J. Cancer Res. Ther. 10 Suppl 1 (Suppl. 1), 38–41. doi:10.4103/0973-1482.139753
He, X., Hu, H., Wu, Y., and Zeng, X. (2012). Urinary Metabolites of Cinobufagin in Rats and Their Antiproliferative Activities. Nat. Prod. Res. 26 (6), 489–499. doi:10.1080/14786419.2010.510798
Huang, H., Yang, Y., Lv, C., Chang, W., Chang, C., Wang, S., et al. (2015). Pharmacokinetics and Tissue Distribution of Five Bufadienolides from the Shexiang Baoxin Pill Following Oral Administration to Mice. J. Ethnopharmacol. 161, 175–185. doi:10.1016/j.jep.2014.07.056i
Huang, J., Chen, F., Zhong, Z., Tan, H. Y., Wang, N., Liu, Y., et al. (2020). Interpreting the Pharmacological Mechanisms of Huachansu Capsules on Hepatocellular Carcinoma through Combining Network Pharmacology and Experimental Evaluation. Front. Pharmacol. 11, 414. doi:10.3389/fphar.2020.00414
Huang, Z., Wang, Y., Chen, J., Wang, R., and Chen, Q. (2013). Effect of Xiaoaiping Injection on Advanced Hepatocellular Carcinoma in Patients. J. Tradit. Chin. Med. 33 (1), 34–38. doi:10.1016/s0254-6272(13)60097-7
Mansouri, V., Beheshtizadeh, N., Gharibshahian, M., Sabouri, L., Varzandeh, M., and Rezaei, N. (2021). Recent Advances in Regenerative Medicine Strategies for Cancer Treatment. Biomed. Pharmacother. 141, 111875. doi:10.1016/j.biopha.2021.111875
Nakata, M., Mori, S., Kamoshida, Y., Kawaguchi, S., Fujita-Yamaguchi, Y., Gao, B., et al. (2015). Toad Skin Extract Cinobufatini Inhibits Migration of Human Breast Carcinoma MDA-MB-231 Cells into a Model Stromal Tissue. Biosci. Trends. 9 (4), 266–269. doi:10.5582/bst.2015.01109
Peng, W., Xu, Y., Feng, F., Gu, C., Wang, Z., Han, D., et al. (2021). Meta-Analysis of Therapy of Cinobufacini Capsule Adjunct with First-Line Platinum-Based Chemotherapy for the Treatment of Advanced NSCLC. Evidence-Based Complementary Altern. Med. 2021, 1–18. doi:10.1155/2021/5596415
Qi, F., Wang, J., Zhao, L., Cai, P., Tang, W., and Wang, Z. (2018a). Cinobufacini Inhibits Epithelial-Mesenchymal Transition of Human Hepatocellular Carcinoma Cells through C-Met/ERK Signaling Pathway. Biosci. Trends. 12 (3), 291–297. doi:10.5582/bst.2018.01082
Qi, J., Zulfiker, A. H. M., Li, C., Good, D., and Wei, M. Q. (2018b). The Development of Toad Toxins as Potential Therapeutic Agents. Toxins (Basel) 10 (8), 336. doi:10.3390/toxins10080336
Shen, Z., Li, Y., Zhao, C., Wang, F., Zhou, R., and Chen, G. (2018). miR 494 BAG1 axis Is Involved in Cinobufacini induced Cell Proliferation and Apoptosis in Gastric Cancer. Mol. Med. Rep. 17 (5), 7435–7441. doi:10.3892/mmr.2018.8788
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Toma, S., Morishita, S., Kuronuma, K., Mishima, Y., Hirai, Y., and Kawakami, M. (1987). Metabolism and Pharmacokinetics of Cinobufagin. Xenobiotica 17 (10), 1195–1202. doi:10.3109/00498258709167411
Wang, J., Cai, H., Liu, Q., Xia, Y., Xing, L., Zuo, Q., et al. (2020). Cinobufacini Inhibits Colon Cancer Invasion and Metastasis via Suppressing Wnt/β-Catenin Signaling Pathway and EMT. Am. J. Chin. Med. 48 (03), 703–718. doi:10.1142/S0192415X20500354
Wang, S., Peng, C., Jiang, P., Fu, P., Tao, J., Han, L., et al. (2014). Simultaneous Determination of Seven Bufadienolides in Rat Plasma after Oral Administration of Shexiang Baoxin Pill by Liquid Chromatography-Electrospray Ionization-Tandem Mass Spectrometry: Application to a Pharmacokinetic Study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 967, 255–263. doi:10.1016/j.jchromb.2014.07.038
Wu, X., Zhao, H., Wang, H., Gao, B., Yang, J., Si, N., et al. (2012). Simultaneous Determination of Eight Bufadienolides in Cinobufacini Injection by HPLC Coupled with Triple Quadrupole Mass Spectrometry. J. Sep. Sci. 35 (15), 1893–1898. doi:10.1002/jssc.201200036
Xie, R. F., Li, Z. C., Gao, B., Shi, Z. N., and Zhou, X. (2012). Bufothionine, a Possible Effective Component in Cinobufocini Injection for Hepatocellular Carcinoma. J. Ethnopharmacol. 141 (2), 692–700. doi:10.1016/j.jep.2011.12.018
Xu, Y., Han, D., Feng, F. C., Wang, Z. C., Gu, C., Peng, W. P., et al. (2019). Meta-analysis of Cinobufacini Injection Combined with Platinum-Contained First-Line Chemotherapy in Treatment of Non-small Cell Lung Cancer. Zhongguo Zhong Yao Za Zhi 44 (21), 4728–4737. doi:10.19540/j.cnki.cjcmm.20190505.501
Yang, M., Shen, C., Zhu, S. J., Zhang, Y., Jiang, H. L., Bao, Y. D., et al. (2022). Chinese Patent Medicine Aidi Injection for Cancer Care: An Overview of Systematic Reviews and Meta-Analyses. J. Ethnopharmacol. 282, 114656. doi:10.1016/j.jep.2021.114656
Yang, T., Shi, R., Chang, L., Tang, K., Chen, K., Yu, G., et al. (2015). Huachansu Suppresses Human Bladder Cancer Cell Growth through the Fas/Fasl and TNF- alpha/TNFR1 Pathway In Vitro and In Vivo. J. Exp. Clin. Cancer Res. 34 (1), 21. doi:10.1186/s13046-015-0134-9
Zhang, X., Liu, T., Zhang, Y., Liu, F., Li, H., Fang, D., et al. (2019). Elucidation of the Differences in Cinobufotalin's Pharmacokinetics between Normal and Diethylnitrosamine-Injured Rats: The Role of P-Glycoprotein. Front. Pharmacol. 10, 521. doi:10.3389/fphar.2019.00521
Zhao, H., Wu, X., Wang, H., Gao, B., Yang, J., Si, N., et al. (2013). Qualitative and Quantitative Analysis of Cinobufacini Injection Using Rapid Separation Liquid Chromatography Coupled with Quadrupole-Time-Of-Flight Mass Spectrometry and HPLC-Photodiode Array Detection, a Feasible Strategy for the Quality Control of Chinese Medicine Injections. J. Sep. Sci. 36 (3), 492–502. doi:10.1002/jssc.201200762
Keywords: comparative pharmacokinetics, cinobufacini capsule, cinobufacini injection, UPLC-MS/MS, bufalin
Citation: Li M, Qin Y, Li Z, Lan J, Zhang T and Ding Y (2022) Comparative Pharmacokinetics of Cinobufacini Capsule and Injection by UPLC-MS/MS. Front. Pharmacol. 13:944041. doi: 10.3389/fphar.2022.944041
Received: 14 May 2022; Accepted: 17 June 2022;
Published: 18 July 2022.
Edited by:
Tomohiro Nabekura, Aichi Gakuin University, JapanReviewed by:
Huimin Gao, China Academy of Chinese Medical Sciences, ChinaPrakash Katakam, Al Zawiya University, Libya
Mohamed Attwa, King Saud University, Saudi Arabia
Copyright © 2022 Li, Qin, Li, Lan, Zhang and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tong Zhang, emhhbmd0b25nc2h1dGNtQGhvdG1haWwuY29t; Yue Ding, ZGluZ3l1ZS0yMDAxQGhvdG1haWwuY29t
 Ming Li
Ming Li Yanhong Qin1,2
Yanhong Qin1,2 Zhe Li
Zhe Li Tong Zhang
Tong Zhang Yue Ding
Yue Ding