
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 12 August 2022
Sec. Neuropharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.943163
This article is part of the Research Topic Current Status of Natural Products Targeting Alzheimer’s Disease View all 11 articles
Alzheimer’s disease (AD) is a neurological illness that causes severe cognitive impairment. AD patients also experience at least one of the neuropsychiatric symptoms including apathy, depression, and anxiety during the course of their life. Acetylcholine esterase inhibitors are the available treatment options to alleviate cognitive deficits, whereas methylphenidate (MPH), a psychostimulant, is considered for the treatment of apathy in AD patients. Rosmarinus officinalis, a perennial herb, has been potentially known to have antioxidant and anti-inflammatory properties. The present study investigated the potential effects of MPH and R. officinalis in comparison with the standard drug, Donepezil, on cognition, anxiety, and depression in the AlCl3-induced mouse model of AD. The animals were divided into eight groups (n = 8, each). The results revealed that the MPH- and R. officinalis-treated groups significantly improved memory impairment, whereas R. officinalis substantially reduced depression and anxiety as compared with other treatment groups. MPH treatment induced an antidepressant effect and increased anxiety-like behavior. Moreover, the AlCl3 exposure led to the formation of amyloid beta (Aβ) plaques in mice hippocampus; however, none of the tested drugs caused a significant reduction in amyloid burden at the selected doses. The present study suggested the potential of R. officinalis to improve memory as well as neuropsychiatric symptoms in AD. Although R. officinalis improved cognitive abilities, it did not reduce the amyloid plaque burden, which indicates that the memory-enhancing effects of R. officinalis are due to some alternate mechanism that needs to be explored further.
Alzheimer’s disease (AD) is a neurological illness that causes neuronal loss and cognitive impairment. The major pathological characteristic of the disease is the successive accumulation of amyloid beta (Aβ) plaques and neurofibrillary tangles (Scheltens et al., 2021). AD has public health burden because of not just cognitive symptoms but also noncognitive neuropsychiatric symptoms including apathy, agitation, depression, aggression, and anxiety. During the course of the disease, most of the AD patients will have at least one of these symptoms (Heilman and Nadeau, 2022). Apathy affects 70% of people with AD, whereas depression and anxiety are also evident in AD patients (Teixeira et al., 2021). The occurrence of depression and anxiety in AD patients has a significant ramification on the patient’s quality of life, wellness of the caregivers, chances of hospitalization, and mortality rate (Botto et al., 2022).
A number of pharmaceutical treatments for treating apathy in AD have also been investigated. Various randomized clinical trials of cholinesterase inhibitors have shown minor improvements in apathy-associated symptoms (Sepehry et al., 2017). Furthermore, antidepressants are also unable to improve the apathy-related symptoms in AD patients, and some studies reported the negative consequences of these medications (Magierski et al., 2020).
Methylphenidate (MPH) is a potent psychostimulant that has been utilized to enhance cognition and promote wakefulness for a range of disorders (Wenthur 2016). It has become the standard treatment for attention deficit hyperactivity disorder with time, because of its ability to decrease impulsivity and improve cognition and executive control (Shellenberg et al., 2020).
Based on clinical anecdotal reports, MPH is being considered for the treatment of apathy in AD, and preliminary trials have shown promising outcomes (Padala et al., 2018; Mintzer et al., 2021).
Plant-originated natural compounds having pharmacological properties have appeared as a promising treatment alternative for AD in recent years (Uddin et al., 2020; Akter et al., 2021; Fernandes et al., 2022). These natural compounds or phytochemicals are largely categorized into alkaloids, terpenoids, and polyphenols, where the terpenoids and polyphenols are the major groups of plant’s secondary metabolites, targeting several signaling pathways in the biological system (Zhu et al., 2018). The pharmacological properties of these compounds are due to their distinctive structures that enable them to interact with different key enzymes, receptors, antioxidant systems, and signaling cascades including transcription factors as well as cytokines (Safe et al., 2021; Santos et al., 2021; Kamran et al., 2022). For instance, the antioxidant activity of flavonoids, a subgroup of polyphenols, is correlated with the number of phenolic groups (Glevitzky et al., 2019), whereas anti-inflammatory and antidepressant effects are also associated with polyphenols (Hussain et al., 2016; Jiang et al., 2019). Moreover, terpenoids are considered for exhibiting anticholinesterase activity and are a promising source for future AD treatment (Lai Shi Min et al., 2022).
Rosmarinus officinalis (R. officinalis), having the common name rosemary, is a member of the Lamiaceae family and is rich in phenolic and terpenoid compounds (Andrade et al., 2018). It is potentially known to have antioxidant, anti-inflammatory, and antidepressant properties (Guo et al., 2018; Dabaghzadeh et al., 2022).
At present, there is no effective medicine that can cure or stop the deterioration of neurons and manage multiple symptoms of AD at a time, although many new drugs are under clinical trials proposed as neuroprotective treatment (Huang et al., 2020). Cholinesterase inhibitors including Donepezil are the only available treatments for managing cognitive symptoms in AD (Haake et al., 2020). However, they are associated with several adverse side effects (Zhang et al., 2022). A recent study demonstrated the potential anticholinesterase activity of R. officinalis to combat cognitive decline disorders (Kamli et al., 2022). By contrast, our earlier findings also highlighted the potential of R. officinalis to enhance memory and affect synaptic regulation in the Aβ1–42-induced AD mouse model (Mirza et al., 2021). Likewise, another study by our group showed the therapeutic potential of R. officinalis and MPH to improve cognition and regulate inflammation, synaptic gene expression, and hippocampal neuronal density in mouse models of AlCl3-induced neurotoxicity (Khalid et al., 2020).
Based on the promising results of our previous work and the available literature, the current study focused on the potential effects of MPH and R. officinalis on anxiety, depression, and cognition through behavioral analysis in an AlCl3-induced mouse model of AD, to explore a better and comprehensive therapeutic regimen that can manage multiple symptoms including memory loss and psychiatric symptoms. The study highlights the potential effects of R. officinalis and MPH on psychological behaviors suggesting it as a better option to treat neuropsychiatric as well cognitive symptoms associated with AD. However, further studies are needed to explore the molecular mechanisms regulated by R. officinalis to help understand its mode of action.
Aluminum chloride hexahydrate (AlCl3·6H2O, Cat #: AL0770) was procured from Pharmpur® Scharlau, Spain. Donepezil hydrochloride (Donecept) was from ATCO Laboratories, Pakistan, and methylphenidate hydrochloride (Ritalin®) was procured from Novartis specs, Pakistan. All chemicals were obtained from Merck, Germany, unless noted otherwise, and were of molecular biology grade.
Male BALB/c mice (6–8 weeks old) were chosen for the study. Animals were bred and resided in the Laboratory Animal House of Atta-ur-Rahman School of Applied Biosciences (ASAB), National University of Science and Technology (NUST). Mice (n = 64) were housed in standard metal cages under standard conditions of constant temperature (25°C ± 2°C) and a regular light–dark cycle (12–12 h). All experimental protocols were conducted in accordance with the rulings of the Institute of Laboratory Animal Research, Division on Earth and Life Sciences, National Institute of Health, United States (Guide for the Care and Use of Laboratory Animal). Internal Review Board of ASAB, NUST approved this study protocol.
Commonly available dried leaves of R. officinalis were collected during the fall of 2020 from a vendor in the local spice market of Islamabad, Pakistan. Verification of the plant was conducted by an experienced botanist prior to the initiation of the procedure. The specimen was stored in Neurobiology Lab, ASAB, NUST, and also submitted at Pakistan Natural History Museum, Islamabad, Pakistan, with voucher no. 42570. R. officinalis extract was prepared according to the protocol described by Khalid et al. (2020). R. officinalis (500 g) leaves were ground to fine powder form and allowed to pass through 80 mesh sieves. It was followed by taking 10 g of fine powder in a thimble and loading the thimble into Soxhlet extractor with a distillation flask containing 100% ethanol as extraction solvent. The process was run for 24 h before filtrate was collected and concentrated using a rotary evaporator (R200 Rotavapor, Buchii) under the pressure of 68°C to attain a crude extract. The crude extract was incubated at 37°C to remove the remaining solvent. The extract was stored at 4°C until further use.
Animals were divided into eight groups, eight mice each. Animals of groups 1, 2, 3, and 4 were given distilled water and normal feed for 15 days. After 15 days, the animals of groups 2, 3, and 4 were administered with intraperitoneal (i.p.) injections (single injection volume of 100 µl) of 2 mg/kg (i.p.) Donepezil (Madani Neishaboori et al., 2021), 10 mg/kg (i.p.) MPH, and 100 mg/kg (i.p.) R. officinalis extract (Khalid et al., 2020) for 5 days. Aluminum chloride (AlCl3) (300 mg/kg) (Amber et al., 2018; Khalid et al., 2020) was given in drinking water with normal feed for 15 days to groups 5, 6, 7, and 8 (n = 32). After the development of AlCl3-induced AD models, the animals of groups 5, 6, 7, and 8 were switched to normal drinking water and those of groups 5, 6, and 7 were administered with intraperitoneal (i.p.) injections (single injection volume of 100 µl) of 2 mg/kg (i.p.) Donepezil, 10 mg/kg MPH, and 100 mg/kg R officinalis extract, respectively. Behavior tests were conducted in the next 10 days followed by dissection, collection of brain tissues, and histological assessment. The treatment timeline is depicted in Figure 1.
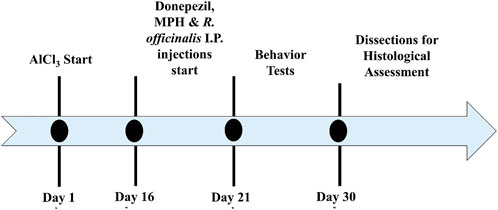
FIGURE 1. Timeline depicting a period for the induction of Alzheimer’s disease (AD), treatment with Donepezil, methylphenidate (MPH), and R. officinalis extract; behavior analysis; and decapitation of animals for histological assessment.
The procedure described by (Bromley-Brits et al., 2011) was used with small modification for the Morris water maze (MWM) test. A circular pool (120 cm × 60 cm), divided equally into four quadrants (east, west, north, and south), was filled with water (21°C ± 2°C). A transparent platform (13 cm × 32 cm) was placed 1 cm below the surface of the water in the north–west quadrant. Five acquisition trials were conducted five times a day for five consecutive days, and before each trial, a minimum of 10 min intertrial interval was kept for each mouse. In each trial, the mice were released in water at one of the four but different quadrant positions with their heads facing the tank. The cut-off time was identified as 90 s, and those who failed to locate the platform in 90 s were manually placed on the platform for 20 s. Those animals who located the platform before 90 s were allowed to sit there for 5 s. The escape latency for all the trials of 5 days was recorded, and the average was calculated. On day 6, the platform was removed and a probe test was conducted. In this test, the mice were allowed to swim in the pool for 90 s and the video was recorded. The animal’s reference memory was monitored by calculating the number of entries, time spent in the target quadrant, and number of crossings made by the subjects over the removed platform position.
A forced swim test was conducted to analyze the depressive behavior of the animals. The protocol of the test was followed as described by (Murad et al., 2014) with few modifications. A transparent container with a diameter of 20 cm and a height of 30 cm was filled with water at 25°C ± 2°C. The depth of the water was adjusted according to the size of the mice, to prevent their hind limbs and tail from touching the bottom of the container. The animals were placed into the water, and their activity was recorded using a camera for 6 min. The water in the container was discarded and refilled for each mouse prior to testing. The time spent immobile, number of immobile episodes, and latency to immobility were calculated for each mouse.
An open field test was conducted to assess the anxiety level and exploratory behavior of the animals. Protocol for the test was followed as described by (Farhat et al., 2017) with slight modifications. A square-shaped arena (40 × 40 × 40) was used, which was divided into center and periphery by drawing a boundary. Each mouse was placed in the center of a wooden box and was allowed to explore the box for 30 min. The behavior of the animals was monitored using a camera and was assessed by calculating the time spent in the center and periphery of the box.
Data were analyzed using GraphPad Prism 8.0.2 by applying a one-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison test. Two-way ANOVA was applied for analyzing escape latency in the MWM test. The data observed are shown as mean ± SEM having a 95% confidence interval, which is considered statistically significant when p < 0.05.
Heart perfusion was conducted to excise the whole brain according to the procedure described by Gage et al. (2012). Tissue sections (5 µm) of the hippocampus were deparaffinized with xylene, rehydrated, and washed with 70% isopropanol and double-distilled water (ddH2O) respectively. The Congo red stain (Cat #C6767, Merck Germany; working solution: 49.5 ml Congo red (Stock) and 0.5 ml 1% NaOH) was poured on the deparaffinized brain sections and retained for 20 min. ddH2O and alkaline alcohol were used to wash sections for 2 min. The sections were then counterstained by hematoxylin for 30 s and further washed with 70% isopropanol for 6 min and then with ddH2O. After air-drying (1 h), the slides were mounted by coverslips and later visualized using B-150, OPTIKA microscope (Italy) at 40×, 10×, and 4× resolution. The images were captured using Optika Vision Lite 2.1 image analysis software.
The MWM test was used to assess the outcomes of MPH and R. officinalis on spatial learning and memory in comparison with Donepezil. Average escape latency to find out the platform directly indicates the effects of the drugs on spatial memory. The AlCl3-treated group showed poor memory retention than the control group by demonstrating an escape latency of 63 s on day 5. A significant improvement (p < 0.01) in spatial memory was observed in the AlCl3 + R. officinalis-treated group. However, better (p < 0.05) restoration of spatial memory was seen in the AlCl3 + MPH- and AlCl3 + Donepezil-treated groups. Escape latency for 5 days is represented graphically in Figure 2A.
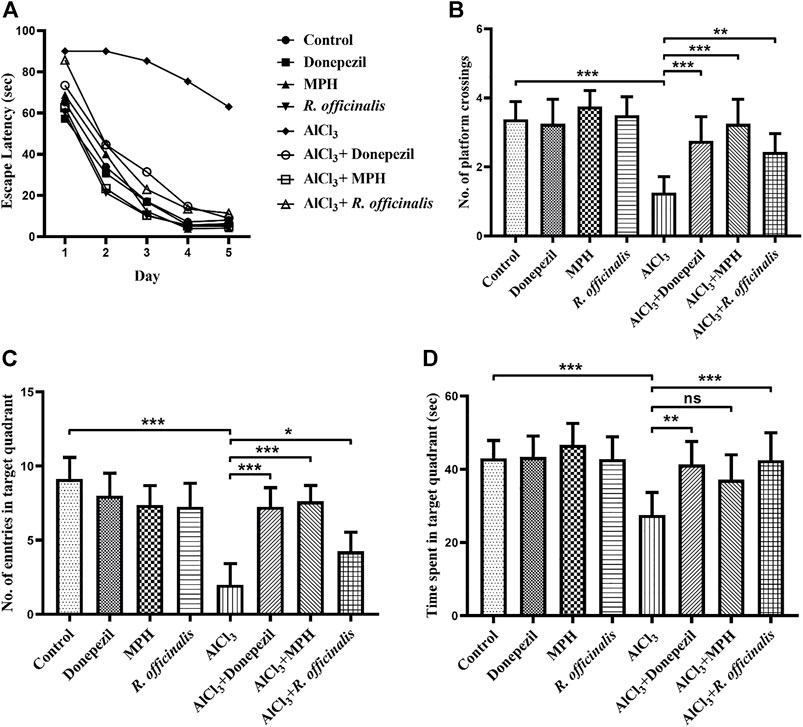
FIGURE 2. Effects of Donepezil, MPH, and R. officinalis on spatial memory in AlCl3-induced mice models using the Morris water maze test. (A) Graph demonstrates escape latency (sec) of the experimental groups, (B) number of platform crossings by the study groups, (C) number of entries in the target quadrant, and (D) time (sec) spent in the target quadrant during the probe trial. One-way ANOVA followed by the Bonferroni comparison test (mean ± SEM) was applied to analyze the data using GraphPad Prism. n = 8. ***p < 0.001, **p < 0.01, *p < 0.05.
A probe trial was conducted for the assessment of reference memory and exploratory behavior of mice for the previously placed invisible platform in the target quadrant. The AlCl3-treated group reflected a significant decrease (p < 0.0001) in reference memory by making a smaller number of crossings over the platform position as compared with the control group. A number of platform crossings were significantly increased in the AlCl3 + R. officinalis-treated group as compared with the AlCl3-treated group; however, the AlCl3 + Donepezil- and AlCl3 + MPH-treated groups made a greater number of crossings than the AlCl3 + R. officinalis-treated group (Figure 2B). A similar trend was seen in the number of entries where the AlCl3-treated group showed the least number of entries in the target quadrant than the control and all the other experimental groups. Again, the AlCl3 + Donepezil- and AlCl3 + MPH-treated groups showed a greater number of entries than the AlCl3 + R. officinalis-treated group (Figure 2C). Likewise, a significant decrease (p < 0.001) in time spent in the target quadrant was observed in the AlCl3-treated group compared with the control group and a significant improvement was observed after treatment with R. officinalis and Donepezil in the AlCl3 + R. officinalis- and AlCl3 + Donepezil-treated groups (Figure 2D). Overall, our results depicted in Figures 2A–D demonstrate that MPH and R. officinalis significantly improved cognition and alleviate the cognitive deficits induced by AlCl3.
The forced swim test was conducted to assess the antidepressant effect of MPH and R. officinalis on an AlCl3-induced AD mice model having impaired cognitive functions. The AlCl3-treated group exhibited depressive behavior by showing the least latency to immobility as compared with the control and other experimental groups. The AlCl3 + MPH-treated group spent significantly more time struggling and hence delayed latency to immobility in comparison with the AlCl3-treated group demonstrating that treatment aided in overcoming the effect of AlCl3 on depression. However, AlCl3 + Donepezil and AlCl3 + R. officinalis comparatively delayed more (p < 0.001) latency to immobility than AlCl3 + MPH (Figure 3A). Likewise, the AlCl3-treated group displayed the highest number of immobile episodes as compared with all other experimental groups, and after treatment, a significant decrease in the number of immobile episodes was observed in the AlCl3 + Donepezil- and AlCl3 + R. officinalis-treated groups with AlCl3 + MPH having the least number of immobile episodes as compared with the other treatment groups (Figure 3B). The AlCl3-treated group also spent the greatest amount of time being immobile, which was significantly decreased via drug treatment in the AlCl3 + Donepezil- and AlCl3 + MPH-treated groups, respectively. A more significant decrease (p < 0.05) in time spent being immobile was observed after treatment with R. officinalis (Figure 3C). The overall findings depicted in Figures 3A–C show that MPH and R. officinalis significantly induce antidepressant effects and reduce depression-like behavior induced by AlCl3.
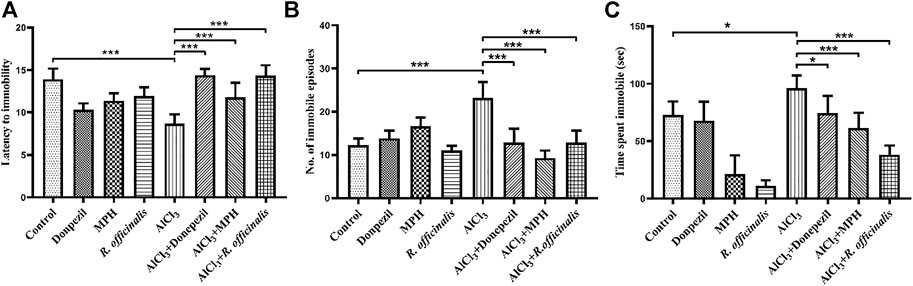
FIGURE 3. Effects of Donepezil, MPH, and R. officinalis on depression-like behavior in the forced swim test. (A) Graph depicting latency to immobility, (B) number of immobile episodes, and (C) time spent immobile. One-way ANOVA followed by the Bonferroni comparison test (mean ± SEM) was applied to analyze the data using GraphPad Prism. n = 8. ***p < 0.001, *p < 0.05, ns nonsignificant.
An open field test was conducted to analyze the anxiety-like behavior in animal models. Animals that tend to spend more time in the center are considered less anxious. The AlCl3-treated group showed more anxiety-like behavior by spending significantly (p < 0.001) lesser time in the center and more time in the periphery as compared with the control group (Figures 4A, B). The AlCl3 + Donepezil- and AlCl3 + R. officinalis-treated groups improved their performance by spending more time in the center as compared with the AlCl3-treated group, demonstrating the antianxiety potential of Donepezil and R. officinalis, whereas the AlCl3 + MPH-treated group spent the least amount of time in the center of the box (Figure 4A). Likewise, treatment with Donepezil and R. officinalis significantly decreased the time spent in the periphery in the AlCl3 + Donepezil- and AlCl3 + R. officinalis-treated groups, respectively, in comparison with the AlCl3-treated group (Figure 4B). However, the AlCl3 + R. officinalis-treated group showed more significant improvement than the AlCl3 + Donepezil-treated group. It is of interest that the most anxious behavior was observed after treatment with MPH (Figures 4A, B). These results indicate the potential of R. officinalis to induce an anxiolytic effect.
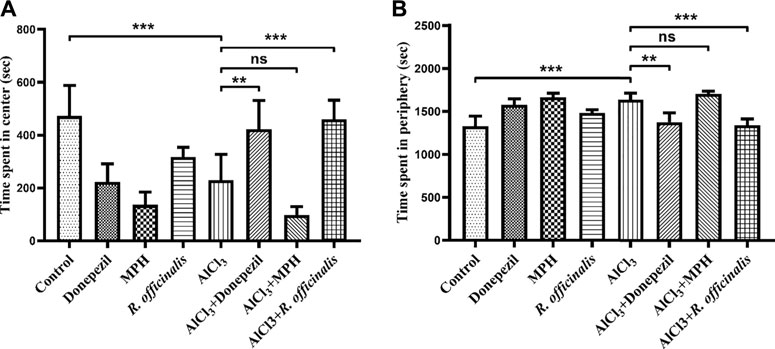
FIGURE 4. Effects of Donepezil, MPH, and R. officinalis on anxiety and exploratory behavior in the open field test. (A) Graph depicting time spent in the center and (B) time spent in the periphery of the open field box. One-way ANOVA followed by the Bonferroni comparison test (mean ± SEM) was applied to analyze the data using GraphPad Prism. n = 8. ***p < 0.001, **p < 0.01.
Congo red staining of the hippocampus showed the presence of Aβ plaques in the AlCl3-treated groups compared with the control group. An almost similar number of plaques are seen in all AlCl3 plus drug-treated groups and the AlCl3-treated group. None of the tested groups showed a significant decrease in Aβ plaque burden (Figure 5). Therefore, it is likely that the selected doses of MPH and R. officinalis are not much effective to reduce the amyloid burden.

FIGURE 5. Histological assessment of hippocampal tissues sections stained with Congo Red. (I) 4×, (II) 10×, (III) 40×. (a) Control, (b) Donepezil-treated, (c) MPH-treated, (d) R. officinalis-treated, (e) AlCl3-treated, (f) AlCl3 + Donepezil-treated, (g) AlCl3 + MPH-treated (h) AlCl3 + R. officinalis-treated.
The current study investigated the effects of MPH and R. officinalis on various parameters including memory and learning, anxiety, and depression in the AlCl3-induced AD mouse model.
Aluminum (Al) is the most abundant element in earth’s crust and is in human use for centuries. Its widespread use and exposure originate from many sources including vaccine adjuvants, processed foods, cosmetics, medical treatments, cooking wares, and pharmaceuticals (Tietz et al., 2019). Chronic exposure to Al develops cellular toxicity and accumulation in various organs including the central nervous system. It binds to plasma transferrin and citrate molecules in the body and is transferred to the brain. The Al accumulation in the brain generates misfolded proteins and facilitates hyperphosphorylation and aggregation (Colomina and Peris-Samperdo, 2017; Niu 2018). AlCl3 is commonly used to develop aluminum-induced animal models for AD, where it is suggested to cause shrinking of hippocampus pyramidal cells (Saeed et al., 2021), neural cell death, and cognitive deficits similar to AD (Zhang 2018).
MPH and other psychostimulants are often used as a therapy to enhance cognitive functions, reduce impulsivity, and induce wakefulness (Carlier et al., 2019). The majority of the studies emphasized that MPH improves cognitive efficiency and influences working memory, inhibitory control, and mental flexibility (Bolfer et al., 2017). Our results also showed a significant effect of MPH in improving spatial learning and memory. We compared the effects of MPH and R. officinalis with Donepezil on the AlCl3-induced AD mouse model and found significant effects of MPH on spatial memory and reference memory in terms of the number of platform crossings and the number of entries in the target quadrant in the MWM test. R. officinalis also exhibit significant improvement in spatial memory as compared with the AlCl3-treated group and demonstrated more significant effects in reference memory especially in comparison with Donepezil in terms of time spent in the target quadrant.
Depression is one of the most prevalent behavioral symptoms of AD (Yang et al., 2020). It is of interest that Donepezil showed antidepressant effects in the mouse forced swim test and chronic mild stress model in rats (Papp et al., 2016; Fitzgerald et al., 2020). However, MPH has also revealed significant improvement in depression symptoms in patients with Asperger syndrome (Golubchik et al., 2017). Our study compared the effects of R. officinalis with both Donepezil and MPH on depression-like behavior in the AlCl3-induced AD model. Comparison of our forced swim results showed that all the tested compounds significantly improved depression-like symptoms in the AlCl3-induced AD model; however, R. officinalis exhibited significantly more antidepressant potential as compared with MPH and Donepezil by delaying more latency to immobility and spending more time being immobile. This can be credited to the antidepressant-like activity of one of the active compounds of R. officinalis, i.e., carnosic acid. Recent evidence reveals carnosic acid as a substantial modulator of the ADPN-FGF9 pathway via activation of PPAR-γ in adipocytes, a recently established factor in the development of depression (Wang X. Q. et al., 2021). Likewise, rosmarinic acid has shown antidepressant effects mediated through its increased antioxidant response (Wang J. et al., 2021) exhibiting neuroprotective effects via activation of GABAA receptors (Wang C. C. et al., 2021). In addition, Lataliza et al. suggested the substantial involvement of cannabinoid receptors/PPAR-γ signaling pathways in exerting the antidepressant-like effect of rosmarinic acid (Lataliza et al., 2021).
Moreover, it has been demonstrated that 60–90% of AD patients develop neuropsychiatric symptoms including anxiety disorder that could be treated with acetylcholinesterase inhibitors (AChEIs) (Cummings et al., 2016; Botto et al., 2022). AChEIs have varying effects on anxiety. Besides several disease-modifying effects of various new derivatives of AChEIs, they also possess promising effects on anxiety-like behavior that can be helpful for disease management (Giménez-Llort et al., 2017). Therefore, the current study also assessed the comparative anxiolytic potential of MPH, R. officinalis, and Donepezil in the AlCl3-induced AD model. Our results showed that treatment with Donepezil and R. officinalis significantly improved anxiety-like behavior in the AlCl3-treated AD model by increasing the time spent in the center arena of the open field box. A comparison of the results showed that R. officinalis displayed the strongest antianxiety potential. R. officinalis reduces the extent of anxiety; however, a substantial decrease in neural activity was also observed in a study by Choukairi et al., which can be attributed to the potential of its specific active compounds exerting their effects through modulation of certain neurotransmitter receptors (Choukairi et al., 2019). For instance, two major compounds of R. officinalis, i.e., rosmarinic acid and ursolic acid, exhibit anxiolytic and antidepressant-like effects observed in animal studies (Colla et al., 2015; Mirza et al., 2021).
Besides anxiolytic and antidepressant effects of R. officinalis, various other therapeutic properties were also documented by various research groups. Some of the recent significant findings are highlighted in Table 1, which represent the potential of R. officinalis as antinociceptive, anti-inflammatory, antiapoptotic, anticancer etc. (Table 1).
Of note, bioactive compounds of R. officinalis have limited ability to cross the blood–brain barrier; therefore, different delivery approaches were experimented with to enhance delivery through the blood–brain barrier. Kuo et al. demonstrated apolipoprotein E-modified liposomes conjugated with phosphatidic acid as a carrier for rosmarinic acid and quercetin to infiltrate the BBB to block Aβ1–42-induced AD (Kuo et al., 2018). Likewise, attachment of specific ligand, targeted delivery by nanoparticles, and nanoemulsion through intranasal administration were used to rescue neurodegeneration (Fachel et al., 2020; Kuo et al., 2020; Long et al., 2020).
It is of interest that MPH exhibited increased anxiety-like behavior in the open field test in the current study. The effects of MPH on anxiety are still not clear. Some studies have shown that MPH either decreases (Jager et al., 2019) or increases anxiety (Zoratto et al., 2019). Different effects of MPH on anxiety have been debated, and such variations indicate the multidimensional and complex nature of emotional behaviors. There is a possibility that the procedures applied to assess anxiety only measure a specific idiosyncratic domain of the tested emotion. For instance, as reported by Boyette-Davis et al., the antianxiety effects of MPH are not affected based on the changes in locomotor behavior (Boyette-Davis et al., 2018). Therefore, various experimental approaches would be needed to completely assess the effects of MPH on anxiety. By contrast, acute administration of MPH has also been found to exhibit anxiolytic effects in the open field test, where MPH was administered 20 min prior to testing (Jager et al., 2019). Differences in dosage and treatment duration could be another possible reason for varying effects on anxiety (Zoratto et al., 2019). Although the physiological and behavioral effects of MPH are generally reversible after intermittent MPH chronic exposure, it is followed by prolonged abstinence (Kalinowski et al., 2020). However, withdrawal effects could also be the possible reason for high anxiety in the MPH-treated group in the present study.
A histopathological assessment revealed the formation of amyloid plaques in the AlCl3-treated groups showing the development of AD pathology. Our results revealed that all the tested compounds failed to reduce the Aβ plaques burden. Although carnosic acid significantly improves cholinergic dysfunction and mitochondrial defects by reducing the Aβ-mediated toxicity (Chen et al., 2022), in our case, no substantial effect was observed by any of the tested drugs or R. officinalis. Perhaps the selected doses of our study are not much effective to reduce the Aβ plaques with a shorter treatment duration, which could be the possible reason for not being effective in reducing the amyloid burden.
These findings provide a preliminary data set on the therapeutic potential of R. officinalis possessing substantial anxiolytic and antidepressant activities. MPH and R. officinalis have significant effects on cognition with MPH being more effective than R. officinalis in restoring spatial memory. Nevertheless, MPH increases the anxiety-like behavior, which needs a further understanding of the complex molecular mechanisms involved in its mode of action.
Memory loss along with neuropsychiatric symptoms in AD leads to the requirement of a drug that can enhance memory and reduce behavioral despair. Donepezil has failed to stop the disease progression and is associated with various side effects, and MPH can aggravate psychological symptoms. Therefore, R. officinalis, a natural plant extract, could be more suitable to treat cognitive decline as well as psychological symptoms with minimal side effects. Further investigation of the mode of action of R. officinalis and the role of its various active compounds is warranted to indicate its therapeutic potential for AD.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by Internal Review Board ASAB-NUST.
SZ, substantial contribution to conception and design of the study and finalization of the manuscript; SA, assistance in intraperitoneal injections, animal handling, behavioral analysis, and editing and formatting the manuscript; NM, all experimental work, data analysis, interpretation, and drafting the article.
This research was supported through an MS student’s research grant by the National University of Sciences and Technology (NUST), Islamabad, Pakistan awarded to NM. The work was partially funded through research grant number 5974 awarded to SZ under National Research Grants Program for Universities-HEC, Pakistan. The funding sources have no involvement in study design, collection, analysis and interpretation of data, writing of the report, and decision to submit the article for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Achour, M., Ben Salem, I., Ferdousi, F., Nouira, M., Ben Fredj, M., Mtiraoui, A., et al. (2022). Rosemary Tea Consumption Alters Peripheral Anxiety and Depression Biomarkers: a Pilot Study in Limited Healthy Volunteers. J. Am. Nutr. Assoc. 41 (3), 240–249. doi:10.1080/07315724.2021.1873871
Akter, R., Rahman, H., Behl, T., Chowdhury, M. A. R., Manirujjaman, M., Bulbul, I. J., et al. (2021). Prospective Role of Polyphenolic Compounds in the Treatment of Neurodegenerative Diseases. CNS Neurol. Disord. Drug Targets. 20 (5), 430–450. doi:10.2174/1871527320666210218084444
Amaral, G. P., Dobrachinski, F., de Carvalho, N. R., Barcelos, R. P., da Silva, M. H., Lugokenski, T. H., et al. (2018). Multiple Mechanistic Action of Rosmarinus Officinalis L. Extract against Ethanol Effects in an Acute Model of Intestinal Damage. Biomed. Pharmacother. 98, 454–459. doi:10.1016/j.biopha.2017.12.091
Andrade, J. M., Faustino, C., Garcia, C., Ladeiras, D., Reis, C. P., and Rijo, P. (2018). Rosmarinus Officinalis L.: an Update Review of its Phytochemistry and Biological Activity. Future Sci. OA. 4 (4), FSO283. doi:10.4155/fsoa-2017-0124
Araki, R., Sasaki, K., Onda, H., Nakamura, S., Kassai, M., Kaneko, T., et al. (2020). Effects of Continuous Intake of Rosemary Extracts on Mental Health in Working Generation Healthy Japanese Men: Post-hoc Testing of a Randomized Controlled Trial. Nutrients 12 (11), 3551. doi:10.3390/nu12113551
Bolfer, C., Pacheco, S. P., Tsunemi, M. H., Carreira, W. S., Casella, B. B., and Casella, E. B. (2017). Attention-deficit/hyperactivity Disorder: the Impact of Methylphenidate on Working Memory, Inhibition Capacity and Mental Flexibility. Arq. Neuropsiquiatr. 75 (4), 204–208. doi:10.1590/0004-282X20170030
Borges, R. S., Lima, E. S., Keita, H., Ferreira, I. M., Fernandes, C. P., Cruz, R. A. S., et al. (2018). Anti-inflammatory and Antialgic Actions of a Nanoemulsion of Rosmarinus Officinalis L. Essential Oil and a Molecular Docking Study of its Major Chemical Constituents. Inflammopharmacology 26 (1), 183–195. doi:10.1007/s10787-017-0374-8
Botto, R., Callai, N., Cermelli, A., Causarano, L., and Rainero, I. (2022). Anxiety and Depression in Alzheimer's Disease: a Systematic Review of Pathogenetic Mechanisms and Relation to Cognitive Decline. Neurol. Sci. 43, 4107–4124. doi:10.1007/s10072-022-06068-x
Boyette-Davis, J. A., Rice, H. R., Shoubaki, R. I., Gonzalez, C. M. F., Kunkel, M. N., Lucero, D. A., et al. (2018). A Recreational Dose of Methylphenidate, but Not Methamphetamine, Decreases Anxiety-like Behavior in Female Rats. Neurosci. Lett. 682, 21–26. doi:10.1016/j.neulet.2018.06.005
Bromley-Brits, K., Deng, Y., and Song, W. (2011). Morris Water Maze Test for Learning and Memory Deficits in Alzheimer's Disease Model Mice. J. Vis. Exp. 53, e2920. doi:10.3791/2920
Carlier, J., Giorgetti, R., Varì, M. R., Pirani, F., Ricci, G., and Busardò, F. P. (2019). Use of Cognitive Enhancers: Methylphenidate and Analogs. Eur. Rev. Med. Pharmacol. Sci. 23 (1), 3–15. doi:10.26355/eurrev_201901_16741
Chen, Y., Wang, Y., Qin, Q., Zhang, Y., Xie, L., Xiao, J., et al. (2022). Carnosic Acid Ameliorated Aβ-Mediated (Amyloid-β Peptide) Toxicity, Cholinergic Dysfunction and Mitochondrial Defect in Caenorhabditis elegans of Alzheimer's Model. Food Funct. 13 (8), 4624–4640. doi:10.1039/d1fo02965g
Choukairi, Z., Hazzaz, T., Lkhider, M., Ferrandez, J. M., and Fechtali, T. (2019). Effect of Salvia Officinalis L. and Rosmarinus Officinalis L. leaves extracts on anxiety and neural activity. Bioinformation 15 (3), 172–178. doi:10.6026/97320630015172
Colla, A. R., Rosa, J. M., Cunha, M. P., and Rodrigues, A. L. (2015). Anxiolytic-like effects of ursolic acid in mice. Eur. J. Pharmacol. 758, 171–176. doi:10.1016/j.ejphar.2015.03.077
Colomina, M. T., and Peris-Sampedro, F. (2017). Aluminum and Alzheimer's disease. Adv. Neurobiol. 18, 183–197. doi:10.1007/978-3-319-60189-2_9
Cummings, J., Lai, T. J., Hemrungrojn, S., Mohandas, E., Yun Kim, S., Nair, G., et al. (2016). Role of donepezil in the management of neuropsychiatric symptoms in Alzheimer's disease and dementia with lewy bodies. CNS Neurosci. Ther. 22 (3), 159–166. doi:10.1111/cns.12484
Dabaghzadeh, F., Mehrabani, M., Abdollahi, H., and Karami-Mohajeri, S. (2022). Antioxidant and anticholinesterase effects of rosemary (Salvia rosmarinus) extract: A double-blind randomized controlled trial. Adv. Integr. Med. 9 (1), 69–74. doi:10.1016/j.aimed.2021.03.002
de Almeida Gonçalves, G., de Sá-Nakanishi, A. B., Comar, J. F., Bracht, L., Dias, M. I., Barros, L., et al. (2018). Water soluble compounds of Rosmarinus officinalis L. improve the oxidative and inflammatory states of rats with adjuvant-induced arthritis. Food Funct. 9 (4), 2328–2340. doi:10.1039/c7fo01928a
El-Demerdash, F. M., El-Sayed, R. A., and Abdel-Daim, M. M. (2021). Rosmarinus officinalis essential oil modulates renal toxicity and oxidative stress induced by potassium dichromate in rats. J. Trace. Elem. Med. Biol. 67, 126791. doi:10.1016/j.jtemb.2021.126791
Elbahnasawy, A. S., Valeeva, E. R., El-Sayed, E. M., and Rakhimov, I. I. (2019). The impact of thyme and rosemary on prevention of osteoporosis in rats. J. Nutr. Metabolism 2019, 1431384. doi:10.1155/2019/1431384
Fachel, F. N. S., Michels, L. R., Azambuja, J. H., Lenz, G. S., Gelsleichter, N. E., Endres, M., et al. (2020). Chitosan-coated rosmarinic acid nanoemulsion nasal administration protects against LPS-induced memory deficit, neuroinflammation, and oxidative stress in Wistar rats. Neurochem. Int. 141, 104875. doi:10.1016/j.neuint.2020.104875
Farhat, S. M., Mahboob, A., and Ahmed, T. (2017). Cortex- and Amygdala-Dependent Learning and Nicotinic Acetylcholine Receptor Gene Expression is Severely Impaired in Mice Orally Treated with AlCl3. Biol. Trace Elem. Res. 179 (1), 91–101. doi:10.1007/s12011-017-0942-1
Fernandes, F., Barroso, M. F., De Simone, A., Emriková, E., Dias-Teixeira, M., Pereira, J. P., et al. (2022). Multi-target neuroprotective effects of herbal medicines for Alzheimer's disease. J. Ethnopharmacol. 290, 115107. doi:10.1016/j.jep.2022.115107
Fitzgerald, P. J., Hale, P. J., Ghimire, A., and Watson, B. O. (2020). The cholinesterase inhibitor donepezil has antidepressant-like properties in the mouse forced swim test. Transl. Psychiatry. 10 (1), 255–313. doi:10.1038/s41398-020-00928-w
Gage, G. J., Kipke, D. R., and Shain, W. (2012). Whole animal perfusion fixation for rodents. JoVE (65), e3564.
Ghasemzadeh Rahbardar, M., Amin, B., Mehri, S., Mirnajafi-Zadeh, S. J., and Hosseinzadeh, H. (2017). Anti-inflammatory effects of ethanolic extract of Rosmarinus officinalis L. and rosmarinic acid in a rat model of neuropathic pain. Biomed Pharmacother. 86, 441–449. doi:10.1016/j.biopha.2016.12.049
Giménez-Llort, L., Ratia, M., Pérez, B., Camps, P., Muñoz-Torrero, D., Badia, A., et al. (2017). Behavioural effects of novel multitarget anticholinesterasic derivatives in Alzheimer's disease. Behav. Pharmacol. 28, 124–131. doi:10.1097/FBP.0000000000000292
Glevitzky, I., Dumitrel, G. A., Glevitzky, M., Pasca, B., Otrisal, P., Bungau, S., et al. (2019). Statistical analysis of the relationship between antioxidant activity and the structure of flavonoid compounds. Rev. Chim. 70 (9), 3103–3107. doi:10.37358/RC.19.9.7497
Golubchik, P., Rapaport, M., and Weizman, A. (2017). The effect of methylphenidate on anxiety and depression symptoms in patients with Asperger syndrome and comorbid attention deficit/hyperactivity disorder. Int. Clin. Psychopharmacol. 32 (5), 289–293. doi:10.1097/YIC.0000000000000175
Guo, Y., Xie, J., Li, X., Yuan, Y., Zhang, L., Hu, W., et al. (2018). Antidepressant effects of rosemary extracts associate with anti-inflammatory effect and rebalance of gut microbiota. Front. Pharmacol. 9, 1126. doi:10.3389/fphar.2018.01126
Haake, A., Nguyen, K., Friedman, L., Chakkamparambil, B., and Grossberg, G. T. (2020). An update on the utility and safety of cholinesterase inhibitors for the treatment of Alzheimer's disease. Expert Opin. Drug Saf. 19 (2), 147–157. doi:10.1080/14740338.2020.1721456
Hamed, H., Boulila, S., Ghrab, F., Kallel, R., Boudawara, T., and El Feki, A. (2020). The preventive effect of aqueous extract of Rosemary (Rosmarinus officinalis) leaves against the nephrotoxicity of carbon tetrachloride in mice. Arch. Physiol. Biochem. 126 (3), 201–208. doi:10.1080/13813455.2018.1508236
Heilman, K. M., and Nadeau, S. E. (2022). Emotional and neuropsychiatric disorders associated with alzheimer’s disease. Neurotherapeutics. 19 (1), 99–116. doi:10.1007/s13311-021-01172-w
Huang, L. K., Chao, S. P., and Hu, C. J. (2020). Clinical trials of new drugs for Alzheimer disease. J. Biomed. Sci. 27 (1), 18. doi:10.1186/s12929-019-0609-7
Hussain, T., Tan, B., Yin, Y., Blachier, F., Tossou, M. C., and Rahu, N. (2016). Oxidative stress and inflammation: what polyphenols can Do for us? Oxid. Med. Cell. Longev. 2016, 7432797. doi:10.1155/2016/7432797
Jager, A., Kanters, D., Geers, F., Buitelaar, J. K., Kozicz, T., and Glennon, J. C. (2019). Methylphenidate dose-dependently affects aggression and improves fear extinction and anxiety in BALB/cJ mice. Front. Psychiatry 10, 768. doi:10.3389/fpsyt.2019.00768
Jiang, C., Sakakibara, E., Lin, W. J., Wang, J., Pasinetti, G. M., and Salton, S. R. (2019). Grape-derived polyphenols produce antidepressant effects via VGF- and BDNF-dependent mechanisms. Ann. N. Y. Acad. Sci. 1455 (1), 196–205. doi:10.1111/nyas.14098
Kalinowski, L., Connor, C., Somanesan, R., Carias, E., Richer, K., Smith, L., et al. (2020). Brief and extended abstinence from chronic oral methylphenidate treatment produces reversible behavioral and physiological effects. Dev. Psychobiol. 62 (2), 170–180. doi:10.1002/dev.21902
Kamli, M. R., Sharaf, A. A. M., Sabir, J. S. M., and Rather, I. A. (2022). Phytochemical Screening of Rosmarinus officinalis L. as a Potential Anticholinesterase and Antioxidant-Medicinal Plant for Cognitive Decline Disorders. Plants (Basel) 11 (4), 514. doi:10.3390/plants11040514
Kamran, S., Sinniah, A., Abdulghani, M. A. M., and Alshawsh, M. A. (2022). Therapeutic potential of certain terpenoids as anticancer agents: a scoping review. Cancers (Basel) 14 (5), 1100. doi:10.3390/cancers14051100
Khalid, A., Abbasi, U. A., Amber, S., Sumera, F. J., Mirza, F. J., Asif, M., et al. (2020). Methylphenidate and Rosmarinus officinalis improves cognition and regulates inflammation and synaptic gene expression in AlCl3-induced neurotoxicity mouse model. Mol. Biol. Rep. 47 (10), 7861–7870. doi:10.1007/s11033-020-05864-y
Khezri, K., Farahpour, M. R., and Mounesi Rad, S. (2019). Accelerated infected wound healing by topical application of encapsulated Rosemary essential oil into nanostructured lipid carriers. Artif. Cells Nanomed. Biotechnol. 47 (1), 980–988. doi:10.1080/21691401.2019.1582539
Kuo, Y.-C., Chen, I.-Y., and Rajesh, R. (2018). Use of functionalized liposomes loaded with antioxidants to permeate the blood-brain barrier and inhibit β-amyloid-induced neurodegeneration in the brain. J. Taiwan Inst. Chem. Eng. 87, 1–14. doi:10.1016/j.jtice.2018.03.001
Kuo, Y. C., Lou, Y. I., and Rajesh, R. (2020). Dual functional liposomes carrying antioxidants against tau hyperphosphorylation and apoptosis of neurons. J. Drug Target 28 (9), 949–960. doi:10.1080/1061186X.2020.1761819
Lai Shi Min, S., Liew, S. Y., Chear, N. J. Y., Goh, B. H., Tan, W. N., and Khaw, K. Y. (2022). Plant terpenoids as the promising source of cholinesterase inhibitors for anti-AD therapy. Biol. (Basel) 11 (2), 307. doi:10.3390/biology11020307
Lataliza, A. A. B., de Assis, P. M., da Rocha Laurindo, L., Gonçalves, E. C. D., Raposo, N. R. B., and Dutra, R. C. (2021). Antidepressant-like effect of rosmarinic acid during LPS-induced neuroinflammatory model: The potential role of cannabinoid receptors/PPAR-γ signaling pathway. Phytother. Res. 35 (12), 6974–6989. doi:10.1002/ptr.7318
Long, Y., Yang, Q., Xiang, Y., Zhang, Y., Wan, J., Liu, S., et al. (2020). Nose to brain drug delivery - A promising strategy for active components from herbal medicine for treating cerebral ischemia reperfusion. Pharmacol. Res. 159, 104795. doi:10.1016/j.phrs.2020.104795
Madani Neishaboori, A., Nasseri Maleki, S., Saberi Pirouz, M., Golmohammadi, S., Nazarinia, D., and Aboutaleb, N. (2021). Donepezil attenuates injury following ischaemic stroke by stimulation of neurogenesis, angiogenesis, and inhibition of inflammation and apoptosis. Inflammopharmacology 29 (1), 153–166. doi:10.1007/s10787-020-00769-5
Magierski, R., Sobow, T., Schwertner, E., and Religa, D. (2020). Pharmacotherapy of behavioral and psychological symptoms of dementia: state of the art and future progress. Front. Pharmacol. 11, 1168. doi:10.3389/fphar.2020.01168
Mintzer, J., Lanctôt, K. L., Scherer, R. W., Rosenberg, P. B., Herrmann, N., van Dyck, C. H., et al. (2021). Effect of methylphenidate on apathy in patients with Alzheimer disease: The ADMET 2 randomized clinical trial. JAMA neurol. 78 (11), 1324–1332. doi:10.1001/jamaneurol.2021.3356
Mirza, F. J., Amber, S., Sumera, D., Hassan, D., Ahmed, T., and Zahid, S. (2021). Rosmarinic acid and ursolic acid alleviate deficits in cognition, synaptic regulation and adult hippocampal neurogenesis in an Aβ1-42-induced mouse model of Alzheimer's disease. Phytomedicine 83, 153490. doi:10.1016/j.phymed.2021.153490
Murad, H. A., Suliaman, M. I., Abdallah, H., and Abdulsattar, M. (2014). Does curcumin or pindolol potentiate fluoxetine's antidepressant effect by a pharmacokinetic or pharmacodynamic interaction? Indian J. Pharm. Sci. 76 (3), 203–210. doi:10.4103/0250-474X.134991
Nasiri, A., and Boroomand, M. M. (2021). The effect of rosemary essential oil inhalation on sleepiness and alertness of shift-working nurses: A randomized, controlled field trial. Complement. Ther. Clin. Pract. 43, 101326. doi:10.1016/j.ctcp.2021.101326
Nematolahi, P., Mehrabani, M., Karami-Mohajeri, S., and Dabaghzadeh, F. (2018). Effects of Rosmarinus officinalis L. on memory performance, anxiety, depression, and sleep quality in university students: A randomized clinical trial. Complement. Ther. Clin. Pract. 30, 24–28. doi:10.1016/j.ctcp.2017.11.004
Niu, Q. (2018). Overview of the Relationship Between Aluminum Exposure and Health of Human Being. Adv. Exp. Med. Biol. 1091, 1–31. doi:10.1007/978-981-13-1370-7_1
Padala, P. R., Padala, K. P., Lensing, S. Y., Ramirez, D., Monga, V., Bopp, M. M., et al. (2018). Methylphenidate for apathy in community-dwelling older veterans with mild Alzheimer's disease: A double-blind, randomized, placebo-controlled trial. Am. J. Psychiatry 175 (2), 159–168. doi:10.1176/appi.ajp.2017.17030316
Papp, M., Gruca, P., Lason-Tyburkiewicz, M., and Willner, P. (2016). Antidepressant, anxiolytic and procognitive effects of rivastigmine and donepezil in the chronic mild stress model in rats. Psychopharmacol. Berl. 233 (7), 1235–1243. doi:10.1007/s00213-016-4206-0
Pusceddu, M. M., Hernandez-Baixauli, J., Puiggrós, F., Arola, L., Caimari, A., Del Bas, J. M., et al. (2022). Mediterranean natural extracts improved cognitive behavior in zebrafish and healthy rats and ameliorated lps-induced cognitive impairment in a sex dependent manner. Behav. Brain Funct. 18 (1), 5–13. doi:10.1186/s12993-022-00190-8
Rasoulian, B., Hajializadeh, Z., Esmaeili-Mahani, S., Rashidipour, M., Fatemi, I., and Kaeidi, A. (2019). Neuroprotective and antinociceptive effects of rosemary (Rosmarinus officinalis L.) extract in rats with painful diabetic neuropathy. J. Physiol. Sci. 69 (1), 57–64. doi:10.1007/s12576-018-0620-x
Rezk, S., Lashen, S., El-Adl, M., Elshopakey, G. E., Elghareeb, M. M., Hendam, B. M., et al. (2022). Effects of Rosemary Oil (Rosmarinus officinalis) supplementation on the fate of the transplanted human olfactory bulb neural stem cells against ibotenic acid-induced neurotoxicity (Alzheimer model) in rat. Metab. Brain Dis. 37, 973–988. doi:10.1007/s11011-021-00890-6
Roohbakhsh, Y., Baradaran Rahimi, V., Silakhori, S., Rajabi, H., Rahmanian-Devin, P., Samzadeh-Kermani, A., et al. (2020). Evaluation of the effects of peritoneal lavage with Rosmarinus officinalis extract against the prevention of postsurgical-induced peritoneal adhesion. Planta Med. 86 (06), 405–414. doi:10.1055/a-1118-3918
Saeed, A., Qusti, S. Y., Almarwani, R. H., Jambi, E. J., Alshammari, E. M., Gusty, N. F., et al. (2021). Effects of aluminum chloride and coenzyme Q10 on the molecular structure of lipids and the morphology of the brain hippocampus cells. RSC Adv. 11 (48), 29925–29933. doi:10.1039/d1ra03786b
Safe, S., Jayaraman, A., Chapkin, R. S., Howard, M., Mohankumar, K., and Shrestha, R. (2021). Flavonoids: structure-function and mechanisms of action and opportunities for drug development. Toxicol. Res. 37 (2), 147–162. doi:10.1007/s43188-020-00080-z
Santos Rodrigues, A. P., Faria E Souza, B. S., Alves Barros, A. S., de Oliveira Carvalho, H., Lobato Duarte, J., Leticia Elizandra Boettger, M., et al. (2020). The effects of Rosmarinus officinalis L. essential oil and its nanoemulsion on dyslipidemic Wistar rats. J. Appl. Biomed. 18 (4), 126–135. doi:10.32725/jab.2020.016
Santos, S. C., Fortes, G. A. C., Camargo, L. T. F. M., Camargo, A. J., and Ferri, P. H. (2021). Antioxidant effects of polyphenolic compounds and structure-activity relationship predicted by multivariate regression tree. LWT 137, 110366. doi:10.1016/j.lwt.2020.110366
Sasaki, K., Ferdousi, F., Fukumitsu, S., Kuwata, H., and Isoda, H. (2021). Antidepressant- and anxiolytic-like activities of Rosmarinus officinalis extract in rodent models: Involvement of oxytocinergic system. Biomed. Pharmacother. 144, 112291. doi:10.1016/j.biopha.2021.112291
Scheltens, P., De Strooper, B., Kivipelto, M., Holstege, H., Chételat, G., Teunissen, C. E., et al. (2021). Alzheimer's disease. Lancet 397 (10284), 1577–1590. doi:10.1016/S0140-6736(20)32205-4
Sepehry, A. A., Sarai, M., and Hsiung, G. R. (2017). Pharmacological therapy for apathy in Alzheimer's disease: a systematic review and meta-analysis. Can. J. Neurol. Sci. 44 (3), 267–275. doi:10.1017/cjn.2016.426
Shellenberg, T. P., Stoops, W. W., Lile, J. A., and Rush, C. R. (2020). An update on the clinical pharmacology of methylphenidate: therapeutic efficacy, abuse potential and future considerations. Expert Rev. Clin. Pharmacol. 13 (8), 825–833. doi:10.1080/17512433.2020.1796636
Teixeira, A. L., Gonzales, M. M., de Souza, L. C., and Weisenbach, S. L. (2021). Revisiting apathy in Alzheimer's disease: from conceptualization to therapeutic approaches. Behav. Neurol. 2021, 6319826. doi:10.1155/2021/6319826
Tietz, T., Lenzner, A., Kolbaum, A. E., Zellmer, S., Riebeling, C., Gürtler, R., et al. (2019). Aggregated aluminium exposure: risk assessment for the general population. Arch. Toxicol. 93 (12), 3503–3521. doi:10.1007/s00204-019-02599-z
Uddin, M. S., Mamun, A. A., Sumsuzzman, D. M., Ashraf, G. M., Perveen, A., Bungau, S. G., et al. (2020). Emerging Promise of cannabinoids for the management of pain and associated neuropathological alterations in Alzheimer's disease. Front. Pharmacol. 11, 1097. doi:10.3389/fphar.2020.01097
Valdés, A., García-Cañas, V., Pérez-Sánchez, A., Barrajón-Catalán, E., Ruiz-Torres, V., Artemenko, K. A., et al. (2017). Shotgun proteomic analysis to study the decrease of xenograft tumor growth after rosemary extract treatment. J. Chromatogr. A 1499, 90–100. doi:10.1016/j.chroma.2017.03.072
Valones, M., Silva, I., Gueiros, L., Leão, J. C., Caldas, A. F., and Carvalho, A. (2019). Clinical assessment of Rosemary-based toothpaste (Rosmarinus officinalis Linn.): A randomized controlled double-blind study. Braz. Dent. J. 30 (2), 146–151. doi:10.1590/0103-6440201902164
Villareal, M. O., Ikeya, A., Sasaki, K., Arfa, A. B., Neffati, M., and Isoda, H. (2017). Anti-stress and neuronal cell differentiation induction effects of Rosmarinus officinalis L. essential oil. BMC Complement. Altern. Med. 17 (1), 1–10. doi:10.1186/s12906-017-2060-1
Wang, C. C., Hsieh, P. W., Kuo, J. R., and Wang, S. J. (2021c). Rosmarinic acid, a bioactive phenolic compound, inhibits glutamate release from rat cerebrocortical synaptosomes through gabaa receptor activation. Biomolecules 11 (7), 1029. doi:10.3390/biom11071029
Wang, J., Wang, S., Guo, H., Li, Y., Jiang, Z., Gu, T., et al. (2021b). Rosmarinic acid protects rats against post-stroke depression after transient focal cerebral ischemic injury through enhancing antioxidant response. Brain Res. 1757, 147336. doi:10.1016/j.brainres.2021.147336
Wang, X. Q., Tang, Y. H., Zeng, G. R., Wu, L. F., Zhou, Y. J., Cheng, Z. N., et al. (2021a). Carnosic acid alleviates depression-like behaviors on chronic mild stressed mice via PPAR-γ-dependent regulation of ADPN/FGF9 pathway. Psychopharmacology 238 (2), 501–516. doi:10.1007/s00213-020-05699-2
Wenthur, C. J. (2016). Classics in Chemical Neuroscience: Methylphenidate. ACS Chem. Neurosci. 7 (8), 1030–1040. doi:10.1021/acschemneuro.6b00199
Yang, H., Hong, W., Chen, L., Tao, Y., Peng, Z., and Zhou, H. (2020). Analysis of risk factors for depression in Alzheimer's disease patients. Int. J. Neurosci. 130 (11), 1136–1141. doi:10.1080/00207454.2020.1730369
Zahid, S., Amber, S., Ali Shah, S., and Ahmed, T. (2018). Syzygium aromaticum ethanol extract reduces AlCl3-induced neurotoxicity in mice brain through regulation of amyloid precursor protein and oxidative stress gene expression. Asian Pac J. Trop. Med. 11 (2), 123. doi:10.4103/1995-7645.225019
Zhang, Q. (2018). Aluminum-induced neural cell death. Adv. Exp. Med. Biol. 1091, 129–160. doi:10.1007/978-981-13-1370-7_8
Zhang, X., Lian, S., Zhang, Y., and Zhao, Q. (2022). Efficacy and safety of donepezil for mild cognitive impairment: A systematic review and meta-analysis. Clin. Neurol. Neurosurg. 213, 107134. doi:10.1016/j.clineuro.2022.107134
Zhu, F., Du, B., and Xu, B. (2018). Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Crit. Rev. Food Sci. Nutr. 58 (8), 1260–1270. doi:10.1080/10408398.2016.1251390
Keywords: Rosmarinus officinalis, methylphenidate (MPH), anxiety, depression, cognition
Citation: Malik N, Amber S and Zahid S (2022) Rosmarinus officinalis and Methylphenidate Exposure Improves Cognition and Depression and Regulates Anxiety-Like Behavior in AlCl3-Induced Mouse Model of Alzheimer’s Disease. Front. Pharmacol. 13:943163. doi: 10.3389/fphar.2022.943163
Received: 13 May 2022; Accepted: 20 June 2022;
Published: 12 August 2022.
Edited by:
Syed Shams Ul Hassan, Shanghai Jiao Tong University, ChinaReviewed by:
Muhammad Asad Farooq, East China Normal University, ChinaCopyright © 2022 Malik, Amber and Zahid. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saadia Zahid, c2FhZGlhLnphaGlkQGFzYWIubnVzdC5lZHUucGs=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.