
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 14 September 2022
Sec. Renal Pharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.940791
Background: Nephrotoxicity of drugs contributes to acute kidney injury with high mortality and morbidity, which crucially limits the application and development of drugs. Although many publications on nephrotoxicity have been conducted globally, there needs to be a scientometric study to systematically analyze the intellectual landscape and frontiers research trends in the future.
Methods: Publications on nephrotoxicity from 2011 to 2021 were collected to perform bibliometric visualization using VOSviewer, CiteSpace, and Scimago Graphica software based on the Web of Science Core Collection.
Results: A total of 9,342 documents were analyzed, which were primarily published in the United States (1,861), China (1,724), and Egypt (701). For institutions, King Saud University (166) had the most publications; Food and Chemical Toxicology, PLOS One, and Antimicrobial Agents and Chemotherapy were productive journals, primarily concentrating on the mechanisms of nephrotoxicity and renoprotective in cisplatin and antibiotics, especially in oxidative stress. Burst detection suggested that cisplatin, piperacillin-tazobactam, vancomycin-induced nephrotoxicity, antioxidants, and new biomaterials are frontiers of research.
Conclusion: This study first provides an updated perspective on nephrotoxicity and renoprotective strategies and mechanisms. This perspective may benefit researchers in choosing suitable journals and collaborators and assisting them in the deep understanding of the nephrotoxicity and renoprotective hotspots and frontiers.
The kidney, as the main excretory organ in the body, is more susceptible to chemical and drug toxicity (Nishinakamura, 2019). Valuable evidence demonstrate that nephrotoxicity is one of the common adverse effects of drugs and a major obstacle to drug development. Acute and chronic kidney injuries are mainly the result of nephrotoxicity, which affects approximately 13.3 million people around the world each year (Kellum et al., 2021; Pickkers et al., 2021), a significant statistic given that 75% of hospitalized patients may suffer nephrotoxicity exposure (Moffett and Goldstein, 2011; Davis-Ajami et al., 2016), which contributes to increasing a patient’s odds of developing acute kidney injury (AKI) by 53% (Ostermann et al., 2018). Importantly, the general population is often exposed to the nephrotoxicity of prescribed and over-the-counter drugs, natural products, supplements, herbal medicines, and imaging agents (Izzedine et al., 2009; Perazella and Reilly, 2011; Yang et al., 2018). For example, cisplatin can induce nephrotoxicity in 30–40% of patients, commonly causing AKI and renal failure (Volarevic et al., 2019). However, nephrotoxicity is a sophisticated toxic reaction, which involves many risks and complex processes, including congenital nephrotoxicity, patient-specific risk factors, metabolism, and excretion of drugs which may be potential causative agents of kidney damage (Perazella, 2018).
Modern toxicology evidence suggest that medications could induce kidney injury through continuous drug accumulation, drug-related immune effects, and drug-metabolite insolubility in the urine (Perazella, 2010; Perazella and Izzedine, 2015). The molecular mechanism of nephrotoxicity is commonly related to oxidative stress (OS), inflammation, apoptosis, and autophagy (Gao et al., 2021). Notably, the latest nephrotoxicity studies have commonly focused on the renoprotective mechanism, early warning, and nanomedicine of nephrotoxicity. Beclin-1, MiR-155, farnesoid X receptor, and mitochondrial homeostasis proteins may be novel therapy target strategies of nephrotoxicity through autophagy blockade, ferroptosis, and OS (Kim et al., 2022; Shi et al., 2022; Yin et al., 2022; Zhang et al., 2022). In addition, phosphatidylcholines and taurine may be early biomarkers of nephrotoxicity using metabolomics and mass spectrometry imaging approaches (Chen et al., 2022; Locci et al., 2022), while nuclear factor erythroid 2-related factor 2 (Nrf2) could predict the severity of nephrotoxicity using deep learning approach (Feng et al., 2022). Moreover, compound nanocapsules, selenium nanoparticles, and renalase agonist nanoparticles could effectively reduce nephrotoxicity by inhibiting cell death and OS (Alotaibi et al., 2022; Guo et al., 2022; Mehanna et al., 2022).
Although a large number of nephrotoxicity studies have been conducted currently, there has been no global study to examine and predict the frontiers of nephrotoxicity research. Therefore, a bibliometric analysis was performed for scientists to systematically review the nephrotoxicity research studies. Bibliometric analysis (Chen, 2004) is used to track developments and explore particular areas of knowledge in medicine fields (Lu et al., 2020; Dong et al., 2021; Huang et al., 2021; Zhu et al., 2021) using CiteSpace (Chen, 2006) and VOSviewer (Waltman, 2007) software. Notably, we intended to visually analyze nephrotoxicity research hotspots through the bibliometric approach to make suggestions and further perspective.
All the data were collected from the SCI-E and SSCI of the Web of Science Core Collection (WoSCC). The final suitable result was chosen by performing different search strategies, including various publication time periods or topics, and any divergences were settled by consultation with external specialists to reach consensus. Then, the retrieval strategy was stated as (topic = nephrotoxicity), (type = article), (year published = 2011–2021), and (language = English). The date of retrieval was 4 March 2022 by two researchers (Figure 1).
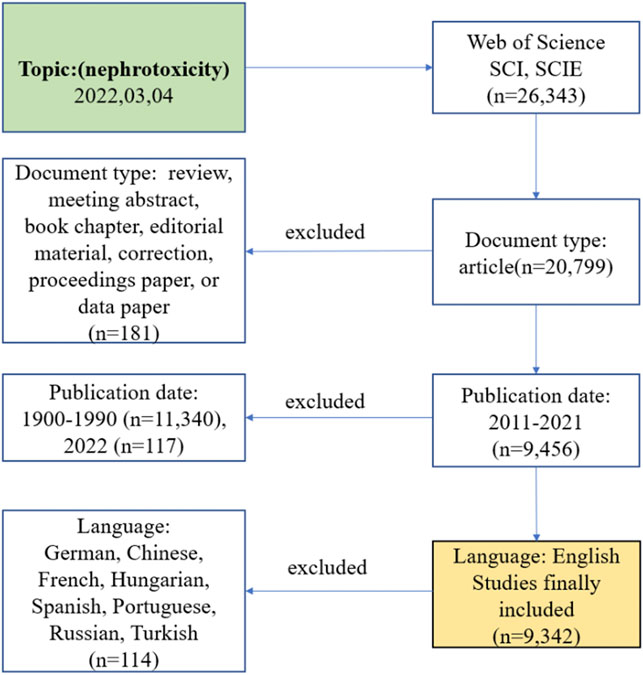
FIGURE 1. Frame flow diagram of nephrotoxicity search strategy from 2011 to 2021 based on Web of Science.
VOSviewer 1.6.16 and CiteSpace 5.8.R3 were applied to identify co-cited articles, keywords, countries, institutions, journals, authors, and reference bursts. The H-index, impact factor (IF), and category quartiles were collected from the Journal Citation Report (2020). The data of publications, citations, and polynomial trend lines were analyzed by Excel software. CiteSpace software was used to construct the hotspots and knowledge base map of nephrotoxicity research, and detect the centrality. VOSviewer was utilized to analyze the authorship and co-occurrence of keywords and visualize the frontiers of the nephrotoxicity field by clustering with different colors. Scimago Graphica 1.0.18 was used to visualize the collaborative relationships in countries/regions.
A total of 9,342 articles on nephrotoxicity were published in 2011–2021 based on WoSCC, and the H-index count was 112. There were 91,626 and 16.46 total and mean citations, respectively, exhibiting an overall upward trend in the past decade (Figure 2A). From 2011 onward, the number of articles on nephrotoxicity increased with a continuous growth till 2021, and the publications in 2021 were nearly double that in 2011. In the past 5 years, research activity on nephrotoxicity peaked in terms of volume, with 4,953 articles being published. Furthermore, the annual number of citations also sharply increased from 2015 onward, with even more than 100 times the number of citations in 2021 than in 2011. The linear fitting of articles in nephrotoxicity showed a significant correlation (R2 = 0.9964) between the year and the citations, attracting widespread attention from scientists in the nephrotoxicity field around the world.
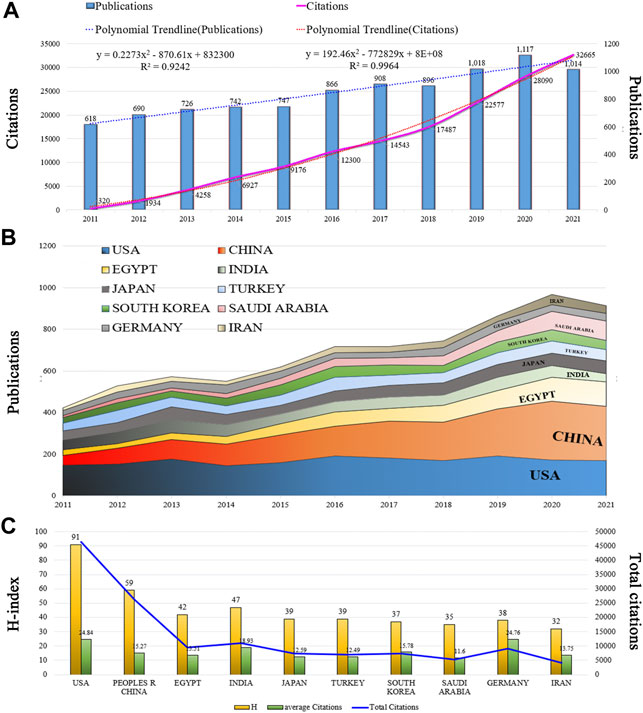
FIGURE 2. Trends in publications and citations of nephrotoxicity research. (A) Annual trends of global publications and citations. (B) Temporal trends of publications from the top 10 countries. (C) H-index, average citations (citations per article), and total citations of the top 10 countries.
All literature was distributed among 123 countries/regions and 6,843 institutions. The top five countries of publications were the United States (1,861, 19.92%), China (1,724, 18.45%), Egypt (701, 7.50%), India (585, 6.26%), and Japan (583.6.24%); in the top 10 countries, 60% of the countries originated from Asia (Table 1). In addition, further effort was made to build the annual national publications (Figure 2B) and citations (Figure 2C) of the countries. The United States has published nearly 20% of all studies with a stable growth of publications in the last decade, but China has aroused the concern for nephrotoxicity research with a sharp increase of publications in nearly 5 years. The top three countries of mean citations were the United States (24.84), Germany (24.76), and India (18.93). Also, the top three countries of the H-index were the United States (H = 91), China (H = 59), and Egypt (H = 42). Among them, the United States, England, Germany, France, Spain, and Belgium were core nodes (centrality>0.1) marked with a purple circle (Figure 3A). The earlier mentioned results demonstrated that nephrotoxicity had received widespread attention from global scholars, and the United States and China were the leading contributors; furthermore, the United States represented a closer cooperation with each other in nephrotoxicity research (Figure 3D).
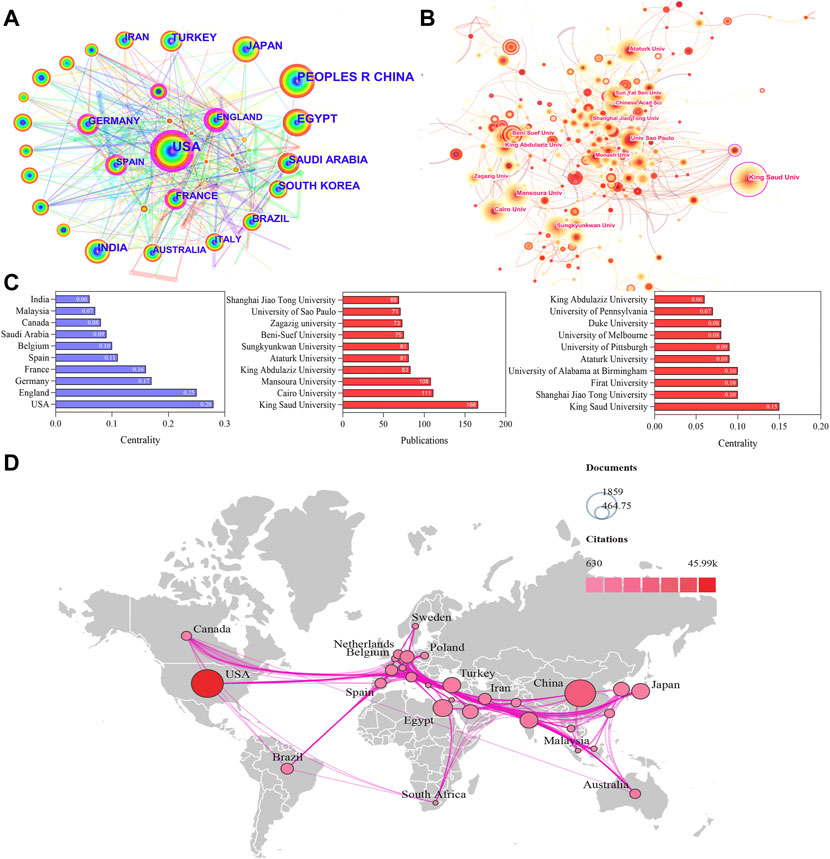
FIGURE 3. Visualization map of countries/regions and institutions involved in nephrotoxicity research. (A) Collaboration network of countries of CiteSpace. N = 123, E = 552. (B) Collaboration network of institutions of CiteSpace. N = 520, E = 978. (N represents the number of network nodes and E represents the number of connections). (C) Publications and centrality of countries/regions and institutions in nephrotoxicity research. (D) Collaborative relationships in countries/regions.
The top five publications of institutions were King Saud University (166), Cairo University (111), Mansoura University (108), King Abdulaziz University (83), and Ataturk University (81), and most of them originated from Egypt and Saudi Arabia (Figure 3C). In addition, King Saud University (0.15), Shanghai Jiao Tong University (0.10), Firat University (0.10), and University of Alabama at Birmingham (0.10) were the core nodes with a high centrality (Figure 3B), and most of them originated from the United States. The aforementioned results suggested that the institutions of Egypt and the United States were the main research forces. However, the centrality values were still low in general; thus, global cooperation should be strengthened in nephrotoxicity research field.
A total of 47,803 authors were obtained for the nephrotoxicity research. Kang, Ki Sung (45), the most productive author from South Korea, followed by Li, Jian (40), Li, Wei (38), Abdel-Daim, Mohamed M (35), and Lee, Dahae (30) (Table 2). In the total citation, the top three most cited authors were Li, Jian (1,497), Abdel-Daim, Mohamed M (1,155), and Dong, Zheng (1,061). Furthermore, Li, Jian’s H-index (25) was the highest (Figures 4C,D), who cooperated closely with Azad, Mohammad A. K and collaborated frequently with Li, Wei and Dong, Zheng (Figure 4A). In the co-citation network (Figure 4B), Pabla, Navjotsingh (831) had the highest co-citations, followed by Ramesh, Ganesan (600), and Ali, Badreldin H (575).
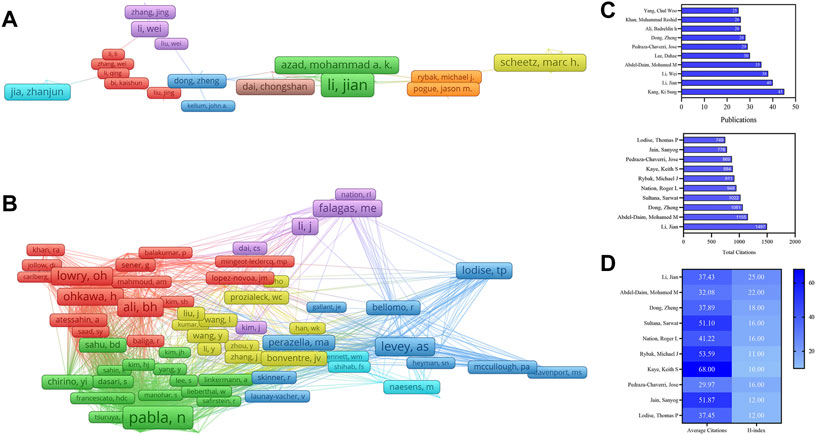
FIGURE 4. VOSviewer visualization map of authors and co-cited authors devoted to nephrotoxicity research. (A) Cooperation network of authors. Of the 47,803 authors, 144 had published at least 10 documents. (B) Co-citation network of authors. Of the 136,917 co-cited authors, 195 had at least 100 citations. Publications, total citations (C), average citations, and H-index (D) of authors in nephrotoxicity research.
A total of 1,756 journals were obtained, including 18 journals with more than 60 articles. The top three prolific and most citations journals were PLOS One (IF3.24), Antimicrobial Agents and Chemotherapy (IF5.191), and Food and Chemical Toxicology (IF6.023) (Figures 5A,B). Also, Clinical infectious diseases (IF9.079) had the highest average citations (91.24); most journals were classified in Q1 or Q2, suggesting that they were highly regarded for their research on nephrotoxicity.
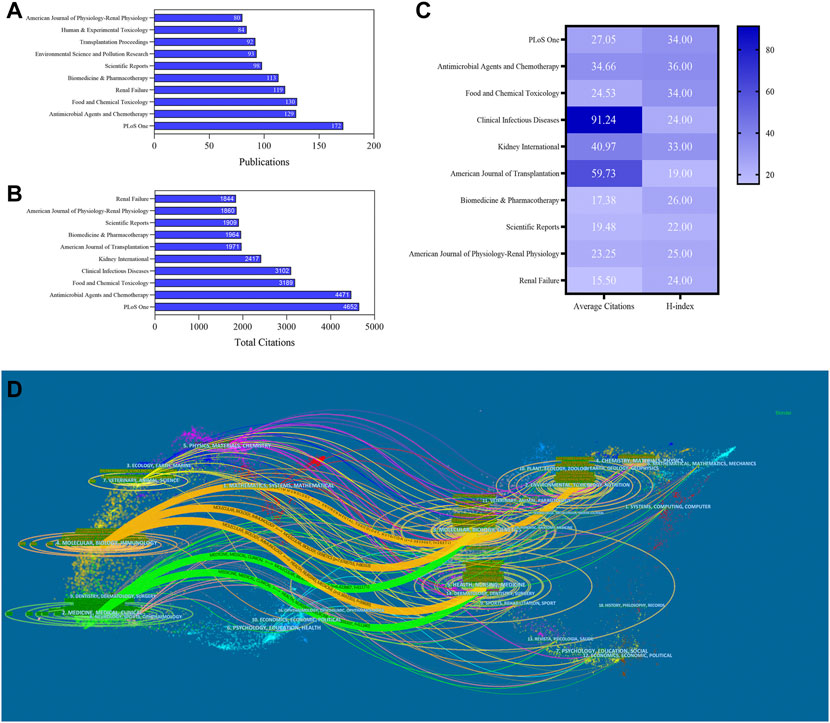
FIGURE 5. Publications (A), total citations (B), average citations, and H-index (C) of journals in nephrotoxicity research. (D) Dual-map overlay of journals in nephrotoxicity research. Left circles were targeted literature, while right circles were source literature.
The dual-map overlays showed the effective collaboration between publications and citing references in diverse fields (Figure 5D). Citing articles primarily centered on three fields: molecular, biology, and immunology; medicine, medical, and clinical; and veterinary, animal, and science. Among these fields, the circles representing the fields of medicine, medical, and clinical were larger, indicating that the numbers of coauthors and the numbers of published publications were relatively large. Also, the circle of health, nursing, and medicine was cited by publications in other fields, which played a crucial role in the cited references of nephrotoxicity research.
A map was then created by VOSviewer with 120 terms (23,900 in total), with at least 100 appearances per term (Figure 6D). Terms with comparable studies were merged under the same catalog, with four major clusters of “#0 acute kidney injury,” “#1 oxidative stress,” “#2 molecular mechanism,” “#3 cisplatin-induced nephrotoxicity,” and “#4 antibiotic” (Figure 6A). The major red cluster #0 was constituted by 45 items, including “acute renal injury,” “acute renal failure,” and “pharmacokinetics,” which mainly focused on the clinical therapy of nephrotoxicity. The green #1 and blue green #2 clusters primarily probed various toxic mechanisms of nephrotoxicity, including OS, inflammation, apoptosis, and autophagy. The time overlay map indicated that the signaling pathways and AKI still dominated in recent nephrotoxicity research (Figure 6B). Also, the frequency of the keywords was constructed to ascertain their density, and OS and AKI occupied the core part (Figure 6C).
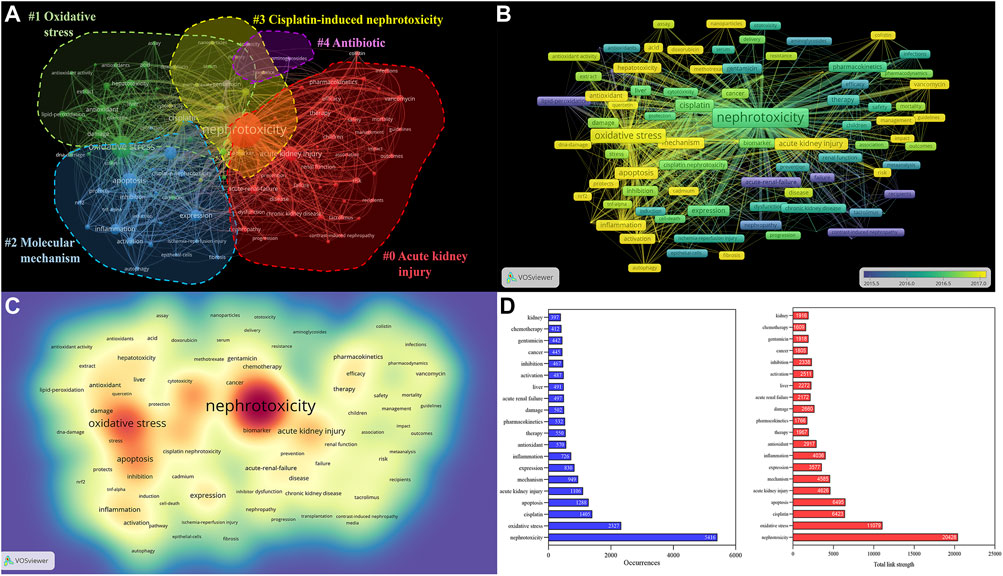
FIGURE 6. Analysis of all keywords in studies related to nephrotoxicity research. (A) VOSviewer visualization map of co-occurring keywords. Of the 23,900 keywords, 120 had at least 100 co-occurrences. (B) Overlay map of keywords. The closer to blue the keyword node color, the earlier the time of its occurrence. (C) Density map of keywords. (D) Occurrences and total link strength of keywords.
The top 10 highly cited literature on nephrotoxicity research were shown in Table 3. These studies mainly focus on antibiotic- and cisplatin-induced nephrotoxicity. Among them, the article published in Nature aroused wide concern, which demonstrated that the human nephrogenesis organoids model was representative of nephrotoxicity screening for future applications (Takasato et al., 2015). Another article proved that cisplatin-induced renal OS and apoptosis could be inhibited by exosomes from human umbilical cord mesenchymal stem cells (Zhou et al., 2013). To avoid the effect of publication year, the influence of time and co-citation were considered in citation analysis (Figure 7A). Interestingly, there were seven highly co-cited references related to cisplatin-induced nephrotoxicity (Table 4), which mainly focused on toxic mechanisms and protection strategies. These research studies (Pabla and Dong, 2008; Miller et al., 2010; Manohar and Leung, 2018) were most frequently co-cited with a higher centrality value, which demonstrated that the disorder of inflammation and cellular transporters could provoke renal tubular epithelial cells toxicity.
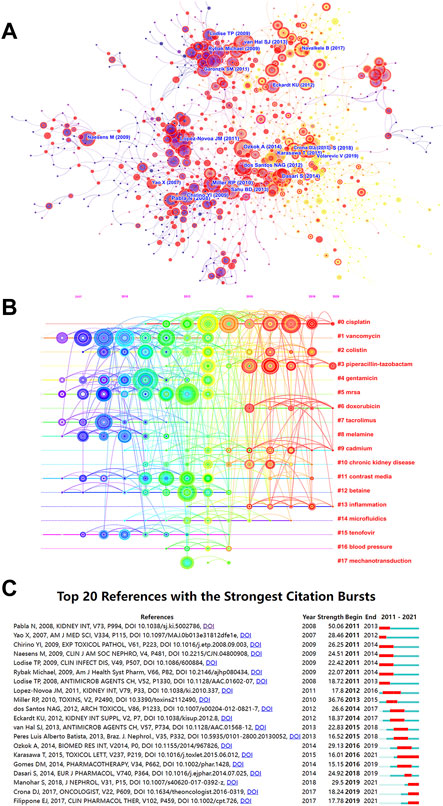
FIGURE 7. Analysis of most commonly cited references related to nephrotoxicity research. (A) Co-citation network of references. (B) Timeline view of reference cluster analysis. (C) Top 20 references with the strongest citation burst.
The high-frequency terms of nephrotoxicity were displayed by the timeline cluster analysis, including 18 categories (Figure 7B). In our study, the mean S of the 20 clusters was 0.9084, indicating that the clusters were convincing. Among them, “#0 cisplatin” was the largest cluster with 133 references, followed by “#1 vancomycin (105),” “#2 colistin (78),” “#3 piperacillin-tazobactam (75),” and “#4 gentamicin (74).” In the past 5 years, #0 cisplatin, #3 piperacillin-tazobactam, #9 cadmium, and #13 inflammation have dominated the core part of nephrotoxicity.
The top 20 references with the strongest strength citation burst represented the frontiers of the nephrotoxicity field, and the burst strength ranged from 15.15 to 50.06 while endurance strength ranged from 3 to 6 years by red squares (Figure 7C). The research focused on cisplatin-induced nephrotoxicity that maintained a long-term level of citation burst, which discussed the toxic mechanism of endoplasmic reticulum stress (ERS), inflammation, and DNA damage in cisplatin (Karasawa and Steyger, 2015). In the last 3 years, citation burst indicated that scientists mainly focused on the renoprotective mechanism and strategies of cisplatin (Crona et al., 2017; Manohar and Leung, 2018) and vancomycin-induced nephrotoxicity with the pharmacokinetics/pharmacodynamics metabolism (Filippone et al., 2017).
It is worth noting that nephrotoxicity constitutes an important factor restricting the development of drugs and clinical therapy, which contributes to AKI in 14.4% of patients and 19% of renal failure in critically ill patients (Uchino et al., 2005; Hoste et al., 2015). Therefore, the development of clinically applicable interventions for nephrotoxicity are particularly important for the prevention of kidney injury and the development of new drugs. Notably, a numerous number more than 20,000 nephrotoxicity articles have been published around the world, which could be tracked back to 1933 (Bell, 1933). To further predict the hotspots precisely and make suggestions for the future perspective, we finally utilized bibliometric technology to analyze the latest nephrotoxicity literature between 2011 and 2021 globally, providing a basic reference for scientists to discover the hotspots and frontiers of nephrotoxicity.
The output of studies is the basic indicator for reflecting the activity degree of nephrotoxicity; 9,342 publications were obtained based on WoSCC, and cisplatin, OS, antibiotics, and AKI were still the dominating player in this field. Our results indicated that the development of nephrotoxicity field showed a significant growth trend in nearly a decade, with the number of publications and citations in 2021 reaching 1,014 and 32,665, respectively. An increasing number of resources (funds, scholars) will be invested in this field, and nephrotoxicity research will gain more momentum.
As the main driving force, the United States and China are highly productive countries with frequent citations and higher H-index of nephrotoxicity literature. Interestingly, Asia had made significant contributions to nephrotoxicity research due to the productive and wide application of natural products, especially in traditional Chinese medicine components, including aristolochic acids, alkaloids, and flavonoids (Yang et al., 2018; Xu et al., 2020). In addition, the United States, England, and Germany were the core nodes, and the nephrotoxicity cooperation network was mainly concentrated in Europe and America, which particularly focused on pharmacology, pharmacy, urology, and nephrology.
However, the most prolific institutions were from Egypt and Saudi Arabia, including King Saud University and Cairo University. The institutions from the United States have closer cooperation with each other, which further strengthened its academic influence on nephrotoxicity research by maximizing its geographical advantages. Although Saudi Arabia ranked eighth in the number of publications, King Saud University ranked first in the number of publications and centrality in the cooperation network, which mainly focused on mechanism analysis of natural product protection against nephrotoxicity underlying OS and apoptosis, suggesting that it is quite professional in this field (Dkhil et al., 2014; El Gamal et al., 2014; Hagar and Al Malki, 2014). But in general, the lack of frequent global cooperation in institutions across different countries limits its development in nephrotoxicity research.
In the authors’ contribution, Kang, Ki Sung from South Korea was the productive author, the research studies mainly focused on the renoprotective of ginsenosides against cisplatin-induced nephrotoxicity through mitigating apoptosis and inflammation (Park et al., 2015; Han et al., 2016). Moreover, Li, Jian with the highest H-index had depth research in colistin-induced nephrotoxicity. Among them, the research of dosing guidance for intravenous colistin to reduce the risk of nephrotoxicity (Nation et al., 2017) and the research studies on the toxic mechanism of nephrotoxicity through ERS, mitochondrial, death receptor, and apoptosis were the highest cited articles (Dai et al., 2014; Park et al., 2015). Another contributor is Abdel-Daim, Mohamed M, who mainly focused on the renoprotective mechanism action of Spirulina platensis in deltamethrin-induced nephrotoxicity through inhibition of lipid peroxidation, NO, and OS (Abdel-Daim et al., 2013; Abdel-Daim et al., 2016). The previously mentioned authors have higher academic reputations in renoprotective research studies and have made a great contribution to the developments and advancements.
The dual-map analysis found that the fields of molecular, biology, and immunology were the major publications in nephrotoxicity research studies. In addition, the Journal Citation Reports (2020) (Khan et al., 2021) showed that most of the productive journals of nephrotoxicity were classified as Q1 or Q2. Among them, Antimicrobial Agents and Chemotherapy and Food and Chemical Toxicology were the most productive journals with higher H-index and IF than five concurrently, indicating that these journals were highly favored for scientists with convincing and mature outcomes to enhance their academic influence on nephrotoxicity.
The highly cited studies mainly focused on clinical dosing suggestions of antibiotics (Garonzik et al., 2011; Neely et al., 2014) and the renoprotective mechanisms of natural products research studies (Arjumand et al., 2011; Domitrovic et al., 2013). Notably, the co-cited network of references constitutes the knowledge base of the field. By combining the frequency and centrality of a reference, these references including mechanisms of nephrotoxicity and renoprotective in antibiotic, cisplatin, and calcineurin inhibitor that performed important knowledge-based functions were obtained. Here are the main findings.
Cisplatin, as a much sought chemotherapeutic agent, was still the dominant player of nephrotoxicity research, inducing apoptosis in tumor cells through DNA damage (Dasari and Tchounwou, 2014). At present, the major toxic mechanism in cisplatin-induced nephrotoxicity includes DNA damage, cytoplasmic organelle dysfunction, mitochondrial dysfunction, ERS, cell apoptosis, OS, and inflammation (Manohar and Leung, 2018). The tubulo-interstitial compartment is the major damage part (Ozkok and Edelstein, 2014). Notably, concurrent use of nephrotoxic medications (such as nonsteroidal anti-inflammatory drugs and iodinated contrast) are also considered risk factors. Many renoprotective measures have been studied, which are mainly employed for hydration/diuresis and monitoring of renal function. In addition, nephroprotective agents mainly included amifostine, amino oxyacetic acid, peroxisome proliferators-activated receptor agonists, and quercetin (dos Santos et al., 2012), which focused on inhibition of p53 targets, g-glutamyl transpeptidase and cysteine-S-conjugate b-lyase metabolism, cell death, OS, and inflammation (Pabla and Dong, 2008). However, it is unclear whether they reduce the cisplatin therapeutic effects in tumors concurrently.
OS is commonly caused by nephrotoxicity. Maintenance of mitochondria homeostasis plays a key role against OS to attenuate renal injury. Studies showed that OS could be activated with ROS accumulation to cause AKI by gap junction protein connexin 32 expression underlying mitochondrial apoptosis (Luo et al., 2015; Chen et al., 2019). In addition, antioxidant therapy had aroused a growing concern about nephrotoxicity. Synthetic and natural antioxidants defended oxidative injury by activating antioxidant enzymes, inhibiting NOSs, oxidases, and decomposing peroxide (Hassan et al., 2017; Forman and Zhang, 2021; Gao et al., 2021). SOD1 is a major antioxidant enzyme against OS through inhibition of ROS production, restoration of mitochondrial function, and attenuation apoptosis (Wang et al., 2021). Combining with targeting gap junction proteins and antioxidant strategies could be a novel perspective in mitochondrial damage of nephrotoxicity research.
As for antibiotics, the nephrotoxicity mechanism of aminoglycoside presents a novel insight, which concentrated on an integrative analysis of tubular obstruction and malfunction, further activated tubuloglomerular feedback, and caused renal vasoconstriction and mesangial contraction in gentamicin-induced nephrotoxicity (Lopez-Novoa et al., 2011). Similarly, concentration and duration of therapy contributed to a key factor in nephrotoxicity exposure. A study showed that patients with vancomycin troughs in excess of 15 mg/L were preferred to generate toxicity (van Hal et al., 2013). In summary, the research studies of nephrotoxicity mechanisms in cisplatin and different antibiotics constitutes the knowledge base of the nephrotoxicity field in the last decade, which has achieved great progress.
The integrative analysis and cluster of high-frequency keywords and references can identify hotspots and frontiers in the nephrotoxicity research field. Among them, research on cisplatin, piperacillin-tazobactam, doxorubicin, and cadmium was the hotspot in the nephrotoxicity field based on timeline cluster.
The frontiers research studies demonstrated that cisplatin-induced nephrotoxicity could be inhibited by preventing mitochondrial fragmentation and subsequent cell injury and death through sirtuin 3-dependent mitochondrial dynamics remodeling underlying honokiol intervention (Mao et al., 2022). Also, the role of the renoprotective effect of leonurine hydrochloride was achieved by inhibition of lipid peroxide-mediated ferroptosis and activation of Nrf2 (Hu et al., 2022). In addition, renalase agonists may be a new tool to enhance the renoprotective effects (Curry and McCormick, 2022). Studies have reported that a combination of vancomycin and piperacillin-tazobactam could induce additive nephrotoxicity through routine creatinine evaluation, but it does not worsen the level of kidney injury molecular-1 biomarker, suggesting that kidney function should be considered by integrative evaluation (Chang et al., 2022). Doxorubicin nephrotoxicity could be mitigated by nano-resveratrol through modulation of Beclin-1 and mammalian TOR (Alhusaini et al., 2022). It could also be protected by pirfenidone and vitamin D through inhibition of c-Jun N-terminal kinase-1 and monocyte chemoattractant protein-1 pathways (Hazem et al., 2022). Cadmium nephrotoxicity was induced by bromodomain-containing protein 4, further mediating lysosomal dysfunction, autophagy blockade, and OS (Gong et al., 2022). Biological oxidation (mainly glucuronidation) and apoptosis were the major stress responses in the cadmium treatment, especially in diabetes (Feng et al., 2022).
By analyzing burst citations of references, we hypothesized that the renoprotective mechanism and strategies of cisplatin-induced nephrotoxicity and antibiotic-induced nephrotoxicity will continuously become an academic trend in nephrotoxicity research (Karasawa and Steyger, 2015; Manohar and Leung, 2018). In addition, the latest research also focused on the subject of cisplatin hydration, suggesting that short-duration, low-volume with magnesium supplementation, mannitol forced diuresis, and oral post-hydration were essential for nephrotoxicity prevention (Crona et al., 2017). In vancomycin-induced nephrotoxicity with attributed rates of >10%, guideline-based trough levels of 15–20 mg/L have greater nephrotoxicity than levels <15 mg/L, and combination with piperacillin-tazobactam should be avoided. It is safe to use vancomycin in patients with exposure risks for AKI with therapeutic drug monitoring and antibiotic stewardship. But the cessation of vancomycin should be considered once patients develop AKI (Filippone et al., 2017).
New biomaterials, such as nanoparticles, dendrimers, micelles, liposomes, and nanogels, including nanomedicine, , which could offer more precise pharmacotherapy options with improved safety profiles, especially in cisplatin and antibiotics, could attenuate nephrotoxicity (Cooper et al., 2014; Desai et al., 2022). Reports showed that cisplatin conjugated to a polyphosphazene, lipid-coated cisplatin nanoparticle, and cholesterol-tethered platinum II-based nanoparticle could increase the antitumor effect and reduce nephrotoxicity (Sengupta et al., 2012; Guo et al., 2013; Patil et al., 2020). Similarly, reduced nephrotoxicity was found in paclitaxel, amphotericin B, and cyclosporine-based nanoparticle application (Wang et al., 2011; Guada et al., 2016; Liu et al., 2020). Collectively, the renoprotective mechanisms and new biomaterials applications in cisplatin and antibiotics constitute the hotspots and frontiers in the future of this field.
Combined with the knowledge base and hotspots, there are several suggestions worth noting for future perspectives against nephrotoxicity. 1) Identifying early new warning biomarkers is a critical research priority for nephrotoxicity prevention using multiomics approaches; for example, mass spectrometry imaging can give an insight into biomarkers on a spatial level, which contributes to biomarker localization and pathological processes combination. 2) Antioxidant therapy strategies should be utilized in further research studies against oxidative injury, which could protect mitochondrial damage to restore disorders of the kidney;. 3) New biomaterials with new technologies, including nanomedicine, are emerging as a new clinical favorite to reduce nephrotoxicity, which could achieve precise guidance for targeted drugs.
However, our research also has some limitations. 1) Although the most commonly used databases in scientometrics are WoSCC, Scopus, and PubMed, databases would be further needed in a comprehensive analysis, exhibiting extreme difficulty in analyzing multiple databases using available bibliometric software. 2) Only English articles were included, and nephrotoxicity related research in 2022 were not included; therefore, we may have missed some research hotspots.
Although the quantity of publications in nephrotoxicity has been growing at a rapid pace, especially in the past decade, nephrotoxicity crucially limits drug development and clinical applications. Our results demonstrated that the recognition of nephrotoxicity has improved significantly as follows. 1) The United States and China are the leading contributors. 2) Main research foci are the mechanism of nephrotoxicity and renoprotective in cisplatin and antibiotics. 3) Future research studies will focus on cisplatin, piperacillin-tazobactam, vancomycin, and cadmium. However, exploring more sensitive diagnostic markers of nephrotoxicity, focusing on antioxidant therapy, and strengthening the management and application of natural product nephrotoxicity are urgently needed globally. Collectively, this study systematically analyzed the literature on nephrotoxicity and reported the research results of the last decade in multiple dimensions, which could lay the foundation for its in-depth development and expand its clinical application globally.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
TH and XFL designed this study. TH and JZ contributed toward manuscript writing. JA performed the search. XML and RY collected and rechecked the data. TH, LL, and CD performed the analysis. All the authors reviewed and revised the manuscript.
This research is supported by the National Natural Science Foundation of China (Grants no. 82060754, 81803838, and 81760746); Education Department of Guizhou Province of China ((2017)078, YJSKYJJ(2021)154); Science and Technology Department of Guizhou province of China ((2020)1Y376 (2022)615, ZK (2021)532); Science and Technology Department of Zunyi city of Guizhou province of China ((2020)7 (2020)17 (2020)39 (2021)3); Key project at central government level: The ability establishment of sustainable use for valuable Chinese medicine resources (2060302); Science and technology project of Guizhou health and Health Committee (gzwkj 2021-441); and the Guizhou Provincial Special research project on science and technology of traditional Chinese medicine and ethnic medicine (QZYY-2021-035).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdel-Daim, M., El-Bialy, B. E., Rahman, H. G., Radi, A. M., Hefny, H. A., and Hassan, A. M. (2016). Antagonistic effects of Spirulina platensis against sub-acute deltamethrin toxicity in mice: Biochemical and histopathological studies. Biomed. Pharmacother. 77, 79–85. doi:10.1016/j.biopha.2015.12.003
Abdel-Daim, M. M., Abuzead, S. M., and Halawa, S. M. (2013). Protective role of Spirulina platensis against acute deltamethrin-induced toxicity in rats. PLoS One 8, e72991. doi:10.1371/journal.pone.0072991
Alhusaini, A. M., Fadda, L. M., Alanazi, A. M., Sarawi, W. S., Alomar, H. A., Ali, H. M., et al. (2022). Nano-resveratrol: A promising candidate for the treatment of renal toxicity induced by doxorubicin in rats through modulation of beclin-1 and mTOR. Front. Pharmacol. 13, 826908. doi:10.3389/fphar.2022.826908
Alotaibi, B., El-Masry, T. A., Elekhnawy, E., El-Kadem, A. H., Saleh, A., Negm, W. A., et al. (2022). Aqueous core epigallocatechin gallate PLGA nanocapsules: Characterization, antibacterial activity against uropathogens, and in vivo reno-protective effect in cisplatin induced nephrotoxicity. Drug Deliv. 29, 1848–1862. doi:10.1080/10717544.2022.2083725
Arjumand, W., Seth, A., and Sultana, S. (2011). Rutin attenuates cisplatin induced renal inflammation and apoptosis by reducing NFκB, TNF-α and caspase-3 expression in wistar rats. Food Chem. Toxicol. 49, 2013–2021. doi:10.1016/j.fct.2011.05.012
Chang, J., Pais, G. M., Valdez, K., Marianski, S., Barreto, E. F., and Scheetz, M. H. (2022). Glomerular function and urinary biomarker changes between vancomycin and vancomycin plus piperacillin-tazobactam in a translational rat model. Antimicrob. Agents Chemother. 66, e0213221. doi:10.1128/aac.02132-21
Chen, C. (2006). CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J. Clin. Microbiol. 57, 359–365. doi:10.1002/asi.20317
Chen, C. (2004). Searching for intellectual turning points: Progressive knowledge domain visualization. Proc. Natl. Acad. Sci. U. S. A. 101, 5303–5310. doi:10.1073/pnas.0307513100
Chen, C., Yao, W., Wu, S., Zhou, S., Ge, M., Gu, Y., et al. (2019). Crosstalk between Connexin32 and mitochondrial apoptotic signaling pathway plays a pivotal role in renal ischemia reperfusion-induced acute kidney injury. Antioxid. Redox Signal. 30, 1521–1538. doi:10.1089/ars.2017.7375
Chen, Y., Jiang, L., Zhang, R., Shi, Z., Xie, C., Hong, Y., et al. (2022). Spatially revealed perfluorooctane sulfonate-induced nephrotoxicity in mouse kidney using atmospheric pressure MALDI mass spectrometry imaging. Sci. Total Environ. 838, 156380. doi:10.1016/j.scitotenv.2022.156380
Cooper, D. L., Conder, C. M., and Harirforoosh, S. (2014). Nanoparticles in drug delivery: Mechanism of action, formulation and clinical application towards reduction in drug-associated nephrotoxicity. Expert Opin. Drug Deliv. 11, 1661–1680. doi:10.1517/17425247.2014.938046
Crona, D. J., Faso, A., Nishijima, T. F., McGraw, K. A., Galsky, M. D., and Milowsky, M. I. (2017). A systematic review of strategies to prevent cisplatin-induced nephrotoxicity. Oncologist 22, 609–619. doi:10.1634/theoncologist.2016-0319
Curry, J. N., and McCormick, J. A. (2022). Cisplatin-induced kidney injury: Delivering the goods. J. Am. Soc. Nephrol. 33, 255–256. doi:10.1681/ASN.2021121591
Dai, C., Li, J., Tang, S., Li, J., and Xiao, X. (2014). Colistin-induced nephrotoxicity in mice involves the mitochondrial, death receptor, and endoplasmic reticulum pathways. Antimicrob. Agents Chemother. 58, 4075–4085. doi:10.1128/AAC.00070-14
Dasari, S., and Tchounwou, P. B. (2014). Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 740, 364–378. doi:10.1016/j.ejphar.2014.07.025
Davis-Ajami, M. L., Fink, J. C., and Wu, J. (2016). Nephrotoxic medication exposure in U.S. Adults with predialysis chronic kidney disease: Health services utilization and cost outcomes. J. Manag. Care Spec. Pharm. 22, 959–968. doi:10.18553/jmcp.2016.22.8.959
Desai, P., Rimal, R., Sahnoun, S. E. M., Mottaghy, F. M., Moller, M., Morgenroth, A., et al. (2022). Radiolabeled nanocarriers as theranostics-advancement from peptides to nanocarriers. Small 18, e2200673. doi:10.1002/smll.202200673
Dkhil, M. A., Al-Quraishy, S., Diab, M. M., Othman, M. S., Aref, A. M., and Abdel Moneim, A. E. (2014). The potential protective role of Physalis peruviana L. fruit in cadmium-induced hepatotoxicity and nephrotoxicity. Food Chem. Toxicol. 74, 98–106. doi:10.1016/j.fct.2014.09.013
Domitrovic, R., Cvijanovic, O., Pernjak-Pugel, E., Skoda, M., Mikelic, L., and Crncevic-Orlic, Z. (2013). Berberine exerts nephroprotective effect against cisplatin-induced kidney damage through inhibition of oxidative/nitrosative stress, inflammation, autophagy and apoptosis. Food Chem. Toxicol. 62, 397–406. doi:10.1016/j.fct.2013.09.003
Dong, Q., Liang, Q., Chen, Y., Li, J., Lu, L., Huang, X., et al. (2021). Bibliometric and visual analysis of vascular calcification research. Front. Pharmacol. 12, 690392. doi:10.3389/fphar.2021.690392
dos Santos, N. A., Carvalho Rodrigues, M. A., Martins, N. M., and dos Santos, A. C. (2012). Cisplatin-induced nephrotoxicity and targets of nephroprotection: An update. Arch. Toxicol. 86, 1233–1250. doi:10.1007/s00204-012-0821-7
El Gamal, A. A., AlSaid, M. S., Raish, M., Al-Sohaibani, M., Al-Massarani, S. M., Ahmad, A., et al. (2014). Beetroot (Beta vulgaris L.) extract ameliorates gentamicin-induced nephrotoxicity associated oxidative stress, inflammation, and apoptosis in rodent model. Mediat. Inflamm. 2014, 983952. doi:10.1155/2014/983952
Feng, X., Jin, X., Zhou, R., Jiang, Q., Wang, Y., Zhang, X., et al. (2022). Deep learning approach identified a gene signature predictive of the severity of renal damage caused by chronic cadmium accumulation. J. Hazard. Mat. 433, 128795. doi:10.1016/j.jhazmat.2022.128795
Filippone, E. J., Kraft, W. K., and Farber, J. L. (2017). The nephrotoxicity of vancomycin. Clin. Pharmacol. Ther. 102, 459–469. doi:10.1002/cpt.726
Forman, H. J., and Zhang, H. (2021). Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 20, 689–709. doi:10.1038/s41573-021-00233-1
Gao, C., Liu, C., Chen, Y., Wang, Q., and Hao, Z. (2021). Protective effects of natural products against drug-induced nephrotoxicity: A review in recent years. Food Chem. Toxicol. 153, 112255. doi:10.1016/j.fct.2021.112255
Garonzik, S. M., Li, J., Thamlikitkul, V., Paterson, D. L., Shoham, S., Jacob, J., et al. (2011). Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob. Agents Chemother. 55, 3284–3294. doi:10.1128/AAC.01733-10
Gong, Z. G., Zhao, Y., Wang, Z. Y., Fan, R. F., Liu, Z. P., and Wang, L. (2022). Epigenetic regulator BRD4 is involved in cadmium-induced acute kidney injury via contributing to lysosomal dysfunction, autophagy blockade and oxidative stress. J. Hazard. Mat. 423, 127110. doi:10.1016/j.jhazmat.2021.127110
Guada, M., Lana, H., Gil, A. G., Dios-Vieitez Mdel, C., and Blanco-Prieto, M. J. (2016). Cyclosporine A lipid nanoparticles for oral administration: Pharmacodynamics and safety evaluation. Eur. J. Pharm. Biopharm. 101, 112–118. doi:10.1016/j.ejpb.2016.01.011
Guo, S., Wang, Y., Miao, L., Xu, Z., Lin, C. M., Zhang, Y., et al. (2013). Lipid-coated Cisplatin nanoparticles induce neighboring effect and exhibit enhanced anticancer efficacy. ACS Nano 7, 9896–9904. doi:10.1021/nn403606m
Guo, X., Xu, L., Velazquez, H., Chen, T. M., Williams, R. M., Heller, D. A., et al. (2022). Kidney-targeted renalase agonist prevents cisplatin-induced chronic kidney disease by inhibiting regulated necrosis and inflammation. J. Am. Soc. Nephrol. 33, 342–356. doi:10.1681/ASN.2021040439
Hagar, H., and Al Malki, W. (2014). Betaine supplementation protects against renal injury induced by cadmium intoxication in rats: Role of oxidative stress and caspase-3. Environ. Toxicol. Pharmacol. 37, 803–811. doi:10.1016/j.etap.2014.02.013
Han, M. S., Han, I. H., Lee, D., An, J. M., Kim, S. N., Shin, M. S., et al. (2016). Beneficial effects of fermented black ginseng and its ginsenoside 20(S)-Rg3 against cisplatin-induced nephrotoxicity in LLC-PK1 cells. J. Ginseng Res. 40, 135–140. doi:10.1016/j.jgr.2015.06.006
Hassan, W., Noreen, H., Rehman, S., Gul, S., Kamal, M. A., Paul Kamdem, J., et al. (2017). Oxidative stress and antioxidant potential of one hundred medicinal plants. Plants. Curr. Top. Med. Chem. 17, 1336–1370. doi:10.2174/1568026617666170102125648
Hazem, R. M., Antar, S. A., Nafea, Y. K., Al-Karmalawy, A. A., Saleh, M. A., and El-Azab, M. F. (2022). Pirfenidone and vitamin D mitigate renal fibrosis induced by doxorubicin in mice with Ehrlich solid tumor. Life Sci. 288, 120185. doi:10.1016/j.lfs.2021.120185
Hoste, E. A., Bagshaw, S. M., Bellomo, R., Cely, C. M., Colman, R., Cruz, D. N., et al. (2015). Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 41, 1411–1423. doi:10.1007/s00134-015-3934-7
Hu, J., Gu, W., Ma, N., Fan, X., and Ci, X. (2022). Leonurine alleviates ferroptosis in cisplatin-induced acute kidney injury by activating the Nrf2 signalling pathway. Br. J. Pharmacol. 179, 3991–4009. doi:10.1111/bph.15834
Huang, Z., Hou, Z., Liu, F., Zhang, M., Hu, W., and Xu, S. (2021). Scientometric analysis of medicinal and edible plant coptis. Front. Pharmacol. 12, 725162. doi:10.3389/fphar.2021.725162
Izzedine, H., Harris, M., and Perazella, M. A. (2009). The nephrotoxic effects of HAART. Nat. Rev. Nephrol. 5, 563–573. doi:10.1038/nrneph.2009.142
Karasawa, T., and Steyger, P. S. (2015). An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol. Lett. 237, 219–227. doi:10.1016/j.toxlet.2015.06.012
Kellum, J. A., Romagnani, P., Ashuntantang, G., Ronco, C., Zarbock, A., and Anders, H. J. (2021). Acute kidney injury. Nat. Rev. Dis. Prim. 7, 52. doi:10.1038/s41572-021-00284-z
Khan, A. S., Ur Rehman, S., Ahmad, S., AlMaimouni, Y. K., Alzamil, M. A. S., and Dummer, P. M. H. (2021). Five decades of the international endodontic journal: Bibliometric overview 1967-2020. Int. Endod. J. 54, 1819–1839. doi:10.1111/iej.13595
Kim, D. H., Choi, H. I., Park, J. S., Kim, C. S., Bae, E. H., Ma, S. K., et al. (2022). Farnesoid X receptor protects against cisplatin-induced acute kidney injury by regulating the transcription of ferroptosis-related genes. Redox Biol. 54, 102382. doi:10.1016/j.redox.2022.102382
Liu, Y., Han, Y., Fang, T., Chen, S. M., Hu, X., Song, L., et al. (2020). Turning weakness into strength: Albumin nanoparticle-redirected amphotericin B biodistribution for reducing nephrotoxicity and enhancing antifungal activity. J. Control. Release 324, 657–668. doi:10.1016/j.jconrel.2020.05.026
Locci, E., Liu, J., Pais, G. M., Chighine, A., Kahnamoei, D. A., Xanthos, T., et al. (2022). Urinary metabolomics from a dose-fractionated polymyxin B rat model of acute kidney injury. Int. J. Antimicrob. Agents 60, 106593. doi:10.1016/j.ijantimicag.2022.106593
Lopez-Novoa, J. M., Quiros, Y., Vicente, L., Morales, A. I., and Lopez-Hernandez, F. J. (2011). New insights into the mechanism of aminoglycoside nephrotoxicity: An integrative point of view. Kidney Int. 79, 33–45. doi:10.1038/ki.2010.337
Lu, C., Liu, M., Shang, W., Yuan, Y., Li, M., Deng, X., et al. (2020). Knowledge mapping of angelica sinensis (oliv.) diels (danggui) research: A scientometric study. Front. Pharmacol. 11, 294. doi:10.3389/fphar.2020.00294
Luo, C., Yuan, D., Li, X., Yao, W., Luo, G., Chi, X., et al. (2015). Propofol attenuated acute kidney injury after orthotopic liver transplantation via inhibiting gap junction composed of connexin 32. Anesthesiology 122, 72–86. doi:10.1097/ALN.0000000000000448
Manohar, S., and Leung, N. (2018). Cisplatin nephrotoxicity: A review of the literature. J. Nephrol. 31, 15–25. doi:10.1007/s40620-017-0392-z
Mao, R. W., He, S. P., Lan, J. G., and Zhu, W. Z. (2022). Honokiol ameliorates cisplatin-induced acute kidney injury via inhibition of mitochondrial fission. Br. J. Pharmacol. 179, 3886–3904. doi:10.1111/bph.15837
Mehanna, E. T., Khalaf, S. S., Mesbah, N. M., Abo-Elmatty, D. M., and Hafez, M. M. (2022). Anti-oxidant, anti-apoptotic, and mitochondrial regulatory effects of selenium nanoparticles against vancomycin induced nephrotoxicity in experimental rats. Life Sci. 288, 120098. doi:10.1016/j.lfs.2021.120098
Miller, R. P., Tadagavadi, R. K., Ramesh, G., and Reeves, W. B. (2010). Mechanisms of cisplatin nephrotoxicity. Toxins (Basel) 2, 2490–2518. doi:10.3390/toxins2112490
Moffett, B. S., and Goldstein, S. L. (2011). Acute kidney injury and increasing nephrotoxic-medication exposure in noncritically-ill children. Clin. J. Am. Soc. Nephrol. 6, 856–863. doi:10.2215/CJN.08110910
Nation, R. L., Garonzik, S. M., Thamlikitkul, V., Giamarellos-Bourboulis, E. J., Forrest, A., Paterson, D. L., et al. (2017). Dosing guidance for intravenous colistin in critically-ill patients. Clin. Infect. Dis. 64, 565–571. doi:10.1093/cid/ciw839
Neely, M. N., Youn, G., Jones, B., Jelliffe, R. W., Drusano, G. L., Rodvold, K. A., et al. (2014). Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob. Agents Chemother. 58, 309–316. doi:10.1128/AAC.01653-13
Nishinakamura, R. (2019). Human kidney organoids: Progress and remaining challenges. Nat. Rev. Nephrol. 15, 613–624. doi:10.1038/s41581-019-0176-x
Ostermann, M., Chawla, L. S., Forni, L. G., Kane-Gill, S. L., Kellum, J. A., Koyner, J., et al. (2018). Drug management in acute kidney disease - Report of the acute disease quality initiative XVI meeting. Br. J. Clin. Pharmacol. 84, 396–403. doi:10.1111/bcp.13449
Ozkok, A., and Edelstein, C. L. (2014). Pathophysiology of cisplatin-induced acute kidney injury. Biomed. Res. Int. 2014, 967826. doi:10.1155/2014/967826
Pabla, N., and Dong, Z. (2008). Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 73, 994–1007. doi:10.1038/sj.ki.5002786
Park, J. Y., Choi, P., Kim, T., Ko, H., Kim, H. K., Kang, K. S., et al. (2015). Protective effects of processed ginseng and its active Ginsenosides on cisplatin-induced nephrotoxicity: In vitro and in vivo studies. J. Agric. Food Chem. 63, 5964–5969. doi:10.1021/acs.jafc.5b00782
Patil, B. R., Kang, S. Y., Jung, D. H., Avaji, P. G., Jun, Y. J., Lee, H. J., et al. (2020). Design of a novel theranostic nanomedicine (III): Synthesis and physicochemical properties of tumor-targeting cisplatin conjugated to a hydrophilic polyphosphazene. Int. J. Nanomedicine 15, 981–990. doi:10.2147/IJN.S235618
Perazella, M. A., and Izzedine, H. (2015). New drug toxicities in the onco-nephrology world. Kidney Int. 87, 909–917. doi:10.1038/ki.2015.30
Perazella, M. A. (2018). Pharmacology behind common drug nephrotoxicities. Clin. J. Am. Soc. Nephrol. 13, 1897–1908. doi:10.2215/CJN.00150118
Perazella, M. A., and Reilly, R. F. (2011). Imaging patients with kidney disease: How do we approach contrast-related toxicity? Am. J. Med. Sci. 341, 215–221. doi:10.1097/MAJ.0b013e3181f016e6
Perazella, M. A. (2010). Tenofovir-induced kidney disease: An acquired renal tubular mitochondriopathy. Kidney Int. 78, 1060–1063. doi:10.1038/ki.2010.344
Pickkers, P., Darmon, M., Hoste, E., Joannidis, M., Legrand, M., Ostermann, M., et al. (2021). Acute kidney injury in the critically ill: An updated review on pathophysiology and management. Intensive Care Med. 47, 835–850. doi:10.1007/s00134-021-06454-7
Sengupta, P., Basu, S., Soni, S., Pandey, A., Roy, B., Oh, M. S., et al. (2012). Cholesterol-tethered platinum II-based supramolecular nanoparticle increases antitumor efficacy and reduces nephrotoxicity. Proc. Natl. Acad. Sci. U. S. A. 109, 11294–11299. doi:10.1073/pnas.1203129109
Shi, M., Maique, J., Shepard, S., Li, P., Seli, O., Moe, O. W., et al. (2022). In vivo evidence for therapeutic applications of beclin 1 to promote recovery and inhibit fibrosis after acute kidney injury. Kidney Int. 101, 63–78. doi:10.1016/j.kint.2021.09.030
Takasato, M., Er, P. X., Chiu, H. S., Maier, B., Baillie, G. J., Ferguson, C., et al. (2015). Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526, 564–568. doi:10.1038/nature15695
Uchino, S., Kellum, J. A., Bellomo, R., Doig, G. S., Morimatsu, H., Morgera, S., et al. (2005). Beginning, ending supportive therapy for the kidney, IAcute renal failure in critically ill patients: A multinational, multicenter study. JAMA 294, 813–818. doi:10.1001/jama.294.7.813
van Hal, S. J., Paterson, D. L., and Lodise, T. P. (2013). Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob. Agents Chemother. 57, 734–744. doi:10.1128/AAC.01568-12
Volarevic, V., Djokovic, B., Jankovic, M. G., Harrell, C. R., Fellabaum, C., Djonov, V., et al. (2019). Molecular mechanisms of cisplatin-induced nephrotoxicity: A balance on the knife edge between renoprotection and tumor toxicity. J. Biomed. Sci. 26, 25. doi:10.1186/s12929-019-0518-9
Waltman, N. J. v. E. L., and Waltman, L. (2007). VOS: A new method for visualizing similarities between objects. Adv. Data Analysis 2007, 299–306. doi:10.1007/978-3-540-70981-7_34
Wang, X. L., Wang, L., Lin, F. L., Li, S. S., Lin, T. X., and Jiang, R. W. (2021). Protective effect of penetratin analogue-tagged SOD1 on cisplatin-induced nephrotoxicity through inhibiting oxidative stress and JNK/p38 MAPK signaling pathway. Oxid. Med. Cell. Longev. 2021, 5526053. doi:10.1155/2021/5526053
Wang, Y., Li, X., Wang, L., Xu, Y., Cheng, X., and Wei, P. (2011). Formulation and pharmacokinetic evaluation of a paclitaxel nanosuspension for intravenous delivery. Int. J. Nanomedicine 6, 1497–1507. doi:10.2147/IJN.S21097
Xu, X., Zhu, R., Ying, J., Zhao, M., Wu, X., Cao, G., et al. (2020). Nephrotoxicity of herbal medicine and its prevention. Front. Pharmacol. 11, 569551. doi:10.3389/fphar.2020.569551
Yang, B., Xie, Y., Guo, M., Rosner, M. H., Yang, H., and Ronco, C. (2018). Nephrotoxicity and Chinese herbal medicine. Clin. J. Am. Soc. Nephrol. 13, 1605–1611. doi:10.2215/CJN.11571017
Yin, Q., Zhao, Y. J., Ni, W. J., Tang, T. T., Wang, Y., Cao, J. Y., et al. (2022). MiR-155 deficiency protects renal tubular epithelial cells from telomeric and genomic DNA damage in cisplatin-induced acute kidney injury. Theranostics 12, 4753–4766. doi:10.7150/thno.72456
Zhang, Q., Luo, P., Chen, J., Yang, C., Xia, F., Zhang, J., et al. (2022). Dissection of targeting molecular mechanisms of aristolochic acid-induced nephrotoxicity via a combined deconvolution strategy of chemoproteomics and metabolomics. Int. J. Biol. Sci. 18, 2003–2017. doi:10.7150/ijbs.69618
Zhou, Y., Xu, H., Xu, W., Wang, B., Wu, H., Tao, Y., et al. (2013). Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res. Ther. 4, 34. doi:10.1186/scrt194
Keywords: acute kidney injury, bibliometrics, nephrotoxicity mechanism, renoprotective strategies, hotspot
Citation: He T, Ao J, Duan C, Yan R, Li X, Liu L, Zhang J and Li X (2022) Bibliometric and visual analysis of nephrotoxicity research worldwide. Front. Pharmacol. 13:940791. doi: 10.3389/fphar.2022.940791
Received: 10 May 2022; Accepted: 24 August 2022;
Published: 14 September 2022.
Edited by:
Elham Ahmadian, Tabriz University of Medical Sciences, IranReviewed by:
Aziz Eftekhari, Joint Ukraine-Azerbaijan Research Center of Nanobiotechnology, AzerbaijanCopyright © 2022 He, Ao, Duan, Yan, Li, Liu, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianyong Zhang, emhhbmdqaWFueW9uZzIwMDZAMTI2LmNvbQ==; Xiaofei Li, bGl4aWFvZmVpQHptdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.