
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 11 July 2022
Sec. Ethnopharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.940572
This article is part of the Research TopicHerbal Medicine for the Treatment of Chronic Metabolic DiseasesView all 16 articles
 Fikile T. Mthiyane1
Fikile T. Mthiyane1 Phiwayinkosi V. Dludla2
Phiwayinkosi V. Dludla2 Khanyisani Ziqubu1
Khanyisani Ziqubu1 Sinenhlanhla X. H. Mthembu1,2
Sinenhlanhla X. H. Mthembu1,2 Ndivhuwo Muvhulawa1
Ndivhuwo Muvhulawa1 Nokulunga Hlengwa3
Nokulunga Hlengwa3 Bongani B. Nkambule4
Bongani B. Nkambule4 Sithandiwe E. Mazibuko-Mbeje1*
Sithandiwe E. Mazibuko-Mbeje1*Moringa oleifera is one of the popular plants that have shown significant health benefits. Certainly, preclinical evidence (predominantly from animal models) summarized in the current review supports the beneficial effects of Moringa oleifera leaf extracts in combating the prominent characteristic features of diabetes mellitus. This includes effective control of blood glucose or insulin levels, enhancement of insulin tissue sensitivity, improvement of blood lipid profiles, and protecting against organ damage under sustained conditions of hyperglycemia. Interestingly, as major complications implicated in the progression of diabetes, including organ damage, Moringa oleifera leaf and seed extracts could efficiently block the detrimental effects of oxidative stress and inflammation in these preclinical models. Notably, these extracts (especially leaf extracts) showed enhanced effects in strengthening intracellular antioxidant defences like catalase, superoxide dismutase, and glutathione to lower lipid peroxidation products and reduce prominent pro-inflammatory markers such as tumor necrosis factor-α, interleukin (1L)-β, IL-6, monocyte chemoattractant protein-1 and nitric oxide synthase. From animal models of diabetes, the common and effective dose of leaf extracts of Moringa oleifera was 100–300 mg/kg, within the treatment duration of 2–8 weeks. Whereas supplementation with approximately 20 g leaf powder of Moringa oleifera for at least 2 weeks could improve postprandial blood glucose in subjects with prediabetes or diabetes. Although limited clinical studies have been conducted on the antidiabetic properties of Moringa oleifera, current findings provide an important platform for future research directed at developing this plant as a functional food to manage diabetic complications.
According to the World Health Organization, diabetes mellitus is amongst the top ten leading causes of mortality and morbidity around the world (World Health Organization, 2022). Diabetes is a metabolic disorder that is characterized by a state of hyperglycemia, that occurs alongside dysregulations in insulin levels and in some cases, it arises concurrently to overweight and obesity (International Diabetes Federation, 2021). Indeed, excessive body fat or obesity remains the major culprits in the development of type 2 diabetes (T2D), which is the predominant form of diabetes (International Diabetes Federation, 2021). The rapid increase in cases of diabetes mellitus, especially T2D, raises concerns, also highlighting an urgent need to investigate effective therapies to curb this disease (Ahmad et al., 2019). Accumulative research has focused on understanding the pathophysiological mechanisms implicated in the development of diabetes-associated complications, which is essential to discover effective therapies that can improve metabolic function and prevent multiple organ failure in those affected by this condition (King and Brownlee, 1996; Fowler, 2007; Wei et al., 2020).
As a prime example, inflammation and oxidative stress, which normally emerge as a result of an abnormal pro-inflammatory response, or an exacerbated production of free radical species are increasingly recognized as the key abnormalities implicated in the development and acceleration of diabetes-linked complications (King and Brownlee, 1996). Notably, oxidative stress is linked with the activation of protein kinase C (PKC), which is normally consistent with impaired insulin signaling and tissue damage in experimental models of diabetes (King and Brownlee, 1996). Importantly, this content supports the hypothesis by Randle and others (Randle et al., 1963) which stated that alteration in the uptake and metabolism of glucose and free fatty acids may be an instrumental process in the pathogenesis of insulin resistance, the major characteristic feature of T2D. Indeed, many diverse biochemical mechanisms, extending beyond inflammation and oxidative stress or activation of PKC, are implicated in the development of T2D (King and Brownlee, 1996).
Literature suggests that effective modulation of energy metabolism and insulin signaling through the regulation of AMP-activated protein kinase (AMPK) or phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) pathways appears to reverse some devastating outcomes of diabetes (Long and Zierath, 2006; Huang et al., 2018; Mazibuko-Mbeje et al., 2018). In fact, plants and their related bioactive compounds are increasingly screened for their antidiabetic properties. Some natural plants have shown anti diabetic properties through lowering blood glucose and modulation of AMPK/PI3K/AKT pathways (Francini et al., 2019; Mazibuko-Mbeje et al., 2019; Costa et al., 2020). Consistently, our group continues to review literature on the impact of plants like Camellia sinensis and Aspalathus linearis, including prominent bioactive compounds from some of these plants such as gallic acid and isoorientin for their ameliorative effects against metabolic complications (Dludla et al., 2019; Ziqubu et al., 2020; Dludla et al., 2021).
Moringa oleifera is a medicinal plant that has gained a lot of interest for its diverse biological properties. Reviewed evidence indicates the biological capabilities of this plant expand to protecting against complications linked with heart disease, cancer, fatty liver, and diabetes mellitus (Paikra et al., 2017; Vergara-Jimenez et al., 2017; Abd Rani et al., 2018). For example, a previously published review supported the beneficial effects of the leaves of the Moringa oleifera in improving blood glucose control in experimental models of diabetes (Ahmad et al., 2019). Notably, this review indicated draw backs such as the limited number of studies that have reported on the potential beneficial effects of this plant, including the fact that summarized literature was mainly conducted in animals, through in vitro and in vivo preclinical models. Nevertheless, while such information already affirms the hypoglycaemic potential of this medicinal plant, data regarding the prominent biochemical mechanisms implicated in the antidiabetic effects of Moringa oleifera have not been critically reviewed. Recently, Louisa and others supported the potential benefits of Moringa oleifera in cardiovascular or metabolic disorders, mainly by ameliorating the undesired pro-inflammatory response and inhibiting oxidative stress by mediating molecular mechanisms such as hindering nuclear factor kappa B (NF-κB) translocation or enhancing the antioxidant response of nuclear factor-erythroid factor 2-related factor 2 (Nrf2) in different preclinical models (Louisa et al., 2022). Thus, there is a need to better understand such intracellular responses of Moringa oleifera within a setting of diabetes or in related metabolic complications. The current study provides a brief overview on Moringa oleifera as medicinal plant, followed by its therapeutic mechanisms in controlling diverse diabetic complications. Mostly focusing on understanding the modulatory effects of this medicinal plant in mechanisms of inflammation and oxidative stress in a diabetic state.
This current review includes evidence that was obtained from a search done (from inception until end of December 2021) on major search engines such as PubMed, Google Scholar and ScienceDirect. The systematic search was conducted using the following Medical Subject Heading (MeSH) terms “Moringa oleifera”, “diabetes mellitus”, “glucose metabolism”, “insulin resistance”, “oxidative stress”, and “inflammation” as well as relevant synonyms. EndNote20 desktop software (Elsevier, Amsterdam, Netherlands) was used for references and identification of duplicated studies. Preclinical and clinical studies reporting on the mechanisms of Moringa oleifera in diabetes models and related metabolic syndrome was included in this review. However, review papers, and encyclopaedias were excluded but screened for primary findings. Notably, critical information related to the portion (part) of the plant that was assessed, as well as effective therapeutic dose and an experimental model used for the investigation is provided to better understand the potential benefits of Moringa oleifera.
Moringa oleifera (shown in Figure 1) is a fast-growing tree that is classified as a vegetable that also serves as a medicinal plant (Gopalakrishnan et al., 2016; Trigo et al., 2020). This miracle tree originates from the sub-Himalayan parts of India, and it can be grown in both tropical and subtropical regions and is able to withstand droughts and mild frosty weather, hence it can be cultivated anywhere in the world (Gopalakrishnan et al., 2016). This plant has gained medical and socioeconomic popularity because it has shown great health benefit and it is easy to cultivate (Alegbeleye, 2018; Zhu et al., 2020). Traditionally, it is applied in diets to maintain healthy skin and it has also been used as a decoction to relieve stress and provide energy (Mishra et al., 2011; Kumar et al., 2018). All the parts of the plant can be utilized in a diet or as medicine since they are rich in minerals, proteins, vitamins, polyphenols, flavonoids, glucosinolates, isothiocyanates, alkaloids (Gopalakrishnan et al., 2016; Trigo et al., 2020). For example, the leaves can be eaten raw, dried or taken as an infusion of an aqueous extract, while the bark is boiled in water or soaked in alcohol to make drinks and infusions that help with toothaches, stomach aches, the same is done to the roots (Leone et al., 2015). Furthermore, the leaves are utilized the most for medicinal purposes and they are a great source of prominent anti-inflammatory and antioxidant flavonoids, namely myricetin, quercetin and kaempferol (Vergara-Jimenez et al., 2017). Interesting, these bioactive compounds are known to contain potential anticancer, hypolipidemic, hypotensive and antidiabetic properties, antioxidant and anti-inflammatory (Vergara-Jimenez et al., 2017). Other documented uses for this medicinal plant include its application as a diuretic, a testosterone stimulant, an antifungal and as an antibacterial (Mishra et al., 2011; Kumar et al., 2018). It can also be used to relieve a sore throat and symptoms of influenza, or as an anti-inflammatory agent (Mishra et al., 2011). Interestingly, evidence has grown that Moringa oleifera contains hypoglycemic effects in diabetic animal models, including its associated complications such as oxidative stress and inflammation (Balakrishnan et al., 2019; Chin et al., 2019; Bao et al., 2020).
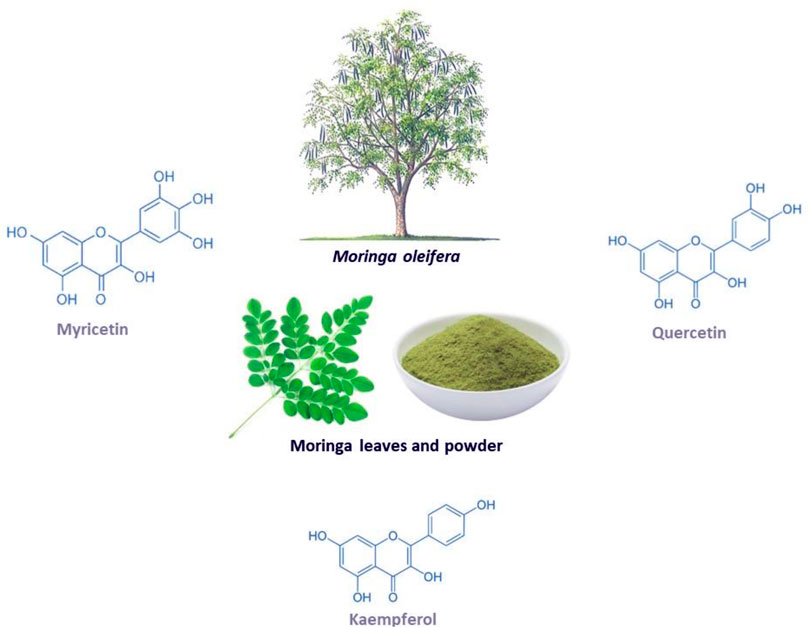
FIGURE 1. The Moringa oleifera plant, including the chemical structures of some of its major flavonoids myricetin, quercetin and kaempferol.
Several pathophysiological mechanisms have been implicated in the aggravation of diabetes-related complications. For instance, individuals with T2D already present with the dyslipidemic feature which is normally characterised by the highly accumulation of lipids and these can easily be attached by free radicals to generate damaging oxidative products (Biswas et al., 2017:; Ito et al., 2019). This consequence is referred to as lipid peroxidation, and it remains as one of the vital parameters used to track the devastating outcomes of oxidative stress in conditions of diabetes or any related metabolic disease (Rahimi et al., 2005; Grotto et al., 2009; Augustine et al., 2021). Within the pathological state, free radicals can be generated through impaired mitochondrial function, or enhanced activities of some enzymes complexes such as NADPH oxidases, in a process like oxidative stress that is known to deplete intracellular antioxidant systems (Mittal et al., 2014; He et al., 2017). Generally, oxidative stress is generated as a disparity in the production of reactive oxygen species (ROS) or reactive nitrogen species, in comparison to counteractive activity of antioxidants in diabetes (Giacco and Brownlee, 2010). Some of the prominent free radical molecules include hydroxyl radical (•OH), superoxide anion (O2•-), peroxynitrite (ONOO−), and all these molecules are crucial for efficient metabolic process in a physiological state (Burgos-mor et al., 2019; Chandra et al., 2019). Also, individuals with T2D display classic signatures of oxidative stress by presenting significantly decreased levels of antioxidant mechanisms such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx), and heme oxygenawe-1 (HO-1) (Matough et al., 2012; Sharma et al., 2012; Dumanović et al., 2021). In diabetes or related metabolic complications, uncontrolled ROS can induce damage to the lipids, proteins, and nucleic acids which lead to impaired signaling mechanisms and activation of pro-inflammatory response (Burgos-mor et al., 2019; Kim et al., 2020). Figure 2 gives an overview of some of the pathophysiological mechanisms implicating the detrimental effects of oxidative stress and inflammation in conditions of diabetes or related metabolic complications.
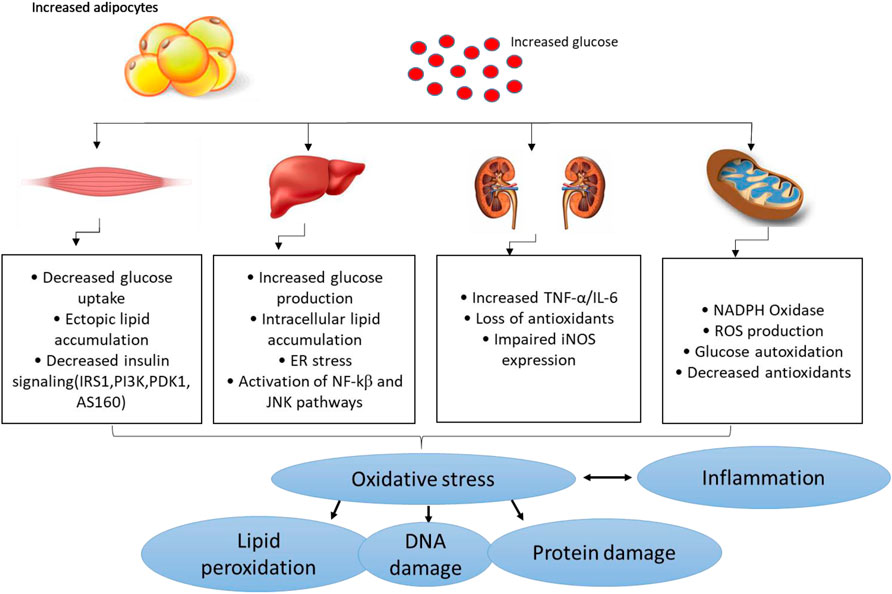
FIGURE 2. An overview of pathological mechanisms implicated in the development of diabetes mellitus or related metabolic complications. Briefly, overnutrition (which may be characterized by increased adipocyte size) and consistent increased levels of glucose (a state of hyperglycemia) may induce detrimental effects in major organs of the body including the skeletal muscle, liver, and kidneys, and thus aggravate metabolic complications through enhanced oxidative stress and exacerbated inflammation. This consequence is predominantly characterized by impaired glucose homeostasis/insulin signaling, ectopic lipid accumulation, mitochondrial dysfunction, endoplasmic reticulum (ER) stress insufficient or decreased antioxidant responses/increased ROS (reactive oxygen species) production and altered actions of inducible nitric oxide synthase (iNOS) and lipid peroxidation/DNA damage. This may occur along with raised pro-inflammatory markers like tumor necrosis factor-alpha (TNF-α), like nuclear factor kappa β (NF-κβ), c-Jun N-terminal kinases (JNK) and interleukin-6 (IL-6).
Currently, both oxidative stress and inflammation have been subject to ongoing research to improve metabolic function in conditions of syndrome (Furman et al., 2019; Monserrat-Mesquida et al., 2020; Oria et al., 2020). Also, accumulative research has evaluated the use of dietary compounds with antioxidant and anti-inflammatory effects such as salvianolic acid, aspalathin and resveratrol in combination with common drugs like metformin to lower glucose as well as attenuate the detrimental effects of oxidative stress and inflammation (Frendo-Cumbo et al., 2016; Wu et al., 2016; Dludla et al., 2018). This has been especially important aspect uncover to improve the long-term protective effects of metformin. The latter is the first line drug for diabetes which works by lowering blood glucose, body weight and lipid levels in the body it also mediates the activation of the AMPK pathway. On the other hand, other antidiabetic drugs like the thiozonidediones have been used to manage T2D, and function by activating peroxisome proliferator activated receptor gamma (PPARγ) and mediate adipogenesis and the uptake of fatty acids in the adipocytes (Greenfield and Chisholm, 2004). This class of drugs improve insulin sensitivity by reducing the circulating fatty acids in the peripheral tissues and can control the production of hormones such as adiponectin to improve metabolic function (Greenfield and Chisholm, 2004). However, like metformin, thiozonidediones are known to present with various side effects (DeFronzo et al., 2016), and their long-term protects effects against deteriorated metabolic function is not proven. This fact, has created opportunities to evaluate alternative regimes, including medicinal plants like Moringa oleifera with antioxidant and anti-inflammatory for their capacity to improve metabolic function in conditions of T2D or metabolic syndrome (Rena et al., 2013; Yendapally et al., 2020). This is especially important since most medicinal plants and bioactive compounds are known to play a major role in cellular detoxifying mechanisms, especially in part activating Nrf2, the major antioxidant response element involved in the attenuation of oxidative stress and an undesired pro-inflammatory response in a disease state (Ma, 2013; Minhaj et al., 2016; Dludla et al., 2017b).
Antioxidants are important substances that aid in eliminating oxidizing agents. Any imbalance of antioxidants caused by oxidative stress may lead to tissue damage (Kurutas, 2016). This may further prompt the disruption of lipids, membranes, nucleic acids and proteins which may further cause detrimental effect and metabolic complications (Phaniendra and Babu, 2015; Pizzino et al., 2017). For years, the first line of drugs for metabolic complications such as diabetes and related metabolic disorders have been metformin, thiazolidinediones and rosiglitazone but literature has proven that plant polyphenols and their bioactive compounds may potentially provide more efficacy in alleviating diabetes, especially through targeting oxidative stress and inflammation to promote human health (Marimoutou et al., 2015; Singh et al., 2016; Taïl et al., 2020; Do et al., 2021). For example, a study showed that Moringa oleifera has great scavenging activity, as measured through the DPPH (1,1-diphenyl-2-picrylhydrazyl (DPPH)-2,2-diphenyl-1-picrylhydrazyl) and ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (Pakade and Chimuka, 1996). However, these results were purely relevant to its potential antioxidant potential, meaning that additional studies, making use of established preclinical and clinical models of diabetes, were still required to confirm the efficacy of this plant. Congruently, the below preclinical and clinical evidence discusses the therapeutic potential of this plant to reduce limit pathological features of oxidative stress and inflammation to alleviate complications linked with diabetes and related metabolic complications, without causing any adverse complications.
Type 1 diabetes mellitus represents approximately 10% of all diagnosed cases of diabetes mellitus, with abnormally increased glucose levels “a state of hyperglycemia” being the major culprit implicated in the detrimental effects associated with this condition (Internationa Diabetes Federation, 2021). Generally accepted as an autoimmune disease that is categorized by immune-mediated damage to pancreatic β-cells, a persistent state of hyperglycemia is accredited for the damaging effects on major organs of the body in those with T1D (Roep et al., 2021). Accordingly, successful establishment of experimental models of T1D, characterized by chronic hyperglycemia, have been predominantly accomplished by employing chemicals that destroy the activity of pancreatic β-cells, triggering impaired insulin secretion (Kottaisamy et al., 2021). Persistent/sustained hyperglycemia is perhaps the main consequence responsible for major organ damage, especially through destructive mechanisms involving activation of oxidative stress and promoting a pro-inflammatory response (Giacco and Brownlee, 2010). Thus far, different animals, mostly rodents have been administering different chemicals such as streptozotocin and alloxan to generate preclinical models of T1D (Kottaisamy et al., 2021). Despite their usefulness in understanding the pathogenesis of T1D, these experimental models have become relevant for screening novel drugs for their potential antidiabetic properties (King, 2012). In fact, besides their potential capacity to reduce the abnormally elevated levels of glucose, increasing research has actively screened novel therapies for their ameliorative effects against oxidative stress and inflammation to alleviate organ damage within a state of T1D (Mima, 2013; Mokgalaboni et al., 2021a; Perreault et al., 2021). This has especially been relevant for plant sources like Moringa oleifera, with known antioxidant and anti-inflammatory properties (Xu et al., 2019).
Evidence summarized in Table 1 reports on the impact of Moringa oleifera extracts on modulating markers of oxidative stress and inflammation in preclinical models of T1D. Importantly, most of these findings indicate that chemical exposure to STZ and alloxan, followed by a state of hyperglycemia, remains a principal method used to induce T1D in these animals. Consequently, most studies showed that Moringa oleifera extracts (at varied doses between 100 and 300 mg/kg) could effectively ameliorate hyperglycemia, when used for a period starting from 2 weeks (Tuorkey, 2016; Oboh et al., 2018), to an average time of 6-weeks (Omodanisi et al., 2017a; Omodanisi et al., 2017b), or even in treatments lasting 8-weeks (Yassa and Tohamy, 2014; Aju et al., 2020). Interestingly, treatment with Moringa oleifera leaf extracts proved effective in wound healing and tissue regeneration in animals exposed to sustained levels of hyperglycemia, when used for an estimated time of 3 weeks (Muhammad et al., 2016; Azevedo et al., 2018). In addition to wound healing properties, the extracts Moringa oleifera showed enhanced protective effects against damage to various organs, including the liver and kidneys, in these preclinical models of T1D (Abd Eldaim et al., 2017; Omodanisi et al., 2017a; Oguntibeju, 2019; Oldoni et al., 2021). The antidiabetic properties of these extracts extend to preventing cognitive or erectile dysfunction in rats, by mainly reducing the activities of enzymes like acetylcholinesterase, angiotensin-I converting enzyme and butyrylcholinesterase (Oboh et al., 2018; Oyeleye et al., 2021). Apparently, the ameliorative effects against oxidative stress or undesired pro-inflammatory response remain the predominant mechanisms by Moringa oleifera extracts protect against complications of T1D in preclinical (animal) models.

TABLE 1. Studies on the effect of Moringa oleifera extracts targeting markers of oxidative stress and inflammation in preclinical models of type 1 diabetes.
For instance, through blockade of lipid peroxidation as well as by reinforcing intracellular antioxidant capacity, as demonstrated by reduced levels of peroxidation products like MDA/thiobarbituric acid reactive species (TBARS) and elevated antioxidant defences such as SOD, GSH, GST and CAT, Moringa oleifera extracts showed enhanced effects in protecting against the detrimental effects of oxidative stress in preclinical models of T1D (Jaiswal et al., 2013; Yassa and Tohamy, 2014; Al-Malki and El Rabey, 2015; Raafat and Hdaib, 2017). In support of this mechanistic insight, it has long been established that induction of diabetes in rats with STZ or alloxan favors uncontrolled availability of lipid peroxidation products, while consequently suppressing the intracellular antioxidant defences (Maritim et al., 2003; Davì et al., 2005). This process prompts excess free radical production, as also observed in patients with T1D (Domínguez et al., 1998), while the accompanied hyperglycemic state may directly contribute to oxidative stress-induced organ damage (Maritim et al., 2003). Besides the harmful effects associated with lipid peroxidation, evidence summarized in Table 1 indicates that treatment with Moringa oleifera extracts for 3 weeks remains effective in targeting other sources of oxidative stress like the mitochondria to ease complications linked with T1D. In actual fact, Alejandra Sánchez-Muñoz and others showed that a leaf extract of this plant improved mitochondrial respiration, while increasing levels of intracellular antioxidants like GSH, glutathione reductase and HO-1 activity, to reduce excess production of ROS liver cells of STZ-induced diabetic rats (Alejandra Sánchez-Muñoz et al., 2018). Generally, these results are of interest as many studies indicate that mitochondria remain one of the major therapeutic targets to ameliorate hyperglycemia-induced oxidative damage (Giacco and Brownlee, 2010; Dludla et al., 2017a; Teodoro et al., 2018).
Consistent with attenuating the destructive effects of oxidative stress, presented evidence showed that Moringa oleifera extracts could effectively reduce the elevated levels of pro-inflammatory mediators such as tumor necrosis factor alpha (TNF-α), IL-1β, IL-6, cyclooxygenase-2 (COX-2), nitric oxide synthase (iNOS), and chemokine (MCP-1) to protect against exacerbated inflammation, under sustained conditions of hyperglycemia (Muhammad et al., 2016; Omodanisi et al., 2017a; Azevedo et al., 2018). Significantly, such positive effects were associated with reduced nephrotoxic and hepatotoxic damage (Oguntibeju, 2019), including upregulation of an angiogenic marker vascular endothelial growth factor (VEGF) to protect against hyperglycemia-induced wound injury in a preclinical model of T1D (Muhammad et al., 2016). This is an essential result since tenacious hyperglycemia, seen in T1D, is already known to mediate iNOS induction, leading to the activation of protein kinase enzymes such as PKC/c-Jun N-terminal kinases (JNKs)/mitogen-activated protein kinase (MAPK) to propagate the detrimental effects of inflammation (Giacco and Brownlee, 2010). Notably, activation of such pro-inflammatory mechanisms can directly cause excess generation of oxidation products that precede the onset of atherosclerosis and endothelial dysfunction, which are major risk factors for the development of cardiovascular diseases (Rose et al., 2010; Mokgalaboni et al., 2020). Although there was limited information on its cardioprotective effects, much evidence suggests Moringa oleifera extracts can significantly decrease pro-inflammatory and apoptotic markers such as TNF-α, IL-1β, IL-6, NF-κβ, caspase 3, caspase 9, and tumor protein (p53) to alleviate the detrimental effects of hyperglycemia in preclinical models of T1D (Muhammad et al., 2016; Omodanisi et al., 2017a; Azevedo et al., 2018; Oguntibeju, 2019). Overall, summarized evidence supports the beneficial effects of Moringa oleifera in lowering hyperglycemia in addition to ameliorating the detrimental effects of oxidative stress and inflammation in preclinical (animal) models of T1D. Some other takeaways from the current results indicate that most studies observed therapeutic effects when using doses between 100 and 300 mg/kg (Al-Malki and El Rabey, 2015; Tuorkey, 2016; Alejandra Sánchez-Muñoz et al., 2018; Azevedo et al., 2018), with an average dose of 250 mg/kg commonly exploited (Abd Eldaim et al., 2017; Omodanisi et al., 2017a; Omodanisi et al., 2017b; Raafat and Hdaib, 2017). Also, most studies reported on the use of leaf extracts over seed extracts of this plant (Table 1). This could be supported by available evidence already supporting the strong antioxidant properties of leaf extracts of over seed extracts (Ilyas et al., 2015; Saini et al., 2016).
Type 2 diabetes, remains the major form of diabetes, contributing to approximately 90% to all global cases of this condition, as regularly reported by the world leading health surveillance organizations (International Diabetes Federation, 2021). Modifiable risk factors, mostly involving sedentary lifestyle, taking place together with overnutrition are to blame for increased cases of T2D, as these factors cause overweight and obesity (Grundy, 2016). In such conditions, increased nutrient availability may drive excessive fat accumulation in key body areas such as the liver, skeletal muscle, blood circulation and heart muscle, leading to the development of pathological complications like non-alcoholic fatty liver disease, muscle wasting or sarcopenia and cardiovascular diseases (Grundy, 2016; Chait and den Hartigh, 2020). Just like in T1D, hyperglycemia remains the major pathological feature of T2D. Besides hyperglycemia, patients with T2D are known to present with insulin resistance and a cluster of other irregularities such as abnormal blood lipid profiles, as observed through aberrant levels of triglycerides, total cholesterol, and low-density lipoprotein cholesterol (Stanford and Goodyear, 2014; Oguntibeju, 2019). However, defects in insulin signaling or a state of insulin resistance has been seen as an early sign of T2D manifestation, which is likely to occur simultaneous to other metabolic dysregulations, including enhanced inflammatory signaling, generation of oxidative products and initiation of endoplasmic reticulum stress pathways (Muoio and Newgard, 2008). These are among the most explored pathological mechanisms in preclinical models of T2D. For example, rodents exposed to a high fat diet (HFD) or its combination with low dose STZ (Waterman et al., 2015; Jaja-Chimedza et al., 2018; Chin et al., 2019; Mohamed et al., 2019; El-Shehawi et al., 2021), as well as gene-defiant mice such as those considered leptin resistance (db/db) (Tang et al., 2017) are known to progressively develop T2D, including its complications involving oxidative stress and inflammation. This explains, the surge use of these preclinical models to test novel treatments against T2D.
Table 2 gives an overview of information on the antidiabetic properties of Moringa oleifera extracts, including its modulatory effects on markers of oxidative stress and inflammation in preclinical models of T2D. Most importantly, summarized evidence showed that these extracts were effective in reducing body weight, body fat mass and fasting plasma glucose levels, which are the major characteristic features of T2D (Tang et al., 2017; Jaja-Chimedza et al., 2018; El-Shehawi et al., 2021). Consistent with evidence summarized in Table 1, blocking hepatic lipid accumulation, in part through effective modulating the makers of oxidative stress and inflammation such as antioxidants like CAT, SOD, MDA content, uncoupling protein 2/3, TNF-α, 1L-β, IL-6, IL-2 and MCP-1 remains the major mechanism of action of these extracts (Joung et al., 2017; Mohamed et al., 2019). Some evidence showed these extracts could improve lipid profiles and reduce the expression of genes involved in energy metabolism or fat synthesis such as fatty-acid synthase, lipoprotein lipase, CCAAT-enhancer-binding protein homologous-α (C/EBPα), sterol regulatory element-binding protein 1c (SREBP1c), within the skeletal muscle (Joung et al., 2017; Tang et al., 2017). Partially indicating that Moringa oleifera extracts may be a potent remedy to decrease excess body fat or ameliorate complications linked with obesity, as reviewed elsewhere (Redha et al., 2021). Of further interest, some evidence indicated that Moringa oleifera extracts could target early pathological signs of T2D, such as improving glucose tolerance and insulin levels, while enhancing insulin sensitivity and glucose homeostasis in tissues of these preclinical models (Jaja-Chimedza et al., 2018; Mohamed et al., 2019). This hypothesis remains to be confirmed in other experimental models of T2D, however provides necessary information to guide future research.
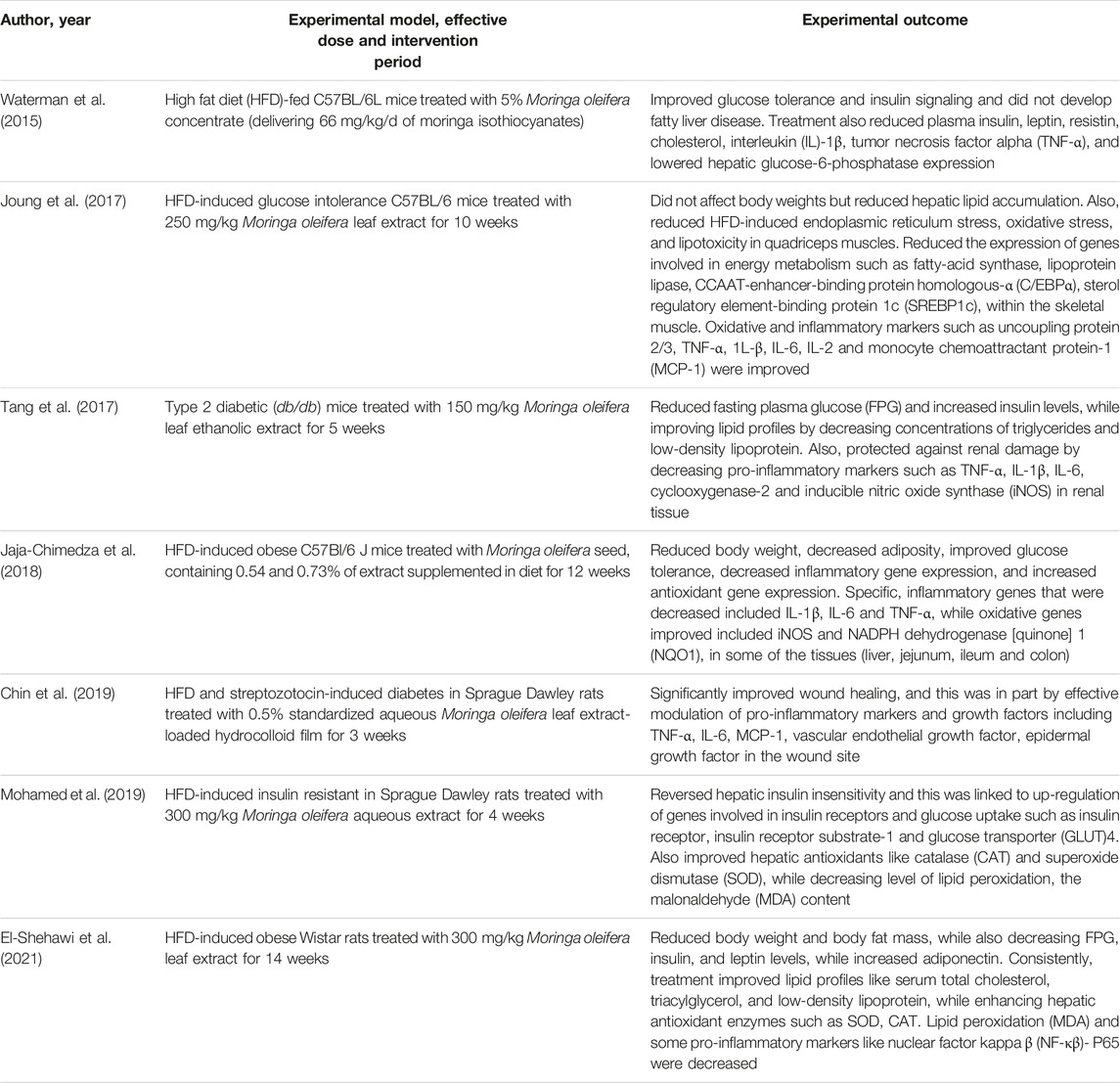
TABLE 2. An overview of studies on the effects of Moringa oleifera extracts targeting markers of oxidative stress and inflammation in preclinical models of type 2 diabetes.
It is currently acknowledged that a large population of people depend on medicinal plants to treat different diseases, which mainly due to ancestral knowledge (Palhares et al., 2015; Muvhulawa et al., 2022). Thus, the general interest in the use of medicinal plants to cure various disease conditions has grown over the years (Rakotoarivelo et al., 2015; Michel et al., 2020). Therefore, it is important for plants to be evaluated for their toxicity to know how safe they are for human use. Accumulative research shows that Moringa oleifera exhibits a lot of important biological properties such as antioxidant, anti-inflammation, anti-hyperglycemic properties over the past years proving that it is a good plant to use as an alternative therapeutic for diabetes (Omodanisi et al., 2017b; Gothai et al., 2017; Paula et al., 2017; Abd et al., 2020; Xiong et al., 2021). In vitro and in vivo studies that have been conducted to show that this plant has no lethal dose and is safe to use. Indeed, work by Villarruel-Lòpez and others showed that the use of Moringa oleifera, at different doses ranging from 100 to 500 mg/kg, is not toxic in rats (Villarruel-López et al., 2018). Albrahim and Binobead also used rats to show that Moringa oleifera alleviates vetsin-induced cytotoxicity, as measured by alterations in liver functions, oxidative stress, DNA damage, and liver injury (Albrahim and Binobead, 2018a). Reviewed evidence from Asare and co-workers revealed that Moringa oleifera is genotoxic at supra-supplementation levels of 3,000 mg/kg body weight, with intake mostly considered is safe at levels ≤1,000 mg/kg (body weight) (Asare et al., 2012). However, other studies have indicated that although available literature is very promising (Awodele et al., 2012; Ajibade et al., 2013; Stohs and Hartman, 2015; Patriota et al., 2020; Siddiqui et al., 2021; Teshome et al., 2021), additional clinical (human) to accomplish standardized extracts of this plant.
Preclinical studies are important to understand the pathophysiological mechanisms implicated in disease development, and this aspect remains essential to explore the therapeutic potential of plant extracts and their derivative compounds (Bonjour et al., 1999; Steinmetz and Spack, 2009). Whereas screening plant extracts and their biological compounds using preclinical models has become a routine procedure to determine effective doses, pharmacokinetic profile and evaluate relevant toxicological aspects before commencement of clinical trials (Bonjour et al., 1999; Steinmetz and Spack, 2009). Although accumulative literature supports the beneficial effects of plant extracts and naturally derived compounds against diabetes (Hung et al., 2012; Jugran et al., 2021), persistent setbacks have been the limited number of studies entering clinical trial phase, which is a vital component in drug development. Likewise, there has been limited number of studies on the antidiabetic properties of Moringa oleifera extracts. At present, only a few randomized controlled trials have been published on the antidiabetic potential (Table 3) of Moringa oleifera. In 2016, Anthanont and co-workers showed that capsules of Moringa oleifera leaf powder (at a dose of 4 g), taken after an overnight fast and every 2 weeks, could significantly increase insulin secretion in healthy subjects (Anthanont et al., 2016). Leone and others demonstrated that randomly giving Saharawi people with diabetes a traditional meal supplemented with 20 g leaf powder of Moringa oleifera on two different days could improve postprandial blood glucose response when compared to nondiabetic controls (Leone et al., 2018). Dixit and co-workers (Dixit et al., 2018) reported that intake of extracts of Moringa oleifera (LI85008F) at 900 mg/day (combined with modest calorie restriction and physical activity) for 16 weeks could reduce waist and hip circumferences, and improved lipid profiles in overweight participants. Also, this was relevant to reduced low-density lipoprotein (LDL) cholesterol decreased, while high-density lipoprotein (HDL) cholesterol increased, resulting in a significantly improved LDL/HDL ratio. While Gómez-Martínez and co-workers reported that giving subjects with prediabetes six daily capsules of Moringa oleifera leaf powder (2,400 mg/day) improved fasting blood glucose and glycated hemoglobin (HbA1c) when compared to the controls. Recently, Díaz-Prieto et al., demonstrated that consumption of 6 × 400 mg capsule/day of Moringa oleifera dry leaf powder for 12 weeks indicated that plasma tumor necrosis factor alpha (TNF-α) was a significant predictor of the subject’s glycated hemoglobin (HbA1c) response in subjects with prediabetes (Díaz-Prieto et al., 2022). These results are consistent with some preclinical findings (Jaja-Chimedza et al., 2018; Mohamed et al., 2019), indicating that Moringa oleifera leaf extracts might be useful complications identified during the early development of T2D. Nonetheless, despite accumulative literature, as reviewed elsewhere (Ba et al., 2020; Watanabe et al., 2021), indicating that this plant might present with important antidiabetic properties, more needs to be done to confirm these in clinical settings. It remains crucial, to evaluate whether Moringa oleifera leaf extracts can prominent biomarkers of oxidative stress and inflammation, to verify preclinical findings.
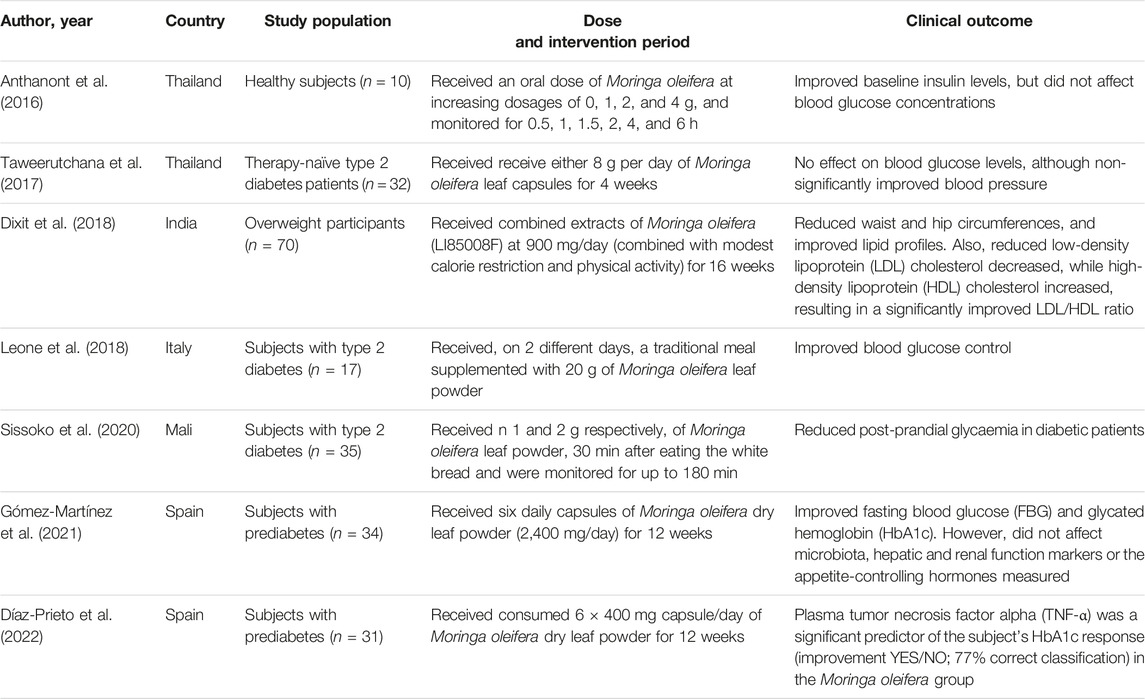
TABLE 3. An overview of clinical studies reporting on the antidiabetic properties of Moringa oleifera.
The swift prevalence of diabetes warrants urgent investigation into novel therapies to protect and better manage this chronic medical condition (International Diabetes Federation, 2021). Metformin and insulin, which are commonly used antidiabetic therapies, have certainly prolonged the lives of patients with diabetes (Joya-Galeana et al., 2011; Foretz et al., 2014; Bailey, 2017). Correspondingly, other effective interventions like physical exercise and caloric striction can be used to manage diabetes (Nyawo et al., 2021; Shakoor et al., 2021; Mthembu et al., 2022), however only a few individuals can constantly adhere to such strenuous interventions. Besides lowering glucose or improving insulin sensitivity, it has become imperative to uncover therapies that can target the amelioration of both oxidative stress and inflammation, as the prime dysregulations implicated in the pathogenesis of diabetes (Vikram et al., 2014; Mahlangu et al., 2020; Mokgalaboni et al., 2021b). This also explains the surge in research investigating the biological properties of nutritional plant sources like rooibos (Aspalathus linearis) and broccoli (Brassica oleracea var. italica) with abundant antioxidants properties in combating metabolic complications like oxidative stress and inflammation (Hwang and Lim, 2014; Dludla et al., 2017a; Orlando et al., 2022).
Plants have been studied for their therapeutic properties and they are also cheap, easily accessible and safer than synthetic conventional drugs (Ahmad et al., 2019). There is growing evidence that plants not only serve as a food source but as medicine, nutraceuticals and so forth (Alegbeleye, 2018). Also, they are a body of polyphenols, vitamins, flavonoids, alkaloids and other important phytochemicals. Moringa oleifera has been proven in a number of studies to alleviate insulin resistance by activating the insulin-independent pathway PI3K/AKT and also through AMPK pathway in the skeletal muscle and it can also improve skeletal muscle oxidative metabolism through the NAD-dependent deacetylase (SIRT1)-PPARα pathway and also through improving fatty acid peroxidation (Bao et al., 2020; Duranti et al., 2021).
In fact, overwhelming evidence summarized in this review supports the beneficial effects of Moringa oleifera in improving blood glucose levels, lipid profiles and insulin sensitivity, in addition to protecting against hepatic or nephrotic damage in preclinical (animal) models of T1D/T2D (Table 1 and 2). Interestingly, these extracts show enhanced effects in strengthening intracellular antioxidants like CAT, SOD, GSH, and GST to lower lipid peroxidation products MDA/TBARS, and reduce prominent pro-inflammatory markers like TNF-α, 1L-β, IL-6, MCP-1, COX-2, and nitric oxide synthase in these animal models. Figure 3 summarizes some therapeutic effects in protecting against oxidative stress and inflammation associated with Moringa oleifera extracts in preclinical models of diabetes. Furthermore, the current literature review indicates the common use of leaf extracts of Moringa oleifera, within a range 100–300 mg/kg, from initial treatment duration of 2 weeks up until 8 weeks (Tables 1, 2). This further sets a platform for future research (which is currently limited) directed at developing Moringa oleifera as a functional food to manage diabetes mellitus. Importantly, additional clinical trials are necessary to evaluate whether Moringa oleifera leaf extracts can prominent biomarkers of oxidative stress and inflammation, to verify preclinical findings.
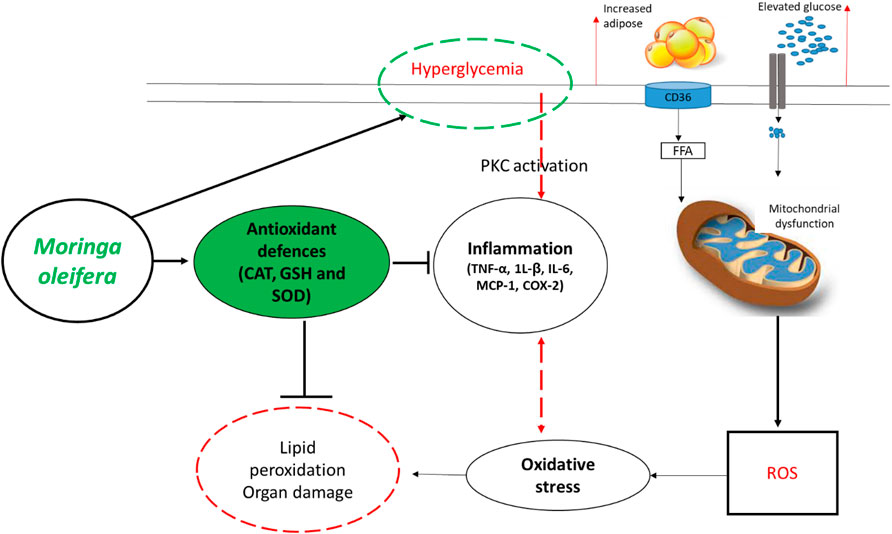
FIGURE 3. An overview of therapeutic mechanisms associated with the ameliorative effects of Moringa oleifera extracts in preclinical (animal) models of diabetes. Briefly, overwhelming evidence supports the beneficial effects of this plant in enhancing intracellular antioxidants such as catalase (CAT), glutathione (GSH) and superoxide dismutase (SOD) to block the detrimental effects reactive oxygen species (ROS), lipid peroxidation and organ damage. This is in part by also improving glucose control (hyperglycemia) and reducing prominent pro-inflammatory markers like tumor necrosis factor-alpha (TNF-α), interleukin (1L)-β, IL-6, monocyte chemoattractant protein-1 (MCP-1) and COX-2-cyclooxygenase-2 (COX-2). Abbreviations: CD36-cluster of differentiation 36; FFA-free fatty acid; PKC-protein kinase C. Indicators: red lines-detrimental effects, bold lines/green lines-protective effects of Moringa oleifera extracts.
FM, PD, and SM-M concept and original draft. All authors, including FM, PD, KZ, SM, NM, NH, BN, and SM-M wrote and approved the final manuscript.
This work was funded by the National Research Foundation (NRF) Thuthuka Programme grant 128296 to SM-M. Funding from North-West University and the University of Zululand is also acknowledged. The work reported herein was made possible through funding by the South African Medical Research Council (SAMRC) through its Division of Research of Capacity Development under the Early Investigators Programme from the South African National Treasury (funding number: HDID8682/MB2022/EIP052). The content hereof is the sole responsibility of the authors and do not necessarily represent the official views of the SAMRC. Also, all the content expressed in this review is the official views of the authors and do not represent that of the North-West University or the University of Zululand. FM acknowledges funding by the NRF, Thuthuka grant UID 128296 linked to SM-M.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Grant holders acknowledge that opinions, findings and conclusions or recommendations expressed in any publication generated by the SAMRC supported research are those of the authors, and that the SAMRC accepts no liability whatsoever in this regard. SM and KZ are funded by the South African Medical Research Council (SAMRC) through its Division of Research Capacity Development under the internship scholarship program from funding received from the South African National Treasury.
Abd Eldaim, M. A., Shaban Abd Elrasoul, A., and Abd Elaziz, S. A. (2017). An Aqueous Extract from Moringa Oleifera Leaves Ameliorates Hepatotoxicity in Alloxan-Induced Diabetic Rats. Biochem. Cell. Biol. 95, 524–530. doi:10.1139/bcb-2016-0256
Abd, H. H., Ahmed, H. A., and Mutar, T. F. (2020). Moringa Oleifera Leaves Extract Modulates Toxicity, Sperms Alterations, Oxidative Stress, and Testicular Damage Induced by Tramadol in Male Rats. Toxicol. Res. (Camb) 9, 101–106. doi:10.1093/toxres/tfaa009
Abd Rani, N. Z., Husain, K., and Kumolosasi, E. (2018). Moringa Genus: A Review of Phytochemistry and Pharmacology. Front. Pharmacol. 9, 108. doi:10.3389/fphar.2018.00108
Ahmad, J., Khan, I., and Blundell, R. (2019). Moringa Oleifera and Glycemic Control: A Review of Current Evidence and Possible Mechanisms. Phytother. Res. 33, 2841–2848. doi:10.1002/ptr.6473
Ajibade, T. O., Arowolo, R., and Olayemi, F. O. (2013). Phytochemical Screening and Toxicity Studies on the Methanol Extract of the Seeds of Moringa Oleifera. J. Complement. Integr. Med. 10. doi:10.1515/jcim-2012-0015
Aju, B. Y., Rajalakshmi, R., and Mini, S. (2020). Corrigendum to "Protective role of Moringa oleifera leaf extract on cardiac antioxidant status and lipid peroxidation in streptozotocin induced diabetic rats" [Heliyon 5, (12), (December 2019), e02935]. [HeliyonHeliyon 56 (12), e02935e03146. doi:10.1016/j.heliyon.2019.e03146
Aju, B. Y., Rajalakshmi, R., and Mini, S. (2019). Protective Role of Moringa Oleifera Leaf Extract on Cardiac Antioxidant Status and Lipid Peroxidation in Streptozotocin Induced Diabetic Rats. Heliyon 5, e02935. doi:10.1016/j.heliyon.2019.e02935
Al-Malki, A. L., and El Rabey, H. A. (2015). The Antidiabetic Effect of Low Doses ofMoringa oleiferaLam. Seeds on Streptozotocin Induced Diabetes and Diabetic Nephropathy in Male Rats. BioMed Res. Int. 2015, 13. doi:10.1155/2015/381040
Albrahim, T., and Binobead, M. A. (2018a). Roles of Moringa Oleifera Leaf Extract in Improving the Impact of High Dietary Intake of Monosodium Glutamate-Induced Liver Toxicity, Oxidative Stress, Genotoxicity, DNA Damage, and PCNA Alterations in Male Rats. Oxidative Med. Cell. Longev. 2018, 1–11. doi:10.1155/2018/4501097
Alegbeleye, O. O. (2018). How Functional Is Moringa Oleifera? A Review of its Nutritive, Medicinal, and Socioeconomic Potential. Food Nutr. Bull. 39, 149–170. doi:10.1177/0379572117749814
Alejandra Sánchez-Muñoz, M., Valdez-Solana, M. A., Campos-Almazán, M. I., Flores-Herrera, Ó., Esparza-Perusquía, M., Olvera-Sánchez, S., et al. (2018). Streptozotocin-induced Adaptive Modification of Mitochondrial Supercomplexes in Liver of Wistar Rats and the Protective Effect of. Moringa oleifera Lam. Biochem. Res. Int. 2018, 5681081. doi:10.1155/2018/5681081
Anthanont, P., Lumlerdkij, N., Akarasereenont, P., Vannasaeng, S., and Sriwijitkamol, A. (2016). Moringa Oleifera Leaf Increases Insulin Secretion after Single Dose Administration: A Preliminary Study in Healthy Subjects. J. Med. Assoc. Thai. 99, 308–313.
Asare, G. A., Gyan, B., Bugyei, K., Adjei, S., Mahama, R., Addo, P., et al. (2012). Toxicity Potentials of the Nutraceutical Moringa Oleifera at Supra-supplementation Levels. J. Ethnopharmacol. 139, 265–272. doi:10.1016/j.jep.2011.11.009
Augustine, R., Hasan, A., Dalvi, Y. B., Rehman, S. R. U., Varghese, R., Unni, R. N., et al. (2021). Growth Factor Loaded In Situ Photocrosslinkable Poly (3-Hydroxybutyrate-Co-3-Hydroxyvalerate)/gelatin Methacryloyl Hybrid Patch for Diabetic Wound Healing. Mat. Sci. Eng. C Mat. Biol. Appl. 118, 111519. doi:10.1016/j.msec.2020.111519
Awodele, O., Oreagba, I. A., Odoma, S., Teixeira Da Silva, J. A., and Osunkalu, V. O. (2012). Toxicological Evaluation of the Aqueous Leaf Extract of Moringa Oleifera Lam. (Moringaceae). J. Ethnopharmacol. 139, 330–336. doi:10.1016/j.jep.2011.10.008
Azevedo, Í. M., Araújo-Filho, I., Teixeira, M. M. A., Moreira, M., and Medeiros, A. C. (2018). Wound Healing of Diabetic Rats Treated with Moringa Oleifera Extract. Acta Cir. Bras. 33, 799–805. doi:10.1590/s0102-865020180090000008
Ba, F, B. M., Sene, M., Sambou, J. K., Gueye, M. M., and Ba, E. H. M. (2020). Antidiabetic Properties of Moringa Oleifera: A Review of the Literature. J. Diabetes Endocrinol. 11, 18–29. doi:10.5897/JDE2019.0136
Bailey, C. J. (2017). Metformin: Historical Overview. Diabetologia 60, 1566–1576. doi:10.1007/s00125-017-4318-z
Balakrishnan, B. B., Krishnasamy, K., Mayakrishnan, V., and Selvaraj, A. (2019). Moringa Concanensis Nimmo Extracts Ameliorates Hyperglycemia-Mediated Oxidative Stress and Upregulates PPARγ and GLUT4 Gene Expression in Liver and Pancreas of Streptozotocin-Nicotinamide Induced Diabetic Rats. Biomed. Pharmacother. 112, 108688. doi:10.1016/j.biopha.2019.108688
Bao, Y., Xiao, J., Weng, Z., Lu, X., Shen, X., and Wang, F. (2020). A Phenolic Glycoside from Moringa Oleifera Lam. Improves the Carbohydrate and Lipid Metabolisms through AMPK in Db/db Mice. Food Chem. 311, 125948. doi:10.1016/j.foodchem.2019.125948
Biswas, S., Das, R., and Banerjee, E. R. (2017). Role of Free Radicals in Human Inflammatory Diseases. AIMS Biophys. 4, 596–614. doi:10.3934/biophy.2017.4.596
Bonjour, J. P., Ammann, P., and Rizzoli, R. (1999). Importance of Preclinical Studies in the Development of Drugs for Treatment of Osteoporosis: a Review Related to the 1998 WHO Guidelines. Osteoporos. Int. 9, 379–393. doi:10.1007/s001980050161
Burgos-Mor, E., Abad-Jim, Z., Mart, A., Marañ, D., Iannantuoni, F., Escribano-L, I., et al. (2019). Relationship between Oxidative Stress , Er Stress , and Inflammation in Type 2 Diabetes : the Battle Continues. J. Clin. Med. 4, 1385. doi:10.3390/jcm809138510.3390/jcm8091385
Chait, A., and Den Hartigh, L. J. (2020). Adipose Tissue Distribution, Inflammation and its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 7, 22. doi:10.3389/fcvm.2020.00022
Chandra, K., Dwivedi, S., and Jain, S. K. (2019). Diabetes Mellitus and Oxidative Stress: A Co-relative and Therapeutic Approach. J. Clin. Diagnostic Res. 13, BE07–BE12. doi:10.7860/JCDR/2019/40628.12878
Chin, C. Y., Ng, P. Y., and Ng, S. F. (2019). Moringa Oleifera Standardised Aqueous Leaf Extract-Loaded Hydrocolloid Film Dressing: In Vivo Dermal Safety and Wound Healing Evaluation in STZ/HFD Diabetic Rat Model. Drug Deliv. Transl. Res. 9, 453–468. doi:10.1007/s13346-018-0510-z
Costa, I. S., Medeiros, A. F., Piuvezam, G., Medeiros, G., Maciel, B. L. L., and Morais, A. H. A. (2020). Insulin-like Proteins in Plant Sources: A Systematic Review. Diabetes Metab. Syndr. Obes. 13, 3421–3431. doi:10.2147/DMSO.S256883
Davì, G., Falco, A., and Patrono, C. (2005). Lipid Peroxidation in Diabetes Mellitus. Antioxid. Redox Signal. 7, 256–268. doi:10.1089/ars.2005.7.256
Defronzo, R., Fleming, G. A., Chen, K., and Bicsak, T. A. (2016). Metformin-associated Lactic Acidosis: Current Perspectives on Causes and Risk. Metabolism 65, 20–29. doi:10.1016/j.metabol.2015.10.014
Díaz-Prieto, L. E., Gómez-Martínez, S., Vicente-Castro, I., Heredia, C., González-Romero, E. A., Martín-Ridaura, M. D. C., et al. (2022). Effects of Moringa Oleifera Lam. Supplementation on Inflammatory and Cardiometabolic Markers in Subjects with Prediabetes. Nutrients 14, 1937. doi:10.3390/nu14091937
Dixit, K., Kamath, D. V., Alluri, K. V., and Davis, B. A. (2018). Efficacy of a Novel Herbal Formulation for Weight Loss Demonstrated in a 16-week Randomized, Double-Blind, Placebo-Controlled Clinical Trial with Healthy Overweight Adults. Diabetes Obes. Metab. 20, 2633–2641. doi:10.1111/dom.13443
Dludla, P. V., Gabuza, K. B., Muller, C. J. F., Joubert, E., Louw, J., and Johnson, R. (2018). Aspalathin, a C-Glucosyl Dihydrochalcone from Rooibos Improves the Hypoglycemic Potential of Metformin in Type 2 Diabetic (Db/db) Mice. Physiol. Res. 67, 813–818. doi:10.33549/physiolres.933891
Dludla, P. V., Joubert, E., Muller, C. J. F., Louw, J., and Johnson, R. (2017a). Hyperglycemia-induced Oxidative Stress and Heart Disease-Cardioprotective Effects of Rooibos Flavonoids and Phenylpyruvic Acid-2-O-β-D-Glucoside. Nutr. Metab. (Lond) 14, 45. doi:10.1186/s12986-017-0200-8
Dludla, P. V., Muller, C. J., Joubert, E., Louw, J., Essop, M. F., Gabuza, K. B., et al. (2017b). Aspalathin Protects the Heart against Hyperglycemia-Induced Oxidative Damage by Up-Regulating Nrf2 Expression. Molecules 22, 129. doi:10.3390/molecules22010129
Dludla, P. V., Nkambule, B. B., Jack, B., Mkandla, Z., Mutize, T., Silvestri, S., et al. (2019). Inflammation and Oxidative Stress in an Obese State and the Protective Effects of Gallic Acid. Nutrients 11, 23. doi:10.3390/nu11010023
Dludla, P. V., Nkambule, B. B., Mazibuko-Mbeje, S. E., Nyambuya, T. M., Mxinwa, V., Mokgalaboni, K., et al. (2021). Adipokines as a Therapeutic Target by Metformin to Improve Metabolic Function: A Systematic Review of Randomized Controlled Trials. Pharmacol. Res. 163, 105219. doi:10.1016/j.phrs.2020.105219
Do, B. H., Hoang, N. S., Nguyen, T. P. T., Ho, N. Q. C., Le, T. L., and Doan, C. C. (2021). Phenolic Extraction of Moringa Oleifera Leaves Induces Caspase-dependent and Caspase-independent Apoptosis through the Generation of Reactive Oxygen Species and the Activation of Intrinsic Mitochondrial Pathway in Human Melanoma Cells. Nut.r Cancer 73, 869–888. doi:10.1080/01635581.2020.1776885
Domínguez, C., Ruiz, E., Gussinye, M., and Carrascosa, A. (1998). Oxidative Stress at Onset and in Early Stages of Type 1 Diabetes in Children and Adolescents. Diabetes Care 21, 1736–1742. doi:10.2337/diacare.21.10.1736
Dumanović, J., Nepovimova, E., Natić, M., Kuča, K., and Jaćević, V. (2021). The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: a Concise Overview. Front. Plant Sci. 11, 2106. doi:10.3389/fpls.2020.552969
Duranti, G., Maldini, M., Crognale, D., Sabatini, S., Corana, F., Horner, K., et al. (2021). Moringa Oleifera Leaf Extract Influences Oxidative Metabolism in C2C12 Myotubes through SIRT1-Pparα Pathway. Phytomedicine Plus 1, 100014. doi:10.1016/j.phyplu.2020.100014
El-Shehawi, A. M., Alkafafy, M., El-Shazly, S., Sayed, S., Farouk, S., Alotaibi, S., et al. (2021). Moringa Oleifera Leaves Ethanolic Extract Ameliorates High Fat Diet-Induced Obesity in Rats. J. King Saud Univ. - Sci. 33, 101552. doi:10.1016/j.jksus.2021.101552
Foretz, M., Guigas, B., Bertrand, L., Pollak, M., and Viollet, B. (2014). Metformin: from Mechanisms of Action to Therapies. Cell Metab. 20, 953–966. doi:10.1016/j.cmet.2014.09.018
Fowler, M. J. (2007). Diabetes: Magnitude and Mechanisms. Clin. Diabetes 28, 42–46. doi:10.2337/diaclin.28.1.42
Francini, F., Schinella, G. R., and Ríos, J. L. (2019). Activation of AMPK by Medicinal Plants and Natural Products: its Role in Type 2 Diabetes Mellitus. Mini Rev. Med. Chem. 19, 880–901. doi:10.2174/1389557519666181128120726
Frendo-Cumbo, S., Macpherson, R. E., and Wright, D. C. (2016). Beneficial Effects of Combined Resveratrol and Metformin Therapy in Treating Diet-Induced Insulin Resistance. Physiol. Rep. 4, e12877. doi:10.14814/phy2.12877
Furman, D., Campisi, J., Verdin, E., Carrera-Bastos, P., Targ, S., Franceschi, C., et al. (2019). Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 25, 1822–1832. doi:10.1038/s41591-019-0675-0
Giacco, F., and Brownlee, M. (2010). Oxidative Stress and Diabetic Complications. Circ. Res. 107, 1058–1070. doi:10.1161/CIRCRESAHA.110.223545
Gómez-Martínez, S., Díaz-Prieto, L. E., Vicente Castro, I., Jurado, C., Iturmendi, N., Martín-Ridaura, M. C., et al. (2021). Moringa Oleifera Leaf Supplementation as a Glycemic Control Strategy in Subjects with Prediabetes. Nutrients 14, 57. doi:10.3390/nu14010057
Gopalakrishnan, L., Doriya, K., and Kumar, D. S. (2016). Moringa Oleifera: A Review on Nutritive Importance and its Medicinal Application. Food Sci. Hum. Wellness 5, 49–56. doi:10.1016/j.fshw.2016.04.001
Gothai, S., Muniandy, K., Zarin, M. A., Sean, T. W., Kumar, S. S., Munusamy, M. A., et al. (2017). Chemical Composition of Moringa Oleifera Ethyl Acetate Fraction and its Biological Activity in Diabetic Human Dermal Fibroblasts. Pharmacogn. Mag. 13, S462–S469. doi:10.4103/pm.pm_368_16
Greenfield, J. R., and Chisholm, D. J. (2004). Experimental and Clinical Pharmacology Thiazolidinediones–Mechanisms of Action. Aust. Prescr. 27 (3), 67–70. doi:10.18773/austprescr.05910.18773/austprescr.2004.059
Grotto, D., Maria, L. S., Valentini, J., Paniz, C., Schmitt, G., Garcia, S. C., et al. (2009). Importance of the Lipid Peroxidation Biomarkers and Methodological Aspects for Malondialdehyde Quantification. Quimica Nova 32, 169–174. doi:10.1590/S0100-40422009000100032
Grundy, S. M. (2016). Metabolic Syndrome Update. Trends cardiovasc. Med. 26, 364–373. doi:10.1016/j.tcm.2015.10.004
He, L., He, T., Farrar, S., Ji, L., Liu, T., and Ma, X. (2017). Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. biochem. 44, 532–553. doi:10.1159/000485089
Huang, X., Liu, G., Guo, J., and Su, Z. (2018). The PI3K/AKT Pathway in Obesity and Type 2 Diabetes. Int. J. Biol. Sci. 14, 1483–1496. doi:10.7150/ijbs.27173
Hung, H. Y., Qian, K., Morris-Natschke, S. L., Hsu, C. S., and Lee, K. H. (2012). Recent Discovery of Plant-Derived Anti-diabetic Natural Products. Nat. Prod. Rep. 29, 580–606. doi:10.1039/c2np00074a
Hwang, J. H., and Lim, S. B. (2014). Antioxidant and Anti-inflammatory Activities of Broccoli Florets in Lps-Stimulated Raw 264.7 Cells. Prev. Nutr. Food Sci. 19, 89–97. doi:10.3746/pnf.2014.19.2.089
Ilyas, M., Arshad, M. U., Saeed, F., and Iqbal, M. (2015). Antioxidant Potential and Nutritional Comparison of Moringa Leaf and Seed Powders and Their Tea Infusions. J. Animal Plant Sci. 25, 226–233.
International Diabetes Federation (IDF) (2021). IDF Diabetes Atlas 2021. Available at: https://diabetesatlas.org/(Accessed February 01, 2022).
Ito, F., Sono, Y., and Ito, T. (2019). Measurement and Clinical Significance of Lipid Peroxidation as a Biomarker of Oxidative Stress: Oxidative Stress in Diabetes, Atherosclerosis, and Chronic Inflammation. Antioxidants 8, 72. doi:10.3390/antiox8030072
Jaiswal, D., Rai, P. K., Mehta, S., Chatterji, S., Shukla, S., Rai, D. K., et al. (2013). Role of Moringa Oleifera in Regulation of Diabetes-Induced Oxidative Stress. Asian pac. J. Trop. Med. 6, 426–432. doi:10.1016/S1995-7645(13)60068-1
Jaja-Chimedza, A., Zhang, L., Wolff, K., Graf, B. L., Kuhn, P., Moskal, K., et al. (2018). A Dietary Isothiocyanate-Enriched Moringa (Moringa Oleifera) Seed Extract Improves Glucose Tolerance in a High-Fat-Diet Mouse Model and Modulates the Gut Microbiome. J. Funct. Foods 47, 376–385. doi:10.1016/j.jff.2018.05.056
Joung, H., Kim, B., Park, H., Lee, K., Kim, H. H., Sim, H. C., et al. (2017). Fermented Moringa Oleifera Decreases Hepatic Adiposity and Ameliorates Glucose Intolerance in High-Fat Diet-Induced Obese Mice. J. Med. Food 20, 439–447. doi:10.1089/jmf.2016.3860
Joya-Galeana, J., Fernandez, M., Cervera, A., Reyna, S., Ghosh, S., Triplitt, C., et al. (2011). Effects of Insulin and Oral Anti-diabetic Agents on Glucose Metabolism, Vascular Dysfunction and Skeletal Muscle Inflammation in Type 2 Diabetic Subjects. Diabetes Metab. Res. Rev. 27, 373–382. doi:10.1002/dmrr.1185
Jugran, A. K., Rawat, S., Devkota, H. P., Bhatt, I. D., and Rawal, R. S. (2021). Diabetes and Plant-Derived Natural Products: From Ethnopharmacological Approaches to Their Potential for Modern Drug Discovery and Development. Phytothe.r Res. 35, 223–245. doi:10.1002/ptr.6821
Kim, J.-H., Cho, J.-H., Kim, S.-R., and Hur, Y. B. (2020). Toxic Effects of Waterborne Ammonia Exposure on Hematological Parameters, Oxidative Stress and Stress Indicators of Juvenile Hybrid Grouper, Epinephelus Lanceolatus × Epinephelus Fuscoguttatus. Environ. Toxicol. Pharmacol. 80, 103453. doi:10.1016/j.etap.2020.103453
King, A. J. (2012). The Use of Animal Models in Diabetes Research. Br. J. Pharmacol. 166, 877–894. doi:10.1111/j.1476-5381.2012.01911.x
King, G. L., and Brownlee, M. (1996). The Cellular and Molecular Mechanisms of Diabetic Complications. Endocrinol. Metab. Clin. North. Am. 25, 255–270. doi:10.1016/s0889-8529(05)70324-8
Kottaisamy, C. P. D., Raj, D. S., Prasanth Kumar, V., and Sankaran, U. (2021). Experimental Animal Models for Diabetes and its Related Complications-A Review. Lab. Anim. Res. 37, 23. doi:10.1186/s42826-021-00101-4
Kumar, R. P. S., Arts, K., Veluswamy, B., Arts, K., and Malayaman, V. (2018). Phytochemical screening of aqueous leaf extract of Sida acuta Burm . F . and its antibacterial activity. J. Emerg. Technol. Innovative Res. (JETIR) 5, 472–478.
Kurutas, E. B. (2016). The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr. J. 15, 71. doi:10.1186/s12937-016-0186-5
Leone, A., Bertoli, S., Di Lello, S., Bassoli, A., Ravasenghi, S., Borgonovo, G., et al. (2018). Effect of Moringa oleifera leaf powder on postprandial blood glucose response: In Vivo study on saharawi people living in refugee camps. Nutrients 10, 1494. doi:10.3390/nu10101494
Leone, A., Spada, A., Battezzati, A., Schiraldi, A., Aristil, J., and Bertoli, S. (2015). Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera Leaves: an overview. Int. J. Mol. Sci. 16, 12791–12835. doi:10.3390/ijms160612791
Long, Y. C., and Zierath, J. R. (2006). AMP-activated protein kinase signaling in metabolic regulation. J. Clin. Invest. 116, 1776–1783. doi:10.1172/JCI29044
Louisa, M., Patintingan, C. G. H., and Wardhani, B. W. K. (2022). Moringa Oleifera Lam. in cardiometabolic disorders: a systematic review of recent studies and possible mechanism of actions. Front. Pharmacol. 13, 792794. doi:10.3389/fphar.2022.792794
Ma, Q. (2013). Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 53, 401–426. doi:10.1146/annurev-pharmtox-011112-140320
Mahlangu, T., Dludla, P. V., Nyambuya, T. M., Mxinwa, V., Mazibuko-Mbeje, S. E., Cirilli, I., et al. (2020). A systematic review on the functional role of Th1/Th2 cytokines in type 2 diabetes and related metabolic complications. Cytokine 126, 154892. doi:10.1016/j.cyto.2019.154892
Marimoutou, M., Le Sage, F., Smadja, J., Lefebvre D’hellencourt, C., Gonthier, M.-P., and Robert-Da Silva, C. (2015). Antioxidant polyphenol-rich extracts from the medicinal plants Antirhea borbonica, Doratoxylon apetalum and Gouania mauritiana protect 3T3-L1 preadipocytes against H2O2, TNFα and LPS inflammatory mediators by regulating the expression of superoxide dismutase and NF-κB genes. J. Inflamm. 12, 10. doi:10.1186/s12950-015-0055-6
Maritim, A. C., Sanders, R. A., and Watkins, J. B. (2003). Diabetes, oxidative stress, and antioxidants: a review. J. Biochem. Mol. Toxicol. 17, 24–38. doi:10.1002/jbt.10058
Matough, F. A., Budin, S. B., Hamid, Z. A., Alwahaibi, N., and Mohamed, J. (2012). The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ. Med. J. 12, 5–18. doi:10.12816/0003082
Mazibuko-Mbeje, S. E., Dludla, P. V., Nkambule, B. B., Obonye, N., and Louw, J. (2018). “The role of glucose and fatty acid metabolism in the development of insulin resistance in skeletal muscle,” in Muscle Cell and Tissue-Current Status of Research Field (London: IntechOpen). doi:10.5772/intechopen.75904
Mazibuko-Mbeje, S. E., Dludla, P. V., Roux, C., Johnson, R., Ghoor, S., Joubert, E., et al. (2019). Aspalathin-enriched green rooibos extract reduces hepatic insulin resistance by modulating PI3K/AKT and AMPK pathways. Int. J. Mol. Sci. 20, 633. doi:10.3390/ijms20030633
Michel, J., Abd Rani, N. Z., and Husain, K. (2020). A review on the potential use of medicinal plants from asteraceae and lamiaceae plant family in cardiovascular diseases. Front. Pharmacol. 11, 852. doi:10.3389/fphar.2020.00852
Mima, A. (2013). Inflammation and oxidative stress in diabetic nephropathy: new insights on its inhibition as new therapeutic targets. J. Diabetes Res. 2013, 248563. doi:10.1155/2013/248563
Minhaj, S., Ahmed, U., Luo, L., Namani, A., Wang, X. J., and Tang, X. (2016). Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 1863 (2), 585–597. doi:10.1016/j.bbadis.2016.11.005
Mishra, G., Singh, P., Verma, R., Kumar, S., Srivastav, S., Jha, K., et al. (2011). Traditional uses, phytochemistry and pharmacological properties of Moringa oleifera plant: An overview. Der Pharm. Lett. 3, 141–164.
Mittal, M., Siddiqui, M. R., Tran, K., Reddy, S. P., and Malik, A. B. (2014). Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 20, 1126–1167. doi:10.1089/ars.2012.5149
Mohamed, M. A., Ahmed, M. A., and El Sayed, R. A. (2019). Molecular effects of Moringa leaf extract on insulin resistance and reproductive function in hyperinsulinemic male rats. J. Diabetes Metab. Disord. 18, 487–494. doi:10.1007/s40200-019-00454-7
Mokgalaboni, K., Dludla, P. V., Nyambuya, T. M., Yakobi, S. H., Mxinwa, V., and Nkambule, B. B. (2020). Monocyte-mediated inflammation and cardiovascular risk factors in type 2 diabetes mellitus: A systematic review and meta-analysis of pre-clinical and clinical studies. JRSM Cardiovasc. Dis. 9, 2048004019900748. doi:10.1177/2048004019900748
Mokgalaboni, K., Nkambule, B. B., Ntamo, Y., Ziqubu, K., Nyambuya, T. M., Mazibuko-Mbeje, S. E., et al. (2021a). Vitamin K: A vital micronutrient with the cardioprotective potential against diabetes-associated complications. Life Sci. 286, 120068. doi:10.1016/j.lfs.2021.120068
Mokgalaboni, K., Ntamo, Y., Ziqubu, K., Nyambuya, T. M., Nkambule, B. B., Mazibuko-Mbeje, S. E., et al. (2021b). Curcumin supplementation improves biomarkers of oxidative stress and inflammation in conditions of obesity, type 2 diabetes and NAFLD: updating the status of clinical evidence. Food Funct. 12, 12235–12249. doi:10.1039/d1fo02696h
Monserrat-Mesquida, M., Quetglas-Llabrés, M., Capó, X., Bouzas, C., Mateos, D., Pons, A., et al. (2020). Metabolic syndrome is associated with oxidative stress and proinflammatory state. Antioxidants 9, 236. doi:10.3390/antiox9030236
Mthembu, S. X. H., Mazibuko-Mbeje, S. E., Ziqubu, K., Nyawo, T. A., Obonye, N., Nyambuya, T. M., et al. (2022). Impact of physical exercise and caloric restriction in patients with type 2 diabetes: Skeletal muscle insulin resistance and mitochondrial dysfunction as ideal therapeutic targets. Life Sci. 297, 120467. doi:10.1016/j.lfs.2022.120467
Muhammad, A. A., Arulselvan, P., Cheah, P. S., Abas, F., and Fakurazi, S. (2016). Evaluation of wound healing properties of bioactive aqueous fraction from Moringa oleifera Lam on experimentally induced diabetic animal model. Drug Des. devel. Ther. 10, 1715–1730. doi:10.2147/DDDT.S96968
Muoio, D. M., and Newgard, C. B. (2008). Mechanisms of disease:Molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat. Rev. Mol. Cell. Biol. 9, 193–205. doi:10.1038/nrm2327
Muvhulawa, N., Dludla, P. V., Ziqubu, K., Mthembu, S. X. H., Mthiyane, F., Nkambule, B. B., et al. (2022). Rutin ameliorates inflammation and improves metabolic function: A comprehensive analysis of scientific literature. Pharmacol. Res. 178, 106163. doi:10.1016/j.phrs.2022.106163
Nyawo, T. A., Pheiffer, C., Mazibuko-Mbeje, S. E., Mthembu, S. X. H., Nyambuya, T. M., Nkambule, B. B., et al. (2021). Physical exercise potentially targets epicardial adipose tissue to reduce cardiovascular disease risk in patients with metabolic diseases: oxidative stress and inflammation emerge as major therapeutic targets. Antioxidants (Basel) 10, 1758. doi:10.3390/antiox10111758
Oboh, G., Oyeleye, S. I., Akintemi, O. A., and Olasehinde, T. A. (2018). Moringa oleifera supplemented diet modulates nootropic-related biomolecules in the brain of STZ-induced diabetic rats treated with acarbose. Metab. Brain Dis. 33, 457–466. doi:10.1007/s11011-018-0198-2
Oguntibeju, Oo, Aboua, G. Y., and Omodanisi, E. I. (2020). Effects of Moringa oleifera on oxidative stress, apoptotic and inflammatory biomarkers in streptozotocin-induced diabetic animal model. S. Afr. J. Bot. 129, 354–365. doi:10.1016/j.sajb.2019.08.039
Oguntibeju, O. O. (2019). Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 11, 45–63.
Oldoni, T. L. C., Merlin, N., Bicas, T. C., Prasniewski, A., Carpes, S. T., Ascari, J., et al. (2021). Antihyperglycemic activity of crude extract and isolation of phenolic compounds with antioxidant activity from Moringa oleifera Lam. leaves grown in Southern Brazil. Food Res. Int. 141, 110082. doi:10.1016/j.foodres.2020.110082
Omodanisi, E. I., Aboua, Y. G., Chegou, N. N., and Oguntibeju, O. O. (2017a). Hepatoprotective, antihyperlipidemic, and anti-inflammatory activity of Moringa oleifera in diabetic-induced damage in male Wistar rats. Pharmacogn. Res. 9, 182–187. doi:10.4103/0974-8490.204651
Omodanisi, E. I., Aboua, Y. G., and Oguntibeju, O. O. (2017b). Assessment of the anti-hyperglycaemic, anti-inflammatory and antioxidant activities of the methanol extract of Moringa Oleifera in diabetes-induced nephrotoxic male wistar rats. Molecules 22, 439. doi:10.3390/molecules22040439
Oria, R. D., Schipani, R., Leonardini, A., Natalicchio, A., Perrini, S., Cignarelli, A., et al. (2020). Review article the role of oxidative stress in cardiac disease : from physiological response to injury factor. Oxid. Med. Cell. Longev. 14, 5732956. doi:10.1155/2020/5732956
Orlando, P., Nartea, A., Silvestri, S., Marcheggiani, F., Cirilli, I., Dludla, P. V., et al. (2022). Bioavailability study of isothiocyanates and other bioactive compounds of Brassica oleracea L. var. italica boiled or steamed: Functional food or dietary supplement? Antioxidants 11, 209. doi:10.3390/antiox11020209
Oyeleye, S. I., Ojo, O. R., and Oboh, G. (2021). Moringa oleifera leaf and seed inclusive diets influenced the restoration of biochemicals associated with erectile dysfunction in the penile tissue of STZ-induced diabetic male rats treated with/without Acarbose drug. J. Food Biochem. 45, e13323. doi:10.1111/jfbc.13323
Paikra, B. K., Dhongade, H. K. J., and Gidwani, B. (2017). Phytochemistry and pharmacology of Moringa oleifera Lam. J. Pharmacopuncture 20, 194–200. doi:10.3831/KPI.2017.20.022
Pakade, V, C. E., and Chimuka, L. (1996). Comparison of antioxidant activity of Moringa oleifera and selected vegetables in South Africa. South Afr. J. Sci. 109.
Palhares, R. M., Gonçalves Drummond, M., Dos Santos Alves Figueiredo Brasil, B., Pereira Cosenza, G., Das Graças Lins Brandão, M., and Oliveira, G. (2015). Medicinal plants recommended by the world health organization: DNA barcode identification associated with chemical analyses guarantees their quality. PLoS One 10, e0127866. doi:10.1371/journal.pone.0127866
Patriota, L. L. S., Ramos, D. B. M., Dos Santos, A., Silva, Y. A., Gama, E. S. M., Torres, D. J. L., et al. (2020). Antitumor activity of Moringa oleifera (drumstick tree) flower trypsin inhibitor (MoFTI) in sarcoma 180-bearing mice. Food Chem. Toxicol. 145, 111691. doi:10.1016/j.fct.2020.111691
Paula, P. C., Sousa, D. O. B., Oliveira, J. T. A., Carvalho, A. F. U., Alves, B. G. T., Pereira, M. L., et al. (2017). A protein isolate from moringa oleifera leaves has hypoglycemic and antioxidant effects in alloxan-induced diabetic mice. Molecules 22, 271. doi:10.3390/molecules22020271
Perreault, L., Skyler, J. S., and Rosenstock, J. (2021). Novel therapies with precision mechanisms for type 2 diabetes mellitus. Nat. Rev. Endocrinol. 17, 364–377. doi:10.1038/s41574-021-00489-y
Phaniendra, A., and Babu, D. (2015). Free radicals : properties , sources , targets , and their implication in various diseases. Indian J. Clin. biochem. 30, 11–26. doi:10.1007/s12291-014-0446-0
Pizzino, G., Irrera, N., Cucinotta, M., Pallio, G., Mannino, F., Arcoraci, V., et al. (2017). Oxidative stress: harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 8416763. doi:10.1155/2017/8416763
Raafat, K., and Hdaib, F. (2017). Neuroprotective effects of Moringa oleifera: Bio-guided GC-MS identification of active compounds in diabetic neuropathic pain model. Chin. J. Integr. Med. 2017. doi:10.1007/s11655-017-2758-4
Rahimi, R., Nikfar, S., Larijani, B., and Abdollahi, M. (2005). A review on the role of antioxidants in the management of diabetes and its complications. Biomed. Pharmacother. 59, 365–373. doi:10.1016/j.biopha.2005.07.002
Rakotoarivelo, N. H., Rakotoarivony, F., Ramarosandratana, A. V., Jeannoda, V. H., Kuhlman, A. R., Randrianasolo, A., et al. (2015). Medicinal plants used to treat the most frequent diseases encountered in Ambalabe rural community, Eastern Madagascar. J. Ethnobiol. Ethnomed. 11, 68. doi:10.1186/s13002-015-0050-2
Randle, P. J., Garland, P. B., Hales, C. N., and Newsholme, E. A. (1963). The glucose fatty-acid cycle its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 281, 785–789. doi:10.1016/s0140-6736(63)91500-9
Redha, A. A., Perna, S., Riva, A., Petrangolini, G., Peroni, G., Nichetti, M., et al. (2021). Novel insights on anti-obesity potential of the miracle tree, Moringa oleifera: A systematic review. J. Funct. Foods 84, 104600. doi:10.1016/j.jff.2021.104600
Rena, G., Pearson, E. R., and Sakamoto, K. (2013). Molecular mechanism of action of metformin : old or new insights. Diabetologia 56, 1898–1906. doi:10.1007/s00125-013-2991-0
Roep, B. O., Thomaidou, S., Van Tienhoven, R., and Zaldumbide, A. (2021). Type 1 diabetes mellitus as a disease of the β-cell (Do Not blame the immune system?). Nat. Rev. Endocrinol. 17, 150–161. doi:10.1038/s41574-020-00443-4
Rose, B. A., Force, T., and Wang, Y. (2010). Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiol. Rev. 90, 1507–1546. doi:10.1152/physrev.00054.2009
Saini, R. K., Sivanesan, I., and Keum, Y. S. (2016). Phytochemicals of Moringa oleifera: a review of their nutritional, therapeutic and industrial significance. 3 Biotech. 6, 203. doi:10.1007/s13205-016-0526-3
Shakoor, H., Apostolopoulos, V., Feehan, J., Ali, H. I., Ismail, L. C., Al Dhaheri, A., et al. (2021). Effect of calorie restriction and exercise on type 2 diabetes. Pril. Makedon. Akad. Nauk. Umet. Odd. Med. Nauki) 42, 109–126. doi:10.2478/prilozi-2021-0010
Sharma, P., Jha, A. B., Dubey, R. S., and Pessarakli, M. (2012). Reactive oxygen species , oxidative damage , and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 26. Article ID 217037. doi:10.1155/2012/217037
Siddiqui, S., Upadhyay, S., Ahmad, I., Hussain, A., and Ahamed, M. (2021). Cytotoxicity of Moringa oleifera fruits on human liver cancer and molecular docking analysis of bioactive constituents against caspase-3 enzyme. J. Food Biochem. 45, e13720. doi:10.1111/jfbc.13720
Sierra-Campos, E., Valdez-Solana, M., Avitia-Domínguez, C., Campos-Almazán, M., Flores-Molina, I., García-Arenas, G., et al. (2020). Effects of Moringa oleifera leaf extract on diabetes-induced alterations in paraoxonase 1 and catalase in rats analyzed through progress kinetic and blind docking. Antioxidants 9, 840. doi:10.3390/antiox9090840
Singh, G., Passsari, A. K., Leo, V. V., Mishra, V. K., Subbarayan, S., Singh, B. P., et al. (2016). Evaluation of phenolic content variability along with antioxidant, antimicrobial, and cytotoxic potential of selected traditional medicinal plants from India. Front. Plant Sci. 7, 407. doi:10.3389/fpls.2016.00407
Sissoko, L., Diarra, N., Nientao, I., Stuart, B., Togola, A., Diallo, D., et al. (2020). Moringa Oleifera leaf powder for type 2diabetes: A pilot clinical trial. Afr. J. Traditional Complementary Altern. Med. 17, 29–36. doi:10.21010/ajtcam.v17i2.3
Stanford, K. I., and Goodyear, L. J. (2014). Exercise and type 2 diabetes: molecular mechanisms regulating glucose uptake in skeletal muscle. Adv. Physiol. Educ. 38, 308–314. doi:10.1152/advan.00080.2014
Steinmetz, K. L., and Spack, E. G. (2009). The basics of preclinical drug development for neurodegenerative disease indications. BMC Neurol. 9 (Suppl. 1), S2. doi:10.1186/1471-2377-9-S1-S2
Stohs, S. J., and Hartman, M. J. (2015). Review of the safety and efficacy of Moringa oleifera. Phytother. Res. 29, 796–804. doi:10.1002/ptr.5325
Taïl, J., Clerc, P., Gauvin-Bialecki, A., and Gonthier, M.-P. (2020). Medicinal plant polyphenols attenuate oxidative stress and improve inflammatory and vasoactive markers in cerebral endothelial cells during hyperglycemic condition. Antioxidants 9, 5731–5827. doi:10.3390/antiox9070573
Tang, Y., Choi, E. J., Han, W. C., Oh, M., Kim, J., Hwang, J. Y., et al. (2017). Moringa oleifera from Cambodia ameliorates oxidative stress, hyperglycemia, and kidney dysfunction in type 2 diabetic mice. J. Med. Food 20, 502–510. doi:10.1089/jmf.2016.3792
Taweerutchana, R., Lumlerdkij, N., Vannasaeng, S., Akarasereenont, P., and Sriwijitkamol, A. (2017). Effect of Moringa oleifera leaf capsules on glycemic control in therapy-naïve type 2 diabetes patients: a randomized placebo controlled study. Evid. Based Complement. Altern. Med. 2017, 6581390. doi:10.1155/2017/6581390
Teodoro, J. S., Nunes, S., Rolo, A. P., Reis, F., and Palmeira, C. M. (2018). Therapeutic options targeting oxidative stress, mitochondrial dysfunction and inflammation to hinder the progression of vascular complications of diabetes. Front. Physiol. 9, 1857. doi:10.3389/fphys.2018.01857
Teshome, D., Tiruneh, C., and Berihun, G. (2021). Toxicity of methanolic extracts of seeds of moringa stenopetala, moringaceae in rat embryos and fetuses. Biomed. Res. Int. 2021, 5291083. doi:10.1155/2021/5291083
Trigo, C., Castelló, M. L., Ortolá, M. D., García-Mares, F. J., and Desamparados Soriano, M. (2020). Moringa oleifera: An unknown crop in developed countries with great potential for industry and adapted to climate change. Foods 10, 31. doi:10.3390/foods10010031
Tuorkey, M. J. (2016). Effects of Moringa oleifera aqueous leaf extract in alloxan induced diabetic mice. Interv. Med. Appl. Sci. 8, 109–117. doi:10.1556/1646.8.2016.3.7
Vergara-Jimenez, M., Almatrafi, M. M., and Fernandez, M. L. (2017). Bioactive components in moringa oleifera leaves protect against chronic disease. Antioxidants 6, 91. doi:10.3390/antiox6040091
Vikram, A., Tripathi, D. N., Kumar, A., and Singh, S. (2014). Oxidative stress and inflammation in diabetic complications. Int. J. Endocrinol. 2014, 679754. doi:10.1155/2014/679754
Villarruel-López, A., López-De La Mora, D. A., Vázquez-Paulino, O. D., Puebla-Mora, A. G., Torres-Vitela, M. R., Guerrero-Quiroz, L. A., et al. (2018). Effect of Moringa oleifera consumption on diabetic rats. BMC Complement. Altern. Med. 18, 127. doi:10.1186/s12906-018-2180-2
Watanabe, S., Okoshi, H., Yamabe, S., and Shimada, M. (2021). Moringa oleifera Lam. in diabetes mellitus: A systematic review and meta-analysis. Molecules 26, 3513. doi:10.3390/molecules26123513
Waterman, C., Rojas-Silva, P., Tumer, T. B., Kuhn, P., Richard, A. J., Wicks, S., et al. (2015). Isothiocyanate-rich Moringa oleifera extract reduces weight gain, insulin resistance, and hepatic gluconeogenesis in mice. Mol. Nutr. Food Res. 59, 1013–1024. doi:10.1002/mnfr.201400679
Wei, Q., Qi, L., Lin, H., Liu, D., Zhu, X., Dai, Y., et al. (2020). Pathological mechanisms in diabetes of the exocrine pancreas: what’s known and what’s to know. Front. Physiol. 11, 1394. doi:10.3389/fphys.2020.570276
World Health Organization (WHO) (2022). The top ten leading causes of death. Available at: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (Accessed February 01, 2022).
Wu, P., Yan, Y., Ma, L.-L., Hou, B.-Y., He, Y.-Y., and Zhang, L. (2016). Effects of the Nrf2 protein modulator salvianolic acid a alone or combined with metformin on diabetes-associated macrovascular and renal injury. J. Biol. Chem. 291, 22288–22301. doi:10.1074/jbc.M115.712703
Xiong, Y., Rajoka, M. S. R., Mehwish, H. M., Zhang, M., Liang, N., Li, C., et al. (2021). Virucidal activity of Moringa A from Moringa oleifera seeds against Influenza A Viruses by regulating TFEB. Int. Immunopharmacol. 95, 107561. doi:10.1016/j.intimp.2021.107561
Xu, Y. B., Chen, G. L., and Guo, M. Q. (2019). Antioxidant and anti-inflammatory activities of the crude extracts of Moringa oleifera from Kenya and their correlations with flavonoids. Antioxidants 8. doi:10.3390/antiox8080296
Yassa, H. D., and Tohamy, A. F. (2014). Extract of Moringa oleifera leaves ameliorates streptozotocin-induced Diabetes mellitus in adult rats. Acta histochem. 116, 844–854. doi:10.1016/j.acthis.2014.02.002
Yendapally, R., Sikazwe, D., Kim, S. S., Ramsinghani, S., Fraser-Spears, R., Witte, A., et al. (2020). A review of phenformin, metformin, and imeglimin. Drug Dev. Res. 81, 390–401. doi:10.1002/ddr.21636
Zhu, Y., Yin, Q., and Yang, Y. (2020). Comprehensive investigation of moringa oleifera from different regions by simultaneous determination of 11 polyphenols using UPLC-ESI-MS/MS. Molecules 25, 676. doi:10.3390/molecules25030676
Ziqubu, K., Dludla, P. V., Joubert, E., Muller, C. J. F., Louw, J., Tiano, L., et al. (2020). Isoorientin: A dietary flavone with the potential to ameliorate diverse metabolic complications. Pharmacol. Res. 158, 104867. doi:10.1016/j.phrs.2020.104867
AMPK-AMP activated protein kinase
AKT protein kinase B
ACOX1 peroxisomal acyl-CoA oxidase 1
BCL-2 B-cell lymphoma 2
CAT catalase
C/EBPα enhancer-binding protein
COX-2 cyclooxygenase-2
GPx glutathione reductase
GST glutathione-S-transferase
GLUT4 glucose transporter 4
GSH glutathione
HbA1C glycated hemoglobin
HDL high density lipoprotein
HO-1 heme oygenase-1
LDL low density lipoprotein
NJK-c-Jun N terminal kinases
MDA malonaldehyde
iNOS inducible nitric oxide synthase
INF-γ interferon gamma
IL interleukin
MAPK mitogen-activated protein kinase
MCP-1 monocyte chemoattractant protein-1
NF-κβ nuclear factor kappa β
T1D/T1D type 1/2 diabetes mellitus
TNF-α tumor necrosis factor alpha
TBARS thiobarbituric acid reactive species
PDE-5 phosphodiesterase type 5
PKC protein kinase C
PPARγ peroxisome proliferator-activated receptor gamma
SIRT1 NAD-dependent deacetylase
SOD superoxide dismutase
SREBP1c sterol regulatory element-binding protein 1c
WHO World Health Organization
VEGF vascular endothelial growth factor
Keywords: diabetes complications, oxidative stress, inflammation, moringa (Moringa oleifera), therapeutic targets
Citation: Mthiyane FT, Dludla PV, Ziqubu K, Mthembu SXH, Muvhulawa N, Hlengwa N, Nkambule BB and Mazibuko-Mbeje SE (2022) A Review on the Antidiabetic Properties of Moringa oleifera Extracts: Focusing on Oxidative Stress and Inflammation as Main Therapeutic Targets. Front. Pharmacol. 13:940572. doi: 10.3389/fphar.2022.940572
Received: 10 May 2022; Accepted: 21 June 2022;
Published: 11 July 2022.
Edited by:
Dongmei Li, Georgetown University, United StatesReviewed by:
Oluwafemi Adeleke Ojo, Bowen University, NigeriaCopyright © 2022 Mthiyane, Dludla, Ziqubu, Mthembu, Muvhulawa, Hlengwa, Nkambule and Mazibuko-Mbeje. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sithandiwe E. Mazibuko-Mbeje, MzY1ODgyOTZAbnd1LmFjLnph
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.