- 1School of Traditional Chinese Medicine & School of Integrated Chinese and Western Medicine, Nanjing University of Chinese Medicine, Nanjing, China
- 2School of Traditional Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
- 3Wangjing Hospital, China Academy of Chinese Medical Sciences, Beijing, China
Background: Oxidative stress (OS) is associated with ferroptosis. Coenzyme Q10 (CoQ10), as an adjuvant treatment, has shown to be beneficial against OS. However, the efficacy of CoQ10 as a therapeutic agent against OS has not been promptly updated and systematically investigated.
Methods: A systematic literature search was performed using the Medline, EMBASE, Web of science, Cochrane Central Register of Controlled Trials, CNKI, CBM, Science direct and clinical trial. gov to identify randomized clinical trials evaluating the efficacy of CoQ10 supplementation on OS parameters. Standard mean differences and 95% confidence intervals were calculated for net changes in OS parameters using a random-effects model.
Results: Twenty-one randomized clinical studies met the eligibility criteria to be included in the meta-analysis. Overall, CoQ10 supplementation increased the levels of antioxidant enzymes [including superoxide dismutase (SOD) (SMD = 0.63; 95% CI: 0.38 to 0.88; p < 0.001), catalase (CAT) (SMD = 0.44; 95% CI:0.16 to 0.72; p = 0.002)] significantly and the levels of malondialdehyde (MDA) (SMD = -0.68; 95% CI: 0.93 to -0.43; p < 0.001) was decreased considerably. However, significant associations were not observed between this supplement and total antioxidant capacity (TAC), glutathione peroxidase (GPx) activity.
Conclusion: CoQ10 can improve OS as indicated by statistical significance in CAT and MDA concentrations, as well as SOD activity. Future studies focusing on long-term results and specific valuation of OS parameters are required to confirm the efficacy of CoQ10 on OS. We also believe that with the further research on ferroptosis, CoQ10 will gain more attention.
Systematic Review Registration: [https://inplasy.com/], identifier [INPLASY2021120123].
Introduction
There are encouraging data showing that life expectancy has been increased for most of the past century. However, with aging, high rates of debilitating diseases become a major public health burden. Aging is a natural and complex physiological process influenced by many factors (Glatt et al., 2007; Flatt, 2012). Actually, one of the most accepted theories to explain aging is damage accumulation driven by oxidative stress (OS) (Finkel, Holbrook; Zhang et al., 2020; Marianna et al., 2020). Numerous studies have shown mitochondrial bioenergetic deterioration, particular with reactive oxygen species- (ROS-) induced mitochondrial DNA damage, is a prominent risk factor in normal physiological aging and in the pathogenesis of various cell and tissue (Nita and Grzybowski, 2016; Srivastava, 2017).
Coenzyme Q10 (CoQ10, ubiquinone), a redox-active lipid, it is mainly found in the inner mitochondrial membrane. All observations allowed the identification of CoQ10 as an obligate component in the electron transport chain and it is associated with the process of oxidative phosphorylation. Tissues with elevated energy demands, such as the heart, skeletal muscle and neurons, cultured with high concentrations of CoQ10. Moreover, the tissue concentration of CoQ10 declines with ageing and oxidative stress. All these observations demonstrated its bioenergetic role and this forms the basis of the clinical recommendation and application for CoQ10 (Lass et al., 1999; Díaz-Casado et al., 2019).
Starting from these premises, the supplementation with CoQ10 to maintain adequate tissue concentrations should be therapeutically promising. Nevertheless, the results obtained were contradictory. Additionally, weak search strategy, not registered in advance, monotonous types of diseases, and lack of assessing CoQ10 impact on comprehensive OS markers such as TAC are limitations in previous systematic reviews and meta-analysis (Jorat et al., 2019; Akbari et al., 2020; Alimohammadi et al., 2021). Hence, it gave us the opportunity to update the relevant evidence and conducted this study. We hope that the effects of CoQ10 supplementation on OS biomarkers can be comprehensively sort out and evaluated, as well as providing more accurate estimates of the overall effect to assess the future role of CoQ10 in the in the field of interest.
Methods
This study was conducted and reported according to the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (Page et al., 2021). We registered the protocol of this study on the International Platform of Registered Systematic Review (Register number: INPLASY2021120123).
Data Sources
Comprehensive computerized systematic searches were performed throughout PubMed/Medline, Web of science, Science direct, EMBASE, Cochrane Central Register of Controlled Trials, Chinese National Knowledge Infrastructure (CNKI), and Chinese Biomedical Literature Database (CBM) from inception until September 2021. In addition, we reviewed the reference lists of eligible reports, previous systematic reviews, guidelines, and contacted industry representatives to identify additional studies.
Data Searches
The combination of MESH (Medical Subject Headings) and non-MESH terms were applied as follow (“Ubiquinone” OR “Coenzyme Q″) AND (“Antioxidants” OR “Reactive Oxygen Species” OR “Reactive Nitrogen Species” OR “Oxidative Stress” OR “Nitric Oxide” OR “Malondialdehyde” OR “Superoxide Dismutase” OR “Glutathione” OR “Glutathione Peroxidase” OR “Glutathione Reductase” OR “Oxidative Stresses” OR “stress oxidative” OR “GR” OR “Glutathione Lipoperoxidase” OR “GPx” OR “GSH-Px” OR “Glutathione” OR “SOD” OR “Malonyldialdehyde” OR “Malonylaldehyde” OR MDA OR “Nitrogen Monoxide” OR “Mononitrogen Mon-oxide” OR “NO” OR “Reactive Nitrogen Species” OR “Active Oxygen”) AND (“Random Allocation” OR “Single-Blind Method” OR “Double-Blind Method” OR “Cross-Over Studies” OR “Clinical Trials as Topic” OR RCT OR “Intervention Studies” OR “intervention” OR “controlled trial” OR “randomized” OR “randomized” OR “random” OR “randomly” OR “placebo” OR “assignment” OR “Cross-Over”). In addition, we manually searched relevant articles and clinical studies in order to find additional pertinent studies.
Selection Criteria
All retrieved studies were evaluated independently by two reviewers (ZYL and HXY) according to the inclusion and exclusion criteria. Studies were included if they: 1) were randomized controlled trials with parallel design; 2) were performed on adult people (≥18 years old); 3) assessed the effects of oral coenzyme CoQ10 supplementation on aforementioned OS biomarkers compared with the placebo. No restrictions on the baseline health status, sex, race of the participants, and date.
Articles were excluded if 1) conducted on healthy people; 2) contained incomplete information about the selected outcomes; 3) surveyed the effects of medications containing CoQ10, where the specific effects of CoQ10 could not be discovered; 4) took CoQ10 for more than 6 months.
Data Extraction
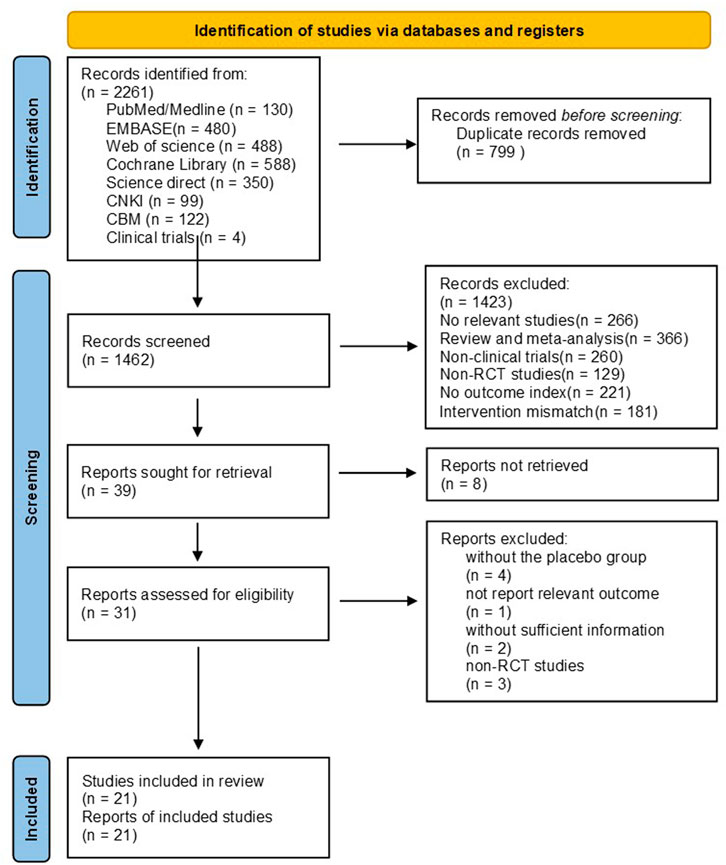
Using a standardized pilot-tested form, paired reviewers screened titles and abstracts of identified citations, as well as full texts of potentially eligible studies (Figure 1). Multi-arm trials were included as a separate article in the meta-analysis. If any studies provided inadequate data for outcomes, we contacted the authors at least by email, and if we did not receive a response, we excluded the article from the analysis or recalculated data from the graphs using digital ruler software. Disagreements were resolved through discussion between the researchers or with an adjudicator’s help if there was a lack of consensus.
Outcomes
MDA, SOD, TAC, NOx, GPx, and CAT were considered as the outcomes.
Quality Assessment
The quality of the included RCTs was assessed independently by two reviewers according to the Cochrane Handbook for Systematic Review of Interventions, Version 5.1.0, including 1) sequence generation, 2) allocation concealment, 3) blinding of participants and personnel, 4) blinding of outcome assessment, 5) incomplete outcome data, 6) selective outcome reporting, and 7) other bias. “Low risk”, “high risk”, or “unclear risk” were the quality status.
Data Synthesis
All analyses were performed using Stata version 16.0 (Stata Corp, College station, TX, United States). The overall effect sizes were assessed using the random-effects model (DerSimonian and Kacker, 2007). Standardized mean difference (SMD) was calculated by generic inverse variance. The heterogeneity was evaluated by using the Cochrane’s Q-test and the I2 index. I2 values < 25–50%, 50–75%, or >75% indicated mild, moderate, or severe heterogeneity, respectively.
Subgroup Analysis and Sensitivity Analysis
Sub-group analysis was used to reduce the heterogeneity and sensitivity analysis was conducted to detect the impact of each study on the pooled effect size using the leave-one-out method (Sahebkar, 2014). The potential sources of heterogeneity were explored identified based on dosage, study duration, and types of diseases. Publication bias was tested by Egger’s test. Moreover, we used Engauge Digitizer version 10.8 to extract numerical estimates from graphs.
Quality of the Evidence
The quality of the evidence would be assessed by the GRADE tool (Guyatt et al., 2008). Based on five key domains (methodology quality, directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias), levels of evidence quality were defined as high, moderate, low and very low (Balshem et al., 2011).
Results
Study Selection and Characteristics
The initial search revealed 2,261 citations (130 from Medline, 480 from EMBASE, 488 from Web of science, 588 from Cochrane Central Register of Controlled Trials, 99 from CNKI, 122 from CBM, 350 from Science direct, and four from clinical trial. gov). After elimination of 799 duplicates, 1,462 records were retained for further evaluation. Articles were further screened by browsing their titles and abstracts, and the process was conducted by three independent authors (ZYL, HXY and LN). 1,423 articles were excluded as irrelevant to the topic, and a total of 39 articles remained for full-text review. 18 studies were excluded for the following reasons: studies did not retrieve (n = 8), studies did not report relevant outcomes (n = 1), trials without the placebo group (n = 4), trials without sufficient information (n = 2), and non-RCT studies (n = 3) (Figure 1).
The detailed characteristics of the 21studies are summarized in Table 1. Totally, 1,132 participants, including 577 cases and 555 controls, were participated in these studies. Published studies from 2000 to 2021, with 14 studies from Iran, four studies from China, two studies from Indonesia, and one study from India. Three trials exclusively included women or men. The mean age of participants varied between 19 and 76 years old.
Risk of Bias in Individual Studies
We used Cochrane risk of bias (ROB) tool for all included studies (Figure 2). There were three studies with high risk of performance bias, two studies with unclear risk of performance bias. There was unclear risk of selection bias in 13 studies, unclear risk of detection bias in 20 studies.
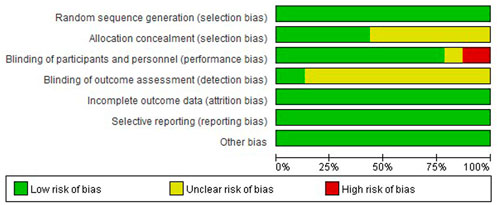
FIGURE 2. Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
Primary Results
MDA
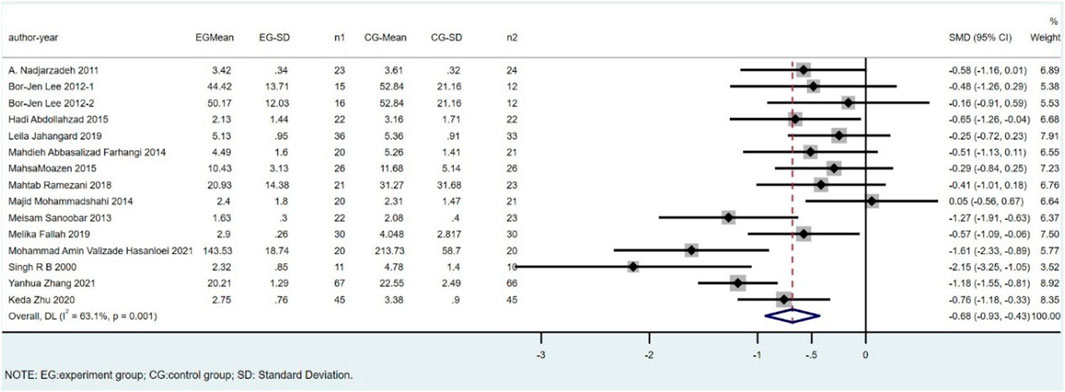
Fifteen studies, including 782 subjects, reported the outcome of MDA. The difference in MDA between the CoQ10 groups and placebo groups was significant (SMD = -0.68; 95% CI: -0.93 to -0.43; p < 0.001; I2 = 63.1%). (Figure 3).
SOD
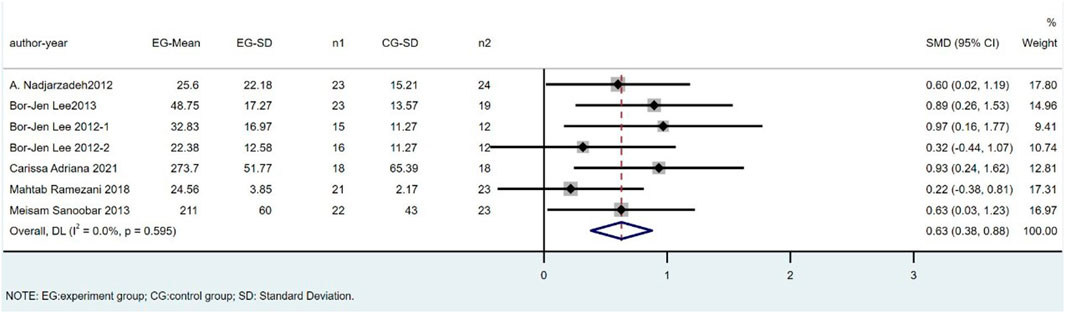
There were seven trials involving 269 individuals (138 cases and 131 controls) that compared SOD levels between CoQ10 and placebo groups. The difference in SOD between the CoQ10 groups and placebo groups was significant as shown in Figure 4 (SMD = 0.63; 95% CI: 0.38 to 0.88; p < 0.001), with no heterogeneity between studies (I2 = 0%; p = 0.60).
TAC
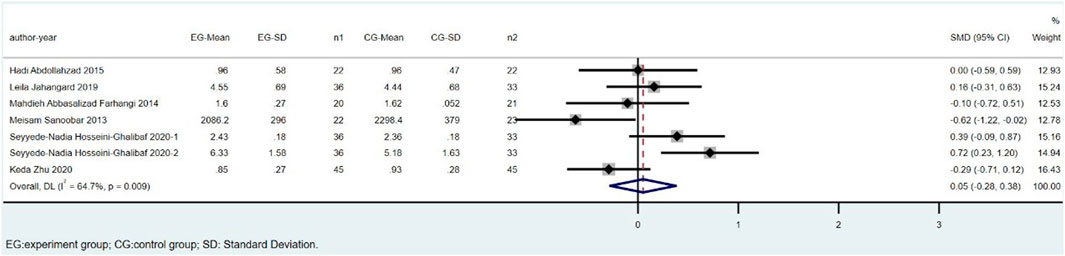
There were eight trials involving 495 individuals (251 cases and 244 controls) that compared TAC levels between CoQ10 and placebo groups. Parvin Zarei’s study was excluded because of the high heterogeneity. The overall estimates showed that TAC levels did not significantly differ between the CoQ10 and placebo groups (SMD = 0.05; 95% CI: -0.28 to 0.38; p = 0.764), with a high heterogeneity between studies (I2 = 64.7%; p = 0.009) (Figure 5).
GPx
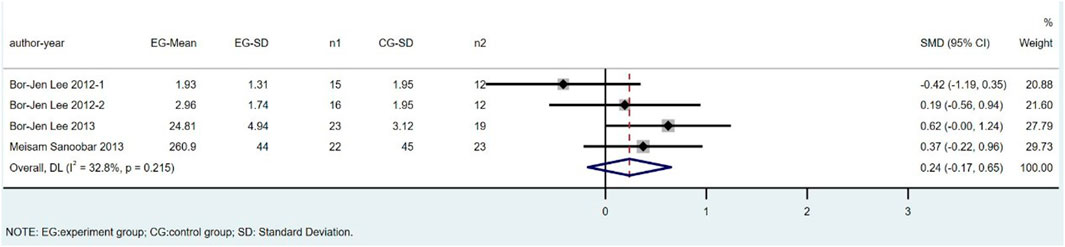
There were four trials involving 142 individuals (76 cases and 66 controls) that compared GPx levels between CoQ10 and placebo groups. The overall estimates showed that GPx levels did not significantly differ between the CoQ10 and placebo groups (SMD = 0.24; 95% CI: 0.17 to 0.65; p = 0.26), with a low heterogeneity between studies (I2 = 32.8%; p = 0.22) (Figure 6).
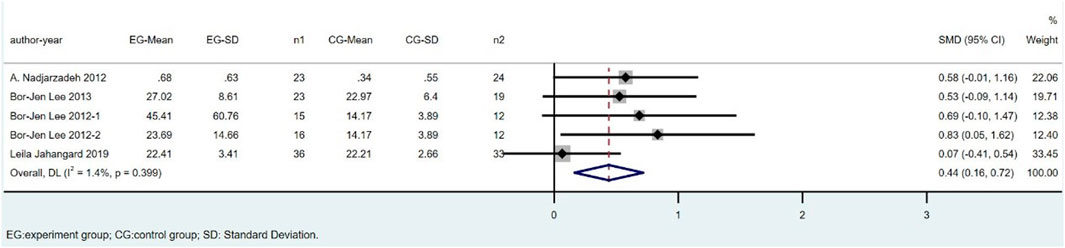
Six studies, including 281 subjects, reported the outcome of CAT. We excluded Parvin Zarei’s study because of the high heterogeneity. The difference in CAT between the CoQ10 groups and placebo groups was significant (SMD = 0.44; 95% CI: 0.16 to 0.72; p = 0.002), with low heterogeneity between studies (I2 = 1.4%; p = 0.399) (Figure 7).
Other Indicators
NOx
Three studies, including 193 subjects, reported the outcome of NOx. The difference in NOx between the CoQ10 groups and placebo groups was significant (SMD = -0.59; 95% CI: -0.88 to -0.30; p < 0.001), with no heterogeneity between studies (I2 = 0%; p = 0.66).
Subgroup Analysis
A subgroup meta-analysis comparing studies with different dosages, duration, and types of diseases was conducted. Patients were investigated at six different kinds of diseases: cardiology (n = 3), rheumatoid arthritis (n = 2), bipolar disorder (n = 1), nonalcoholic fatty liver disease (n = 2), diabetes mellitus type 2 (n = 2) and other diseases (n = 5). The results suggested that different diseases may be responsible for heterogeneity among MDA, TAC, CAT, and GPx. The detailed results for subgroup analyses are summarized in Supplementary Table S1; Supplementary Figures S1–S4.
Sensitivity Analysis and Publication Bias
Sensitivity analysis indicated that the exclusion of any primary studies did not influence the pooled estimates other than GPx (Supplementary Figures S5–S8).
The Egger test for MDA index showed no evidence of publication bias (t = -0.11, p = 0.918).
GRADE Assessment
We used the GRADE assessment to evaluate the evidence regarding the effect of CoQ10 supplementations compared with placebo (Supplementary Table S2). According to this evaluation, there was evidence that CoQ10 supplementation decreased the level of MDA in various population, as well as increased the level of SOD. Although the supplementary CoQ10 had a low impact on all indicators, this was due to the inconsistencies and indirectness of the results.
Discussion
The Main Findings and Strength of This Study
The study illustrated that CoQ10 supplementation can directly or indirectly through increasing SOD and CAT levels significantly, decreasing the levels of MDA considerably and may thereby affect OS and subsequent pathophysiological events. However, significant associations were not observed between this supplement and TAC, GPx activity, which differs from some previous studies (Alimohammadi et al., 2012; Akbari et al., 2020; Sangsefidi et al., 2020) and may be attributed to the generalizability of the included population and the diversity of disease types. Additionally, we also conducted subgroup meta-analysis comparing studies with different dosages, duration, and types of diseases. The findings revealed the value of CoQ10 in different scope of application more accurately. Also, the differences in study population’s characteristics, the dosage of CoQ10 used and the duration of intervention might explain the discrepancies to a certain extent among current evidence.
To our best knowledge, although our meta-analysis is not the first study investigating the effect of CoQ10 on OS biomarkers in human, we believed that the application of robust search strategy and study design, a wide variety of population (disease) as well as the transparency of the entire study process (the protocol registration has been completed on INSPLASY) were among the strengths of this study.
Of note, in order to better guide clinical practice, further evaluation of the evidence was carried out by the GRADE approach. Due to the moderate and high quality of included trials and the above outcomes from individual trials, we are confident that the diagnosis and treatment of oxidative stress in the future will be addressed in the guidelines.
Coenzyme Q10 Affects Antioxidant Enzyme Activity
In the systematic review and meta-analysis, we confirmed that the antioxidant capacity of CoQ10 has an important role in reducing the production of free radicals, which can ultimately lead to a reduction in MDA levels. Furthermore, antioxidant enzymes such as SOD and CAT are responsible for neutralizing the free radicals. The activity of these enzymes increases in body followed by the consumption of antioxidants, such as CoQ10.
MDA is the best product to evaluate lipid peroxidation (Sepidarkish et al., 2020), and it has been shown that CoQ10 can prevent lipid peroxidation and reduce MDA in vitro and in vitro (Talevi et al., 2013; Gholnari et al., 2018; Hormozi et al., 2019). CoQ10 supplementation may reduce MDA levels via several mechanisms. Firstly, CoQ10, as a part of the mitochondrial respiratory chain, inhibits mitochondrial endogenous ROS production (Jing et al., 2015), and lower ROS is associated with reduced MDA levels (Karajibani et al., 2009). Besides, lipid metabolism was regulated by CoQ10, which can prevent lipid oxidation in a variety of cellular and gene expression ways.
The results of our study confirmed that CoQ10 significantly increases SOD activity. SOD is known to be one of the main detoxifying enzymes in the mitochondria (Zou et al., 2017). One of the possible mechanisms is that CoQ10, as a mitochondrial antioxidant, works by decreasing ROS in the mitochondria, so increasing the activity of SOD (Sourris et al., 2012). Besides, CoQ10 is also able to promote the expression of FOXO3a, which has been shown to increase SOD gene expression (Abd El-Aal et al., 2017).
The results of our meta-analysis also indicated that CoQ10 supplementation increased CAT activity in a non-significant manner. In addition, the results showed that CoQ10 supplementation had no significant effect on NO levels. Primary studies of high heterogenicity and low numbers maybe the reasons that we could not find a significant effect of CoQ10 supplementation on these indicators. However, the results of other studies investigating the effect of CoQ10 supplementation on NO concentrations are also controversial (Erol et al., 2010), and there are no concrete conclusions in these regards.
The Rise of Ferroptosis Concept and Potential Relationship With OS and CoQ10
Cell death has an important role in the process of development, homeostasis, and pathogenesis of acute and chronic diseases (Van Opdenbosch and Lamkanfi, 2019). Ferroptosis is a new kind of regulated cell death involving various biology processes, such as iron metabolism, lipid metabolism, OS and CoQ10 (Qiu et al., 2020).
Iron, a potentially toxic molecule, can catalyze reactive oxygen species (ROS) radical formation through the Fenton reaction, in which H2O2 is reduced by a single electron to produce hydroxyl radicals (Bresgen and Eckl, 2015); this ultimately leads to damage to various cellular structures.
OS is a common pathogenesis of many chronic diseases. Iron metabolism could be the basis for the dynamic interplay between oxidative stress and antioxidants in many pathophysiological processes. The redox state is affected by iron deficiency and iron overload, which can be restored using iron supplementation and iron chelation, respectively. Likewise, antioxidants combining with basis treatment has been suggested for attenuating tissue damage caused by oxidative stress.
Therefore, antioxidants could be useful for protecting cells and tissues as well as reversing oxidative damage. CoQ10 is an endogenous antioxidant produced by the mevalonate pathway, which is important component of the mitochondrial respiratory chain. Multiple studies have shown that the regulatory role of CoQ10 in uncoupling proteins’ activation (Parikh et al., 2009), cell signaling (Groneberg et al., 2005), cell growth (Crane, 1999), and cell death (Alleva et al., 2001). In recent research of ferroptosis with CoQ10, ferroptosis suppressor protein 1 (FSP1) has been recognized as an oxidoreductase of CoQ10 to reduce it at the plasma membrane and can strengthen the resistance of cells to ferroptosis (Bersuker et al., 2019). Notably, CoQ10 deficiency currently is the only treatable mitochondrial disorder, however, little is known about how it may affect other organelles. High concentrations of CoQ10 have been found on lysosomal membranes, which has been proposed to be associated with normal acidification of the lysosomal lumen (Heaton et al., 2020). Additionally, Rizzardi N et al. in a study of cultured cells demonstrated that, protecting of membrane lipids from peroxidation and increasing cellular resistance to ferroptotic stimuli can be achieved by supplementation with exogenous CoQ10 (Rizzardi et al., 2021).
Greater interest has been shown in improving our understanding of the relationship between ferroptosis and various diseases, but there are few studies on the downstream effects of ferroptosis; that is, how it triggers disease occurrence and progression. Hence, it is vital to elucidate the mechanisms of ferroptosis in different diseases. Future studies are needed to investigate the basis of ferroptosis and to identify relationship between ferroptosis and other forms of cell death in dynamic environments. This would improve our understanding of the occurrence, progression, diagnosis, and treatment of ferroptosis-related disorders. We believe that it will provide new ideas for the treatment of ferrroptosis-related diseases via focuses on the relationship between antioxidant systems and ferroptosis and reveals the inhibitory role of antioxidant system in ferroptosis.
Future Direction and the Limitations of This Study
There are multiple studies combining CoQ10 with other drugs, making it difficult to assess CoQ10 specific effect. Another limitation is the poor oral bioavailability of CoQ10. However, due to the relatively easy availability and high safety profile of CoQ10, more randomized studies are required to assess its’ role in various diseases. The development of inhalator CoQ10 is a promising approach and may prove useful in the future.
It is vital to acknowledge some limitations of our study. Firstly, information on the formulation of CoQ10 supplementation used in clinical trials was not available, and different pharmacokinetic properties may affect the bioavailability of various formulations, thus affecting the effect of CoQ10. Secondly, the heterogeneity within the studied factors may be due to various diseases, study durations (1–12 weeks), supplemental doses (30–500 mg/day), patients’ initial antioxidant serum levels, and patients’ other characteristics, such as gender and age. Moreover, the clinical trials included in this meta-analysis had limited sample sizes and follow-up periods.
Conclusion
We concluded that CoQ10 can improve OS by statistical significance in CAT and MDA concentrations, as well as SOD activity, compared with placebo. Future studies should confirm its efficacy of CoQ10 on OS by assessing the long-term results and specific evaluation of OS parameters. With the increasing maturity of ferroptosis phenotype in the pathogenesis of various diseases, we believe that CoQ10 will have more promising scientific and clinical applications in the future.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
YZ: Writing—Original Draft, Data Curation and Software; XH: Writing—Original Draft and Data Curation; NL: Data Curation and software; ML: Data Curation and Visualization; CS: Formal analysis; BQ: Resources; KS: Visualization; XW: Conceptualization and Project administration; YM: Conceptualization and Writing—Review and Editing; LZ: Funding acquisition and Supervision.
Funding
This study was funded by the Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (Grant number: ZYYCXTD-C-202003), the Fundamental Research Funds for the Central Public Welfare Research Institutes (Grant number: ZZ13-YQ-039, 2020YJSZX-4 and CI 2021A02013). In addition, this work was also financially supported in part by the grants from a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.936233/full#supplementary-material
References
Abd El-Aal, S. A., Abd El-Fattah, M. A., and El-Abhar, H. S. (2017). CoQ10 Augments Rosuvastatin Neuroprotective Effect in a Model of Global Ischemia via Inhibition of NF-κB/JNK3/Bax and Activation of Akt/FOXO3A/Bim Cues. Front. Pharmacol. 8, 735. doi:10.3389/fphar.2017.00735
Abdollahzad, H., Aghdashi, M. A., Asghari Jafarabadi, M., and Alipour, B. (2015). Effects of Coenzyme Q10 Supplementation on Inflammatory Cytokines (TNF-α, IL-6) and Oxidative Stress in Rheumatoid Arthritis Patients: A Randomized Controlled Trial. Arch. Med. Res. 46 (7), 527–533. doi:10.1016/j.arcmed.2015.08.006
Adriana, C., Budiastuti, A., Kabulrachman, K., Indar Widayati, R., Riyanto, P., and Muslimin, M.. Coenzyme Q10 Supplementation as an Adjuvant Therapy Potentially Increase Serum Superoxide Dismutase Levels in Acne Vulgaris Patients. Maced. J. Med. Sci. 9 (B), 444–450. doi:10.3889/oamjms.2021.6048
Akbari, A., Mobini, G. R., Agah, S., Morvaridzadeh, M., Omidi, A., Potter, E., et al. (2020). Coenzyme Q10 Supplementation and Oxidative Stress Parameters: a Systematic Review and Meta-Analysis of Clinical Trials. Eur. J. Clin. Pharmacol. 76, 1483–1499. doi:10.1007/s00228-020-02919-8
Alimohammadi, M., Rahimi, A., Faramarzi, F., Golpour, M., Jafari-Shakib, R., Alizadeh-Navaei, R., et al. (2012). Effects of Coenzyme Q10 Supplementation on Inflammation, Angiogenesis, and Oxidative Stress in Breast Cancer Patients: a Systematic Review and Meta-Analysis of Randomized Controlled- Trials. Inflammopharmacology 29 (3), 579–593. doi:10.1007/s10787-021-00817-8
Alimohammadi, M., Rahimi, A., Faramarzi, F., Golpour, M., Jafari-Shakib, R., Alizadeh-Navaei, R., et al. (2021). Effects of Coenzyme Q10 Supplementation on Inflammation, Angiogenesis, and Oxidative Stress in Breast Cancer Patients: a Systematic Review and Meta-Analysis of Randomized Controlled- Trials. Inflammopharmacology 29, 579–593. doi:10.1007/s10787-021-00817-8
Alleva, R., Tomasetti, M., Andera, L., Gellert, N., Borghi, B., Weber, C., et al. (2001). Coenzyme Q Blocks Biochemical but Not Receptor-Mediated Apoptosis by Increasing Mitochondrial Antioxidant Protection. FEBS Lett. 503 (1), 46–50. doi:10.1016/s0014-5793(01)02694-1
Balshem, H., Helfand, M., Schünemann, H. J., Oxman, A. D., Kunz, R., Brozek, J., et al. (2011). GRADE Guidelines: 3. Rating the Quality of Evidence. J. Clin. Epidemiol. 64 (4), 401–406. doi:10.1016/j.jclinepi.2010.07.015
Bersuker, K., Hendricks, J. M., Li, Z., Magtanong, L., Ford, B., Tang, P. H., et al. (2019). The CoQ Oxidoreductase FSP1 Acts Parallel to GPX4 to Inhibit Ferroptosis. Nature 575 (7784), 688–692. doi:10.1038/s41586-019-1705-2
Bresgen, N., and Eckl, P. M. (2015). Oxidative Stress and the Homeodynamics of Iron Metabolism. Biomolecules 5, 808–847. doi:10.3390/biom5020808
DerSimonian, R., and Kacker, R. (2007). Random-effects Model for Meta-Analysis of Clinical Trials: an Update. Contemp. Clin. Trials 28 (2), 105–114. doi:10.1016/j.cct.2006.04.004
Díaz-Casado, M. E., Quiles, J. L., Barriocanal-Casado, E., González-García, P., Battino, M., López, L. C., et al. (2019). The Paradox of Coenzyme Q10 in Aging. Nutrients 11, 2221. doi:10.3390/nu11092221
Erol, B., Bozlu, M., Hanci, V., Tokgoz, H., Bektas, S., and Mungan, G. (2010). Coenzyme Q10 Treatment Reduces Lipid Peroxidation, Inducible and Endothelial Nitric Oxide Synthases, and Germ Cell-specific Apoptosis in a Rat Model of Testicular Ischemia/reperfusion Injury. Fertil. Steril. 93 (1), 280–282. doi:10.1016/j.fertnstert.2009.07.981
Fallah, M., Askari, G., Soleimani, A., Feizi, A., and Asemi, Z. (2019). Clinical Trial of the Effects of Coenzyme Q10 Supplementation on Biomarkers of Inflammation and Oxidative Stress in Diabetic Hemodialysis Patients. Int. J. Prev. Med. 10 (1), 12–11. doi:10.4103/ijpvm.IJPVM_418_18
Farhangi, M. A., Alipour, B., Jafarvand, E., and Khoshbaten, M. (2014). Oral Coenzyme Q10 Supplementation in Patients with Nonalcoholic Fatty Liver Disease: Effects on Serum Vaspin, Chemerin, Pentraxin 3, Insulin Resistance and Oxidative Stress. Arch. Med. Res. 45 (7), 589–595. doi:10.1016/j.arcmed.2014.11.001
Finkel, T., and Holbrook, N. J. (200). Oxidants, Oxidative Stress and the Biology of Ageing. Nature 408 (6809), 239–247. doi:10.1038/35041687
Gholnari, T., Aghadavod, E., Soleimani, A., Hamidi, G. A., Sharifi, N., and Asemi, Z. (2018). The Effects of Coenzyme Q10 Supplementation on Glucose Metabolism, Lipid Profiles, Inflammation, and Oxidative Stress in Patients with Diabetic Nephropathy: a Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Coll. Nutr. 37 (3), 188–193. doi:10.1080/07315724.2017.1386140
Glatt, S. J., Chayavichitsilp, P., Depp, C., Schork, N. J., and Jeste, D. V. (2007). Successful Aging: from Phenotype to Genotype. Biol. Psychiatry 62 (4), 282–293. doi:10.1016/j.biopsych.2006.09.015
Groneberg, D. A., Kindermann, B., Althammer, M., Klapper, M., Vormann, J., Littarru, G. P., et al. (2005). Coenzyme Q10 Affects Expression of Genes Involved in Cell Signalling, Metabolism and Transport in Human CaCo-2 Cells. Int. J. Biochem. Cell. Biol. 37 (6), 1208–1218. doi:10.1016/j.biocel.2004.11.017
Guyatt, G. H., Oxman, A. D., Vist, G. E., Kunz, R., Falck-Ytter, Y., Alonso-Coello, P., et al. (2008). GRADE: an Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ 336 (7650), 924–926. doi:10.1136/bmj.39489.470347.AD
Hasanloei, M. A. V., Zeinaly, A., Rahimlou, M., Houshyar, H., Moonesirad, S., and Hashemi, R. (2021). Effect of Coenzyme Q10 Supplementation on Oxidative Stress and Clinical Outcomes in Patients with Low Levels of Coenzyme Q10 Admitted to the Intensive Care Unit. J. Nutr. Sci. 10, e48. doi:10.1017/jns.2021.39
Heaton, R. A., Heales, S., Rahman, K., Sexton, D. W., and Hargreaves, I. (2020). The Effect of Cellular Coenzyme Q10 Deficiency on Lysosomal Acidification. J. Clin. Med. 9 (6), undefined. doi:10.3390/jcm9061923
Hormozi, M., Mirzaei, R., Nakhaee, A., Payandeh, A., Izadi, S., and Haghighi, J. D. (2019). Effects of Coenzyme Q10 Supplementation on Oxidative Stress and Antioxidant Enzyme Activity in Glazers with Occupational Cadmium Exposure: a Randomized, Double-Blind, Placebo-Controlled Crossover Clinical Trial. Toxicol. Ind. Health 35 (1), 32–42. doi:10.1177/0748233718809256
Hosseini-Ghalibaf, S. N., Ranjbar, A., Yasrebifar, F., Mirzaei, E., Mirjalili, M., Mohammadi, Y., et al. (2020). Effect of Coenzyme Q10 Supplementation on Urinary and Salivary Oxidative Stress Biomarkers in Bipolar Patients during the Depressive Episode. Nat. Prod. J. 10 (5), 664–672. doi:10.2174/2210315509666190624102012
Hosseinzadeh-Attar, M., Kolahdouz Mohammadi, R., Eshraghian, M., Nakhjavani, M., Khorrami, E., Ebadi, M., et al. (2015). Reduction in Asymmetric Dimethylarginine Plasma Levels by Coenzyme Q10 Supplementation in Patients with Type 2 Diabetes Mellitus. Minerva Endocrinol. 40 (4), 259–266.
Jahangard, L., Yasrebifar, F., Haghighi, M., Ranjbar, A., and Mehrpooya, M. (2019). Influence of Adjuvant Coenzyme Q10 on Inflammatory and Oxidative Stress Biomarkers in Patients with Bipolar Disorders during the Depressive Episode. Mol. Biol. Rep. 46 (2), 5333–5343. doi:10.1007/s11033-019-04989-z
Jing, L., He, M. T., Chang, Y., Mehta, S. L., He, Q. P., Zhang, J. Z., et al. (2015). Coenzyme Q10 Protects Astrocytes from ROS-Induced Damage through Inhibition of Mitochondria-Mediated Cell Death Pathway. Int. J. Biol. Sci. 11 (1), 59–66. doi:10.7150/ijbs.10174
Jorat, M. V., Tabrizi, R., Kolahdooz, F., Akbari, M., Salami, M., Heydari, S. T., et al. (2019). The Effects of Coenzyme Q10 Supplementation on Biomarkers of Inflammation and Oxidative Stress in Among Coronary Artery Disease: a Systematic Review and Meta-Analysis of Randomized Controlled Trials. Inflammopharmacology 27 (2), 233–248. doi:10.1007/s10787-019-00572-x
Karajibani, M., Hashemi, M., Montazerifar, F., Bolouri, A., and Dikshit, M. (2009). The Status of Glutathione Peroxidase, Superoxide Dismutase, Vitamins A, C, E and Malondialdehyde in Patients with Cardiovascular Disease in Zahedan, Southeast Iran. J. Nutr. Sci. Vitaminol. (Tokyo) 55 (4), 309–316. doi:10.3177/jnsv.55.309
Lass, A., Kwong, L., and Sohal, R. S. (1999). Mitochondrial Coenzyme Q Content and Aging. Biofactors 9, 199–205. doi:10.1002/biof.5520090215
Lee, B. J., Huang, Y. C., Chen, S. J., and Lin, P. T. (2012). Coenzyme Q10 Supplementation Reduces Oxidative Stress and Increases Antioxidant Enzyme Activity in Patients with Coronary Artery Disease. Nutrition 28, 250–255. doi:10.1016/j.nut.2011.06.004
Lee, B. J., Tseng, Y. F., Yen, C. H., and Lin, P. T. (2013). Effects of Coenzyme Q10 Supplementation (300 Mg/day) on Antioxidation and Anti-inflammation in Coronary Artery Disease Patients during Statins Therapy: a Randomized, Placebo-Controlled Trial. Nutr. J. 12 (1), 142–147. doi:10.1186/1475-2891-12-142
Leleury, M., Adriani, D., Widayati, R., Kabulrachman, fnm, Budiastuti, A., and Muslimin, fnm (2020). The Effect of Coenzyme Q10 on Serum Glutathione Peroxidase Levels and Severity of Acne Vulgaris. Turk J. Dermatol 14, 71–75. doi:10.4103/tjd.tjd_51_20
Marianna, G., Radana, G., Janka, B., and Ľubomíra, T. (2020). Oxidative Stress in the Pathophysiology of Kidney Disease: Implications for Noninvasive Monitoring and Identification of Biomarkers. Oxid. Med. Cell. Longev. 2020, 5478708. doi:10.1155/2020/5478708
Moazen, M., Mazloom, Z., Ahmadi, A., Dabbaghmanesh, M. H., and Roosta, S. (2015). Effect of Coenzyme Q10 on Glycaemic Control, Oxidative Stress and Adiponectin in Type 2 Diabetes. J. Pak Med. Assoc. 65, 404–408.
Mohammadshahi, M., Farsi, F., Nejad, P. A., Hajiani, E., Zarei, M., and Engali, K. A. (2014). The Coenzyme Q10 Supplementation Effects on Lipid Profile, Fasting Blood Sugar, Blood Pressure and Oxidative Stress Status Among Non-alcoholic Fatty Liver Disease Patients: A Randomized, Placebo-Controlled. Pilot Study 3 (6), 1108–1113.
Nadjarzadeh, A., Sadeghi, M. R., Amirjannati, N., Vafa, M. R., Motevalian, S. A., Gohari, M. R., et al. (2011). Coenzyme Q10 Improves Seminal Oxidative Defense but Does Not Affect on Semen Parameters in Idiopathic Oligoasthenoteratozoospermia: a Randomized Double-Blind, Placebo Controlled Trial. J. Endocrinol. Invest. 34 (8), e224–8. doi:10.3275/7572
Nadjarzadeh, A., Shidfar, F., Amirjannati, N., Vafa, M. R., Motevalian, S. A., Gohari, M. R., et al. (2014). Effect of Coenzyme Q10 Supplementation on Antioxidant Enzymes Activity and Oxidative Stress of Seminal Plasma: a Double-Blind Randomised Clinical Trial. Andrologia 46 (2), 177–183. doi:10.1111/and.12062
Nita, M., and Grzybowski, A. (2016). The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell. Longev. 2016, 3164734. doi:10.1155/2016/3164734
Page, M. J., Moher, D., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 372, n160. doi:10.1136/bmj.n160
Parikh, S., Saneto, R., Falk, M. J., Anselm, I., Cohen, B. H., Haas, R., et al. (2009). A Modern Approach to the Treatment of Mitochondrial Disease. Curr. Treat. Options Neurol. 11 (6), 414–430. doi:10.1007/s11940-009-0046-0
Qiu, Y., Cao, Y., Cao, W., Jia, Y., and Lu, N. (2020). The Application of Ferroptosis in Diseases. Pharmacol. Res. 159, 104919. doi:10.1016/j.phrs.2020.104919
Ramezani, M., Sahraei, Z., Simani, L., Heydari, K., and Shahidi, F. (2020). Coenzyme Q10 Supplementation in Acute Ischemic Stroke: Is it Beneficial in Short-Term Administration? Nutr. Neurosci. 23 (8), 640–645. doi:10.1080/1028415X.2018.1541269
Rizzardi, N., Liparulo, I., Antonelli, G., Orsini, F., Riva, A., Bergamini, C., et al. (2021). Coenzyme Q10 Phytosome Formulation Improves CoQ10 Bioavailability and Mitochondrial Functionality in Cultured Cells. Antioxidants (Basel) 10, 927. doi:10.3390/antiox10060927
Sahebkar, A. (2014). Are Curcuminoids Effective C-Reactive Protein-Lowering Agents in Clinical Practice? Evidence from a Meta-Analysis. Phytother. ResPTR 28 (5), 633–642. doi:10.1002/ptr.5045
Sangsefidi, Z. S., Yaghoubi, F., Hajiahmadi, S., and Hosseinzadeh, M. (2020). The Effect of Coenzyme Q10 Supplementation on Oxidative Stress: A Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. Food Sci. Nutr. 8 (4), 1766–1776. doi:10.1002/fsn3.1492
Sanoobar, M., Eghtesadi, S., Azimi, A., Khalili, M., Jazayeri, S., and Reza Gohari, M. (2013). Coenzyme Q10 Supplementation Reduces Oxidative Stress and Increases Antioxidant Enzyme Activity in Patients with Relapsing-Remitting Multiple Sclerosis. Int. J. Neurosci. 123, 776–782. doi:10.3109/00207454.2013.801844
Sepidarkish, M., Akbari-Fakhrabadi, M., Daneshzad, E., Yavari, M., Rezaeinejad, M., Morvaridzadeh, M., et al. (2020). Effect of Omega-3 Fatty Acid Plus Vitamin E Co-supplementation on Oxidative Stress Parameters: A Systematic Review and Meta-Analysis. Clin. Nutr. 39 (4), 1019–1025. doi:10.1016/j.clnu.2019.05.004
Singh, R. B., Khanna, H. K., and Niaz, M. A. (2009). Randomized, Double-Blind Placebo-Controlled Trial of Coenzyme Q10 in Chronic Renal Failure: Discovery of a New Role. J. Nutr. Environ. Med. 10 (4), 281–288. doi:10.1080/13590840020013266
Sourris, K. C., Harcourt, B. E., Tang, P. H., Morley, A. L., Huynh, K., Penfold, S. A., et al. (2012). Ubiquinone (Coenzyme Q10) Prevents Renal Mitochondrial Dysfunction in an Experimental Model of Type 2 Diabetes. Free Radic. Biol. Med. 52 (3), 716–723. doi:10.1016/j.freeradbiomed.2011.11.017
Srivastava, S. (2017). The Mitochondrial Basis of Aging and Age-Related Disorders. Genes (Basel) 8. undefined. doi:10.3390/genes8120398
Talevi, R., Barbato, V., Fiorentino, I., Braun, S., Longobardi, S., and Gualtieri, R. (2013). Protective Effects of In Vitro Treatment with Zinc, D-Aspartate and Coenzyme Q10 on Human Sperm Motility, Lipid Peroxidation and DNA Fragmentation. Reprod. Biol. Endocrinol. 11 (1), 81. doi:10.1186/1477-7827-11-81
Van Opdenbosch, N., and Lamkanfi, M. (2019). Caspases in Cell Death, Inflammation, and Disease. Immunity 50, 1352–1364. doi:10.1016/j.immuni.2019.05.020
Zarei, P., Rezvanfar, M. R., Ansarihadipour, H., Delavar, M., Abdollahi, M., and Khosrowbeygi, A. (2018). Effects of Coenzyme Q10 Supplementation on the Serum Levels of Amylase, Adenosine Deaminase, Catalase, and Total Antioxidant Capacity in Women with Type 2 Diabetes Mellitus: A Randomized, Double-Blind Placebo-Controlled Trial. J. Res. Med. Sci. 23, 91. doi:10.4103/jrms.JRMS_970_17
Zhang, P., Li, T., Wu, X., Nice, E. C., Huang, C., and Zhang, Y. (2020). Oxidative Stress and Diabetes: Antioxidative Strategies. Front. Med. 14, 583–600. doi:10.1007/s11684-019-0729-1
Zhang, Y., Zhang, J., and Zhai, D. (2019). Co-enzyme Q10 and Vitamin E Supplementation Improve Lipid Metabolism and Lipid Peroxidation in Patients with Polycystic Ovary Syndrome. Chin. J. Pract. Med. 16 (11), 23–26.
Zhu, K., Wang, R., and Liu, F. (2020). Effects of Coenzyme Q (10) on the Levels of Pro-inflammatory Cytokines and Oxidative Stress in Patients with Rheumatoid Arthritis. Guangxi Med. J. 42 (04), 417–420.
Keywords: oxidative stress, ferroptosis, evidence-based medicine, coenzyme Q10, mechanism
Citation: Zhang Y, Huang X, Liu N, Liu M, Sun C, Qi B, Sun K, Wei X, Ma Y and Zhu L (2022) Discovering the Potential Value of Coenzyme Q10 in Oxidative Stress: Enlightenment From a Synthesis of Clinical Evidence Based on Various Population. Front. Pharmacol. 13:936233. doi: 10.3389/fphar.2022.936233
Received: 09 May 2022; Accepted: 09 June 2022;
Published: 14 July 2022.
Edited by:
Elham Ahmadian, Tabriz University of Medical Sciences, IranReviewed by:
Gvozden Luka Rosic, University of Kragujevac, SerbiaIain Hargreaves, University of Liverpool, United Kingdom
Copyright © 2022 Zhang, Huang, Liu, Liu, Sun, Qi, Sun, Wei, Ma and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu Wei, d2VpeHUuMDA3QDE2My5jb20=; Yong Ma, MjYwNzU4QG5qdWNtLmVkdS5jbg==; Liguo Zhu, dGNtc3BpbmVAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Yili Zhang
Yili Zhang Xinyi Huang2†
Xinyi Huang2† Ning Liu
Ning Liu Mengmin Liu
Mengmin Liu Chuanrui Sun
Chuanrui Sun Baoyu Qi
Baoyu Qi Xu Wei
Xu Wei