
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 28 November 2022
Sec. Drugs Outcomes Research and Policies
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.934275
Background: Esophageal cancer has a poor prognosis and currently ranks sixth in global cancer mortality rates. The ORIENT-15 trial showed sintilimab plus chemotherapy significantly improved survival when compared to chemotherapy alone. This study aimed to evaluate the cost-effectiveness of sintilimab, a programmed death-ligand 1 (PD-L1) inhibitor, plus chemotherapy in treating patients with esophageal cancer compared with chemotherapy alone.
Methods: A Markov model with a 10-year horizon was developed based on the perspective of the Chinese healthcare payers. We conducted a cost-effectiveness analysis for sintilimab combined with chemotherapy based on a questionnaire. Patients were grouped into the sintilimab group based on a positive score of 10 or more (combined positive score (CPS)
Results: In the base-case analysis, compared with chemotherapy alone, the ICER of sintilimab plus chemotherapy for all patients was $21024.05 per QALY, and in the CPS≥10 group, it was $20974.23 per QALY. This was lower than $37653 per QALY. One-way sensitivity analysis demonstrated that ICERs were most sensitive to the price of sintilimab.
Conclusion: The study demonstrated that sintilimab plus chemotherapy for advanced esophageal cancer as its first-line treatment would be more cost-effective than chemotherapy alone in Chinese patients.
Esophageal carcinoma is a prevalent and fatal malignancy consisting of two major histological types: adenocarcinoma and squamous cell carcinoma (SCC). The latter accounts for approximately 90
In recent years, immune checkpoint inhibitors targeting programmed cell death protein 1 (PD-1) and programmed cell death ligand 1 (PD-L1) have attracted global attention as a novel therapy in treating numerous malignancies. They are able to effectively reduce regulatory T-cell apoptosis and block the immune escape mechanism of tumors (Iwai et al., 2002; Sun et al., 2018). Among them, sintilimab, a fully recombinant human immunoglobulin G4 (IgG4) anti-PD-1 monoclonal antibody, has been approved as treatment for non-small-cell lung cancer, classical Hodgkin’s lymphoma, and hepatocellular cancer by the National Medical Products Administration of China (Shi et al., 2019; Yang et al., 2020; Ren et al., 2021; Zhou et al., 2021). Sintilimab and several other PD-1 monoclonal antibodies have been proven to act as single-agent activity in patients with esophageal squamous cell carcinoma and given as their first-line chemotherapy, leading to an overall improvement of their outcome (Kato et al., 2019; Huang et al., 2020; Kojima et al., 2020; Xu et al., 2020). ORIENT-15, a phase III clinical trial, demonstrated that the combination of sintilimab with chemotherapy outperformed ongoing first-line treatment (cisplatin plus 5-fluorouracil chemotherapy) in patient survival (Lu et al., 2022). In the clinical trial, the sintilimab plus chemotherapy group displayed better progression-free survival (PFS) with a hazard ratio of 0.56 (95
The target cohort for this study was based on the patient characteristics from the population studied in the phase III ORIENT-15 clinical trial. The factors included patients
The study used TreeAge software 2021 (TreeAge Software, Inc., Williamstown, Massachusetts) to program a multi-state Markov model to evaluate the cost-effectiveness of sintilimab plus chemotherapy compared with chemotherapy. The multiple health states include PFS, progressive disease state (PD), and death (Supplementary Figure S1). If patients in a certain state only make one state transition in a cycle, then patients in the PD state cannot return to the PFS state. Similarly, if patients have died then they cannot transition to other states (Ding et al., 2021). The specific transition relationships are shown in Supplementary Figure S1. We assumed that all patients included in this study were in a healthy PFS state at the initial stage of the model. Patients were randomized into two groups: sintilimab with or without chemotherapy. When the disease progressed, the follow-up treatment plan in the ORIENT-15 clinical trial was used as an additional treatment until the patient’s death.
The Markov cohort was used to simulate the patients’ entire live courses. With reference to the dosing cycle from the ORIENT-15 clinical trial, we set the cycle of the Markov model to three weeks, and the time horizon of the model was set at 10 years. Approximately 99
We extracted survival data from the ORIENT-15 trial for model building. The GetData Graph Digitizer (version 2.26; http://getdata-graph-digitizer.com/download.php) was used to obtain the Kaplan–Meier (KM) curve based on the PFS and OS of sintilimab combined with chemotherapy versus chemotherapy alone. We also referred to the algorithm of Guyot et al. who used the pseudo-individual patient’s data reconstructed by R software (version 4.1.0; https://www.r-project.org/) (Guyot et al., 2012). This was combined with the Akaike information criterion (AIC), Bayesian information criterion (BIC), and the visual method to select the optimal distribution from gamma, Weibull, exponential, log-normal, and log-logistic distributions after the reconstruction (Liu et al., 2019). Log-logistic and log-normal distribution can better simulate long-term survival for the survival curve (Supplementary Table S1). Details of model extrapolation are shown in Supplementary Figures S2,S3. Referring to the formula of Liu et al. (2021) to calculate the transition probability, we combined specific parameters of the model to estimate the dynamic transition probability between states for each cycle.
During the follow-up period, the ORIENT-15 trial used the Quality of Life Questionnaire-Core 30 (QLQ-C30), the Quality of Life Questionnaire-Esophageal Cancer Module 18 (QLQ-OES18), and the Five Level EuroQol Five-Dimensional Questionnaire (EQ-5D-5L) to evaluate the patients’ quality of health. Since no specific questionnaire data were previously published, we referred to past studies to obtain the average health utility in terms of PFS and PD (PFS
Only the direct costs of the medical expenses were considered. This included the cost of the drugs, subsequent treatment costs, management costs, follow-up costs, laboratory examination costs, and the major Grade 3/4 AEs. The drug prices were adjusted according to the local drug pricing and medical insurance policies after consulting with drug suppliers. The calculated drug costs were based on actual clinical trials. Once every three weeks, patients received immunotherapy (sintilimab, 3 mg/kg for patients weighing
A one-way sensitivity analysis was performed to explore the influence of uncertain parameters on the ICER. The baseline value and 95
Based on the data from patients with combined positive scores of
From the perspective of the Chinese healthcare payers, the incremental cost of sintilimab plus chemotherapy for all patients was $21024.05, and in the CPS
A one-way sensitivity analysis was used to test the robustness of the model. The influence of each parameter on the results was discussed within the variation range of input model parameters. The results are presented in the tornado diagram (Figure 1). The sensitivity analysis results demonstrated that the cost of sintilimab, the utility of PD, and the utility of PFS were the three primary factors with the greatest impact on the results for all the patients. Under the condition of a payment threshold of $37653 per QALY, when parameters varied within a given range, the ICER was still lower than the WTP of Chinese payers.
PSA was applied to test the bias of the multiple model parameters on the analysis results when the multiple model parameters changed simultaneously. The incremental cost-benefit scatter chart (Figure 3) displayed the results of Monte Carlo simulation. The cost-effectiveness acceptability curves (Figure 2) showed that the randomized patient group is compared with chemotherapy under the condition of a payment threshold of $37653 per QALY. For the combination therapy, the probability of sintilimab plus chemotherapy being cost-effective was 99.36
In ORIENT-15, the use of sintilimab varied according to patient weight. Therefore, in this study, we assessed patients weighing
In addition, we set the WTP to three times China’s GDP per capita in 2021. However, we wanted to explore whether the scenario can be cost-effective under different WTP thresholds. Therefore, we additionally assumed a WTP of twice ($25102 per QALY) China’s GDP per capita in 2021 ($12551). The probability of sintilimab plus chemotherapy being cost-effective was 83.12
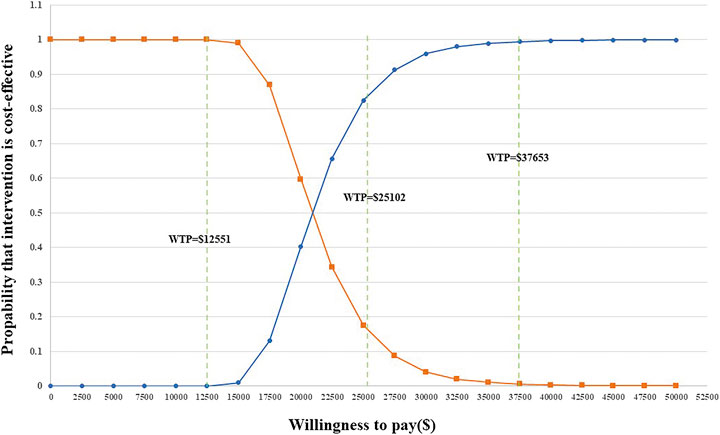
FIGURE 3. Acceptability curves for the choice of sintilimab + chemotherapy versus chemotherapy at different WTP thresholds in any PD-L1 expression group.
Sintilimab is an IgG4 monoclonal antibody that specifically binds to the PD-1 molecule on the surface of T cells, thereby inhibiting the PD-1/PD-L1 pathway, which prevents tumor immune tolerance and reactivates the anti-tumor activity of lymphocytes for the purpose of tumor treatment. The Chinese Society of Clinical Oncology (CSCO) Guidelines Conference 2022 included sintilimab in combination with chemotherapy as a potential first-line therapeutic approach for advanced gastric cancer and advanced esophageal squamous cancer.
In ORIENT-15, the clinical benefits of sintilimab combined with chemotherapy were demonstrated in patients with advanced esophageal cancer. This was regarded as a breakthrough treatment for esophageal cancer (Lu et al., 2022). However, the high cost of immunotherapy remains out of reach for most middle-class families. Finding a balance between price and effectiveness remains a key challenge. Clinicians may be discouraged from using immunotherapy with patients since it is often restricted to certain affluent groups. The income level of a patient was often considered when deciding whether to use immunotherapy (Elkin and Bach, 2010). An economic evaluation of immunotherapy, including sintilimab, could help to avoid squandering healthcare resources. In addition, it will guide physicians in selecting the best treatment options for this specific patient population.
Previous studies have analyzed the economic benefits of sintilimab combined with chemotherapy compared with chemotherapy alone as the first-line treatment of unresectable hepatocellular carcinoma, locally advanced or metastatic non-squamous (Peng et al., 2022; Rui et al., 2022). The economic evaluation of sintilimab as the primary treatment for advanced esophageal cancer was lacking. Therefore, a detailed evaluation of its costs and health outcomes was required which we aimed to provide.
From the perspective of the Chinese healthcare payers, based on the clinical trial results of ORIENT-15, we established a multi-state Markov model to evaluate the economic differences of sintilimab plus chemotherapy and platinum bimodal drug therapy. In ORIENT-15, KM survival curves for all patients (any PD-L1 expression) and PD-L1 expression positive (CPS≥10) were not significantly different in OS and PFS. In our study, for locally advanced or metastatic esophageal squamous cell carcinoma, the combination of sintilimab and chemotherapy was cost-effective in the total population and in PD-L1-positive population at a WTP threshold of $37,653. Sensitivity analysis suggested that the price of sintilimab had a large effect on the results. Therefore, we conducted a scenario analysis of the factors that may influence the price of sintilimab, such as weight, and the results suggested that it would be cost-effective regardless of weight. The cost-effectiveness analysis has different results under different WTP criteria, but it is encouraging to note that even at two to three times China’s GDP in 2021, there was still at least an 83.12
We did not find other studies on the cost-effectiveness analysis related to sintilimab and esophageal cancer, but there were three cost-effectiveness analyses of PD-L1 inhibitors related to esophageal cancer.
Two studies suggest that pembrolizumab was not cost-effective in advanced esophageal cancer, with Zhan et al. (2022) suggesting an increased cost of $37,201.68 for pembrolizumab compared to chemotherapy alone while obtaining a QALY of 0.23. Zhu et al. (2022) suggested an ICER per QALY for pembrolizumab plus chemotherapy compared to chemotherapy of $550,211 in the United States and China were $244,580/QALY and $258,261/QALY, respectively. Pembrolizumab plus chemotherapy yielded 0.386–0.607 QALYs (0.781–1.195 LYs) compared with chemotherapy alone, and both studies had well above the standard WTP. These two studies had no cost effect due to the much higher price of pembrolizumab than the price of the chemotherapy group. In our study, sintilimab plus chemotherapy obtained QALY values of 0.64–0.67 compared to chemotherapy alone, and with the low price of sintilimab relative to pembrolizumab, sintilimab plus chemotherapy was therefore more cost-effective for first-line recommendations in locally advanced or metastatic esophageal squamous cell carcinoma. In addition, Cai et al. (2021) demonstrated that camrelizumab was cost-effective as a second-line regimen compared to chemotherapy in locally advanced or metastatic esophageal squamous cell carcinoma (incremental cost of $1,439.64; added 0.36 QALYs; ICER of $3,999 per QALY). According to his findings, the price of the drug was not significant. The main reason for its cost-effectiveness was the low price of camrelizumab ($432 at a dose of 200 mg) due to its inclusion in China’s national health insurance reimbursement (http://www.nhsa.gov.cn/art/2020/12/28/art_14_4221.html) and the similarly low price of its control chemotherapy drugs (docetaxel: $1.77; irinotecan: $1.64). Unfortunately, the study was based on second-line treatment for locally advanced/metastatic esophageal squamous cell carcinoma, and the chemotherapy regimens used as controls were inconsistent. Therefore, we were unable to make a direct comparison between camrelizumab and sintilimab to determine which was more cost-effective. Large-scale future clinical trials with long follow-up periods are needed to facilitate a comparison of the advantages and disadvantages of the two immunotherapies.
This study had some limitations. First, ORIENT-15 was a phase III randomized controlled trial, and we used this model to simplify the study. For instance, regarding the AEs, we selected the topmost three to four main AEs that grade 3 or higher. Second, the data originated from the ORIENT-15 trial. Due to the limitation of the number of patients included in the trial, we could not perform a larger-scale analysis and the trial did not provide follow-up survival data for patients. We relied on the survival data from the trial and performed a reasonable extrapolation to predict the long-term survival of patients. This will inevitably vary from the data of real-world patients obtained through regular follow-ups. Third, since ORIENT-15 does not disclose the specific health data of patients, our PFS and PD utility were derived from previously published related studies. This may be different from the real-life situation. Fourth, we only considered the cost impact and utility reduction caused by the three main AEs. The utility reduction caused by specific AEs was derived from other published literature works. Fifth, the treatment plan of the trial, and especially the follow-up treatment of patients, will be adjusted appropriately according to the specific situation. For instance, we did not find specific information about follow-up treatment in the ORIENT-15 data, so we assumed several follow-up treatment options, which affected the treatment impact of the two groups to a certain extent. The results of the study were inadequate due to several factors, and more accurate data could be obtained in the future by increasing the sample size and with a longer follow-up period. Therefore, more clinical trials are required in the future to reduce the study population, follow-up treatment, and other factors that impact the results.
Overall, from the perspective of the Chinese health-care payers, sintilimab plus chemotherapy should be considered as the first-line treatment for patients with locally advanced or metastatic esophageal squamous cell carcinoma. Compared with chemotherapy, the combination therapy would be a more cost-effective choice.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Z-MY and ZX: conceptualized the study design, collected the data, interpreted the data, and performed data analysis; Z-MY, ZX, and F-YZ: conducted statistical analysis; Z-QT and Z-MY: writing of the manuscript; QZ: supervised the study, obtained funding, and revised the manuscript; F-YZ and Z-QT: provided administrative, technical, and material support.
This study was supported by Science Foundation of Xiangya Hospital for Young Scholar (No.2015Q09) and the Natural Science Foundation of Hunan Province for Young Scholars (No. 2020JJ5957; project recipient: QZ).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.934275/full#supplementary-material
Abnet, C. C., Arnold, M., and Wei, W. Q. (2018). Epidemiology of esophageal squamous cell carcinoma. Gastroenterology 154 (2), 360–373. doi:10.1053/j.gastro.2017.08.023
Cai, H., Xu, B., Li, N., Zheng, B., Zheng, Z., and Liu, M. (2021). Cost-effectiveness analysis of camrelizumab versus chemotherapy as second-line treatment of advanced or metastatic esophageal squamous cell carcinoma. Front. Pharmacol. 12, 732912. doi:10.3389/fphar.2021.732912
Ding, D., Hu, H., Li, S., Zhu, Y., Shi, Y., Liao, M., et al. (2021). Cost-effectiveness analysis of durvalumab plus chemotherapy in the first-line treatment of extensive-stage small cell lung cancer. J. Natl. Compr. Canc. Netw. 19, 1141–1147. doi:10.6004/jnccn.2020.7796
Ding, D., Hu, H., Liao, M., Shi, Y., She, L., Yao, L., et al. (2020). Cost-effectiveness analysis of Atezolizumab plus chemotherapy in the first-line treatment of metastatic non-squamous non-small cell lung cancer. Adv. Ther. 37 (5), 2116–2126. doi:10.1007/s12325-020-01292-3
Elkin, E. B., and Bach, P. B. (2010). Cancer's next frontier: Addressing high and increasing costs. Jama 303 (11), 1086–1087. doi:10.1001/jama.2010.283
Fatehi Hassanabad, A., Chehade, R., Breadner, D., and Raphael, J. (2020). Esophageal carcinoma: Towards targeted therapies. Cell. Oncol. 43 (2), 195–209. doi:10.1007/s13402-019-00488-2
Guyot, P., Ades, A. E., Ouwens, M. J., and Welton, N. J. (2012). Enhanced secondary analysis of survival data: Reconstructing the data from published kaplan-meier survival curves. BMC Med. Res. Methodol. 12, 9. doi:10.1186/1471-2288-12-9
Haddad, R., Cohen, E. E. W., Venkatachalam, M., Young, K., Singh, P., Shaw, J. W., et al. (2020). Cost-effectiveness analysis of nivolumab for the treatment of squamous cell carcinoma of the head and neck in the United States. J. Med. Econ. 23 (5), 442–447. doi:10.1080/13696998.2020.1715414
Huang, J., Xu, J., Chen, Y., Zhuang, W., Zhang, Y., Chen, Z., et al. (2020). Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): A multicentre, randomised, open-label, phase 3 study. Lancet. Oncol. 21 (6), 832–842. doi:10.1016/S1470-2045(20)30110-8
Husereau, D., Drummond, M., Petrou, S., Carswell, C., Moher, D., Greenberg, D., et al. (2013). Consolidated health economic evaluation reporting standards (CHEERS)-explanation and elaboration: A report of the ISPOR health economic evaluation publication guidelines good reporting practices task Force. Value Health 16 (2), 231–250. doi:10.1016/j.jval.2013.02.002
Ilson, D. H., and van Hillegersberg, R. (2018). Management of patients with adenocarcinoma or squamous cancer of the esophagus. Gastroenterology 154 (2), 437–451. doi:10.1053/j.gastro.2017.09.048
Iwai, Y., Ishida, M., Tanaka, Y., Okazaki, T., Honjo, T., and Minato, N. (2002). Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. U. S. A. 99 (19), 12293–12297. doi:10.1073/pnas.192461099
Kato, K., Cho, B. C., Takahashi, M., Okada, M., Lin, C. Y., Chin, K., et al. (2019). Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet. Oncol. 20 (11), 1506–1517. doi:10.1016/S1470-2045(19)30626-6
Kelly, R. J. (2019). Emerging multimodality approaches to treat localized esophageal cancer. J. Natl. Compr. Canc. Netw. 17 (8), 1009–1014. doi:10.6004/jnccn.2019.7337
Kojima, T., Shah, M. A., Muro, K., Francois, E., Adenis, A., Hsu, C. H., et al. (2020). Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J. Clin. Oncol. 38 (35), 4138–4148. doi:10.1200/JCO.20.01888
Lagergren, J., Smyth, E., Cunningham, D., and Lagergren, P. (2017). Oesophageal cancer. Lancet (London, Engl. 390 (10110), 2383–2396. doi:10.1016/S0140-6736(17)31462-9
Liu, G., Kang, S., Wang, X., and Shang, F. (2021). Cost-effectiveness analysis of Atezolizumab versus chemotherapy as first-line treatment for metastatic non-small-cell lung cancer with different PD-L1 expression status. Front. Oncol. 11, 669195. doi:10.3389/fonc.2021.669195
Liu, M., Zhang, L., Huang, Q., Li, N., Zheng, B., and Cai, H. (2019). Cost-effectiveness analysis of ceritinib and alectinib versus crizotinib in the treatment of anaplastic lymphoma kinase-positive advanced non-small cell lung cancer. Cancer Manag. Res. 11, 9195–9202. doi:10.2147/CMAR.S223441
Lu, Z., Wang, J., Shu, Y., Liu, L., Kong, L., Yang, L., et al. (2022). Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): Multicentre, randomised, double blind, phase 3 trial. BMJ Clin. Res. ed) 377, e068714. doi:10.1136/bmj-2021-068714
Murray, C. J., Evans, D. B., Acharya, A., and Baltussen, R. M. (2000). Development of WHO guidelines on generalized cost-effectiveness analysis. Health Econ. 9 (3), 235–251. doi:10.1002/(sici)1099-1050(200004)9:3<235:aid-hec502>3.0.co;2-o
Nafees, B., Stafford, M., Gavriel, S., Bhalla, S., and Watkins, J. (2008). Health state utilities for non small cell lung cancer. Health Qual. Life Outcomes 6, 84. doi:10.1186/1477-7525-6-84
Napier, K. J., Scheerer, M., and Misra, S. (2014). Esophageal cancer: A review of epidemiology, pathogenesis, staging workup and treatment modalities. World J. Gastrointest. Oncol. 6 (5), 112–120. doi:10.4251/wjgo.v6.i5.112
NCCN (2021). NCCN Guidelines,Esophageal and esophagogastric junction cancers. Available at: https://www.nccn.org/guidelines/guidelines-detail.
Pei, R., Shi, Y., Lv, S., Dai, T., Zhang, F., Liu, S., et al. (2021). Nivolumab vs pembrolizumab for treatment of US patients with platinum-refractory recurrent or metastatic head and neck squamous cell carcinoma: A Network meta-analysis and cost-effectiveness analysis. JAMA Netw. Open 4 (5), e218065. doi:10.1001/jamanetworkopen.2021.8065
Peng, Y., Zeng, X., Peng, L., Liu, Q., Yi, L., Luo, X., et al. (2022). Sintilimab plus bevacizumab biosimilar versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma: A cost-effectiveness analysis. Front. Pharmacol. 13, 778505. doi:10.3389/fphar.2022.778505
Pongchaiyakul, C., Nanagara, R., Songpatanasilp, T., and Unnanuntana, A. (2020). Cost-effectiveness of denosumab for high-risk postmenopausal women with osteoporosis in Thailand. J. Med. Econ. 23 (7), 776–785. doi:10.1080/13696998.2020.1730381
Ren, Z., Xu, J., Bai, Y., Xu, A., Cang, S., Du, C., et al. (2021). Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2-3 study. Lancet. Oncol. 22 (7), 977–990. doi:10.1016/S1470-2045(21)00252-7
Rui, M., Fei, Z., Wang, Y., Zhang, X., Ma, A., Sun, H., et al. (2022). Cost-effectiveness analysis of sintilimab + chemotherapy versus camrelizumab + chemotherapy for the treatment of first-line locally advanced or metastatic nonsquamous NSCLC in China. J. Med. Econ. 25, 618–629. doi:10.1080/13696998.2022.2071066
Rustgi, A. K., and El-Serag, H. B. (2014). Esophageal carcinoma. N. Engl. J. Med. 371 (26), 2499–2509. doi:10.1056/NEJMra1314530
Sanders, G. D., Neumann, P. J., Basu, A., Brock, D. W., Feeny, D., Krahn, M., et al. (2016). Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second panel on cost-effectiveness in health and medicine. Jama 316 (10), 1093–1103. doi:10.1001/jama.2016.12195
She, L., Hu, H., Liao, M., Xia, X., Shi, Y., Yao, L., et al. (2019). Cost-effectiveness analysis of pembrolizumab versus chemotherapy as first-line treatment in locally advanced or metastatic non-small cell lung cancer with PD-L1 tumor proportion score 1% or greater. Lung Cancer 138, 88–94. doi:10.1016/j.lungcan.2019.10.017
Shi, Y., Su, H., Song, Y., Jiang, W., Sun, X., Qian, W., et al. (2019). Safety and activity of sintilimab in patients with relapsed or refractory classical Hodgkin lymphoma (ORIENT-1): A multicentre, single-arm, phase 2 trial. Lancet. Haematol. 6 (1), e12–e19. doi:10.1016/S2352-3026(18)30192-3
Sun, C., Mezzadra, R., and Schumacher, T. N. (2018). Regulation and function of the PD-L1 checkpoint. Immunity 48 (3), 434–452. doi:10.1016/j.immuni.2018.03.014
Uhlenhopp, D. J., Then, E. O., Sunkara, T., and Gaduputi, V. (2020). Epidemiology of esophageal cancer: Update in global trends, etiology and risk factors. Clin. J. Gastroenterol. 13 (6), 1010–1021. doi:10.1007/s12328-020-01237-x
Ward, M. C., Shah, C., Adelstein, D. J., Geiger, J. L., Miller, J. A., Koyfman, S. A., et al. (2017). Cost-effectiveness of nivolumab for recurrent or metastatic head and neck cancer. Oral Oncol. 74, 49–55. doi:10.1016/j.oraloncology.2017.09.017
Wu, B., Ye, M., Chen, H., and Shen, J. F. (2012). Costs of trastuzumab in combination with chemotherapy for HER2-positive advanced gastric or gastroesophageal junction cancer: An economic evaluation in the Chinese context. Clin. Ther. 34 (2), 468–479. doi:10.1016/j.clinthera.2012.01.012
Xu, J., Li, Y., Fan, Q., Shu, Y., Wu, Z., Cui, T., et al. (2020). Sintilimab in patients with advanced esophageal squamous cell carcinoma refractory to previous chemotherapy: A randomized, open-label phase II trial (ORIENT-2). J. Clin. Oncol. 38 (15), 4511. doi:10.1200/jco.2020.38.15_suppl.4511
Yang, F., Fu, Y., Kumar, A., Chen, M., Si, L., and Rojanasarot, S. (2021). Cost-effectiveness analysis of camrelizumab in the second-line treatment for advanced or metastatic esophageal squamous cell carcinoma in China. Ann. Transl. Med. 9 (15), 1226. doi:10.21037/atm-21-1803
Yang, Y., Wang, Z., Fang, J., Yu, Q., Han, B., Cang, S., et al. (2020). Efficacy and safety of sintilimab plus pemetrexed and platinum as first-line treatment for locally advanced or metastatic nonsquamous NSCLC: A randomized, double-blind, phase 3 study (Oncology pRogram by InnovENT anti-PD-1-11). J. Thorac. Oncol. 15 (10), 1636–1646. doi:10.1016/j.jtho.2020.07.014
Zhan, M., Xu, T., Zheng, H., and He, Z. (2022). Cost-effectiveness analysis of pembrolizumab in patients with advanced esophageal cancer based on the KEYNOTE-181 study. Front. Public Health 10, 790225. doi:10.3389/fpubh.2022.790225
Zhang, P. F., Xie, D., and Li, Q. (2020). Cost-effectiveness analysis of nivolumab in the second-line treatment for advanced esophageal squamous cell carcinoma. Future Oncol. 16 (17), 1189–1198. doi:10.2217/fon-2019-0821
Zhou, C., Wu, L., Fan, Y., Wang, Z., Liu, L., Chen, G., et al. (2021). Sintilimab plus platinum and gemcitabine as first-line treatment for advanced or metastatic squamous NSCLC: Results from a randomized, double-blind, phase 3 trial (ORIENT-12). J. Thorac. Oncol. 16 (9), 1501–1511. doi:10.1016/j.jtho.2021.04.011
Zhou, T., Cao, Y., Wang, X., Yang, L., Wang, Z., et al. (2022). Economic evaluation of sintilimab plus bevacizumab versus sorafenib as a first-line treatment for unresectable hepatocellular carcinoma. Adv. Ther. 39 (5), 2165–2177. doi:10.1007/s12325-022-02079-4
Keywords: cost-effectiveness, sintilimab, esophageal cancer, Markov model, PD-L1
Citation: Ye Z-M, Xu Z, Zeng F-Y, Tang Z-Q and Zhou Q (2022) Cost-Effectiveness Analysis of Sintilimab Combined with Chemotherapy Versus Chemotherapy Alone as the First-Line Treatment for Advanced Esophageal Cancer. Front. Pharmacol. 13:934275. doi: 10.3389/fphar.2022.934275
Received: 02 May 2022; Accepted: 24 May 2022;
Published: 28 November 2022.
Edited by:
Ming Yi, Zhejiang University, ChinaReviewed by:
Shiyu Li, Huazhong University of Science and Technology, ChinaCopyright © 2022 Ye, Xu, Zeng, Tang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qin Zhou, emhvdXFpbjU3OTZAY3N1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.