
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 06 October 2022
Sec. Ethnopharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.933519
This article is part of the Research Topic Multi-Component and Multi-Target Drugs in the Therapy of Chronic Complex Diseases View all 8 articles
 Linda LD. Zhong1,2,3*†
Linda LD. Zhong1,2,3*† Rongsheng Wang4,5‡
Rongsheng Wang4,5‡ Wai Ching Lam1‡
Wai Ching Lam1‡ Qi Zhu4,5‡
Qi Zhu4,5‡ Peipei Du1
Peipei Du1 Pei Hua Cao1
Pei Hua Cao1 Ting Jiang4,5
Ting Jiang4,5 Yuan Yuan Zhang4,5
Yuan Yuan Zhang4,5 Jie Shen4,5
Jie Shen4,5 Xiao Su6
Xiao Su6 Luan Xue7
Luan Xue7 Jianchun Mao8
Jianchun Mao8 Yong Fei Fang9
Yong Fei Fang9 Ming Li Gao10
Ming Li Gao10 Chun Rong Hu11
Chun Rong Hu11 Jiang Yun Peng12
Jiang Yun Peng12 Ying Gu13
Ying Gu13 Qianghua Wei14
Qianghua Wei14 Runyue Huang3,15,16,17
Runyue Huang3,15,16,17 Aiping Lyu1,3
Aiping Lyu1,3 Hongxia Liu18*†
Hongxia Liu18*† Dongyi He4,5*†
Dongyi He4,5*†Objective: To investigate the efficacy of Integrative medicine (IM), compare with Western medicine (WM), in the treatment of rheumatoid arthritis (RA) in a cohort study.
Methods: This is a cohort study with recruitment of RA patients from 10 hospitals in China. The primary outcome was change in disease activity score 28 (DAS28) during 4 follow-up visits. Generalized estimating equation (GEE) models that controlled for variables were used to investigate a time trend and assess group differences in the primary outcome and secondary outcomes after propensity score matching (PSM).
Results: A total of 3195 patients with RA received IM (n = 1379, 43.2%) or WM (n = 1816, 56.8%). Following 1:1 propensity score matching, 1,331 eligible patients prescribed IM were compared to 1,331 matched patients prescribed WM. The GEE analysis with PSM showed that the IM was more beneficial to significantly decrease the levels of VAS, PGA and PhGA (VAS: odds ratio (OR), 0.76; 95% CI, 0.63–0.92; p = 0.004; PGA: OR, 0.76; 95% CI, 0.64–0.92; p = 0.007; and PhGA: OR, 0.77; 95% CI, 0.64, 0.93; p = 0.004), and reduce DAS28 (OR, 0.84; 95% CI, 0.73–0.98; p = 0.030) in the per-protocol population.
Conclusion: This study suggests that compare to WM, IM has advantages in improving RA-related outcomes. However, the statistical significance might not reveal significant clinical difference. Further studies should be focused on specific treatment strategies and/or disease stages.
Management of rheumatoid arthritis (RA) symptoms using Integrative medicine (IM), such as a combination of Chinese medicine (CM) and Western medicine (WM), has been widely adopted among Chinese populations (Zhao et al., 2013). With growing needs in the public and interests among investigators, number of clinical studies, including trials and reviews, have been trending up in recent decades.
Although existing clinical trials provided evidence for the efficacy and safety of integrative approaches in treating RA (Xing et al., 2020; Lu et al., 2022), the studies only involved limited kinds of interventions and cannot reflect the situations happening in the real-world. Moreover, clinical trials are not feasible given the large numbers of participants needed and high adherence with long follow-up (Zhang et al., 2015).
Currently in China, the prevalence of RA patients is estimated to be tens of million across 23 provinces (Langley et al., 2011). Rather than biologic therapies, patients are prescribed IM or WM as they visit the primary health-care system. The majority of WM prescribed covers steroids, non-steroidal anti-inflammatory drugs (NSAIDs), and disease-modifying antirheumatic drugs (DMARDs). On the other hand, the prescriptions of Chinese herbal medicine can be variable according to the CM diagnosis. While the use of IM for RA has been the norm in recent decades, there are still lack of large-scale studies to review the efficacy. An overview of whether the combined use of CM and WM could be beneficial to the prognosis of RA becomes critical.
Given the complexity in clinical settings and limited evidence on the advantages of using IM in alleviating RA related symptoms, we proposed this cohort study to investigate the efficacy of IM in the treatment of RA.
This cohort study was conducted through the National Integrative Medicine Network for Rheumatoid Diseases from February 2014 to August 2018. The 10 hospitals in the network included I) ShangHai GuangHua Hospital of Integrated Traditional Chinese and Western Medicine, II) Mianyang Hospital of Traditional Chinese Medicine, III) The Ninth People’s Hospital of Chongqing, IV) Shanghai Municipal Hospital of Traditional Chinese Medicine, V) Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, VI) Southwest Hospital, VII) LongHua Hospital Shanghai University of Traditional Chinese Medicine, VIII) Liaoning Hospital of Traditional Chinese Medicine, IX) Yunnan Provincial Hospital of Traditional Chinese Medicine, and X) Shanghai General Hospital. The study was approved by the Research Ethics Committee of ShangHai GuangHua Hospital specifically for this research (No. 2014-K-04) and the ethics approval has been circulated and endorsed by the other nine hospital ethics committees before initiating the research. Public promotion was adopted within the hospitals and affiliated outpatient clinics. Both inpatients and outpatients of Rheumatology were able to sign up for the study.
Eligible patients aged 18 years or older with RA for at least 3 months who fulfilled the 1987 American College of Rheumatology (ACR) or 2010 European Alliance of Associations for Rheumatology (EULAR) diagnostic criteria diagnosed by physicians (Britsemmer et al., 2011; Kay and Upchurch, 2012). Patients were excluded if they had little or were lack of ability for self-care; confusion in diagnosis caused by acute and chronic infections; been diagnosed with severe, progressive, or uncontrolled diseases on heart, liver, kidney, gastroenterology, endocrinology, hematology, or cancer; history of joint surgery; medical history of neurological diseases or psychiatric disorders; been currently participating in clinical trials. All the participants gave written informed consent. Patients included in the study received either 1) IM or 2) WM during the treatment based on clinical considerations of physicians. The IM is defined as combined treatment of WM and CM. WM consists of pharmacotherapy for RA including steroids, NSAIDs, and DMARDs. On the other hand, CM involved Chinese herbal decoctions, or tablets/capsules composed solely of Chinese herbs and their extracts. The types of medications used by patients with RA at baseline and during follow-up were shown in Table 1. All participants were then followed up 1 year with 3-month intervals: at 3 months, 6 months, 9 months, and 12 months from the baseline visit. Since there were cases with a discrepancy between the medication at each follow-up, we defined study populations as follows to delineate the genuine effect of IM or WM treatment; intention-to-treat (ITT) and per-protocol (PP) populations. The ITT population was defined by patients who received medication at baseline. The PP population was restricted to the population who received the same medication at baseline and at follow-up.
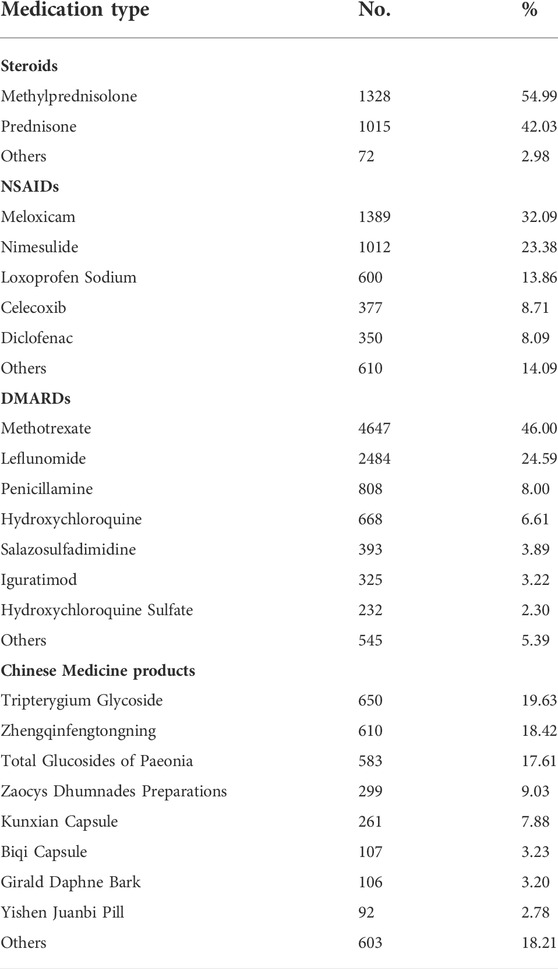
TABLE 1. Types of medications used by patients with Rheumatoid Arthritis at baseline and during follow-up.
The following baseline variables were gathered from designed questionnaire: patient demographic characteristics (age, gender, body mass index (BMI)), family history of rheumatic immunity (RI)-related, operation history of RI-related, smoking, alcohol use, comorbidities (hypertension, diabetes, et al.), duration of RA.
To reduce the potential biases in selection and lost to follow-up, we used propensity score matching (PSM) to match the comparative groups in ITT and PP population. Propensity scores for the type of medicine were estimated using a logistic regression model including all the above-mentioned baseline variables. Participants in the IM group were matched 1:1 to the WM group, by propensity score using the nearest neighbor algorithm, with a caliper width of 0.05.
The primary outcome was change in disease activity score 28 (DAS28) during 4 follow-up visits. The secondary outcomes included change in tender joint count (TJC), swollen joint count (SJC), morning stiffness (MS), visual analog scale (VAS), patient’s and physician’s global assessment of disease activity based on visual analogue scale (PGA, PhGA), erythrocyte sedimentation rate (ESR), c-reactive protein (CRP), rheumatoid factor (RF), anti-cyclic citrullinated peptide (Anti-CCP), simplified disease activity index (SDAI), clinical disease activity index (CDAI), health assessment questionnaire (HAQ) during 4 follow-up visits.
Continuous variables were expressed as mean (standard deviation) or medians (interquartile range, IQR), depending on the data distribution pattern. Categorical variables were described using frequencies and percentages. The Multiple Imputation (MI) by chained equations method was used to interpolate the missing data. Baseline characteristics before and after PSM were compared between IM and WM groups using the variance analysis Kruskal–Wallis rank-sum test for continuous variables and Chi-square test for categorical variables. Generalized estimating equation (GEE) models that controlled for variables were used to investigate a time trend and assess group differences in the primary outcome and secondary outcomes after PSM. This was done using a GEE autoregressive time-lag model that correlates the IM or WM on RA related clinical outcomes 1 year later. The IM or WM was used as an independent variable, each RA related clinical outcomes at baseline was used as control variable, and the corresponding continuous clinical outcomes were used as dependent variables. Significance levels were set at a 2-tailed p < 0.05. Statistical analyses were performed using R (Version 4.1.0).
A total of 3195 patients with RA received IM (n = 1379, 43.2%) or WM (n = 1816, 56.8%) and were included in the ITT population (Figure 1). During the 1-year observational period, 996 patients lost to follow up, and a comparison of baseline characteristics between the lost and follow-up groups was shown in Supplementary Table S1. 1619 (50.7%) patients continued the same medication until 12 months and were considered as the PP population. During the 1-year follow-up, a total of 1576 ITT patients changed the type of medication from WM to IM or vis versa. The comparison of baseline characteristics between the unchanged medication group and the medication changed group was shown in Supplementary Table S2. The frequency of missing baseline information of the unchanged medication patients was 38 (1.19%) cells (Supplementary Table S3). Throughout the study, we recorded abnormal values from the patients based on their laboratory test results. However, we are not able to judge whether the abnormalities were caused by the disease or/and the treatments. In the study, no serious adverse event was observed. There was no direct evidence showing a significant difference in the rate of self-reporting adverse events in the PP population between the two groups (p = 0.713), which was 1.35% and 1.72% in the WM and IM groups, respectively.
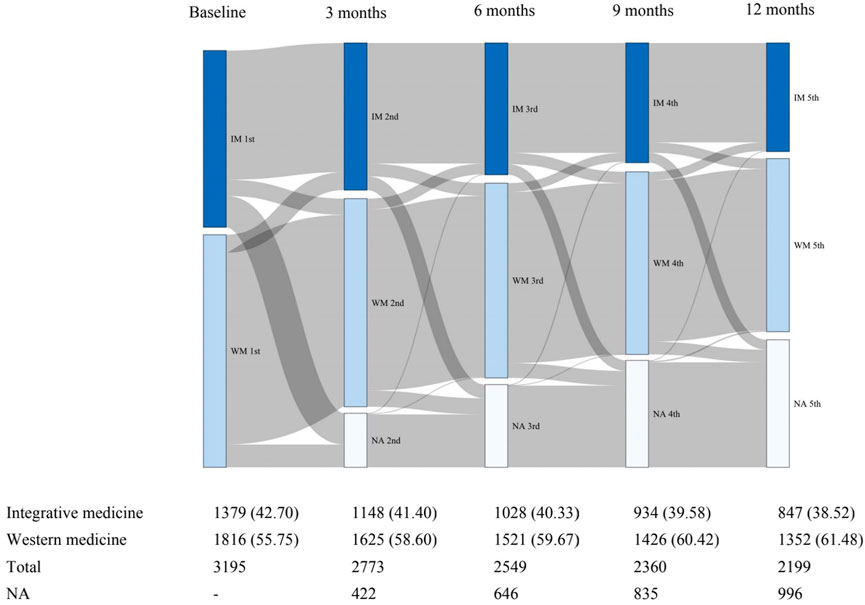
FIGURE 1. Sankey diagram of changes in clinical medicine during the first year of follow-up. IM: Integrative medicine, WM: Western medicine.
In the unmatched groups (1,816 patients treated by WM compared to 1,379 patients treated by IM), the IM group was older (mean age 62.02 versus 60.34 years, p < 0.001) and had a higher level of BMI (22.09 versus 21.89 kg/m2, p = 0.036) compared to the WM group in the ITT population (Supplementary Table S4). Compared to the WM group, the IM group had similar proportion of males, family history and operation history of RI-related. The IM group had higher rates of smoking and drinking. 256 (18.6%) patients reported at least one comorbidity in the IM group which was slightly higher than the WM group (p = 0.044). However, the prevalence of hypertension and diabetes was similar in both groups. The median duration of RA of both groups was about 6 years.
Following 1:1 propensity score matching, 1,331 eligible patients prescribed IM were compared to 1,331 matched patients prescribed WM. After matching, the demographic and clinical characteristics of both groups were well balanced (Supplementary Table S4).
Comparisons of treatment groups in the PP population before PSM showed that the IM group were older than the WM group (mean age 64.66 versus 60.96 years, p < 0.001). The proportions of male patients were about 19% in both groups. 56 (5.4%) patients in the WM group had family history of RI-related which was higher than the IM group (p = 0.018). Patients in the WM group had higher smoking rates (2.4% versus 1.0%, p = 0.050). 553 eligible patients prescribed IM were compared to 553 matched patients prescribed WM after PSM. No statistically significant differences were found in baseline variables after matching between groups (Table 2).
Changes in clinical manifestations measures of RA in the ITT and PP population from baseline to 4 follow-up visits were shown in Supplementary Table S5 and Table 3, respectively. The comparison in different rheumatoid arthritis clinical manifestations between baseline and visit 4 in medication changed group was shown in Supplementary Table S6. In the ITT population, the time × group interaction for all outcomes was not significant (p > 0.05). Figure 2 shows the changes of outcomes in six domains related to RA, including joint, morning stiffness, and pain, between baseline and the fourth follow-up, and overall decreased in both IM and WM group in PP population. The results indicated a significant time × group interaction for MS (p = 0.049), PGA (p = 0.049), and PhGA (p = 0.047), indicating that the scores for these three domains in the 2 groups had different trends over the 5 time points. Compared with the WM, the IM significantly decreased the levels of VAS, PGA and PhGA in the PP analysis (VAS: odds ratio (OR), 0.76; 95%CI, 0.63–0.92; p = 0.004; PGA: OR, 0.76; 95% CI, 0.64–0.92; p = 0.007; and PhGA: OR, 0.77; 95% CI, 0.64, 0.93; p = 0.004).
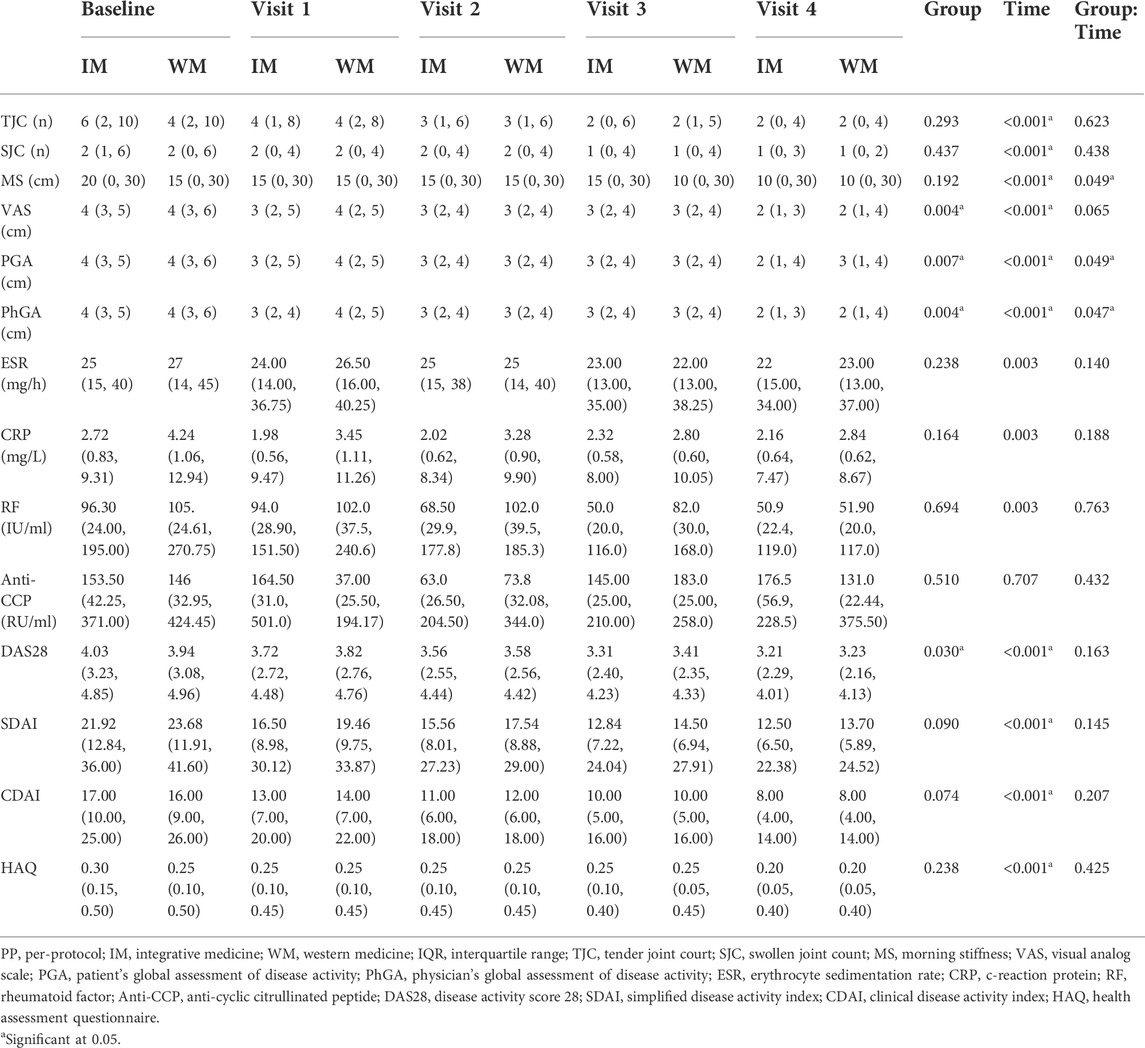
TABLE 3. Change in different rheumatoid arthritis clinical manifestations at different visits in PP population, median (IQR) unless otherwise stated.
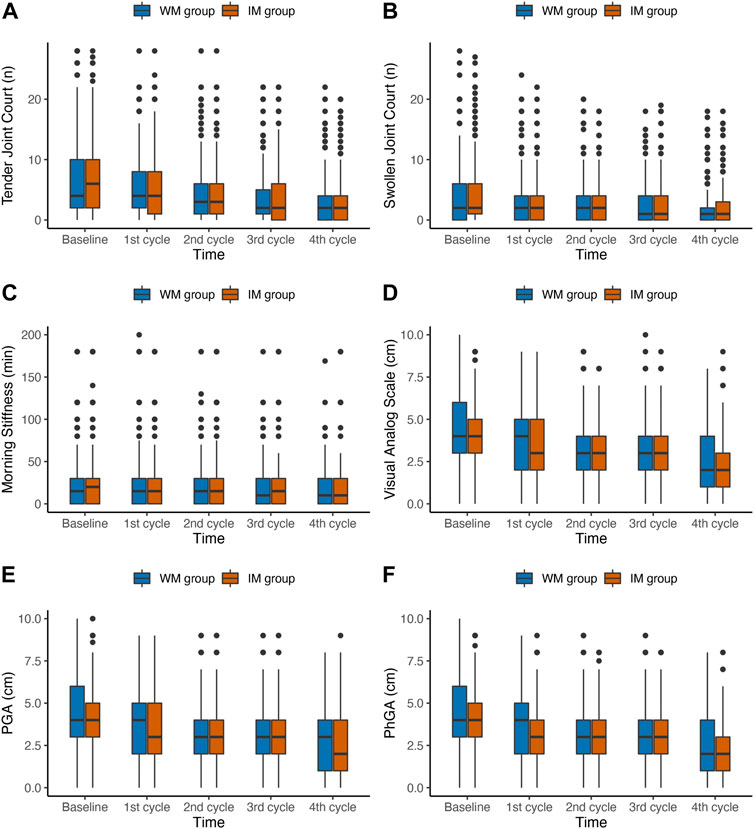
FIGURE 2. Box plots of changes in joint, morning stiffness, and pain outcomes during the first year of follow-up in PP population. IM: Integrative medicine, WM: Western medicine, PGA, PhGA, patient’s and doctor’s global assessment of disease activity based on visual analogue scale.
The variation patterns of laboratory outcomes in the IM and WM groups in PP population at baseline and follow-up were shown in Figure 3. The average level of RA related laboratory indicators in each domain in both two groups gradually decreased over time (Table 3). The result indicated a significant time × group interaction for ESR (p = 0.032). The ESR level of patients in the IM and WM groups decreased gradually from baseline 28 mm/h to 21 and 22 mm/h, respectively. However, the level of CRP, RF and CCP reduced by IM was not significantly higher than that of WM (p > 0.05).
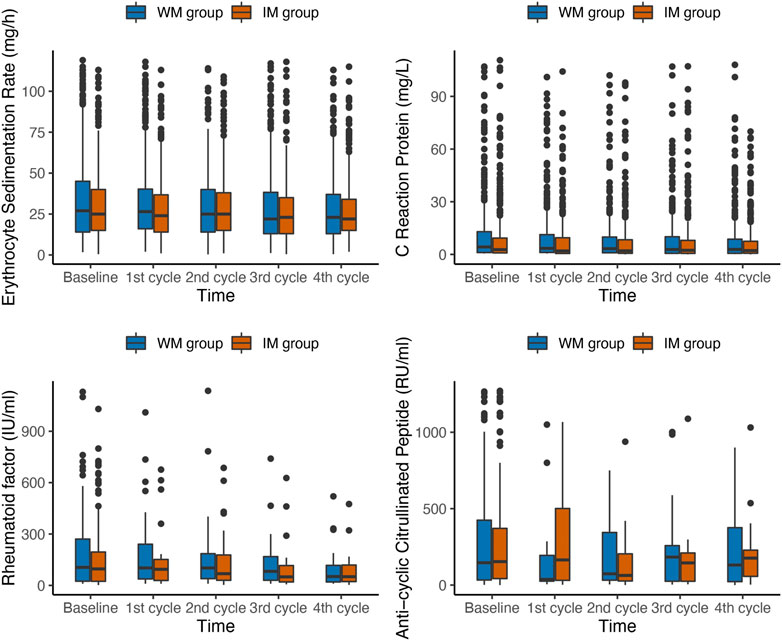
FIGURE 3. Box plots of changes in laboratory outcomes during the first year of follow-up in PP population. IM: Integrative medicine, WM: Western medicine, PGA, PhGA, patient’s and doctor’s global assessment of disease activity based on visual analogue scale.
In the PP population, the scores of DAS28, SDAI, CDAI and HAQ from patients were gradually reduced from baseline among groups over time in 1 year (Figure 4). The median DAS28 score decreased from 4.07 at baseline to 3.21 at the fourth follow-up in the IM group and from 3.94 to 3.23 in the WM. The GEE analysis with PSM showed that the IM was more beneficial to significantly reduce DAS28 in the PP population (OR, 0.84; 95% CI, 0.73–0.98; p = 0.030; Table 3). Both IM and WM were beneficial to reduce the scores of SDAI, CDAI and HAQ, and there was no statistical significance between the two groups (p > 0.05).
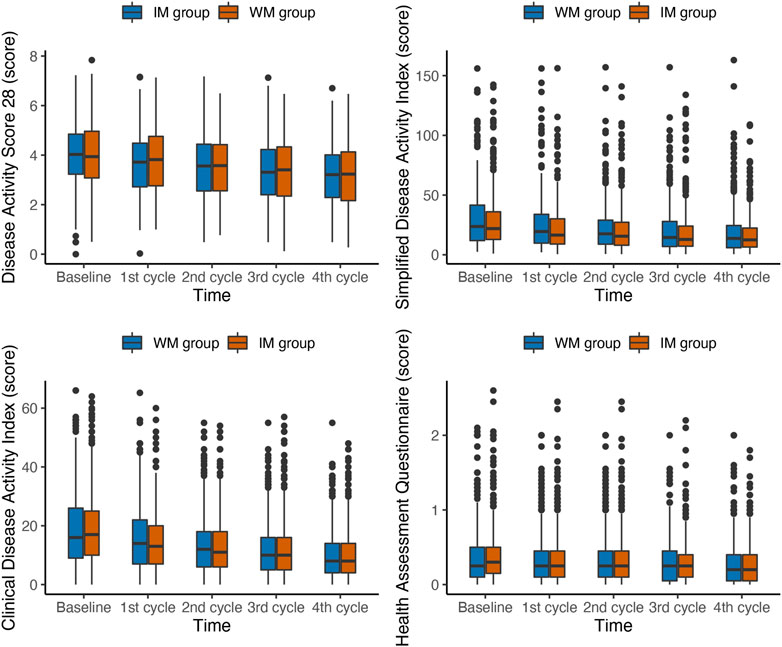
FIGURE 4. Box plots of changes in Composite outcomes during the first year of follow-up in PP population. IM: Integrative medicine, WM: Western medicine, PGA, PhGA, patient’s and doctor’s global assessment of disease activity based on visual analogue scale.
This is the first study compared the use of IM and WM for treatment of RA in real-world clinical settings with proper design, participants in the study had been followed-up for months with continuous collection of variables through blood tests, interviews, and questionnaires. The analysis with matching showed that IM could significantly reduce levels of VAS, PGA, PhGA, and DAS28 in the population who received the same medication at baseline and at follow-up.
A possible explanation for the finding is that the application of IM allows physicians to have further treatment options for alleviating global symptoms. Under the primary health-care system in mainland China, the diversity of WM drugs provided in hospitals is limited, and Chinese herbal medicine can be used as complementary approach. Chinese herbal medicine not only can be applied to treat RA-related symptoms, but also help to relief adverse effects caused by the WM (Xing et al., 2020). In this study, however, we were not able to specify the potential adverse effects which could be avoided by the use of IM.
Biologically, the involvement of Chinese herbal medicine induces multiple treatment pathways and mechanisms especially single herb can contain various kinds of active ingredients targeting RA-related receptors and biomarkers (Chen et al., 2004; van der Greef et al., 2010; Seca and Franconi, 2018). With human clinical trials and animal studies suggested the efficacy and safety of IM with Chinese herbal medicine and their extractions (Liu et al., 2018; Wang et al., 2019), IM approaches are expected to motivate improvements in RA treatments strategies and outcomes. Moreover, as biological agents for rheumatoid arthritis are gradually under consideration for national health insurance coverage, we look forward to investigating the role of biological agents in IM treatment strategies in future studies.
Prevalence of RA is estimated to be 0.2–0.3% in China with approximately 3-million patients (Zeng et al., 2008). Data provided by this perspective cohort will play a key role in reflection of the health service in China by revealing the medical treatments given to the RA patients. In recent years, IM approaches have been receiving attention globally not only among the patients, but also among physicians, researchers, and decision-makers. However, in many diseases, the benefits brought by IM over WM are still unclear. As a result, we hope our analysis could facilitate other teams/nations to carry out further IM-related research for RA in particular through clinical trials with investigation of underlying treatment mechanisms, and eventually, facilitate development of corresponding IM clinical practice guidelines.
Our study has strengths in large sample size, months of follow-up period, high response rate, high compliance of participants, and the study design. With interviews and medical visits to provide complete and accurate information, patients were expected to be relatively easy to follow over time and more likely to maintain participation in long-term. Aimed to provide an overview of IM and WM treatments in RA patients, this study is unique with generalizability as covered patients in multiple provinces. In limitations, dosages and formula of the medicines and disease stages were not involved in the analysis. Then, adverse events were not clearly identified and analyzed due to limitations in medical records and data collection. Finally, residual confounding cannot be ignored in cohort studies due to incomplete control of confounders.
This study suggests that compare to WM, IM has advantages in improving RA-related outcomes. However, the statistical significance might not reveal significant clinical difference. Further studies should be focused on specific treatment strategies and/or disease stages.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Research Ethics Committee of ShangHai GuangHua Hospital. The patients/participants provided their written informed consent to participate in this study.
LZ, AL, DH designed the study, RW, QZ, TJ, YZ, JS, XS, LX, JM, YF, MG, CH, JP, YG, QW conducted the research in different centers. PD, PC and HL analyzed the data. LZ, WL, and PD drafted the paper. DH, AL and RH gave critical advice. All the authors revised and approved the final manuscript.
This work was supported by following funding programs: Leading Talents in Shanghai; Shanghai Municipal Health Commission, and East China Region based Chinese and Western Medicine Joint Disease Specialist Alliance [ZY (2021-2023)-0302]; The 2020 Guangdong Provincial Science and Technology Innovation Strategy Special Fund (Guangdong-Hong Kong-Macau Joint Lab), [No: 2020B1212030006]; and Science and Technology Commission of Shanghai Municipality [STCSM Q12 19401934600].
We thank all the patients participated for data contribution and our colleagues for their dedicated work on the cohort. We would also like to thank Shu Yang, School of Intelligent Medicine, Chengdu University of Traditional Chinese Medicine, for the support in statistical analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.933519/full#supplementary-material
RA, Rheumatoid arthritis; IM, Integrative medicine; CM, Chinese medicine; WM, Western medicine; NSAIDs, non-steroidal anti-inflammatory drugs; DMARDs, disease-modifying antirheumatic drugs; ACR, American College of Rheumatology; EULAR, European Alliance of Associations for Rheumatology; ITT, intention-to-treat; PP, per-protocol; BMI, body mass index; RI, rheumatic immunity; PSM, propensity score matching; DAS28, disease activity score 28; TJC, tender joint count; SJC, swollen joint count; MS, morning stiffness; VAS, visual analog scale; PGA, patient’s global assessment of disease activity; PhGA, physician’s global assessment of disease activity; ESR, erythrocyte sedimentation rate; CRP, c-reactive protein; RF, rheumatoid factor; Anti-CCP, anti-cyclic citrullinated peptide; SDAI, simplified disease activity index; CDAI, clinical disease activity index; HAQ, health assessment questionnaire; MI, multiple imputation.
Britsemmer, K., Ursum, J., Gerritsen, M., van Tuyl, L. H., van Schaardenburg, D., and van Schaardenburg, D. (2011). Validation of the 2010 ACR/EULAR classification criteria for rheumatoid arthritis: Slight improvement over the 1987 ACR criteria. Ann. Rheum. Dis. 70 (8), 1468–1470. doi:10.1136/ard.2010.148619
Chen, X., Oppenheim, J. J., and Howard, O. M. (2004). Chemokines and chemokine receptors as novel therapeutic targets in rheumatoid arthritis (RA): Inhibitory effects of traditional Chinese medicinal components. Cell. Mol. Immunol. 1 (5), 336
Kay, J., and Upchurch, K. S. (2012). ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatol. Oxf. 51 (6), vi5–9. doi:10.1093/rheumatology/kes279
Langley, P. C., Mu, R., Wu, M., Dong, P., and Tang, B. (2011). The impact of rheumatoid arthritis on the burden of disease in urban China. J. Med. Econ. 14 (6), 709–719. doi:10.3111/13696998.2011.611201
Liu, W., Zhang, Y., Zhu, W., Ma, C., Ruan, J., Long, H., et al. (2018). Sinomenine inhibits the progression of rheumatoid arthritis by regulating the secretion of inflammatory cytokines and monocyte/macrophage subsets. Front. Immunol. 9, 2228. doi:10.3389/fimmu.2018.02228
Lu, H. L., Chang, C. M., Hsieh, P. C., Wang, J. C., and Kung, Y. Y. (2022). The effects of acupuncture and related techniques on patients with rheumatoid arthritis: A systematic review and meta-analysis. J. Chin. Med. Assoc. 85 (3), 388–400. doi:10.1097/JCMA.0000000000000659
Seca, S., and Franconi, G. (2018). Understanding Chinese medicine patterns of rheumatoid arthritis and related biomarkers. Med. (Basel) 5 (1), 17. doi:10.3390/medicines5010017
van der Greef, J., van Wietmarschen, H., Schroën, J., Wang, M., Hankemeier, T., and Xu, G. (2010). Systems biology-based diagnostic principles as pillars of the bridge between Chinese and Western medicine. Planta Med. 76 (17), 2036–2047. doi:10.1055/s-0030-1250450
Wang, Y., Liu, Y., Xi, Z., Yu, Y., Liu, L., Mao, J., et al. (2019). A multicenter, randomized, double-blind, placebo-controlled trial evaluating the efficacy and safety of Huangqi Guizhi Wuwutang granule in patients with rheumatoid arthritis. Med. Baltim. 98 (11), e14888. doi:10.1097/MD.0000000000014888
Xing, Q., Fu, L., Yu, Z., and Zhou, X. (2020). Efficacy and safety of integrated traditional Chinese medicine and western medicine on the treatment of rheumatoid arthritis: A meta-analysis. Evid. Based. Complement. Altern. Med. 2020, 4348709. doi:10.1155/2020/4348709
Zeng, Q. Y., Chen, R., Darmawan, J., Xiao, Z. Y., Chen, S. B., Wigley, R., et al. (2008). Rheumatic diseases in China. Arthritis Res. Ther. 10 (1), R17. doi:10.1186/ar2368
Zhang, C., Jiang, M., He, X. J., and Lu, A. P. (2015). Clinical trials of integrative medicine for rheumatoid arthritis: Issues and recommendations. Chin. J. Integr. Med. 21 (6), 403–407. doi:10.1007/s11655-015-2041-5
Keywords: cohort study, integrative medicine, rheumatoid arthritis, autoimmune disease, clinical study
Citation: Zhong LL, Wang R, Lam WC, Zhu Q, Du P, Cao PH, Jiang T, Zhang YY, Shen J, Su X, Xue L, Mao J, Fang YF, Gao ML, Hu CR, Peng JY, Gu Y, Wei Q, Huang R, Lyu A, Liu H and He D (2022) The combination of Chinese and Western Medicine in the management of rheumatoid arthritis: A real-world cohort study across China. Front. Pharmacol. 13:933519. doi: 10.3389/fphar.2022.933519
Received: 01 May 2022; Accepted: 21 September 2022;
Published: 06 October 2022.
Edited by:
Mingbao Lin, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaReviewed by:
Muhammad Nisar, NHS England, United KingdomCopyright © 2022 Zhong, Wang, Lam, Zhu, Du, Cao, Jiang, Zhang, Shen, Su, Xue, Mao, Fang, Gao, Hu, Peng, Gu, Wei, Huang, Lyu, Liu and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linda LD. Zhong, bGR6aG9uZ0Boa2J1LmVkdS5oaw==; Linda LD. Zhong, bGluZGEuemhvbmdAbnR1LmVkdS5zZw==; Hongxia Liu, aG9uZ3hpYS5saXVAZHJ1Z2NoaW5hLm5ldA==; Dongyi He, aGVkb25neWkxOTY3QHNodXRjbS5lZHUuY24=
†These authors have contributed equally to this work
‡These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.