
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 19 July 2022
Sec. Obstetric and Pediatric Pharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.932776
 Yueyue Li1†
Yueyue Li1† Jingjie Li2†
Jingjie Li2† Yuhuan Shi1
Yuhuan Shi1 Xuhui Zhou2
Xuhui Zhou2 Wanqing Feng2
Wanqing Feng2 Lu Han1
Lu Han1 Daqing Ma3
Daqing Ma3 Hong Jiang2*
Hong Jiang2* Yongfang Yuan1*
Yongfang Yuan1*Background: Emergence agitation (EA) is very common in paediatric patients during recovery from general anaesthesia, but underlying mechanisms remain unknown. This prospective study was designed to profile preoperative urine metabolites and identify potential biomarkers that can predict the occurrence of EA.
Methods: A total of 224 patients were screened for recruitment; of those, preoperative morning urine samples from 33 paediatric patients with EA and 33 non-EA gender- and age-matched patients after being given sevoflurane general anaesthesia were analysed by ultra-high-performance liquid chromatography (UHPLC) coupled with a Q Exactive Plus mass spectrometer. Univariate analysis and orthogonal projection to latent structures squares-discriminant analysis (OPLS-DA) were used to analyse these metabolites. The least absolute shrinkage and selection operator (LASSO) regression was used to identify predictive variables. The predictive model was evaluated through the receiver operating characteristic (ROC) analysis and then further assessed with 10-fold cross-validation.
Results: Seventy-seven patients completed the study, of which 33 (42.9%) patients developed EA. EA and non-EA patients had many differences in preoperative urine metabolic profiling. Sixteen metabolites including nine aromatic amino acid metabolites, acylcarnitines, pyridoxamine, porphobilinogen, 7-methylxanthine, and 5′-methylthioadenosine were found associated with an increased risk of EA, and they all exhibited higher levels in the EA group than in the non-EA group. The main metabolic pathways involved in these metabolic changes included phenylalanine, tyrosine and tryptophan metabolisms. Among these potential biomarkers, L-tyrosine had the best predictive value with an odds ratio (OR) (95% CI) of 5.27 (2.20–12.63) and the AUC value of 0.81 (0.70–0.91) and was robust with internal 10-fold cross-validation.
Conclusion: Urinary aromatic amino acid metabolites are closely associated with EA in paediatric patients, and further validation with larger cohorts and mechanistic studies is needed.
Clinical Trial Registration: clinicaltrials.gov, identifier NCT04807998
Emergence agitation (EA), also known as emergence delirium, is common in paediatric patients during recovery from general anaesthesia. EA is characterized by crying, screaming, agitation, disorientation, and altered responses to their environment (Koch et al., 2018). EA paediatric patients may cause themselves physical injury and stress to care workers and their patients and prolong postoperative recovery time. Furthermore, young patients who had EA after general anaesthesia and surgery were reported to have more likely behavioural changes that may last for several weeks (Lee and Sung, 2020).
The mechanisms of paediatric EA remain unclear. Several risk factors including age, preoperative anxiety, anaesthesia type and postoperative pain were reported (Voepel-Lewis et al., 2003). Indeed, sevoflurane, an inhaled anaesthetic commonly used in paediatric surgery, was found to be closely related to the incidence of EA (Cohen et al., 2003; Chandler et al., 2013). Sevoflurane stimulated locus coeruleus neurons, increased lactic acid and glucose concentrations in the parietal cortex and induced asymptomatic epileptogenic activity (Vlajkovic and Sindjelic, 2007; Gibert et al., 2012; Jacob et al., 2012; Stoicea et al., 2014), all of which may trigger EA, but these are assumptions and further study is needed.
Metabolomics is an emerging analytical system biology methodology that studies the metabolic changes of endogenous metabolites in living systems under pathological conditions or in response to medications (Carneiro et al., 2019). Metabolomics has been commonly used to explore biomarkers in paediatric neurological diseases such as epilepsy, autism, attention deficit and hyperactivity disorder (Kennedy et al., 2019; Zhou et al., 2019; Wang L. J. et al., 2021). Metabolomics was also applied to preclinical studies of anaesthetic-induced neurotoxicity in paediatric patients, which was considered to be an effective approach to identify biomarkers of adverse events associated with anaesthesia (Liu et al., 2015; Wang et al., 2018). Wang Q et al. (2021) used the metabolomics method to explore the preoperative serum biomarkers of emergence agitation after general anaesthesia in adult patients and found that choline, cytidine, glycophosphorophocholine, L-phenylalanine, oleamide and inosine may have a predictive value for EA diagnosis. In addition, metabolomic techniques have also been applied to the discovery of predictive biomarkers for postoperative delirium in elderly patients. Abnormal changes in the levels of multiple metabolites in the preoperative serum or cerebrospinal fluid in elderly patients were found to be associated with the occurrence of postoperative delirium (Watne et al., 2016; Guo et al., 2017; Han et al., 2020). Postoperative emergence agitation is the postoperative delirium phenotype in the young population. Up to date, it remains unknown whether metabolic changes in young patients are associated with EA after anaesthesia and surgery. The aim of this study, therefore, was to determine metabolites associated with an increased risk of EA in paediatric patients through preoperative urine metabolomic profiling analyses.
HPLC-grade acetonitrile and formic acid were purchased from Tedia (Fairfield, OH, United States). 3-Hydroxykynurenine and 3-hydroxyanthranilic acid were obtained from Sigma-Aldrich (St. Louis, MO). Tyrosine and xanthurenic acid were purchased from Shanghai Aladdin Bio-Chem Technology Co., LTD.
This prospective observational cohort study protocol (SH9H-2020-T149) was approved by the Institutional Review Board of the Ninth People’s Hospital of Shanghai. The study was part II of the prospective study entitled “Risk factors for paediatric emergence agitation and postoperative pain following maxillofacial surgery and analysis of serum or urine metabolomics in children with agitation—an exploratory study” and was registered at ClinicalTrials.gov (NCT04807998). The study was conducted at the Departments of Anaesthesiology, Otolaryngology, and Oral and Maxillofacial Surgery of the Ninth People’s Hospital of Shanghai Jiaotong University’s School of Medicine from April 2021 to September 2021. It was in compliance with the Declaration of Helsinki for Medical Research. Written informed consent was obtained from parents or guardians of all patients before recruitment.
Paediatric patients aged 3–7 years with an American Society of Anaesthesiologists (ASA) physical status of I or II who were scheduled for tonsillectomy and/or adenoidectomy under sevoflurane-based anaesthesia were recruited. The exclusion criteria included the presence of 1) developmental delays or intellectual disability; 2) a history of neurological or psychiatric diseases; 3) severe liver and kidney dysfunction or other heart and lung diseases; 4) metabolic diseases or inherited diseases; 5) surgery with total intravenous anaesthesia or combined intravenous-inhalation anaesthesia and; 6) under medications or healthy supplements, e.g. vitamins.
All patients had fasted from 22:00 onward before surgery. They all received routine premedications of dolasetron 0.3–0.6 mg/kg and dexamethasone 0.25–0.3 mg/kg (i.v. injection). Non-invasive blood pressure, ECG and pulse oximetry were continuously monitored throughout anaesthesia and surgery. Anaesthetic induction included the sequential administration of intravenous midazolam, propofol, fentanyl and rocuronium to facilitate endotracheal intubation. Anaesthesia maintenance was 1.5%–2.5% sevoflurane supplemented with an intravenous administration of remifentanil (0.05–0.3 μg/kgmin) when necessary to maintain a bispectral index range from 40 to 60. Patients were transferred to the post-anaesthetic care unit (PACU) after surgery completion. The tracheal tube was removed in PACU when spontaneous breathing and swallowing cough reflexes were fully recovered.
During recovery, emergence agitation and postoperative pain were assessed and recorded according to the Paediatric Assessment of Emergence Delirium (PAED) score and the Face, Legs, Activity, Cry and Consolability scale (FLACC) by a researcher who was blinded with the study protocol. PAED and FLACC scores were assessed every 10 min until the patient returned to the ward. Emergence agitation was considered once the patient had a PAED score equal to or greater than 12. Once EA occurred, they were treated with esketamine 0.1–0.25 mg/kg, sufentanil 0.05–0.1 μg/kg or fentanyl 0.5–1 μg/kg administered intravenously as necessary.
Once EA patients were identified, gender- and age-matching non-EA patients, who had the same type of surgery in a similar time period, were randomly selected to match the same number of EA patients for metabolomics analysing comparisons (Figure 1). Patients’ demographics in both groups including age, gender, height, weight, BMI, ASA physical status classification, length of surgery, anaesthesia or anaesthetic dose were collected.
The primary outcome of our study was the usefulness of preoperative urine in metabolomics in predicting the occurrence of postoperative EA. The secondary outcomes were FLACC scores and post-operative complications or adverse events including postoperative nausea and vomiting (PONV), respiratory (e.g., cough, laryngospasm and bronchospasm) and cardiovascular (bradycardia and hypotension) complications.
A first morning urine sample from each patient was collected on the operation day. Urine samples were centrifuged at 13,000 g at 4 C for 10 min, and then, the supernatants were kept at −80°C for further analyses. Prior to liquid chromatography–mass spectrometry (LC–MS) analysis, 400 μL acetonitrile was added to 100 μL aliquots of freshly thawed urine samples. The mixture was, then, vortexed for 2 min and centrifuged at 13,000 g for 10 min at 4°C. The supernatant was transferred to vials for the LC–MS analysis. To monitor the stability of instrument analysis, a quality control (QC) sample was made by combining 10 μL aliquots of each urine sample. Before analysing the entire list of samples, the QC sample was injected six times consecutively to balance the system. Then, the QC sample was injected into every ten samples throughout the entire sample analysis. Variables with a coefficient of variation (CV) ≥ 20% in QC samples were excluded from the data matrix.
The metabolite analysis was performed using a Vanquish UHPLC system (Thermo Fisher) coupled with a Q Exactive Plus mass spectrometer (Thermo Fisher, Milan, Italy) (Lavarello et al., 2021). An InfinityLab Poroshell 120 EC-C18 (2.1 × 100 mm, 2.7 μm, Agilent) column was used for chromatographic separation. The column temperature was set at 45°C. Ultrapure water with 0.1% formic acid (v/v) and acetonitrile (Tedia, Fairfield, OH, United States) with 0.1% formic acid (v/v) constituted phase A and phase B of the mobile phase, respectively. The gradient elution programme for urine analysis was as follows: 1% B (0–1 min), 1%–20% B (1–5.5 min), 20%–30% B (5.5–6 min), 30%–35% B (6–8.5 min), 35%–70% B (8.5–10.5 min), 70%–100% B (10.5–11 min), 100% B (11–13.5 min) and 1% B (13.5–15.5 min). The flow rate was 0.4 ml/min, and the sample injection volume was 1 μL.
Mass detection was performed using a heated electrospray source probe both in positive and negative modes with the following settings: scan mode: DDA mode, one full scan followed by five MS/MS scans. The scan range was set from 67 to 1,000 m/z. The scanning resolutions of MS1 and MS2 were 70,000 (at m/z 200) and 17,500, automatic gain control was 1 × 106 and 5 × 105, and the maximum injection times were 100 ms and 50 ms, respectively. The spray voltages in positive and negative modes were 3,200 and 2800 V, respectively. The auxiliary gas temperature was 300°C, and the capillary temperature was 320°C. Sheath gas and auxiliary gas were set at 30 and 10 a.u., respectively. The s-lens RF level was 50 V. The collision energy was set to 15 and 30 to fragment the ions.
Samples were analysed in a random manner. All the data were acquired by Xcalibur 3.0 (Thermo Fisher). Then, the LC–MS raw data were converted via Progenesis QI v2.3 software (Waters Corp., Milford, MA, United States) for peak finding and alignment. Compound identification was achieved by comparing the precision m/z, MS2, RT and other information collected by mass spectrometry with authentic standards or searching the Human Metabolome Database (http://hmdb.ca/) or METLIN (https://metlin.scripps.edu/).
Due to the complexity of untargeted metabolomics, there are currently no standard methods for sample size estimation. Given that this was a prospective preliminary study, we randomly recruited and collected samples with 33 patients per group, which met the requirement for adequate statistical modelling of metabolomics study (Nyamundanda et al., 2013).
Continuous variables such as age, weight, BMI, duration of anaesthesia and surgery, and anaesthetic dose were reported as the mean ± SD. Categorical variables including demographic variables were reported as frequencies and percentages. PAED scores were reported as median (interquartile range; IQR). Data were analysed with the chi-squared test, Student t-tests or Mann–Whitney U test with SPSS 23.0 software (SPSS, Inc.) where appropriate. A p-value less than 0.05 was considered to be of statistical significance.
The urine creatinine level of each sample was measured and used to normalize the urinary metabolomics data. Any metabolites that were not detected in more than 50% urine samples were excluded from further analysis (Hackstadt and Hess, 2009). Then, metabolites for which data had zero values (due to below of the threshold of MS detection, for example) were imputed with the minimum detection level of that metabolite (Dehaven et al., 2010). The univariate analysis by independent Student’s t test was applied to analyse differences of variables between EA and non-EA groups with SPSS 23.0 software (SPSS, Inc.). The p-values were corrected with the false discovery rate (FDR) calculated with the “fdrtool” package in R 4.1.2. The principal component analysis (PCA) and the orthogonal projection to latent structures squares-discriminant analysis (OPLS-DA) were performed with the data matrix via SIMCA-P 14.1 (Umetrics, Umea, Sweden) software. PCA was used to obtain an overview of metabolic profiling. OPLS-DA was conducted to identify differential biomarkers between EA and non-EA groups. R2Y (the interpretation rate parameter), Q2 (the predictive ability parameter) and a random permutation test were used to indicate the quality of the OPLS-DA model. The variables with variable importance for projection (VIP) scores >1 in the OPLS-DA model, a p-value <0.05 (FDR <5%) for independent sample t test and the FC value (the ratio of mean values of EA and non-EA groups) > 1.5 were selected as differential metabolites. Metabolic pathways of differential metabolites were analysed by MetaboAnalyst 4.0 and KEGG.
Variable selection was performed with the differential metabolites obtained through univariate analysis and OPLS-DA modelling. The levels of metabolites were log-transformed (base 10) and mean-centred and divided by the standard deviation of each variable. The least absolute shrinkage and selection operator (LASSO) regression using a 10-fold cross-validation was used to select the most predictive metabolites to EA (Zhu, et al., 2022). The selection was judged from the lambda with 1 SE of the minimum partial likelihood of deviance. The LASSO regression method was performed in R with the “glmnet” package. The performance of the metabolite biomarker model was evaluated through the receiver operating characteristic (ROC) analysis and underwent internal validation using the 10-fold cross-validation. The area under the ROC curve (AUC), sensitivity and specificity values, as well as predictive values were used to assess the predictive power of potential metabolite biomarker panels.
A total of 224 paediatric patients were enrolled in the study; however, 147 patients were excluded for the following reasons: 4 declined, 1 had a history of neurological disease, 1 was given drug treatment after admission, 2 were not obtained urine samples, 26 with total intravenous anaesthesia, 112 with combined intravenous-inhalation anaesthesia and 1 failed to score PAED due to post-operative complication. Ultimately, 77 patients completed the study, and their morning urine samples were obtained (Figure 1). Among them, 33 (42.9%) patients developed EA in PACU. Another 33 age- and gender-matched patients without EA were selected to comprise the non-EA group. There were no significant differences in age, gender, height, weight, BMI, length of surgery, anaesthesia or anaesthetic dose between patients in EA and non-EA groups (Table 1). PAED scores and FLACC scores in the EA group were significantly higher than those of the non-EA group (p < 0.01). The incidence of post-operative adverse reactions that occurred in PACU such as PONV, cough, laryngospasm and bronchospasm was not statistically different between the two groups (p > 0.05).
A typical urine LC–MS total ion chromatogram is shown in Supplementary Figure S1. As shown in PCA score plots (Supplementary Figure S2), QC samples were clustered closely together, indicating the high stability of the LC–MS system during the entire sequence. As shown in Figure 2, the urine samples of the EA group and the non-EA group were clearly divided into two clusters in the OPLS-DA score plots. In the positive ion mode, OPLS-DA model parameters R2Y and Q2 were 0.81 and 0.40, respectively. In the negative ion mode, OPLS-DA model parameters R2Y and Q2 were 0.71 and 0.22, respectively. The corresponding 200-fold randomization permutation test results showed that OPLS-DA models were valid without overfitting.
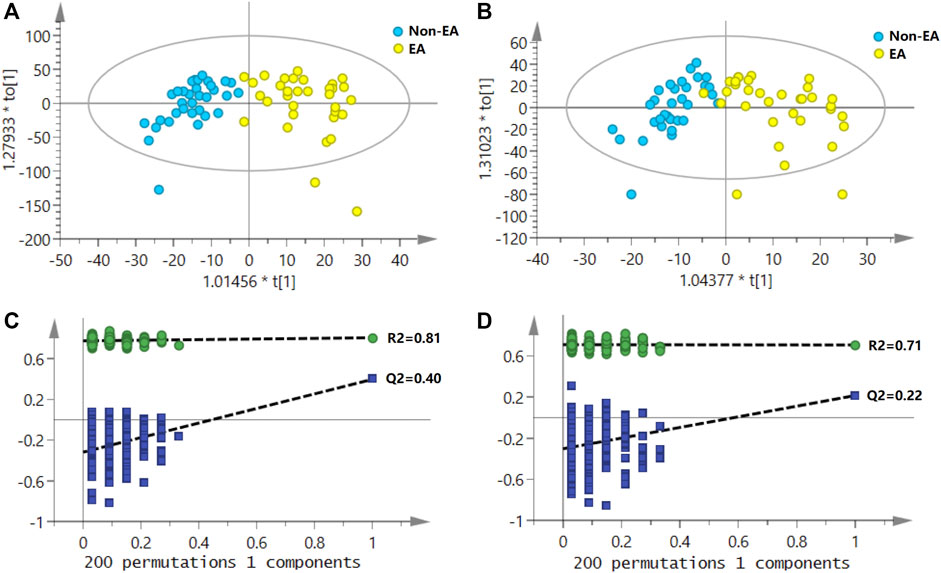
FIGURE 2. Score plots of OPLS-DA for the urine metabolomics of emergence agitation (EA) versus the non-EA patients; (A) the positive ionization mode; (B) the negative ionization mode. The permutation test (200 tests total) for the OPLS-DA model; (C) in the positive ionization mode, R2Y and Q2Y were 0.81 and 0.40, respectively; (D) in the negative ionization mode, R2Y and Q2Y were 0.71 and 0.22, respectively.
In our study, metabolites with VIP >1, p < 0.05 (FDR<5%) and FC > 1.5 were selected as potential biomarkers. As listed in Table 2, 16 metabolites associated with an increased risk of EA were identified, of which nine metabolites were aromatic amino acids and their metabolites. Others included several acylcarnitine, pyridoxamine, porphobilinogen, 7-methylxanthine and 5′-methylthioadenosine. The heatmap of differential metabolites between EA and non-EA groups is shown in Figure 3. All the 16 metabolites exhibited higher levels in the EA group than in the non-EA group. Using the metabolic pathway analysis function of MetaboAnalyst 5.0, several related metabolic pathways with p < 0.05 were found, including phenylalanine metabolism, phenylalanine, tyrosine and tryptophan biosynthesis, and tryptophan metabolism.
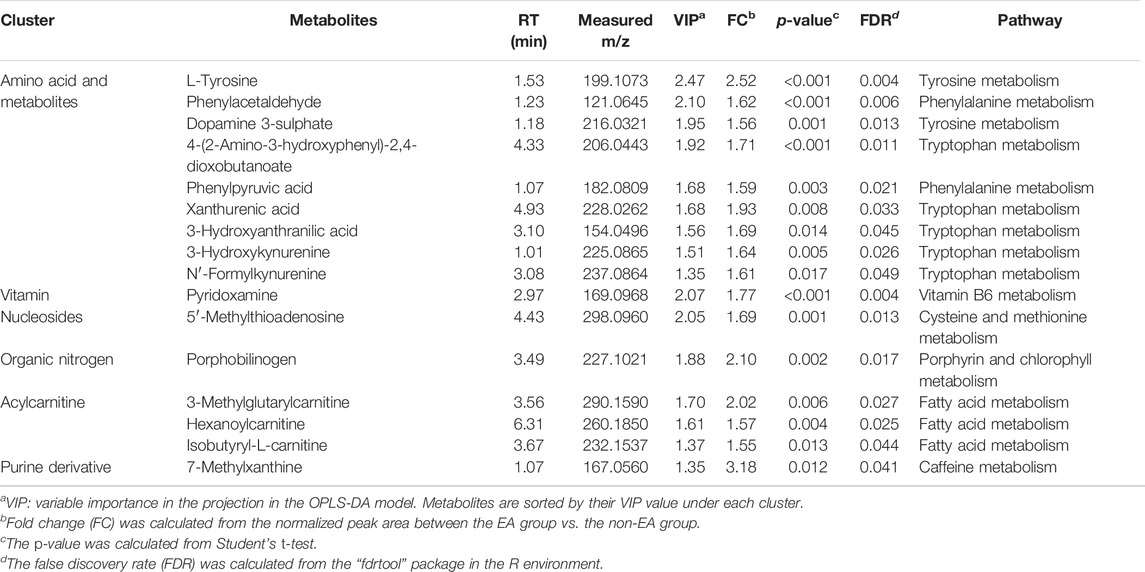
TABLE 2. Potential biomarkers of urine samples from postoperative emergence agitation (EA) and non-EA patients.
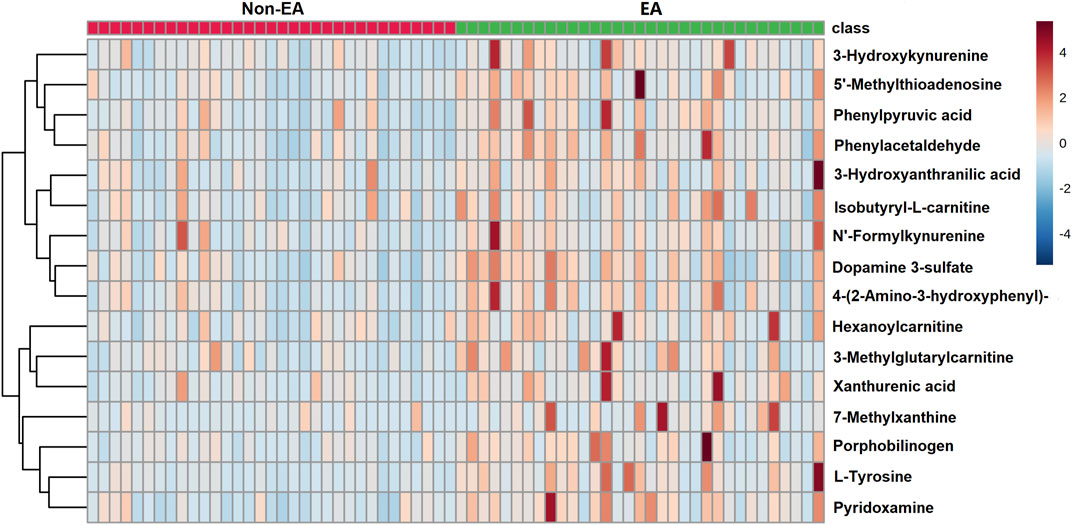
FIGURE 3. Heat map of differential metabolites between emergence agitation (EA) and non-EA patients.
The LASSO regression selected the two most predictive metabolites, L-tyrosine and pyridoxamine (Supplementary Figure S3). Then, the backward stepwise logistic regression was used to examine the association between the two metabolites and occurrence of EA. The result showed that only L-tyrosine (p = 2.0 × 10–4) was retained in the model. The odds ratio (OR) (95% CI) was 5.27 (2.20–12.63). The AUC of ROC (95% CI) was 0.81 (0.70–0.91). The sensitivity and specificity were 0.67 (0.51–0.83) and 0.88 (0.77–0.99), respectively. The positive predictive value (PPV) and negative predictive value (NPV) were 0.846 and 0.725, respectively. At internal 10-fold cross-validation, the model was shown to be robust with an AUC of 0.78 (0.67–0.90) (Figure 4).
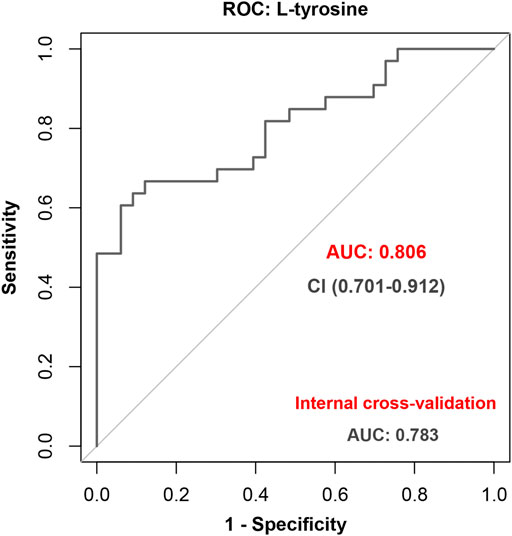
FIGURE 4. Performance of L-tyrosine to predict emergence agitation (EA) with the receiver operating characteristic (ROC) analysis and further internal cross-validation.
This is the first metabolomics study that demonstrated that EA patients and non-EA patients can be distinguished by pre-operative urine metabolic profiling. We found 16 metabolites including aromatic amino acids, acylcarnitines, pyridoxamine, porphobilinogen, 7-methylxanthine and 5′-methylthioadenosine were associated with the EA incidence, implying that the occurrence of EA in paediatric patients was associated with different neuroactive metabolites and the disturbance of the overall balance of metabolic pathways. Furthermore, L-tyrosine was found to be effectively predicting EA in paediatric patients.
Of 5,005 metabolites in urine, 16 metabolites were identified to be associated with an increased risk of EA in paediatric patients; nine metabolites of those were aromatic amino acids and their metabolites. The main metabolic pathways involved in these metabolic changes included phenylalanine, tyrosine and tryptophan metabolisms. These three are all essential aromatic amino acids that are precursors of monoamine neurotransmitters such as catecholamines and serotonin. It has been reported that the neuroactive metabolite level changes, and the disturbance of the overall balance of metabolic pathways were closely related to the occurrence of neurological diseases (Fernstrom and Fernstrom, 2007; Pan et al., 2018).
In view of the limited accessibility to the central nervous system (CNS) in patients, peripheral biomarkers in blood or body fluids related to nervous system diseases were increasingly explored to indirectly reflect the disease-associated changes in the CNS. As a non-invasive and easily available body fluid, urine has been widely used for these purposes. Indeed, previous studies have indicated a relationship between urinary neurotransmitter metabolites and neurotransmitter levels in the CNS in rats (Chekhonin et al., 2000; Lynn-Bullock et al., 2004). The usefulness of urinary neurotransmitter metabolites as biomarkers of neurological diseases or disorders was documented in paediatric patients (Gevi et al., 2016; Yu et al., 2021). Sevoflurane anaesthesia was reported to be closely related to the incidence of EA (Cohen et al., 2003; Chandler et al., 2013). The underlying mechanisms of this association remain unknown, but it may relate to neurotransmitter imbalances including serotonin (5-HT) and neuropeptide Y (NPY) induced by inhalational agents such as isoflurane or sevoflurane administration reported in the pre-clinical studies (Westphalen et al., 2013; Kang et al., 2020).
The kynurenine pathway (KP) is the major metabolic pathway of tryptophan. Along KP, tryptophan is the first to be metabolised to N′-formyl-l-kynurenine, which is then further metabolised to be L-kynurenine (KYN) and 3-hydroxy-l-kynurenine (3-HK). According to our data, the levels of 3-HK and its further metabolites 3-hydroxyanthranilic acid (3-HAA), 4-(2-amino-3-hydroxyphenyl)-2,4-dioxobutanoate and xanthurenic acid in urine of the EA group were higher than those of the non-EA group. Both 3-HK and 3-HAA are considered to be neurotoxic metabolites. They inhibit the T-cell response through the formation of intracellular reactive oxygen species or glutathione depletion or activate glutamate receptors to cause headache (Lee et al., 2010; Curto et al., 2015). Xanthurenic acid can indirectly activate the mGlu2/3 metabotropic glutamate receptor and inhibit the vesicular glutamate transporter (Fazio et al., 2017). Increasing evidence indicated that the activation of tryptophan metabolites, especially those in KP, was involved in neurocognitive disorders under inflammatory conditions of the central nervous system (Watzlawik et al., 2016).
Phenylalanine is a precursor of catecholamines and indispensable for the biosynthesis of these neurotransmitters. In the present study, we noted that the amounts of phenylpyruvic acid and phenylacetaldehyde, by-products of phenylalanine, were increased in urine in the EA group patients. Phenylpyruvic acid inhibited the activity of glucose-6-phosphate dehydrogenase, hindered NADPH production and likely changed the redox status of brain cells (Rosa et al., 2012). These results indicated that dysregulated phenylalanine metabolism may likely be one of the pathogeneses of EA.
The excretion of tyrosine was also increased in the urine of the EA group in our study. The increased tyrosine level led to an increase in dopamine (DA) formation (Woods and Meyer, 1991). We found an increased production of DA metabolite dopamine 3-sulphate in the urine of the EA group, indicating that the concentration of DA was likely increased in our young patients’ blood. The increase in DA transmission accompanied by a decrease of cholinergic transmission is one of the most common neurotransmitter imbalances in the pathogenesis of delirium (Maldonado, 2013). Compared with the non-EA group, the urine excretion of aromatic amino acids and their metabolites were increased in the EA group, indicating the high monoaminergic activity of the EA group. Interestingly, in line with our data, the increase levels of aromatic amino acids in the cerebrospinal fluid were also found in elderly patients who developed delirium post-operatively (Watne et al., 2016; Han et al., 2020), whilst post-operative emergence agitation is the post-operative delirium phenotype in the young population.
In addition, several other metabolites, for example, pyridoxamine, were found to be increased in the urine of the EA group. Pyridoxamine is one of the three interconvertible members of vitamin B6. All three forms are bio-transformed into physiologically active pyridoxal-5-phosphate which has multiple functions. It is an essential coenzyme/cofactor in processes including metabolism of essential amino acids, glycogen and lipid metabolism, and synthesis of neurotransmitters such as serotonin, dopamine and GABA (Parra et al., 2018). 3-Methylglutarylcarnitine, isobutyryl-L-carnitine and hexanoylcarnitine are acylcarnitine. Acylcarnitine and carnitine supplementation had beneficial effects in the treatment of various neurological diseases. Neuroprotective benefits of brain acylcarnitines included improving mitochondrial function, increasing the antioxidant activity, stabilizing membrane composition, modulating protein and genes and enhancing cholinergic neurotransmission (Zanelli et al., 2005; Silva-Adaya et al., 2008). However, how much of these metabolites contribute to the EA development in our patients remains unknown and warrants further study.
Our study has several limitations. The number of subjects included in this study was relatively small. In addition, the patients we included in the study were mainly OSAS patients. It has been reported that urinary neurotransmitter metabolites were altered in children with OSAS and may predict cognitive morbidity (Kheirandish-Gozal et al., 2013). All these may induce the less generalizability of our findings. However, despite the aforementioned limitations, the strictly matched analysis between EA and non-EA patients was carried out, and the metabolite profile in urine reported here for predicting paediatric patients with EA may help understand the underlying mechanisms of postoperative EA for preventive strategy development in the future.
In conclusion, we found certain metabolites including aromatic amino acids, acylcarnitines, pyridoxamine, porphobilinogen, 7-methylxanthine and 5′-methylthioadenosine were closely associated with the EA incidence, and L-tyrosine was found to be effectively predicting EA in paediatric patients. The clinical significance of our work is subjected to further study in a large cohort of patients.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The Institutional Review Board of the Ninth People’s Hospital of Shanghai. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
YL and JL contributed equally to this work. YL, JL, HJ, and YY conceived the project. YL, JL, YS, XZ, WF, and LH completed patient recruitment and data collection. YL, JL, YS, and WF analysed the data. YL, JL, and DM wrote the manuscript. All authors revised the manuscript and approved the final manuscript.
This work was supported by the National Natural Science Foundation of China (Grant No. 82104347) and the Seed Founding of Shanghai Ninth People’s Hospital, Shanghai JiaoTong University School of Medicine (Grant No. JYZZ112).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We gratefully acknowledge the patients and family members who participated in this study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.932776/full#supplementary-material
Carneiro, G., Radcenco, A. L., Evaristo, J., and Monnerat, G. (2019). Novel Strategies for Clinical Investigation and Biomarker Discovery: a Guide to Applied Metabolomics. Horm. Mol. Biosl. Clin. Investig. 38, e0045. doi:10.1515/hmbci-2018-0045
Chandler, J. R., Myers, D., Mehta, D., Whyte, E., Groberman, M. K., Montgomery, C. J., et al. (2013). Emergence Delirium in Children: a Randomized Trial to Compare Total Intravenous Anesthesia with Propofol and Remifentanil to Inhalational Sevoflurane Anesthesia. Paediatr. Anaesth. 23 (4), 309–315. doi:10.1111/pan.12090
Chekhonin, V. P., Baklaushev, V. P., Kogan, B. M., Savchenko, E. A., Lebedev, S. V., Man'kovskaya, I. V., et al. (2000). Catecholamines and Their Metabolites in the Brain and Urine of Rats with Experimental Parkinson's Disease. Bull. Exp. Biol. Med. 130 (8), 805–809. doi:10.1007/BF02766101
Cohen, I. T., Finkel, J. C., Hannallah, R. S., Hummer, K. A., and Patel, K. M. (2003). Rapid Emergence Does Not Explain Agitation Following Sevoflurane Anaesthesia in Infants and Children: a Comparison with Propofol. Paediatr. Anaesth. 13 (1), 63–67. doi:10.1046/j.1460-9592.2003.00948.x
Curto, M., Lionetto, L., Fazio, F., Mitsikostas, D. D., and Martelletti, P. (2015). Fathoming the Kynurenine Pathway in Migraine: Why Understanding the Enzymatic Cascades Is Still Critically Important. Intern Emerg. Med. 10 (4), 413–421. doi:10.1007/s11739-015-1208-6
Dehaven, C. D., Evans, A. M., Dai, H., and Lawton, K. A. (2010). Organization of GC/MS and LC/MS Metabolomics Data into Chemical Libraries. J. Cheminform 2 (1), 9. doi:10.1186/1758-2946-2-9
Fazio, F., Lionetto, L., Curto, M., Iacovelli, L., Copeland, C. S., Neale, S. A., et al. (2017). Cinnabarinic Acid and Xanthurenic Acid: Two Kynurenine Metabolites that Interact with Metabotropic Glutamate Receptors. Neuropharmacology 112 (Pt B), 365–372. doi:10.1016/j.neuropharm.2016.06.020
Fernstrom, J. D., and Fernstrom, M. H. (2007). Tyrosine, Phenylalanine, and Catecholamine Synthesis and Function in the Brain. J. Nutr. 137 (6 Suppl. 1), 1539S–1548S. doi:10.1093/jn/137.6.1539S
Gevi, F., Zolla, L., Gabriele, S., and Persico, A. M. (2016). Urinary Metabolomics of Young Italian Autistic Children Supports Abnormal Tryptophan and Purine Metabolism. Mol. Autism 7 (7), 47. doi:10.1186/s13229-016-0109-5
Gibert, S., Sabourdin, N., Louvet, N., Moutard, M. L., Piat, V., Guye, M. L., et al. (2012). Epileptogenic Effect of Sevoflurane: Determination of the Minimal Alveolar Concentration of Sevoflurane Associated with Major Epileptoid Signs in Children. Anesthesiology 117 (6), 1253–1261. doi:10.1097/ALN.0b013e318273e272
Guo, Y., Zhang, Y., Jia, P., Wang, W., Zhou, Q., Sun, L., et al. (2017). Preoperative Serum Metabolites Are Associated with Postoperative Delirium in Elderly Hip-Fracture Patients. J. Gerontol. A Biol. Sci. Med. Sci. 72 (12), 1689–1696. doi:10.1093/gerona/glx001
Hackstadt, A. J., and Hess, A. M. (2009). Filtering for Increased Power for Microarray Data Analysis. BMC Bioinforma. 10, 11. doi:10.1186/1471-2105-10-11
Han, Y., Zhang, W., Liu, J., Song, Y., Liu, T., Li, Z., et al. (2020). Metabolomic and Lipidomic Profiling of Preoperative CSF in Elderly Hip Fracture Patients with Postoperative Delirium. Front. Aging Neurosci. 12 (12), 570210. doi:10.3389/fnagi.2020.570210
Jacob, Z., Li, H., Makaryus, R., Zhang, S., Reinsel, R., Lee, H., et al. (2012). Metabolomic Profiling of Children's Brains Undergoing General Anesthesia with Sevoflurane and Propofol. Anesthesiology 117, 1062–1071. doi:10.1097/ALN.0b013e31826be417
Kang, W., Lu, D., Yang, X., Ma, W., Chen, X., Chen, K., et al. (2020). Sevoflurane Induces Hippocampal Neuronal Apoptosis by Altering the Level of Neuropeptide Y in Neonatal Rats. Neurochem. Res. 45 (9), 1986–1996. doi:10.1007/s11064-020-03028-9
Kennedy, A. D., Pappan, K. L., Donti, T., Delgado, M. R., Shinawi, M., Pearson, T. S., et al. (2019). 2-Pyrrolidinone and Succinimide as Clinical Screening Biomarkers for GABA-Transaminase Deficiency: Anti-seizure Medications Impact Accurate Diagnosis. Front. Neurosci. 13, 394. doi:10.3389/fnins.2019.00394
Kheirandish-Gozal, L., McManus, C. J. T., Kellermann, G. H., Samiei, A., and Gozal, D. (2013). Urinary Neurotransmitters Are Selectively Altered in Children with Obstructive Sleep Apnea and Predict Cognitive Morbidity. Chest 143 (6), 1576–1583. doi:10.1378/chest.12-2606
Koch, S., Rupp, L., Prager, C., Wernecke, K. D., Kramer, S., Fahlenkamp, A., et al. (2018). Emergence Delirium in Children Is Related to Epileptiform Discharges during Anaesthesia Induction: An Observational Study. Eur. J. Anaesthesiol. 35 (12), 929–936. doi:10.1097/EJA.0000000000000867
Lavarello, C., Barco, S., Bartolucci, M., Panfoli, I., Magi, E., Tripodi, G., et al. (2021). Development of an Accurate Mass Retention Time Database for Untargeted Metabolomic Analysis and its Application to Plasma and Urine Pediatric Samples. Molecules 26 (14), 4256. doi:10.3390/molecules26144256
Lee, S. J., and Sung, T. Y. (2020). Emergence Agitation: Current Knowledge and Unresolved Questions. Korean J. Anesthesiol. 73 (6), 471–485. doi:10.4097/kja.20097
Lee, S. M., Lee, Y. S., Choi, J. H., Park, S. G., Choi, I. W., Joo, Y. D., et al. (2010). Tryptophan Metabolite 3-hydroxyanthranilic Acid Selectively Induces Activated T Cell Death via Intracellular GSH Depletion. Immunol. Lett. 132 (1-2), 53–60. doi:10.1016/j.imlet.2010.05.008
Liu, F., Rainosek, S. W., Frisch-Daiello, J. L., Patterson, T. A., Paule, M. G., Slikker, W., et al. (2015). Potential Adverse Effects of Prolonged Sevoflurane Exposure on Developing Monkey Brain: From Abnormal Lipid Metabolism to Neuronal Damage. Toxicol. Sci. 147 (2), 562–572. doi:10.1093/toxsci/kfv150
Lynn-Bullock, C. P., Welshhans, K., Pallas, S. L., and Katz, P. S. (2004). The Effect of Oral 5-HTP Administration on 5-HTP and 5-HT Immunoreactivity in Monoaminergic Brain Regions of Rats. J. Chem. Neuroanat. 27 (2), 129–138. doi:10.1016/j.jchemneu.2004.02.003
Maldonado, J. R. (2013). Neuropathogenesis of Delirium: Review of Current Etiologic Theories and Common Pathways. Am. J. Geriatr. Psychiatry 21 (12), 1190–1222. doi:10.1016/j.jagp.2013.09.005
Nyamundanda, G., Gormley, I. C., Fan, Y., Gallagher, W. M., and Brennan, L. (2013). MetSizeR: Selecting the Optimal Sample Size for Metabolomic Studies Using an Analysis Based Approach. BMC Bioinforma. 14, 338. doi:10.1186/1471-2105-14-338
Pan, J. X., Xia, J. J., Deng, F. L., Liang, W. W., Wu, J., Yin, B. M., et al. (2018). Diagnosis of Major Depressive Disorder Based on Changes in Multiple Plasma Neurotransmitters: a Targeted Metabolomics Study. Transl. Psychiatry 8 (1), 130. doi:10.1038/s41398-018-0183-x
Parra, M., Stahl, S., and Hellmann, H. (2018). Vitamin B₆ and its Role in Cell Metabolism and Physiology. Cells 7 (7), 84. doi:10.3390/cells7070084
Rosa, A. P., Jacques, C. E., Moraes, T. B., Wannmacher, C. M., Dutra, A. M., and Dutra-Filho, C. S. (2012). Phenylpyruvic Acid Decreases Glucose-6-Phosphate Dehydrogenase Activity in Rat Brain. Cell. Mol. Neurobiol. 32 (7), 1113–1118. doi:10.1007/s10571-012-9834-2
Silva-Adaya, D., Pérez-De La CruzCruz, V. V., Herrera-Mundo, M. N., Mendoza-Macedo, K., Villeda-Hernández, J., Binienda, Z., et al. (2008). Excitotoxic Damage, Disrupted Energy Metabolism, and Oxidative Stress in the Rat Brain: Antioxidant and Neuroprotective Effects of L-Carnitine. J. Neurochem. 105 (3), 677–689. doi:10.1111/j.1471-4159.2007.05174.x
Stoicea, N., McVicker, S., Quinones, A., Agbenyefia, P., and Bergese, S. D. (2014). Delirium-biomarkers and Genetic Variance. Front. Pharmacol. 5, 75. doi:10.3389/fphar.2014.00075
Vlajkovic, G. P., and Sindjelic, R. P. (2007). Emergence Delirium in Children: Many Questions, Few Answers. Anesth. Analg. 104, 84–91. doi:10.1213/01.ane.0000250914.91881.a8
Voepel-Lewis, T., Malviya, S., and Tait, A. R. (2003). A Prospective Cohort Study of Emergence Agitation in the Pediatric Postanesthesia Care Unit. Anesth. Analg. 96 (6), 1625. doi:10.1213/01.ANE.0000062522.21048.61
Wang, C., Liu, F., Frisch-Daiello, J. L., Martin, S., Patterson, T. A., Gu, Q., et al. (2018). Lipidomics Reveals a Systemic Energy Deficient State that Precedes Neurotoxicity in Neonatal Monkeys after Sevoflurane Exposure. Anal. Chim. Acta 1037, 87–96. doi:10.1016/j.aca.2017.11.052
Wang, L. J., Chou, W. J., Tsai, C. S., Lee, M. J., Lee, S. Y., Hsu, C. W., et al. (2021). Novel Plasma Metabolite Markers of Attention-Deficit/hyperactivity Disorder Identified Using High-Performance Chemical Isotope Labelling-Based Liquid Chromatography-Mass Spectrometry. World J. Biol. Psychiatry 22 (2), 139–148. doi:10.1080/15622975.2020.1762930
Wang, Q., Zhou, J., Liu, T., Yang, N., Mi, X., Han, D., et al. (2021). Predictive Value of Preoperative Profiling of Serum Metabolites for Emergence Agitation after General Anesthesia in Adult Patients. Front. Mol. Biosci. 8, 739227. doi:10.3389/fmolb.2021.739227
Watne, L. O., Idland, A. V., Fekkes, D., Raeder, J., Frihagen, F., Ranhoff, A. H., et al. (2016). Increased CSF Levels of Aromatic Amino Acids in Hip Fracture Patients with Delirium Suggests Higher Monoaminergic Activity. BMC Geriatr. 16, 16149. doi:10.1186/s12877-016-0324-0
Watzlawik, J. O., Wootla, B., and Rodriguez, M. (2016). Tryptophan Catabolites and Their Impact on Multiple Sclerosis Progression. Curr. Pharm. Des. 22 (8), 1049–1059. doi:10.2174/1381612822666151215095940
Westphalen, R. I., Desai, K. M., and Hemmings, H. C. (2013). Presynaptic Inhibition of the Release of Multiple Major Central Nervous System Neurotransmitter Types by the Inhaled Anaesthetic Isoflurane. Br. J. Anaesth. 110 (4), 592–599. doi:10.1093/bja/aes448
Woods, S. K., and Meyer, J. S. (1991). Exogenous Tyrosine Potentiates the Methylphenidate-Induced Increase in Extracellular Dopamine in the Nucleus Accumbens: a Microdialysis Study. Brain Res. 560 (1-2), 97–105. doi:10.1016/0006-8993(91)91220-u
Yu, M. C., Wang, T. M., Chiou, Y. H., Yu, M. K., Lin, C. F., and Chiu, C. Y. (2021). Urine Metabolic Phenotyping in Children with Nocturnal Enuresis and Comorbid Neurobehavioral Disorders. Sci. Rep. 11 (1), 16592. doi:10.1038/s41598-021-96104-1
Zanelli, S. A., Solenski, N. J., Rosenthal, R. E., and Fiskum, G. (2005). Mechanisms of Ischemic Neuroprotection by Acetyl-L-Carnitine. Ann. N. Y. Acad. Sci. 1053, 153–161. doi:10.1196/annals.1344.013
Zhou, X., Liu, L., Lan, X., Cohen, D., Zhang, Y., Ravindran, A. V., et al. (2019). Polyunsaturated Fatty Acids Metabolism, Purine Metabolism and Inosine as Potential Independent Diagnostic Biomarkers for Major Depressive Disorder in Children and Adolescents. Mol. Psychiatry 24 (10), 1478–1488. doi:10.1038/s41380-018-0047-z
Keywords: aromatic amino acids, emergence agitation, metabolomics, paediatric anaesthesia, prediction model, urine neurotransmitters
Citation: Li Y, Li J, Shi Y, Zhou X, Feng W, Han L, Ma D, Jiang H and Yuan Y (2022) Urinary Aromatic Amino Acid Metabolites Associated With Postoperative Emergence Agitation in Paediatric Patients After General Anaesthesia: Urine Metabolomics Study. Front. Pharmacol. 13:932776. doi: 10.3389/fphar.2022.932776
Received: 30 April 2022; Accepted: 22 June 2022;
Published: 19 July 2022.
Edited by:
Matitiahu Berkovitch, Yitzhak Shamir Medical Center, IsraelReviewed by:
Halil Ibrahim Ulusoy, Cumhuriyet University, TurkeyCopyright © 2022 Li, Li, Shi, Zhou, Feng, Han, Ma, Jiang and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Jiang, ZHJfamlhbmdob25nQDE2My5jb20=; Yongfang Yuan, bm14eXlmQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.