
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 23 June 2022
Sec. Pharmacology of Anti-Cancer Drugs
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.931495
This article is part of the Research Topic Therapeutic Drug Monitoring and Clinical Toxicology of Anti-Cancer Drugs, volume II View all 13 articles
 Zaiwei Song1,2†
Zaiwei Song1,2† Lan Ma3†
Lan Ma3† Li Bao4
Li Bao4 Yi Ma1,2
Yi Ma1,2 Ping Yang1,2
Ping Yang1,2 Dan Jiang1,2
Dan Jiang1,2 Aijun Liu5
Aijun Liu5 Lu Zhang6
Lu Zhang6 Yan Li3
Yan Li3 Yinchu Cheng1,2
Yinchu Cheng1,2 Fei Dong3
Fei Dong3 Rongsheng Zhao1,2*
Rongsheng Zhao1,2* Hongmei Jing3*
Hongmei Jing3*Objective: Continuous lenalidomide (LEN) therapy is important to achieve a therapeutic effect in patients with multiple myeloma (MM) and non-Hodgkin lymphoma (NHL). However, despite dose adjustment according to kidney function, many patients discontinue LEN therapy because of hematological toxicity. To date, therapeutic drug monitoring (TDM) of LEN has not been performed in oncology, and no target concentration level has been yet defined. The aim of this study was to evaluate the exposure-safety relationship of LEN and determine the target concentration for toxicity.
Materials and Methods: A prospective observational study was designed and implemented. Blood samples were collected at 0.5 h (trough concentration, Cmin) before oral administration and 1 h (C1h) thereafter on the day. Clinical data were gathered from patients’ medical records and laboratory reports. Outcome measures of hematological toxicity were defined by the Common Terminology Criteria for Adverse Events. The concentration values were dichotomized by receiver operating characteristic (ROC) curve analysis, and the association between exposure and outcome was determined using the logistic regression model.
Results: Out of the 61 patients enrolled in this study, 40 (65.57%) had MM, and 21 (34.43%) had NHL. Hematological toxicity was reported in 15 (24.59%) patients. The LEN Cmin showed remarkable differences (p = 0.031) among patients with or without hematological toxicity, while no association between C1h values and toxicity was noted (p>0.05). By ROC analysis, a Cmin threshold of 10.95 ng/mL was associated with the best sensitivity/specificity for toxicity events (AUC = 0.687; sensitivity = 0.40; specificity = 0.935). By multivariate logistic regression, an LEN Cmin below 10.95 ng/mL was associated with a markedly decreased risk of hematological toxicity (<10.95 ng/mL vs. >10.95 ng/mL: OR = 0.023, 95% CI = 0.002–0.269; p = 0.003).
Conclusions: We demonstrate that the LEN trough concentration correlates with hematological toxicity, and the Cmin threshold for hematological toxicity (10.95 ng/mL) is proposed. Altogether, LEN TDM appears to be a new approach to improve medication safety and achieve continuous treatment for patients with NHL or MM in routine clinical care.
Non-Hodgkin’s lymphoma (NHL) is a cancer that develops in white blood cells called lymphocytes, representing the most frequent hematological malignancy. It is estimated that NHL was responsible for 544,000 new cases and 260,000 deaths worldwide in 2020 (Sung et al., 2021). Multiple myeloma (MM), the second most common hematologic malignancy, is a plasma cell malignancy in which monoclonal plasma cells proliferate in bone marrow (Mitsiades et al., 2004). Currently, projections suggest that, as the two most common hematologic malignancies, the incidence of both NHL and MM will continue to increase (Cai et al., 2021; Zhou et al., 2021).
The past years have witnessed a dramatic shift in the treatment of NHL and MM, from chemotherapy to chemoimmunotherapeutic regimens, and now biological and targeted strategies. One such treatment option is lenalidomide (LEN), which is a thalidomide derivative known as an immunomodulatory drug (List et al., 2005). LEN’s significant activity in hematological malignancy has led to its incorporation into multiple treatment regimens (Gribben et al., 2015), such as the LEN plus rituximab (R2) regimen for NHL (Leonard et al., 2019), as well as regimens based on LEN plus dexamethasone for MM (Mateos et al., 2013). As an oral targeted antineoplastic agent, long-term and continuous LEN treatment is important to achieve a therapeutic effect (Kado et al., 2020).
However, a close link has been established between LEN therapy and severe hematological toxicities, including neutropenia, thrombocytopenia, anemia, and leukopenia. Almost half of the patients experienced hematological toxicity of any grade across studies, and the incidence of high-grade hematologic toxicity might be 30% or higher (Cheson et al., 2020). During real clinical practice, despite dose adjustments according to baseline kidney function, unacceptable hematological toxicity is still the most common factor preventing continuous therapy with LEN. In addition to possibly causing treatment interruption, LEN-related hematological toxicity can affect patient adherence to treatment, increase the relapse risk and increase healthcare costs.
Therapeutic drug monitoring (TDM) is the clinical practice of measuring drug exposure at designated intervals to tailor drug doses, thereby optimizing outcomes in individual patients. The process of TDM is predicated on the assumption that there is a definable relationship between concentration and therapeutic or adverse effects (Kang and Lee, 2009). In terms of LEN, dose-limiting hematological toxicity has been observed, and dose modification or treatment interruption according to the severity of myelosuppression is recommended (Ludwig et al., 2018). Furthermore, a high area under the plasma concentration-time curve (AUC) of LEN has been shown to result in severe adverse events (Chen et al., 2013; Kobayashi et al., 2018). However, accurate measurement of the AUC requires collecting and analyzing multiple blood samples, which is both costly and time consuming for patients and clinical staff. Consequently, AUC-based TDM could be difficult to implement in clinical practice. There still exists a gap in the optimal index for TDM of hematological toxicity that can be used in clinical settings.
Herein, in the present study, we aimed to investigate the association between LEN exposure and its hematological toxicity and to determine concentration targets, which could be used in the TDM of LEN in patients with NHL or MM.
This was a prospective observational study that was in compliance with the Declaration of Helsinki and approved by the hospital medical science research ethics committee (No. M2021573). Patients provided written informed consent prior to enrollment. Adult patients with MM and NHL taking LEN capsules (Revlimid®, Celgene Corporation) for at least 3 days (steady state) and performing LEN concentration measurements during therapy between October 2021 and February 2022 were enrolled in this study. Patients taking any dose of LEN, pretreated with or without LEN, were eligible.
Patients were excluded if they had incomplete data, making it unable to assess whether the outcome event occurred; the clinical diagnosis was unclear; they had impaired kidney function with creatinine clearance (CCr) < 45 mL/min; they had impaired liver function with alanine aminotransferase (ALT) or aspartate aminotransferase (AST) greater than 5 times the upper limits of normal (ULN); they failed to take medicine as prescribed; they did not perform blood sample collection according to the prescribed time; and the plasma concentration of LEN was below the detection limit of 1 ng/mL.
Data were gathered from patients’ medical records and laboratory reports, which included patients’ demographics, clinical data on the diagnosis (subtype and stage of MM or NHL), history of previous chemotherapy, details of LEN therapy (including number of courses, duration days, dosage, antineoplastic agents combined), other concomitant drugs, and biological results. The biological data of complete blood count (CBC, including white cells, neutrophils, platelets, hemoglobin) were tested and collected on the day blood plasma concentration was measured, whereas the baseline CBC was collected before taking LEN of this cycle. In addition, other laboratory test data, including serum creatinine (SCr), total protein, albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) values, were also collected. The creatinine clearance (CCr) was estimated using the Cockcroft-Gault formula.
Given that the half-life of LEN is approximately 3–5 h (Chen et al., 2017), LEN was considered to have achieved a steady-state plasma concentration after 3 days. Only patients who achieved steady state were included in the analysis; thus, we accepted that the blood samples could be collected on day 3 after starting the LEN therapy. Blood samples were collected at 0.5 h (trough concentration, Cmin) before oral administration and 1 h (C1h) thereafter on the day. The two blood samples need to be collected before meals in the morning to avoid the influence of food on drug absorption and plasma concentration, although LEN was administered without regard to food intake in daily clinical practice. Blood samples were drawn into EDTA tubes.
The plasma was stored at −80°C until analysis. LEN concentrations were measured using a validated high-performance liquid chromatography tandem-mass spectrometry method (HPLC–MS/MS). LEN-13C5 was used as the internal standard. The analyte was separated on a Waters Atlantis® HILIC silica column (5 μm, 2.1 mm × 150 mm). The selected reaction monitoring transitions were 260.1→149.1 for LEN and 265.1→149.1 for the internal standard. The lower limit of quantification is 1 ng/mL for LEN. This method was developed and validated according to regulatory requirements. The inter-run precision and accuracy were less than 11.8% and 5.0%, respectively.
Attending physicians and pharmacists evaluated and graded the hematological toxicity according to the Common Terminology Criteria for Adverse Events Version 5.0 (NCI-CTCAE), and the highest grade of all decreased blood cells was defined as the grade of severity of hematological toxicity in this study. The clinical outcome was classified into two categories: a group with hematological toxicity and a group with no hematological toxicity.
In patients with normal baseline counts, hematological toxicity was defined as grade 2 or higher hematological adverse events, including leukopenia (decreased white blood cell count), neutropenia (decreased neutrophil count), thrombocytopenia (decreased platelet count) and anemia (decreased hemoglobin count). In patients with abnormal baseline counts, hematological toxicity was defined as a count <75% of baseline and grade 2 or higher hematological adverse events.
The statistical analyses were performed with SPSS software version 27.0 (IBM Corp., Armonk, NY, United States). For quantitative data following a normal distribution, we calculated the mean with standard deviations (mean ± SD) and used a T test to determine the difference between the groups. For nonnormally distributed data, we calculated the median with interquartile range [median (IQR)] and used the Mann–Whitney U test to compare the difference between the groups. Categorical variables were expressed as frequencies and proportions (%), and the chi-squared test was used to compare the differences between the groups.
If a statistically significant difference in LEN concentrations was observed between the groups, receiver operating characteristic (ROC) curves were constructed to determine a concentration threshold associated with hematological toxicity. The best threshold was chosen using the Youden index, identifying the target concentration that might result in hematological toxicity. Patients’ exposure to LEN was dichotomized depending on this threshold.
To account for various potential risk factors for developing hematological toxicity and to reduce other potential bias, the associations between concentrations and hematological toxicity were further adjusted by the logistic regression model. First, univariate analysis was performed to identify possible factors. Variables with statistical significance (defined as p <0.05) in the univariate analysis, as well as those determined by reading relevant literature and combining clinical experience, including gender, age, weight, CCr, albumin, diagnosis (NHL or MM), and co-administration of cytotoxic antitumor drugs, were then included in the multivariate logistic regression using the Enter method. All statistical analyses were two-tailed, and p <0.05 was considered statistically significant.
A total of 75 patients were screened initially, and only 61 (34 male, 55.74%) were included in the study. The other 14 patients were excluded as a result of unclear diagnosis, impaired kidney function with CCr less than 45 mL/min, missing clinical data, or LEN concentration below the detection limit of 1 ng/mL (Figure 1).
The demographic and clinical characteristics of the included patients are listed in Table 1. Out of the 61 patients enrolled in this study, hematological toxicity was observed in 15 (24.59%) patients. Patient demographics of the no-hematology toxicity group and hematology toxicity group were similar. Regarding the clinical diagnosis, 40 (65.57%) patients had MM, of which 26 were newly diagnosed; 21 (34.43%) patients had NHL, of which 5 were newly diagnosed. The median number of previous courses of immunochemotherapy was 0 (IQR, 0–5). Among 40 MM patients, the most common type was IgG (n = 19), followed by light chain (n = 15), IgA (n = 5), and IgD (n = 1). The most common Durie-Salmon (DS) stage was stage III (n = 33), followed by stage II (n = 5) and stage I (n = 2). Regarding the International Staging System (ISS), 23 patients had stage I disease, followed by stage III (n = 9) and stage I (n = 8) disease. Among 21 NHL patients, most had diffuse large B-cell lymphoma (DLBCL) (n = 11) and follicular lymphoma (FL) (n = 9), and only one had high-grade B-cell lymphoma (HGBL). According to the Ann Arbor Staging Classification, 19 and 2 patients had stage IV and stage II disease, respectively, of which almost half (n = 10) had B symptoms.
The median number of LEN treatment courses was 1 (range 1–15), and the median days of LEN duration in this current cycle was 5 (range 3–21). The LEN dosage was classified into four categories: 10 mg QD, 12.5 mg QD, 25 mg QD, and 25 mg QOD. In terms of antineoplastic agents combined, LEN was combined with other targeted therapies (e.g., bortezomib, ibrutinib, rituximab) and glucocorticoids in most patients, and LEN monotherapy was administered in only 7 patients. In addition, patients were concomitant with other medications, including aspirin, antiviral agents, antibacterial agents, proton pump inhibitors (PPI) or H2 receptor antagonists (H2RA) and other drugs.
Figure 2 shows the comparison of LEN plasma concentrations between the groups. The trough concentration of LEN at 0.5 h before oral administration (Cmin), expressed as the median (IQR), was significantly higher in the toxicity group [5.53 (3.97–13.05) ng/mL] than in the no-toxicity group [4.17 (1.03–6.33) ng/mL; p = 0.031]. Regarding the plasma concentration of LEN at 1 h after oral administration (C1h), expressed as the mean (SD), there was no significant difference between the toxicity group [396.67 (206.73) ng/mL] and the no-toxicity group [416.98 (254.05) ng/mL; p = 0.78].
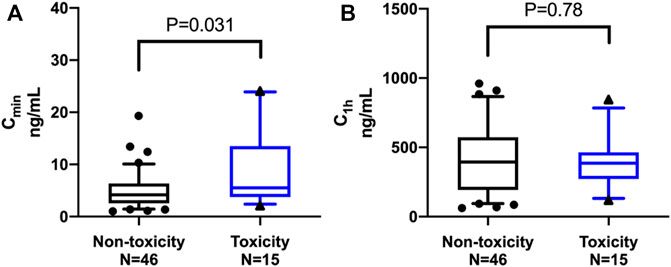
FIGURE 2. Comparison of lenalidomide plasma concentrations between the groups. The middle line represents the median in each group. (A) The trough concentration (Cmin), expressed as the median (IQR), was significantly higher in the toxicity group than in the non-toxicity group [5.53 (3.97–13.05) ng/mL versus 4.17 (1.03–6.33) ng/mL; p = 0.031]. (B) The plasma concentration at 1 h (C1h) after oral administration, expressed as the mean (SD), showed no significant difference between the toxicity group and the non-toxicity group [396.67 (206.73) ng/mL versus 416.98 (254.05) ng/mL; p = 0.78].
Using the ROC curve (Figure 3), the LEN Cmin threshold predicting hematological toxicity was 10.95 ng/mL with an area under the curve (AUC) of 0.687 [95% confidence interval (95% CI) = 0.527–0.847; p = 0.031]. Considering the threshold value of 10.95 ng/mL, the sensitivity and specificity were 0.4 and 0.935, respectively.
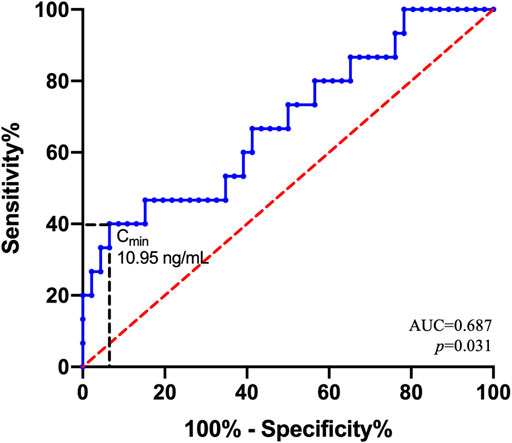
FIGURE 3. Receiver operating characteristic (ROC) curve for hematological toxicity. ROC curve estimates for the 61 patients. AUC is the area under the ROC curve. With regard to the Cmin threshold value of 10.95 ng/mL, the sensitivity and specificity were 0.4 and 0.935, respectively.
Then, the LEN Cmin was binarized according to the 10.95 ng/mL threshold as “high exposure” when Cmin was above this value and as “low exposure” when below. Compared to the “low exposure” group (n = 9/52, 17.31%), there was a significantly increased risk of toxicity in the “high exposure” group (n = 6/9, 66.67%; p = 0.006).
Logistic regression analysis was used to identify independent factors influencing hematological toxicity. The results of the univariate and multivariate analyses are presented in Table 2. The dichotomized LEN Cmin was retained in the final model. In line with previous analysis, a LEN Cmin threshold below 10.95 ng/mL was significantly associated with a decreased risk of hematological toxicity (<10.95 ng/mL vs. >10.95 ng/mL: OR = 0.023, 95% CI = 0.002–0.269; p = 0.003). In other words, compared to “low exposure” (Cmin<10.95 ng/mL), “high exposure” (Cmin>10.95 ng/mL) was associated with an apparent increase in the odds of developing hematological toxicity.
LEN, a non-chemotherapy immunomodulator, has been extensively used in the treatment of MM and NHL, and the mechanism involves direct cytotoxicity as well as indirect effects on tumor immunity (Gribben et al., 2015). With the expanding role of LEN in hematologic malignancies, the management of its hematological toxicity has become a wide clinical concern and research focus (Cheson et al., 2020). Despite dose adjustments according to the severity of myelosuppression, unacceptable hematological toxicity is still the most common factor preventing continuous therapy with LEN. To date, there is no established and feasible marker that can be used as a predictive factor in routine clinical practice to inform a high risk of developing hematological toxicity. Therefore, we paid more attention to the association between hematological toxicity and its plasma concentration in this study.
This current study revealed that only Cmin was independently associated with hematological toxicity. We found that LEN over-exposure contributed to its hematological toxicity, which is similar to previous investigations on the cumulative exposure (AUC) of LEN (Komrokji et al., 2012; Chen et al., 2013; Kobayashi et al., 2018). Given that a Cmin higher than 10.95 ng/mL was associated with a remarkable increase in the risk of developing toxicity, a Cmin of 10.95 ng/mL was determined as the upper limit to prevent hematological toxicity. This threshold was associated with a specificity of 93.5% and a sensitivity of 40%.
Conversely, no apparent association was observed between LEN C1h and its hematological toxicity in our study, which corresponds to the findings in a previous investigation on the relationship of peak serum concentration (Cmax) and hematological toxicity (Bridoux et al., 2016). However, it is notable that the inter-individual variability of the time to reach Cmax (Tmax) could not be excluded; thus, C1h cannot be equal to Cmax in our study.
As similar pharmacokinetic profiles of LEN have been previously shown in patients with various types of hematological malignancies (Chen et al., 2017), the two most common hematologic malignancies, MM and NHL, were simultaneously included in our study population. Interestingly, the results of univariate analysis suggested that patients with MM might have a lower risk of hematological toxicity than those with NHL, which needs to be studied further. In addition, the univariate analysis suggested a possible tendency toward an increased risk of hematological toxicity in patients co-administered cytotoxic antitumor drugs. However, it was not an independent factor affecting hematological toxicity in the multivariate analysis, which also enhances the reliability of the observed association between Cmin and hematological toxicity.
With regard to TDM, sampling trough concentrations at steady state (Cmin) is often performed in clinical practice and is currently the most precise approach, as it avoids the shrinkage of individual information to the population mean (Mueller- Schoell et al., 2021). To the best of our knowledge, the present study is the first to establish and highlight a relationship between LEN Cmin and its hematological toxicity. Furthermore, multivariate logistic analysis confirmed the significance of this cut-off value of LEN Cmin, and we propose it as an optimal index for TDM of LEN hematological toxicity. In comparison, TDM based on cumulated AUC requires collecting and analyzing multiple blood samples, whereas dense blood sampling is rarely feasible in the clinical setting.
Of note, despite TDM’s value in oncology becoming more recognized, it is still not commonly used in antineoplastic treatment compared to other therapeutic areas (e.g., antimicrobial and antiepileptic) (Wicha et al., 2015; Velghe and Stove, 2018; Mueller- Schoell et al., 2021). The present study provides exploratory evidence for LEN TDM in patients with NHL and MM, which contributes to further promoting the extensive use of TDM in the field of oncology. Additionally, the exposure-safety relationship was established based on real-world data from patients’ medical records in our study. Evidence from the real-world setting can help to establish a broad picture of TDM implementation in everyday clinical practice.
From a clinician’s or pharmacist’s point of view, the hematological toxicity of LEN has become a wide clinical concern. Herein, we discuss recommendations regarding the clinical management of the hematological toxicity of LEN. First, prior to the initiation of LEN treatment, the assessment of patients’ baseline kidney function, complete blood count (CBC), and other concomitant drugs causing myelosuppression (e.g., cytotoxic drugs) should be performed. These aforementioned factors should be taken into consideration when determining the initial dose. Second, early measurement of LEN Cmin on at least day 3 after starting LEN therapy (that is, at least 2 days of dosing) would help to inform a high risk of developing hematological toxicity. Then, individual dose adjustments can be made if necessary. Last but not least, patients should be well trained and motivated to take their medication correctly. Additionally, patients should have CBC assessment regularly to monitor for hematological toxicity, particularly neutropenia.
Several limitations should be considered for our study. First, we included only a relatively small number of patients; thus, the findings need to be confirmed in a larger and independent population. The outcome measure of hematology toxicity was defined as grade 2 and a higher level in this study, whereas the relationship between exposure and severe hematology toxicity (grade 3 and above) still deserves further consideration. Second, it was unfeasible to assess the exposure-response relationship due to lack of follow-up on long-term efficacy. More particularly, efficacy is a multifactorial and more complex process than toxicity events. Future well-designed studies are warranted to explore the exposure-response relationship. Third, patients were required to delay the breakfast on the morning of the blood sample, but it was difficult to be sure that these instructions were followed. However, it is unlikely that this would alter our conclusions of Cmin.
In conclusion, we demonstrate that the LEN trough concentration correlates with hematological toxicity, and the Cmin threshold for hematological toxicity (10.95 ng/mL) is proposed. These findings provide the first elements of proof in favor of Cmin-based TDM in NHL or MM patients receiving LEN therapy. Altogether, LEN TDM appears to be a new approach to improve medication safety and achieve continuous treatment for NHL or MM patients in routine clinical care.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Peking University Third Hospital Medical Science Research Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
RZ, HJ, and ZS conceived and designed the study. LM, LB, AL, LZ, YL and, FD enrolled patients. ZS, YM, DJ, and YC collected the data and performed the statistical analysis. PY measured concentrations. ZS and LM wrote the article. ZS and DJ prepared the pictures and tables. RZ, HJ, and FD provided suggestions and participated in the revision of the article. All authors read and approved the final manuscript.
This work was supported by the National Natural Science Foundation of China (NSFC) (72074005).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to express our gratitude to Wei Zhao, Qihui Li, Ping Yang, Wei Wan, Fang Bao and Lingli Wang from Peking University Third Hospital for help with enrolling patients. We are also grateful to Libo Zhao and Xianhua Zhang from Peking University Third Hospital for consultation, and Yang Hu from Peking University Third Hospital for help with editing.
Bridoux, F., Chen, N., Moreau, S., Arnulf, B., Moumas, E., Abraham, J., et al. (2016). Pharmacokinetics, Safety, and Efficacy of Lenalidomide Plus Dexamethasone in Patients with Multiple Myeloma and Renal Impairment. Cancer Chemother. Pharmacol. 78, 173–182. doi:10.1007/s00280-016-3068-9
Cai, W., Zeng, Q., Zhang, X., and Ruan, W. (2021). Trends Analysis of Non-hodgkin Lymphoma at the National, Regional, and Global Level, 1990-2019: Results from the Global Burden of Disease Study 2019. Front. Med. 8, 738693. doi:10.3389/fmed.2021.738693
Chen, N., Zhou, S., and Palmisano, M. (2017). Clinical Pharmacokinetics and Pharmacodynamics of Lenalidomide. Clin. Pharmacokinet. 56, 139–152. doi:10.1007/s40262-016-0432-1
Chen, N., Ette, E., Zhou, S., Weiss, D., and Palmisano, M. (2013). Population Pharmacokinetics and Exposure-Safety of Lenalidomide in Patients with Multiple Myeloma, Myelodysplastic Syndromes and Mantle Cell Lymphoma. Blood 122, 3234. doi:10.1182/blood.v122.21.3234.3234
Cheson, B. D., Morschhauser, F., and Martin, P. (2020). Management of Adverse Events from the Combination of Rituximab and Lenalidomide in the Treatment of Patients with Follicular and Low-Grade Non-hodgkin Lymphoma. Clin. Lymphoma Myeloma Leuk. 20, 563–571. doi:10.1016/j.clml.2020.03.009
Gribben, J. G., Fowler, N., and Morschhauser, F. (2015). Mechanisms of Action of Lenalidomide in B-Cell Non-hodgkin Lymphoma. J. Clin. Oncol. 33, 2803–2811. doi:10.1200/JCO.2014.59.5363
Kado, Y., Tsujimoto, M., Fuchida, S. I., Okano, A., Hatsuse, M., Murakami, S., et al. (2020). Factors Associated with Dose Modification of Lenalidomide Plus Dexamethasone Therapy in Multiple Myeloma. Biol. Pharm. Bull. 43, 1253–1258. doi:10.1248/bpb.b20-00337
Kang, J. S., and Lee, M. H. (2009). Overview of Therapeutic Drug Monitoring. Korean J. Intern Med. 24, 1–10. doi:10.3904/kjim.2009.24.1.1
Kobayashi, T., Miura, M., Niioka, T., Abumiya, M., Ito, F., Kobayashi, I., et al. (2018). Phase II Clinical Trial of Lenalidomide and Dexamethasone Therapy in Japanese Elderly Patients with Newly Diagnosed Multiple Myeloma to Determine Optimal Plasma Concentration of Lenalidomide. Ther. Drug Monit. 40, 301–309. doi:10.1097/FTD.0000000000000499
Komrokji, R. S., Lancet, J. E., Swern, A. S., Chen, N., Paleveda, J., Lush, R., et al. (2012). Combined Treatment with Lenalidomide and Epoetin Alfa in Lower-Risk Patients with Myelodysplastic Syndrome. Blood 120, 3419–3424. doi:10.1182/blood-2012-03-415661
Leonard, J. P., Trneny, M., Izutsu, K., Fowler, N. H., Hong, X., Zhu, J., et al. (2019). AUGMENT: A Phase III Study of Lenalidomide Plus Rituximab Versus Placebo Plus Rituximab in Relapsed or Refractory Indolent Lymphoma. J. Clin. Oncol. 37, 1188–1199. doi:10.1200/JCO.19.00010
List, A., Kurtin, S., Roe, D. J., Buresh, A., Mahadevan, D., Fuchs, D., et al. (2005). Efficacy of Lenalidomide in Myelodysplastic Syndromes. N. Engl. J. Med. 352, 549–557. doi:10.1056/NEJMoa041668
Ludwig, H., Delforge, M., Facon, T., Einsele, H., Gay, F., Moreau, P., et al. (2018). Prevention and Management of Adverse Events of Novel Agents in Multiple Myeloma: a Consensus of the European Myeloma Network. Leukemia 32, 1542–1560. doi:10.1038/s41375-018-0040-1
Mateos, M. V., Hernández, M. T., Giraldo, P., de la Rubia, J., de Arriba, F., López Corral, L., et al. (2013). Lenalidomide Plus Dexamethasone for High-Risk Smoldering Multiple Myeloma. N. Engl. J. Med. 369, 438–447. doi:10.1056/NEJMoa1300439
Mitsiades, C. S., Mitsiades, N., Munshi, N. C., and Anderson, K. C. (2004). Focus on Multiple Myeloma. Cancer Cell 6, 439–444. doi:10.1016/j.ccr.2004.10.020
Mueller-Schoell, A., Groenland, S. L., Scherf-Clavel, O., van Dyk, M., Huisinga, W., Michelet, R., et al. (2021). Therapeutic Drug Monitoring of Oral Targeted Antineoplastic Drugs. Eur. J. Clin. Pharmacol. 77, 441–464. doi:10.1007/s00228-020-03014-8
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
Velghe, S., and Stove, C. P. (2018). Volumetric Absorptive Microsampling as an Alternative Tool for Therapeutic Drug Monitoring of First-Generation Anti-epileptic Drugs. Anal. Bioanal. Chem. 410, 2331–2341. doi:10.1007/s00216-018-0866-4
Wicha, S. G., Kees, M. G., Solms, A., Minichmayr, I. K., Kratzer, A., and Kloft, C. (2015). TDMx: a Novel Web-Based Open-Access Support Tool for Optimising Antimicrobial Dosing Regimens in Clinical Routine. Int. J. Antimicrob. Agents 45, 442–444. doi:10.1016/j.ijantimicag.2014.12.010
Keywords: lenalidomide, hematological toxicity, therapeutic drug monitoring, trough concentration, hematological malignancies
Citation: Song Z, Ma L, Bao L, Ma Y, Yang P, Jiang D, Liu A, Zhang L, Li Y, Cheng Y, Dong F, Zhao R and Jing H (2022) Toward Therapeutic Drug Monitoring of Lenalidomide in Hematological Malignancy? Results of an Observational Study of the Exposure-Safety Relationship. Front. Pharmacol. 13:931495. doi: 10.3389/fphar.2022.931495
Received: 29 April 2022; Accepted: 31 May 2022;
Published: 23 June 2022.
Edited by:
Miao Yan, Central South University, ChinaReviewed by:
Samuel Yamshon, Weill Cornell Medical Center, NewYork-Presbyterian, United StatesCopyright © 2022 Song, Ma, Bao, Ma, Yang, Jiang, Liu, Zhang, Li, Cheng, Dong, Zhao and Jing. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rongsheng Zhao, emhhb3JvbmdzaGVuZ0Biam11LmVkdS5jbg==; Hongmei Jing, aG9uZ21laWppbmdAYmptdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.