- 1College of Pharmacy, Gansu University of Chinese Medicine, Lanzhou, China
- 2Key Laboratory for Chemistry and Quality of Traditional Chinese Medicine and Tibetan Medicine of Gansu Provincial Colleges, Lanzhou, China
Ulcerative colitis (UC) is an inflammatory bowel disease that is persistent and nonspecific. There are several medications available for the treatment of UC. However, conventional UC medications have substantial adverse effects, low clinical effectiveness, and a high recurrence rate. Therefore, it is critical to discover new medicines that are both safe and effective for UC patients. Natural polysaccharides offer a wide range of pharmacological benefits, including anti-inflammatory, anti-virus, anti-tumor, anti-aging, immune enhancement, and gut flora regulation. In the therapy of UC, natural polysaccharides can modulate inflammatory factors, the immune system, and intestinal flora, and preserve the intestinal mucosa. It demonstrates a good curative effect and is of safety to use, thereby being a potential treatment for UC patients. This paper covers the structure, the pharmacological effects on UC, and the mechanisms of natural polysaccharides. Finally, limitations, challenges, and perspectives are discussed. It is hoped that the findings of this publication will inspire more natural polysaccharides research and provide a theoretical foundation for the creation of new UC medications.
Introduction
Inflammatory bowel disease is a special chronic intestinal inflammatory disease, includes Ulcerative colitis (UC) and Crohn’s disease. UC is a chronic, non-specific inflammatory bowel diseases that affects large number of people (Du et al., 2020). The lesion is found in the large intestine’s intestinal mucosa and submucosa. The distal colon is the primary site of the UC, and there is a backflow development from the rectum to the colon, which can be used to distinguish from Crohn’s disease. There is an inflammatory response throughout the beginning process, with stomach discomfort, diarrhea, mucus, and bloody stool as the predominant symptoms, which are frequently accompanied by mucosal tissue congestion and edema, as well as ulceration (Keshteli et al., 2019; Kaenkumchorn et al., 2020). Currently, although the particular pathophysiology remains unknown, several variables including inheritance, environment, psychology, and food, are thought to have a role in the pathophysiology of UC (Alastair et al., 2011; Lee, 2020). Most researchers now believe that the destruction of intestinal immune balance is caused by a combination of factors, such as such as the impairment of some barrier functions of the intestinal mucosa, a change in the permeability of mucosal epithelial cells, a disorder of neuroendocrine regulation, an ectopic phenomenon of intestinal flora, and harmful substances produced by the body (Tahan et al., 2011; Sun et al., 2021). The imbalance of intestinal immune activity is a major cause of UC. Immunological damage occurs when the equilibrium between immune response and immune tolerance is disrupted. It was demonstrated that when intestinal tolerance is diminished, it leads to a malfunction in T cell regulatory function, resulting in UC. The intestinal flora is also crucial in UC (Wang et al., 2011). When the variety of intestinal flora is diminished and the microbial composition and structure are uneven, the intestinal homeostasis is disrupted and the inflammatory response in the gut is getting worse (Kozik et al., 2019; Monif, 2019). Furthermore, UC has family aggregation and hereditary susceptibility (Kubota et al., 2019). Notably, if UC is not treated in a timely manner, it can cause anemia, perforation, cancer, and other complications. The primary goal of clinical therapy is to minimize the inflammatory response and heal the intestinal mucosal ulcer (Yashiro, 2014; Bopanna et al., 2017). Therefore, immunosuppressants, aminosalicylic acid medicines, antibiotics, and monoclonal antibodies are the most often used pharmaceuticals in modern medicine (Maul et al., 2012; Burri et al., 2020; Le Berre et al., 2020). However, these medications frequently have severe adverse effects, poor clinical effectiveness, and a high recurrence rate (Yan and Li, 2021). As a result, there is an urgent need to discover new treatments that are both curative and tolerable for UC patients. Based on the shortcomings of traditional drugs, NPs have been research and developed in the field of UC treatment.
Natural polysaccharides (NPs) isolated from plants have been demonstrated to have a wide variety of pharmacological benefits, including anti-inflammatory, anti-virus, anti-tumor, anti-aging, increasing body immunity, and controlling gastrointestinal flora. NPs oral absorption though paracellular pathway, transcellular pathways, the microfold cells (M cell) mediated transport, and so on (Chen et al., 2020; Feng et al., 2020; Xiang et al., 2020). It is useful in the treatment of diabetes, inflammation, cancer, and other disorders (Figure 1). It also offers great safety, a good curative effect, a low cost, and strong biocompatibility (Wang et al., 2016; Chen et al., 2019; Hou et al., 2020; Zhao et al., 2020). The Auricularia auricula polysaccharides could lower blood sugar levels and had a positive therapeutic impact in the treatment of diabetes (Chen et al., 2022). Ganoderma lucidum polysaccharides could inhibit breast and prostate cancer cells in cancer (Jiao et al., 2029), and Candida polysaccharides could inhibit colon tumor by changing intestinal flora structure (Guo et al., 2019). In terms of anti-inflammatory activity, Hedysari polysaccharide, Astragalus polysaccharide, and Rhubarb polysaccharide were all effective. It was discovered in the study of NPs that a variety of NPs, including Hedysari polysaccharide, Astragalus polysaccharide, Rhubarb polysaccharide, Codonopsis pilosula (C. pilosula) polysaccharide, and Purslane polysaccharide, have good anti-inflammatory and immunomodulatory activities, and have significant therapeutic effects on UC. Therefore, this study examines the advancement of NPs research in the treatment of UC, in order to give a theoretical foundation for the research and development of novel medications for UC.
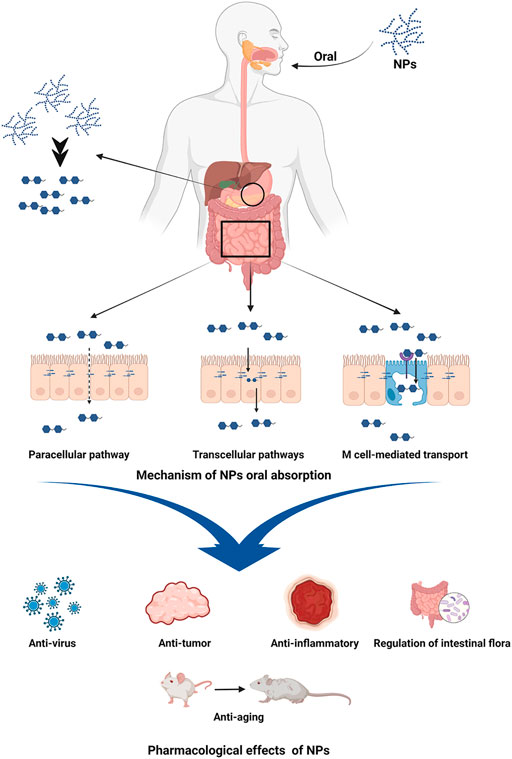
FIGURE 1. Oral absorption mechanism and pharmacological effects of NPs. Image was made in BioRender (biorender.com).
Structural Characteristics of NPs
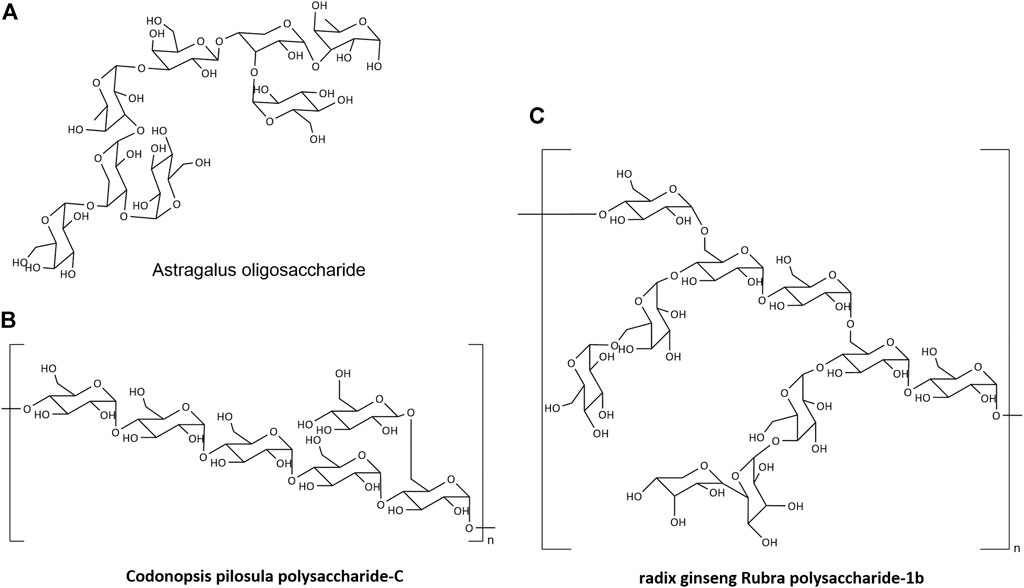
Natural products generated by linking monosaccharide residues with numerous glycosidic linkages are known as NPs. It may be found in plants, animals, bacteria, fungus, algae, and other organisms as structural components of biological membranes and cell walls, as well as reserve cytoplasm compounds. NPs have a complicated structure and a wide range of components (Cheng et al., 2020). Polymerization of glucose (Glu), arabinose (Ara), xylose (Xyl), galactose (Gal), mannose (Man), galactose (Gal), or more than one monosaccharide is commonly used to produce the main chain (Figure 2) (Zhu C et al., 2017; Li H et al., 2020; Zhang et al., 2021).
There are several ways for extracting, separating, and purifying NPs. Water extraction, enzyme extraction, ultrasonic extraction, and microwave extraction are routinely utilized. After that, proteins are removed using the Savage technique, the protease method, or the trichloroacetic acid method. Finally, NPs are purified by precipitation, gel chromatography, anion exchange chromatography, and ultrafiltration. The total sugar content is measured by phenol-sulfuric acid method, and the uronic acid content is determined by m-hydroxybiphenyl assay. The molecular weights of polysaccharide are usually determined by high-performance liquid chromatography (HPLC) equipped with size-exclusion chromatography (SEC) and refractive index detector/evaporative light scattering detector. For monosaccharide compositional analysis, the saccharides of polysaccharide are normally subjected to acid hydrolysis followed by conversion into derivatives. The derivative products can be analyzed by gas chromatography (GC) equipped with flame ionization detector (FID) or ion trap mass spectrometry. Besides, the hydrolyzed monosaccharides can be also converted into 1-phenyl-3-methyl-5-pyrazo-lone (PMP) derivatives and analyzed by HPLC equipped with UV detector. The structures of polysaccharide–polyphenolic conjugates can be further characterized by UV–vis, Fourier-transform infrared (FTIR) and nuclear magnetic resonance (NMR) spectroscopy. Among NPs available for UC treatment, the structure of Hedysarum polysaccharide, Astragalus polysaccharide, Rhubarb polysaccharide, C. pilosula polysaccharide has been thoroughly studied, relatively.
Hedysarum Polysaccharide
Water extraction and alcohol precipitation were used to extract the entire polysaccharide from Hedysarum. The crude product was deproteinized using the Sevage method, and the polysaccharides HG-2, HG-3, and HG-4 were separated with greater than 95% purity using Sephadex G-25, Sephadex G-100, and Sephadex G-75 column chromatography, respectively (Zhang, 2019; Zhou, 2021; Zhou et al., 2021a).
White powders with good solubility include HG-2, HG-3, and HG-4. The Ninhydrin and iodine iodide assays revealed that they were non starch polysaccharides. They had the distinctive functional group of polysaccharides, which may be Pyran sugar, and demonstrate a certain antioxidant capacity, according to Fourier Transform Infrared spectroscopy (FTIR), and DPPH radical scavenging ability. The mass average molar mass (Mw) of HG-2, HG-3, HG-4 was 32.7 kDa (PDI, 8.83), 28.9 kDa (PDI, 6.79), 32.0 kDa (PDI, 5.89), respectively. HG-G consists of Glu, Ara, Gal, Rha, and Xyl. HG-3 was a homogenous polysaccharide with only one Glu component, while HG-4 was made up of Glu, Ara, and Gal (in the molar ratio Glu: Ara: Gal = 4.8:0.4:0.5) (Zhou, 2021).
Astragalus Polysaccharide
The molecular weight of Astragalus polysaccharides (APS) in Astragalus membranaceus was 1.77 × 103 kDa, with Glc, Gal, and Ara, respectively. The monosaccharide composition was Glc, Gal, and Ara, and the molar ratio was 27.92:5.20:2.86 (Liang, 2019). The structure of Astragalus membranaceus low molecular weight polysaccharide (LMW-ASP) was investigated. The molecular weight of LMW-ASP was 5.6 kDa, and the molar ratio was Glu: Gal: Ara: Xyl: Gal A = 10.0:1.3:1.7:1.0:0.9 (Yang et al., 2016). After FTIR and GC analysis, the APS was shown to be constituted of Ara, Man, Glu, and Gal, with a molar ratio of 0.0992:1.26:1.00:0.0115 (Yan et al., 2010).
A preliminary investigation on the connecting method of Astragalus polysaccharides has been undertaken by several researchers. The Astragalus polysaccharide breakdown product (Astragalus oligosaccharide) was isolated and examined using monosaccharide composition analysis, periodate oxidation and Smith degradation, methylation analysis, ESI-MS, FTIR, and NMR. It was made up of Rha linked by (3→3), Rha linked by (1→3), Araf linked by (1→3, 4), Gal linked by (1→3), and terminal residues linked by - Gal and–Glu (Figure 2A) (Zhu ZY et al., 2017).
Rhubarb Polysaccharide
Alcohol was used to extract Rhubarb and precipitate crude polysaccharide. After eliminating protein with three chloroacetic acid washes, RP-1 (molecular weight 11 kDa), RP-2 (molecular weight 20 kDa), and RP-3 (molecular weight 69 kDa) were separated by DE-52 chromatography column and purified by Sephacryl S-200 Gel column. RP-1 was a homogenous polymer that exclusively contains glucose, while RP-2 and RP-3 were the acidic heteropolysaccharides with galacturonic acid and glucuronic acid as uronic acids, respectively. RP-2 was made up of Rha, Ara, Xyl, Man, Glu, and Gal in the following molar ratios: 0.5:1.0:1.3:1.4:12.4:3.8. Rha, Ara, Xyl, Man, and Glu were present in the molar ratios 0.9:3.9:4.7:1.0:2.6 in RP-3 (Hu et al., 2011).
Guo et al. identified RTP-2, a polysaccharide from Rhubarb with a molecular weight of 100–200 kDa. It consisted of Xyl, Ara, Glu, Rha, Gal, Man, Glu A, and Gal A, with a molar ratio of 1.00:23.27:15.93:2.95:1.34:49.07:2.62:4.31. However, because RTP-2 includes anthraquinones, RTP-2A with a molecular weight of 7–11 kDa and no anthraquinones was produced by enzymatic hydrolysis, separation, and purification (Guo, 2013).
C. pilosula Polysaccharide
After extraction, separation, and purification, C. pilosula polysaccharide was recovered. Membrane separation was used to purify inulin fructose CP-A with a molecular weight of 3.6 kDa, fructan CP-B with a molecular weight of 1.70 × 103 kDa, and homogenous polysaccharide CP-C with a molecular weight of 1.698 kDa (Wang, 2021). According to the FTIR data, CP-C was α-Polysaccharide with a skeleton of pyranose. Methylation, along with GC-MS and NMR detection, revealed that the structure of CP-A was (2→1)-D-furan fructan (Li et al., 2017). The CP-B structure was made up of—D-fructofuranyl—(2→3)-β- D furan fructose linkage; the CPC structure was made up of Glcp—(1→, 4→)—Glcp—(1→ and →4,4)—Glcp—(1 three connection modes) (Figure 2B) (Li J et al., 2020).
Water-soluble polysaccharides extracted from C. pilosula were CPP1a and CPP1c. CPP1a was composed of Rha, Ara, Glu, Gal, and Gal A in the molar ratios 1.34:12.30:3.49:10.44:1.18, with a relative molecular weight of 1.01 × 102 kDa CPP1c was composed of Rha, Ara, Gal, and Gal A in the molar ratios 2.99:1.15:1.94:33.29, with a relative molecular weight of 1.26 × 102 kDa (Bai et al., 2018). WCP-Ia was a C. pilosula acidic polysaccharide. The GC analysis revealed that WCP-Ia was mostly constituted of Ara, Rha, Man, Gal, Glu, and Gal A in the following molar ratios: 5.5:6.4:0.7:17.6:0.2:69.6 (Zou et al., 2019).
The study of NPs in C. pilosula, Astragalus, and other plants has been rather extensive. We noticed that various researchers use different extraction, separation, and purification procedures to get distinct types of NPs. The structure of NPs could be classified as primary, secondary, tertiary, or quaternary (Zeng et al., 2019). The intricacy of the NPs, from a chemical standpoint, surely complicates structural investigation. These NPs differ in their nature, composition, and activity. One of the major constraints of NPs research is the difficulty to define the precise structure of NPs.
Therapeutic Impact and Mechanism of NPs on Ulcerative Colitis
NPs had a strong therapeutic impact on ulcerative colitis, which is mostly due to its role in controlling cytokines, maintaining intestinal environmental homeostasis, boosting intestinal immune function, and protecting colonic mucosa. UC lacks a properly matched animal model. There are now two recognized UC Animal Models, 2,4,6-trinitrobenzenesulfonic acid solution (TNBS) model and dextran sulfate sodium (DSS) model.
Regulation of Intestinal Mucosal Immune Cell Differentiation
Immune regulatory dysfunction is a critical element in the pathophysiology of UC. Inflammation occurs, develops, and migrates as a result of both innate and adaptive immune control. NPs aid in the therapy of UC by influencing the development of intestinal mucosal immune cells and the release of immune cytokines.
By modulating the expression of T-bet and GATA-3 proteins (p < 0.05) in the small intestine mucosa, APS could maintain the dynamic balance of Th1/Th-2 cells and control immunological activity (Li J et al., 2020).
A polysaccharide derived from cultivated mycelium of Hericium, C. pilosula polysaccharide, and Astragalus polysaccharide might suppress Th17 cell activity and prevent Th17 cell differentiation, hence controlling disease progression (Guo et al., 2014; Zhao et al., 2016; Wang et al., 2018). Angelica polysaccharide might increase CD3+, CD4+, and CD8+ peripheral blood T cells (p < 0.05) while decreasing the CD4/CD8 ratio (p < 0.05), so reducing colon damage (Liu et al., 2003; Li and Liu, 2005).
Furthermore, APS might ameliorate intestinal mucosa damage and reduce inflammatory responses by downregulating dendritic cells, enhancing macrophage shape, and restoring macrophage proliferation (Dai et al., 2011; Lu et al., 2018).
Cytokines With Regulatory Function
Cytokines, which are proteins or tiny molecular peptides that carry information between cells, have a variety of biological impacts, including increasing inflammation, immunomodulation, and tissue healing. Interferon (IFN), growth factor (GF), interleukin (IL), tumor necrosis factor (TNF), chemokine, and other cytokines are examples of common cytokines. These variables have a significant role in the pathological progression of UC and are also critical in the therapy of UC (Ardizzone et al., 2012).
Hedysarum polysaccharide, HG-2, HG-3, and HG-4 might increase mice body weight and fecal characteristics to varied degrees in the UC mouse model generated by TNBS (p < 0.01). Meanwhile, the macroscopic morphology of the colon and the damage of colonic mucosa can also be improved to varied degrees, promoting intestinal mucosa repair and improving the CMDI score of UC mice (p < 0.05). TNF-α, IL-6 dropped and IL-10 increased considerably (p < 0.01), and HG-4 was more effective than HG-2 and HG-3 (Yan et al., 2019; Zhou et al., 2021b).
APS demonstrated promising therapeutic benefits in both the TNBS-induced mouse model and the DSS-induced rat model (Xi et al., 2019). It has been shown to lower MPO activity and TNF-α levels in the colon of UC rats (p < 0.01), as well as to delay inflammatory reactions, reduce inflammatory cell infiltration, minimize mucosal damage, and control colon EGF and EGF-β (p < 0.01). APS is also capable of improving mucosal barrier function, promoting ulcer healing, and reducing the development of UC by increasing the expression of intestinal occludin and ZO-1 protein (p < 0.05) (Zang et al., 2018).
Rhubarb polysaccharide (RTP) helps lower blood glucose levels, protect the liver, and heal stress ulcers. RTP might successfully minimize UC mouse weight loss, lower the incidence of diarrhea and bloody stool, ameliorate colonic tissue damage, and has a positive effect on UC therapy. RTP might cure UC by raising anti-inflammatory factors like IL-10 and IL-4 (p < 0.05) while decreasing pro-inflammatory factors like TNF-α and IL-8 (p < 0.05) (Zhang et al., 2006). The mechanism might be to diminish the immunological response by blocking the CD4+ propensity of immune T cells to the gut, decreasing caspase3 expression, and promoting PMN apoptosis (p < 0.01) (Wang et al., 2006).
Ge et al. (2019) discovered that IFN was down regulated in colon tissue of UC mice treated with Lycium barbarum polysaccharide by gavage, IFN-γ miRNA was down regulated (p < 0.05), indicating suppression of proinflammatory factor interferon-γ. Its expression might be a method for decreasing inflammatory infiltration of the intestinal wall and alleviating tissue damage.
Regulation of Signaling Pathways
Multiple signal pathways are activated and inhibited during the formation and progression of UC.
Inhibition of the NF-κB signaling pathway. NF-κB is a transcription factor that promotes the expression of proinflammatory factor genes. NF-κB inhibitor protein (IKB) is phosphorylated and then degraded in response to pro-inflammatory stimulation, culminating in NF-κB release and nuclear translocation. NF-κB activation governs the expression of a number of pro-inflammatory genes (Kaushik et al., 2014).
C. pilosula polysaccharide inhibited the TLR4-NF-κB pathway, lowering TLR4, IL-6, and NF-B in UC mRNA expression and thus alleviating the TNBS-induced UC model in rats (Tian et al., 2016). APS inhibits the expression of Toll-like receptor, TLR4, and myeloid differentiation factor 88 and MyD88, thereby inhibiting the NF-κB signaling pathway and lowering the production of inflammatory factors and inflammatory mediators (Luo et al., 2015). RTP-2 and RTP-2A both decreased NF-κB activation and TNF-α levels. Simultaneously, it was ingested into macrophages, where it lowered p-p65 expression and blocked NF-κB-p65. In order to reduce UC symptoms (Guo, 2013).
Inhibition of JAK-STAT signaling pathway. The Janus Kinase-Signal Transducer and Activator of Tranions (JAK-STAT) signaling pathway is important in controlling T cell development. Dysfunction of the JAK-STAT signaling system results in aberrant T cell differentiation and impaired memory Treg activity, which is thought to be a key role in the pathophysiology of inflammatory bowel disease (Cordes et al., 2020). Portulaca oleracea polysaccharide regulated the TNF-/NF-κB and IL-6/STAT3 signaling pathways, thereby decreasing the contents of STL-6agp130 and MPO, NF-κB level in colon, inhibiting the expression of key proteins such as p-STAT3, COX-2, and IKBA, inhibiting the expression of CNC-1 and its receptor, and influencing the tendency of neutrophils to inflammatory sites (Wang, 2017). APS might diminish the phosphorylation of JAK and STAT proteins, as well as activate and inhibit the production of this pathway’s SOCS system, therefore alleviating mucositis symptoms in UC mice (Zhao et al., 2018).
Inhibition of the MAPK signaling pathway. Mitogen-Activated Protein Kinases (MAPKs) are critical players in the interplay between intracellular and extracellular responses. The activation of p38 kinase and c-Jun N-terminal kinase, JNK, can speed up cell death and exacerbate the inflammatory response in the intestine (Wei and Feng, 2010). P38 and JNK inhibitors are effective in the treatment of intestinal inflammatory disorders (Wang et al., 2021). APS might reduce p38 and MAPK phosphorylation, hence inhibiting MAPK pathway activation (Dong et al., 2020).
Regulation of Intestinal Flora
Intestinal flora has steadily become one of the hotspots in UC research in recent years. The intestinal flora of patients has been altered by UC, as evidenced by a decrease in the variety of microorganisms and metabolites and a rise in the kinds of pathogenic bacteria and intestinal adhesion bacteria (Franzosa et al., 2019). Wen et al. (2021) employed fecal microbiota transplantation to ameliorate DSS-induced colitis in mice by regulating intestinal flora and T cell modulation. This study provided direct evidence for the role of gut flora control in the therapy of UC.
RTP-2 and RTP-2A, two types of RTP with and without anthraquinone, were isolated from Rheum polysaccharides in a sequential manner. The two components’ effects on the intestinal flora of UC rats were compared. RTP-2 and RTP-2A exhibited the same reversal impact on the flora imbalance (raising the reproduction of Bifidobacterium and Lactobacillus probiotics while suppressing the reproduction of Bacteroides fragilis and Enterococcus harmful bacteria), but RTP-2 was superior than RTP-2A (Guo, 2013).
In UC model rats, APS could increase the number of intestinal bifidobacteria and Lactobacillus, decrease the number of intestinal bacteria, improve the proportion of intestinal microorganisms, increase the content of the intestinal anaerobic metabolite acetic acid, improve the intestinal environment, and alleviate pathological injury to the intestinal mucosa (Liang et al., 2013).
Baicalin polysaccharide can minimize DSS-induced UC mouse weight loss, DAI, ameliorate colonic histological damage, and MPO activity. SP2-1 also reduces the amount of proinflammatory cytokines in the body. Furthermore, the intestinal barrier was restored as a result of increased expression of ZO-1, occludin, and claudin-5. SP2-1 dramatically raised acetic acid, propionic acid, and butyric acid levels in DSS-treated mice. Furthermore, as compared to the control group, SP2-1 therapy increased the number of Firmicutes, Bifidobacteria, Lactobacillus, and Roseobacteria. SP2-1 had the ability to drastically reduce the levels of Bacteroides, Proteus, and Staphylococcus (Cui et al., 2020).
C. pilosula polysaccharide might perform a therapeutic function in the treatment of UC by causing SCFA to generate related bacteria and regulating intestinal flora (Chen, 2016). Lentinan, a polysaccharide derived from Hawthorn, has been shown to balance intestinal probiotics and harmful bacteria in UC rats by increasing intestinal Bifidobacteria and Lactobacillus while decreasing Escherichia coli and Enterococcus. To rectify the dysregulated intestinal flora, Longan polysaccharide fermentation broth can reduce the relative abundance of Helicobacter in the colon of UC mice (Han et al., 2011; Guo et al., 2021).
Modified Polysaccharide Used in the Treatment of Ulcerative Colitis
Targeted Therapy
After being modified, NPs can be utilized as drug carriers, combining with other medications to regulate drug release in the colon, increase drug targeting and affinity, and provide targeted therapeutic effects. For example, starch hydrogels (10%, w/v) can be used as targeted colonic drug delivery vehicles in UC (Koev et al., 2022).
Zhu and co-workers constructed Selenium nanoparticles coated with Ulva lactuca polysaccharide (ULP-SeNPs). They found that ULP-SeNPs showed strong protective benefits against DSS-induced acute colitis in mice, including weight loss and colitis damage (Zhu C et al., 2017). ULP-SeNPs increased macrophage infiltration, as shown by a drop in CD68 levels in colon tissue sections. ULP-SeNPs have anti-inflammatory properties that have been discovered and controlled, including IL-6 and TNF-α related cytokines. ULP-SeNPs limit NF-κB nuclear translocation, which inhibits macrophage activation. NF-κB is responsible for the production of these proinflammatory cytokines.
To create PLGA-RMP (PR) nanoparticles, Ramulus Mori polysaccharide (RMP) was encapsulated in poly (lactic-co-glycolic acid) (PLGA). These PR nanoparticles had substantial therapeutic benefits in a colitis mouse model, as evidenced by a reduction in body weight loss, a drop in the DAI score, and a restoration of colon length. These findings suggested that PR nanoparticles might be employed as an effective nanomedicine to treat UC and as possible prebiotics (Feng et al., 2021).
Gels can not only govern the regeneration process of intestinal tissue, but it can also engage in intestinal tissue remodeling in response to pathological triggering stimuli. Ju and colleagues shown that the cells targeted by grape exon like nanoparticles (GELNs) are intestinal stem cells, and their response is the foundation of GELN mediated intestinal tissue remodeling and protection against sodium dextran sulfate (DSS) induced colitis (Ju et al., 2013).
Prevent Colitis From Becoming Cancerous
The use of modified apple polysaccharide may minimize the occurrence of carcinogenesis in colitis. Its mechanism may be to block galectin-3 and its ligand interaction, hence promoting apoptosis and preventing carcinogenesis (Li et al., 2012).
Discussion
NPs are effective in the treatment of UC. There are numerous NPs that are not mentioned in the article. The polysaccharide isolated from Robinia hainanensis substantially reduced the disease activity index and TNF-α, IL-17 of UC mice, and prevented neutrophil aggregation in colonic mucosa (Miao et al., 2015). Cranberry polysaccharide has been shown to lower the macroscopic score and damage area of the colon, down regulate MPO and MDA, greatly stimulate mucosal repair and regeneration, and diminish experimental colitis produced by DSS and TNBS (Ishisono et al., 2019). Ganoderma lucidum polysaccharide, Purslane polysaccharide, Arctium lappa polysaccharide, and Angelica polysaccharide might successfully alleviate the inflammatory symptoms of UC rats, and the therapeutic effect is consistent (Wang et al., 2019; Zhang et al., 2019; Chen et al., 2020; Wang et al., 2020). The NPs listed above can be used to treat UC with minimal adverse effects. They have promising promise in the research and development of novel UC medications.
When administered alone or in combination, NPs have excellent curative properties. In colitis animals, co-administration of ARP and CRP at precise levels might ameliorate clinical symptoms, restore immunological balance, and reduce colonic mucosal damage. The particular effectiveness of co-administration was due to the activation of the aryl hydrocarbon receptor as well as the upregulation of isovaleric acid and butyrate. Furthermore, the structure of the gut flora was restored in the co-administration group (Tang et al., 2021). Co-administration of Coptis chinensis crude polysaccharide and berberine increases the expression of tight junction protein in the colon of UC mice, therefore repairing colonic mucosal barrier damage in UC animals (Xue et al., 2022). The anti-inflammatory and antioxidant effects of DSS caused UC animals were improved by co-administration of Lycium barbarum polysaccharide and Capsaicin. It reduced serum IL-6 and colon TNF-α but enhanced serum SOD activity (Chen et al., 2021). Bone marrow mesenchymal stem cell (BMSCs) could markedly alleviate injury according to histological analysis and regulate inflammatory cytokine in TNBS-induced UC. And Atractylodes macrocephala polysaccharide could potentiated the effects of BMSCs on preventing TNBS-induced UC, though homing to the injured tissue and regulated cytokines (Zheng and Wang, 2022). The combination of NPs and other drugs has the potential to increase the therapeutic impact on UC to some level, which will be a key research focus in the future.
As a new preparation, probiotics (e.g., probiotics, prebiotics, synbiotics, and so on) have also been used to treat UC. We can alter the intestinal flora, enhance the intestinal barrier, and ease the symptoms of UC by providing typical probiotics in the gut (Roselli et al., 2020). Compared with probiotics, NPs have more ways to treat UC. With few adverse effects, NPs had good therapeutic benefits in UC through regulating cytokines, inhibiting/activating signaling pathways, regulating intestinal flora, and other features, and are progressively becoming a research and development hotspot (Figure 3). It is unfortunate that this work did not discover a link between the structure and effectiveness of NPs throughout the summarization phase. In addition, we have to admit it, the therapeutic effect of single polysaccharide is not as good as that of traditional drugs. However, the combination of polysaccharides and modified polysaccharides provide new ideas for NPs in the field of UC treatment. According to the findings of the research on the published papers, we found that the study of mechanism and intestinal flora were relatively independent, the relationship between mechanism and intestinal flora needs to be addressed.
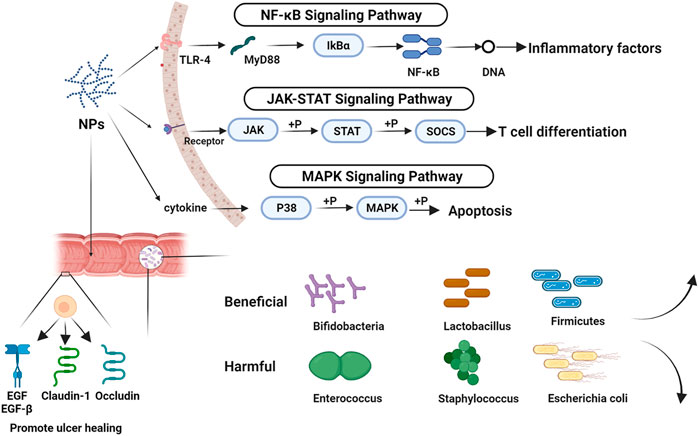
FIGURE 3. The mechanism of NPs in the treatment of UC. Image was made in BioRender (biorender.com).
First of all, the determination of the structure of NPs. There are several ways for the extraction and purification of NPs, thereby leading to a variety of components of polysaccharides. At the moment, the methods for structural identification of polysaccharides are primarily confined to determining the molecular weight and the monosaccharide composition, and there is no mature way for determining the connection sequence and connection mode between monosaccharides. However, if the structure of polysaccharide cannot be precisely recognized, the content of polysaccharide extracted from the same plant will be highly different, which will be the primary challenge in polysaccharide development and application. Secondly, the methodologies for evaluating the therapeutic impact of UC are not consistent. Currently, the most widely employed macro markers are the DAI score, the CI value, and the presence of colonic tissue lesions. They are relatively subjective and lack a consistent quantitative norm, which makes research and development of novel UC therapies difficult. Thirdly, NPs are primarily restricted to experimental studies in the absence of clinical evidence. The gap between experimental animal data and clinical data is unknown, which is also a significant challenge for the future development of natural polysaccharides. It is desired that subsequent researchers would investigate these aspects more in order to encourage NPs to visit clinics as soon as feasible and assist UC patients.
Conclusion
NPs can treat UC by regulating immune cell differentiation, regulating cytokines, regulating signal pathway and regulating intestinal flora. It has definite curative effect and little side effects, and has a good development prospect.
Author Contributions
YG, YL, and MG conceived the project. MG supervised the project. YG and YL carried out most experiments and wrote the manuscript. QC, LY, and JW analyzed the project and revised the manuscript. All authors discussed the results and revised the manuscript.
Funding
This work was supported by the key Project of Joint Funds of the National Natural Science Foundation of China (U21A20412); the Provincial Natural Science Foundation of Gansu Province, China (21JR7RA558); the “Double First-Class” key Scientific Research Project of the Department of Education of Gansu Province, China (GSSYLXM-05); the Project of Central Government Guides the Development of Local Science and Technology, Gansu Province, China.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alastair, F., Emma, G., and Emma, P. (2011). Nutrition in Inflammatory Bowel Disease. J. Parenter. Enter. Nutr. 35, 571–580. doi:10.1177/0148607111413599
Ardizzone, S., Cassinotti, A., and de Franchis, R. (2012). Immunosuppressive and Biologic Therapy for Ulcerative Colitis. Expert. Opin. Emerg. Drugs. 17, 449–467. doi:10.1517/14728214.2012.744820
Bai, R., Li, W., Li, Y., Ma, M., Wang, Y., Zhang, J., et al. (2018). Cytotoxicity of Two Water-Soluble Polysaccharides from Codonopsis Pilosula Nannf. Var. Modesta (Nannf.) L.T.Shen against Human Hepatocellular Carcinoma HepG2 Cells and its Mechanism. Int. J. Biol. Macromol. 120, 1544–1550. doi:10.1016/j.ijbiomac.2018.09.123
Bopanna, S., Ananthakrishnan, A. N., Kedia, S., Yajnik, V., and Ahuja, V. (2017). Risk of Colorectal Cancer in Asian Patients with Ulcerative Colitis: A Systematic Review and Meta-Analysis. Lancet. Gastroenterol. Hepatol. 2, 269–276. doi:10.1016/S2468-1253(17)30004-3
Burri, E., Maillard, M. H., Schoepfer, A. M., Seibold, F., Van Assche, G., Rivière, P., et al. (2020). Treatment Algorithm for Mild and Moderate-To-Severe Ulcerative Colitis: An Update. Digestion 101 (1), 2–15. doi:10.1159/000504092
Chen, L., Ge, M. D., Zhu, Y. J., Song, Y., Cheung, P. C. K., Zhang, B. B., et al. (2019). Structure, Bioactivity and Applications of Natural Hyperbranched Polysaccharides. Carbohydr. Polym. 223, 115076. doi:10.1016/j.carbpol.2019.115076
Chen, Q., Ren, R., Zhang, Q., Wu, J., Zhang, Y., Xue, M., et al. (2020). Coptis Chinensis Franch Polysaccharides Provide a Dynamically Regulation on Intestinal Microenvironment, Based on the Intestinal Flora and Mucosal Immunity. J. Ethnopharmacol. 267, 113542. doi:10.1016/j.jep.2020.113542
Chen, Y.-S., Lian, Y. Z., and Chao, J. (2021). Protective Effect of Lycium Barbarum Polysaccharides and Capsaicin in Rats with Dextran Sulfate Sodium-Induced Ulcerative Colitis via Anti-inflammation and Antioxidation. Curr. Dev. Nutr. 5, 306. doi:10.1093/CDN/NZAB037_016
Chen, Y., Zhang, S., Li, W., Yu, W., Zhao, X., and Liu, T. (2022). Characterization of Phosphorylated Structure of Auricularia Auriculata Polysaccharide and its Hypoglycemic Effect In Vitro. Food Sci. 43 (08), 29–35. doi:10.7506/spkx1002-6630-20210908-091
Chen, X. (2016). Sijunzi Decoction and Codonopsis Pilosula Polysaccharide Alleviate DSS-induced Mice Colitis by Modulating Gut Microbiota. Dissertation. Lanzhou: Lanzhou University.
Cheng, F., Zhang, Y., Li, Q., Zeng, F., and Wang, K. (2020). Inhibition of Dextran Sodium Sulfate-Induced Experimental Colitis in Mice by Angelica Sinensis Polysaccharide. J. Med. Food. 23, 584–592. doi:10.1089/jmf.2019.4607
Cordes, F., Foell, D., Ding, J. N., Varga, G., and Bettenworth, D. (2020). Differential Regulation of JAK/STAT-signaling in Patients with Ulcerative Colitis and Crohn's Disease. World J. Gastroenterol. 26, 4055–4075. doi:10.3748/wjg.v26.i28.4055
Cui, L., Guan, X., Ding, W., Luo, Y., Wang, W., Bu, W., et al. (2020). Scutellaria Baicalensis Georgi Polysaccharide Ameliorates DSS-Induced Ulcerative Colitis by Improving Intestinal Barrier Function and Modulating Gut Microbiota. Int. J. Biol. Macromol. 166, 1035–1045. doi:10.1016/j.ijbiomac.2020.10.259
Dai, J., Gao, Y., and Zhu, F. (2011). Phenotypic Changes of Dendritic Cells in Rats with Experimental Colitis and the Effect of Astragalus Polysaccharide. Chin. J. Clin. Nutri. 02, 93–97. doi:10.3760/cma.j.issn.1674-635X.2011.02.006
Dong, N., Li, X., Xue, C., Zhang, L., Wang, C., Xu, X., et al. (2020). Astragalus Polysaccharides Alleviates LPS-Induced Inflammation via the NF-Κb/MAPK Signaling Pathway. J. Cell Physiol. 235, 5525–5540. doi:10.1002/jcp.29452
Du, L., and Ha, C. (2020). Epidemiology and Pathogenesis of Ulcerative Colitis. Gastroenterol. Clin. North Am. 49, 643–654. doi:10.1016/j.gtc.2020.07.005
Feng, L., Xiao, X., Liu, J., Wang, J., Zhang, N., Bing, T., et al. (2020). Immunomodulatory Effects of Lycium Barbarum Polysaccharide Extract and its Uptake Behaviors at the Cellular Level. Molecules 25, 1351. doi:10.3390/molecules25061351
Feng, Z., Peng, S., Wu, Z., Jiao, L., Xu, S., Wu, Y., et al. (2021). Ramulus Mori Polysaccharide-Loaded PLGA Nanoparticles and Their Anti-inflammatory Effects In Vivo. Int. J. Biol. Macromol. 182, 2024–2036. doi:10.1016/j.ijbiomac.2021.05.200
Franzosa, E. A., Sirota-Madi, A., Avila-Pacheco, J., Fornelos, N., Haiser, H. J., Reinker, S., et al. (2019). Gut Microbiome Structure and Metabolic Activity in Inflammatory Bowel Disease. Nat. Microbiol. 4, 293–305. doi:10.1038/s41564-018-0306-4
Ge, J., Huang, W. Y., Yang, F., Ma, L., and Zhai, H. (2019). Lycium Barbarum Polysaccharide Down-Regulates the Expression of IFN-γ in Ulcerative Colitis. J. Ningxia Med. Univ. 41, 24–27+32. doi:10.16050/j.cnki.issn1674-6309.2019.01.005
Guo, Y., Shi, D., Wan, C., Yao, H., and Feng, G. (2014). Level of T Helper 17 Cell of Experimental Colitis in Rats and Effect of Astragalus Polysacharide. Chin. Arch. Tradi. Chin. Med. 32, 2265–2267. doi:10.13193/j.issn.1673-7717.2014.09.069
Guo, M., Li, Z., Huang, Y., and Shi, M. (2019). Polysaccharides from Nostoc Commune Vaucher Activate Macrophages via NF-Κb and AKT/JNK1/2 Pathways to Suppress Colorectal Cancer Growth In Vivo. Food Funct. 10, 4269–4279. doi:10.1039/c9fo00595a
Guo, C., Wang, Y., Zhang, S., Zhang, X., Du, Z., Li, M., et al. (2021). Crataegus Pinnatifida Polysaccharide Alleviates Colitis via Modulation of Gut Microbiota and SCFAs Metabolism. Int. J. Biol. Macromol. 181, 357–368. doi:10.1016/j.ijbiomac.2021.03.137
Guo, Z. (2013). Investigation of the Therapeutical Mechanisms on the Ulcerative Colitis by Rheum Tanguticum Polysaccharide. Dissertation. Xi'an: Air Force Medical University.
Han, W., Li, L., Ma, S., and Yang, J. (2011). The Regulatory Effect of Lentinan on Dysbacteria in Rats with Ulcerative Colitis. Chin. J. Microecol. 23, 423–425. doi:10.13381/j.cnki.cjm.2011.05.010
Hou, C., Chen, L., Yang, L., and Ji, X. (2020). An Insight into Anti-inflammatory Effects of Natural Polysaccharides. Int. J. Biol. Macromol. 153, 248–255. doi:10.1016/j.ijbiomac.2020.02.315
Hu, P., Zhao, H., Zhang, M., Zhang, H., and Wang, Y. (2011). Structural Analysis of Three Polysaccharides RP-1,RP-2 and RP-3 Isolated from Rheum Palmatum L. Shanghai J. Tradit. Chin. Med. 45, 69–73. doi:10.16305/j.1007-1334.2011.04.029
Ishisono, K., Mano, T., Yabe, T., and Kitaguchi, K. (2019). Dietary Fiber Pectin Ameliorates Experimental Colitis in a Neutral Sugar Side Chain-dependent Manner. Front. Immunol. 10, 2979. doi:10.3389/fimmu.2019.02979
Jiao, C., Chen, W., Tan, X., Liang, H., Li, J., Yun, H., et al. (2019). Ganoderma Lucidum Spore Oil Induces Apoptosis of Breast Cancer Cells In Vitro and In Vivo by Activating Caspase-3 and Caspase-9. J. Ethnopharmacol. 247, 112256. doi:10.1016/j.jep.2019.112256
Ju, S., Mu, J., Dokland, T., Zhuang, X., Wang, Q., Jiang, H., et al. (2013). Grape Exosome-like Nanoparticles Induce Intestinal Stem Cells and Protect Mice from DSS-Induced Colitis. Mol. Ther. 21, 1345–1357. doi:10.1038/mt.2013.64
Kaenkumchorn, T., and Wahbeh, G. (2020). Ulcerative Colitis: Making the Diagnosis. Gastroenterol. Clin. North Am. 49, 655–669. doi:10.1016/j.gtc.2020.07.001
Kaushik, D. K., Thounaojam, M. C., Mitra, A., and Basu, A. (2014). Vespa Tropica Venom Suppresses Lipopolysaccharide-Mediated Secretion of Pro-inflammatory Cyto-Chemokines by Abrogating Nuclear Factor-κ B Activation in Microglia. Inflamm. Res. 63 (8), 657–665. doi:10.1007/s00011-014-0738-0
Keshteli, A. H., Madsen, K. L., and Dieleman, L. A. (2019). Diet in the Pathogenesis and Management of Ulcerative Colitis; A Review of Randomized Controlled Dietary Interventions. Nutrients 11, 1498. doi:10.3390/nu11071498
Koev, T. T., Harris, H. C., Kiamehr, S., Khimyak, Y. Z., and Warren, F. J. (2022). Starch Hydrogels as Targeted Colonic Drug Delivery Vehicles. Carbohydr. Polym. 289, 119413. doi:10.1016/j.carbpol.2022.119413
Kozik, A. J., Nakatsu, C. H., Chun, H., and Jones-Hall, Y. L. (2019). Comparison of the Fecal, Cecal, and Mucus Microbiome in Male and Female Mice after TNBS-Induced Colitis. PLoS. One. 14, e0225079. doi:10.1371/journal.pone.0225079
Kubota, M., Kakimoto, K., Nakagawa, T., Koubayashi, E., Nakazawa, K., Tawa, H., et al. (2019). Autophagy Deficiency Exacerbates Colitis through Excessive Oxidative Stress and Mapk Signaling Pathway Activation. PLoS ONE 14, e0225066. doi:10.1371/journal.pone.0225066
Le Berre, C., Roda, G., Nedeljkovic Protic, M., Danese, S., and Peyrin-Biroulet, L. (2020). Modern Use of 5-aminosalicylic Acid Compounds for Ulcerative Colitis. Expert. Opin. Biol. Ther. 20, 363–378. doi:10.1080/14712598.2019.1666101
Lee, S. D. (2020). Health Maintenance in Ulcerative Colitis. Gastroenterol. Clin. North Am. 49, xv–xvi. doi:10.1016/j.gtc.2020.09.002
Li, X., and Liu, Z. (2005). Effects of Angelica Sinensis Polysaccharides on the Immunological Funtion of the Rats with Colitis. Pract. Clin. Med. 07, 11–13+16. doi:10.3969/j.issn.1009-8194.2005.07.004
Li, Y., Liu, L., Niu, Y., Feng, J., Sun, Y., Kong, X., et al. (2012). Modified Apple Polysaccharide Prevents against Tumorigenesis in a Mouse Model of Colitis-Associated Colon Cancer: Role of Galectin-3 and Apoptosis in Cancer Prevention. Eur. J. Nutr. 51, 107–117. doi:10.1007/s00394-011-0194-3
Li, J., Wang, T., Zhu, Z., Yang, F., Cao, L., and Gao, J. (2017). Structure Features and Anti-gastric Ulcer Effects of Inulin-type Fructan CP-A from the Roots of Codonopsis Pilosula (Franch.) Nannf. Molecules. 22, 2258. doi:10.3390/molecules22122258
Li H, H., Chen, L., Li, Y., Li, Q., and Liu, S. (2020). Effect of astragalus Polysaccharide on Intestinal Mucosal Immunity in Immunosuppressed Mice. Chin. J. Clin. Pharmacol. 36, 3465–3468. doi:10.13699/j.cnki.1001-6821.2020.21.017
Li J, J., Wang, Y., Zhang, X., Cao, L., Ji, J., Zheng, Q., et al. (2020). Isolation and Structural Identification of a Novel Fructan from Radix Codonopsis. J. Carbohydr. Chem. 39, 163–174. doi:10.1080/07328303.2020.1772278
Liang, J., Zheng, K., and Sun, L. (2013). Explore the Regulative Action of Astragalus Polysaccharide for Intestinal Dysbacteriosis in Ulcerative Colitis Rat Models. Stud. Trace Elem. Health 02, 1–3. CNKI:SUN:WYJK.0.2013-02-003.
Liang, Z. (2019). Structural Characterization of Fermented Astragalus Membranaceus Polysaccharides and its Improvement Effect on Intestinal Mucosal Immunity in Mice. Dissertation. Hangzhou: Chinese Academy of Agricultural Sciences.
Liu, S., Dong, W., Wu, D., Xu, Y., Luo, H., and Yu, B. (2003). Study on the Protective Effects of Angelica Sinensis Polysaccharides on the Colon Injury in Immunological Colitis Rats. Chin. Pharmacol. Bull. 06, 693–696. doi:10.3321/j.issn:1001-1978.2003.06.026
Lu, X., Yue, H., Liu, Y., Liu, X., Liu, D., Huang, X., et al. (2018). Regulation of Astragalus Polysaccharides on the Expression of Costimulatory Molecules on the Surface of Dendritic Cells in Mice with Colitis. Chin. Tradi. Pat. Med. 40, 2296–2298. doi:10.3969/j.issn.1001-1528.2018.10.037
Luo, T., Qin, J., Liu, M., Luo, J., Ding, F., Wang, M., et al. (2015). Astragalus Polysaccharide Attenuates Lipopolysaccharide-Induced Inflammatory Responses in Microglial Cells: Regulation of Protein Kinase B and Nuclear Factor-Κb Signaling. Inflamm. Res. 64, 205–212. doi:10.1007/s00011-015-0798-9
Maul, J., and Zeitz, M. (2012). Ulcerative Colitis: Immune Function, Tissue Fibrosis and Current Therapeutic Considerations. Langenbecks Arch. Surg. 397, 1–10. doi:10.1007/s00423-011-0789-4
Miao, X. P., Sun, X. N., Cui, L. J., Cao, Q. F., Zhuang, G. F., Deng, T. Z., et al. (2015). Suppressive Effect of Pectic Polysaccharides Extracted from Rauwolfia Verticillata (Lour.) Baill.var.Hainanensis Tsiang on Inflammation by Regulation of NF- κB Pathway and Interleukin-17 in Mice with Dextran Sulphatesodium-Induced Ulcerative Colitis. Asian. Pac. J. Trop. Med. 8, 147–152. doi:10.1016/S1995-7645(14)60306-0
Monif, G. R. G. (2019). Is Ulcerative Colitis a Disease of a Dysfunctional Microbiota? Med. Hypotheses 131, 109300. doi:10.1016/j.mehy.2019.109300
Roselli, M., and Finamore, A. (2020). Use of Synbiotics for Ulcerative Colitis Treatment. Curr. Clin. Pharmacol. 15, 174–182. doi:10.2174/1574884715666191226120322
Sun, Y., Zhang, Z., Zheng, C. Q., and Sang, L. X. (2021). Mucosal Lesions of the Upper Gastrointestinal Tract in Patients with Ulcerative Colitis: A Review. World J. Gastroenterol. 27, 2963–2978. doi:10.3748/wjg.v27.i22.2963
Tahan, G., Aytac, E., Aytekin, H., Gunduz, F., Dogusoy, G., Aydin, S., et al. (2011). Vitamin E Has a Dual Effect of Anti-inflammatory and Antioxidant Activities in Acetic Acid-Induced Ulcerative Colitis in Rats. Can. J. Surg. 54, 333–338. doi:10.1503/cjs.013610
Tang, S., Liu, W., Zhao, Q., Li, K., Zhu, J., Yao, W., et al. (2021). Combination of Polysaccharides from Astragalus Membranaceus and Codonopsis Pilosula Ameliorated Mice Colitis and Underlying Mechanisms. J. Ethnopharmacol. 264, 113280. doi:10.1016/j.jep.2020.113280
Tian, X., Zhao, X., Wu, Y., Tan, Y., Hu, J., and Zheng, G. (2016). Preventive and Therapeutic Effect of Banqiao Codonopsis Radix Polysaccharide against Ulcerative Colitis in Rats. Chin. J. Exp. Tradi. Med. Form. 22, 107–112. doi:10.13422/j.cnki.syfjx.2016100107
Wang, Z.-P., Zhang, R., Liu, L., Mei, Q.-B., and Liu, L.-N. (2006). Effects of Rhubarb Polysacchrides on Apoptosis of Colonic Epithelial Cells and Peripheral Blood Polymorphonuclear Neutrophils in Mice with Ulcerative Colitis. World China J. Digestol. 14, 29–34. CNKI:SUN:XXHB.0.2006-01-006. doi:10.11569/wcjd.v14.i1.29
Wang, Y., Liu, X. P., Zhao, Z. B., Chen, J. H., and Yu, C. G. (2011). Expression of CD4+ Forkhead Box P3 (FOXP3)+ Regulatory T Cells in Inflammatory Bowel Disease. J. Dig. Dis. 12, 286–294. doi:10.1111/j.1751-2980.2011.00505.x
Wang, P. C., Zhao, S., Yang, B. Y., Wang, Q. H., and Kuang, H. X. (2016). Anti-diabetic Polysaccharides from Natural Sources: A Review. Carbohydr. Polym. 148, 86–97. doi:10.1016/j.carbpol.2016.02.060
Wang, D., Zhang, Y., Yang, S., Zhao, D., and Wang, M. (2018). A Polysaccharide from Cultured Mycelium of Hericium erinaceus Relieves Ulcerative Colitis by Counteracting Oxidative Stress and Improving Mitochondrial Function. Int. J. Biol. Macromol. 125, 572–579. doi:10.1016/j.ijbiomac.2018.12.092
Wang, Y., Zhang, N., Kan, J., Zhang, X., Wu, X., Sun, R., et al. (2019). Structural Characterization of Water-Soluble Polysaccharide from Arctium Lappa and its Effects on Colitis Mice. Carbohydr. Polym. 213, 89–99. doi:10.1016/j.carbpol.2019.02.090
Wang, Z., Liang, Y., Zhang, D., Wu, X., Yu, J., Zhang, Z., et al. (2020). Protective Effects of Polysaccharide Extracted from Portulacae Oleracea L. On Colitis Induced by Dextran Sulfate Sodium. J. Med. Food. 23, 125–131. doi:10.1089/jmf.2019.4414
Wang, J., Wang, J., Li, Y., Ren, M., Fu, Y., Yang, X., et al. (2021). Research Progress of Effect of Berberine in Treatment of Ulcerative Colitis Based on Cell Signaling Pathway. Chin. J. Chin. Mat. Med. 46, 33–40. doi:10.19540/j.cnki.cjcmm.20201002.601
Wang, L. (2017). Extraction and Isolation of Polysaccharide from Portula Caoleracea and Evaluation of its Therapeutic Effect on Ulcerative Colitis and its Mechanisms. Dissertation. Xianyang: Shaanxi University of Chinese Medicine.
Wang, Y. (2021). Structural Characterization and Immunomodulatory Activity of Codonopsis Polysaccharide CPC. Dissertation. Taiyuan: Shanxi Medical University.
Wei, J., and Feng, J. (2010). Signaling Pathways Associated with Inflammatory Bowel Disease. Recent Pat. Inflamm. Allergy. Drug Discov. 4 (2), 105–117. doi:10.2174/187221310791163071
Wen, X., Wang, H. G., Zhang, M. N., Zhang, M. H., Wang, H., and Yang, X. Z. (2021). Fecal Microbiota Transplantation Ameliorates Experimental Colitis via Gut Microbiota and T-Cell Modulation. World J. Gastroenterol. 27, 2834–2849. doi:10.3748/wjg.v27.i21.2834
Qinhua, X., Yajie, T., Yueqin, L., Juan, D., Guangbo, Z., and Weichang, C. (2019). Empirical Study on the Therapeutic Effect of Astragalus Polysaccharides in a Mouse Model of Ulcerative Colitis. Am. J. Biom. Life Sci. 7, 143. doi:10.11648/j.ajbls.20190706.13
Xiang, Q., Zhang, W., Li, Q., Zhao, J., Feng, W., Zhao, T., et al. (2020). Investigation of the Uptake and Transport of Polysaccharide from Se-Enriched Grifola Frondosa in Caco-2 Cells Model. Int. J. Biol. Macromol. 24, S0141–S8130. doi:10.1016/j.ijbiomac.2020.04.160
Xue, M., Zheng, Y., Zhang, Y., Tao, Q., Yang, H., and Yin, D. (2022). Synergistic Effect of Crude Coptis Polysaccharide and Berberine on Restoring the Intestinal Mucosal Barrier Damage in Ulcerative Colitis. Chin. J. Exp. Tradi. Med. Form., 1–7. doi:10.13422/j.cnki.syfjx.20220702
Yan, P. G., and Li, J. N. (2021). Standard Diagnosis and Treatment of Ulcerative Colitis. Chin. J. Intern. Med. 60, 567–570. doi:10.3760/cma.j.cn112138-20210316-00216
Yan, H., Xie, Y., Sun, S., Sun, X., Ren, F., Shi, Q., et al. (2010). Chemical Analysis of Astragalus Mongholicus Polysaccharides and Antioxidant Activity of the Polysaccharides. Carbohydr. Polym. 82, 636–640. doi:10.1016/j.carbpol.2010.05.026
Yan, Y., Wang, Z., Guo, M., Zhou, S., and Wang, J. (2019). Effect of Radix Hedysari on Mice Ulcerative Colitis and its Material Basis. China J. Clin. Pharmacol. 35, 2698–2701. doi:10.13699/j.cnki.1001-6821.2019.21.014
Yang, F., Xiao, C., Qu, J., and Wang, G. (2016). Structural Characterization of Low Molecular Weight Polysaccharide from Astragalus Membranaceus and its Immunologic Enhancement in Recombinant Protein Vaccine against Systemic Candidiasis. Carbohydr. Polym. 145, 48–55. doi:10.1016/j.carbpol.2016.03.024
Yashiro, M. (2014). Ulcerative Colitis-Associated Colorectal Cancer. World J. Gastroenterol. 20, 16389–16397. doi:10.3748/wjg.v20.i44.16389
Zang, K., Li, Y., Zhu, L., Li, T., Chen, L., Yue, J., et al. (2018). Study on the Repair Effect on Colon Mucosa and Mechanism of astragalus Polysaccharides in Rats with Ulcerative Colitis. J. Gansu Univ. Chin. Med. 35, 5–10. doi:10.16841/j.issn1003-8450.2018.03.02
Zeng, P., Li, J., Chen, Y., and Zhang, L. (2019). The Structures and Biological Functions of Polysaccharides from Traditional Chinese Herbs. Prog. Mol. Biol. Transl. Sci. 163, 423–444. doi:10.1016/bs.pmbts.2019.03.003
Zhang, R., Wang, Z., Liu, L., and Mei, Q. (2006). Effects of Rheum Tanguticum Polysaccharides on Ulcerative Colitis Induced by TNBS in Mice. Chin. J. Clin. Pharmacol. Ther. 11, 995–998. doi:10.3969/j.issn.1009-2501.2006.09.008
Zhang, J., Liu, Y., Tang, Q., Zhou, S., Feng, J., and Chen, H. (2019). Polysaccharide of Ganoderma and its Bioactivities. Adv. Exp. Med. Biol. 1181, 107–134. doi:10.1007/978-981-13-9867-4_4
Zhang, X., Liu, Z., Zhong, C., Pu, Y., Yang, Z., and Bao, Y. (2021). Structure Characteristics and Immunomodulatory Activities of a Polysaccharide RGRP-1b from Radix Ginseng Rubra. Int. J. Biol. Macromol. 189, 980–992. doi:10.1016/j.ijbiomac.2021.08.176
Zhang, Y. (2019). Study on the Preparation of Radix Hedysari Polysaccharide HG-2 and the Effect of Hyaluronic Acid Hydrogel on Osteoarthritis in Rats. Dissertation. Lanzhou: Gansu University of Chinese Medicine.
Zhao, H. M., Wang, Y., Huang, X. Y., Huang, M. F., Xu, R., Yue, H. Y., et al. (2016). Astragalus Polysaccharide Attenuates Rat Experimental Colitis by Inducing Regulatory T Cells in Intestinal Peyer's Patches. World J. Gastroenterol. 22, 3175–3185. doi:10.3748/wjg.v22.i11.3175
Zhao, H., Liu, X., Yue, H., Liu, Y., Liu, X., Huang, X., et al. (2018). Regulation of Astragalus Polysaccharide Treated Colitis Mice by JAK/STAT Signaling Pathway. Chin. Pharmacol. Bull. 34 (01), 145–146. doi:10.3969/j.issn.1001-1978.2018.01.030
Zhao, Y., Yan, B., Wang, Z., Li, M., and Zhao, W. (2020). Natural Polysaccharides with Immunomodulatory Activities. Mini Rev. Med. Chem. 20, 96–106. doi:10.2174/1389557519666190913151632
Zheng, Z., and Wang, J. (2022). Bone Marrow Mesenchymal Stem Cells Combined with Atractylodes Macrocephala Polysaccharide Attenuate Ulcerative Colitis. Bioengineered 13 (1), 824–833. doi:10.1080/21655979.2021.2012954
Zhou, S., Guo, M., Wang, J., Yan, Y., Zhang, Y., and Shao, J. (2021a). Preparation and Structural Characteristics of Hedysari Radix Polysaccharides and its Effects on 1,1-Diphenyl-2-Picrylhydrazyl Free Radical Scavenging Ability. J. Gansu Univ. Chin. Med. 38, 1–6. doi:10.16841/j.issn1003-8450.2021.04.01
Zhou, S., Guo, M., Wang, Z., Wang, J., Yan, Y., and Zhang, Y. (2021b). Protective Effect of Hedysari Polysaccharides with Different Molecular Weights on Ulcerative Colitis Mice. Chin. J. Clin. Pharmacol. 37, 1346–1350. doi:10.13699/j.cnki.1001-6821.2021.11.011
Zhou, S. (2021). Study on the Preparation of Radix Hedysari Polysaccharide with Different Molecular Weights and its Effect on UC Mice. Dissertation. Lanzhou: Gansu University of Chinese Medicine.
Zhu C, C., Zhang, S., Song, C., Zhang, Y., Ling, Q., Hoffmann, P. R., et al. (2017). Selenium Nanoparticles Decorated with Ulva Lactuca Polysaccharide Potentially Attenuate Colitis by Inhibiting NF-Κb Mediated Hyper Inflammation. J. Nanobiotechnology 15, 20. doi:10.1186/s12951-017-0252-y
Zhu ZY, Z. Y., Zhang, J. Y., Liu, F., Chen, L., Chen, L. J., and Tang, Y. (2017). Characterization and Lymphocyte Proliferation Activity of an Oligosaccharide Degraded from Astragalus Polysaccharide. Medchemcomm 8, 1521–1530. doi:10.1039/c7md00148g
Keywords: natural polysaccharides, ulcerative colitis, structure, mechanism, gut flora
Citation: Guo Y, Li Y, Cao Q, Ye L, Wang J and Guo M (2022) The Function of Natural Polysaccharides in the Treatment of Ulcerative Colitis. Front. Pharmacol. 13:927855. doi: 10.3389/fphar.2022.927855
Received: 05 May 2022; Accepted: 02 June 2022;
Published: 04 July 2022.
Edited by:
Qian Feng, Chongqing University, ChinaReviewed by:
Jiang Meng, Guangdong Pharmaceutical University, ChinaDi Duolong, Lanzhou Institute of Chemical Physics, (CAS), China
Copyright © 2022 Guo, Li, Cao, Ye, Wang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mei Guo, Z3VvbWVpQGdzenkuZWR1LmNu
†These authors have contributed equally to this work
 Yafei Guo
Yafei Guo Yang Li1†
Yang Li1† Qiang Cao
Qiang Cao Mei Guo
Mei Guo