
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Pharmacol. , 25 August 2022
Sec. Pharmacology of Anti-Cancer Drugs
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.927083
U0126, as an inhibitor of the MAPK signaling pathway, is closely related to various biological processes, such as differentiation, cell growth, autophagy, apoptosis, and stress responses. It makes U0126 play an essential role in balancing cellular homeostasis. Although U0126 has been suggested to inhibit various cancers, its complete mechanisms have not been clarified in cancers. This review summarized the most recent and relevant research on the many applications of U0126 and described its role and mechanisms in different cancer cell types. Moreover, some acknowledged functions of U0126 researched in the laboratory were listed in our review. We discussed the probability of using U0126 to restain cancers or suppress the MAPK pathway as a novel way of cancer treatment.
MAPK signaling pathway plays a vital role in cancer cell dissemination, proliferation, and drug resistance (De Luca et al., 2012). MAPK pathways were composed mainly of four families: 1) MAPK/ERK family or classical pathway; 2) Big MAP kinase-1(BMK-1); 3) c-Jun N-terminal kinase (JNK); 4) p38 signaling families (De Luca et al., 2012; Cossa et al., 2013) (Figure 1). In MAPK/ERK family, the carcinogenesis of ERK1/2 relates to upstream the activation of ERK1/2, which includes overexpression of RTKs (receptor tyrosine kinases) (Lu and Xu, 2006; Low and Zhang, 2016). Aberrant ERK1/2 activation is existed in various malignancies, including renal cell carcinoma, hepatocellular carcinoma, and gastric adenocarcinoma. The carcinogenesis of JNK mainly depends on the process of the phosphorylated c-Jun and activated AP-1 induced by JNK (Cellurale et al., 2011). JNK has two different proteins, JNK1 and JNK2, which make the JNK pathway dual role in cancer cells. Many studies have indicated that the JNK pathway can exert pro- and anti-oncogenic effects in different cancers and stages of cancer development (Wagner and Nebreda, 2009). In addition, JNK and p38 collectively have upstream activators and synergistically influence cancer cell survival (Svensson et al., 2011; Ruan et al., 2015). Recent studies have verified that increased phosphorylated p38 has been linked to various malignant tumors such as lung cancer, thyroid cancer, breast carcinoma, follicular lymphoma, and glioma. Nevertheless, the effect of p38 on cancer is complex and controversial at this stage (Wagner and Nebreda, 2009; Low and Zhang, 2016).
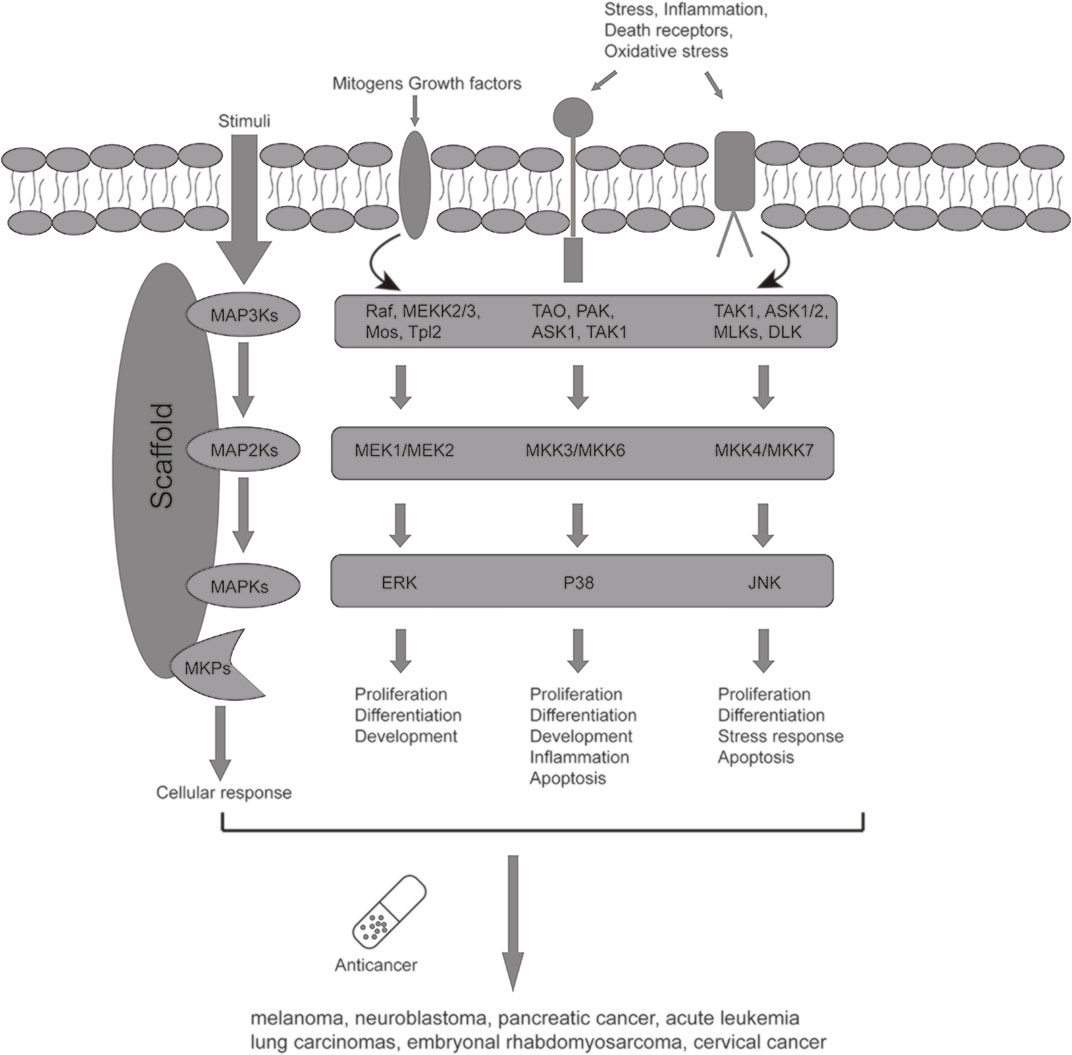
FIGURE 1. The known MAPK signaling pathways downstream target cell receptor signaling, working cooperatively to regulate cell physiology.
Over the last few years, U0126, as a signal transduction inhibitor of the MAPK (mitogen-activated protein kinase) pathway, has become the focus of relevant studies because of its impact on the development of malignancies (Ma et al., 2013). Many reports have shown that U0126 can inhibit tumor proliferation and enhance the anticancer effects of drugs or gene silencing treatments (Fukazawa et al., 2002; Wiesenauer et al., 2004; Marampon et al., 2006; Takayama et al., 2008; Marampon et al., 2009; Ito et al., 2010). U0126 mainly targets the RAF/MEK/ERK pathway in animal cells (Favata et al., 1998). It inhibits the activation of ERK1/2 by blocking the activation of the upstream MEK1/MEK2 and affecting p38 MAPK activity (Duncia et al., 1998; Wang and Studzinski, 2001; Hotokezaka et al., 2002). The RAF/MEK/ERK pathway and p38 pathway belong to MAPK signal pathways involving cell survival, differentiation, proliferation, apoptosis, and stress adaptation (Pagès et al., 1993; Matsumoto et al., 2002; Lefloch et al., 2008; Lefloch et al., 2009; Wu et al., 2011).
In this review, we described the role of U0126 in the MAPK signaling pathway and its biological activities, such as apoptosis, cell survival, and autophagy. At the same time, we concluded some new applications of this inhibitor from its molecular mechanism. Furthermore, we describe some well-recognized functions of U0126 stayed in the laboratory. Finally, we discussed the role of U0126 in different types of cancers and emphasized it as a potential ant-cancer drug that can improve the therapeutic effect on tumors.
W. J. Middleton first synthesized U0126 in the late 1950s. U0126 can keep the crystalline state stable even for decades. There mainly include three possible isomers of U0126: Z, Z-isomer (Figure 2A); Z, E-isomer (Figure 2B); E, E-isomer (Figure 2C). As research progresses, Stephen et al. discovered that U0126 exerts its effects on cells via suppressing the activation of MEK1 (MAPK kinase 1; also known as MKK1) and not by blocking the activity. Therefore, U0126 has been widely researched in anti-tumor research as a MAPK inhibitor, but, in addition, U0126 also has biological effects in other aspects.
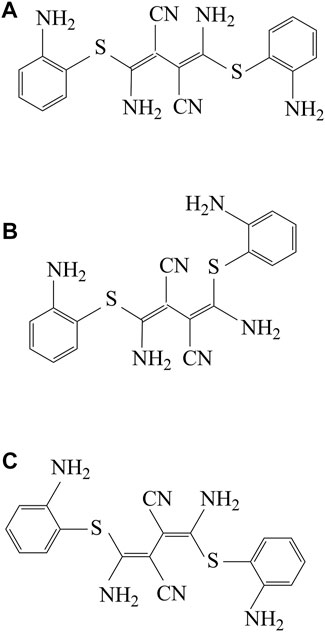
FIGURE 2. The structure and chemical characteristics of three isomers of U0126. (A) U0126, Z,Z-isomer; (B) U0126, Z,E-isomer; (C) U0126, E,E-isomer.
Our review of U0126 studies found that U0126 is more than just a MAPK inhibitor. We started from the anti-tumor aspect of U0126. We found that U0126 can affect multiple molecular signaling pathways, including MEK/ERK, JNK, KRAS, P44/42, JAK/STAT, PI3K/AKT/mTOR, and Ras/Raf/ERK signaling pathways. Moreover, U0126 can act on the following targets: ERK 1/2, MEK 1/2, C-JNK, μPA, MMP-9, P44/42, P21, p53, P27, and so on. The relevant results are summarized in Table 1. In the meantime, the roles of U0126 in several biological processes are described in detail below.
Many findings have supported that chemical inhibitors suppressing signal transduction are potent tools in exploring signaling pathways. As a MAPK inhibitor, U0126 is widely used in investigating what pathways are involved in cell responses, such as growth and differentiation. It has been demonstrated that U0126 can inhibit MEK leading to an apparent decrease of phosphorylated ERK, accelerating the differentiation of RAW264.7 cells into osteoclast-like cells (Hotokezaka et al., 2002). Moreover, Xu et al. (2015) demonstrated that U0126 could promote osteogenic differentiation of rat MSCs model by activating the BMP/Smad pathway.
Apoptosis is an important mode of cell death that is no longer needed or is a pathologic status to the organism, including nuclear chromatin condensation, cell shrinkage, and caspase activation (Patel et al., 1996; Thornberry, 1998). U0126 is a potent anti-apoptotic agent. Jo et al. found that U0126 decreases apoptosis and the activation of caspase 3 through inhibition of ERK1/2, suggesting pretreatment of U0126 has significant functional and histologic protection to attenuate cisplatin-induced renal injury (Jo et al., 2005). Interestingly, U0126 can also induce apoptosis to inhibit the proliferation of tumors. For example, U0126 can cause apoptosis in leukemic blast cells, especially in the KG1a cell line (Kerr et al., 2003). Accordingly, U0126 can potentiate or antagonize apoptosis, depending on the drug or the target cells. It is still indistinct whether these different regulations on apoptosis are due to a direct effect of U0126 or whether it is the only result of the inhibition of the MAPK pathway, or whether there exist other pathways. Therefore, further studies are needed to explore the existence of potential mechanisms of U0126 in the apoptosis pathway.
Autophagy is a process of self-degradation. Autophagosome degradation removes damaged cell organelles and misfolded or aggregated proteins (Glick et al., 2010). This pathway is important for limiting tumor initiation because it can inhibit genomic instability and oxidative stress (Eskelinen, 2011). Cells can use this process to balance energy sources in response to nutrient stress such as serum starvation or glucose (Glick et al., 2010). Interestingly, many studies have reported that U0126 is involved in suppressing autophagy. Wang et al. verified that U0126 inhibits cisplatin-induced autophagy in HEI-OC1 and cochlear hair cells (Wang et al., 2021). Moreover, Wang et al. found that U0126 reduces ischemia/reperfusion-induced autophagy in the myocardium (Wang et al., 2016). However, there is no evidence of the activation of autophagy by U0126. Due to limited data, the effect of U0126 on autophagy needs further research, especially in different cells.
Inflammation occurs when tissues respond to injury. The various cell types’ expression and diverse mediators’ reactions both play a role in inflammation. Significantly, The ERK 1/2 pathway, as the most extensively occurred member of the MAPK pathway, is related to inflammation (Mohammad et al., 2013). U0126, a potent and selective MAPK inhibitor, can decrease ERK 1/2 activation. According to these theories, many studies have further verified that U0126 has anti-inflammatory effects. For example, U0126 reduces diabetes-induced upregulation of MMP-9 and biomarkers of inflammation in the retina (Mohammad et al., 2013). In the asthma mouse model, U0126 attenuates pulmonary eosinophilia, OVA-induced Th2 cytokine production, serum IgG1, IgE synthesis, mucus hypersecretion, and AHR to restrain allergic airway inflammation (Duan et al., 2004).
U0126 has been found to play an antiproliferative role in cancers. Interestingly, there are different antitumor mechanisms of U0126 in each tumor. To achieve clear comprehension, we summarized existing studies and listed known antitumor mechanisms of U0126 in the following section (Table 1).
Tumor cell invasiveness is a multistep process including cell adhesion, matrix proteolysis, and cell migration. The extracellular matrix’s degradation needs invasive proteases secreted by the invading cells, including uPA (urokinase plasminogen activator) and MMP (matrix metalloproteinase) (Aguirre Ghiso et al., 1999; Jesionowska et al., 2015). In human melanoma, U0126 not only inhibits phosphorylation of MEK 1/2 and ERK 1/2 but also decreases the expression of c-Jun, a significant component of the transcription factor AP-1 (Ge et al., 2002). Because the gene promoter regions of uPA and MMP-9 contain AP-1, the decrease of c-Jun suppresses the expression of uPA and MMP-9. Therefore, U0126 can significantly inhibit melanoma invasion via decreasing uPA and MMP-9 concentrations (De Petro et al., 1998; Lakka et al., 2000).
There are three unique cell phenotypes in neuroblastoma cells: neuroblastic (N-type), intermediate (I-type), and substrate-adherent (S-type) (Ross et al., 2003). Those cell types differentiate into another type in culture, and the proportions of each type of tumor are different (Ross and Spengler, 2004). Because cell growth and differentiation involve the MAPK pathway, many researchers believe in the feasibility of MAPK-targeted therapies in tumors (Giroux et al., 1999; Mattingly et al., 2001; Ross and Spengler, 2004). Eppstein et al. (2006) found that all three cell types exhibit the expression of p-ERK decreased after processing by MEK inhibition. However, only I-type cells exhibit significantly decreased proliferation with U0126 treatment. Although U0126 has the promise of targeting I-type cells, neuroblastoma treatments may need to combine agents against N-type and S-type cells. It may be a new point to explore tumor-targeted therapeutic strategies further.
Pancreatic cancer is a common malignancy worldwide, with a median survival time of fewer than 6 months (Hidalgo, 2010). Several targeted therapies have been used in researching pancreatic cancer (Vaccaro et al., 2011). Pancreatic cancer cell growth depends on the activity of the mutated KRAS gene. Therefore, silencing the KRAS gene can control pancreatic cancer cell line proliferation (Réjiba et al., 2007). It makes the components in the KRAS pathway become promising targets for identifying novel therapies (McCubrey et al., 2007). In addition, the ERK signaling pathway is not regulated in pancreatic carcinoma cells despite KRAS gene expression, and the reason is that increased MKP-2 (MAP kinase phosphatase-2) inactivates ERK. The results showed that both KARs gene expression and the MAPK-ERK pathways are involved in the occurrence of pancreatic cancer. Similarly, if the MEK-ERK signaling pathway is necessary for the growth of the pancreatic cell, it may be a potential therapeutic target alone or with other cellular pathways (Yip-Schneider et al., 1999; Yip-Schneider et al., 2001).
Interestingly, these hypotheses have been confirmed in subsequent studies. For example, U0126 effectively controls pancreatic cancer cell line proliferation via targeting the downstream effectors of KRAS signaling in a zebrafish xenotransplantation model (Guo et al., 2015). In addition, Yip-Schneider and Schmidt (2003) confirmed that U0126 dependently inhibits the growth of three human pancreatic carcinoma cell lines (PANC-1, BxPC-3, and MIA PaCa-2).
It is challenging to diagnose gallbladder cancer in clinical practice because the symptoms and the manifestations of gallbladder cancer are similar to benign gallbladder disease. Therefore, most cases of gallbladder cancer are found at an advanced stage, accompanied by metastases to the lymph nodes, invasion of the liver, and distant organs. Most of the time, the tumor is unresectable despite radical surgery (Mizuguchi et al., 1997; Levin, 1999). Oncogenic mutation of KRAS is associated with gallbladder carcinogenesis (Watanabe et al., 1994; Ajiki et al., 1996; Hidaka et al., 2000). Activation of KRAS can induce the constitutive activation of the MAPK pathway and PI3K-AKT pathway, which rapidly develops the growth of the gallbladder epithelium (Leevers and Marshall, 1992; Thomas et al., 1992; Rubio et al., 1997). Furthermore, point mutation of P53 is also related to gallbladder cancer carcinogenesis (Hanada et al., 1997; Watanabe et al., 1999). Therefore, U0126 as a MAPK inhibitor has the potential to inhibit gallbladder cancer proliferation. Moreover, a recent study has verified that U0126 observably prolongs the survival duration of mice with gallbladder tumors. The major organs such as kidneys, liver, small intestine, colon, stomach, brain, lungs, and heart do not have apparent histopathological abnormality after U0126 treatment in mice bearing gallbladder cancer cells with KRAS mutation (Horiuchi et al., 2003). However, the underlying mechanisms of U0126 inhibiting gallbladder cancer need to be further explored.
The control of cell proliferation, differentiation, and apoptosis depends on the balance between a series of signaling cascades. In acute leukemia, this delicate balance is frequently deranged. Blockade of proliferative pathways by inhibiting MEK is growth inhibitory or pro-apoptotic in some acute myeloblastic leukemia (AML) cell lines and some AML patients (Berra et al., 1998; Jarvis et al., 1998; Milella et al., 2001; Morgan et al., 2001; Baines et al., 2002). It has been reported that the MEK inhibitor U0126 induces significant levels of apoptosis in three acute leukemia cell lines, KG1a, THP-1, and M-07e (James et al., 2003). Although the sensitivity of different cell lines is variable, U0126 seems to offer a potential alternative to standard chemotherapeutic agents in treating acute leukemia.
A recent study has reported that U0126 inhibits chemically-induced pulmonary carcinomas’ growth and improves tumor-free survival rates in mice with inoculated lung carcinomas. Among them, the antitumor effect of U0126 mainly depends on the activation of IFN-γ production (Ma et al., 2015). IFNs (Interferons) are a family of pleiotropic cytokines including three major groups: Type I, Type II, and Type III IFNs (Maher et al., 2007). IFN-γ is the sole member of Type II IFN, and it has multiple biological functions in defense and immune systems, just like the antiviral, antimicrobial, antiproliferative, and antitumor activity (Schroder et al., 2004; Schoenborn and Wilson, 2007). However, more and more evidence has shown that IFN-γ can also induce tumor progression. It makes the role of IFN-γ in regulating antitumor immunity appear complex and paradoxical. Related literature reports that IFN-γ can promote lung cancer progression via the JAK/STAT3 signaling pathway and PI3K/AKT signaling pathway in lung carcinomas (Zhang et al., 2017). Thus it can be seen that the relationship between U0126 and INF-γ and the effect of INF-γ on lung cancer are skeptical. Therefore, we think that the role of U0126 in lung cancer is unclear.
It is worth noting that if U0126 has an inhibitory effect on lung cancer, it may be associated with inhibition of cell cycle and proliferation. The explanation is as follows: U0126 can exert its effects on G0-G1 arrest via up-regulating p21, p53, and p27. Meanwhile, cyclin D1 and cyclin E1 are down-regulation (Zou et al., 2012).
In childhood, RMS (malignant tumors of skeletal muscle rhabdomyosarcoma) is the most common soft-tissue sarcoma (Merlino and Helman, 1999). C-Myc, N-Myc, and MYCL1 play an important role in human cancer (Adhikary and Eilers, 2005). In conditional transgenic models on Myc inactivation, tumors can regress (Jain et al., 2002; Shachaf et al., 2004). Ras mutation activating MEKs and ERKs occurs in various tumors (Kohno and Pouyssegur, 2003; Faivre et al., 2006; Liu et al., 2007). C-Myc is targeted by ERKs that stabilize C-Myc, whereas GSK-3β induces C-Myc degradation (Sears et al., 2000; Yeh et al., 2004). Ras activation can induce chronic MEK/ERK activation and phosphatidylinositol 3-kinase/AKT-mediated GSK-3β inactivation leading to C-Myc aberrant accumulation (Bachireddy et al., 2005). According to the above basic theory, Marampon et al. used the MEK/ERK inhibitor U0126 and embryonal rhabdomyosarcoma cell line-xenotransplanted mice to verify whether MEK//ERK inhibitions affect C-Myc protein level and growth of RMS tumor. They found that U0126 significantly reduces RMS tumor growth via disrupting C-Myc (Marampon et al., 2009). Thus, U0126 could be used in a signal transduction-based therapy for RMS and warrants testing in RMS trials.
Cervical cancer is a common malignant tumor in females (Chan and So, 2015). ERK1/2 signaling pathway plays an essential role in cervical cancer differentiation (Chang et al., 2014). ERK 1/2 is expressed in cervical cancer tissues in cytoplasm and nuclei (Kake et al., 2017). Moreover, ERK can facilitate cancer cell growth via promoting cell movement from the G1 phase to the S phase. U0126, an ERK inhibitor, can decrease cell content in the S phase, which may restrain breast cancer proliferation by blocking the cell cycle (Zhao et al., 2009). In addition, U0126 can induce apoptosis to suppress cervical cancer. The induced-apoptosis mechanism of U0126 in cervical cancer relates to the inhibition of the ERK signaling pathway and the suppression of the JAK-STAT pathway (Zhao et al., 2009; Ye et al., 2017) .(de Tomaso Portaz et al., 2015)
Currently, the effects of U0126 are limited in treating human cancers unless particular cancer proliferates mainly relies on the MAPK signaling pathway. Moreover, the anticancer effects of U0126 often depend on cytostatic rather than cytotoxic. Although it is an effective anticancer agent in a single treatment setting, existing research is restricted to a laboratory experiment state.
However, many studies have confirmed that U0126 may be more effective when combined with chemotherapies or radiotherapies (Yacoub et al., 2003; Gao et al., 2005; Ito et al., 2010). It means U0126 could overcome the resistance to chemotherapeutic drugs (Shi et al., 2014). Thus, combination therapy with either a traditional drug/physical treatment or U0126 is also a meaningful way to improve the effectiveness and usefulness of U0126.
As the technology develops, more and more data support the role of molecular signaling pathways in cancer biology. While on a single tumor, molecular “tailored” treatment is still the most ambitious goal, but to build a new, based on the combination mechanism, may bypass the escape mechanism, and in patients with relatively not choose groups to overcome the resistance of single channel inhibitors, seems to have in our ability range, and possible treatment of substantial progress soon. In general, MEK inhibitors are well tolerated, with only rash edema and transient blurred vision being common side effects. Importantly, plasma concentrations of each compound are sufficient to inhibit MEK in vitro tumor tissues. Although the concentration of U0126 is relatively high in vitro, it is still an optimistic assumption that subsequent studies can combine U0126 with other drugs to achieve the maximum tumor suppressive effect at the minimum concentration and apply it in clinical practice.
Researchers and doctors sometimes have a purposely narrow view of a particular topic. For example, cancer researchers think that U0126 can suppress the growth of cancer cells. Nevertheless, U0126 may also help treat inflammatory and tissue injury with abnormal cellular proliferation (Clemons et al., 2002; Cho et al., 2004; Christensen et al., 2019). These research topics, such as ischemic brain injury (Farrokhnia et al., 2008), myocardial ischemia (Wang et al., 2016), and asthma (Duan et al., 2004), significantly improve the potential clinical uses of U0126.
In summary, U0126, a drug discovered a long time ago, has been reported to have anti-tumor effects, but it may not be paid too much attention due to force majeure reasons. With the development of science and technology, we do not want U0126 to be buried in history. Therefore, in this article, we will review its functions and mechanisms again, hoping to arouse people’s attention. If combined with the existing new technology, U0126 can solve the previous legacy problems and become a powerful tool not only for anti-tumor but also for treating other diseases. We believe it will provide hope for the majority of patients.
In this review, we described that U0126 is an inhibitor in the cell proliferation of many cancers, mainly through its role in blocking the MAPK pathway. If one tumor depends on the MAPK pathway, it may be sensitive to U0126. In addition, U0126 will only exhibit antitumor effects combined with cytotoxic chemotherapeutic drugs or radiation. However, recent studies also describe other mechanisms of U0126, which makes U0126 more critical to research how it can become a drug of anticancer therapies. At the same time, U0126 may also be considered for development in treating other diseases due to its ability to affect apoptosis, autophagy, and inflammation.
YY conceived and designed the study and drafted the manuscript. YY and YN acquired the data and performed data extraction. YY interpreted the data, and performed extensive research on the topic. YY performed the statistical analysis and made all tables and figures. PD, JZ, and SH checked the data. XW funded the project. FS and XW supervised the project. All authors contributed to the accomplishment of the manuscript.
The study was supported by the General project of Xinhua Hospital Chongming Branch (No.2019YA-04 to XW) and Shanghai Chongming District “Sustainable Development of science and technology innovation action Plan” project plan (No. CKY2021-18 to XW).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adhikary, S., and Eilers, M. (2005). Transcriptional regulation and transformation by Myc proteins. Nat. Rev. Mol. Cell Biol. 6, 635–645. doi:10.1038/nrm1703
Aguirre Ghiso, J. A., Alonso, D. F., Farías, E. F., Gomez, D. E., and de Kier Joffè, E. B. (1999). Deregulation of the signaling pathways controlling urokinase production. Its relationship with the invasive phenotype. Eur. J. Biochem. 263, 295–304. doi:10.1046/j.1432-1327.1999.00507.x
Ajiki, T., Fujimori, T., Onoyama, H., Yamamoto, M., Kitazawa, S., Maeda, S., et al. (1996). K-ras gene mutation in gall bladder carcinomas and dysplasia. Gut 38, 426–429. doi:10.1136/gut.38.3.426
Bachireddy, P., Bendapudi, P. K., and Felsher, D. W. (2005). Getting at MYC through RAS. Clin. cancer Res. official J. Am. Assoc. Cancer Res. 11, 4278–4281. doi:10.1158/1078-0432.ccr-05-0534
Baines, P., Fisher J Fau - Truran, L., TruranFau - Davies, L. E., Davies E Fau - Hallett, M., Hallett M Fau - Hoy, T., Hoy T Fau - Burnett, A. K., et al. (2002). The MEK inhibitor, PD98059, reduces survival but does not block acute myeloid leukemia blast maturation in vitro. Eur. J. Haematol. 64, 211–218. doi:10.1034/j.1600-0609.2000.90139.x
Berra, E., Diaz-Meco Mt Fau - Moscat, J., and Moscat, J. (1998). The activation of p38 and apoptosis by the inhibition of Erk is antagonized by the phosphoinositide 3-kinase/Akt pathway. J. Biol. Chem. 273, 10792–10797. doi:10.1074/jbc.273.17.10792
Cellurale, C., Sabio, G., Kennedy, N. J., Das, M., Barlow, M., Sandy, P., et al. (2011). Requirement of c-Jun NH(2)-terminal kinase for Ras-initiated tumor formation. Mol. Cell Biol. 31, 1565–1576. doi:10.1128/MCB.01122-10
Chan, D. N., and So, W. K. (2015). A systematic review of randomised controlled trials examining the effectiveness of breast and cervical cancer screening interventions for ethnic minority women. Eur. J. Oncol. Nurs. 19, 536–553. doi:10.1016/j.ejon.2015.02.015
Chang, H., Shi, Y., Tuokan, T., Chen, R., and Wang, X. (2014). Expression of aquaporin 8 and phosphorylation of erk1/2 in cervical epithelial carcinogenesis: Correlation with clinicopathological parameters. Int. J. Clin. Exp. Pathol. 7, 3928–3937.
Cho, D. G., Mulloy, M. R., Chang, P. A., Johnson, M. D., Aharon, A. S., Robison, T. A., et al. (2004). Blockade of the extracellular signal-regulated kinase pathway by U0126 attenuates neuronal damage following circulatory arrest. J. Thorac. Cardiovasc Surg. 127, 1033–1040. doi:10.1016/j.jtcvs.2003.09.038
Christensen, S. T., Johansson, S. E., Radziwon-Balicka, A., Warfvinge, K., Haanes, K. A., and Edvinsson, L. (2019). MEK1/2 inhibitor U0126, but not nimodipine, reduces upregulation of cerebrovascular contractile receptors after subarachnoid haemorrhage in rats. PloS one 14, e0215398. doi:10.1371/journal.pone.0215398
Clemons, A. P., Holstein, D. M., Galli, A., and Saunders, C. (2002). Cerulein-induced acute pancreatitis in the rat is significantly ameliorated by treatment with MEK1/2 inhibitors U0126 and PD98059. Pancreas 25, 251–259. doi:10.1097/00006676-200210000-00007
Cossa, G., Gatti, L., Cassinelli, G., Lanzi, C., Zaffaroni, N., and Perego, P. (2013). Modulation of sensitivity to antitumor agents by targeting the MAPK survival pathway. Curr. Pharm. Des. 19, 883–894. doi:10.2174/138161213804547187
De Luca, A., Maiello, M. R., D'Alessio, A., Pergameno, M., and Normanno, N. (2012). The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: Role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin. Ther. Targets 16 Suppl 2 (2), S17–S27. doi:10.1517/14728222.2011.639361
De Petro, G., Tavian, D., Copeta, A., Portolani, N., Giulini, S. M., and Barlati, S. (1998). Expression of urokinase-type plasminogen activator (u-PA), u-PA receptor, and tissue-type PA messenger RNAs in human hepatocellular carcinoma. Cancer Res. 58, 2234–2239.
de Tomaso Portaz, A. C., Caimi, G. R., Sánchez, M., Chiappini, F., Randi, A. S., Kleiman de Pisarev, D. L., et al. (2015). Hexachlorobenzene induces cell proliferation, and aryl hydrocarbon receptor expression (AhR) in rat liver preneoplastic foci, and in the human hepatoma cell line HepG2. AhR is a mediator of ERK1/2 signaling, and cell cycle regulation in HCB-treated HepG2 cells. Toxicology 336, 36–47. doi:10.1016/j.tox.2015.07.013
Duan, W., Chan, J. H., Wong, C. H., Leung, B. P., and Wong, W. S. (2004). Anti-inflammatory effects of mitogen-activated protein kinase kinase inhibitor U0126 in an asthma mouse model, J. Immunol. 172. 7053–7059. doi:10.4049/jimmunol.172.11.7053
Duncia, J. V., Santella, J. B., Higley, C. A., Pitts, W. J., Wityak, J., Frietze, W. E., et al. (1998). MEK inhibitors: The chemistry and biological activity of U0126, its analogs, and cyclization products. Bioorg. Med. Chem. Lett. 8, 2839–2844. doi:10.1016/s0960-894x(98)00522-8
Eppstein, A. C., Sandoval, J. A., Klein, P. J., Woodruff, H. A., Grosfeld, J. L., Hickey, R. J., et al. (2006). Differential sensitivity of chemoresistant neuroblastoma subtypes to MAPK-targeted treatment correlates with ERK, p53 expression, and signaling response to U0126. J. Pediatr. Surg. 41, 252–259. doi:10.1016/j.jpedsurg.2005.10.047
Eskelinen, E. L. (2011). The dual role of autophagy in cancer. Curr. Opin. Pharmacol. 11, 294–300. doi:10.1016/j.coph.2011.03.009
Faivre, S., Djelloul, S., and Raymond, E. (2006). New paradigms in anticancer therapy: Targeting multiple signaling pathways with kinase inhibitors. Semin. Oncol. 33, 407–420. doi:10.1053/j.seminoncol.2006.04.005
Farrokhnia, N., Ericsson, A., Terént, A., and Lennmyr, F. (2008). MEK-inhibitor U0126 in hyperglycaemic focal ischaemic brain injury in the rat. Eur. J. Clin. Invest. 38, 679–685. doi:10.1111/j.1365-2362.2008.01990.x
Favata, M. F., Horiuchi, K. Y., Manos, E. J., Daulerio, A. J., Stradley, D. A., Feeser, W. S., et al. (1998). Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 273, 18623–18632. doi:10.1074/jbc.273.29.18623
Fukazawa, H., Noguchi, K., Murakami, Y., and Uehara, Y. (2002). Mitogen-activated protein/extracellular signal-regulated kinase kinase (MEK) inhibitors restore anoikis sensitivity in human breast cancer cell lines with a constitutively activated extracellular-regulated kinase (ERK) pathway. Mol. Cancer Ther. 1, 303–309.
Gao, J., Niwa, K., Takemura, M., Sun, W., Onogi, K., Wu, Y., et al. (2005). Significant anti-proliferation of human endometrial cancer cells by combined treatment with a selective COX-2 inhibitor NS398 and specific MEK inhibitor U0126. Int. J. Oncol. 26, 737–744. doi:10.3892/ijo.26.3.737
Ge, X., Fu, Y. M., and Meadows, G. G. (2002). U0126, a mitogen-activated protein kinase kinase inhibitor, inhibits the invasion of human A375 melanoma cells. Cancer Lett. 179, 133–140. doi:10.1016/s0304-3835(02)00004-6
Giroux, S., Tremblay, M., Bernard, D., Cardin-Girard, J. F., Aubry, S., Larouche, L., et al. (1999). Embryonic death of Mek1-deficient mice reveals a role for this kinase in angiogenesis in the labyrinthine region of the placenta. Curr. Biol. 9, 369–372. doi:10.1016/s0960-9822(99)80164-x
Glick, D., Barth, S., and Macleod, K. F. (2010). Autophagy: Cellular and molecular mechanisms. J. Pathol. 221, 3–12. doi:10.1002/path.2697
Guo, M., Wei, H., Hu, J., Sun, S., Long, J., and Wang, X. (2015). U0126 inhibits pancreatic cancer progression via the KRAS signaling pathway in a zebrafish xenotransplantation model. Oncol. Rep. 34, 699–706. doi:10.3892/or.2015.4019
Hanada, K., Itoh, M., Fujii, K., Tsuchida, A., Hirata, M., Iwao, T., et al. (1997). TP53 mutations in stage I gallbladder carcinoma with special attention to growth patterns. Eur. J. Cancer 33, 1136–1140. doi:10.1016/s0959-8049(97)00080-4
Hidaka, E., Yanagisawa, A., Seki, M., Takano, K., Setoguchi, T., and Kato, Y. (2000). High frequency of K-ras mutations in biliary duct carcinomas of cases with a long common channel in the papilla of Vater. Cancer Res. 60, 522–524.
Horiuchi, H., Kawamata, H., Fujimori, T., and Kuroda, Y. (2003). A MEK inhibitor (U0126) prolongs survival in nude mice bearing human gallbladder cancer cells with K-ras mutation: Analysis in a novel orthotopic inoculation model. Int. J. Oncol. 23, 957–963. doi:10.3892/ijo.23.4.957
Hotokezaka, H., Sakai, E., Kanaoka, K., Saito, K., Matsuo, K., Kitaura, H., et al. (2002). U0126 and PD98059, specific inhibitors of MEK, accelerate differentiation of RAW264.7 cells into osteoclast-like cells. J. Biol. Chem. 277, 47366–47372. doi:10.1074/jbc.M208284200
Ito, M., Zhao, N., Zeng, Z., Chang, C. C., and Zu, Y. (2010). Synergistic growth inhibition of anaplastic large cell lymphoma cells by combining cellular ALK gene silencing and a low dose of the kinase inhibitor U0126. Cancer Gene Ther. 17, 633–644. doi:10.1038/cgt.2010.20
Jain, M., Arvanitis, C., Chu, K., Dewey, W., Leonhardt, E., Trinh, M., et al. (2002). Sustained loss of a neoplastic phenotype by brief inactivation of MYC. Science 297, 102–104. doi:10.1126/science.1071489
James, J. A., Smith, M. A., Court, E. L., Yew Ching, C., Willson, Y., Graham Smith, C., et al. (2003). An investigation of the effects of the MEK inhibitor U0126 on apoptosis in acute leukemia. Hematol. J. 4, 427–432. doi:10.1038/sj.thj.6200327
Jarvis, W. D., FaFau - Tombes, F. R. M., Tombes Rm Fau - Erukulla, R. K., Erukulla Rk Fau - Bittman, R., Bittman R Fau - Schwartz, G. K., Schwartz Gk Fau - Dent, P., et al. (1998). Evidence for involvement of mitogen-activated protein kinase, rather than stress-activated protein kinase, in potentiation of 1-beta-D-arabinofuranosylcytosine-induced apoptosis by interruption of protein kinase C signaling. Mol. Pharmacol. 54 (5), 844–856. doi:10.1124/mol.54.5.844
Jesionowska, A., Cecerska-Heryć, E., Marczuk, N., Safranow, K., and Dołęgowska, B. (2015). Lysophosphatidic acid and malignant neoplasms. Postepy Biochem. 61, 381–387.
Jo, S. K., Cho, W. Y., Sung, S. A., Kim, H. K., and Won, N. H. (2005). MEK inhibitor, U0126, attenuates cisplatin-induced renal injury by decreasing inflammation and apoptosis. Kidney Int. 67, 458–466. doi:10.1111/j.1523-1755.2005.67102.x
Kake, S., Usui, T., Ohama, T., Yamawaki, H., and Sato, K. (2017). Death-associated protein kinase 3 controls the tumor progression of A549 cells through ERK MAPK/c-Myc signaling. Oncol. Rep. 37, 1100–1106. doi:10.3892/or.2017.5359
Kerr, A. H., James, J. A., Smith, M. A., Willson, C., Court, E. L., and Smith, J. G. (2003). An investigation of the MEK/ERK inhibitor U0126 in acute myeloid leukemia. Ann. N. Y. Acad. Sci. 1010, 86–89. doi:10.1196/annals.1299.013
Kohno, M., and Pouyssegur, J. (2003). Pharmacological inhibitors of the ERK signaling pathway: Application as anticancer drugs. Prog. Cell Cycle Res. 5, 219–224.
Lakka, S. S., Jasti, S. L., Kyritsis, A. P., Yung, W. K., Ali-Osman, F., Nicolson, G. L., et al. (2000). Regulation of MMP-9 (type IV collagenase) production and invasiveness in gliomas by the extracellular signal-regulated kinase and jun amino-terminal kinase signaling cascades. Clin. Exp. metastasis 18, 245–252. doi:10.1023/a:1006724826083
Leevers, S. J., and Marshall, C. J. (1992). Activation of extracellular signal-regulated kinase, ERK2, by p21ras oncoprotein. EMBO J. 11, 569–574. doi:10.1002/j.1460-2075.1992.tb05088.x
Lefloch, R., Pouysségur, J., and Lenormand, P. (2008). Single and combined silencing of ERK1 and ERK2 reveals their positive contribution to growth signaling depending on their expression levels. Mol. Cell Biol. 28, 511–527. doi:10.1128/MCB.00800-07
Lefloch, R., Pouysségur, J., and Lenormand, P. (2009). Total ERK1/2 activity regulates cell proliferation. Cell Cycle 8, 705–711. doi:10.4161/cc.8.5.7734
Levin, B. (1999). Gallbladder carcinoma. Ann. Oncol. 10, S129–S130. doi:10.1093/annonc/10.suppl_4.s129
Liu, D., Liu, Z., Condouris, S., and Xing, M. (2007). BRAF V600E maintains proliferation, transformation, and tumorigenicity of BRAF-mutant papillary thyroid cancer cells. J. Clin. Endocrinol. Metab. 92, 2264–2271. doi:10.1210/jc.2006-1613
Low, H. B., and Zhang, Y. (2016). Regulatory roles of MAPK phosphatases in cancer. Immune Netw. 16, 85–98. doi:10.4110/in.2016.16.2.85
Lu, Z., and Xu, S. (2006). ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB life 58, 621–631. doi:10.1080/15216540600957438
Ma, D.-m., Ji, Y.-j., Yang, F., Liu, W., Wan, Z., and Li, R.-y. (2013). Effects of U0126 on growth and activation of mitogen-activated protein kinases in Aspergillus fumigatus. Chin. Med. J. Engl. 126, 220–225.
Ma, X., Wang, Q., Liu, Y., Chen, Y., Zhang, L., Jiang, M., et al. (2015). Inhibition of tumor growth by U0126 is associated with induction of interferon-γ production. Int. J. Cancer 136, 771–783. doi:10.1002/ijc.29038
Maher, S. G., Romero-Weaver, A. L., Scarzello, A. J., and Gamero, A. M. (2007). Interferon: Cellular executioner or white knight? Curr. Med. Chem. 14, 1279–1289. doi:10.2174/092986707780597907
Marampon, F., Bossi, G., Ciccarelli, C., Di Rocco, A., Sacchi, A., Pestell, R. G., et al. (2009). MEK/ERK inhibitor U0126 affects in vitro and in vivo growth of embryonal rhabdomyosarcoma. Mol. Cancer Ther. 8, 543–551. doi:10.1158/1535-7163.MCT-08-0570
Marampon, F., Ciccarelli, C., and Zani, B. M. (2006). Down-regulation of c-Myc following MEK/ERK inhibition halts the expression of malignant phenotype in rhabdomyosarcoma and in non muscle-derived human tumors. Mol. Cancer 5, 31. doi:10.1186/1476-4598-5-31
Matsumoto, T., Turesson, I., Book, M., Gerwins, P., and Claesson-Welsh, L. (2002). p38 MAP kinase negatively regulates endothelial cell survival, proliferation, and differentiation in FGF-2-stimulated angiogenesis. J. Cell Biol. 156, 149–160. doi:10.1083/jcb.200103096
Mattingly, R. R., Milstein, M. L., and Mirkin, B. L. (2001). Down-regulation of growth factor-stimulated MAP kinase signaling in cytotoxic drug-resistant human neuroblastoma cells. Cell Signal 13, 499–505. doi:10.1016/s0898-6568(01)00173-5
McCubrey, J. A., Steelman, L. S., Chappell, W. H., Abrams, S. L., Wong, E. W., Chang, F., et al. (2007). Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta 1773, 1263–1284. doi:10.1016/j.bbamcr.2006.10.001
Merlino, G., and Helman, L. J. (1999). Rhabdomyosarcoma--working out the pathways. Oncogene 18, 5340–5348. doi:10.1038/sj.onc.1203038
Milella, M., Kornblau Sm Fau - Estrov, Z., EstrovFau - Carter, Z. B. Z., Carter Bz Fau - Lapillonne, H., Fau - Harris, D., Harris D Fau - Konopleva, M., et al. (2001). Therapeutic targeting of the MEK/MAPK signal transduction module in acute myeloid leukemia. J. Clin. Invest. 108, 851–859. doi:10.1172/JCI12807
Mizuguchi, M., Kudo, S., Fukahori, T., Matsuo, Y., Miyazaki, K., Tokunaga, O., et al. (1997). Endoscopic ultrasonography for demonstrating loss of multiple-layer pattern of the thickened gallbladder wall in the preoperative diagnosis of gallbladder cancer. Eur. Radiol. 7, 1323–1327. doi:10.1007/s003300050296
Mohammad, G., Mairaj Siddiquei, M., Imtiaz Nawaz, M., and Abu El-Asrar, A. M. (2013). The ERK1/2 inhibitor U0126 attenuates diabetes-induced upregulation of MMP-9 and biomarkers of inflammation in the retina. J. diabetes Res. 2013, 658548. doi:10.1155/2013/658548
Morgan, M. A., Dolp O Fau - Reuter, C. W., and Reuter, C. W. (2001). Cell-cycle-dependent activation of mitogen-activated protein kinase kinase (MEK-1/2) in myeloid leukemia cell lines and induction of growth inhibition and apoptosis by inhibitors of RAS signaling. Blood 97, 1823–1834. doi:10.1182/blood.v97.6.1823
Pagès, G., Lenormand, P., L'Allemain, G., Chambard, J. C., Meloche, S., and Pouysségur, J. (1993). Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc. Natl. Acad. Sci. U. S. A. 90, 8319–8323. doi:10.1073/pnas.90.18.8319
Patel, T., Gores, G. J., and Kaufmann, S. H. (1996). The role of proteases during apoptosis. FASEB J. 10, 587–597. doi:10.1096/fasebj.10.5.8621058
Réjiba, S., Wack, S., Aprahamian, M., and Hajri, A. (2007). K-ras oncogene silencing strategy reduces tumor growth and enhances gemcitabine chemotherapy efficacy for pancreatic cancer treatment. Cancer Sci. 98, 1128–1136. doi:10.1111/j.1349-7006.2007.00506.x
Ross, R. A., Biedler, J. L., and Spengler, B. A. (2003). A role for distinct cell types in determining malignancy in human neuroblastoma cell lines and tumors. Cancer Lett. 197, 35–39. doi:10.1016/s0304-3835(03)00079-x
Ross, R. A., and Spengler, B. A. (2004). The conundrum posed by cellular heterogeneity in analysis of human neuroblastoma. J. Natl. Cancer Inst. 96, 1192–1193. doi:10.1093/jnci/djh262
Ruan, J., Qi, Z., Shen, L., Jiang, Y., Xu, Y., Lan, L., et al. (2015). Crosstalk between JNK and NF-κB signaling pathways via HSP27 phosphorylation in HepG2 cells. Biochem. Biophys. Res. Commun. 456, 122–128. doi:10.1016/j.bbrc.2014.11.045
Rubio, I., Rodriguez-Viciana, P., Downward, J., and Wetzker, R. (1997). Interaction of Ras with phosphoinositide 3-kinase gamma. Biochem. J. 326 (3), 891–895. doi:10.1042/bj3260891
Schoenborn, J. R., and Wilson, C. B. (2007). Regulation of interferon-gamma during innate and adaptive immune responses. Adv. Immunol. 96, 41–101. doi:10.1016/S0065-2776(07)96002-2
Schroder, K., Hertzog, P. J., Ravasi, T., and Hume, D. A. (2004). Interferon-gamma: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 75, 163–189. doi:10.1189/jlb.0603252
Sears, R., Nuckolls, F., Haura, E., Taya, Y., Tamai, K., and Nevins, J. R. (2000). Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 14, 2501–2514. doi:10.1101/gad.836800
Shachaf, C. M., Kopelman, A. M., Arvanitis, C., Karlsson, A., Beer, S., Mandl, S., et al. (2004). MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature 431, 1112–1117. doi:10.1038/nature03043
Shi, R., Lin, J., Guo, Y., and Gong, Y. P. (2014). The MEK1/2 inhibitor U0126 reverses imatinib resistance through down-regulating activation of Lyn/ERK signaling pathway in imatinib-resistant K562R leukemia cells. Pharmazie 69, 346–352.
Svensson, C., Part, K., Künnis-Beres, K., Kaldmäe, M., Fernaeus, S. Z., and Land, T. (2011). Pro-survival effects of JNK and p38 MAPK pathways in LPS-induced activation of BV-2 cells. Biochem. Biophys. Res. Commun. 406, 488–492. doi:10.1016/j.bbrc.2011.02.083
Takayama, Y., Kokuryo, T., Yokoyama, Y., Nagino, M., Nimura, Y., Senga, T., et al. (2008). MEK inhibitor enhances the inhibitory effect of imatinib on pancreatic cancer cell growth. Cancer Lett. 264, 241–249. doi:10.1016/j.canlet.2008.01.035
Thomas, S. M., DeMarco, M., D'Arcangelo, G., Halegoua, S., and Brugge, J. S. (1992). Ras is essential for nerve growth factor- and phorbol ester-induced tyrosine phosphorylation of MAP kinases. Cell 68, 1031–1040. doi:10.1016/0092-8674(92)90075-n
Thornberry, N. A. (1998). Caspases: Key mediators of apoptosis. Chem. Biol. 5, R97–R103. doi:10.1016/s1074-5521(98)90615-9
Vaccaro, V., Melisi, D., Bria, E., Cuppone, F., Ciuffreda, L., Pino, M. S., et al. (2011). Emerging pathways and future targets for the molecular therapy of pancreatic cancer. Expert Opin. Ther. Targets 15, 1183–1196. doi:10.1517/14728222.2011.607438
Wagner, E. F., and Nebreda, A. R. (2009). Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 9, 537–549. doi:10.1038/nrc2694
Wang, A., Zhang, H., Liang, Z., Xu, K., Qiu, W., Tian, Y., et al. (2016). U0126 attenuates ischemia/reperfusion-induced apoptosis and autophagy in myocardium through MEK/ERK/EGR-1 pathway. Eur. J. Pharmacol. 788, 280–285. doi:10.1016/j.ejphar.2016.06.038
Wang, D., Shi, S., Ren, T., Zhang, Y., Guo, P., Wang, J., et al. (2021). U0126 pretreatment inhibits cisplatin-induced apoptosis and autophagy in HEI-OC1 cells and cochlear hair cells. Toxicol. Appl. Pharmacol. 415, 115447. doi:10.1016/j.taap.2021.115447
Wang, X., and Studzinski, G. P. (2001). Phosphorylation of raf-1 by kinase suppressor of ras is inhibited by "MEK-specific" inhibitors PD 098059 and U0126 in differentiating HL60 cells. Exp. Cell Res. 268, 294–300. doi:10.1006/excr.2001.5292
Watanabe, H., Date, K., Itoi, T., Matsubayashi, H., Yokoyama, N., Yamano, M., et al. (1999). Histological and genetic changes in malignant transformation of gallbladder adenoma. Ann. Oncol. official J. Eur. Soc. Med. Oncol. 10 (4), 136–139. doi:10.1093/annonc/10.suppl_4.s136
Watanabe, M., Asaka, M., Tanaka, J., Kurosawa, M., Kasai, M., and Miyazaki, T. (1994). Point mutation of K-ras gene codon 12 in biliary tract tumors. Gastroenterology 107, 1147–1153. doi:10.1016/0016-5085(94)90240-2
Wiesenauer, C. A., Yip-Schneider, M. T., Wang, Y., and Schmidt, C. M. (2004). Multiple anticancer effects of blocking MEK-ERK signaling in hepatocellular carcinoma. J. Am. Coll. Surg. 198, 410–421. doi:10.1016/j.jamcollsurg.2003.10.004
Wu, R. W., Yow, C. M., Wong, C. K., and Lam, Y. H. (2011). Photodynamic therapy (PDT) - initiation of apoptosis via activation of stress-activated p38 MAPK and JNK signal pathway in H460 cell lines. Photodiagnosis Photodyn. Ther. 8, 254–263. doi:10.1016/j.pdpdt.2010.12.002
Xu, L., Liu, Y., Hou, Y., Wang, K., Wong, Y., Lin, S., et al. (2015). U0126 promotes osteogenesis of rat bone-marrow-derived mesenchymal stem cells by activating BMP/Smad signaling pathway. Cell Tissue Res. 359, 537–545. doi:10.1007/s00441-014-2025-3
Yacoub, A., Han, S. I., Caron, R., Gilfor, D., Mooberry, S., Grant, S., et al. (2003). Sequence dependent exposure of mammary carcinoma cells to Taxotere and the MEK1/2 inhibitor U0126 causes enhanced cell killing in vitro. Cancer Biol. Ther. 2, 670–676. doi:10.4161/cbt.2.6.534
Ye, H., Zhang, Y., Wang, Y., Xia, J., Mao, X., and Yu, X. (2017). The restraining effect of baicalein and U0126 on human cervical cancer cell line HeLa. Mol. Med. Rep. 16, 957–963. doi:10.3892/mmr.2017.6648
Yeh, E., Cunningham, M., Arnold, H., Chasse, D., Monteith, T., Ivaldi, G., et al. (2004). A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells. Nat. Cell Biol. 6, 308–318. doi:10.1038/ncb1110
Yip-Schneider, M. T., Lin, A., Barnard, D., Sweeney, C. J., and Marshall, M. S. (1999). Lack of elevated MAP kinase (Erk) activity in pancreatic carcinomas despite oncogenic K-ras expression. Int. J. Oncol. 15, 271–279. doi:10.3892/ijo.15.2.271
Yip-Schneider, M. T., Lin, A., and Marshall, M. S. (2001). Pancreatic tumor cells with mutant K-ras suppress ERK activity by MEK-dependent induction of MAP kinase phosphatase-2. Biochem. Biophys. Res. Commun. 280, 992–997. doi:10.1006/bbrc.2001.4243
Yip-Schneider, M. T., and Schmidt, C. M. (2003). MEK inhibition of pancreatic carcinoma cells by U0126 and its effect in combination with sulindac. Pancreas 27, 337–344. doi:10.1097/00006676-200311000-00012
Zhang, X., Zeng, Y., Qu, Q., Zhu, J., Liu, Z., Ning, W., et al. (2017). PD-L1 induced by IFN-γ from tumor-associated macrophages via the JAK/STAT3 and PI3K/AKT signaling pathways promoted progression of lung cancer. Int. J. Clin. Oncol. 22, 1026–1033. doi:10.1007/s10147-017-1161-7
Zhao, L. Y., Huang, C., Li, Z. F., Liu, L., Ni, L., and Song, T. S. (2009). STAT1/2 is involved in the inhibition of cell growth induced by U0126 in HeLa cells. Cell. Mol. Biol. (Noisy-le-Grand, France) 55, Ol1168–1174.
Keywords: U0126, MAPK inhibitors, MAPK signal pathway, cancer, cancer therapy
Citation: You Y, Niu Y, Zhang J, Huang S, Ding P, Sun F and Wang X (2022) U0126: Not only a MAPK kinase inhibitor. Front. Pharmacol. 13:927083. doi: 10.3389/fphar.2022.927083
Received: 23 April 2022; Accepted: 25 July 2022;
Published: 25 August 2022.
Edited by:
Sungpil Yoon, Sungkyunkwan University, South KoreaReviewed by:
Lars Edvinsson, Lund University, SwedenCopyright © 2022 You, Niu, Zhang, Huang, Ding, Sun and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fengbing Sun, Y21kcnN1bkAxNjMuY29t; Xuhui Wang, dGd5eHl5akBmb3htYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.