
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 26 September 2022
Sec. Drugs Outcomes Research and Policies
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.926431
This article is part of the Research Topic Rising Stars in Drugs Outcomes Research and Policies: 2021 View all 9 articles
Part of this article's content has been mentioned in:
Editorial: Rising stars in drugs outcomes research and policies: 2021
Objective: The objective of this study is to evaluate the cost-effectiveness of different knee OA care sequences compared to standard treatment reimbursed by the major health insurance payer in Thailand.
Method: We used decision analytical modeling to evaluate the effect of either adding etoricoxib or crystalline glucosamine sulfate compared to standard treatment from a societal perspective over patients’ lifetimes. Data were analyzed based on efficacy, whereas adverse events were considered as a substate. Model input data were retrieved from relevant published literature and the Standard Cost Lists for Health Technology Assessment, Thailand. All health outcomes were measured in a unit of quality-adjusted life-year (QALY). An incremental cost-effectiveness ratio (ICER) was applied to examine the costs and QALYs. Sensitivity analysis was performed to investigate the robustness of the model.
Result: The results demonstrated that adding crystalline glucosamine sulfate (before diclofenac plus proton pump inhibitors, PPI) into the standard care sequence was a dominant strategy compared to the standard care sequence. Adding etoricoxib alone or including crystalline glucosamine sulfate (after diclofenac plus PPI) was dominated by adding crystalline glucosamine sulfate (before diclofenac plus PPI), whereas in a willingness-to-pay (WTP) threshold in Thailand, adding of both crystalline glucosamine sulfate (before diclofenac plus PPI) and etoricoxib were cost-effective when compared to adding crystalline glucosamine sulfate alone with ICER of 125,547 Thai baht/QALY (3,472 US dollars/QALY).
Conclusion: The addition of crystalline glucosamine sulfate and etoricoxib into standard knee OA treatment were cost-effective at the WTP threshold in Thailand. In addition, early initiation of crystalline glucosamine sulfate would be less costly and more effective than delayed treatment or the use of standard treatment alone.
Osteoarthritis (OA), the most common chronic condition of the joints, is the deterioration of the cartilage in joints resulting in bones rubbing together and leading cause of pain, stiffness, swelling, and disability. The global prevalence of knee OA was 22.9% in people of 40 years and over (Cui et al., 2020), whereas in Thailand, the prevalence of knee OA in 2018 was approximately 8.64% (Department of Thai Traditional and Alternative Medicine, 2019). The number of knee OA patients have been gradually increasing as the aging society becomes an issue globally. The economic burden of knee OA is also high in not only direct medical costs associated with OA but also indirect costs (Centers for Disease Control Prevention (CDC), 2007; Murphy and Helmick, 2012; Hatoum et al., 2014).
The treatment for OA is broadly divided into five groups: acetaminophen, oral non-steroidal anti-inflammatory drugs (NSAIDs), symptomatic slow-acting drugs for osteoarthritis (SYSADOA), intra-articular steroid injection, and surgery (Royal College of Orthopedic Surgeons of Thailand (RCOST), 2011). The most common treatment for knee OA is the use of NSAIDs because of its efficacy for pain relief. Nevertheless, the risk of developing NSAID‐related gastrointestinal and cardiovascular complications are concerned (Mosler, 2014). Glucosamine sulfate, a SYSADOA treatment, is one of an alternative treatments for mild to moderate knee OA. Glucosamine has a very good safety profile; however, its efficacy is still an arguable issue resulted in differences in regulatory status as well as the reimbursement of the product (Luksameesate and Taychakhoonavudh, 2021).
In Thailand, the reimbursement policy of knee OA treatments among three major health insurance schemes, namely, Civil Servant Medical Benefit Scheme (CSMBS), Universal Coverage Scheme (UC), and Social Security Scheme (SSS), varies as some medicines are being reimbursed by one health insurance payers but not the others. For example, glucosamine is reimbursable with some restrictions under CSMBS but is not reimbursed under UC and SSS (Tantivess and Tangcharoensathien, 2016).
Several economic evaluation studies of knee OA treatment were conducted to compare NSAIDs either selective COX-2 NSAIDs or non-selective NSAIDs (Yen et al., 2004; Contreras-Hernández et al., 2008) as well as glucosamine sulfate (Black et al., 2009; Chaiyakunapruk et al., 2010; Scholtissen et al., 2010; Bruyère et al., 2019). However, most of these studies compared between two knee OA treatments and focused only on efficacy or adverse events but not on both aspects of knee OA treatment together. The issue of knee OA treatments become complex as it is not a question of choosing the most effective or cost-effective treatment over the other but rather the decision to incorporate the treatment into the care sequence in which the efficacy is to delayed progression with the optimal tolerable side effects of the treatment itself. To our understanding, no research has been done to systematically evaluate knee OA treatment in which all OA treatments including acetaminophen, NSAIDs, SYSADOA, intra-articular corticosteroid, and total knee arthroplasty (TKA) are being compared altogether. The objective of this study is to evaluate the cost-effectiveness of different knee OA care sequences compared to standard treatment reimbursed by the major health insurance payer in Thailand from a societal perspective.
A cost-utility analysis was conducted to compare among treatment options for knee OA patients aged 45 or over with mild to moderate pain and no comorbidities. All health outcomes were measured in a unit of quality-adjusted life-year (QALY). We analyzed based on the standard treatment while considering the adverse events of all care sequences. Model input data were obtained from the Drug and Medical Supply Information Center (DMSIC), Ministry of Public Health (MoPH) and relevant published literature. The analysis was done from a societal perspective. Costs and QALYs were each discounted at 3% as recommended by the Guideline for Health Technology Assessment (Guideline Development Working Group, 2019). The willingness-to-pay threshold in Thailand was determined as 160,000 Thai baht (THB)/QALY (Chaikledkaew and Teerawattananon, 2013). The cycle of the study was 6 months and a lifetime horizon.
A Markov model was used in our decision analytical modeling. The core concept of three previous literatures were applied to our Markov model structure which incorporates all knee OA treatments and its adverse events (Losina et al., 2013; Capel et al., 2014; Katz et al., 2016). The standard care sequence was based on the clinical guideline of knee OA treatment published by the Royal College of Orthopedic Surgeons of Thailand (RCOST) 2011 (Royal College of Orthopedic Surgeons of Thailand (RCOST), 2011), available evidence, and experts’ opinions. Either etoricoxib or crystalline glucosamine sulfate or both were incorporated into the standard care sequence as an intervention to compare the results of delaying disease progression and to calculate cost-effectiveness.
In this study, five treatment states were used as a standard treatment to model OA prognosis based on the knee OA treatments including acetaminophen, diclofenac plus a proton pump inhibitors (PPI), triamcinolone acetonide (TA) injection, total knee arthroplasty (TKA), and death (Figure 1). A total of five alternatives of either etoricoxib or crystalline glucosamine sulfate or both were added into standard treatment to compare with the standard treatment alone as follows:
1) the addition of etoricoxib;
2) the addition of crystalline glucosamine sulfate (after diclofenac plus PPI state);
3) the addition of crystalline glucosamine sulfate (before diclofenac plus PPI state);
4) the addition of crystalline glucosamine sulfate (after diclofenac plus PPI state) and etoricoxib;
5) the addition of crystalline glucosamine sulfate (before diclofenac plus PPI state) and etoricoxib.
We also took adverse events into account as sub-state including gastrointestinal (GI) discomfort, symptomatic ulcer, myocardial infarction (MI), heart failure (HF), and stroke.
In each cycle of the model, patients could move either to the next progressive state where advanced treatment is needed or the death state (absorbing state) according to the transition probabilities.
An incremental cost-effectiveness ratio (ICER) was applied to examine the costs and QALYs of knee OA treatments. All patients in the study began the model from the initial “acetaminophen” state to the “death” state and transition probabilities were used for each cycle of the model.
The main assumption of our study was that patients received only assigned treatment in those states and the risks of adverse events were constant over time. Transition to the next treatment option was also irreversible.
Regarding the literature review of economic evaluation of knee osteoarthritis, all studies including our study use the Markov model in which either sequence of treatment or adverse events are designed as model states. This is due to the nature of osteoarthritis disease in which disease status is hard to identify (Bessette et al., 2009; Latimer et al., 2009; Brereton et al., 2012; Losina et al., 2013; Capel et al., 2014; Katz et al., 2016; Karasawa et al., 2021). The treatment of osteoarthritis thus usually depends on the pain, symptoms, and activities of daily living of patients. If the pain persists, treatment will be changed to higher potency and thus next model states. Therefore, we assume that treatment failure in which the patient move to the next state is when the patient reports the persistence of pain. The subject whose pain was not relieved on those states was failure resulting in discontinuation and the subject would move to the next treatment state.
A structured literature review was conducted to explore relevant published studies in Thailand. Where local data were unavailable, data from the randomized controlled trial (RCT), meta-analysis, systematic review, and real-world evidence studies were considered. Base-case probability estimates and ranges over a period of 6 months are presented in Table 1, and the model parameter is shown in Table 2.
All efficacy data were identified by RCT studies worldwide due to limited efficacy data available in Thailand (Fortin et al., 1999; Zacher et al., 2003; Herrero‐Beaumont et al., 2007; Reginster et al., 2007; McAlindon et al., 2017). The inclusion criteria of selected studies were knee OA patients who had most relevant characteristic to the Thai population and received the treatment. Nevertheless, the treatment or daily dose which was differently defined from our study was excluded. Crystalline glucosamine sulfate was the sole formulation included in the study for estimation. The efficacy of pain relief for each treatment state was derived from the outcome measurement of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score, a self-administered health status assessing pain for patients with painful arthritis of the knee. Efficacy was then converted to probability for use in the model.
Adverse events of acetaminophen were retrieved from the cost-effectiveness study (Latimer et al., 2009), whereas large RCT studies estimated adverse events of diclofenac, etoricoxib, glucosamine, and TA injection (Cannon et al., 2006; Sawitzke et al., 2010; McAlindon et al., 2017). A total of three obsevational study were used to extracted each adverse event of TKA (Bullock et al., 2003; Husted et al., 2010; Bohm et al., 2012).
To deal with the heterogeneity of multiple data sources, we applied the most appropriate method to parameterize this model as a base-case analysis, and sensitivity analysis was performed for the remaining data.
For the base-case analysis, direct and indirect costs were included in the study. Direct medical costs consisted of the cost of therapies, cost of treatments due to adverse events, laboratory cost, outpatient visit cost, inpatient visit cost, and doctor fees. Direct non-medical costs consisted of traveling costs and additional food costs. Indirect costs included productivity loss due to pain. All cost data were converted at the rate of 36.16 THB/US dollars (USD) (Bank of Thailand, 2022).
Costs of the drugs in this model were calculated by assuming that patients received treatment with the maximum dose for knee OA treatments (Thai Rheumatism Association, 2010; Wells et al., 2015). The hospital purchasing prices that were available from the Drug and Medical Supply Information Centre of the Ministry of Public Health were used to calculate drug cost (Drug And Medical Supply Information Center MoPH, 2018). Data from the generic drug cost data were used for the base-case analysis, and the original drug cost was used for the sensitivity analysis. Cost of TKA was obtained from the database of Chiangrai Prachanukroh Hospital from October 2009 to September 2011 (Vivattanavarang, 2011). Costs of treatment for adverse events including GI discomfort, symptomatic ulcer, stroke, MI, and HF were obtained from the cost-effectiveness study in Thailand.
Direct non-medical costs consisted of traveling costs and additional food costs. Indirect costs included productivity loss due to pain. The data was retrieved from the Standard Cost Lists for Health Technology Assessment, Thailand (The Health Intervention and Technology Assessment Program (HITAP), 2017).
The utility of each health state was measured by the efficacy of the drug to relieve pain. Patients who moved to the next treatment regimen due to uncontrollable pain were given a utility of 0.56 in the previous health state since the medication given could not control the OA symptoms. The utility data, one local data availability, were retrieved from the data collected by Wajajamroen (2016) using EQ-5D-3L index scores for knee OA patients at Nopparat Rajathanee Hospital, Thailand. Since no disutility of data was done in Thailand, the disutilities of adverse events including GI events and Cardiovascular (CV) events from Sullivan and Ghushchyan (2006) were collected using preference-based EQ-5D index scores for chronic conditions in the United States.
A sensitivity analysis was performed to investigate the robustness of the model. A deterministic sensitivity analysis (DSA) was used to examine the model’s results when the changed value in the specific input parameters. The key parameters were selected from the literature including the cost of crystalline glucosamine sulfate, cost of TKA, transition probability of diclofenac plus PPI, transition probability of TA injection, transition probability of TKA, and utility of knee OA pain. A probabilistic sensitivity analysis (PSA) was performed using Monte Carlo simulation. The 1,000 iterations generated a different value of cost and QALYs, which were demonstrated by the incremental cost-effectiveness plane and cost-effectiveness acceptability curve (CEAC). Costs were underlying gamma distributions. Probabilities and utilities were underlying beta distributions. Transition probabilities were underlying log-normal distributions.
The base-case analysis was done in patients of 45 years or over with mild to moderate pain and no comorbidities. The results demonstrated that adding crystalline glucosamine sulfate (before diclofenac plus PPI state) into the standard care sequence was a dominant strategy compared to the standard care sequence. Adding etoricoxib alone or including crystalline glucosamine sulfate (after diclofenac plus PPI state) was dominated by adding crystalline glucosamine sulfate (before diclofenac plus PPI). When comparing the rest of the alternatives we found that adding of both crystalline glucosamine sulfate (before diclofenac plus PPI state) and etoricoxib incurred a higher total cost [366,819 THB (10,144 USD) vs. 150,878 THB (5,325 USD)] and gained more QALYs (4.98 vs. 3.26) than adding crystalline glucosamine sulfate alone. This yielded an ICER of 125,547 THB/QALY (3,472 USD/QALY) and was cost-effective under the willingness-to-pay (WTP) threshold in Thailand (160,000 THB/QALY). The total costs, total QALYs, and ICER in six treatment alternatives under base-case conditions are given in detail in Table 3.
Based on the one-way sensitivity analysis, the effect of the utility of moderate pain had the highest impact of all six alternative results when varying values between 0.35 and 0.77. The cost-effectiveness ratio was less sensitive to the cost of TKA results when varying values between 78,533 THB (2,172 USD) and 79,316 THB (2,193 USD). The tornado diagram is presented in Figure 2.
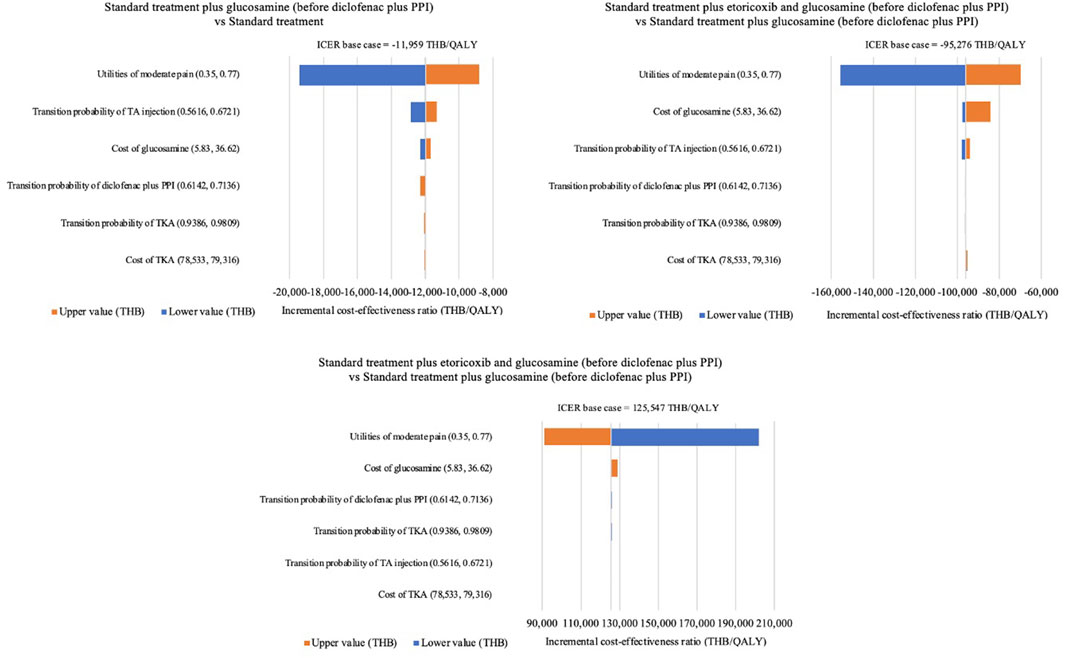
FIGURE 2. Tornado diagram. PPI, proton pump inhibitors; TA, triamcinolone acetonide; TKA, total knee arthroplasty; QALYs, quality-adjusted life-years; THB, Thai Baht.
The probabilistic sensitivity analysis of 1,000 Monte Carlo simulations was shown in the incremental cost-effectiveness plane (Figure 3). At a WTP of 160,000 THB/QALY, a WTP threshold in Thailand (Tanvejsilp et al., 2019), addition of both crystalline glucosamine sulfate (before diclofenac plus PPI state) and etoricoxib had approximately a 98.8% chance of being cost-effective when compared to addition of crystalline glucosamine sulfate alone. When adding crystalline glucosamine sulfate (before diclofenac plus PPI state) into the standard care sequence, all 1,000 iterations of ICERs fell in the lower-right quadrant, demonstrating that crystalline glucosamine sulfate treatment incurred lower costs and improved QALYs. CEAC was indicated to access the impact of WTP on the probability of cost-effectiveness. The addition of crystalline glucosamine sulfate (before diclofenac plus PPI state) and etoricoxib was cost-effective when compared to adding crystalline glucosamine sulfate alone at the WTP threshold at least 125,547THB (3,472 USD), as shown in Figure 4.
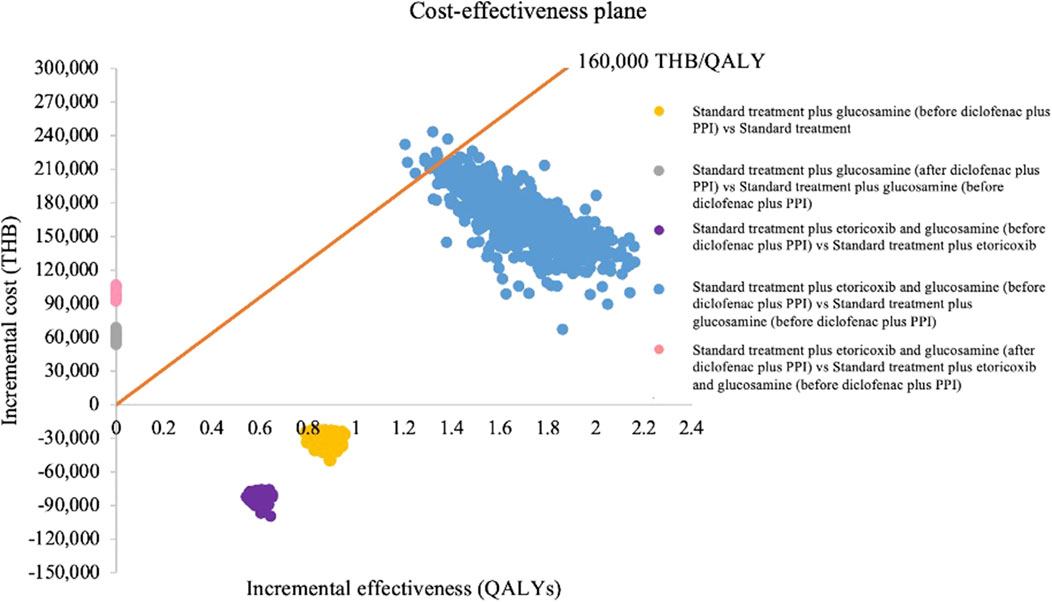
FIGURE 3. Incremental cost-effectiveness plane. PPI, proton pump inhibitors; QALYs, quality-adjusted life-years; THB, Thai Baht.

FIGURE 4. Cost-effectiveness acceptablity curve for standard treatment plus crystalline glucosamine sulfate (before diclofenac plus PPI) and etoricoxib vs. standard treatment plus crystalline glucosamine sulfate (before diclofenac plus PPI). PPI, proton pump inhibitors; QALYs, quality-adjusted life-years; THB, Thai Baht.
To finding the most efficient alternative of knee OA treatment, including pain relief and adverse events into an analysis is crucial. Our Markov model evaluated the cost-effectiveness of standard treatment compared to adding either crystalline glucosamine sulfate or etoricoxib or both and switching care sequence. As the WTP threshold of 160,000 THB, the results indicated that adding crystalline glucosamine sulfate (before diclofenac plus PPI) and etoricoxib into standard treatment was cost-effective. It is also interesting to note that early initiation of crystalline glucosamine sulfate would be less costly and more effective than delayed treatment or the use of standard treatment alone.
According to our all analysis, the order of care sequence had an impact on the patient’s treatment outcome. One reason might be that crystalline glucosamine sulfate has been proposed as one of the treatment choices for treating mild to moderate knee OA. It affected efficacy in terms of clinical improvement, retardation of disease progression, and decreased risk of undergoing total joint replacement surgery while adverse events were lower than other treatments (Bruyère et al., 2016). These effects when taken into consideration resulted in making an early initiation of crystalline glucosamine sulfate before the use of NSAIDs a dominant strategy.
Recent cost-effectiveness models for etoricoxib treating knee OA have been founded in two studies. Moore A et al. (Moore et al., 2004) concluded that etoricoxib was cost-effective and superior over non-selective NSAIDs plus a PPI or misoprostol. In contrast with another study, Spiegel BMR et al. (Spiegel et al., 2005) reported that non-selective NSAIDs plus a PPI was a dominant strategy and less costly than coxib in patients with high risk of GI or CV events. The different results might be due to assumptions and data used in the analysis. Both studies assumed equivalent efficacy and emphasized only adverse events, whereas our analysis considered these two aspects that would have an impact on the cost and outcomes of each treatment alternative.
For glucosamine sulfate, our findings were consistent with two previous studies which concluded that glucosamine sulfate was a cost-effective therapy. Scholtissen et al. (Scholtissen et al., 2010) reported that crystalline glucosamine sulfate was a highly cost-effective dominant over paracetamol and placebo. Bruyere et al. (Bruyère et al., 2019) showed that crystalline glucosamine sulfate was cost-effective compared to placebo. Inconsistency with two studies’ findings, Black et al. (Black et al., 2009) concluded that glucosamine presented some clinical effectiveness in the treatment of knee OA but not clearly demonstrated about cost-effectiveness which related to the magnitude and duration of quality of life gain. Chaiyakunapruk N et al. (Chaiyakunapruk et al., 2010) estimated the cost-effectiveness of glucosamine sulfate compared with current care and concluded that glucosamine sulfate may not be cost-effective. The contradictory results might be due to the difference of the state model, perspective, and focusing only on efficacy. To the best of our knowledge, there has been no published study evaluating the cost-effectiveness of adding crystalline glucosamine sulfate and etoricoxib into standard treatment compared to standard treatment alone.
Currently, glucosamine sulfate formulation was the only licensed product available in Thailand. It was classified as a nonessential drug (NEDs) and authorized to reimburse in Civil Servant Medical Benefit Scheme (CSMBS) with restriction under the reimbursement protocol (Tantivess and Tangcharoensathien, 2016), whereas other national health insurance schemes, universal Coverage Scheme (UCS), and Social Security Scheme (SSS), did not cover. Consequently, access to crystalline glucosamine sulfate may be limited. According to the results of this study, early initiation of crystalline glucosamine sulfate into the standard treatment is a good alternative to enhance the health outcomes of knee OA patients. This strategy might be used as one evidence integrate with clinical outcome evidence to support policymakers in their decision process of the reimbursement protocol development so that patients can access the appropriate treatment available equally when needed.
There were some limitations of this study. First, combining multiple published data sources from other countries were used when data was unavailable in Thailand. There were probability estimates of pain relief, adverse events, and disutility which might incompatible real situations in Thailand. Second, all cost data were collected in Thailand, even so, the data of adverse events-related costs was published many years later. Finally, our model did not cover all real-world knee OA treatments. We had to select the most appropriate treatment and feasibility to represent the model. Therefore, we recommend increasing all possible treatments and adverse events for further study.
In conclusion, the addition of crystalline glucosamine sulfate and etoricoxib into standard knee OA treatment were cost-effective at the WTP threshold in Thailand. In addition, early initiation of crystalline glucosamine sulfate would be less costly and more effective than delayed treatment or the use of standard treatment alone.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
PL, AT, and ST: conception and design of the work. AT: provided resources. PL: analysis and interpretation of studies for the work. PL: drafted the work. ST: revised the manuscript critically for important intellectual content. ST: provided approval for publication of the content. All authors contributed to the article and approved the submitted version.
This work was supported by the 100th Anniversary Chulalongkorn University Fund for Doctoral Scholarships; and the 90th Anniversary of Chulalongkorn University Fund (Ratchadaphiseksomphot Endowment Fund) (grant number GCUGR1125623022D).
The authors express great appreciation to Associate Professor Puree Anantachoti for valuable suggestion on this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Anukoolsawat, P., Sritara, P., and Teerawattananon, Y. (2006). Costs of lifetime treatment of acute coronary syndrome at Ramathibodi Hospital. Thai Heart J. 19, 132–143.
Bank of Thailand (2022). Foreign exchange rates. Available at https://www.bot.or.th/english/statistics/financialmarkets/exchangerate/_layouts/application/exchangerate/ExchangeRate.aspx (Accessed Aug 24, 2022).
Bessette, L., Risebrough, N., Mittmann, N., Roussy, J-P., Ho, J., and Zlateva, G. (2009). Cost-utility of celecoxib use in different treatment strategies for osteoarthritis and rheumatoid arthritis from the Quebec healthcare system perspective. J. Med. Econ. 12 (3), 246–258. doi:10.3111/13696990903288970
Black, C., Clar, C., Henderson, R., MacEachern, C., McNamee, P., Quayyum, Z., et al. (2009). NIHR health technology assessment programme: Executive summaries.The clinical effectiveness of glucosamine and chondroitin supplements in slowing or arresting progression of osteoarthritis of the knee: A systematic review and economic evaluation
Bohm, E. R., Dunbar, M. J., Frood, J. J., Johnson, T. M., and Morris, K. A. (2012). Rehospitalizations, early revisions, infections, and hospital resource use in the first year after hip and knee arthroplasties. J. Arthroplasty 27 (2), 232–237. doi:10.1016/j.arth.2011.05.004
Brereton, N., Winn, B., and Akehurst, R. (2012). The cost-effectiveness of celecoxib vs diclofenac in the treatment of osteoarthritis in the UK; an update to the NICE model using data from the CONDOR trial. J. Med. Econ. 15 (3), 465–472. doi:10.3111/13696998.2012.659778
Bruyère, O., Altman, R. D., and Reginster, J-Y. (2016). Efficacy and safety of glucosamine sulfate in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Semin. Arthritis Rheum. 45 (4), S12–S17. doi:10.1016/j.semarthrit.2015.11.011
Bruyère, O., Reginster, J-Y., Honvo, G., and Detilleux, J. (2019). Cost-effectiveness evaluation of glucosamine for osteoarthritis based on simulation of individual patient data obtained from aggregated data in published studies. Aging Clin. Exp. Res. 31 (6), 881–887. doi:10.1007/s40520-019-01138-1
Bullock, D. P., Sporer, S. M., and Shirreffs, T. G. (2003). Comparison of simultaneous bilateral with unilateral total knee arthroplasty in terms of perioperative complications. J. Bone Jt. Surg. Am. 85 (10), 1981–1986. doi:10.2106/00004623-200310000-00018
Cannon, C. P., Curtis, S. P., FitzGerald, G. A., Krum, H., Kaur, A., Bolognese, J. A., et al. (2006). Cardiovascular outcomes with etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the multinational etoricoxib and diclofenac arthritis long-term (MEDAL) programme: A randomised comparison. Lancet 368 (9549), 1771–1781. doi:10.1016/S0140-6736(06)69666-9
Capel, M., Tornero, J., Zamorano, J. L., Oyagüez, I., Casado, M. Á., Sánchez-Covisa, J., et al. (2014). Efficiency of naproxen/esomeprazole in association for osteoarthrosis treatment in Spain. Reumatol. Clin. 10 (4), 210–217. doi:10.1016/j.reuma.2013.11.009
Centers for Disease Control Prevention (CDC) (2007). National and state medical expenditures and lost earnings attributable to arthritis and other rheumatic conditions--United States (2003). MMWR. Morb. Mortal. Wkly. Rep. 56 (1), 4–7.
Chaikledkaew, U., and Teerawattananon, Y. (2013). Guidelines for health technology assessment in Thailand. Nonthaburi: Wacharin Publications.
Chaiyakunapruk, N., Saokaew, S., and Pansang, S. (2010). Cost-effectiveness analysis of glucosamine sulphate for the treatment of osteoarthritis in Thailand. Value Health 13 (7), A502. doi:10.1111/j.1524-4733.2010.00793_1.x
Contreras-Hernández, I., Mould-Quevedo, J. F., Torres-González, R., Goycochea-Robles, M. V., Pacheco-Domínguez, R. L., Sánchez-García, S., et al. (2008). Cost-effectiveness analysis for joint pain treatment in patients with osteoarthritis treated at the Instituto Mexicano del Seguro Social (IMSS): Comparison of nonsteroidal anti-inflammatory drugs (NSAIDs) vs. cyclooxygenase-2 selective inhibitors. Cost. Eff. Resour. Alloc. 6 (1), 21. doi:10.1186/1478-7547-6-21
Cui, A., Li, H., Wang, D., Zhong, J., Chen, Y., and Lu, H. (2020). Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine 29, 100587. doi:10.1016/j.eclinm.2020.100587
Department of Thai Traditional and Alternative Medicine (2019). Sevice development and treatment of knee osteoarthritis in national health security office (NHSO). Available at https://dhes.moph.go.th/wp-content/uploads/2019/11/4.1.35.1 (Accessed May 15, 2021).
Drug And Medical Supply Information Center MoPH (2018). Drug reference price. Available at http://dmsic.moph.go.th/dmsic/index.php?p=1&id=1 (Accessed May 07, 2018).
Fortin, P. R., Clarke, A. E., Joseph, L., Liang, M. H., Tanzer, M., Ferland, D., et al. (1999). Outcomes of total hip and knee replacement: Preoperative functional status predicts outcomes at six months after surgery. Arthritis Rheum. 42 (8), 1722–1728. doi:10.1002/1529-0131(199908)42:8<1722::AID-ANR22>3.0.CO;2-R
Guideline Development Working Group (2019). Guideline for health technology assessment in Thailand. Updated Edition, 271.
Hatoum, H. T., Fierlinger, A. L., Lin, S-J., and Altman, R. D. (2014). Cost-effectiveness analysis of intra-articular injections of a high molecular weight bioengineered hyaluronic acid for the treatment of osteoarthritis knee pain. J. Med. Econ. 17 (5), 326–337. doi:10.3111/13696998.2014.902843
Herrero‐Beaumont, G., Ivorra, J. A. R., del Carmen Trabado, M., Blanco, F. J., Benito, P., Martín‐Mola, E., et al. (2007). Glucosamine sulfate in the treatment of knee osteoarthritis symptoms: A randomized, double‐blind, placebo‐controlled study using acetaminophen as a side comparator. Arthritis Rheum. 56 (2), 555–567. doi:10.1002/art.22371
Husted, H., Otte, K. S., Kristensen, B. B., Ørsnes, T., and Kehlet, H. (2010). Readmissions after fast-track hip and knee arthroplasty. Arch. Orthop. Trauma Surg. 130 (9), 1185–1191. doi:10.1007/s00402-010-1131-2
Karasawa, Y., Kamae, I., Nozawa, K., Zeniya, S., Murata, T., Soen, S., et al. (2021). Cost-effectiveness analysis of branded and authorized generic celecoxib for patients with chronic pain in Japan. Plos one 16 (7), e0253547. doi:10.1371/journal.pone.0253547
Katz, J. N., Smith, S. R., Collins, J. E., Solomon, D. H., Jordan, J. M., Hunter, D. J., et al. (2016). Cost-effectiveness of nonsteroidal anti-inflammatory drugs and opioids in the treatment of knee osteoarthritis in older patients with multiple comorbidities. Osteoarthr. Cartil. 24 (3), 409–418. doi:10.1016/j.joca.2015.10.006
Latimer, N., Lord, J., Grant, R. L., O’Mahony, R., Dickson, J., Conaghan, P. G., et al. (2009). Cost effectiveness of COX 2 selective inhibitors and traditional NSAIDs alone or in combination with a proton pump inhibitor for people with osteoarthritis. BMJ, b2538. doi:10.1136/bmj.b2538
Latimer, N., Lord, J., Grant, R. L., O’Mahony, R., Dickson, J., and Conaghan, P. G. (2011). Value of information in the osteoarthritis setting: Cost effectiveness of COX-2 selective inhibitors, traditional NSAIDs and proton pump inhibitors. Pharmacoeconomics 29 (3), 225–237. doi:10.2165/11584200-000000000-00000
Limwattananon, S., Limwattananon, C., Srisuk, P., and Sakolchai, S. (2005). Cost-effectiveness analysis of celecoxib on an avoidance of gastrointestinal adverse events in patients with osteoarthritis. Isan J. Pharm. Sci. 1 (2), 15–29.
Losina, E., Daigle, M. E., Suter, L., Hunter, D., Solomon, D., Walensky, R., et al. (2013). Disease-modifying drugs for knee osteoarthritis: Can they be cost-effective? Osteoarthr. Cartil. 21 (5), 655–667. doi:10.1016/j.joca.2013.01.016
Luksameesate, P., and Taychakhoonavudh, S. (2021). Health Technology assessment (HTA) evidence, regulatory classification and reimbursement of medicine: The case of glucosamine. J. Pharm. Health Serv. Res. 12 (4), 600–606. doi:10.1093/jphsr/rmab058
McAlindon, T. E., LaValley, M. P., Harvey, W. F., Price, L. L., Driban, J. B., Zhang, M., et al. (2017). Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: A randomized clinical trial. Jama 317 (19), 1967–1975. doi:10.1001/jama.2017.5283
Moore, A., Phillips, C., Hunsche, E., Pellissier, J., and Crespi, S. (2004). Economic evaluation of etoricoxib versus non-selective NSAIDs in the treatment of osteoarthritis and rheumatoid arthritis patients in the UK. Pharmacoeconomics 22 (10), 643–660. doi:10.2165/00019053-200422100-00003
Mosler, C. (2014). Cardiovascular risk associated with NSAIDs and COX-2 inhibitors. U. S. Pharm. 39 (3), 35–38.
Murphy, L., and Helmick, C. G. (2012). The impact of osteoarthritis in the United States: A population-health perspective: A population-based review of the fourth most common cause of hospitalization in US adults. Orthop. Nurs. 31 (2), 85–91. doi:10.1097/NOR.0b013e31824fcd42
Permpanicha, A., Kulsomboon, V., and Udol, K. (2015). Cost-effectiveness analysis of highly concentrated n-3 polyunsaturated fatty acids in secondary prevention after myocardial infarction. Asian Biomed. 9 (1), 21–30. doi:10.5372/1905-7415.0901.364
Reginster, J-Y., Malmstrom, K., Mehta, A., Bergman, G., Ko, A., Curtis, S., et al. (2007). Evaluation of the efficacy and safety of etoricoxib compared with naproxen in two, 138-week randomised studies of patients with osteoarthritis. Ann. Rheum. Dis. 66 (7), 945–951. doi:10.1136/ard.2006.059162
Royal College of Orthopedic Surgeons of Thailand (RCOST) (2011). Clinical practice guideline of Knee osteoarthritis. Thailand: Bangkok, 76.
Sawitzke, A. D., Shi, H., Finco, M. F., Dunlop, D. D., Harris, C. L., Singer, N. G., et al. (2010). Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann. Rheum. Dis. 69 (8), 1459–1464. doi:10.1136/ard.2009.120469
Scholtissen, S., Bruyère, O., Neuprez, A., Severens, J., Herrero‐Beaumont, G., Rovati, L., et al. (2010). Glucosamine sulphate in the treatment of knee osteoarthritis: Cost‐effectiveness comparison with paracetamol. Int. J. Clin. Pract. 64 (6), 756–762. doi:10.1111/j.1742-1241.2010.02362.x
Spiegel, B. M., Chiou, C. F., and Ofman, J. J. (2005). Minimizing complications from nonsteroidal antiinflammatory drugs: Cost‐effectiveness of competing strategies in varying risk groups. Arthritis Rheum. 53 (2), 185–197. doi:10.1002/art.21065
Sullivan, P. W., and Ghushchyan, V. (2006). Preference-based EQ-5D index scores for chronic conditions in the United States. Med. Decis. Mak. 26 (4), 410–420. doi:10.1177/0272989X06290495
Tamteeranon, Y., Khonputsa, P., Chaikledkaew, U., Teerawattananon, Y., and Lim, S. (2007). “Economic evaluation of HMG-CoA reductase inhibitors (statin) for primary prevention of cardiovascular diseases among Thai population,” in Health intervention and Technology assessment Program (HITAP) (Bangkok: Ministry of Public Health).
Tantivess, S., and Tangcharoensathien, V. (2016). Coverage decisions and the court: A public health perspective on glucosamine reimbursement in Thailand. Health Syst. Reform 2 (2), 106–111. doi:10.1080/23288604.2016.1128514
Tanvejsilp, P., Taychakhoonavudh, S., Chaikledkaew, U., Chaiyakunapruk, N., and Ngorsuraches, S. (2019). Revisit roles of HTA on drug policy in universal health coverage in Thailand: Where are we? And what is next? Value Health Reg. Issues 18, 78–82. doi:10.1016/j.vhri.2018.11.004
Thai Rheumatism Association (2010). Guideline for the treatment of osteoarthritis of knee. Available at http://www.thairheumatology.org/wp-content/uploads/2016/08/Guideline-for-Management-of-OA-knee.pdf (Accessed Dec 2, 2017).
The Health Intervention and Technology Assessment Program (HITAP) (2017). Standard cost list for health Technology assessment. Available at http://costingmenu.hitap.net/(Accessed Sep 05, 2017).
Torio, CM, and Andrews, RM (2013).National inpatient hospital costs: the most expensive conditions by payer.
Vivattanavarang, N. (2011). Direct unit cost of total knee replacement in ChiangRai prachanukroh hospital. Chiangrai Med. J. 3 (2), 61.
Wajajamroen, B. (2016). Factors affecting health utilities in patients with knee osteoarthitis at Nopparat Rajathanee hospital [Doctoral dissertation]. Silpakorn University.
Wells, B. G., DiPiro, J. T., Schwinghammer, T. L., and DiPiro, C. V. (2015). “Osteoarthritis,” in Pharmacotherapy handbook. Editor T. La Schwinghammer. 9 ed. (United States: McGraw-Hill Education), 11.
Yen, Z-S., Lai, M-S., Wang, C-T., Chen, L-S., Chen, S-C., Chen, W-J., et al. (2004). Cost-effectiveness of treatment strategies for osteoarthritis of the knee in Taiwan. J. Rheumatol. 31 (9), 1797–1803.
Keywords: knee osteoarthritis, economic evaluation, cost-utility analysis, glucosamine, etoricoxib
Citation: Luksameesate P, Tanavalee A and Taychakhoonavudh S (2022) An economic evaluation of knee osteoarthritis treatments in Thailand. Front. Pharmacol. 13:926431. doi: 10.3389/fphar.2022.926431
Received: 22 April 2022; Accepted: 30 August 2022;
Published: 26 September 2022.
Edited by:
Robert L. Lins, Independent researcher, BelgiumReviewed by:
Yong Yang, Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, ChinaCopyright © 2022 Luksameesate, Tanavalee and Taychakhoonavudh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suthira Taychakhoonavudh, c3V0aGlyYS50QGNodWxhLmFjLnRo
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.