- 1Department of Pharmacology, Physiology and Physiopathology, Faculty of Pharmacy, “Iuliu Haţieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania
- 2Department of Medical Informatics and Biostatistics, “Iuliu Hatieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania
- 3Pharmacovigilance Research Center, “Iuliu Hatieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania
- 4Department of Internal Medicine, “Iuliu Hațieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania
Background: The Food and Drug Administration issued a warning on the risk of acute kidney injury and a signal of nephrolithiasis for patients using sodium–glucose co-transporter 2 inhibitors (SGLT2i). We performed a descriptive analysis on acute renal failure (ARF) and nephrolithiasis cases reported to SGLT2i in the VigiBase®, in the scope of characterizing the patients and reactions and to report on the disproportionality analysis.
Methods: We analyzed all ARF and nephrolithiasis reports for SGLT2i in VigiBase from inception to September 2021. ARF cases were defined as reports containing at least one of the preferred terms (PTs) included in the ARF narrow Medical Dictionary for Regulatory Activities Standardised Queries (MedDRA SMQ). SGLT2i exposure was considered for reports with at least one gliflozin as a suspected/interacting drug. We characterized the patients, reporters, and reactions, and we present the proportional reporting ratio (PRR).
Results: Of 27,370,413 total reports in VigiBase, we found 3,972 ARF reactions to gliflozins as suspected/interacting drugs in 3,751 patients and 231 nephrolithiasis reactions in 227 patients. Most cases were reported from American regions (3057; 81.49%), for patients of age group 45–64 years (1590; 59%). About 30% (1156) of the ARF reports were registered in 2018, most from spontaneous reporting, and from consumers followed by healthcare professionals (2,235; 61% and 1440; 38%, respectively). Canagliflozin was the most involved gliflozin in the ARF and nephrolithiasis cases (2,640; 67% and 109; 47%, respectively). The great majority of ARF and nephrolithiasis reports were serious (3,761; 95% and 182; 79%, respectively). Of the total ARF cases reported, 51 had fatal outcome, while 152 had not recovered/not resolved outcome. No fatal outcome was reported for nephrolithiasis. Disproportionality analysis in full database showed a PRR of 4.68 (95% CI 4.53–4.83) for all gliflozins–ARF and a PRR of 3.44 (95% CI 3.00–3.95) for all gliflozins–nephrolithiasis.
Conclusion: Most of ARF reports associated with gliflozins were serious, with an important number of cases with fatal outcome. A drug safety signal was found between ARF narrow SMQ and gliflozins. Also, gliflozins were associated with an increase in the proportion of nephrolithiasis reports compared to other medications.
1 Introduction
The benefit–risk balance of sodium–glucose co-transporter 2 inhibitors (SGLT2i, gliflozins), a relatively new glucose-lowering agent (GLA) class, was extensively studied over the last years. SGLT2i have proven to have beneficial effects beyond glycemic control, on metabolic, cardiovascular, and renal outcomes (Minze et al., 2018). Three cardiovascular clinical trials, (EMPA-REG, CANVAS, and DECLARE-TIMI 58 trial), reported beneficial renal outcomes (AY et al., 2018). Two kidney clinical trials (CREDENCE and DAPA_CKD) demonstrated that SGLT2 inhibitors can reduce the risk of worsening chronic kidney disease (CKD) (Giorgino et al., 2020). In contrast to this renoprotective benefit proven during clinical trials, several case reports on acute kidney injury (AKI) associated with SGLT2i were reported and triggered a strengthening of the warning on the risk of AKI issued by the Food and Drug Administration (FDA) (U.S. Food and Drug Administration, 2015; U.S. Food and Drug Administration, 2016). In addition, nephrolithiasis is another possible adverse drug reaction (ADR) of SGLT2i at the renal level. FDA also issued a warning on this potential risk, raising concerns regarding renal safety of these medications (U.S. Food and Drug Administration, 2017).
AKI is defined by an abrupt decrease in kidney function with important clinical consequences, including increased risk of death. AKI includes, but is not limited to, acute renal failure (ARF) and other, less severe conditions (Kellum et al., 2012). As a syndrome, AKI includes patients without actual damage to the kidney but with functional impairment (Kellum et al., 2012). Patients with diabetes are known to have a higher susceptibility to AKI/ARF (Wang et al., 2021), but also medications are a common cause of AKI/ARF, especially for patients admitted to hospital wards and the intensive care unit (Perazella and Rosner, 2022a).
The risk of developing renal impairment with SGLT2i and the role of plausible mechanisms have to be established yet (Szalat et al., 2018). SGLT2i may induce excessive diuresis which can lead to intravascular volume depletion, particularly in hemodynamically unstable and volume-depleted patients (Perlman et al., 2017; Szalat et al., 2018). Reduced trans-glomerular pressure with a modest decline in kidney function, a characteristic of SGLT2i, is on the long-term renal protective. The acute decrease in estimated glomerular filtration rate (eGFR) was attributed to the effect of proximal tubular natriuresis on tubuloglomerular feedback, leading to reversible intrarenal hemodynamic effects, including afferent arteriole vasoconstriction (Cherney et al., 2014; Sridhar et al., 2020; Meraz-Muñoz et al., 2021). Lastly, SLGT2i increase medullary oxygen consumption, increasing the risk for hypoxia (Perlman et al., 2017), especially with concomitant use of agents impairing medullary oxygenation, such as nonsteroidal antiinflammatory drugs (NSAIDs) and radiocontrast agents (Szalat et al., 2018).
The clinical implications of the acute decrease in eGFR were unknown and led to concerns about the safety of SGLT2i because observational reports suggested an increase in the risk of AKI (Perlman et al., 2017). On the other hand, a retrospective cohort study found that there were no differences observed in the incident AKI in SGLT2i versus other GLAs (Rampersad et al., 2020). A systematic review and meta-analysis showed that SGLT2i even reduced the odds of suffering AKI with and without hospitalization in randomized trials and in the real-world setting, despite the fact that more AEs related to hypovolemia are reported (Menne et al., 2019). In addition, some propensity score analysis comparing SGLT2i with other antidiabetics (dipeptidyl peptidase 4 inhibitors) reported that AKI risk was reduced in SGLT2i users, but the mechanism is unknown yet (Nadkarni et al., 2017; Sridhar et al., 2020).
In addition, SGLT2i treatment could lower the risk for incident and recurrent kidney stones in people with type 2 diabetes, as recent evidence suggests (Kristensen et al., 2021). SGLT2i induce osmotic diuresis, increased urinary flow, polyuria, and urine dilution that may even reduce the risk of nephrolithiasis (Kristensen et al., 2021). However, a potential signal of nephrolithiasis was detected earlier by the FDA in patients using SGLT2 inhibitors (U.S. Food and Drug Administration, 2017). It was also suggested that SGLT2i can cause hyperuricosuria (Chino et al., 2014; Novikov et al., 2019), which may confer a greater risk for specific types of kidney stones. Diabetes mellitus is also a known risk factor for nephrolithiasis (Aune et al., 2018).
Despite the fact that the risk of AKI and nephrolithiasis was not confirmed by observational database studies of large cohorts, reports of AKI and nephrolithiasis associated with SGLT2i use continue to be submitted to VigiBase (2487 and 231 reactions, respectively, by September 2021), the World Health Organization (WHO) unique global database of individual case safety reports (ICSRs). VigiBase includes reports from around 140 countries representing over 90% of the world’s population and is maintained by the Uppsala Monitoring Centre (Uppsala, Sweden). The database contained more than 27 million ICSRs that has been submitted by national pharmacovigilance centers since 1967. Although VigiBase data cannot offer evidence on the causality relationship between the gliflozins and the events, analysis on the disproportionality of events reported for a particular drug versus the rest of the database and versus a restrictive diabetic therapeutic area can still be informative and add value to better characterize the safety profile of this class of drugs. Also, specific information on patients and reactions reported may help in contouring the common ground of the reactions reported. The objective of our study was to perform such analyses in the VigiBase®, on ARF and nephrolithiasis reported to SGLT2i versus all ADRs and ADRs of all antidiabetics, in the scope of characterizing the patients and reactions and to investigate their relationship through disproportionality analysis in the largest international pharmacovigilance database.
2 Methods
2.1 Study Population and Design
This is an observational study that characterizes the reported ARF and nephrolithiasis reactions related to gliflozins use and presents their disproportionality analysis when compared to all ADRs and to ADRs of all antidiabetics (ATC A10) in the VigiBase from inception to 31 August 2021.
The adverse events captured in the ICSRs have been coded according to the latest version of the Medical Dictionary for Regulatory Activities (MedDRA) at the time of reporting.
2.2 Information
Following variables on the reports were received from WHO: region of origin, date of report, reporter qualification, report source, serious, seriousness criteria per reaction, patient’s characteristics (sex and age group), drugs (indication, start and end dates, dosage, and route of administration), and reactions reported (MedDRA terms, onset date and end date, time to onset, reaction outcome, and dechallenge/rechallenge action). One ICSR can include more than one adverse reaction and more than one suspected or interacting drugs.
2.3 Data Management
All ARF and nephrolithiasis reactions with canagliflozin, dapagliflozin, empagliflozin, ertugliflozin, ipragliflozin, and/or luseogliflozin as suspected or interacting drugs until 31 August 2021 were received from VigiBase. In addition, the disproportionality analysis of each preferred term (PT, the term used to describe an adverse event in MedDRA) associated with each of the gliflozins as compared to all ADR reports and compared to all antidiabetics reports were received from VigiBase. We hypothesized that it is possible that some cases could be reported with a PT different from AKI or ARF, and decided to use MedDRA Standardised Medical Queries (SMQs), which are validated, predetermined sets of MedDRA terms grouped together to support safety analysis.1 We identified the acute renal failure SMQ broad and narrow and we decided to use the narrow search as this is more specific (cases highly likely to be related to a specific condition). Narrow search on SMQ “Acute Renal Failure” yielded 19 PTs as follows: Acute kidney injury, Acute phosphate nephropathy, Anuria, Azothemia, Continuous hemodiafiltration, Dialysis, Fetal renal impairment, Hemodialysis, Hemofiltration, Neonatal anuria, Nephropathy toxic, Oliguria, Peritoneal dialysis, Prerenal failure, Renal failure, Renal failure neonatal, Renal impairment, Renal impairment neonatal, and Subacute kidney injury. All these PTs were included in the analysis.
The reports of ARF with fatal outcome were further analyzed with the aim of identifying and characterizing co-suspect/concomitant medications, concurrent conditions and potential risk factors for fatal ARF.
Regarding nephrolithiasis, we used only the PT with the same name.
Exposure to gliflozins was defined as the mention in the report of at least one of the following: canagliflozin, dapagliflozin, empagliflozin, ertugliflozin, ipragliflozin, and/or luseogliflozin as suspect or interacting medication.
2.4 Data Analysis
Descriptive statistics were used to summarize the baseline characteristics of the reports, patients, drugs, and reactions for the ICSRs containing adverse events from the ARF SMQ narrow (ARF reactions hereafter) and nephrolithiasis reactions. The comorbidities of the patients were retrieved from the field of indication for all drugs included in the analyzed ICSRs.
The disproportionality of selected reactions associated with the use of the six gliflozins was studied in a case–non-case analysis individually for each PT. The proportion of ARF/nephrolithiasis reactions reported for gliflozins was compared with the proportion of the ARF/nephrolithiasis reactions reported for all other drugs in VigiBase. Additionally, as diabetes mellitus itself is a known risk factor for development of kidney disease, a sensitivity analysis was performed and the database was restricted to the diabetic therapeutic area (i.e., considering only medicines used in diabetes) as the background, to take into account the increased baseline risk of ARF and nephrolithiasis. Furthermore, we excluded from the database all reactions with co-suspected drugs to limit the innocent bystander effect with gliflozins.
The proportional reporting ratio (PRR) was used as a measure of disproportionate reporting. The data were provided by the Uppsala Monitoring Centre for individual gliflozins and PTs. We calculated the PRR for the gliflozin class and ARF reactions and nephrolithiasis as described in the Supplementary Tables 1, 2 on the data provided by the WHO Uppsala Monitoring Center(Proportional Reporting Ratio, 2022).
A PRR value ≥ 1 associated with ≥5 cases was considered a positive association between the reaction and gliflozins (Slattery et al., 2013).
Microsoft Excel was used to compute the PRR values that were not provided by WHO Uppsala Monitoring Center and to tabulate relevant data.
3 Results
3.1 Case Selection
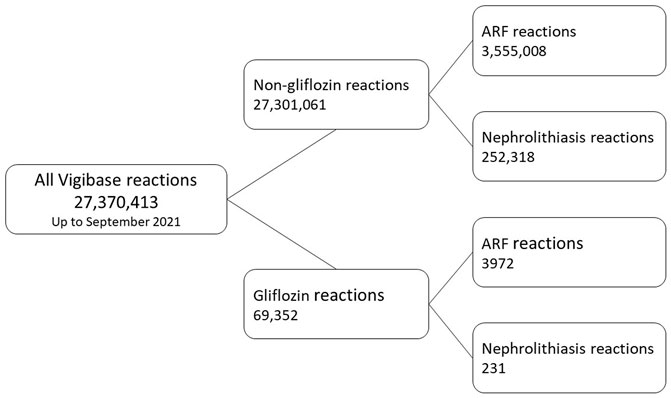
A flowchart of the study is presented in Figure 1. VigiBase contained 27,370,413 reports until 31 August 2021. Exposure to gliflozins as suspected/interacting drug was found in 69,352 reports.
3.2 Reports and ADR Characteristics
We identified 3972 ARF reports to gliflozins as suspected/interacting drugs in 3751 patients and 231 nephrolithiasis reactions in 227 patients (more than one gliflozin may be suspected for one reaction and more than one PT may be reported in case of ARF SMQ).
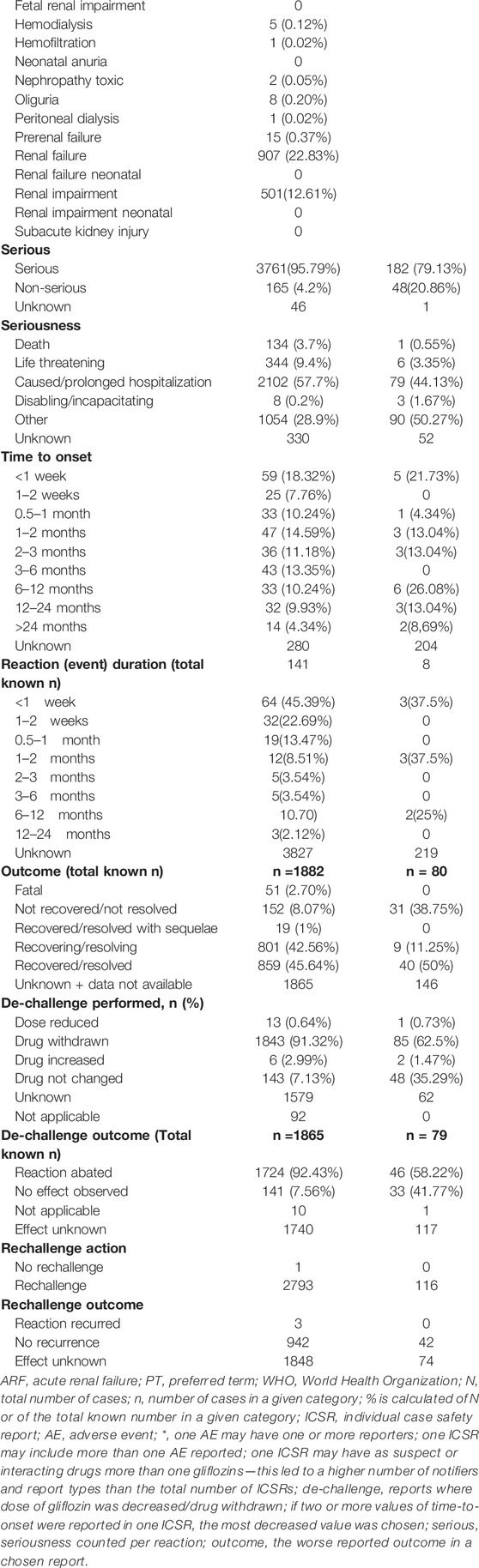
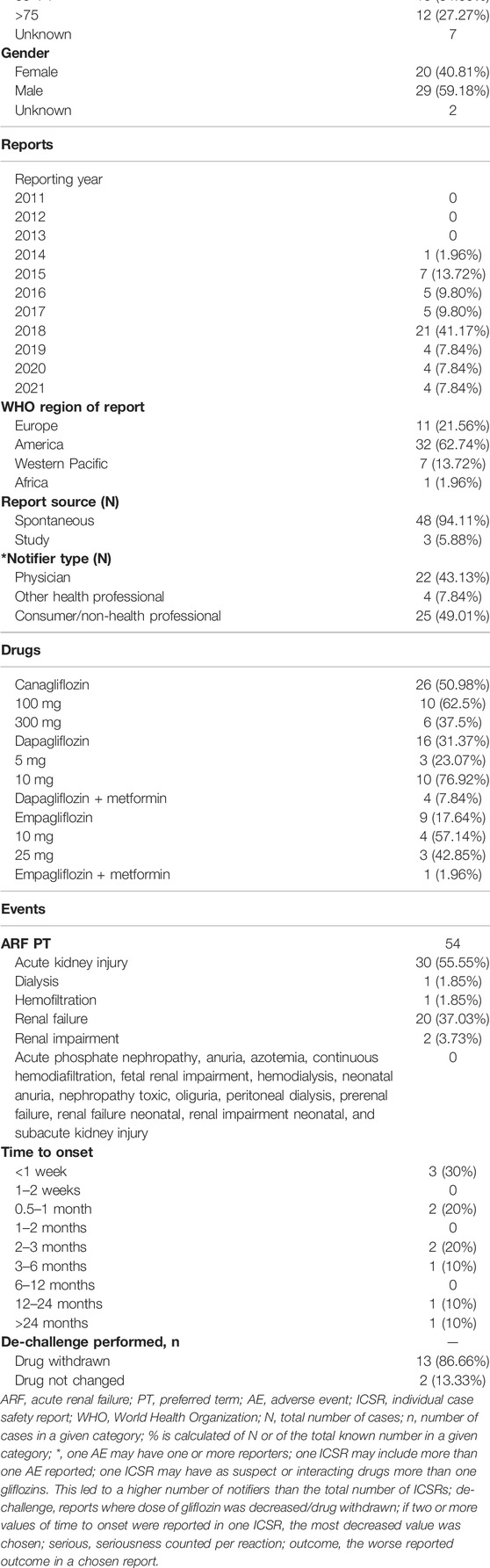
The characteristics of reports, patients, and reactions are shown in Table 1. The most cases with available information were reported in the regions of America (3057; 81.49%), in the category of 45–64 years of age (1590; 59%) and male gender was more frequent (1852; 57% and 114; 56% in ARF and nephrolithiasis reports, respectively).
Most of the ARF reports came from spontaneous reporting, the majority from consumers followed by healthcare professionals (2235; 60.86% and 1.440; 38.09% from available data, respectively with 59.57% coming from consumers only). The first ARF reports were registered in 2011 and their number increased until 2018 (1156, around 31% of the total reports) and then declined until 2021 (169 reports by September) (Table 1). For nephrolithiasis, the highest number of reports were registered in 2015 (53 cases) and slightly decreased in the years after.
For the ARF analysis, we found that canagliflozin was the most frequently involved gliflozin (2640 cases; 66.81%) followed by dapagliflozin (622 cases; 15.74%) and empagliflozin (606 cases; 15.33%). Canagliflozin was the most involved gliflozin in the nephrolithiasis cases as well (109 cases; 47.18%), followed by empagliflozin (65 cases; 28.13%) and dapagliflozin (55 cases; 23.80%) (Table 1).
Of the 19 PTs included in the ARF SMQ narrow, we found 12 PTs reported for gliflozins. AKI (2387: 62.61%), renal failure (907; 22.83%), and renal impairment (501; 12.61%) were the most reported PTs for each gliflozin. Most of the reports were with canagliflozin (1750 AKI, 599 renal failure and 160 renal impairment), followed by empagliflozin (280 AKI, 128 renal impairment and 122 renal failure) and dapagliflozin (245 AKI, 138 renal impairment and 136 renal failure), (Table 1; Figure 2).
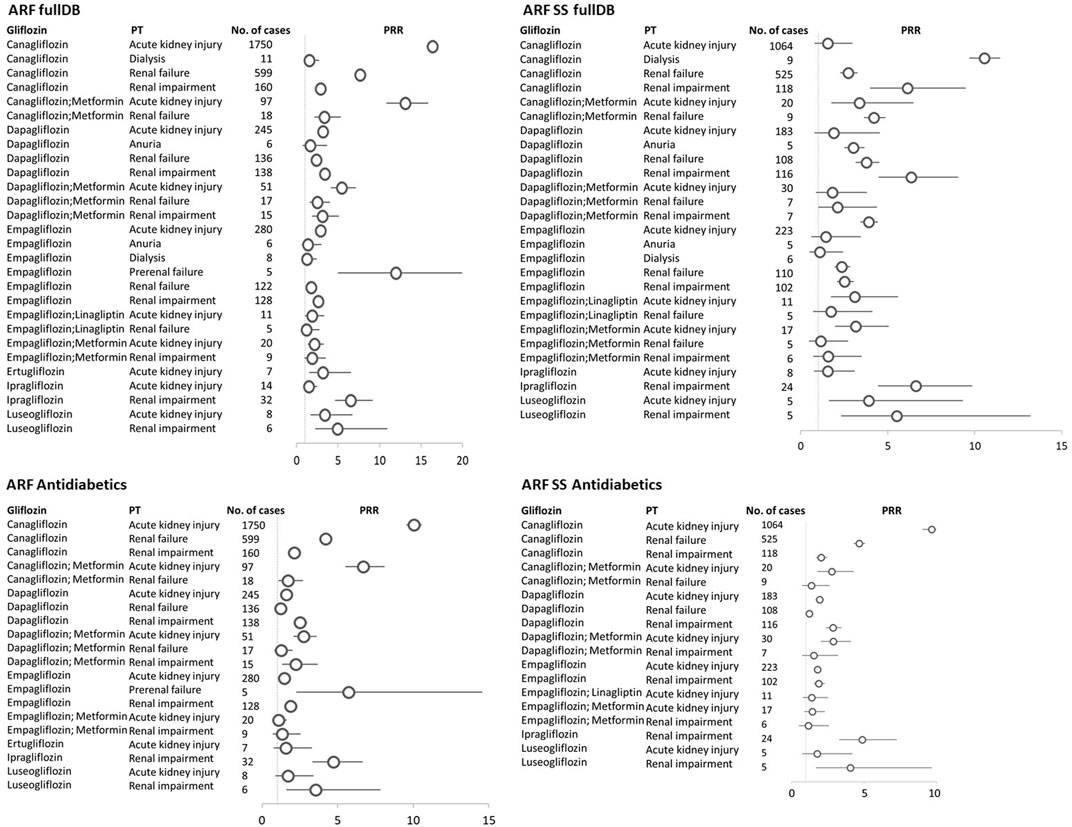
FIGURE 2. Gliflozins-ARF narrow SMQ reactions with the proportional reporting ratio (PRR) greater than 1 and minimum 5 reactions were reported. ARF fullDB, acute renal failure narrow SMQ reactions with PRR calculated against all reports in VigiBase; ARF SS fullDB, acute renal failure narrow SMQ reactions with PRR calculated against all reports in VigiBase where gliflozins are the only suspect in the respective reaction; ARF Antidiabetics, acute renal failure narrow SMQ reactions with PRR calculated against all reports to antidiabetics in VigiBase; ARF SS Antidiabetics, acute renal failure narrow SMQ reactions with PRR calculated against all reports to antidiabetics in VigiBase where gliflozins are the only suspect in the respective reaction; PT, MedDRA preferred term; circles represent PRR, and horizontal lines represent 95% confidence interval.
Information on time-to-onset of the reaction was available in 322 (8%) cases for ARF and in 158 reports ARF occurred after the first 8-week period of starting therapy (49% of the cases with this information available). In 45% of the 141 cases with available information, the reaction duration was less than 1 week for ARF. In 23 cases with information available for nephrolithiasis, we found the most cases reported within 6–12 months from treatment start (6 cases) followed by the interval within first week (5 cases) (Table 1).
The great majority of ARF reports were serious (95.79%) with more than half (57.7%) causing/prolonging hospitalization. Of the total ARF reported, 51 had fatal outcome (2.7% from available data), while 152 (8.07%) had not recovered/not resolved outcome. However, the vast majority of the cases have a favorable outcome, recovered or recovering at the moment of reporting (88.2% of known data). In 1724 of the ARF reactions, reaction abated after gliflozin withdrawal (positive de-challenge in 43.40% of the total ARF reactions) and also 3 cases with positive re-challenge were reported (Table 1).
The most frequent co-suspect and interacting drugs reported in the 3751 ARF ICSRs were: other antidiabetics (biguanide, insulin, DPP-4 inhibitors, GLP-1 receptor agonists, and sulphonylurea), diuretics and antihypertensive drugs. We found an important number of patients using diuretics (n = 125), ACE inhibitors and ARB blockers (n = 163), and NSAIDs (n = 43) who had ARF (Table 2) The most frequent comorbidities of the patients with ARF were hypertension, cardiac disorders, depression and anxiety, hyperlipidemia, and pain. Associated ketoacidosis was frequently reported (27.87% of reports with ARF reactions), followed by different types of infections. If we cumulate the number of infections, these become the most frequently reported PTs (30.84% of reports with ARF reactions) (Table 2) Regarding associated conditions with ARF that induce plasmatic volume depletion, we found dehydration, diarrhea, and vomiting (n = 437 associated PTs), cardiac failure (n = 86 associated diseases), hypovolemia (n = 25 associated PTs), or concomitant use of diuretics (n = 124).
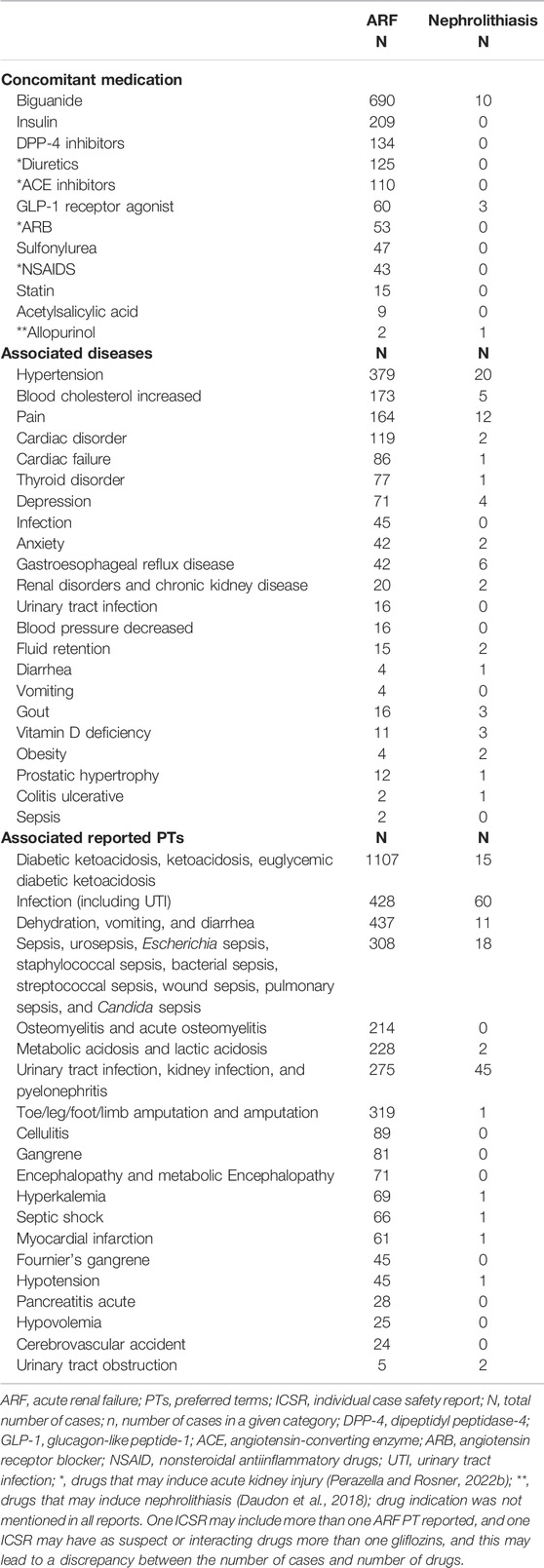
TABLE 2. Concomitant medication (co-suspect and interacting) and associated conditions in ARF and nephrolithiasis reports.
For nephrolithiasis, the majority of reports were also serious (79%) and 50% of the cases with this information available had a favorable outcome. No fatal cases were reported. We found 46 nephrolithiasis reactions with positive de-challenge (58.22% of the available data), but no cases with positive re-challenge were reported (Table 1). Urinary/renal infections were the most frequent associated adverse event with nephrolithiasis (52 reports, 22.51%).
No dose–response pattern was observed for ARF and nephrolithiasis (Table 1).
3.3 Fatal Outcome Analysis
Of the total of 3972 ARF ICSRs, 134 (3.7%) deaths were reported as seriousness criteria and a fatal outcome due to ARF reaction was reported for 51 patients (1.35%).
The characteristics of reports, patients, and reactions are shown in Table 3. Most of the reports came from spontaneous reporting and half from healthcare professionals. Therefore, half of the fatal ARF cases were considered medically confirmed.
In 32 fatal ARF reports (62.74%), the only suspect drug was one of the gliflozins. Most of the ARF cases were reported as AKI (n = 30) followed by renal failure (n = 20), renal impairment (n = 2), hemofiltration (n = 1), and dialysis (n = 1). In 16 (31%) cases, the only fatal reaction reported was as an ARF PT: AKI (n = 9), renal failure (n = 6), or renal impairment (n = 1).
In the majority of the reports (n = 35, 68%), other associated conditions were also considered responsible for this fatal outcome along with ARF. The most frequent associated causes of death were metabolic acidosis (including ketoacidosis) in 11 patients (21.56%) and sepsis in 10 patients (19.60%) (Table 4).
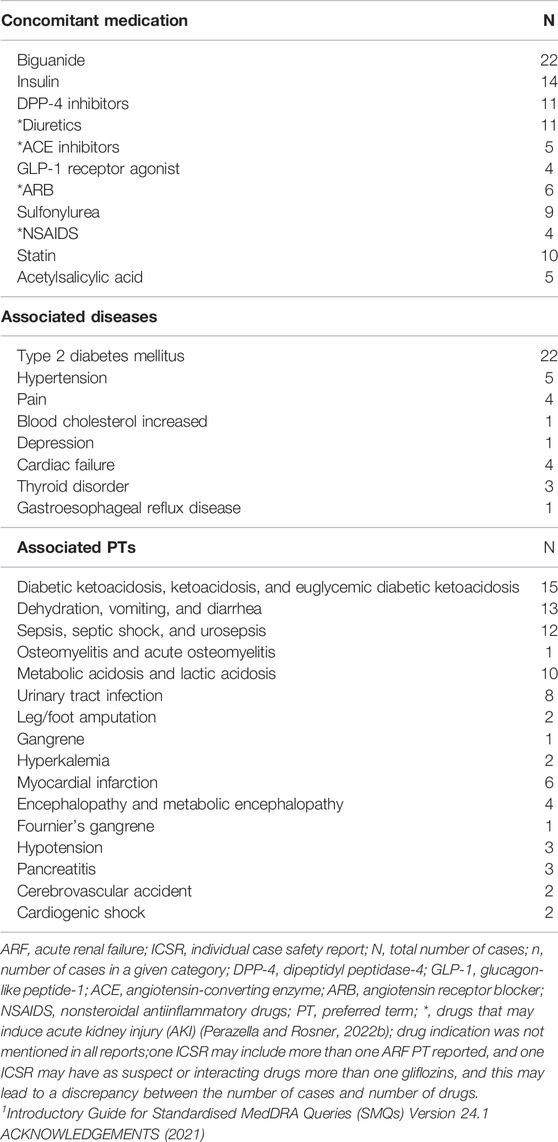
TABLE 4. Concomitant medication (co-suspect, interacting, and concomitant) and associated conditions in fatal ARF reports.
3.4 Disproportionality Analysis
3.4.1 ARF
A PRR of 4.68 (95% CI 4.53–4.83) for the association ARF narrow SMQ–gliflozins (all) as compared to ARF–other drugs was found. When we used all database as reference, we found 28 significant associations between different gliflozins use and acute kidney injury, renal failure, renal impairment, dialysis, anuria, and prerenal failure PTs (Figure 2).
In the sensitivity analysis, the significance (as defined by PRR≥1, associated with≥5 cases) of these association was kept for most of the pairs 27) when taking into account only ARF cases where gliflozins were the single suspected drug. When reference was set as all reactions reported to antidiabetics instead of the entire database, 20 of the aforementioned associations were significant in terms of PRR. Narrowing further the analysis to gliflozins as single suspected drugs versus the reports to antidiabetics, we found significant PRRs in 18 pairs (Figure 2).
3.4.2 Nephrolithiasis
For nephrolithiasis, we found a PRR of 3.44 (95% CI 3.00–3.95) for all gliflozins calculated versus the entire database. When we took into account all reports where gliflozins were deemed suspected/interacting drugs, we found six pairs of gliflozins–nephrolithiasis with significant PRRs (against both the entire database and reports to antidiabetics). When narrowing to reports where gliflozins were the only suspected drug, we found significant PRRs only for dapagliflozin + metformin and empagliflozin + linagliptin combinations (Figure 3).
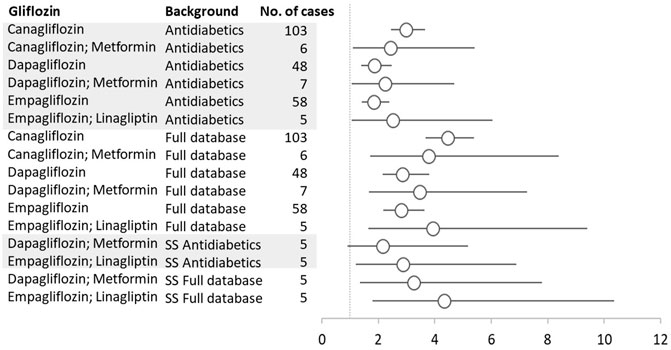
FIGURE 3. Gliflozins–Nephrolithiasis reactions with the proportional reporting ratio (PRR) greater than 1 and minimum five reactions were reported. SS, single suspect; circles represent PRR, and vertical lines represent 95% confidence.
4 Discussion
Renal safety of SGLTi is much debated in the medical literature. While the benefits of gliflozins in the chronic mild or moderate kidney disease are widely recognized, they are also suspected of inducing adverse renal effects, like acute kidney injury and nephrolithiasis.
Our research focused on the association of gliflozins and ARF and nephrolithiasis in the world’s largest pharmacovigilance database (VigiBase), with the aim to characterize the patients, reports, and drugs involved and also to look into the disproportionality analysis.
Most of both the ARF and nephrolithiasis reactions were reported in patients within the 45–64 years of age group and slightly more in males. This is in line with results of Perlman et al. (2017) who reported a mean age of 59 years and 49% females in their FDA adverse event report system (FAERS) database for the ARF broad SMQ.
Some articles reported that SGLT2 inhibitors were not observed to be associated with increased risk for AKI. The JADER database study (Katsuhara and Ogawa, 2020) did not find a significant association between SGLT2 inhibitors and ARF, but 1) the database is smaller (only 126 ARF cases reported to SGLT2) and also 2) they used ARF broad definition and included 50 PTs, which may have affected their ROR. One network meta-analysis (Zhao et al., 2020) only included clinical trials on patients with established or at risk of cardiovascular disease or CKD, limiting its generalizability to other patient populations without these risks, and since the renal risk was not the primary objective, the AKI events were more likely to be underreported. Also, the definitions of ARF may differ between the trials included. On the other hand, case reports of ARF due to SGLT2i with proper documentation are published, and ADR reports continue to be submitted to VigiBase, despite the FDA strengthening the warning on the risk of AKI in October 2015, so its effect should be faded.
The FDA warning on the risk of AKI associated with gliflozins was issued in June 2016 and probably impacted the reporting of ARF in the VigiBase. The number of ARF reports doubled from 2015 to 2017 (341–720), but the highest number of ARF cases was in 2018 (1186 reports). Perlman et al. (2017) found a rise of 1 in ROR after the FDA warning and by September 2016 (a period of approximately 2 months. Interestingly, for ARF, a lot of the reports came from the consumers only (59.57% of the notifiers with available information), although typically this is not a reaction that can be recognized by consumers. We could not identify the reason for this reporting and how much this impacted the disproportionality analysis. A dose–response relationship could not be established, as variation in the number of cases per dose was probably due to the patterns of the gliflozins’ use (e.g., approximately the same number of cases for canagliflozin 100 and 300 mg, more case for dapagliflozin 10 versus 5 mg, but also more cases for empagliflozin 10 versus 25 mg). This is consistent with the dose trend of reports from another analysis that looked at the gliflozins reactions in VigiBase during the same period (Frent et al., 2021).
The exact mechanism for gliflozins induced AKI is not fully understood. Decreased glomerular pressure is a likely potential explanation, but development of osmotic nephropathy may be also an alternative explanation at least in some reports. Some case reports with biopsy evidence of osmotic nephropathy were published (Sia et al., 2021; Perazella and Juncos, 2022). A possible explanation is that proximal tubules are exposed to significant amounts of filtered glucose, as there are some experimental studies and in humans exposed to glucose 10% solutions that leads to osmotic nephropathy. Probably, the excessive amount of urinary glucose undergoes tubular cell pinocytosis. Most of the AKI cases occurring in clinical practice were not biopsied; therefore, drug-induced osmotic nephropathy as a possible mechanism for AKI induced by SGLT2i is probably under-recognized (Perazella and Juncos, 2022). A possible approach mentioned in the literature is to undertake a kidney biopsy in patients with prolonged AKI (>5 days) or dialysis-dependent AKI that persists despite withdrawing SGLT2i, as such a clinical presentation is unlikely only due to a reduction in glomerular pressure or water and sodium depletion (Phadke et al., 2020). In our analysis, in 92.43%% of patients with ARF where gliflozin was discontinued, the reaction abated and most patients improved after stopping the drug. This is in line with the strengthening warning of the FDA (U.S. Food and Drug Administration, 2016).
The FDA warning was based on the analysis of 101 cases of AKI reported during March 2013–October 2015 with approximately half of the AKI occurring within 1 month of starting the drug (U.S. Food and Drug Administration, 2016). In our analysis, only 36.32% of the cases with available information occurred within 1 month and another 14.59% occurred during 1–2 months of starting therapy. It is well known that the initiation of SGLT2i may cause an increase in serum creatinine and decrease in eGFR during the first 6 weeks of therapy. In patients with moderate renal impairment, the increase in serum creatinine generally does not exceed 0.2 mg/dl, occurs within the first 6 weeks of starting therapy, and then stabilizes. Increases that do not fit this pattern should prompt further evaluation to exclude the possibility of acute kidney injury (AstraZeneca Pharmaceuticals, 2021; Janssen Pharmaceuticals, 2021; Boehringer Ingelheim Pharmaceuticals, 2022). However, in VigiBase almost half of the cases (49%) with available data occurred after 2 months of SGLT2i therapy. A descriptive study of clinical spectrum and mechanism of AKI in patients with diabetes mellitus on SGLT2i found a wide interval of 7–365 days period after initiation of SGLT2i for AKI (Pearlman et al., 2018).
There are some known risk factors that may predispose patients with SGLT2i treatment to AKI, like decreased blood volume, chronic kidney insufficiency, congestive heart failure, and concomitant medications such as diuretics, ACE inhibitors, ARBs, and NSAIDs (U.S. Food and Drug Administration, 2016).
Since the cause of AKI due to gliflozins is generally attributed to osmotic diuresis that induce plasmatic volume depletion (AstraZeneca Pharmaceuticals, 2021; Janssen Pharmaceuticals, 2021; Boehringer Ingelheim Pharmaceuticals, 2022), we looked at the associated conditions of the patients like dehydration, diarrhea and vomiting, cardiac failure, hypovolemia, or concomitant use of diuretics. Regarding other medication that can cause/contribute to ARF, we found a significant number of patients with diuretics, ACE inhibitors, ARB blockers, and NSAIDs. This is still far from the findings of Pearlman et al. (2018), where all patients with gliflozins-associated AKI had been prescribed RAAS blockers and most had also injection of contrast-product or NSAIDs).
The most associated reaction in ARF cases was ketoacidosis in 27.87% of patients. Diabetic ketoacidosis (DKA) is a known rare but potentially life-threatening condition also related to SGLT2i (AstraZeneca Pharmaceuticals, 2021; Janssen Pharmaceuticals, 2021; Boehringer Ingelheim Pharmaceuticals, 2022); Plewa et al., 2022). Due to glucose-induced osmotic polyuria and even emesis, volume depletion is a major cause of AKI in DKA patients. AKI is a complication of diabetic ketoacidosis and an independent risk factor for poor long-term renal outcomes and mortality in DKA patients (Orban et al., 2014; Chen et al., 2020; Janssen Pharmaceuticals, 2021).
Sepsis and septic shock were the second most common reactions reported together with ARF in our analysis (n = 366 reactions reported). Sepsis is a known complication of diabetes mellitus and a major cause of AKI. Diabetes was also identified as an independent risk factor for AKI. Moreover, the risk of AKI during sepsis is increased in patients with diabetes. AKI develops in one-fourth of patients with sepsis and half of the patients with shock and is associated with high-mortality (up to 70%) (Venot et al., 2015) ARF with fatal outcome.
In our study, the reported data for time-to-onset was limited, only 10 cases out of 54 cases of fatal ARF, with 5 cases reported within 6 weeks of treatment with gliflozin. Fatal ARF may occur also within 6 weeks of the treatment with gliflozin, when an acute reversible decrease in eGFR is expected. AKI that may lead to hospitalization or dialysis was previously reported in SGLT2i US Prescribing Information (US PI), but AKI with fatal outcome was not considered as expected with gliflozins (fatalities were not mentioned in the FDA strengthened warning or in the SGLT2i US PI) (U.S. Food and Drug Administration, 2016; Janssen Pharmaceuticals, 2021; AstraZeneca Pharmaceuticals, 2021; Boehringer Ingelheim Pharmaceuticals, 2022). Medical literature presents though scattered reports suggesting that the risk for AKI may occasionally be fatal or might require renal replacement therapy (Szalat et al., 2018) and we provide in our analysis additional evidence showing that fatal ARF associated with the use of gliflozins is possible, and even more, this may also occur within first 6 weeks of gliflozin treatment.
4.1 Nephrolithiasis
SGLT2i treatment could lower the risk for incident and recurrent kidney stones in people with type 2 diabetes, as recent evidence suggests (Kristensen et al., 2021; Balasubramanian et al., 2022). However, it was also suggested that SGLT2i can cause hyperuricosuria (Chino et al., 2014; Novikov et al., 2019), which may confer a greater risk for specific types of kidney stones. Moreover, a recent study of pooled analysis of data from clinical studies found that in 19% of patients, treatment with luseogliflozin resulted in increased serum uric acid, which may be due to reduced glomerular filtration of uric acid via the tubuloglomerular feedback (Chino et al., 2022). Yet, another meta-analysis found no association of SGLT-2 inhibitors with nephrolithiasis (Cosentino et al., 2019).
In 2017, a potential signal of nephrolithiasis in patients using SGLT2 inhibitors was detected by the FDA, but the decision was not to update the product information based on available information at that time (U.S. Food and Drug Administration, 2017). Our analysis does not contradict previous studies with conflicting results; it only adds the global perspective from VigiBase, the largest database of spontaneous reported ADRs and we included data until September 2021; therefore, data reported from four additional years were included. In fact, the highest evidence till present is the one the meta-analyses of randomized controlled trials like, where no association has been observed (Cosentino et al., 2019). In VigiBase, fewer nephrolithiasis reactions (231) were reported than ARF reactions (3,972), and these did not seem to have been impacted by the FDA potential signal from beginning of 2017. Positive de-challenge was noticed in 20% from the total of 231 reactions, but no cases with positive re-challenge were reported. The most frequently associated adverse event with nephrolithiasis was urinary/renal infection (52 reports, 22.51%). Kidney stone formers are at increased risk of developing urinary tract infections. Some potential mechanisms have been postulated, like bacteria, which increase aggregation of crystals or increase expression of a stone matrix (Brain et al., 2021).
Although we found disproportionality for gliflozins–nephrolithiasis reactions, the low number of reactions reported and the fact that most of them come from the USA, where all adverse events are reported, whether or not implying a causality relationship, firm conclusions regarding SGLT2i-induced nephrolithiasis are still debatable.
4.2 Disproportionality Analysis
The disproportionality analysis showed an increased risk of ARF and gliflozins (PRR = 4.67). From the total of 28 gliflozin-ARF PTs associations found significant in the general analysis, 20 kept their significance when the background was restricted to antidiabetics’ events (considering only reactions to antidiabetics as background). This analysis was done in order to take into account the increased baseline risk of ARF in diabetes patients. In the scope of eliminating alternative causes’ risk, we calculated the PRRs for reactions where only gliflozins were suspected and we found that almost all pairs from the general analysis kept their significance. We can therefore say that our findings for the ARF SMQ narrow are consistent with the ones from the 2016 study (Perlman et al., 2017). Likewise, for nephrolithiasis, our case–non case analysis showed a significant disproportionality (PRR = 3.44) in the general analysis and the same pairs kept significance with antidiabetics as the background. However, it is important to notice that only fixed combinations of gliflozins and metformin/linagliptin kept their significant PRR when excluding nephrolithiasis reports with other suspected drugs. More research is needed to investigate these associations.
Our analysis adds to the current knowledge the description of the gliflozins-related ARF cases from VigiBase and the description of fatal cases. We would like to emphasize here the judicious usage of gliflozins in patients with factors that may predispose patients to ARF. Treating physicians should be aware that AKI is a serious adverse event with potential fatalities when gliflozin class is used. Fatal ARF may occur also within 6 weeks of the treatment with gliflozin, when an acute reversible decrease in eGFR is expected; however, our observation is based on limited time-to-onset data. A high prevalence of severe infections (sepsis) was found in the patients with ARF, and this finding strengthens the link between sepsis and ARF.
4.3 Limitations
1. Another study on ARF reports related to gliflozins was published and contained data from the FDA adverse event report system, FAERS database, until September 2016 (Perlman et al., 2017). We are adding a global perspective, as the information is from VigiBase, the largest database of spontaneous reported ADRs and for an extended reporting period, until 31 August 2021.
2. Most of the ARF reports came from spontaneous reporting, the majority from consumers followed by healthcare professionals (60.86% and 38.09% from available data, respectively with 59.57% coming from consumers only). Therefore, almost 60% of the cases were not medically confirmed. Meanwhile, half of the fatal ARF cases were considered medically confirmed.
3. The disproportionality method: The real risk of ADRs cannot be measured, but only the difference in the rate of notifications by calculating the PRR. However, this method is useful for detecting health risks that need further investigation.
4. Underreporting, lack of reported clinical details, including laboratory results, and reporting bias (more likely to report severe cases).
5. The information contained in the VigiBase comes from a variety of sources, the amount of information in each report varies between reports, and the probability that the suspected adverse effect is drug-related is not the same in all cases. Variation in the causality relation between the drug and the ADR may have affected our analysis since approximately 80% of ARF reports and 84% of nephrolithiasis reports come from the regions of America of which the United States of America (United States) may have a great contribution. The United States collects any adverse event associated with the use of a drug in humans, whether or not considered drug-related (eCFR—Code of Federal Regulations, 2020), while other countries collect only suspected ADRs with at least a possibility of a causal relationship between the drug and reported (Vigiaccess, 2022) Also, the frequency of reporting to VigiBase may vary over time and between countries. This could be the case for increased reporting of AKI following the FDA warning (U.S. Food and Drug Administration, 2016), which could have resulted in increased recognition and reporting of AKI (notoriety bias).
5 Conclusion
SGLT2i are considered safe and provide multiple benefits for patients with type 2 diabetes mellitus. Exposure of patients to SGLT2i is expected to increase due to the recent evidence of cardiovascular and renal protection that led to extension of their indications. ARF cases are rarely reported and the benefit–risk balance of these antidiabetics remains favorable. Nevertheless, healthcare professionals and patients should be aware that: 1) AKI may occur following the use of SGLT2i and may produce serious and even life-threatening consequences, 2) especially in the context of concomitant diseases or medications affecting the renal mechanisms, and 3) also fatal ARF may occur within the first 6 weeks (however, our observation is based on limited time-to-onset data) of treatment with gliflozin, when an acute reversible decrease in eGFR is expected. The observational nature of this study precludes firm statements, but the importance of the findings demands future in-depth analyses to explore the relation with the fatal outcome.
Data Availability Statement
The data that support the findings of this study are available from the WHO Uppsala Monitoring Center, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Requests to access these datasets should be directed to: Q3VzdG9tU2VhcmNoZXNAd2hvLXVtYy5vcmc=.
Author Contributions
CB, IF and CM conceived the study. All authors (IF, CB, DL, AF, FC, and CM) were involved substantially in the design and planning of the study. IF, DL, and CB were involved in data analysis. IF and CB prepared the manuscript. All authors interpreted the results, contributed to later drafts of the manuscript, and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank the WHO Uppsala Monitoring Centre for providing the data. They also confirm that the information does not represent the opinion of the Uppsala Monitoring Centre or the WHO.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.925805/full#supplementary-material
Footnotes
1Introductory Guide for Standardised MedDRA Queries (SMQs) Version 24.1 ACKNOWLEDGEMENTS (2021)
References
AstraZeneca Pharmaceuticals (2021). DailyMed - FARXIGA- Dapagliflozin Tablet, Film Coated, Dailymed - U.S. Natl. Libr. Med., Available at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=72ad22ae-efe6-4cd6-a302-98aaee423d69 (Accessed April 21, 2022).
Aune, D., Mahamat-Saleh, Y., Norat, T., and Riboli, E. (2018). Body Fatness, Diabetes, Physical Activity and Risk of Kidney Stones: a Systematic Review and Meta-Analysis of Cohort Studies. Eur. J. Epidemiol. 33, 1033–1047. doi:10.1007/s10654-018-0426-4
Kluger, A. Y., Tecson, K. M., Barbin, C. M., Lee, A. Y., Lerma, E. V., Rosol, Z. P., et al. (2018). Cardiorenal Outcomes in the CANVAS, DECLARE-TIMI 58, and EMPA-REG OUTCOME Trials: A Systematic Review. Rev. Cardiovasc. Med. 19 (2), 41–49. doi:10.31083/J.RCM.2018.02.907
Balasubramanian, P., Wanner, C., Ferreira, J. P., Ofstad, A. P., Elsaesser, A., Zinman, B., et al. (2022). Empagliflozin and Decreased Risk of Nephrolithiasis: A Potential New Role for SGLT2 Inhibition? J. Clin. Endocrinol. Metab. 107 (7), e3003–e3007. doi:10.1210/CLINEM/DGAC154
Boehringer Ingelheim Pharmaceuticals (2022). DailyMed - JARDIANCE- Empagliflozin Tablet, Film Coated. Dailymed - U.S. Natl. Libr. Med. Available at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=faf3dd6a-9cd0-39c2-0d2e-232cb3f67565 (Accessed April 21, 2022).
Brain, E., Geraghty, R. M., Cook, P., Roderick, P., and Somani, B. (2021). Risk of UTI in Kidney Stone Formers: a Matched-Cohort Study over a Median Follow-Up of 19 Years. World J. Urol. 39, 3095–3101. doi:10.1007/s00345-020-03564-7
Chen, J., Zeng, H., Ouyang, X., Zhu, M., Huang, Q., Yu, W., et al. (2020). The Incidence, Risk Factors, and Long-Term Outcomes of Acute Kidney Injury in Hospitalized Diabetic Ketoacidosis Patients. BMC Nephrol. 21, 48. doi:10.1186/s12882-020-1709-z
Cherney, D. Z., Perkins, B. A., Soleymanlou, N., Maione, M., Lai, V., Lee, A., et al. (2014). Renal Hemodynamic Effect of Sodium-Glucose Cotransporter 2 Inhibition in Patients with Type 1 Diabetes Mellitus. Circulation 129, 587–597. doi:10.1161/CIRCULATIONAHA.113.005081
Chino, Y., Kuwabara, M., and Hisatome, I. (2022). Factors Influencing Change in Serum Uric Acid after Administration of the Sodium-Glucose Cotransporter 2 Inhibitor Luseogliflozin in Patients with Type 2 Diabetes Mellitus. J. Clin. Pharmacol. 62, 366–375. doi:10.1002/JCPH.1970
Chino, Y., Samukawa, Y., Sakai, S., Nakai, Y., Yamaguchi, J., Nakanishi, T., et al. (2014). SGLT2 Inhibitor Lowers Serum Uric Acid through Alteration of Uric Acid Transport Activity in Renal Tubule by Increased Glycosuria. Biopharm. Drug Dispos. 35, 391–404. doi:10.1002/bdd.1909
Cosentino, C., Dicembrini, I., Nreu, B., Mannucci, E., and Monami, M. (2019). Nephrolithiasis and Sodium-Glucose Co-transporter-2 (SGLT-2) Inhibitors: A Meta-Analysis of Randomized Controlled Trials. Diabetes Res. Clin. Pract. 155, 107808. doi:10.1016/j.diabres.2019.107808
Daudon, M., Frochot, V., Bazin, D., and Jungers, P. (2018). Drug-Induced Kidney Stones and Crystalline Nephropathy: Pathophysiology, Prevention and Treatment. Drugs 78, 163–201. doi:10.1007/s40265-017-0853-7
eCFR—Code of Federal Regulations (2020). eCFR—Code of Federal Regulations. Available at: https://www.ecfr.gov/cgi-bin/text-idx?SID=77821c5f7ae204cd5251ed82639d876a&mc=true&node=se21.5.314_180&rgn=div8 (Accessed May 8, 2020).
Frent, I., Bucsa, C., Leucuta, D., Farcas, A., and Mogosan, C. (2021). An Investigation on the Association between Sodium Glucose Co-transporter 2 Inhibitors Use and Acute Pancreatitis: A VigiBase Study. Pharmacoepidemiol Drug Saf. 30, 1428–1440. doi:10.1002/pds.5313
Giorgino, F., Vora, J., Fenici, P., and Solini, A. (2020). Renoprotection with SGLT2 Inhibitors in Type 2 Diabetes over a Spectrum of Cardiovascular and Renal Risk. Cardiovasc Diabetol. 19, 196. doi:10.1186/s12933-020-01163-9
Janssen Pharmaceuticals (2021). DailyMed - INVOKANA- Canagliflozin Tablet, Film Coated. Dailymed - U.S. Natl. Libr. Med. Available at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b9057d3b-b104-4f09-8a61-c61ef9d4a3f3 (Accessed April 21, 2022).
Katsuhara, Y., and Ogawa, T. (2020). Acute Renal Failure, Ketoacidosis, and Urogenital Tract Infections with SGLT2 Inhibitors: Signal Detection Using a Japanese Spontaneous Reporting Database. Clin. Drug Investig. 40, 645–652. doi:10.1007/s40261-020-00925-3
Kellum, J. A., Lameire, N., Aspelin, P., Barsoum, R. S., Burdmann, E. A., Goldstein, S. L., et al. (2012). Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. Suppl. 2, 1–138. doi:10.1038/kisup.2012.1
Kristensen, K. B., Henriksen, D. P., Hallas, J., Pottegård, A., and Lund, L. C. (2021). Sodium-glucose Cotransporter 2 Inhibitors and Risk of Nephrolithiasis. Diabetologia 64, 1563–1571. doi:10.1007/s00125-021-05424-4
Menne, J., Dumann, E., Haller, H., and Schmidt, B. M. W. (2019). Acute Kidney Injury and Adverse Renal Events in Patients Receiving SGLT2-Inhibitors: A Systematic Review and Meta-Analysis. PLoS Med. 16, e1002983. doi:10.1371/JOURNAL.PMED.1002983
Meraz-Muñoz, A. Y., Weinstein, J., and Wald, R. (2021). eGFR Decline after SGLT2 Inhibitor Initiation: The Tortoise and the Hare Reimagined. Kidney360 2, 1042–1047. doi:10.34067/KID.0001172021
Minze, M. G., Will, K. J., Terrell, B. T., Black, R. L., and Irons, B. K. (2018). Benefits of SGLT2 Inhibitors Beyond Glycemic Control - A Focus on Metabolic, Cardiovascular and Renal Outcomes. Curr. Diabetes Rev. 141, 509–517. doi:10.2174/1573399813666170816142351
Nadkarni, G. N., Ferrandino, R., Chang, A., Surapaneni, A., Chauhan, K., Poojary, P., et al. (2017). Acute Kidney Injury in Patients on SGLT2 Inhibitors: A Propensity-Matched Analysis. Diabetes Care 40, 1479–1485. doi:10.2337/dc17-1011
Novikov, A., Fu, Y., Huang, W., Freeman, B., Patel, R., van Ginkel, C., et al. (2019). SGLT2 Inhibition and Renal Urate Excretion: Role of Luminal Glucose, GLUT9, and URAT1. Am. J. Physiol. Ren. Physiol. 316, F173–F185. doi: doi:10.1152/ajprenal.00462.2018
Orban, J. C., Maizière, E. M., Ghaddab, A., van Obberghen, E., and Ichai, C. (2014). Incidence and Characteristics of Acute Kidney Injury in Severe Diabetic Ketoacidosis. PLoS ONE 9, e110925. doi:10.1371/journal.pone.0110925
Perazella, M. A., and Juncos, L. A. (2022). Drug-Induced Osmotic Nephropathy: Add SGLT2-Inhibitors to the List? Kidney360 3, 550–553. doi:10.34067/kid.0007882021
Perazella, M. A., and Rosner, M. H. (2022a). Drug-Induced Acute Kidney Injury Clin. J. Am. Soc. Nephrol., 17. CJN.11290821. doi:10.2215/CJN.11290821
Perazella, M. A., and Rosner, M. H. (2022b). Drug-Induced Acute Kidney Injury. Clin. J. Am. Soc. Nephrol., 17. CJN.11290821. doi:10.2215/CJN.11290821
Perlman, A., Heyman, S. N., Matok, I., Stokar, J., Muszkat, M., and Szalat, A. (2017). Acute Renal Failure with Sodium-Glucose-Cotransporter-2 Inhibitors: Analysis of the FDA Adverse Event Report System Database. Nutr. Metab. Cardiovasc Dis. 27, 1108–1113. doi:10.1016/j.numecd.2017.10.011
Perlman, A., Heyman, S. N., Stokar, J., Darmon, D., Muszkat, M., and Szalat, A. (2018). Clinical Spectrum and Mechanism of Acute Kidney Injury in Patients with Diabetes Mellitus on SGLT-2 Inhibitors. Isr. Med. Assoc. J. 20, 513–516. Available at: https://pubmed.ncbi.nlm.nih.gov/30084579/(Accessed April 21, 2022).
Phadke, G., Kaushal, A., Tolan, D. R., Hahn, K., Jensen, T., Bjornstad, P., et al. (2020). Osmotic Nephrosis and Acute Kidney Injury Associated with SGLT2 Inhibitor Use: A Case Report. Am. J. Kidney Dis. 76, 144–147. doi:10.1053/j.ajkd.2020.01.015
Plewa, M. C., Bryant, M., and King-Thiele, R. (2022). “Euglycemic Diabetic Ketoacidosis,” in StatPearls. Treasure Island (FL) (StatPearls Publishing). Available at: https://pubmed.ncbi.nlm.nih.gov/32119457/(Accessed April 21, 2022).
Proportional Reporting Ratio (2022). Proportional Reporting Ratio. Available at: https://www.rxmd.com/proportional-reporting-ratio/(Accessed May 31, 2022).
Rampersad, C., Kraut, E., Whitlock, R. H., Komenda, P., Woo, V., Rigatto, C., et al. (2020). Acute Kidney Injury Events in Patients with Type 2 Diabetes Using SGLT2 Inhibitors versus Other Glucose-Lowering Drugs: A Retrospective Cohort Study. Am. J. Kidney Dis. 76, 471–e1. doi:10.1053/j.ajkd.2020.03.019
Sia, C. S. M., Leong, L. C. Y., Wong, E. T. Y., Goh, G. H., and Leo, C. C. H. (2021). Empagliflozin-induced Severe Osmotic Nephrosis and Acute Renal Injury in Advanced Chronic Kidney Disease. Ann. Acad. Med. Singap 50, 255–257. doi:10.47102/ANNALS-ACADMEDSG.2020526
Slattery, J., Alvarez, Y., and Hidalgo, A. (2013). Choosing Thresholds for Statistical Signal Detection with the Proportional Reporting Ratio. Drug Saf. 36, 687–692. doi:10.1007/s40264-013-0075-1
Sridhar, V. S., Tuttle, K. R., and Cherney, D. Z. I. (2020). We Can Finally Stop Worrying about SGLT2 Inhibitors and Acute Kidney Injury. Am. J. Kidney Dis. 76, 454–456. doi:10.1053/j.ajkd.2020.05.014
Szalat, A., Perlman, A., Muszkat, M., Khamaisi, M., Abassi, Z., and Heyman, S. N. (2018). Can SGLT2 Inhibitors Cause Acute Renal Failure? Plausible Role for Altered Glomerular Hemodynamics and Medullary Hypoxia. Drug Saf. 41, 239–252. doi:10.1007/s40264-017-0602-6
U.S. Food and Drug Administration (2016). FDA Drug Safety Communication: FDA Strengthens Kidney Warnings for Diabetes Medicines Canagliflozin (Invokana, Invokamet) and Dapagliflozin (Farxiga, Xigduo XR) | FDA. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-strengthens-kidney-warnings-diabetes-medicines-canagliflozin (Accessed April 21, 2022).
U.S. Food and Drug Administration (2017). January - March 2017 | Potential Signals of Serious Risks/New Safety Information Identified by the FDA Adverse Event Reporting System (FAERS) | FDA. Available at: https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/january-march-2017-potential-signals-serious-risksnew-safety-information-identified-fda-adverse (Accessed April 21, 2022).
U.S. Food and Drug Administration (2015). October - December 2015 | Potential Signals of Serious Risks/New Safety Information Identified by the FDA Adverse Event Reporting System (FAERS) | FDA. Available at: https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/october-december-2015-potential-signals-serious-risksnew-safety-information-identified-fda-adverse (Accessed April 21, 2022).
Venot, M., Weis, L., Clec'h, C., Darmon, M., Allaouchiche, B., Goldgran-Tolédano, D., et al. (2015). Acute Kidney Injury in Severe Sepsis and Septic Shock in Patients with and without Diabetes Mellitus: A Multicenter Study. PLoS ONE 10, e0127411. doi:10.1371/journal.pone.0127411
Vigiaccess (2022). Guideline for Using VigiBase Data in Studies. Available at: www.vigiaccess.org (Accessed April 21, 2022).
Wang, Y. J., Li, J. H., Guan, Y., Xie, Q. H., Hao, C. M., and Wang, Z. X. (2021). Diabetes Mellitus Is a Risk Factor of Acute Kidney Injury in Liver Transplantation Patients. Hepatobiliary Pancreat. Dis. Int. 20, 215–221. doi:10.1016/j.hbpd.2021.02.006
Keywords: SGLT2I, acute kidney injury, acute renal failure, nephrolithiasis, drug-induced acute kidney injury, drug-induced nephrolithiasis, disproportionality analysis, VigiBase
Citation: Frent I, Leucuta D, Bucsa C, Farcas A, Casoinic F and Mogosan C (2022) A Description of Acute Renal Failure and Nephrolithiasis Associated With Sodium–Glucose Co-Transporter 2 Inhibitor Use: A VigiBase Study. Front. Pharmacol. 13:925805. doi: 10.3389/fphar.2022.925805
Received: 21 April 2022; Accepted: 15 June 2022;
Published: 08 August 2022.
Edited by:
Bita Mesgarpour, National Institute for Medical Research and Development, IranReviewed by:
Lin Zhang, Sichuan University, ChinaMalahat Khalili, Kerman University of Medical Sciences, Iran
Copyright © 2022 Frent, Leucuta, Bucsa, Farcas, Casoinic and Mogosan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel Leucuta, ZGxldWN1dGFAdW1mY2x1ai5ybw==
†These authors have contributed equally to this work
 Ioana Frent
Ioana Frent Daniel Leucuta
Daniel Leucuta Camelia Bucsa
Camelia Bucsa Andreea Farcas
Andreea Farcas Florin Casoinic4
Florin Casoinic4 Cristina Mogosan
Cristina Mogosan