
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 22 August 2022
Sec. Drugs Outcomes Research and Policies
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.923939
This article is part of the Research Topic Medication Safety and Interventions to Reduce Patient Harm in Low- and Middle-Income Countries View all 20 articles
 Huaqiao Jiang1
Huaqiao Jiang1 Yanhua Lin2
Yanhua Lin2 Weifang Ren1
Weifang Ren1 Zhonghong Fang1
Zhonghong Fang1 Yujuan Liu1
Yujuan Liu1 Xiaofang Tan1
Xiaofang Tan1 Xiaoqun Lv1*†‡
Xiaoqun Lv1*†‡ Ning Zhang1*†‡
Ning Zhang1*†‡Introduction: Adverse drug reactions (ADRs) represent a public health problem worldwide that deserves attention due to the impact on mortality, morbidity, and healthcare costs. Drug–drug interactions (DDIs) are an important contributor to ADRs. Most of the studies focused only on potential DDIs (pDDIs), while the detailed data are limited regarding the ADRs associated with actual DDIs.
Methods: This retrospective study evaluated ADRs reported between 2011 and 2020 in a tertiary hospital. The causality and severity of ADRs were evaluated through the Naranjo Algorithm and Hartwig’s scale, respectively. Preventability classification was based on the modified Schoumock and Thornton scale. For ADRs with at least two suspected drugs, pDDIs were identified according to the Lexi-Interact. We further checked whether the ADR description in the reports corresponded to the clinical consequences of the pDDIs.
Results: A total of 1,803 ADRs were reported, of which 36.77% ADRs were classified as mild, 43.26% as moderate, and 19.97% as severe. The assessment of causality showed that the distributions of definite, probable, and possible categories were 0.33%, 58.68%, and 40.99%, respectively. A total of 53.97% of ADRs were identified as preventable ADRs, while 46.03% were recognized as unpreventable. The severity of ADRs was significantly correlated with age, the number of suspected drugs and preventability. Antimicrobial agents were the most common implicated pharmacological group, and the most frequently affected system was the gastrointestinal system. Considering individual drugs, aspirin was the most frequently reported drug. Among 573 ADRs with at least two suspected drugs, 105 ADRs were caused by actual DDIs, of which only 59 and 6 ADRs were caused by actual DDIs in category D and X, respectively. The most frequent drugs involved in actual DDIs of category D were aspirin and heparin, with the majority of ADRs being gastrointestinal bleeding.
Conclusion: This study analyzed the pattern of ADRs in detail and obtained clinical evidence about ADRs associated with actual DDIs. These findings may be useful to compare patterns between different centers and to design preventive strategies for ADRs. Continuous education and training should be provided for physicians regarding the knowledge and recognition of ADRs associated with DDIs.
According to the World Health Organization (WHO), an adverse drug reaction (ADR) is an unintended and noxious response that is detected in patients after the use of drugs for the prophylaxis, diagnosis or treatment of a disease at doses normally used (Edwards and Aronson, 2000). ADRs, as a major threat in the healthcare system, contribute significantly to mortality, morbidity, extended hospital stays, and increased healthcare costs (Khan, 2013; Angamo et al., 2016). A meta-analysis showed that the percentage of ADR-induced admissions in patients over 60 years old was accurately estimated to be 8.7% (Oscanoa et al., 2017). To minimize the consequences of ADRs, it is necessary to study ADRs in terms of their early identification and prevention and to motivate healthcare professionals to report ADRs (Arulappen et al., 2018).
According to a WHO report, 60% of ADRs are preventable (Lau et al., 2003). Drug–drug interactions (DDIs) are an important cause of preventable ADRs. The increasing number of patients with multimorbidity and the growing complexity of therapeutic agents have led to widespread polypharmacy, which could result in the rising numbers of potential DDIs (pDDIs), especially in elderly individuals (Obreli-Neto et al., 2012a; Scondotto et al., 2018). Although there are several databases available that could be used to evaluate pDDIs, the clinical relevance and actual clinical importance of majority pDDIs remain insufficiently characterized and underestimated (Roblek et al., 2015). Actual DDIs are identified on the basis of clinical evidence, such as laboratory test results or symptoms, consequently, the frequency of actual DDIs is much lower than that of pDDIs (Magro et al., 2012; Zheng et al., 2018). Over the past years, a substantial number of articles have been published about ADRs due to DDIs (Leone et al., 2010; Obreli-Neto et al., 2012a; Obreli Neto et al., 2012b; Kovacevic et al., 2019; Letinier et al., 2020; Magro et al., 2020). A 6-year retrospective study in Bengbu in China showed that among the ADRs reported between nervous system drugs in hospitalized patients, 12.14% of the ADRs were associated with potential and actual DDIs, and actual DDIs were present in 6.21% of all ADRs (Shi et al., 2014). However, the incidence of ADRs resulting from DDIs could not be accurately estimated primarily because of differences in study designs and populations (Mirosevic Skvrce et al., 2011).
In this context, the present study aimed to describe the distribution of ADRs, assess causality, preventability and severity of ADRs, and determine factors involved in the severity of ADRs in a tertiary hospital between 2011 and 2020. Additionally, we described and analyzed the most frequent drugs suspected to cause ADRs and the organ system classes affected by ADRs. Furthermore, we evaluated the pDDIs among the ADRs with more than one suspected drug, estimated the incidence of ADRs due to actual DDIs and characterized ADRs caused by actual DDIs.
In this retrospective single-center study, all the ADRs was collected from the National ADR Monitoring system in Jinshan Hospital of Fudan University, between 01 January 2011 and 31 December 2020. Jinshan Hospital is a tertiary general hospital with a 700-bed capacity in the Jinshan district of Shanghai. In 2020, there were 28,533 hospital admissions, and 1.28 million outpatient and emergency department visits. ADR reports were filled out according to a specific ADR report format and submitted in paper based or electronic way by healthcare professionals, including physicians, pharmacists, and nurses.
Once received, the reported ADRs were reviewed and evaluated by ADR surveillance unit of the pharmacy department. Only the reported ADRs followed the WHO definition (Edwards and Aronson, 2000) and without any uncertainty or mistakes were accepted after exclusion of duplicates and uploaded to ADR Monitoring system. A series of exclusion criteria were applied to ensure a robust data set for analysis. Exclusion criteria included the following: 1) ADRs with doubtful causality with Naranjo’s algorithm (Naranjo et al., 1981). 2) ADR forms with insufficient information 3) ADRs symptoms similar to the original disease.
The demographic and other information relevant to ADRs were documented, including gender, age, diagnosis, admission department, suspected drugs, concomitant medications, drug details, organ system involved in the ADR, the management and outcome of the ADRs, and the type of reporter. One report could describe one or more ADRs. The incriminated drugs were classified by pharmacological group according to the WHO Anatomical Therapeutic Chemical Classification (ATC). The involved system organ classes were determined according to WHO Adverse Reaction Terminologies (WHO-ART). Two investigators cross checked the data for accuracy. Flowchart depicting the study process was shown in Figure 1.
Each ADR was further evaluated for various parameters, such as causality, severity and preventability, using previously validated and recognized approaches. The assessment of causality was performed using the Naranjo Algorithm, which consists of 10 individually scored criteria. ADRs were categorized as possible ADRs (1–4), probable ADRs (5–8) or definite ADRs (≥9) based on the total score (Naranjo et al., 1981). Severity classification was based on Hartwig’s scale, which showed the criteria and matched levels used for ADR severity assessment. ADRs were considered as severe if they resulted in one of the following outcomes: the requirement for intensive medical care, permanent harm to the patient, or the death of the patient (Hartwig et al., 1992). The preventability of ADRs was assessed by the modified Schoumock and Thornton scale and classified into definitely preventable, probably preventable and not preventable reactions (Schumock and Thornton, 1992). In our study, both definitely and probably preventable ADRs were considered as one category of preventable reactions.
For ADRs caused by two or more suspected drugs, pDDIs were identified by the software Lexi-Interact in UpToDate. The evaluation results of pDDIs were classified into five levels of risk as no known interaction (A), no action needed (B), monitor therapy (C), consider therapy modification (D), and avoid combination (X). We further verified whether the clinical consequences of pDDIs corresponded to the description of the ADR in the report, and if consistent, the pDDI was considered the actual DDI. Two clinical pharmacists independently assessed the probability, severity and preventability of ADRs as well as the consistency between ADRs and pDDIs. Any discrepancies were resolved by discussion.
Descriptive statistics were applied to describe the population as well as the clinical characteristics of ADRs and pDDIs. The categorical data were presented as numbers and proportions. Sankey diagrams of severity in preventable and unpreventable ADRs were plotted with the R package alluvial. The Mann–Whitney U test was used to evaluate the correlation between gender and the severity of ADRs. Spearman’s rank tests were performed to determine the association of age, the number of suspected drugs and the category of preventability with the severity of ADRs. The Kruskal–Wallis H test was performed to evaluate the correlation between the route of administration and the severity of ADRs. Statistical analysis was performed using IBM SPSS Statistics version 25. A p-value < 0.05 was considered statistically significant.
From January 2011 to December 2020, a total of 1,803 ADRs were reported by healthcare professionals in our hospital, although the number of ADRs reported was relatively small between 2011 and 2013. During this 10-year period, pharmacists contributed 55.69% of all ADR reports, followed by physicians (43.98%). The frequency of ADRs reported by nurses was low, accounting for only 0.33%. The annual number of reports was no more than 221 during 2011–2018, however, this number subsequently increased significantly over the next 2 years, reaching 388 in 2020 (Figure 2). A small proportion of ADRs were reported by pharmacists between 2011 and 2013, however, since 2014, more than half of ADR reports have been submitted by pharmacists. Detailed data by the year and distribution of reporters were shown in Figure 2. In our study, the highest percentage of ADRs was collected from the gastroenterology department (26.8%), followed by the departments of emergency and critical care medicine (11.4%), cardiology department (7.9%), and neurology department (7.8%) (Figure 3). The proportions of ADRs collected from clinical departments were presented in Figure 3.
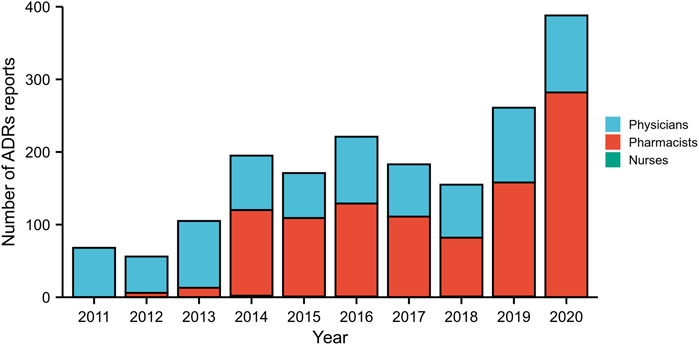
FIGURE 2. The total number of adverse drug reaction (ADR) reports and the distribution of reporters from different occupations by year during 2011–2020.
ADRs were further analyzed for causality, preventability and severity, as shown in Table 1. The assessment of causality according to the Naranjo Algorithm showed that the numbers of definite, probable and possible ADRs were 6 (0.33%), 1,058 (58.68%), and 739 (40.99%), respectively. According to Hartwig’s Severity Assessment Scale, 663 (36.77%) ADRs were classified as mild, 780 (43.26%) as moderate, and 360 (19.97%) as severe. The evaluation of the preventability of ADRs using the modified Schumock and Thornton criteria revealed that 973 (53.97%) ADRs were identified as preventable ADRs, including 93 as definitely preventable and 880 as probably preventable, while 830 (46.03%) ADRs were recognized as unpreventable. Symptomatic or specific treatment was given for 1,045 (57.96%) ADRs. According to the records of ADR reports, the majority of ADRs (81.09%) had improved, 238 (13.20%) patients had recovered from their ADRs, and 103 (5.71%) ADRs continued or their status was unclear. Suspected drugs were withdrawn in 1,700 (94.29%) ADR reports, but an altered dose or no change in therapy was observed in 103 (5.71%) reports. The visual design follows the principle of the Sankey diagram, which links the ADR characteristics by lines and signifies the quantities via line width, stratified by preventability (Figure 4).
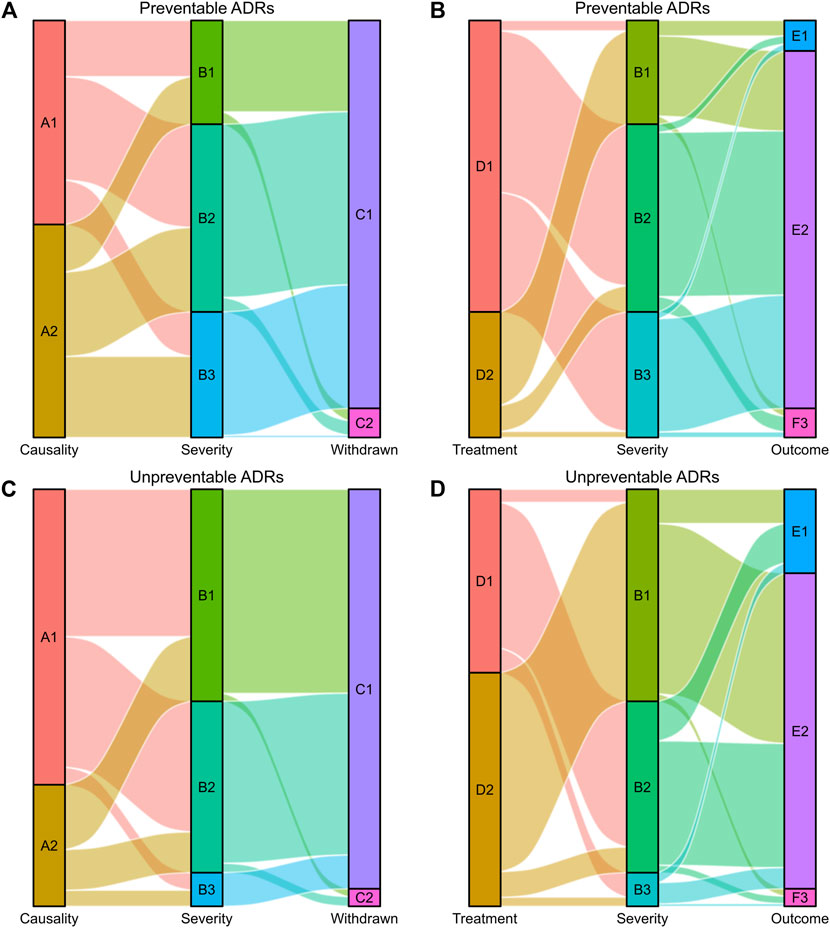
FIGURE 4. Sankey diagram of severity in preventable and unpreventable ADRs. (A,B) The causality assessment, fate of the suspected drug, treatment given and outcome of ADRs matched with ADR severity in preventable ADRs. (C,D) The causality assessment, fate of the suspected drug, treatment given and outcome of ADRs matched with ADR severity in unpreventable ADRs. The causality assessment of ADRs (A1 and A2), A1: Definite/Probable, A2: Possible. ADR severity (B1, B2, and B3), B1: Mild, B2: Moderate, B3: Severe. Fate of the suspected drug (C1 and C2), C1: Drug withdrawn, C2: Dose altered/No change. Treatment given (D1 and D2), D1: Treatment given, D2: No treatment. Outcome of ADRs (E1, E2, and E3), E1: Recovered, E2: Improved, E3: Continuing/Unclear.
The characteristics of ADRs according to severity assessment were shown in Table 2. A total of 1,803 ADRs were identified among 1,779 patients. Multiple ADRs in the same patient may be identified with different severity scale, so Table 2 depicted the distribution of mild, moderate, and severe reactions between different gender and age based on ADRs rather than patients. Concerning patient gender and ADRs, 46.87% males and 53.13% females experienced ADRs over the past 10 years. The proportion of mild ADRs was higher in females (60.33%) than in males (39.67%), however, the ratio of males to females was approximately 1:1 among those experiencing moderate and severe ADRs. The Mann–Whitney U test revealed significant differences in the mild, moderate, and severe ADR distributions between the males and females.
As shown in Table 2, the percentage of ADRs was highest among elderly individuals over 65 years of age (42.87%), followed by the 41–64-year (38.38%) and 18–40-year (14.03%) age groups. The minimum number of ADRs was observed in the age group under 18 years (4.71%). More than half of severe ADRs occurred in elderly individuals over 65 years of age. The majority of ADRs (67.72%) were identified with only one suspected drug, followed by 24.35% with two suspected drugs, and only 143 (7.93%) ADRs were found with ≥3 suspected drugs. According to Hartwig’s scale, 46.03% of ADRs were classified as unpreventable ADRs, 48.81% as probably preventable ADRs and 5.16% as definitely preventable ADRs. The percentage of unpreventable ADRs significantly decreased with ADR severity (mild 63.65% vs. moderate 43.72% vs. severe 18.61%). The statistical results revealed significant positive correlations of ADR severity with age (Spearman’s R = 0.167, p < 0.001), the number of suspected drugs (Spearman’s R = 0.136, p < 0.001) and ADR preventability (Spearman’s R = 0.299, p < 0.001).
The route of administration was classified according to the first suspected drug in the ADR reports. More than half of ADRs were associated with oral medicines regardless of their severity rating. Unexpectedly, the proportion of ADRs associated with intravenous drugs gradually decreased with increasing severity. The Kruskal–Wallis H test was further carried out and showed a significant association between the route of administration and the severity of ADRs (Table 2).
The pharmacological groups implicated in the ADRs were summarized in Table 3. Systemic antimicrobial agents were the most commonly implicated drugs (22.75%), with 14.53% and 39.32% of their associated ADRs being classified as severe and preventable ADRs, respectively. Cardiovascular agents were the second most frequently reported class of drugs responsible for ADRs (12.41%), followed by medications for the alimentary tract and metabolism (12.06%). Drugs acting on the blood and blood-forming organs represented 11.75% of the reports (32.45% severe and 83.11% preventable ADRs). Drugs acting on the musculoskeletal system were implicated in 10.77% of the ADRs (39.71% severe and 71.48% preventable ADRs). Traditional Chinese medicines were implicated in 10.07% of the reports (18.53% severe ADRs and 54.44% preventable ADRs).
The frequency of commonly prescribed drugs among total and severe ADRs was shown in Table 4. When individual drugs were considered, aspirin was responsible for a maximum number of both total and severe ADRs, far more than any other drugs. Among the total ADRs, levofloxacin (82) was the second most frequent causative drug, followed by compound pseudoephedrine hydrochloride (65) and clopidogrel (48). In addition to aspirin, the drugs most frequently involved in severe ADRs were clopidogrel (17), levofloxacin (16), compound pseudoephedrine hydrochloride (14), and diclofenac sodium (13).
Upon a review of the outcomes of ADRs, the most frequently affected system was the gastrointestinal system (30.83%), with the clinical symptoms of nausea, vomiting, abdominal pain, diarrhea, abdominal distention, and so on. In addition, the commonly reported reactions were skin and appendage disorders (22.44%) and liver and biliary system disorders (14.19%). A more detailed description was presented in Table 5.
pDDIs were evaluated in 573 of 1,803 ADR reports (31.78%) involving more than one suspected drug. 156 ADRs were identified with pDDIs of category C, D, and X, of which 100 ADRs were identified with only one pDDI and 56 ADRs with multiple pDDIs. Table 6 showed that 208 pDDIs of category C were identified in 112 ADRs, 74 pDDIs of category D in 58 ADRs, and 11 pDDIs of category X in 10 ADRs. Furthermore, we checked whether the reported ADRs were consistent with the potential clinical consequences of pDDIs. The results showed 105 ADRs were caused by actual DDIs, accounting for 18.32% of the ADR reports with more than one suspected drug. Among them, 59 and 6 ADRs were caused by actual DDIs in the category D and X, respectively.
Tables 7, 8 summarized the ADRs caused by actual DDIs belonging to category X and D, respectively. Potassium chloride and promethazine were the drug–drug combination most involved in ADRs caused by actual DDIs in category X, with severe and adverse clinical consequences to the gastrointestinal system. The most frequent drugs involved in actual DDIs of category D were aspirin (n = 34) and heparin (n = 26), and the great majority of ADRs caused by DDIs were associated with gastrointestinal bleeding. Aspirin/heparin (n = 10) and heparin/clopidogrel (n = 10), followed by aspirin/warfarin (n = 6) and aspirin/ibuprofen (n = 5), were the drug–drug combinations most involved in ADRs caused by DDIs of category D.
In this study, physicians and pharmacists were the groups that reported the great majority of ADRs, and the frequency of ADRs reported by nursing staff was low, which may be due to their extensive workload in everyday practice, inattention and unawareness toward ADR reporting or worry about legal implications (Singh et al., 2017). The reporter distribution of ADRs varies widely in different studies because of differences in healthcare structures as well as the awareness and motivation of healthcare professionals. The number of ADRs was relatively small, especially for ADRs reported by pharmacists between 2011 and 2013, indicating underreporting in pharmacovigilance. The key to improving ADR reporting rates is adequate pharmacovigilance education and training for healthcare professionals (Barzaga Arencibia et al., 2012).
In the present study, we analyzed the pattern of ADRs based on the causality, severity, and preventability in our hospital, all of which vary among different hospitals due to differences in the population characteristics and hospital specialties. Naranjo’s causality assessment showed that only 0.33% of reports were definite because of limited use of dechallenge and rechallenge processes for ethical reasons as well as the retrospective study design without the ability to assess the ADR completely. The suspected drugs were withdrawn among 94.29% of ADRs, and for the remaining 5.71% of ADRs, the suspected drug doses were altered or rechallenge processes were initiated. In this study, 19.97% of ADRs were classified as severe. Severe ADRs, as major concerns for public health, are a contributing factor of hospitalizations and morbidity (Rottenkolber et al., 2011; Marques et al., 2014). The analysis indicated a preventability rate of 53.97% among ADRs, comparable with the results of studies conducted in Romania and Jordan showing that 41% and 44.7% of ADRs were preventable, respectively (Farcas et al., 2014; Al Damen and Basheti, 2019). However, the data from a study showed lower preventability for ADRs (12%) compared with our finding (Dequito et al., 2011). As described in previous studies, insufficient monitoring, inappropriate dosing, and DDIs were the most frequent factors involved in ADR preventability (Farcas et al., 2014; Al Damen and Basheti, 2019). Incriminated drugs were withdrawn in 94.29% of the reports, which is in line with a previous study in a psychiatric department of a tertiary care teaching hospital in India (Patel et al., 2015). The high proportion of withdrawal may be due to the reporting nature of ADRs that troublesome ADRs are more likely to be detected.
There may be significant difference between male and female regarding the ADR prevalence due to factors such as body mass index, fat composition, hormonal effects, drug susceptibility, or genetic differences in the levels of enzymes (Haile et al., 2013; Rukmangathen et al., 2020). However, we demonstrated that females had only slightly higher incidence of ADRs than males in the present study. The frequency of ADRs increased with age, with the highest prevalence of ADRs in elderly individuals over 65 years (42.87%), followed by individuals 41–64 years of age (38.38%), which is in concordance with the findings of a previous study (Shepherd et al., 2012). Older patients are particularly vulnerable to ADRs owing to the multiple-drug regimens used for chronic diseases and physiological changes in this population, such as reduced gastrointestinal motility and gastric blood flow, impaired repair mechanisms, and lower mucosal protection (Marusic et al., 2014). A systematic review of ADRs in elderly individuals revealed that comorbid complexity was positively associated with ADR occurrence (Alhawassi et al., 2014). In the present study, there were statistically significant differences in the incidence of severe ADRs in the different gender and age groups, and polypharmacy increased the proportion of severe ADRs.
Anti-infectives for systemic use were the most common pharmacological group, accounting for 22.75% of total ADRs in our study, which is in line with previous studies (Haile et al., 2013; Marques et al., 2014). The excessive use of antibiotics may be responsible for the increased risk of ADRs. Cardiovascular system agents (12.41%) were the second most frequently incriminated pharmacological class of ADRs in our study, among them, 65.20% were preventable ADRs. A systematic review showed that cardiovascular medicines were commonly associated with preventable drug-related admissions (Howard et al., 2007). In another study, cardiovascular agents were identified as the second most frequently responsible drugs linked to preventable ADRs (Farcas et al., 2014).
The system most frequently affected by ADRs in this study was the gastrointestinal system, accounting for 30.83%, probably due to more than half of the suspected drugs being administered orally. This was followed by skin and appendage disorders (22.44%). This observation is consistent with the findings of a prospective observational study of hospitalized pediatric patients, which reported gastrointestinal system disorders (51.56%) and skin and appendage disorders (18.75%) as the most frequent manifestations of ADRs (Kurian et al., 2016).
As DDIs are usually predictable and manageable, ADRs caused by DDIs may be prevented by monitoring the patient closely or replacing the responsible drugs with other medications. To reduce the risk of DDIs and improve patient safety, it is essential that healthcare professionals regularly review the medication regimens, recognize potentially interacting drug pairs, and withdraw unnecessary drugs (Magro et al., 2020). A prospective study showed that the number of patients with pDDIs and actual DDIs decreased by 18% and 43%, respectively, with an intervention based on a computerized clinical decision support system containing information on drug combinations (Bertsche et al., 2010). However, reporters less frequently recognize actual DDIs due to the limited availability of DDI databases or alerting drug-interaction systems (Mirosevic Skvrce et al., 2011). Therefore, it is important to increase the knowledge of pharmacovigilance through the additional education of healthcare providers.
In a previous study, we investigated the prevalence of pDDIs and their association with characteristics in outpatient prescriptions (Ren et al., 2020). However, to assess the clinical impact of DDIs on public health, only ADRs associated with DDIs should be considered. In our study, 105 ADR reports were induced by actual DDIs, accounting for 18.32% of the ADR reports with more than one suspected drug. This percentage was close to the proportion reported by Magro et al. (2020). According to the online version of DRUGDEX® system, they verified DDI among serious ADRs containing at least two suspected or concomitant drugs in the National Pharmacovigilance database from Veneto Region, and identified 17.4% ADR reports associated with a DDI. However, the results of another study performed in an Italian spontaneous reporting database showed that regarding patients treated with at least two drugs, 6.5% of ADR reports was associated with a DDI using the DRUGDEX® system (Leone et al., 2010). Similarly, a prospective cohort study conducted in the primary public health system of the Ourinhos microregion in Brazil revealed that the incidence of DDI-related ADRs was 6% in elderly outpatients using DDI-checker programs (DrugDigest®, Drugs®, Micromedex®, and Medscape®) (Obreli-Neto et al., 2012b).
In the present study, aspirin and heparin were the drugs most frequently associated with actual DDIs of category D, with symptom of gastrointestinal bleeding. Similarly, a prospective observational study conducted in the cardiology unit of an Indian hospital showed that heparin and aspirin were the most common drugs responsible for DDIs, and bleeding was the most frequent clinical consequence (Mateti et al., 2011). Furthermore, aspirin, which is widely used for the prevention of vascular events, was reported to increase the baseline risk of gastrointestinal bleeding by approximately 60% among older persons aged over 70 years in a randomized controlled trial (Mahady et al., 2021).
Although the study had important findings regarding the pattern of ADRs and the role of actual DDIs in ADRs over the past decade along with a large sample size, several limitations should be taken into consideration. First, as a retrospective study, data were collected from the clinical records of ADRs always with incomplete information, such as information on concomitant drugs, comorbidities, lifestyle, diet, and so on. Prospective studies will be carried out to clarify and reduce this limitation in the future. Second, this study was conducted at a single institution, limiting the generalizability of its findings due to the differences in population characteristics and prescribing patterns. Last, the single source of the DDI screening database used in this study may hinder the identification of DDIs because consistent criteria for DDI identification and assessment are currently lacking.
This study of ADR data collected over 10 years revealed that almost all ADRs were reported by pharmacists and physicians in our hospital, and the severity of ADRs was significantly correlated with age, the number of suspected drugs and preventability. Systemic antimicrobial agents were the most frequently incriminated pharmacological group, and aspirin was responsible for the largest proportion of total and severe ADRs. The gastrointestinal system was the system most frequently affected by ADRs. As observed in this study, aspirin and heparin were the most common drugs in actual DDIs of category D, resulting in gastrointestinal bleeding.
Active pharmacovigilance programs are important to accurately identify and assess ADRs in the clinical setting, further minimize drug-induced harm and improve the quality of patient care. Our findings obtained clinical evidence about ADRs associated with actual DDIs in our hospital. It will be necessary to make clinicians aware of the possibility of DDI-related ADRs and achieve a clear understanding of drug pairs resulting in DDI-related ADRs, in order to guide the prescribing practices and minimize the harms from actual DDIs. Moreover, rigorous prescription and frequent monitoring of drug therapy are essential for reducing the risk of ADRs.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
This study was approved by the Ethics Committee of Jinshan Hospital, Fudan University at Shanghai, China (approval No. JIEC 2022-S29).
HJ, ZF, XL, and NZ contributed to the conception and design of the study. HJ, YL, WR, YL, and XT contributed to the recording and statistical analysis of the data. HJ and XL wrote the first draft of the manuscript. XL and NZ made critical revisions to the manuscript. All authors approved the final version of the manuscript.
This work was supported by the Fourth Training Program for the Outstanding Young Talents, Jinshan Health Commission (#JSYQ201904), Key Construction Project on Clinical Pharmacy of Shanghai (#2019-1229).
The authors acknowledge the ADR reporting system of Jinshan Hospital, Fudan University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Al Damen, L., and Basheti, I. (2019). Preventability analysis of adverse drug reactions in a Jordanian hospital: A prospective observational study. Int. J. Clin. Pharm. 41 (6), 1599–1610. doi:10.1007/s11096-019-00925-0
Alhawassi, T. M., Krass, I., Bajorek, B. V., and Pont, L. G. (2014). A systematic review of the prevalence and risk factors for adverse drug reactions in the elderly in the acute care setting. Clin. Interv. Aging 9, 2079–2086. doi:10.2147/CIA.S71178
Angamo, M. T., Chalmers, L., Curtain, C. M., and Bereznicki, L. R. (2016). Adverse-drug-reaction-related hospitalisations in developed and developing countries: A review of prevalence and contributing factors. Drug Saf. 39 (9), 847–857. doi:10.1007/s40264-016-0444-7
Arulappen, A. L., Danial, M., and Sulaiman, S. A. S. (2018). Evaluation of reported adverse drug reactions in antibiotic usage: A retrospective study from a tertiary care hospital, Malaysia. Front. Pharmacol. 9, 809. doi:10.3389/fphar.2018.00809
Barzaga Arencibia, Z., Lopez Leyva, A., Mejias Pena, Y., Gonzalez Reyes, A. R., Fernandez Manzano, E., Choonara, I., et al. (2012). Pharmacovigilance in children in camaguey province, Cuba. Eur. J. Clin. Pharmacol. 68 (7), 1079–1084. doi:10.1007/s00228-012-1222-9
Bertsche, T., Pfaff, J., Schiller, P., Kaltschmidt, J., Pruszydlo, M. G., Stremmel, W., et al. (2010). Prevention of adverse drug reactions in intensive care patients by personal intervention based on an electronic clinical decision support system. Intensive Care Med. 36 (4), 665–672. doi:10.1007/s00134-010-1778-8
Dequito, A. B., Mol, P. G., van Doormaal, J. E., Zaal, R. J., van den Bemt, P. M., Haaijer-Ruskamp, F. M., et al. (2011). Preventable and non-preventable adverse drug events in hospitalized patients: A prospective chart review in The Netherlands. Drug Saf. 34 (11), 1089–1100. doi:10.2165/11592030-000000000-00000
Edwards, I. R., and Aronson, J. K. (2000). Adverse drug reactions: Definitions, diagnosis, and management. Lancet 356 (9237), 1255–1259. doi:10.1016/s0140-6736(00)02799-9
Farcas, A., Bucsa, C., Sinpetrean, A., Leucuta, D., Mogosan, C., Dumitrascu, D., et al. (2014). Preventability analysis of adverse drug reactions detected in two internal medicine departments in Romania. Intern. Emerg. Med. 9 (2), 187–193. doi:10.1007/s11739-012-0843-4
Haile, D. B., Ayen, W. Y., and Tiwari, P. (2013). Prevalence and assessment of factors contributing to adverse drug reactions in wards of a tertiary care hospital, India. Ethiop. J. Health Sci. 23 (1), 39–48.
Hartwig, S. C., Siegel, J., and Schneider, P. J. (1992). Preventability and severity assessment in reporting adverse drug reactions. Am. J. Health. Syst. Pharm. 49 (9), 2229–2232. doi:10.1093/ajhp/49.9.2229
Howard, R. L., Avery, A. J., Slavenburg, S., Royal, S., Pipe, G., Lucassen, P., et al. (2007). Which drugs cause preventable admissions to hospital? A systematic review. Br. J. Clin. Pharmacol. 63 (2), 136–147. doi:10.1111/j.1365-2125.2006.02698.x
Khan, L. M. (2013). Comparative epidemiology of hospital-acquired adverse drug reactions in adults and children and their impact on cost and hospital stay – A systematic review. Eur. J. Clin. Pharmacol. 69 (12), 1985–1996. doi:10.1007/s00228-013-1563-z
Kovacevic, M., Vezmar Kovacevic, S., Radovanovic, S., Stevanovic, P., and Miljkovic, B. (2019). Adverse drug reactions caused by drug-drug interactions in cardiovascular disease patients: Introduction of a simple prediction tool using electronic screening database items. Curr. Med. Res. Opin. 35 (11), 1873–1883. doi:10.1080/03007995.2019.1647021
Kurian, J., Mathew, J., Sowjanya, K., Chaitanya, K. R., Ramesh, M., Sebastian, J., et al. (2016). Adverse drug reactions in hospitalized pediatric patients: A prospective observational study. Indian J. Pediatr. 83 (5), 414–419. doi:10.1007/s12098-015-2002-1
Lau, P. M., Stewart, K., and Dooley, M. J. (2003). Comment: Hospital admissions resulting from preventable adverse drug reactions. Ann. Pharmacother. 37 (2), 303–304. doi:10.1177/106002800303700229
Leone, R., Magro, L., Moretti, U., Cutroneo, P., Moschini, M., Motola, D., et al. (2010). Identifying adverse drug reactions associated with drug-drug interactions: Data mining of a spontaneous reporting database in Italy. Drug Saf. 33 (8), 667–675. doi:10.2165/11534400-000000000-00000
Letinier, L., Ferreira, A., Marceron, A., Babin, M., Micallef, J., Miremont-Salame, G., et al. (2020). Spontaneous reports of serious adverse drug reactions resulting from drug-drug interactions: An analysis from the French pharmacovigilance database. Front. Pharmacol. 11, 624562. doi:10.3389/fphar.2020.624562
Magro, L., Arzenton, E., Leone, R., Stano, M. G., Vezzaro, M., Rudolph, A., et al. (2020). Identifying and characterizing serious adverse drug reactions associated with drug-drug interactions in a spontaneous reporting database. Front. Pharmacol. 11, 622862. doi:10.3389/fphar.2020.622862
Magro, L., Moretti, U., and Leone, R. (2012). Epidemiology and characteristics of adverse drug reactions caused by drug-drug interactions. Expert Opin. Drug Saf. 11 (1), 83–94. doi:10.1517/14740338.2012.631910
Mahady, S. E., Margolis, K. L., Chan, A., Polekhina, G., Woods, R. L., Wolfe, R., et al. (2021). Major GI bleeding in older persons using aspirin: Incidence and risk factors in the ASPREE randomised controlled trial. Gut 70 (4), 717–724. doi:10.1136/gutjnl-2020-321585
Marques, J., Ribeiro-Vaz, I., Pereira, A. C., and Polonia, J. (2014). A survey of spontaneous reporting of adverse drug reactions in 10 years of activity in a pharmacovigilance centre in Portugal. Int. J. Pharm. Pract. 22 (4), 275–282. doi:10.1111/ijpp.12078
Marusic, S., Sicaja, M., Obreli Neto, P. R., Franic, M., Marinovic, I., and Bacic-Vrca, V. (2014). Adverse drug reactions in elderly patients following discharge from an internal medicine clinic. Int. J. Clin. Pharmacol. Ther. 52 (10), 906–913. doi:10.5414/CP202041
Mateti, U., Rajakannan, T., Nekkanti, H., Rajesh, V., Mallaysamy, S., and Ramachandran, P. (2011). Drug-drug interactions in hospitalized cardiac patients. J. Young Pharm. 3 (4), 329–333. doi:10.4103/0975-1483.90246
Mirosevic Skvrce, N., Macolic Sarinic, V., Mucalo, I., Krnic, D., Bozina, N., and Tomic, S. (2011). Adverse drug reactions caused by drug-drug interactions reported to Croatian agency for medicinal products and medical devices: A retrospective observational study. Croat. Med. J. 52 (5), 604–614. doi:10.3325/cmj.2011.52.604
Naranjo, C. A., Busto, U., Sellers, E. M., Sandor, P., Ruiz, I., Roberts, E. A., et al. (1981). A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 30 (2), 239–245. doi:10.1038/clpt.1981.154
Obreli-Neto, P. R., Nobili, A., de Lyra, D. P., Pilger, D., Guidoni, C. M., de Oliveira Baldoni, A., et al. (2012b). Incidence and predictors of adverse drug reactions caused by drug-drug interactions in elderly outpatients: A prospective cohort study. J. Pharm. Pharm. Sci. 15 (2), 332–343. doi:10.18433/j3cc86
Obreli-Neto, P. R., Nobili, A., de Oliveira Baldoni, A., Guidoni, C. M., de Lyra Junior, D. P., Pilger, D., et al. (2012a). Adverse drug reactions caused by drug-drug interactions in elderly outpatients: A prospective cohort study. Eur. J. Clin. Pharmacol. 68 (12), 1667–1676. doi:10.1007/s00228-012-1309-3
Oscanoa, T. J., Lizaraso, F., and Carvajal, A. (2017). Hospital admissions due to adverse drug reactions in the elderly. A meta-analysis. Eur. J. Clin. Pharmacol. 73 (6), 759–770. doi:10.1007/s00228-017-2225-3
Patel, T. K., Bhabhor, P. H., Desai, N., Shah, S., Patel, P. B., Vatsala, E., et al. (2015). Adverse drug reactions in a psychiatric department of tertiary care teaching hospital in India: Analysis of spontaneously reported cases. Asian J. Psychiatr. 17, 42–49. doi:10.1016/j.ajp.2015.07.003
Ren, W., Liu, Y., Zhang, J., Fang, Z., Fang, H., Gong, Y., et al. (2020). Prevalence of potential drug-drug interactions in outpatients of a general hospital in China: A retrospective investigation. Int. J. Clin. Pharm. 42 (4), 1190–1196. doi:10.1007/s11096-020-01068-3
Roblek, T., Vaupotic, T., Mrhar, A., and Lainscak, M. (2015). Drug-drug interaction software in clinical practice: A systematic review. Eur. J. Clin. Pharmacol. 71 (2), 131–142. doi:10.1007/s00228-014-1786-7
Rottenkolber, D., Schmiedl, S., Rottenkolber, M., Farker, K., Salje, K., Mueller, S., et al. (2011). Adverse drug reactions in Germany: Direct costs of internal medicine hospitalizations. Pharmacoepidemiol. Drug Saf. 20 (6), 626–634. doi:10.1002/pds.2118
Rukmangathen, R., Brahmanapalli, V. D., Thammisetty, D. P., Pemmasani, D., Gali, S. D., and Atmakuru, R. B. (2020). Study of adverse drug reactions to antiretroviral therapy in a tertiary care hospital, Tirupati. Perspect. Clin. Res. 11 (4), 158–163. doi:10.4103/picr.PICR_133_18
Schumock, G. T., and Thornton, J. P. (1992). Focusing on the preventability of adverse drug reactions. Hosp. Pharm. 27 (6), 538.
Scondotto, G., Pojero, F., Pollina Addario, S., Ferrante, M., Pastorello, M., Visconti, M., et al. (2018). The impact of polypharmacy and drug interactions among the elderly population in Western Sicily, Italy. Aging Clin. Exp. Res. 30 (1), 81–87. doi:10.1007/s40520-017-0755-2
Shepherd, G., Mohorn, P., Yacoub, K., and May, D. W. (2012). Adverse drug reaction deaths reported in United States vital statistics, 1999-2006. Ann. Pharmacother. 46 (2), 169–175. doi:10.1345/aph.1P592
Shi, Q. P., He, X. D., Yu, M. L., Zhu, J. X., Liu, Y., Ding, F., et al. (2014). A 6-year retrospective study of adverse drug reactions due to drug-drug interactions between nervous system drugs. Int. J. Clin. Pharmacol. Ther. 52 (5), 392–401. doi:10.5414/CP202050
Singh, P., Agrawal, M., Hishikar, R., Joshi, U., Maheshwari, B., and Halwai, A. (2017). Adverse drug reactions at adverse drug reaction monitoring center in Raipur: Analysis of spontaneous reports during 1 year. Indian J. Pharmacol. 49 (6), 432–437. doi:10.4103/ijp.IJP_781_16
Keywords: adverse drug reactions, drug–drug interactions, causality, severity, preventability
Citation: Jiang H, Lin Y, Ren W, Fang Z, Liu Y, Tan X, Lv X and Zhang N (2022) Adverse drug reactions and correlations with drug–drug interactions: A retrospective study of reports from 2011 to 2020. Front. Pharmacol. 13:923939. doi: 10.3389/fphar.2022.923939
Received: 19 April 2022; Accepted: 19 July 2022;
Published: 22 August 2022.
Edited by:
Ahmed Awaisu, Qatar University, QatarReviewed by:
Yaw Owusu, Qatar University, QatarCopyright © 2022 Jiang, Lin, Ren, Fang, Liu, Tan, Lv and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoqun Lv, bHZ4aWFvcXVuMTk4NUAxNjMuY29t; Ning Zhang, MTc3MDk4NzAwNzdAMTYzLmNvbQ==
†These authors have contributed equally to this work and share last authorship
‡ORCID: Xiaoqun Lv, orcid.org/0000-0002-6130-9904; Ning Zhang, orcid.org/0000-0002-8376-1037
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.