
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 13 June 2022
Sec. Pharmacology of Anti-Cancer Drugs
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.919652
 Hong-Shuai Li1†
Hong-Shuai Li1† Guang-Jian Yang2†
Guang-Jian Yang2† Yi Cai3†
Yi Cai3† Jun-Ling Li1
Jun-Ling Li1 Hai-Yan Xu1
Hai-Yan Xu1 Tao Zhang1
Tao Zhang1 Li-Qiang Zhou1
Li-Qiang Zhou1 Yu-Ying Wang4
Yu-Ying Wang4 Jin-Liang Wang4
Jin-Liang Wang4 Xing-Sheng Hu1
Xing-Sheng Hu1 Xiang Yan4*
Xiang Yan4* Yan Wang1*
Yan Wang1*Objective: Dacomitinib has been approved for non-small-cell lung cancer (NSCLC) patients harboring classical epidermal growth factor receptor (EGFR) mutations; however, clinical evidence of its activity on major uncommon EGFR mutations is currently limited.
Materials and methods: This was a dual-center, single-arm, ambispective cohort study in China. Patients with histologically confirmed metastatic or recurrent NSCLC harboring major uncommon EGFR mutations were eligible for the study. The objective response rate and disease control rate were determined by RECIST 1.1 every 1–2 months. Adverse events were assessed by CTCAE 5.0.
Results: In total, 32 NSCLC patients were enrolled between July 2020 and January 2022, and 18 (56.3%) patients received dacomitinib as first-line therapy. Median age was 64 years, and 20 (62.5%) were female. The mutations identified were G719X (n = 24; 75%), followed by L861X (n = 10; 31.3%), and S768I (n = 8; 25%). In the first-line setting, 72.2% of patients (13/18) had a confirmed partial response and 100% (18/18) had disease control, and the median progression-free survival (PFS) and overall survival (OS) were unreached. In the whole cohort, 56.3% of patients (18/32) had a confirmed partial response and 90.6% (29/32) had disease control, and the median PFS was 10.3 months (95% confidence interval, 6.1–14.5) and the median OS was 36.5 months. Except for one case not available for brain re-evaluation, control of the intracranial metastases was observed in 13 patients (13/14, 92.9%). No grade 4–5 adverse events (AEs) occurred, but all patients had grade 1–2 AEs, and 12.5% (4/32) patients required a dosage reduction due to intolerable AEs.
Conclusions: Dacomitinib demonstrated favorable activity with manageable toxicity in patients with NSCLC harboring major uncommon EGFR mutations.
The in-frame deletion in exon 19 (19del; 49–72%) and one nucleotide substitution within codon 858 of exon 21 (L858R; 28–43%) are two predominant epidermal growth factor receptor (EGFR) alterations in non-small cell lung cancer (NSCLC), called “common mutations” or “classic mutations” (Wang and Li, 2019). Patients with NSCLC harboring these tyrosine kinase inhibitor (TKI)-sensitive mutations respond remarkably to EGFR-TKIs. Other EGFR mutations (10–20%), also called “uncommon mutations,” include any mutations other than 19del or L858R. These include exon 18 point mutations (e.g., E709X and G719X), exon 19 insertion mutations, exon 20 insertion mutations, exon 20 point mutations such as S768I, and exon 21 point mutations (e.g., L861Q) (Baek et al., 2015; Cho et al., 2020; Gristina et al., 2020; Wu et al., 2020; Passaro et al., 2021). These uncommon mutations represent a subset of NSCLC with poorer prognosis and a less favorable EGFR-TKI efficacy (Chiu et al., 2015a). Among them, exon 20 insertion mutations are the most prevalent and are largely insensitive to EGFR-TKIs (Remon et al., 2020; Huang et al., 2021). However, major uncommon mutations (including G719X, S768I, and L861Q) have also been found to be sensitive to EGFR-TKIs, especially the second-generation (2G) TKI afatinib. Recent National Comprehensive Cancer Network guidelines recommend afatinib as the preferred TKI for NSCLC harboring major uncommon mutations.
Dacomitinib is a potent, irreversible, highly selective 2G EGFR-TKI that inhibits signaling from both heterodimers and homodimers of all members of the human EGFR family. The significantly superior progression-free survival (PFS) benefit of dacomitinib over gefitinib in the ARCHER 1050 trial provided the basis for its use as a standard first-line option in EGFR-positive advanced NSCLC (Wu et al., 2017). A recent case series study of 14 patients has shown that dacomitinib is also effective against central nervous system (CNS) metastasis in EGFR-positive NSCLC (Peng et al., 2021). In addition, dacomitinib has potential applications in patients harboring uncommon mutations (Jänne et al., 2011; Reckamp et al., 2014; Kris et al., 2015; Nishino et al., 2018). In our previous study, we presented the later-line efficacy of dacomitinib on patients with NSCLC harboring uncommon EGFR mutations in a relatively small scale (n = 11) retrospectively, and the findings indicated a promising activity of dacomitinib (Li et al., 2022).
In this ambispective cohort study, we aimed to evaluate the efficacy and safety of dacomitinib in patients with NSCLC harboring major uncommon EGFR mutations in a relatively large scale.
Chinese patients with NSCLC harboring major uncommon EGFR mutations treated with dacomitinib between July 2020 and January 2022 were ambispectively recruited from the Chinese PLA General Hospital and the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. The inclusion criteria were as follows: 1) Cytologically or histologically confirmed diagnosis of metastatic or recurrent NSCLC; 2) Harbor major uncommon EGFR mutations (including G719X, L861X, and S768I); and 3) Cell-free DNA from plasma, cerebrospinal fluid, or pleural effusion samples or tumor tissue before dacomitinib initiation were analyzed using the next-generation sequencing (NGS) method, which was conducted by our hospital and third-party qualified inspection institutions accredited by the College of American Pathologists.
All patients were treated with dacomitinib monotherapy. Unlike the standard dacomitinib dosing regimen of 45 mg used in the ARCHER 1050 study, in this real-world study, the starting dose was determined by the physician based on the patient’s condition. In general, the starting dose was 45 mg for patients with an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 and weight equal or greater than 60 kg; 30 mg for patients with an ECOG PS of one and weight less than 60 kg; 30 mg for patients with an ECOG PS of one and weight equal or greater than 60 kg, with an increase to 45 mg if well-tolerated by the patient; and 15 mg for patients with an ECOG PS of equal or greater than 2. A stepwise dose reduction of dacomitinib was conducted according to drug tolerance based on the above principles.
Imaging evaluation, including chest and abdomen computed tomography and brain magnetic resonance imaging (MRI), was performed every 1–2 months after drug administration. The objective tumor response was determined according to the Response Evaluation Criteria in Solid Tumors (version 1.1) guidelines. Objective response was categorized as complete response (CR) or partial response (PR), and disease control was categorized as CR, PR, or stable disease (SD). Toxicity was evaluated per the Common Terminology Criteria for Adverse Events version 5.0.
For patient’s characteristics, categorical variables were reported as absolute numbers and percentages. Survival curves were constructed by the Kaplan-Meier method (Log-rank test). The data cutoff date was January 15, 2022, when the disease status of the patients was determined. PFS was defined as the period from dacomitinib initiation to disease progression or any-cause death. Overall survival (OS) was defined as the period from dacomitinib initiation to any-cause death. Patients who were lost to follow-up were censored, and the last determinable time of survival was used as the time of termination of follow-up. All statistical analyses were performed and all pictures were created using GraphPad Prism nine software (GraphPad Software, San Diego, CA, United States). p values < 0.05 denoted statistically significant differences.
A total of 32 patients with EGFR-mutant NSCLC treated with dacomitinib were enrolled. The clinical, demographic, pathological, and molecular characteristics of the cohort are shown in Table 1. More than 60% of the patients were women and never-smokers. The median age was 64 years. Overall, 15 patients (46.9%) had one or more nodules in the brain, and two patients (P4, P6) of them also had leptomeningeal metastasis (Figure 1A). Dacomitinib was applied as first-line treatment in more than half (18, 56.3%) of patients, and the median application line was 1 (range, 1–6). Most patients (65.5%) had an ECOG PS of 1. More than 60% of patients received 30 mg of dacomitinib as the starting dose.
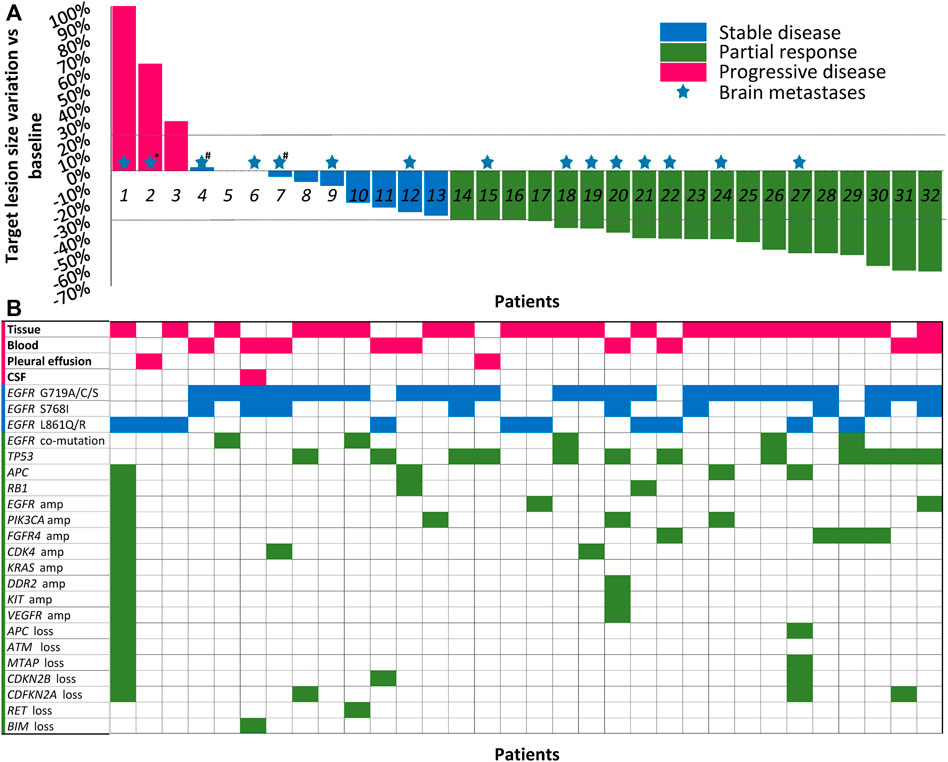
FIGURE 1. Best shrinkage of target lesion size and corresponding mutation profile by patient (N = 32). (A) Treatment responses of dacomitinib in all 32 patients are shown in the waterfall plot. Patients with compound mutations and brain metastases were marked with colored pentagrams. *Brain metastases not evaluated due to patient’s poor condition. #The patients also received whole brain radiotherapy before dacomitinib initiation. Dashed lines represent 20% progression (progressive disease) and 30% tumor regression (partial response). (B) The corresponding mutation profile of each patient was displayed under the waterfall plot. CSF, cerebrospinal fluid; amp, amplification.
Cell-free DNA from plasma, cerebrospinal fluid, or pleural effusion samples or tumor tissue before dacomitinib initiation were analyzed utilizing the NGS method. Figure 1B shows the details of the major uncommon EGFR mutations and accompanying mutations. For specific mutation subtypes, 24 patients harbored G719X; 10 patients, L861X; eight patients, S768I (Table 1). G719A and L861Q were the two most frequent single uncommon mutations, and 11 patients had compound mutations, with G719C + S768I being the predominant (36.4%, 4/11). The most common concomitant mutations were detected in tumor protein p53 (TP53) (37.5%), cyclin-dependent kinase inhibitor 2A (12.5%), phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (12.5%), adenomatous polyposis coli (12.5%), cyclin-dependent kinase inhibitor 2B (9.4%), RB transcriptional corepressor 1 (9.4%), EGFR amplification (9.4%), fibroblast growth factor receptor 4 (9.4%), and cyclin-dependent kinase 4 (9.4%) genes (Figure 1B).
All the enrolled patients received dacomitinib monotherapy. Nine patients received a dacomitinib starting dose of 45 mg once daily, while 21 patients received 30 mg, and two patients received 15 mg once daily until disease progression or intolerable toxicity. The specific dosing regimen was described in the Methods section.
In the overall cohort, 18 (56.3%) patients had PR, 11 (34.4%) had SD, and another three (9.4%) patients had de novo resistance to dacomitinib with PD as the best response (Figure 1A). Previous treatments before dacomitinib were shown in Figure 2A. The objective response rate (ORR) was 56.3% (18/32), and the disease control rate (DCR) was 90.6% (29/32). The median follow-up duration was 11.4 months (95% confidence interval [CI]: 7.4–15.4). The PFS was mature in 14 patients, and the tumors in 18 patients remained under control (Figure 2B).
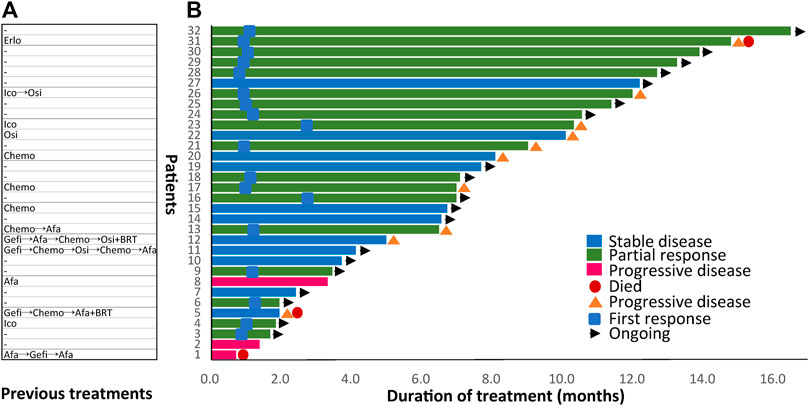
FIGURE 2. Previous treatments before dacomitinib initiation and duration of dacomitinib treatment by patient (N = 32). (A) Treatment history of each patient was displayed. (B) Duration of dacomitinib treatment of each patient was demonstrated correspondingly. Afa, afatinib; Gefi, gefitinib; Erlo, erlotinib; Ico, icotinib; Osi, osimertinib; Chemo, chemotherapy; BRT, whole brain radiotherapy.
At the data cutoff date, the median PFS was 10.3 months (95% CI, 6.1–14.5) (Figure 3A), and median OS was 36.5 months (Figure 3B). Swimming plots of different mutation subgroups (Supplementary Figure S1A) and dose subgroups (Supplementary Figure S1A) were also generated. Subgroup analysis by mutation subtypes showed differences in response rates, with the L861X subgroup demonstrating the worst ORR (L861X vs G719X vs S768I: 44.4% vs 56.5% vs 62.5%) and DCR (66.7% vs 100% vs 100%). However, there was no significant difference in the ORR (p = 0.737) (Table 2).
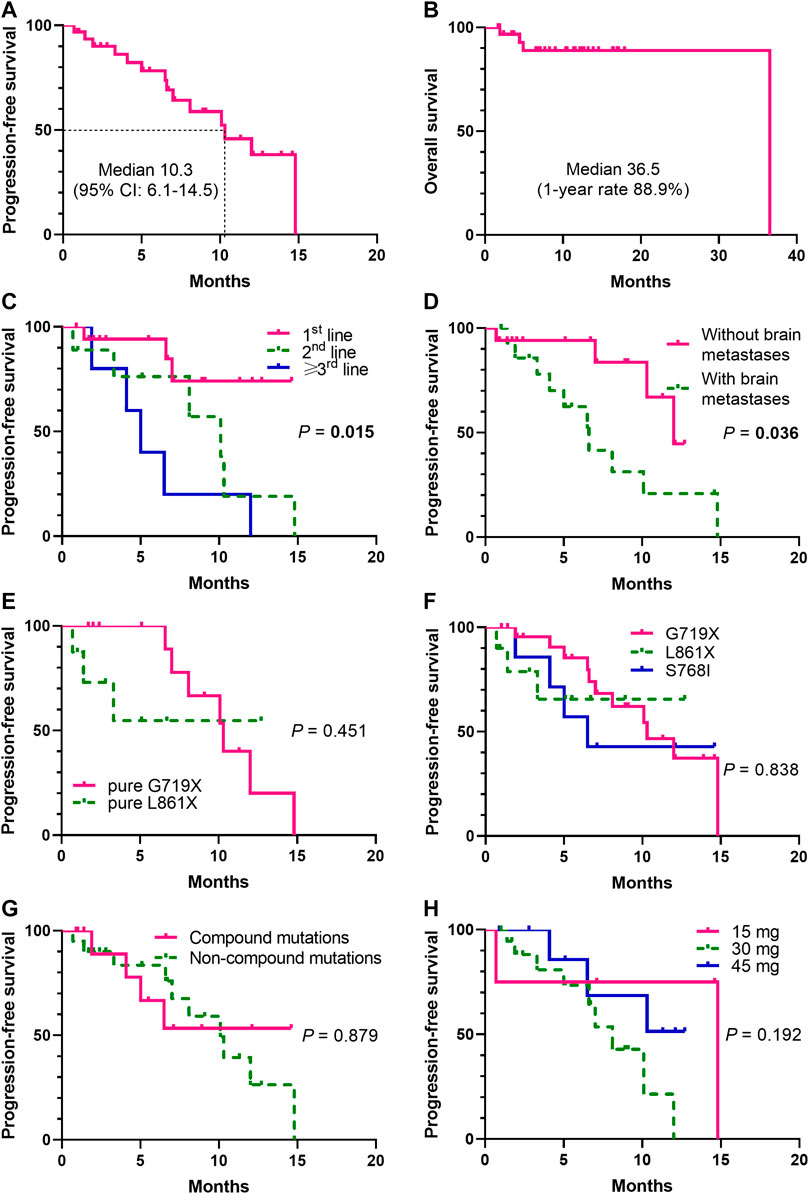
FIGURE 3. Kaplan-Meier analysis of progression-free survival (PFS) and overall survival (OS) (N= 32). The Kaplan-Meier curves were shown for PFS (Figure 3A) and OS (Figure 3B). Significant difference was observed (p = 0.015) in the median PFS (mPFS) of patients treated with dacomitinib in different lines (Figure 3C). Significant unfavorable effect of brain metastases on PFS (p = 0.036) (Figure 3D) was shown. Patients harboring different mutation subtypes demonstrated different mPFS (Figures 3E,F), though no significant difference was observed. No significant difference between compound mutations and non-compound mutations groups was found (p = 0.879) (Figure 3G). Patients received different dacomitinib doses showed no different mPFS (p = 0.192) (Figure 3H).
Among 18 (56.3%) prospectively enrolled patients who received dacomitinib as the first-line therapy, after at least one radiologic review, 5 (27.8%) patients showed SD and 13 (72.2%) showed PR, achieving an ORR of 72.2% and a DCR of 100%. The median PFS was not reached (NR) (Figure 3C). Specifically, compared with the other patients with the G719A mutation, the third patient (P5) (Figure 1A) did not experience any shrinkage of the tumor lesion after treatment, and NGS testing suggested S899N and D1083V co-mutations of EGFR; however, the significance of these co-mutations is currently unknown.
In total, 14 (43.8%) retrospectively enrolled patients received dacomitinib as later-line therapy and previous treatments of each patient were displayed in Figure 2A. Five patients received dacomitinib after progression from first-line 2G TKI afatinib treatment (”2+2 mode”). Among them, two patients had PD, two patients had SD, and one patient achieved PR. Three patients were given dacomitinib after progression from third-generation TKI (”3+2 mode”) without T790M. Among them, two patients achieved SD and one patient developed PR. The PFS of the three patients was 5, 10.1, and 12 months, respectively.
A total of 15 patients were diagnosed with brain parenchymal metastases before dacomitinib treatment, and 2 (P4, P6) of them also had concurrent leptomeningeal metastases (Figure 1A). Seven patients had symptoms associated with brain lesions, including dizziness, headache, and ambiopia, while the remaining eight patients had asymptomatic brain lesions. Two patients received whole brain radiotherapy before dacomitinib initiation. Except for one patient who was unable to undergo a brain examination due to obvious chest progression and poor ECOG PS, enhanced MRI confirmed disease control in 13 of the 14 patients (92.9%) (Figure 4). Five symptomatic patients experienced symptom relief. In addition, among the 10 patients with brain metastases who developed disease progression, four patients (40%) progressed again due to brain metastases.
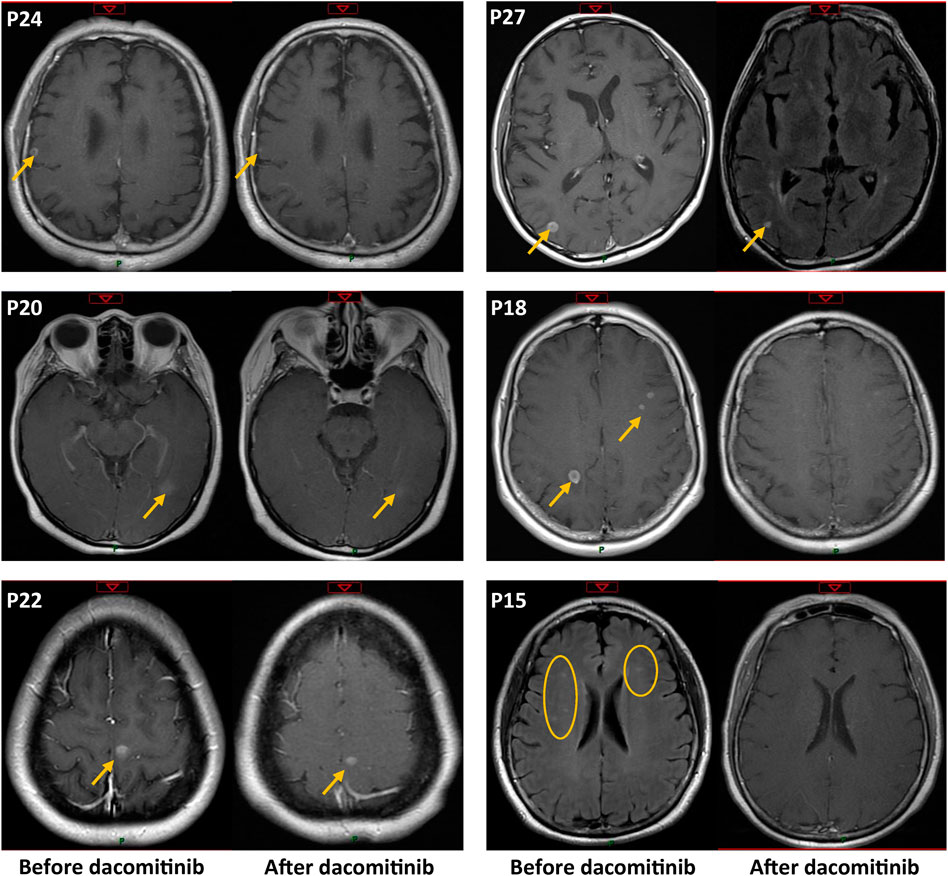
FIGURE 4. Enhanced MRI confirmed intracranial metastasis control before and after dacomitinib treatment in six representative patients. The patient number is consistent with Figure 1 but not Figure 2.
In the overall cohort, the most common adverse events (AEs) were rash (86.7%), diarrhea (80%), and oral mucositis (60%). Three patients required a dosage reduction from 45 mg to 30 mg or 15 mg, one patient required a dosage reduction from 30 to 15 mg due to intolerable grade 3 diarrhea or rash, and two patients required a dosage escalation from 30 to 45 mg owing to good tolerance. All patients completed dose adjustment within 4 weeks. All patients (100%) experienced grade 1–2 AEs, but no grade 4–5 treatment-emergent AEs occurred (Table 3).
The median PFS (mPFS) was significantly among patients treated with dacomitinib in the first-, second-, and third-line setting (NR vs 10.1 vs 5 months, p = 0.015) (Figure 3C). Brain metastases significantly affected PFS (HR = 2.997, 95% CI: 1.047–8.583; p = 0.036) (Figure 3D). The mPFS was different among the mutation subtypes pure G719X and pure L861X, although the difference did not reach statistical significance (10.3 vs NR; p = 0.451) (Figure 3E). The mPFS was also different among the mutation subtypes G719X, L861X, and S768I, although the difference did not reach statistical significance (10.3 vs NR vs 6.5; p = 0.838) (Figure 3F). There were also no significant differences between the compound and non-compound mutation subgroups (p = 0.879) (Figure 3G). In addition, the mPFS did not significantly differ according to the dacomitinib dose (p = 0.192) (Figure 3H). Survival comparisons according to age, gender, smoking history, ECOG PS, total tumor burden, and TP53 co-mutation status showed no significant differences (Supplementary Figure S2).
There have been few studies on the efficacy and safety of dacomitinib for uncommon EGFR mutations (Lau et al., 2019; Lavacchi et al., 2019; Wang and Li, 2019). In our previous study, we retrospectively presented the later-line efficacy of dacomitinib on 11 patients with NSCLC harboring uncommon EGFR mutations, and the findings indicated a promising activity of dacomitinib (Li et al., 2022). Further, in this study of 32 Chinese patients with NSCLC harboring major uncommon EGFR mutations treated with dacomitinib, 14 patients (46.7%) showed disease progression within a median follow-up duration of 11.4 months. Particularly, in the first-line setting, the patients obtained an ORR of 72.2% and a DCR of 100%, and the median PFS was unreached and the median OS was 36.5 months.
Common EGFR mutations are highly responsive to different types of EGFR-TKIs. However, uncommon mutations (especially exon 20 insertions) are less sensitive, with less satisfactory response rates and survival in previous studies (Wu et al., 2011; Watanabe et al., 2014; Baek et al., 2015; Chiu et al., 2015b; Kuiper et al., 2016; Krawczyk et al., 2017). Nevertheless, major uncommon EGFR mutations, including L861Q, G719X, and S768I, are still sensitive to first-generation (1G) EGFR-TKIs (gefitinib/erlotinib), with ORRs ranging from 41.6% to 53.8%; DCR, 62.9%–86.7%; mPFS, 2.2 months–7.7 months; and median OS (mOS), 11.9 months–19.0 months (Wu et al., 2011; Chiu et al., 2015b; Kuiper et al., 2016; Krawczyk et al., 2017). However, evidence suggests that 2G TKIs are more favorable in patients with NSCLC harboring uncommon mutations. Wu et al. (Wu et al., 2020) demonstrated that for patients with major uncommon EGFR mutations, the mPFS of afatinib was significantly longer than that of gefitinib and erlotinib. A combined post-hoc analysis of three LUX-Lung trials demonstrated that for the most frequent uncommon mutations (i.e., G719X, L861Q, and S768I), the ORRs were 77.8%, 56.3%, and 100%, and the mPFS were 13.8 months, 8.2 months, and 14.7 months, respectively (Yang et al., 2015).
Based on these promising results, afatinib was approved by the Food and Drug Administration in 2018 for lung cancer with L861Q, G719X, and S768I mutations. Nevertheless, efforts to explore more effective drugs for uncommon mutations have continued. In the KCSG-LU15-09 trial conducted by Cho et al. (Cho et al., 2020), osimertinib achieved an ORR of 53%, 78%, 38% and an mPFS of 8.2 months, 15.2 months, and 12.3 months in 32 patients (mostly first-line and all TKI-naïve) harboring major uncommon EGFR mutations of G719X, L861Q, and S768I, respectively. Dacomitinib is another 2G TKI that is an irreversible pan-human epidermal growth factor receptor TKI. Despite its potential, data on its usefulness in the treatment of NSCLC patients with major uncommon EGFR mutations are rare (1). In the current study, dacomitinib in the first-line setting achieved an ORR of 72.2% and a DCR of 100%. These values are close to the post-hoc analysis data for the 2G TKI afatinib from the LUX-Lung trial (24). Specifically, the ORRs for different mutation subtypes (G719X vs L861X vs S768I) were 66.7%, 50%, and 100%, respectively, which are also similar to the data reported by Yang et al. Collectively, these findings indicate that, compared to osimertinib, 2G TKIs yield a better treatment response for G719X and S768I; however, they have a worse therapeutic efficacy for L861X, consistent with the findings by Robichaux et al. (Robichaux et al., 2021).
In the new structure-based classification developed by Robichaux et al., G719X and S768I are categorized as the “P-loop C-helix compressing” type, where changes in the orientation of the P-loop could alter the position of TKI stabilization points, tilting the indole ring of osimertinib away from the P-loop and destabilizing drug binding. It is known that 2G TKIs do not interact with the P-loop of EGFR and maintain interaction points in the hydrophobic cleft, thus retaining full effectiveness for G719X and S768I, as supported by patient-derived xenograft model experiments. However, the L861Q mutation, categorized as the “classical-like” type, is distal from the drug-binding pocket and has lower impact on the overall structure of EGFR than the wild-type. In this mutation, osimertinib has better efficacy than 2G TKIs and is thus worthy of further research. The current study involved a higher proportion of females and never smokers, and this trend is similar to that seen in patients harboring common mutations. This is in contrast to previous studies on uncommon EGFR mutations (Wang et al., 2013; Chiu et al., 2015a; Tu et al., 2017; Yamada et al., 2020). For specific mutation subtypes, G719X (40.6%) and L861Q (21.9%) were the two most frequent single uncommon mutations. Overall, 34.4% of patients (11/32) had compound mutations, and G719X + S768I was the most common compound mutation (81.8%, 9/11), consistent with the mutation profiles reported by Yamada et al. (Yamada et al., 2020) and by Brindel et al. (Brindel et al., 2020).
Patients with compound uncommon mutations have better tumor response and prognosis than patients with single uncommon mutations (Brindel et al., 2020; Yamada et al., 2020; Yang et al., 2020; Passaro et al., 2021; Tan et al., 2021). In the study by Yang et al., afatinib yielded better anti-tumor activity against compound uncommon mutations than against major uncommon mutations in both TKI-naïve patients and TKI-pretreated patients (Yang et al., 2020). However, we found no significant difference in the ORR and mPFS between compound mutation and non-compound mutation groups. We speculate that this may be due to differences in ethnicity, study size, and mutation-type distribution. A recent case series by Peng et al. (Peng et al., 2021) showed that dacomitinib has potent efficacy against CNS metastasis in EGFR-positive NSCLC, with an ORR of 92.9% and a DCR of 100%. One of the patients with brain metastases carrying G719A mutation also achieved PR, indicating the potential efficacy of dacomitinib for NSCLC harboring uncommon mutations with brain metastases. In our study, disease control of brain metastatic lesions was confirmed in 13 patients (13/14, 92.9%). In addition, 5/8 (62.5%) patients with symptomatic brain metastatic experienced symptom relief, supporting the efficacy of dacomitinib for patients harboring uncommon mutations with brain metastases.
Interestingly, five patients who progressed with 2G TKI afatinib were treated with dacomitinib. In the OLCSG trial 1403, afatinib re-administration for sensitive EGFR-mutant NSCLC without T790M after resistance to 1G or 2G EGFR-TKIs yielded modest activity, with an ORR and DCR of 17% and 84%, respectively. However, no successful rechallenge with dacomitinib after afatinib progression has been reported to date, especially in patients harboring uncommon mutations. Masuda et al. (Masuda et al., 2020) reported long-term survival of NSCLC patients harboring concomitant G719C and S768I mutations who received afatinib rechallenge, indicating the potential of 2G TKI rechallenge. In our study, of the five patients treated with 2G afatinib followed by dacomitinib, two patients developed PD, two achieved SD, and one achieved PR, suggesting the possibility dacomitinib rechallenge after afatinib progression. However, additional clinical data are required to explore and confirm this strategy.
In the ARCHER 1050 study (Wu et al., 2017), dacomitinib treatment had to be discontinued in 7% of the patients due to toxic effects, and dose reductions due to intolerable AEs were required in 66% of the patients. However, Cheng et al. (Cheng et al., 2021) reported that the OS benefit was maintained in patients who had a stepwise dose reduction of dacomitinib (from 45 mg/day to 30 mg/day or 15 mg/day). Based on data from previous clinical trials, we adjusted the dose according to the patient’s physical status, weight, and comorbidities to ensure that the patient tolerated the AEs as soon as possible. No patient required treatment discontinuation due to serious AEs, and most patients were able to adapt to the therapy within 4 weeks. In addition, only 12.5% (4/32) of the patients required a dosage reduction due to intolerable AEs. However, due to the limited study scale, no definite conclusions could be made on whether different doses affect the efficacy and survival benefit of dacomitinib.
This study had some limitations. The sample size was small, and the patients were only enrolled from two medical centers in China. Therefore, the possibility of selection bias, which may have resulted in potentially compromised results, could not be ruled out. Thus, the results should be interpreted cautiously. Furthermore, the mechanisms of dacomitinib resistance have not been investigated. Our findings need to be confirmed in prospective clinical trials.
This ambiospective cohort study shows that dacomitinib has potential efficacy in advanced NSCLC patients harboring major uncommon EGFR mutations. In addition, dacomitinib has favorable efficacy for brain metastases and has a good safety profile. Dacomitinib may be a new paradigm and expectation for patients with major uncommon EGFR mutations, and more prospective data are warranted.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the Chinese PLA hospital and the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (NO. 18-070/1648; NO. 20-232/2428). Considering the ambispective nature of the study, the need for informed consent was waived for the retrospectively enrolled patients, while it was obtained from prospectively enrolled patients.
Conceptualization H-SL, XY, YW; methodology H-SL, G-JY, YC; software G-JY, YC; validation J-LL, H-YX, TZ, L-QZ, YYW, J-LW, X-SH; formal analysis H-SL, G-JY, YC; investigation J-LL, H-YX, TZ, L-QZ, Y-YW, J-LW, X-SH; resources J-LL, H-YX, TZ, L-QZ, Y-YW, J-LW, X-SH; data curation J-LL, H-YX, TZ, L-QZ, Y-YW, J-LW, X-SH.; writing—original draft preparation H-SL, G-JY, YC; writing—review and editing, J-LL, H-YX, TZ, L-QZ, Y-YW, J-LW, X-SH, XY, YW; visualization G-JY, YC; supervision XY, YW; project administration H-SL, G-JY, YC; funding acquisition XY, YW. All authors have read and agreed to the published version of the manuscript.
This research was funded by the National Natural Science Foundation of China (Grant No. 82072590), Beijing Health Promotion Association (Grant No. 2021–053-ZZ), and the National Natural Science Foundation of China (Grant No. 82172864).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank Editage (www.editage.cn) for English language editing.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.919652/full#supplementary-material
Baek, J. H., Sun, J. M., Min, Y. J., Cho, E. K., Cho, B. C., Kim, J. H., et al. (2015). Efficacy of EGFR Tyrosine Kinase Inhibitors in Patients with EGFR-Mutated Non-small Cell Lung Cancer except Both Exon 19 Deletion and Exon 21 L858R: a Retrospective Analysis in Korea. Lung Cancer 87 (2), 148–154. doi:10.1016/j.lungcan.2014.11.013
Brindel, A., Althakfi, W., Barritault, M., Watkin, E., Maury, J. M., Bringuier, P. P., et al. (2020). Uncommon EGFR Mutations in Lung Adenocarcinoma: Features and Response to Tyrosine Kinase Inhibitors. J. Thorac. Dis. 12 (9), 4643–4650. doi:10.21037/jtd-19-3790
Cheng, Y., Mok, T. S., Zhou, X., Lu, S., Zhou, Q., Zhou, J., et al. (2021). Safety and Efficacy of First-Line Dacomitinib in Asian Patients with EGFR Mutation-Positive Non-small Cell Lung Cancer: Results from a Randomized, Open-Label, Phase 3 Trial (ARCHER 1050). Lung Cancer 154, 176–185. doi:10.1016/j.lungcan.2021.02.025
Chiu, C. H., Yang, C. T., Shih, J. Y., Huang, M. S., Su, W. C., Lai, R. S., et al. (2015). Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Treatment Response in Advanced Lung Adenocarcinomas with G719X/L861Q/S768I Mutations. J. Thorac. Oncol. 10 (5), 793–799. doi:10.1097/JTO.0000000000000504
Chiu, C. H., Yang, C. T., Shih, J. Y., Huang, M. S., Su, W. C., Lai, R. S., et al. (2015). Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Treatment Response in Advanced Lung Adenocarcinomas with G719X/L861Q/S768I Mutations. J. Thorac. Oncol. 10 (5), 793–799. doi:10.1097/JTO.0000000000000504
Cho, J. H., Lim, S. H., An, H. J., Kim, K. H., Park, K. U., Kang, E. J., et al. (2020). Osimertinib for Patients with Non-small-cell Lung Cancer Harboring Uncommon EGFR Mutations: A Multicenter, Open-Label, Phase II Trial (KCSG-LU15-09). J. Clin. Oncol. 38 (5), 488–495. doi:10.1200/JCO.19.00931
Gristina, V., Malapelle, U., Galvano, A., Pisapia, P., Pepe, F., Rolfo, C., et al. (2020). The Significance of Epidermal Growth Factor Receptor Uncommon Mutations in Non-small Cell Lung Cancer: A Systematic Review and Critical Appraisal. Cancer Treat. Rev. 85, 101994. doi:10.1016/j.ctrv.2020.101994
Huang, C. H., Ju, J. S., Chiu, T. H., Huang, A. C., Tung, P. H., Wang, C. C., et al. (2022). Afatinib Treatment in a Large Real-World Cohort of Non-small Cell Lung Cancer Patients with Common and Uncommon Epidermal Growth Factor Receptor Mutation. Int. J. Cancer 150 (4), 626–635. doi:10.1016/j.ctrv.2020.101994
Jänne, P. A., Boss, D. S., Camidge, D. R., Britten, C. D., Engelman, J. A., Garon, E. B., et al. (2011). Phase I Dose-Escalation Study of the Pan-HER Inhibitor, PF299804, in Patients with Advanced Malignant Solid Tumors. Clin. Cancer Res. 17 (5), 1131–1139. doi:10.1158/1078-0432.CCR-10-1220
Krawczyk, P., Kowalski, D. M., Ramlau, R., Kalinka-Warzocha, E., Winiarczyk, K., Stencel, K., et al. (2017). Comparison of the Effectiveness of Erlotinib, Gefitinib, and Afatinib for Treatment of Non-small Cell Lung Cancer in Patients with Common and Rare EGFR Gene Mutations. Oncol. Lett. 13 (6), 4433–4444. doi:10.3892/ol.2017.5980
Kris, M. G., Camidge, D. R., Giaccone, G., Hida, T., Li, B. T., O'Connell, J., et al. (2015). Targeting HER2 Aberrations as Actionable Drivers in Lung Cancers: Phase II Trial of the Pan-HER Tyrosine Kinase Inhibitor Dacomitinib in Patients with HER2-Mutant or Amplified Tumors. Ann. Oncol. 26 (7), 1421–1427. doi:10.1093/annonc/mdv186
Kuiper, J. L., Hashemi, S. M., Thunnissen, E., Snijders, P. J., Grünberg, K., Bloemena, E., et al. (2016). Non-classic EGFR Mutations in a Cohort of Dutch EGFR-Mutated NSCLC Patients and Outcomes Following EGFR-TKI Treatment. Br. J. Cancer 115 (12), 1504–1512. doi:10.1038/bjc.2016.372
Lau, S. C. M., Batra, U., Mok, T. S. K., and Loong, H. H. (2019). Dacomitinib in the Management of Advanced Non-small-cell Lung Cancer. Drugs 79 (8), 823–831. doi:10.1007/s40265-019-01115-y
Lavacchi, D., Mazzoni, F., and Giaccone, G. (2019). Clinical Evaluation of Dacomitinib for the Treatment of Metastatic Non-small Cell Lung Cancer (NSCLC): Current Perspectives. Drug Des. Devel Ther. 13, 3187–3198. doi:10.2147/DDDT.S194231
Li, H. S., Zhang, J. Y., Yan, X., Xu, H. Y., Hao, X. Z., Xing, P. Y., et al. (2022). A Real-World Study of Dacomitinib in Later-Line Settings for Advanced Non-small Cell Lung Cancer Patients Harboring EGFR Mutations. Cancer Med. 11 (4), 1026–1036. doi:10.1002/cam4.4495
Masuda, T., Sunaga, N., Kasahara, N., Takehara, K., Yatomi, M., Hara, K., et al. (2020). Successful Afatinib Rechallenge in a Patient with Non-small Cell Lung Cancer Harboring EGFR G719C and S768I Mutations. Thorac. Cancer 11 (8), 2351–2356. doi:10.1111/1759-7714.13532
Nishino, M., Suda, K., Kobayashi, Y., Ohara, S., Fujino, T., Koga, T., et al. (2018). Effects of Secondary EGFR Mutations on Resistance against Upfront Osimertinib in Cells with EGFR-Activating Mutations In Vitro. Lung Cancer 126, 149–155. doi:10.1016/j.lungcan.2018.10.026
Passaro, A., Mok, T., Peters, S., Popat, S., Ahn, M. J., and de Marinis, F. (2021). Recent Advances on the Role of EGFR Tyrosine Kinase Inhibitors in the Management of NSCLC with Uncommon, Non Exon 20 Insertions, EGFR Mutations. J. Thorac. Oncol. 16 (5), 764–773. doi:10.1016/j.jtho.2020.12.002
Peng, W., Pu, X., Jiang, M., Wang, J., Li, J., Li, K., et al. (2021). Dacomitinib Induces Objective Responses in Metastatic Brain Lesions of Patients with EGFR-Mutant Non-small-cell Lung Cancer: A Brief Report. Lung Cancer 152, 66–70. doi:10.1016/j.lungcan.2020.12.008
Reckamp, K. L., Giaccone, G., Camidge, D. R., Gadgeel, S. M., Khuri, F. R., Engelman, J. A., et al. (2014). A Phase 2 Trial of Dacomitinib (PF-00299804), an Oral, Irreversible Pan-HER (Human Epidermal Growth Factor Receptor) Inhibitor, in Patients with Advanced Non-small Cell Lung Cancer after Failure of Prior Chemotherapy and Erlotinib. Cancer 120 (8), 1145–1154. doi:10.1002/cncr.28561
Remon, J., Hendriks, L. E. L., Cardona, A. F., and Besse, B. (2020). EGFR Exon 20 Insertions in Advanced Non-small Cell Lung Cancer: A New History Begins. Cancer Treat. Rev. 90, 102105. doi:10.1016/j.ctrv.2020.102105
Robichaux, J. P., Le, X., Vijayan, R. S. K., Hicks, J. K., Heeke, S., Elamin, Y. Y., et al. (2021). Structure-based Classification Predicts Drug Response in EGFR-Mutant NSCLC. Nature 597 (7878), 732–737. doi:10.1038/s41586-021-03898-1
Tan, J., Hu, C., Deng, P., Wan, R., Cao, L., Li, M., et al. (2021). The Predictive Values of Advanced Non-small Cell Lung Cancer Patients Harboring Uncommon EGFR Mutations-The Mutation Patterns, Use of Different Generations of EGFR-TKIs, and Concurrent Genetic Alterations. Front. Oncol. 11, 646577. doi:10.3389/fonc.2021.646577
Tu, H. Y., Ke, E. E., Yang, J. J., Sun, Y. L., Yan, H. H., Zheng, M. Y., et al. (2017). A Comprehensive Review of Uncommon EGFR Mutations in Patients with Non-small Cell Lung Cancer. Lung Cancer 114, 96–102. doi:10.1016/j.lungcan.2017.11.005
Wang, Q., Mou, J., Yang, X., He, Y., Li, Z., Luo, Q., et al. (2013). EGFR Mutations in Patients with Lung Adenocarcinoma in Southwest China: Are G719S/A and L861Q More Likely Detected in Tumors Derived from Smokers? Lung Cancer (Auckl) 4, 27–33. doi:10.2147/LCTT.S44825
Wang, S., and Li, J. (2019). Second-generation EGFR and ErbB Tyrosine Kinase Inhibitors as First-Line Treatments for Non-small Cell Lung Cancer. Onco Targets Ther. 12, 6535–6548. doi:10.2147/OTT.S198945
Watanabe, S., Minegishi, Y., Yoshizawa, H., Maemondo, M., Inoue, A., Sugawara, S., et al. (2014). Effectiveness of Gefitinib against Non-small-cell Lung Cancer with the Uncommon EGFR Mutations G719X and L861Q. J. Thorac. Oncol. 9 (2), 189–194. doi:10.1097/JTO.0000000000000048
Wu, J. Y., Yu, C. J., Chang, Y. C., Yang, C. H., Shih, J. Y., and Yang, P. C. (2011). Effectiveness of Tyrosine Kinase Inhibitors on "uncommon" Epidermal Growth Factor Receptor Mutations of Unknown Clinical Significance in Non-small Cell Lung Cancer. Clin. Cancer Res. 17 (11), 3812–3821. doi:10.1158/1078-0432.CCR-10-3408
Wu, S. G., Yu, C. J., Yang, J. C., and Shih, J. Y. (2020). The Effectiveness of Afatinib in Patients with Lung Adenocarcinoma Harboring Complex Epidermal Growth Factor Receptor Mutation. Ther. Adv. Med. Oncol. 12, 1758835920946156. doi:10.1177/1758835920946156
Wu, Y. L., Cheng, Y., Zhou, X., Lee, K. H., Nakagawa, K., Niho, S., et al. (2017). Dacomitinib versus Gefitinib as First-Line Treatment for Patients with EGFR-Mutation-Positive Non-small-cell Lung Cancer (ARCHER 1050): a Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 18 (11), 1454–1466. doi:10.1016/S1470-2045(17)30608-3
Yamada, Y., Tamura, T., Yamamoto, Y., Ichimura, H., Hayashihara, K., Saito, T., et al. (2020). Treatment of Patients with Non-small-cell Lung Cancer with Uncommon EGFR Mutations in Clinical Practice. Anticancer Res. 40 (10), 5757–5764. doi:10.21873/anticanres.14592
Yang, J. C., Schuler, M., Popat, S., Miura, S., Heeke, S., Park, K., et al. (2020). Afatinib for the Treatment of NSCLC Harboring Uncommon EGFR Mutations: A Database of 693 Cases. J. Thorac. Oncol. 15 (5), 803–815. doi:10.1016/j.jtho.2019.12.126
Yang, J. C., Sequist, L. V., Geater, S. L., Tsai, C. M., Mok, T. S., Schuler, M., et al. (2015). Clinical Activity of Afatinib in Patients with Advanced Non-small-cell Lung Cancer Harbouring Uncommon EGFR Mutations: a Combined Post-hoc Analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 16 (7), 830–838. doi:10.1016/S1470-2045(15)00026-1
Keywords: dacomitinib, non-small cell lung cancer, major uncommon EGFR mutations, efficacy, safety
Citation: Li H-S, Yang G-J, Cai Y, Li J-L, Xu H-Y, Zhang T, Zhou L-Q, Wang Y-Y, Wang J-L, Hu X-S, Yan X and Wang Y (2022) Dacomitinib for Advanced Non-small Cell Lung Cancer Patients Harboring Major Uncommon EGFR Alterations: A Dual-Center, Single-Arm, Ambispective Cohort Study in China. Front. Pharmacol. 13:919652. doi: 10.3389/fphar.2022.919652
Received: 13 April 2022; Accepted: 18 May 2022;
Published: 13 June 2022.
Edited by:
Lele Song, Eighth Medical Center of the General Hospital of the Chinese People’s Liberation Army, ChinaReviewed by:
Yongfeng Yu, Shanghai Jiao Tong University, ChinaCopyright © 2022 Li, Yang, Cai, Li, Xu, Zhang, Zhou, Wang, Wang, Hu, Yan and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiang Yan, eXhpYW5nMzAxQHNpbmEuY29t; Yan Wang, d2FuZ3lhbnlpZnVAMTYzLmNvbQ==
†These authors have contributed equally to this work and share co-first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.