- 1Department of Epidemiology and Health Statistics, College of Integrated Traditional Chinese and Western Medicine, Hunan University of Chinese Medicine, Changsha, China
- 2Department of Scientific Research, Xiangya Hospital, Central South University, Changsha, China
- 3Department of Anesthesiology, Shandong Provincial Qianfoshan Hospital, the First Hospital Affiliated with Shandong First Medical University, Ji’nan, China
- 4Xiangya School of Public Health, Central South University, Changsha, China
- 5Department of Nephrology, Xiangya Hospital, Central South University, Changsha, China
- 6Department of Science and Education, The First People’s Hospital of Changde City, Changde, China
- 7Department of Acupuncture and Massage Rehabilitation, First Affiliated Hospital of Hunan University of Chinese Medicine, Changsha, China
Background: The safety of prescribing angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) during acute kidney injury (AKI) remains unclear. We aimed to investigate the associations of ACEI/ARB therapy in AKI with the risk of mortality, acute kidney disease (AKD), and hyperkalemia.
Methods: We conducted a retrospective monocentric study, which included patients in Massachusetts between 2008 and 2019 from the Medical Information Mart for Intensive Care IV (MIMIC-IV) database. Propensity score matching was performed for the endpoint analysis. The association between ACEI/ARB therapy and mortality was assessed using Cox proportional hazards regression models. Logistic regression was used to assess the risk of AKD and hyperkalemia.
Results: Among the 19,074 individuals with AKI admitted to the intensive care unit (ICU), 3,244 (17.0%) received ACEI/ARBs, while 15,830 (83.0%) did not. In the propensity score-matched sample of 6,358 individuals, we found a decreased risk of mortality in those who received ACEI/ARBs compared to those who did not (hazard ratio [HR] for ICU mortality: 0.34, 95% confidence interval [CI]: 0.27–0.42); HR for in-hospital mortality: 0.47, 95% CI: 0.39–0.56; HR for 30-day mortality: 0.47, 95% CI: 0.40–0.56; HR for 180-day mortality: 0.53, 95% CI: 0.45–0.62). However, the use of ACEI/ARBs was associated with a higher risk of AKD (risk ratio [RR]: 1.81; 95% CI: 1.55–2.12). There was no significant association between ACEI/ARBs and an increased risk of hyperkalemia (RR: 1.21; 95% CI: 0.96–1.51).
Conclusions: ACEI/ARB treatment during an episode of AKI may decrease all-cause mortality, but increases the risk of AKD. Future randomized controlled trials are warranted to validate these findings.
Introduction
Acute kidney injury (AKI) manifests as an elevated serum creatinine (SCr) level and/or decreased urine output, attributable to the abrupt deterioration of kidney function (Waikar and Bonventre, 2009). Many patients, especially those in intensive care units (ICUs), are at risk for AKI (Nash et al., 2002; Bellomo et al., 2012; Lewington et al., 2013). AKI is independently associated with an increased risk of in-hospital and long-term mortality, subsequent chronic kidney disease (CKD), end-stage renal disease, and cardiovascular events (Coca et al., 2009; Coca et al., 2012).
Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) are widely used in the treatment of hypertension, heart failure, coronary artery disease, and CKD. ACEI/ARBs have been shown to reduce proteinuria (Levin et al., 2013). Studies have shown that ACEI/ARBs could slow CKD progression, decrease the risk of dialysis dependence, preserve cardiovascular function, and reduce the rates of cardiovascular events and mortality in CKD patients (Balamuthusamy et al., 2008; Wang et al., 2018).
ACEI/ARBs may cause or exacerbate AKI in acute conditions, especially in patients with acute hypovolemia or sepsis. A previous study found that, on one hand, chronic ACEI/ARB treatment was associated with a small increase in AKI risk over a period of 4 years (Mansfield et al., 2016). On the other hand, in AKI patients who survived from the ICU, ACEI/ARB prescription at discharge was associated with a significant reduction in 1-year mortality (Gayat et al., 2018). In AKI patients discharged from the hospital, ACEI/ARB treatment was associated with a reduced mortality risk over a 2-year follow up (Brar et al., 2018). The risks and benefits of ACEI/ARB use in AKI remain unclear. Acute kidney disease (AKD) is defined as an AKI event persisting for more than 7 days (up to 90 days) (Ostermann et al., 2020). A previous study of AKI survivors found that acute exposure to ACEI/ARBs before or during an episode of AKI was not associated with an increased risk of AKD (Hines et al., 2020). The role of ACEI/ARBs in the conversion of AKI to AKD requires further investigation.
There is limited evidence regarding the risk of mortality and kidney outcomes in people who received ACEI/ARBs during an episode of AKI. Therefore, we aimed to evaluate whether exposure to ACEI/ARBs in AKI reduced mortality, and to determine the risk of adverse outcomes, including AKD and hyperkalemia.
Materials and methods
Data sources
The data analyzed in this retrospective observational study were extracted from the Medical Information Mart for Intensive Care IV (MIMIC-IV) database, which is a freely accessible critical care database consisting of over 60,000 patients admitted to ICUs at a US tertiary academic medical center between 2008 and 2019 (Johnson et al., 2020). The MIMIC-IV database contains comprehensive high-quality de-identified data, including demographic characteristics, vital signs, laboratory parameters, intake and output records, treatment information, and survival status. To gain access to the database, one of the authors completed the Collaborative Institutional Training Initiative examination (all data were retrieved by Xu Zhu; certification number: 40063205). The project was approved by the institutional review boards of Beth Israel Deaconess Medical Center and Massachusetts Institute of Technology. All participants in this study remained anonymous, so the ethics committee’s approval was not required for this study. The article was reported in compliance with the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) statement (von Elm et al., 2007).
Selection criteria
We compared the AKI patients who received ACEI/ARBs in the ICU with those who did not. We identified the AKI patients according to the Kidney Disease Improving Global Outcome (KDIGO) criteria, as follows (Kellum et al., 2012): An increase in SCr of more than 1.5-fold from the baseline level within 7 days; an increase of SCr >0.3 mg/dl within 48 h; or urine volume <0.5 ml/kg/h for ≥6 h. Baseline SCr was defined as the minimum SCr level within 7 days before admission, or the first SCr obtained at the ICU admission if pre-admission SCr was not available. If a patient had multiple ICU admissions, only the data from the first admission were included in the analysis. Patients who were discharged or those who died within 48 h after ICU admission were excluded. Patients aged <18 years were also excluded.
Data collection
PostgreSQL (ver. 9.6) was used to extract all the variables and outcomes in a Structured Query Language (SQL) format. Demographic information included age, sex, and ethnicity. Vital signs recorded at admission included systolic and diastolic blood pressures, heart rate, temperature, oxygen saturation (SpO2), and respiratory rate. Co-morbidities included hypertension, congestive heart failure, myocardial infarction, cerebrovascular disease, diabetes mellitus, CKD, sepsis, and shock. All co-morbidities were diagnosed using the International Classification of Diseases (ICD) code 9 or 10. Laboratory parameters included white blood cells (WBCs), hemoglobin, SCr, blood urea nitrogen (BUN), sodium, chloride, and bicarbonate. Laboratory parameters were obtained within 48 h of ICU admission. The Sequential Organ Failure Assessment (SOFA) score was used to assess illness severity. SOFA scores were recorded at the time of ICU admission. In terms of treatments, the following data were extracted: use of diuretics, vasopressors, and glucocorticoids, as well as mechanical ventilation, renal replacement therapy, and cardiac surgery. Fluid balance was calculated by subtracting the fluid output from the total fluid intake within 48 h after ICU admission.
The AKI stages were defined using both SCr and urine output during the first 48 h after ICU admission (Zhao et al., 2020). AKI stages based on changes in SCr were diagnosed as follows: SCr greater than or equal to 1.5 times the baseline SCr; or an increase in SCr greater than or equal to 0.3 mg/dl from baseline SCr constitutes AKI stage 1; SCr greater than or equal to 2.0 times the baseline SCr constitutes AKI stage 2; SCr greater than or equal to 3.0 times the baseline SCr, or increase in the SCr concentration no less than 4.0 mg/dl, or initiation of renal replacement therapy constitutes stage 3. AKI stages based on urine output were extracted directly from the MIMIC database.
The defined daily dose (DDD) is a statistical measure of drug consumption defined by the World Health Organization. The DDD is the assumed average daily maintenance dose of a drug product used for its primary indication in adults. It is used to standardize the comparison of drug usage between different drugs or between different healthcare settings. We compared ACEI/ARBs based on the same standard by using the following formula (total amount of drug)/(amount of drug in a DDD) = number of DDDs (WHOCC-ATC and Index, 2013). The cumulative defined daily dose (cDDD), which was calculated as the sum of the dispensed DDD of any specific ACEI/ARB in the ICU, was used to examine the dose–response relationship between ACEI/ARBs and all-cause mortality.
Endpoints
The primary outcomes were all-cause ICU mortality, in-hospital mortality, 30-day all-cause mortality, and 180-day all-cause mortality. The secondary outcomes were AKD and hyperkalemia. AKD was diagnosed according to the Acute Disease Quality Initiative-16 (ADQI-16) of 2017 and was defined as an AKI for >7 days after an AKI-initiating event (Chawla et al., 2017). Hyperkalemia was defined as a serum potassium level > 5.5 mEq/L.
Statistical analysis
Patients were categorized as treated or untreated with ACEI/ARBs during the ICU stay. Continuous variables with a normal distribution and homogeneity of variance are expressed as means ± standard deviation. The skewed distributed variables are presented as medians with an inter-quartile range. Categorical variables are expressed as numbers with proportions.
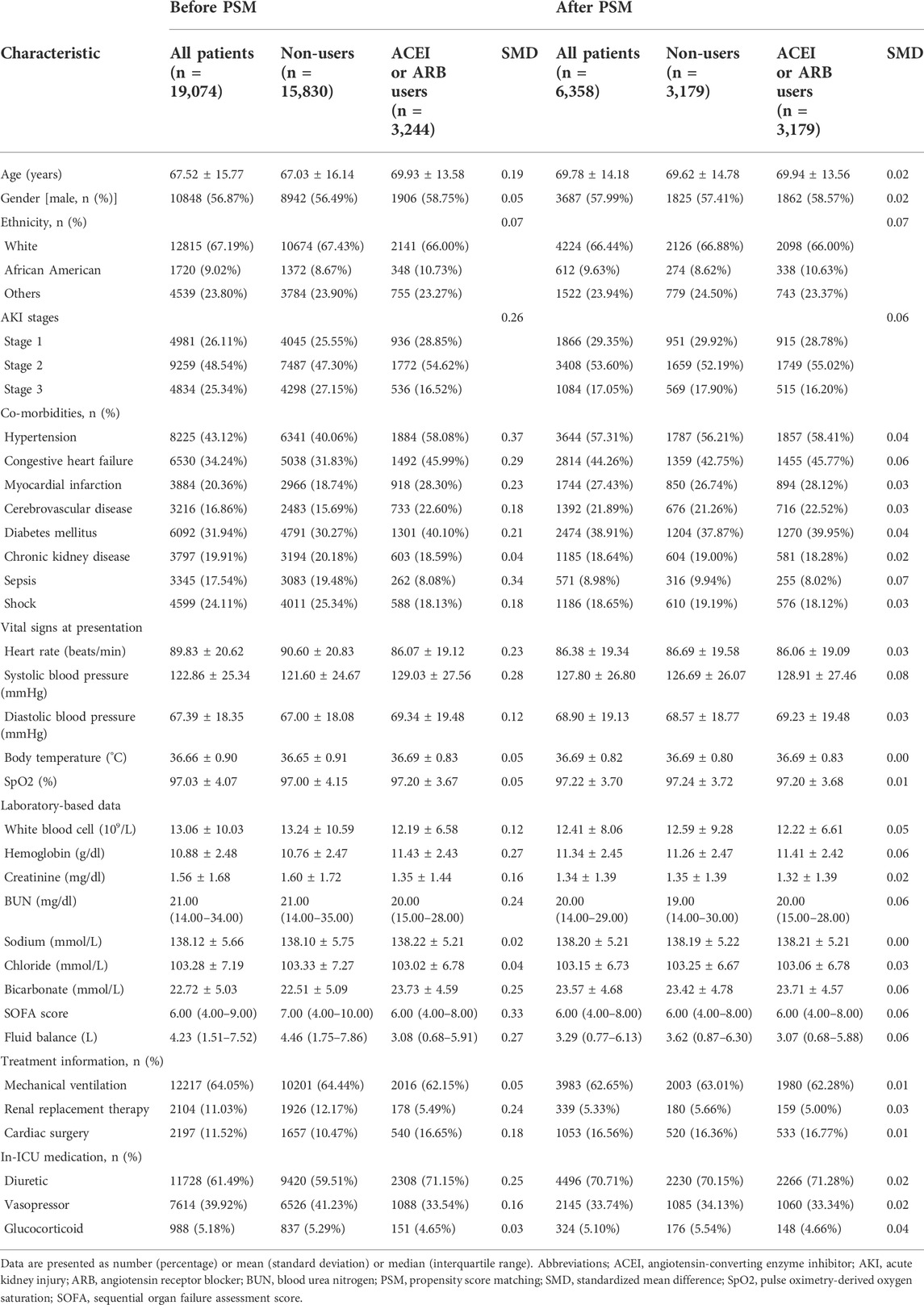
To maximize comparability between the two groups, propensity score-matching (PSM) analyses were performed. The propensity scores were estimated using a multivariable logistic regression model adjusted for variables including age, sex, ethnicity, vital signs, co-morbidities, laboratory data, and treatments. We implemented the 1:1 or 1:2 nearest neighbor PSM without replacement, and used a caliper width of 0.02 for the pooled standard deviation of the logit of the propensity score. The standardized mean difference was used to compare covariates between groups; a value of 10% or less indicated a high degree of similarity between the groups. Cox proportional-hazards models were used to estimate the hazard ratios (HRs) for associations between ACEI/ARB use and mortality risk in both univariate and multivariate analyses. Logistic regression models were used to examine the association of ACEI/ARBs with AKD and hyperkalemia. Kaplan–Meier survival curves and log-rank tests were used to compare the cumulative survival rates according to ACEI/ARB use (Efron, 1988). Restricted cubic spline models with five knots and the non-linear test were used to assess the potential non-linear relationship between ACEI/ARBs use and all-cause mortality (Harrell et al., 1988).
Subgroup analyses were performed, stratified by sex, AKI stage, co-morbidity status, the use of vasopressors, diuretics, mechanical ventilation, and SOFA scores. We also performed a subgroup analysis according to whether or not patients received ACEI/ARBs before ICU admission. We performed sensitivity analyses to compare the outcomes among patients who took only ACEIs or only ARBs to determine whether the type of medication influenced the outcomes. A two-tailed p-value < 0.05 was considered statistically significant. All statistical analyses were performed using the R software (ver. 3.4.3; R Foundation for Statistical Computing, Vienna, Austria) and Empower (X & Y Solutions, Inc., Boston, MA, United States).
Results
Patient characteristics
The subject selection procedure is shown in Figure 1. After applying the inclusion and exclusion criteria, a total of 19,074 AKI patients were included in the analysis. The baseline characteristics of the ACEI/ARB users and non-users are shown below. There were 3,244 patients (17.0%) who received ACEI/ARBs and 15,830 (83.0%) who did not. The average age of the participants was 67.52 years. Among them, 4,981 patients were classified as AKI stage 1, 9,259 as stage 2, and 4,834 as stage 3. Large numbers of the participants had hypertension [n = 8,225 (43.12%)], congestive heart failure [n = 6,530 (34.24%)], and diabetes mellitus [n = 6,092 (31.94%)]. Compared to patients who did not use ACEI/ARBs, those who received ACEI/ARBs had higher rates of hypertension, congestive heart failure, and lower rates of sepsis. Covariates were balanced by applying the PSM method. In total, 3,179 ACEI/ARB users were matched 1:1 to similar ACEI/ARB non-users, resulting in a final study cohort of 6,358 patients. The baseline characteristics of the propensity-matched patients with and without ACEI/ARBs were similar (Table 1).
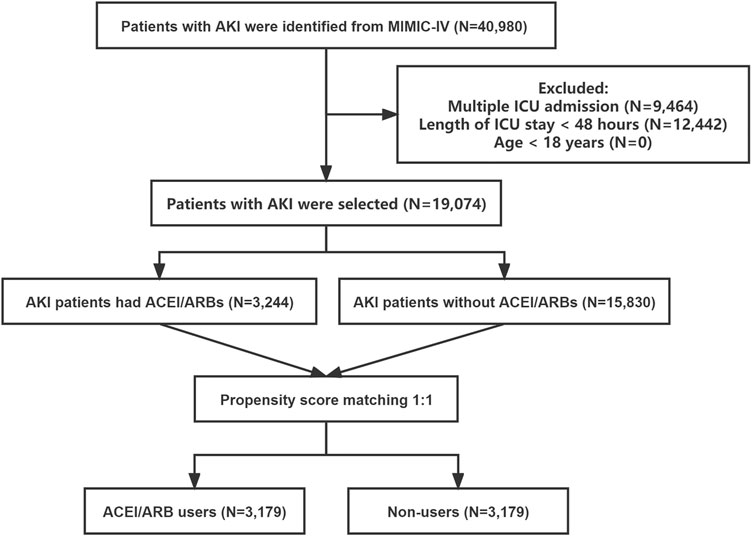
FIGURE 1. The flowchart of patient selection. Abbreviations: AKI, acute kidney injury; ICU, intensive care unit; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; MIMIC-IV, the Medical Information Mart for Intensive Care IV.
Association of ACEI/ARBs with mortality outcomes
Among the PSM AKI patients, the risk of ICU mortality was significantly lower in those who received ACEI/ARBs compared to those who did not [adjusted HR: 0.34; 95% confidence interval (CI): 0.27–0.42]. Compared to ACEI/ARB non-users, the adjusted HR for in-hospital mortality associated with ACEI/ARB use was 0.47 (95% CI: 0.39–0.56). The 30- and 180-day mortality risks remained significantly lower in the ACEI/ARB prescription group compared to those not prescribed ACEI/ARBs (adjusted HR: 0.47 and 0.53, 95% CI: 0.40–0.56 and 0.45–0.62, respectively) (Table 2). Figure 2 shows the Kaplan–Meier curves for 180-day survival. Log-rank analyses indicated significant group differences in the survival curves (p < 0.0001). Figures 3, 4 present the non-linear dose-response association between cDDD and the ICU/180-day mortality risk. The results showed an inverse dose-response association between the cumulative dose of ACEI/ARBs and mortality risk.
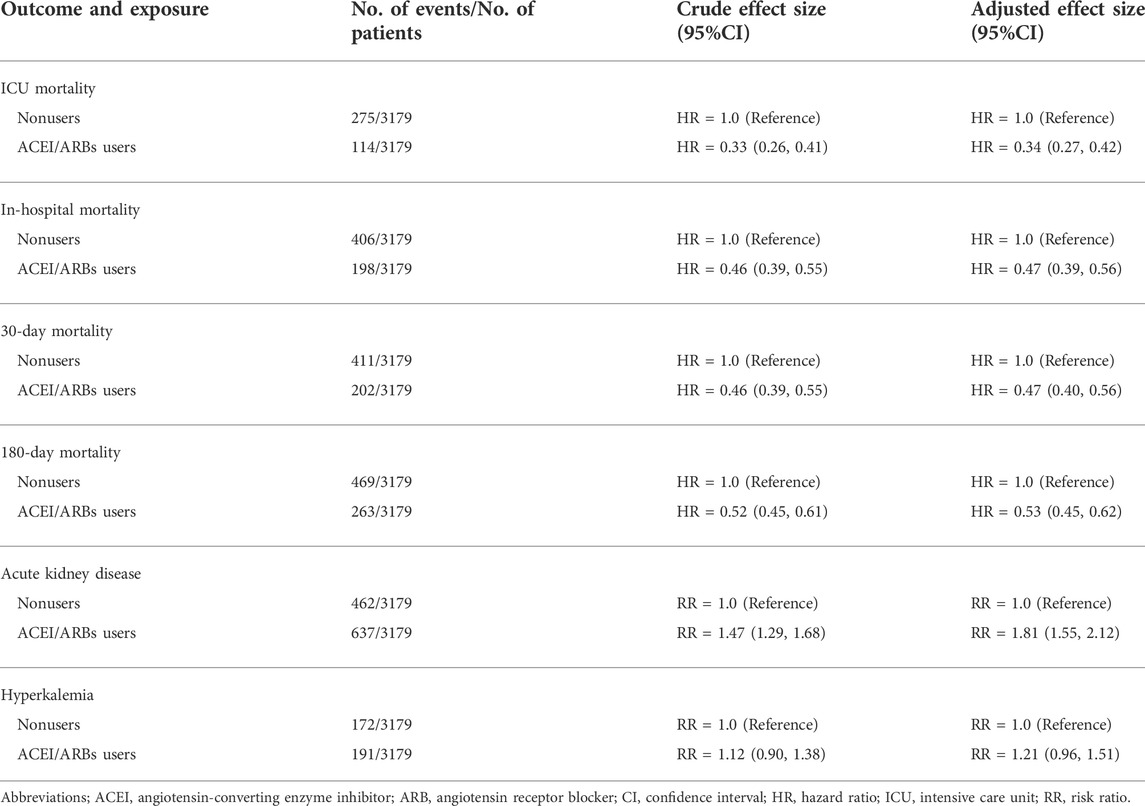
TABLE 2. Survival and composite outcomes associated with ACEI/ARB use in propensity score–matched patients with AKI.
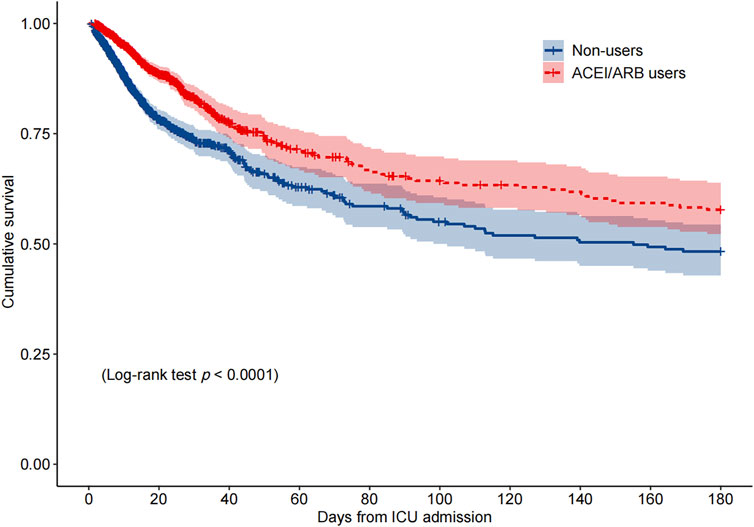
FIGURE 2. Kaplan–Meier survival curve for 180-day mortality by the ACEI/ARB status. p < 0.0001 by log-rank test. Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ICU, intensive care unit.
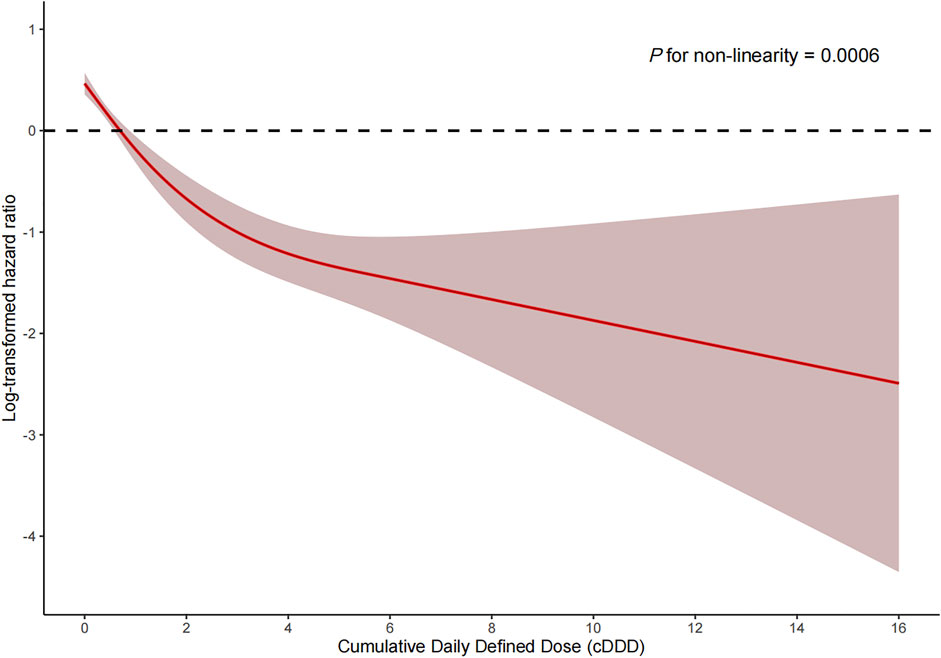
FIGURE 3. Dose-response association between the cumulative daily defined dose of ACEI/ARBs and ICU mortality. p for non-linearity = 0.0006. The solid red lines represent the effect estimates and the shaded area represents 95% confidence intervals. Abbreviations: cDDD, cumulative daily defined dose.
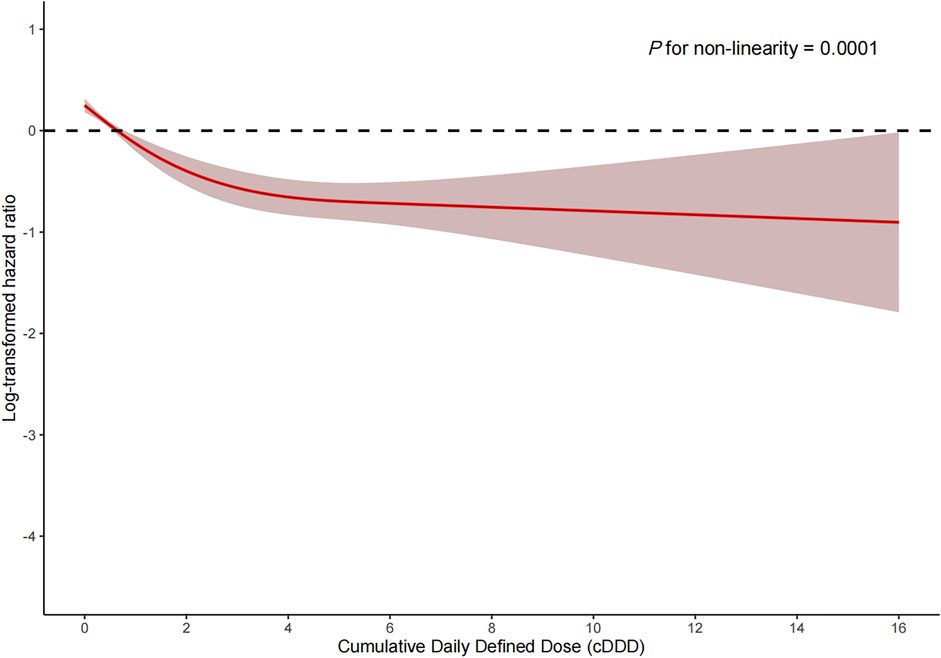
FIGURE 4. Dose-response association between the cumulative daily defined dose of ACEI/ARBs and 180-day mortality. p for non-linearity = 0.0001. The solid red lines represent the effect estimates and the shaded area represents 95% confidence intervals. Abbreviations: cDDD, cumulative daily defined dose.
Association of ACEI/ARBs with adverse outcomes
In AKI patients, the use of ACEI/ARBs was associated with a higher risk of AKD (adjusted risk ratio [RR]: 1.81; 95% CI: 1.55–2.12) (Table 2). Patients who received ACEI/ARBs during their ICU stay were more likely to have a higher risk of hyperkalemia compared to those who did not (adjusted RR: 1.21; 95% CI: 0.96–1.51).
Subgroup analyses
The associations between in-hospital mortality and the use of ACEI/ARBs found in the subgroup analyses are presented in Figure 5. The associations were similar in every stratum, and there were no interactions (p > 0.05). Figure 6 shows the association between ACEI/ARB use and AKD, which was similar in all subgroup analyses except the one stratified by ACEI/ARB use prior to ICU admission. New users who started to use ACEI/ARBs after ICU admission were at a significantly higher risk of AKD than users who did not use ACEI/ARBs before or after ICU admission (adjusted RR: 1.86; 95% CI: 1.58–2.20). There was no significant association between continued ACEI/ARBs treatment after ICU admission and AKD (adjusted RR: 1.40; 95% CI: 0.82–2.39). The results of the subgroup analysis of the association between ACEI/ARB use and hyperkalemia are presented in Figure 7. ACEI/ARB use was associated with a higher risk of hyperkalemia in participants with sepsis (RR: 2.06; 95% CI: 1.11–3.82) compared to those without sepsis (RR: 1.10; 95% CI: 0.86–1.41).
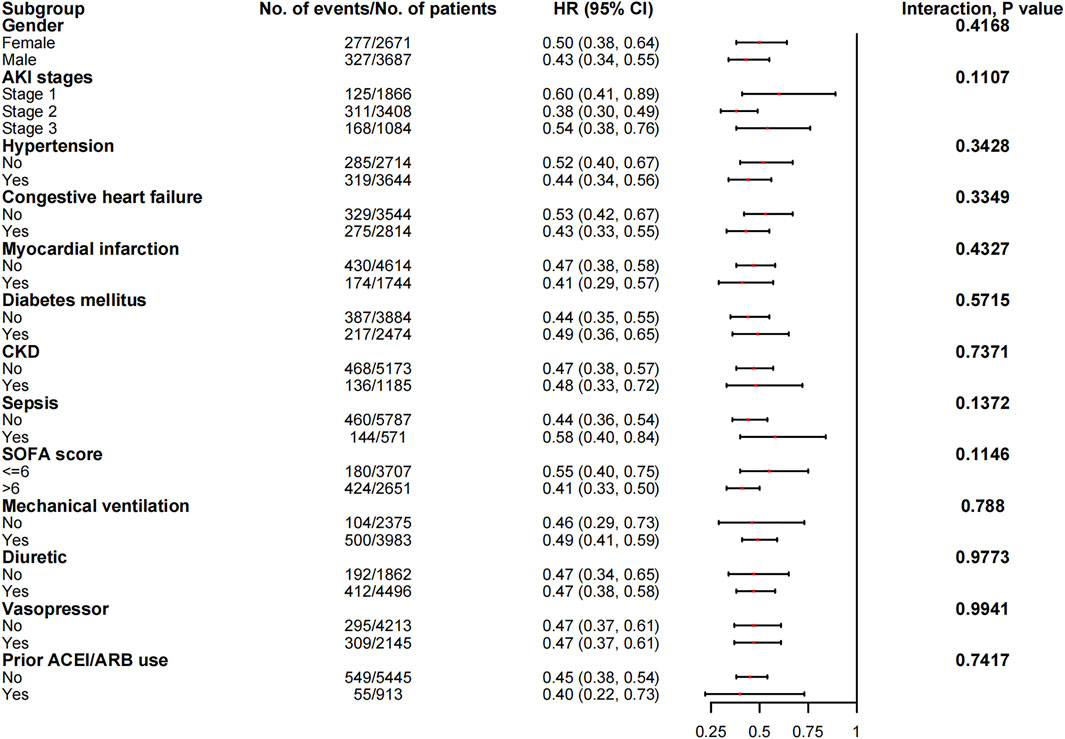
FIGURE 5. The association between ACEI/ARB use and in-hospital mortality in the subgroups. Abbreviations: AKI, acute kidney injury; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CI, confidence interval; CKD, chronic kidney disease; HR, hazard ratio; SOFA, sequential organ failure assessment score.
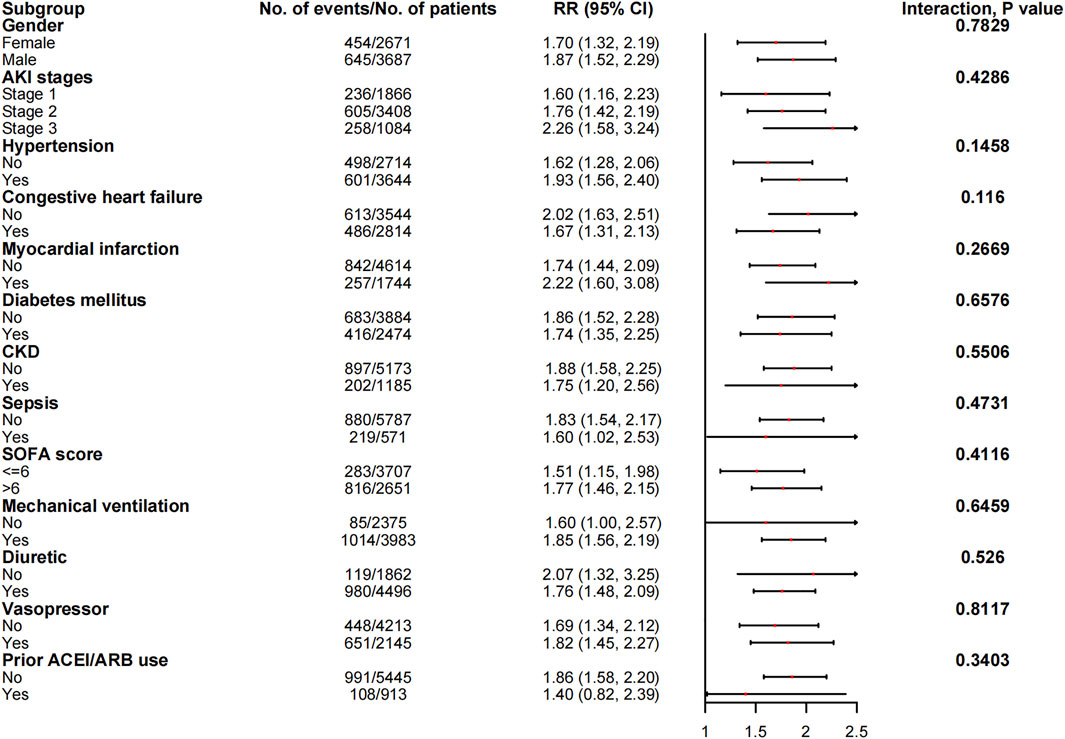
FIGURE 6. The association between ACEI/ARB use and AKD in the subgroups. Abbreviations: AKI, acute kidney injury; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; AKD, acute kidney disease; CI, confidence interval; CKD, chronic kidney disease; RR, risk ratio; SOFA, sequential organ failure assessment score.
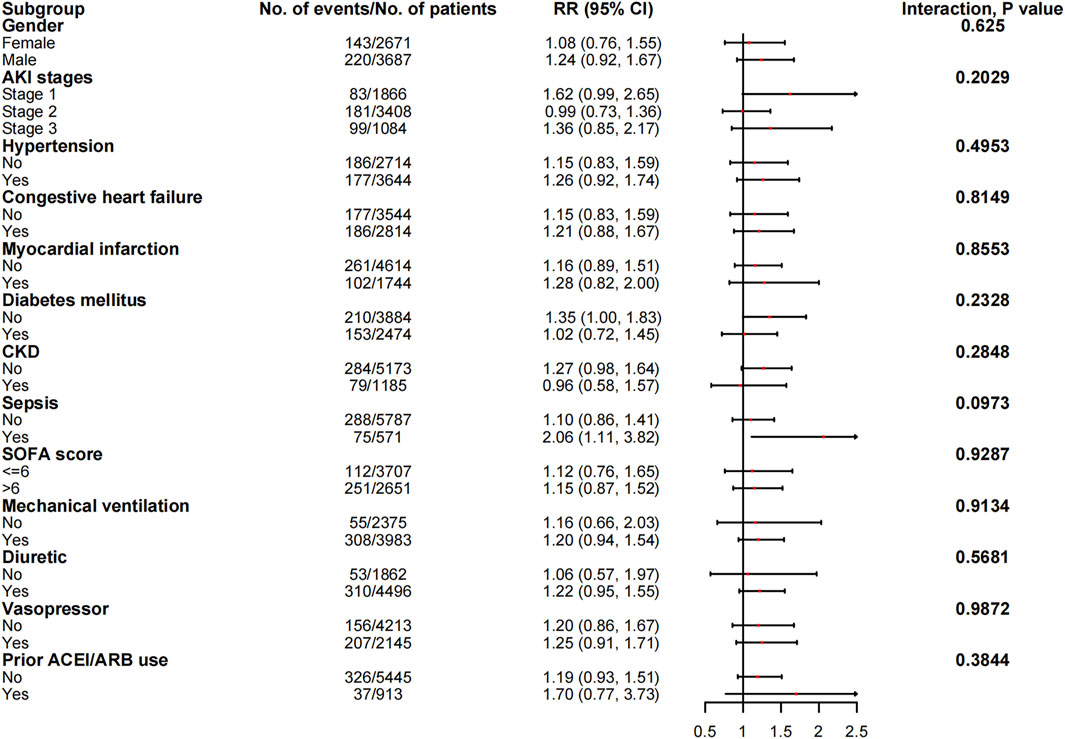
FIGURE 7. The association between ACEI/ARB use and hyperkalemia in subgroups. Abbreviations: AKI, acute kidney injury; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CI, confidence interval; CKD, chronic kidney disease; RR, risk ratio; SOFA, sequential organ failure assessment score.
Sensitivity Analysis
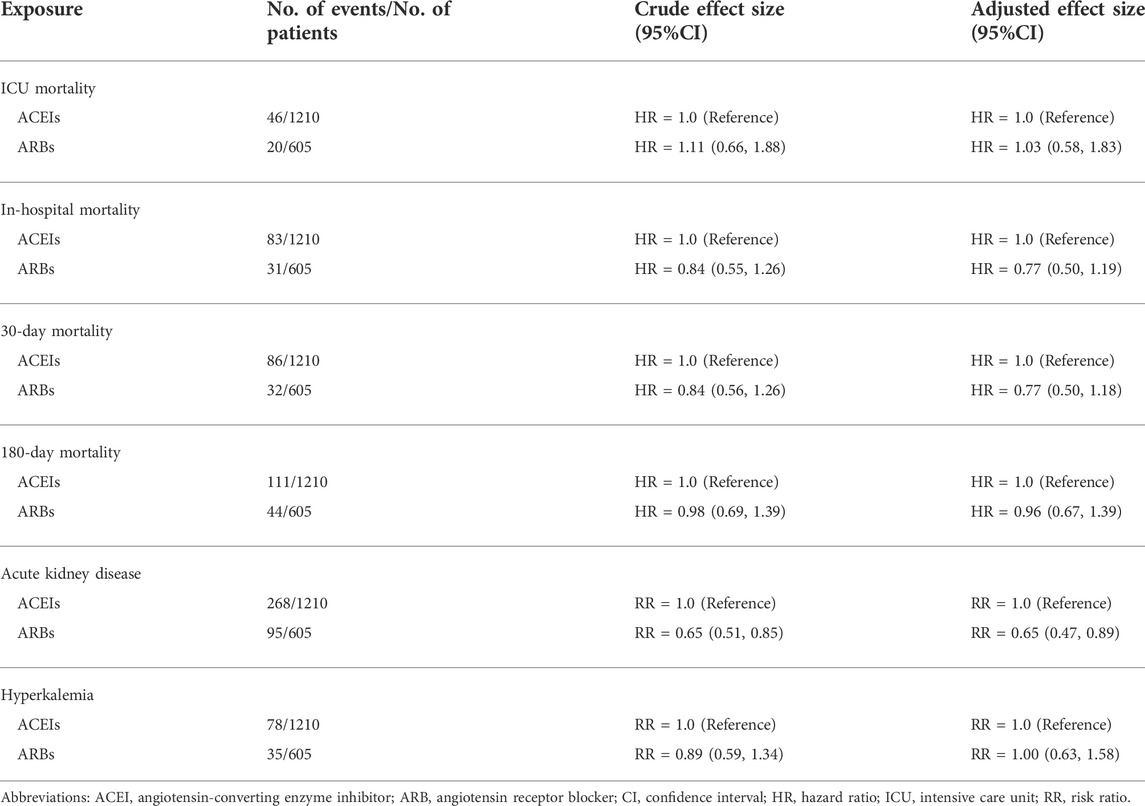
After 1: 2 propensity score matching, patients who received ARBs (n = 605) and those who received ACEIs (n = 1,210) were included in analyses to evaluate the different types of ACEI/ARBs on the outcomes (Table 3). ARB users had similar survival benefits compared with those of ACEI users (adjusted HR for ICU mortality: 1.03; 95% CI: 0.58–1.83; HR for in-hospital mortality: 0.77; 95% CI: 0.50–1.19; HR for 30-day mortality: 0.77; 95% CI: 0.50–1.18; HR for 180-day mortality: 0.96; 95% CI: 0.67–1.39). The risk of AKD was lower in ARB users compared to ACEI users (RR: 0.65; 95% CI: 0.47–0.89). There was no significant difference in the risk of hyperkalemia between ARB users and ACEI users (RR: 1.00; 95% CI: 0.63–1.58).
Discussion
To the best of our knowledge, this was the first study to evaluate whether ACEI/ARB exposure is associated with better outcomes in ICU patients with AKI. Our findings demonstrated the survival benefits of ACEI/ARBs during AKI. However, ACEI/ARB users had a higher risk of AKD compared to ACEI/ARB non-users.
AKI incidence in hospitalized adults is estimated to range from 3,000 to 100,000 person–years (Liaño and Pascual, 1996). AKI is an independent risk factor for long-term cardiovascular events and mortality (Wu et al., 2014). McCallum et al. reported that enalapril use decreased the risk of all-cause and cardiovascular mortality, regardless of the degree of decline in the estimated glomerular filtration rate (eGFR) in patients with heart failure and reduced ejection fraction (McCallum et al., 2019). A retrospective cohort study of 46,253 adults who experienced AKI during hospitalization but survived reported that ACEI/ARB use was associated with a lower mortality risk after 2 years (HR: 0.85; 95% CI: 0.81–0.89) (Brar et al., 2018). However, patients who received ACEI/ARBs had a higher risk of hospitalization due to renal problems, including acute renal failure, congestive heart failure, hypervolemia, hyperkalemia, and malignant hypertension (HR: 1.28; 95% CI: 1.12–1.46) (Brar et al., 2018). A cohort study involving 21 European ICUs and 1,551 patients demonstrated that an ACEI/ARB prescription at discharge for patients who survived an AKI episode was associated with a lower 1-year mortality risk (HR: 0.48; 95% CI: 0.27–0.85) (Gayat et al., 2018). A meta-analysis found that the use of ACEI/ARBs after AKI was associated with a lower rate of all-cause mortality, along with a lower risk of AKI recurrence and progression to CKD (Chen et al., 2021). Bidulka et al. demonstrated that, in patients with a history of ACEI/ARB use, the continued use of ACEI/ARBs after an episode of AKI did not increase the risk of heart failure or subsequent AKI compared to the discontinuation of ACEI/ARB use (Bidulka et al., 2020).
The consequences of ACEI/ARB use during an AKI episode are still unknown. Our study demonstrated that treatment with ACEI/ARBs in AKI might improve the survival outcomes, including during hospitalization and after discharge. ACEI/ARBs reduced the mortality rate of cardiovascular diseases, including heart failure and myocardial infarction (Yusuf et al., 1991; Ambrosioni et al., 1995; Køber et al., 1995). The patients in our cohort were critically ill, and most had cardiovascular risk factors. A large number of patients had co-morbidities, including hypertension, heart failure, and myocardial infarction. These patients were at higher risk for cardiovascular-related mortality. The protective effects of ACEI/ARBs in AKI patients might be related to their beneficial effects on the cardiovascular system. The underlying mechanism still needs further investigation.
Patients with CKD and hypotension, as well as those undergoing surgery, were more likely to develop AKI while receiving ACEI/ARBs (Alabdan et al., 2017). An increased risk of AKI was observed in patients who received ACEI/ARBs in combination with non-steroidal anti-inflammatory drugs and diuretics (Lapi et al., 2013). Patients with ACEI/ARBs had a higher risk of AKI during admission for medical emergencies (Feidakis et al., 2021). It is common clinical practice to discontinue or avoid the use of ACEI/ARBs in the context of AKI and other acute conditions because of concerns regarding altered renal hemodynamics. This has been called the “sick day rule” (Blakeman et al., 2013; Whiting et al., 2017). ACEI/ARBs cause efferent arteriolar dilation in the kidneys, leading to an acute decline in the eGFR and delayed renal recovery. However, ACEI/ARBs also have anti-inflammatory effects and increase the peritubular blood flow, which protects against acute tubular necrosis (Perazella and Coca, 2013; Tomson and Tomlinson, 2019). Although our results showed that ACEI/ARB use in AKI patients was associated with an increased risk of AKD during ICU stays, the long-term impact on renal function remains unclear and warrants further studies. Our results demonstrated that patients who received ACEI/ARBs during AKI might be at a higher risk for hyperkalemia compared with those who did not (RR: 1.21; 95% CI: 0.96–1.51). AKI and ACEI/ARBs are both associated with hyperkalemia, which is a potentially life-threatening electrolyte abnormality (Chang et al., 2016; Palmer and Clegg, 2018). Therefore, close monitoring of serum potassium levels is needed in AKI patients, especially in those using ACEI/ARBs.
ACEIs and ARBs have similar clinical effects, and show comparable efficacy in the treatment of hypertension, heart failure, cardiovascular diseases, and CKD (Messerli et al., 2018). The use of ARBs was associated with lower mortality rates compared to ACEIs in patients with chronic obstructive pulmonary diseases (Lai et al., 2019). A case-control study comparing the effects of ACEI with ARB in AKD patients found that ARB users had a lower mortality than ACEI users (HR: 0.824; p = 0.017). New ACEI users had an increased risk of re-dialysis compared with patients without ACEI/ARBs (HR: 1.82; p < 0.001) (Wu et al., 2022). Their respective beneficial and adverse effects on the risk of mortality and adverse outcomes in AKI patients have not been investigated before. ACEI and ARB suppress different parts of the renin–angiotensin–aldosterone system (RAS). ARBs may have stronger anti-inflammatory effects than ACEI (Gamboa et al., 2012). In this study, we noticed the lower risk of AKD events in patients with ARBs compared with those with ACEIs.
This study had several limitations. First, treatment allocation was not random due to the observational study design. We used PSM to minimize allocation bias, thus allowing the comparison of ACEI/ARB users with non-users having similar characteristics. Residual confounding might have undermined the associations between ACEI/ARB use and its outcomes. Second, we were unable to confirm the causality of the associations in this observational study. Interventional randomized controlled trials are warranted to investigate the role of ACEI/ARBs in AKI. Third, true baseline SCr measurements were not available for all patients, which might have influenced the AKI grading. Fourth, we were unable to investigate the effects on cardiovascular events and long-term renal outcomes due to a lack of relevant information. Fifth, the information about the initial indications for prescribing ACEI/ARBs and the causes for changing their prescriptions during the whole study period was not available. Thus, further prospective randomized studies are needed to address these limitations.
Conclusion
ACEI/ARB treatment during an episode of AKI may decrease all-cause mortality, but increase the risk of AKD. Future randomized controlled trials are warranted to validate these findings.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: All data and material are available at https://mimic.mit.edu/.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Study concept: WC; Acquisition of data: XZ, ZL; Analysis and interpretation of data: XZ, JX; Drafting of the manuscript: WC, WD, JX, XW; Study supervision: HX, QLZ, QZ; Critical revision of the manuscript for important intellectual content; All authors have read and approved the final manuscript.
Funding
Supported by Changsha Municipal Natural Science Foundation (No. kq2014276), Scientific Research Project of Hunan Provincial Health Commission (No. 202103050756), Provincial Natural Science Foundation of Hunan (No. 2021JJ40972), National Youth Science Foundation of China (No. 81600536).
Acknowledgments
The authors are particularly grateful to all the researchers who built and maintained the MIMIC database.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alabdan, N., Gosmanova, E. O., Tran, N. Q., Oliphant, C. S., Pan, H., Broyles, J. E., et al. (2017). Acute kidney injury in patients continued on renin-angiotensin system blockers during hospitalization. Am. J. Med. Sci. 353 (2), 172–177. doi:10.1016/j.amjms.2016.09.012
Ambrosioni, E., Borghi, C., and Magnani, B. (1995). The effect of the angiotensin-converting-enzyme inhibitor zofenopril on mortality and morbidity after anterior myocardial infarction. The Survival of Myocardial Infarction Long-Term Evaluation (SMILE) Study Investigators. N. Engl. J. Med. 332 (2), 80–85. doi:10.1056/NEJM199501123320203
Balamuthusamy, S., Srinivasan, L., Verma, M., Adigopula, S., Jalandhara, N., Hathiwala, S., et al. (2008). Renin angiotensin system blockade and cardiovascular outcomes in patients with chronic kidney disease and proteinuria: A meta-analysis. Am. Heart J. 155 (5), 791–805. doi:10.1016/j.ahj.2008.01.031
Bellomo, R., Kellum, J. A., and Ronco, C. (2012). Acute kidney injury. Lancet 380 (9843), 756–766. doi:10.1016/S0140-6736(11)61454-2
Bidulka, P., Fu, E. L., Leyrat, C., Kalogirou, F., McAllister, K. S. L., Kingdon, E. J., et al. (2020). Stopping renin-angiotensin system blockers after acute kidney injury and risk of adverse outcomes: Parallel population-based cohort studies in English and Swedish routine care. BMC Med. 18 (1), 195. doi:10.1186/s12916-020-01659-x
Blakeman, T., Harding, S., and O'Donoghue, D. (2013). Acute kidney injury in the community: Why primary care has an important role. Br. J. Gen. Pract. 63 (609), 173–174. doi:10.3399/bjgp13X664207
Brar, S., Ye, F., James, M. T., Hemmelgarn, B., Klarenbach, S., Pannu, N., et al. (2018). Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with outcomes after acute kidney injury. JAMA Intern. Med. 178 (12), 1681–1690. doi:10.1001/jamainternmed.2018.4749
Chang, A. R., Sang, Y., Leddy, J., Yahya, T., Kirchner, H. L., Inker, L. A., et al. (2016). Antihypertensive medications and the prevalence of hyperkalemia in a large health system. Hypertension 67 (6), 1181–1188. doi:10.1161/HYPERTENSIONAHA.116.07363
Chawla, L. S., Bellomo, R., Bihorac, A., Goldstein, S. L., Siew, E. D., Bagshaw, S. M., et al. (2017). Acute kidney disease and renal recovery: Consensus report of the acute disease quality initiative (ADQI) 16 workgroup. Nat. Rev. Nephrol. 13 (4), 241–257. doi:10.1038/nrneph.2017.2
Chen, J. Y., Tsai, I. J., Pan, H. C., Liao, H. W., Neyra, J. A., Wu, V. C., et al. (2021). The impact of angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers on clinical outcomes of acute kidney disease patients: A systematic review and meta-analysis. Front. Pharmacol. 12, 665250. doi:10.3389/fphar.2021.665250
Coca, S. G., Singanamala, S., and Parikh, C. R. (2012). Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int. 81 (5), 442–448. doi:10.1038/ki.2011.379
Coca, S. G., Yusuf, B., Shlipak, M. G., Garg, A. X., and Parikh, C. R. (2009). Long-term risk of mortality and other adverse outcomes after acute kidney injury: A systematic review and meta-analysis. Am. J. Kidney Dis. 53 (6), 961–973. doi:10.1053/j.ajkd.2008.11.034
Efron, B. (1988). Logistic regression, survival analysis, and the Kaplan-Meier curve. J. Am. Stat. Assoc. 83 (402), 414–425. doi:10.1080/01621459.1988.10478612
Feidakis, A., Panagiotou, M. R., Tsoukakis, E., Bacharaki, D., Gounari, P., Nikolopoulos, P., et al. (2021). Impact of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers on acute kidney injury in emergency medical admissions. J. Clin. Med. 10 (3), 412. doi:10.3390/jcm10030412
Gamboa, J. L., Pretorius, M., Todd-Tzanetos, D. R., Luther, J. M., Yu, C., Ikizler, T. A., et al. (2012). Comparative effects of angiotensin-converting enzyme inhibition and angiotensin-receptor blockade on inflammation during hemodialysis. J. Am. Soc. Nephrol. 23 (2), 334–342. doi:10.1681/ASN.2011030287
Gayat, E., Hollinger, A., Cariou, A., Deye, N., Vieillard-Baron, A., Jaber, S., et al. (2018). Impact of angiotensin-converting enzyme inhibitors or receptor blockers on post-ICU discharge outcome in patients with acute kidney injury. Intensive Care Med. 44 (5), 598–605. doi:10.1007/s00134-018-5160-6
Harrell, F. E., Lee, K. L., and Pollock, B. G. (1988). Regression models in clinical studies: Determining relationships between predictors and response. J. Natl. Cancer Inst. 80 (15), 1198–1202. doi:10.1093/jnci/80.15.1198
Hines, A., Li, X., Ortiz-Soriano, V., Saleh, S., Litteral, J., Ruiz-Conejo, M., et al. (2020). Use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers and acute kidney disease after an episode of AKI: A multicenter prospective cohort study. Am. J. Nephrol. 51 (4), 266–275. doi:10.1159/000505893
Johnson, A., Bulgarelli, L., Pollard, T., Horng, S., Celi, L. A., and Mark, I. V. R. (2020). MIMIC-IV (version 1.0). PhysioNet. doi:10.13026/s6n6-xd98
Kellum, J. A., Lameire, N., Aspelin, P., Barsoum, R. S., Burdmann, E. A., Goldstein, S. L., et al. (2012). Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2 (1), 1–138. doi:10.1038/kisup.2012.1
Køber, L., Torp-Pedersen, C., Carlsen, J. E., Bagger, H., Eliasen, P., Lyngborg, K., et al. (1995). A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) Study Group. N. Engl. J. Med. 333 (25), 1670–1676. doi:10.1056/NEJM199512213332503
Lai, C. C., Wang, Y. H., Wang, C. Y., Wang, H. C., Yu, C. J., Chen, L., et al. (2019). Risk of sepsis and mortality among patients with chronic obstructive pulmonary disease treated with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Crit. Care Med. 47 (1), e14–e20. doi:10.1097/CCM.0000000000003475
Lapi, F., Azoulay, L., Yin, H., Nessim, S. J., and Suissa, S. (2013). Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non-steroidal anti-inflammatory drugs and risk of acute kidney injury: Nested case-control study. Bmj 346, e8525. doi:10.1136/bmj.e8525
Levin, A., Stevens, P. E., Bilous, R. W., Coresh, J., De Francisco, A. L., De Jong, P. E., et al. (2013). Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 3 (1), 1–150. doi:10.1038/kisup.2012.73
Lewington, A. J., Cerdá, J., and Mehta, R. L. (2013). Raising awareness of acute kidney injury: A global perspective of a silent killer. Kidney Int. 84 (3), 457–467. doi:10.1038/ki.2013.153
Liaño, F., and Pascual, J. (1996). Epidemiology of acute renal failure: A prospective, multicenter, community-based study. Madrid acute renal failure study group. Kidney Int. 50 (3), 811–818. doi:10.1038/ki.1996.380
Mansfield, K. E., Nitsch, D., Smeeth, L., Bhaskaran, K., and Tomlinson, L. A. (2016). Prescription of renin-angiotensin system blockers and risk of acute kidney injury: A population-based cohort study. BMJ Open 6 (12), e012690. doi:10.1136/bmjopen-2016-012690
McCallum, W., Tighiouart, H., Ku, E., Salem, D., and Sarnak, M. J. (2019). Acute declines in estimated glomerular filtration rate on enalapril and mortality and cardiovascular outcomes in patients with heart failure with reduced ejection fraction. Kidney Int. 96 (5), 1185–1194. doi:10.1016/j.kint.2019.05.019
Messerli, F. H., Bangalore, S., Bavishi, C., and Rimoldi, S. F. (2018). Angiotensin-converting enzyme inhibitors in hypertension: To use or not to use? J. Am. Coll. Cardiol. 71 (13), 1474–1482. doi:10.1016/j.jacc.2018.01.058
Nash, K., Hafeez, A., and Hou, S. (2002). Hospital-acquired renal insufficiency. Am. J. Kidney Dis. 39 (5), 930–936. doi:10.1053/ajkd.2002.32766
Ostermann, M., Bellomo, R., Burdmann, E. A., Doi, K., Endre, Z. H., Goldstein, S. L., et al. (2020). Controversies in acute kidney injury: Conclusions from a kidney disease: Improving global outcomes (KDIGO) conference. Kidney Int. 98 (2), 294–309. doi:10.1016/j.kint.2020.04.020
Palmer, B. F., and Clegg, D. J. (2018). Hyperkalemia across the continuum of kidney function. Clin. J. Am. Soc. Nephrol. 13 (1), 155–157. doi:10.2215/CJN.09340817
Perazella, M. A., and Coca, S. G. (2013). Three feasible strategies to minimize kidney injury in 'incipient AKI. Nat. Rev. Nephrol. 9 (8), 484–490. doi:10.1038/nrneph.2013.80
Tomson, C., and Tomlinson, L. A. (2019). Stopping RAS inhibitors to minimize AKI: More harm than good? Clin. J. Am. Soc. Nephrol. 14 (4), 617–619. doi:10.2215/CJN.14021118
von Elm, E., Altman, D. G., Egger, M., Pocock, S. J., Gøtzsche, P. C., Vandenbroucke, J. P., et al. (2007). The strengthening the reporting of observational studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 370 (9596), 1453–1457. doi:10.1016/S0140-6736(07)61602-X
Waikar, S. S., and Bonventre, J. V. (2009). Creatinine kinetics and the definition of acute kidney injury. J. Am. Soc. Nephrol. 20 (3), 672–679. doi:10.1681/ASN.2008070669
Wang, K., Hu, J., Luo, T., Wang, Y., Yang, S., Qing, H., et al. (2018). Effects of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on all-cause mortality and renal outcomes in patients with diabetes and albuminuria: A systematic review and meta-analysis. Kidney Blood Press. Res. 43 (3), 768–779. doi:10.1159/000489913
Whiting, P., Morden, A., Tomlinson, L. A., Caskey, F., Blakeman, T., Tomson, C., et al. (2017). What are the risks and benefits of temporarily discontinuing medications to prevent acute kidney injury? A systematic review and meta-analysis. BMJ Open 7 (4), e012674. doi:10.1136/bmjopen-2016-012674
World Health Organization (2018). WHO Collaborating Centre for Drug Statistics Methodology. World Health Organization. [Online] Available at: https://www.whocc.no/.
Wu, V. C., Lin, Y. F., Teng, N. C., Yang, S. Y., Chou, N. K., Tsao, C. H., et al. (2022). Angiotensin II receptor blocker associated with less outcome risk in patients with acute kidney disease. Front. Pharmacol. 13, 714658. doi:10.3389/fphar.2022.714658
Wu, V. C., Wu, C. H., Huang, T. M., Wang, C. Y., Lai, C. F., Shiao, C. C., et al. (2014). Long-term risk of coronary events after AKI. J. Am. Soc. Nephrol. 25 (3), 595–605. doi:10.1681/ASN.2013060610
Yusuf, S., Pitt, B., Davis, C. E., Hood, W. B., and Cohn, J. N. (1991). Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N. Engl. J. Med. 325 (5), 293–302. doi:10.1056/NEJM199108013250501
Keywords: acute kidney injury, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, critically ill, mortality
Citation: Zhu X, Xue J, Liu Z, Dai W, Xiang J, Xu H, Zhou Q, Zhou Q, Wei X and Chen W (2022) The effects of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in critically ill patients with acute kidney injury: An observational study using the MIMIC database. Front. Pharmacol. 13:918385. doi: 10.3389/fphar.2022.918385
Received: 12 April 2022; Accepted: 02 August 2022;
Published: 29 August 2022.
Edited by:
Norberto Perico, Mario Negri Pharmacological Research Institute (IRCCS), ItalyReviewed by:
Shao-Yu Yang, National Taiwan University Hospital, TaiwanMichel Marre, INSERM U1138 Centre de Recherche des Cordeliers (CRC), France
Copyright © 2022 Zhu, Xue, Liu, Dai, Xiang, Xu, Zhou, Zhou, Wei and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenhang Chen, wenhangchen@csu.edu.cn
 Xu Zhu
Xu Zhu