- 1Center for Health Regulatory Policies, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy
- 2Department of Pharmacoepidemiology, Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, Glasgow, United Kingdom
- 3Centre of Medical and Bio-allied Health Sciences Research, Ajman University, Ajman, United Arab Emirates
- 4Division of Public Health Pharmacy and Management, School of Pharmacy, Sefako Makgatho Health Sciences University, Ga-Rankuwa, South Africa
- 5Laboratory of Pharmacodynamics and Pharmacokinetics, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy
- 6Presidency, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy
Biological medicines have improved patients’ outcomes, but their high costs may limit access. Biosimilars, alternatives that have demonstrated high similarity in terms of quality, safety, and efficacy to an already licensed originator biological product, could increase competition and decrease prices. Given the expanding number of biosimilars, patients may switch from originator to biosimilar or among biosimilars. Randomized trials and observational studies conducted with multiple biosimilars over many disease areas confirmed the safety and efficacy of switching from originator to biosimilar. This study summarizes evidence on switching between biosimilars for which there are concerns to provide future guidance. A systematic search (MEDLINE, Embase, and Cochrane Library) for studies on anti-TNF agents, assessing clinical efficacy and safety of biosimilar-to-biosimilar switch in chronic inflammatory diseases, was performed. We retrieved 320 records and included 19 clinical studies. One study with historical control compared switching between biosimilars to maintenance of the same biosimilar. Ten were controlled cohort studies comparing switching between two biosimilars vs. switching from originator to a biosimilar or vs. multiple switches. Eight were single-arm cohort studies, where participants switched from one biosimilar to another, and the outcomes were compared before and after the switch. Overall, these studies did not highlight significant concerns in switching between biosimilars. Therefore, switching studies seem difficult to perform and unnecessary with the body of evidence suggesting no real problems in practice coupled with stringent regulatory requirements. Monitoring the use of biosimilars in clinical practice could support clinical decision-making, rational use of biological medicines, and help to further realize possible savings.
Introduction
Biological medicines have appreciably improved the outcomes for patients with immunological diseases including rheumatoid arthritis, psoriatic arthritis, and inflammatory bowel diseases as well as many neoplasms (Alfonso-Cristancho et al., 2017; Nam et al., 2014; Ruyssen-Witrand et al., 2020; Cholapranee et al., 2017; Wilson et al., 2018; da Silva et al., 2018). However, their high costs have limited their use especially in low- and middle-income countries including Central and Eastern European countries (Putrik et al., 2014; Kostić et al., 2017; Baumgart et al., 2019; Gershon et al., 2019; Tubic et al., 2021). The expiration of patents allows the production of biosimilars, alternatives that have demonstrated high similarity in terms of quality, safety, and efficacy to an already licensed originator biological product (Glintborg et al., 2017; Jørgensen et al., 2017; Fiorino et al., 2019b; Meyer et al., 2019; Yang et al., 2019).
Regulatory approval of biosimilars by the European Medicines Agency (EMA) and Food and Drug Administration (FDA) is a rigorous process requiring an extensive comparability exercise based on the assessment of quality, structural, functional, preclinical, and clinical similarity with respect to the originator. While the EMA does not regulate interchangeability between the reference product and biosimilars (European Medicinal Agency and the European commission, 2019), in the USA, the FDA considers the originator and its biosimilars therapeutically interchangeable if the manufacturer has demonstrated no clinically meaningful differences from the reference product (US FDA, 2017).
The expanding number of available biosimilars, and national procurement and reimbursement policies aiming to save costs with ever increasing demands on available resources, inevitably leads to strategies to encourage switching from the originator to less expensive biosimilar(s) in chronic conditions, especially if there are substantial price differences between originators and biosimilars and no differences in effectiveness or safety (Huoponen et al., 2020; Jensen et al., 2020; Moorkens et al., 2020; Godman et al., 2021a; MacBride-Stewart et al., 2021; Vogler et al., 2021). To reduce concerns with switching, many randomized controlled trials (RCTs), real world data in routine clinical care, and systematic reviews have been conducted across countries with multiple biosimilars over many disease areas. These typically show similar effectiveness, safety, and immunogenicity between biosimilars and originators (Danese et al., 2017; Glintborg et al., 2017; Griffiths et al., 2017; Jørgensen et al., 2017; Park et al., 2017; Yoo et al., 2017; Cohen et al., 2018; Matucci-Cerinic et al., 2018; Ratnakumaran et al., 2018; Cohen et al., 2019; Gisondi et al., 2019; Goll et al., 2019; Pegram et al., 2019; Yang et al., 2019; Barbier et al., 2020; Barberio et al., 2021; Bruni et al., 2021; Cingolani et al., 2021; Li et al., 2022). For instance, the NOR-Switch study conducted in Norway provided reassurance that a nonmedical switch from infliximab originator to its biosimilar was not associated with worse outcomes (Jørgensen et al., 2017; Goll et al., 2019). Studies such as these have enhanced the acceptance of biosimilars among clinicians, which is resulting in their more rapid uptake across a number of countries to realize appreciable savings (Matusewicz et al., 2015; Jensen et al., 2020; Godman et al., 2021a; MacBride-Stewart et al., 2021). However, most studies have addressed a single switch from originator to biosimilar with few evaluating multiple or “back and forth” switching between originators and biosimilars or between biosimilars (Blauvelt et al., 2018).
These findings resulted in the World Health Organization (WHO)-in its 2021 Essential Medicine Model List recommending that quality-assured biosimilars should be considered interchangeable (substitution and switching) and eligible for selection and procurement at the country level for national essential medicines lists (World Health Organization, 2021). In addition, competition between biosimilars leading to lower prices will increasingly mean patients potentially being switched between different biosimilars in addition to switching from an originator to a biosimilar.
However, the practice of switching from one biosimilar to another is not presently recommended by a number of scientific societies as well as regulatory agencies as there are still concerns. These include a lack of information regarding potential immunogenicity and the risk of side effects (Cohen et al., 2017; Danese et al., 2017; Position Statement on Biosimilars, 2017; Medicines for Europe, 2019). This may be due to the lack of convincing evidence regarding switching from one biosimilar to another of the same biologic medicine or multiple switches, that is, a treatment sequence including more than one switch between an originator and one or more biosimilars. However, at the same time, regulatory agencies accept multiple changes in the manufacturing of originators without requiring any additional studies even with some changes described as either high or moderate risk (Vezér et al., 2016; Jiménez-Pichardo et al., 2018; Godman et al., 2019).
Consequently, there is a need to further evaluate current evidence regarding switching between biosimilars, sometimes referred to as cross-switching (Mysler et al., 2021), to dispel concerns among key stakeholder groups.
Methodology
To this aim, we updated the systematic searches launched in October 2021 for the WHO report (Allocati and Gerardi, 2020). We searched MEDLINE, Embase and the Cochrane Library from 2021 to March 2022 for studies on anti-TNF agents assessing clinical efficacy and safety of biosimilar-to-biosimilar switch in chronic inflammatory diseases including Crohn’s disease, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, and psoriasis. We included studies on anti-TNF agents as multiple biosimilars have been marked in the European Union for infliximab, adalimumab, and etanercept. We also chose the anti-TNF agents as there have been multiple activities across countries to increase the use of their biosimilars (Moorkens et al., 2017; Jensen et al., 2020; Moorkens et al., 2020; Godman et al., 2021a). For instance, in Norway, price reductions for biosimilar infliximab were already approximately 70% lower than the originator price soon after the launch of the biosimilar (Matusewicz et al., 2015; Godman et al., 2021a). In Denmark, expenditure on adalimumab decreased by 83% following aggressive contracting with multiple biosimilars, with similar expectations for the United Kingdom with estimated savings of over GB£300 million per year (Jensen et al., 2020; Godman et al., 2021a).
We included comparative and single-arm studies. We applied search term for three categories of keywords: “switch/substitution,” “biological medicine/biosimilar,” and “anti-TNF agents” and adapted the search strategy to the three databases (Full search available in the Supplementary Material S1).
One reviewer retrieved the eligible studies and extracted the key information (EA), including the study design, target condition, biological medicine and biosimilars assessed, sample size, and main study outcomes. A second reviewer (RB) checked the data extraction. Studies were described narratively.
Clinical Evidence of Switching Between Biosimilars
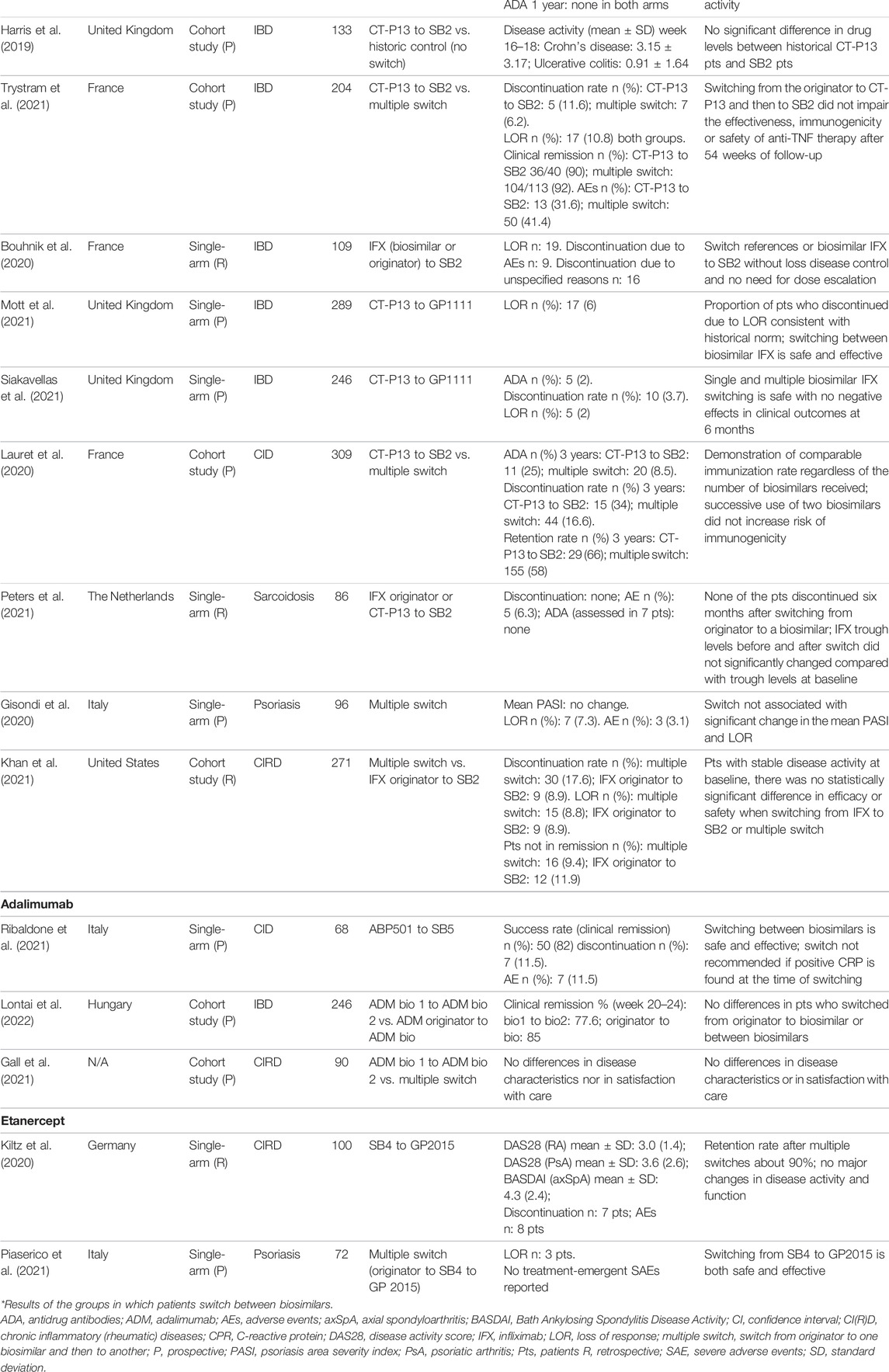
We retrieved 189 records from MEDLINE, 141 from Embase, and none from the Cochrane Library. From the screening of titles and abstract, we selected 20 eligible publications (full articles and posters), corresponding to a total of 18 clinical studies. All were included in the analysis. Another publication (abstract) was retrieved by checking the references of other articles and included in the study sample (Bouhnik et al., 2020). Thus, we included a total number of 19 studies. None of them directly compared switching from a biosimilar to another of the same biologic medicine vs. the maintenance of the same biosimilar, either as RCTs or observational studies. These would have been the optimal study designs to assess the efficacy and possible risks of switching between biosimilars (vs. nonswitch), as for the switch between originators to biosimilars. One study, published as poster, compared a group of patients with inflammatory bowel diseases switching from infliximab CT-P13 to SB2 to an historical cohort of patients treated with CT-P13 (Harris et al., 2019). These preliminary data that did not suggest switching had an impact on drug persistence. Ten controlled cohort studies compared switching between two biosimilars vs. switching from originator to a biosimilar or vs. multiple switches, for example, from an originator to biosimilar A to biosimilar B (Lauret et al., 2020; Gall et al., 2021; Hanzel et al., 2021; Khan et al., 2021; Lovero et al., 2021; Luber et al., 2021; Macaluso et al., 2021; Trystram et al., 2021; Lontai et al., 2022; Mazza et al., 2022). Eight were single-arm cohort studies, where participants switched from one biosimilar to another and outcome were compared before and after the switch (Bouhnik et al., 2020; Gisondi et al., 2020; Kiltz et al., 2020; Mott et al., 2021; Peters et al., 2021; Piaserico et al., 2021; Ribaldone et al., 2021; Siakavellas et al., 2021).
Overall, 12 studies adopted a prospective design, six were retrospective, and one (Harris et al., 2019) was a prospective observational study with a retrospective control group. Table 1 shows the details of the included studies and their main results. The total number of participants included in these studies was 3111, with a median number of 133 (range: 36–309). The median follow-up of the included studies was 12 months (range: 4–21 months).
As shown in Figure 1, most of the studies (74%, 14 out of 19) involved infliximab (originator and the biosimilars CT-P13 and SB2). This is likely to be due to the immunogenicity concerns regarding infliximab, which is a chimeric human/murine IgG1 monoclonal antibody (mAb) able to induce the production of human anti-infliximab antibodies (Pecoraro et al., 2017). Moreover, infliximab is among the most prescribed biosimilars worldwide.
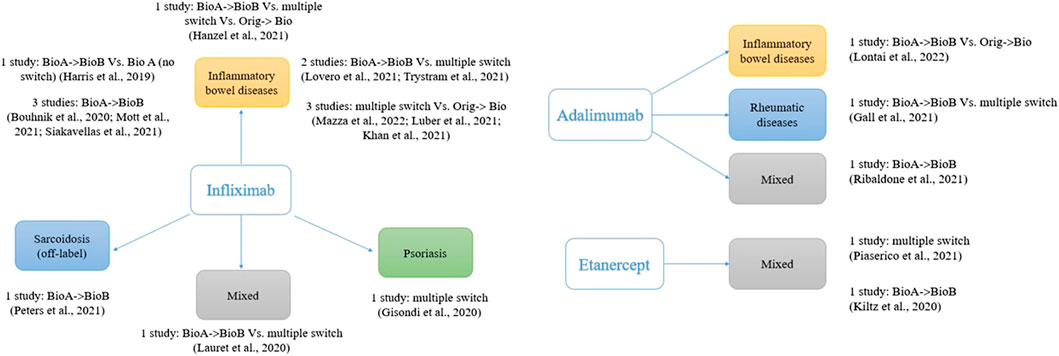
FIGURE 1. Studies assessing switch between biosimilars of anti-TNF; one study with five comparisons not included (Macaluso et al., 2021).
Most of the studies (63%, 12 out of 19) assessed anti-TNF for the management of inflammatory bowel diseases (IBD), ulcerative colitis, or Crohn’s disease in clinical practice setting. The first biosimilar for the treatment of IBD was introduced in 2013, and by the end of March 2022, 14 anti-TNF alpha biosimilar active principles (three for infliximab, eight for adalimumab, and three for etanercept) have been licensed by the EMA (European Medicinal Agency Biosimilars, 2022). The pivotal studies supporting the authorization of these biosimilars all included participants with chronic conditions other than inflammatory bowel diseases, but they were licensed for these indications following the principle of extrapolation of indications (Allocati and Gerardi, 2020; European Medicinal Agency Biosimilars, 2022).
This approach caused some reluctance among gastroenterologists regarding the use of biosimilars, which resulted in the instigation of several clinical studies with biosimilars for IBD in different countries and settings (Jørgensen et al., 2017; Ratnakumaran et al., 2018; Ye et al., 2019; Iniesta Navalón et al., 2021; Schreiber et al., 2021). These studies, coupled with the real-life clinical experiences, have progressively changed the point of view of physicians (Fiorino et al., 2019a; Bhat and Qazi, 2021).
It is worth noting that one study analyzed the switching between two infliximab biosimilars in patients with sarcoidosis, an inflammatory disorder characterized by a heightened granulomatous immune response (Peters et al., 2021). Infliximab is used off-label to treat this condition, as multiple studies demonstrated a clinical improvement, possibly because of the cytokine TNF-α role in the inflammatory process and granuloma formation.
In terms of outcome, all the included studies evaluated whether the switch between biosimilars impacted on the safety and efficacy of anti-TNF agents. Safety was typically measured as the frequency of adverse events and discontinuations, while efficacy was assessed by measuring clinical responses or worsening of the disease, steroid-free clinical remission, or loss of response, through standard metrics applied to the different diseases. For instance, serum C-reactive protein levels were measured in inflammatory disease and American College of Rheumatology (ACR) criteria used in rheumatic disorders. Less than a third of the included studies (26%, 5 out of 19) specifically addressed the impact on immunogenicity, by measuring infliximab trough levels and antidrug antibodies using ELISA assay (Lauret et al., 2020; Hanzel et al., 2021; Luber et al., 2021; Peters et al., 2021; Trystram et al., 2021).
Overall, these studies suggest that switching from biosimilar (infliximab, adalimumab, or etanercept) to another biosimilar of the same medicinal biologic medicine in patients with chronic inflammatory diseases is safe and effective in terms of disease activity, remission rate, loss of response, adverse events, and immunogenicity (when analyzed). Similar conclusion can be drawn from studies assessing multiple switches, that is, studies in which patients already on treatment with the originator are switched to one biosimilar and then to another one. None of the studies assessing immunogenicity demonstrated that switching between biosimilars leads to a change in the immune response, with similar antidrug antibodies trough levels either soon after switching or after longer follow-up (Table 1).
Discussion and Potential Next Steps
The lack of studies that directly compared switching from a biosimilar to another of the same biologic medicine vs. the maintenance of the same biosimilar could lead to a call for further (high-quality) studies to dispel concerns about switching between biosimilars. However, a serious reflection on the relevance of this research is needed in light of our findings. It is true that the medical community have expressed some reservations about interchangeability and switching, with immunogenicity frequently raised as main concern. However, clinical studies to date that have focused on switching between the originator reference product and biosimilars have been able to reassure the prescribers through confirming substantial equivalence. Moreover, the increasing number of biosimilars available on the market makes it extremely challenging to conduct standard parallel trials comparing all the possible sequence combinations. This heterogeneity is clear observing the fragmentation of the treatment sequences (Figure 1). The analysis is limited to anti-TNF drugs for chronic inflammatory diseases. Although we cannot exclude different scenarios, it is likely that similar reflections apply to other biologics or disease areas.
Switching is typically triggered by nonmedical decisions including cost or procurement issues given the typically high and growing cost of new biological medicines especially in disease areas such as cancer and orphan diseases (Luzzatto et al., 2018; Godman et al., 2021b; Mysler et al., 2021). Hurdles in the development of biosimilars including the request for studies demonstrating their efficacy and safety after switching can appear disproportionate and may discourage companies from developing biosimilars, which will be detrimental to key stakeholder groups in the future. The greater the number of companies that develop biosimilars, the greater the potential price discounts, which is the ultimate goal of health authorities with increasing pressures on their budgets.
In the rapidly evolving scenario of currently available biosimilars for inflammatory chronic diseases and given that RCTs are unfeasible, disease registries and prescription monitoring may be feasible alternatives with providing relevant information for physicians in everyday practice. Data collected during clinical practice in well-conducted observational studies (the so-called real-world data) can provide relevant and valuable evidence, complementary to those derived from RCTs, on the effectiveness and safety of biosimilars across multiple indications and treatment setting. Moreover, therapeutic drug and immunogenicity monitoring (TDIM), that is, the measurement of drug and antidrug antibodies to individualize treatment strategy, has been proposed as a method to maximize efficacy, safety, and cost-effectiveness of anti-TNF therapy (Bloem et al., 2017; Medina et al., 2017; Ricciuto et al., 2018; Ma et al., 2019; Papamichael et al., 2019). This is particularly important when switching patients from originators to considerably less expensive biosimilars and when there are concerns with the effectiveness in practice. The envisaged availability and convenience of TDIM may help ascertain the rationale for any decrease in effectiveness with switching and avoid automatic switch back to the originator in patients with a loss of response, approximately 25–30% patients (Qiu et al., 2017). Recently, a RCT conducted among 20 Norwegian hospitals showed that proactive TDIM during maintenance therapy with infliximab (the originator or a biosimilar product) was more likely to lead to sustained disease control in patients with immune-mediated inflammatory diseases (Syversen et al., 2021; Wallace and Sparks, 2021). However, proactive monitoring is currently not routinely offered to patients treated with biological medicines across countries. Despite the promising results of the Norwegian trial, other studies assessing the clinical utility of TDIM over empirical decisions have reported conflicting results (Ricciuto et al., 2018; Borren et al., 2021). The variety of analytical methods and thresholds may be one of the key drivers of these contradictions. Various immunoassay approaches have been used to detect and quantify ADA (Beeg et al., 2021), and the comparison of different techniques highlighted different results in terms of ADA titers (Steenholdt et al., 2013). As regards ELISA, that is, the most common assay, a diagnostic guidance of NICE, comparing commercial and in house ELISA kits, raised concerns on their analytical performance (NICE, 2016). More recent data suggested that ELISA can result in an underestimation, or even the lack of detection, of ADA (Beeg et al., 2021). A recent survey of 80 studies showed that the proportion of ADA-positive patients varies widely, from 4.8 to 79%, depending on the assay (Gorovits et al., 2018). These data call for unified and validated analytical approaches to increase the reliability of ADA measurements during treatment with anti-TNF agents.
While some clinical guidance recommends TDIM when patients loss response to treatment (reactive monitoring) (Feuerstein et al., 2017; Gomollón et al., 2017), it has not widely been adopted and currently not typically reimbursed by national health services, as seen, for example, in Italy. If the usefulness of TDIM to support clinical decisions, and thereby improving patients’ outcomes and the rational use of biologic agents, can be confirmed, it may become a key tool for the management of the increasing number of patients undergoing switching between originators and biosimilars as well as between biosimilars.
Routine patient monitoring may also have a positive impact on discontinuation or adverse events from biosimilars where these are caused by patients’ negative perception of biosimilars or any change in therapy, the so-called nocebo effect. In particular, the emergence of side effects after switching and their resolution after reverting to the formulation previously prescribed (originator or another biosimilar) may have been a result of the nocebo effect (Odinet et al., 2018; Rezk and Pieper, 2018; Colloca et al., 2019).
Patient information remains essential to strengthen their relationship with the doctor and to accept biosimilars, including switching between biosimilars, and TDIM can help in this respect along with general patient information.
Final Remarks
There is a need to increase physicians’ and patients’ confidence in biosimilar medicines, including switching between biosimilars, to increase the availability and use of biological medicines especially where there are issues of affordability.
The findings from the 19 identified studies show that whether switching for the first or second time, there was no significant difference in the efficacy and safety of biosimilars, particularly if patients are in remission at the time of the switch. This is similar to the multiple studies that have shown similar effectiveness, safety, and immunogenicity between biosimilars and originators (Danese et al., 2017; Glintborg et al., 2017; Griffiths et al., 2017; Jørgensen et al., 2017; Park et al., 2017; Yoo et al., 2017; Cohen et al., 2018; Matucci-Cerinic et al., 2018; Ratnakumaran et al., 2018; Cohen et al., 2019; Gisondi et al., 2019; Goll et al., 2019; Pegram et al., 2019; Yang et al., 2019; Barbier et al., 2020; Barberio et al., 2021; Bruni et al., 2021; Cingolani et al., 2021; Li et al., 2022). In addition to data supporting biosimilarity at the time of approval, these data should reassure professional societies and patient groups who strongly advocate that any decision to exchange an originator with a biosimilar should remain the responsibility of the physicians in consultation with their patients.
Potential savings, enhanced by increasing competition between biosimilar manufacturers, with competition potentially further increased by WHO prequalification scheme (Davio, 2019; Hagen, 2020; Godman et al., 2021a; Haque et al., 2021), can subsequently be used to enhance the number of patients receiving biologicals to manage their disease (Dutta et al., 2020).
In view of our findings, healthcare professional expectations for routine switching studies now seem unnecessary with the growing body of evidence suggesting no real problems in practice coupled with stringent regulatory requirements. Increased monitoring of patients prescribed biosimilars in clinical practice through increased use of TDIM that could offer an additional tool to support interchangeability and help to further realize possible savings.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author Contributions
EA, RB, and BG were involved in study conception; EA searched for studies, selected studies, and extracted data; RB checked study selection and data extraction; EA analyzed data; EA, RB, and BG drafted the manuscript; all the authors were involved in data interpretation and manuscript revision.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank the Essential Medicine List Secretariat for the general suggestions on the project and Vittorio Bertele’ for the critical revision of the manuscript. This analysis was part of a broader assessment of the evidence regarding the benefits and harms of switching from originator and its biosimilars commissioned and paid by the World Health Organization. The authors though were totally responsible for the views expressed in this article, and they do not necessarily represent the decisions, policy, or views of the World Health Organization.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.917814/full#supplementary-material
References
Alfonso-Cristancho, R., Armstrong, N., Arjunji, R., Riemsma, R., Worthy, G., Ganguly, R., et al. (2017). Comparative Effectiveness of Biologics for the Management of Rheumatoid Arthritis: Systematic Review and Network Meta-Analysis. Clin. Rheumatol. 36 (1), 25–34. doi:10.1007/s10067-016-3435-2
Allocati, E., and Gerardi, C. (2020). OUTCOMES OF SWITCHING FROM ANTI-TNF BIOLOGIC DRUGS TO THEIR BIOSIMILARS: A SYSTEMATIC REVIEW. Available at URL: https://cdn.who.int/media/docs/default-source/essential-medicines/2021-eml-expert-committee/other-matters/o.7_switching-anti-tnf.pdf?sfvrsn=ddae8024_6. (Accessed July 7, 2022).
Barberio, B., Cingolani, L., Canova, C., Barbieri, G., Sablich, R., Urbano, M. T., et al. (2021). A Propensity Score-Weighted Comparison between Adalimumab Originator and its Biosimilars, ABP501 and SB5, in Inflammatory Bowel Disease: a Multicenter Italian Study. Ther. Adv. Gastroenterol. 14, 17562848211031420. doi:10.1177/17562848211031420
Barbier, L., Ebbers, H. C., Declerck, P., Simoens, S., Vulto, A. G., and Huys, I. (2020). The Efficacy, Safety, and Immunogenicity of Switching between Reference Biopharmaceuticals and Biosimilars: A Systematic Review. Clin. Pharmacol. Ther. 108 (4), 734–755. doi:10.1002/cpt.1836
Baumgart, D. C., Misery, L., Naeyaert, S., and Taylor, P. C. (2019). Biological Therapies in Immune-Mediated Inflammatory Diseases: Can Biosimilars Reduce Access Inequities? Front. Pharmacol. 10, 279. doi:10.3389/fphar.2019.00279
Beeg, M., Burti, C., Allocati, E., Ciafardini, C., Banzi, R., Nobili, A., et al. (2021). Surface Plasmon Resonance Unveils Important Pitfalls of Enzyme-Linked Immunoassay for the Detection of Anti-infliximab Antibodies in Patients' Sera. Sci. Rep. 11 (1), 14976. doi:10.1038/s41598-021-94431-x
Bhat, S., and Qazi, T. (2021). Switching from Infliximab to Biosimilar in Inflammatory Bowel Disease: A Review of Existing Literature and Best Practices. Crohn’s Colitis 3 (1), 1–6. doi:10.1093/crocol/otaa093
Blauvelt, A., Lacour, J. P., Fowler, J. F., Weinberg, J. M., Gospodinov, D., Schuck, E., et al. (2018). Phase III Randomized Study of the Proposed Adalimumab Biosimilar GP2017 in Psoriasis: Impact of Multiple Switches. Br. J. Dermatol 179 (3), 623–631. doi:10.1111/bjd.16890
Bloem, K., Hernández-Breijo, B., Martínez-Feito, A., and Rispens, T. (2017). Immunogenicity of Therapeutic Antibodies: Monitoring Antidrug Antibodies in a Clinical Context. Ther. Drug Monit. 39 (4), 327–332. doi:10.1097/FTD.0000000000000404
Borren, N. Z., Paulides, E., Frinack, J. L., Olson, R. N., Willrich, M. A. V., van der Woude, C. J., et al. (2021). Infliximab Trough Levels Are Not Predictive of Relapse in Patients with IBD in Endoscopic Remission: A Multicenter Cohort Study. Dig. Dis. Sci. 66 (10), 3548–3554. doi:10.1007/s10620-020-06645-0
Bouhnik, Y., Fautrel, B., Desjeux, G., Freudensprung, U., Brigui, A., and Addison, J. (2020). P637 PERFUSE: A French Non-interventional Cohort Study of Infliximab-Naive and Transitioned Patients Receiving Infliximab Biosimilar SB2; an Interim Analysis. J. Crohn's Colitis 14 (Suppl. ment_1), S529. doi:10.1093/ecco-jcc/jjz203.765
Bruni, C., Bitti, R., Nacci, F., Cometi, L., Tofani, L., Bartoli, F., et al. (2021). Efficacy and Safety of Switching from Reference Adalimumab to SB5 in a Real-Life Cohort of Inflammatory Rheumatic Joint Diseases. Clin. Rheumatol. 40 (1), 85–91. doi:10.1007/s10067-020-05199-w
Cholapranee, A., Hazlewood, G. S., Kaplan, G. G., Peyrin-Biroulet, L., and Ananthakrishnan, A. N. (2017). Systematic Review with Meta-Analysis: Comparative Efficacy of Biologics for Induction and Maintenance of Mucosal Healing in Crohn's Disease and Ulcerative Colitis Controlled Trials. Aliment. Pharmacol. Ther. 45 (10), 1291–1302. doi:10.1111/apt.14030
Cingolani, L., Barberio, B., Zingone, F., Ferronato, A., Bertani, L., Costa, F., et al. (2021). Adalimumab Biosimilars, ABP501 and SB5, Are Equally Effective and Safe as Adalimumab Originator. Sci. Rep. 11 (1), 10368. doi:10.1038/s41598-021-89790-4
Cohen, H., Beydoun, D., Chien, D., Lessor, T., McCabe, D., Muenzberg, M., et al. (2017). Awareness, Knowledge, and Perceptions of Biosimilars Among Specialty Physicians. Adv. Ther. 33 (12), 2160–2172. doi:10.1007/s12325-016-0431-5
Cohen, S. B., Burgos-Vargas, R., Emery, P., Jin, B., Cronenberger, C., and Vázquez-Abad, M. D. (2018). Extension Study of PF-05280586, a Potential Rituximab Biosimilar, versus Rituximab in Subjects with Active Rheumatoid Arthritis. Arthritis Care Res. Hob. 70 (11), 1598–1606. doi:10.1002/acr.23586
Cohen, S. B., Czeloth, N., Lee, E., Klimiuk, P. A., Peter, N., and Jayadeva, G. (2019). Long-term Safety, Efficacy, and Immunogenicity of Adalimumab Biosimilar BI 695501 and Adalimumab Reference Product in Patients with Moderately-To-Severely Active Rheumatoid Arthritis: Results from a Phase 3b Extension Study (VOLTAIRE-RAext). Expert Opin. Biol. Ther. 19 (10), 1097–1105. doi:10.1080/14712598.2019.1645114
Colloca, L., Panaccione, R., and Murphy, T. K. (2019). The Clinical Implications of Nocebo Effects for Biosimilar Therapy. Front. Pharmacol. 10, 1372. doi:10.3389/fphar.2019.01372
da Silva, W. C., de Araujo, V. E., Lima, E. M. E. A., Dos Santos, J. B. R., Silva, M. R. R. D., Almeida, P. H. R. F., et al. (2018). Comparative Effectiveness and Safety of Monoclonal Antibodies (Bevacizumab, Cetuximab, and Panitumumab) in Combination with Chemotherapy for Metastatic Colorectal Cancer: A Systematic Review and Meta-Analysis. BioDrugs 32 (6), 585–606. doi:10.1007/s40259-018-0322-1
Danese, S., Fiorino, G., Raine, T., Ferrante, M., Kemp, K., Kierkus, J., et al. (2017). ECCO Position Statement on the Use of Biosimilars for Inflammatory Bowel Disease-An Update. J. Crohns Colitis 11 (1), 26–34. doi:10.1093/ecco-jcc/jjw198
Davio, K. (2019). WHO Prequalifies Samsung Bioepis' Biosimilar Trastuzumab. Available at https://www.centerforbiosimilars.com/view/who-prequalifies-samsung-bioepis-biosimilar-trastuzumab (Accessed July 7, 2022).
Dutta, B., Huys, I., Vulto, A. G., and Simoens, S. (2020). Identifying Key Benefits in European Off-Patent Biologics and Biosimilar Markets: It Is Not Only about Price. BioDrugs 34 (2), 159–170. doi:10.1007/s40259-019-00395-w
European Medicinal Agency Biosimilars (2022). EMA. Biosimilars. Available at URL: https://www.ema.europa.eu/en/medicines/search_api_aggregation_ema_medicine_types/field_ema_med_biosimilar/ema_group_types/ema_medicine (Accessed July 7, 2022).
European Medicinal Agency and the European commission (2019). Biosimilars in the EU Information Guide for Healthcare Professionals. Available at URL: https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf (Accessed July 7, 2022).
Feuerstein, J. D., Nguyen, G. C., Kupfer, S. S., Falck-Ytter, Y., and Singh, S. (2017). American Gastroenterological Association Institute Guideline on Therapeutic Drug Monitoring in Inflammatory Bowel Disease. Gastroenterology 153 (3), 827–834. doi:10.1053/j.gastro.2017.07.032
Fiorino, G., Caprioli, F., Daperno, M., Mocciaro, F., Principi, M., Viscido, A., et al. (2019a). Use of Biosimilars in Inflammatory Bowel Disease: a Position Update of the Italian Group for the Study of Inflammatory Bowel Disease (IG-IBD). Dig. Liver Dis. 51 (5), 632–639. doi:10.1016/j.dld.2019.02.004
Fiorino, G., Peyrin-Biroulet, L., and Danese, S. (2019b). Effectiveness of Infliximab Biosimilar in Crohn's Disease: A Dime, A Dozen. Gastroenterology 156 (8), 2349–2351. doi:10.1053/j.gastro.2019.04.029
Gall, S., Kiltz, U., Kobylinski, T. P., Andreica, I., Vaupel, K., Baraliakos, X., et al. (2021). Pos0301 no Major Differences Between Patients With Chronic Inflammatory Rheumatic Disease Who Underwent Mono- or Multiswitching of Biosimilars in Routine Care (Perception Study). Ann. rheumatic Dis. 80, 1872. doi:10.1136/annrheumdis-2021-eular.1742
Gershon, N., Berchenko, Y., Hall, P. S., and Goldstein, D. A. (2019). Cost Effectiveness and Affordability of Trastuzumab in Sub-saharan Africa for Early Stage HER2-Positive Breast Cancer. Cost. Eff. Resour. Alloc. 17, 5. doi:10.1186/s12962-019-0174-7
Gisondi, P., Bianchi, L., Calzavara-Pinton, P., Conti, A., Chiricozzi, A., Fimiani, M., et al. (2019). Etanercept Biosimilar SB4 in the Treatment of Chronic Plaque Psoriasis: Data from the Psobiosimilars Registry. Br. J. Dermatol 180 (2), 409–410. doi:10.1111/bjd.17133
Gisondi, P., Virga, C., Piaserico, S., Meneguzzo, A., Odorici, G., Conti, A., et al. (2020). Switching from One Infliximab Biosimilar (CT-P13) to Another Infliximab Biosimilar (SB2) in Patients with Chronic Plaque Psoriasis. Br. J. Dermatol 183 (2), 397–398. doi:10.1111/bjd.19013
Glintborg, B., Sørensen, I. J., Loft, A. G., Lindegaard, H., Linauskas, A., Hendricks, O., et al. (2017). A Nationwide Non-medical Switch from Originator Infliximab to Biosimilar CT-P13 in 802 Patients with Inflammatory Arthritis: 1-year Clinical Outcomes from the DANBIO Registry. Ann. Rheum. Dis. 76 (8), 1426–1431. doi:10.1136/annrheumdis-2016-210742
Godman, B., Fadare, J., Kwon, H. Y., Dias, C. Z., Kurdi, A., Dias Godói, I. P., et al. (2021a). Evidence-based Public Policy Making for Medicines across Countries: Findings and Implications for the Future. J. Comp. Eff. Res. 10 (12), 1019–1052. doi:10.2217/cer-2020-0273
Godman, B., Hill, A., Simoens, S., Selke, G., Selke Krulichová, I., Zampirolli Dias, C., et al. (2021b). Potential Approaches for the Pricing of Cancer Medicines across Europe to Enhance the Sustainability of Healthcare Systems and the Implications. Expert Rev. Pharmacoecon Outcomes Res. 21 (4), 527–540. doi:10.1080/14737167.2021.1884546
Godman, B., Allocati, E., and Moorkens, E. (2019). Ever-changing Landscape of Biosimilars in Canada; Findings and Implications from a Global Perspective. GaBI J. 8 (3), 93–97. doi:10.5639/gabij.2019.0803.012
Goll, G. L., Jørgensen, K. K., Sexton, J., Olsen, I. C., Bolstad, N., Haavardsholm, E. A., et al. (2019). Long-term Efficacy and Safety of Biosimilar Infliximab (CT-P13) after Switching from Originator Infliximab: Open-Label Extension of the NOR-SWITCH Trial. J. Intern Med. 285 (6), 653–669. doi:10.1111/joim.12880
Gomollón, F., Dignass, A., Annese, V., Tilg, H., Van Assche, G., Lindsay, J. O., et al. (2017). 3rd European Evidence-Based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 1: Diagnosis and Medical Management. J. Crohns Colitis 11 (1), 3–25. doi:10.1093/ecco-jcc/jjw168
Gorovits, B., Baltrukonis, D. J., Bhattacharya, I., Birchler, M. A., Finco, D., Sikkema, D., et al. (2018). Immunoassay Methods Used in Clinical Studies for the Detection of Anti-drug Antibodies to Adalimumab and Infliximab. Clin. Exp. Immunol. 192 (3), 348–365. doi:10.1111/cei.13112
Griffiths, C. E. M., Thaçi, D., Gerdes, S., Arenberger, P., Pulka, G., Kingo, K., et al. (2017). The EGALITY study: a confirmatory, randomized, double-blind study comparing the efficacy, safety and immunogenicity of GP2015, a proposed etanercept biosimilar, vs. the originator product in patients with moderate-to-severe chronic plaque-type psoriasis. Br. J. Dermatol 176 (4), 928–938. doi:10.1111/bjd.15152
Hagen, T. (2020). WHO Clears Celltrion Rituximab for Use. Available at URL: https://www.centerforbiosimilars.com/view/who-clears-celltrion-rituximab-for-use (Accessed July 7, 2022).
Hanzel, J., Jansen, J. M., Ter Steege, R. W. F., Gecse, K. B., and D'Haens, G. R. (2021). Multiple Switches From the Originator Infliximab to Biosimilars Is Effective and Safe in Inflammatory Bowel Disease: A Prospective Multicenter Cohort Study. Inflamm. Bowel Dis. 28 (4), 495–501. doi:10.1093/ibd/izab099
Haque, M., Islam, S., Kamal, Z. M., Akter, F., Jahan, I., Rahim, M. S. A., et al. (2021). Ongoing efforts to improve the management of patients with diabetes in Bangladesh and the implications. Hosp. Pract. (1995) 49 (4), 266–272. doi:10.1080/21548331.2021.1906083
Harris, C., Harris, R., Young, D., McDonnell, M., Harvey, J., Felwick, R., et al. (2019). IBD biosimilar to biosimilar infliximab switching study: preliminary results. United Eur. Gastroenterol. J. 7:8 (361 Suppl.).
Huoponen, S., Eberl, A., Räsänen, P., Roine, R. P., Sipponen, T., Arkkila, P., et al. (2020). Health-related quality of life and costs of switching originator infliximab to biosimilar one in treatment of inflammatory bowel disease. Med. Baltim. 99 (2), e18723. doi:10.1097/MD.0000000000018723
Iniesta Navalón, C., Gil Candel, M., Salar Valverde, I., Nicolás de Prado, I., Gómez Espín, R., and Rentero Redondo, L. (2021). Biosimilar infliximab CPT-13 for inflammatory bowel disease in a real clinical setting: pharmacokinetic outcomes, immunogenicity, and drug survival. Rev. Esp. Enferm. Dig. 113 (11), 770–775. doi:10.17235/reed.2021.7638/2020
Jensen, T. B., Kim, S. C., Jimenez-Solem, E., Bartels, D., Christensen, H. R., and Andersen, J. T. (2020). Shift From Adalimumab Originator to Biosimilars in Denmark. JAMA Intern Med. 180 (6), 902–903. doi:10.1001/jamainternmed.2020.0338
Jiménez-Pichardo, L., Gázquez-Pérez, R., and Sierra-Sánchez, J. F. (2018). Degree of prescriber's knowledge about variability in biological drugs "innovators" in manufacturing process. Eur. J. Clin. Pharmacol. 74 (4), 505–511. doi:10.1007/s00228-017-2397-x
Jørgensen, K. K., Olsen, I. C., Goll, G. L., Lorentzen, M., Bolstad, N., Haavardsholm, E. A., et al. (2017). Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet 389 (10086), 2304–2316. doi:10.1016/S0140-6736(17)30068-5
Khan, N., Patel, D., Pernes, T., Patel, M., Trivedi, C., Medvedeva, E., et al. (2021). The Efficacy and Safety of Switching From Originator Infliximab to Single or Double Switch Biosimilar Among a Nationwide Cohort of Inflammatory Bowel Disease Patients. Crohn's Colitis 3603 (2), otab037. doi:10.1093/crocol/otab022
Kiltz, U., Tsiami, S., Baraliakos, X., and Braun, J. (2020). AB1171 Effects of successive switches of two different biosimilars of etanercept on outcomes in inflammatory rheumatic diseases in daily practice. Ann. rheumatic Dis. 79, 1876. doi:10.1136/annrheumdis-2020-eular.3640
Kostić, M., Djakovic, L., Šujić, R., Godman, B., and Janković, S. M. (2017). Inflammatory Bowel Diseases (Crohn´s Disease and Ulcerative Colitis): Cost of Treatment in Serbia and the Implications. Appl. Health Econ. Health Policy 15 (1), 85–93. doi:10.1007/s40258-016-0272-z
Lauret, A., Moltó, A., Abitbol, V., Gutermann, L., Conort, O., Chast, F., et al. (2020). Effects of successive switches to different biosimilars infliximab on immunogenicity in chronic inflammatory diseases in daily clinical practice. Semin. Arthritis Rheum. 50 (6), 1449–1456. doi:10.1016/j.semarthrit.2020.02.007
Li, R. K., Tokunaga, E., Adamchuk, H., Vladimirov, V., Yanez, E., Lee, K. S., et al. (2022). Long-Term Safety and Effectiveness of PF-05280014 (a Trastuzumab Biosimilar) Treatment in Patients with HER2-Positive Metastatic Breast Cancer: Updated Results of a Randomized, Double-Blind Study. BioDrugs 36 (1), 55–69. doi:10.1007/s40259-021-00513-7
Lontai, L., Gonczi, L., Balogh, F., Komlodi, N., Resal, T., Farkas, K., et al. (2022). P578 Non-medical switch between adalimumab biosimilars and from the originator adalimumab to biosimilars in inflammatory bowel disease patients - a multicentre study on efficacy and drug sustainability. J. Crohn's Colitis 16 (Suppl. ment_1), i518–i519. doi:10.1093/ecco-jcc/jjab232.704
Lovero, R., Losurdo, G., La Fortezza, R. F., Terracciano, F., Biscaglia, G., Martino, G., et al. (2021). Safety and efficacy of switching from infliximab biosimilar CT-P13 to infliximab biosimilar SB2 in patients with inflammatory bowel disease. Eur. J. gastroenterology hepatology 32 (2), 201–207. doi:10.1097/meg.0000000000001988
Luber, R. P., O'Neill, R., Singh, S., Sharma, E., Cunningham, G., Honap, S., et al. (2021). An observational study of switching infliximab biosimilar: no adverse impact on inflammatory bowel disease control or drug levels with first or second switch. Aliment. Pharmacol. Ther. 54 (5), 678–688. doi:10.1111/apt.16497
Luzzatto, L., Hyry, H. I., Schieppati, A., Costa, E., Simoens, S., Schaefer, F., et al. (2018). Outrageous prices of orphan drugs: a call for collaboration. Lancet 392 (10149), 791–794. doi:10.1016/S0140-6736(18)31069-9
Ma, C., Battat, R., Jairath, V., and Vande Casteele, N. (2019). Advances in Therapeutic Drug Monitoring for Small-Molecule and Biologic Therapies in Inflammatory Bowel Disease. Curr. Treat. Options Gastroenterol. 17 (1), 127–145. doi:10.1007/s11938-019-00222-9
Macaluso, F. S., Fries, W., Viola, A., Centritto, A., Cappello, M., Giuffrida, E., et al. (2021). The SPOSIB SB2 Sicilian Cohort: Safety and Effectiveness of Infliximab Biosimilar SB2 in Inflammatory Bowel Diseases, Including Multiple Switches. Inflamm. Bowel Dis. 27 (2), 182–189. doi:10.1093/ibd/izaa036
MacBride-Stewart, S., McTaggart, S., Kurdi, A., Sneddon, J., McBurney, S., do Nascimento, R. C. R. M., et al. (2021). Initiatives and reforms across Scotland in recent years to improve prescribing; findings and global implications of drug prescriptions. Int. J. Clin. Exp. Med. 14 (12), 2563–2586.
Matucci-Cerinic, M., Schulze-Koops, H., Buch, M. H., Kavanaugh, A., Allanore, Y., Kucharz, E. J., et al. (2018). FRI012Y Switch between reference etanercept (ETN) and gp2015, an etanercept biosimilar, did Not impact efficacy and safety in patients with moderate-to-severe rheumatoid arthritis: 48-week results from the phase 3 equira study. Ann. Rheumatic Dis. 77 (609 Suppl. 2).
Matusewicz, W., Godman, B., Pedersen, H. B., Fürst, J., Gulbinovič, J., Mack, A., et al. (2015). Improving the managed introduction of new medicines: sharing experiences to aid authorities across Europe. Expert Rev. Pharmacoecon Outcomes Res. 15 (5), 755–758. doi:10.1586/14737167.2015.1085803
Mazza, S., Piazza O Sed, N., Conforti, F. S., Fascì, A., Rimondi, A., Marinoni, B., et al. (2022). Safety and clinical efficacy of the double switch from originator infliximab to biosimilars CT-P13 and SB2 in patients with inflammatory bowel diseases (SCESICS): A multicenter cohort study. Clin. Transl. Sci. 15 (1), 172–181. doi:10.1111/cts.13131
Medicines for Europe (2019). Positioning statements on physician-led switching for biosimilar medicines. Available at URL: https://www.medicinesforeurope.com/wp-content/uploads/2017/03/M-Biosimilars-Overview-of-positions-on-physician-led-switching.pdf (Accessed July 7, 2022).
Medina, F., Plasencia, C., Goupille, P., Ternant, D., Balsa, A., and Mulleman, D. (2017). Current Practice for Therapeutic Drug Monitoring of Biopharmaceuticals in Rheumatoid Arthritis. Ther. Drug Monit. 39 (4), 364–369. doi:10.1097/FTD.0000000000000421
Meyer, A., Rudant, J., Drouin, J., Weill, A., Carbonnel, F., and Coste, J. (2019). Effectiveness and Safety of Reference Infliximab and Biosimilar in Crohn Disease: A French Equivalence Study. Ann. Intern Med. 170 (2), 99–107. doi:10.7326/M18-1512
Moorkens, E., Godman, B., Huys, I., Hoxha, I., Malaj, A., Keuerleber, S., et al. (2020). The Expiry of Humira® Market Exclusivity and the Entry of Adalimumab Biosimilars in Europe: An Overview of Pricing and National Policy Measures. Front. Pharmacol. 11, 591134. doi:10.3389/fphar.2020.591134
Moorkens, E., Vulto, A. G., Huys, I., Dylst, P., Godman, B., Keuerleber, S., et al. (2017). Policies for biosimilar uptake in Europe: An overview. PLoS One 12 (12), e0190147. doi:10.1371/journal.pone.0190147
Mott, A., Taherzadeh, N., Shah, T., Whitley, L., Murray, C., and Jani, Y. PMO-29 Switching between infliximab biosimilars: experience from two inflammatory bowel disease centres2021. A92.1-A p 70, A92. doi:10.1136/gutjnl-2021-BSG.168
Mysler, E., Azevedo, V. F., Danese, S., Alvarez, D., Iikuni, N., Ingram, B., et al. (2021). Biosimilar-to-Biosimilar Switching: What is the Rationale and Current Experience? Drugs 81 (16), 1859–1879. doi:10.1007/s40265-021-01610-1
Nam, J. L., Ramiro, S., Gaujoux-Viala, C., Takase, K., Leon-Garcia, M., Emery, P., et al. (2014). Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2013 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann. Rheum. Dis. 73 (3), 516–528. doi:10.1136/annrheumdis-2013-204577
NICE (2016). Therapeutic monitoring of TNF-alpha inhibitors in Crohn’s disease (LISA-TRACKER ELISA kits, IDKmonitor ELISA kits, and Promonitor ELISA kits). Available at URL: https://www.nice.org.uk/guidance/dg22 (Accessed July 7, 2022).
Odinet, J. S., Day, C. E., Cruz, J. L., and Heindel, G. A. (2018). The Biosimilar Nocebo Effect? A Systematic Review of Double-Blinded Versus Open-Label Studies. J. Manag. Care Spec. Pharm. 24 (10), 952–959. doi:10.18553/jmcp.2018.24.10.952
Papamichael, K., Vogelzang, E. H., Lambert, J., Wolbink, G., and Cheifetz, A. S. (2019). Therapeutic drug monitoring with biologic agents in immune mediated inflammatory diseases. Expert Rev. Clin. Immunol. 15 (8), 837–848. doi:10.1080/1744666X.2019.1630273
Park, W., Yoo, D. H., Miranda, P., Brzosko, M., Wiland, P., Gutierrez-Ureña, S., et al. (2017). Efficacy and safety of switching from reference infliximab to CT-P13 compared with maintenance of CT-P13 in ankylosing spondylitis: 102-week data from the PLANETAS extension study. Ann. Rheum. Dis. 76 (2), 346–354. doi:10.1136/annrheumdis-2015-208783
Pecoraro, V., De Santis, E., Melegari, A., and Trenti, T. (2017). The impact of immunogenicity of TNFα inhibitors in autoimmune inflammatory disease. A systematic review and meta-analysis. Autoimmun. Rev. 16 (6), 564–575. doi:10.1016/j.autrev.2017.04.002
Pegram, M. D., Bondarenko, I., Zorzetto, M. M. C., Hingmire, S., Iwase, H., Krivorotko, P. V., et al. (2019). PF-05280014 (a trastuzumab biosimilar) plus paclitaxel compared with reference trastuzumab plus paclitaxel for HER2-positive metastatic breast cancer: a randomised, double-blind study. Br. J. Cancer 120 (2), 172–182. doi:10.1038/s41416-018-0340-2
Peters, B. J. M., Bhatoe, A., Vorselaars, A. D. M., and Veltkamp, M. (2021). Switching to an Infliximab Biosimilar Was Safe and Effective in Dutch Sarcoidosis Patients. Cells 10 (2), 441. doi:10.3390/cells10020441
Piaserico, S., Conti, A., Messina, F., Meneguzzo, A., Odorici, G., Bellinato, F., et al. (2021). Cross-Switch from Etanercept Originator to Biosimilar SB4 and to GP2015 in Patients with Chronic Plaque Psoriasis. BioDrugs 35 (4), 469–471. doi:10.1007/s40259-021-00485-8
Position Statement on Biosimilars (2017). British Association of Dermatologists’ Position Statement on Biosimilars. Available at URL: https://www.bad.org.uk/shared/get-file.ashx?itemtype=document&id=5842 (Accessed July 7, 2022).
Putrik, P., Ramiro, S., Kvien, T. K., Sokka, T., Pavlova, M., Uhlig, T., et al. (2014). Inequities in access to biologic and synthetic DMARDs across 46 European countries. Ann. Rheum. Dis. 73 (1), 198–206. doi:10.1136/annrheumdis-2012-202603
Qiu, Y., Chen, B. L., Mao, R., Zhang, S. H., He, Y., Zeng, Z. R., et al. (2017). Systematic review with meta-analysis: loss of response and requirement of anti-TNFα dose intensification in Crohn's disease. J. Gastroenterol. 52 (5), 535–554. doi:10.1007/s00535-017-1324-3
Ratnakumaran, R., To, N., Gracie, D. J., Selinger, C. P., O'Connor, A., Clark, T., et al. (2018). Efficacy and tolerability of initiating, or switching to, infliximab biosimilar CT-P13 in inflammatory bowel disease (IBD): a large single-centre experience. Scand. J. Gastroenterol. 53 (6), 700–707. doi:10.1080/00365521.2018.1464203
Rezk, M. F., and Pieper, B. (2018). To See or NOsee: The Debate on the Nocebo Effect and Optimizing the Use of Biosimilars. Adv. Ther. 35 (6), 749–753. doi:10.1007/s12325-018-0719-8
Ribaldone, D. G., Tribocco, E., Rosso, C., Armandi, A., Vernero, M., Bugianesi, E., et al. (2021). Switching from Biosimilar to Biosimilar Adalimumab, Including Multiple Switching, in Crohn's Disease: A Prospective Study. J. Clin. Med. 10 (15), 3387. doi:10.3390/jcm10153387
Ricciuto, A., Dhaliwal, J., Walters, T. D., Griffiths, A. M., and Church, P. C. (2018). Clinical Outcomes With Therapeutic Drug Monitoring in Inflammatory Bowel Disease: A Systematic Review With Meta-Analysis. J. Crohns Colitis 12 (11), 1302–1315. doi:10.1093/ecco-jcc/jjy109
Ruyssen-Witrand, A., Perry, R., Watkins, C., Braileanu, G., Kumar, G., Kiri, S., et al. (2020). Efficacy and safety of biologics in psoriatic arthritis: a systematic literature review and network meta-analysis. RMD Open 6 (1), e001117. doi:10.1136/rmdopen-2019-001117
Schreiber, S., Ben-Horin, S., Leszczyszyn, J., Dudkowiak, R., Lahat, A., Gawdis-Wojnarska, B., et al. (2021). Randomized Controlled Trial: Subcutaneous vs Intravenous Infliximab CT-P13 Maintenance in Inflammatory Bowel Disease. Gastroenterology 160 (7), 2340–2353. doi:10.1053/j.gastro.2021.02.068
Siakavellas, S., Barrett, R., Plevris, N., Derikx, L., Gauci, J., Lucaciu, L., et al. (2021). 610 Both SINGLE AND MULTIPLE SWITCHING BETWEEN INFLIXIMAB BIOSIMILARS CAN BE SAFE AND EFFECTIVE IN INFLAMMATORY BOWEL DISEASE (IBD): REAL WORLD OUTCOMES FROM THE EDINBURGH IBD UNIT. Gastroenterology 160, S120. doi:10.1016/s0016-5085(21)01042-8
Steenholdt, C., Ainsworth, M. A., Tovey, M., Klausen, T. W., Thomsen, O. O., Brynskov, J., et al. (2013). Comparison of techniques for monitoring infliximab and antibodies against infliximab in Crohn's disease. Ther. Drug Monit. 35 (4), 530–538. doi:10.1097/FTD.0b013e31828d23c3
Syversen, S. W., Jørgensen, K. K., Goll, G. L., Brun, M. K., Sandanger, Ø., Bjørlykke, K. H., et al. (2021). Effect of Therapeutic Drug Monitoring vs Standard Therapy During Maintenance Infliximab Therapy on Disease Control in Patients With Immune-Mediated Inflammatory Diseases: A Randomized Clinical Trial. Jama 326 (23), 2375–2384. doi:10.1001/jama.2021.21316
Trystram, N., Abitbol, V., Tannoury, J., Lecomte, M., Assaraf, J., Malamut, G., et al. (2021). Outcomes after double switching from originator Infliximab to biosimilar CT-P13 and biosimilar SB2 in patients with inflammatory bowel disease: a 12-month prospective cohort study. Aliment. Pharmacol. Ther. 53 (8), 887–899. doi:10.1111/apt.16312
Tubic, B. M-P. V., Jungić, S., Allocati, E., and Godman, B. (2021). Availability and accessibility of monoclonal antibodies in Bosnia and Herzegovina: Findings and implications. Med. Access @ Point Care 5, 1–7. doi:10.1177/23992026211027692
Us, F. D. A. (2017). Biosimilar and Interchangeable Products. Available at URL: https://www.fda.gov/drugs/biosimilars/biosimilar-and-interchangeable-products (Accessed July 7, 2022).
Vezér, B., Buzás, Z., Sebeszta, M., and Zrubka, Z. (2016). Authorized manufacturing changes for therapeutic monoclonal antibodies (mAbs) in European Public Assessment Report (EPAR) documents. Curr. Med. Res. Opin. 32 (5), 829–834. doi:10.1185/03007995.2016.1145579
Vogler, S., Schneider, P., Zuba, M., Busse, R., and Panteli, D. (2021). Policies to Encourage the Use of Biosimilars in European Countries and Their Potential Impact on Pharmaceutical Expenditure. Front. Pharmacol. 12, 625296. doi:10.3389/fphar.2021.625296
Wallace, Z. S., and Sparks, J. A. (2021). Therapeutic Drug Monitoring for Immune-Mediated Inflammatory Diseases. Jama 326 (23), 2370–2372. doi:10.1001/jama.2021.21315
Wilson, F. R., Coombes, M. E., Brezden-Masley, C., Yurchenko, M., Wylie, Q., Douma, R., et al. (2018). Herceptin® (trastuzumab) in HER2-positive early breast cancer: a systematic review and cumulative network meta-analysis. Syst. Rev. 7 (1), 191. doi:10.1186/s13643-018-0854-y
World Health Organization (2021). WHO Expert Committee on Selection and Use of Essential Medicines, 2021 (Including the 22nd WHO Model List of Essential Medicines and the 8th WHO Model List of Essential Medicines for Children). Geneva: World Health Organization. WHO Technical Report Series, No. 1035. Licence: CC BY-NC-SA 3.0 IGO. Available at URL: https://apps.who.int/iris/bitstream/handle/10665/351172/9789240041134-eng.pdf?sequence=1&isAllowed=y. (Accessed July 7, 2022).
Yang, J., Yu, S., Yang, Z., Yan, Y., Chen, Y., Zeng, H., et al. (2019). Efficacy and Safety of Anti-cancer Biosimilars Compared to Reference Biologics in Oncology: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. BioDrugs 33 (4), 357–371. doi:10.1007/s40259-019-00358-1
Ye, B. D., Pesegova, M., Alexeeva, O., Osipenko, M., Lahat, A., Dorofeyev, A., et al. (2019). Efficacy and safety of biosimilar CT-P13 compared with originator infliximab in patients with active Crohn's disease: an international, randomised, double-blind, phase 3 non-inferiority study. Lancet 393 (10182), 1699–1707. doi:10.1016/S0140-6736(18)32196-2
Yoo, D. H., Prodanovic, N., Jaworski, J., Miranda, P., Ramiterre, E., Lanzon, A., et al. (2017). Efficacy and safety of CT-P13 (biosimilar infliximab) in patients with rheumatoid arthritis: comparison between switching from reference infliximab to CT-P13 and continuing CT-P13 in the PLANETRA extension study. Ann. Rheum. Dis. 76 (2), 355–363. doi:10.1136/annrheumdis-2015-208786
Keywords: biosimilar, switch, infliximab, adalimumab, etanercept, therapeutic drug monitoring
Citation: Allocati E, Godman B, Gobbi M, Garattini S and Banzi R (2022) Switching Among Biosimilars: A Review of Clinical Evidence. Front. Pharmacol. 13:917814. doi: 10.3389/fphar.2022.917814
Received: 11 April 2022; Accepted: 15 June 2022;
Published: 24 August 2022.
Edited by:
Helder Mota-Filipe, University of Lisbon, PortugalReviewed by:
Valderilio Feijó Azevedo, Federal University of Paraná, BrazilCopyright © 2022 Allocati, Godman, Gobbi, Garattini and Banzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eleonora Allocati, ZWxlb25vcmEuYWxsb2NhdGlAbWFyaW9uZWdyaS5pdA==
 Eleonora Allocati
Eleonora Allocati Brian Godman
Brian Godman Marco Gobbi
Marco Gobbi Silvio Garattini6
Silvio Garattini6 Rita Banzi
Rita Banzi