- 1School of Clinical Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
Berberine, the main bioactive component of Coptis chinensis Franch., is widely used in the treatment of diabetes. Previous studies have reported that berberine supplementation may play a multitarget therapeutic role in diabetes-related cognitive impairment (DCI). This systematic review and meta-analysis evaluated the effect and possible mechanisms of berberine in animal models of DCI. Relevant studies were searched through PubMed, Web of Science, Embase, and three Chinese databases (CNKI, Wanfang, and VIP) until March 2022. Twenty studies involving 442 animals were included, and SYRCLE’s risk of bias tool was used to assess methodological quality. The statistical analysis was performed using STATA 15.0 to calculate the weighted standard mean difference (SMD) with a 95% confidence interval (CI). The fasting blood glucose (FBG) and Morris water maze test (MWM) were the main outcomes to be analyzed. The overall results showed that berberine could significantly improve FBG, escape latency, the times of crossing the platform, the time spent in the target quadrant, serum insulin, 2hBG of oral glucose tolerance test (OGTT), amyloid β (Aβ), acetylcholinesterase (AChE), oxidative stress, and inflammation levels. The present meta-analysis demonstrated that berberine could not only lower blood glucose levels but also improve learning and memory in DCI animal models, which might involve regulating glucose and lipid metabolism, improving insulin resistance, anti-oxidation, anti-neuroinflammation, inhibiting endoplasmic reticulum (ER) stress; and improving the cholinergic system. However, additional attention should be paid to these outcomes due to the significant heterogeneity.
1 Introduction
Diabetes mellitus (DM) has become one of the worldwide public health challenges in the twenty-first century (Giannini et al., 2014). According to the International Diabetes Federation Diabetes Atlas, in 2021, more than half a billion people worldwide suffer from diabetes, and the number is estimated to reach 782 million by 2045 (Sun et al., 2022). Diabetes-related cognitive impairment (DCI), a cognitive impairment caused by diabetes, is mainly characterized by declining learning and memory function (Mijnhout et al., 2006; Biessels and Reijmer, 2014). This is one of the chronic complications of diabetes. Studies showed that chronic diabetes could accelerate and promote the onset of neurodegeneration, and 29% of patients with DM will eventually develop severe cognitive decline and neurodegeneration (de Matos et al., 2018). A meta-analysis of 144 studies showed that DM increased the risk of mild cognitive impairment and dementia in the elderly by 1.25–1.91-fold (Xue et al., 2019). Multiple lines of evidence indicate that cognitive impairment is directly associated with DM and is a serious complication of diabetes (Cukierman et al., 2005; Biessels and Despa, 2018). The pathogenesis of DCI has been widely reported in recent years, including disorders of glucose and lipid metabolism, insulin resistance, oxidative stress, inflammatory response, endoplasmic reticulum (ER) stress, and blood–brain barrier damage (Karvani et al., 2019; Zhang et al., 2020). Unfortunately, there have been no specific drugs or therapies to delay the progression of cognitive impairment in diabetes so far. New therapies and drugs are urgently needed to prevent and treat diabetes-induced cognitive impairment.
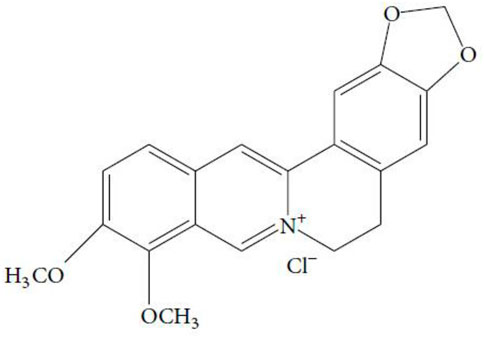
Coptis chinensis Franch. (Ranunculaceae), a recognized traditional herb in China, has been widely used in the treatment of type 2 diabetes mellitus (T2DM), hyperlipidemia, and gastrointestinal tract infection (Pang et al., 2015; Liu R. Y. et al., 2018). Berberine (C20H18NO4+, Figure 1) is the main bioactive component of Coptis chinensis Franch. (Zhao et al., 2021). In the modern pharmacological studies, due to its potential therapeutic applications, berberine has been extensively studied and has been shown to have multiple bioactivities, such as regulating glucose and lipid metabolism (Yin et al., 2008), anti-oxidation and anti-inflammation (Ma et al., 2018), and anti-cancer and neuroprotective effects (Song et al., 2020). As an isoquinoline alkaloid extracted from Coptis chinensis, berberine can effectively improve the cognitive function of DCI and Alzheimer’s disease (AD) (Shinjyo et al., 2020; Akbar et al., 2021). However, there is no meta-analysis based on preclinical studies to synthesize the evidence relating to berberine for the treatment of cognitive dysfunction in diabetes. This study aimed to conduct a systematic review and meta-analysis to analyze the neuroprotective effect and mechanisms of berberine on DCI by pooling relevant animal studies.
2 Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) was followed to design and conduct the current systematic review and meta-analysis (Page et al., 2021).
2.1 Search Strategy
The databases of PubMed, Web of Science, Embase, China National Knowledge Infrastructure (CNKI), Wanfang Data Knowledge Service Platform, and VIP Periodical Service Platform were searched from database inception until March 2022. The search had no language restrictions. The combination of MeSH and free-text terms was used to identify the disease, intervention drug, and animals: [(“Diabetes Mellitus” OR “Diabetes, cognitive impairment” OR “Diabetes, cognitive dysfunction”) AND (“Berberine” OR “Umbellatine”) AND (“Animals” OR “Animal model” OR “Rat” OR “Mice”)].
2.2 Inclusion and Exclusion Criteria
The pre-determined inclusion criteria were as follows: 1) model: DM was selected as the research model. The success criterion of the models was blood glucose (BG) ≥ 11.1 mmol/L. 2) Intervention: the treatment group only received any dose of berberine. The control group was administered with an equal dose volume of nonfunctional sterile liquids or no treatment. 3) Outcome: the primary outcomes were fasting blood glucose (FBG) and behavioral tests. Behavioral tests were used to assess animal learning and memory function, such as the Morris water maze test (MWM), Y-maze test, passive avoidance test, and conditioned fear test. The secondary outcomes were serum insulin, hemoglobin A1c (HbA1c), 2 h blood glucose (2hBG) of oral glucose tolerance test (OGTT), 2hBG of insulin tolerance test (ITT), and the related mechanisms of berberine against DCI. The exclusion criteria were as follows: 1) experimental studies unrelated to impairment of learning, memory, and cognitive function; 2) no control group or treatment group without berberine or combined with other intervention; 3) non-in vivo studies; 4) no full texts, and 5) duplicate data or publications.
2.3 Data Extraction
All retrieved literature was imported into EndNote X9 and eliminated duplicates. Two researchers (L.J.X and W.S.F) independently performed literature collection based on the inclusion and exclusion criteria. First, the title and abstract were screened to exclude irrelevant articles. Second, the remaining articles were reviewed by obtaining the full text. The following details were extracted from selected studies: 1) name of first author and year of publication; 2) characteristics of experimental animals, including animal species, sex, sample size, age, and weight; 3) modeling methods and the criteria for modeling successfully; 4) drug intervention, including administration method, drug dose, and duration of treatment; and 5) outcome indicators and intergroup differences, including blood glucose index, cognitive assessment, and mechanism of action. We created a database and manually extracted data from the included literature. If the results were shown only by graphs, we tried to contact the author for more information. The graph data were quantified by WebPlotDigitizer 4.5 software (https://automeris.io/WebPlotDigitizer) if there was no response. For the results obtained at multiple time points, only the data of the last time point before the animals were sacrificed were extracted. If the drug involved several doses in the treatment group, we only extracted data for the highest dose.
2.4 Risk of Bias Assessment
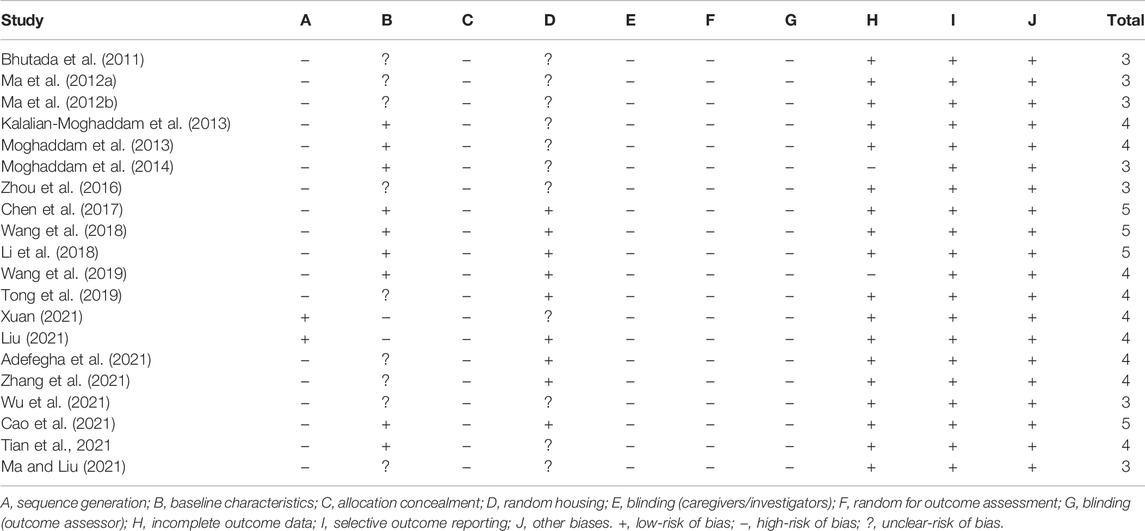
We used the SYRCLE risk of bias tool based on the Cochrane risk of bias tool to assess the methodological quality of the included studies (Hooijmans et al., 2014). The tool mainly covers selection bias, performance bias, detection bias, reporting bias, and other biases. The maximum score is 10 for each study. Any divergences in the process of quality assessment were finally resolved by consultation with the correspondence author.
2.5 Data Synthesis and Analysis
The statistical analysis was performed using STATA software version 15.0. The outcome of each indicator was considered a continuous variable, and the combined overall effect sizes of outcome were expressed using the standard mean difference (SMD) and 95% confidence interval (CI). It is considered that there is a significant difference between the treatment group and the control group when p < 0.05. I-square (I2) was used to evaluate statistical heterogeneity. The fixed effects model was used to combine the effect size when I2 ≤ 50%, and the random effects model was used to combine the effect size when I2 > 50%. In order to recognize the source of heterogeneity among included studies, subgroup analysis based on year of publication, animal species, the dose of STZ (≤ 40 and > 40 mg/kg), dose of berberine (≤ 100 and > 100 mg/kg), and duration of treatment (≤ 8 and > 8 weeks) were conducted. Then, sensitivity analysis was performed to evaluate the stability of the overall results. Potential publication bias was assessed using Egger’s test if the number of included datasets was 10 or higher. Moreover, the trim and fill method was performed in the presence of publication bias.
3 Results
3.1 Study Inclusion
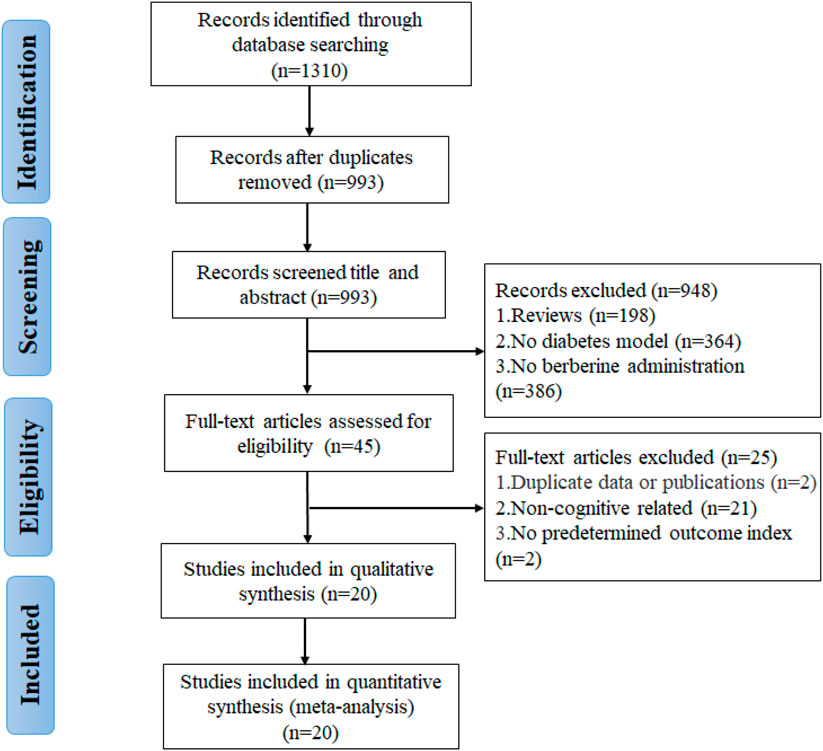
A total of 1,310 potentially relevant articles were found through database searching, including 283 from PubMed, 97 from Web of Science, 179 from Embase, 279 from CNKI, 284 from Wanfang, and 188 from VIP. After incorporating all searches and removing duplicates, 993 records are retained. Among the remaining articles, 948 records were eliminated by reading the titles and abstracts. Finally, through the full-text assessment, 20 studies met the eligibility requirements and were included in this systematic review and meta-analysis (Figure 2). All included studies were published during the past decade (2011–2021), indicating that the neuroprotective effects of berberine on DCI have attracted intense interest in recent years.
3.2 Characteristics of Included Studies
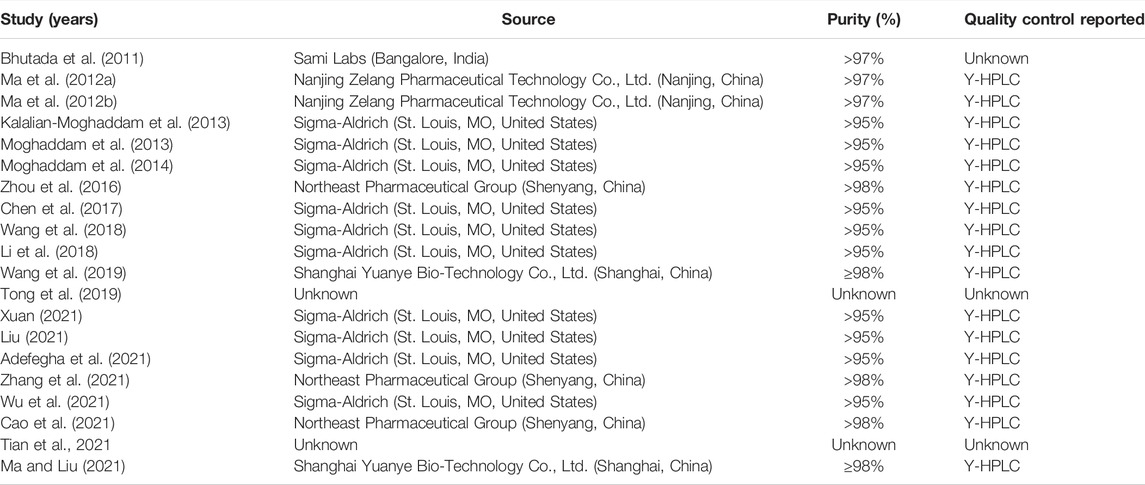
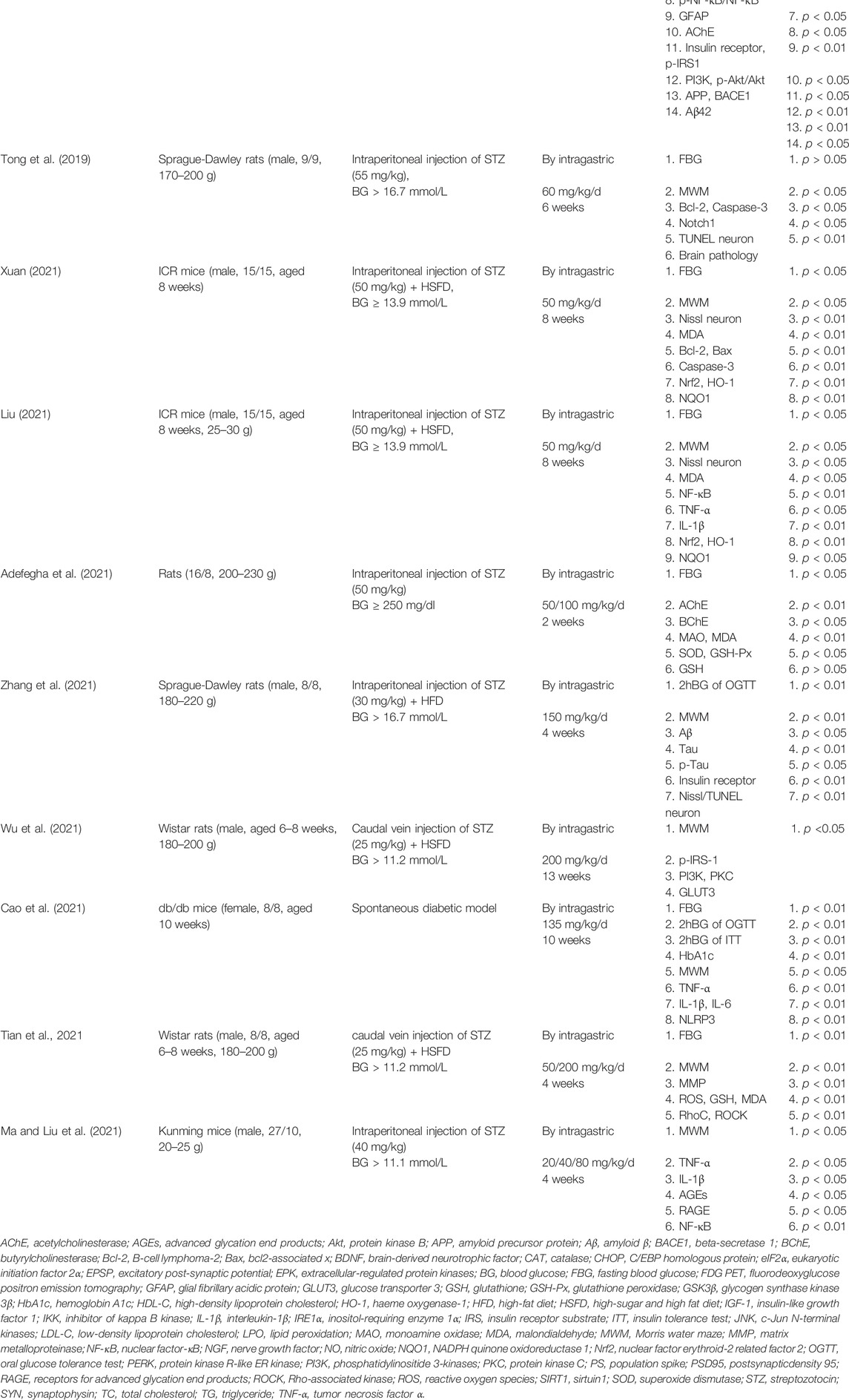
In the 20 included studies, 13 studies were published in English (Bhutada et al., 2011; Kalalian-Moghaddam et al., 2013; Moghaddam et al., 2013; Moghaddam et al., 2014; Zhou et al., 2016; Chen et al., 2017; Li et al., 2018; Wang et al., 2018; Wang et al., 2019; Adefegha et al., 2021; Tian et al., 2021; Wu et al., 2021; Zhang et al., 2021) and 7 studies were published in Chinese (Ma et al., 2012a; Ma et al., 2012b; Tong et al., 2019; Cao et al., 2021; Liu, 2021; Ma and Liu, 2021; Xuan, 2021). A total of 442 diabetic model animals were enrolled, including 278 in the treatment group and 164 in the control group. Ten studies used Wistar rats (268/442, 60.6%), four used Sprague Dawley rats (82/442, 18.6%), two used db/db mice (32/442, 7.2%), two used ICR mice (60/442, 13.6%), one used the Kunming mice (36/442, 8.1%), and one did not specify which species of rats were used. All the animal models included in the studies were rats or mice. Seventeen studies used male animals, two used female animals, and one did not specify the sex of the animals. The initial weight of animals was 170–220 g in 11 studies, 200–230 g in 1 study, 225–285 g in 3 studies, 20–30 g in 2 studies, and not mentioned in 3 studies. Only eight studies reported the age of animals, ranging from 4 to 10 weeks. In the preparation of diabetes models, seven studies established animal models by intraperitoneal injection of streptozotocin (STZ) only, and the dose range for STZ injections was 40–60 mg/kg (Bhutada et al., 2011; Kalalian-Moghaddam et al., 2013; Moghaddam et al., 2013; Moghaddam et al., 2014; Tong et al., 2019; Adefegha et al., 2021; Ma and Liu, 2021). Six studies were to induced diabetes models by intraperitoneal injection of STZ combined with a high-sugar and high-fat diet (HSFD) or high-fat diet (HFD), and the dose range of STZ was 30–50 mg/kg (Ma et al., 2012a; Ma et al., 2012b; Zhou et al., 2016; Liu, 2021; Xuan, 2021; Zhang et al., 2021). Five studies were to induce diabetes models by caudal vein injection of STZ (25 mg/kg) combined with HSFD or HFD (Chen et al., 2017; Wang et al., 2018; Wang et al., 2019; Tian et al., 2021; Wu et al., 2021). Two studies were spontaneous diabetes models (Li et al., 2018; Cao et al., 2021). All studies used FBG > 11.1 mmol/L or 2hBG > 11.1 mmol/L of OGTT as the standard to evaluate model success. The maximum duration of administration was 23 weeks (range, 2–23 weeks), and the maximum dose of berberine was 200 mg/kg/d (range, 20–200 mg/kg/d). In order to assess the effect of berberine on blood glucose in diabetic models, 15 studies recorded the FBG level (Bhutada et al., 2011; Ma et al., 2012a; Ma et al., 2012b; Kalalian-Moghaddam et al., 2013; Moghaddam et al., 2013; Moghaddam et al., 2014; Zhou et al., 2016; Wang et al., 2018; Tong et al., 2019; Wang et al., 2019; Adefegha et al., 2021; Cao et al., 2021; Liu, 2021; Tian et al., 2021; Xuan, 2021), 3 studies recorded serum insulin (Li et al., 2018; Wang et al., 2018; Wang et al., 2019), 3 studies recorded the OGTT level (Wang et al., 2019; Cao et al., 2021; Zhang et al., 2021), 2 studies recorded the ITT level (Li et al., 2018; Cao et al., 2021), and 1 study recorded the HbA1c level (Zhou et al., 2016). Behavioral tests were used to evaluate the learning and memory function of animals. For further assessment of the neuroprotective effect of berberine on DCI model animals, 14 studies conducted the MWM test (Bhutada et al., 2011; Ma et al., 2012b; Zhou et al., 2016; Li et al., 2018; Wang et al., 2018; Tong et al., 2019; Wang et al., 2019; Cao et al., 2021; Liu, 2021; Ma and Liu, 2021; Tian et al., 2021; Wu et al., 2021; Xuan, 2021; Zhang et al., 2021), 2 studies conducted the Y-maze test (Kalalian-Moghaddam et al., 2013; Li et al., 2018), 2 studies conducted the conditioned fear test (Chen et al., 2017; Li et al., 2018), and 1 study conducted the passive avoidance test (Kalalian-Moghaddam et al., 2013). Detailed information on berberine in each study is displayed in Table 1. The characteristics and details of the included studies are presented in Table 2.
3.3 Quality of Included Studies
We evaluated the quality of all the included studies. The scores ranged from 3 to 5 for each study, and the overall quality was low. Four studies got 5 points (Chen et al., 2017; Li et al., 2018; Wang et al., 2018; Cao et al., 2021), nine studies got 4 points (Kalalian-Moghaddam et al., 2013; Moghaddam et al., 2013; Tong et al., 2019; Wang et al., 2019; Adefegha et al., 2021; Liu, 2021; Tian et al., 2021; Xuan, 2021; Zhang et al., 2021), and seven studies got 3 points (Bhutada et al., 2011; Ma et al., 2012a; Ma et al., 2012b; Moghaddam et al., 2014; Zhou et al., 2016; Ma and Liu, 2021; Wu et al., 2021). Among the 20 included studies, only two studies reported random sequence generation (Liu, 2021; Xuan, 2021), and nine studies fully reported the baseline characteristics (Kalalian-Moghaddam et al., 2013; Moghaddam et al., 2013; Moghaddam et al., 2014; Chen et al., 2017; Li et al., 2018; Wang et al., 2018; Wang et al., 2019; Cao et al., 2021; Tian et al., 2021), and nine studies described randomness of housing (Chen et al., 2017; Li et al., 2018; Wang et al., 2018; Tong et al., 2019; Wang et al., 2019; Adefegha et al., 2021; Cao et al., 2021; Liu, 2021; Zhang et al., 2021). Allocation concealment, blind intervention, random for outcome evaluation, and blinding for outcomes were not reported in any studies. The methodological quality of the included studies is displayed in Table 3.
3.4 Effectiveness
3.4.1 Primary Outcomes
3.4.1.1 Effect of Berberine on Fasting Blood Glucose
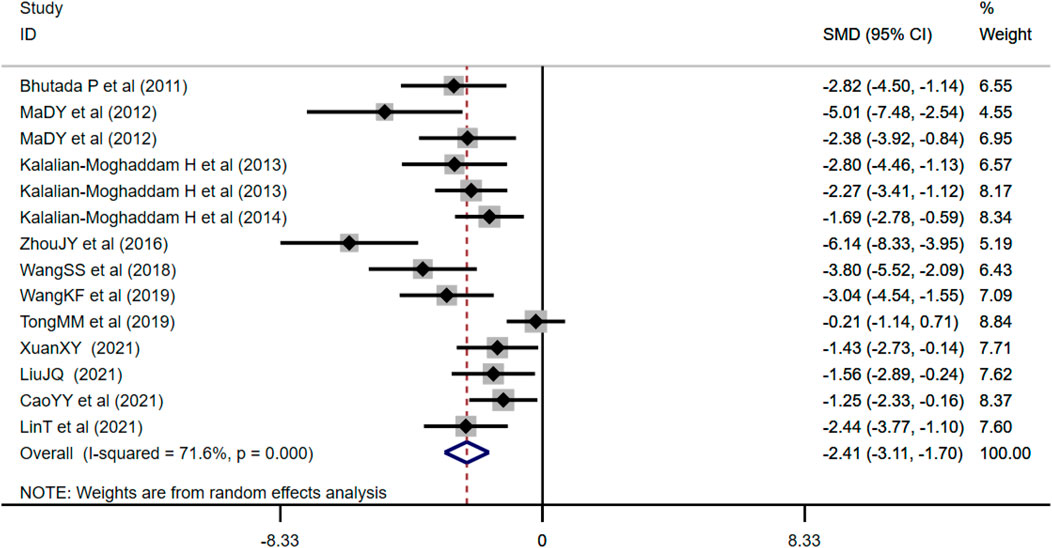
FBG was adopted as the outcome measure in 14 studies and used random effects analysis combined with an effect size. The overall outcome showed berberine could reduce FBG level compared with the control group (n = 212; SMD: −2.41 [95% CI: −3.11, −1.70], p < 0.001; heterogeneity: χ2 = 45.84, df = 13, p < 0.001, I2 = 71.6%, Figure 3).
3.4.1.2 Effect of Berberine on Morris Water Maze Test
As one of the primary outcome measures, the MWM could reflect changes in learning memory of diabetic animals. Among the 14 studies included, 12 studies reported the escape latency, 7 studies reported times of platform crossing, and 9 studies reported the time spent in the target quadrant. Compared with the control group, berberine treatment group could significantly shorten escape latency (n = 184; SMD: −2.59 [95% CI: −3.48, −1.70], p < 0.001; heterogeneity: χ2 = 51.53, df = 11, p < 0.001, I2 = 78.7%, Figure 4A). Compared with the control group, the times of crossing the platform increased in the berberine treatment group (n = 102; SMD: 2.05 [95% CI: 1.20, 2.90], p < 0.001; heterogeneity: χ2 = 16.61, df = 6, p = 0.011, I2 = 63.9%, Figure 4B). Compared with the control group, the time spent in the target quadrant increased in the berberine treatment group (n = 134; SMD: 2.28 [95% CI: 1.49, 3.06], p < 0.001; heterogeneity: χ2 = 23.17, df = 8, p = 0.003, I2 = 65.5%, Figure 4C).
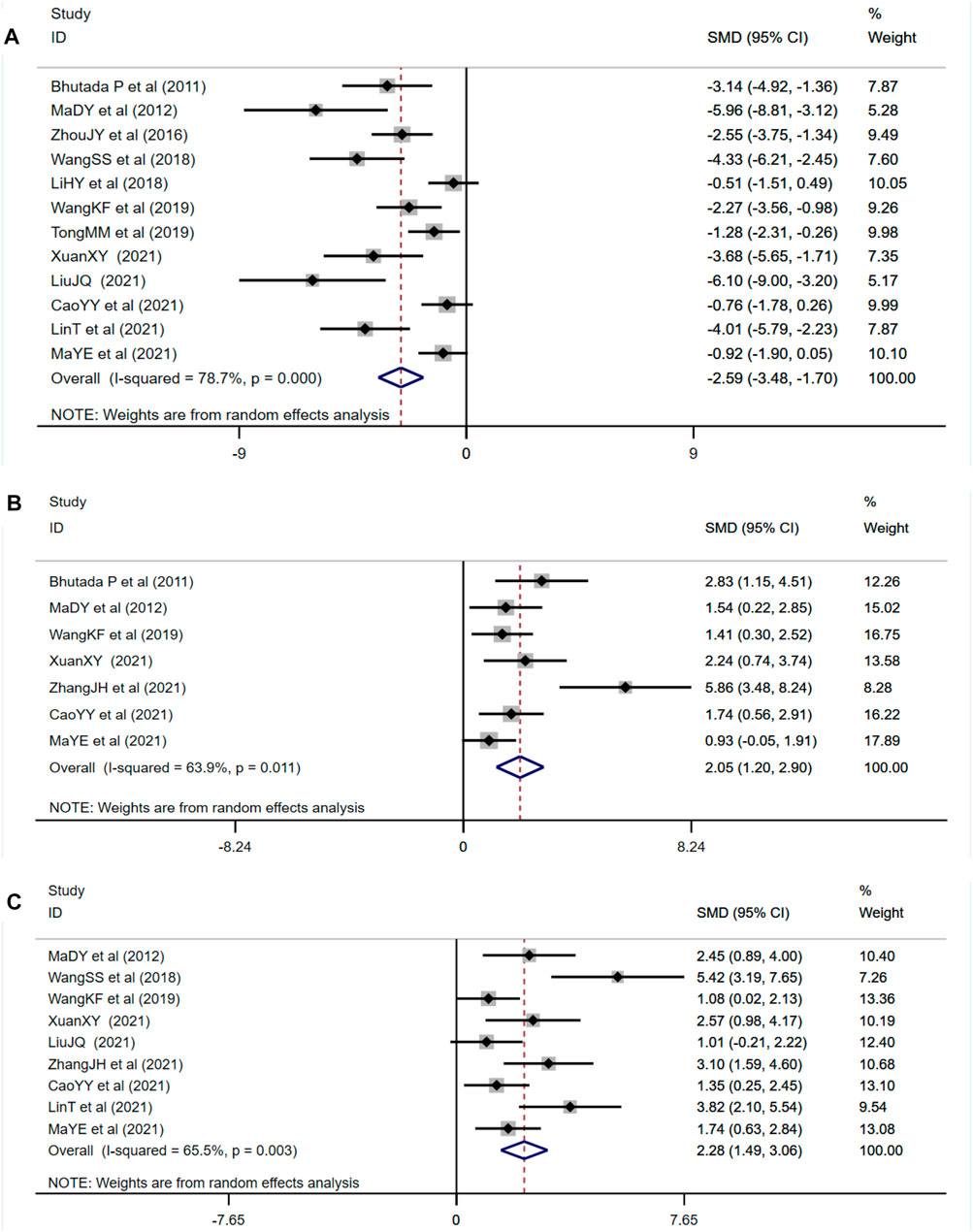
FIGURE 4. Forest plot: effect of berberine on (A) escape latency, (B) times of crossing the platform, and (C) time spent in the target quadrant.
3.4.2 Secondary Outcomes
3.4.2.1 Effect of Berberine on Serum Insulin
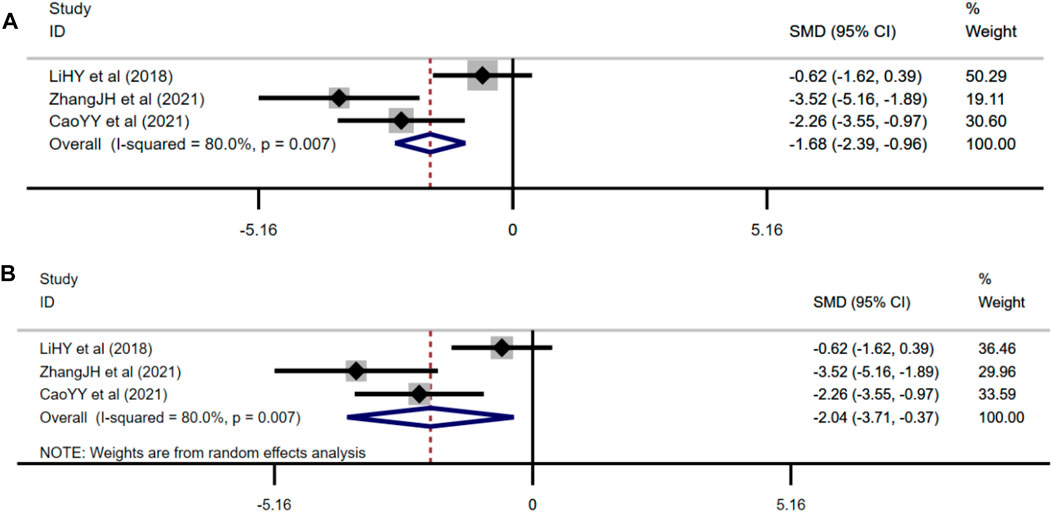
Three studies adopted serum insulin as one of the outcome measures and used fixed effects model combined with an effect size. The results indicated berberine could reduce serum insulin level compared with the control group (n = 48; SMD: −2.14 [95% CI: −2.88, −1.40], p < 0.001; heterogeneity: χ2 = 2.52, df = 2, p = 0.284, I2 = 20.6%, Figure 5A).
3.4.2.2 Effect of Berberine on 2hBG of Oral Glucose Tolerance Test
Three studies used 2hBG of OGTT as one of the outcome measures and used random effects analysis combined with an effect size. The overall outcome showed berberine could reduce 2hBG of the OGTT level compared with the control group (n = 48; SMD: −2.04 [95% CI: −3.71, −0.37], p = 0.016; heterogeneity: χ2 = 10, df = 2, p = 0.007, I2 = 80%, Figure 5B).
3.4.2.3 Effect of Berberine on Amyloid β
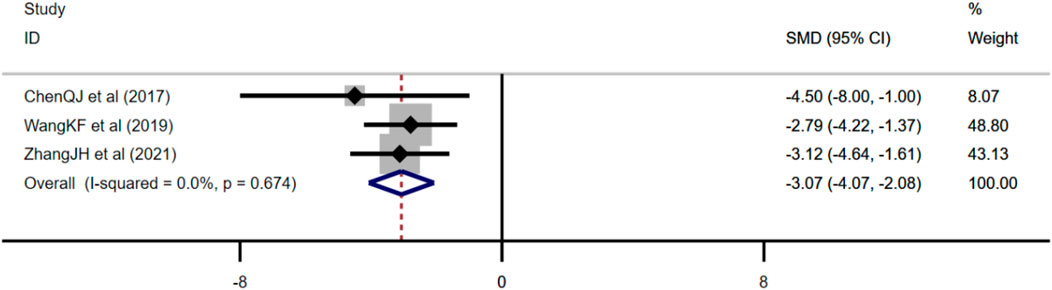
Three studies adopted Aβ as an outcome measure and used fixed effects model combined with an effect size. The results indicated berberine could reduce the Aβ level compared with the control group (n = 38; SMD: −3.07 [95% CI: −4.07, −2.08], p < 0.001; heterogeneity: χ2 = 0.79, df = 2, p = 0.674, I2 = 0, Figure 6).
3.4.2.4 Effect of Berberine on Oxidative Stress Index
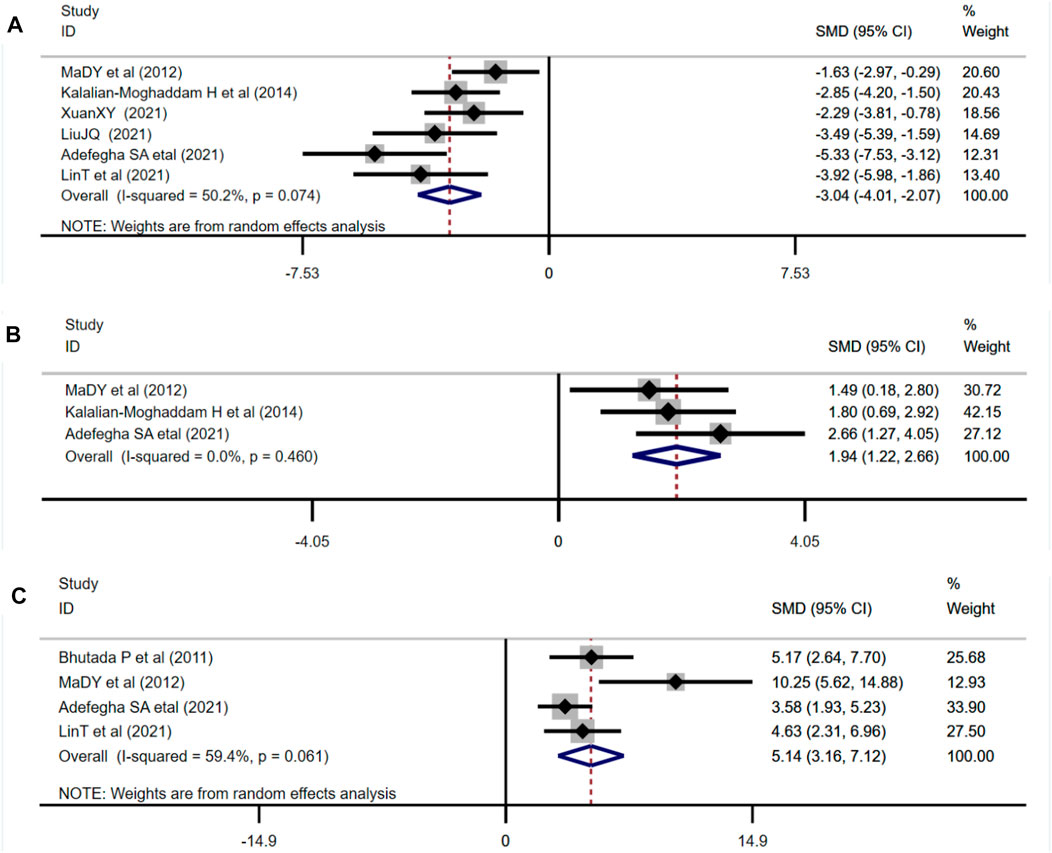
Six studies adopted MDA as an outcome measure and used random effects analysis combined with an effect size. The results indicated berberine could reduce the MDA level compared with the control group (n = 82; SMD: −3.04 [95% CI: −4.01, −2.07], p < 0.001; heterogeneity: χ2 = 10.05, df = 5, p = 0.074, I2 = 50.2%, Figure 7A). Three studies adopted SOD as an outcome measure and used fixed effects model combined with an effect size. The results indicated berberine could increase SOD level compared with the control group (n = 46; SMD: 1.94 [95% CI: 1.22, 2.66], p < 0.001; heterogeneity: χ2 = 1.55, df = 2, p = 0.460, I2 = 0, Figure 7B). Four studies adopted GSH as an outcome measure and used random effects analysis combined with an effect size. The results indicated berberine could increase GSH level compared with the control group (n = 52; SMD: 5.14 [95% CI: 3,16, 7.12], p < 0.001; heterogeneity: χ2 = 7.38, df = 3, p = 0.061, I2 = 59.4%, Figure 7C).
3.4.2.5 Effect of Berberine on Inflammation Levels
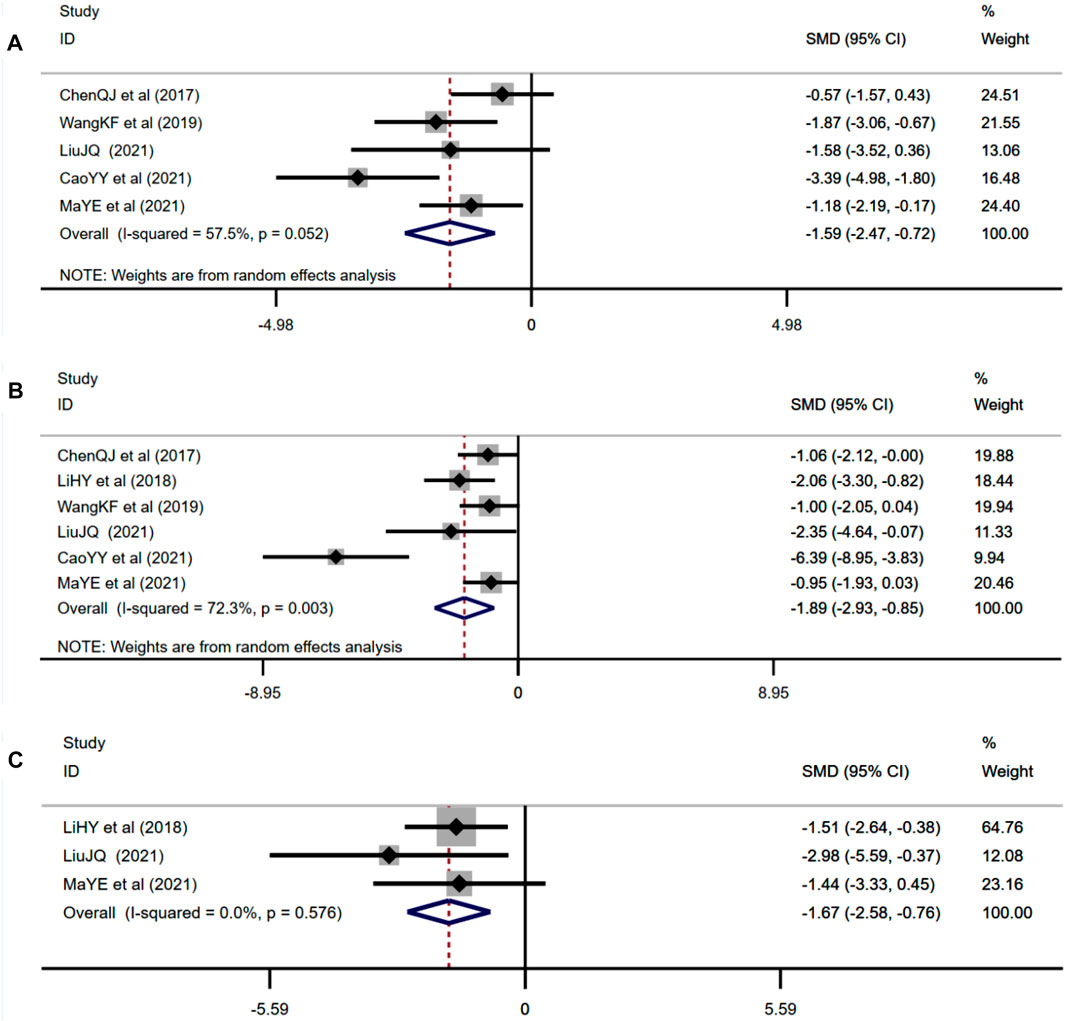
Five studies adopted IL-1β as an outcome measure and used random effects analysis combined with an effect size. The results indicated berberine could reduce the IL-1β level compared with the control group (n = 72; SMD: −1.59 [95% CI: −2.47, −0.72], p < 0.001; heterogeneity: χ2 = 9.41, df = 4, p = 0.052, I2 = 57.5%, Figure 8A). Six studies adopted TNF-α as an outcome measure and used random effects analysis combined with an effect size. The results indicated berberine could reduce the TNF-α level compared with the control group (n = 88; SMD: −1.89 [95% CI: −2.93, −0.85], p < 0.001; heterogeneity: χ2 = 18.02, df = 5, p = 0.003, I2 = 72.3%, Figure 8B). Three studies adopted NF-κB as an outcome measure and used the fixed effects model combined with an effect size. The results indicated berberine could reduce the NF-κB level compared with the control group (n = 28; SMD: −1.67 [95% CI: −2.58, −0.76], p < 0.001; heterogeneity: χ2 = 1.10, df = 2, p = 0.576, I2 = 0, Figure 8C).
3.4.2.6 Effect of Berberine on Acetylcholinesterase
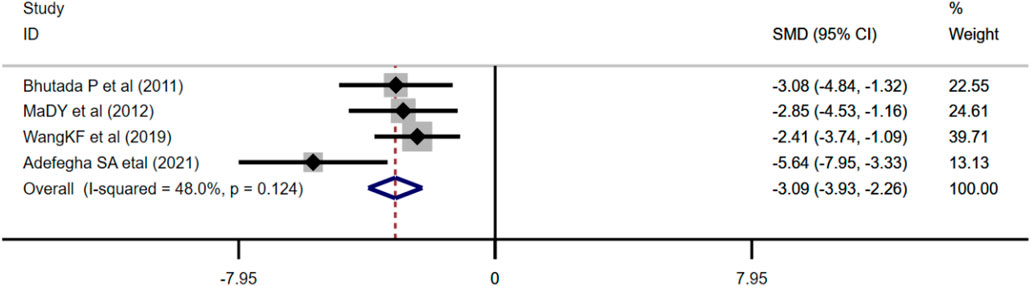
Four studies adopted AChE as an outcome measure and used the fixed effects model combined with an effect size. The results indicated berberine could reduce the AChE level compared with the control group (n = 76; SMD: −3.09 [95% CI: −3.93, −2.26], p < 0.001; heterogeneity: χ2 = 5.76, df = 3, p = 0.124, I2 = 48%, Figure 9).
3.5 Subgroup Analysis
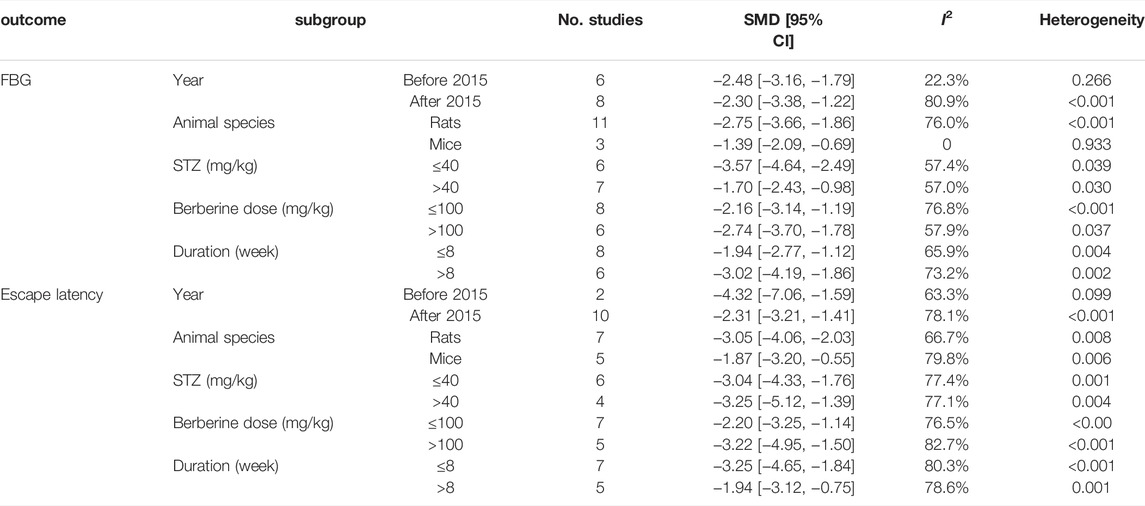
Due to the high heterogeneity among the studies, we evaluated five subgroups for FBG and escape latency depending on the year of publication, animal species, the dose of STZ, the dose of berberine, and the duration of treatment. The results showed that animal species and year of publication could be the source of heterogeneity for FBG. For escape latency, the subgroup analysis based on year of publication, animal species, dose of STZ, dose of berberine, and duration of treatment did not reveal the sources of heterogeneity among the studies. There was still significant heterogeneity among studies. The results are presented in Table 4.
3.6 Sensitivity Analysis
After excluding each trial in turn from the meta-analysis, the results remained consistent with the overall effect size for the effect of berberine supplementation on FBG and escape latency. Although there was no significant effect of any single study on the combined effect size of FBG and escape latency, sensitivity analysis of FBG revealed that the overall effect size was (SMD: −2.15, 95% CI [−2.77, −1.54]) and (SMD: −2.56, 95% CI [−3.19, −1.93]) after omitting ZhouJY et al. and TongMM et al., respectively. For escape latency, omitting any single study had no significant effect on the overall effect size.
3.7 Publication Bias
According to the results of Egger’s test, there was a significant publication bias for FBG and escape latency of MWM (PEgger < 0.0001). Asymmetry was then corrected using the trim and fill method, with imputing five studies that may have been missed for FBG and escape latency, respectively. The trim and fill analysis indicated that these missed studies did not obviously change the magnitude of the overall pooled effect size. The results from Egger’s test and trim and fill analysis are presented in Table 5.
4 Discussion
In this systematic review and meta-analysis, we evaluated the effects and mechanisms of berberine on DCI model animals. A total of 20 studies involving 422 animals were included. According to the results derived from the meta-analysis, berberine could significantly reduce FBG levels and improve learning and memory abilities. The mechanisms underlying the effects of berberine on cognitive function in animals with DCI include regulating glucose and lipid metabolism, improving insulin resistance, anti-oxidative stress, anti-neuroinflammation, inhibiting ER stress, and protecting the cholinergic system. We further conducted a subgroup analysis of the primary outcomes, FBG, and escape latency. The results of the subgroup analysis indicated that animal species and year of publication could be the sources of heterogeneity for FBG. However, because of the small sample sizes, these findings should be prudently interpreted, and more studies are needed to provide more precise evidence. Escape latency, animal species, dose of berberine, duration of treatment, dose of STZ, and year of publication may not be the sources of heterogeneity among studies. Therefore, we speculate that the heterogeneity probably resulted from other differences in the studies, including the design of the research scheme, the standard of successful modeling, characteristics of the sample, and the experimental sample size.
The behavioral test MWM has been widely applied to evaluate the cognitive and behavioral abilities of rodents (Vorhees and Williams, 2006), which usually consists of two parts: the learning trials and the probe trials. Escape latency, defined as the time required to arrive at the platform, is the main content of learning trials. In the probe trials, times of platform crossing and time spent in the target quadrant are used to assess memory ability after removing the platform. Studies have shown that, in the diabetic model animals, the escape latency was significantly prolonged and times of platform crossing and the time spent in the target quadrant were reduced. It was implied that the cognitive function and spatial memory of diabetic model animals were impaired. While berberine supplementation obviously shortened the escape latency, the times of crossing the platform and the time in the target quadrant increased, which indicated that berberine had good neuroprotective potential.
Hyperglycemia is the hallmark of T2DM and the long-term hyperglycemia may have toxicities on neurons through the formation of advanced glycation end products (AGEs) (Lovestone and Smith, 2014). AGEs are products of reactions between proteins or lipids with sugars and accumulate rapidly with aging. Additionally, AGEs can bind to the receptor for advanced glycation end products (RAGE) to produce reactive oxygen species (ROS), which could cause oxidative stress in neurons, activate the p38 mitogen-activated protein kinase (MAPK) pathway, upregulate the NF-κB expression, and lead to an inflammatory response (Adhikary et al., 2004). Studies have demonstrated that berberine can significantly reduce FBG, 2hBG of OGTT, and 2hBG of ITT, thereby reducing the production of AGEs. In addition, berberine can also downregulate the expression of RAGE and p38 MAPK and reduce oxidative stress and inflammatory damage. Insulin resistance (IR) is an important pathogenesis of T2DM. In the central nervous system (CNS), insulin, insulin-like growth factor 1 (IGF-1), and their associated receptors are highly concentrated within the hippocampus, hypothalamus, and cortex, and their combination leads to IR substrate protein (IRS-1) phosphorylation (Bedse et al., 2015). However, chronic peripheral hyperinsulinemia downregulates blood–brain barrier insulin receptors and decreases the amount of insulin transported into the CNS (Kellar and Craft, 2020). Then, the IRS-activated phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) pathway is inhibited, and the activity of glycogen synthase kinase (GSK-3β) in downstream sites of the pathway is elevated, thus affecting the transport of glucose transporter 3 (GLUT3) into the brain, which impairs amyloid clearance and results in cognitive decline (Zaulkffali et al., 2019; Leao et al., 2020). Current studies suggested that the serum insulin index of experimental diabetic animals decreased significantly, and the phosphorylation levels of PI3K/Akt/GSK-3β were reversed after berberine supplementation. Berberine could promote glucose uptake and metabolism in the brain by upregulating the expression of GLUT3. However, only one study reported berberine elevated central insulin levels compared with diabetic model animals (Wang et al., 2019). Therefore, it may be difficult to determine the effects of berberine on central insulin levels.
Lipid metabolism plays an important role in maintaining the structure and function of brain neurons. DM patients are usually accompanied by lipid dysmetabolism, which can induce the occurrence of neurodegenerative diseases, such as AD (Chornenkyy et al., 2019). The brain is one of the most lipid-rich organs, and the increase in total cholesterol (TC) is a significant risk factor for cognitive impairment (Anstey et al., 2017; Chornenkyy et al., 2019). Studies demonstrated that high levels of cholesterol modulate Aβ levels by affecting the activities of beta-secretase 1 (BACE1) (Liu et al., 2007; Beel et al., 2010). Experimental data suggested that berberine can ameliorate lipid metabolism by reducing the levels of TC, triglyceride (TG), and low-density lipoprotein cholesterol (LDL-C) and accelerate the clearance of APP, Aβ, and BACE1 in the brain.
Oxidative stress is closely related to the pathogenesis of DCI. When the body is exposed to harmful stimulation, ROS are overproduced. It causes lipid peroxidation of nerve cells and impairs the ability of antioxidant damage. Multiple studies showed that the levels of ROS and MDA increased significantly while the levels of GSH and SOD decreased significantly in the hippocampi of diabetic animals (Ma et al., 2012b; Moghaddam et al., 2014; Adefegha et al., 2021; Tian et al., 2021). Meanwhile, nuclear factor erythroid-2 related factor 2 (Nrf2) plays a pivotal defensive role against oxidative stress via eliciting the transcription of anti-oxidative genes encoding heme oxygenase-1 (HO-1) and NADPH quinine oxidoreductase 1 (NQO1) (Ghanim et al., 2021). This study found that berberine can reduce oxidative stress by activating the Nrf2/HO-1/NQO1 pathway. Moreover, the inflammatory response is present throughout the development of diabetes and its complications. It is generally thought that inflammation causes neurodegeneration because microglia and astrocytes in the hippocampus are activated and release large amounts of inflammatory factors (Kaur et al., 2019). In the physiological state, microglia and astrocytes are inactive and only release a small amount of cytokines. Once stimulated by hyperglycemia, neuroglia are activated rapidly and release many pro-inflammatory and cytotoxic factors, leading to the activation of MAPK and AGEs/RAGE/NF-κB signal pathways (Tobon-Velasco et al., 2014; Liu Y. et al., 2018). Besides, chronic inflammation will change the permeability of the blood–brain barrier, resulting in Aβ deposition and tau protein phosphorylation, which further exacerbates inflammatory response and synaptic damage (Michalicova et al., 2020; McLarnon, 2021). Berberine can regulate the expression of AGEs, RAGE, and NF-κB; reduce the release of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6; and attenuate Aβ deposition and p-tau protein expression to delay cognitive impairment. Moreover, the occurrence and development of various inflammatory responses are closely correlated to endoplasmic reticulum (ER) stress, which is considered the pathogenic factor of many chronic neurodegenerative diseases (Zhang et al., 2015; Liu et al., 2020). It is reported that high glucose increases ER stress levels, and activation of ER stress pathway could participate in diabetes-induced neuronal apoptosis and cognitive decline (Kong et al., 2018; Escribano-Lopez et al., 2019). The specific mechanism is as follows: ER stress induces the dissociation of protein kinase R-like ER kinase (PERK), inositol-requiring enzyme 1α (IRE1α), and glucose-regulated protein 78 (GRP78), further activating the c-Jun N-terminal kinase (JNK) and PERK signaling pathway. In addition, PERK phosphorylates its substrate eukaryotic translation initiation factor 2α (eIF2α), then activating pro-apoptotic factor C/EBP homologous protein (CHOP), which eventually leads to neuronal apoptosis (Ettcheto et al., 2019). Our findings showed that berberine can decrease the expression of IRE1α, CHOP, and p-PERK by affecting the signal transduction of PERK and regulate the neuronal apoptosis by inhibiting the expression of ER stress to improve the ability of learning and memory of diabetic animals.
In addition to the above, the disorder of the central cholinergic nervous system is one of the important causes of DCI. The cholinergic system of CNS is involved in memory, and acetylcholine (ACh) is the main neurotransmitter responsible for learning and memory in the CNS. The expression of acetylcholinesterase (AChE) is partly regulated by insulin. Under the condition of long-term IR, the activity of AChE in the hippocampus is significantly enhanced (Hamed, 2017; Kim et al., 2017). Our data suggested that berberine can prevent the damage to the cholinergic system by decreasing the level of AChE, thereby improving the ability to learn and memory.
In the current review, we included all of the conducted meta-analyses focusing on the effects of berberine on blood glucose, cognitive function, and relevant mechanisms. However, there are some limitations of this study that should be noted. First, in terms of the quality of studies, none of the studies have described the method used to conceal the allocation sequence, blind intervention, and random outcome assessment. Some studies did not provide detailed baseline characteristics. In addition, given that the methodological quality of some studies was not sufficient to make definitive judgments about the risk of bias, the results of this study should be interpreted with caution, and more high-quality studies will be needed in the future. Second, the high degree of heterogeneity in the combined results reduces the reliability of the results (Prady et al., 2014). Although we attempted to explore potential sources of heterogeneity using our prespecified subgroup analysis, this did not appear to work well. The different study protocols and intervention processes in animal experiments may be key potential factors contributing to high heterogeneity. Therefore, we suggest that, in future studies, researchers should pay more attention to the rigor of research design and provide adequate information for experimental research. Third, because available data for some indicators existed only in individual studies, we did not conduct further meta-analysis on the relevant indicators. These indicators will require more attention in the future. Obviously, much work still needs to be done to adequately address the shortcomings of the available literature.
5 Conclusion
The present meta-analysis revealed that berberine could lower blood glucose levels and improve learning and memory abilities. The underlying mechanisms of these protective effects include regulating glucose and lipid metabolism, improving insulin resistance, anti-oxidation, anti-neuroinflammation, inhibiting ER stress, and protecting the cholinergic system. However, the validity of the research results may be undermined by the low methodological quality and publication bias in the included studies. More rigorous experiment designs and more comprehensive studies are required to test the protection of berberine against DCI in the future.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
BL and YH designed the whole research. YH and SY formulated the search strategy and searched the databases. JL and SW screened the articles and extracted the data. JL and YH completed data analysis and quality assessment. YH and SH contributed to manuscript drafting. SY revised the manuscript carefully.
Funding
This work was supported by the National Natural Science Foundation of China 82174345) and the Sichuan Department of Science and Technology Programs (2021YJ0435).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adefegha, S. A., Dada, F. A., Oyeleye, S. I., and Oboh, G. (2021). Effects of Berberine on Cholinesterases and Monoamine Oxidase Activities, and Antioxidant Status in the Brain of Streptozotocin (STZ)-induced Diabetic Rats. J. Basic Clin. Physiol. Pharmacol. 32, 1–9. doi:10.1515/jbcpp-2020-0173
Adhikary, L., Chow, F., Nikolic-Paterson, D. J., Stambe, C., Dowling, J., Atkins, R. C., et al. (2004). Abnormal P38 Mitogen-Activated Protein Kinase Signalling in Human and Experimental Diabetic Nephropathy. Diabetologia 47 (7), 1210–1222. doi:10.1007/s00125-004-1437-0
Akbar, M., Shabbir, A., Rehman, K., Akash, M. S. H., and Shah, M. A. (2021). Neuroprotective Potential of Berberine in Modulating Alzheimer's Disease via Multiple Signaling Pathways. J. Food Biochem. 45 (10), e13936. doi:10.1111/jfbc.13936
Anstey, K. J., Ashby-Mitchell, K., and Peters, R. (2017). Updating the Evidence on the Association between Serum Cholesterol and Risk of Late-Life Dementia: Review and Meta-Analysis. J. Alzheimers Dis. 56 (1), 215–228. doi:10.3233/JAD-160826
Bedse, G., Di Domenico, F., Serviddio, G., and Cassano, T. (2015). Aberrant Insulin Signaling in Alzheimer's Disease: Current Knowledge. Front. Neurosci. 9, 204. doi:10.3389/fnins.2015.00204
Beel, A. J., Sakakura, M., Barrett, P. J., and Sanders, C. R. (2010). Direct Binding of Cholesterol to the Amyloid Precursor Protein: An Important Interaction in Lipid-Alzheimer's Disease Relationships? Biochim. Biophys. Acta 1801 (8), 975–982. doi:10.1016/j.bbalip.2010.03.008
Bhutada, P., Mundhada, Y., Bansod, K., Tawari, S., Patil, S., Dixit, P., et al. (2011). Protection of Cholinergic and Antioxidant System Contributes to the Effect of Berberine Ameliorating Memory Dysfunction in Rat Model of Streptozotocin-Induced Diabetes. Behav. Brain Res. 220 (1), 30–41. doi:10.1016/j.bbr.2011.01.022
Biessels, G. J., and Despa, F. (2018). Cognitive Decline and Dementia in Diabetes Mellitus: Mechanisms and Clinical Implications. Nat. Rev. Endocrinol. 14 (10), 591–604. doi:10.1038/s41574-018-0048-7
Biessels, G. J., and Reijmer, Y. D. (2014). Brain Changes Underlying Cognitive Dysfunction in Diabetes: what Can We Learn from MRI? Diabetes 63 (7), 2244–2252. doi:10.2337/db14-0348
Cao, Y. Y., Meng, X. B., Sun, G. B., Sun, X. B., Sun, Y., Zhao, X., et al. (2021). Metformin Plus Berberine Alleviates Cognitive Dysfunction in Db/db Mice. Chin. J. New Drugs 30 (8), 690–700. doi:10.3969/j.issn.1003-3734.2021.08.004
Chen, Q., Mo, R., Wu, N., Zou, X., Shi, C., Gong, J., et al. (2017). Berberine Ameliorates Diabetes-Associated Cognitive Decline through Modulation of Aberrant Inflammation Response and Insulin Signaling Pathway in DM Rats. Front. Pharmacol. 8 (JUN), 334. doi:10.3389/fphar.2017.00334
Chornenkyy, Y., Wang, W. X., Wei, A., and Nelson, P. T. (2019). Alzheimer's Disease and Type 2 Diabetes Mellitus Are Distinct Diseases with Potential Overlapping Metabolic Dysfunction Upstream of Observed Cognitive Decline. Brain Pathol. 29 (1), 3–17. doi:10.1111/bpa.12655
Cukierman, T., Gerstein, H. C., and Williamson, J. D. (2005). Cognitive Decline and Dementia in Diabetes-Ssystematic Overview of Prospective Observational Studies. Diabetologia 48 (12), 2460–2469. doi:10.1007/s00125-005-0023-4
de Matos, A. M., de Macedo, M. P., and Rauter, A. P. (2018). Bridging Type 2 Diabetes and Alzheimer's Disease: Assembling the Puzzle Pieces in the Quest for the Molecules with Therapeutic and Preventive Potential. Med. Res. Rev. 38 (1), 261–324. doi:10.1002/med.21440
Escribano-Lopez, I., Bañuls, C., Diaz-Morales, N., Iannantuoni, F., Rovira-Llopis, S., Gomis, R., et al. (2019). The Mitochondria-Targeted Antioxidant MitoQ Modulates Mitochondrial Function and Endoplasmic Reticulum Stress in Pancreatic β Cells Exposed to Hyperglycaemia. Cell. Physiol. Biochem. 52 (2), 186–197. doi:10.33594/000000013
Ettcheto, M., Cano, A., Busquets, O., Manzine, P. R., Sánchez-López, E., Castro-Torres, R. D., et al. (2019). A Metabolic Perspective of Late Onset Alzheimer's Disease. Pharmacol. Res. 145, 104255. doi:10.1016/j.phrs.2019.104255
Ghanim, A. M. H., Farag, M. R. T., Anwar, M. A., Ali, N. A. M., Hawas, M. A., Elsallab, H. M. E., et al. (2022). Taurine Alleviates Kidney Injury in a Thioacetamide Rat Model by Mediating Nrf2/HO-1, NQO-1, and MAPK/NF-κB Signaling Pathways. Can. J. Physiol. Pharmacol. 100, 352–360. doi:10.1139/cjpp-2021-0488
Giannini, C., Dalla Man, C., Groop, L., Cobelli, C., Zhao, H., Shaw, M. M., et al. (2014). Co-occurrence of Risk Alleles in or Near Genes Modulating Insulin Secretion Predisposes Obese Youth to Prediabetes. Diabetes Care 37 (2), 475–482. doi:10.2337/dc13-1458
Hamed, S. A. (2017). Brain Injury with Diabetes Mellitus: Evidence, Mechanisms and Treatment Implications. Expert Rev. Clin. Pharmacol. 10 (4), 409–428. doi:10.1080/17512433.2017.1293521
Hooijmans, C. R., Rovers, M. M., de Vries, R. B., Leenaars, M., Ritskes-Hoitinga, M., and Langendam, M. W. (2014). SYRCLE's Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 14, 43. doi:10.1186/1471-2288-14-43
Kalalian-Moghaddam, H., Baluchnejadmojarad, T., Roghani, M., Goshadrou, F., and Ronaghi, A. (2013). Hippocampal Synaptic Plasticity Restoration and Anti-apoptotic Effect Underlie Berberine Improvement of Learning and Memory in Streptozotocin-Diabetic Rats. Eur. J. Pharmacol. 698 (1-3), 259–266. doi:10.1016/j.ejphar.2012.10.020
Karvani, M., Simos, P., Stavrakaki, S., and Kapoukranidou, D. (2019). Neurocognitive Impairment in Type 2 Diabetes Mellitus. Horm. (Athens) 18 (4), 523–534. doi:10.1007/s42000-019-00128-2
Kaur, D., Sharma, V., and Deshmukh, R. (2019). Activation of Microglia and Astrocytes: a Roadway to Neuroinflammation and Alzheimer's Disease. Inflammopharmacology 27 (4), 663–677. doi:10.1007/s10787-019-00580-x
Kellar, D., and Craft, S. (2020). Brain Insulin Resistance in Alzheimer's Disease and Related Disorders: Mechanisms and Therapeutic Approaches. Lancet Neurol. 19 (9), 758–766. doi:10.1016/S1474-4422(20)30231-3
Kim, J. M., Park, C. H., Park, S. K., Seung, T. W., Kang, J. Y., Ha, J. S., et al. (2017). Ginsenoside Re Ameliorates Brain Insulin Resistance and Cognitive Dysfunction in High Fat Diet-Induced C57BL/6 Mice. J. Agric. Food Chem. 65 (13), 2719–2729. doi:10.1021/acs.jafc.7b00297
Kong, F. J., Ma, L. L., Guo, J. J., Xu, L. H., Li, Y., and Qu, S. (2018). Endoplasmic Reticulum Stress/autophagy Pathway Is Involved in Diabetes-Induced Neuronal Apoptosis and Cognitive Decline in Mice. Clin. Sci. (Lond) 132 (1), 111–125. doi:10.1042/CS20171432
Leão, L. L., Tangen, G., Barca, M. L., Engedal, K., Santos, S. H. S., Machado, F. S. M., et al. (2020). Does Hyperglycemia Downregulate Glucose Transporters in the Brain? Med. Hypotheses 139, 109614. doi:10.1016/j.mehy.2020.109614
Li, H. Y., Wang, X. C., Xu, Y. M., Luo, N. C., Luo, S., Hao, X. Y., et al. (2018). Berberine Improves Diabetic Encephalopathy through the SIRT1/ER Stress Pathway in Db/db Mice. Rejuvenation Res. 21 (3), 200–209. doi:10.1089/rej.2017.1972
Liu, J. Q. (2021). Berberine Activates Nrf2 Pathway to Reduce Inflammation and Improve Cognitive Function in Type 2 Diabetic Mice (Langfang: North China University of Science and Technology). Master’s thesis.
Liu, Q., Körner, H., Wu, H., and Wei, W. (2020). Endoplasmic Reticulum Stress in Autoimmune Diseases. Immunobiology 225 (2), 151881. doi:10.1016/j.imbio.2019.11.016
Liu, Q., Zerbinatti, C. V., Zhang, J., Hoe, H. S., Wang, B., Cole, S. L., et al. (2007). Amyloid Precursor Protein Regulates Brain Apolipoprotein E and Cholesterol Metabolism through Lipoprotein Receptor LRP1. Neuron 56 (1), 66–78. doi:10.1016/j.neuron.2007.08.008
Liu, R. Y., Zhang, S. Y., Ren, B., He, Y. M., Gu, J., and Tan, R. (2018). Hypolipidemic Effect of Coptis Chinensis and C. Deltoidea on Type 2 Diabetes Rats through SCAP/SREBP-1c Signal Pathway. Zhongguo Zhong Yao Za Zhi 43 (10), 2129–2133. doi:10.19540/j.cnki.cjcmm.20180208.003
Liu, Y., Li, M., Zhang, Z., Ye, Y., and Zhou, J. (2018). Role of Microglia-Neuron Interactions in Diabetic Encephalopathy. Ageing Res. Rev. 42, 28–39. doi:10.1016/j.arr.2017.12.005
Lovestone, S., and Smith, U. (2014). Advanced Glycation End Products, Dementia, and Diabetes. Proc. Natl. Acad. Sci. U. S. A. 111 (13), 4743–4744. doi:10.1073/pnas.1402277111
Ma, D. Y., Liu, J. P., Ji, W. W., Fu, Q., and Ma, S. P. (2012a). Effects of Berberine on Oxidative Stress and BDNF Expression in hippocampus of Diabetic Encephalopathy Rats. Traditional Chin. Drug Res. Clin. Pharmacol. 28 (05), 39–41. doi:10.13412/j.cnki.zyyl.2012.05.018
Ma, D. Y., Liu, J. P., Wu, J. J., Tan, X., Fu, Q., and Ma, S. P. (2012b). Effects of Berberine on Cognitive Impairment and Hippocampal Pathological Changes in Diabetic Rats. Traditional Chin. Drug Res. Clin. Pharmacol. 23 (6), 631–636. doi:10.3969/j.issn.1003-9783.2012.06.010
Ma, X., Chen, Z., Wang, L., Wang, G., Wang, Z., Dong, X., et al. (2018). The Pathogenesis of Diabetes Mellitus by Oxidative Stress and Inflammation: Its Inhibition by Berberine. Front. Pharmacol. 9, 782. doi:10.3389/fphar.2018.00782
Ma, Y. E., and Liu, C. (2021). Effects of Berberine on AGEs/RAGE/NF-κB Signaling Pathway in Diabetic Mice with Cognitive Impairment. West. J. Traditional Chin. Med. 34 (2), 26–31. doi:10.12174/j.issn.2096-9600.2021.02.07
McLarnon, J. G. (2021). A Leaky Blood-Brain Barrier to Fibrinogen Contributes to Oxidative Damage in Alzheimer's Disease. Antioxidants (Basel) 11 (1), 102. doi:10.3390/antiox11010102
Michalicova, A., Majerova, P., and Kovac, A. (2020). Tau Protein and its Role in Blood-Brain Barrier Dysfunction. Front. Mol. Neurosci. 13, 570045. doi:10.3389/fnmol.2020.570045
Mijnhout, G. S., Scheltens, P., Diamant, M., Biessels, G. J., Wessels, A. M., Simsek, S., et al. (2006). Diabetic Encephalopathy: A Concept in Need of a Definition. Diabetologia 49 (6), 1447–1448. doi:10.1007/s00125-006-0221-8
Moghaddam, H. K., Baluchnejadmojarad, T., Roghani, M., Goshadrou, F., and Ronaghi, A. (2013). Berberine Chloride Improved Synaptic Plasticity in STZ Induced Diabetic Rats. Metab. Brain Dis. 28 (3), 421–428. doi:10.1007/s11011-013-9411-5
Moghaddam, H. K., Baluchnejadmojarad, T., Roghani, M., Khaksari, M., Norouzi, P., Ahooie, M., et al. (2014). Berberine Ameliorate Oxidative Stress and Astrogliosis in the hippocampus of STZ-Induced Diabetic Rats. Mol. Neurobiol. 49 (2), 820–826. doi:10.1007/s12035-013-8559-7
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 Statement: an Updated Guideline for Reporting Systematic Reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Pang, B., Yu, X. T., Zhou, Q., Zhao, T. Y., Wang, H., Gu, C. J., et al. (2015). Effect of Rhizoma Coptidis (Huang Lian) on Treating Diabetes Mellitus. Evid. Based Complement. Altern. Med. 2015, 921416. doi:10.1155/2015/921416
Prady, S. L., Burch, J., Crouch, S., and MacPherson, H. (2014). Problems Caused by Heterogeneity in Meta-Analysis: a Case Study of Acupuncture Trials. Acupunct. Med. 32 (1), 56–61. doi:10.1136/acupmed-2013-010364
Shinjyo, N., Parkinson, J., Bell, J., Katsuno, T., and Bligh, A. (2020). Berberine for Prevention of Dementia Associated with Diabetes and its Comorbidities: A Systematic Review. J. Integr. Med. 18 (2), 125–151. doi:10.1016/j.joim.2020.01.004
Song, D., Hao, J., and Fan, D. (2020). Biological Properties and Clinical Applications of Berberine. Front. Med. 14 (5), 564–582. doi:10.1007/s11684-019-0724-6
Sun, H., Saeedi, P., Karuranga, S., Pinkepank, M., Ogurtsova, K., Duncan, B. B., et al. (2022). IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 183, 109119. doi:10.1016/j.diabres.2021.109119
Tian, L., Ri, H., Qi, J., and Fu, P. (2021). Berberine Elevates Mitochondrial Membrane Potential and Decreases Reactive Oxygen Species by Inhibiting the Rho/ROCK Pathway in Rats with Diabetic Encephalopathy. Mol. Pain 17, 1744806921996101. doi:10.1177/1744806921996101
Tóbon-Velasco, J. C., Cuevas, E., and Torres-Ramos, M. A. (2014). Receptor for AGEs (RAGE) as Mediator of NF-kB Pathway Activation in Neuroinflammation and Oxidative Stress. CNS Neurol. Disord. Drug Targets 13 (9), 1615–1626. doi:10.2174/1871527313666140806144831
Tong, M. M., Liu, G. S., Zhang, N., Cui, J. W., Hou, C. C., Zhang, Y. Q., et al. (2019). Effect of Berberine on Cognitive Function and Expression of Notch1 and Hes1 in Diabetic Rats. Liaoning J. Traditional Chin. Med. 46 (4), 839–842. doi:10.13192/j.issn.1000-1719.2019.04.050
Vorhees, C. V., and Williams, M. T. (2006). Morris Water Maze: Procedures for Assessing Spatial and Related Forms of Learning and Memory. Nat. Protoc. 1 (2), 848–858. doi:10.1038/nprot.2006.116
Wang, K., Chen, Q., Wu, N., Li, Y., Zhang, R., Wang, J., et al. (2019). Berberine Ameliorates Spatial Learning Memory Impairment and Modulates Cholinergic Anti-inflammatory Pathway in Diabetic Rats. Front. Pharmacol. 10, 1003. doi:10.3389/fphar.2019.01003
Wang, S., He, B., Hang, W., Wu, N., Xia, L., Wang, X., et al. (2018). Berberine Alleviates Tau Hyperphosphorylation and Axonopathy-Associated with Diabetic Encephalopathy via Restoring PI3K/Akt/GSK3β Pathway. J. Alzheimers Dis. 65 (4), 1385–1400. doi:10.3233/jad-180497
Wu, N., Liu, W., Wang, J., Han, Y., Ye, Y., Liu, X., et al. (2021). Berberine Ameliorates Neuronal AD-like Change via Activating Pi3k/PGCε Pathway. Biofactors 47 (4), 587–599. doi:10.1002/biof.1725
Xuan, X. Y. (2021). Effect of Berberine on Cognitive Function and its Mechanism in Type 2 Diabetes Mellitus (Langfang: North China University of Science and Technology). Master’s thesis.
Xue, M., Xu, W., Ou, Y. N., Cao, X. P., Tan, M. S., Tan, L., et al. (2019). Diabetes Mellitus and Risks of Cognitive Impairment and Dementia: A Systematic Review and Meta-Analysis of 144 Prospective Studies. Ageing Res. Rev. 55, 100944. doi:10.1016/j.arr.2019.100944
Yin, J., Xing, H., and Ye, J. (2008). Efficacy of Berberine in Patients with Type 2 Diabetes Mellitus. Metabolism 57 (5), 712–717. doi:10.1016/j.metabol.2008.01.013
Zaulkffali, A. S., Md Razip, N. N., Syed Alwi, S. S., Abd Jalil, A., Abd Mutalib, M. S., Gopalsamy, B., et al. (2019). Vitamins D and E Stimulate the PI3K-AKT Signalling Pathway in Insulin-Resistant SK-N-SH Neuronal Cells. Nutrients 11 (10). doi:10.3390/nu11102525
Zhang, H. Y., Wang, Z. G., Lu, X. H., Kong, X. X., Wu, F. Z., Lin, L., et al. (2015). Endoplasmic Reticulum Stress: Relevance and Therapeutics in Central Nervous System Diseases. Mol. Neurobiol. 51 (3), 1343–1352. doi:10.1007/s12035-014-8813-7
Zhang, J.-H., Zhang, J.-F., Song, J., Bai, Y., Deng, L., Feng, C.-P., et al. (2021). Effects of Berberine on Diabetes and Cognitive Impairment in an Animal Model: The Mechanisms of Action. Am. J. Chin. Med. 49 (06), 1399–1415. doi:10.1142/s0192415x21500658
Zhang, S., Xue, R., and Hu, R. (2020). The Neuroprotective Effect and Action Mechanism of Polyphenols in Diabetes Mellitus-Related Cognitive Dysfunction. Eur. J. Nutr. 59 (4), 1295–1311. doi:10.1007/s00394-019-02078-2
Zhao, M. M., Lu, J., Li, S., Wang, H., Cao, X., Li, Q., et al. (2021). Berberine Is an Insulin Secretagogue Targeting the KCNH6 Potassium Channel. Nat. Commun. 12 (1), 5616. doi:10.1038/s41467-021-25952-2
Keywords: diabetes, cognitive impairment, berberine, animal models, systematic review, meta-analysis
Citation: Hao Y, Li J, Yue S, Wang S, Hu S and Li B (2022) Neuroprotective Effect and Possible Mechanisms of Berberine in Diabetes-Related Cognitive Impairment: A Systematic Review and Meta-Analysis of Animal Studies. Front. Pharmacol. 13:917375. doi: 10.3389/fphar.2022.917375
Received: 11 April 2022; Accepted: 02 May 2022;
Published: 06 June 2022.
Edited by:
Mohammad Reza Khazdair, Birjand University of Medical Sciences, IranReviewed by:
Daniela Giacomazza, National Research Council (CNR), ItalyAngel Agis-Torres, Universidad Complutense de Madrid, Spain
Copyright © 2022 Hao, Li, Yue, Wang, Hu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Li, bGliaW5AY2R1dGNtLmVkdS5jbg==
 Yanwei Hao
Yanwei Hao Jiaxin Li1
Jiaxin Li1 Bin Li
Bin Li