- 1School of Public Health, Bengbu Medical College, Bengbu, China
- 2Division of Life Sciences and Medicine, The Stroke Center and Department of Neurology, The First Affiliated Hospital of USTC, University of Science and Technology of China, Hefei, China
- 3Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 4Department of Neurology, The Hefei Affiliated Hospital of Anhui Medical University (No. 2 People’s Hospital of Hefei), Hefei, China
- 5Department of Neurosurgery, The First Affiliated Hospital of Anhui Medical University, Hefei, China
- 6Department of Neurology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, China
Immune and inflammatory mechanisms play key roles in the development and outcome of acute ischemic stroke (AIS). β2-Microglobulin (β2M) is the light chain of major histocompatibility complex-1 (MHC-1), which can directly and quickly reflect the immune and inflammatory state of the body. Previous studies have shown a close relationship between β2M and AIS, but its relationship with the recurrence of AIS has not been reported. This study attempted to explore the relationship between β2M and the recurrence of AIS. A single-center AIS cohort involving 135 patients was followed for approximately 26–46 months. Clinical and laboratory data from the patients were collected when hospitalized. The endpoint was the occurrence of recurrent AIS after patients were discharged. Propensity score matching was used to match cohort groups. Cox regression analysis was used to predict risk factors for recurrent AIS, and receiver operating characteristic curve (ROC) analysis was used to calculate the optimal cutoff value for discriminating recurrence in patients with AIS. The rate of recurrence was 29.6% [95% CI, 21.8%–37.3%] in the follow-up group. Patients with higher levels of serum β2M had a higher risk of AIS recurrence than patients with lower levels of β2M (adjusted hazard ratio, 3.214 [95% CI, 1.557–6.633]; adjusted hazard ratio after matching, 5.831, [95% CI, 2.052–16.572]). A β2M value of 2.31 mg/L was calculated by ROC analysis as the optimal cutoff value for AIS recurrence (area under the curve 0.770, [95% CI, 0.687–0.853]). As a quick responder to the body’s immune and inflammatory states, β2M may be a novel and reliable biomarker in predicting AIS recurrence.
Introduction
Ischemic stroke (IS) is the leading cause of death and disability among adults worldwide (O'Donnell et al., 2016). With the aging of society and the acceleration of urbanization, the burden of IS in our country has exploded, and it has become an important public health problem that seriously affects the national economy and people’s livelihoods. In parallel with its high incidence, IS currently lacks effective prevention and treatment (Wu et al., 2019). Therefore, the etiology and pathology of IS have become the focus of research in recent years.
Chronic inflammation and an aberrant immune response are characteristic features of atherosclerosis, a leading cause of cardiovascular diseases and IS (Solanki et al., 2018). Additionally, immunity and inflammation are key factors in the pathobiology of AIS. For example, the inflammatory signaling pathway is associated with the ischemic cascade, and stroke is associated with immunosuppression and infection (Iadecola and Anrather, 2011). Immunity and inflammation are considered to be the core pathological mechanisms of IS.
β2-microglobulin (β2M) is the light chain of major histocompatibility complex-1 (MHC-1), a small-molecular-weight protein of 11.8 kD that is secreted by nucleated cells. β2M is an important structural protein by which CD8+ T lymphocytes regulate host immune recognition of self and nonself antigens as well as immunoglobulin transport (Nomura et al., 2014) and is closely associated with the innate and adaptive immune systems (Ardeniz et al., 2015), possibly being a potential initiator of inflammatory responses (Xie and Yi, 2003).
There are many reports about the relationship between β2M and peripheral organ diseases (including tumors) related to immunity and inflammation (Wilson et al., 2007; Josson et al., 2011; Hermansen et al., 2012; Mao et al., 2020), and the central nervous system (CNS) has traditionally been regarded as immune-privileged, though this has been questioned (Piehl and Lidman, 2001). The mechanism of β2M and poststroke immunity and inflammation may be complex, and there are relatively few reports.
Previous studies have found that high levels of β2M were associated with an increased risk of IS among women (Rist et al., 2017). Our previous work also found that serum β2M levels are positively correlated with an increased risk of AIS and adverse prognosis, and are strongly associated with the risk score for IS recurrence (Essen Stroke Risk Score, ESRS) (Hu et al., 2019; Qun et al., 2019). However, due to the lack of long-term follow-up, its relationship with IS recurrence has not been specifically studied. In this study, we conducted a detailed analysis of the relationship between β2M and the recurrence of cerebral infarction through 26–46 months of follow-up.
Materials and Methods
Ethical Statements
This study was approved by the Research Ethics Committee of the First Affiliated Hospital of the University of Science and Technology of China (USTC). Written informed consent was obtained from all participants and their guardians while involved in this study.
Participant Recruitment
Patients admitted to the hospital for AIS were recruited from September 2015 to July 2017 and diagnosed as per their medical history, symptoms and signs, and diffusion-weighted magnetic resonance imaging (DWI). The patients were treated with antiplatelet drugs and statin therapy. Anticoagulant therapy was given for cardiac embolism and etiological treatment was given for other determined strokes. All patients were discharged in a stable condition.
The exclusion criteria for participants were as follows (O'Donnell et al., 2016): serious systemic diseases, such as acute or chronic renal dysfunction, or endocrine diseases (other than diabetes mellitus) (Wu et al., 2019); the use of immunosuppressant drugs (steroids) (Solanki et al., 2018); the presence of cancer (Iadecola and Anrather, 2011), trauma (Nomura et al., 2014), infectious diseases, or (Ardeniz et al., 2015) hematological disorders.
Laboratory Assays and Clinical Information
We collected blood samples for laboratory tests in the morning (between 6:00 and 7:00 a.m.) following an overnight fast. Serum β2M was tested by the particle-enhanced turbidimetric immunoassay method. The intra-assay coefficient of variation ranged from 2.4 to 3.8%, meanwhile, the inter-assay coefficient of variation ranged from 1.7 to 2.2%. C-reactive protein (CRP) was tested by the immune transmission turbidity method. Other biochemical parameters, including fasting blood glucose (GLU), homocysteine (Hcy), creatinine (Crea), urea nitrogen (BUN), uric acid (UA), low-density lipoprotein cholesterol (LDL), triglyceride (TG), total cholesterol (CHOL), high-density lipoprotein cholesterol (HDL), very low-density lipoprotein cholesterol (VLDL), cystatin C (CysC), were measured by enzymatic method. All serum biochemical parameters were assayed by an automatic biochemical analyzer (HITACHI Automatic Analyzer 7600-020, Japan). Laboratory personnel was blinded to clinical data as well as clinicians to laboratory data.
Information on patient demographic characteristics, including age, sex, stroke risk factors for hypertension referenced by systolic blood pressure (SBP) or diastolic blood pressure (DBP), type 2 diabetes, stroke history, coronary heart disease (CHD), and smoking and alcohol consumption was collected.
ESRS and the National Institute of Health Stroke Scale (NIHSS) were used to evaluate the patient situation (Weimar et al., 2009), (Lyden et al., 1994). The Trial of ORG 10172 in Acute Stroke Treatment (TOAST) was used to classify the five subtypes of acute ischemic stroke: large-artery atherosclerosis (LAA), cardiac embolism (CE), small-artery occlusion (SAO), a stroke of other determined cause (SOC), and stroke of undetermined cause (SUC) (Chen et al., 2012).
Endpoint
The participants were followed up after discharge from the hospital until the following events occurred: AIS, death, and loss to follow-up. The deadline was set on 26 July 2019.
Propensity Score Matching
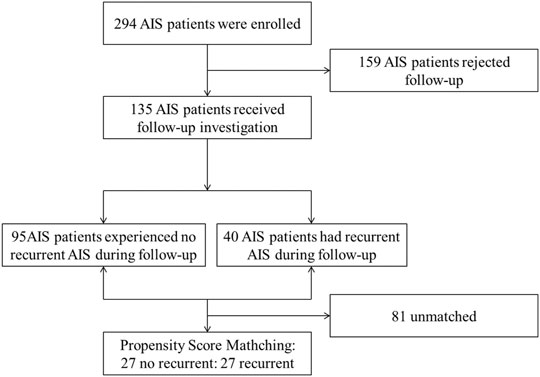
The propensity score was calculated for each patient based on a multivariable logistic regression model. We only included age and CHD according to the significant differences in demographic characteristics between recurrent and no recurrent groups. A 1:1 ratio using a greedy nearest neighbor method with a tolerance of 0.02 was performed. Figure 1 presents the participants selection.
Statistical Analysis
Statistical analysis was conducted with the Statistical Package for the Social Sciences version (SPSS) 22.0 (IBM Corp., Armonk, NY, USA). Quantitative data were tested for normality using the Shapiro–Wilk test. Variables that followed a normal distribution are expressed as mean ± standard deviation or as the median and interquartile range (IQR). Categorical variables are presented as frequencies and percentages. Differences between recurrent and no recurrent groups were assessed by the independent-samples t test, the Mann–Whitney U-test, the Chi-square test, or Fisher’s exact test, as appropriate. The optimal cutoff value of β2M for stroke recurrence was evaluated by the ROC analysis. Cox regression was used to develop a recurrence risk model.
Results
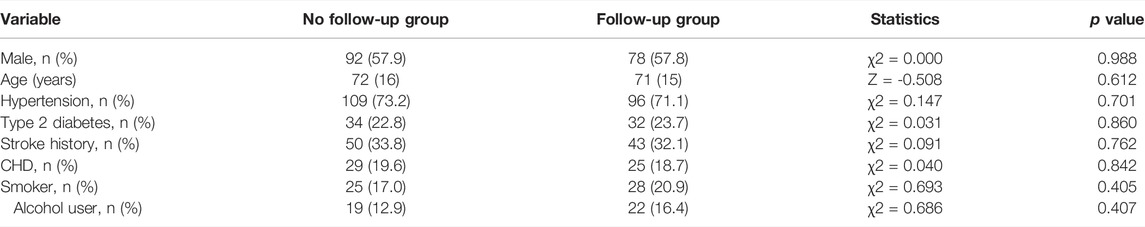
A total of 294 AIS patients were enrolled in this study, among those discharged patients, 135 received follow-up investigation, and 159 (54.1%) rejected follow-up. Table 1 shows baseline characteristics of no follow-up group and follow-up group, and there were no significant differences between the two groups (p > 0.05).
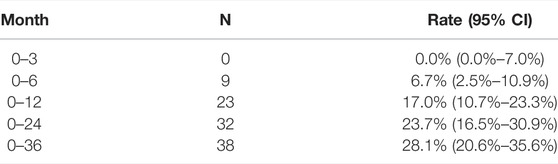
Up to the deadline, a total of 40 patients had recurrent AIS, and the rate of recurrence was 29.6% [95% CI, 21.8%–37.3%]. The recurrence rates were 0% [95% CI, 0.0%–7.0%] at 3 months, 6.7% [95% CI, 2.5%–10.9%] at 6 months, 17.0% [95% CI, 10.7%–23.3%] at 12 months, 23.7% [95% CI, 16.5%–30.9%] at 2 years, and 28.1% [95% CI, 20.6%–35.6%] at 3 years (Table 2). By propensity score matching, 27 no recurrent and 27 recurrent patients were matched.
Comparison of Patients With AIS Recurrence and Those With no Recurrence
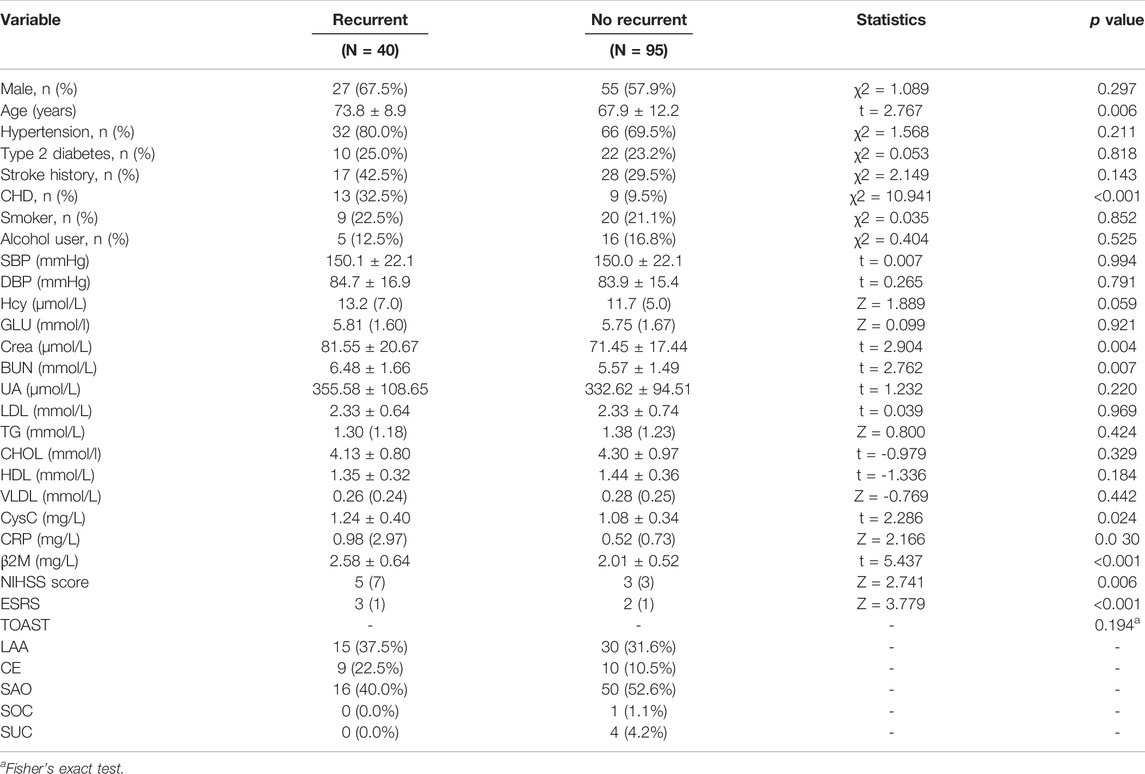
Compared to the patients with no recurrence, those with recurrence had higher age, an increased prevalence of CHD, and increased levels of Crea, BUN, CysC, CRP, β2M, NIHSS, and ESRS (p < 0.05). Other variables were not significantly different between the two groups (Table 3).
β2M is a Reliable Predictive Risk Factor for Recurrent AIS
A β2M value of 2.31 mg/L was calculated by ROC analysis as the optimal cutoff value for AIS recurrence (area under the curve 0.770 [95% CI, 0.687–0.853]). The cutoff value had a sensitivity of 65.0% and a specificity of 75.8% (Figure 2A).
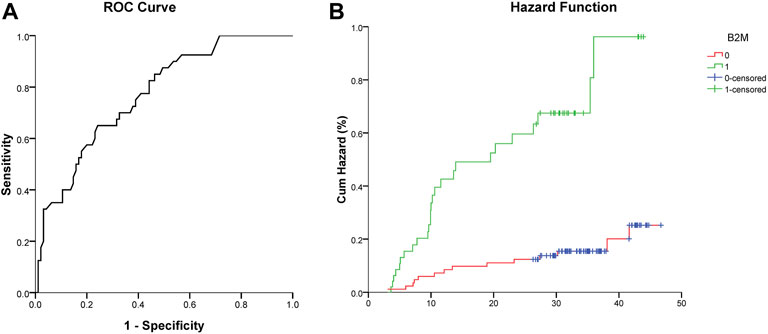
FIGURE 2. (A) ROC curve analysis showed a predictive serum level of β2M for stroke recurrence. (B) Cumulative hazard (%) of recurrent stroke between the low (0, β2M ≤ 2.31 mg/L) and high (1, β2M > 2.31 mg/L) β2M groups (Log-rank test, χ2 = 23.840, p < 0.001).
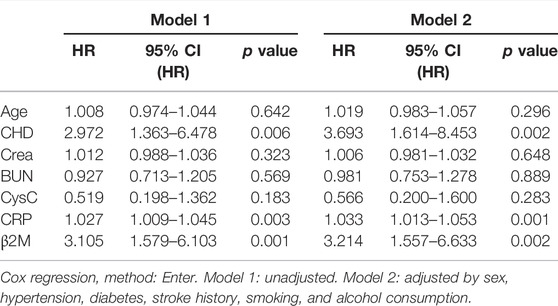
A Cox regression hazard model was used to predict the risk factors for recurrent AIS. The possible variables tested by univariate analysis (Table 3) were put into this model. Patients with higher levels of serum CRP (hazard ratio (HR) 1.033 [95% CI, 1.013–1.053]), β2M (HR 3.214 [95% CI, 1.557–6.633]), and attacked with CHD (HR 3.693 [95% CI, 1.614–8.453]) had higher risks of recurrent AIS, even after adjustments for other variables. The Kaplan–Meier curve showed the same result for β2M (Figure 2B). Variables including age, Crea, BUN, and CysC were not significantly different in terms of recurrent AIS (Table 4).
Comparison of Patients With AIS Recurrence and Those With no Recurrence After Propensity Score Matching
Table 5 shows that after propensity score matching, compared to patients with no recurrence, those with recurrence had a higher level of Hcy, Crea, CRP, and β2M (p < 0.05), and other variables were not a significant difference. In the multivariable Cox proportional hazard regression model, Table 6 shows that only a higher level of β2M indicated a higher risk of AIS recurrence (HR 5.831, [95% CI, 2.052–16.572]).
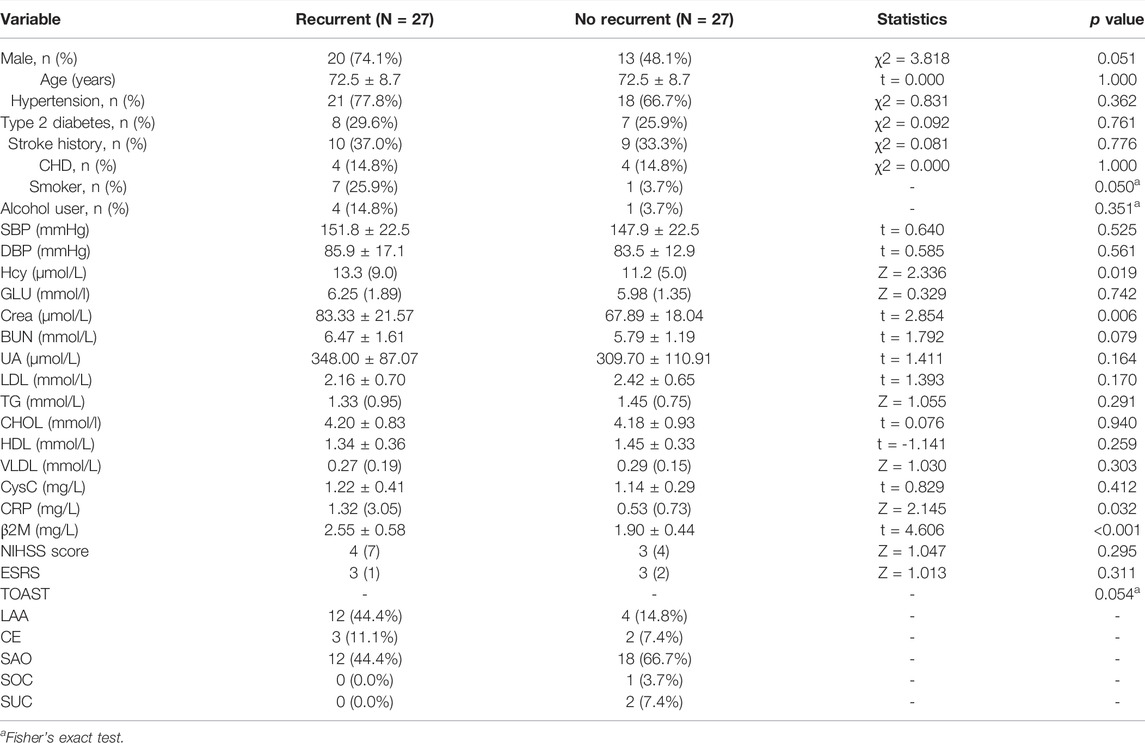
TABLE 5. Clinical and laboratory findings in recurrent and no recurrent patients after propensity score matching.
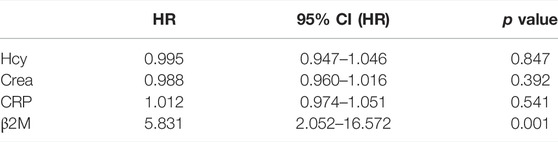
TABLE 6. Predictive risk factors for recurrent AIS using Cox proportional hazard regression after propensity score matching.
Discussion
Ten risk factors are associated with 90% of the risk of AIS (O'Donnell et al., 2010), and nine out of 10 AIS are due to modifiable risk factors (Boehme et al., 2017). Thus, IS prevention has generally focused on modifiable risk factors. Hypertension, dyslipidemia, diabetes, smoking, alcohol consumption, air pollution, diets low in fruit and vegetables, and high sodium intake are the most common and modifiable risk factors for AIS in China (Guan et al., 2017; Wang et al., 2017). In view of these traditional risk factors, a series of prevention strategies have been developed. However, the incidence of IS is still expected to increase in China, and recurrence of IS may be the main cause (Del Brutto et al., 2019).
AIS survivors have a high risk of recurrence, and recurrent IS patients make up nearly one-third of all AIS (Bushnell et al., 2009; Pennlert et al., 2014). Recurrent AIS causes substantially higher morbidity and mortality than first-time AIS (Aarnio et al., 2014; Pennlert et al., 2014). Therefore, the prediction of recurrent IS may be a key strategy for IS prevention and treatment. Rapid identification of etiology is important for the prediction of recurrence of IS. Due to the complex etiology of ischemic stroke, reliable biomarkers of AIS recurrence are the focus of our attention.
In our cohort study, we found a strong correlation between plasma β2M levels and recurrence of AIS. After 26–46 months of follow-up, we found that a high level of serum β2M had nearly three times the risk of recurrent AIS (HR 3.105, 95% CI, 1.579–6.103) by means of the Cox proportional hazard regression model, suggesting that β2M is a reliable predictive risk factor for recurrent AIS. A β2M value of 2.31 mg/L was calculated by ROC analysis as the optimal cutoff value for stroke recurrence. We used propensity score matching to control the potential confounders. Considering the sample size, we only included covariates of age and CHD. After matching, the basic characteristics had no difference between the two groups, especially the NIHSS score. The result of Cox regression after matching still showed the positive relationship between β2M and recurrence of AIS.
Different from the above traditional risk factors, β2M is closely related to the innate and adaptive immune systems (Ardeniz et al., 2015), in addition, may be an initiator of inflammation (Xie and Yi, 2003). Atherosclerosis is a chronic inflammatory disease, and a previous study showed that β2M was independently and significantly associated with adverse cardiovascular outcomes in patients with prevalent asymptomatic carotid atherosclerosis (Amighi et al., 2011). Serum β2M may reflect levels of chronic inflammation in the body and thus serve as a risk marker for IS. However, the entire course of disease surrounding the occurrence, development, and outcome of IS is a vast array of immune-stimulating and inflammatory events (Iadecola and Anrather, 2011; Solanki et al., 2018). The serum level of β2M in our cohort may reflect a combination of two determinants: the basal level of the body and the emergency response state of the acute stage of AIS. As the basal state of the body’s immunity and inflammation, chronic inflammation is the core factor in the formation mechanism of atherosclerosis and the main cause of cardiovascular and cerebrovascular diseases (Diomedi et al., 2005; Ferrucci and Fabbri, 2018). Additionally, activation of both innate and adaptive immunity actively contributes to the initiation and progression of atherogenesis, from early endothelial dysfunction to the development of acute thrombotic complications triggered by plaque rupture or erosion (Ruparelia et al., 2017). On the other hand, AIS events are a concentrated expression process of immune stress and the inflammatory response: the immune system participates in the brain damage produced by ischemia. Subsequently, the brain actively promotes immunosuppression to avoid further brain damage but may promote stroke-associated infection and increase the mortality rate. Finally, regeneration and repair of ischemic brain tissue are regulated by adaptive immunity (Iadecola and Anrather, 2011). Serum β2M levels are also a concentrated reflection of immune and inflammatory states associated with post-AIS. As previously mentioned, IS-related immunity and inflammation are the results of the expression of systemic immune and inflammatory states before, during, and after stroke events. The physiological characteristics of β2M just meet the above requirements, so it can reflect the prognosis of stroke from the essential mechanism level of AIS. These studies provide a good explanation for β2M as a novel biological indicator of AIS recurrence. In the future, we will conduct relevant studies on β2M as a target to intervene in the recurrence of ischemic stroke, especially the relationship between β2M and the modifiable risk factors of AIS.
Limitations
First, the relatively low number of research subjects is an important limitation of this study. Second, many patients are lost to follow-up. Third, data from multiple medical centers are needed to confirm our results. In the future, we will increase the sample size and reduce the loss of follow-up rate and conduct a multicenter case-control study to overcome the limitations of the current study.
Conclusions
Following an AIS cohort of 26–46 months, we found that as a rapid responder of the immune and inflammatory state of the body, the level of peripheral blood β2M in patients with AIS has an important predictive value for the recurrence of AIS, suggesting that β2M is a novel and reliable predictor of recurrent AIS.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Research Ethics Committee of the First Affiliated Hospital of the University of Science and Technology of China (USTC). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SQ was involved in the design of the study, data collection, and manuscript writing and was a recipient of the obtained funding. F-yH took part in the design of the study, and the statistical analysis and was a recipient of the obtained funding. JW was a recipient of the obtained funding and was involved in the interpretation of the data and the manuscript revision. WW, HG, JY, KJ, GW, ZW, and QL were involved in data collection. WG and YQ participated in data analysis, interpretation of the data, and manuscript revision.
Funding
This work was supported by the Key Research and Development Project of Anhui Province (No. 202004j07020014); the Hefei Science and Technology Bureau “Borrow, Transfer, and Supplement” Project (No. J2019Y01); the Natural Science Foundation for the Higher Education institutions of Anhui Province (KJ 2018A0991); Natural Science Key Project of Bengbu Medical College (2020byzd042).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all participants in this study.
References
Aarnio, K., Haapaniemi, E., Melkas, S., Kaste, M., Tatlisumak, T., and Putaala, J. (2014). Long-term Mortality after First-Ever and Recurrent Stroke in Young Adults. Stroke 45 (9), 2670–2676. doi:10.1161/STROKEAHA.114.005648
Amighi, J., Hoke, M., Mlekusch, W., Schlager, O., Exner, M., Haumer, M., et al. (2011). Beta 2 Microglobulin and the Risk for Cardiovascular Events in Patients with Asymptomatic Carotid Atherosclerosis. Stroke 42 (7), 1826–1833. doi:10.1161/STROKEAHA.110.600312
Ardeniz, Ö., Unger, S., Onay, H., Ammann, S., Keck, C., Cianga, C., et al. (2015). β2-Microglobulin Deficiency Causes a Complex Immunodeficiency of the Innate and Adaptive Immune System. J. Allergy Clin. Immunol. 136 (2), 392–401. doi:10.1016/j.jaci.2014.12.1937
Boehme, A. K., Esenwa, C., and Elkind, M. S. (2017). Stroke Risk Factors, Genetics, and Prevention. Circ. Res. 120 (3), 472–495. doi:10.1161/CIRCRESAHA.116.308398
Bushnell, C., Zimmer, L., Schwamm, L., Goldstein, L. B., Clapp-Channing, N., Harding, T., et al. (2009). The Adherence eValuation after Ischemic Stroke Longitudinal (AVAIL) Registry: Design, Rationale, and Baseline Patient Characteristics. Am. Heart J. 157 (3), 428–e2. doi:10.1016/j.ahj.2008.11.002
Chen, P. H., Gao, S., Wang, Y. J., Xu, A. D., Li, Y. S., and Wang, D. (2012). Classifying Ischemic Stroke, from TOAST to CISS. CNS Neurosci. Ther. 18 (6), 452–456. doi:10.1111/j.1755-5949.2011.00292.x
Del Brutto, V. J., Chaturvedi, S., Diener, H. C., Romano, J. G., and Sacco, R. L. (2019). Antithrombotic Therapy to Prevent Recurrent Strokes in Ischemic Cerebrovascular Disease: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 74 (6), 786–803. doi:10.1016/j.jacc.2019.06.039
Diomedi, M., Leone, G., and Renna, A. (2005). The Role of Chronic Infection and Inflammation in the Pathogenesis of Cardiovascular and Cerebrovascular Disease. Drugs Today (Barc) 41 (11), 745–753. doi:10.1358/dot.2005.41.11.917342
Ferrucci, L., and Fabbri, E. (2018). Inflammageing: Chronic Inflammation in Ageing, Cardiovascular Disease, and Frailty. Nat. Rev. Cardiol. 15 (9), 505–522. doi:10.1038/s41569-018-0064-2
Guan, T., Ma, J., Li, M., Xue, T., Lan, Z., Guo, J., et al. (2017). Rapid Transitions in the Epidemiology of Stroke and its Risk Factors in China from 2002 to 2013. Neurology 89 (1), 53–61. doi:10.1212/WNL.0000000000004056
Hermansen, M. L., Hummelshøj, L., Lundsgaard, D., Hornum, L., Keller, P., Fleckner, J., et al. (2012). Increased Serum β2-microglobulin Is Associated with Clinical and Immunological Markers of Disease Activity in Systemic Lupus Erythematosus Patients. Lupus 21 (10), 1098–1104. doi:10.1177/0961203312447668
Hu, F. Y., Wu, J., Tang, Q., Zhang, J., Chen, Z., Wang, X., et al. (2019). Serum β2-Microglobulin Is Closely Associated with the Recurrence Risk and 3-Month Outcome of Acute Ischemic Stroke. Front. Neurol. 10, 1334. doi:10.3389/fneur.2019.01334
Iadecola, C., and Anrather, J. (2011). The Immunology of Stroke: from Mechanisms to Translation. Nat. Med. 17 (7), 796–808. doi:10.1038/nm.2399
Josson, S., Nomura, T., Lin, J. T., Huang, W. C., Wu, D., Zhau, H. E., et al. (2011). β2-microglobulin Induces Epithelial to Mesenchymal Transition and Confers Cancer Lethality and Bone Metastasis in Human Cancer Cells. Cancer Res. 71 (7), 2600–2610. doi:10.1158/0008-5472.CAN-10-3382
Lyden, P., Brott, T., Tilley, B., Welch, K. M., Mascha, E. J., Levine, S., et al. (1994). Improved Reliability of the NIH Stroke Scale Using Video Training. NINDS TPA Stroke Study Group. Stroke 25 (11), 2220–2226. doi:10.1161/01.str.25.11.2220
Mao, W., Wang, J., Zhang, L., Wang, Y., Wang, W., Zeng, N., et al. (2020). Serum β2-Microglobulin Is Associated with Mortality in Hospitalized Patients with Exacerbated Chronic Obstructive Pulmonary Disease. Int. J. Chron. Obstruct Pulmon Dis. 15, 723–732. doi:10.2147/COPD.S243905
Nomura, T., Huang, W. C., Zhau, H. E., Josson, S., Mimata, H., and Chung, L. W. (2014). β2-Microglobulin-mediated Signaling as a Target for Cancer Therapy. Anticancer Agents Med. Chem. 14 (3), 343–352. doi:10.2174/18715206113139990092
O'Donnell, M. J., Chin, S. L., Rangarajan, S., Xavier, D., Liu, L., Zhang, H., et al. (2016). Global and Regional Effects of Potentially Modifiable Risk Factors Associated with Acute Stroke in 32 Countries (INTERSTROKE): a Case-Control Study. Lancet 388 (10046), 761–775. doi:10.1016/S0140-6736(16)30506-2
O'Donnell, M. J., Xavier, D., Liu, L., Zhang, H., Chin, S. L., Rao-Melacini, P., et al. (2010). Risk Factors for Ischaemic and Intracerebral Haemorrhagic Stroke in 22 Countries (The INTERSTROKE Study): a Case-Control Study. Lancet 376 (9735), 112–123. doi:10.1016/S0140-6736(10)60834-3
Pennlert, J., Eriksson, M., Carlberg, B., and Wiklund, P. G. (2014). Long-term Risk and Predictors of Recurrent Stroke beyond the Acute Phase. Stroke 45 (6), 1839–1841. doi:10.1161/STROKEAHA.114.005060
Piehl, F., and Lidman, O. (2001). Neuroinflammation in the Rat--CNS Cells and Their Role in the Regulation of Immune Reactions. Immunol. Rev. 184, 212–225. doi:10.1034/j.1600-065x.2001.1840119.x
Qun, S., Hu, F., Wang, G., Wu, J., Tang, Q., Zhang, J., et al. (2019). Serum Beta2-Microglobulin Levels Are Highly Associated with the Risk of Acute Ischemic Stroke. Sci. Rep. 9 (1), 6883. doi:10.1038/s41598-019-43370-9
Rist, P. M., Jiménez, M. C., and Rexrode, K. M. (2017). Prospective Association between β2-microglobulin Levels and Ischemic Stroke Risk Among Women. Neurology 88 (23), 2176–2182. doi:10.1212/WNL.0000000000004006
Ruparelia, N., Chai, J. T., Fisher, E. A., and Choudhury, R. P. (2017). Inflammatory Processes in Cardiovascular Disease: a Route to Targeted Therapies. Nat. Rev. Cardiol. 14 (3), 314–344. doi:10.1038/nrcardio.2017.33
Solanki, A., Bhatt, L. K., and Johnston, T. P. (2018). Evolving Targets for the Treatment of Atherosclerosis. Pharmacol. Ther. 187, 1–12. doi:10.1016/j.pharmthera.2018.02.002
Wang, W., Jiang, B., Sun, H., Ru, X., Sun, D., Wang, L., et al. (2017). Prevalence, Incidence, and Mortality of Stroke in China: Results from a Nationwide Population-Based Survey of 480 687 Adults. Circulation 135 (8), 759–771. doi:10.1161/CIRCULATIONAHA.116.025250
Weimar, C., Diener, H. C., Alberts, M. J., Steg, P. G., Bhatt, D. L., Wilson, P. W., et al. (2009). The Essen Stroke Risk Score Predicts Recurrent Cardiovascular Events: a Validation within the REduction of Atherothrombosis for Continued Health (REACH) Registry. Stroke 40 (2), 350–354. doi:10.1161/STROKEAHA.108.521419
Wilson, A. M., Kimura, E., Harada, R. K., Nair, N., Narasimhan, B., Meng, X. Y., et al. (2007). Beta2-microglobulin as a Biomarker in Peripheral Arterial Disease: Proteomic Profiling and Clinical Studies. Circulation 116 (12), 1396–1403. doi:10.1161/CIRCULATIONAHA.106.683722
Wu, S., Wu, B., Liu, M., Chen, Z., Wang, W., Anderson, C. S., et al. (2019). Stroke in China: Advances and Challenges in Epidemiology, Prevention, and Management. Lancet Neurol. 18 (4), 394–405. doi:10.1016/S1474-4422(18)30500-3
Keywords: β2-microglobulin, acute ischemic stroke, recurrence, immunity and inflammation, propensity score matching
Citation: Hu F-y, Wu W, Liu Q, Wu J, Guo H, Yang J, Wu Z, Jiang K, Wang G, Qian Y, Ge W and Qun S (2022) β2-Microglobulin is a Novel and Reliable Biomarker for Predicting Ischemic Stroke Recurrence: A Prospective Cohort Study. Front. Pharmacol. 13:916769. doi: 10.3389/fphar.2022.916769
Received: 10 April 2022; Accepted: 05 May 2022;
Published: 17 June 2022.
Edited by:
Tao Xu, Anhui Medical University, ChinaCopyright © 2022 Hu, Wu, Liu, Wu, Guo, Yang, Wu, Jiang, Wang, Qian, Ge and Qun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Qian, Zmx5aGVhcnRfMDAxQHFxLmNvbQ==; Wei Ge, Z3cxMDAzQDE2My5jb20=; Sen Qun, cXVuc2VuNjE2M0AxNjMuY29t
†These authors have contributed equally to this work and share the first authorship
 Fu-yong Hu
Fu-yong Hu Wentao Wu
Wentao Wu Qiuwan Liu
Qiuwan Liu Juncang Wu4
Juncang Wu4 Sen Qun
Sen Qun