- Department of Physiology and Biophysics, Case Western Reserve University School of Medicine, Cleveland, OH, United States
Neuroinflammation is a protective mechanism against insults from exogenous pathogens and endogenous cellular debris and is essential for reestablishing homeostasis in the brain. However, excessive prolonged neuroinflammation inevitably leads to lesions and disease. The use of natural compounds targeting pathways involved in neuroinflammation remains a promising strategy for treating different neurological and neurodegenerative diseases. Astaxanthin, a natural xanthophyll carotenoid, is a well known antioxidant. Mounting evidence has revealed that astaxanthin is neuroprotective and has therapeutic potential by inhibiting neuroinflammation, however, its functional roles and underlying mechanisms in modulating neuroinflammation have not been systematically summarized. Hence, this review summarizes recent progress in this field and provides an update on the medical value of astaxanthin. Astaxanthin modulates neuroinflammation by alleviating oxidative stress, reducing the production of neuroinflammatory factors, inhibiting peripheral inflammation and maintaining the integrity of the blood-brain barrier. Mechanistically, astaxanthin scavenges radicals, triggers the Nrf2-induced activation of the antioxidant system, and suppresses the activation of the NF-κB and mitogen-activated protein kinase pathways. With its good biosafety and high bioavailability, astaxanthin has strong potential for modulating neuroinflammation, although some outstanding issues still require further investigation.
Introduction
The initiation of neuroinflammation is physiologically responsible for phagocytosis and the clearance of cellular debris, aberrant proteins, and exogenous pathogens. This process is beneficial because it maintains the homeostatic environment and defends against exogenous insults in the brain. However, chronic or aberrantly prolonged inflammation can also cause devastating injury to resident cells of the central nervous system (CNS). Regulation of neuroinflammatory processes to maintain balanced innate immunity is crucial for brain homeostasis and intervening in CNS disorders (Marques-Deak et al., 2005; DiSabato et al., 2016; Fung et al., 2017).
Natural compounds with anti-inflammatory properties have sparked substantial interest as they can enhance neuroprotection. Carotenoids, a group of natural tetraterpenes that are the most abundant lipophilic pigments in nature, show great potential in medical applications (Milani et al., 2017; Sauer et al., 2019). They are found in various organisms, including plants, algae, bacteria, and fungi, and play vital roles in photosynthesis, photoprotection, anti-oxidation, biosynthesis of phytohormones, and signal transduction. Carotenoids are also crucial metabolic components and essential dietary supplements for animals with a deficiency in de novo carotenoid biosynthesis.
There are two types of carotenoids oxygen-free carotenes and oxygen-containing xanthophylls. Astaxanthin is one of the most common xanthophylls with an oxygen-containing group in its structure. Since its first isolation from a lobster in 1938 (Kuhn, 1938), astaxanthin has been used as a pigment and food additive for its good coloring properties. The fundamental structural feature of astaxanthin that resembles other carotenoids is a polyene chain comprising a battery of conjugated C=C bonds. Based on its molecular structure, astaxanthin is a 3,3′-dihydroxy-β,β′-carotene-4,4′-dione, containing two identical asymmetric carbon atoms at the 3 and 3′ positions of the β-ionone ring with a hydroxyl group at both ends. The 3,3′ asymmetric carbons allow astaxanthin to form three possible optical isomers with an all-trans configuration of the chain: 3R,3′R, 3S,3′S, and 3R,3′S (Figure 1). The ratios of astaxanthin stereoisomers vary widely in different organisms (Turujman et al., 1997; Osterlie et al., 1999; Coral-Hinostroza and Bjerkeng, 2002; Hussein et al., 2006), while synthetic astaxanthin is universally a racemic mixture composed of 25% 3R,3′R, 25% 3S,3′S, and 50% 3R,3′S isomers (Ambati et al., 2014). Astaxanthin can form monoesters and diesters, which is attributed to the reaction of its hydroxyl groups with fatty acids, such as palmitic, stearic, oleic, and linoleic acids. The esterified form generally dominates in different organisms, such as Antarctic krill, marine copepods and shrimps, and algae, while Phaffia rhodozyma predominantly contains its free form (Ambati et al., 2014).
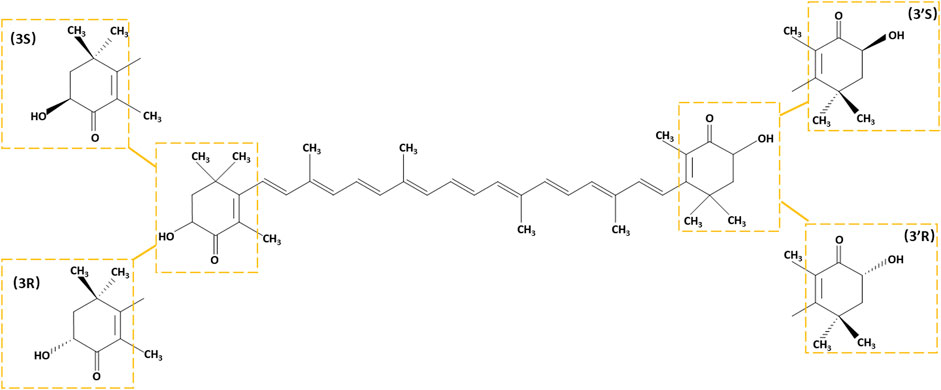
FIGURE 1. The chemical structure of astaxanthin. Stereoisomeric units are indicated with yellow boxes.
Astaxanthin has been commercially developed for various applications in food ingredients, cosmetics, nutritional supplements, and pharmaceuticals due to its varied beneficial health effects that counter inflammatory, cancerous, diabetic, and cardiac diseases (Yuan et al., 2011). In recent years, an increasing number of studies have shown that astaxanthin can modulate neuroinflammation and be neuroprotective. In this review, we summarize the functional roles and mechanisms of action of astaxanthin in neuroinflammation and discuss the prospects and challenges for its potential therapeutic application in modulating neuroinflammation and protecting against neuroinflammation-associated disorders.
Astaxanthin Modulates Neuroinflammation by Alleviating Oxidative Stress
Oxidative Stress and Neuroinflammation
Neuroinflammation is generally recognized as an intriguingly complex process involving synergistic actions between neurons and different types of glial cells, including microglia, astrocytes, oligodendrocytes, and oligodendrocyte precursor cells. The coordinated interplay of these cells is mediated by neurotransmitters, ions, neurotrophic factors, and cytokines. Microglia are the most acute cells and usually the first to sense abnormalities in the brain microenvironment, even in their presumed resting state (Kreutzberg, 1996; Davalos et al., 2005; Nimmerjahn et al., 2005; Prinz et al., 2019). Acting as resident macrophages in the brain, microglia primarily play pivotal roles in initiating neuroinflammation. Under stress (e.g., local ischemia, mechanical injury, epilepsy, or exogenous pathogens) (Konat et al., 2006; Lehnardt, 2010; Fitzgerald and Kagan, 2020), injured neurons or oligodendrocytes can release neurotransmitters (i.e., ATP, glutamate, and nitric oxide) to activate microglia. In an inflammatory model, microglia were recruited to the injury site with the activation of intracellular inflammasomes and the production of pro-inflammatory cytokines (Liu GJ. et al., 2009; Duan et al., 2009; Dibaj et al., 2010; Gundersen et al., 2015; Song et al., 2021). Activated microglia can be broadly categorized into two subtypes, M1 and M2, which have pro- and anti-inflammatory roles, respectively. The traditional M1/M2 terminology for microglia was referenced from a classical macrophage polarization mode, which helped deduce different phenotypes of activated microglia in neuroinflammation processes. However, this biphasic partition appears to be an oversimplification as activated microglia also display mixed phenotypes and intermediate states (Hu et al., 2012; Nakagawa and Chiba, 2015; Orihuela et al., 2016; Ransohoff, 2016).
A dynamic redox equilibrium based on a balance between the production of reactive oxygen/nitrogen species (RONS) and the antioxidant defense system is crucial for maintaining normal cellular processes in the brain. Once excessive RONS overwhelm the defense system comprised of a series of antioxidant molecules and enzymes, oxidative stress occurs, with detrimental effects on various physiological processes. The brain is particularly susceptible to oxidative stress as elevated RONS can cause oxidative damage to brain resident cells, especially neurons and oligodendrocytes. A vast body of evidence shows that oxidative stress and neuroinflammation are inseparable and closely interrelated. Oxidative stress-induced neuronal damage or apoptosis promotes the release of neurotransmitters, such as ATP and nitrogen monoxide (NO), which trigger the initiation of neuroinflammation (Yang and Zhou, 2019). Moreover, reactive oxygen species (ROS) act as secondary messengers to evoke immune activation, while persistent inflammation can also facilitate oxidative stress (Simpson and Oliver, 2020). Reactive nitrogen species (RNS) can activate matrix metalloproteinases (MMPs) to trigger blood-brain barrier (BBB) disruption and neuroinflammation (Chen HS. et al., 2018; Hannocks et al., 2019). Consequently, the interplay of RONS generation and neuroinflammation leads to a vicious circle, resulting in persistent damage or degeneration of the brain (Dias et al., 2013; Agrawal and Jha, 2020; Teixeira-Santos et al., 2020; Tewari et al., 2021).
Mechanisms by Which Astaxanthin Defends Against Oxidative Stress
Astaxanthin is a superior antioxidant for neutralizing RONS. ROS are defined as highly reactive oxidizing free radical agents, consisting of superoxide anions (O2•), hydroxyl (OH•), peroxyl (ROO•), and hydrogen peroxide (H2O2) radicals. RNS mostly consists of NO, nitrogen dioxide (NO2), and peroxynitrite (ONOO−). They all exhibit high reactivity to proteins, lipids, and DNA (Valko et al., 2006; Ryter et al., 2007; Valko et al., 2007). Therefore, the aberrant accumulation of RONS can lead to the impairment of cellular components associated with cellular senescence and various diseases (Gorrini et al., 2013; Sies, 2015; Bisht et al., 2017; van der Pol et al., 2019). Carotenoids have attracted considerable interest for their potent antioxidant activity. Several studies published almost 30 years ago revealed that the anti-oxidative activity of carotenoids is mediated by quenching singlet oxygen and free radicals (Palozza and Krinsky, 1992b; a; Tsuchiya et al., 1992). Astaxanthin has higher antioxidant activity by scavenging peroxyl radicals than other carotenoids, such as lycopene, β-carotene, α-carotene, and lutein (Naguib, 2000). It is about 550 times more capable of neutralizing singlet oxygen than α-tocopherol (Shimidzu et al., 2008). The powerful antioxidant capacity of astaxanthin depends both on the polyene system found in other carotenoids and on the terminal rings that are unique to its structure (Britton, 1995; Miller et al., 1996). Its polar β-ionone ring with a hydroxyl group at either end gives it a higher capacity to neutralize free radicals. It is postulated that astaxanthin in a dihydroxy-conjugated polyene form possesses a hydrogen atom suitable for blocking free radical reactions like that of α-tocopherol (Higuera-Ciapara et al., 2006).
In addition to direct radical scavenging, astaxanthin can also regulate the cellular enzymatic system to defend against excessive ROS production. Nuclear factor erythroid 2-related factor (Nrf2) is a pivotal transcription factor acting as the guardian of redox homeostasis and is considered a prospective therapeutic target for oxidative stress- and inflammation-associated diseases (Innamorato et al., 2008; Ahmed et al., 2017; Zhang R. et al., 2017). Nrf2 regulates the transcriptional activation of many cytoprotective genes, such as those encoding NADPH quinone dehydrogenase 1 (Nqo1), glutathione-S-transferase-α1 (GST-α1), and heme oxygenase-1 (H O -1), which protect against oxidative stress and inflammation (Telakowski-Hopkins et al., 1988; Rushmore et al., 1991; Friling et al., 1992; Lu et al., 2016). For instance, HO-1 catalyzes the degradation of heme into carbon monoxide, free iron, and biliverdin. Monoxide functions as an inhibitor of the nuclear factor-κB (NF-κB) pathway, contributing to the decreased expression of pro-inflammatory cytokines. It can also directly inhibit pro-inflammatory cytokines and activate anti-inflammatory cytokines, alleviating inflammation (Ahmed et al., 2017).
Astaxanthin can activate the Nrf2 pathway by promoting the activity of phosphoinositol-3 kinase/protein kinase B (PI3K/Akt) and extracellular signal-regulated protein kinase (ERK) pathways (Wang et al., 2010; Li et al., 2013). PI3K/Akt and ERK can promote the nuclear translocation of Nrf2, although the underlying mechanism has not been completely elucidated. Several E3 ligase adaptor proteins, such as Kelch-like ECH-associated protein 1 (Keap1) (Nguyen et al., 2003; Stewart et al., 2003), β-transducing repeat-containing protein (β-TrCP) (Rada et al., 2011; Cuadrado, 2015), and synoviolin 1 (Hrd1), tightly regulate Nrf2 levels (Wu et al., 2014). Some studies and reviews have suggested that astaxanthin may inhibit Nrf2 degradation via the Keap1 pathway (Wu et al., 2015; Fakhri et al., 2019). However, whether astaxanthin regulates Keap1 expression remains unclear as different studies presented controversial conclusions (Li L. et al., 2020; Ma et al., 2020). Although astaxanthin can promote ERK activity (Wang et al., 2010), disruption of the Keap1 from Nrf2 is not dependent on ERK activation, suggesting that astaxanthin activates Nrf2 via another pathway (Zipper and Mulcahy, 2003). The phosphorylation of Nrf2 mediated by glycogen synthase kinase 3β (GSK3β) can facilitate its ubiquitination and proteasomal degradation via β-TrCP (Cuadrado, 2015; Mathur et al., 2018). Moreover, GSK3β can activate the Fyn tyrosine kinase to induce the nuclear export of Nrf2 for its ubiquitination and degradation (Jain and Jaiswal, 2007; Niture et al., 2014). Considering that PI3K/Akt and ERK inhibit the activity of GSK3β (Ding et al., 2005; Kaidanovich-Beilin and Woodgett, 2011; Manning and Toker, 2017), we speculate that astaxanthin enhances the stability of Nrf2 by inactivating the GSK3β/β-TrCP or GSK3β/Fyn pathway (Figure 2).

FIGURE 2. Mechanisms by which astaxanthin defends against oxidative stress. Astaxanthin mitigates oxidative stress by directly scavenging radicals and regulating the cellular antioxidative enzymatic system via the Nrf2 pathway. The black arrows represent positive regulation, while the lines with T-shaped ends represent inhibition. A dotted line indicates an inconclusive pathway. The gray lines indicate phosphorylation, ubiquitination, or nuclear translocation. P and Ub represent the phosphorylation and ubiquitination of target proteins, respectively.
Astaxanthin can also regulate mitochondrial function in response to oxidation stress (Kim and Kim, 2018). Astaxanthin pretreatment can restored mitochondrial membrane potential (MMP) and significantly inhibit hydrogen peroxide-induced apoptosis of primary cortical neurons (Lu et al., 2010). Similarly, astaxanthin can improve mitochondrial function in a reduced state under oxidative stress (Wolf et al., 2010). Some evidence indicates that astaxanthin contributes to mitochondrial quality control and promotes mitochondrial biogenesis through the AMP-activated protein kinase (AMPK) pathway, however, the underlying mechanism remains unclear (Nishida et al., 2020; Nishida et al., 2021).
Neuroprotective Effect of Astaxanthin is Mediated by Inhibiting Oxidative Stress
Astaxanthin inhibits neuroinflammation by alleviating oxidative stress, thereby exerting a beneficial neuroprotective effect. Oxidative stress is a major cause of neuronal damage-induced neuroinflammation. Astaxanthin protects against neuronal loss in the rat hippocampus caused by epilepsy by attenuating oxidative damage (Lu et al., 2015). Moreover, the treatment of rats with astaxanthin attenuated epilepticus-induced cognitive dysfunction by inhibiting oxidative stress and neuroinflammation and mitigating a decrease in Nrf2 levels (Deng et al., 2019). Similarly, astaxanthin can prevent lanthanum oxide nanoparticle-induced hippocampal injury by reducing oxidative stress and neuroinflammation via the PI3K/AKT/Nrf-2 pathway (Yuan et al., 2020). Furthermore, astaxanthin ameliorates lipopolysaccharide (LPS)-induced oxidative stress, neuroinflammation, and memory dysfunction (Han et al., 2019). Astaxanthin also significantly protects against doxorubicin-induced memory impairment by blocking oxidative, inflammatory, and pro-apoptotic insults (El-Agamy et al., 2018). The ROS accumulating during oxidative stress are crucial triggers of microglial polarization (Simpson and Oliver, 2020). Astaxanthin treatment can halt M1 and promote M2 microglial polarization in response to LPS, suppressing neuroinflammation in BV2 microglial cells (Wen et al., 2017; Zhou et al., 2021). Consistent with its inhibitory role against microglial activation, astaxanthin can suppress the release of ATP from microglia by reducing the P2X7 receptor levels, although the mechanism underlying this remains elusive (Wang M. et al., 2020).
Astaxanthin Modulates Neuroinflammation by Inhibiting Pro-Inflammatory Cytokine Production
The aberrant production of pro-inflammatory cytokines in the CNS is a representative feature of neuroinflammation. Astaxanthin can inhibit the production of several pro-inflammatory cytokines, such as interleukin 1β (IL-1β), interleukin 6 (IL-6), and tumor necrosis factor-α (TNF-α), via repressing the NF-κB and mitogen-activated protein kinase (MAPK) pathways.
NF-κB Pathway
The NF-κB transcription factor family, a prototypical mediator of inflammation, is crucial for innate and adaptive immune responses. NF-κB family members, namely RelA (p65), RelB, c-Rel, NF-κB1 (p50), and NF-κB2 (p52), form homo- or heterodimers that activate the transcription of target genes by binding to a specific DNA element (Wan and Lenardo, 2009). NF-κB pathways can be classified into canonical and non-canonical (alternative) signaling pathways, which are induced by different pro-inflammatory cytokines through the participation of different family members. In the canonical NF-κB pathway, diverse stimuli, (e.g., LPS, TNFα, and IL-1) can trigger the activation of the multi-subunit IκB kinase (IKK) complex that further phosphorylates IκBα at two N-terminal serines and induces ubiquitin-mediated IκBα degradation. In most quiescent conditions, NF-κB signaling is inactivated because the dimers, (e.g., p50/RelA and p50/c-Rel) are bound to the inhibitory protein, IκBα (Senftleben et al., 2001; Hayden and Ghosh, 2008; Liu et al., 2017). The induced degradation of IκBα alleviates this inhibition, resulting in the transient nuclear translocation of the NF-κB dimers and subsequent expression of various pro-inflammatory factors, including cytokines, chemokines, and adhesion molecules (Hoesel and Schmid, 2013). In contrast, the non-canonical NF-κB pathway involves the processing of the NF-κB2 precursor protein (p100) by TNF receptor (TNFR) superfamily receptors (Sun, 2012; 2017). NF-κB-inducing kinase and IKKα mediate p100 phosphorylation and processing into p52, which then induces the transcriptional activation of target genes by forming a heterodimer with RelB (Senftleben et al., 2001; Xiao et al., 2001). Functionally, both NF-κB pathways are important in regulating different aspects of the innate and adaptive immune responses (Sun, 2011; Liu et al., 2017; Sun, 2017).
Mounting evidence indicates that astaxanthin inhibits neuroinflammation by halting NF-κB activation through the canonical NF-κB pathway. For example, administration of astaxanthin after the onset of status epilepticus in a rat model abrogated the induced expression of several inflammatory factors (e.g., cytochrome c oxidase subunit II [Cox-2], IL-1β, and TNFα) and p65 phosphorylation in the hippocampus and parahippocampal cortex (Deng et al., 2019). In addition, trans-astaxanthin could effectively antagonized LPS-induced TNF-α, IL-1β, and IL-6 expression in the hippocampus and the prefrontal cortex by regulating the NF-κB pathway (Jiang et al., 2016). Moreover, Zhang et al. (2014a) reported administration of a high dose of astaxanthin after subarachnoid hemorrhage significantly downregulated NF-κB DNA binding activity and the expression of inflammatory cytokines and intercellular adhesion molecule. Mechanistically, astaxanthin can effectively reduce NF-κB-related inflammation by suppressing IKKβ phosphorylation and the nuclear translocation of the p65 subunit (Bhuvaneswari et al., 2014). Moreover, astaxanthin can decrease p65 phosphorylation, which may impair the nuclear translocation and DNA binding activity of p50/p65 dimers (Figure 3) (Terazawa et al., 2012; Zhang et al., 2014a).
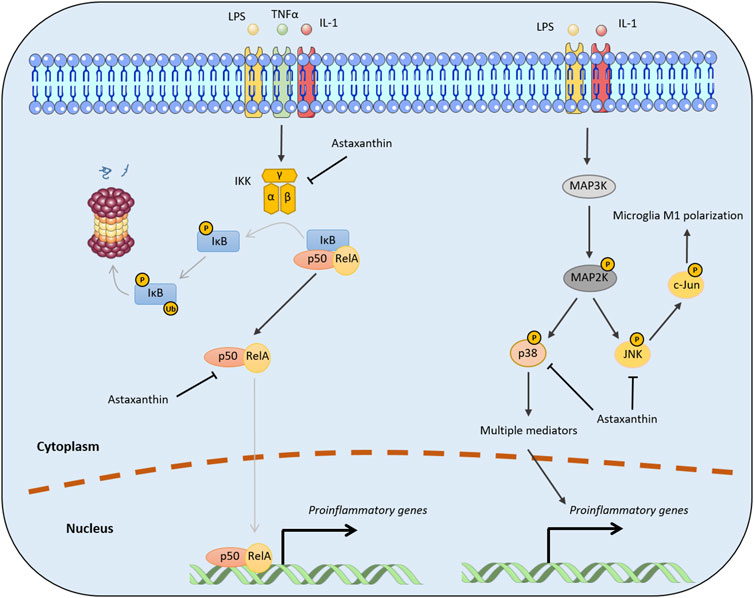
FIGURE 3. Molecular mechanisms by which astaxanthin inhibits pro-inflammatory cytokine production. The black arrows represent positive regulation, while the lines with T-shaped ends represent inhibition. Gray lines indicate the processes of phosphorylation, ubiquitination, or nuclear translocation. P and Ub represent the phosphorylation and ubiquitination of target proteins, respectively.
MAPK Pathway
The MAPK family of serine/threonine kinases is involved in the immune response (Arthur and Ley, 2013). There are 14 known MAPK proteins in mammalian cells, that function in seven distinct signaling pathways (Mathien et al., 2021). Based on structural and functional features, MAPK family members can be divided into classic MAPKs, consisting of the ERK1/2, c-Jun N-terminal kinases (JNK1/2/3), p38 (p38a/b/c/d), and ERK5 subfamilies, and atypical MAPKs, which include the ERK3/4, ERK7, and Nemo-like kinase subfamilies (Coulombe and Meloche, 2007; Mathien et al., 2021). The MAPK signaling pathway involves a cascade of three kinases that lead to the successive phosphorylation of different kinase targets. Briefly, MAPK kinase (MAP3K) is first activated in response to pathogen infection or tissue damage through the Toll-like or interleukin-1 receptors. Accordingly, MAP3K activates a MAPK kinase (MAP2K), which activates MAPK subfamily members by dual phosphorylation of the Thr–X–Tyr activation motif. Finally, MAPK proteins promote inflammatory reactions by producing different pro-inflammatory cytokines (Dumitru et al., 2000) and modulating macrophage polarization (Rincon and Davis, 2009).
The mechanism by which astaxanthin affects MAPK signaling pathways is intrinsically linked to its regulation of MAPK proteins, which act as inflammatory signaling mediators. Consistent with this mechanism, a molecular docking study identified an interaction between astaxanthin and human p38. Astaxanthin inhibits p38 by occupying its active site and interacting with surrounding amino acid residues (Yang et al., 2019). Moreover, astaxanthin can promote M2 polarization of BV2 cells and suppress neuroinflammation by inhibiting NF-κB and JNK signaling (Figure 3). Specifically, astaxanthin can reduce phosphorylated c-Jun levels which is indicative of the inactivation of JNK signaling, although the mechanism underlying this remains unclear (Wen et al., 2017).
Astaxanthin Modulates Neuroinflammation by Maintaining Blood–Brain Barrier Integrity and Inhibiting Peripheral Inflammation
BBB, Peripheral inflammation, and Neuroinflammation in the CNS
Disruption of the BBB and peripheral inflammation are two factors contributing to neuroinflammation. The mammalian brain has an intricate system of blood vessels; cerebral blood vessels measure ∼640 km with an endothelial surface area of ∼12 m2 (Abbott et al., 2010) ensuring efficient molecular exchange. The BBB is formed by vascular endothelial cells, mural cells (e.g., pericytes and smooth muscle cells), and perivascular astrocytic end-feet around capillaries (or glial limitans ensheathing the penetrating arterioles (Yao et al., 2014)). Its permeability is also regulated by surrounding microglia and neurons (Zhao et al., 2015; Sweeney et al., 2019). The BBB can prevent blood cells, neurotoxic components, and pathogens from entering the brain, while its breakdown and dysfunction can lead to various neurological deficits (Obermeier et al., 2013; Liebner et al., 2018). Peripheral inflammation involves the activation of the immune system outside of the CNS and the release of pro-inflammatory cytokines against various pathological stimuli in the peripheral blood. Peripheral inflammation is a crucial trigger of BBB disruption because increasing the levels of pro-inflammatory cytokines and cytotoxic pathogens or molecules in the plasma during infections damages its integrity (Huang et al., 2021). Impaired BBB permeability allows peripheral inflammatory molecules and signals to access the brain, leading to neuroinflammation. Moreover, neuroinflammation can further contribute to BBB disruption by damaging endothelial tight junction proteins (Schreibelt et al., 2007). Leukocytes (e.g.,monocytes, neutrophils, and T- and B-lymphocytes) and secreted inflammatory cytokines in the peripheral blood can infiltrate the brain due to BBB dysfunction, triggering or exacerbating the progression of neuroinflammation (Kim et al., 2016; Gimenez-Arnau et al., 2021; Pluta et al., 2021). Therefore, improving BBB integrity and mitigating peripheral inflammation can be neuroprotective by inhibiting neuroinflammation.
Astaxanthin Protects BBB integrity
Astaxanthin has enormous potential for protecting the BBB from disruption or dysfunction. Astaxanthin treatment after subarachnoid hemorrhage significantly reduces brain edema, BBB dysfunction, and concomitant neuroinflammation in rat and rabbit models (Zhang et al., 2014a; Zhang et al., 2014c; Zhang et al., 2015). Similarly, pretreatment with astaxanthin prevents the BBB disruption and neuroinflammation caused by kaliotoxin (Sifi et al., 2016). Mechanistically, astaxanthin can restore the survival rate, increase oxidative stress resistance, and maintain the tight junction stability of rodent brain microvascular endothelial cells in response to oxygen-glucose deprivation/reperfusion treatment or subarachnoid hemorrhage, indicating its effectiveness in protecting the BBB (Zhang et al., 2015; Kuo et al., 2019) (Figure 4).
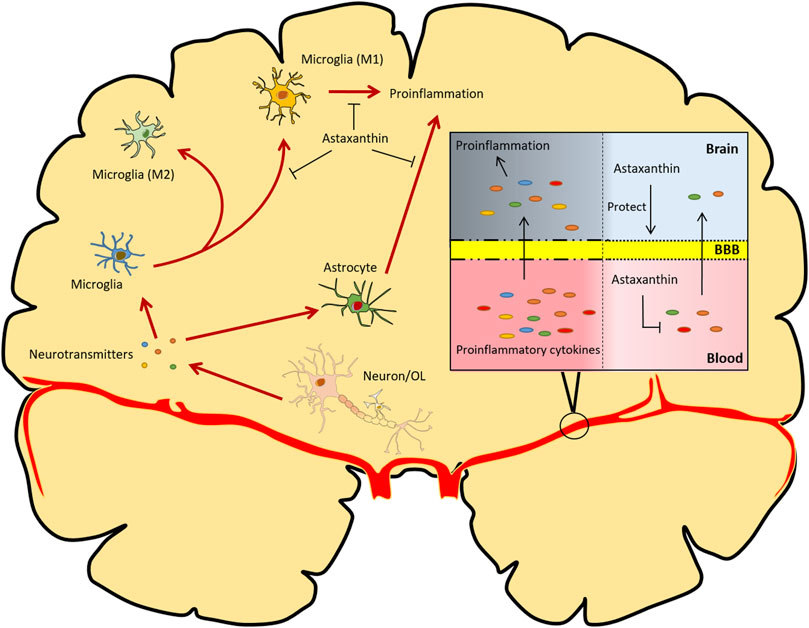
FIGURE 4. Overview of the functional implications of astaxanthin in modulating neuroinflammation. Astaxanthin counteracts oxidative stress-induced damage to neurons and glial cells. Astaxanthin can also reduce the production of pro-inflammatory cytokines in the brain and retard the M1 polarization of microglia. Additionally, astaxanthin protects BBB integrity and inhibits the production and infiltration of inflammatory cytokines derived from peripheral inflammation. OL, oligodendrocytes. The red arrows represent processes of neuroinflammation; the black arrows represent promotion, protection or transportation; the lines with T-shaped ends represent inhibition.
Astaxanthin inhibits Peripheral inflammation
The anti-inflammatory properties of astaxanthin have been demonstrated in various in vivo and in vitro studies. For example, astaxanthin decreased mRNA and protein expression levels of pro-inflammatory genes in macrophages, including TNF-α, transforming growth factor (TGF-β), IL-1β, IL-6, COX-2, and inducible nitric oxide synthase (iNOS) (Kishimoto et al., 2010; Farruggia et al., 2018; Cai et al., 2019; Kang et al., 2020; Binatti et al., 2021). It could also inhibit the expression of pro-inflammatory cytokines in human corneal epithelial cells (Li H. et al., 2020) and keratinocytes (Terazawa et al., 2012). Similarly, astaxanthin could suppress the activation of the NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome in macrophages (Peng et al., 2020). Moreover, multiple in vivo studies have revealed the anti-inflammatory effects of astaxanthin in different disease models, including non-alcoholic fatty liver (Bhuvaneswari et al., 2014; Ni et al., 2015; Chiu et al., 2016; Jia et al., 2016), hepatic injury or fibrosis (Zhang J. et al., 2017; Han et al., 2018; Liu et al., 2018; Zhang Z. et al., 2020), kidney injury (Guo et al., 2021), myocardial injury (Xie et al., 2020), diabetes mellitus (Feng et al., 2020; Liu et al., 2020; Zhuge et al., 2021), arthritis (Park MH. et al., 2020; Kumar et al., 2020), gastroenteritis inflammation (Han et al., 2020; Chen Y. et al., 2021), acute pancreatitis (Yasui et al., 2011), asthma (Hwang et al., 2017), atopic dermatitis (Park et al., 2018; Park et al., 2019), and hyperosmoticity-induced dry eye (Li H. et al., 2020). As described earlier, astaxanthin counters inflammation primarily by blocking the NF-κB-dependent signaling pathways. However, astaxanthin can also promote cyclooxygenase inhibition and downregulation of prostaglandin and TNF-α by decreasing nitric oxide (NO) production and iNOS activity in an NF-κB pathway-independent manner (Ohgami et al., 2003). Additionally, dietary astaxanthin supplementation in females decreases DNA oxidative damage and lipid-peroxidation, reduces C-reactive protein concentrations, enhances natural killer cell cytotoxic activity, and increases total T- and B-cell subpopulations, indicating the beneficial effects of astaxanthin in ameliorating oxidative stress and inflammation and in improving the immune response, although the mechanisms involved require further elucidation (Park et al., 2010).
Therapeutic Benefits of Astaxanthin in Neuroinflammation-Associated Disorders
Neuroinflammation is prevalent in neurodegenerative and neurodevelopmental diseases and metabolic neuropathy. Notably, astaxanthin has shown efficacy in modulating neuroinflammation in different disease models. Because astaxanthin has good biosafety and high bioavailability, its medical use, especially in modulating neuroinflammation, has always been a hot topic that warrants further investigation. In this section, the therapeutic benefits of astaxanthin against neurological disorders are summarized concerning neuroinflammation modulation. Its effects and potential mechanisms of action in inhibiting neuroinflammation in different diseases are shown in Figure 5 and Table 1.
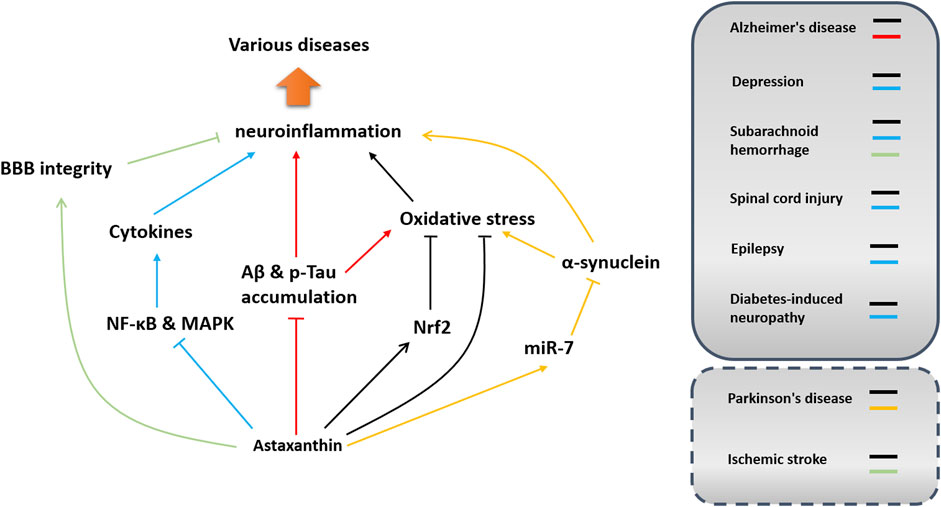
FIGURE 5. Implications and potential mechanisms of astaxanthin in neuroinflammation-associated disorders. Potential pathways involved in neuroinflammation modulation in different disease models are depicted in different colors. Arrows indicate acceleration or promotion, while lines with T-shaped ends represent inhibition or blocking. Dashed boxes indicate that the function of astaxanthin in modulating neuroinflammation has not been confirmed in these diseases.
Alzheimer’s Disease
Chronic, aberrant neuroinflammation is a hallmark of different neurodegenerative diseases. Alzheimer’s disease (AD), a progressive neurodegenerative disorder characterized by memory loss and dementia, is always combined with neuroinflammation in the brain. Considering the beneficial effects of astaxanthin in modulating neuroinflammation, this carotenoid could be developed as a therapeutic agent for preventing or alleviating neurodegeneration. A randomized, double-blind clinical trial showed that daily supplementation with a combination of 3 mg astaxanthin and 5 mg sesamin for 6 weeks improved cognitive function in those with mild cognitive impairment (Ito et al., 2018).
AD is characterized by the aggregation of neurotoxic proteins, such as β-amyloid (Aβ) and hyperphosphorylated Tau (p-Tau), in the central nervous system, leading to chronic neuroinflammation by triggering microglial activation (Leng and Edison, 2021). Some studies have shown that astaxanthin (or astaxanthin-derived diesters) could reduce Aβ42 deposition and Tau phosphorylation, resulting in the suppression of neuroinflammation and enhancement of learning and memory in APP/SP1 transgenic mice, APPNL−G-F mice, or Aβ-infused AD rat (Che et al., 2018; Rahman et al., 2019; Taksima et al., 2019; Hongo et al., 2020). Astaxanthin likely reduces Aβ generation and Tau phosphorylation by inhibiting GSK3β activity (Figure 2) (Rahman et al., 2019). GSK3 can phosphorylate Tau at more than 42 sites (Toral-Rios et al., 2020) and its activity strongly correlates with the number of neurofibrillary tangles in AD brains (Leroy et al., 2002). GSK3β can also enhance Aβ production by promoting BACE1 transcription (Ly et al., 2013), which can partially explain the inhibitory effects of astaxanthin on BACE1 expression in AlCl3-induced AD-like rats (Hafez et al., 2021).
Moreover, impaired proteostasis caused by Aβ and p-Tau accumulation in the AD brain further contributes to oxidative stress, producing excessive reactive oxygen and nitrogen species. Oxidative stress has been detected in the early stages of AD, shown by a reduction in the levels of detoxifying enzymes, including superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase enzymes (GPx) (Cioanca et al., 2013; Hritcu et al., 2014). Astaxanthin can suppress oxidative stress by improving GPx activity, inhibiting lipid peroxidation, and reducing products of protein oxidation and superoxide anion in the cortex and the hippocampus of AD rats (Taksima et al., 2019). Therefore, astaxanthin can potentially suppress neuroinflammation by alleviating oxidative stress in the context of AD. Astaxanthin significantly elevated Nrf2 in an AD-like rat model (Hafez et al., 2021), consistent with its augmentative effect on Ho-1 enzyme expression (Wang et al., 2010; Wen et al., 2015). These data suggest that astaxanthin might also attenuate oxidation stress and subsequent neuroinflammation by regulating the Nrf2 pathway in the context of AD. Astaxanthin was also reported to decrease neuroinflammation, restore choline acetyltransferase positive fibers, increase the spine numbers of pyramidal neurons in the hippocampal CA1 region, and ameliorate the behavioral deficits in a ferrous amyloid buthionine (FAB)-infused sporadic AD rat model (Chen MH. et al., 2021). Astaxanthin likely modulates cholinergic decline by increasing the expression of nerve growth factor (NGF), which could prevent the degeneration of cholinergic neurons (Counts and Mufson, 2005; Nai et al., 2018). Thus, astaxanthin represents a potential therapeutic agent for slowing AD progression by inhibiting neuroinflammation.
In the AD brain, increased levels of pro-inflammatory cytokines (e.g., TNF-α, IL-1, and IL-12) may contribute to aberrant neuroinflammation and neurological impairment by triggering sustained microglial activation (Ahmad et al., 2022). Impaired BBB integrity in AD facilitates the infiltration of immune cells and cytokines from the peripheral blood that function as adverse factors or accomplices with resident immune cells to trigger neuroinflammation (Zenaro et al., 2017). Astaxanthin can reduce the release of pro-inflammatory factors in the BV2 cell line by regulating the NF-κB and MAPK pathways and can also protect BBB integrity and inhibit peripheral inflammation. However, whether astaxanthin can ameliorate AD neuropathy by modulating neuroinflammation through these mechanisms remains unknown.
Parkinson’s Disease
Neuroinflammatory processes are also involved in the pathogenesis of Parkinson’s disease (PD). Increased pro-inflammatory cytokines levels in the brain, cerebrospinal fluid (CSF), and blood have been found postmortem in PD patients (Nagatsu et al., 2000). Abnormal neuroinflammation has also been observed in PD models induced by 6-hydroxydopamine, MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine), and α-synuclein (Hirsch and Hunot, 2009). Chronic, prolonged inflammation in microglia, which induces the death and dysfunction of neighboring dopaminergic neurons, is a crucial mechanism underlying the pathogenesis of PD. Hence, immune-based neuroprotection is widely considered an effective approach in PD therapy (Hirsch and Standaert, 2021). Astaxanthin has shown promising therapeutic effects in PD, which have been discussed in other reviews (Galasso et al., 2018; Park HA. et al., 2020; Bahbah et al., 2021). Whether astaxanthin can modulate neuroinflammation in the context of PD remains elusive. Focusing specifically on modulating neuroinflammation, astaxanthin deserves more in-depth investigation for PD treatment. Aggregated α-synuclein can stimulate the expression of pro-inflammatory cytokines (e.g., IL-1β, TNF-α, and IFN-γ) through activation of the NF-κB pathway in microglia (Reynolds et al., 2008). Astaxanthin significantly reduces α-synuclein expression and apoptosis in SH-SY5Y cells treated with 1-methyl-4-phenylpyridinium (MPP+), which resembles PD by initiating neuron death via a miR-7-dependent pathway (Shen et al., 2021). Because astaxanthin can attenuate NF-κB activation (Figure 3), it may act by suppressing neuroinflammation induced by α-synuclein in PD. Similarly, astaxanthin can attenuate PD progression by reducing apoptosis in dopamine neurons through the P38 MAPK pathway (Wang CC. et al., 2020) while also triggering the production of pro-inflammatory cytokines (Figure 3).
Oxidative stress in the PD brain has been observed in postmortem human samples and animals and is another risk factor leading to the dysfunction of dopaminergic neurons (Gilgun-Sherki et al., 2001; Gaki and Papavassiliou, 2014). Mitochondrial dysfunction-induced oxidative stress can trigger neuroinflammation-associated dopaminergic neuronal cell death and parkinsonism (Shulman et al., 2011). Considering astaxanthin can regulate mitochondrial function that prevents the excessive production of ROS, it could be used as a protective agent to suppress neuroinflammation-associated parkinsonism. Astaxanthin has exhibited an anti-oxidative effect in MPP-induced PC12 cells through the HO-1/NOX2 and Sp1/NR1 pathways (Ye et al., 2012; Ye et al., 2013) and an anti-apoptosis effect in 6-hydroxydopamine-induced SH-SY5Y cells via a mitochondria-targeted protective mechanism (Ikeda et al., 2008; Liu X. et al., 2009).
Depression
Dysfunctional immune and endocrine systems are two contributors to depression. Neuroinflammation in the brain is associated with the pathophysiology of depression, although the mechanism remains unsolved (Kim et al., 2016). The P2x7-Nlrp3 inflammasome cascade acts as a key mechanism underlying depression. The NF-κB and MAPK pathways, through their control of pro-inflammatory cytokine production and NLRP3 inflammasome activation, are recognized as two significant mechanisms in the pathogenesis of depression (Troubat et al., 2021). Trans-astaxanthin can rescue LPS-induced depressive-like behavior by antagonizing neuroinflammation in an NF-κB-dependent manner (Jiang et al., 2016). Moreover, astaxanthin has anti-depressant effects on diabetic mice by inhibiting inflammation. Astaxanthin can significantly reduce the number of GFAP-positive cells (mostly astrocytes) in the hippocampus and hypothalamus and downregulate the expression of IL-6, IL-1β and COX-2 in the hippocampus of depressive mice (Zhou et al., 2017). Taken together, these data suggest that astaxanthin could be an effective therapeutic agent for depression by targeting the associated inflammation.
Depression is also accompanied by aberrant oxidative stress in the brain, manifested as increased lipid peroxidation and free radicals, an abnormal antioxidant system, oxidative damage,e and pro-inflammatory cytokine overproduction (Liu et al., 2015; Vavakova et al., 2015). Impaired activation of the Nrf2-dependent antioxidant system can lead to stress-induced vulnerability to depression. Several drugs targeting the Nrf2 pathway (e.g., melatonin and edaravone) have beneficial effects against neuroinflammation and depressive-like behaviors (Maes et al., 2012; Arioz et al., 2019; Dang et al., 2022). Considering astaxanthin as a potential neuroinflammation modulator counteracting oxidative stress via the Nrf2 pathway, it may also be effective in treating depression.
Cardiac-Cerebral Vascular Diseases
Subarachnoid hemorrhage (SAH) caused by traumatic or non-traumatic cerebral angiorrhexis is associated with profound systemic complications, leading to high mortality rates and long-term neurological disabilities (Macdonald, 2014; Macdonald and Schweizer, 2017). Systemic immune responses always occur after SAH, which is commonly manifested by high levels of pro-inflammatory cytokines (IL-1, IL-6, and TNF-α) in peripheral blood and brain (Jung et al., 2013). Moreover, resident immune responses mediated by microglia activation in the brain can cause secondary brain damage after SAH (Heinz et al., 2021). Therefore immunosuppressive treatment is effective and fundamental to improving SAH prognosis. Astaxanthin antagonizes neuronal apoptosis by counteracting the neuroinflammatory responses after subarachnoid hemorrhage (SAH). The neuroprotective effect of astaxanthin in SAH is mediated by regulating mitochondrial function through the phosphorylation-dependent inactivation of BCL2, a crucial agonist of cell death. Astaxanthin may regulate BCL2 phosphorylation through the PI3K/Akt pathway (Zhang et al., 2014b). Moreover, astaxanthin could attenuate neuroinflammation by rescuing BBB disruption and inhibiting NF-κB-dependent expression of inflammatory cytokines in a SAH rat model (Zhang et al., 2014a).
Ischemic stroke caused by cerebral infarction is a common cerebrovascular disease and a striking cause of death and severe disability worldwide (Feske, 2021). Pro-inflammatory signals can rapidly activate resident immune cells in the brain in response to ischemic stroke and promote the infiltration of a wide range of peripheral inflammatory cells into the ischemic region, exacerbating brain damage (Jayaraj et al., 2019). Neuroinflammation, which can be both beneficial and detrimental, has become a widely studied target for therapeutic intervention for ischemic stroke. Notably, astaxanthin has shown a neuroprotective effect after ischemic stroke by attenuating oxidative stress. For example, astaxanthin could reduce brain injury in a rat ischemic stroke model by decreasing oxidative stress and inhibiting glutamate overflow (Shen et al., 2009). In other studies, astaxanthin attenuated oxidative stress and promoted axon regeneration and reconnection after ischemic stroke (Lee et al., 2010; Wang et al., 2019). In an oxygen and glucose deprivation (OGD) model, astaxanthin treatment protected cultured SH-SY5Y cells against OGD-induced cytotoxicity by modulating oxidative stress (Zhang J. et al., 2020). Impaired BBB permeability after ischemic stroke is an underlying cause of the invasion of peripheral inflammatory cells into the brain (Jayaraj et al., 2019). RNS are reactive molecules that trigger BBB disruption following cerebral ischemia-reperfusion injury (Chen et al., 2013). They are recognized as important therapeutic targets for identifying drug candidates for attenuating brain injury (Chen H. et al., 2018; Chen HS. et al., 2018; Feng et al., 2018; Chen et al., 2020). Astaxanthin can quench RNS, such as peroxynitrite (Rodrigues et al., 2012) and nitrogen monoxide (Khan et al., 2010), which might reduce RNS-induced BBB impairment under some pathological conditions. The protective effect of astaxanthin on BBB integrity provides another mechanism for anti-inflammation in ischemic stroke; however, the mechanism requires further elucidation. The available evidence indicates that astaxanthin could be used as a potential neuroinflammation modulator in response to ischemic stroke-induced brain damage.
The interaction between neuroinflammation and cardiovascular disease has recently become a research focus. Neuroinflammation is both a cause and a consequence of cardiovascular disease (Richards et al., 2022). Neuroinflammation has been implicated in hypertension (in animal models and humans) through multiple mechanisms (Mowry and Biancardi, 2019; Sharma et al., 2019). It is particularly apparent in the hypothalamic paraventricular nucleus (PVN) of hypertensive rodents (Sklerov et al., 2019). Moreover, increased expression of pro-inflammatory cytokines has been reported in cardio-regulatory brain regions in hypertensive animals (Shi et al., 2010b; Qi et al., 2016) and angiotensin II-induced hypertension was found to trigger microglial activation predominantly in the PVN (Li Y. et al., 2020). In contrast, the administration of anti-inflammatory cytokines (e.g., IL-10) and minocycline induced an antihypertensive response by alleviating microglial activation (Shi et al., 2010a).
Astaxanthin is recognized as a potential therapeutic agent against cardiovascular disease. Disodium disuccinate astaxanthin protected the myocardium in a myocardial ischemia-reperfusion model by reducing inflammation and myocardial apoptosis (Gross and Lockwood, 2005; Lauver et al., 2005; Gross et al., 2006). In addition, astaxanthin improved arterial hypertension by decreasing the production of superoxide anions in rodents (Monroy-Ruiz et al., 2011). Moreover, astaxanthin may be protective against atherosclerotic cardiovascular disease by reducing oxidative stress and inflammation and improving glucose metabolism (Fassett and Coombes, 2011; Kishimoto et al., 2016). Astaxanthin is a likely candidate for treating cardiovascular disease, considering its cardiovascular protective effects and its therapeutic effects against neuroinflammation.
Spinal Cord injury
Spinal cord injury (SCI) is a devastating condition associated with impaired motor ability and long-term comorbidity. Neural restoration in the spinal cord remains the most effective treatment for SCI, while aberrant neuroinflammation contributes to a poor prognosis (Brockie et al., 2021). Astaxanthin can decrease the production of TNF-α by modulating the phosphorylation of MAPK p38 (threonine 180 and tyrosine 182) and improve sensory and motor function following rat spinal cord injury (Fakhri et al., 2018), indicating that this agent can inhibit inflammatory reactions in the secondary phase of SCI. Thus, it represents a promising candidate for enhancing functional recovery after SCI. Inhibiting oxidative stress and Nrf2 activation may also promote functional recovery after SCI (Jin et al., 2021). Therefore, astaxanthin treatment might improve neural restoration by counteracting oxidative stress-induced neuroinflammation.
Epilepsy
Alleviating neuroinflammation can also reduce cellular damage caused by epilepsy (Jayaraj et al., 2019; Parsons et al., 2022). Neuroinflammation also acts as a trigger for epilepsy. Pro-inflammatory cytokines (e.g., IL-1β, IL-6, and TNF-α) can induce this disorder (Vezzani et al., 2016). Indeed, increased levels of these cytokines have been found in the cerebrospinal fluid (CSF) and blood serum of patients with epilepsy (Dupuis and Auvin, 2015). Thus, astaxanthin is a potential agent for improving the prognosis of epilepsy patients by inhibiting the neuroinflammation-associated injury following a seizure (Lu et al., 2015; Deng et al., 2019).
Diabetes-Induced Neuropathy
Diabetes-induced neurological complications, such as vascular pathogenesis in the brain, impaired neuronal regeneration, neurodegeneration, and peripheral neuropathy, are major obstacles to improving the quality of life of diabetes patients (Asslih et al., 2021). Aberrant neuroinflammation is an important mechanism triggering these complications. Therefore, inhibitors of neuroinflammation (e.g., astaxanthin) could serve as auxiliary supplements or drugs for alleviating diabetic neuropathy (Asslih et al., 2021). Astaxanthin can prevent diabetic nephropathy and renal cell damage by reducing oxidative stress and inflammation (Naito et al., 2004; Manabe et al., 2008; Kim et al., 2009). In addition, insulin sensitivity is enhanced after feeding mice astaxanthin (Bhuvaneswari et al., 2010). Moreover, it has provided a significant beneficial effect in the treatment of diabetes. For example, astaxanthin can reduce oxidative stress-induced hyperglycemia in pancreatic β-cells, improving serum insulin and glucose levels (Uchiyama et al., 2002; Nakano et al., 2008). However, whether the improved blood glucose levels generated by astaxanthin can inhibit neuroinflammation remains unclear.
Challenges and Prospects for the Medical Application of Astaxanthin
Source, Safety, and Bioavailability
Astaxanthin is commercially used as both a food additive and supplement. Synthetic astaxanthin is mostly consumed as a food additive for animals, while only natural astaxanthin can be used as a food ingredient for humans. In 1987, the United States Food and Drug Administration (FDA) approved synthetic astaxanthin produced by Roche as a food additive in the aquaculture industry, while natural astaxanthin was approved as a nutraceutical for humans in 1999. The chemical process by which astaxanthin is synthesized can inevitably generate detrimental by-products, which reduce its biosafety and bioactivity. Natural astaxanthin can be sourced from microalgae, yeast, shrimp, krill, and plankton; however, it is primarily commercially produced from Phaffia rhodozyma and H. pluvialis due to their advantages of astaxanthin content, growth rate, and cost of cultivation. H. pluvialis, a green freshwater microalga, is widely recognized as the optimal natural microbial factory for astaxanthin production due to its high carotenoid content and easy extraction method. H. pluvialis can synthesize and accumulate astaxanthin up to 4% of total cellular dry weight. The high protein content of microalgae is another reason for the development of H. pluvialis as a source of astaxanthin production (Hu, 2019). There are currently commercial producers of astaxanthin-rich H. pluvialis worldwide, with a large output capacity. Optimizing the cultivation strategy and developing an effective extraction method has reduced the cost of industrial production, promoting natural astaxanthin, which is more accepted for public consumption in various countries. Moreover, the increasing awareness of the multifunctional benefits of astaxanthin should induce rapid growth in this market. According to Grand View Research, a market research and consulting company in the US, the compound annual growth rate of the astaxanthin market is expected to increase by 19.3% from 2021 to 2028.
The safety of astaxanthin for use in humans is well documented. The FDA has approved astaxanthin sourced from H. pluvialis as a food ingredient since 2010 (GRAS [Generally Recognized as Safe] No. 294 and No. 580). In Europe, astaxanthin from H. pluvialis is authorized by the European Food Safety Authority Commission as a food supplement at levels up to 8 mg/day for adults (EFSA Panel on Nutrition Novel Foods and Food Allergens et al., 2020). In 2010, astaxanthin-rich H. pluvialis was also listed as an edible strain by the Ministry of Public Health of China. Taking these developments together, astaxanthin is generally accepted as safe for food and is in growing demand. Thus, astaxanthin products have great potential to be integrated into everyday life, bringing benefits to public health.
Astaxanthin is a fat-soluble compound incorporated into micelles with lipids for absorption by mucosal cells in the intestinum tenue. In the form of chylomicrons, it is transported into the liver via the lymphatic system and subsequently transported by lipoproteins to different organs and tissues via the circulation (Parker, 1996; Coral-Hinostroza et al., 2004). The form of astaxanthin can affect its absorption efficiency. According to clinical research, the maximum plasma level of astaxanthin can reach up to 1.3 ± 0.1 mg/L with a plasma elimination half-life of 21 ± 11 h after a single dose of 100 mg of free astaxanthin (Osterlie et al., 2000). In contrast, ingestion of 100 mg of astaxanthin diesters results in a maximum plasma astaxanthin level of 0.28 ± 0.12 mg/L with a plasma elimination half-life of 52 ± 40 h, indicating the complicated absorption of astaxanthin due to the additional hydrolysis of astaxanthin esters (Coral-Hinostroza et al., 2004). The bioavailability of astaxanthin is also influenced by concomitant diet and lifestyle. Its consumption in combination with oil or an oil-based formulation can enhance the absorption of astaxanthin (Mercke Odeberg et al., 2003), while smoking can reduce its bioavailability by decreasing its half-life (Okada et al., 2009).
Unresolved Issues in the Medical Application of Astaxanthin
The development of astaxanthin as an effective modulator to relieve dysregulated neuroinflammation may be an effective neuroprotective strategy; however, challenges remain. First, neuroinflammation functions as a double-edged sword for maintaining the homeostasis of the nervous system. Acute neuroinflammation is beneficial in rebuilding balanced metabolism by clearing cellular debris and pathogens, but aberrantly prolonged or chronic neuroinflammation may be harmful. Hence, it may be an oversimplification to use astaxanthin as an inhibitor of neuroinflammation in all situations. Furthermore, animal experiments and clinical research have indicated that the long-term consumption of high-dose astaxanthin is associated with its medical efficacy. Therefore, the likelihood of incidental adverse effects, such as pigmentation and allergies (Nutrition and Allergies EFSA Panel on Dietetic Products, 2014), need to be studied. Moreover, the role of astaxanthin in abrogating neuroinflammation is complex, and side effects due to the targeting of the NF-κB, Nrf2, and MAPK pathways remain a concern for any potential clinical applications (Sporn and Liby, 2012; Ramadass et al., 2020; Zang et al., 2020; Moreira et al., 2021). Therefore, the molecular mechanisms of astaxanthin in these pathways require further investigation. Thus far, astaxanthin has only been shown to directly interact with p38 MAPK (Yang et al., 2019), while other direct targets for astaxanthin in different pathways remain elusive.
In summary, astaxanthin is currently only used as a food ingredient. The potential efficacy of astaxanthin as a drug that modulates neuroinflammation requires further elucidation, although its neuroprotective effects are well-documented. First, there is a need to explore the specific target(s) of astaxanthin in different pathways to further understand the underlying mechanisms of its modulation of neuroinflammation. In addition, the combination of astaxanthin with other existing chemicals may more effectively counteract neuroinflammation (Polotow et al., 2015; Qiao et al., 2017; Ito et al., 2018). Moreover, improved formulations (e.g., microencapsulation) or modified forms (e.g., docosahexaenoic-acid-acylated astaxanthin) may enhance the bioavailability and bioactivity of this agent (Yang et al., 2021; Zhao et al., 2021).
Some issues with the potential adverse effects of astaxanthin may provide more misgivings during its medical application, although they have not been reported in animal studies or clinical research using a reasonable dose range according to FDA GRAS notices. Some ingredients (e.g., sunflower or krill oil) may be used in astaxanthin production. Thus, allergenicity may remain uncertain, especially for immunocompromised individuals. In addition, astaxanthin can also induce cytochrome P450 enzymes in rats (Ohno et al., 2011) and primary human hepatocytes (Kistler et al., 2002). However, an FDA GRAS panel has concluded that astaxanthin at human exposure levels is unlikely to affect cytochrome P450 enzymes. Pigmentation in human tissues has also been raised as a concern (Petri and Lundebye, 2007); however, the FDA GRAS panel has also clarified this issue by concluding that the proposed astaxanthin dose levels do not raise safety concerns for pigmentation in humans.
Conclusion
Neuroinflammation functions as a defense mechanism to protect the central nervous system from different insults; however, it is also a pathological hallmark of numerous neurological and neurodegenerative diseases. Astaxanthin, a natural carotenoid with marked antioxidant capacity, suppresses neuroinflammation and is thus neuroprotective. First and foremost, astaxanthin can effectively counteract the oxidative stress-induced cell injury and death known to trigger neuroinflammation, in part, by inhibiting the production of pro-inflammatory cytokines via the NF-κB and MAPK pathways. It can also potentially modulate neuroinflammation in the brain by maintaining the integrity of the BBB and alleviating peripheral inflammation. To date, astaxanthin has been developed as a commercial food ingredient with well-documented biosafety, numerous bioactivities, and a reasonable price. At the same time, it also exhibits abundant therapeutic benefits for modulating neuroinflammation. Although several issues concerning its medical efficacy and mechanisms for treating neuroinflammation-associated diseases require further elucidation, astaxanthin remains a prospective medicinal component for the modulation of neuroinflammation.
Author Contributions
SW: conceptualization and writing of the original draft. XQ: conceptualization, supervision, validation, writing, review and editing, and funding acquisition.
Funding
This study was supported by grants from the United States National Institutes of Health (R01AG065240, R01NS115903, R01AG076051, and R21NS107897 to XQ), a Dr. Ralph and Marian Falk Medical Research Trust-Transformative Award (XQ), and a Harrington Rare Disease Scholar Award (XQ).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbott, N. J., Patabendige, A. A., Dolman, D. E., Yusof, S. R., and Begley, D. J. (2010). Structure and Function of the Blood-Brain Barrier. Neurobiol. Dis. 37 (1), 13–25. doi:10.1016/j.nbd.2009.07.030
Agrawal, I., and Jha, S. (2020). Mitochondrial Dysfunction and Alzheimer's Disease: Role of Microglia. Front. Aging Neurosci. 12, 252. doi:10.3389/fnagi.2020.00252
Ahmad, M. A., Kareem, O., Khushtar, M., Akbar, M., Haque, M. R., Iqubal, A., et al. (2022). Neuroinflammation: A Potential Risk for Dementia. Int. J. Mol. Sci. 23 (2), 616. doi:10.3390/ijms23020616
Ahmed, S. M., Luo, L., Namani, A., Wang, X. J., and Tang, X. (2017). Nrf2 Signaling Pathway: Pivotal Roles in Inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 1863 (2), 585–597. doi:10.1016/j.bbadis.2016.11.005
Ambati, R. R., Phang, S. M., Ravi, S., and Aswathanarayana, R. G. (2014). Astaxanthin: Sources, Extraction, Stability, Biological Activities and its Commercial Applications-Aa Review. Mar. Drugs 12 (1), 128–152. doi:10.3390/md12010128
Arioz, B. I., Tastan, B., Tarakcioglu, E., Tufekci, K. U., Olcum, M., Ersoy, N., et al. (2019). Melatonin Attenuates LPS-Induced Acute Depressive-like Behaviors and Microglial NLRP3 Inflammasome Activation through the SIRT1/Nrf2 Pathway. Front. Immunol. 10, 1511. doi:10.3389/fimmu.2019.01511
Arthur, J. S., and Ley, S. C. (2013). Mitogen-activated Protein Kinases in Innate Immunity. Nat. Rev. Immunol. 13 (9), 679–692. doi:10.1038/nri3495
Asslih, S., Damri, O., and Agam, G. (2021). Neuroinflammation as a Common Denominator of Complex Diseases (Cancer, Diabetes Type 2, and Neuropsychiatric Disorders). Int. J. Mol. Sci. 22 (11), 6138. doi:10.3390/ijms22116138
Bahbah, E. I., Ghozy, S., Attia, M. S., Negida, A., Emran, T. B., Mitra, S., et al. (2021). Molecular Mechanisms of Astaxanthin as a Potential Neurotherapeutic Agent. Mar. Drugs 19 (4), 201. doi:10.3390/md19040201
Bhuvaneswari, S., Yogalakshmi, B., Sreeja, S., and Anuradha, C. V. (2014). Astaxanthin Reduces Hepatic Endoplasmic Reticulum Stress and Nuclear Factor-Κb-Mediated Inflammation in High Fructose and High Fat Diet-Fed Mice. Cell Stress Chaperones 19 (2), 183–191. doi:10.1007/s12192-013-0443-x
Bhuvaneswari, S., Arunkumar, E., Viswanathan, P., and Anuradha, C. V. (2010). Astaxanthin Restricts Weight Gain, Promotes Insulin Sensitivity and Curtails Fatty Liver Disease in Mice Fed a Obesity-Promoting Diet. Process Biochem. 45 (8), 1406–1414. doi:10.1016/j.procbio.2010.05.016
Binatti, E., Zoccatelli, G., Zanoni, F., Donà, G., Mainente, F., and Chignola, R. (2021). Phagocytosis of Astaxanthin-Loaded Microparticles Modulates TGFβ Production and Intracellular ROS Levels in J774A.1 Macrophages. Mar. Drugs 19 (3), 163. doi:10.3390/md19030163
Bisht, S., Faiq, M., Tolahunase, M., and Dada, R. (2017). Oxidative Stress and Male Infertility. Nat. Rev. Urol. 14 (8), 470–485. doi:10.1038/nrurol.2017.69
Britton, G. (1995). Structure and Properties of Carotenoids in Relation to Function. FASEB J. 9 (15), 1551–1558. doi:10.1096/fasebj.9.15.8529834
Brockie, S., Hong, J., and Fehlings, M. G. (2021). The Role of Microglia in Modulating Neuroinflammation after Spinal Cord Injury. Int. J. Mol. Sci. 22 (18), 9706. doi:10.3390/ijms22189706
Cai, X., Chen, Y., Xie, X., Yao, D., Ding, C., and Chen, M. (2019). Astaxanthin Prevents against Lipopolysaccharide-Induced Acute Lung Injury and Sepsis via Inhibiting Activation of MAPK/NF-κB. Am. J. Transl. Res. 11 (3), 1884–1894.
Che, H., Li, Q., Zhang, T., Wang, D., Yang, L., Xu, J., et al. (2018). Effects of Astaxanthin and Docosahexaenoic-Acid-Acylated Astaxanthin on Alzheimer's Disease in APP/PS1 Double-Transgenic Mice. J. Agric. Food Chem. 66 (19), 4948–4957. doi:10.1021/acs.jafc.8b00988
Chen, H., Guan, B., Chen, X., Chen, X., Li, C., Qiu, J., et al. (2018a). Baicalin Attenuates Blood-Brain Barrier Disruption and Hemorrhagic Transformation and Improves Neurological Outcome in Ischemic Stroke Rats with Delayed T-PA Treatment: Involvement of ONOO--MMP-9 Pathway. Transl. Stroke Res. 9 (5), 515–529. doi:10.1007/s12975-017-0598-3
Chen, H., Guan, B., Wang, B., Pu, H., Bai, X., Chen, X., et al. (2020). Glycyrrhizin Prevents Hemorrhagic Transformation and Improves Neurological Outcome in Ischemic Stroke with Delayed Thrombolysis through Targeting Peroxynitrite-Mediated HMGB1 Signaling. Transl. Stroke Res. 11 (5), 967–982. doi:10.1007/s12975-019-00772-1
Chen, H. S., Chen, X., Li, W. T., and Shen, J. G. (2018b). Targeting RNS/caveolin-1/MMP Signaling Cascades to Protect against Cerebral Ischemia-Reperfusion Injuries: Potential Application for Drug Discovery. Acta Pharmacol. Sin. 39 (5), 669–682. doi:10.1038/aps.2018.27
Chen, M. H., Wang, T. J., Chen, L. J., Jiang, M. Y., Wang, Y. J., Tseng, G. F., et al. (2021a). The Effects of Astaxanthin Treatment on a Rat Model of Alzheimer's Disease. Brain Res. Bull. 172, 151–163. doi:10.1016/j.brainresbull.2021.04.020
Chen, X. M., Chen, H. S., Xu, M. J., and Shen, J. G. (2013). Targeting Reactive Nitrogen Species: a Promising Therapeutic Strategy for Cerebral Ischemia-Reperfusion Injury. Acta Pharmacol. Sin. 34 (1), 67–77. doi:10.1038/aps.2012.82
Chen, Y., Zhao, S., Jiao, D., Yao, B., Yang, S., Li, P., et al. (2021b). Astaxanthin Alleviates Ochratoxin A-Induced Cecum Injury and Inflammation in Mice by Regulating the Diversity of Cecal Microbiota and TLR4/MyD88/NF-Κb Signaling Pathway. Oxidative Med. Cell. Longev. 2021, 8894491. doi:10.1155/2021/8894491
Chiu, C. H., Chang, C. C., Lin, S. T., Chyau, C. C., and Peng, R. Y. (2016). Improved Hepatoprotective Effect of Liposome-Encapsulated Astaxanthin in Lipopolysaccharide-Induced Acute Hepatotoxicity. Int. J. Mol. Sci. 17 (7), 1128. doi:10.3390/ijms17071128
Cioanca, O., Hritcu, L., Mihasan, M., and Hancianu, M. (2013). Cognitive-enhancing and Antioxidant Activities of Inhaled Coriander Volatile Oil in Amyloid β(1-42) Rat Model of Alzheimer's Disease. Physiol. Behav. 120, 193–202. doi:10.1016/j.physbeh.2013.08.006
Coral-Hinostroza, G. N., and Bjerkeng, B. (2002). Astaxanthin from the Red Crab Langostilla (Pleuroncodes Planipes): Optical R/S Isomers and Fatty Acid Moieties of Astaxanthin Esters. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 133 (3), 437–444. doi:10.1016/s1096-4959(02)00186-0
Coral-Hinostroza, G. N., Ytrestøyl, T., Ruyter, B., and Bjerkeng, B. (2004). Plasma Appearance of Unesterified Astaxanthin Geometrical E/Z and Optical R/S Isomers in Men Given Single Doses of a Mixture of Optical 3 and 3'R/S Isomers of Astaxanthin Fatty Acyl Diesters. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 139 (1-3), 99–110. doi:10.1016/j.cca.2004.09.011
Coulombe, P., and Meloche, S. (2007). Atypical Mitogen-Activated Protein Kinases: Structure, Regulation and Functions. Biochim. Biophys. Acta 1773 (8), 1376–1387. doi:10.1016/j.bbamcr.2006.11.001
Counts, S. E., and Mufson, E. J. (2005). The Role of Nerve Growth Factor Receptors in Cholinergic Basal Forebrain Degeneration in Prodromal Alzheimer Disease. J. Neuropathol. Exp. Neurol. 64 (4), 263–272. doi:10.1093/jnen/64.4.263
Cuadrado, A. (2015). Structural and Functional Characterization of Nrf2 Degradation by Glycogen Synthase Kinase 3/β-TrCP. Free Radic. Biol. Med. 88 (Pt B), 147–157. doi:10.1016/j.freeradbiomed.2015.04.029
Dang, R., Wang, M., Li, X., Wang, H., Liu, L., Wu, Q., et al. (2022). Edaravone Ameliorates Depressive and Anxiety-like Behaviors via Sirt1/Nrf2/HO-1/Gpx4 Pathway. J. Neuroinflammation 19 (1), 41. doi:10.1186/s12974-022-02400-6
Davalos, D., Grutzendler, J., Yang, G., Kim, J. V., Zuo, Y., Jung, S., et al. (2005). ATP Mediates Rapid Microglial Response to Local Brain Injury In Vivo. Nat. Neurosci. 8 (6), 752–758. doi:10.1038/nn1472
Deng, X., Wang, M., Hu, S., Feng, Y., Shao, Y., Xie, Y., et al. (2019). The Neuroprotective Effect of Astaxanthin on Pilocarpine-Induced Status Epilepticus in Rats. Front. Cell Neurosci. 13, 123. doi:10.3389/fncel.2019.00123
Dias, V., Junn, E., and Mouradian, M. M. (2013). The Role of Oxidative Stress in Parkinson's Disease. J. Park. Dis. 3 (4), 461–491. doi:10.3233/JPD-130230
Dibaj, P., Nadrigny, F., Steffens, H., Scheller, A., Hirrlinger, J., Schomburg, E. D., et al. (2010). NO Mediates Microglial Response to Acute Spinal Cord Injury under ATP Control In Vivo. Glia 58 (9), 1133–1144. doi:10.1002/glia.20993
Ding, Q., Xia, W., Liu, J. C., Yang, J. Y., Lee, D. F., Xia, J., et al. (2005). Erk Associates with and Primes GSK-3beta for its Inactivation Resulting in Upregulation of Beta-Catenin. Mol. Cell 19 (2), 159–170. doi:10.1016/j.molcel.2005.06.009
DiSabato, D. J., Quan, N., and Godbout, J. P. (2016). Neuroinflammation: the Devil Is in the Details. J. Neurochem. 139 (Suppl. 2), 136–153. doi:10.1111/jnc.13607
Duan, Y., Sahley, C. L., and Muller, K. J. (2009). ATP and NO Dually Control Migration of Microglia to Nerve Lesions. Dev. Neurobiol. 69 (1), 60–72. doi:10.1002/dneu.20689
Dumitru, C. D., Ceci, J. D., Tsatsanis, C., Kontoyiannis, D., Stamatakis, K., Lin, J. H., et al. (2000). TNF-alpha Induction by LPS Is Regulated Posttranscriptionally via a Tpl2/ERK-dependent Pathway. Cell 103 (7), 1071–1083. doi:10.1016/s0092-8674(00)00210-5
Dupuis, N., and Auvin, S. (2015). Inflammation and Epilepsy in the Developing Brain: Clinical and Experimental Evidence. CNS Neurosci. Ther. 21 (2), 141–151. doi:10.1111/cns.12371
El-Agamy, S. E., Abdel-Aziz, A. K., Wahdan, S., Esmat, A., and Azab, S. S. (2018). Astaxanthin Ameliorates Doxorubicin-Induced Cognitive Impairment (Chemobrain) in Experimental Rat Model: Impact on Oxidative, Inflammatory, and Apoptotic Machineries. Mol. Neurobiol. 55 (7), 5727–5740. doi:10.1007/s12035-017-0797-7
Fakhri, S., Aneva, I. Y., Farzaei, M. H., and Sobarzo-Sánchez, E. (2019). The Neuroprotective Effects of Astaxanthin: Therapeutic Targets and Clinical Perspective. Molecules 24 (14), 2640. doi:10.3390/molecules24142640
Fakhri, S., Dargahi, L., Abbaszadeh, F., and Jorjani, M. (2018). Astaxanthin Attenuates Neuroinflammation Contributed to the Neuropathic Pain and Motor Dysfunction Following Compression Spinal Cord Injury. Brain Res. Bull. 143, 217–224. doi:10.1016/j.brainresbull.2018.09.011
Farruggia, C., Kim, M. B., Bae, M., Lee, Y., Pham, T. X., Yang, Y., et al. (2018). Astaxanthin Exerts Anti-inflammatory and Antioxidant Effects in Macrophages in NRF2-dependent and Independent Manners. J. Nutr. Biochem. 62, 202–209. doi:10.1016/j.jnutbio.2018.09.005
Fassett, R. G., and Coombes, J. S. (2011). Astaxanthin: a Potential Therapeutic Agent in Cardiovascular Disease. Mar. Drugs 9 (3), 447–465. doi:10.3390/md9030447
Feng, J., Chen, X., Guan, B., Li, C., Qiu, J., and Shen, J. (2018). Inhibition of Peroxynitrite-Induced Mitophagy Activation Attenuates Cerebral Ischemia-Reperfusion Injury. Mol. Neurobiol. 55 (8), 6369–6386. doi:10.1007/s12035-017-0859-x
Feng, W., Wang, Y., Guo, N., Huang, P., and Mi, Y. (2020). Effects of Astaxanthin on Inflammation and Insulin Resistance in a Mouse Model of Gestational Diabetes Mellitus. Dose Response 18 (2), 1. doi:10.1177/1559325820926765
Feske, S. K. (2021). Ischemic Stroke. Am. J. Med. 134 (12), 1457–1464. doi:10.1016/j.amjmed.2021.07.027
Fitzgerald, K. A., and Kagan, J. C. (2020). Toll-like Receptors and the Control of Immunity. Cell 180 (6), 1044–1066. doi:10.1016/j.cell.2020.02.041
Friling, R. S., Bergelson, S., and Daniel, V. (1992). Two Adjacent AP-1-like Binding Sites Form the Electrophile-Responsive Element of the Murine Glutathione S-Transferase Ya Subunit Gene. Proc. Natl. Acad. Sci. U. S. A. 89 (2), 668–672. doi:10.1073/pnas.89.2.668
Fung, T. C., Olson, C. A., and Hsiao, E. Y. (2017). Interactions between the Microbiota, Immune and Nervous Systems in Health and Disease. Nat. Neurosci. 20 (2), 145–155. doi:10.1038/nn.4476
Gaki, G. S., and Papavassiliou, A. G. (2014). Oxidative Stress-Induced Signaling Pathways Implicated in the Pathogenesis of Parkinson's Disease. Neuromolecular Med. 16 (2), 217–230. doi:10.1007/s12017-014-8294-x
Galasso, C., Orefice, I., Pellone, P., Cirino, P., Miele, R., Ianora, A., et al. (2018). On the Neuroprotective Role of Astaxanthin: New Perspectives? Mar. Drugs 16 (8), 247. doi:10.3390/md16080247
Gilgun-Sherki, Y., Melamed, E., and Offen, D. (2001). Oxidative Stress Induced-Neurodegenerative Diseases: the Need for Antioxidants that Penetrate the Blood Brain Barrier. Neuropharmacology 40 (8), 959–975. doi:10.1016/s0028-3908(01)00019-3
Giménez-Arnau, A. M., DeMontojoye, L., Asero, R., Cugno, M., Kulthanan, K., Yanase, Y., et al. (2021). The Pathogenesis of Chronic Spontaneous Urticaria: The Role of Infiltrating Cells. J. Allergy Clin. Immunol. Pract. 9 (6), 2195–2208. doi:10.1016/j.jaip.2021.03.033
Gorrini, C., Harris, I. S., and Mak, T. W. (2013). Modulation of Oxidative Stress as an Anticancer Strategy. Nat. Rev. Drug Discov. 12 (12), 931–947. doi:10.1038/nrd4002
Gross, G. J., Hazen, S. L., and Lockwood, S. F. (2006). Seven Day Oral Supplementation with Cardax (Disodium Disuccinate Astaxanthin) Provides Significant Cardioprotection and Reduces Oxidative Stress in Rats. Mol. Cell Biochem. 283 (1-2), 23–30. doi:10.1007/s11010-006-2217-6
Gross, G. J., and Lockwood, S. F. (2005). Acute and Chronic Administration of Disodium Disuccinate Astaxanthin (Cardax) Produces Marked Cardioprotection in Dog Hearts. Mol. Cell Biochem. 272 (1-2), 221–227. doi:10.1007/s11010-005-7555-2
Gundersen, V., Storm-Mathisen, J., and Bergersen, L. H. (2015). Neuroglial Transmission. Physiol. Rev. 95 (3), 695–726. doi:10.1152/physrev.00024.2014
Guo, S., Guo, L., Fang, Q., Yu, M., Zhang, L., You, C., et al. (2021). Astaxanthin Protects against Early Acute Kidney Injury in Severely Burned Rats by Inactivating the TLR4/MyD88/NF-Κb axis and Upregulating Heme Oxygenase-1. Sci. Rep. 11 (1), 6679. doi:10.1038/s41598-021-86146-w
Hafez, H. A., Kamel, M. A., Osman, M. Y., Osman, H. M., Elblehi, S. S., and Mahmoud, S. A. (2021). Ameliorative Effects of Astaxanthin on Brain Tissues of Alzheimer's Disease-like Model: Cross Talk between Neuronal-specific microRNA-124 and Related Pathways. Mol. Cell Biochem. 476 (5), 2233–2249. doi:10.1007/s11010-021-04079-4
Han, H., Lim, J. W., and Kim, H. (2020). Astaxanthin Inhibits Helicobacter Pylori-Induced Inflammatory and Oncogenic Responses in Gastric Mucosal Tissues of Mice. J. Cancer Prev. 25 (4), 244–251. doi:10.15430/JCP.2020.25.4.244
Han, J. H., Ju, J. H., Lee, Y. S., Park, J. H., Yeo, I. J., Park, M. H., et al. (2018). Astaxanthin Alleviated Ethanol-Induced Liver Injury by Inhibition of Oxidative Stress and Inflammatory Responses via Blocking of STAT3 Activity. Sci. Rep. 8 (1), 14090. doi:10.1038/s41598-018-32497-w
Han, J. H., Lee, Y. S., Im, J. H., Ham, Y. W., Lee, H. P., Han, S. B., et al. (2019). Astaxanthin Ameliorates Lipopolysaccharide-Induced Neuroinflammation, Oxidative Stress and Memory Dysfunction through Inactivation of the Signal Transducer and Activator of Transcription 3 Pathway. Mar. Drugs 17 (2), 123. doi:10.3390/md17020123
Hannocks, M. J., Zhang, X., Gerwien, H., Chashchina, A., Burmeister, M., Korpos, E., et al. (2019). The Gelatinases, MMP-2 and MMP-9, as Fine Tuners of Neuroinflammatory Processes. Matrix Biol. 75-76, 102–113. doi:10.1016/j.matbio.2017.11.007
Hayden, M. S., and Ghosh, S. (2008). Shared Principles in NF-kappaB Signaling. Cell 132 (3), 344–362. doi:10.1016/j.cell.2008.01.020
Heinz, R., Brandenburg, S., Nieminen-Kelhä, M., Kremenetskaia, I., Boehm-Sturm, P., Vajkoczy, P., et al. (2021). Microglia as Target for Anti-inflammatory Approaches to Prevent Secondary Brain Injury after Subarachnoid Hemorrhage (SAH). J. Neuroinflammation 18 (1), 36. doi:10.1186/s12974-021-02085-3
Higuera-Ciapara, I., Félix-Valenzuela, L., and Goycoolea, F. M. (2006). Astaxanthin: A Review of its Chemistry and Applications. Crit. Rev. Food Sci. Nutr. 46 (2), 185–196. doi:10.1080/10408690590957188
Hirsch, E. C., and Hunot, S. (2009). Neuroinflammation in Parkinson's Disease: a Target for Neuroprotection? Lancet Neurol. 8 (4), 382–397. doi:10.1016/S1474-4422(09)70062-6
Hirsch, E. C., and Standaert, D. G. (2021). Ten Unsolved Questions about Neuroinflammation in Parkinson's Disease. Mov. Disord. 36 (1), 16–24. doi:10.1002/mds.28075
Hoesel, B., and Schmid, J. A. (2013). The Complexity of NF-Κb Signaling in Inflammation and Cancer. Mol. Cancer 12, 86. doi:10.1186/1476-4598-12-86
Hongo, N., Takamura, Y., Nishimaru, H., Matsumoto, J., Tobe, K., Saito, T., et al. (2020). Astaxanthin Ameliorated Parvalbumin-Positive Neuron Deficits and Alzheimer's Disease-Related Pathological Progression in the Hippocampus of AppNL-G-F/NL-G-F Mice. Front. Pharmacol. 11, 307. doi:10.3389/fphar.2020.00307
Hritcu, L., Noumedem, J. A., Cioanca, O., Hancianu, M., Kuete, V., and Mihasan, M. (2014). Methanolic Extract of Piper Nigrum Fruits Improves Memory Impairment by Decreasing Brain Oxidative Stress in Amyloid Beta(1-42) Rat Model of Alzheimer's Disease. Cell Mol. Neurobiol. 34 (3), 437–449. doi:10.1007/s10571-014-0028-y
Hu, I.-C. (2019). “Production of Potential Coproducts from Microalgae,” in Biofuels from Algae. Editors A. Pandey, J.-S. Chang, C. R. Soccol, D.-J. Lee, and Y. Chisti. Second Edition (Elsevier), 345–358. doi:10.1016/b978-0-444-64192-2.00014-7
Hu, X., Li, P., Guo, Y., Wang, H., Leak, R. K., Chen, S., et al. (2012). Microglia/macrophage Polarization Dynamics Reveal Novel Mechanism of Injury Expansion after Focal Cerebral Ischemia. Stroke 43 (11), 3063–3070. doi:10.1161/STROKEAHA.112.659656
Huang, X., Hussain, B., and Chang, J. (2021). Peripheral Inflammation and Blood-Brain Barrier Disruption: Effects and Mechanisms. CNS Neurosci. Ther. 27 (1), 36–47. doi:10.1111/cns.13569
Hussein, G., Sankawa, U., Goto, H., Matsumoto, K., and Watanabe, H. (2006). Astaxanthin, a Carotenoid with Potential in Human Health and Nutrition. J. Nat. Prod. 69 (3), 443–449. doi:10.1021/np050354+
Hwang, Y. H., Hong, S. G., Mun, S. K., Kim, S. J., Lee, S. J., Kim, J. J., et al. (2017). The Protective Effects of Astaxanthin on the OVA-Induced Asthma Mice Model. Molecules 22 (11), 2019. doi:10.3390/molecules22112019
Ikeda, Y., Tsuji, S., Satoh, A., Ishikura, M., Shirasawa, T., and Shimizu, T. (2008). Protective Effects of Astaxanthin on 6-Hydroxydopamine-Induced Apoptosis in Human Neuroblastoma SH-Sy5y Cells. J. Neurochem. 107 (6), 1730–1740. doi:10.1111/j.1471-4159.2008.05743.x
Innamorato, N. G., Rojo, A. I., García-Yagüe, A. J., Yamamoto, M., de Ceballos, M. L., and Cuadrado, A. (2008). The Transcription Factor Nrf2 Is a Therapeutic Target against Brain Inflammation. J. Immunol. 181 (1), 680–689. doi:10.4049/jimmunol.181.1.680
Ito, N., Saito, H., Seki, S., Ueda, F., and Asada, T. (2018). Effects of Composite Supplement Containing Astaxanthin and Sesamin on Cognitive Functions in People with Mild Cognitive Impairment: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Alzheimers Dis. 62 (4), 1767–1775. doi:10.3233/JAD-170969
Jain, A. K., and Jaiswal, A. K. (2007). GSK-3beta Acts Upstream of Fyn Kinase in Regulation of Nuclear Export and Degradation of NF-E2 Related Factor 2. J. Biol. Chem. 282 (22), 16502–16510. doi:10.1074/jbc.M611336200
Jayaraj, R. L., Azimullah, S., Beiram, R., Jalal, F. Y., and Rosenberg, G. A. (2019). Neuroinflammation: Friend and Foe for Ischemic Stroke. J. Neuroinflammation 16 (1), 142. doi:10.1186/s12974-019-1516-2
Jia, Y., Wu, C., Kim, J., Kim, B., and Lee, S. J. (2016). Astaxanthin Reduces Hepatic Lipid Accumulations in High-Fat-Fed C57BL/6J Mice via Activation of Peroxisome Proliferator-Activated Receptor (PPAR) Alpha and Inhibition of PPAR Gamma and Akt. J. Nutr. Biochem. 28, 9–18. doi:10.1016/j.jnutbio.2015.09.015
Jiang, X., Chen, L., Shen, L., Chen, Z., Xu, L., Zhang, J., et al. (2016). Trans-astaxanthin Attenuates Lipopolysaccharide-Induced Neuroinflammation and Depressive-like Behavior in Mice. Brain Res. 1649 (Pt A), 30–37. doi:10.1016/j.brainres.2016.08.029
Jin, W., Botchway, B. O. A., and Liu, X. (2021). Curcumin Can Activate the Nrf2/HO-1 Signaling Pathway and Scavenge Free Radicals in Spinal Cord Injury Treatment. Neurorehabil Neural Repair 35 (7), 576–584. doi:10.1177/15459683211011232
Jung, C. S., Lange, B., Zimmermann, M., and Seifert, V. (2013). CSF and Serum Biomarkers Focusing on Cerebral Vasospasm and Ischemia after Subarachnoid Hemorrhage. Stroke Res. Treat. 2013, 560305. doi:10.1155/2013/560305
Kaidanovich-Beilin, O., and Woodgett, J. R. (2011). GSK-3: Functional Insights from Cell Biology and Animal Models. Front. Mol. Neurosci. 4, 40. doi:10.3389/fnmol.2011.00040
Kang, H., Lee, Y., Bae, M., Park, Y. K., and Lee, J. Y. (2020). Astaxanthin Inhibits Alcohol-Induced Inflammation and Oxidative Stress in Macrophages in a Sirtuin 1-dependent Manner. J. Nutr. Biochem. 85, 108477. doi:10.1016/j.jnutbio.2020.108477
Khan, S. K., Malinski, T., Mason, R. P., Kubant, R., Jacob, R. F., Fujioka, K., et al. (2010). Novel Astaxanthin Prodrug (CDX-085) Attenuates Thrombosis in a Mouse Model. Thromb. Res. 126 (4), 299–305. doi:10.1016/j.thromres.2010.07.003
Kim, J. Y., Park, J., Chang, J. Y., Kim, S. H., and Lee, J. E. (2016). Inflammation after Ischemic Stroke: The Role of Leukocytes and Glial Cells. Exp. Neurobiol. 25 (5), 241–251. doi:10.5607/en.2016.25.5.241
Kim, S. H., and Kim, H. (2018). Inhibitory Effect of Astaxanthin on Oxidative Stress-Induced Mitochondrial Dysfunction-A Mini-Review. Nutrients 10 (9), 1137. doi:10.3390/nu10091137
Kim, Y. J., Kim, Y. A., and Yokozawa, T. (2009). Protection against Oxidative Stress, Inflammation, and Apoptosis of High-Glucose-Exposed Proximal Tubular Epithelial Cells by Astaxanthin. J. Agric. Food Chem. 57 (19), 8793–8797. doi:10.1021/jf9019745
Kishimoto, Y., Tani, M., Uto-Kondo, H., Iizuka, M., Saita, E., Sone, H., et al. (2010). Astaxanthin Suppresses Scavenger Receptor Expression and Matrix Metalloproteinase Activity in Macrophages. Eur. J. Nutr. 49 (2), 119–126. doi:10.1007/s00394-009-0056-4
Kishimoto, Y., Yoshida, H., and Kondo, K. (2016). Potential Anti-atherosclerotic Properties of Astaxanthin. Mar. Drugs 14 (2), 35. doi:10.3390/md14020035
Kistler, A., Liechti, H., Pichard, L., Wolz, E., Oesterhelt, G., Hayes, A., et al. (2002). Metabolism and CYP-Inducer Properties of Astaxanthin in Man and Primary Human Hepatocytes. Arch. Toxicol. 75 (11-12), 665–675. doi:10.1007/s00204-001-0287-5
Konat, G. W., Kielian, T., and Marriott, I. (2006). The Role of Toll-like Receptors in CNS Response to Microbial Challenge. J. Neurochem. 99 (1), 1–12. doi:10.1111/j.1471-4159.2006.04076.x
Kreutzberg, G. W. (1996). Microglia: a Sensor for Pathological Events in the CNS. Trends Neurosci. 19 (8), 312–318. doi:10.1016/0166-2236(96)10049-7
Kuhn, R., and Sörensen, N. A. (1938). Über die Farbstoffe des Hummers (Astacus gammarus L.). Angew. Chem. 51, 465–466. doi:10.1002/ange.19380512703
Kumar, A., Dhaliwal, N., Dhaliwal, J., Dharavath, R. N., and Chopra, K. (2020). Astaxanthin Attenuates Oxidative Stress and Inflammatory Responses in Complete Freund-Adjuvant-Induced Arthritis in Rats. Pharmacol. Rep. 72 (1), 104–114. doi:10.1007/s43440-019-00022-z
Kuo, M. H., Lee, H. F., Tu, Y. F., Lin, L. H., Cheng, Y. Y., and Lee, H. T. (2019). Astaxanthin Ameliorates Ischemic-Hypoxic-Induced Neurotrophin Receptor P75 Upregulation in the Endothelial Cells of Neonatal Mouse Brains. Int. J. Mol. Sci. 20 (24), 6168. doi:10.3390/ijms20246168
Lauver, D. A., Lockwood, S. F., and Lucchesi, B. R. (2005). Disodium Disuccinate Astaxanthin (Cardax) Attenuates Complement Activation and Reduces Myocardial Injury Following Ischemia/reperfusion. J. Pharmacol. Exp. Ther. 314 (2), 686–692. doi:10.1124/jpet.105.087114
Lee, D. H., Lee, Y. J., and Kwon, K. H. (2010). Neuroprotective Effects of Astaxanthin in Oxygen-Glucose Deprivation in SH-Sy5y Cells and Global Cerebral Ischemia in Rat. J. Clin. Biochem. Nutr. 47 (2), 121–129. doi:10.3164/jcbn.10-29
Lehnardt, S. (2010). Innate Immunity and Neuroinflammation in the CNS: the Role of Microglia in Toll-like Receptor-Mediated Neuronal Injury. Glia 58 (3), 253–263. doi:10.1002/glia.20928
Leng, F., and Edison, P. (2021). Neuroinflammation and Microglial Activation in Alzheimer Disease: where Do We Go from Here? Nat. Rev. Neurol. 17 (3), 157–172. doi:10.1038/s41582-020-00435-y
Leroy, K., Boutajangout, A., Authelet, M., Woodgett, J. R., Anderton, B. H., and Brion, J. P. (2002). The Active Form of Glycogen Synthase Kinase-3beta Is Associated with Granulovacuolar Degeneration in Neurons in Alzheimer's Disease. Acta Neuropathol. 103 (2), 91–99. doi:10.1007/s004010100435
Li, H., Li, J., Hou, C., Li, J., Peng, H., and Wang, Q. (2020a). The Effect of Astaxanthin on Inflammation in Hyperosmolarity of Experimental Dry Eye Model In Vitro and In Vivo. Exp. Eye Res. 197, 108113. doi:10.1016/j.exer.2020.108113
Li, L., Chen, Y., Jiao, D., Yang, S., Li, L., and Li, P. (2020b). Protective Effect of Astaxanthin on Ochratoxin A-Induced Kidney Injury to Mice by Regulating Oxidative Stress-Related NRF2/KEAP1 Pathway. Molecules 25 (6), 1386. doi:10.3390/molecules25061386
Li, Y., Wei, B., Liu, X., Shen, X. Z., and Shi, P. (2020c). Microglia, Autonomic Nervous System, Immunity and Hypertension: Is There a Link? Pharmacol. Res. 155, 104451. doi:10.1016/j.phrs.2019.104451
Li, Z., Dong, X., Liu, H., Chen, X., Shi, H., Fan, Y., et al. (2013). Astaxanthin Protects ARPE-19 Cells from Oxidative Stress via Upregulation of Nrf2-Regulated Phase II Enzymes through Activation of PI3K/Akt. Mol. Vis. 19, 1656–1666.
Liebner, S., Dijkhuizen, R. M., Reiss, Y., Plate, K. H., Agalliu, D., and Constantin, G. (2018). Functional Morphology of the Blood-Brain Barrier in Health and Disease. Acta Neuropathol. 135 (3), 311–336. doi:10.1007/s00401-018-1815-1
Liu, G. J., Nagarajah, R., Banati, R. B., and Bennett, M. R. (2009a). Glutamate Induces Directed Chemotaxis of Microglia. Eur. J. Neurosci. 29 (6), 1108–1118. doi:10.1111/j.1460-9568.2009.06659.x
Liu, H., Liu, M., Fu, X., Zhang, Z., Zhu, L., Zheng, X., et al. (2018). Astaxanthin Prevents Alcoholic Fatty Liver Disease by Modulating Mouse Gut Microbiota. Nutrients 10 (9), 1298. doi:10.3390/nu10091298
Liu, T., Zhang, L., Joo, D., and Sun, S. C. (2017). NF-κB Signaling in Inflammation. Signal Transduct. Target Ther. 2 (1), 17023. doi:10.1038/sigtrans.2017.23
Liu, T., Zhong, S., Liao, X., Chen, J., He, T., Lai, S., et al. (2015). A Meta-Analysis of Oxidative Stress Markers in Depression. PLoS One 10 (10), e0138904. doi:10.1371/journal.pone.0138904
Liu, X., Shibata, T., Hisaka, S., and Osawa, T. (2009b). Astaxanthin Inhibits Reactive Oxygen Species-Mediated Cellular Toxicity in Dopaminergic SH-Sy5y Cells via Mitochondria-Targeted Protective Mechanism. Brain Res. 1254, 18–27. doi:10.1016/j.brainres.2008.11.076
Liu, Y., Yang, L., Guo, Y., Zhang, T., Qiao, X., Wang, J., et al. (2020). Hydrophilic Astaxanthin: PEGylated Astaxanthin Fights Diabetes by Enhancing the Solubility and Oral Absorbability. J. Agric. Food Chem. 68 (11), 3649–3655. doi:10.1021/acs.jafc.0c00784
Lu, M. C., Ji, J. A., Jiang, Z. Y., and You, Q. D. (2016). The Keap1-Nrf2-ARE Pathway as a Potential Preventive and Therapeutic Target: An Update. Med. Res. Rev. 36 (5), 924–963. doi:10.1002/med.21396
Lu, Y., Xie, T., He, X. X., Mao, Z. F., Jia, L. J., Wang, W. P., et al. (2015). Astaxanthin Rescues Neuron Loss and Attenuates Oxidative Stress Induced by Amygdala Kindling in Adult Rat hippocampus. Neurosci. Lett. 597, 49–53. doi:10.1016/j.neulet.2015.04.018
Lu, Y. P., Liu, S. Y., Sun, H., Wu, X. M., Li, J. J., and Zhu, L. (2010). Neuroprotective Effect of Astaxanthin on H(2)O(2)-induced Neurotoxicity In Vitro and on Focal Cerebral Ischemia In Vivo. Brain Res. 1360, 40–48. doi:10.1016/j.brainres.2010.09.016
Ly, P. T., Wu, Y., Zou, H., Wang, R., Zhou, W., Kinoshita, A., et al. (2013). Inhibition of GSK3β-Mediated BACE1 Expression Reduces Alzheimer-Associated Phenotypes. J. Clin. Invest 123 (1), 224–235. doi:10.1172/JCI64516
Ma, H., Chen, S., Xiong, H., Wang, M., Hang, W., Zhu, X., et al. (2020). Astaxanthin from Haematococcus pluvialis Ameliorates the Chemotherapeutic Drug (Doxorubicin) Induced Liver Injury through the Keap1/Nrf2/HO-1 Pathway in Mice. Food Funct. 11 (5), 4659–4671. doi:10.1039/c9fo02429h
Macdonald, R. L. (2014). Delayed Neurological Deterioration after Subarachnoid Haemorrhage. Nat. Rev. Neurol. 10 (1), 44–58. doi:10.1038/nrneurol.2013.246
Macdonald, R. L., and Schweizer, T. A. (2017). Spontaneous Subarachnoid Haemorrhage. Lancet 389 (10069), 655–666. doi:10.1016/S0140-6736(16)30668-7
Maes, M., Fišar, Z., Medina, M., Scapagnini, G., Nowak, G., and Berk, M. (2012). New Drug Targets in Depression: Inflammatory, Cell-Mediated Immune, Oxidative and Nitrosative Stress, Mitochondrial, Antioxidant, and Neuroprogressive Pathways. And New Drug Candidates--Nrf2 Activators and GSK-3 Inhibitors. Inflammopharmacology 20 (3), 127–150. doi:10.1007/s10787-011-0111-7
Manabe, E., Handa, O., Naito, Y., Mizushima, K., Akagiri, S., Adachi, S., et al. (2008). Astaxanthin Protects Mesangial Cells from Hyperglycemia-Induced Oxidative Signaling. J. Cell Biochem. 103 (6), 1925–1937. doi:10.1002/jcb.21583
Manning, B. D., and Toker, A. (2017). AKT/PKB Signaling: Navigating the Network. Cell 169 (3), 381–405. doi:10.1016/j.cell.2017.04.001
Marques-Deak, A., Cizza, G., and Sternberg, E. (2005). Brain-immune Interactions and Disease Susceptibility. Mol. Psychiatry 10 (3), 239–250. doi:10.1038/sj.mp.4001643
Mathien, S., Tesnière, C., and Meloche, S. (2021). Regulation of Mitogen-Activated Protein Kinase Signaling Pathways by the Ubiquitin-Proteasome System and its Pharmacological Potential. Pharmacol. Rev. 73 (4), 263–296. doi:10.1124/pharmrev.120.000170
Mathur, A., Pandey, V. K., and Kakkar, P. (2018). Activation of GSK3β/β-TrCP axis via PHLPP1 Exacerbates Nrf2 Degradation Leading to Impairment in Cell Survival Pathway during Diabetic Nephropathy. Free Radic. Biol. Med. 120, 414–424. doi:10.1016/j.freeradbiomed.2018.04.550
Mercke Odeberg, J., Lignell, A., Pettersson, A., and Höglund, P. (2003). Oral Bioavailability of the Antioxidant Astaxanthin in Humans Is Enhanced by Incorporation of Lipid Based Formulations. Eur. J. Pharm. Sci. 19 (4), 299–304. doi:10.1016/s0928-0987(03)00135-0
Milani, A., Basirnejad, M., Shahbazi, S., and Bolhassani, A. (2017). Carotenoids: Biochemistry, Pharmacology and Treatment. Br. J. Pharmacol. 174 (11), 1290–1324. doi:10.1111/bph.13625
Miller, N. J., Sampson, J., Candeias, L. P., Bramley, P. M., and Rice-Evans, C. A. (1996). Antioxidant Activities of Carotenes and Xanthophylls. FEBS Lett. 384 (3), 240–242. doi:10.1016/0014-5793(96)00323-7
Monroy-Ruiz, J., Sevilla, M. Á., Carrón, R., and Montero, M. J. (2011). Astaxanthin-enriched-diet Reduces Blood Pressure and Improves Cardiovascular Parameters in Spontaneously Hypertensive Rats. Pharmacol. Res. 63 (1), 44–50. doi:10.1016/j.phrs.2010.09.003
Moreira, A., Lebbé, C., and Heinzerling, L. (2021). MAPK Blockade, Toxicities, Pathogenesis and Management. Curr. Opin. Oncol. 33 (2), 139–145. doi:10.1097/CCO.0000000000000710
Mowry, F. E., and Biancardi, V. C. (2019). Neuroinflammation in Hypertension: the Renin-Angiotensin System versus Pro-resolution Pathways. Pharmacol. Res. 144, 279–291. doi:10.1016/j.phrs.2019.04.029
Nagatsu, T., Mogi, M., Ichinose, H., and Togari, A. (2000). Changes in Cytokines and Neurotrophins in Parkinson's Disease. J. Neural Transm. Suppl. 1 (60), 277–290. doi:10.1007/978-3-7091-6301-6_19
Naguib, Y. M. (2000). Antioxidant Activities of Astaxanthin and Related Carotenoids. J. Agric. Food Chem. 48 (4), 1150–1154. doi:10.1021/jf991106k
Nai, Y., Liu, H., Bi, X., Gao, H., and Ren, C. (2018). Protective Effect of Astaxanthin on Acute Cerebral Infarction in Rats. Hum. Exp. Toxicol. 37 (9), 929–936. doi:10.1177/0960327117745693
Naito, Y., Uchiyama, K., Aoi, W., Hasegawa, G., Nakamura, N., Yoshida, N., et al. (2004). Prevention of Diabetic Nephropathy by Treatment with Astaxanthin in Diabetic Db/db Mice. Biofactors 20 (1), 49–59. doi:10.1002/biof.5520200105
Nakagawa, Y., and Chiba, K. (2015). Diversity and Plasticity of Microglial Cells in Psychiatric and Neurological Disorders. Pharmacol. Ther. 154, 21–35. doi:10.1016/j.pharmthera.2015.06.010
Nakano, M., Onodera, A., Saito, E., Tanabe, M., Yajima, K., Takahashi, J., et al. (2008). Effect of Astaxanthin in Combination with Alpha-Tocopherol or Ascorbic Acid against Oxidative Damage in Diabetic ODS Rats. J. Nutr. Sci. Vitaminol. (Tokyo) 54 (4), 329–334. doi:10.3177/jnsv.54.329
Nguyen, T., Sherratt, P. J., Huang, H. C., Yang, C. S., and Pickett, C. B. (2003). Increased Protein Stability as a Mechanism that Enhances Nrf2-Mediated Transcriptional Activation of the Antioxidant Response Element. Degradation of Nrf2 by the 26 S Proteasome. J. Biol. Chem. 278 (7), 4536–4541. doi:10.1074/jbc.M207293200
Ni, Y., Nagashimada, M., Zhuge, F., Zhan, L., Nagata, N., Tsutsui, A., et al. (2015). Astaxanthin Prevents and Reverses Diet-Induced Insulin Resistance and Steatohepatitis in Mice: A Comparison with Vitamin E. Sci. Rep. 5, 17192. doi:10.1038/srep17192
Nimmerjahn, A., Kirchhoff, F., and Helmchen, F. (2005). Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma In Vivo. Science 308 (5726), 1314–1318. doi:10.1126/science.1110647
Nishida, Y., Nawaz, A., Hecht, K., and Tobe, K. (2021). Astaxanthin as a Novel Mitochondrial Regulator: A New Aspect of Carotenoids, beyond Antioxidants. Nutrients 14 (1), 107. doi:10.3390/nu14010107
Nishida, Y., Nawaz, A., Kado, T., Takikawa, A., Igarashi, Y., Onogi, Y., et al. (2020). Astaxanthin Stimulates Mitochondrial Biogenesis in Insulin Resistant Muscle via Activation of AMPK Pathway. J. Cachexia Sarcopenia Muscle 11 (1), 241–258. doi:10.1002/jcsm.12530
Niture, S. K., Khatri, R., and Jaiswal, A. K. (2014). Regulation of Nrf2-An Update. Free Radic. Biol. Med. 66, 36–44. doi:10.1016/j.freeradbiomed.2013.02.008
Nutrition and Allergies Efsa Panel on Dietetic Products, (2014). Scientific Opinion on the Safety of Astaxanthin-Rich Ingredients (AstaREAL A1010 and AstaREAL L10) as Novel Food Ingredients. EFSA J. 12, 3757. doi:10.2903/j.efsa.2014.3757
Obermeier, B., Daneman, R., and Ransohoff, R. M. (2013). Development, Maintenance and Disruption of the Blood-Brain Barrier. Nat. Med. 19 (12), 1584–1596. doi:10.1038/nm.3407
Ohgami, K., Shiratori, K., Kotake, S., Nishida, T., Mizuki, N., Yazawa, K., et al. (2003). Effects of Astaxanthin on Lipopolysaccharide-Induced Inflammation In Vitro and In Vivo. Invest Ophthalmol. Vis. Sci. 44 (6), 2694–2701. doi:10.1167/iovs.02-0822
Ohno, M., Darwish, W. S., Ikenaka, Y., Miki, W., and Ishizuka, M. (2011). Astaxanthin Can Alter CYP1A-dependent Activities via Two Different Mechanisms: Induction of Protein Expression and Inhibition of NADPH P450 Reductase Dependent Electron Transfer. Food Chem. Toxicol. 49 (6), 1285–1291. doi:10.1016/j.fct.2011.03.009
Okada, Y., Ishikura, M., and Maoka, T. (2009). Bioavailability of Astaxanthin in Haematococcus Algal Extract: the Effects of Timing of Diet and Smoking Habits. Biosci. Biotechnol. Biochem. 73 (9), 1928–1932. doi:10.1271/bbb.90078
Orihuela, R., McPherson, C. A., and Harry, G. J. (2016). Microglial M1/M2 Polarization and Metabolic States. Br. J. Pharmacol. 173 (4), 649–665. doi:10.1111/bph.13139
Osterlie, M., Bjerkeng, B., and Liaaen-Jensen, S. (1999). Accumulation of Astaxanthin All-E, 9Z and 13Z Geometrical Isomers and 3 and 3' RS Optical Isomers in Rainbow Trout (Oncorhynchus mykiss) Is Selective. J. Nutr. 129 (2), 391–398. doi:10.1093/jn/129.2.391
Østerlie, M., Bjerkeng, B., and Liaaen-Jensen, S. (2000). Plasma Appearance and Distribution of Astaxanthin E/Z and R/S Isomers in Plasma Lipoproteins of Men after Single Dose Administration of Astaxanthin. J. Nutr. Biochem. 11 (10), 482–490. doi:10.1016/s0955-2863(00)00104-2
Palozza, P., and Krinsky, N. I. (1992a). Antioxidant Effects of Carotenoids In Vivo and In Vitro: an Overview. Methods Enzymol. 213, 403–420. doi:10.1016/0076-6879(92)13142-k
Palozza, P., and Krinsky, N. I. (1992b). Astaxanthin and Canthaxanthin Are Potent Antioxidants in a Membrane Model. Arch. Biochem. Biophys. 297 (2), 291–295. doi:10.1016/0003-9861(92)90675-m
Park, H. A., Hayden, M. M., Bannerman, S., Jansen, J., and Crowe-White, K. M. (2020a). Anti-Apoptotic Effects of Carotenoids in Neurodegeneration. Molecules 25 (15), 3453. doi:10.3390/molecules25153453
Park, J. H., Yeo, I. J., Han, J. H., Suh, J. W., Lee, H. P., and Hong, J. T. (2018). Anti-inflammatory Effect of Astaxanthin in Phthalic Anhydride-Induced Atopic Dermatitis Animal Model. Exp. Dermatol 27 (4), 378–385. doi:10.1111/exd.13437
Park, J. H., Yeo, I. J., Jang, J. S., Kim, K. C., Park, M. H., Lee, H. P., et al. (2019). Combination Effect of Titrated Extract of Centella asiatica and Astaxanthin in a Mouse Model of Phthalic Anhydride-Induced Atopic Dermatitis. Allergy Asthma Immunol. Res. 11 (4), 548–559. doi:10.4168/aair.2019.11.4.548
Park, J. S., Chyun, J. H., Kim, Y. K., Line, L. L., and Chew, B. P. (2010). Astaxanthin Decreased Oxidative Stress and Inflammation and Enhanced Immune Response in Humans. Nutr. Metab. (Lond) 7, 18. doi:10.1186/1743-7075-7-18
Park, M. H., Jung, J. C., Hill, S., Cartwright, E., Dohnalek, M. H., Yu, M., et al. (2020b). FlexPro MD®, a Combination of Krill Oil, Astaxanthin and Hyaluronic Acid, Reduces Pain Behavior and Inhibits Inflammatory Response in Monosodium Iodoacetate-Induced Osteoarthritis in Rats. Nutrients 12 (4), 956. doi:10.3390/nu12040956
Parker, R. S. (1996). Absorption, Metabolism, and Transport of Carotenoids. FASEB J. 10 (5), 542–551. doi:10.1096/fasebj.10.5.8621054
Parsons, A. L. M., Bucknor, E. M. V., Castroflorio, E., Soares, T. R., Oliver, P. L., and Rial, D. (2022). The Interconnected Mechanisms of Oxidative Stress and Neuroinflammation in Epilepsy. Antioxidants (Basel) 11 (1), 157. doi:10.3390/antiox11010157
Peng, Y. J., Lu, J. W., Liu, F. C., Lee, C. H., Lee, H. S., Ho, Y. J., et al. (2020). Astaxanthin Attenuates Joint Inflammation Induced by Monosodium Urate Crystals. FASEB J. 34 (8), 11215–11226. doi:10.1096/fj.202000558RR
Petri, D., and Lundebye, A. K. (2007). Tissue Distribution of Astaxanthin in Rats Following Exposure to Graded Levels in the Feed. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 145 (2), 202–209. doi:10.1016/j.cbpc.2006.12.008
Pluta, R., Januszewski, S., and Czuczwar, S. J. (2021). Neuroinflammation in Post-Ischemic Neurodegeneration of the Brain: Friend, Foe, or Both? Ijms 22 (9), 4405. doi:10.3390/ijms22094405
Polotow, T. G., Poppe, S. C., Vardaris, C. V., Ganini, D., Guariroba, M., Mattei, R., et al. (2015). Redox Status and Neuro Inflammation Indexes in Cerebellum and Motor Cortex of Wistar Rats Supplemented with Natural Sources of Omega-3 Fatty Acids and Astaxanthin: Fish Oil, Krill Oil, and Algal Biomass. Mar. Drugs 13 (10), 6117–6137. doi:10.3390/md13106117
Prinz, M., Jung, S., and Priller, J. (2019). Microglia Biology: One Century of Evolving Concepts. Cell 179 (2), 292–311. doi:10.1016/j.cell.2019.08.053
Qi, J., Zhao, X. F., Yu, X. J., Yi, Q. Y., Shi, X. L., Tan, H., et al. (2016). Targeting Interleukin-1 Beta to Suppress Sympathoexcitation in Hypothalamic Paraventricular Nucleus in Dahl Salt-Sensitive Hypertensive Rats. Cardiovasc Toxicol. 16 (3), 298–306. doi:10.1007/s12012-015-9338-7
Qiao, J., Rong, L., Wang, Z., and Zhang, M. (2017). Involvement of Akt/GSK3β/CREB Signaling Pathway on Chronic Omethoate Induced Depressive-like Behavior and Improvement Effects of Combined Lithium Chloride and Astaxanthin Treatment. Neurosci. Lett. 649, 55–61. doi:10.1016/j.neulet.2017.03.048
Rada, P., Rojo, A. I., Chowdhry, S., McMahon, M., Hayes, J. D., and Cuadrado, A. (2011). SCF/{beta}-TrCP Promotes Glycogen Synthase Kinase 3-dependent Degradation of the Nrf2 Transcription Factor in a Keap1-independent Manner. Mol. Cell Biol. 31 (6), 1121–1133. doi:10.1128/MCB.01204-10
Rahman, S. O., Panda, B. P., Parvez, S., Kaundal, M., Hussain, S., Akhtar, M., et al. (2019). Neuroprotective Role of Astaxanthin in Hippocampal Insulin Resistance Induced by Aβ Peptides in Animal Model of Alzheimer's Disease. Biomed. Pharmacother. 110, 47–58. doi:10.1016/j.biopha.2018.11.043
Ramadass, V., Vaiyapuri, T., and Tergaonkar, V. (2020). Small Molecule NF-Κb Pathway Inhibitors in Clinic. Int. J. Mol. Sci. 21 (14), 5164. doi:10.3390/ijms21145164
Ransohoff, R. M. (2016). A Polarizing Question: Do M1 and M2 Microglia Exist? Nat. Neurosci. 19 (8), 987–991. doi:10.1038/nn.4338
Reynolds, A. D., Glanzer, J. G., Kadiu, I., Ricardo-Dukelow, M., Chaudhuri, A., Ciborowski, P., et al. (2008). Nitrated Alpha-Synuclein-Activated Microglial Profiling for Parkinson's Disease. J. Neurochem. 104 (6), 1504–1525. doi:10.1111/j.1471-4159.2007.05087.x
Richards, E. M., Li, J., Stevens, B. R., Pepine, C. J., and Raizada, M. K. (2022). Gut Microbiome and Neuroinflammation in Hypertension. Circ. Res. 130 (3), 401–417. doi:10.1161/CIRCRESAHA.121.319816
Rincón, M., and Davis, R. J. (2009). Regulation of the Immune Response by Stress-Activated Protein Kinases. Immunol. Rev. 228 (1), 212–224. doi:10.1111/j.1600-065X.2008.00744.x
Rodrigues, E., Mariutti, L. R., and Mercadante, A. Z. (2012). Scavenging Capacity of Marine Carotenoids against Reactive Oxygen and Nitrogen Species in a Membrane-Mimicking System. Mar. Drugs 10 (8), 1784–1798. doi:10.3390/md10081784
Rushmore, T. H., Morton, M. R., and Pickett, C. B. (1991). The Antioxidant Responsive Element. Activation by Oxidative Stress and Identification of the DNA Consensus Sequence Required for Functional Activity. J. Biol. Chem. 266 (18), 11632–11639. doi:10.1016/s0021-9258(18)99004-6
Ryter, S. W., Kim, H. P., Hoetzel, A., Park, J. W., Nakahira, K., Wang, X., et al. (2007). Mechanisms of Cell Death in Oxidative Stress. Antioxid. Redox Signal 9 (1), 49–89. doi:10.1089/ars.2007.9.49
Sauer, L., Li, B., and Bernstein, P. S. (2019). Ocular Carotenoid Status in Health and Disease. Annu. Rev. Nutr. 39, 95–120. doi:10.1146/annurev-nutr-082018-124555
Schreibelt, G., Kooij, G., Reijerkerk, A., van Doorn, R., Gringhuis, S. I., van der Pol, S., et al. (2007). Reactive Oxygen Species Alter Brain Endothelial Tight Junction Dynamics via RhoA, PI3 Kinase, and PKB Signaling. FASEB J. 21 (13), 3666–3676. doi:10.1096/fj.07-8329com
Senftleben, U., Cao, Y., Xiao, G., Greten, F. R., Krähn, G., Bonizzi, G., et al. (2001). Activation by IKKalpha of a Second, Evolutionary Conserved, NF-Kappa B Signaling Pathway. Science 293 (5534), 1495–1499. doi:10.1126/science.1062677
Sharma, R. K., Yang, T., Oliveira, A. C., Lobaton, G. O., Aquino, V., Kim, S., et al. (2019). Microglial Cells Impact Gut Microbiota and Gut Pathology in Angiotensin II-Induced Hypertension. Circ. Res. 124 (5), 727–736. doi:10.1161/CIRCRESAHA.118.313882
Shen, D. F., Qi, H. P., Ma, C., Chang, M. X., Zhang, W. N., and Song, R. R. (2021). Astaxanthin Suppresses Endoplasmic Reticulum Stress and Protects against Neuron Damage in Parkinson's Disease by Regulating miR-7/SNCA axis. Neurosci. Res. 165, 51–60. doi:10.1016/j.neures.2020.04.003
Shen, H., Kuo, C. C., Chou, J., Delvolve, A., Jackson, S. N., Post, J., et al. (2009). Astaxanthin Reduces Ischemic Brain Injury in Adult Rats. FASEB J. 23 (6), 1958–1968. doi:10.1096/fj.08-123281
Shi, P., Diez-Freire, C., Jun, J. Y., Qi, Y., Katovich, M. J., Li, Q., et al. (2010a). Brain Microglial Cytokines in Neurogenic Hypertension. Hypertension 56 (2), 297–303. doi:10.1161/HYPERTENSIONAHA.110.150409
Shi, P., Raizada, M. K., and Sumners, C. (2010b). Brain Cytokines as Neuromodulators in Cardiovascular Control. Clin. Exp. Pharmacol. Physiol. 37 (2), e52–7. doi:10.1111/j.1440-1681.2009.05234.x
Shimidzu, N., Goto, M., and Miki, W. (2008). Carotenoids as Singlet Oxygen Quenchers in Marine Organisms. Fish. Sci. 62 (1), 134–137.
Shulman, J. M., De Jager, P. L., and Feany, M. B. (2011). Parkinson's Disease: Genetics and Pathogenesis. Annu. Rev. Pathol. 6, 193–222. doi:10.1146/annurev-pathol-011110-130242
Sies, H. (2015). Oxidative Stress: a Concept in Redox Biology and Medicine. Redox Biol. 4, 180–183. doi:10.1016/j.redox.2015.01.002
Sifi, N., Martin-Eauclaire, M. F., and Laraba-Djebari, F. (2016). K(+) Channel Blocker-Induced Neuroinflammatory Response and Neurological Disorders: Immunomodulatory Effects of Astaxanthin. Inflamm. Res. 65 (8), 623–634. doi:10.1007/s00011-016-0945-y
Simpson, D. S. A., and Oliver, P. L. (2020). ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants (Basel) 9 (8), 743. doi:10.3390/antiox9080743
Sklerov, M., Dayan, E., and Browner, N. (2019). Functional Neuroimaging of the Central Autonomic Network: Recent Developments and Clinical Implications. Clin. Auton. Res. 29 (6), 555–566. doi:10.1007/s10286-018-0577-0
Song, B., Lee, S.-J., and Kim, C.-H. (2021). Roles of Cytokines in the Temporal Changes of Microglial Membrane Currents and Neuronal Excitability and Synaptic Efficacy in ATP-Induced Cortical Injury Model. Ijms 22 (13), 6853. doi:10.3390/ijms22136853
Sporn, M. B., and Liby, K. T. (2012). NRF2 and Cancer: the Good, the Bad and the Importance of Context. Nat. Rev. Cancer 12 (8), 564–571. doi:10.1038/nrc3278
Stewart, D., Killeen, E., Naquin, R., Alam, S., and Alam, J. (2003). Degradation of Transcription Factor Nrf2 via the Ubiquitin-Proteasome Pathway and Stabilization by Cadmium. J. Biol. Chem. 278 (4), 2396–2402. doi:10.1074/jbc.M209195200
Sun, S. C. (2011). Non-canonical NF-Κb Signaling Pathway. Cell Res. 21 (1), 71–85. doi:10.1038/cr.2010.177
Sun, S. C. (2017). The Non-canonical NF-Κb Pathway in Immunity and Inflammation. Nat. Rev. Immunol. 17 (9), 545–558. doi:10.1038/nri.2017.52
Sun, S. C. (2012). The Noncanonical NF-Κb Pathway. Immunol. Rev. 246 (1), 125–140. doi:10.1111/j.1600-065X.2011.01088.x
Sweeney, M. D., Zhao, Z., Montagne, A., Nelson, A. R., and Zlokovic, B. V. (2019). Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 99 (1), 21–78. doi:10.1152/physrev.00050.2017
Taksima, T., Chonpathompikunlert, P., Sroyraya, M., Hutamekalin, P., Limpawattana, M., and Klaypradit, W. (2019). Effects of Astaxanthin from Shrimp Shell on Oxidative Stress and Behavior in Animal Model of Alzheimer's Disease. Mar. Drugs 17 (11), 628. doi:10.3390/md17110628
Teixeira-Santos, L., Albino-Teixeira, A., and Pinho, D. (2020). Neuroinflammation, Oxidative Stress and Their Interplay in Neuropathic Pain: Focus on Specialized Pro-resolving Mediators and NADPH Oxidase Inhibitors as Potential Therapeutic Strategies. Pharmacol. Res. 162, 105280. doi:10.1016/j.phrs.2020.105280
Telakowski-Hopkins, C. A., King, R. G., and Pickett, C. B. (1988). Glutathione S-Transferase Ya Subunit Gene: Identification of Regulatory Elements Required for Basal Level and Inducible Expression. Proc. Natl. Acad. Sci. U. S. A. 85 (4), 1000–1004. doi:10.1073/pnas.85.4.1000
Terazawa, S., Nakajima, H., Shingo, M., Niwano, T., and Imokawa, G. (2012). Astaxanthin Attenuates the UVB-Induced Secretion of Prostaglandin E2 and Interleukin-8 in Human Keratinocytes by Interrupting MSK1 Phosphorylation in a ROS Depletion-independent Manner. Exp. Dermatol 21 (Suppl. 1), 11–17. doi:10.1111/j.1600-0625.2012.01496.x
Tewari, D., Sah, A. N., Bawari, S., Nabavi, S. F., Dehpour, A. R., Shirooie, S., et al. (2020). Role of Nitric Oxide in Neurodegeneration: Function, Regulation, and Inhibition. Curr. Neuropharmacol. 19 (2), 114–126. doi:10.2174/1570159X18666200429001549
Toral-Rios, D., Pichardo-Rojas, P. S., Alonso-Vanegas, M., and Campos-Peña, V. (2020). GSK3β and Tau Protein in Alzheimer's Disease and Epilepsy. Front. Cell Neurosci. 14, 19. doi:10.3389/fncel.2020.00019
Troubat, R., Barone, P., Leman, S., Desmidt, T., Cressant, A., Atanasova, B., et al. (2021). Neuroinflammation and Depression: A Review. Eur. J. Neurosci. 53 (1), 151–171. doi:10.1111/ejn.14720
Tsuchiya, M., Scita, G., Freisleben, H. J., Kagan, V. E., and Packer, L. (1992). Antioxidant Radical-Scavenging Activity of Carotenoids and Retinoids Compared to Alpha-Tocopherol. Methods Enzymol. 213, 460–472. doi:10.1016/0076-6879(92)13148-q
EFSA Panel on Nutrition,Novel Foods and Food Allergens, Turck, D., Castenmiller, J., de Henauw, S., Hirsch-Ernst, K. I., Kearney, J., Maciuk, A., et al. (2020). Safety of Astaxanthin for its Use as a Novel Food in Food Supplements. EFSA J. 18 (2), e05993. doi:10.2903/j.efsa.2020.5993
Turujman, S. A., Wamer, W. G., Wei, R. R., and Albert, R. H. (1997). Rapid Liquid Chromatographic Method to Distinguish Wild Salmon from Aquacultured Salmon Fed Synthetic Astaxanthin. J. AOAC Int. 80 (3), 622–632. doi:10.1093/jaoac/80.3.622
Uchiyama, K., Naito, Y., Hasegawa, G., Nakamura, N., Takahashi, J., and Yoshikawa, T. (2002). Astaxanthin Protects Beta-Cells against Glucose Toxicity in Diabetic Db/db Mice. Redox Rep. 7 (5), 290–293. doi:10.1179/135100002125000811
Valko, M., Leibfritz, D., Moncol, J., Cronin, M. T., Mazur, M., and Telser, J. (2007). Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 39 (1), 44–84. doi:10.1016/j.biocel.2006.07.001
Valko, M., Rhodes, C. J., Moncol, J., Izakovic, M., and Mazur, M. (2006). Free Radicals, Metals and Antioxidants in Oxidative Stress-Induced Cancer. Chem. Biol. Interact. 160 (1), 1–40. doi:10.1016/j.cbi.2005.12.009
van der Pol, A., van Gilst, W. H., Voors, A. A., and van der Meer, P. (2019). Treating Oxidative Stress in Heart Failure: Past, Present and Future. Eur. J. Heart Fail 21 (4), 425–435. doi:10.1002/ejhf.1320
Vaváková, M., Ďuračková, Z., and Trebatická, J. (2015). Markers of Oxidative Stress and Neuroprogression in Depression Disorder. Oxid. Med. Cell Longev. 2015, 898393. doi:10.1155/2015/898393
Vezzani, A., Fujinami, R. S., White, H. S., Preux, P. M., Blümcke, I., Sander, J. W., et al. (2016). Infections, Inflammation and Epilepsy. Acta Neuropathol. 131 (2), 211–234. doi:10.1007/s00401-015-1481-5
Wan, F., and Lenardo, M. J. (2009). Specification of DNA Binding Activity of NF-kappaB Proteins. Cold Spring Harb. Perspect. Biol. 1 (4), a000067. doi:10.1101/cshperspect.a000067
Wang, C. C., Shi, H. H., Xu, J., Yanagita, T., Xue, C. H., Zhang, T. T., et al. (2020a). Docosahexaenoic Acid-Acylated Astaxanthin Ester Exhibits Superior Performance over Non-esterified Astaxanthin in Preventing Behavioral Deficits Coupled with Apoptosis in MPTP-Induced Mice with Parkinson's Disease. Food Funct. 11 (9), 8038–8050. doi:10.1039/d0fo01176b
Wang, H. Q., Sun, X. B., Xu, Y. X., Zhao, H., Zhu, Q. Y., and Zhu, C. Q. (2010). Astaxanthin Upregulates Heme Oxygenase-1 Expression through ERK1/2 Pathway and its Protective Effect against Beta-Amyloid-Induced Cytotoxicity in SH-Sy5y Cells. Brain Res. 1360, 159–167. doi:10.1016/j.brainres.2010.08.100
Wang, M., Deng, X., Xie, Y., and Chen, Y. (2020b). Astaxanthin Attenuates Neuroinflammation in Status Epilepticus Rats by Regulating the ATP-P2x7r Signal. Drug Des. Devel Ther. 14, 1651–1662. doi:10.2147/DDDT.S249162
Wang, Y. L., Zhu, X. L., Sun, M. H., and Dang, Y. K. (2019). Effects of Astaxanthin Onaxonal Regeneration via cAMP/PKA Signaling Pathway in Mice with Focal Cerebral Infarction. Eur. Rev. Med. Pharmacol. Sci. 23 (3 Suppl. l), 135–143. doi:10.26355/eurrev_201908_18640
Wen, X., Huang, A., Hu, J., Zhong, Z., Liu, Y., Li, Z., et al. (2015). Neuroprotective Effect of Astaxanthin against Glutamate-Induced Cytotoxicity in HT22 Cells: Involvement of the Akt/GSK-3β Pathway. Neuroscience 303, 558–568. doi:10.1016/j.neuroscience.2015.07.034
Wen, X., Xiao, L., Zhong, Z., Wang, L., Li, Z., Pan, X., et al. (2017). Astaxanthin Acts via LRP-1 to Inhibit Inflammation and Reverse Lipopolysaccharide-Induced M1/M2 Polarization of Microglial Cells. Oncotarget 8 (41), 69370–69385. doi:10.18632/oncotarget.20628
Wolf, A. M., Asoh, S., Hiranuma, H., Ohsawa, I., Iio, K., Satou, A., et al. (2010). Astaxanthin Protects Mitochondrial Redox State and Functional Integrity against Oxidative Stress. J. Nutr. Biochem. 21 (5), 381–389. doi:10.1016/j.jnutbio.2009.01.011
Wu, H., Niu, H., Shao, A., Wu, C., Dixon, B. J., Zhang, J., et al. (2015). Astaxanthin as a Potential Neuroprotective Agent for Neurological Diseases. Mar. Drugs 13 (9), 5750–5766. doi:10.3390/md13095750
Wu, T., Zhao, F., Gao, B., Tan, C., Yagishita, N., Nakajima, T., et al. (2014). Hrd1 Suppresses Nrf2-Mediated Cellular Protection during Liver Cirrhosis. Genes Dev. 28 (7), 708–722. doi:10.1101/gad.238246.114
Xiao, G., Harhaj, E. W., and Sun, S. C. (2001). NF-kappaB-inducing Kinase Regulates the Processing of NF-kappaB2 P100. Mol. Cell 7 (2), 401–409. doi:10.1016/s1097-2765(01)00187-3
Xie, W. J., Hou, G., Wang, L., Wang, S. S., and Xiong, X. X. (2020). Astaxanthin Suppresses Lipopolysaccharide Induced Myocardial Injury by Regulating MAPK and PI3K/AKT/mTOR/GSK3β Signaling. Mol. Med. Rep. 22 (4), 3338–3346. doi:10.3892/mmr.2020.11443
Yang, L., Li, F., Cao, X., Qiao, X., Xue, C., and Xu, J. (2021). Stability and Bioavailability of Protein Matrix‐encapsulated Astaxanthin Ester Microcapsules. J. Sci. Food Agric. 102, 2144–2152. doi:10.1002/jsfa.11556
Yang, Q. Q., and Zhou, J. W. (2019). Neuroinflammation in the Central Nervous System: Symphony of Glial Cells. Glia 67 (6), 1017–1035. doi:10.1002/glia.23571
Yang, X., Guo, A. L., Pang, Y. P., Cheng, X. J., Xu, T., Li, X. R., et al. (2019). Astaxanthin Attenuates Environmental Tobacco Smoke-Induced Cognitive Deficits: A Critical Role of P38 MAPK. Mar. Drugs 17 (1), 24. doi:10.3390/md17010024
Yao, Y., Chen, Z. L., Norris, E. H., and Strickland, S. (2014). Astrocytic Laminin Regulates Pericyte Differentiation and Maintains Blood Brain Barrier Integrity. Nat. Commun. 5, 3413. doi:10.1038/ncomms4413
Yasui, Y., Hosokawa, M., Mikami, N., Miyashita, K., and Tanaka, T. (2011). Dietary Astaxanthin Inhibits Colitis and Colitis-Associated Colon Carcinogenesis in Mice via Modulation of the Inflammatory Cytokines. Chem. Biol. Interact. 193 (1), 79–87. doi:10.1016/j.cbi.2011.05.006
Ye, Q., Huang, B., Zhang, X., Zhu, Y., and Chen, X. (2012). Astaxanthin Protects against MPP(+)-induced Oxidative Stress in PC12 Cells via the HO-1/NOX2 axis. BMC Neurosci. 13, 156. doi:10.1186/1471-2202-13-156
Ye, Q., Zhang, X., Huang, B., Zhu, Y., and Chen, X. (2013). Astaxanthin Suppresses MPP(+)-induced Oxidative Damage in PC12 Cells through a Sp1/NR1 Signaling Pathway. Mar. Drugs 11 (4), 1019–1034. doi:10.3390/md11041019
Yuan, J. P., Peng, J., Yin, K., and Wang, J. H. (2011). Potential Health-Promoting Effects of Astaxanthin: a High-Value Carotenoid Mostly from Microalgae. Mol. Nutr. Food Res. 55 (1), 150–165. doi:10.1002/mnfr.201000414
Yuan, L., Qu, Y., Li, Q., An, T., Chen, Z., Chen, Y., et al. (2020). Protective Effect of Astaxanthin against La2O3 Nanoparticles Induced Neurotoxicity by Activating PI3K/AKT/Nrf-2 Signaling in Mice. Food Chem. Toxicol. 144, 111582. doi:10.1016/j.fct.2020.111582
Zang, H., Mathew, R. O., and Cui, T. (2020). The Dark Side of Nrf2 in the Heart. Front. Physiol. 11, 722. doi:10.3389/fphys.2020.00722
Zenaro, E., Piacentino, G., and Constantin, G. (2017). The Blood-Brain Barrier in Alzheimer's Disease. Neurobiol. Dis. 107, 41–56. doi:10.1016/j.nbd.2016.07.007
Zhang, J., Ding, C., Zhang, S., and Xu, Y. (2020a). Neuroprotective Effects of Astaxanthin against Oxygen and Glucose Deprivation Damage via the PI3K/Akt/GSK3β/Nrf2 Signalling Pathway In Vitro. J. Cell Mol. Med. 24 (16), 8977–8985. doi:10.1111/jcmm.15531
Zhang, J., Zhang, S., Bi, J., Gu, J., Deng, Y., and Liu, C. (2017a). Astaxanthin Pretreatment Attenuates Acetaminophen-Induced Liver Injury in Mice. Int. Immunopharmacol. 45, 26–33. doi:10.1016/j.intimp.2017.01.028
Zhang, R., Xu, M., Wang, Y., Xie, F., Zhang, G., and Qin, X. (2017b). Nrf2-a Promising Therapeutic Target for Defensing against Oxidative Stress in Stroke. Mol. Neurobiol. 54 (8), 6006–6017. doi:10.1007/s12035-016-0111-0
Zhang, X. S., Zhang, X., Wu, Q., Li, W., Wang, C. X., Xie, G. B., et al. (2014a). Astaxanthin Offers Neuroprotection and Reduces Neuroinflammation in Experimental Subarachnoid Hemorrhage. J. Surg. Res. 192 (1), 206–213. doi:10.1016/j.jss.2014.05.029
Zhang, X. S., Zhang, X., Wu, Q., Li, W., Zhang, Q. R., Wang, C. X., et al. (2014b). Astaxanthin Alleviates Early Brain Injury Following Subarachnoid Hemorrhage in Rats: Possible Involvement of Akt/bad Signaling. Mar. Drugs 12 (8), 4291–4310. doi:10.3390/md12084291
Zhang, X. S., Zhang, X., Zhang, Q. R., Wu, Q., Li, W., Jiang, T. W., et al. (2015). Astaxanthin Reduces Matrix Metalloproteinase-9 Expression and Activity in the Brain after Experimental Subarachnoid Hemorrhage in Rats. Brain Res. 1624, 113–124. doi:10.1016/j.brainres.2015.07.020
Zhang, X. S., Zhang, X., Zhou, M. L., Zhou, X. M., Li, N., Li, W., et al. (2014c). Amelioration of Oxidative Stress and Protection against Early Brain Injury by Astaxanthin after Experimental Subarachnoid Hemorrhage. J. Neurosurg. 121 (1), 42–54. doi:10.3171/2014.2.JNS13730
Zhang, Z., Guo, C., Jiang, H., Han, B., Wang, X., Li, S., et al. (2020b). Inflammation Response after the Cessation of Chronic Arsenic Exposure and Post-treatment of Natural Astaxanthin in Liver: Potential Role of Cytokine-Mediated Cell-Cell Interactions. Food Funct. 11 (10), 9252–9262. doi:10.1039/d0fo01223h
Zhao, T., Ma, D., Mulati, A., Zhao, B., Liu, F., and Liu, X. (2021). Development of Astaxanthin-Loaded Layer-By-Layer Emulsions: Physicochemical Properties and Improvement of LPS-Induced Neuroinflammation in Mice. Food Funct. 12 (12), 5333–5350. doi:10.1039/d0fo03018j
Zhao, Z., Nelson, A. R., Betsholtz, C., and Zlokovic, B. V. (2015). Establishment and Dysfunction of the Blood-Brain Barrier. Cell 163 (5), 1064–1078. doi:10.1016/j.cell.2015.10.067
Zhou, X., Zhang, F., Hu, X., Chen, J., Wen, X., Sun, Y., et al. (2015). Inhibition of Inflammation by Astaxanthin Alleviates Cognition Deficits in Diabetic Mice. Physiol. Behav. 151, 412–420. doi:10.1016/j.physbeh.2015.08.015
Zhou, X. Y., Zhang, F., Hu, X. T., Chen, J., Tang, R. X., Zheng, K. Y., et al. (2017). Depression Can Be Prevented by Astaxanthin through Inhibition of Hippocampal Inflammation in Diabetic Mice. Brain Res. 1657, 262–268. doi:10.1016/j.brainres.2016.12.018
Zhou, X., Zhang, J., Li, Y., Cui, L., Wu, K., and Luo, H. (2021). Astaxanthin Inhibits Microglia M1 Activation against Inflammatory Injury Triggered by Lipopolysaccharide through Down-Regulating miR-31-5p. Life Sci. 267, 118943. doi:10.1016/j.lfs.2020.118943
Zhuge, F., Ni, Y., Wan, C., Liu, F., and Fu, Z. (2021). Anti-diabetic Effects of Astaxanthin on an STZ-Induced Diabetic Model in Rats. Endocr. J. 68 (4), 451–459. doi:10.1507/endocrj.EJ20-0699
Keywords: neuroinflammation, astaxanthin, medical application, antioxidation, neuroinflammatory factors, blood-brain barrier
Citation: Wang S and Qi X (2022) The Putative Role of Astaxanthin in Neuroinflammation Modulation: Mechanisms and Therapeutic Potential. Front. Pharmacol. 13:916653. doi: 10.3389/fphar.2022.916653
Received: 09 April 2022; Accepted: 07 June 2022;
Published: 24 June 2022.
Edited by:
Hansen Chen, Stanford University, United StatesReviewed by:
Muhammad Ikram, Khyber Medical University, PakistanQingkun Liu, Mount Sinai Hospital, United States
Chenjie Pan, Stanford University, United States
Copyright © 2022 Wang and Qi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Qi, eHhxMzhAY2FzZS5lZHU=
 Shuai Wang
Shuai Wang Xin Qi
Xin Qi