- 1Jiaxing University Medical College, Jiaxing, China
- 2Department of Clinical Medicine, Jiaxing University Medical College, Jiaxing, China
- 3Research Center of Neuroscience, Jiaxing University Medical College, Jiaxing, China
Panax ginseng and Panax notoginseng, two well-known herbs with enormous medical value in Asian countries, have a long usage history in China for the therapy of some diseases, such as stroke. Ginsenoside Rb1 is one of most important active ingredients in Panax ginseng and Panax notoginseng. In the last two decades, more attention has focused on ginsenoside Rb1 as an antioxidative, anti-apoptotic and anti-inflammatory agent that can protect the nervous system. In the review, we summarize the neuroprotective roles of ginsenoside Rb1 and its potential mechanisms in central nervous system diseases (CNSDs), including neurodegenerative diseases, cerebral ischemia injury, depression and spinal cord injury. In conclusion, ginsenoside Rb1 has a potential neuroprotection due to its inhibition of oxidative stress, apoptosis, neuroinflammation and autophagy in CNSDs and may be a promising candidate agent for clinical therapy of CNSDs in the future.
Introduction
Panax ginseng and Panax notoginseng are two valuable medicinal herbs in the genus Panax, family Araliaceae (Wang et al., 2016). The curative effects of Panax ginseng and Panax notoginseng were first detailedly recorded by Shizhen Li in “Compendium of Materia Medica,” which was praised by Darwin as “the Encyclopedia of ancient China.” Panax ginseng and Panax notoginseng have a wide and significant application in medicinal purposes and economic values (Qiao et al., 2018). In China Panax ginseng has a more than 5,000 years of application history (Yun, 2001) and was recorded in the world’s oldest pharmacopeia of medicinal herbs and plants, “Shennong’s Herbal Classic” (Shi et al., 2019). Panax ginseng mainly grows in the mountains of East Asia countries, particularly in China, Korea and Japan (Baeg and So, 2013). Shizhen Li described Panax ginseng as a magic medicine that can almost cure all diseases. Since the content of active ingredient in Panax notoginseng is higher than that in Panax ginseng, Panax notoginseng enjoys a reputation for “The King in Panax.” In modem times, they have received considerable interest due to their extensive application in healthcare products, clinical therapy, and as foods and food additives in the whole world (Yang et al., 2014) because they could relieve stress and fatigue, prevent aging, increase vigor and strengthen the body and mind (Choi, 2008; Kim et al., 2015). Panax ginseng and Panax notoginseng are often used to slow down the symptoms, such as traumatic injury, blood stasis, swelling and pain (Tianhong et al., 2014; Chen et al., 2017; Wu et al., 2018). The ginsenosides, chemical constituents found in Panax ginseng and Panax notoginseng, can inhibit the effects of inflammatory cytokines, block signaling pathways that induce inflammation, and inhibit cells that participate in inflammatory processes (de Oliveira Zanuso et al., 2022). What’s more, increasing evidence has demonstrated that ginsenosides are involved in neuroprotective effects in the central nervous system diseases due to their antioxidant, anti-apoptotic, and anti-inflammatory features (Lu et al., 2022).
Ginsenoside Rb1
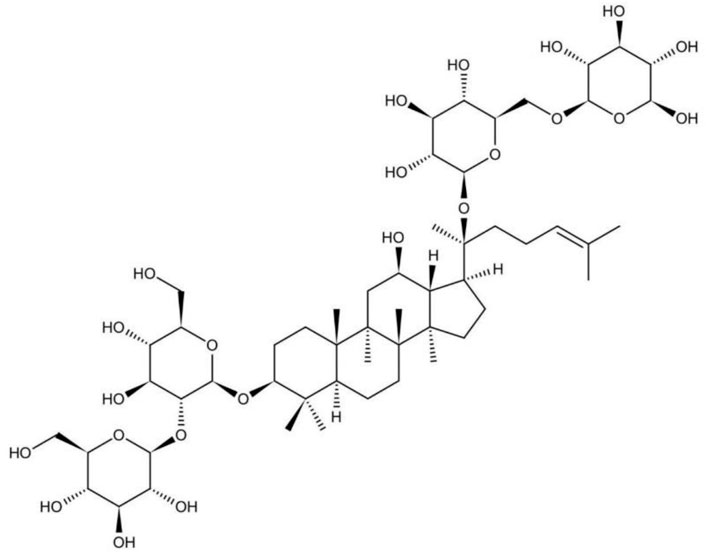
Many studies support that the beneficial effects of Panax ginseng attributed to ginsenosides (Qi et al., 2011). Triterpenoid plays an important role in the medical value of Panax ginseng and Panax notoginseng (Ng, 2006). Triterpenoid is constituted mainly by ginsenoside Rb1, ginsenoside Rb2, and notoginsenoside R1, among which ginsenoside Rb1 takes up a tremendous part. Ginsenoside is a kind of steroids, also known as triterpenoid saponins. All the ginsenosides have the similarity in the basic structure, containing sterane steroid nuclei arranged into four rings by 30 carbon atoms (Cheng et al., 2019). Results also indicate that the function of ginsenoside Rb1 is superior to the function of ginsenoside Rb2, because ginsenoside Rb1 is a panaxtriol with two sugars and ginsenoside Rb2 is a panaxtriol with four sugars (Kim et al., 2014). The structure of ginsenoside Rb1 is shown in Figure 1.
Pharmacological Effects of Ginsenoside Rb1
With the development of the study, ginsenoside Rb1 presents antioxidant, anti-apoptotic, and anti-inflammatory properties. In a cell free system, ginsenoside Rb1 can significantly and selectively scavenge hydroxyl radical and hypochlorous acid, two of the strongest reactive oxygen species (ROS), and protect biomacromolecules from oxidative damage (Lu et al., 2012). Ginsenoside Rb1 could inhibit mitochondria-, endoplasmic reticulum stress- and death receptor-mediated apoptotic pathways (Ke et al., 2021; Shaukat et al., 2021). Our previous study demonstrated that ginsenoside Rb1 inhibited oxidative stress-induced endoplasmic reticulum stress in rat PC12 cells (Zeng et al., 2015). What’s more, treatment with ginsenoside Rb1 attenuated tumor necrosis factor-α (TNF-α)-induced inflammation by inhibiting the activation of c-Jun N-terminal kinase (JNK) and p38 pathways in human umbilical vein endothelial cells and further suppressed the nuclear factor-kappa B (NF-κB) signaling and downregulated the expression of inflammatory factors (Zhou et al., 2017). In addition, ginsenoside Rb1 has the ability to regulate autophagy (Liu et al., 2020). It’s believed that oxidative stress, apoptosis, inflammation and autophagic dysfunction contribute to a variety of diseases, especially the central nervous system diseases (CNSDs). CNSDs, including neurodegenerative diseases, cerebral ischemia injury, depression and spinal cord injury, which are always difficult to cure clinically. Therapeutic drugs used clinically fail to block the development of diseases or are proved to produce severe side effects. Thus, there is an urgent need to develop new drugs to treat these diseases. In the last two decades, ginsenoside Rb1 is reported to play potent neuroprotection in rodent models of CNSDs. In this review, we summarize the neuroprotective roles of ginsenoside Rb1 and highlight its potential molecular mechanisms.
Ginsenoside Rb1 in Neurodegenerative Diseases
Alzheimer’s Disease
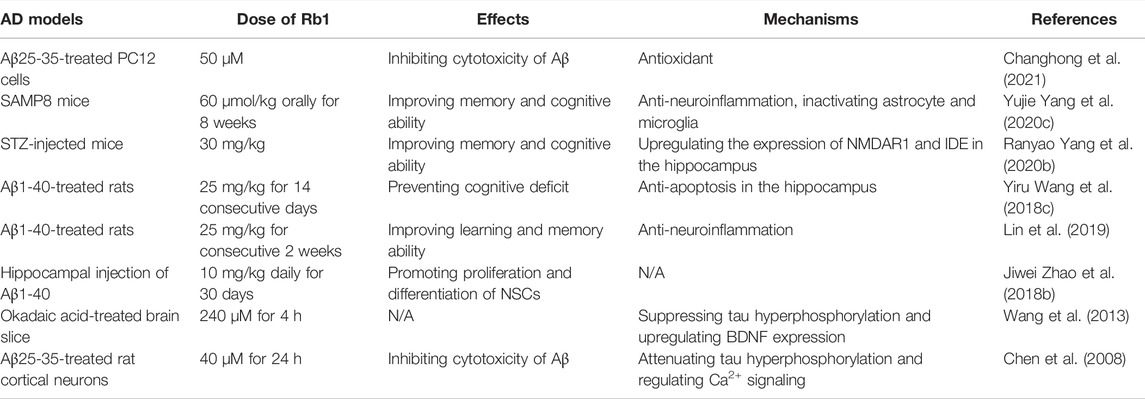
Alzheimer’s disease (AD) is the most common neurodegenerative disease, characterized by progressive cognitive and behavioral impairment (Mao et al., 2018). The pathological features of AD are Amyloid β (Aβ) deposition (Harrison et al., 2021), tau protein hyperphosphorylation (Xia et al., 2021) and loss of hippocampal neurons (Edler et al., 2017).
Some in vitro studies showed that ginsenoside Rb1 protected against Aβ-induced cytotoxicity in various cells (Qian et al., 2009; Xie et al., 2010). Changhong and others found that ginsenoside Rb1 treatment could serve as an activator of peroxisom proliferator-activated receptor-γ (PPARγ) and reduce the level of cholesterol and further lowered the cytotoxicity of Aβ25-35 in PC12 cells (Changhong et al., 2021). Several in vivo studies revealed the neuroprotective roles and potential mechanisms in various AD models. Oral administration of ginsenoside Rb1 significantly shortened the escape latency in the Morris water maze (MWM) test and reduced the number of errors in the passive avoidance task by decreasing protein expression levels of ASC, caspase-1 and Aβ, repairing neuronal cells loss and inhibiting the activation of astrocyte and microglia in hippocampus of SAMP8 mice (Yang et al., 2020c). More importantly, ginsenoside Rb1 was more effective than another ginsenoside, Rg1, in SAMP8 mice. One interesting study showed that ginsenoside Rb1 given orally is completely metabolized to 20-O-beta-D-glucopyranosyl-20(S)-protopanaxadiol (M1) to exert neuroprotective role (Tohda et al., 2004). Ginsenoside Rb1 improved memory and cognitive ability of streptozotocin (STZ)- injected mice and also relieved glucose intolerance induced by STZ injection by enhancing insulin sensitivity (Yang et al., 2020b). These beneficial effects of ginsenoside Rb1 is most likely mediated by upregulating the expression of NMDAR1 and IDE in the hippocampus through inhibiting the activity of CDK5/p35. Ginsenoside Rb1 treatment decreased the levels of Bax and cleaved caspase-3, upregulated the level of Bcl-2 in the hippocampus (Wang et al., 2018c), suggesting that ginsenoside Rb1 inhibited Aβ1-40-induced mitochondrial apoptosis pathway.
The abnormal deposition of Aβ, a common induction pathway in AD (Hardy and Selkoe, 2002), exhibits neurotoxicity and may lead to a complex array of responses, including an inflammatory cascade (Streit, 2004). AD inflammation and thrombosis are promoted by Aβ through interaction with circulating protein XII and fibrinogen (Zamolodchikov and Strickland, 2016). Pro-inflammatory cytokines such as interleukin-1β (IL-1β), IL-6, IL-10, and TNF-β were found to have increased expression in the brain and cerebrospinal fluid of AD patients (Mrak and Griffin, 2005; Jiang et al., 2011). Interestingly, ginsenoside Rb1 mitigated the isoflurane/surgery-induced cognitive impairment- and synapse dysfunction via decreasing levels of ROS, TNF-α and IL-6 in the mice hippocampus, suggesting that the mechanisms refer to inhibiting oxidative stress and neuroinflammation (Miao et al., 2017). Glial fibrillary acidic protein (GFAP), an astrocyte marker, is associated with memory impairment and neuronal reduction (Murphy et al., 2003). In a Aβ1-40-induced AD model, the expression of inflammation-related genes Aβ, IL-1β, and GFAP were decreased in the hippocampus of rats after ginsenoside Rb1 injection (Lin et al., 2019), indicating that ginsenoside Rb1 can reduce the neuroinflammation in AD. The increased number of hippocampal neurons in CA1 area may be involved with the ability of ginsenoside Rb1 promoting the proliferation and differentiation of neural stem cells (NSC) in AD model (Zhao et al., 2018b). (Table 1).
Neurofibrillary tangles (NFTs), composed of hyperphosphorylated microtubule-associated protein tau, are a defining pathological feature of AD. It is hypothesized that hyperphosphorylation of tau impairs the microtubule-stabilizing function of tau, leading to the formation of paired helical filaments and neuronal death (Liu et al., 2003). In spite of the fact that Aβ aggregation is considered an important causative factor of AD, there are great correlations between clinical symptoms, atrophy and brain damage and the appearance of tau aggregation (Bennett et al., 2017). Ginsenoside Rb1 can reduce the expression of phosphorylated tau protein in brain slices of rat model of AD and effectively reduce the formation of NFTs (Wang et al., 2013). Overexpression of glycogen synthase kinase-3β (GSK3β), a tau protein kinase, induced hyperphosphorylation of tau protein in cellular and animal models (Lucas et al., 2001). Brain-derived neurotrophic factor (BDNF) inhibited tau protein phosphorylation by suppressing the activity of GSK3β (Liu et al., 2003). Ginsenoside Rb1 inhibits tau protein phosphorylation by upregulating BDNF and therefore has a preventive effect in AD (Wang et al., 2013). Increasing studies have shown that Aβ induced the hyperphosphorylation of tau. Ginsenoside Rb1 inhibited fibrillar Aβ25-35-induced tau hyperphosphorylation in primary cultured cortical neurons via inactivating Ca2+/calpain/CDK5 signal pathway (Chen et al., 2008) (Table 1).
Several omics researches revealed the neuroprotection of ginsenoside Rb1 in AD. An RNA sequencing study demonstrated ginsenoside Rb1 could regulate the expression of genes related to nervous system development and mitogen-activated protein kinase (MAPK) signaling pathway in SAMP8 mice (Zhang et al., 2017). Ginsenoside Rb1 treatment also showed a potential to upregulate the expression of proteins, such as CAP1, CAPZB, TOMM40, and DATN which are essential for growth cone morphology and neurite outgrowth according to results of a proteomics study (Hwang et al., 2016). In addition, a metabolomic study revealed that ginsenoside Rb1 displayed anti-AD effects through regulating lecithin and amino acid metabolism (Li et al., 2015).
These researches demonstrated that ginsenoside Rb1 have potent effects in alleviating the pathological features of AD, suggesting ginsenoside Rb1 may be a competitive candidate for AD therapy.
Parkinson Disease
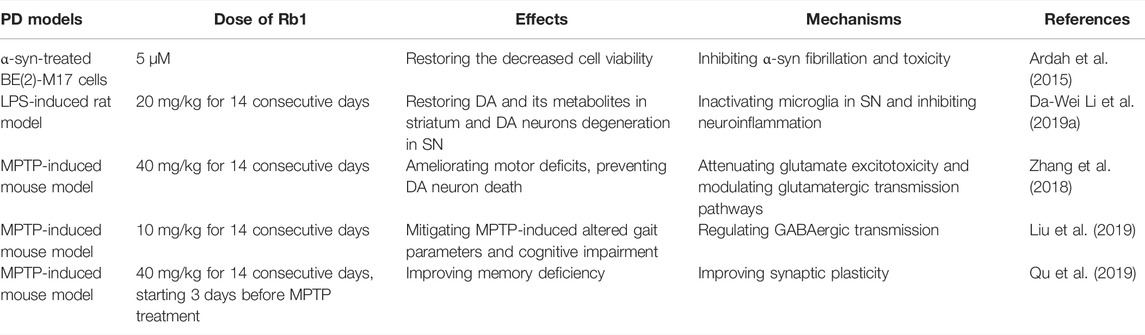
Parkinson Disease (PD), one of the most common neurodegenerative diseases (Obeso et al., 2017), is characterized by the loss of dopaminergic neurons in the substantia nigra of midbrain (Zeng et al., 2014a). Actually, increasing studies have showed that both genetic and environmental factors may lead to PD inspite of the unknown pathology of PD (Kim and Alcalay, 2017). Based on current studies, the mutations of several genes, including α-synuclein, LRRK2, PINK1, Parkin, DJ-1, VPS35, and GBA1, lead to the onset of PD (Zeng et al., 2018c). Various biological processes, such as dopamine metabolism, mitochondrial dysfunction, endoplasmic reticulum stress, impaired autophagy, and deregulation of immunity could lead to the loss of dopaminergic neurons (Zeng et al., 2018c).
Normally, pathologic changes of PD contain the appearance of Lewy bodies and the mass death of dopaminergic neurons (Simon et al., 2020). The misfolded of α-synuclein (α-syn) is a classic sign in PD. Study shows that the initial α-syn aggregations is neurotoxic since they cause the death of the cells (Mehra et al., 2019). Ardah et al. (2015) found that ginsenoside Rb1 could inhibit the toxicity and aggregation of α-syn; What’s more, ginsenoside Rb1 exhibited a strong ability to decompose preformed fibrils. Mechanistically, ginsenoside Rb1 bonds to soluble non-toxic oligomers with no β-sheet content, making it susceptible to proteinase K digestion. Ginsenoside Rb1 attenuated the lipopolysaccharide (LPS)-induced depletion of dopamine and its metabolites in the striatum, inhibiting dopamine (DA)rgic neuron degeneration in the substantial nigra via inhibiting the activation of microglia in substantial nigra by downregulating LPS-induced activation of NF-κB signaling pathway (Li et al., 2019a). An earlier study showed that ginsenoside Rb1 significantly increased the numbers and lengths of neurites of surviving DA neurons although it could not prevent cell death by glutamate challenge (Radad et al., 2004). 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is a commonly used reagent to build PD model in mice (Zeng et al., 2018b), which could cross the blood-brain barrier (BBB, acting as the gatekeeper of the CNS in maintaining the delicate homeostasis of the brain), and be metabolized into the potent dopaminergic neurotoxin 1-methyl-4-phenylpyridinium ion (MPP+) by monoamine oxidase B in glial cells (Langston, 2017). It was reported for the first time that ginsenoside Rb1 treatment ameliorated motor deficits, prevented DA neuron death, and suppressed the expression of α-synuclein and astrogliosis in the MPTP mouse model of PD (Zhang et al., 2018). Glutamate plays a role in exciting neurons and promoting them to produce action potentials in CNS. In vivo, the appropriate level of glutamate is maintained by astrocytic glutamate transporters. However, too much endogenous glutamate may cause the death of the excitotoxic neurons, which may be connected with PD (Pajarillo et al., 2019). Zhang et al. (2018) found that ginsenoside Rb1 could suppress the excitotoxicity of glutamate by increasing glutamate transporter expression via nuclear translocation of NF-κB and modulates synaptic transmission in MPTP model of PD. In addition, ginsenoside Rb1 attenuated MPTP-induced cognitive impairment and dysfunctional gait dynamic via regulating gamma-aminobutyric acid (GABA)ergic transmission in the prefrontal cortex (PFC) (Liu et al., 2019). Administration of ginsenoside Rb1 also improved the memory deficiency of MPTP-treated mice via promoting hippocampal CA3 α-syn monomer expression, restoring the glutamate in the CA3-schaffer collateral-CA1 pathway, and sequentially increasing postsynaptic density-95 (PSD-95) expression (Qu et al., 2019) (Table 2). These researches suggest that ginsenoside Rb1 may serve as a potential therapeutic agent for PD.
Other Neurodegenerative Diseases
A few studies also found that ginsenoside Rb1 showed neuroprotection in other neurodegenerative diseases, such as epilepsy and Huntington’s disease (HD). Ginsenoside Rb1 ameliorated pentylenetetrazol (PTZ)-injured longer seizure duration and shorter seizure latency, as well as the cognitive deficits and neuronal damage in rats via Rb1 dose-dependently increasing GSH levels, decreasing MDA levels, and activating Nrf2/ARE signaling (Shi et al., 2018). Its metabolic production, compound K, exerted anti-epileptic effects by promoting the hippocampal release of GABA and enhancing the GABAA receptor-mediated inhibitory synaptic transmission (Zeng et al., 2018). Nanomolar concentrations of ginsenoside Rb1 effectively protected YAC128 medium spiny striatal neurons from YAC128 HD model mice against glutamate-induced apoptosis and Ca2+ responses (Wu et al., 2009). Notably, the protective effect of ginsenoside Rb1 is stronger than others ginsenosides.
Ginsenoside Rb1 in Cerebral Ischemia
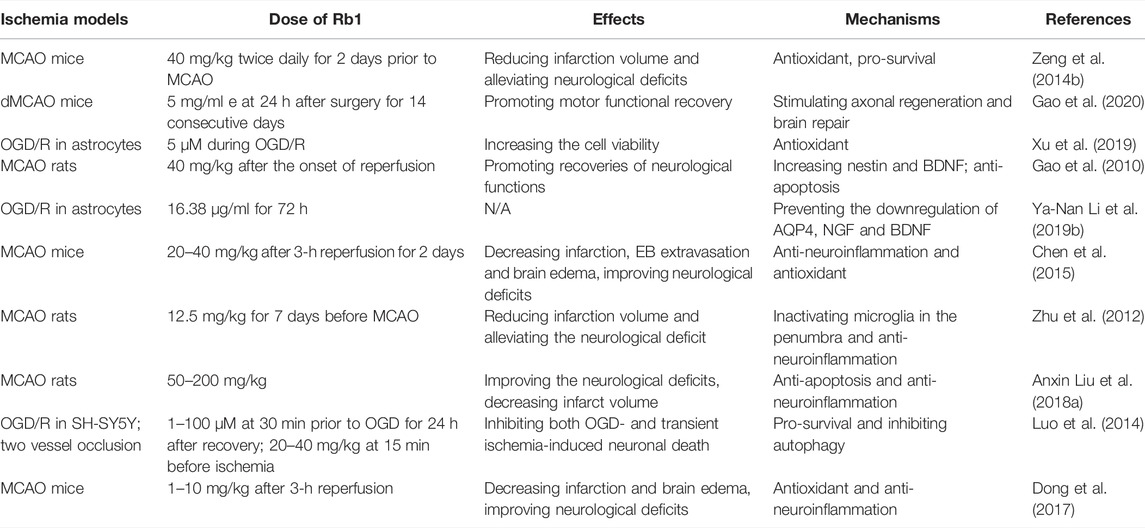
Ischemic stroke is one of the leading causes of adult disability and death all over the world. Cerebral ischemia, caused by a significant blockage in cerebral blood flow, results in various pathological events such as oxidative stress, neuroinflammation response, excitatory neurotransmitter release and energy failure, which eventually lead to neuronal apoptosis and brain tissue necrosis (Kamel and Iadecola, 2012; Khoshnam et al., 2017). What’s more, the recovery of blood supply after a certain period of brain tissue ischemia caused the secondary cerebral ischemia-reperfusion (I/R) injury. Currently, only a limited number of drugs commonly used in clinic are available for treating ischemic stroke and the therapeutic efficacy of these drugs is still limited (Cheng et al., 2019). Therefore, exploring and developing other potential strategies and agents for preventing and treating ischemic stroke are extremely and urgently needed.
Ginsenoside Rb1 could effectively reduce the levels of oxidative stress to protect against cerebral ischemia-induced neuronal injury. Our previous study demonstrated that ginsenoside Rb1 notably inhibited the increase in malondialdehyde (MDA) concentration and restored the expression of thioredoxin-1 (Trx-1) and copper-zinc superoxide dismutase (SOD-1) (Zeng et al., 2014b). As one of the two redox systems in cells, Trx plays crucial roles in maintaining the intracellular redox state. Trx-1 may serve as a therapeutic target in cerebral ischemia (Zeng et al., 2018a). Nrf2 is a primary antioxidant pathway and targets intracellular antioxidant genes (Carpenter et al., 2022). Ginsenoside Rb1 treatment could activate Keap1-Nrf2/ARE signaling pathway (Yang et al., 2020a). What’s more, ginsenoside Rb1 treatment at 24 h after surgery for 14 consecutive days activated cAMP/PKA/CREB signaling pathway in a distal middle cerebral artery occlusion (dMCAO) mouse model and promoted motor functional recovery and axonal regeneration (Gao et al., 2020), suggesting that ginsenoside Rb1 may serve as a potential clinically therapy drug during the recovery stage of stroke. Nrf2 and CREB are the transcription factor of Trx-1, which upregulate Trx-1 expression under stress (Im et al., 2012; Jia et al., 2016). Ginsenoside Rb1 treatment is able to improve the mitochondrial function in astrocytes in an oxygen-glucose deprivation/reoxygenation (OGD/R) model by inhibiting reactive oxygen species (ROS) formation and mitochondrial membrane potential (MMP) depolarization, increasing mtDNA content, activities of catalase (CAT), complexes I, II, III, and V and the level of ATP (Xu et al., 2019). (Table 3).
Administration of ginsenoside Rb1 could upregulate the expression of neurotrophic factor, which is beneficial for the neural survival. In a rat MCAO model, injection of ginsenoside Rb1 immediately after the onset of reperfusion significantly upregulated expression of BDNF, which induced the neurogenesis and promoted the recovery of neurological functions (Gao et al., 2010). In addition, Ginsenoside Rb1 also significantly increased the expression of nerve growth factor (NGF) and BDNF, as well as the expression of aquaporin 4 (AQP4), in OGD/R-induced rat astrocytes (Li et al., 2019b) (Table 3).
Increasing studies have suggested that neuroinflammation play an important role in the pathology of stroke. The main aim of acute stroke treatment is to rescue the ischemic penumbra or nonfunctional, yet still viable tissue surrounding the infarcted core. During ischemic stroke, the integrity of BBB tight junctions can be seriously destroyed due to blocked or reduced circulating blood flow (Meyers et al., 2011). Ginsenoside Rb1 protects loss of BBB integrity in ischemic stroke by suppressing neuroinflammation induction of matrix metalloproteinase-9 (MMP-9) and nicotinamide adenine dinucleotide phosphate oxidase 4 (NOX4)-derived free radical (Chen et al., 2015). A study reported that ginsenoside Rb1 administration markedly mitigated the activation of microglia in the ischemic penumbra by inhibiting the expression of TNF-α and IL-6 and the activation of NF-κB (Zhu et al., 2012), suggesting that ginsenoside Rb1 treatment is beneficial for salvaging the ischemic penumbra via inhibiting microglia-mediated neuroinflammation. High-mobility group box 1 (HMGB1) is released after focal cerebral I/R and aggravates brain tissue damage. Ginsenoside Rb1 treatment significantly inhibited the release of HMGB1 in MCAO rats, accompanied by decreasing the levels of NF-κB, TNFα, IL-6, and inducible nitric oxide synthase (iNOS) (Liu et al., 2018a), suggesting that the effects of ginsenoside Rb1 may be associated with the inhibition of HMGB1 inflammatory signals (Table 3).
The function of ginsenoside Rb1 in cerebral ischemia is related to common pro-survival pathways in cells. Ginsenoside Rb1 treatment upregulated the protein level of p-Akt at Ser473 and inhibited the elevation in protein levels of LC3II and Beclin1 and protected against OGD and transient global ischemia (two vessels occlusion)-induced injury (Luo et al., 2014). The recovery of brain damage after MCAO is significantly impaired in aged mice compared with young mice. Interestingly, long-term oral administration of ginsenoside Rb1 greatly prevented the injury in a dose-dependent manner by inhibiting oxidative stress and the activation of extracellular signal-regulated kinase 1/2 (ERK1/2) in aged mice (Dong et al., 2017). ERK1/2 activation is also a well-known pro-survival pathway, however, activation of mitogen-activated protein kinase/ERK may contribute to premature aging, and its inhibition has potential use in preventing cellular senescence as well as aging (Chappell et al., 2011) (Table 3).
These researches suggest that ginsenoside Rb1 may serve as a potential therapeutic agent for cerebral ischemia.
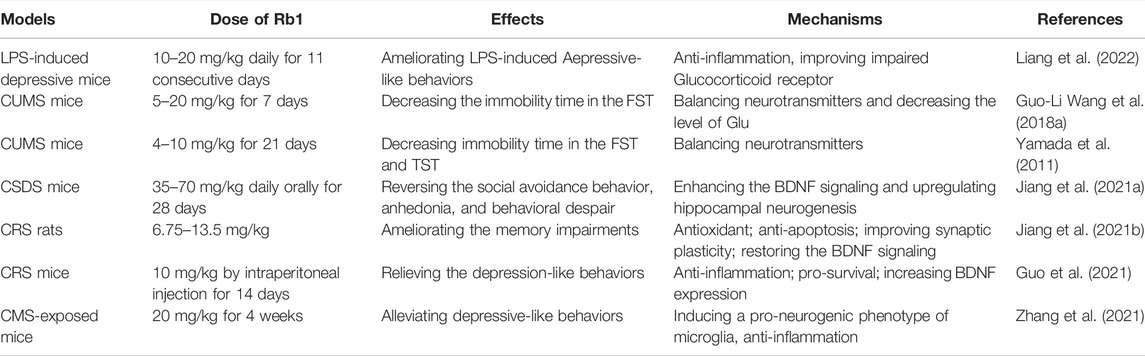
Ginsenoside Rb1 in Depression
Depression, a common psychiatric disease and the leading cause of disability worldwide (Lopez and Murray, 1998), is a heterogenous diagnostic concept consisting of a set of different symptoms, with decreased mood, anhedonia, and reduced energy defined as core symptomatologies in ICD-10 and depressed mood and loss of interest and pleasure in DSM-5 (Hoflich et al., 2019). Depression is often associated with chronic illnesses (Evans et al., 2005) or other mood disorders such as co-morbid anxiety (Menard et al., 2016). At present, it is generally believed that dysfunction of monoamine neurotransmitters and their receptors, decrease in neurotrophic factors, neuroinflammation, activity enhancement of HPA axis are involved in the pathogenesis of depression. Monoamine oxidase inhibitors (MAOI), selective serotonin reuptake inhibitors (SSRI) or serotonin norepinephrine reuptake inhibitors (SNRI) are used clinically to treat moderate to severe depression (Walker, 2013). Unfortunately, these inhibitors are well recognized to produce a number of undesirable side effects that often lead to patient noncompliance. With the in-depth study of traditional Chinese medicine, people began to seek active ingredients from natural products for the treatment of depression. Increasing evidence demonstrated that ginsenoside Rb1 could significantly improve the depressive-like behaviors in various rodent models.
In a lipopolysaccharide (LPS)-induced depressive model, ginsenoside Rb1 significantly suppressed peripheral and hippocampal inflammation via MAPK/NF-κB signaling, improved impaired glucocorticoid receptor and inhibited activity of indoleamine 2,3-dioxygenase (Liang et al., 2022). The authors also found that ginsenoside Rb1 increased the levels of 5-HT and expression of 5-HT1A receptor. The treatment with 5-HT1A receptor antagonist, NAN190 or 5-HT2A receptor antagonist, ritanserin, reversed the antidepressant-like effect of ginsenoside Rb1 in chronic unpredictable mild stress (CUMS) mice (Yamada et al., 2011; Wang et al., 2018a), suggesting that serotonergic receptor may be involved in the antidepressant-like effect of ginsenoside Rb1. Besides, dopaminergic and noradrenergic systems are also involved in the antidepressant-like effects of ginsenoside Rb1 (Wang et al., 2017). Rd, F2, compound K, Rh2, and Rg3 were identified as the metabolites of ginsenoside Rb1. Liang et al. (2022) found that F2 exerted antidepressant-like effects and it showed higher activity than ginsenoside Rb1 against depression (Table 4).
Ginsenoside Rb1 exerted promising antidepressant-like effects in mice with chronic social defeat stress (CSDS)-induced depression by enhancing the BDNF/Trkb signaling cascade, which increased the hippocampal neurogenesis (Jiang et al., 2021a). Similarly, ginsenoside Rb1 treatment ameliorated chronic restraint stress (CRS)-induced memory impairments in rats by improving synaptic plasticity and restoring the BDNF/TrkB signalling pathway (Jiang et al., 2021b). Guo et al. (2021) found that ginsenoside Rb1 not only increased the protein expression of BDNF, but also activated the pro-survival Akt pathway in CRS mice. In addition, ginsenoside Rb1 alleviated the inflammation induced by CRS, including decrease in the protein expression of IL-1β, TNF-α and ionized calcium binding adapter molecule 1 in hippocampus, and reduce in the levels of IL-1β and TNF-α in serum (Guo et al., 2021). Intervention with ginsenoside Rb1 for 2 weeks induced a pro-neurogenic phenotype of microglia via activating peroxisome proliferator-activated receptor γ (PPARγ), inhibited chronic mild stress (CMS)-induced inflammation and increased the proliferation and differentiation of neural precursor cells (Zhang et al., 2021) (Table 4).
These studies have demonstrated that ginsenoside Rb1 could improve the depressive-like behaviors through regulating the balance of neurotransmitters, inhibiting the neuroinflammation and promoting neuronal survival. Thus, ginsenoside Rb1 may be a promising candidate in the therapy of depression.
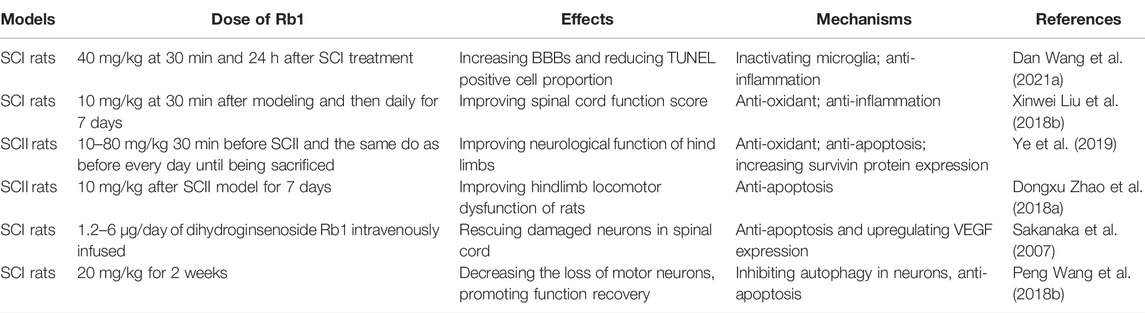
Ginsenoside Rb1 in Spinal Cord Injury
Spinal cord, located in the spinal canal, is one part of the central nervous system. The spinal cord is comprised of white matter and gray matter, which includes cerebral nuclei and fiber conduction bundle. Spinal cord injury (SCI) is a central nervous system disease, which is classified as traumatic SCI (TSCI) and non-traumatic SCI (NTSCI), and this disease can lead to the permanent disability (Gedde et al., 2019). Currently, the prevalence of SCI witnesses a rise which makes a burden to the individual and the society because it is still incurable (Ahuja et al., 2017b). Since the functions of the spinal cord contain the motor adjustment and the sensory transmission, the position and the degree about SCI have different effect on the consequences. The causes of TSCI contain recreational activities, violence falls, traffic accident and so on (McDonald and Sadowsky, 2002) and that of NTSCI include compression of tumor, spinal canal stenosis, vascular ischemia, inflammatory conditions and so on (New and Sundararajan, 2008). Broadly speaking, the treatments of SCI contain neuroprotection and nerve regeneration (Kim et al., 2017). At present, the clinical treatment of SCI includes surgical decompression, drug therapy and early definitive care and so on (Ahuja et al., 2017a). Recently, more and more researches showed that ginsenoside Rb1 may have fine effects on the recovery of SCI.
Wang and others found that in SCI rats ginsenoside Rb1 treatment facilitated the expression of miR-130b-5p, which inactivated Toll-like receptor 4 (TLR4)/NF-κB in microglia and further increased Basso, Beattie, and Bresnahan score (BBBs) and reduced TUNEL-positive cell proportion and inflammation (Wang et al., 2021), suggesting that ginsenoside Rb1 alleviated SCI through reducing activated microglia-induced neuronal injury via miR-130b-5p/TLR4/NF-κB axis. Administration of ginsenoside Rb1 also significantly inhibited oxidative stress, including decreasing serum MDA content and increasing the activity of SOD, CAT and GSH, at least partly via the eNOS/Nrf2/HO-1 pathway in SCI rats, and improved spinal cord function score (Liu et al., 2018b). Ginsenoside Rb1 could improve neurological function of hind limbs and reduce the cell apoptosis through decreasing MDA content in serum and spinal cord tissue and increasing activity of SOD, as well as inhibit apoptosis by promoting the expression of Survivin protein in spinal cord I/R injury (SCII) rats (Ye et al., 2019). Importantly, Zhao et al., 2018a found that ginsenoside Rb1 injection after surgical operation also prevented neural cell apoptosis in the spinal cord and improved hindlimb locomotor dysfunction of SCII rats via inhibiting the activation of caspase-3 and apoptosis signal-regulating kinase (ASK 1), and the Bax/Bcl-2 ratio, suggesting that ginsenoside Rb1 may be a potential drug for SCII treatment. Sakanaka et al. (2007) produced dihydroginsenoside Rb1 and found that its effective dose to improve SCI was ten times lower than that of ginsenoside Rb1. Dihydroginsenoside Rb1 rescued SCI-induced neuronal damage through upregulating the expression of Bcl-x(L) and vascular endothelial growth factor (VEGF) by activating hypoxia response element (HRE) and signal transducers and activators of transcription 5 (Stat5), respectively. (Table 5).
Demyelination occurs after SCI due to the injury and the imbalance of the microenvironment while myelin promotes facilitate axon signal conduction. Study shows that the unceasing loss of oligodendrocytes may lead to the barrier to the function recovery, while the reason why the loss of oligodendrocytes happens owes to the imbalance between the remyelination and the demyelination (Fan et al., 2018). Ginsenoside compound K, a metabolite of ginsenoside Rb1, promoted the proliferation, migration and differentiation of Schwann cells, which are critical for the remyelination of injured peripheral nerve, via activating MEK/ERK1/2 and PI3K/Akt pathways (Wang et al., 2021). Autophagy is the process of engulfing unwanted cells and maintaining homeostasis. A study shows that autophagy may lead to the death of the oligodendrocytes while functional recovery requires the participation of oligodendrocytes (Fan et al., 2018). The maintenance of neurons requires the participation of autophagy. However, high levels of autophagy may lead to neuronal cell death and further cause neurodegeneration. Bisicchia and his others demonstrated that inhibition of autophagy improved the survival rate of rats with spinal cord hemisection (Bisicchia et al., 2017). Wang et al. (2018b) found ginsenoside Rb1 treatment decreased the death of motor neurons and promoted the recovery of motor function, as well as restored the expression of LC3-II/I, Beclin-1, and p62 in the SCI rats, suggesting that ginsenoside Rb1 may play neuroprotective role via inhibiting autophagy and autophagic cell death. (Table 5). These data suggest that ginsenoside Rb1 may be a promising candidate in the therapy of SCI.
Conclusion and Expectation
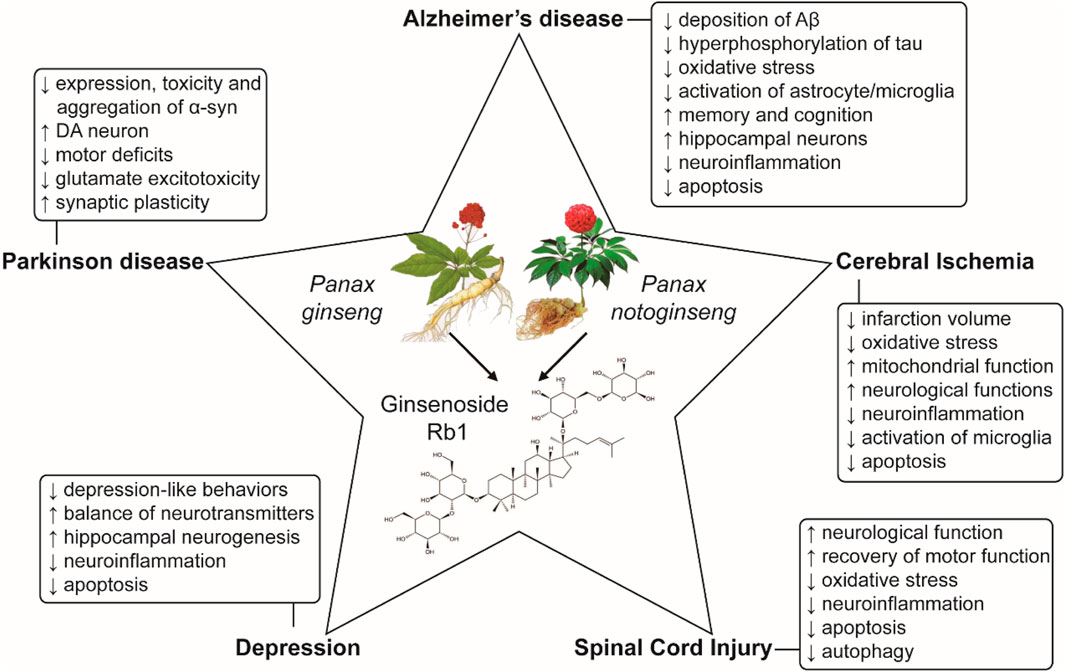
In summary, current studies have demonstrated that ginsenoside Rb1 exerts neuroprotective roles through inhibiting oxidative stress, apoptosis and neuroinflammation and regulating the autophagy in neurodegenerative diseases, cerebral ischemia injury, depression and spinal cord injury (Figure 2). More importantly, the effects of ginsenoside Rb1 seems to be much stronger than other ginsenosides, suggesting that may be a promising candidate agent for clinical therapy of CNSDs.
At this stage, the associated studies are extremely few and limited to focus on the neuroprotective effects of ginsenoside Rb1 in CNSDs in cellular and rodent models. The experiments in nonhuman primate models of CNSDs and the clinical trials should be also successively carried out to accelerate the clinical application of ginsenoside Rb1 for CNSDs treatment in the future. There are different routes of administration and the dosage used in rodents is in a large range. Further studies are also needed to performed to confirm the optimal administration route and dosage for individual disease.
Author Contributions
XZ and JJ designed the article contents. LG, JY, YZ, RH, and HJ wrote the original paper. YL and XZ drew the figure. XZ, JJ, and LS revised the paper. All authors reviewed the paper and approved the submitted version.
Funding
This study was supported by the Natural Science Foundation of Zhejiang Province (LQ22H090003), Natural Science Foundation of Henan Province (202300410332), Sci-Tech Planning Project of Jiaxing (2021AY30001, 2022AY30020), Scientific Research Training Plan of Jiaxing University (CD8517211493) and Research Foundation for Advanced Talents of Jiaxing University (CD70520017 and CD70520018).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahuja, C. S., Nori, S., Tetreault, L., Wilson, J., Kwon, B., Harrop, J., et al. (2017a). Traumatic Spinal Cord Injury-Repair and Regeneration. Neurosurgery 80 (3S), S9–S22. doi:10.1093/neuros/nyw080
Ahuja, C. S., Wilson, J. R., Nori, S., Kotter, M. R. N., Druschel, C., Curt, A., et al. (2017b). Traumatic Spinal Cord Injury. Nat. Rev. Dis. Prim. 3, 17018. doi:10.1038/nrdp.2017.18
Ardah, M. T., Paleologou, K. E., Lv, G., Menon, S. A., Abul Khair, S. B., Lu, J. H., et al. (2015). Ginsenoside Rb1 Inhibits Fibrillation and Toxicity of Alpha-Synuclein and Disaggregates Preformed Fibrils. Neurobiol. Dis. 74, 89–101. doi:10.1016/j.nbd.2014.11.007
Baeg, I. H., and So, S. H. (2013). The World Ginseng Market and the Ginseng (Korea). J. Ginseng Res. 37 (1), 1–7. doi:10.5142/jgr.2013.37.1
Bennett, R. E., DeVos, S. L., Dujardin, S., Corjuc, B., Gor, R., Gonzalez, J., et al. (2017). Enhanced Tau Aggregation in the Presence of Amyloid β. Am. J. Pathol. 187 (7), 1601–1612. doi:10.1016/j.ajpath.2017.03.011
Bisicchia, E., Latini, L., Cavallucci, V., Sasso, V., Nicolin, V., Molinari, M., et al. (2017). Autophagy Inhibition Favors Survival of Rubrospinal Neurons after Spinal Cord Hemisection. Mol. Neurobiol. 54 (7), 4896–4907. doi:10.1007/s12035-016-0031-z
Carpenter, E. L., Becker, A. L., and Indra, A. K. (2022). NRF2 and Key Transcriptional Targets in Melanoma Redox Manipulation. Cancers (Basel) 14 (6), 1531. doi:10.3390/cancers14061531
Chappell, W. H., Steelman, L. S., Long, J. M., Kempf, R. C., Abrams, S. L., Franklin, R. A., et al. (2011). Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR Inhibitors: Rationale and Importance to Inhibiting These Pathways in Human Health. Oncotarget 2 (3), 135–164. doi:10.18632/oncotarget.240
Chen, B., Qin, M. C., Huang, J. L., Wu, D. P., Guo, E. C., Liu, Z. P., et al. (2017). Preliminary Establishment of Integration of Alzheimer's Disease and Blood Stasis Syndrome Tree Shrew Model and Evaluation of Intervention of Panax Notoginseng Saponins. Zhongguo Zhong Yao Za Zhi 42 (6), 1175–1182. doi:10.19540/j.cnki.cjcmm.20170121.034
Chen, W., Guo, Y., Yang, W., Zheng, P., Zeng, J., and Tong, W. (2015). Protective Effect of Ginsenoside Rb1 on Integrity of Blood-Brain Barrier Following Cerebral Ischemia. Exp. Brain Res. 233 (10), 2823–2831. doi:10.1007/s00221-015-4352-3
Chen, X., Huang, T., Zhang, J., Song, J., Chen, L., and Zhu, Y. (2008). Involvement of Calpain and P25 of CDK5 Pathway in Ginsenoside Rb1's Attenuation of Beta-Amyloid Peptide25-35-Induced Tau Hyperphosphorylation in Cortical Neurons. Brain Res. 1200, 99–106. doi:10.1016/j.brainres.2007.12.029
Cheng, Z., Zhang, M., Ling, C., Zhu, Y., Ren, H., Hong, C., et al. (2019). Neuroprotective Effects of Ginsenosides against Cerebral Ischemia. Molecules 24 (6), 1102. doi:10.3390/molecules24061102
Choi, K. T. (2008). Botanical Characteristics, Pharmacological Effects and Medicinal Components of Korean Panax Ginseng C A Meyer. Acta Pharmacol. Sin. 29 (9), 1109–1118. doi:10.1111/j.1745-7254.2008.00869.x
de Oliveira Zanuso, B., de Oliveira Dos Santos, A. R., Miola, V. F. B., Guissoni Campos, L. M., Spilla, C. S. G., and Barbalho, S. M. (2022). Panax Ginseng and Aging Related Disorders: A Systematic Review. Exp. Gerontol. 161, 111731. doi:10.1016/j.exger.2022.111731
Dong, X., Zheng, L., Lu, S., and Yang, Y. (2017). Neuroprotective Effects of Pretreatment of Ginsenoside Rb1 on Severe Cerebral Ischemia-Induced Injuries in Aged Mice: Involvement of Anti-oxidant Signaling. Geriatr. Gerontol. Int. 17 (2), 338–345. doi:10.1111/ggi.12699
Edler, M. K., Sherwood, C. C., Meindl, R. S., Hopkins, W. D., Ely, J. J., Erwin, J. M., et al. (2017). Aged Chimpanzees Exhibit Pathologic Hallmarks of Alzheimer's Disease. Neurobiol. Aging 59, 107–120. doi:10.1016/j.neurobiolaging.2017.07.006
Evans, D. L., Charney, D. S., Lewis, L., Golden, R. N., Gorman, J. M., Krishnan, K. R., et al. (2005). Mood Disorders in the Medically Ill: Scientific Review and Recommendations. Biol. Psychiatry 58 (3), 175–189. doi:10.1016/j.biopsych.2005.05.001
Fan, B., Wei, Z., Yao, X., Shi, G., Cheng, X., Zhou, X., et al. (2018). Microenvironment Imbalance of Spinal Cord Injury. Cell Transpl. 27 (6), 853–866. doi:10.1177/0963689718755778
Gao, X., Zhang, X., Cui, L., Chen, R., Zhang, C., Xue, J., et al. (2020). Ginsenoside Rb1 Promotes Motor Functional Recovery and Axonal Regeneration in Post-stroke Mice through cAMP/PKA/CREB Signaling Pathway. Brain Res. Bull. 154, 51–60. doi:10.1016/j.brainresbull.2019.10.006
Gao, X. Q., Yang, C. X., Chen, G. J., Wang, G. Y., Chen, B., Tan, S. K., et al. (2010). Ginsenoside Rb1 Regulates the Expressions of Brain-Derived Neurotrophic Factor and Caspase-3 and Induces Neurogenesis in Rats with Experimental Cerebral Ischemia. J. Ethnopharmacol. 132 (2), 393–399. doi:10.1016/j.jep.2010.07.033
Gedde, M. H., Lilleberg, H. S., Assmus, J., Gilhus, N. E., and Rekand, T. (2019). Traumatic vs Non-traumatic Spinal Cord Injury: A Comparison of Primary Rehabilitation Outcomes and Complications during Hospitalization. J. Spinal Cord. Med. 42 (6), 695–701. doi:10.1080/10790268.2019.1598698
Guo, Y., Xie, J., Zhang, L., Yang, L., Ma, J., Bai, Y., et al. (2021). Ginsenoside Rb1 Exerts Antidepressant-like Effects via Suppression Inflammation and Activation of AKT Pathway. Neurosci. Lett. 744, 135561. doi:10.1016/j.neulet.2020.135561
Hardy, J., and Selkoe, D. J. (2002). The Amyloid Hypothesis of Alzheimer's Disease: Progress and Problems on the Road to Therapeutics. Science 297 (5580), 353–356. doi:10.1126/science.1072994
Harrison, T. M., Du, R., Klencklen, G., Baker, S. L., and Jagust, W. J. (2021). Distinct Effects of Beta-Amyloid and Tau on Cortical Thickness in Cognitively Healthy Older Adults. Alzheimers Dement. 17 (7), 1085–1096. doi:10.1002/alz.12249
Höflich, A., Michenthaler, P., Kasper, S., and Lanzenberger, R. (2019). Circuit Mechanisms of Reward, Anhedonia, and Depression. Int. J. Neuropsychopharmacol. 22 (2), 105–118. doi:10.1093/ijnp/pyy081
Hwang, J. Y., Shim, J. S., Song, M. Y., Yim, S. V., Lee, S. E., and Park, K. S. (2016). Proteomic Analysis Reveals that the Protective Effects of Ginsenoside Rb1 Are Associated with the Actin Cytoskeleton in β-amyloid-treated Neuronal Cells. J. Ginseng Res. 40 (3), 278–284. doi:10.1016/j.jgr.2015.09.004
Im, J. Y., Lee, K. W., Woo, J. M., Junn, E., and Mouradian, M. M. (2012). DJ-1 Induces Thioredoxin 1 Expression through the Nrf2 Pathway. Hum. Mol. Genet. 21 (13), 3013–3024. doi:10.1093/hmg/dds131
Jia, J. J., Zeng, X. S., Li, K., Ma, L. F., Chen, L., and Song, X. Q. (2016). The Expression of Thioredoxin-1 in Acute Epinephrine Stressed Mice. Cell Stress Chaperones 21 (5), 935–941. doi:10.1007/s12192-016-0722-4
Jiang, H., Hampel, H., Prvulovic, D., Wallin, A., Blennow, K., Li, R., et al. (2011). Elevated CSF Levels of TACE Activity and Soluble TNF Receptors in Subjects with Mild Cognitive Impairment and Patients with Alzheimer's Disease. Mol. Neurodegener. 6, 69. doi:10.1186/1750-1326-6-69
Jiang, N., Huang, H., Zhang, Y., Lv, J., Wang, Q., He, Q., et al. (2021a). Ginsenoside Rb1 Produces Antidepressant-like Effects in a Chronic Social Defeat Stress Model of Depression through the BDNF-Trkb Signaling Pathway. Front. Pharmacol. 12, 680903. doi:10.3389/fphar.2021.680903
Jiang, N., Wang, K., Zhang, Y., Huang, H., Lv, J. W., Wang, Q., et al. (2021b). Protective Effect of Ginsenoside Rb1 against Chronic Restraint Stress (CRS)-induced Memory Impairments in Rats. Behav. Brain Res. 405, 113146. doi:10.1016/j.bbr.2021.113146
Kamel, H., and Iadecola, C. (2012). Brain-immune Interactions and Ischemic Stroke: Clinical Implications. Arch. Neurol. 69 (5), 576–581. doi:10.1001/archneurol.2011.3590
Ke, C., Peng, Y., Yuan, Z., and Cai, J. (2021). Ginsenoside Rb1 Protected PC12 Cells from Aβ25-35-Induced Cytotoxicity via PPARγ Activation and Cholesterol Reduction. Eur. J. Pharmacol. 893, 173835. doi:10.1016/j.ejphar.2020.173835
Ke, S. Y., Yu, S. J., Liu, D. H., Shi, G. Y., Wang, M., Zhou, B., et al. (2021). Ginsenoside Rb1 Protects Human Umbilical Vein Endothelial Cells against High Glucose-Induced Mitochondria-Related Apoptosis through Activating SIRT3 Signalling Pathway. Chin. J. Integr. Med. 27 (5), 336–344. doi:10.1007/s11655-020-3478-8
Khoshnam, S. E., Winlow, W., Farzaneh, M., Farbood, Y., and Moghaddam, H. F. (2017). Pathogenic Mechanisms Following Ischemic Stroke. Neurol. Sci. 38 (7), 1167–1186. doi:10.1007/s10072-017-2938-1
Kim, C. Y., and Alcalay, R. N. (2017). Genetic Forms of Parkinson's Disease. Semin. Neurol. 37 (2), 135–146. doi:10.1055/s-0037-1601567
Kim, M., Kim, S. O., Lee, M., Park, Y., Kim, D., Cho, K. H., et al. (2014). Effects of Ginsenoside Rb1 on the Stress-Induced Changes of BDNF and HSP70 Expression in Rat hippocampus. Environ. Toxicol. Pharmacol. 38 (1), 257–262. doi:10.1016/j.etap.2014.06.004
Kim, Y. H., Ha, K. Y., and Kim, S. I. (2017). Spinal Cord Injury and Related Clinical Trials. Clin. Orthop. Surg. 9 (1), 1–9. doi:10.4055/cios.2017.9.1.1
Kim, Y. J., Zhang, D., and Yang, D. C. (2015). Biosynthesis and Biotechnological Production of Ginsenosides. Biotechnol. Adv. 33 (6 Pt 1), 717–735. doi:10.1016/j.biotechadv.2015.03.001
Li, D. W., Zhou, F. Z., Sun, X. C., Li, S. C., Yang, J. B., Sun, H. H., et al. (2019a). Ginsenoside Rb1 Protects Dopaminergic Neurons from Inflammatory Injury Induced by Intranigral Lipopolysaccharide Injection. Neural Regen. Res. 14 (10), 1814–1822. doi:10.4103/1673-5374.257536
Li, N., Zhou, L., Li, W., Liu, Y., Wang, J., and He, P. (2015). Protective Effects of Ginsenosides Rg1 and Rb1 on an Alzheimer's Disease Mouse Model: a Metabolomics Study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 985, 54–61. doi:10.1016/j.jchromb.2015.01.016
Li, Y. N., Gao, Z. W., Li, R., Zhang, Y. F., Zhu, Q. S., and Huang, F. (2019). Aquaporin 4 Regulation by Ginsenoside Rb1 Intervenes with Oxygen-Glucose Deprivation/reoxygenation-Induced Astrocyte Injury. Med. Baltim. 98 (42), e17591. doi:10.1097/MD.0000000000017591
Liang, W., Liu, Y., Zhou, K., Jian, P., Zhang, Q., Chang, Z., et al. (2022). Ginsenoside Rb1 Prevents Lipopolysaccharide-Induced Depressive-like Behavior by Inhibiting Inflammation and Neural Dysfunction and F2 Elicits a Novel Antidepressant-like Effect: A Metabolite-Based Network Pharmacology Study. J. Ethnopharmacol. 282, 114655. doi:10.1016/j.jep.2021.114655
Lin, J., Gao, S., Wang, T., Shen, Y., Yang, W., Li, Y., et al. (2019). Ginsenoside Rb1 Improves Learning and Memory Ability through its Anti-inflammatory Effect in Aβ1-40 Induced Alzheimer's Disease of Rats. Am. J. Transl. Res. 11 (5), 2955–2968.
Liu, A., Zhu, W., Sun, L., Han, G., Liu, H., Chen, Z., et al. (2018). Ginsenoside Rb1 Administration Attenuates Focal Cerebral Ischemic Reperfusion Injury through Inhibition of HMGB1 and Inflammation Signals. Exp. Ther. Med. 16 (4), 3020–3026. doi:10.3892/etm.2018.6523
Liu, S. J., Zhang, A. H., Li, H. L., Wang, Q., Deng, H. M., Netzer, W. J., et al. (2003). Overactivation of Glycogen Synthase Kinase-3 by Inhibition of Phosphoinositol-3 Kinase and Protein Kinase C Leads to Hyperphosphorylation of Tau and Impairment of Spatial Memory. J. Neurochem. 87 (6), 1333–1344. doi:10.1046/j.1471-4159.2003.02070.x
Liu, X., Chen, J., Sun, N., Li, N., Zhang, Z., Zheng, T., et al. (2020). Ginsenoside Rb1 Ameliorates Autophagy via the AMPK/mTOR Pathway in Renal Tubular Epithelial Cells In Vitro and In Vivo. Int. J. Biol. Macromol. 163, 996–1009. doi:10.1016/j.ijbiomac.2020.07.060
Liu, X., Gu, X., Yu, M., Zi, Y., Yu, H., Wang, Y., et al. (2018). Effects of Ginsenoside Rb1 on Oxidative Stress Injury in Rat Spinal Cords by Regulating the eNOS/Nrf2/HO-1 Signaling Pathway. Exp. Ther. Med. 16 (2), 1079–1086. doi:10.3892/etm.2018.6286
Liu, Y., Zong, X., Huang, J., Guan, Y., Li, Y., Du, T., et al. (2019). Ginsenoside Rb1 Regulates Prefrontal Cortical GABAergic Transmission in MPTP-Treated Mice. Aging (Albany NY) 11 (14), 5008–5034. doi:10.18632/aging.102095
Lopez, A. D., and Murray, C. C. (1998). The Global Burden of Disease, 1990-2020. Nat. Med. 4 (11), 1241–1243. doi:10.1038/3218
Lü, J. M., Weakley, S. M., Yang, Z., Hu, M., Yao, Q., and Chen, C. (2012). Ginsenoside Rb1 Directly Scavenges Hydroxyl Radical and Hypochlorous Acid. Curr. Pharm. Des. 18 (38), 6339–6347. doi:10.2174/138161212803832254
Lu, J., Wang, X., Wu, A., Cao, Y., Dai, X., Liang, Y., et al. (2022). Ginsenosides in Central Nervous System Diseases: Pharmacological Actions, Mechanisms, and Therapeutics. Phytotherapy Res. 36, 1523–1544. doi:10.1002/ptr.7395
Lucas, J. J., Hernández, F., Gómez-Ramos, P., Morán, M. A., Hen, R., and Avila, J. (2001). Decreased Nuclear Beta-Catenin, Tau Hyperphosphorylation and Neurodegeneration in GSK-3beta Conditional Transgenic Mice. EMBO J. 20 (1-2), 27–39. doi:10.1093/emboj/20.1.27
Luo, T., Liu, G., Ma, H., Lu, B., Xu, H., Wang, Y., et al. (2014). Inhibition of Autophagy via Activation of PI3K/Akt Pathway Contributes to the Protection of Ginsenoside Rb1 against Neuronal Death Caused by Ischemic Insults. Int. J. Mol. Sci. 15 (9), 15426–15442. doi:10.3390/ijms150915426
Mao, D. D., Yang, W. Y., Li, Y., Lin, J. W., Gao, S. Y., Wang, Y. R., et al. (2018). Effect of Qingxin Kaiqiao Fang on Hippocampus mRNA Expression of the Inflammation-Related Genes IL-1β, GFAP, and Aβ in an Alzheimer's Disease Rat Model. Evid. Based Complement. Altern. Med. 2018, 9267653. doi:10.1155/2018/9267653
McDonald, J. W., and Sadowsky, C. (2002). Spinal-cord Injury. Lancet 359 (9304), 417–425. doi:10.1016/S0140-6736(02)07603-1
Mehra, S., Sahay, S., and Maji, S. K. (2019). α-Synuclein Misfolding and Aggregation: Implications in Parkinson's Disease Pathogenesis. Biochim. Biophys. Acta Proteins Proteom 1867 (10), 890–908. doi:10.1016/j.bbapap.2019.03.001
Ménard, C., Hodes, G. E., and Russo, S. J. (2016). Pathogenesis of Depression: Insights from Human and Rodent Studies. Neuroscience 321, 138–162. doi:10.1016/j.neuroscience.2015.05.053
Meyers, P. M., Schumacher, H. C., Connolly, E. S., Heyer, E. J., Gray, W. A., and Higashida, R. T. (2011). Current Status of Endovascular Stroke Treatment. Circulation 123 (22), 2591–2601. doi:10.1161/CIRCULATIONAHA.110.971564
Miao, H. H., Zhang, Y., Ding, G. N., Hong, F. X., Dong, P., and Tian, M. (2017). Ginsenoside Rb1 Attenuates Isoflurane/surgery-Induced Cognitive Dysfunction via Inhibiting Neuroinflammation and Oxidative Stress. Biomed. Environ. Sci. 30 (5), 363–372. doi:10.3967/bes2017.047
Mrak, R. E., and Griffin, W. S. (2005). Potential Inflammatory Biomarkers in Alzheimer's Disease. J. Alzheimers Dis. 8 (4), 369–375. doi:10.3233/jad-2005-8406
Murphy, M. P., Das, P., Nyborg, A. C., Rochette, M. J., Dodson, M. W., Loosbrock, N. M., et al. (2003). Overexpression of Nicastrin Increases Abeta Production. FASEB J. 17 (9), 1138–1140. doi:10.1096/fj.02-1050fje
New, P. W., and Sundararajan, V. (2008). Incidence of Non-traumatic Spinal Cord Injury in Victoria, Australia: a Population-Based Study and Literature Review. Spinal Cord. 46 (6), 406–411. doi:10.1038/sj.sc.3102152
Ng, T. B. (2006). Pharmacological Activity of Sanchi Ginseng (Panax Notoginseng). J. Pharm. Pharmacol. 58 (8), 1007–1019. doi:10.1211/jpp.58.8.0001
Obeso, J. A., Stamelou, M., Goetz, C. G., Poewe, W., Lang, A. E., Weintraub, D., et al. (2017). Past, Present, and Future of Parkinson's Disease: A Special Essay on the 200th Anniversary of the Shaking Palsy. Mov. Disord. 32 (9), 1264–1310. doi:10.1002/mds.27115
Pajarillo, E., Rizor, A., Lee, J., Aschner, M., and Lee, E. (2019). The Role of Astrocytic Glutamate Transporters GLT-1 and GLAST in Neurological Disorders: Potential Targets for Neurotherapeutics. Neuropharmacology 161, 107559. doi:10.1016/j.neuropharm.2019.03.002
Qi, L. W., Wang, C. Z., and Yuan, C. S. (2011). Isolation and Analysis of Ginseng: Advances and Challenges. Nat. Prod. Rep. 28 (3), 467–495. doi:10.1039/c0np00057d
Qian, Y. H., Han, H., Hu, X. D., and Shi, L. L. (2009). Protective Effect of Ginsenoside Rb1 on Beta-Amyloid Protein(1-42)-Induced Neurotoxicity in Cortical Neurons. Neurol. Res. 31 (7), 663–667. doi:10.1179/174313209X385572
Qiao, Y. J., Shang, J. H., Wang, D., Zhu, H. T., Yang, C. R., and Zhang, Y. J. (2018). Research of Panax Spp. In Kunming Institute of Botany, CAS. Nat. Prod. Bioprospect 8 (4), 245–263. doi:10.1007/s13659-018-0176-8
Qu, S., Meng, X., Liu, Y., Zhang, X., and Zhang, Y. (2019). Ginsenoside Rb1 Prevents MPTP-Induced Changes in Hippocampal Memory via Regulation of the α-synuclein/PSD-95 Pathway. Aging (Albany NY) 11 (7), 1934–1964. doi:10.18632/aging.101884
Radad, K., Gille, G., Moldzio, R., Saito, H., and Rausch, W. D. (2004). Ginsenosides Rb1 and Rg1 Effects on Mesencephalic Dopaminergic Cells Stressed with Glutamate. Brain Res. 1021 (1), 41–53. doi:10.1016/j.brainres.2004.06.030
Sakanaka, M., Zhu, P., Zhang, B., Wen, T. C., Cao, F., Ma, Y. J., et al. (2007). Intravenous Infusion of Dihydroginsenoside Rb1 Prevents Compressive Spinal Cord Injury and Ischemic Brain Damage through Upregulation of VEGF and Bcl-XL. J. Neurotrauma 24 (6), 1037–1054. doi:10.1089/neu.2006.0182
Shaukat, A., Shaukat, I., Rajput, S. A., Shukat, R., Hanif, S., Jiang, K., et al. (2021). Ginsenoside Rb1 Protects from Staphylococcus Aureus-Induced Oxidative Damage and Apoptosis through Endoplasmic Reticulum-Stress and Death Receptor-Mediated Pathways. Ecotoxicol. Environ. Saf. 219, 112353. doi:10.1016/j.ecoenv.2021.112353
Shi, Y., Miao, W., Teng, J., and Zhang, L. (2018). Ginsenoside Rb1 Protects the Brain from Damage Induced by Epileptic Seizure via Nrf2/ARE Signaling. Cell Physiol. Biochem. 45 (1), 212–225. doi:10.1159/000486768
Shi, Z. Y., Zeng, J. Z., and Wong, A. S. T. (2019). Chemical Structures and Pharmacological Profiles of Ginseng Saponins. Molecules 24 (13), 2443. doi:10.3390/molecules24132443
Simon, D. K., Tanner, C. M., and Brundin, P. (2020). Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clin. Geriatr. Med. 36 (1), 1–12. doi:10.1016/j.cger.2019.08.002
Streit, W. J. (2004). Microglia and Alzheimer's Disease Pathogenesis. J. Neurosci. Res. 77 (1), 1–8. doi:10.1002/jnr.20093
Tianhong, M., Yuxi, Q., Zhimin, W., and Baili, Y. (2014). Effect of Panax Notoginseng in Patients with Multiple Fractured Ribs and Pulmonary Contusions Caused by the 2008 Wenchuan Earthquake. Forsch Komplementmed 21 (6), 360–364. doi:10.1159/000370011
Tohda, C., Matsumoto, N., Zou, K., Meselhy, M. R., and Komatsu, K. (2004). Abeta(25-35)-induced Memory Impairment, Axonal Atrophy, and Synaptic Loss Are Ameliorated by M1, A Metabolite of Protopanaxadiol-type Saponins. Neuropsychopharmacology 29 (5), 860–868. doi:10.1038/sj.npp.1300388
Walker, F. R. (2013). A Critical Review of the Mechanism of Action for the Selective Serotonin Reuptake Inhibitors: Do These Drugs Possess Anti-inflammatory Properties and How Relevant Is This in the Treatment of Depression? Neuropharmacology 67, 304–317. doi:10.1016/j.neuropharm.2012.10.002
Wang, C. Z., Anderson, S., Du, W., He, T. C., and Yuan, C. S. (2016). Red Ginseng and Cancer Treatment. Chin. J. Nat. Med. 14 (1), 7–16. doi:10.3724/SP.J.1009.2016.00007
Wang, D., Zhao, S., Pan, J., Wang, Z., Li, Y., Xu, X., et al. (2021). Ginsenoside Rb1 Attenuates Microglia Activation to Improve Spinal Cord Injury via microRNA-130b-5p/TLR4/NF-Κb axis. J. Cell Physiol. 236 (3), 2144–2155. doi:10.1002/jcp.30001
Wang, G. L., He, Z. M., Zhu, H. Y., Gao, Y. G., Zhao, Y., Yang, H., et al. (2017). Involvement of Serotonergic, Noradrenergic and Dopaminergic Systems in the Antidepressant-like Effect of Ginsenoside Rb1, a Major Active Ingredient of Panax Ginseng C.A. Meyer. J. Ethnopharmacol. 204, 118–124. doi:10.1016/j.jep.2017.04.009
Wang, G. L., Wang, Y. P., Zheng, J. Y., and Zhang, L. X. (2018). Monoaminergic and Aminoacidergic Receptors Are Involved in the Antidepressant-like Effect of Ginsenoside Rb1 in Mouse hippocampus (CA3) and Prefrontal Cortex. Brain Res. 1699, 44–53. doi:10.1016/j.brainres.2018.05.035
Wang, H., Qu, F., Xin, T., Sun, W., He, H., and Du, L. (2021). Ginsenoside Compound K Promotes Proliferation, Migration and Differentiation of Schwann Cells via the Activation of MEK/ERK1/2 and PI3K/AKT Pathways. Neurochem. Res. 46 (6), 1400–1409. doi:10.1007/s11064-021-03279-0
Wang, P., Lin, C., Wu, S., Huang, K., Wang, Y., Bao, X., et al. (2018b). Inhibition of Autophagy Is Involved in the Protective Effects of Ginsenoside Rb1 on Spinal Cord Injury. Cell Mol. Neurobiol. 38 (3), 679–690. doi:10.1007/s10571-017-0527-8
Wang, Y., Feng, Y., Fu, Q., and Li, L. (2013). Panax Notoginsenoside Rb1 Ameliorates Alzheimer's Disease by Upregulating Brain-Derived Neurotrophic Factor and Downregulating Tau Protein Expression. Exp. Ther. Med. 6 (3), 826–830. doi:10.3892/etm.2013.1215
Wang, Y., Li, Y., Yang, W., Gao, S., Lin, J., Wang, T., et al. (2018). Ginsenoside Rb1 Inhibit Apoptosis in Rat Model of Alzheimer's Disease Induced by Aβ1-40. Am. J. Transl. Res. 10 (3), 796–805.
Wu, J., Jeong, H. K., Bulin, S. E., Kwon, S. W., Park, J. H., and Bezprozvanny, I. (2009). Ginsenosides Protect Striatal Neurons in a Cellular Model of Huntington's Disease. J. Neurosci. Res. 87 (8), 1904–1912. doi:10.1002/jnr.22017
Wu, M. H., Zhang, Y., Ma, Z. G., Cao, H., and Liu, G. P. (2018). Historical Evolution of Panax Notoginseng (Sanqi) Processing Methods. Zhongguo Zhong Yao Za Zhi 43 (24), 4923–4928. doi:10.19540/j.cnki.cjcmm.20181025.007
Xia, Y., Wang, Z. H., Zhang, J., Liu, X., Yu, S. P., Ye, K. X., et al. (2021). C/EBPβ Is a Key Transcription Factor for APOE and Preferentially Mediates ApoE4 Expression in Alzheimer's Disease. Mol. Psychiatry 26 (10), 6002–6022. doi:10.1038/s41380-020-00956-4
Xie, X., Wang, H. T., Li, C. L., Gao, X. H., Ding, J. L., Zhao, H. H., et al. (2010). Ginsenoside Rb1 Protects PC12 Cells against β-amyloid-induced Cell Injury. Mol. Med. Rep. 3 (4), 635–639. doi:10.3892/mmr_00000308
Xu, M., Ma, Q., Fan, C., Chen, X., Zhang, H., and Tang, M. (2019). Ginsenosides Rb1 and Rg1 Protect Primary Cultured Astrocytes against Oxygen-Glucose Deprivation/Reoxygenation-Induced Injury via Improving Mitochondrial Function. Int. J. Mol. Sci. 20 (23), 6086. doi:10.3390/ijms20236086
Yamada, N., Araki, H., and Yoshimura, H. (2011). Identification of Antidepressant-like Ingredients in Ginseng Root (Panax Ginseng C.A. Meyer) Using a Menopausal Depressive-like State in Female Mice: Participation of 5-HT2A Receptors. Psychopharmacol. Berl. 216 (4), 589–599. doi:10.1007/s00213-011-2252-1
Yang, J. E., Jia, N., Wang, D., He, Y., Dong, L., and Yang, A. G. (2020). Ginsenoside Rb1 Regulates Neuronal Injury and Keap1-Nrf2/ARE Signaling Pathway in Cerebral Infarction Rats. J. Biol. Regul. Homeost. Agents 34 (3), 1091–1095. doi:10.23812/20-143-L-7
Yang, R., Jiang, X., He, X., Liang, D., Sun, S., and Zhou, G. (2020). Ginsenoside Rb1 Improves Cognitive Impairment Induced by Insulin Resistance through Cdk5/p35-NMDAR-IDE Pathway. Biomed. Res. Int. 2020, 3905719. doi:10.1155/2020/3905719
Yang, W. Z., Hu, Y., Wu, W. Y., Ye, M., and Guo, D. A. (2014). Saponins in the Genus Panax L. (Araliaceae): a Systematic Review of Their Chemical Diversity. Phytochemistry 106, 7–24. doi:10.1016/j.phytochem.2014.07.012
Yang, Y., Li, S., Huang, H., Lv, J., Chen, S., Pires Dias, A. C., et al. (2020). Comparison of the Protective Effects of Ginsenosides Rb1 and Rg1 on Improving Cognitive Deficits in SAMP8 Mice Based on Anti-neuroinflammation Mechanism. Front. Pharmacol. 11, 834. doi:10.3389/fphar.2020.00834
Ye, J. T., Li, F. T., Huang, S. L., Xue, J. L., Aihaiti, Y., Wu, H., et al. (2019). Effects of Ginsenoside Rb1 on Spinal Cord Ischemia-Reperfusion Injury in Rats. J. Orthop. Surg. Res. 14 (1), 259. doi:10.1186/s13018-019-1299-2
Yun, T. K. (2001). Brief Introduction of Panax Ginseng C.A. Meyer. J. Korean Med. Sci. 16 (Suppl. l), S3–S5. doi:10.3346/jkms.2001.16.S.S3
Zamolodchikov, D., and Strickland, S. (2016). A Possible New Role for Aβ in Vascular and Inflammatory Dysfunction in Alzheimer's Disease. Thromb. Res. 141 (Suppl. 2), S59–S61. doi:10.1016/S0049-3848(16)30367-X
Zeng, X., Hu, K., Chen, L., Zhou, L., Luo, W., Li, C., et al. (2018). The Effects of Ginsenoside Compound K against Epilepsy by Enhancing the γ-Aminobutyric Acid Signaling Pathway. Front. Pharmacol. 9, 1020. doi:10.3389/fphar.2018.01020
Zeng, X. S., Geng, W. S., Chen, L., and Jia, J. J. (2018a). Thioredoxin as a Therapeutic Target in Cerebral Ischemia. Curr. Pharm. Des. 24 (25), 2986–2992. doi:10.2174/1381612824666180820143853
Zeng, X. S., Geng, W. S., and Jia, J. J. (2018b). Neurotoxin-Induced Animal Models of Parkinson Disease: Pathogenic Mechanism and Assessment. ASN Neuro 10, 1759091418777438. doi:10.1177/1759091418777438
Zeng, X. S., Geng, W. S., Jia, J. J., Chen, L., and Zhang, P. P. (2018c). Cellular and Molecular Basis of Neurodegeneration in Parkinson Disease. Front. Aging Neurosci. 10, 109. doi:10.3389/fnagi.2018.00109
Zeng, X. S., Jia, J. J., Kwon, Y., Wang, S. D., and Bai, J. (2014a). The Role of Thioredoxin-1 in Suppression of Endoplasmic Reticulum Stress in Parkinson Disease. Free Radic. Biol. Med. 67, 10–18. doi:10.1016/j.freeradbiomed.2013.10.013
Zeng, X. S., Jia, J. J., and Ma, L. F. (2015). Gensenoside Rb1 Protects Rat PC12 Cells from Oxidative Stress-Induced Endoplasmic Reticulum Stress: the Involvement of Thioredoxin-1. Mol. Cell Biochem. 410 (1-2), 239–246. doi:10.1007/s11010-015-2557-1
Zeng, X. S., Zhou, X. S., Luo, F. C., Jia, J. J., Qi, L., Yang, Z. X., et al. (2014b). Comparative Analysis of the Neuroprotective Effects of Ginsenosides Rg1 and Rb1 Extracted from Panax Notoginseng against Cerebral Ischemia. Can. J. Physiol. Pharmacol. 92 (2), 102–108. doi:10.1139/cjpp-2013-0274
Zhang, L., Tang, M., Xie, X., Zhao, Q., Hu, N., He, H., et al. (2021). Ginsenoside Rb1 Induces a Pro-neurogenic Microglial Phenotype via PPARγ Activation in Male Mice Exposed to Chronic Mild Stress. J. Neuroinflammation 18 (1), 171. doi:10.1186/s12974-021-02185-0
Zhang, S., Zhu, D., Li, H., Zhang, H., Feng, C., and Zhang, W. (2017). Analyses of mRNA Profiling through RNA Sequencing on a SAMP8 Mouse Model in Response to Ginsenoside Rg1 and Rb1 Treatment. Front. Pharmacol. 8, 88. doi:10.3389/fphar.2017.00088
Zhang, Y. L., Liu, Y., Kang, X. P., Dou, C. Y., Zhuo, R. G., Huang, S. Q., et al. (2018). Ginsenoside Rb1 Confers Neuroprotection via Promotion of Glutamate Transporters in a Mouse Model of Parkinson's Disease. Neuropharmacology 131, 223–237. doi:10.1016/j.neuropharm.2017.12.012
Zhao, D., Zhang, M., Yuan, H., Meng, C., Zhang, B., and Wu, H. (2018). Ginsenoside Rb1 Protects against Spinal Cord Ischemia-Reperfusion Injury in Rats by Downregulating the Bax/Bcl-2 Ratio and Caspase-3 and P-Ask-1 Levels. Exp. Mol. Pathol. 105 (3), 229–235. doi:10.1016/j.yexmp.2018.09.001
Zhao, J., Lu, S., Yu, H., Duan, S., and Zhao, J. (2018). Baicalin and Ginsenoside Rb1 Promote the Proliferation and Differentiation of Neural Stem Cells in Alzheimer's Disease Model Rats. Brain Res. 1678, 187–194. doi:10.1016/j.brainres.2017.10.003
Zhou, P., Lu, S., Luo, Y., Wang, S., Yang, K., Zhai, Y., et al. (2017). Attenuation of TNF-α-Induced Inflammatory Injury in Endothelial Cells by Ginsenoside Rb1 via Inhibiting NF-Κb, JNK and P38 Signaling Pathways. Front. Pharmacol. 8, 464. doi:10.3389/fphar.2017.00464
Keywords: central nervous system diseases, ginsenoside Rb1, neuroprotection, mechanisms, antioxidant
Citation: Gong L, Yin J, Zhang Y, Huang R, Lou Y, Jiang H, Sun L, Jia J and Zeng X (2022) Neuroprotective Mechanisms of Ginsenoside Rb1 in Central Nervous System Diseases. Front. Pharmacol. 13:914352. doi: 10.3389/fphar.2022.914352
Received: 06 April 2022; Accepted: 19 May 2022;
Published: 02 June 2022.
Edited by:
Young-Su Yi, Kyonggi University, South KoreaReviewed by:
Hyun-jeong Yang, Korea Institute of Brain Science, South KoreaIk-Hyun Cho, Kyung Hee University, South Korea
Copyright © 2022 Gong, Yin, Zhang, Huang, Lou, Jiang, Sun, Jia and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiansi Zeng, enhzLTIwMDVAdmlwLjE2My5jb20=; Jinjing Jia, amlhamluamluZzE5ODZAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Liang Gong
Liang Gong Jiayi Yin1†
Jiayi Yin1† Liyan Sun
Liyan Sun Jinjing Jia
Jinjing Jia Xiansi Zeng
Xiansi Zeng