
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 26 July 2022
Sec. Inflammation Pharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.912029
This article is part of the Research Topic Acute phase proteins as biomarkers and therapeutics in acute and chronic inflammatory conditions View all 5 articles
 Fei Geng1,2
Fei Geng1,2 Mengying Xu1†
Mengying Xu1† Lan Zhao1
Lan Zhao1 Haoming Zhang3
Haoming Zhang3 Jiarui Li2
Jiarui Li2 Fuyu Jin1
Fuyu Jin1 Yaqian Li1
Yaqian Li1 Tian Li1
Tian Li1 Xinyu Yang2
Xinyu Yang2 Shifeng Li1
Shifeng Li1 Xuemin Gao1
Xuemin Gao1 Wenchen Cai1
Wenchen Cai1 Na Mao1
Na Mao1 Ying Sun2
Ying Sun2 Heliang Liu1
Heliang Liu1 Hong Xu1
Hong Xu1 Zhongqiu Wei2*
Zhongqiu Wei2* Fang Yang1*
Fang Yang1*Quercetin exerts anti-inflammatory, anti-oxidant and other protective effects. Previous studies have shown that senescent cells, such as fibroblasts and type II airway epithelial cells, are strongly implicated in the development of pulmonary fibrosis pathology. However, the role of senescent macrophages during silicosis remains unclear. We investigated the effects of quercetin on macrophage senescence and pulmonary fibrosis, and explored underlying mechanisms. Mice were randomized to six model groups. Vitro model was also established by culturing RAW264.7 macrophages with silica (SiO2). We examined the effects of quercetin on fibrosis, senescence-associated β-galactosidase (SA-β-Gal) activity, and senescence-specific genes (p16, p21, and p53). We showed that quercetin reduced pulmonary fibrosis and inhibited extracellular matrix (ECM) formation. Quercetin also attenuated macrophage senescence induced by SiO2 both in vitro and in vivo. In addition, quercetin significantly decreased the expressions of the senescence-associated secretory phenotype (SASP), including proinflammatory factors (interleukin-1α (Il-1α), Il-6, tumor necrosis factor-α (TNF-α), and transforming growth factor-β1 (TGF-β1)) and matrix metalloproteinases (MMP2, MMP9, and MMP12). In conclusion, quercetin mediated its anti-fibrotic effects by inhibiting macrophage senescence, possibly via SASP.
Silicosis is a chronic and progressive disease mainly caused by prolonged exposure to free silica dust, and is characterized by excessive nodular fibrosis in the lung (Hoy and Chambers, 2020; Kimberly and John, 2021). The precise underlying pathogenesis remains unclearly, therefore, comprehensive molecular investigations are required to identify treatment strategies for this disease.
Alveolar macrophages are a major cell type residing in alveoli, and function as first line defenses against inhaled infectious agents and environmental pollutants (Kim and Nair, 2019; Chen et al., 2020; Wen et al., 2021). Recent studies reported that senescent macrophages reduced proliferation, elevated senescence-associated β-galactosidase (SA-β-Gal) activity, increased senescence-associated secretory phenotype (SASP) mRNA expression, and contributed to aged disease development such as fibrosis (Sonia and Elaine, 2020; Jacques and Jesús, 2021; Su et al., 2021).
Quercetin exerts anti-oxidant, anti-inflammatory, and other protective effects (Li et al., 2016; Liang et al., 2018). Previous studies reported that quercetin reduced senescent fibroblasts and lung fibrosis in bleomycin treated mice (Liu et al., 2020; Martel et al., 2020; Li et al., 2021). Therefore, the impact of quercetin on senescence macrophages may be a key research strategy to identify mechanisms of fibrosis.
To establish an early therapeutic model of quercetin administration, mice were intragastric administration of quercetin for 4 weeks at the time of silica (SiO2) via one-off non-invasive instillation (Figure 1A). From hematoxylin and eosin (HE) staining, more infiltrating inflammatory cells and silicotic nodules were observed in silicosis group at 4 weeks. These alterations were ameliorated after treatment with quercetin (Figure 1B). After silica exposure, the expression of the fibrotic indicator, collagen was examined in lung tissues. Van Gieson (VG) and elastin staining showed excessive collagen deposition in pulmonary mesenchyme in silicosis group. Nonetheless, collagen deposition areas were diminished after quercetin administration (Figure 1C). Western blot showed that collagen I (Col Ⅰ) levels were significantly higher in silicosis group compared with control group. In quercetin treatment group, a marked decrease of Col Ⅰ levels was observed (Figure 1D). Thus, early therapeutic administration of quercetin ameliorated silicosis in vivo.
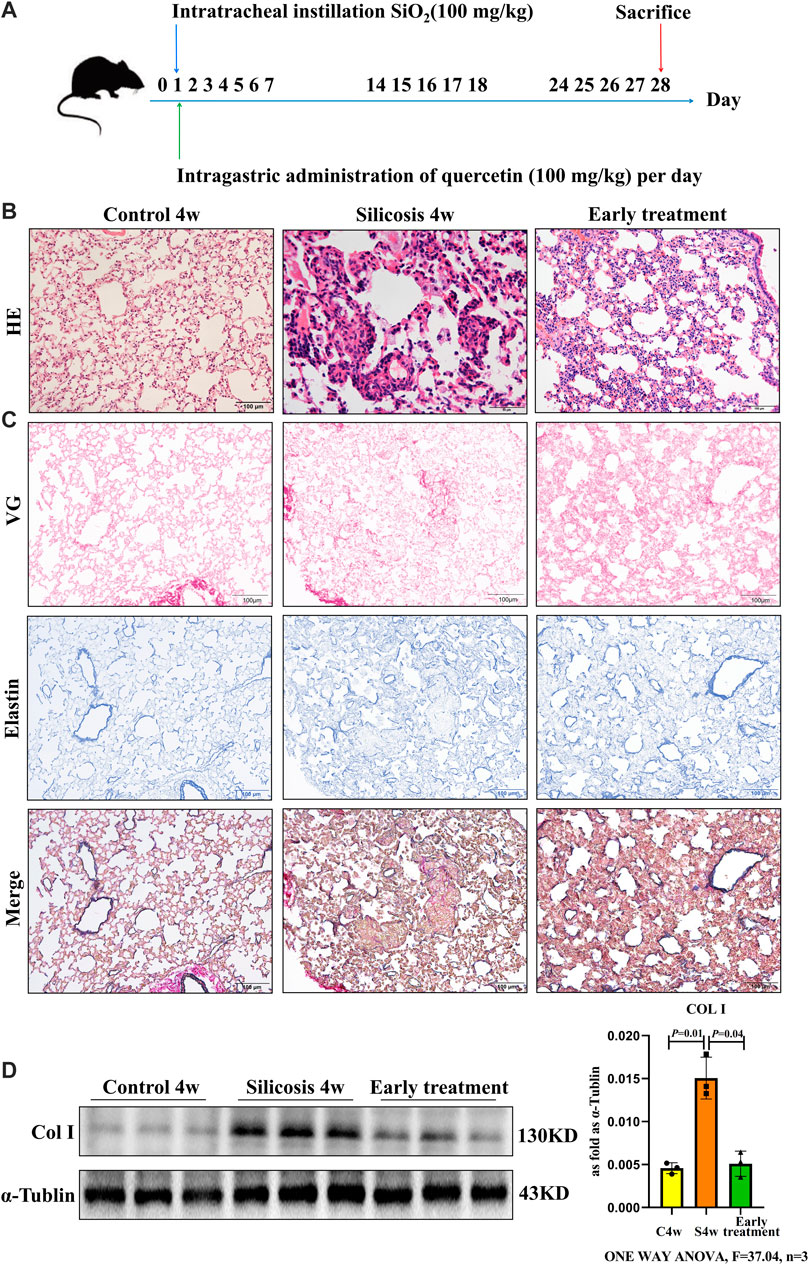
FIGURE 1. Quercetin attenuates lung injury in an early therapeutic silicosis model. (A) Schematic of quercetin administration in an early therapeutic silicosis model. (B) Representative micrographs showing lung injury by HE staining. (C) VG and elastin staining of lung tissue sections. (D) Col I expression by western blot. Data are presented as the mean ± SD. n = 3 per group.
We next investigated the late therapeutic model of quercetin administration after the formation of silicotic nodules in silicosis group, and mice were also intragastric administration of quercetin for 4 weeks beginning at 2 weeks after SiO2 exposure (Figure 2A). HE staining showed the anti-fibrotic effects of quercetin treatment for the onset of silicotic nodules (Figure 2B). In addition, reduced Col Ⅰ levels by western blot were consistent with the results using VG and elastin staining (Figures 2C,D). Collectively, these data suggest that quercrtin has improved effects on pulmonary injury when administered after onset insilicosis mice alsoly, and subsequent mechanism experiments were also conducted in this late therapeutic silicosis model.
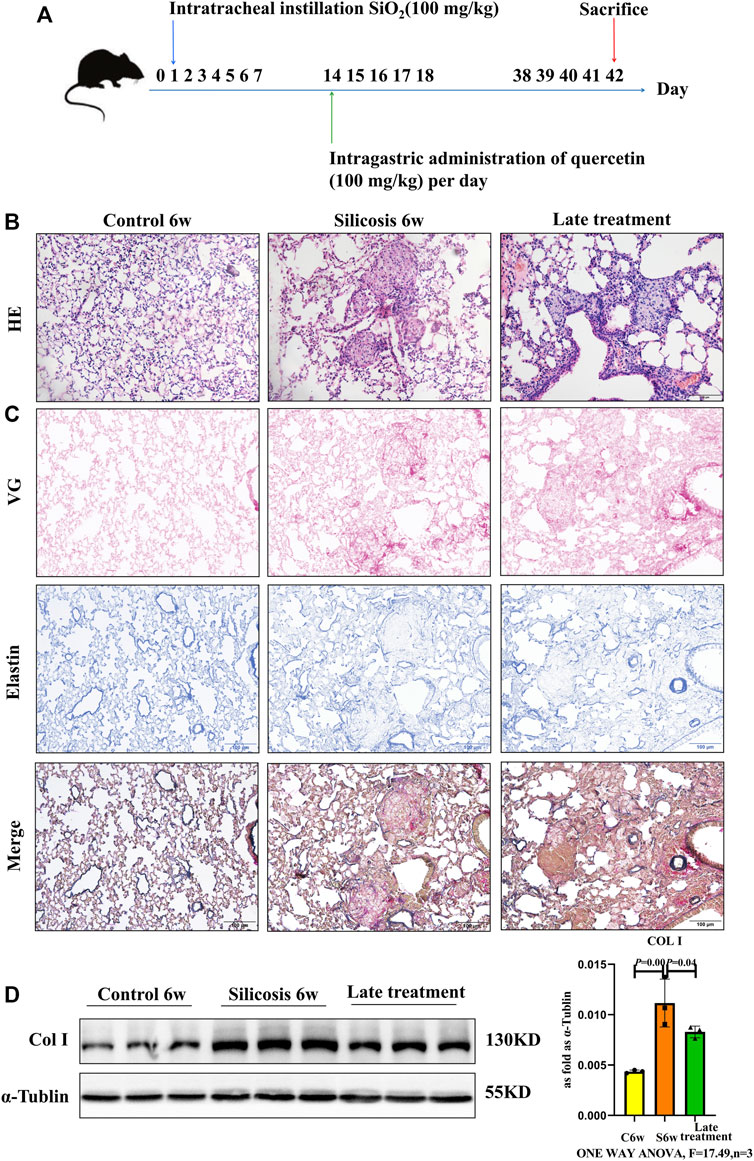
FIGURE 2. Quercetin attenuates lung injury in a late therapeutic silicosis model. (A) Schematic of quercetin administration in a late therapeutic silicosis model. (B) Representative micrographs showing lung injury by HE staining. (C) VG and elastin staining of lung tissue sections. (D) Col I expression by western blot. Data are presented as the mean ± SD. n = 3 per group.
Western blot showed that quercetin administration downregulated the higher level of senescence markers, p16, p21 and p53 in silicosis group (Figure 3A).
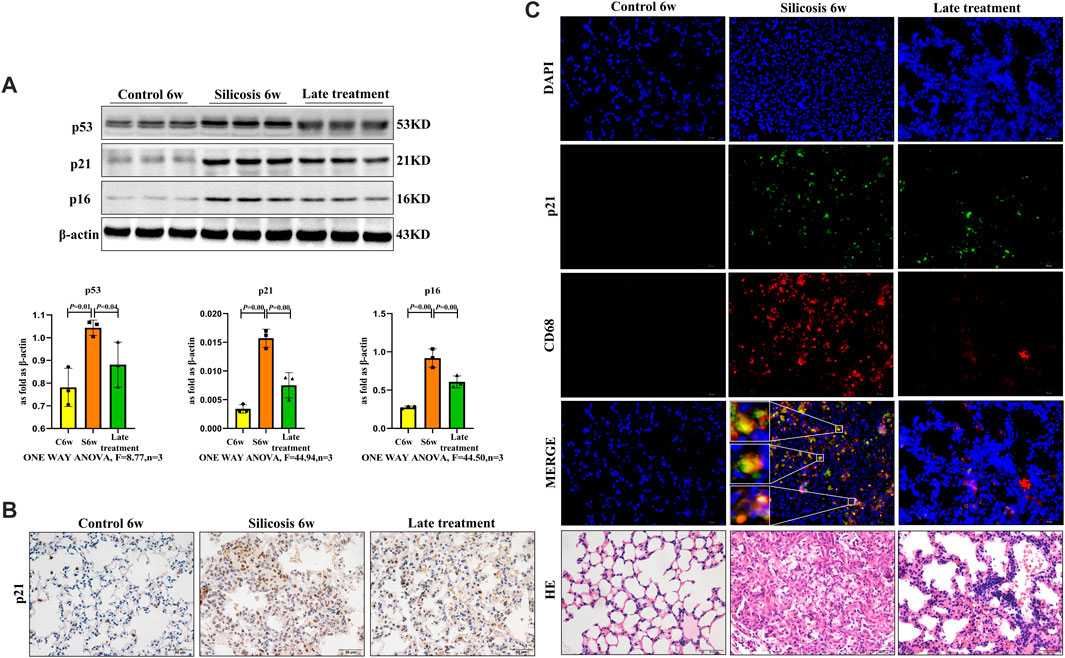
FIGURE 3. Quercetin attenuates macrophage senescence in silicosis model. (A) p16, p21 and p53 expression by western blot. (B) Immunohistochemistry staining of p21 in the lung. (C) Immunofluorescence staining for p21 and CD68 in the lung. Data are presented as the mean ± SD. n = 3 per group.
While previous studies showed that mice exposed to SiO2 induced a significant increase in senescent cells and contributed to silicosis, the role of senescent macrophages requires clarification. To further assess if quercetin was associated with reduced macrophage senescence and mediated in silicosis, the senescence marker-p21 and macrophage marker-CD68 were used to examine macrophages senescence in silicosis. We observed increased numbers of p21+\CD68+ cells after SiO2 administration, thereby supporting the role of macrophages in senescence-associated silicosis. The percentage of p21+macrophages was significantly decreased in the quercetin group compared with the silicosis group (Figures 3B,C). Thus, quercetin treatment inhibited SiO2-induced macrophage senescence.
To further verify if SiO2 induced macrophage senescence, RAW264.7 cells were examined at different times after 100, 200 or 300 μg/ml SiO2 exposure. Senescence-associated proteins of p16, p21 and p53 were gradually upregulated in a time- and dose-dependent manner. The levels of p16, p21 and p53 induced by the 200 μg/ml SiO2 displayed higher expression in 24 and 48 h groups compared with control (Figure 4A). In addition, the levels of p16, p21 and p53 for 24 h displayed higher expressions in 200 μg/ml and 300 μg/ml groups compared with control (Figure 4B). Thus, macrophages could be induced senescent by 200 μg/ml SiO2 after 24 h, suggesting senescent macrophages may be involved in silicosis development.rcetin Inhibits SiO2-Induced Macrophage Senescence
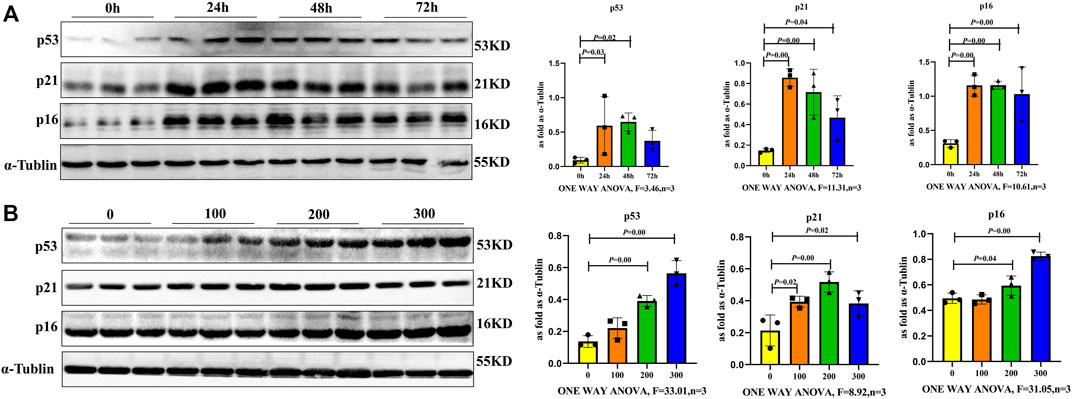
FIGURE 4. Macrophages exhibited a senescent phenotype after SiO2 exposure. (A) p16, p21 and p53 expression levels in RAW264.7 cells following 200 μg/ml SiO2 exposure for 24, 48 and 72 h by western blot. (B) p16, p21 and p53 expression levels in RAW264.7 cells following SiO2 exposure (100, 200, or 300 μg/ml) for 24 h by western blot. Data are presented as the mean ± SD. n = 3 per group.
To investigate the effect of quercetin on SiO2 induced macrophage senescence, RAW264.7 cells were treated with SiO2 (200 μg/ml) and/or quercetin (25 μmol/L) for 24 h. As shown in Figure 5A, the expressions of p16, p21, and p53 were significantly increased by SiO2 stimulated for 24 h, however, p16, p21, and p53 expressions were decreased after quercetin treatment. Cytoplasmic SA-β-gal activity, which is a marker of cell senescence, was examined by SA-β-gal staining. These assays showed that positive staining cell numbers in Quercetin + SiO2 group were significantly lower than SiO2 group (Figure 5B), confirming our western blot findings. Thus, quercetin administration significantly delayed macrophage senescence induced by SiO2.

FIGURE 5. Quercetin inhibits SiO2-induced macrophage senescence. (A) p16, p21 and p53 expressions by western blot. (B) SA-β-gal staining in RAW264.7 cells. Data are presented as the mean ± SD. n = 3 per group.
To clarify quercetin mechanisms related to senescence, we performed a network pharmacology analysis. In total, 223 potential quercetin targets were identified using Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) and Swiss Target Prediction Database. We also identified 251 silicosis disease targets in GeneCards and Diagene. The GeneCards Database revealed 4,780 potential targets for senescence. Using a Venn diagram of quercetin, silicosis and senescence target intersections, 38 overlapping targets were identified (Figure 6A).
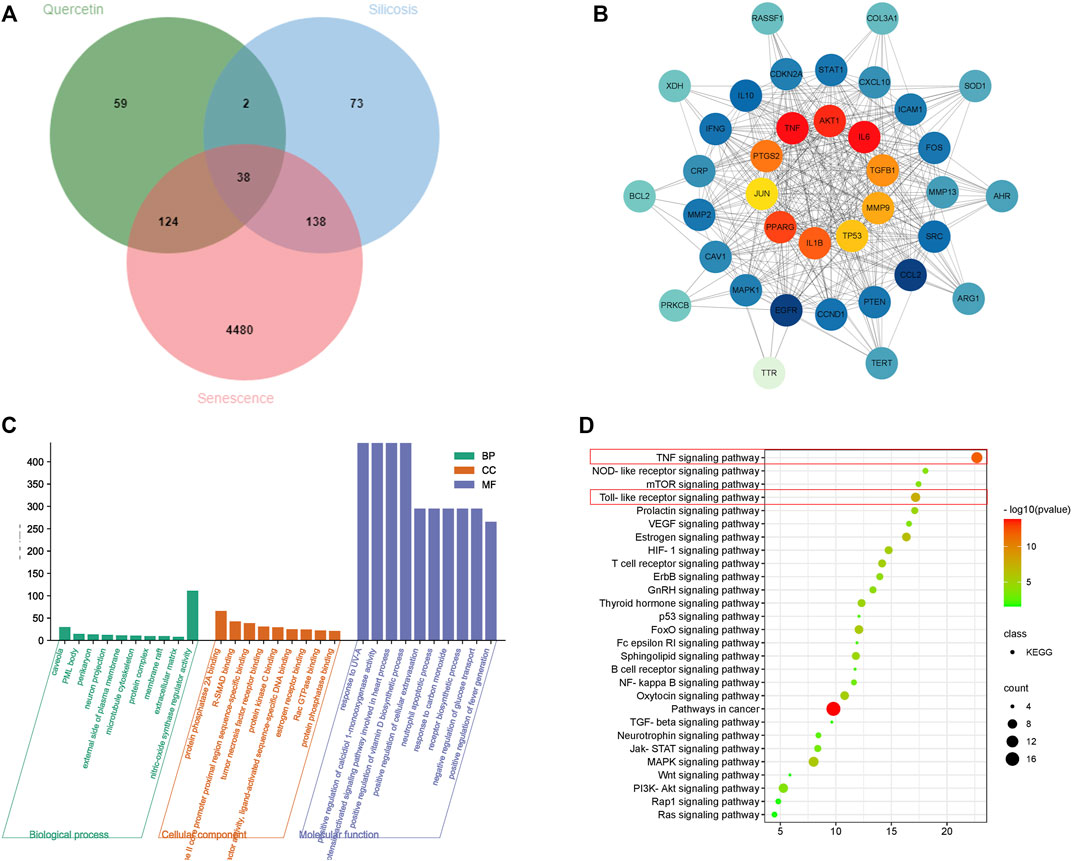
FIGURE 6. Network pharmacology analysis of a quercetin-senescence-silicosis intersection target network. (A) Venn diagram showing a quercetin-senescence-silicosis intersection target network. (B) PPI network of quercetin-senescence-silicosis intersection targets. (C) GO enrichment analyses. (D) KEGG enrichment analyses showing the top 10 pathways.
These 38 targets were then imported into the STRING Database to establish a protein-protein interaction (PPI) network. Next, cytoscape software was used to generate a quercetin-senescence-silicosis target network diagram, showing the top 10 molecular targets: interleukin-6 (IL-6), protein kinase B (AKT1), tumor necrosis factor (TNF), prostaglandin-endoperoxide synthase 2 (PTGS2), peroxisome proliferator activated receptor gama (PPARG), IL-1β, transforming growth factor-β1 (TGF-β1), matrix metalloproteinases-9 (MMP9), TP53, and c-Jun N-terminal kinase (JUN) (Figure 6B).
After gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses, 343 biological processes, 34 molecular functions, 26 cell components and 28 signal pathways were identified (Figures 6C,D). According to adjusted p values, the top ten pathways were selected, including pathways in cancer, TNF signaling pathway, Toll-like receptor signaling pathway, Estrogen signaling pathway, Mitogen-activated protein kinase (MAPK) signaling pathway, Forkhead box protein O (FoxO) signaling pathway, Hypoxia inducible factor 1(HIF-1) signaling pathway, oxytocin signaling pathway, T cell receptor signaling pathway, and thyroid hormone signaling pathway.
To explore the role of senescent macrophages during silicosis, dynamic changes in SASP components were determined after macrophages were exposed to SiO2, including the analysis of growth and matrix remodeling factors. Firstly, the SASP proinflammatory factors, including IL-1β, IL-6, TNF-α, and TGF-β1 were significantly upregulated in senescent macrophages at 24 h after 200 μg/ml SiO2 exposure (Figures 7A,C). Secondly, MMPs including MMP2, MMP9 and MMP12 of the SASP matrix in macrophages were increased after macrophages exposure to SiO2 (Figures 7B,D). Taken together, our results supported the notion that SiO2 promoted SASP secretion in senescent macrophages.
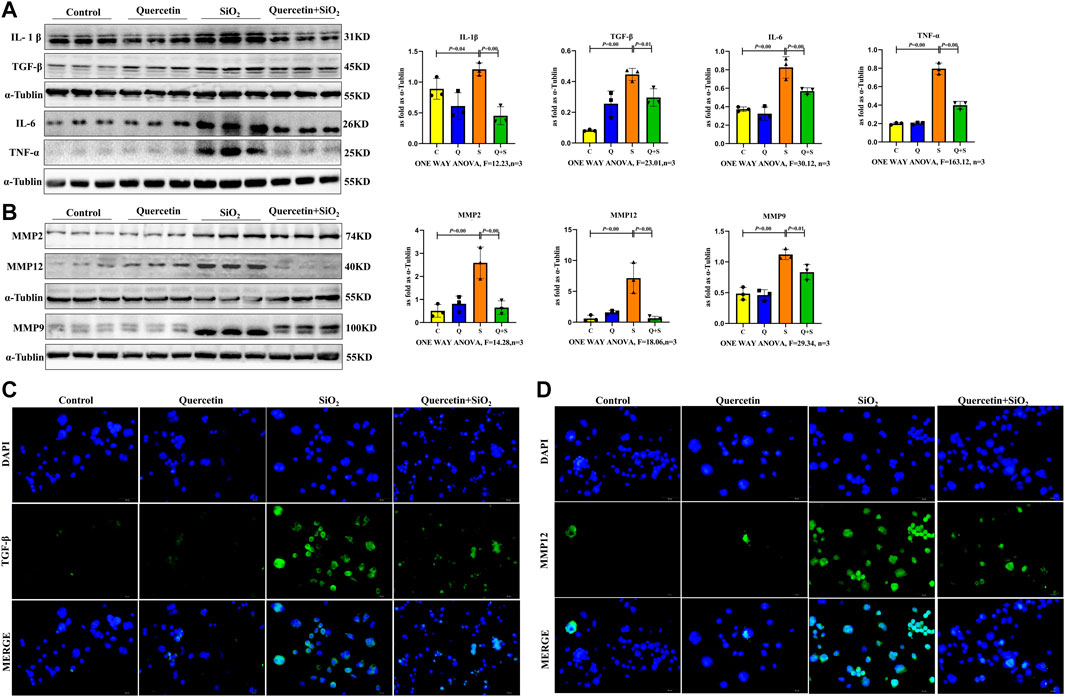
FIGURE 7. Elevated fibrotic and SASP protein expression in senescent macrophages. (A) IL-1β, IL-6, TNF-α and TGF-β1 expressions by western blot. (B) MMP2, MMP9 and MMP12 expressions by western blot. (C) Immunofluorescence staining for TGF-β1. (D) Immunofluorescence staining for MMP12. Data are presented as the mean ± SD. n = 3 per group.
Importantly, SASP proinflammatory factor secretion and MMPs in the SASP matrix were decreased after quercetin treatment. (Figure 7).
Fibrotic and SASP protein expression levels in the lungs of the silicosis model were analyzed. Western blot showed that the expression levels of IL-1β, IL-6, TNF-α and TGF-β1 in lung tissue from the silicosis group were significantly elevated (Figures 8A,C). Additionally, increased MMP2, MMP9 and MMP12 expression levels were also detected after SiO2 exposure (Figures 8B,D).
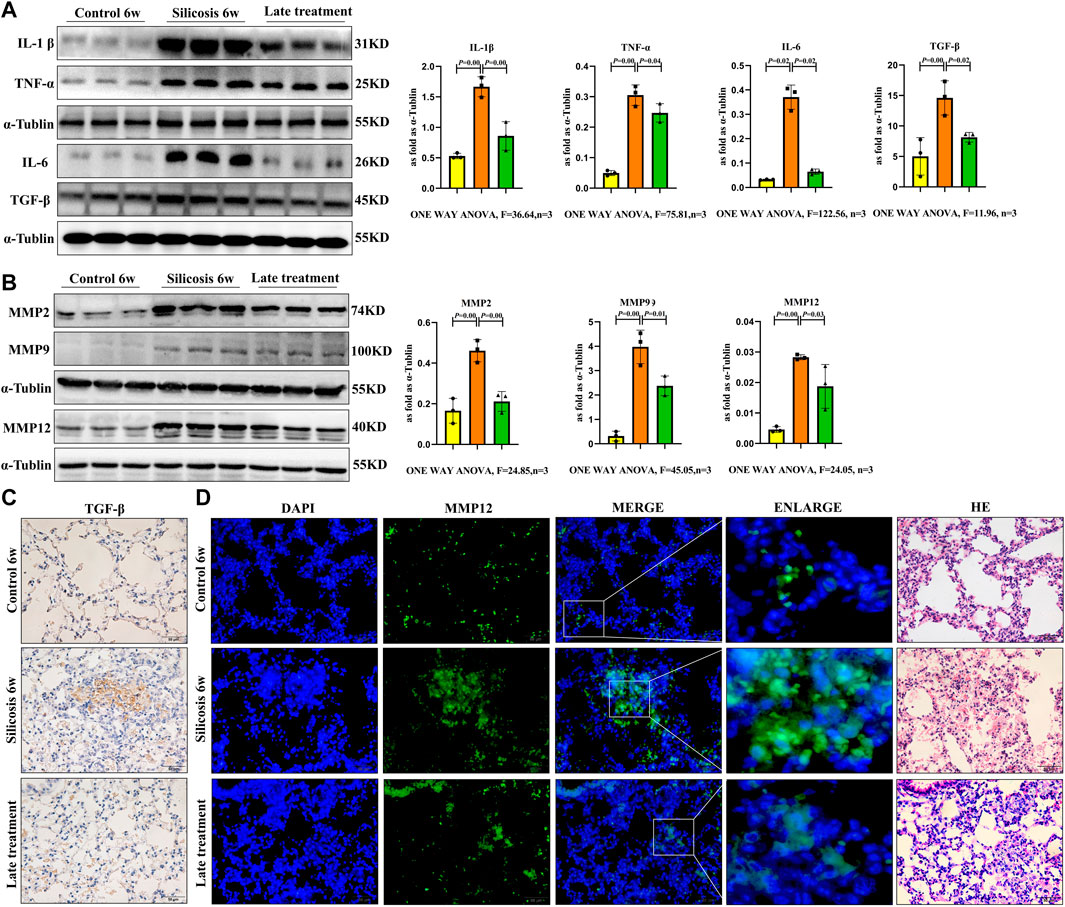
FIGURE 8. SiO2 significantly elevated fibrotic and SASP protein expression levels in the lung tissue of silicosis model. (A) IL-1β, IL-6, TNF-α and TGF-β1 expression levels were detected by western blot. (B) MMP2, MMP9 and MMP12 expression levels were detected by western blot. (C) Immunohistochemistry staining for TGF-β1 in the lung. (D) Immunofluorescence staining for MMP12 in the lung. Data are presented as the mean ± SD. n = 3 per group.
Quercetin treatment significantly decreased fibrotic and SASP protein expression levels in vivo. Thus, quercetin administration inhibited silicosis activity by attenuating SASP levels in senescent macrophages in a silicosis model.
Silicosis has a chronic, progressive and ultimately fatal fibrotic course, thus effective preventative and therapeutic measures are required. The disease is characterized by fibroblast proliferation, ECM accumulation, and ultimately leading to respiratory failure (Wei et al., 2019; Li et al., 2020). Although the disease pathogenesis has been widely investigated, detailed molecular and cellular pathogenic mechanisms are unclear with current therapeutic strategies largely ineffective.
Quercetin is a natural plant flavonoid in many fruits, vegetables and grains, which displays numerous beneficial biological activities including anti-inflammatory, anti-oxidant, immune regulation and other effects (Fontana and Partridge, 2015). Quercetin suppressed bleomycin induced epithelial-mesenchymal transition by suppressing reactive oxygen species via Smad and β-catenin signaling pathways (Takano et al., 2020). Quercetin also exerted anti-fibrogenic and anti-inflammatory effects, possibly by modulating the redox balance via nuclear erythroid factor 2-related factor 2 (Nrf2) induction (Boots et al., 2020). Quercetin (100 mg/kg) markedly reduced the extent of tissue damage in bile duct ligation (BDL) - and carbon tetrachloride (CCl4) -liver fibrosis model mice, confirming its protective effect (Wu et al., 2017). In our study, we showed that both early and late therapeutic administration of quercetin (100 mg/kg) protected against SiO2-induced silicosis in mice.
Cellular senescence involves DNA damage responses which activate the tumor suppressors including p16, p21 and p53, and the subsequent induction of SA-β-gal, which has key roles in embryogenesis, wound healing and protection against cancer (Birch et al., 2017). Previous studies reported that senescent cells including senescent fibroblasts and type II airway epithelial cells are contributed to the development of pulmonary fibrosis and (Schafer et al., 2017). Pulmonary macrophages are predominant effector cells with vital roles in lung fibrosis pathogenic mechanisms. However, the precise role of these macrophages remains largely unexplored. Here, we showed that similar trait combinations were acquired by macrophages following SiO2 exposure both in vivo and in vitro. In the silicosis model, SiO2 increased the expressions of the senescence-specific proteins, p16, p21 and p53 in lung tissue, and numbers of p21-expressing macrophages by immunohistochemistry staining. In vitro, RAW264.7 cell elevated SA-β-Gal activity after SiO2 exposure, suggesting pulmonary macrophages exhibited senescence characteristics during silicosis.
Quercetin inhibited phosphatidylinositol-3-hydroxykinase (PI3K) and Akt to reduce senescent cells and delay aging as indicated by improved cardiac function and exercise capacity (Zhu et al., 2015). Quercetin (20 µmoL/L) also alleviated kidney fibrosis by reducing renal tubular epithelial cell senescence via mitophagy, possibly by activating the Sirtuin-1(SIRT1)/PTEN-induced kinase 1 (PINK1)/Parkin axis (Liu et al., 2020). 25 µmoL/L of quercetin inhibited the formation of foam cells induced by oxidized low density lipoprotein (ox-LDL) and delayed RAW264.7 cell senescence (Cao et al., 2019). Our findings showed that quercetin (25 µmoL/L) effectively delayed macrophage senescence after SiO2 exposure, thereby confirming the anti-silicosis and anti-aging effects of quercetin.
Generally, senescent cells contribute to tissue dysfunction via SASP, which comprises pro-inflammatory cytokines (e.g., IL‐1β and IL‐6) and MMPs (Daniel and Manuel, 2014). Senescent cells and SASP may fuel increasingly fibrotic responses, driving lung deterioration and severe functional decline during silicosis.
To screen for key quercetin targets against senescence and silicosis, our network pharmacology approach identified 38 overlapping targets, with 10 hub genes identified: IL-6, AKT1, TNF, PTGS2, PPARG, IL-1β, TGF-β1, MMP9, TP53 and JUN. Based on KEGG pathway analyses of target proteins, both TNF and Toll-like receptor signaling pathways were related to senescence responses and silicosis. Thus, TNF, IL-6, IL-1β, TGF-β1 and MMP9 modulation may be key pharmacological mechanisms underpinning quercetin action against senescence. Our findings support the hypothesis that SiO2 induced macrophage senescence and promoted the SASP secretion of profibrotic factors, chemokines and MMPs leading to irreversible lung fibrosis.
Also, SiO2 induced macrophage senescence and silicosis in lung tissue, both of which were accompanied by increased fibrosis-associated factors (IL-1β, IL-6, TNF, and TGF-β1) and MMPs (MMP2, MMP9 and MMP12). Importantly, quercetin appeared to restrain SASP secretion.
Animal studies were approved by the Institutional Animal Care and Use Committee of the North China University of Science and Technology. Animals received ad libitum access to food and water according to the guidelines of the National Institutes of Health (NIH, MD, United States).
Female C57BL/6 mice (6–8 weeks old) were purchased from Beijing HFK Bioscience Co. Ltd. (Beijing, China). The silicotic model was established by given silica (SiO2) (100 mg/kg) via one-off non-invasive intratracheal instillation. The quercetin therapeutic group was intragastric administration of quercetin (100 mg/kg) once a day.
To establish both early and late therapeutic model of quercetin administration, sixty mice were randomly divided into six groups: 1) Control 4w group (placebo treatment for 4 weeks); 2) Silicosis 4w group (given SiO2 for 4 weeks); 3) Quercetin 4w groups (given SiO2 and simultaneously administration of quercetin for 4 weeks); 4) Control 6w group (placebo treatment for 6 weeks); 5) Silicosis 6w group (given SiO2 for 6 weeks); 3) Quercetin 6w groups (given SiO2 and administration of quercetin for 4 weeks beginning at 2 weeks after silica exposure).
Mice were humanely anaesthetized, and sera and lung samples collected and frozen at −80°C until required.
RAW264.7 mouse macrophages were purchased from the Chinese Academy of Sciences Cell Library (Shanghai, China). Cells were cultured in F12 K media (BOSTER; California, United States) containing 10% fetal bovine serum (Bovogen Biologicals; Melbourne, Australia) at 37°C in 5% CO2. After 8 h starvation in serum-free F12K, cells were randomly divided into four groups: Control; Quercetin (25 μmol/L); SiO2 (200 μg/ml); Quercetin + SiO2 (added of quercetin (25 μmol/L) for 2 h before SiO2 (200 μg/ml) stimulation).
Briefly, lung tissue slides were stained in HE to assess silicosis. VG and elastin stains were used to assess collagen fiber deposition.
Total protein was extracted from lung tissue or cells using RIPA buffer (Cat. No. JFR0150; JFBiotech, Shanghai, China) containing a protease inhibitor (Cat. No. RP-WA0102; Report Biotech, China). Protein concentrations were measured using the Bradford assay (Cat. No. RP-WA0201; Report Biotech). Then, 15 μg protein was separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 10% gels. Proteins were transferred to polyvinylidene difluoride membranes (Sigma-Aldrich; Shanghai, China) and membranes blocked in 5% non-fat milk at room temperature for 1 h. Then, membranes were incubated overnight at 4°C with primary antibodies (anti-collagen type I [Cat. No. Ab34710; Abcam, Cambridge, United Kingdom], anti-p16 [Cat. No. A0262; Abclonal, Wuhan, China], anti-p21 [Cat. No. 5556431; BD Pharmingen, Guangzhou, China], anti-p53 [Cat. No. SC-6243; Santa Cruz Biotechnology, CA, United States], anti-interleukin-1α (anti-IL-1β) [Cat. No. DF6251; Affinity, Cincinnati, OH, United States], anti-IL-6 [Cat. No. A0286; Abclonal], anti-tumor necrosis factor-α (anti-TNF-α) [Cat. No. A11534; Abclonal], anti-transforming growth factor-β1 (anti-TGF-β1) [Cat. No. ARG56429; Arigo, Taiwan, China], anti-matrix metalloproteinase 2 (anti-MMP2) [Cat. No. AF0577; Affinity], anti-MMP9 [Cat. No. AF5228; Affinity], and anti-MMP12 [Cat. No. ET1602-42; HUABIO, Hangzhou, China]).
Membranes were washed three times in TBS-T and incubated with diluted (1:5,000) peroxidase-labelled affinity-purified antibodies targeting rabbit/mouse IgG (H + L) (Cat. No. 5220-0336(074-1506) and Cat. No. 5220-0341(074-1806); Seracare, United States) at 37°C for 1 h. After washing three times in TBS-T, membranes were visualized by adding enhanced chemiluminescent Prime Western Blot Detection Reagent (Cat. No. ZD310A-1; ZOMANBIO, China). Grey values were analyzed using ImageJ software (NIH). α-Tubulin and β-actin was used as a loading control.
These techniques were performed on lung tissue sections and RAW264.7 cells. Non-specific binding sites were blocked for 30 min in blocking solution (phosphate-buffered saline (PBS) plus 1% bovine serum albumin). Samples were incubated overnight at 4°C with primary antibodies against p21 and TGF-β1, and then incubated with secondary antibodies (Cat. Mo. PV-6000; ZSGB-BIO, Beijing, China) at 37°C for 30 min. Immunoreactivity was visualized with diaminobenzidine (Cat. No. ZLI-9018; ZSGB-BIO). Brown staining was considered a positive result.
SA-β-gal activity staining was performed as per manufacturer’s instructions (Cat. No. C0602; Beyotime, Shanghai, China). Briefly, cells were washed in PBS (pH 7.2), fixed in fixative solution (provided by the kit), and stained in a 5-bromo-4-Chloro-3-Indolyl β-d-Galactopyranoside (X-gal) solution for 48 h at 37°C. Imaging was performed using bright-field microscopy where senescent cells stained blue.
Identify protein targets of quercetin were used TCMSP (http://tcmspw.com/)and Swiss Target Prediction (http://www.swisstargetsprediction.ch/). Targets for silicosis and senescence were collected from Gene Cards database (https://www.genecards.org/) and Disgene (http://www.disgenet.org/). The PPI network of these targets was obtained from the String (https://string-db.org). Finally, we used Cytoscape software to open the results, created quercetin-silicosis-senescence targets network.
Statistical analyses were conducted in SPSS statistical software and performed with one‐way analysis of variance, followed by post-hoc analysis with Bonferroni’s tests for multiple comparisons. Data were presented as the mean ± standard deviation. p values <0.05 were statistically significant.
In summary, we suggest that senescent macrophages and SASP mechanisms may be treatment targets for silicosis, with quercetin functioning to eliminate senescent macrophages and block SASP activity, thereby acting as a novel therapeutic approach for silicosis.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The animal study was reviewed and approved by North China University of Science and Technology. Written informed consent was obtained from the owners for the participation of their animals in this study.
Conceptualization, FG, MX, ZW, and FY; methodology, FG and MX; software, FG, LZ, HZ, and FJ; validation, TL, YL, and XY; formal analysis, FG and XY; investigation, YL; resources, FY and HX; data curation, MX and JL; writing—original draft preparation, FG; writing—review and editing, ZW and FY; visualization, XG, NM, and WC; supervision, YS and HL; project administration, ZW; funding acquisition, SL and ZW. All authors have read and agreed to the published version of the manuscript.
This work was funded by the National Natural Science Foundation of China (No. 82003406), the National Natural Science Foundation of Hebei Province (H2020209292), Innovative experiment Program for College students (No. R2020006), Science and Technology Project of Hebei Education Department (No. JQN2020010).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank Editor (https://www.editorbar.com) for editing the English text of a draft of this manuscript.
Birch, J., Barnes, P. J., and Passos, J. F. (2017). Mitochondria, Telomeres and Cell Senescence: Implications for Lung Ageing and Disease. Pharmacol. Ther. 183, 34–49. doi:10.1016/j.pharmthera.2017.10.005
Boots, A. W., Veith, C., Albrecht, C., Bartholome, R., Drittij, M. J., Claessen, S. M. H., et al. (2020). The Dietary Antioxidant Quercetin Reduces Hallmarks of Bleomycin-Induced Lung Fibrogenesis in Mice. BMC Pulm. Med. 20 (1), 112. doi:10.1186/s12890-020-1142-x
Cao, H., Jia, Q., Yan, L., Chen, C., Xing, S., and Shen, D. (2019). Quercetin Suppresses the Progression of Atherosclerosis by Regulating MST1-Mediated Autophagy in Ox-LDL-Induced RAW264.7 Macrophage Foam Cells. Int. J. Mol. Sci. 20 (23), 6093. doi:10.3390/ijms20236093
Chen, X., Tang, J., Shuai, W., Meng, J., Feng, J., and Han, Z. (2020). Macrophage Polarization and its Role in the Pathogenesis of Acute Lung Injury/acute Respiratory Distress Syndrome. Inflamm. Res. 69 (9), 883–895. doi:10.1007/s00011-020-01378-2
Daniel, M. E., and Manuel, S. (2014). Cellular Senescence: from Physiology to Pathology. Nat. Rev. Mol. Cell Biol. 15 (7), 482–496. doi:10.1038/nrm3823
Fontana, L., and Partridge, L. (2015). Promoting Health and Longevity through Diet: from Model Organisms to Humans. Cell 161 (1), 106–118. doi:10.1016/j.cell.2015.02.020
Hoy, R. F., and Chambers, D. C. (2020). Silica-related Diseases in the Modern World. Allergy 75 (11), 2805–2817. doi:10.1111/all.14202
Jacques, B., and Jesús, G. (2021). Similarities and Interplay between Senescent Cells and Macrophages. J. Cell Biol. 220 (2), 1–8. doi:10.1083/jcb.202010162
Kim, S. Y., and Nair, M. G. (2019). Macrophages in Wound Healing: Activation and Plasticity. Immunol. Cell Biol. 97 (3), 258–267. doi:10.1111/imcb.12236
Kimberly, R., and John, J. (2021). Silicosis. Workplace Health Saf. 69 (1), 51. doi:10.1177/2165079920967923
Li, Y., Yao, J., Han, C., Yang, J., Chaudhry, M. T., Wang, S., et al. (2016). Quercetin, Inflammation and Immunity. Nutrients 8 (3), 167. doi:10.3390/nu8030167
Li, S., Wei, Z., Li, G., Zhang, Q., Niu, S., Xu, D., et al. (2020). Silica Perturbs Primary Cilia and Causes Myofibroblast Differentiation during Silicosis by Reduction of the KIF3A-Repressor GLI3 Complex. Theranostics 10 (4), 1719–1732. doi:10.7150/thno.37049
Li, C. Z., Shen, Y. T., Huang, L. W., Liu, C., and Wang, J. (2021). Senolytic Therapy Ameliorates Renal Fibrosis Postacute Kidney Injury by Alleviating Renal Senescence. FASEB J. 35 (1), e21229. doi:10.1096/fj.202001855rr
Liang, Q., Chen, Y., Li, C., and Lu, L. (2018). Quercetin Attenuates Ox-LDL-Induced Calcification in Vascular Smooth Muscle Cells by Regulating ROS-TLR4 Signaling Pathway. Nan Fang. Yi Ke Da Xue Xue Bao 38 (8), 980–985. doi:10.3969/j.issn.1673-4254.2018.08.13
Liu, T., Yang, Q., Zhang, X., Qin, R., Shan, W., Zhang, H., et al. (2020). Quercetin Alleviates Kidney Fibrosis by Reducing Renal Tubular Epithelial Cell Senescence through the SIRT1/PINK1/mitophagy axis. Life Sci. 257, 118116. doi:10.1016/j.lfs.2020.118116
Martel, J., Ojcius, D. M., Wu, C. Y., Peng, H. H., Voisin, L., Perfettini, J. L., et al. (2020). Emerging Use of Senolytics and Senomorphics against Aging and Chronic Diseases. Med. Res. Rev. 40, 2114–2131. doi:10.1002/med.21702
Schafer, M. J., White, T. A., Iijima, K., Haak, A. J., Ligresti, G., Atkinson, E. J., et al. (2017). Cellular Senescence Mediates Fibrotic Pulmonary Disease. Nat. Commun. 8, 14532. doi:10.1038/ncomms14532
Sonia, S. E., and Elaine, E. (2020). Senescent Cells and Macrophages: Key Players for Regeneration? Open Biol. 10 (12), 200309. doi:10.1098/rsob.200309
Su, L., Dong, Y., Wang, Y., Wang, Y., Guan, B., Lu, Y., et al. (2021). Potential Role of Senescent Macrophages in Radiation-Induced Pulmonary Fibrosis. Cell Death Dis. 12 (6), 527. doi:10.1038/s41419-021-03811-8
Takano, M., Deguchi, J., Senoo, S., Izumi, M., Kawami, M., and Yumoto, R. (2020). Suppressive Effect of Quercetin against Bleomycin-Induced Epithelial-Mesenchymal Transition in Alveolar Epithelial Cells. Drug Metab. Pharmacokinet. 35 (6), 522–526. doi:10.1016/j.dmpk.2020.08.001
Wei, Z., Xu, H., Zhang, Y., Yi, X., Yang, X., Chen, Y., et al. (2019). Rho GDP Dissociation Inhibitor α Silencing Attenuates Silicosis by Inhibiting RhoA/Rho Kinase Signalling. Exp. Cell Res. 380 (2), 131–140. doi:10.1016/j.yexcr.2019.04.026
Wen, Y., Yan, H. R., Wang, B., and Liu, B. C. (2021). Macrophage Heterogeneity in Kidney Injury and Fibrosis. Front. Immunol. 12, 681748. doi:10.3389/fimmu.2021.681748
Wu, L., Zhang, Q., Mo, W., Feng, J., Li, S., Li, J., et al. (2017). Quercetin Prevents Hepatic Fibrosis by Inhibiting Hepatic Stellate Cell Activation and Reducing Autophagy via the TGF-β1/Smads and PI3K/Akt Pathways. Sci. Rep. 7 (1), 9289. doi:10.1038/s41598-017-09673-5
Keywords: silicosis, quercetin, macrophage, senescence, SASP
Citation: Geng F, Xu M, Zhao L, Zhang H, Li J, Jin F, Li Y, Li T, Yang X, Li S, Gao X, Cai W, Mao N, Sun Y, Liu H, Xu H, Wei Z and Yang F (2022) Quercetin Alleviates Pulmonary Fibrosis in Mice Exposed to Silica by Inhibiting Macrophage Senescence. Front. Pharmacol. 13:912029. doi: 10.3389/fphar.2022.912029
Received: 03 April 2022; Accepted: 08 June 2022;
Published: 26 July 2022.
Edited by:
Christina Maria Pabelick, Mayo Clinic, United StatesReviewed by:
Swarna Bale, National Institute of Pharmaceutical Education and Research, IndiaCopyright © 2022 Geng, Xu, Zhao, Zhang, Li, Jin, Li, Li, Yang, Li, Gao, Cai, Mao, Sun, Liu, Xu, Wei and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongqiu Wei, d2VpemhvbmdxaXVAbmNzdC5lZHUuY24=; Fang Yang, ZmFuZ3lhbmdAbmNzdC5lZHUuY24=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.