
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 09 June 2022
Sec. Neuropharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.907516
This article is part of the Research Topic Emerging Inner Ear Therapies View all 5 articles
Hearing loss affects roughly 466 million people worldwide. While the causes of hearing loss are diverse, mechanistically, inflammation and oxidative stress have been identified as major players in hearing loss regardless of pathogenesis. Treatment options remain extremely limited and there is currently no FDA approved drug therapy. Studies indicate that antioxidants such as d-Methionine have shown some protective effects; however, these studies involved systemic or invasive localized delivery methods and highlighted the need for the development of minimally invasive localized therapeutic approaches. Described herein is the development of an antioxidant-conjugated system that shows prophylactic potential against oxidative damage and appears suitable for topical delivery. Specifically, our covalent conjugate of hyaluronan with d-Methionine shows cytocompatibility and protection from oxidative stress in two mouse cochlear cell lines (HEI-OC1 and SV-k1). Mechanistically, the data indicate that the protective effects of the conjugate are due to the hyaluronan-mediated cellular internalization of the antioxidant. Most notably, the conjugate can efficiently permeate through an in vitro round window membrane model without the loss of the attached antioxidant, for subsequent delivery of the therapeutic cargo to the hearing sensory cells. Collectively these findings show that the novel conjugate could be a potential topical preventive agent against hearing loss.
Worldwide, hearing loss is reportedly affecting more than half a billion people and is the fourth leading contributor to living with a disability; the costs associated with it are greater than $750 billion each year (Brown et al., 2018). Hearing loss can occur for a variety of reasons and can be age related, noise-induced, drug-induced, or caused by viral, bacterial, or chemical agents. Nevertheless, currently there are no Food and Drug Administration (FDA)-approved drugs to prevent it (Ingersoll et al., 2020). Mechanistically, inflammation and oxidative stress have been identified as major players in this pathology regardless of its etiology (Kamogashira et al., 2015; Kurabi et al., 2017).
Animal studies highlighted the protective role of antioxidants against noise-, virus-, age- or drug-induced hearing loss (Le Prell et al., 2007; Tavanai and Mohammadkhani, 2017; Pecha et al., 2020). In noise-induced hearing loss, one study underlined the mitochondrial generation of reactive oxygens species (ROS) and showed that d-Methionine (D-Met) treatment protected animals from temporary threshold shifts (Cheng et al., 2008). Mechanistically, noise-initiated oxidative stress was shown to occur through the release of calcium ions from the endoplasmic reticulum or potential entry from extracellular fluid leading to the release of ROS from mitochondria, and via activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (Kurabi et al., 2017). A separate study revealed that excessive ROS is associated with cytomegalovirus (CMV)-induced hearing loss, and intraperitoneal treatment of hearing-impaired mice with D-Met and ACE-Mg (combination of vitamins A, C, E and magnesium) attenuated the extent of hearing loss (Pecha et al., 2020). In presbycusis or age-related hearing loss supplementation with antioxidants was shown to reduce the progression of the disease (Fujimoto and Yamasoba, 2014). Aging, according to the free radical or oxidative stress theory, is considered to be the result of increased oxidative damage (Lin and Flint Beal, 2003). Drug-induced ototoxicity was also linked to oxidative stress. Cisplatin and aminoglycoside antibiotics were shown to drastically increase cellular ROS production (Ravi et al., 1995; Sha and Schacht, 1999).
While animal studies targeting hearing loss via oxidative stress reduction reported successful outcomes, human data lag (Kurabi et al., 2017; Pak et al., 2020). Interestingly, while hereditary deafness was reported to benefit from dietary antioxidant supplementation (Green et al., 2016), other studies have shown that antioxidant-enriched diets did not delay the progression of age-related hearing loss (Cheng et al., 2008). We believe that, in contrast to orally administered antioxidants, their topical delivery would result in increased cochlear bioavailability and enhanced therapeutic outcomes. Therefore, our approach for this study was to develop a topically deliverable antioxidant system that could prevent or treat oxidative damage typically associated with hearing loss. Our rationale was to employ an antioxidant ‘carrier’ to permeate ear-specific biological membranes (such as the tympanic membrane or the round window membrane) with the drug cargo and deliver it to the cochlear structures typically affected by oxidative stress.
For this, hyaluronic acid (HA) was selected as the carrier, as this macromolecule has been previously reported to aid in the delivery of drugs into cochlear structures (Chaulagain et al., 2018; Altman et al., 2019; Yu et al., 2021). Specifically, one study reported that pre-treatment of the round window membrane with HA resulted in increased cochlear gene delivery in a guinea pig model (Shibata et al., 2012). A separate clinical study reported that patients treated with a mix of dexamethasone/HA led to better therapeutic outcomes for sudden sensorineural hearing loss compared to patients treated with only dexamethasone (Rogha and Kalkoo, 2017). Moreover, HA has been widely explored as a transdermal drug delivery enhancer, and shown to increase drug permeation through normal skin, seemingly by a combination of cotransport, increased tissue hydration, and modifications of the stratum corneum properties (Witting et al., 2015). HA is a naturally occurring, high-molecular weight polymer consisting of repeating disaccharide units, ubiquitous in the mammalian extracellular matrix (Gupta et al., 2019). Its chemical structure readily lends itself to well characterized chemical modifications and generation of highly biocompatible derivatives (Serban and Skardal, 2019). d-Methionine (D-Met) was selected as the antioxidant as its protective effects against cochlear oxidative stress are well documented (Cheng et al., 2008; Wang et al., 2019; Pecha et al., 2020; Campbell et al., 2021) Additionally, its structure contains a primary amine group suitable for carbodiimide mediated conjugation to carboxyl groups present on the HA backbone (Serban and Skardal, 2019). Presented herein are the syntheses and characterization of hyaluronan-d-Methionine conjugates (HAM) and their effects on cochlear cells exposed to oxidative stress.
The following reagents and consumables have been used for this study: hyaluronic acid (HA) MW 850 kDa (Novozymes, Denmark), sodium hydroxide (NaOH, VWR, Radnor, PA), iodoacetic acid (ThermoScientific, Waltham, MA), isopropanol (VWR, Radnor, PA), Whatman filter paper (Fisher, Waltham, MA), hydrochloric acid 6N (HCl, Fisher, Waltham, MA), Slide-A-Lyzer Dialysis Cassettes, 3500 MWCO (ThermoScientific, Waltham, MA), deuterated water (D2O, ThermoScientific, Waltham, MA), d-Methionine (D-Met, Waltham, MA), 2-(N-morpholino)ethanesulfonic acid buffer (MES, ThermoScientific, Waltham, MA), 1-ethyl-3-(3- dimethylaminopropyl) carbodiimide (EDC, ThermoScientific, Waltham, MA), lithium citrate buffer (FUJIFILM Wako, Osaka, Japan), Agilent PL Aquagel-OH Mixed H larger molecular weight SEC column (Agilent Technologies, Santa Clara, CA), Agilent PL Aquagel-OH SEC column (Agilent Technologies, Santa Clara, CA).
The following cell lines, cell culture reagents and assay kits have been used for this study: House Ear Institute-Organ of Corti (HEI-OC1) and Stria Vascularis k1 (SV-k1) (Kalinec Lab, UCLA, Los Angeles, CA), three-dimensional (3D) in vitro tissue models, Epi-Airway day 7 (MatTek, Ashland, MA), fetal bovine serum (FBS, Corning, Corning, NY), Dulbecco’s Modified Eagle Medium (DMEM, Corning, Corning, NY), Cell-Titer 96 Aqueous One Solution Cell Proliferation Assay (Promega, Madison, WI), CyQUANT NF Cell Proliferation Assay Kit (Molecular Probes, Eugene, OR), phosphate buffered-saline (PBS, Corning, Corning, NY), hydrogen peroxide (H2O2, Millipore Sigma, St. Louis, MO), chloromethyl derivative of 2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA, Invitrogen, Waltham, MA), CellEvent Caspase-3/7 Green Detection Reagent (ThermoFisher, Waltham, MA), Trypsin-ethylenediaminetetraacetic acid (trypsin-EDTA, Corning, Corning, NY), NADP/NADPH Assay Kit (Abcam, Waltham, MA), 10 kDa column (Biovision, Milpitas, CA), fluorometric methionine assay kit (Biovision, CA), Ibidi
The following instruments have been used for this study: Varian VNMRS 500 MHz nuclear magnetic resonance spectrometer (Agilent Technologies, Palo Alto, CA), High Speed Amino Acid Analyzer L-8900 (Hitachi, Tokyo, Japan), FilterMax F5 multimode microplate reader (Molecular Devices, San Jose, CA), Leica Stellaris 5 Confocal Microscope (Leica, Germany), Agilent High Pressure Liquid Chromatography instrument with a UV-VIS detector (Agilent, Santa Clara, CA) additionally equipped with an OptiLab differential refractive index (RI) detector (Wyatt Technologies, Santa Barbara, CA), and a miniDawn 3-angle/multi-angle light scattering (MALS) detector (Wyatt Technologies, Santa Barbara, CA).
HA was derivatized with carboxymethyl functionalities to increase the conjugation sites available for the covalent attachment of molecules containing primary amines. Briefly, 2 g of HA, MW 850 kDa were added to 20 ml of 45% w/v NaOH and allowed to activate at room temperature (RT) for 2 h. In parallel, 2 g of iodoacetic acid were dissolved in 50 ml of isopropanol. The viscous HA solution was added to 150 ml of isopropanol, then the iodoacetic acid solution was added to the activated HA/isopropanol solution. The reaction mix was allowed to react for 2 h and was then filtered using a Buchner funnel with a #2 Whatman filter paper. The white filter cake obtained after filtration was dissolved in 200 ml of deionized water (diH2O) and the pH of the resulting solution was neutralized using 6N HCl. The resulting CMHA solution was then loaded into 3500 MWCO Slide-A-Lyzer Dialysis Cassettes and dialyzed for 72 h with a minimum of five water changes per 24 h to remove residual reagent and salts. After dialysis the CMHA solution was removed from the cassettes, frozen by placement in a −80°C freezer for a minimum of 4 h and subsequently lyophilized. The reaction yielded 1.580 g of CMHA. The carboxymethylation was confirmed by proton nuclear magnetic resonance (1H-NMR).
D-Met was chemically conjugated to CMHA using carbodiimide chemistry. Briefly, 1 g of CMHA was dissolved into 200 ml of MES buffer in a 450 ml beaker covered with parafilm. The solution was stirred for approximately 25 min until CMHA was fully dissolved. Next, 4.4 g of D-Met were added to the solution and allowed to dissolve (∼5 min). For the covalent conjugation of the antioxidant to CMHA, 2 g of EDC, a zero-length crosslinker, was added to the solution and allowed to react at RT for 20 h. Subsequently, the reaction was neutralized to a pH of 7.0 with an NaOH solution. The reaction mix was then transferred to 3500 MWCO Slide-A-Lyzer Dialysis Cassettes and dialyzed for 72 h with a minimum of five water changes per 24 h to remove residual D-Met. After dialysis the HAM solution was removed from the cassettes, frozen by placement in a −80 °C freezer for a minimum of 4 h and subsequently lyophilized. The reaction yielded 1.214 g of HAM. The conjugation was confirmed by 1H-NMR.
To confirm the structures and purity of CMHA and HAM, 1H-NMR spectral data were obtained using a Varian VNMRS 500 MHz at 20°C. For the analyses, samples were dissolved in deuterium oxide at a concentration of 5 mg/ml and all spectra were referenced to the residual solvent peak (H2O) at
HEI-OC1 and SV-k1 were seeded in a 96-well plate with a seeding density of 1.5 × 105 cells/mL in
HEI-OC1 and SV-k1 cells were used to assess the proliferation of cells treated with the HAM conjugate and controls. Cells were seeded in a 96-well plate with a seeding density of 1.5 × 105 cells/mL in
HEI-OC1 and SV-k1 cells, respectively, were seeded in a 96-well plate at a seeding density of 2 × 105 cells/mL in DMEM media with 10% FBS and incubated at 33°C/10% CO2. The cells were allowed to adhere 24 h, then the media was aspirated and replaced with sterile filtered media containing HAM conjugates and controls, followed by incubation for 24 h. The media was then aspirated and replaced with
HEI-OC1 and SV-k1, respectively, were seeded in a 96-well plate with a seeding density of 2 × 105 cells/mL in DMEM media with 10% FBS and incubated at 33°C/10% CO2. The cells were allowed to adhere 24 h, then the media was aspirated and replaced with sterile filtered media containing HAM conjugate and controls and then incubated for 24 h. The media was then aspirated and replaced with either PBS for control or 0.5 mM H2O2 in PBS and incubated for 30 min. Media was then aspirated, and wells were washed 2 times with PBS. DMEM with 10% FBS was added to each well and the plate was incubated for 48 h at 33°C/10% CO2. The amount of apoptosis was assessed with
HEI-OC1 and SV-k1, respectively, were seeded in a 75 cm2 flask with a seeding density of 1 × 106 cells/mL in DMEM media with 10% FBS and incubated at 33°C/10% CO2 for 48 h. Media was then aspirated and replaced with sterile filtered media containing HAM conjugate and controls and incubated for 24 h. Treatment solutions were aspirated, and flasks were washed with 1x PBS two times. Cells were then stressed with fresh 0.5 mM H2O2 or PBS only for controls for 30 min. Stressing solution was then aspirated and flasks were washed two times with 1x PBS. Cells were detached using 0.25% trypsin-EDTA. Cells and and trypsin solution was centrifuged at 300 RCF for 5 min and cells were counted using Countess Cell Counter. Cell numbers for each group were normalized to 4 × 106 cells. Cells were washed two times with 1x PBS to remove any remaining trypsin then processed according to the NADP/NADPH Assay Kit manufacture’s protocol for cellular lysates. Sample quantification was performed via absorbance detection at 450 nm with a microplate reader.
A fluorometric methionine assay kit was used for this. HEI-OC1 and SV-k1, respectively, were seeded in a 12 well plate with 0.5 ml of cell solution seeded at 1 × 106 cells/mL in DMEM media with 10% FBS and incubated at 33°C/10% CO2 for 48 h. Media was then aspirated and replaced with sterile filtered media containing HAM conjugate and controls and incubated for 24 h. Cells were detached with 0.25% trypsin-EDTA and pelleted via centrifugation at 300 RCF. The cell pellets were resuspended in 1x PBS two times to remove any traces of trypsin. Cells were then counted with Countess Cell Counter, and each treatment group was normalized to 2 × 106 cells. Subsequently,
HEI-OC1 and SV-k1, respectively, were plated at 5 × 104 cells/mL with
An Epi-Airway Day 7 3D in vitro tissue model was used as mimetic of the round window membrane (Rask-Andersen et al., 2015; Liebesny et al., 2018). HAM conjugate solution at 50 mg/ml and individual controls diluted in 1x PBS, respectively, were added to the top of Epi-Airway Day 7 3D tissues in transwell plates, with
The filtered samples were immediately frozen until ready to use. For analyses,
All experiments were run in triplicates or greater and all data were presented as mean ± standard deviation of the mean. Student t-tests were performed for 2-group comparisons while one-way ANOVA with multiple comparisons were performed for more than 2-group comparisons with confidence limits of 95% considered significant.
For the conjugate syntheses, D-Met was selected as the antioxidant of choice based on its reported cochlea-specific antioxidant protection and chemical structure. Considering HA’s chemical structure (Figure 1A), our strategy for chemical conjugation was to use carbodiimide chemistry to create a covalent bond between the carboxyl functionalities existing on the HA backbone and the primary amine groups of D-Met. This synthetic scheme is well characterized, uses a zero-length crosslinker that directly couples the two compounds of interest without adding any additional bulk, and requires mild reaction conditions. The HA backbone, that consists of repeating, identical disaccharide units, intrinsically contains one carboxyl functionality per repeat (Figure 1A). To increase the amount of D-Met that could be covalently attached to the HA backbone, in a first step the HA backbone was enriched in carboxyl functionalities. This was achieved via the addition of carboxymethyl groups to the primary hydroxyl functionalities of the disaccharide units to yield CMHA. The successful structural change was confirmed by 1H-NMR (Supplementary Figure S1), and based on the spectra, the extent of the functionalization was assessed to be approximately 58% (Supplementary Figure S2). The CMHA was then used as a substrate for D-Met attachment according to the reaction scheme illustrated in Figure 1B. Three different HA to D-Met stoichiometries were used (2X, 10X, 20X molar excess of D-Met to HA disaccharide unit), and the amount of conjugated D-Met was quantified by amino acid analysis.
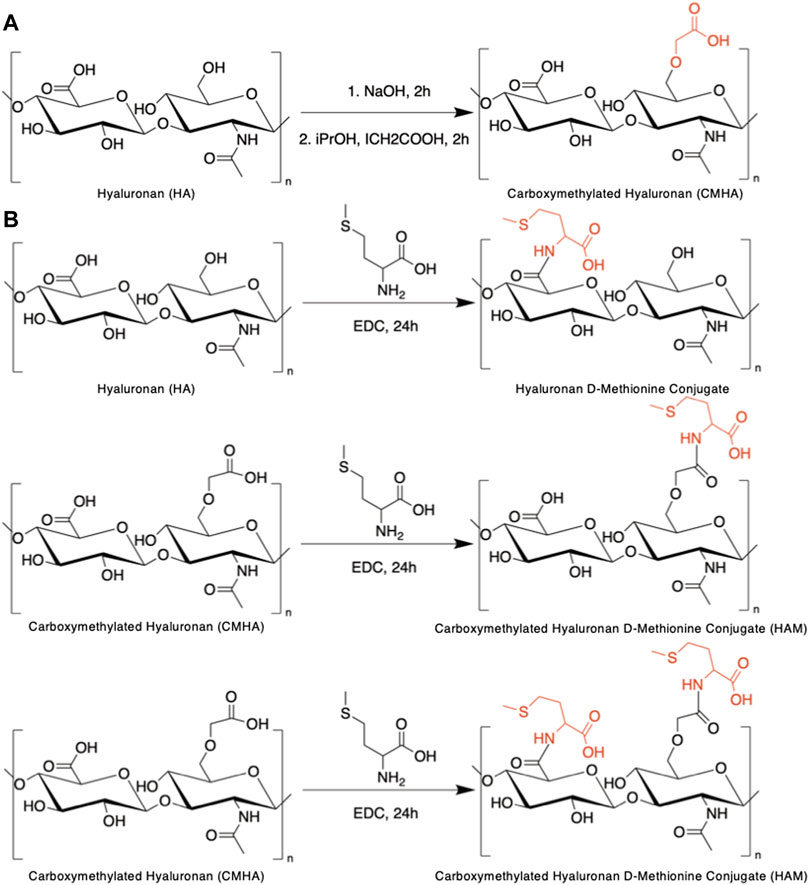
FIGURE 1. Syntheses of HAM conjugate. (A) Reaction scheme for the chemical functionalization of hyaluronic acid (HA) to yield carboxymethylated hyaluronic acid (CMHA). (B) Conjugation of d-Methionine to CMHA to yield the hyaluronan-d-Methionine conjugate (HAM).
Our data (Supplementary Table S1) indicated that the amount of conjugated D-Met was higher for the 10X reaction stoichiometry compared to the 2X and was comparable with the 20X reaction mix (within the detection method variability range). We therefore conducted all our subsequent experimental data with the 10X reaction product, which was determined to contain ∼427 nmol/mg of D-Met. As controls, 10X HAM equivalent amounts of CMHA and D-Met were used either alone or as blend (Supplementary Table S2).
To evaluate the potential applicability of HAM as a prophylactic or therapeutic against cochlear oxidative cell damage, typically associated with hearing loss, HEI-OC1 and SV-k1 cochlear cell lines were treated with HAM, CMHA, D-Met and CMHA + D-Met blend, respectively, and assayed for cell viability. Two different assays were used for this. The first one was a colorimetric methyl tetrazolium salt (MTS)-based assay that quantifies cell viability as a function of the cellular enzymatic reduction of the MTS which results in the development of a red color in the assay solution. The intensity of the color typically directly correlates with the number of viable cells. The second assay used was a more sensitive quantification method that relies on the binding of a cell-permeant fluorescent dye to the nucleic acid of live cells. The colorimetric assay results (Figure 2A) appear to indicate increased cell numbers/proliferation in cells treated with HAM, compared to those treated with CMHA, D-Met and CMHA + D-Met, or control, media-treated ones. Specifically, compared to the control group, the number of HAM treated cells appeared significantly higher, while CMHA and CMHA + D-Met, treated cells exhibited slightly lower viability. Only D-Met treatment did not seem to affect cell viability/number compared to the control. The colorimetric assay, however, is based on the enzymatical reduction of the assay reagent, particularly, it reflects the activity of mitochondrial enzymes. A validation of the MTS assay-observed trends was expected through the CyQuant assay. Interestingly, the fluorescent CyQuant assay results appear to indicate no statistically significant differences in cell numbers/viability for any of the treatment groups compared to the control, media-treated ones (Figure 2B). Cumulatively, these results seem to indicate that HAM is cytocompatible, and when added to cells it impacts the activity of mitochondrial enzymes but does not induce cell proliferation in HEI-OC1 or SV-k1 cells.
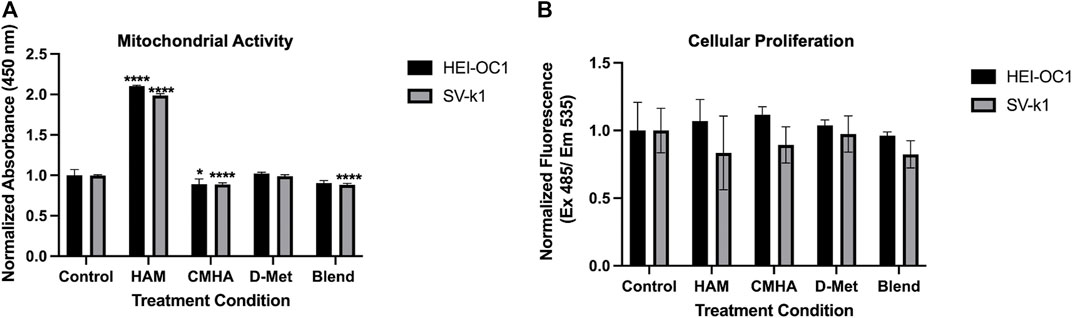
FIGURE 2. Cytocompatibility of the HAM conjugate. (A) MTS assay data indicating that when HAM is added to cells it impacts the activity of mitochondrial enzymes. Data presented are mean ± SD. One-way ANOVA with Tukey’s multiple comparisons run for each cell type individually. HEI-OC1 ANOVA results compared to the control: HAM p < 0.0001 for significant increase, CMHA p = 0.0305, D-Met p = 0.9607, and blend p = 0.0623. SV-k1 ANOVA results compared to the control: HAM p < 0.0001 for significant increase, CMHA p < 0.0001 for significant decrease, D-Met p = 0.9586, and blend p < 0.0001 for significant decrease. ****p < 0.0001, *p < 0.05. (B) CyQuant Cellular Proliferation assay data indicating that HAM addition to the cells does not induce cell proliferation. Data presented are mean ± SD. One-way ANOVA with Tukey’s multiple comparisons run for each cell type individually. HEI-OC1 ANOVA results compared to the control: HAM p = 0.9288, CMHA p = 0.6659, D-Met p = 0.9919 and blend p = 0.9915. SV-k1 ANOVA results compared to the control: HAM p = 0.6557, CMHA p = 0.8984, D-Met p = 0.9995, and blend p = 0.6025.
With an understanding of HAM cytocompatibility and cellular compartmentalization, we next sought to assess its potential prophylactic and/or therapeutic effect on oxidatively stressed cochlear cells. As oxidative stress is one of the major players in sensorineural hearing loss, these experiments are expected to serve as initial indicators of the potential prophylactic and/or therapeutic value of HAM against hearing loss. Both HEI-OC1 and SV-k1 cells were pre-treated with HAM, D-Met, or just tissue culture medium (control) and the amount of reactive oxygen species (ROS) present in each treatment group was quantified. As anticipated, stressing of the cells with H2O2 resulted in a significant increase in ROS in both cell types, with significantly higher levels detected in SV-k1 cells (Figure 3). The pre-treatment of cells with HAM yielded cellular ROS levels comparable to the unstressed controls for both HEI-OC1 and SV-k1. All the other pre-treatment conditions (D-Met or CMHA + D-Met blend) significantly reduced the amount of ROS produced by the cells, but to a significantly lesser extent than the HAM pre-treatment, and the cell line-specific differences observed in the stressed cells remained present, with SV-k1 cells displaying higher ROS levels than their HEI-OC1 counterparts. Post-stress treatment of cells with HAM, D-Met or CMHA + D-Met, did not result in any significant protective effects indicating that HAM could be potentially used as a prophylactic but not as a therapeutic (Supplementary Figure S3A). Considering that ROS are produced in the mitochondria and, as our MTS assay data indicated an increase in mitochondrial enzymatic activity in HAM-treated cells, we also sought to confirm that the observed mitochondrial enzymatic upregulation would not result in ROS production. In agreement with our assumption, our data indicated no change in ROS levels in unstressed cells, when pre-treated with HAM, D-Met or CMHA + D-Met (Supplementary Figure S3B).
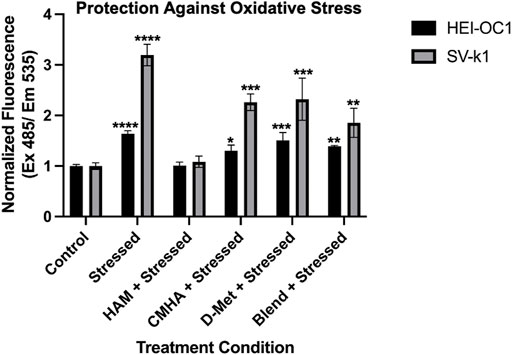
FIGURE 3. Oxidative stress protective effects of the conjugate in cochlear cell lines. Data presented are mean ± SD. One-way ANOVA with Tukey’s multiple comparisons run for each cell type individually. HEI-OC1 ANOVA results compared to unstressed untreated control: stressed p < 0.0001, HAM p > 0.9999, CMHA p = 0.0118, D-Met p = 0.0001, and blend p = 0.0016. ****p < 0.0001, ***p = 0.0001, **p = 0.01, and *p = 0.05. SV-k1 ANOVA compared to control: Stressed p < 0.0001, HAM p = 0.9974, CMHA p = 0.0004, D-Met p = 0.0002, and blend p = 0.0091. ****p < 0.0001, ***p = 0.0001, **p = 0.01, and *p = 0.05.
As oxidative stress typically leads to cell death via apoptosis, we next evaluated the effect of HAM treatment on oxidatively-induced apoptosis in HEI-OC1 and SV-k1 cells via caspase 3/7 detection. In agreement with our ROS results, the data indicated cell type dependent levels of caspase 3 and 7 (Figure 4). Interestingly, even though SV-k1 cells expressed higher levels of ROS in response to oxidative stress, the apoptosis marker levels were lower than those in HEI-OC1. All treatment conditions appeared to reduce the amount of apoptosis markers in HEI-OC1 cells, but in SV-k1 cells, CMHA, D-Met or CMHA + D-Met blend treatments appeared to slightly increase the apoptotic marker levels. It is important to note here that overall SV-k1 cells did not appear to be particularly susceptible to oxidative stress-induced apoptosis.
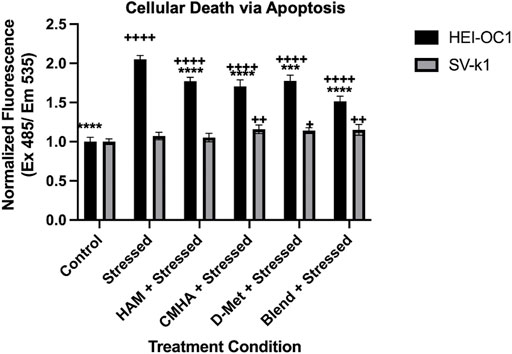
FIGURE 4. HAM effects on cellular death/apoptosis in response to oxidative stress. Data presented are mean ± SD. One-way ANOVA with Tukey’s multiple comparisons run for each cell type individually. HEI-OC1 ANOVA results compared to stressed positive control: control p < 0.0001, HAM p < 0.0001, CMHA p < 0.0001, D-Met p = 0.0001, and blend p < 0.0001. ****p < 0.0001 and ***p < 0.001. HEI-OC1 ANOVA results compared to control cells: stressed p < 0.0001, HAM p < 0.0001, CMHA p < 0.0001, D-Met p < 0.0001, and blend p < 0.0001. Indicated with ++++ p< 0.0001. SV-k1 ANOVA compared to stressed positive control: control p = 0.3932, HAM p = 0.9952, CMHA p0.2071, D-Met p = 0.4274, and blend p = 0.2887. ****p < 0.0001. SV-k1 ANOVA results compared to control cells: stressed p = 0.3932, HAM p = 0.6887, CMHA = 0.0042, D-Met p = 0.0118, and blend p = 0.0065. Indicated with ++ p< 0.01 and ++ p< 0.05.
Methionine has been extensively demonstrated to have antioxidant properties by directly targeting ROS and undergoing oxidation to methionine sulfoxide and methionine sulfone respectively (Figure 5A). Therefore, we sought to further investigate the insignificant D-Met protective effects observed in our ROS and apoptosis assays in both HEI-OC1 and SV-k1 cells. To assess if the enantiomeric form or oxidative status of the methionine were important, cells were treated with D-Met, L-Met, L-Met sulfoxide, L-Met sulfone and D, L-Met sulfone (Figure 5B). The results revealed several different findings. First, in agreement with the ROS data, there was no substantial reduction in the ROS levels as a result of D-Met treatment. L-Met treatment had the same effect as D-Met treatment, indicating no enantiomeric specificity in the cellular responses. Interestingly, pre-treatment of cells with methionine sulfoxide or sulfone, appeared to increase the amount of ROS detected in the cells. We hypothesize that this might be caused by the oxidative interaction of the assay reagent with the sulfoxide and sulfone. These data indicate that treatment with unconjugated D-Met or L-Met is ineffective against ROS and that methionine’s oxidative state does not have a positive impact on cellular ROS. Cumulatively, these findings appear to indicate that D-Met might not be internalized enough to trigger protective cellular effects.
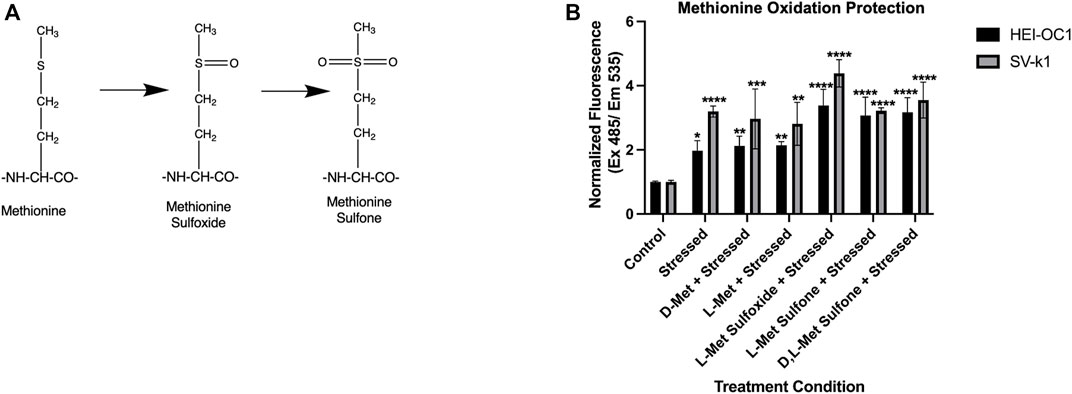
FIGURE 5. Enantiomeric and oxidative state effects of methionine. (A) Potential oxidative states of methionine. (B) Evaluation of the effects of enantiomeric and oxidative state effects of methionine on oxidatively stressed cochlear cells. Data presented are mean ± SD. One-way ANOVA with Tukey’s multiple comparisons run for each cell type individually. HEI-OC1 ANOVA versus unstressed control: stressed p = 0.0209, D-Met p = 0.0061, L-Met p = 0.0051, L- Met Sulfoxide p < 0.0001, L-Met Sulfone p < 0.0001, and D, L-Met Sulfone p = 0.0001. *p < 0.05, **p < 0.01, ****p < 0.0001. SV-k1 ANOVA versus unstressed control: stressed p < 0.0001, D-Met p = 0.0004, L-Met p = 0.0011, L-Met Sulfoxide p < 0.0001, L-Met Sulfone p < 0.0001, and D, L-Met Sulfone p < 0.0001.
The above-mentioned results appeared to indicate that the covalent conjugation of D-Met to CMHA was crucial for protective cellular effects, as CMHA or D-Met treatments alone or as a blend (CMHA + D-Met) did not induce similar changes in the two cochlear cell lines. HA is known to be able to internalize intracellularly via receptor-mediated endocytosis in various tissues. With that consideration, our assumption was that conjugation of D-Met to HA might be able to more effectively internalize the antioxidant to induce the observed changes in mitochondrial enzymatic activity and trigger cell protective effects. To test this, first, the potential internalization of unconjugated HA in the two cochlear cell lines was interrogated, via the use of fluorescently labeled-HA (HA-BODIPY) and microscopical visualization of the compound’s cellular distribution. Our data indicate that in both cell types, HA was able to relocate inside the cells and appeared to co-localize with lysosomes (stained with LysoTracker) and mitochondria (stained with MitoTracker), although its exact organelle-specific location could not be pinpointed at this resolution (Figure 6A). In the context of these observations, however, a mitochondrial localization would be in line with our observed MTS assay results.
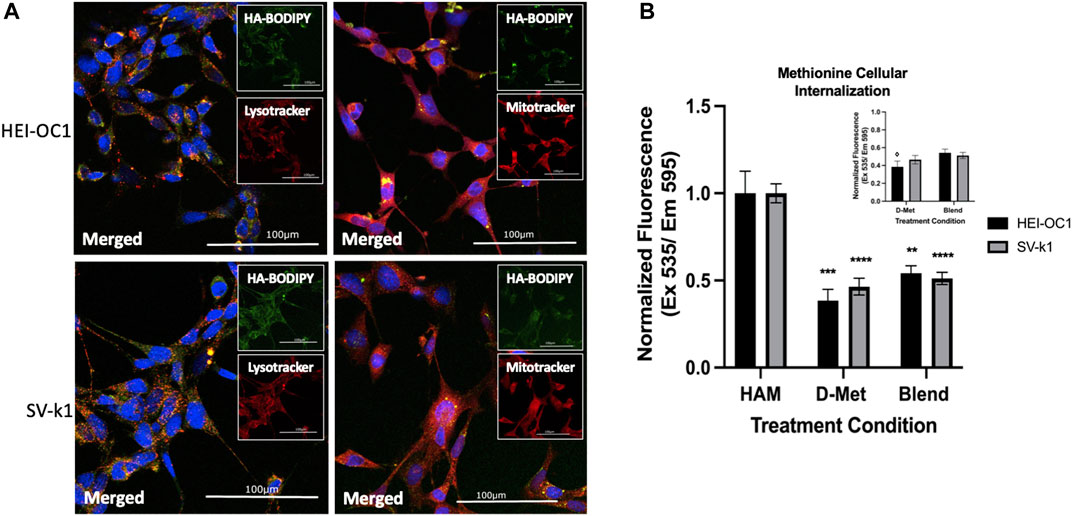
FIGURE 6. Cellular internalization of HAM. (A) Intracellular localization of internalized HA in HEI-OC1 and SV-k1 cells, top and bottom respectively. All scale bars
Second, we sought to assess the amount of D-Met internalized when cells were treated with conjugated versus unconjugated antioxidant. For this HEI-OC1 and SV-k1 cells were treated with HAM, D-Met or CMHA + D-Met and the content of intracellular methionine was quantified. The obtained data indicate that treatment of cells with D-Met alone or as blend with CMHA resulted in significantly less intracellular D-Met than in the cell lysates treated with HAM (Figure 6B). Interestingly, the data also seem to indicate that in the HEI-OC1 cells the blend of D-Met and CMHA did increase the intracellular concentration of D-Met compared to the D-Met alone treatment (Figure 6B inset). Nevertheless, these changes were insignificant compared to the effects of HAM treatment on the amount of intracellular D-Met. Overall, these results further highlight the impact of the chemical conjugation between D-Met to CMHA in the context of the development of a novel compound targeting hearing loss.
With the D-Met cellular internalization confirmed, the next step was to interrogate the potential mechanism of protection employed by the internalized antioxidant. One such mechanism could involve the modulation of the NADP/NADPH ratio in the pentose phosphate pathway (PPP). To investigate this potential mechanism, HEI-OC1 and SV-k1 cells were treated with HAM, D-Met or CMHA + D-Met and the NADP/NADPH ratio was quantified. Both cell lines showed no significant difference in the NADP/NADPH ratio in any of the treatment groups or control groups (Figure 7). These results suggest that mechanisms other than upregulation of the NADP/NADPH ratio in the PPP pathway are responsible for the observed protective effects; however, further mechanistic investigations were out of the scope of this study.
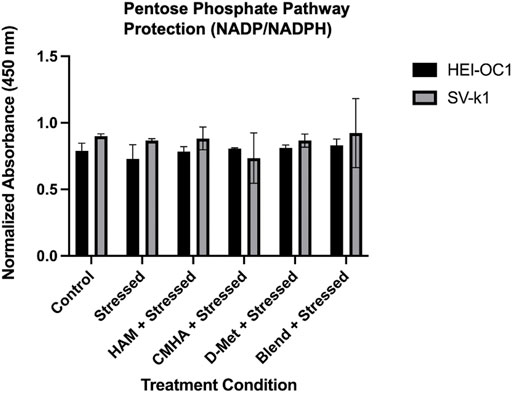
FIGURE 7. Investigation of the potential mechanism of HAM protection. Data presented are mean ± SD. One-way ANOVA with Tukey’s multiple comparisons run for each cell type individually. HEI-OC1 ANOVA versus unstressed control: stressed p = 0.7694, HAM p > 0.9999, CMHA p = 0.9989, D-Met p = 0.9964, and blend p = 0.9391. SV-k1 ANOVA versus unstressed control: stressed p = 0.9996, HAM p > 0.9999, CMHA p = 0.6927, D-Met p = 0.9997, and blend p > 0.9999.
The original intent of this study was to develop a topically applicable prophylactic or therapeutic against hearing loss. Therefore, the next step in HAM’s characterization process was to assess its ability to permeate through biological membranes typical for ear structures. Specifically, a therapeutic targeting the inner ear would be expected to permeate at a minimum the round window membrane (RWM). To test this, we used a three-dimensional tissue model of RWM, consisting of underdeveloped airway cells.
Therefore, HAM and controls were then placed on top of the tissues (donor side) and the amount that traveled through the tissue (receiver side) was quantified (Figure 8A). The receiver side solutions were then analyzed with two SEC columns: one for higher molecular weights (6 kDa–10 MDa) capable of detecting HAM, and CMHA and one for lower molecular weights (100 Da–20 kDa) capable of detecting free D-Met (Figure 8B). Our data indicate that HAM and CMHA were both able to permeate the RWM model, at 2.93% and 0.77%, respectively, of the donor side-applied amount (Figure 8C). Interestingly, the CMHA amount that permeated the model when applied to the donor side as blend, was slightly higher, at 0.90%, than when applied individually. For D-Met detection a standard curve based on antioxidant-specific absorbance A235 values were created to ensure proper chromatographic detection and quantification of the antioxidant (Supplementary Figure S4). However, all treatment conditions resulted in no detectable levels of D-Met in the receiver solution. These results could indicate that the amount of antioxidant able to penetrate the RWM model was either insignificant or below the detection threshold of our instrument (0.01 mg/ml) in the D-Met only or CMHA + D-Met treated samples, and that D-Met was most likely still covalently attached to the CMHA backbone in the HAM treated sample (Supplementary Figure S5). To further assess the latter, we used absorbance A235 detection on the large molecular weight range SEC column and quantified the area under the curve (AUC) for HAM and CMHA, before and after permeation through the tissue models. The analyses indicated a significantly higher AUC of 958.419 for HAM compared to the CMHA only treated group, that had an AUC of 12.945. These values were similar to those obtained for control HAM and CMHA that have not been permeated through the tissue models (Supplementary Table S3). Overall, our data indicate that a significantly higher amount of HAM permeated the tissue compared to CMHA only or CMHA + D-Met blend, and it appears that D-Met remains covalently attached to the CMHA backbone throughout the RWM model permeation.
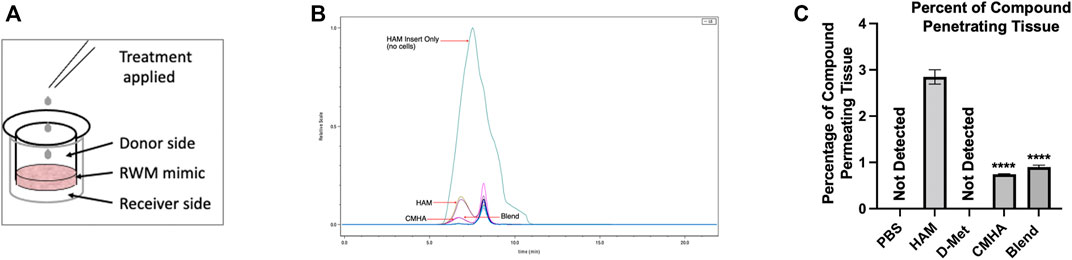
FIGURE 8. Investigation of the transmembrane permeation ability of HAM. (A) Illustration of the permeation experiment setup. (B) Chromatogram of SEC analyzed samples detected with an RI detector. The teal (largest) curve corresponds to HAM passed through a blank/no tissue insert. The mustard green and purple traces correspond to HAM passed through inserts with Epi-Airway tissues as round window membrane (RWM) models. The magenta trace corresponds to the blend treatment passed through an insert with Epi-Airway, and the dark purple trace corresponds to CMHA passed through an insert with Epi-Airway. (C) Quantification of the amount of HAM or controls detected after permeation of Epi-Airway tissues. Data presented are mean ± SD. One-way ANOVA with Tukey’s multiple comparisons run. ANOVA results compared to HAM group: CMHA p < 0.0001 and blend p < 0.0001.
The goal of this study was to assess the feasibility of developing a topically applicable prophylactic or therapeutic against hearing loss. As oxidative stress is one of the major factors associated with hearing loss, regardless of its pathogenesis (Kamogashira et al., 2015; Kurabi et al., 2017), our approach was to use a well characterized antioxidant (D-Met) (Tavanai and Mohammadkhani, 2017; Pecha et al., 2020) and chemically attach it to a “carrier” to facilitate the permeation of ear specific biological barriers such as the round window membrane and delivery to the sensory cells of the cochlea. As a carrier we selected HA, as previous literature reports indicate that drug blends with HA had increased permeation rates across the tympanic and/or round window membrane (Shibata et al., 2012; Queisser et al., 2021). Additionally, the polymeric structure of HA and the presence of readily modifiable chemical functionalities translate this macromolecule into an excellent building block for more complex structures.
From a practical, clinically applicable product perspective, our rationale was to develop a liquid formulation that could be topically deployed into the middle ear of patients (i.e., via intratympanic injections), from where it could permeate across the RWM to access the cochlear structures susceptible to oxidative damage (Figure 9). As a first step in our approach, the HA backbone was enriched in carboxyl functionalities to obtain CMHA, which contained additional functionalization sites (58% increase in -COOH groups) (Serban and Kaplan, 2010; Love et al., 2019). Next, we chose a well characterized carbodiimide mediated crosslinking strategy to covalently connect -COOH (from the CMHA backbone) and -NH2 groups (from the antioxidant) (Serban and Prestwich, 2008; Serban and Skardal, 2019) and showed that we can tailor the amount of covalently conjugated D-Met simply by changing the reaction stoichiometry. As the 10X and 20X stoichiometries generated comparable degrees of CMHA functionalization, the 10X reaction product was further characterized as it was expected to reflect the same biological effects as the 20X version but with lower cost of production. For cellular effects evaluations of two different mouse cochlear cell lines were used: HEI-OC1, derived from the organ of Corti, and SV-k1, derived from the stria vacularis, as both cell types were shown to be responsible for normal hearing (Liu et al., 2016). HEI-OC1 cells are organ of Corti-derived and have been used to investigate cellular events such as drug-activated apoptotic pathways, senescence, mechanism of cell protection, inflammatory responses, effects of hypoxia, oxidative and endoplasmic reticulum stress, and expression of molecular channels and receptors (Kalinec et al., 2003; Kalinec et al., 2016). SV-k1 cells are derived from the Immortomouse™ stria vascularis and have been used as models for strial capillary basement membrane studies (Gratton et al., 2002; Rivolta and Holley, 2002; Kalinec et al., 2016). Cytocompatibility evaluation assays revealed that HAM was well-tolerated by both types of cochlear cells; however, interestingly, in both cell lines HAM appeared to upregulate the activity of mitochondrial enzymes, although to a significantly higher extent in the SV-k1 cells. When evaluated for oxidative protection effects in response to hydrogen peroxide-induced oxidative stress, HAM pretreatment of the cells kept ROS levels similar to those in the control/unstressed cells. Interestingly, our data revealed that the two cell lines have different susceptibility to oxidative stress, with SV-k1 cells generating a significantly higher amount of ROS in response to oxidative stress, compared to HEI-OC1 cells. Additionally, treatment of cochlear cells with HAM after oxidative stress did not show a decrease in ROS levels, suggesting that HAM could be used as a prophylactic but not as therapeutic.
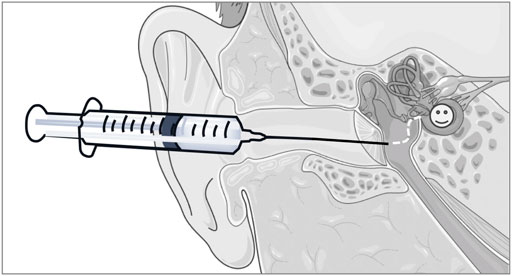
FIGURE 9. Illustration of the rationale for the development of a liquid formulation that could be topically deployed into the middle ear of patients, from where it could subsequently, permeate across the RWM to access the cochlear structures susceptible to oxidative damage. The schematic art pieces used in this figure were created by using Servier Medical Art (http://servier.com/Powerpoint-image-bank). Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License.
Oxidative stress is typically associated with apoptosis (Huang et al., 2000), therefore our expectation was that the two cochlear cell lines used would undergo apoptosis in response to hydrogen peroxide treatment. We chose to monitor this by the assessment of caspase 3/7 levels in response to oxidative stress. Caspase 3 and caspase 7 are considered universal apoptosis markers that are both activated regardless of the specific cell death inducing stimulus (Walsh et al., 2008). Surprisingly, the levels of apoptotic markers were similar to the unstressed controls in SV-k1 cells while in HEI-OC1 cells the apoptotic marker levels were significantly higher than in unstressed cells. However, HAM pre-treatment of the cells significantly reduced the amount of Caspase 3/7 in HEI-OC1 cells, although they were still much higher than in the unstressed controls. All the aforementioned biological effects were specific to HAM and were not observed with CMHA, D-Met and CMHA + D-Met blend pre-treated samples, highlighting the importance of the covalent attachment of D-Met to the CMHA backbone for protective effects initiation.
In most tissue types, HA is cellularly internalized via HA-specific receptor mediated processes (Misra et al., 2015). The main HA receptor responsible for internalization is Cluster of Differentiation 44 (CD44); however, currently there are very limited data available on the presence of CD44 or endogenous HA in cochlear structures (Vilstrup and Jensen, 1954; Friberg et al., 1989; Anniko and Arnold, 1995; Hertzano et al., 2010). A survey of the gEAR multi-omic database (Orvis et al., 2021) indicated that CD44 is expressed in murine cochlear structures at the mRNA level (Hertzano et al., 2010). Therefore, based on our previous findings that HAM pre-treatment increased cochlear cell mitochondrial enzymatic activity, and that chemical conjugation was needed for protective effects, we postulated that HAM was internalizing D-Met to trigger oxidative protection responses. We tested this hypothesis through two different approaches. First, we showed that, at the concentrations tested, D-Met by itself was not able to enter cochlear cells at relevant levels and this effect was independent of its enantiomeric specificity or oxidation status. Second, we showed that HAM did facilitate the internalization of D-Met and that this is most likely due to the natural propensity of (CM)HA to be internalized via HA-specific receptors. Our data (MTS assay and HA-BODIPY cellular tracking) indicate that HA and HAM are co-localizing with the mitochondria and potentially with mitochondrial-lysosomal complexes (Todkar et al., 2017). In other tissues, HA has been reported to localize in the lysosome (Xue et al., 2020); therefore, further investigation of the exact location of HAM in cellular structures would be warranted, especially given that the amount of information available on the role of HA in cochlear structures and hearing is extremely scarce. We were unable to pinpoint the exact mechanism of protection, although we excluded the involvement of the PPP pathway, or at least the involvement of the PPP-associated NADP/NADPH ratio (Kuehne et al., 2015). Collectively, our data revealed that HAM has promising prophylactic and protective properties if applied to cochlear cells prior to oxidative stress.
Circling back to our original study goal, the remaining step was to test the ability of HAM to permeate ear-specific biological membranes unaltered, specifically without “loosing” the attached antioxidant. A previous study (Liebesny et al., 2018) suggested that airway cells could be a viable model for the RWM. Therefore, we used three-dimensional underdeveloped (prior to development of cilia) airway tissues similar in thickness to the physiological RWM, to assess HAM’s ability to effectively carry across its antioxidant cargo. Our data suggest that not only is HAM able to efficiently permeate the RWM model at higher rates than CMHA or D-Met only, or CMHA + D-Met blend respectively, but D-Met remains covalently attached throughout the process. Cumulatively, our data seem to indicate that if HAM would be delivered into the middle ear space (i.e., via intratympanic injections), it would be able to then carry its antioxidant cargo effectively across the RWM as conjugated HAM, and protect cochlear cells from oxidative damage via D-Met internalization as HAM and subsequent intracellular D-Met release, as indicated by our HEI-OC1 and SV-k1 data.
In summary, we have successfully synthesized and characterized a novel HA derivative that shows promise as a prophylactic against oxidatively induced hearing loss, in addition to being able to carry the attached antioxidant cargo across ear-specific anatomical barriers. Further in vivo studies in animals with age, noise or drug-induced hearing loss will be needed to confirm our findings, and the overall role of HA in the cochlea would further need to be investigated; however, we believe that we have successfully shown that covalent conjugation of a therapeutic molecule to an HA carrier is a viable approach to subsequent prophylactics and therapeutics development against hearing loss.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
EA performed the experimental work, data analyses and drafted manuscript; MS designed the study, supervised the work and data analyses, and finalized manuscript. Both authors critically reviewed the content and approved the final version before submission.
Funding for this project was provided by a Center for Biomolecular Structure and Dynamics COBRE Junior Investigator grant to MS, NIH/NIGMS P20GM103546.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor AL is currently organizing a Research Topic with the author MS.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Drs. Keiji Numata and Takaaki Miyamoto, from the Riken Institute, Japan, for their support with the amino acid analyses. Figure 9 and Graphical abstract artwork was prepared by using Servier Medical Art (ServierMedical art—http://servier.com/Powerpoint-image-bank).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.907516/full#supplementary-material
Altman, R., Bedi, A., Manjoo, A., Niazi, F., Shaw, P., and Mease, P. (2019). Anti-Inflammatory Effects of Intra-articular Hyaluronic Acid: A Systematic Review. Cartilage 10 (1), 43–52. doi:10.1177/1947603517749919
Anniko, M., and Arnold, W. (1995). Hyaluronic Acid as a Molecular Filter and Friction-Reducing Lubricant in the Human Inner Ear. ORL J. Otorhinolaryngol. Relat. Spec. 57 (2), 82–86. doi:10.1159/000276716
Brown, C. S., Emmett, S. D., Robler, S. K., and Tucci, D. L. (2018). Global Hearing Loss Prevention. Otolaryngol. Clin. North Am. 51 (3), 575–592. doi:10.1016/j.otc.2018.01.006
Campbell, K. C., Rehemtulla, A., Sunkara, P., Hamstra, D., Buhnerkempe, M., and Ross, B. (2021). Oral D-Methionine Protects against Cisplatin-Induced Hearing Loss in Humans: Phase 2 Randomized Clinical Trial in India. Int. J. Audiol., 1–11. doi:10.1080/14992027.2021.1983215
Chaulagain, B., Jain, A., Tiwari, A., Verma, A., and Jain, S. K. (2018). Passive Delivery of Protein Drugs through Transdermal Route. Artif. Cells Nanomed. Biotechnol. 46 (Suppl. 1), 472–487. doi:10.1080/21691401.2018.1430695
Cheng, P. W., Liu, S. H., Young, Y. H., Hsu, C. J., and Lin-Shiau, S. Y. (2008). Protection from Noise-Induced Temporary Threshold Shift by D-Methionine Is Associated with Preservation of ATPase Activities. Ear Hear 29 (1), 65–75. doi:10.1097/AUD.0b013e31815d635b
Friberg, U., Erwall, C., Bagger-Sjöbäck, D., and Rask-Andersen, H. (1989). Hyaluronan Content in Human Inner Ear Fluids. Acta Otolaryngol. 108 (1–2), 62–67. doi:10.3109/00016488909107393
Fujimoto, C., and Yamasoba, T. (2014). Oxidative Stresses and Mitochondrial Dysfunction in Age-Related Hearing Loss. Oxid. Med. Cell. Longev. 2014, 582849. doi:10.1155/2014/582849
Gratton, M. A., Meehan, D. T., Smyth, B. J., and Cosgrove, D. (2002). Strial Marginal Cells Play a Role in Basement Membrane Homeostasis: In Vitro and In Vivo Evidence. Hear. Res. 163 (1), 27–36. doi:10.1016/S0378-5955(01)00358-6
Green, K. L., Swiderski, D. L., Prieskorn, D. M., DeRemer, S. J., Beyer, L. A., Miller, J. M., et al. (2016). ACEMg Diet Supplement Modifies Progression of Hereditary Deafness. Sci. Rep. 6 (1), 22690. doi:10.1038/srep22690
Gupta, R. C., Lall, R., Srivastava, A., and Sinha, A. (2019). Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci. 6, 192. doi:10.3389/fvets.2019.00192
Hertzano, R., Puligilla, C., Chan, S. L., Timothy, C., Depireux, D. A., Ahmed, Z., et al. (2010). CD44 Is a Marker for the Outer Pillar Cells in the Early Postnatal Mouse Inner Ear. J. Assoc. Res. Otolaryngol. 11 (3), 407–418. doi:10.1007/s10162-010-0211-x
Huang, T., Cheng, A. G., Stupak, H., Liu, W., Kim, A., Staecker, H., et al. (2000). Oxidative Stress-Induced Apoptosis of Cochlear Sensory Cells: Otoprotective Strategies. Int. J. Dev. Neurosci. 18 (2–3), 259–270. doi:10.1016/s0736-5748(99)00094-5
Ingersoll, M. A., Malloy, E. A., Caster, L. E., Holland, E. M., Xu, Z., Zallocchi, M., et al. (2020). BRAF Inhibition Protects against Hearing Loss in Mice. Sci. Adv. 6 (49), eabd0561. doi:10.1126/sciadv.abd0561
Kalinec, G. M., Webster, P., Lim, D. J., and Kalinec, F. (2003). A Cochlear Cell Line as an In Vitro System for Drug Ototoxicity Screening. Audiol. Neurootol. 8 (4), 177–189. doi:10.1159/000071059
Kalinec, G. M., Park, C., Thein, P., and Kalinec, F. (2016). Working with Auditory HEI-OC1 Cells. J. Vis. Exp. 115, 54425. doi:10.3791/54425
Kamogashira, T., Fujimoto, C., and Yamasoba, T. (2015). Reactive Oxygen Species, Apoptosis, and Mitochondrial Dysfunction in Hearing Loss. Biomed. Res. Int. 2015, 617207. doi:10.1155/2015/617207
Kuehne, A., Emmert, H., Soehle, J., Winnefeld, M., Fischer, F., Wenck, H., et al. (2015). Acute Activation of Oxidative Pentose Phosphate Pathway as First-Line Response to Oxidative Stress in Human Skin Cells. Mol. Cell 59 (3), 359–371. doi:10.1016/j.molcel.2015.06.017
Kurabi, A., Keithley, E. M., Housley, G. D., Ryan, A. F., and Wong, A. C. (2017). Cellular Mechanisms of Noise-Induced Hearing Loss. Hear. Res. 349, 129–137. doi:10.1016/j.heares.2016.11.013
Le Prell, C. G., Hughes, L. F., and Miller, J. M. (2007). Free Radical Scavengers Vitamins A, C, and E Plus Magnesium Reduce Noise Trauma. Free Radic. Biol. Med. 42 (9), 1454–1463. doi:10.1016/j.freeradbiomed.2007.02.008
Liebesny, P., Arsenault, B., Moon, C., Pregernig, G., So, K., Shi, F., et al. (2018). “A Surrogate In Vitro Model for Assessing Compound Permeability through the Round Window Membrane,” in Poster presented at: Midwinter Meeting of the Association for Research in Otolaryngology, San Diego, CA, 2018 Feb 10-14.
Lin, M. T., and Flint Beal, M. (2003). The Oxidative Damage Theory of Aging. Clin. Neurosci. Res. 2 (5), 305–315. doi:10.1016/S1566-2772(03)00007-0
Liu, H., Li, Y., Chen, L., Zhang, Q., Pan, N., Nichols, D. H., et al. (2016). Organ of Corti and Stria Vascularis: Is There an Interdependence for Survival? PLoS ONE 11 (12), e0168953. doi:10.1371/journal.pone.0168953
Love, C. J., Serban, B. A., Katashima, T., Numata, K., and Serban, M. A. (2019). Mechanistic Insights into Silk Fibroin’s Adhesive Properties via Chemical Functionalization of Serine Side Chains. ACS Biomater. Sci. Eng. 5 (11), 5960–5967. doi:10.1021/acsbiomaterials.9b01014
Misra, S., Hascall, V. C., Markwald, R. R., and Ghatak, S. (2015). Interactions between Hyaluronan and its Receptors (CD44, RHAMM) Regulate the Activities of Inflammation and Cancer. Front. Immunol. 6, 201. doi:10.3389/fimmu.2015.00201
Orvis, J., Gottfried, B., Kancherla, J., Adkins, R. S., Song, Y., Dror, A. A., et al. (2021). GEAR: Gene Expression Analysis Resource Portal for Community-Driven, Multi-Omic Data Exploration. Nat. Methods 18 (8), 843–844. doi:10.1038/s41592-021-01200-9
Pak, J. H., Kim, Y., Yi, J., and Chung, J. W. (2020). Antioxidant Therapy against Oxidative Damage of the Inner Ear: Protection and Preconditioning. Antioxidants (Basel) 9 (11), 1076. doi:10.3390/antiox9111076
Pecha, P. P., Almishaal, A. A., Mathur, P. D., Hillas, E., Johnson, T., Price, M. S., et al. (2020). Role of Free Radical Formation in Murine Cytomegalovirus-Induced Hearing Loss. Otolaryngol. Head. Neck Surg. 162 (5), 709–717. doi:10.1177/0194599820901485
Queisser, K. A., Mellema, R. A., and Petrey, A. C. (2021). Hyaluronan and its Receptors as Regulatory Molecules of the Endothelial Interface. J. Histochem. Cytochem. 69 (1), 25–34. doi:10.1369/0022155420954296
Rask-Andersen, H. E., Liu, W., Schrott-Fischer, A., and Glueckert, R. (2015). Anatomical Considerations for the Design and Applications of Cochlear Implants. J. Acoust. Soc. Am. 137 (4), 2297. doi:10.1121/1.4920386
Ravi, R., Somani, S. M., and Rybak, L. P. (1995). Mechanism of Cisplatin Ototoxicity: Antioxidant System. Pharmacol. Toxicol. 76 (6), 386–394. doi:10.1111/j.1600-0773.1995.tb00167.x
Rivolta, M. N., and Holley, M. C. (2002). Cell Lines in Inner Ear Research. J. Neurobiol. 53 (2), 306–318. doi:10.1002/neu.10111
Rogha, M., and Kalkoo, A. (2017). Therapeutic Effect of Intra-tympanic Dexamethasone–Hyaluronic Acid Combination in Sudden Sensorineural Hearing Loss. Iran. J. Otorhinolaryngol. 29 (94), 255–260.
Serban, M. A., and Kaplan, D. L. (2010). PH-Sensitive Ionomeric Particles Obtained via Chemical Conjugation of Silk with Poly(Amino Acid)s. Biomacromolecules 11 (12), 3406–3412. doi:10.1021/bm100925s
Serban, M. A., and Prestwich, G. D. (2008). Modular Extracellular Matrices: Solutions for the Puzzle. Methods 45 (1), 93–98. doi:10.1016/j.ymeth.2008.01.010
Serban, M. A., and Skardal, A. (2019). Hyaluronan Chemistries for Three-Dimensional Matrix Applications. Matrix Biol. 78-79, 337–345. doi:10.1016/j.matbio.2018.02.010
Sha, S. H., and Schacht, J. (1999). Formation of Reactive Oxygen Species Following Bioactivation of Gentamicin. Free Radic. Biol. Med. 26 (3), 341–347. doi:10.1016/S0891-5849(98)00207-X
Shibata, S. B., Cortez, S. R., Wiler, J. A., Swiderski, D. L., and Raphael, Y. (2012). Hyaluronic Acid Enhances Gene Delivery into the Cochlea. Hum. Gene Ther. 23 (3), 302–310. doi:10.1089/hum.2011.086
Tavanai, E., and Mohammadkhani, G. (2017). Role of Antioxidants in Prevention of Age-Related Hearing Loss: A Review of Literature. Eur. Arch. Otorhinolaryngol. 274 (4), 1821–1834. doi:10.1007/s00405-016-4378-6
Todkar, K., Ilamathi, H. S., and Germain, M. (2017). Mitochondria and Lysosomes: Discovering Bonds. Front. Cell Dev. Biol. 5, 106. doi:10.3389/fcell.2017.00106
Vilstrup, T., and Jensen, C. E. (1954). XIV Three Reports on the Chemical Composition of the Fluids of the Labyrinth. Ann. Otol. Rhinol. Laryngol. 63 (1), 151–156. doi:10.1177/000348945406300114
Walsh, J. G., Cullen, S. P., Sheridan, C., Lüthi, A. U., Gerner, C., and Martin, S. J. (2008). Executioner Caspase-3 and Caspase-7 Are Functionally Distinct Proteases. Proc. Natl. Acad. Sci. U. S. A. 105 (35), 12815–12819. doi:10.1073/pnas.0707715105
Wang, Y., Qu, Y., Chen, X., Zhang, P., Su, D., Wang, L., et al. (2019). Effects of D-Methionine in Mice with Noise-Induced Hearing Loss Mice. J. Int. Med. Res. 47 (8), 3874–3885. doi:10.1177/0300060519860679
Witting, M., Boreham, A., Brodwolf, R., Vávrová, K., Alexiev, U., Friess, W., et al. (2015). Interactions of Hyaluronic Acid with the Skin and Implications for the Dermal Delivery of Biomacromolecules. Mol. Pharm. 12 (5), 1391–1401. doi:10.1021/mp500676e
Xue, J., Chen, J., Shen, Q., Chan, D., Li, J., Tanguay, A. P., et al. (2020). Addition of High Molecular Weight Hyaluronic Acid to Fibroblast-Like Stromal Cells Modulates Endogenous Hyaluronic Acid Metabolism and Enhances Proteolytic Processing and Secretion of Versican. Cells 9 (7), 1681. doi:10.3390/cells9071681
Keywords: cochlear cells, prophylactic, oxidative stress, hyaluronan, antioxidant
Citation: Arrigali EM and Serban MA (2022) Development and Characterization of a Topically Deliverable Prophylactic Against Oxidative Damage in Cochlear Cells. Front. Pharmacol. 13:907516. doi: 10.3389/fphar.2022.907516
Received: 29 March 2022; Accepted: 23 May 2022;
Published: 09 June 2022.
Edited by:
Andrei N. Lukashkin, University of Brighton, United KingdomReviewed by:
Sarath Vijayakumar, Creighton University, United StatesCopyright © 2022 Arrigali and Serban. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Monica A. Serban, bW9uaWNhLnNlcmJhbkB1bW9udGFuYS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.