
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 28 April 2022
Sec. Ethnopharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.893602
 Jingjing Wei1,2†
Jingjing Wei1,2† Bin Li1†
Bin Li1† Xinlu Wang1†
Xinlu Wang1† Xingyuan Li2
Xingyuan Li2 Yucai Hu2
Yucai Hu2 Lijie Qiao2
Lijie Qiao2 Cheng Zhou2
Cheng Zhou2 Peng Yu2
Peng Yu2 Tianqing Sang2
Tianqing Sang2 Mingjun Zhu1*
Mingjun Zhu1* Yongxia Wang1*
Yongxia Wang1*Objective: Qili Qiangxin Capsule (QQC), a Chinese patent medicine, is clinically effective in treating dilated cardiomyopathy (DCM). However, the meta-analysis of QCC combined with conventional western medicine (CWM) on DCM remains unexplored. This study aimed to systematically evaluate the efficacy and safety of QCC in the treatment of DCM.
Methods: Searched the studies of the combination of QQC and CWM in the treatment of DCM, from databases like PubMed, Cochrane Library, Web of Science, Wan Fang Databases, Chinese Biomedical Literature Database, China Science and Technology Journal Database, China National Knowledge Infrastructure, prior to 15 January 2022. Two reviewers respectively regulated research selection, data extraction, and risk of bias assessment. Review Manager Software 5.4 was used for meta-analysis. Furthermore, GRADE pro3.6.1 software was selected to grade the current evidence in our findings. This meta-analysis has been registered in PROSPERO (CRD42022297906).
Results: There were 35 studies pertaining to 3,334 patients included. The meta-analysis showed compared with CWM alone, the combination therapy had significant advantages in improving the clinical efficiency rate (RR = 1.24, 95% CI: 1.19 to 1.29, p < 0.00001), 6 min walking distance (6MWD) (MD = 41.93, 95%CI: 39.82 to 44.04, p < 0.00001), superior in ameliorating the left ventricular ejection fraction (LVEF) (MD = 5.73, 95%CI: 4.70 to 6.77, p < 0.00001), left ventricular end-diastolic dimension (LVEDD) (MD = −4.09, 95%CI: −4.91 to −3.27), p < 0.00001), left ventricular end-systolic diameter (LVESD) (MD = −4.73, 95%CI: −5.63 to −3.84), p < 0.00001) and BNP (MD = −101.09, 95%CI: -132.99 to −69.18), p < 0.00001), and also superior in reducing hypersensitive-C-Reactive Protein (hs-CRP) (MD = −3.78, 95%CI: −4.35 to −3.21), p < 0.00001), Interleukin- 6 (IL-6) (MD = −25.92, 95%CI: −31.35 to -20.50), p < 0.00001), tumor necrosis factor-α (TNF-α) (MD = -5.04, 95%CI: −6.13 to −3.95), p < 0.00001), high mobility group protein B1 (HMGB1) (MD = −4.34, 95%CI: −5.22 to −3.46), p < 0.00001), and adverse reactions (ARs) (RR = 0.70, 95%CI: 0.51–0.97), p = 0.03). The GRADE evidence quality rating presented with moderate or low quality of evidence for the available data.
Conclusion: Compared with the control group, QQC combined with CWM may be effective in treating DCM. However, the conclusion of this study must be interpreted carefully due to the inferior quality and ambiguity of bias in the included trials.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero, identifier [CRD42022297906].
Dilated cardiomyopathy (DCM) is a heterogeneous cardiomyopathy characterized by enlarged ventricles and reduced myocardial systolic function (Reichart et al., 2019). DCM usually results from myocarditis, genetic and environmental insults, metabolic or endocrine disturbances, and neuromuscular disease (Japp et al., 2016). Notably, DCM is a major cause of heart failure, leading to sudden cardiac death in severe cases, and is the most common indication for heart transplantation worldwide. Of concern, DCM predominantly affects younger adults, with an incidence of 5–7 cases per 100,000 people per year (Weintraub et al., 2017). A report from China in 2014 showed that 767 cases of DCM had a mortality rate of 42.24% at 52 months of follow-up (Liu et al., 2014). More importantly, DCM not only significantly reduces the quality of life but also results in high admission and readmission accompanied by a heavy burden on society and families.
Current treatments for dilated cardiomyopathy, such as beta-blockers, angiotensin-converting enzyme inhibitors (ACE-I)/angiotensin II receptor antagonists (ARB), digoxin, spirolactone, and mechanotherapy, are aimed at reducing the rate of damage to the myocardium and not increasing its regenerating potential (Reichart et al., 2019). Consequently, it is highly required to seek out new therapeutic methods for this significant, unmet medical need. The growing utilization of complementary and alternative medicine, consisting of Chinese patent medicine in treating DCM, has recently attracted widespread attention.
Qili Qiangxin Capsule (QQC) is a drug clearly recommended in the “Chinese guidelines for the diagnosis and treatment of dilated cardiomyopathy,” which has been collected by the Pharmacopoeia of the People’s Republic of China (Chinese Society of Cardiology and Chinese myocarditis cardiomyopathy cooperative group, 2018). QQC comprises 11 crude herbs (Table 1). The results of pharmacological experiments showed that QQC can improve the heart function, inhibits cardiomyocyte apoptosis via activating peroxisome proliferation-activated receptor γ, reduce the high expression of MMP-2 and MMP-9 in cardiomyocytes, control the activation of the RAAS system in the paraventricular nucleus of the hypothalamus, reduce the activity of renal sympathetic nerves (Ma L. Y et al., 2016; Wu et al., 2021).
In recent years, a large number of randomized controlled trials (RCTs) have shown that QQC has a definite effect in the treatment of DCM. Nonetheless, the clinical evidence of QCC for DCM has not been established until now. This study aims to systematically evaluate the safety and effectiveness of QQC in the treatment of DCM.
This meta-analysis was carried out strictly in accordance with the PRISMA guidelines (see Supplementary Table S1) and has been registered in PROSPERO (CRD42022297906).
Only RCTs of QQC for patients with dilated cardiomyopathy were included and not restricted by language or publication status.
All subjects of the included study meet the diagnostic criteria for DCM established by the American Heart Association or the Chinese Medical Association (Maron et al., 2006; Chinese Society of Cardiology and Chinese myocarditis cardiomyopathy cooperative group, 2018). Literature required complete safety and efficacy data with a balanced baseline and comparability. The patients’ age was over 18 years. Gender, nationality, and race were not limited.
Participants in the control group received conventional western medicine (CWM), including ACE-I/ARB, Beta-blockers, mineralocorticoid receptor antagonists, angiotensin receptor-neprilysin inhibitor, diuretics, digoxin, and other drugs recommended by the guidelines. The experimental group received QCC in addition to the CWM of the control group.
The primary outcomes were the clinical efficiency rate, left ventricular ejection fraction (LVEF), left ventricular end-diastolic dimension (LVEDD), left ventricular end-systolic diameter (LVESD), and adverse reactions (ARs). The secondary outcomes were brain natriuretic peptide (BNP), 6 min walking distance (6MWD), hypersensitive-C-Reactive Protein (hs-CRP), Interleukin- 6 (IL-6), tumor necrosis factor-α (TNF-α), and high mobility group protein B1 (HMGB1).
The exclusion criteria included studies with incomplete data, repeated publications, or mismatched outcome indicators.
PubMed, Cochrane Library, Web of Science, Wan Fang Databases, Chinese Biomedical Literature Database (CBM), China Science and Technology Journal Database (VIP), China National Knowledge Infrastructure (CNKI) were searched and collected RCT studies of QQC in the treatment of DCM, from the date of establishment of each database to 15 January 2022. The search terms included “Qili Qiangxin capsule”, “Qili Qiangxin”, “qiliqiangxin”, “dilated cardiomyopathy”, “DCM”, “dilative cardiomyopathy.” The search strategies are presented in Supplementary Table S2. In addition, the reference lists of the identified original articles were manually checked for other eligible studies.
Two investigators independently screened according to the title and abstract of the records retrieved in all databases and further assessed the full text for potentially eligible studies. For studies with missing information, the investigators contacted the authors for confirmation. Two researchers separately extracted literature data, including the author, publication year, sample size, age, gender, intervention measures, treatment methods, and treatment time. In the event of a disagreement, the third party shall participate in the negotiation and settlement.
Two independent authors assessed the methodological quality and risk of bias of the included RCTs using the Cochrane Collaboration Tool (Higgins et al., 2011). The evaluation includes seven types: random sequence generation, incomplete outcome data, allocation concealment, selective reporting, blinding of participants and personnel, blinding of outcome assessment, and other bias. Then, Review Manager 5.3 should be used to display the bias risk assessment chart drawn.
Revman 5.3 software was used for Meta-analysis. Binary variables were statistically analyzed by relative risk (RR), and continuous variables were statistically analyzed by mean difference (MD). Each effect size was evaluated with a 95% confidence interval (CI). Measure the statistical heterogeneity according to the value of I2, and the evaluation results are shown in forest maps. The random-effects model is used for analysis if I2>50%. Otherwise, the fixed effects model is used for analysis. Sensitivity analysis or subgroup analysis was performed to explore potential sources of heterogeneity. Finally, funnel plots and Egger’s test examined the publication bias effect if more than ten studies were included in the meta-analysis.
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) criteria were employed to assess the quality of the evidence (Balshem et al., 2011). The quality of evidence of the meta-analysis outcomes was categorized as either very low, low, moderate, or high. Initially, the RCT outcomes were ranked as high-quality evidence. The quality of each outcome independently assessed by two authors was de-graded due to the following factors: risk of bias, inconsistency, indirectness, imprecision, and publication bias. Data analysis and synthesis were performed using GRADE pro3.6.1 software.
Overall, 287 potentially relevant articles from seven electronic databases were retrieved after the literature search. After the elimination of duplicates, 156 articles were identified. After a detailed screening of the titles and abstracts, 91 articles including case reports, reviews, conference papers, and animal studies irrelevant to DCM were excluded. After reading the full text of the remaining 65 articles, 30 studies were further removed for at least one of the following reasons: non-RCTs (n = 15); inconsistent interventions (n = 24); inconsistent study purpose and outcome indicators (n = 7); and duplicate publications (n = 2). The final review included 35 studies (Liu et al., 2009; Zhu et al., 2010; Sun et al., 2012; Yuan, 2012; Yang et al., 2013; Zhang et al., 2013; Wu et al., 2014; Yi and Ou-yang, 2014; Ren, 2015; Zhou and Sun, 2015; Han, 2016; Leng, 2016; Ma X. Y et al., 2016; Jing et al., 2017; Fang, 2018; Hang, 2018; Wang, 2018; Wu et al., 2018; Yao, 2018; Zhao, 2018; Dai and Wang, 2019; Dong, 2019; Liu and He, 2019; Wang and Chang, 2019; Yang et al., 2019; Zheng et al., 2019; Li et al., 2020; Meng, 2020; Niu et al., 2020; Shi, 2020; Wang et al., 2020; Wang and Hu, 2020; Yao et al., 2020; Fan et al., 2021; Niu, 2021). The study selection process is shown in Figure 1.
All of the trials were double-arm randomized clinical trials. The enrolled 35 articles were published between 2009 and 2021, in which all studies related to the comparison of QCC combined with CWM vs. CWM. The standard, type, and dose of conventional western medicine in the QCC group were the same as those in the control group. The composition of QQC was the same in all included studies in our study, and the dosage of QQC in the trial group was 1.2 g three times a day. A total of 3,334 patients were randomly divided into a QCC group and a control group, all from China, including 1850 men. The mean age of the participants ranged from 37.72 to 68.77 years. Treatment duration lasted 2 weeks to 12 months, and most of them lasted 4 weeks (8/35, 23%). The primary outcomes measure was reported. Among them, the clinical efficiency rate was reported in twenty-three studies (Liu et al., 2009; Sun et al., 2012; Zhang et al., 2013; Han, 2016; Ma X. Y et al., 2016; Jing et al., 2017; Fang, 2018; Hang, 2018; Wang, 2018; Yao, 2018; Dai and Wang, 2019; Dong, 2019; Liu and He, 2019; Wang and Chang, 2019; Zheng et al., 2019; Meng, 2020; Niu et al., 2020; Shi, 2020; Wang et al., 2020; Wang and Hu, 2020; Yao et al., 2020; Fan et al., 2021; Niu, 2021), LVEF in 28 (Liu et al., 2009; Zhu et al., 2010; Yuan, 2012; Yang et al., 2013; Zhang et al., 2013; Wu et al., 2014; Yi and Ou-yang, 2014; Ren, 2015; Zhou and Sun, 2015; Leng, 2016; Ma X. Y et al., 2016; Jing et al., 2017; Hang, 2018; Wu et al., 2018; Zhao, 2018; Dai and Wang, 2019; Dong, 2019; Liu and He, 2019; Wang and Chang, 2019; Yang et al., 2019; Zheng et al., 2019; Li et al., 2020; Meng, 2020; Niu et al., 2020; Shi, 2020; Wang et al., 2020; Wang and Hu, 2020; Yao et al., 2020; Fan et al., 2021), LVEDD in 20 (Liu et al., 2009; Zhu et al., 2010; Yuan, 2012; Yang et al., 2013; Zhang et al., 2013; Yi and Ou-yang, 2014; Zhou and Sun, 2015; Leng, 2016; Jing et al., 2017; Wu et al., 2018; Zhao, 2018; Dong, 2019; Liu and He, 2019; Yang et al., 2019; Zheng et al., 2019; Li et al., 2020; Meng, 2020; Wang et al., 2020; Wang and Hu, 2020; Fan et al., 2021), LVESD in 14 (Zhang et al., 2013; Yi and Ou-yang, 2014; Jing et al., 2017; Wu et al., 2018; Zhao, 2018; Dong, 2019; Liu and He, 2019; Yang et al., 2019; Zheng et al., 2019; Li et al., 2020; Meng, 2020; Wang et al., 2020; Wang and Hu, 2020; Fan et al., 2021), ARs in 11 (Liu et al., 2009; Yang et al., 2013; Leng, 2016; Fang, 2018,; Wang, 2018; Wu et al., 2018; Niu et al., 2020; Wang et al., 2020; Wang and Hu, 2020; Fan et al., 2021; Niu, 2021) (Table 2).
Nineteen RCTs specifically described methods for generating random sequences: 17 RCTs utilized the random number table (Yang et al., 2013; Leng, 2016; Jing et al., 2017; Wang, 2018; Yao, 2018; Liu and He, 2019; Wang and Chang, 2019; Yang et al., 2019; Zheng et al., 2019; Li et al., 2020; Niu et al., 2020; Shi, 2020; Wang et al., 2020; Wang and Hu, 2020; Yao et al., 2020; Fan et al., 2021; Niu, 2021), 2 RCTs utilized random lottery (Hang, 2018; Meng, 2020), which were considered to be low risk of bias. The other RCTs did not introduce the randomization method in detail and were rated as unclear risk of bias. All studies did not adequately report allocation concealment details, which was considered the unclear risk of bias. Only one trial (Wu et al., 2018) reported the use of double-blinding, and performance bias was evaluated as “low risk.” The remaining 34 studies did not provide information on blinding, so the performance bias was assessed as “high risk.” Because of the objectivity of outcome measures, the detection bias should be considered low risk of bias, regardless of whether the blinding was reported. All studies reported test indicators as planned, and there was no incomplete outcome data or selective reporting of research results. It is unclear whether there is another bias (Figure 2).
Twenty-three articles reported the clinical efficacy rate, including a total of 1996 patients. The heterogeneity test result suggests that the fixed-effects model should be used for Meta-analysis (I2 = 0%, p = 0.87). The outcome shows that the clinical efficiency rate for the experimental group (QCC plus CWM) was significantly higher than that of CWM alone (RR = 1.24, 95% CI (1.19, 1.29), p < 0.00001; Figure 3).
Twenty-eight articles reported LVEF, including a total of 2,617 patients. The heterogeneity test result suggests that the random-effects model should be used for meta-analysis (I2 = 84%, p < 0.00001). The results showed that the experimental group had significant advantages in ameliorating LVEF over the control group (MD = 5.73, 95%CI (4.70, 6.77), p < 0.00001; Figure 4).
According to the treatment duration, twenty-eight trials were subdivided into two subgroups (less than 12 weeks subgroup and more than or equal to 12 weeks subgroup). There was relatively high heterogeneity in each subgroup (less than 12 weeks: I2 = 86%, p < 0.00001; more than or equal to 12 weeks: I2 = 59%, p = 0.01). The random-effects model was used. According to Figure 4, when the treatment duration was less than 12 weeks, the LVEF in the experimental group was higher than that in the control group (MD = 6.47, 95%CI (5.18, 7.76), p < 0.00001). When the treatment duration was more than or equal to 12 weeks, the LVEF in the experimental group was also higher than that in the control group (MD = 4.03, 95%CI (2.76, 5.27), p < 0.00001). The result of subgroup analysis was limited by substantial heterogeneity. More trials with good homogeneity would be needed to demonstrate the results.
A total of twenty studies pertaining to 1970 patients reported LVEDD. The heterogeneity test results showed that the random-effects model should be used (I2 = 66%, p < 0.0001). The results showed that compared with the control group, the experimental group had more advantages in the treatment of LVEDD in DCM patients (MD = −4.09, 95%CI (−4.91, −3.27), p < 0.00001).
According to the treatment duration, they were subdivided into two subgroups (less than 12 weeks subgroup and more than or equal to 12 weeks subgroup). There was low heterogeneity in each subgroup (less than 12 weeks: I2 = 48%, p = 0.02; more than or equal to 12 weeks: I2 = 61%, p = 0.02). The random-effects model was used. According to Figure 5, when the treatment duration was less than 12 weeks, the LVEDD in the experimental group was lower than that in the control group (MD = −4.71, 95%CI (−5.45, −3.97), p < 0.00001). When the treatment duration was more than or equal to 12 weeks, the LVEDD in the experimental group was also lower than that in the control group (MD = −2.39, 95%CI (−4.06, −0.73), p = 0.005). The results of subgroup analysis indicated that the treatment duration might be the source of heterogeneity.
Fourteen trials with 1,613 patients reported the treatment effects on LVESD. The results demonstrated that QCC combined with CWM showed a weighty decrease in the LVESD level compared with CWM alone (MD = -4.73, 95%CI (-5.63, -3.84), p < 0.00001; Figure 6). However, significant heterogeneity was identified among the studies (I2 = 65%, p = 0.0003).
According to the treatment duration, they were subdivided into two subgroups (less than 12 weeks subgroup and more than or equal to 12 weeks subgroup). There was moderate heterogeneity in each subgroup (less than 12 weeks: I2 = 49%, p = 0.03; more than or equal to 12 weeks: I2 = 90%, p = 0.002). The random-effects model was used. As shown in Figure 6, the LVESD in the experimental group was lower than that in the control group when the treatment duration was less than 12 weeks (MD = −4.90, 95%CI (−5.70, −4.09), p < 0.00001). When the treatment duration was more than or equal to 12 weeks, the LVESD in the experimental group was also lower than that in the control group (MD = 4.03, 95%CI (2.76, 5.27), p < 0.00001).
Eight studies with 684 patients reported the treatment effects on BNP. We use the random-effects model for statistical analysis based on the heterogeneity (I2 = 95%, p < 0.00001). The results showed that the experimental group was significantly better than the control group in ameliorating the BNP of DCM patients (MD = −101.09, 95%CI (−132.99, −69.18), p < 0.00001; Figure 7).
According to the treatment duration, eight studies were subdivided into three subgroups (equal to 2 weeks subgroup, equal to 4 weeks subgroup, and more than 4 weeks subgroup). There was low heterogeneity in each subgroup (equal to 2 weeks: I2 = 0%, p = 0.78; equal to 2 weeks: I2 = 0%, p = 0.39; more than 4 weeks: I2 = 72%, p = 0.06). The random-effects model was used. According to Figure 7, when the treatment duration was equal to 2 weeks, the experimental group was significantly better than the control group in improving the BNP (MD = −129.15, 95%CI (−183.22, −75.09), p < 0.00001). When the treatment duration was equal to 4 weeks, the experimental group was significantly better than the control group in improving the BNP (MD = -58.97, 95%CI (−64.47, −53.46), p < 0.00001). When the treatment duration was more than 4 weeks, the BNP in the experimental group was also lower than that in the control group (MD = −66.18, 95%CI (−250.85, −81.51), p = 0.0001). The results of subgroup analysis indicated that the treatment duration might be the source of heterogeneity.
A total of 828 patients from eleven trials reported on 6MWD. The fixed-effects model was used for statistical analysis (I2 = 0%, p = 0.81). The results showed that the experimental group was significantly better than the control group in improving 6MWD in DCM patients (MD = 41.93, 95%CI (39.82, 44.04), p < 0.00001; Figure 8).
We performed a subgroup analysis according to the treatment duration and selected fixed-effects model (less than 12 weeks: I2 = 0%, p = 0.69; more than or equal to 12 weeks: I2 = 0%, p = 0.79). As shown in Figure 8, the experiment group had significant advantages in ameliorating the 6MWD than the control group when the treatment duration was less than 12 weeks (MD = 42.04, 95%CI (39.92, 44.16), p < 0.00001). When the treatment duration was more than or equal to 12 weeks, the experiment group also had significant advantages in ameliorating the 6MWD over the control group (MD = 32.67, 95%CI (13.49, 51.85), p = 0.0008).
Four trials, including 307 patients, reported the treatment effects on hs-CRP. The heterogeneity test results showed that the random-effects model should be used (I2 = 89%, p < 0.00001). Compared with CWM alone, QCC could significantly reduce the hs-CRP levels (MD = −3.78, 95%CI (−4.35, −3.21), p < 0.00001). We performed subgroup analysis according to the treatment duration. There was low heterogeneity in each subgroup (less than 12 weeks: I2 = 0%, p = 1.00; more than or equal to 12 weeks: I2 = 0%, p = 0.87) among the RCTs, the fixed-effects model was used. According to Figure 9, when the treatment duration was less than 12 weeks, the hs-CRP in the experimental group was lower than that in the control group (MD = −5.20, 95%CI (−5.98, −4.42), p < 0.00001). When the treatment duration was more than or equal to 12 weeks, the hs-CRP in the experimental group was also lower than that in the control group (MD = −2.22, 95%CI (−3.04, −1.39), p < 0.00001). The results of subgroup analysis indicated that the treatment duration might be the source of heterogeneity.
Two trials, including 128 patients, reported the treatment effects on IL-6. We found that its heterogeneity was low (I2 = 0%, p = 0.45). We selected a fixed-effect model. The results showed that compared with CWM alone, QCC could significantly reduce the IL-6 levels (MD = −25.92, 95%CI (−31.35, −20.50), p < 0.00001, Figure 10). Four trials contained 268 patients reported TNF-α, and the fixed-effect model was used for analysis (I2 = 45%, p = 0.14; Figure 10). MD = -5.04, 95%CI (-6.13, -3.95), p < 0.00001, means that the experimental group had an advantage in ameliorating the TNF-α of patients with DCM. A total of 178 patients from three trials reported on HMGB1. The fixed-effect model was used for statistical analysis (I2 = 0%, p = 0.47). The results showed that the experimental group was significantly better than the control group in improving HMGB1 in DCM patients (MD = -4.34, 95%CI (−5.22, −3.46), p < 0.00001; Figure 10).
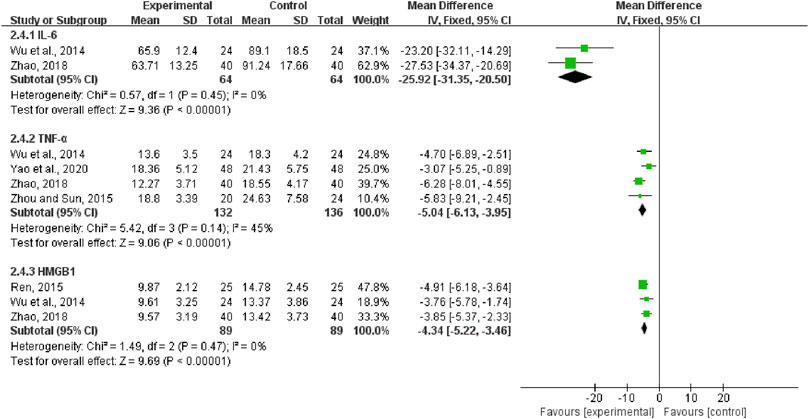
FIGURE 10. Forest plots of Interleukin- 6, tumor necrosis factor-α, and high mobility group protein B1.
Eleven studies with a total of 1,394 patients reported ARs, included dizziness (Wang et al., 2020; Wang and Hu, 2020; Wu et al., 2018), headache (Fang, 2018; Wang, 2018; Wu et al., 2018; Niu et al., 2020; Niu, 2021), hypotension (Wang et al., 2020; Fan et al., 2021), fatigue (Fang, 2018; Niu et al., 2020), dry cough (Liu et al., 2009; Yang et al., 2013; Wu et al., 2018; Wang et al., 2020; Fan et al., 2021), palpitations (Yang et al., 2013; Leng, 2016; Niu et al., 2020; Wang et al., 2020; Wang and Hu, 2020; Fan et al., 2021), and gastrointestinal adverse reactions such as nausea and vomiting (Liu et al., 2009; Yang et al., 2013; Leng, 2016; Fang, 2018; Wang, 2018; Wu et al., 2018; Wang et al., 2020; Wang and Hu, 2020; Niu, 2021). Moreover, the fixed-effects model was used for statistical analysis (I2 = 0%, p = 0.47). The results showed that the experimental group had advantages over the control group in reducing adverse reactions (RR = 0.70, 95%CI (0.51, 0.97), p = 0.03; Figure 11). We performed a subgroup analysis and selected fixed-effects model (less than 12 weeks: I2 = 0%, p = 0.58; more than or equal to 12 weeks: I2 = 8%, p = 0.79). As shown in Figure 11, the experimental group had advantages over the control group in reducing ARs when the treatment duration was less than 12 weeks [RR = 0.45, 95%CI (0.23, 0.86), p = 0.02]. When the treatment duration was more than or equal to 12 weeks, the experimental group had no advantage over the control group in reducing adverse events (MD = 0.84, 95%CI (0.58, 1.22), p = 0.37).
We used the funnel plots (Figure 12) and Egger’s test (Supplementary Figure S1) to examine the possible publication bias for the outcomes of the clinical efficiency rate, LVEF, LVEDD, LVESD, 6MWD, and ARs in this meta-analysis. Consequently, the symmetrical shape of the funnel plots, as well as the p-values from Egger’s tests, revealed that there was no remarkable publication bias for LVEF, LVEDD, LVESD, 6MWD, and ARs (p = 0.225; p = 0.799; p = 0.213; p = 0.079; p = 0.635) and there may be publication bias for the clinical efficiency rate (p < 0.001).
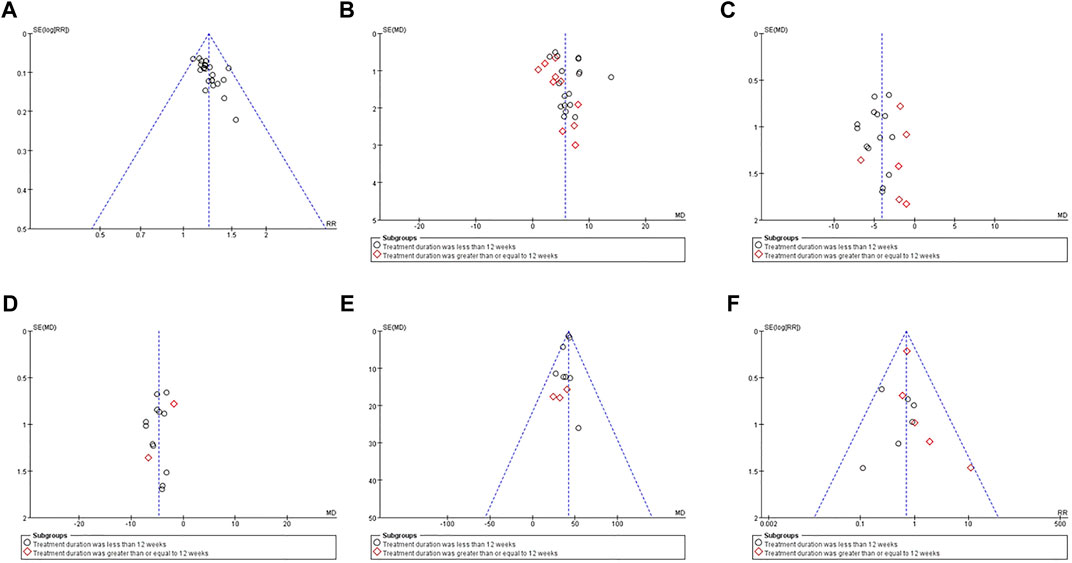
FIGURE 12. Funnel plots of between QCC combined with conventional western medicine and conventional western medicine alone for the clinical efficiency rate (A), LVEF (B), LVEDD (C), LVESD (D), 6MWD (E), and ARs (F).
The GRADE approach was employed to explore the quality of evidence for the six outcomes, which exhibited moderate or low quality with severe methodological problems and a heterogeneity problem. Table 3 presents an overview of the GRADE evidence.
Generally, DCM patients present with typical signs of heart failure (progressive dyspnoea, fatigue, physical exertion, ankle swelling, orthopnea, mood disorders), ventricular arrhythmia, thromboembolic events, and sudden cardiogenic death (Prasad and Halliday, 2021). Patients have a 1-year survival rate of 70%–75% without aggressive treatment and a 5-years survival rate as low as 50% (Reichart et al., 2019). Based on the original effective neuroendocrine suppression, the role of drugs in further reducing the mortality and disability rate is more and more limited, while non-drug treatment is challenging to promote due to issues including technology, cost, and indication. Thus, it is essential to develop new therapies to treat patients with DCM. Integrated traditional Chinese and Western medicine has been developed as a novel therapeutic method for DCM treatment. It has the unique advantage of improving clinical symptoms and reducing the ARs compared with CWM alone. However, no meta-analysis and systematic review on the efficacy and safety of QCC combined with CWM in DCM treatment has been established. Therefore, we performed this study to systematically evaluate the safety and effectiveness of QQC in the treatment of DCM.
Through the statistical analysis of a total of 3,334 patients in the 35 included studies, QQC combined with CWM had advantages in the treatment of DCM, which is reflected in the clinical efficiency rate, the improvement of 6-MWT, LVEF, LVEDD, LVESD, and BNP, and the difference had statistical advantages. The clinical efficiency rate is the sum of the percentage of cardiac function in patients who achieve a complete or partial response. The clinical efficiency rate in patients who had been treated with QCC plus CWM was 92.4% (926/1002). The clinical efficiency rate in patients who had been treated with CWM alone was 74.5% (741/994). The results indicated that the clinical efficiency rate of QCC combined with CWM was remarkably higher than CWM independently. Ultrasound cardiogram (UCG) is a robust test method for cardiac function or structure assessment in diagnosing and evaluating dilated cardiomyopathy. UCG can detect ventricular systolic and diastolic function, wall motion, and mural thrombus. Many experiments show that left ventricular dysfunction is closely related to the prognosis of DCM patients (Sreenivasan and Jain, 2021). Parameters like LVEF, LVEDD, and LVESD reflect left ventricular function and size and provide diagnostic and prognostic value for patients. 6MWD, a good indicator of exercise tolerance, asks the patient to walk as fast as possible in a straight corridor and measures the walking distance of 6 min 6MWD clearly shows improvement in exercise tolerance in patients before and after treatment. BNP testing is recommended for screening, diagnosis and differential diagnosis, and assessment of disease severity and prognosis (Halliday et al., 2019). In the present study, 28 articles with 2,617 patients reported LVEF, in which the experimental group had significant advantages in ameliorating LVEF over the control group. Nevertheless, the result was limited by unidentified heterogeneity. Limited by the existence of substantial heterogeneity, it is necessary to increase the sample size to verify whether the use of QCC in patients with dilated cardiomyopathy actually increases LVEF levels.
Meta-analysis in this study proves that QCC could remarkably reduce the levels of hs-CRP, IL-6, TNF-α, and HMGB1 in DCM patients. IL-6, TNF-α, and other inflammatory markers are prevalent in patients with DCM, especially in patients with disease progression, and inflammatory markers levels are directly correlated with cardiac function levels (Anzai, 2018). HMGB1, which serves as an early promoter and facilitator of inflammation, can cause various inflammatory responses, tissue regeneration, and heart failure. Clinical studies have shown that HMGB1 and its associated inflammatory cytokines (IL-6, TNF-α) may be involved in the pathophysiological processes of dilated cardiomyopathy and accelerated heart function decline (Wu et al., 2013).
In this study, eleven articles with 1,394 patients were observed for the incidence of ARs. The incidence of ARs in patients who had been treated with QCC plus CWM was 7.5% (56/707). The incidence of ARs in patients who had been treated with CWM solely was 11.1% (76/687). The result concluded that a significantly lower incidence rate of ARs in the experimental group compared with the control group suggested that adding the use of QCC combined with CWM was safe. Hence, we provide supporting evidence that, to a remarkable extent, QCC can potentially be recommended for planned use for DCM patients. However, in our meta-analysis, LVEDD and hs-CRP showed high heterogeneity, and we identified sources of heterogeneity through subgroup analysis. The results of subgroup analysis indicated that the treatment duration might be the source of heterogeneity.
Multiple meta-analyses and systematic reviews have confirmed the effectiveness and safety of traditional Chinese medicine (TCM) in treating DCM. A meta-analysis constituting 21 RCTs involving 1,566 patients revealed that oral Chinese herbal medicine effectively improves overall efficacy, LVEF, LVEDD, stroke volume (SV), BNP, and 6MWD in the treatment of DCM (Zhu et al., 2016), which was consistent with our results. However, BNP, LVEF, SV, and 6MWD represented high heterogeneity in their meta-analysis. The reason may be numerous varieties of Chinese herbal compounds and control groups, insufficient clinical samples, and follow-up time. The findings of another systematic review and meta-analysis indicated that five Chinese medicine injections significantly improved performance compared with CWM solely in treating DCM (Cao et al., 2022). This study suggested that the clinical selection of Chinese medicine injections should consider the individual patient’s circumstances, as different types of Chinese medicine injections have different advantages. Besides, a randomized controlled trial confirmed that QCC treatment showed superior performance compared to the placebo concerning cardiac function classification, LVEF, 6MWD, and quality of life (Li et al., 2013).
This study has the following limitations: 19 of the 35 studies described the specific randomization method adequately, accounting for 54.28%, while the rest only mentioned randomization and did not specifically describe the randomization method, and all did not mention the use of allocation concealment, thus potentially creating a risk of bias in the study. At the same time, some of the included studies had small sample sizes, with a total of 14 studies with a sample size of less than 80, accounting for 40%, which makes the results of the included studies unreliable. The efficacy measures lacked endpoint event measures, such as the incidence rate of cardiovascular events, and there was little safety data. The occurrence of adverse reactions was reported only in seven studies. The use of conventional western medicine was not the same in all studies. The span of treatment courses was large in different studies (for a minimum of 2 weeks or up to 12 months), which may lead to an increase in clinical heterogeneity. None of the 35 included studies mentioned the sample size calculation method, reflecting the lack of rigorous sample size estimation, resulting in less reliable study results.
Firstly, it is evident that strategies that improve the methodological quality of RCTs are urgently needed. Going forward, we recommend establishing and reporting the randomized controlled trial of QCC strictly under the CONSORT 2010 statement (Cheng et al., 2017), clearly implementing allocation concealment and blinding, clarify the specific randomization method, record the cases lost to follow-up in detail, and strengthen quality control. Secondly, we propose to establish a curative effect evaluation system that conforms to the characteristics of traditional Chinese medicine, excavate sensitive and practical indicators of TCM, and highlight the advantages of TCM. Thirdly, the safety of QCC for dilated cardiomyopathy needs further investigation. We suggest improving reporting of adverse events and adverse drug reactions related to Qili Qiangxin Capsules according to the standard reporting format (Bian et al., 2010). Fourthly, the overall quality of evidence was graded as low or moderate in our study. Consistent recommendations can be reached through a rigorous expert consensus method in the absence of high-level, evidence-based findings. We propose to revise TCM clinical practice guidelines of QCC combined with CWM for DCM based on the formed expert consensus.
In summary, the available evidence shows that Qili Qiangxin Capsule combined with conventional western medicine can significantly improve the clinical efficacy rate, LVEF, LVEDD, LVESD, BNP, and 6-MWT, decrease the levels of hs-CRP, IL-6, TNF-α, and HMGB1 in DCM patients, and reduce the occurrence of adverse reactions. However, due to some limitations in the methodology of the included trials, high-quality RCT studies with more samples, scientific design, multiple centers and strict implementation are still needed to further demonstrate this conclusion in the future, providing higher quality evidence support for the application of Qili Qiangxin Capsule in clinical practice.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
MZ and YW conceived and designed this study. JW wrote the paper and analyzed the data. BL and XW provided essential methodological suggestions and revised the manuscript. XL and YH collected data. LQ and CZ jointly completed literature retrieval and data extraction. PY and TS were responsible for coordinating the study. All authors read and approved the final manuscript.
The Ministry of Science and Technology of the People’s Republic of China’s Key Projects during the 13th Five-Year Plan Period (2019YFC1710003), the National Natural Science Foundation of China (82074226 and 82030120), the Chinese Medicine Evidence-Based Capacity Building Project of State Administration of Traditional Chinese Medicine (2019XZZX-XXG003) supported the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.893602/full#supplementary-material
Anzai, T. (2018). Inflammatory Mechanisms of Cardiovascular Remodeling. Circ. J. 82 (3), 629–635. doi:10.1253/circj.CJ-18-0063
Balshem, H., Helfand, M., Schünemann, H. J., Oxman, A. D., Kunz, R., Brozek, J., et al. (2011). GRADE Guidelines: 3. Rating the Quality of Evidence. J. Clin. Epidemiol. 64 (4), 401–406. doi:10.1016/j.jclinepi.2010.07.015
Bian, Z. X., Tian, H. Y., Gao, L., Shang, H. C., Wu, T. X., Li, Y. P., et al. (2010). Improving Reporting of Adverse Events and Adverse Drug Reactions Following Injections of Chinese Materia Medica. J. Evid. Based Med. 3 (1), 5–10. doi:10.1111/j.1756-5391.2010.01055.x
Cao, X., Liu, H., Zhou, M., Chen, X., and Long, D. (2022). Comparative Efficacy of Five Chinese Medicine Injections for Treating Dilated Cardiomyopathy with Heart Failure: A Bayesian Network Meta-Analysis. J. Ethnopharmacol 282, 282114604. doi:10.1016/j.jep.2021.114604
Cheng, C. W., Wu, T. X., Shang, H. C., Li, Y. P., Altman, D. G., Moher, D., et al. (2017). CONSORT Extension for Chinese Herbal Medicine Formulas 2017: Recommendations, Explanation, and Elaboration (Traditional Chinese Version). Ann. Intern. Med. 167 (2), W7–W20. doi:10.7326/M16-297710.7326/IsTranslatedFrom_M17-2977_1
Chinese Society of Cardiology, and Chinese myocarditis cardiomyopathy cooperative group (2018). Chinese Guidelines for the Diagnosis and Treatment of Dilated Cardiomyopathy. J. Clin. Cardiol. (China) 34 (05), 421–434. doi:10.13201/j.issn.1001-1439.2018.05.001
Dai, C., and Wang, H. (2019). Clinical Observation of Qili Qiangxin Capsule in the Treatment of Dilated Cardiomyopathy and Heart Failure. China Heal Care Nutr. 29 (18), 242244.
Dong, J. (2019). Analysis on the Therapeutic Effect of Qili Qiangxin Capsule Combined with Recombinant Human Brain Natriuretic Peptide in the Treatment of Dilated Cardiomyopathy Complicated with Heart Failure. China Mod. Dr 57 (34), 109–112.
Fan, H. Y., Chen, X. M., and Zhao, F. Q. (2021). Efficacy and Safety of Qili Qiangxin Capsule Combined with Valsartan in the Treatment of Dilated Cardiomyopathy Heart Failure. Health Manage. 2021 (7), 28.
Fang, J. Y. (2018). Effect of Qiliqiangxin Capsule Combined with Digoxin on Cardiac Function of Patients with Dilated Cardiomyopathy with Mild and Moderate Heart Failure. Drug Eval. 15 (11), 27–29.
Halliday, B. P., Wassall, R., Lota, A. S., Khalique, Z., Gregson, J., Newsome, S., et al. (2019). Withdrawal of Pharmacological Treatment for Heart Failure in Patients with Recovered Dilated Cardiomyopathy (TRED-HF): an Open-Label, Pilot, Randomised Trial. Lancet 393 (10166), 61–73. doi:10.1016/S0140-6736(18)32484-X
Han, B. (2016). Clinical Observation of Qili Qiangxin Capsule in the Treatment of 30 Cases of Dilated Cardiomyopathy and Heart Failure. World Latest Med. Infor 16 (61), 205–07.
Hang, S. N. (2018). Analysis of the Effect of Qili Qiangxin Capsule in the Treatment of Dilated Cardiomyopathy Heart Failure. J. Clin. Med. Lit. Electron. 5 (73), 169–170. doi:10.16281/j.cnki.jocml.2018.73.116
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised Trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Japp, A. G., Gulati, A., Cook, S. A., Cowie, M. R., and Prasad, S. K. (2016). The Diagnosis and Evaluation of Dilated Cardiomyopathy. J. Am. Coll. Cardiol. 67 (25), 2996–3010. doi:10.1016/j.jacc.2016.03.590
Jing, Y. Y., Bai, H, X., Liang, Y. H., Xue, E. Z., Jing, Q, Q., and Liu, C. L. (2017). Clinical Study on Qili Qiangxin Capsules Combined with Recombinant Human Brain Natriuretic Peptide in Treatment of Dilated Cardiomyopathy with Heart Failure. Drugs Clin. 32 (10), 1840–1843.
Leng, Y. P. (2016). Effect of Qili Qiangxin Capsule Combined with Anti-heart Failure in the Treatment of Dilated Cardiomyopathy Heart Failure on Plasma Brain Natriuretic Peptide Level and Cardiac Function. J. Hunan Univ. Tradit Chin. Med. 36 (6), 103–104.
Li, Q. J., Wu, Q. F., Liang, Y., Long, Q. X., and Li, J. (2020). Effects of Qili Qiangxin Capsule on NT-proBNP Level and Cardiac Function in Patients with Dilated Cardiomyopathy. Home Med. 2020 (6), 87.
Li, X., Zhang, J., Huang, J., Ma, A., Yang, J., Li, W., et al. (2013). A Multicenter, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Study of the Effects of Qili Qiangxin Capsules in Patients with Chronic Heart Failure. J. Am. Coll. Cardiol. 6262 (1212), 10651065–10721072. doi:10.1016/j.jacc.2013.05.035
Liu, J., Liu, G., Liu, J. T., and Wang, T. J. (2009). Observation on the Curative Effect of Qili Qiangxin Capsule in Adjuvant Treatment of Dilated Cardiomyopathy with Cardiac Insufficiency. Chin. J. Diffic Compl Cas 8 (03), 159–160.
Liu, L., and He, Y. (2019). Clinical Study of Qili Qiangxin Capsule Combined with Western Medicine in the Treatment of Dilated Cardiomyopathy-Induced Heart Failure. Contemp. Med. 25 (09), 158–159.
Liu, X., Yu, H., Pei, J., Chu, J., Pu, J., and Zhang, S. (2014). Clinical Characteristics and Long-Term Prognosis in Patients with Chronic Heart Failure and Reduced Ejection Fraction in China. Heart Lung Circ. 23 (9), 818–826. doi:10.1016/j.hlc.2014.02.022
Ma, L. Y., Yin, Y. J., Zhang, J. F., Liu, H., and Jia, Z. H. (2016). Effects of Qili Qiangxin Capsule on the RAS System and Sympathetic Nervous System of the Hypothalamic Paraventricular Nucleus in Rats with Chronic Heart Failure. Chin. Pharmacol. Bull. 32 (04), 575–580. doi:10.3969/j.issn.1001-1978.2016.04.026
Ma, X. Y., Yu, N., Chi, H. K., and Jin, X. (2016). Observation on the Effect of Qili Qiangxin Capsule in Adjuvant Treatment of Dilated Cardiomyopathy. Women’s Heal Res. 2016 (11), 41–42.
Maron, B. J., Towbin, J. A., Thiene, G., Antzelevitch, C., Corrado, D., Arnett, D., et al. (2006). Contemporary Definitions and Classification of the Cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 113 (14), 1807–1816. doi:10.1161/CIRCULATIONAHA.106.174287
Meng, X. M. (2020). Effects of Qili Qiangxin Capsule Combined with Recombinant Human Brain Natriuretic Peptide on Cardiac Function and Daily Living Ability in Patients with Dilated Cardiomyopathy and Heart Failure. Chang Shou 116 (7), 118.
Niu, T. X. (2021). Analysis of the Effect of Qili Qiangxin Capsule Combined with Telmisartan in the Treatment of Dilated Cardiomyopathy. Health Horizon 2021 (22), 53.
Niu, Y. G., Niu, G. T., Shang, Z. H., and Wei, G. Y. (2020). Efficacy Analysis of Qili Qiangxin Capsule Combined with Metoprolol in the Treatment of Patients with Dilated Cardiomyopathy. Mod. Diagn. Treat. 31 (18), 2879–2880.
Prasad, S. K., and Halliday, B. P. (2021). Myocardial Fibrosis in Dilated Cardiomyopathy: Moving from Stratifying Risk to Improving Outcomes. JACC Cardiovasc. Imaging 14 (7), 1351–1353. doi:10.1016/j.jcmg.2021.03.015
Reichart, D., Magnussen, C., Zeller, T., and Blankenberg, S. (2019). Dilated Cardiomyopathy: from Epidemiologic to Genetic Phenotypes: A Translational Review of Current Literature. J. Intern. Med. 286 (4), 362–372. doi:10.1111/joim.12944
Ren, C. Q. (2015). Effects of Qili Qiangxin Capsule on Cardiac Function and Expression of High Mobility Group Box Protein B1 in Patients with Dilated Cardiomyopathy. Med. Heal Care 2015 (4), 102103.
Shi, Z. (2020). Analysis of the Effect of Qili Qiangxin Capsule Combined with Immunoglobulin in the Treatment of Elderly Patients with Dilated Cardiomyopathy. Health Lit. 2020 (2), 245–246. doi:10.3969/j.issn.1671-5217.2020.02.124
Sreenivasan, J., and Jain, D. (2021). Parameters of Left Ventricular Systolic and Diastolic Dyssynchrony on Radionuclide Imaging to Improve Cardiac Resynchronization Therapy in Heart Failure Patients with Dilated Cardiomyopathy. J. Nucl. Cardiol. 28 (3), 1037–1039. doi:10.1007/s12350-020-02202-4
Sun, Y. L., Wang, Y. N., Wei, Y. J., Hao, X. M., Bai, H. M., and Wang, Y. Z. (2012). Clinical Observation of Qili Qiangxin Capsule in Treating Dilated Cardiomyopathy Heart Failure. J. Emerg. Tradit Chin. Med. 21 (05), 831–832.
Wang, C. Y., and Hu, X. W. (2020). Clinical Study on Qili Qiangxin Capsules Combined with Levosimendan in Treatment of Dilated Cardiomyopathy Complicated with Heart Failure. Drugs Clin. 35 (11), 2238–2242.
Wang, H. (2018). Clinical Observation of Qili Qiangxin Capsule in the Treatment of Dilated Cardiomyopathy. Health Forefront 27 (11), 201. doi:10.3969/j.issn.9128-6509.2018.11.198
Wang, S. K., Zhang, Z., Wang, C. M., Zhou, M., Chen, L. D., Peng, H., et al. (2020). Clinical Study of Sacubitril-Valsartan Combined with Qiliqiangxin Capsule in the Treatment of Dilated Cardiomyopathy Heart Failure. Chin. J. Integr. Med. Cardio-/Cerebrovascuiar Dis. 18 (21), 3620–3622.
Wang, Y., and Chang, F. J. (2019). Efficacy Observation of Qili Qiangxin Capsule Combined with Immunoglobulin in the Treatment of Senile Dilated Cardiomyopathy. Mod. J. Integrat Trad Chin. West. Med. 28 (14), 1568–1570.
Weintraub, R. G., Semsarian, C., and Macdonald, P. (2017). Dilated Cardiomyopathy. Lancet 390 (10092), 400–414. doi:10.1016/S0140-6736(16)31713-5
Wu, H., Yang, J., Ding, J. W., Li, S., Yang, J., Chen, Y., et al. (2013). Expression of High Mobility Group Box 1 Protein, Interleukin-6 and Tumor Necrosis Factor-α in Serum of Patients with Dilated Cardiomyopathy. J. Clin. Cardiol. (China) 29 (02), 88–90. doi:10.13201/j.issn.1001-1439.2013.02.004
Wu, H., Yang, J., Ding, J. W., Li, S., Yang, J., Dong, W. S., et al. (2014). Influences of Qili Qiangxin Capsules on Heart Function and Expression of High Mobility Group Box 1 Protein (HMGB1) in Patients with Dilated Cardiomyopathy. Chin. J. Evid-based Cardiovasc. Med. 6 (03), 271–273.
Wu, X., Zhang, T., Lyu, P., Chen, M., Ni, G., Cheng, H., et al. (2021). Traditional Chinese Medication Qiliqiangxin Attenuates Diabetic Cardiomyopathy via Activating PPARγ. Front. Cardiovasc. Med. 8, 698056. doi:10.3389/fcvm.2021.698056
Wu, X. J., Zhao, B. L., Han, S. L., and Zhang, X. F. (2018). The Clinical Study of the Influence of Qiliqiangxin Capsule to the Heart Function and Vascular Endothelial Function of the Patients with Dilated Cardiomyopathy. Chin. J. Diffic Compl Cas 17 (04), 329–333.
Yang, X. H., Liu, T. S., Guo, H. W., and Zhang, A. J. (2013). Effect of Qiliqiangxin Capsule on Cardiac Function and β1, M2 Receptor Autoantibodies in Patients with Dilated Cardiomyopathy. Chin. J. Diffic Compl Cas 12 (04), 273–275.
Yang, Y., Jin, M. L., Song, L. Y., Guo, Y. F., and Song, C. Y. (2019). Effect Research Antiventricular Remodeling of Qili Qiangxin Capsules in Treating Dilated Cardiomyopathy. Chin. J. Exp. Tradit Med. Formulae 25 (13), 99–104. doi:10.13422/j.cnki.syfjx.20191332
Yao, S. M. (2018). Observation on the Effect of Qili Qiangxin Capsule on Chronic Heart Failure Caused by Dilated Cardiomyopathy. Henan Med. Res. 27 (06), 1059–1060.
Yao, Z. F., Shen, H., Cui, Z. Q., and Ge, J. B. (2020). Influences of QiliQiangxin Capsule Combined with Telmisartanon Cardiac Function of Patients with Dilated Cardiomyopathy. Chin. J. Integr. Med. Cardio-/Cerebrovascuiar Dis. 18 (09), 1363–1366.
Yi, N., and Ou-yang, A. D. (2014). Effects of Qili Qiangxin Capsule on Cardiac Function and Plasma Brain Natriuretic Peptide Level in Patients with Dilated Cardiomyopathy. Chin. Med. Guide 12 (35), 254–256. doi:10.15912/j.cnki.gocm.2014.35.200
Yuan, J. K. (2012). Effects of Qiliqiangxin Capsule on Cardiac Function and Levels of Serum Brain Natriuretic Peptide in Dilated Cardiomyopathy Patients with Heart Failure. J. Chin. Mod. Med. 14 (11), 40–43.
Zhang, Y., Zhu, M., Zhang, F., Zhang, S., Du, W., and Xiao, X. (2019). Integrating Pharmacokinetics Study, Network Analysis, and Experimental Validation to Uncover the Mechanism of Qiliqiangxin Capsule against Chronic Heart Failure. Front. Pharmacol. 10, 1046. doi:10.3389/fphar.2019.01046
Zhang, Z. X., Zhou, X., and Liu, Z. Y. (2013). Clinical Observation of Qili Qiangxin Capsule in the Treatment of Dilated Cardiomyopathy Heart Failure. Chin. J. Trauma Disabil. Med. 21 (06), 221–222.
Zhao, H. H. (2018). Application of Qili Qiangxin Capsule in the Treatment of Patients with Dilated Cardiomyopathy and Heart Failure. Ningxia Med. J. 40 (10), 1214–1216. doi:10.16050/j.cnki.issn1674-6309.2018.10.026
Zheng, W. W., Zhang, Y. Z., and Liu, B. L. (2019). Effects of Qili Qiangxin Capsule Combined with Recombinant Human Brain Natriuretic Peptide on Cardiac Function and Daily Living Ability in Patients with Dilated Cardiomyopathy and Heart Failure. Heilongjiang J. Tradit Chin. Med. 48 (06), 112–113.
Zhou, L. P., and Sun, Y. X. (2015). Effects of Qili Qiangxin Capsule on Cardiac Function and Cold-Induced RNA-Binding Protein Expression in Patients with Dilated Cardiomyopathy. Zhejiang J. Integr. Tradit Chin. West. Med. 25 (09), 837–839.
Zhu, X. L., Chang, K. L., Yu, J., and Tang, L. L. (2010). 40 Cases of Dilated Cardiomyopathy Treated by Qili Qiangxin Capsule. Shanxi Trad Chin. Med. 31 (05), 549–551.
Keywords: Qili Qiangxin capsule, dilated cardiomyopathy, randomized controlled trials, meta-analysis, grade
Citation: Wei J, Li B, Wang X, Li X, Hu Y, Qiao L, Zhou C, Yu P, Sang T, Zhu M and Wang Y (2022) Efficacy and Safety of Qili Qiangxin Capsule on Dilated Cardiomyopathy: A Systematic Review and Meta-Analysis of 35 Randomized Controlled Trials. Front. Pharmacol. 13:893602. doi: 10.3389/fphar.2022.893602
Received: 10 March 2022; Accepted: 12 12 April 20222022;
Published: 28 April 2022.
Edited by:
Adolfo Andrade-Cetto, National Autonomous University of Mexico, MexicoReviewed by:
Lei Wang, Guangzhou University of Chinese Medicine, ChinaCopyright © 2022 Wei, Li, Wang, Li, Hu, Qiao, Zhou, Yu, Sang, Zhu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingjun Zhu, emh1bWluZ2p1bjMxN0AxNjMuY29t; Yongxia Wang, d3l4Y2h6aHFAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.