
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 18 July 2022
Sec. Pharmacoepidemiology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.891178
 Mingqi Wang1,2,3†
Mingqi Wang1,2,3† Wen Wang1,2,3†
Wen Wang1,2,3† Xue Jia1,4
Xue Jia1,4 Qiao He1,2,3
Qiao He1,2,3 Shichao Zhu5
Shichao Zhu5 Yan Kang6
Yan Kang6 Rui Zhang7
Rui Zhang7 Yan Ren1,2,3
Yan Ren1,2,3 Ling Li1,2,3
Ling Li1,2,3 Kang Zou1,2,3
Kang Zou1,2,3 Zhiyong Zong5,8*
Zhiyong Zong5,8* Xin Sun1,2,3*
Xin Sun1,2,3*Background: The effect of thromboembolism prophylaxis on clinical outcomes, such as ventilator-associated events (VAEs), ICU stays, and mortality, remains controversial. This study was conducted to evaluate the effect of pharmacological thromboprophylaxis on VAEs, ICU stays, and ICU mortality among patients receiving mechanical ventilation (MV).
Materials and Methods: A retrospective cohort study was conducted based on a well-established registry of healthcare-associated infections at ICUs in the West China Hospital system. Patients who consistently received MV for at least 4 days from 1 April 2015 to 31 December 2018 were included. Hazard ratios (HRs) were compared for three tiers of VAEs, ICU stays, and ICU mortality among patients receiving pharmacological thromboprophylaxis versus those without using the time-dependent Cox model. For the analyses of ICU stays and ICU mortality, we also used Fine-Gray models to disentangle the competing risks and outcomes of interest.
Results: Overall, 6,140 patients were included. Of these, 3,805 received at least one prescription of antithrombosis agents. Treatments with antithrombosis agents were associated with lower risk of VAEs (HR: 0.87, 95% CI: 0.77, 0.98) and ICU mortality (HR: 0.72, 95% CI: 0.61, 0.86) than those without. Anticoagulants but not antiplatelet agents were associated with decreased risk of VAEs (HR: 0.86, 95% CI: 0.75, 0.98), ICU mortality (HR: 0.62, 95% CI: 0.51, 0.76), and less time to ICU discharge (HR: 1.15, 95% CI: 1.04, 1.28). Antithrombosis may be associated with decreased risk of VAEs in patients with D-dimer >5 mg/LFEU (HR: 0.84, 95%CI: 0.72, 0.98).
Conclusions: Pharmacological thromboprophylaxis was associated with lower risk of VAEs and ICU mortality. Similar effects were observed between unfractionated heparins versus low-molecular-weight heparins.
Ventilator-associated event (VAE) and ventilator-associated pneumonia (VAP) are important causes of morbidity and mortality in the ICU (Bouadma et al., 2015; Magill et al., 2016; He et al., 2021; Zhu et al., 2021). To improve clinical outcomes of patients receiving mechanical ventilation, ventilator bundles have been implemented by hospitals worldwide. Although ventilator bundles have been implemented by hospitals worldwide to prevent adverse outcomes (Marra et al., 2009; Croce et al., 2013), the components often vary remarkably, which may be associated with adverse outcomes.
As a core constituent of ventilator bundles, thromboembolism prophylaxis has been advocated by the Institute for Healthcare Improvement and widely used by most hospitals (Klompas, 2010; Klompas et al., 2016). Owing to immobilization, venous stasis, and vascular injury, venous thromboembolism (VTE) is common among intensive care unit (ICU) patients (Attia et al., 2001; Boddi and Peris, 2017; Ejaz et al., 2018). Patient prognoses are often poor with VTE, especially deep vein thrombosis (DVT) and pulmonary embolism (PE) (Jain and Schmidt, 1997; Laporte and Mismetti, 2010; Ejaz et al., 2018). Thromboembolism prophylaxis has been proven to reduce risk of VTE (Jain and Schmidt, 1997; Boonyawat and Crowther, 2015; Boddi and Peris, 2017). In particular, pharmacological thromboprophylaxis has been recommended for ICU patients to lower risk of VTE (Boddi and Peris, 2017).
However, the effects of thromboembolism prophylaxis on other adverse outcomes such as VAE and mortality have not been fully understood (Al Yami et al., 2017; Bajaj et al., 2019). The benefit of reducing VTE should be reappraised given the increased risk of adverse outcomes, such as major bleeding. Especially for patients with MV more than 48 h, the risk of major bleeding significantly increased (Goodwin and Hoffman, 2011), which may further result in poor prognosis including infection and mortality. Few studies addressed these important outcomes, and these studies draw inconsistent conclusions (Barrera et al., 2010; Klemen et al., 2020). An observational study involved 630 patients with at least 2 days of MV, of which 210 patients developed ventilator-associated pneumonia (VAP). The findings of this study have showed that thromboprophylaxis was significantly associated with lower risk of VAP and death (Croce et al., 2013). Several other studies reported no association between pharmacological thromboprophylaxis on VAP and VAE (Berenholtz et al., 2011; Lewis et al., 2014; Klompas et al., 2016). Most of these studies had relatively small sample size and only a few events occurred, which may have led to a limited inference. For instance, a case–control study only included 110 ventilator-associated condition (VAC) and 38 infection-related ventilator-associated complication (IVAC) to investigate the risk factors for VACs and IVACs, respectively (Lewis et al., 2014). In addition, most studies have exclusively considered implementing aggregate bundle components together, rather than considering day-to-day implementation of bundles (Croce et al., 2013; Harris et al., 2018). Indeed, the implementation of bundle components varied on a day-to-day basis (Klompas et al., 2016).
Therefore, we conducted a cohort study with a large sample size and handled with time-dependent variates to evaluate the effect of prophylactic antithrombosis agents on clinical outcomes.
This cohort study was reported according to the Reporting of studies Conducted using Observational Routinely collected health Data (Benchimol et al., 2015). This study was approved by the Ethics Committee of West China Hospital (WCH) in 2018 (WCH2018-409).
This study was conducted based on a registry of healthcare-associated infections (HAI) at ICUs in WCH system. The WCH is a major healthcare system in west China, which comprises three independent healthcare organizations (Main Campus, Wenjiang Campus, and Shangjin Campus). A total of 5.73 million outpatients and 279,000 inpatients visited to WCH in 2019. As a national critical care center in west China, the WCH has six ICUs (general ICU, surgical ICU, neurological ICU, respiratory ICU, thoracic surgery ICU, and pediatric ICU) with more than 8,000 inpatient ICU admissions annually.
The ICU-HAI registry contained three databases—ICU system, electronic medical record (EMR), and ICU-HAI system. The ICU-HAI system is an active surveillance system, which is a unique system undertaking routine VAE surveillance in China. Information regarding catheterization, hospital-acquired infection, prevention, and control are collected by a team of experienced infection control practitioners every day. Every year, there were more than 5,000 patients undertake VAE surveillance. Of these, 2,000 patients received MV for at least four consecutive day and 500 patients were judged as VAE cases annually. By integrating these three databases with unique and encoded identifiers, we developed the ICU-HAI registry (Li et al., 2018; He et al., 2021).
Patients who were admitted to the six ICUs in WCH since 1 April 2015 were included into the registry. The registry included 28,848 patients until 31 December 2018 and contained 110 GB of data with 245, 311, 294 original records (Wang et al., 2019). Quality assessment showed that the accuracy of data extraction and linkage was 100%, and the completeness of important laboratory tests such as routine blood tests, serum glucose, and serum creatinine was >98% (Wang et al., 2019).
Patients who consistently received MV for at least 4 days from 1 April 2015 to 31 December 2018 were included in the study. The exclusion criteria were as follows: age <18 years, patients admitted to PICU, without sufficient information regarding age, sex, and diagnosis at discharge, and with a diagnosis related to venous thrombosis at ICU admission. The clinical characteristics differed among non-VAE cases with and without consecutive stable or improved respiratory status. Therefore, we also excluded patients with consecutive unstable or increasing daily minimum positive end-expiratory pressure (PEEP) or fraction of inspired oxygen (FiO2) during MV treatment. To minimize indication bias, we additionally excluded patients with extremely long ICU stays (>90 days) and patients with more than one episode of MV treatment for the analysis of ICU mortality and ICU stays.
We defined antithrombosis agent exposure as a prescription for antiplatelet or anticoagulant agents. We calculated antithrombosis agent exposure as a time-dependent variable. Daily exposure to antiplatelet agents or anticoagulant agents were coded as prescribed or not prescribed from initiation of MV to VAE occurrence or extubation and from ICU admission to ICU discharge, respectively. We additionally evaluated the type of antithrombosis agents (antiplatelet and anticoagulant agent) and type of anticoagulant agents [unfractionated heparin (UFH) and low molecular weight heparin (LMWH)] used.
The clinical outcomes of interest included VAEs, ICU mortality, and ICU stays. We measured clinical outcomes as time-to-event variables. We defined VAEs as at least 2 calendar days of increased daily minimum FiO2 (≥0.20) or PEEP (≥3 cm H2O) greater than after at least 2 calendar days of stable or decreasing daily minimum FiO2 or PEEP according to the criteria proposed by CDC’s National Healthcare Safety Network (CDC-NHSN) (Klompas, 2013). Once the value reached the threshold, an alarm would be triggered. Then, infection control practitioners would judge and classify the suspected VAE cases as VACs, IVACs, and possible ventilator-associated pneumonia (PVAP). The accuracy of PVAP has been validated in a previous study, and the proportion was 96.2% (Wang et al., 2019). Patients died within one calendar day after ICU discharge were defined as ICU mortality.
We assessed hazards ratio (HR) with confidence interval (CI) for antithrombosis agents and risk of VAEs by using time-dependent Cox model. This model is used to analyze studies with complex time-varying variates, and data were converted as counting process form to deal with time-varying variates. We assessed the impact of antithrombosis agents on ICU mortality and ICU stays using Fine‐Gray competing risks model to measure the competing risks for ICU discharge alive versus ICU mortality. The reason for ICU discharge was depended on patient’s health condition: clinical improvement or death. Through generating separate hazard ratios for each competing events, Fine‐Gray competing risks model can disentangle effects of competing risks and outcomes of interest (Fine and Gray, 1999; Berger et al., 2018).
All analyses were adjusted for fixed and time-varying covariates. We defined ICU type, demographic characteristics, comorbidities or acute condition (diabetes, hypertension, heart failure, kidney failure, liver failure, ischemic heart disease, cerebrovascular diseases, chronic obstructive pulmonary disease, pulmonary vascular diseases, malignant tumor, trauma, acute respiratory distress syndrome, shock, gastrointestinal bleeding, pneumonia, and intra-abdominal infection), acute physiology and chronic health evaluation (APACHE) Ⅱ score, surgery, fiberoptic bronchoscopy examination, tracheotomy, and laboratory test at admission (D-dimer, prothrombin time, platelet count, antithrombin III, activated partial thromboplastin time) as fixed covariates. Daily medication exposure and processes of care (sedative, acid inhibitors, blood transfusion, mandatory ventilation, and head-of-bed elevation, gastrointestinal decompression, and rehabilitation exercise) and medications (sedatives, opioids, neuromuscular blockers, immunosuppressive agent, neuroleptic agents, antibiotics, expectorants, vasopressors, intestinal probiotics, and neuroleptic agents) were defined as time-varying variables. Time-varying variables were measured as daily exposure from initiation of MV to the event of interested for the model of VAEs and each day from ICU admission to ICU discharge for the model of ICU discharge and ICU mortality. For the analysis of ICU discharge and ICU mortality, we additionally adjusted VAE, days from ICU admission to initiation of MV, and the duration of mechanical ventilation. We also measured mandatory ventilation and prone position ventilation as fixed-time variables.
To evaluate the effect of different types of antithrombosis agents on clinical outcomes, we further calculated HRs for antiplatelet agents vs. regimens without antithrombosis agents, anticoagulant agents vs. regimens without antithrombosis agents, antiplatelet agents vs. anticoagulant agents, and UFH vs. LMWH. We also calculated HRs regarding VAEs, ICU mortality, and ICU stays for patients with D-dimer ≤5 mg/L and >5 mg/L, respectively. The HRs for VAEs were assessed using time-dependent Cox model. We also used Fine‐Gray competing risks model to estimate HRs for ICU mortality and length of ICU stay. Missing data of APACHE II, prothrombin time, D-dimer, platelet count, and lipid load were processed using multilevel multiple imputation. Through pooling results from different imputed datasets, multiple imputations can help reduce bias and obtain more precise results compared with complete-case analysis.
Significance is 0.05 for all analysis, and all data were analyzed using R version 4.0.3, the packages used mainly included “survival,” “miceadds,” “mice.”
We conducted the following sensitivity analyses to examine the robustness of effect estimates: 1) alternative statistical models: without adjusting prothrombin time, platelet count, antithrombin III, activated partial thromboplastin time; 2) alternative approach for missing data: missing data without imputation; and 3) alternative definition of comparison: regimens without anticoagulant agents and regimens without antiplatelet agents.
To further evaluate the effect of antithrombosis agents on VTE, we conducted an additional analysis. We excluded patients who developed VTE within 3 days after ICU admission for latency purpose and to minimize reverse causality. We assessed HRs for antithrombosis agents and risk of VTE using time-dependent Cox model.
Our study included 6,140 patients consistently received MV for at least 4 days. Of these, 5,679 patients with ICU stay less than 90 days and one episode of MV treatment was additionally included into the cohort for ICU stays and ICU mortality analysis (Figure 1). Of included 6,140 patients, 3,805 received at least one prescription of antithrombosis agents and 2,335 did not receive any prescription of antithrombosis agents.
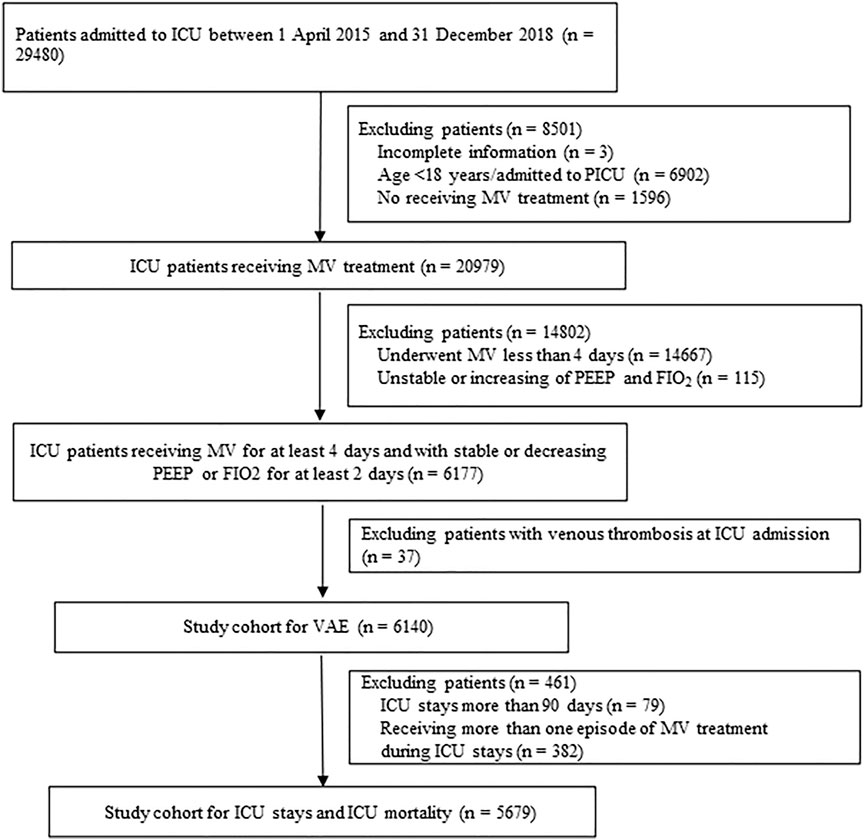
FIGURE 1. Study flow chart. ICU: intensive care units; MV: Mechanical ventilation; PEEP: Positive end-expiratory pressure; FIO2: Fraction of inspired oxygen; VAE: Ventilator-associated events
Patients’ characteristics are showed in Table1. The median age of all included patients was 58 years, and female patients accounted for 36.8% of subjects among included patients. The distribution of patients with and without antithrombosis agents treatment in different ICU wards showed a significant difference (p < 0.001). For instance, 29.2 and 38.7% patients with and without antithrombosis agents treatment, respectively, were admitted to the general ICU. The median APACHEII score was lower among patients treated with antithrombosis agents than those without [19 (15, 24) vs. 21 (16, 26), p < 0.001]. Among all included patients, the most common comorbidity was hypertension (21.5%) and the most common acute condition was pneumonia (10.2%). Compared to patients without antithrombosis agents treatment, the proportions of patients with heart failure (12.7 vs. 2.1%), ischemic heart disease (2.1 vs. 1.2%), pulmonary vascular disease (9.2 vs. 5.7%), and intra-abdominal infection (5.9 vs. 2.8%) were higher among patients receiving antithrombosis agents (p < 0.05). Among patients treated with antithrombosis agents, the activated partial thromboplastin time [33.6 s (28.9, 41.9) vs. 34.7 s (29.1, 44.5)] was higher, and antithrombin III [66.4 s (50.5, 81.3) vs. 62.0 s (46.6, 76.4)] was lower than patients who did not receive antithrombosis agents (p < 0.05).
Among 6,140 included patients, 1,723 patients experienced at least one episode of VAEs. Of these, 498 were classified as IVACs and 168 as PVAPs. The median of ICU stays was 13 days [8, 22], and the crude ICU mortality was 14.3% among included patients.
A total of 3,805 patients (62.0%) received at least one prescription of antithrombosis agent among all included patients. Of those, 691 patients (11.3%) were treated with antiplatelet agents and 3,517 (57.3%) were treated with anticoagulants. Furthermore, 2,577 (42.0%) patients received UFH treatment, and 1,396 (22.7%) received LMWH treatment (Table 2).
Adjusted HRs for antithrombosis agents and VAEs are summarized in Table 3. Regimens with antithrombotic (HR: 0.87, 95% CI: 0.77, 0.98) and anticoagulant (HR: 0.86, 95% CI: 0.75, 0.98) agents were associated with lower risk of VAEs than regimens without. However, no statistically significant decrease in VAEs was found for antiplatelet agents (HR: 0.92, 95% CI: 0.76, 1.11). There were no significant differences between anticoagulants and antiplatelets (HR: 0.92, 95% CI: 0.72, 1.17) and between LMWH and UFH (HR: 1.09, 95% CI: 0.85, 1.39), regarding hazards for VAEs.
Compared to regimens without antithrombosis agent, no statistically significant decrease in IVACs and PVAPs was found for antithrombotic, anticoagulation, and antiplatelet agent. There were no statistically differences regarding the comparisons of antiplatelet agents vs. regimens without antithrombosis agents, anticoagulation agents vs. antiplatelet agents, and LMWH vs. UFH (Table 3).
Adjusted HRs for antithrombosis agents and ICU mortality and length of stay are summarized in Table 4. Compared to regimens without antithrombosis agent, antithrombosis agent (HR: 0.72, 95% CI: 0.61, 0.86) and anticoagulant (HR: 0.62, 95%CI: 0.51, 0.76) agents were associated with lower risk of ICU mortality. Regimens with anticoagulant agents were associated an increased hazard for ICU discharge (HR: 1.15, 95%CI: 1.04, 1.27) than those without antithrombosis agent, suggesting that anticoagulant agents were associated with shorter time of ICU stays. Anticoagulant agents were associated with decreased risk of ICU mortality (HR:0.52, 95%CI: 0.37, 0.74) and less time to ICU discharge (HR:1.43, 95%CI: 1.18, 1.73) relative to antiplatelets agent. No statistically differences were found between LMWH and UFH regarding hazards for ICU mortality and ICU stays (Table 4).
Among patients with D-dimer >5 mg/LFEU, regimens with antithrombotic (HR: 0.84, 95% CI: 0.72, 0.98) and anticoagulant (HR: 0.81, 95% CI: 0.68, 0.98) were associated with lower risk of VAEs than regimens without. However, among patients with D-dimer ≤5 mg/LFEU, the effect of antithrombotic, anticoagulant, and antiplatelet agent on VAEs was not statistically significant (Supplementary Table S1). With regarding to ICU mortality, regimens with antithrombosis agents and regimens with anticoagulant were associated with decreased risk of ICU mortality both among patients with D-dimer >5 mg/LFEU and ≤5 mg/LFEU (Supplementary Table S1).
Sensitivity analyses using alternative statistical models did not show change in interpretation. The result of complete cases analysis was consistent with primary analysis for the comparison of regimens with and without antithrombosis agents regarding hazards for VAEs, but with wider confidence intervals that crossed one (HR 0.88, 95% CI: 0.78, 1.01). The sensitivity analyses of ICU stays using alternative definition of comparison and alternative approach for missing data showed similar results, but the confidence interval less than one regarding the comparison of regimens with and without antiplatelet agents (Supplementary Table S2).
Additional analysis showed that antithrombosis agents were associated with decreased risk of VTE (HR 0.85, 95% CI:0.52, 1.39).
The results of this large observational study showed that pharmacological thromboprophylaxis was commonly administered in ICU patients on MV. Pharmacological thromboprophylaxis was associated with lower risk of VAEs and ICU mortality. The effects of different type of pharmacological thromboprophylaxis on clinical outcomes may differ: treatment of antithrombotic, anticoagulant, but not antiplatelet appear beneficial. Antithrombotic may be associated with decreased risk of VAEs among patients with D-dimer >5 mg/LFEU; however, the effect was not statistically significant among patients with D-dimer ≤5 mg/LFEU.
Similar to ours, several other studies have suggested that thrombosis prophylaxis may decrease the risk of adverse outcomes. A multicenter prospective observational study that included 630 patients receiving MV for at least 48 h showed that DVT prophylaxis was significantly associated with lower incidences of VAP (Croce et al., 2013). Another study on 175,665 ICU patients found that omission of early thromboprophylaxis may increase the risk of mortality for patients with critical illness (Ho et al., 2011). A possible reason may be that pharmacological thromboprophylaxis could decrease the risk of VTE, which may further result in adverse outcomes, such as respiratory deterioration, prolonged MV, and mortality. Two systematic review and meta-analyses showed significantly lower risk of DVT and PE in patients receiving thromboprophylaxis than those without (Alhazzani et al., 2013; Park et al., 2016). The in-hospital mortality was 50% higher among patients with DVT than those without (Jain and Schmidt, 1997; Ejaz et al., 2018), and PE was attributable to 4–11% cause of deaths (Laporte and Mismetti, 2010).
However, several other studies have not found a significant association between thromboprophylaxis and adverse outcomes. A case–control study including 110 VAE cases suggested that thrombosis prophylaxis did not reduce the risk of VAEs and IVACs (Lewis et al., 2014). Klompas et al. conducted a retrospective cohort study and showed that thromboembolism prophylaxis was not associated with decreased risk of VAEs, VAP, hospital stays, and mortality (Klompas et al., 2016). There are several potential reasons for these apparent inconsistencies in results. One reason may be due to the varied outcome definitions, especially for VAPs. For instance, Klompas et al. defined VAPs according to the new surveillance criteria proposed by US CDC, whereas Croce et al. used the traditional definition of VAP (Croce et al., 2013; Klompas et al., 2016). The correlation between VAE and VAP has been proved to be poor (Klompas, 2019). Secondly, the strategies for thromboembolism prophylaxis varied among studies and individual patients. Thromboprophylaxis included pharmacological and mechanical prophylaxis, and the effect of these two measures on preventing adverse events may differ (Park et al., 2016; Boddi and Peris, 2017). A meta-analysis including 12 trials suggested that pharmacological prophylaxis with UFH and LMWH significantly decreased the incidence of DVT. Mechanical thromboprophylaxis, however, was not associated with a significant reduction of DVT risk (Park et al., 2016). Although most previous studies involved both pharmacological and mechanical prophylaxis, the detailed measures and compliance with thromboprophylaxis among studies likely varied (Manoucheri and Fallahi, 2015; Ejaz et al., 2018). In addition, these studies involved patients with different clinical features, such as coagulation function. The effect of thromboprophylaxis on clinical outcomes may differ among patients with different coagulation function (Goodwin and Hoffman, 2011). Patients with hypercoagulability may more likely to benefit from thromboprophylaxis. Our study also showed that antithrombotic was associated with lower risk VAEs among patients with elevated D-dimer.
This study has several strengths. First, our study was conducted based on a multisource database, which contained complete and accurate information regarding HAI in the ICU setting. We included a relatively large number of patients in addition to identifying a large number of VAEs. Validation of outcomes showed a high level of accuracy. Second, we considered time-varying terms for daily pharmacological thromboprophylaxis exposures and used competing risk models to handling the competing risks for ICU discharge alive versus ICU mortality.
Our study also has some limitations. First, the results should be interpreted with caution given the nature of the retrospective observational study. Although we adjusted an extensive array of factors, unmeasured residual confounding factors may still be present. Second, data for some variables were missing. Third, this study was conducted using data from a homogeneous healthcare system, and the findings of our study may not be generalizable to other healthcare settings. Fourthly, only few patients developed VTE, and the inference on the effect of VTE is weakened.
Antithrombosis agents were associated with a lower risk of VAEs and ICU mortality than regimens without antithrombosis agents. Anticoagulation but not antiplatelet agents appeared beneficial. The effects appeared comparable on comparing UFH vs. LMWH. Large, rigorous, randomized trials are needed to validate these results.
The original contributions presented in the study are included in the article/Supplementary Material, and further inquiries can be directed to the corresponding authors.
This study was approved by the Ethical Committee of West China Hospital in 2018 (WCH2018-409), and the need for patient consent was waived.
XS and ZZ conceived and designed the research and assisted in revision of manuscript; MW cleared and analyzed research data and wrote the initial manuscript; WW assisted in the design of the research, interpreted research results, and revised the manuscript; QH, SZ and XJ collected research data and assisted in the interpretation of research results; YK collected the research data; RZ assisted in acquisition and clean data; LL and YR assisted the statistical analysis; and KZ assisted in the interpretation of research results. All authors read and approved the final manuscript.
This study was supported by funding from the National Natural Science Foundation of China (Grant No. 72104155), National Key Research and Development Program (Grant No. 2020YFC2009003), Sichuan Youth Science and Technology Innovation Research Team (Grant No. 2020JDTD0015), and 1·3·5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (Grant Nos. ZYYC08003 and ZYYC08006).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.891178/full#supplementary-material
APACHE II, Acute physiology and chronic health evaluation; CDC, Centers for Disease Control and Prevention; CI, Confidence interval; DVT, Deep vein thrombosis; EMR, Electronic medical record; FiO2, Fraction of inspired oxygen; HAI, Healthcare-associated infections; HR, Hazard ratio; ICU, Intensive care units; IVAC, Infection-related ventilator-associated complication; LMWH, Low molecular weight heparin; MV, Mechanical ventilation; PE, Pulmonary embolism; PEEP, Positive end-expiratory pressure; PVAP, Possible ventilator-associated pneumonia; UFH, Unfractionated heparin; VAC, Ventilator-associated condition; VAEs, Ventilator-associated events; VAP, Ventilator-associated pneumonia; VTE, Venous thromboembolism; WCH, West China Hospital.
Al Yami, M. S., Silva, M. A., Donovan, J. L., and Kanaan, A. O. (2017). Venous Thromboembolism Prophylaxis in Medically Ill Patients: a Mixed Treatment Comparison Meta-Analysis. J. Thromb. Thrombolysis 45 (1), 36–47. doi:10.1007/s11239-017-1562-5
Alhazzani, W., Lim, W., Jaeschke, R. Z., Murad, M. H., Cade, J., and Cook, D. J. (2013). Heparin Thromboprophylaxis in Medical-Surgical Critically Ill Patients: a Systematic Review and Meta-Analysis of Randomized Trials. Crit. Care Med. 41 (9), 2088–2098. doi:10.1097/CCM.0b013e31828cf104
Attia, J., Ray, J. G., Cook, D. J., Douketis, J., Ginsberg, J. S., and Geerts, W. H. (2001). Deep Vein Thrombosis and its Prevention in Critically Ill Adults. Arch. Intern Med. 161 (10), 1268–1279. doi:10.1001/archinte.161.10.1268
Bajaj, N. S., Vaduganathan, M., Qamar, A., Gupta, K., Gupta, A., Golwala, H., et al. (2019). Extended Prophylaxis for Venous Thromboembolism after Hospitalization for Medical Illness: A Trial Sequential and Cumulative Meta-Analysis. PLoS Med. 16, e1002797. doi:10.1371/journal.pmed.1002797
Barrera, L. M., Perel, P., Ker, K., Cirocchi, R., Farinella, E., and Morales Uribe, C. H. (2010). Thromboprophylaxis for Trauma Patients. Cochrane Database Syst. Rev. 2010 (3), CD008303. doi:10.1002/14651858.CD008303.pub2
Benchimol, E. I., Smeeth, L., Guttmann, A., Harron, K., Moher, D., Petersen, I., et al. (2015). The REporting of Studies Conducted Using Observational Routinely-Collected Health Data (RECORD) Statement. PLoS Med. 12 (10), e1001885. doi:10.1371/journal.pmed.1001885
Berenholtz, S. M, Pham, J. C., Thompson, D. A, Needham, D. M., Lubomski, L. H., Hyzy, R. C., et al. (2011). Collaborative Cohort Study of an Intervention to Reduce Ventilator-Associated Pneumonia in the Intensive Care Unit. Infect. Control Hosp. Epidemiol. 32 (4), 305–314. doi:10.1086/658938
Berger, M., Schmid, M., Welchowski, T., Schmitz-Valckenberg, S., and Beyersmann, J. (2018). Subdistribution Hazard Models for Competing Risks in Discrete Time. Biostatistics 21 (3), 449–466. doi:10.1093/biostatistics/kxy069
Boddi, M., and Peris, A. (2017). Deep Vein Thrombosis in Intensive Care. Adv. Exp. Med. Biol. 906, 167. doi:10.1007/5584_2016_114
Boonyawat, K., and Crowther, M. (2015). Venous Thromboembolism Prophylaxis in Critically Ill Patients. Semin. Thromb. Hemost. 41 (01), 068–074. doi:10.1055/s-0034-1398386
Bouadma, L., Sonneville, R., Garrouste-Orgeas, M., Darmon, M., Souweine, B., Voiriot, G., et al. (2015). Ventilator-Associated Events: Prevalence, Outcome, and Relationship with Ventilator-Associated Pneumonia. Crit. Care Med. 43 (9), 1798–1806. doi:10.1097/CCM.0000000000001091
Croce, M. A., Brasel, K. J., Coimbra, R., Adams, C. A., Miller, P. R., Pasquale, M. D., et al. (2013). National Trauma Institute Prospective Evaluation of the Ventilator Bundle in Trauma Patients: Does it Really Work? J. Trauma Acute Care Surg. 74 (2), 354–362. doi:10.1097/TA.0b013e31827a0c65
Ejaz, A., Ahmed, M. M., Tasleem, A., Rafay Khan Niazi, M., Ahsraf, M. F., Ahmad, I., et al. (2018). Thromboprophylaxis in Intensive Care Unit Patients: A Literature Review. Cureus 10 (9), e3341. doi:10.7759/cureus.3341
Fine, J. P., and Gray, R. J. (1999). A Proportional Hazards Model for the Subdistribution of a Competing Risk. J. Am. Stat. Assoc. 94 (446), 496–509. doi:10.1080/01621459.1999.10474144
Goodwin, C. M., and Hoffman, J. A. (2011). Deep Vein Thrombosis and Stress Ulcer Prophylaxis in the Intensive Care Unit. J. Pharm. Pract. 24 (1), 78–88. doi:10.1177/0897190010393851
Harris, B. D., Thomas, G. A., Greene, M. H., Spires, S. S., and Talbot, T. R. (2018). Ventilator Bundle Compliance and Risk of Ventilator-Associated Events. Infect. Control Hosp. Epidemiol. 39 (6), 637–643. doi:10.1017/ice.2018.30
He, Q., Wang, W., Zhu, S., Wang, M., Kang, Y., Zhang, R., et al. (2021). The Epidemiology and Clinical Outcomes of Ventilator-Associated Events Among 20,769 Mechanically Ventilated Patients at Intensive Care Units: An Observational Study. Crit. Care 25 (1), 44. doi:10.1186/s13054-021-03484-x
Ho, K. M., Chavan, S., and Pilcher, D. (2011). Omission of Early Thromboprophylaxis and Mortality in Critically Ill Patients: A Multicenter Registry Study. Chest 140 (6), 1436–1446. doi:10.1378/chest.11-1444
Jain, M., and Schmidt, G. (1997). Venous Thromboembolism: Prevention and Prophylaxis. Semin. Respir. Crit. Care Med. 18 (01), 79–90. doi:10.1055/s-2007-1009334
Klemen, N. D., Feingold, P. L., Hashimoto, B., Wang, M., Kleyman, S., Brackett, A., et al. (2020). Mortality Risk Associated with Venous Thromboembolism: A Systematic Review and Bayesian Meta-Analysis. Lancet Haematol. 7 (8), e583–e93. doi:10.1016/S2352-3026(20)30211-8
Klompas, M., Li, L., Kleinman, K., Szumita, P. M., and Massaro, A. F. (2016). Associations between Ventilator Bundle Components and Outcomes. JAMA Intern Med. 176 (9), 1277–1283. doi:10.1001/jamainternmed.2016.2427
Klompas, M. (2010). Ventilator-Associated Pneumonia: Is Zero Possible? Clin. Infect. Dis. 51 (10), 1123–1126. doi:10.1086/656738
Klompas, M. (2013). Complications of Mechanical Ventilation-Tthe CDC's New Surveillance Paradigm. N. Engl. J. Med. 368 (16), 1472–1475. doi:10.1056/NEJMp1300633
Klompas, M. (2019). Barriers to the Adoption of Ventilator-Associated Events Surveillance and Prevention. Clin. Microbiol. Infect. 25 (10), 1180–1185. doi:10.1016/j.cmi.2019.03.027
Laporte, S., and Mismetti, P. (2010). Epidemiology of Thrombotic Risk Factors: the Difficulty in Using Clinical Trials to Develop a Risk Assessment Model. Crit. Care Med. 38 (2 Suppl. l), S10–S17. doi:10.1097/CCM.0b013e3181c9cc3b
Lewis, S. C., Li, L., Murphy, M. V., and Klompas, M. (2014). Risk Factors for Ventilator-Associated Events: A Case-Control Multivariable Analysis. Crit. Care Med. 42 (8), 1839–1848. doi:10.1097/CCM.0000000000000338
Li, S., Yu, C., Li, Y., Li, Q., Zhang, R., Hou, Q., et al. (2018). Study Design and Baseline Characteristics of Inpatients with Diabetes Mellitus in a Tertiary Hospital in China: A Database Study Based on Electronic Medical Records. J. Evid. Based Med. 11 (3), 152–157. doi:10.1111/jebm.12291
Magill, S. S., Li, Q., Gross, C., Dudeck, M., Allen-Bridson, K., and Edwards, J. R. (2016). Incidence and Characteristics of Ventilator-Associated Events Reported to the National Healthcare Safety Network in 2014. Crit. Care Med. 44 (12), 2154–2162. doi:10.1097/CCM.0000000000001871
Manoucheri, R., and Fallahi, M. J. (2015). Adherence to Venous Thromboprophylaxis Guidelines for Medical and Surgical Inpatients of Teaching Hospitals, Shiraz-Iran. Tanaffos 14 (1), 17–26.
Marra, A. R., Cal, R. G., Silva, C. V., Caserta, R. A., Paes, A. T., Moura, D. F., et al. (2009). Successful Prevention of Ventilator-Associated Pneumonia in an Intensive Care Setting. Am. J. Infect. Control 37 (8), 619–625. doi:10.1016/j.ajic.2009.03.009
Park, J., Lee, J. M., Lee, J. S., and Cho, Y. J. (2016). Pharmacological and Mechanical Thromboprophylaxis in Critically Ill Patients: A Network Meta-Analysis of 12 Trials. J. Korean Med. Sci. 31 (11), 1828–1837. doi:10.3346/jkms.2016.31.11.1828
Wang, W., Zhu, S., He, Q., Zhang, R., Kang, Y., Wang, M., et al. (2019). Developing a Registry of Healthcare-Associated Infections at Intensive Care Units in West China: Study Rationale and Patient Characteristics. Clin. Epidemiol. 11, 1035–1045. doi:10.2147/CLEP.S226935
Keywords: antithrombosis prophylaxis, ventilator-associated events, ventilator-associated pneumonia, ICU mortality, patients receiving mechanical ventilation
Citation: Wang M, Wang W, Jia X, He Q, Zhu S, Kang Y, Zhang R, Ren Y, Li L, Zou K, Zong Z and Sun X (2022) Associations Between Antithrombosis and Ventilator-Associated Events, ICU Stays, and Mortality Among Mechanically Ventilated Patients: A Registry-Based Cohort Study. Front. Pharmacol. 13:891178. doi: 10.3389/fphar.2022.891178
Received: 07 March 2022; Accepted: 21 June 2022;
Published: 18 July 2022.
Edited by:
Robert L. Lins, Independent researcher, Antwerp, BelgiumReviewed by:
Shengping Yang, Pennington Biomedical Research Center, United StatesCopyright © 2022 Wang, Wang, Jia, He, Zhu, Kang, Zhang, Ren, Li, Zou, Zong and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Sun, c3VueGluQHdjaHNjdS5jbg==; Zhiyong Zong, em9uZ3poaXlvbmdAZ21haWwuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.