
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 05 July 2022
Sec. Pharmacology of Anti-Cancer Drugs
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.891171
Cancer ranks as a primary reason for death worldwide. Conventional anticancer therapies can cause severe side effects, and thus natural products may be promising drug candidates for cancer therapy. Accumulating evidence has verified the prominent anticancer properties of Ganoderma polysaccharides, suggesting that Ganoderma polysaccharides may be effective chemopreventive agents of natural origin. Based on their abilities to prevent cancer development by regulating the DNA damage response, cancer cell proliferation, apoptosis, host immunity, gut microbiota and therapeutic sensitivity, there has been increasing interest in elucidating the clinical implication of Ganoderma polysaccharides in cancer therapy. In this review, we summarize recent findings pertaining to the roles of bioactive polysaccharides from Ganoderma in cancer pathogenesis, discuss the multifarious mechanisms involved and propose future directions for research. A more sophisticated understanding of the anticancer benefits of Ganoderma polysaccharides will be helpful for improving current treatments and developing novel therapeutic interventions for human malignancies.
Ganoderma, named lingzhi in China, is one of the most well-known medicinal herbs worldwide (Loyd et al., 2018). Ganoderma is a large, diverse and widely distributed genus in the family Ganodermataceae that comprises at least 300 species (Winska et al., 2019). The common species of Ganoderma include G. applanatum, G. atrum, G. formosanum, G. lucidum, G. sinense, and G. tsugae (Cao et al., 2018). Ganoderma is considered as the “marvelous herb” and has been widely applied for centuries as a drug or nutraceutical to treat many forms of diseases, including autoimmune diseases, diabetes, gastrointestinal disorders and hepatitis (Cao et al., 2018). Particularly, Ganoderma can be an alternative adjunctive therapy for cancer (Zhang Y. et al., 2019). Ganoderma harbors a variety of components including alkaloids, benzoic acid derivatives, glycoproteins, meroterpenoids, polysaccharides, steroids, and triterpenoids (Sohretoglu and Huang, 2018). Among these, polysaccharides are the main bioactive compound in Ganoderma and contribute to its health benefits (Wen et al., 2022).
The most common classes of polysaccharides from Ganoderma spp. are glucans, glycopeptides, glycoproteins, and water-soluble heteropolysaccharides made up of different monosaccharides linked by α-1, 3, α-1, 4, α-1, 6 or β-glycosidic bonds (Cor et al., 2018). Polysaccharides from Ganoderma have been widely studied for their versatile pharmacological activities, such as anticancer, anti-inflammatory, antimicrobial, antioxidant, and immunoregulatory properties (Seweryn et al., 2021). Ganoderma polysaccharides exert anticancer actions through various mechanisms, including impairment of the DNA damage repair pathway, inhibition of cell proliferation and migration, activation of cell death pathways, reinforcement of antitumor immunity, amelioration of gut microbiota dysbiosis and enhancement of cancer cell susceptibility to anticancer therapies. A comprehensive perception of the biological functions of Ganoderma polysaccharides will likely bring significant advances in cancer treatment. However, there are a very few literatures that provide an in-depth landscape of the mechanisms of action of Ganoderma polysaccharides in cancer. In this review, we provide an overview of the anticancer effects of bioactive polysaccharides isolated from Ganoderma spp., with a focus on the underlying mechanisms responsible for their activities.
Ganoderma harbors 59% crude fiber, 26–28% carbohydrate, 7–8% crude protein and 3–5% crude fat (Wang J. et al., 2017). Polysaccharides from Ganoderma are structurally diverse biomacromolecules with molecular weights ranging from thousands to millions. So far, over 200 polysaccharides have been separated from spores, mycelia and fruiting bodies from Ganoderma spp., including α-D-glucans, α-D-mannans, β-D-glucans, and polysaccharide-protein complexes (Ahmad, 2018; Hwang et al., 2018). Polysaccharides have a linear to highly branched conformation (Zeidan et al., 2017). Polysaccharides from Ganoderma are water-soluble while insoluble in alcohol (Lu et al., 2020). Polysaccharides are mainly categorized into two types according to the count of different monomers, homopolysaccharides and heteropolysaccharides (Ferreira et al., 2015). Most polysaccharides from Ganoderma are heteropolysaccharide consisting of various monosaccharides including arabinose, galactose, glucose, mannose and xylose in diverse conformations, while a few are homopolysaccharide, including dextran and galactosan (Wang J. et al., 2017). The first step in the isolation of polysaccharides is pulverization of Ganoderma, followed by the removal of impurities such as compounds with low weights and pigments (Ren et al., 2021). Based on the distributed location and solubility of polysaccharides in cell wall of Ganoderma, hot water or organic solutions including dilute acid, dilute alkalis and salt solvents can be used to extract polysaccharides (Shi, 2016). Among these, hot water extraction is the most popular approach due to its convenience, ease of operation and safety. Enzymolysis, Sevag method, lead acetate precipitation, salting out, and trichloroacetic acid (TCA) precipitation are applied in the purification of polysaccharide (Zeng et al., 2019). In addition, column chromatography methods (e.g., affinity chromatography, anion exchange column chromatography and gel permeation chromatography) can also be used to remove impurities from the polysaccharides (Lu et al., 2020). The most frequent classes of polysaccharides extracted from Ganoderma spp. include glucans, glycopeptides, glycoproteins, and water-soluble heteropolysaccharides (Kladar et al., 2016). Ganoderma polysaccharides exert a broad range of pharmacological effects, including anti-inflammatory, immunostimulating and anticancer activities (Sohretoglu and Huang, 2018). It is proposed that branching conformation and solubility features may be vital parameters in determining the anticarcinogenic capabilities of Ganoderma polysaccharides.
Glucans are glucose polymers that are mainly divided into α-glucans and β-glucans based on the nature of chemically glycosidic bounds as either being α- or β-linked (Cerletti et al., 2021). β-glucans are a heterogeneous group of natural polysaccharides and comprise D-glucose monomers linked by β-glycosidic bonds (Han et al., 2020). β-glucans are the main polysaccharide present in Ganoderma (Ren et al., 2021). The pharmacokinetic profiles of three water-soluble polysaccharides (glucan phosphate, laminarin and scleroglucan) were previously characterized following intravenous administration in rats (Rice et al., 2004). Because of the variations in branching frequency, molecular size and solution conformation, pharmacokinetics for these polysaccharides were distinct. Glucan phosphate showed a smaller volume of distribution and lower clearance, leading to an elimination half-life similar to that of laminarin and scleroglucan. In case of continual administration, plasma glucan levels at steady state were inversely correlated with clearance, and differences in clearance might cause higher plasma levels of glucans with specific physicochemical properties. The pharmacokinetics after oral administration showed differences among these three glucans (Rice et al., 2005). Pharmacokinetic investigation from rats indicated that the peak plasma concentration of glucan phosphate was reached at 4 h after oral administration. By contrast, maximum plasma contents of laminarin occurred at 3 h and again at 12 h. Plasma scleroglucan levels rapidly increased after oral administration and achieved peak concentration at 15 min and again at 3 h. The plasma level of glucan phosphate was 27 ± 3% of maximum at 24 h. There was 20 ± 7% of laminarin remained in the serum at 24 h. Unlike glucan phosphate and laminarin, scleroglucan was cleared from the systemic circulation after 12 h. The bioavailability for laminarin (4.9%) or scleroglucan (4.0%) was apparently higher than for glucan phosphate (0.5%). Vetvicka et al. (Vetvicka et al., 2007) assessed the absorption and tissue distribution of enterally administrated β-glucans in a suckling rat model. The authors revealed that the majority of β-glucans could be detected in the duodenum and stomach 5 minutes following administration. Their contents rapidly declined during first 30 min. A remarkable level of β-glucans entered the proximal intestine. The shuttling of β-glucans through proximal intestine decreased with time, accompanied by an increased amount of β-glucans in the ileum.
Further study suggests that β-glucans enter the proximal small intestine and are internalized by gastrointestinal macrophages after oral administration (Zhang et al., 2018). This may be followed by the transit of β-glucans to the bone marrow and endothelial reticular system and their breakdown within the marrow. The small biologically active β-glucan fragments are secreted by the macrophages and captured by the circulating granulocytes, monocytes, and dendritic cells. However, there is limited evidence supporting this presumptive pharmacokinetics for glucans occurred in humans. The bioavailability and mechanism of glucans in human subjects deserve further study. Despite the pharmacokinetics of some fungal polysaccharides have been determined, the bioavailability of Ganoderma polysaccharides remains to be investigated.
A growing body of evidence has indicated the anticarcinogenic property of Ganoderma polysaccharides (Table 1). Ganoderma polysaccharides repress carcinogenesis and cancer development through their action on intracellular signaling cascades, enhancement of antitumor immunity and modulation of gut microbiota. In addition, Ganoderma polysaccharides augment the efficacy of anticancer therapies, stressing that Ganoderma polysaccharides may act as a complement to existing cancer treatments. The detailed mechanisms of action of Ganoderma polysaccharides are discussed in the following sections.
DNA damage response plays an important role in maintaining genomic stability (Urulangodi and Mohanty, 2020). Dysregulated DNA damage repair pathways are linked with cancer occurrence and development (Li LY. et al., 2020). Targeting DNA repair pathways could enhance cancer cell sensitiveness to anticancer therapies and is a potential therapeutic option for cancer intervention. G. lucidum polysaccharide (GLP) was shown to augment radiation-mediated growth inhibition and apoptosis promotion in hepatocellular carcinoma (HCC) (Yu et al., 2017). As one of mainstream anticancer regimens, radiation can induce cancer cell death by triggering DNA damage (Li LY. et al., 2020). Ataxia-telangiectasia mutated (ATM) and DNA dependent-protein kinase (DNA-PK) are responsible for the repair of radiation-induced DNA lesions and double-strand break (DSB) (Bannik et al., 2021). GLP impaired the DNA damage repair pathway in radiation-treated HCC cells by restraining the function of ATM and DNA-PK (Yu et al., 2017). The protein kinase B (Akt) signaling pathway is involved in DNA damage repair (Alemi et al., 2022). As expected, the Akt repressor increased the activities of ATM and DNA-PK and weakened the anticancer effects of GLP on HCC cells. It could be inferred that the Akt signaling pathway mediated GLP-induced HCC radiosensitization. GLP might be a promising radiation sensitizer to enhance the therapeutic effectiveness of ionizing radiation in HCC patients. Because of its antioxidant property, GLP exhibited radioprotective activity and supported DNA damage repair in human lymphocytes (Pillai et al., 2014). It is possible that there exists a complex interplay between the promotion and repair of radiation-induced DNA damage in GLP-treated cells. The DNA repairing potential of GLP may occupy a dominant position in normal cells, contributing to the prevention of genomic instability and cancer formation. Conversely, GLP facilitates cancer cell death by enhancing DNA damage. However, considerable efforts are required to clarify the knowledge gaps concerning the context-dependent effects of GLP on DNA damage repair.
The DNA damage repair pathway plays a pivotal role in the development of chemoresistance or radioresistance in cancer (Lagunas-Rangel and Bermudez-Cruz, 2020). Induction of DNA damage prevents cancer growth and initiates cancer cell apoptosis. Targeting the DNA damage repair pathway may enhance the efficacy of traditional therapeutic strategies. Ganoderma polysaccharides can target DSB repair kinases ATM and DNA-PK, thus inhibiting cancer pathogenesis (Figure 1). DSB is a major threat to genomic integrity and may arise from the presence of genotoxic agents (Karl et al., 2022). Cells adopt several DSB repair mechanisms including homologous recombination (HR), non-homologous end-joining (NHEJ) and single strand annealing (SSA) that involve a number of mediators and effectors [e.g., ATM, breast cancer susceptibility gene 1/2 (BRCA1/2), checkpoint kinase 1/2 (CHK1/2) and DNA-PK] (Blasiak, 2021). The contribution of Ganoderma polysaccharides to the disruption of particular DSB repair mechanisms awaits further confirmation. Cells also evolve distinct mechanisms involved in repairing other types of DNA lesions, such as replication errors and single-strand break (SSB) (Chatterjee and Walker, 2017). An in-depth investigation on the crosstalk among diverse DNA repair systems will be conducive to the development of more effective therapies that target specific repair pathways. Currently, there are very few literatures on the effect of Ganoderma polysaccharides on the highly intricate DNA repair machineries. Therefore, it is of great necessity to fully understand the roles of Ganoderma polysaccharides in the regulation of DNA repair systems.

FIGURE 1. Schematic of the main anticancer molecular mechanisms of Ganoderma polysaccharides. Ganoderma polysaccharides retard cell cycle progression by targeting the PRMT6/p21/CDK2 signaling pathway. Ganoderma polysaccharides inhibit cancer cell proliferation by suppressing the TGF-β/SMAD2 signaling cascade or downregulating PCNA and Ki67. Ganoderma polysaccharides exert pro-apoptotic actions through modulation of several signal transduction cascades, including the JNK, p38 and JAK/STAT5 signaling pathways. Moreover, they can aggrandize cellular apoptotic response by altering the expression of apoptosis-relevant proteins, such as Bax, Bcl-2, caspase-9, caspase-3 and PARP. Ganoderma polysaccharides impede the EMT program in cancer cells by inactivating the ERK signaling pathway. Intriguingly, Ganoderma polysaccharides interfere with the DNA damage repair response via restricting the activities of DNA-PK and ATM through suppression of the upstream PI3K/Akt signaling cascade. Ganoderma polysaccharides increase the expression of Beclin-1 and LC3 to initiate early autophagic flux, which subsequently facilitates cancer cell apoptosis. In addition, Ganoderma polysaccharides augment the pro-apoptotic effect of chemotherapeutic drugs and lower the expression of the drug efflux pump ABCB1, which reinforce the anticancer efficacy of chemotherapeutic drugs. PRMT6, protein arginine methyltransferase 6; CDK2, cyclin-dependent kinase 2; TGF-β, transforming growth factor-β; TGFβR, transforming growth factor-β receptor; SMAD, small mother against decapentaplegic protein; PCNA, proliferating cell nuclear antigen; TAK1, transforming growth factor-β-activated kinase 1; TAB, TAK1-binding protein; TRAF6, tumor necrosis factor receptor-associated factor 6; MKK, mitogen-activated protein kinase; JNK, c-Jun N-terminal kinase; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; RAS, rat sarcoma virus; RAF, rapidly accelerated fibrosarcoma; MEK, mitogen-activated protein/extracellular signal-regulated kinase; ERK, extracellular signal-regulated kinase; JAK, Janus kinase; RTK, receptor tyrosine kinase; STAT5, signal transducer and activator of transcription 5; PI3K, phosphatidylinositol 3-kinase; PIP3, phosphatidylinositol 3,4,5-triphosphate; Akt, protein kinase B; Bcl-xL, B-cell lymphoma-extra large; DNA-PK, DNA-dependent protein kinase; ATM, ataxia-telangiectasia mutated; Bcl-2, B-cell lymphoma-2; Bax, Bcl-2-associated X protein; LC3, microtubule-associated protein light chain 3; COX-2, cyclooxygenase-2; cleaved PARP, cleaved poly (ADP-ribose) polymerase; ABCB1, adenosine triphosphate (ATP)-binding cassette subfamily B member 1.
Ganoderma polysaccharides can affect cancer cell proliferation and malignancy. The water soluble polysaccharides from G. lucidum (WSG) significantly reduced the viability and mobility of lung cancer cells (Hsu et al., 2020). WSG impeded the phosphorylation of multiple intracellular signaling molecules [e.g., extracellular signal-regulated kinase 1/2 (ERK1/2), focal adhesion kinase (FAK), Akt and SMAD2] upon epidermal growth factor (EGF) and transforming growth factor-β1 (TGF-β1) stimulation, thereby blocking EGF- and TGF-β-activated intracellular signaling pathways (Figure 1). Mechanistically, WSG accelerated the degradation of epidermal growth factor receptor (EGFR) and transforming growth factor-β receptors (TGFβRs) by mobilizing proteasome- and lysosome-mediated protein degradation pathways. It remains to explore how WSG affects the ubiquitination of EGFR. The molecular mechanisms underlying WSG-mediated TGFβR degradation also warrant special attention. It is necessary to verify whether WSG can directly combine with TGFβRs or whether WSG promotes the transport of TGFβRs to the lysosome. Furthermore, WSG repressed lung cancer growth and metastasis in vivo and prolonged the survival of tumor-bearing mice (Hsu et al., 2020). WSG might act as a potential chemopreventive agent against lung cancer. Clinical studies are still required to assess the safety and effectiveness of WSG used alone or co-treatment with chemotherapeutic agents in patients with lung cancer.
Coix seed oil has been used to treat cancer in clinical practice (Wang Y. et al., 2017). GLP-integrated coix oil-based microemulsion (MEs(PS-GLP)) showed a dose-dependent cytotoxicity toward lung cancer cells (Guo et al., 2018). In vivo experimental studies demonstrated that MEs(PS-GLP) exhibited significant tumor-suppressing capabilities in nude mice bearing lung cancer xenografts and might be an oral anticancer agent delivery system. Particularly, the integration of GLP extended the intestinal retention of MEs (PS-GLP), which might be attributable to the enhancement of intestinal attachment caused by GLP. The connection of GLP also promoted the accumulation of MEs (PS-GLP) in tumor tissues. These results supported further studies to substantiate the therapeutic efficacy of MEs (PS-GLP) in patients with lung cancer. In addition, the mechanisms of action of MEs (PS-GLP) in tumor suppression need to be thoroughly defined.
Ganoderan B (GDNB), a component of GLP, repressed the proliferation of lung cancer cells by downregulating Ki67 and proliferating cell nuclear antigen (PCNA) (Wang et al., 2019). Moreover, GDNB inhibited lung cancer cell invasion and migration by reducing the expression of N-cadherin, vimentin and Snail and increasing the expression of E-cadherin. In addition, GDNB fostered lung cancer cell apoptosis by altering the expression levels of apoptosis-related proteins including B-cell lymphoma-2 (Bcl-2), Bcl-2-associated X protein (Bax), cleaved caspase-3, and cleaved poly (ADP-ribose) polymerase (PARP). In vivo experimental evidence verified that GDNB suppressed the growth and motility and promoted the apoptosis of lung cancer cells by inactivating the ERK signaling cascade in tumor-bearing mice. These findings demonstrated that GDNB had clinical implication in cancer treatment. However, only the effect of GDNB on the expression levels of marker proteins in the ERK signaling pathway was determined in this study. The involvement of the ERK signaling pathway in GDNB-mediated tumor suppression warrants additional investigation. Besides, there is still a need for further evidence with respect to the role of GDNB in lung cancer.
GLP repressed cell growth and migration, and induced cell cycle arrest in prostate cancer cells (Zhao et al., 2018). Mechanistical investigation showed that GLP impeded the oncogenic protein arginine methyltransferase 6 (PRMT6) signaling pathway and inhibited the expression of migration-associated proteins FAK and florigen repression complex (FRC). P21 is a vital downstream molecule of PRMT6 and can induce cell cycle arrest (Feng et al., 2019). Cyclin-dependent kinase 2 (CDK2) is also a key mediator of cell cycle progression and plays a crucial role in the migration and metastasis of cancer cells (Lukasik et al., 2021). PRMT6 depletion apparently increased the expression of p21 while decreased that of CDK2. Thus, GLP was effective in inhibiting the progression of prostate cancer by orchestrating cell cycle progression. Further work is required to determine whether GLP has a direct effect on the activity of these cell cycle-related proteins.
Cell cycle dysregulation and sustained cell proliferation are key hallmarks of cancer (Kappel et al., 2019). Unsurprisingly, the aberrant expression of cell cycle-relevant genes is causative to carcinogenesis (Guo H. et al., 2021). Previous studies have demonstrated that Ganoderma polysaccharides can prevent cancer growth by affecting the expression of cell cycle-related genes p21 and CDK2. Moreover, Ganoderma polysaccharides directly modulate the activities of cellular proteins and signaling cascades that are involved in cell proliferation and motility. Much work is still required to understand the detailed mechanisms by which Ganoderma polysaccharides regulate cancer development. Multiple intracellular signal transduction cascades may act synergistically on Ganoderma polysaccharide-mediated tumor inhibition. It is intriguing whether Ganoderma polysaccharides have an impact on the crosstalk between cancer-associated signaling pathways. The profound effect of Ganoderma polysaccharides on intracellular signaling networks is worthy of further study.
Induction of cell death is currently the chief therapeutic purpose of anticancer therapies. Many studies have revealed the promotive action of Ganoderma polysaccharides on cell death in cancer. Reportedly, WSG strikingly decreased cell viability and colony formation of tongue cancer cells (Hsu et al., 2021). WSG promoted tongue cancer cell apoptosis by elevating the ratio of Bax to Bcl-2. WSG inhibited the phosphorylation of EGFR and Akt. WSG had a synergistic effect in combination with cisplatin on cell viability inhibition and apoptosis promotion. Particularly, WSG alleviated the cytotoxicity of cisplatin toward normal oral epithelial cells. Thus, WSG inhibited the development of tongue cancer by blocking the EGFR/Akt signaling pathway and inducing cell apoptosis. WSG might be a safe and effective adjuvant chemotherapy for tongue cancer.
The medicinal fungus G. applanatum polysaccharide (GAP) consisted of arabinose, fucose, glucose, and rhamnose (Hanyu et al., 2020). GAP suppressed the proliferation, invasion and migration of breast cancer cells in a time-/dose-dependent manner. GAP induced chromatin condensation and promoted apoptosis in breast cancer cells. GAP increased the expression of autophagy-associated markers Beclin-1 and microtubule-associated protein light chain 3 (LC3). The combination of GAP with chloroquine (CQ), an inhibitor of late autophagy, further increased the levels of LC3-II, suggesting that GAP stimulated early autophagy to induce breast cancer cell death. The mitogen-activated protein kinase (MAPK)/ERK signaling pathway has been associated with cell proliferation, apoptosis, autophagy and migration (Asl et al., 2021). Mechanistic investigation showed that GAP lowered the expression of phosphorylated ERK1/2 (p-ERK1/2) while increased the expression of phosphorylated p38 (p-p38) and phosphorylated c-Jun N-terminal kinase (p-JNK). Altogether, GAP exerted inhibitory roles in the development of breast cancer by favoring apoptosis and autophagy via the MAPK/ERK signaling cascade. In vivo experimental studies are required to confirm the anticancerous property of GAP. It is also necessary to clarify whether GAP promotes breast cancer cell apoptosis by inducing early autophagy. Further work is still warranted to ascertain whether other signaling pathways mediate the anticarcinogenic actions of GAP.
Zhong et al. Zhong et al. (2021) compared the anticancer activities of polysaccharides from sporoderm-removed spores (RSGLP) and sporoderm-broken spores of G. lucidum (BSGLP). RSGLP showed stronger inhibitory activity against gastric cancer cell viability than BSGLP. In terms of mechanism, RSGLP markedly enhanced the apoptosis of gastric cancer cells by downregulating Bcl-2 and pro-caspase-3 and upregulating cleaved PARP. Moreover, RSGLP increased the expression of autophagy-related proteins (LC3-II and p62). CQ further enhanced RSGLP-induced upregulation of LC3-II and p62, and the autophagy inducer rapamycin co-treatment with RSGLP elevated p62 expression. RSGLP facilitated autophagy initiation and autophagosome accumulation, and repressed autophagosome-lysosome function in gastric cancer cells. RSGLP-mediated regulation of autophagy is a highly fine-tuned process that awaits in-depth investigations. The effects of RSGLP on autophagy was partially responsible for its induction of apoptosis in gastric cancer cells. In summary, RSGLP could act as a potential autophagy suppressor in the intervention of gastric cancer.
5-Fluorouracil (5-FU) stabilized wild-type p53 in colorectal cancer (CRC) cells and thus induced apoptosis (Jiang et al., 2017). GLP alone or in combination with 5-FU suppressed CRC cell proliferation by reactivating mutant p53. GLP also restored the ability of p53 to transactivate the downstream genes encoding pro-apoptotic proteins Bax and p21. 5-FU induced the translocation of mutant p53 to the mitochondria. GLP further enhanced 5-FU-induced p53 mitochondrial translocation, caused mitochondrial membrane permeabilization (MMP) and led to cytochrome c release from the mitochondria. These events resulted in the activation of the downstream caspase cascade to initiate CRC cell apoptosis. GLP might severe as a potential chemotherapeutic agent for the treatment of cancer with mutant p53. Enzymatically hydrolyzed GLP (EGLP) showed cytotoxic activities against CRC cells (Bai et al., 2020). EGLP enhanced CRC cell apoptosis by upregulating Bax, p-ERK and cleaved caspase-3 and downregulating Bcl-2, phosphorylated Akt1 (p-Akt1) and cyclooxygenase-2 (COX-2). These findings provided scientific evidence supporting the therapeutic potential of EGLP in CRC treatment. GLP could initiate CRC cell autophagy by upregulating the levels of LC3-II and p62 and promoting the production of autophagosomes (Pan et al., 2019). Supplementation of CQ further raised the expression level of LC3-II and p62 and enhanced the accumulation of autophagosomes. GLP blocked the autophagosome-lysosome fusion through inhibition of lysosome acidification and lysosomal cathepsin activities. GLP-induced autophagosome accumulation contributed to its promotion effects on CRC cell apoptosis. The addition of 3-methyladenine (3-MA), an inhibitor of early autophagy, restrained GLP-induced cell apoptosis. On the contrary, suppression of late stage autophagy by CQ strengthened the anticancer actions of GLP. In addition, the MAPK/ERK signaling pathway was involved in GLP-induced autophagosome accumulation and apoptosis in CRC cells. Consistently, GLP apparently suppressed tumor growth and autophagy flux in a nude mouse xenograft model of CRC. These observations provide a solid basis for potential application of GLP in CRC treatment. In short, GLP possesses the ability to target diverse molecules and signaling pathways that are associated with apoptosis and autophagy in CRC. The interconnection between these signaling networks is worthy of continuous exploration.
EGLP retarded the growth of cervical carcinoma in tumor-bearing mice (Kong et al., 2019). The chemotherapeutic agent cyclophosphamide (CTX) showed cytotoxicity toward immune organs. In contrast, EGLP protected the immune organs of tumor-bearing mice. Chemotherapy-induced oxidative stress represents a critical cause of anticancer medication-associated adverse effects (Zhang et al., 2022). CTX induced oxidative stresses in tumor-bearing mice by lowering the activities of antioxidant enzymes. EGLP exerted an opposite effect on antioxidant enzymes, suggesting that EGLP could alleviate oxidative stress in vivo. Further study showed that EGLP downregulated the expression of Bcl-2 and COX-2 and upregulated the expression of Bax and cleaved caspase-3. These observations demonstrated that EGLP was capable of promoting cervical cancer cell apoptosis. EGLP exhibited strong anticancer potential with little side effect and held the promise as a chemotherapeutic drug for the treatment of cervical carcinoma. GLP decreased the invasive and migratory abilities of cervical cancer cells (Jin et al., 2020). GLP elevated the expression of Bax, cleaved caspase-3 and cleaved caspase-9 and reduced the expression of Bcl-2. It also enhanced the expression of E-cadherin and decreased the expression of N-cadherin, vimentin and Slug. Meanwhile, GLP lowered the expression of phosphorylated Janus kinase (p-JAK) and signal transducer and activator of transcription 5 (STAT5) in cervical cancer cells. Epithelial-to-mesenchymal transition (EMT) is a vital process in cancer progression and plays an important role in tumor stemness, metastasis and chemoresistance (Loh et al., 2019). Loss of E-cadherin and the concomitant gain of N-cadherin expression are hallmark features of the EMT process (Luu, 2021). These results suggested that GLP provoked the apoptosis of cervical cancer cells, and inhibited cell aggressiveness by retarding the EMT program. It is generally accepted that Ganoderma polysaccharides have the potential to be developed as an adjuvant therapy for cancer. The anticancer potency of chemotherapeutic agents in conjunction with GLP was previously investigated. It turned out that combined treatment with GLP and cisplatin was superior to cisplatin in inhibiting the growth of cervical carcinoma in vivo (Zhu et al., 2019). GLP/cisplatin combined therapy had little toxicological effects, and improved hepatic function and renal function in tumor-bearing mice. GLP/cisplatin promoted the apoptosis of cervical carcinoma cells by upregulating Bax and downregulating Bcl-2. This combination treatment could be used as an efficacious therapeutic option for cervical carcinoma.
GLP obviously suppressed the viability and induced the apoptosis of prostate cancer cells, as evidenced by PARP cleavage and downregulation of pro-caspase-3, -6, and -9 proteins (Wu et al., 2018). GLP increased the expression levels of non-steroidal anti-inflammatory drug-activated gene-1 (NAG-1) and its transcription factor early growth response-1 (Egr-1). GLP enhanced the promoter activity of NAG-1, suggesting that NAG-1 was transcriptionally modulated by GLP. GLP could facilitate the extracellular secretion of NAG-1 proteins. Suppression of NAG-1 expression remarkably prevented GLP-induced apoptosis and counteracted the roles of GLP in regulating PARP and pro-caspase expression. In addition, GLP suppressed Akt phosphorylation and the MAPK/ERK signaling pathway in prostate cancer cells. Therefore, the chemopreventive potential of GLP could be explained by its promotive effects on NAG-1 expression. Nevertheless, considerable research efforts are still needed to corroborate the exact function of NAG-1 in GLP-induced apoptosis and tumor suppression in prostate cancer.
The two programmed cell death pathways, apoptosis and autophagy, have become a topic of intense research in the context of cancer (Das et al., 2021). Generally, apoptosis functions as a tumor-suppressive pathway. Autophagy is an evolutionarily conserved cellular recycling mechanism that leads to internal hemostasis in response to varying environmental stimuli (Klionsky et al., 2021). Autophagy promotes the degradation of oncogenic molecules, hence inhibiting cancer onset and development. It should be noted that autophagy tends to act as a promoter of cancer cell survival under some circumstances, such as hypoxia and nutrition deprivation. Stress-activated autophagy may foster cancer cell survival by degrading cellular building blocks to supply cancer cells with nutrients. Thus, autophagy exerts opposite functions in cancer and can be classified into protective autophagy and lethal autophagy (Xie et al., 2020). Apoptosis and autophagy usually take place within the same cancer cell. Protective autophagy commonly counteracts apoptosis and contributes to a survival mechanism for cancer cells. In contrast, lethal autophagy has an antagonistic role. The effects of autophagy on cancer development may differ depending on tumor stage, tissue/cell type and extent of autophagy activity (Kaluzki et al., 2019). At present, there is limited evidence on the role of the apoptosis pathway in coordination of autophagy in cancer. The two cell death pathways are deemed to be interconnected, and elucidating the interaction between apoptosis and autophagy is essential for the improvement in the therapeutic efficacy of anticancer treatments. Ganoderma polysaccharides can simultaneously regulate the apoptotic and autophagic cell death pathways (Figure 1). Blockade of late autophagy enhances the pro-apoptotic activity of Ganoderma polysaccharides, hinting the occurrence of protective autophagy in Ganoderma polysaccharide-treated cancer. It has been hypothesized that the interruption of autophagy-mediated degradation may lead to the activation of apoptosis-relevant factors (e.g., caspases and p53), thereby enhancing cancer cell apoptosis (Petroni et al., 2020). Improving the anticancer effect through autophagy induction may be a potentially therapeutic option for cancer treatment. Ganoderma polysaccharides in combination with late autophagy inhibitors hold great promise as an effective anticancer strategy. Apoptosis and autophagy can exert synergistic, promoting or antagonistic effects in cancer (Xie et al., 2020). Nevertheless, the exact regulatory role of Ganoderma polysaccharides in the interaction pattern between apoptosis and autophagy deserves in-depth research.
Given their prominent immunoregulatory function, it is suggestive that Ganoderma polysaccharides are implicated in antitumor immune responses. Polysaccharide nano-formulation can enhance stability and prolong immunoactivity of bioactive polysaccharides. Gold nanocomposites containing GLP (GLP-Au) was able to induce dendritic cell (DC) activation and enhanced the production of cytokines in DC including interleukin (IL)-1β, IL-6, IL-12, interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α) (Zhang S. et al., 2019). GLP-Au-activated DC facilitated the proliferation of CD4+ and CD8+ T cells in murine spleen. GLP-Au could improve the function of antigen-presenting system and cellular immunity. DC activation plays a key role in cancer immunotherapy. Accordingly, GLP-Au in combination with doxorubicin markedly suppressed breast cancer growth and metastasis in vivo. GLP-Au co-treatment with doxorubicin increased the count of memory T cells in splenocytes from tumor-bearing mice. Moreover, GLP-Au exerted stronger immunostimulatory effects on T cell proliferation through DC activation and on memory T cell response compared with GLP. GLP-Au might have the potential to improve the efficacy of DC-based cancer immunotherapy.
GLP-conjugated bismuth sulfide nanoparticles (GLP-BiNPs) were shown to activate DCs and enhance GLP accumulation within tumor tissues of breast cancer-bearing mice (Yu et al., 2019). GLP-BiNPs in combination with radiation significantly inhibited tumor growth in vivo by stimulating cell apoptosis. GLP-BiNPs remodeled the tumor immunosuppression microenvironment by elevating the count of intratumor CD8+ T cells and the ratio of IFN-γ to IL-4 in serum. Particularly, GLP conjugation could reduce kidney injury caused by bare BiNP, which might be attributable to the anti-inflammatory benefit of GLP. GLP connection induced the radiosensitivity and antitumor immune responses in breast cancer cells, and reduced BiNP nephrotoxicity. The incorporation of immunoactive polysaccharides into nanoradiosensitizer might have the potential to aggrandize the safety and efficacy of nanoparticle-based anticancer therapeutics.
GLP elevated the levels of serum IL-2, TNF-α, and IFN-γ and augmented the cytotoxic activity of natural killer (NK) cells and T cells in glioma-bearing rats (Wang et al., 2018). IL-2, TNF-α, and IFN-γ act as critical participants in the development of antitumor immune responses. These cytokines might mediate the immune activating effect of GLP. GLP was capable of inducing the maturity of DCs and favored the proliferation of spleen lymphocytes. As a result, GLP suppressed glioma growth and prolonged the survival of tumor-bearing rats. Notably, high concentrations of GLP might result in excessive infiltration of both immunoactive and immunosuppressive cells, hence contributing to inflammation and cancer development. Additional efforts are necessary to determine the optimal dose or dose-range of GLP for cancer treatment. GLP might emerge as a potential treatment option for the immunotherapy of glioma.
GLP prevented the development of lung cancer in tumor-bearing mice (Wang et al., 2020). Mechanistically, GLP promoted the differentiation of myeloid-derived suppressor cells (MDSCs) and inhibited MDSC accumulation in spleen and tumor tissues through the caspase recruitment domain-containing protein 9 (CARD9)/nuclear factor-κB (NF-κB)/indoleamine 2,3-dioxygenase (IDO) signaling pathway. Consequently, GLP enriched the population of CD4+ and CD8+ T cells and enhanced the production of type 1 T helper (Th1)-type cytokines (IFN-γ and IL-12) in the spleen of tumor-bearing mice. The anticancer action of GLP was attributable to its induction of antitumor immune responses. The accumulation of MDSCs is a main inhibitor of the immune system, which hinders effective immunotherapeutic measures. Blockade of MSDC deposition may be an attractive approach for cancer intervention. GLP can stimulate antitumor immune responses by clearance of MDSCs, which demonstrates that GLP may be a MDSC-targeting drug in chemotherapy.
CD68+ macrophages have the ability to secrete inflammatory growth factors to facilitate cancer progression (Chen et al., 2017). GLP decreased the count of CD68+ macrophages in melanoma tissues and inhibited melanoma growth in vivo, thus prolonging the survival rate of tumor-bearing mice (Liu H. et al., 2021). GLP might represent an effective therapeutic option for the treatment of melanoma. GLP may cause autophagy in CD68+ macrophages or transform CD68+ macrophages to other phenotypes. However, the exact mechanism responsible for GLP-associated reduction in CD68+ macrophages needs to be illuminated in future studies. It was reported that GLP fostered primary macrophage polarization to M1 type and increased the production of TGF-β1, TNF-α, IL-1β, and IL-6 (Song et al., 2021). GLP-intervened macrophages evidently repressed HCC cell proliferation by inducing cell cycle arrest and activating the mitochondrial apoptosis pathway via the PI3K/Akt signaling cascade. Therefore, GLP induced antitumor immunity and favored HCC cell apoptosis by stimulating macrophages. GLP might be a promising anticancer agent for HCC treatment. The roles of GLP in altering macrophage polarity and restraining HCC progression remain to be validated in vivo.
GLP has been used as an adjuvant agent to strengthen the immunity of human body in clinical practice. The infiltration of activated immune cells in tumors partially contributes to better prognosis in cancer patients. Beyond cancer cells, the tumor microenvironment is a complex ecosystem that encompasses a diversity of non-epithelial cells that make up the blood vasculature (e.g., endothelial cells and smooth muscle cells), immune cells (e.g., lymphocytes, macrophages and mast cells) and stromal cells (Labani-Motlagh et al., 2020). Undoubtedly, the dynamic interaction between cancer cells and other cells within the tumor microenvironment controls cancer development and progression. Cancer cells create an immunosuppressive niche with diverse mechanisms to escape immune surveillance, such as blockade of antigen presentation, suppression of T cell function and activation of immune tolerance-related pathways (Asiry et al., 2021). Immune cells and their released mediators have emerged as a double-edged sword in cancer. It is unambiguously accepted that immune scrutiny is necessary for tumor growth inhibition. On the contrary, excessive immune responses exert a promotive role in cancer growth and metastasis. Within the tumor microenvironment, CD4+ and CD8+ T cell-mediated Th1 immune responses act as the dominant antitumor immune mechanisms (Dai et al., 2021). Oppositely, MDSCs, type 2 tumor-associated macrophages (TAMs) and their secreted cytokines act as tumor promoters. Accumulating evidence has indicated that GLP activates antitumor Th1 immune responses and impedes macrophage-mediated tumor immune evasion (Figure 2). These findings underlie the theoretical feasibility for subsequent studies of GLP as an immune adjuvant. The exploitation of effective drugs that target the immunoregulatory factors present in the tumor microenvironment would be a promising research direction. Therefore, significant research efforts should be dedicated to revealing the influence of GLP on the tumor microenvironment. In addition, it remains to ascertain whether GLP affects the reciprocal and continuous interactions between cancer cells and the tumor microenvironment.
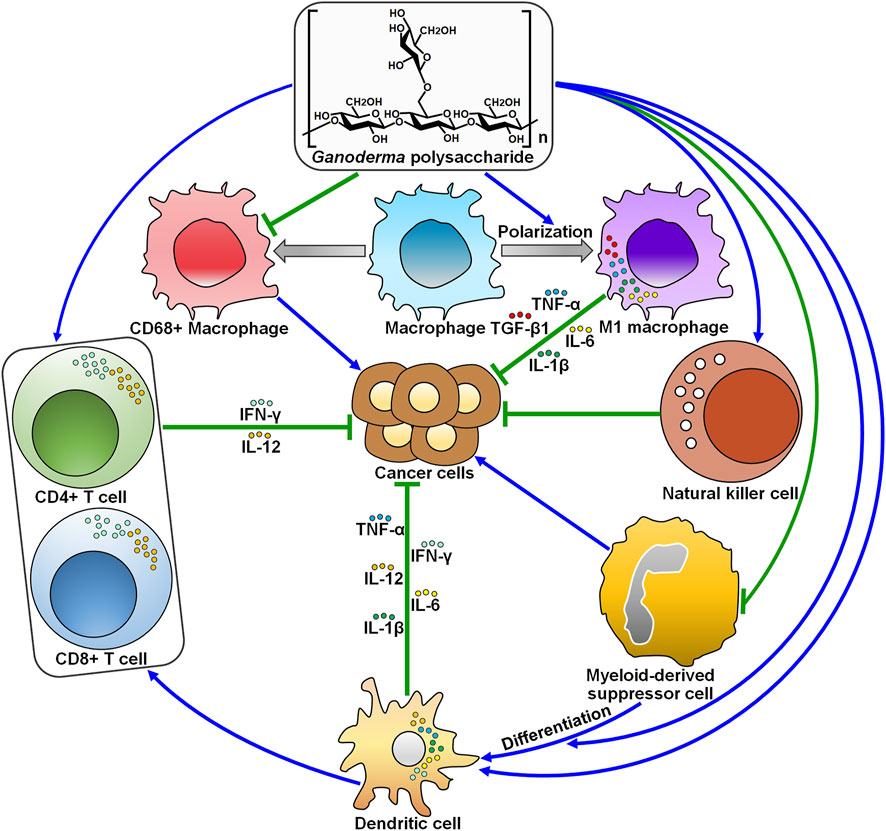
FIGURE 2. The roles of Ganoderma polysaccharides in modifying antitumor immunity. Ganoderma polysaccharides inhibit the proliferation of pro-inflammatory CD68+ macrophages, while they promote macrophage polarization toward the antitumoral M1 phenotype. Ganoderma polysaccharides can enhance the cytotoxic activity of natural killer cells. Ganoderma polysaccharides suppress the accumulation of myeloid-derived suppressor cells and foster their differentiation into mature dendritic cells. On the other hand, Ganoderma polysaccharides directly activate dendritic cells and favor the production of cytokines (e.g., IL-1β, IL-6, IL-12, IFN-γ, and TNF-α). Dendritic cells then present cancer-specific antigens to CD4+ T cells, thus recruiting CD8+ T cells to eliminate cancer cells. IFN-γ, interferon-γ; IL-12, interleukin-12; TNF-α, tumor necrosis factor-α; TGF-β1, transforming growth factor-β1; IL-6, interleukin-6; IL-1β, interleukin-1β.
Ganoderma polysaccharides remain indigestible until they reach the intestine. Polysaccharides could be metabolized by intestinal microbes and change the composition of gut microbiota. GLP improved the intestinal barrier function of ApcMin/+ mice by decreasing polyps, inducing the transition of colonic M1 to M2 macrophages, elevating E-cadherin/N-cadherin ratio and reducing oncogenic signaling molecules (e.g., p-ERK, p-AKT, p-STAT3, Src, and iNOS) (Khan et al., 2019). GLP could increase the abundance of short-chain fatty acid (SCFA)-producing bacteria (e.g., Alloprevotella rava, Bifidobacterium choerinum and Prevotella spp.). SCFAs have been found to play an anticarcinogenic role in CRC (Sivamaruthi et al., 2020). SCFAs regulate host cellular responses through the activation of SCFA-sensing G-protein coupled-receptors (GPCRs) and the suppression of histone deacetylases (HDACs) (Parada Venegas et al., 2019). As expected, GLP attenuated the expression of HDACs, and increased the expression of GPCRs and their relevant signaling molecules including the anticancer peptide tyrosine-tyrosine (PYY) and peroxisome proliferator-activated receptor γ (PPARγ) (Khan et al., 2019). GPCRs are able to transduce signals through attaching to PPAPγ (Wang et al., 2015). PPAPγ then alters intestinal immunity by converting the pro-inflammatory M1 to an anti-inflammatory M2 phenotype. Moreover, GLP decreased the abundance of sulfate-reducing bacteria (e.g., Desulfosporosinus spp. and Desulfotomaculum spp.) that had been associated with cancer pathogenesis. Altogether, GLP antagonized colorectal carcinogenesis by regulating the gut microbiota and host immune responses. GLP possessed the potential to be exploited as an adjuvant treatment for CRC.
GLP was capable of repressing azoxymethane (AOM)/dextran sulfate sodium (DSS)-induced colitis and colorectal carcinogenesis in mice (Guo C. et al., 2021). Mechanistically, GLP reversed gut microbiota dysbiosis caused by AOM/DSS. Firmicutes may exert an anti-CRC role, while Bacteroidetes has a close relationship with cancer development. Bifidobacterium and Lactobacillus encompass probiotic strains for CRC prevention and therapy. GLP increased the ratio of Firmicutes to Bacteroidetes and enriched the population of Bifidobacterium and Lactobacillus. Conversely, GLP diminished the abundance of cancer-associated genera, including Alistipes, Desulfovibrio, Lachnoclostridium, Oscillibacter and Parasutterella. GLP also decreased the amount of Bifidobacterium_pseudolongum and Lactobacillus_reuteri while raised that of Alistipes_finegoldii and Bacteroides_acidifaciens. The metabolites of intestinal microbes including SCFAs play a critical role in preserving intestinal homeostasis. The fecal levels of SCFAs including acetate, butyrate and propionate were reduced in AOM/DSS-treated mice, whereas GLP reversed their downregulation (Guo C. et al., 2021). Besides, GLP treatment markedly elevated the expression of G protein-coupled receptor 43 (GPR43) in colonic cells that specifically recognized SCFAs. It was thus proposed that GLP induced anti-inflammatory and anticarcinogenic effects by increasing GPR43 expression in response to altered intestinal microbiota composition and elevated SCFA production. Therefore, GLP inhibited AOM/DSS-elicited colorectal carcinogenesis by altering gut microbiota. The detailed mechanisms of GLP in coordination of gut microbiota are worthy of in-depth study. It is also essential to better understand how varied gut microbiota adapted by GLP affects CRC progression.
Firmicutes and Bacteroidetes are two ubiquitous phyla in mammalian gut. The increased ratio of Firmicutes to Bacteroidetes was reported to correlate with breast carcinogenesis (Wu et al., 2020). GLP and polysaccharides from G. sinense (GSP) reversed this tendency, contributing to tumor suppression (Li et al., 2018). They also reduced the populations of Bacteroides and Lactobacillus, while increased the abundance of the SCFA-producing Alistipe and the tumor-suppressive Prevotellaceae_UCG-001. Consistently, in vivo studies verified that GLP and GSP exerted inhibitory effects on tumor growth in breast cancer-bearing mice. Despite these intriguing results, the genuine contribution of altered gut microbiota to Ganoderma polysaccharide-induced tumor suppression requires further evidence for verification.
Polysaccharide derived from spore of G. lucidum (SGP) relieved paclitaxel (PTX)-induced gut microbiota dysbiosis by elevating the count of Bacteroides and Ruminococcus and reducing the count of cancer-related genera Desulfovibrio and Odoribacter (Su et al., 2018). Meanwhile, the combination of SGP and PTX showed synergistic effects against breast cancer in vivo. Collectively, SGP restored gut microbiota dysbiosis and thus alleviated adverse effects of chemotherapy, suggesting its potential implication as a supplement for the treatment of breast cancer. Another study revealed that SGP relieved PTX-caused body weight lost and ameliorated PTX-induced intestinal barrier injury in breast cancer-bearing mice (Li D. et al., 2020). The combined administration of SGP and PTX could improve intestinal barrier function by suppressing endotoxemia and upregulating tight junction proteins, such as E-cadherin, β-catenin, occludin and ZO-1. SGP conferred protection against PTX-induced apoptosis in intestinal cells by restraining microtubule polymerization. Collectively, SGP could repair PTX-induced small intestinal barrier injury, stressing its clinical implication as an adjunctive chemotherapy for breast cancer. The potential of Ganoderma polysaccharides as protective agents in cancer treatment has been increasingly acknowledged. Further exploration of the interaction between Ganoderma polysaccharides and gut microbiota will be helpful in disclosing the mechanisms associated with protective benefits offered by Ganoderma polysaccharides during chemotherapy.
G. lucidum polysaccharide peptide (GL-pp) markedly inhibited sleep fragmentation-induced tumor metastasis in melanoma-bearing mice (Xian et al., 2021). GL-pp altered the composition of gut microbiota in melanoma-bearing mice by increasing the number of Bacteroides and decreasing the number of Christensenellaceae, Desulfovibrio, Odoribacter, and Parvibacter. GL-pp promoted macrophage polarization and increased the serum level of TNF-α. GL-pp exhibited antimetastatic activities against breast cancer partially through mitigation of gut microbiota dysbiosis. It is obscure whether GL-pp has similar effects on intestinal dysbiosis in cancer patients, which could be a subject of future studies.
Gut microbiota is a diverse community of microbes that preserve intestinal homeostasis (Fan et al., 2021). Gut microbiota plays dual roles in cancer formation. On the one hand, certain intestinal microbes may expand during pathological dysbiosis and induce inflammation and carcinogenesis via various mechanisms including production of pro-carcinogenic toxins, regulation of cellular survival, proliferative and invasive pathways, elicitation of oxidative stress, promotion of tumor immune escape (Torres-Maravilla et al., 2021). On the other hand, beneficial bacteria, also named probiotics, can produce a variety of metabolites capable to prevent tumor genesis (Vivarelli et al., 2019). Probiotics also stimulate anticancer immune responses, foster cancer cell apoptosis and prevent chronic inflammation. The bidirectional interplay between gut microbiota and host immune system has been correlated with cancer development. At present, gut microbiota has become a new frontier in cancer research. Nevertheless, the causal relationship between gut microbiota and cancer pathogenesis needs to be further defined. Increasing knowledge about the linkage between gut microbiota and cancer will open novel perspectives for alternative therapeutic strategies for cancer prevention and treatment. Accumulating evidence has demonstrated that Ganoderma polysaccharides are able to reverse the dysbiosis of gut microbiota, facilitate the production of anticancerous metabolites from commensal gut microbes, inhibit pro-inflammatory responses and enhance immune surveillance (Figure 3). The studies concerning the roles of Ganoderma polysaccharides in regulation of gut microbiota are just the tip of the iceberg. Continual investigations are needed to comprehensively characterize Ganoderma polysaccharide-induced changes in gut microbiota composition and associated metabolic profile. Gut microbiota can affect the pharmacokinetics, efficacy and toxicity of chemotherapeutic agents (Wilson and Nicholson, 2017). Manipulation of gut microbiota may afford opportunities to improve clinical outcomes of cancer patients. The impact of Ganoderma polysaccharides on drug metabolism through regulation of gut microbiota would be an important research area that is worthy of more attention. Gut microbiota is considered to have a profound impact on host immune system (Gopalakrishnan et al., 2018). However, it has yet to be determined what composition of gut microbiota boosts anticancer immune responses. It is imperative to clarify the contribution of different members of gut microbiota to altering host immune reactions. An increasingly sophisticated comprehension of the immunoregulatory function of gut microbiota is a vital prerequisite for the development of gut microbiota-targeting anticancer therapeutic approaches. Collectively, Ganoderma polysaccharides remodel gut microbiota to strengthen antitumor immunity and thus may be exploited as pharmaceuticals capable to enhance therapeutic actions of cancer immunotherapy.
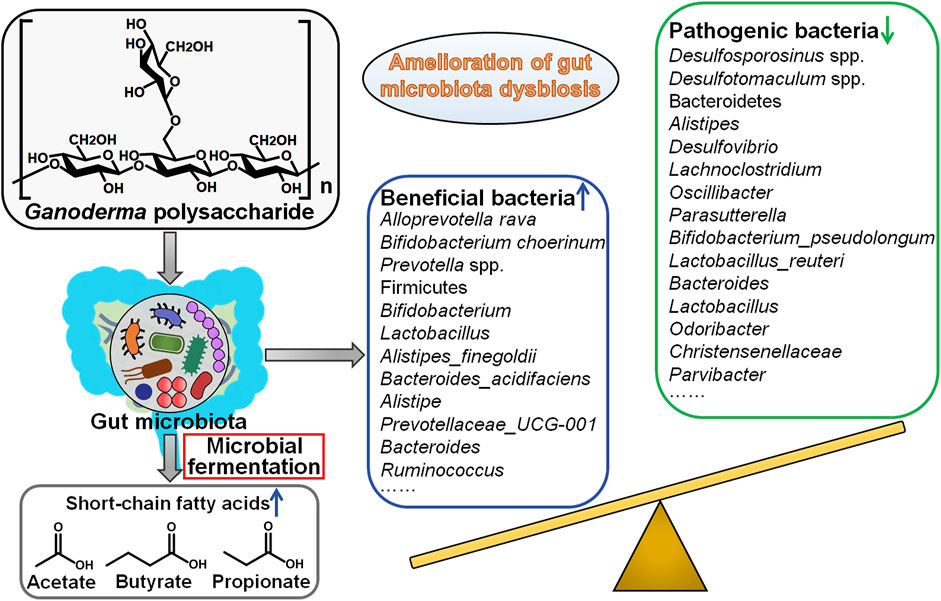
FIGURE 3. Mechanistic implications of intestinal microbiota in Ganoderma polysaccharides’ protective effects against cancer. Gut microbiota dysbiosis has been associated with carcinogenesis and cancer development. Ganoderma polysaccharides can affect gut microbiota composition. Particularly, Ganoderma polysaccharides enrich beneficial commensal microorganisms (e.g., Alloprevotella rava, Bifidobacterium choerinum and Prevotella spp.) while diminish the quantity of pathogenic microbial species (e.g., Desulfosporosinus spp., Desulfotomaculum spp. and Bacteroidetes), thereby leading to the amelioration of gut microbiota dysbiosis and tumor inhibition. Furthermore, Ganoderma polysaccharides increase the abundance of short-chain fatty acid (SCFA) producers and enhance the generation of SCFAs, which have been found to possess anticancerous activities.
Cancer cells usually develop resistance to chemotherapeutic agents, which make it arduous to eradicate tumors. The presence of cancer stem cells (CSCs) is responsible for tumor chemoresistance and recurrence (Gaggianesi et al., 2021). GLP suppressed the proliferation, metabolic activity and migration of oral squamous cell carcinoma (OSCC) (de Camargo et al., 2022). Importantly, GLP impeded CSC properties and eventually enhanced the therapeutic responsiveness of OSCC cells by lowering the expression of CSC, EMT and chemosensitivity markers (e.g., BMI1, p75NGFR, twist, N-cadherin, and ABCB1) (de Camargo et al., 2022). GLP might act as therapeutic co-adjuvants to promote CSC elimination, hence suppressing OSCC malignancy and chemosensitizing OSCC cells to traditional treatments.
It is known that cisplatin-based chemotherapy is one of the most prevalent treatments for lung cancer. A recent study revealed that the combined administration of WSG and cisplatin exerted synergistic inhibitory effects on the growth and metastasis of lung cancer in vitro and in vivo (Qiu et al., 2021). WSG potentiated cisplatin-induced apoptotic responses in lung cancer cells but alleviated its cytotoxicity toward macrophages and normal lung fibroblasts. This result implied that WSG had distinct roles in cisplatin-exposed lung cancer and normal cells. WSG may respond to different signals in cancerous and normal cells, or only respond to a specific signal in cancer cells. The mechanisms determining the fate of WSG-treated cells need to be further elucidated. Taken together, WSG augmented the anticarcinogenic activity of cisplatin against lung cancer, suggesting that WSG could be a potential adjuvant or dietary supplement to increase the effectiveness of cisplatin-based chemotherapy in patients with lung cancer.
Ganoderma polysaccharides and current treatments have synergistic anticancer roles. Ganoderma polysaccharides exert a promoting effect on the tumor-suppressive activity of chemotherapeutic agents and may act as potential chemosensitizing agents for cancer. The acquirement of cancer chemoresistance has become a major hurdle in effective cancer treatment. The development of chemoresistance involves complex mechanisms, such as promotion of DNA repair, regulation of drug efflux and metabolism, suppression of cell apoptosis, activation of cellular survival signaling cascades and enhancement of cancer stemness (Liu C. et al., 2021). Current evidence supports the bioactivity of Ganoderma polysaccharides in inhibiting drug efflux pump in cancer. It is still unclear whether Ganoderma polysaccharides interfere with other chemoresistance-associated mechanisms. Much more work is necessary to delve into the mechanisms of action of Ganoderma polysaccharide in regulation of cancer cell chemosensitivity.
Ganoderma is a highly appreciated vitality-promoting medical herb for over 2000 years. Ganoderma polysaccharides are one of the most pharmacologically active components. Our knowledge regarding the biological actives of Ganoderma polysaccharides is continually expanding. Ganoderma polysaccharides have a wide range of pharmacological activities, including anticarcinogenic, antidiabetic, anti-inflammatory, antimicrobial, and immunoregulatory actions (Seweryn et al., 2021). Ganoderma polysaccharides are capable of suppressing cancer development and progression through blockade of the DNA damage repair pathway, repression of cancer cell proliferation and malignancy, alternation of cell death pathways, reinforcement of antitumor immunity, restoration of gut microbiota homeostasis and potentiation of chemotherapy-mediated cytotoxicity toward cancer cells. In a randomized, double-blind placebo-controlled crossover study, 42 healthy subjects were divided into two groups that received placebo or GLP, respectively (Chiu et al., 2017). It turned out that GLP exhibited antioxidative, anti-aging and hepatoprotective efficacy in healthy volunteers by controlling oxidative stresses. Thus, Ganoderma polysaccharides have significant clinical value due to their anticancer efficacy and low toxicity. Ganoderma polysaccharides can diminish adverse reactions caused by chemotherapy. GSP has been approved as an adjuvant therapeutic agent in China for ameliorating chemotherapy- or radiotherapy-elicited leukopenia and hematopoietic damage (Zhang Y. et al., 2019). Future researches defining the clinical implication of Ganoderma polysaccharides as anticancer adjuvant therapies may contribute to an improvement in therapeutic options for cancer treatment.
Nevertheless, several issues must be addressed before translating promising Ganoderma polysaccharide-based therapies to cancer patients. First, given that Ganoderma polysaccharide is a mixture of glycans, the molecular mechanisms of action of different glycan components in cancer deserve further investigation in future studies. An in-depth investigation into Ganoderma polysaccharides could facilitate the development of efficacious chemotherapeutic agents of natural origin. Second, supplementation with Ganoderma polysaccharides poses a potential risk of interplay with conventional chemotherapeutic drugs. The combination of chemotherapy and immunotherapy has become a research hotspot in clinical cancer treatment. Available therapies show limited effectiveness, low tolerance and severe adverse effects in cancer patients. Thus, more effective agents are needed for chemotherapeutic strategies in cancer patients. Ganoderma has emerged as a valuable source of nutraceuticals with great therapeutic potential. Interest has been increasing in the use of Ganoderma related natural products combined with conventional therapies in cancer management. A growing body of evidence has revealed the efficacy of Ganoderma polysaccharides combined with anticancer agents in cancer treatment. Anticancer agents perform their functions by inducing DNA damage and cell apoptosis. Some chemotherapeutic agents contribute to an excessive accumulation of reactive oxygen species (ROS) via induction of ROS generation or inhibition of their clearance in cancer cells (Idelchik et al., 2017). Chemotherapeutic agent-induced apoptosis can be repressed by components that retard ROS production. Ganoderma polysaccharides are effective in scavenging ROS and protect cellular DNA from oxidative damage (Hu et al., 2019). The antioxidative activity of Ganoderma polysaccharides has been correlated with its potential implication as an anticancer agent. It is possible that Ganoderma polysaccharides could antagonize DNA damage-inducing activity of chemotherapeutic agents in cancer cells by acting as ROS scavengers. The precise function of Ganoderma polysaccharides in DNA damage response needs to be fully deciphered. The intricate interaction between Ganoderma polysaccharides and anticancer agents remains largely equivocal, and thus more researches on this topic require to be undertaken. Third, clinical evidence for the anticancer efficacy of Ganoderma polysaccharides is limited so far. The anticancerous actions of Ganoderma polysaccharides have been substantiated in experimental studies. Even with encouraging published results, follow-up efforts should be put into evaluating the therapeutic benefits of Ganoderma polysaccharides in cancer patients. Particularly, their optimal dosage, duration of action, safety, pharmacokinetic profile and interaction with administrated anticancer agents need to be comprehensively explored in properly designed randomized clinical trials. Moreover, it is essential to confirm whether Ganoderma polysaccharides are effective in treating all kinds of cancers or they only exhibit anticancer effects on specific cancers. In addition, rational clinical combination strategies, the sequence of drug administration and the appropriate time of supplementation of Ganoderma polysaccharides to therapy regimens should be taken into account. Although there are few clinical trials employing Ganoderma polysaccharide-based natural therapies, it can be concluded that they possess the advantage in boosting the therapeutic efficacy of conventional treatments and improve the quality of life in cancer patients.
In vitro and in vivo experimental studies have revealed that Ganoderma polysaccharides present anti-proliferative, anti-migratory, pro-apoptotic and immunomodulatory activities in cancer. These bioactive compounds also prevent cancer pathogenesis by reversing intestinal dysbiosis to trigger a variety of reactions, such as inhibition of chronic inflammation, production of antineoplastic metabolites, restoration of intestinal barrier function and potentiation of antitumor immunity. Importantly, Ganoderma polysaccharides can aggrandize the anticancer potential of mainstream therapies and alleviate their adverse effects. Therefore, Ganoderma polysaccharides may be effective and safe chemotherapeutic agents against cancer. However, future studies are warranted to completely elucidate the anticarcinogenic mechanisms of Ganoderma polysaccharides as well as their clinical potency as an adjunctive cancer treatment.
MW conceived this article, drew the figures and wrote the manuscript. FY collected the related papers and revised the manuscript. All authors have read and approved the final version of the manuscript.
This work was supported by the Natural Science Foundation of Shandong Province, China (Grant No. ZR2021MH018).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahmad, M. F. (2018). Ganoderma Lucidum: Persuasive Biologically Active Constituents and Their Health Endorsement. Biomed. Pharmacother. 107, 507–519. doi:10.1016/j.biopha.2018.08.036
Alemi, F., Raei Sadigh, A., Malakoti, F., Elhaei, Y., Ghaffari, S. H., Maleki, M., et al. (2022). Molecular Mechanisms Involved in DNA Repair in Human Cancers: An Overview of PI3k/Akt Signaling and PIKKs Crosstalk. J. Cell. Physiol. 237 (1), 313–328. doi:10.1002/jcp.30573
Asiry, S., Kim, G., Filippou, P. S., Sanchez, L. R., Entenberg, D., Marks, D. K., et al. (2021). The Cancer Cell Dissemination Machinery as an Immunosuppressive Niche: A New Obstacle towards the Era of Cancer Immunotherapy. Front. Immunol. 12, 654877. doi:10.3389/fimmu.2021.654877
Asl, E. R., Amini, M., Najafi, S., Mansoori, B., Mokhtarzadeh, A., Mohammadi, A., et al. (2021). Interplay between MAPK/ERK Signaling Pathway and MicroRNAs: A Crucial Mechanism Regulating Cancer Cell Metabolism and Tumor Progression. Life Sci. 278, 119499. doi:10.1016/j.lfs.2021.119499
Bai, J. H., Xu, J., Zhao, J., and Zhang, R. (2020). Ganoderma Lucidum Polysaccharide Enzymatic Hydrolysate Suppresses the Growth of Human Colon Cancer Cells via Inducing Apoptosis. Cell. Transpl. 29, 963689720931435. doi:10.1177/0963689720931435
Bannik, K., Madas, B., Jarke, S., Sutter, A., Siemeister, G., Schatz, C., et al. (2021). DNA Repair Inhibitors Sensitize Cells Differently to High and Low LET Radiation. Sci. Rep. 11 (1), 23257. doi:10.1038/s41598-021-02719-9
Blasiak, J. (2021). Single-Strand Annealing in Cancer. Int. J. Mol. Sci. 22 (4), 2167. doi:10.3390/ijms22042167
Cao, Y., Xu, X., Liu, S., Huang, L., and Gu, J. (2018). Ganoderma: A Cancer Immunotherapy Review. Front. Pharmacol. 9, 1217. doi:10.3389/fphar.2018.01217
Cerletti, C., Esposito, S., and Iacoviello, L. (2021). Edible Mushrooms and Beta-Glucans: Impact on Human Health. Nutrients 13 (7), 2195. doi:10.3390/nu13072195
Chatterjee, N., and Walker, G. C. (2017). Mechanisms of DNA Damage, Repair, and Mutagenesis. Environ. Mol. Mutagen 58 (5), 235–263. doi:10.1002/em.22087
Chen, X. J., Han, L. F., Wu, X. G., Wei, W. F., Wu, L. F., Yi, H. Y., et al. (2017). Clinical Significance of CD163+ and CD68+ Tumor-Associated Macrophages in High-Risk HPV-Related Cervical Cancer. J. Cancer 8 (18), 3868–3875. doi:10.7150/jca.21444
Chiu, H. F., Fu, H. Y., Lu, Y. Y., Han, Y. C., Shen, Y. C., Venkatakrishnan, K., et al. (2017). Triterpenoids and Polysaccharide Peptides-Enriched Ganoderma Lucidum: a Randomized, Double-Blind Placebo-Controlled Crossover Study of its Antioxidation and Hepatoprotective Efficacy in Healthy Volunteers. Pharm. Biol. 55 (1), 1041–1046. doi:10.1080/13880209.2017.1288750
Cör, D., Knez, Ž., and Knez Hrnčič, M. (2018). Antitumour, Antimicrobial, Antioxidant and Antiacetylcholinesterase Effect of Ganoderma Lucidum Terpenoids and Polysaccharides: A Review. Molecules 23 (3), 649. doi:10.3390/molecules23030649
Dai, E., Zhu, Z., Wahed, S., Qu, Z., Storkus, W. J., and Guo, Z. S. (2021). Epigenetic Modulation of Antitumor Immunity for Improved Cancer Immunotherapy. Mol. Cancer 20 (1), 171. doi:10.1186/s12943-021-01464-x
Das, S., Shukla, N., Singh, S. S., Kushwaha, S., and Shrivastava, R. (2021). Mechanism of Interaction between Autophagy and Apoptosis in Cancer. Apoptosis 26 (9-10), 512–533. doi:10.1007/s10495-021-01687-9
de Camargo, M. R., Frazon, T. F., Inacio, K. K., Smiderle, F. R., Amôr, N. G., Dionísio, T. J., et al. (2022). Ganoderma Lucidum Polysaccharides Inhibit In Vitro Tumorigenesis, Cancer Stem Cell Properties and Epithelial-Mesenchymal Transition in Oral Squamous Cell Carcinoma. J. Ethnopharmacol. 286, 114891. doi:10.1016/j.jep.2021.114891
Fan, X., Jin, Y., Chen, G., Ma, X., and Zhang, L. (2021). Gut Microbiota Dysbiosis Drives the Development of Colorectal Cancer. Digestion 102 (4), 508–515. doi:10.1159/000508328
Feng, J., Dang, Y., Zhang, W., Zhao, X., Zhang, C., Hou, Z., et al. (2019). PTEN Arginine Methylation by PRMT6 Suppresses PI3K-AKT Signaling and Modulates Pre-mRNA Splicing. Proc. Natl. Acad. Sci. U. S. A. 116 (14), 6868–6877. doi:10.1073/pnas.1811028116
Ferreira, I. C., Heleno, S. A., Reis, F. S., Stojkovic, D., Queiroz, M. J., Vasconcelos, M. H., et al. (2015). Chemical Features of Ganoderma Polysaccharides with Antioxidant, Antitumor and Antimicrobial Activities. Phytochemistry 114, 38–55. doi:10.1016/j.phytochem.2014.10.011
Gaggianesi, M., Di Franco, S., Pantina, V. D., Porcelli, G., D'Accardo, C., Verona, F., et al. (2021). Messing up the Cancer Stem Cell Chemoresistance Mechanisms Supported by Tumor Microenvironment. Front. Oncol. 11, 702642. doi:10.3389/fonc.2021.702642
Gopalakrishnan, V., Helmink, B. A., Spencer, C. N., Reuben, A., and Wargo, J. A. (2018). The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell. 33 (4), 570–580. doi:10.1016/j.ccell.2018.03.015
Guo, C., Guo, D., Fang, L., Sang, T., Wu, J., Guo, C., et al. (2021a). Ganoderma Lucidum Polysaccharide Modulates Gut Microbiota and Immune Cell Function to Inhibit Inflammation and Tumorigenesis in Colon. Carbohydr. Polym. 267, 118231. doi:10.1016/j.carbpol.2021.118231
Guo, H., Deng, H., Liu, H., Jian, Z., Cui, H., Fang, J., et al. (2021b). Nickel Carcinogenesis Mechanism: Cell Cycle Dysregulation. Environ. Sci. Pollut. Res. Int. 28 (5), 4893–4901. doi:10.1007/s11356-020-11764-2
Guo, J., Yuan, C., Huang, M., Liu, Y., Chen, Y., Liu, C., et al. (2018). Ganoderma Lucidum-Derived Polysaccharide Enhances Coix Oil-Based Microemulsion on Stability and Lung Cancer-Targeted Therapy. Drug Deliv. 25 (1), 1802–1810. doi:10.1080/10717544.2018.1516006
Han, B., Baruah, K., Cox, E., Vanrompay, D., and Bossier, P. (2020). Structure-Functional Activity Relationship of β-Glucans from the Perspective of Immunomodulation: A Mini-Review. Front. Immunol. 11, 658. doi:10.3389/fimmu.2020.00658
Hanyu, X., Lanyue, L., Miao, D., Wentao, F., Cangran, C., and Hui, S. (2020). Effect of Ganoderma Applanatum Polysaccharides on MAPK/ERK Pathway Affecting Autophagy in Breast Cancer MCF-7 Cells. Int. J. Biol. Macromol. 146, 353–362. doi:10.1016/j.ijbiomac.2020.01.010
Hsu, W. H., Hua, W. J., Qiu, W. L., Tseng, A. J., Cheng, H. C., and Lin, T. Y. (2021). WSG, a Glucose-Enriched Polysaccharide from Ganoderma Lucidum, Suppresses Tongue Cancer Cells via Inhibition of EGFR-Mediated Signaling and Potentiates Cisplatin-Induced Apoptosis. Int. J. Biol. Macromol. 193 (Pt B), 1201–1208. doi:10.1016/j.ijbiomac.2021.10.146
Hsu, W. H., Qiu, W. L., Tsao, S. M., Tseng, A. J., Lu, M. K., Hua, W. J., et al. (2020). Effects of WSG, a Polysaccharide from Ganoderma Lucidum, on Suppressing Cell Growth and Mobility of Lung Cancer. Int. J. Biol. Macromol. 165 (Pt A), 1604–1613. doi:10.1016/j.ijbiomac.2020.09.227
Hu, S., Huang, J., Pei, S., Ouyang, Y., Ding, Y., Jiang, L., et al. (2019). Ganoderma Lucidum Polysaccharide Inhibits UVB-Induced Melanogenesis by Antagonizing cAMP/PKA and ROS/MAPK Signaling Pathways. J. Cell. Physiol. 234 (5), 7330–7340. doi:10.1002/jcp.27492
Hwang, I.-W., Kim, B.-M., Kim, Y.-C., Lee, S.-H., and Chung, S.-K. (2018). Improvement in β-glucan Extraction from Ganoderma Lucidum with High-Pressure Steaming and Enzymatic Pre-treatment. Appl. Biol. Chem. 61 (2), 235–242. doi:10.1007/s13765-018-0350-z
Idelchik, M. D. P. S., Begley, U., Begley, T. J., and Melendez, J. A. (2017). Mitochondrial ROS Control of Cancer. Semin. Cancer Biol. 47, 57–66. doi:10.1016/j.semcancer.2017.04.005
Jiang, D., Wang, L., Zhao, T., Zhang, Z., Zhang, R., Jin, J., et al. (2017). Restoration of the Tumor-Suppressor Function to Mutant P53 by Ganoderma Lucidum Polysaccharides in Colorectal Cancer Cells. Oncol. Rep. 37 (1), 594–600. doi:10.3892/or.2016.5246
Jin, H., Song, C., Zhao, Z., and Zhou, G. (2020). Ganoderma Lucidum Polysaccharide, an Extract from Ganoderma Lucidum, Exerts Suppressive Effect on Cervical Cancer Cell Malignancy through Mitigating Epithelial-Mesenchymal and JAK/STAT5 Signaling Pathway. Pharmacology 105 (7-8), 461–470. doi:10.1159/000505461
Kaluzki, I., Hailemariam-Jahn, T., Doll, M., Kaufmann, R., Balermpas, P., Zöller, N., et al. (2019). Dimethylfumarate Inhibits Colorectal Carcinoma Cell Proliferation: Evidence for Cell Cycle Arrest, Apoptosis and Autophagy. Cells 8 (11), 1329. doi:10.3390/cells8111329
Kappel, S., Stokłosa, P., Hauert, B., Ross-Kaschitza, D., Borgström, A., Baur, R., et al. (2019). TRPM4 Is Highly Expressed in Human Colorectal Tumor Buds and Contributes to Proliferation, Cell Cycle, and Invasion of Colorectal Cancer Cells. Mol. Oncol. 13 (11), 2393–2405. doi:10.1002/1878-0261.12566
Karl, L. A., Peritore, M., Galanti, L., and Pfander, B. (2021). DNA Double Strand Break Repair and its Control by Nucleosome Remodeling. Front. Genet. 12, 821543. doi:10.3389/fgene.2021.821543
Khan, I., Huang, G., Li, X. A., Liao, W., Leong, W. K., Xia, W., et al. (2019). Mushroom Polysaccharides and Jiaogulan Saponins Exert Cancer Preventive Effects by Shaping the Gut Microbiota and Microenvironment in ApcMin/+ Mice. Pharmacol. Res. 148, 104448. doi:10.1016/j.phrs.2019.104448
Kladar, N. V., Gavarić, N. S., and Božin, B. N. (2016). Ganoderma: Insights into Anticancer Effects. Eur. J. Cancer Prev. 25 (5), 462–471. doi:10.1097/CEJ.0000000000000204
Klionsky, D. J., Petroni, G., Amaravadi, R. K., Baehrecke, E. H., Ballabio, A., Boya, P., et al. (2021). Autophagy in Major Human Diseases. EMBO J. 40 (19), e108863. doi:10.15252/embj.2021108863
Kong, M., Yao, Y., and Zhang, H. (2019). Antitumor Activity of Enzymatically Hydrolyzed Ganoderma Lucidum Polysaccharide on U14 Cervical Carcinoma-Bearing Mice. Int. J. Immunopathol. Pharmacol. 33, 2058738419869489. doi:10.1177/2058738419869489
Labani-Motlagh, A., Ashja-Mahdavi, M., and Loskog, A. (2020). The Tumor Microenvironment: A Milieu Hindering and Obstructing Antitumor Immune Responses. Front. Immunol. 11, 940. doi:10.3389/fimmu.2020.00940
Lagunas-Rangel, F. A., and Bermúdez-Cruz, R. M. (2020). Natural Compounds that Target DNA Repair Pathways and Their Therapeutic Potential to Counteract Cancer Cells. Front. Oncol. 10, 598174. doi:10.3389/fonc.2020.598174
Li, D., Gao, L., Li, M., Luo, Y., Xie, Y., Luo, T., et al. (2020a). Polysaccharide from Spore of Ganoderma Lucidum Ameliorates Paclitaxel-Induced Intestinal Barrier Injury: Apoptosis Inhibition by Reversing Microtubule Polymerization. Biomed. Pharmacother. 130, 110539. doi:10.1016/j.biopha.2020.110539
Li, L. F., Liu, H. B., Zhang, Q. W., Li, Z. P., Wong, T. L., Fung, H. Y., et al. (2018). Comprehensive Comparison of Polysaccharides from Ganoderma Lucidum and G. Sinense: Chemical, Antitumor, Immunomodulating and Gut-Microbiota Modulatory Properties. Sci. Rep. 8 (1), 6172. doi:10.1038/s41598-018-22885-7
Li, L. Y., Guan, Y. D., Chen, X. S., Yang, J. M., and Cheng, Y. (2020b). DNA Repair Pathways in Cancer Therapy and Resistance. Front. Pharmacol. 11, 629266. doi:10.3389/fphar.2020.629266
Liu, C., Jin, Y., and Fan, Z. (2021a). The Mechanism of Warburg Effect-Induced Chemoresistance in Cancer. Front. Oncol. 11, 698023. doi:10.3389/fonc.2021.698023
Liu, H., Amakye, W. K., and Ren, J. (2021b). Codonopsis Pilosula Polysaccharide in Synergy with Dacarbazine Inhibits Mouse Melanoma by Repolarizing M2-like Tumor-Associated Macrophages into M1-like Tumor-Associated Macrophages. Biomed. Pharmacother. 142, 112016. doi:10.1016/j.biopha.2021.112016
Loh, C. Y., Chai, J. Y., Tang, T. F., Wong, W. F., Sethi, G., Shanmugam, M. K., et al. (2019). The E-Cadherin and N-Cadherin Switch in Epithelial-To-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 8 (10), 1118. doi:10.3390/cells8101118
Loyd, A. L., Richter, B. S., Jusino, M. A., Truong, C., Smith, M. E., Blanchette, R. A., et al. (2018). Identifying the "Mushroom of Immortality": Assessing the Ganoderma Species Composition in Commercial Reishi Products. Front. Microbiol. 9, 1557. doi:10.3389/fmicb.2018.01557
Lu, J., He, R., Sun, P., Zhang, F., Linhardt, R. J., and Zhang, A. (2020). Molecular Mechanisms of Bioactive Polysaccharides from Ganoderma Lucidum (Lingzhi), a Review. Int. J. Biol. Macromol. 150, 765–774. doi:10.1016/j.ijbiomac.2020.02.035
Lukasik, P., Zaluski, M., and Gutowska, I. (2021). Cyclin-Dependent Kinases (CDK) and Their Role in Diseases Development-Review. Int. J. Mol. Sci. 22 (6), 2935. doi:10.3390/ijms22062935
Luu, T. (2021). Epithelial-Mesenchymal Transition and its Regulation Mechanisms in Pancreatic Cancer. Front. Oncol. 11, 646399. doi:10.3389/fonc.2021.646399
Pan, H., Wang, Y., Na, K., Wang, Y., Wang, L., Li, Z., et al. (2019). Autophagic Flux Disruption Contributes to Ganoderma Lucidum Polysaccharide-Induced Apoptosis in Human Colorectal Cancer Cells via MAPK/ERK Activation. Cell. Death Dis. 10 (6), 456. doi:10.1038/s41419-019-1653-7
Parada Venegas, D., De la Fuente, M. K., Landskron, G., González, M. J., Quera, R., Dijkstra, G., et al. (2019). Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 10, 277. doi:10.3389/fimmu.2019.00277
Petroni, G., Bagni, G., Iorio, J., Duranti, C., Lottini, T., Stefanini, M., et al. (2020). Clarithromycin Inhibits Autophagy in Colorectal Cancer by Regulating the hERG1 Potassium Channel Interaction with PI3K. Cell. Death Dis. 11 (3), 161. doi:10.1038/s41419-020-2349-8
Pillai, T. G., Maurya, D. K., Salvi, V. P., Janardhanan, K. K., and Nair, C. K. (2014). Fungal Beta Glucan Protects Radiation Induced DNA Damage in Human Lymphocytes. Ann. Transl. Med. 2 (2), 13. doi:10.3978/j.issn.2305-5839.2014.02.02
Qiu, W.-L., Hsu, W.-H., Tsao, S.-M., Tseng, A.-J., Lin, Z.-H., Hua, W.-J., et al. (2021). WSG, a Glucose-Rich Polysaccharide from Ganoderma Lucidum, Combined with Cisplatin Potentiates Inhibition of Lung Cancer In Vitro and In Vivo. Polymers 13 (24), 4353. doi:10.3390/polym13244353
Ren, L., Zhang, J., and Zhang, T. (2021). Immunomodulatory Activities of Polysaccharides from Ganoderma on Immune Effector Cells. Food Chem. 340, 127933. doi:10.1016/j.foodchem.2020.127933
Rice, P. J., Adams, E. L., Ozment-Skelton, T., Gonzalez, A. J., Goldman, M. P., Lockhart, B. E., et al. (2005). Oral Delivery and Gastrointestinal Absorption of Soluble Glucans Stimulate Increased Resistance to Infectious Challenge. J. Pharmacol. Exp. Ther. 314 (3), 1079–1086. doi:10.1124/jpet.105.085415
Rice, P. J., Lockhart, B. E., Barker, L. A., Adams, E. L., Ensley, H. E., and Williams, D. L. (2004). Pharmacokinetics of Fungal (1-3)-Beta-D-Glucans Following Intravenous Administration in Rats. Int. Immunopharmacol. 4 (9), 1209–1215. doi:10.1016/j.intimp.2004.05.013
Seweryn, E., Ziała, A., and Gamian, A. (2021). Health-Promoting of Polysaccharides Extracted from Ganoderma Lucidum. Nutrients 13 (8), 2725. doi:10.3390/nu13082725
Shi, L. (2016). Bioactivities, Isolation and Purification Methods of Polysaccharides from Natural Products: A Review. Int. J. Biol. Macromol. 92, 37–48. doi:10.1016/j.ijbiomac.2016.06.100
Sivamaruthi, B. S., Kesika, P., and Chaiyasut, C. (2020). The Role of Probiotics in Colorectal Cancer Management. Evid. Based Complement. Altern. Med. 2020, 3535982. doi:10.1155/2020/3535982
Sohretoglu, D., and Huang, S. (2018). Ganoderma Lucidum Polysaccharides as an Anti-cancer Agent. Anticancer Agents Med. Chem. 18 (5), 667–674. doi:10.2174/1871520617666171113121246
Song, M., Li, Z. H., Gu, H. S., Tang, R. Y., Zhang, R., Zhu, Y. L., et al. (2021). Ganoderma Lucidum Spore Polysaccharide Inhibits the Growth of Hepatocellular Carcinoma Cells by Altering Macrophage Polarity and Induction of Apoptosis. J. Immunol. Res. 2021, 6696606. doi:10.1155/2021/6696606
Su, J., Li, D., Chen, Q., Li, M., Su, L., Luo, T., et al. (2018). Anti-breast Cancer Enhancement of a Polysaccharide from Spore of Ganoderma Lucidum with Paclitaxel: Suppression on Tumor Metabolism with Gut Microbiota Reshaping. Front. Microbiol. 9, 3099. doi:10.3389/fmicb.2018.03099
Torres-Maravilla, E., Boucard, A. S., Mohseni, A. H., Taghinezhad-S, S., Cortes-Perez, N. G., and Bermúdez-Humarán, L. G. (2021). Role of Gut Microbiota and Probiotics in Colorectal Cancer: Onset and Progression. Microorganisms 9 (5), 1021. doi:10.3390/microorganisms9051021
Urulangodi, M., and Mohanty, A. (2020). DNA Damage Response and Repair Pathway Modulation by Non-histone Protein Methylation: Implications in Neurodegeneration. J. Cell. Commun. Signal 14 (1), 31–45. doi:10.1007/s12079-019-00538-2
Vetvicka, V., Dvorak, B., Vetvickova, J., Richter, J., Krizan, J., Sima, P., et al. (2007). Orally Administered Marine (1-->3)Bbeta-DGglucan Phycarine Stimulates Both Humoral and Cellular Immunity. Int. J. Biol. Macromol. 40 (4), 291–298. doi:10.1016/j.ijbiomac.2006.08.009
Vivarelli, S., Salemi, R., Candido, S., Falzone, L., Santagati, M., Stefani, S., et al. (2019). Gut Microbiota and Cancer: From Pathogenesis to Therapy. Cancers (Basel) 11 (1), 38. doi:10.3390/cancers11010038
Wang, C., Shi, S., Chen, Q., Lin, S., Wang, R., Wang, S., et al. (2018). Antitumor and Immunomodulatory Activities of Ganoderma Lucidum Polysaccharides in Glioma-Bearing Rats. Integr. Cancer Ther. 17 (3), 674–683. doi:10.1177/1534735418762537
Wang, J., Cao, B., Zhao, H., and Feng, J. (2017a). Emerging Roles of Ganoderma Lucidum in Anti-aging. Aging Dis. 8 (6), 691–707. doi:10.14336/AD.2017.0410
Wang, S., Awad, K. S., Elinoff, J. M., Dougherty, E. J., Ferreyra, G. A., Wang, J. Y., et al. (2015). G Protein-Coupled Receptor 40 (GPR40) and Peroxisome Proliferator-Activated Receptor γ (PPARγ): AN INTEGRATED TWO-RECEPTOR SIGNALING PATHWAY. J. Biol. Chem. 290 (32), 19544–19557. doi:10.1074/jbc.M115.638924
Wang, W., Gou, X., Xue, H., and Liu, K. (2019). Ganoderan (GDN) Regulates the Growth, Motility and Apoptosis of Non-small Cell Lung Cancer Cells through ERK Signaling Pathway In Vitro and In Vivo. Onco Targets Ther. 12, 8821–8832. doi:10.2147/OTT.S221161
Wang, Y., Zhang, C., Zhang, S., Zhao, Z., Wang, J., Song, J., et al. (2017b). Kanglaite Sensitizes Colorectal Cancer Cells to Taxol via NF-Κβ Inhibition and Connexin 43 Upregulation. Sci. Rep. 7 (1), 1280. doi:10.1038/s41598-017-01480-2
Wang, Y., Fan, X., and Wu, X. (2020). Ganoderma Lucidum Polysaccharide (GLP) Enhances Antitumor Immune Response by Regulating Differentiation and Inhibition of MDSCs via a CARD9-NF-kappaB-Ido Pathway. Biosci. Rep. 40 (6), BSR20201170. doi:10.1042/BSR20201170
Wen, L., Sheng, Z., Wang, J., Jiang, Y., and Yang, B. (2022). Structure of Water-Soluble Polysaccharides in Spore of Ganoderma Lucidum and Their Anti-inflammatory Activity. Food Chem. 373 (Pt A), 131374. doi:10.1016/j.foodchem.2021.131374
Wilson, I. D., and Nicholson, J. K. (2017). Gut Microbiome Interactions with Drug Metabolism, Efficacy, and Toxicity. Transl. Res. 179, 204–222. doi:10.1016/j.trsl.2016.08.002
Winska, K., Maczka, W., Gabryelska, K., and Grabarczyk, M. (2019). Mushrooms of the Genus Ganoderma Used to Treat Diabetes and Insulin Resistance. Molecules 24 (22), 4075. doi:10.3390/molecules24224075
Wu, A. H., Tseng, C., Vigen, C., Yu, Y., Cozen, W., Garcia, A. A., et al. (2020). Gut Microbiome Associations with Breast Cancer Risk Factors and Tumor Characteristics: a Pilot Study. Breast Cancer Res. Treat. 182 (2), 451–463. doi:10.1007/s10549-020-05702-6
Wu, K., Na, K., Chen, D., Wang, Y., Pan, H., and Wang, X. (2018). Effects of Non-steroidal Anti-inflammatory Drug-Activated Gene-1 on Ganoderma Lucidum Polysaccharides-Induced Apoptosis of Human Prostate Cancer PC-3 Cells. Int. J. Oncol. 53 (6), 2356–2368. doi:10.3892/ijo.2018.4578
Xian, H., Li, J., Zhang, Y., Li, D., Zhu, Y., Li, S., et al. (2021). Antimetastatic Effects of Ganoderma Lucidum Polysaccharide Peptide on B16-F10-Luc-G5 Melanoma Mice with Sleep Fragmentation. Front. Pharmacol. 12, 650216. doi:10.3389/fphar.2021.650216
Xie, Q., Liu, Y., and Li, X. (2020). The Interaction Mechanism between Autophagy and Apoptosis in Colon Cancer. Transl. Oncol. 13 (12), 100871. doi:10.1016/j.tranon.2020.100871
Yu, H., Yang, Y., Jiang, T., Zhang, X., Zhao, Y., Pang, G., et al. (2019). Effective Radiotherapy in Tumor Assisted by Ganoderma Lucidum Polysaccharide-Conjugated Bismuth Sulfide Nanoparticles through Radiosensitization and Dendritic Cell Activation. ACS Appl. Mater Interfaces 11 (31), 27536–27547. doi:10.1021/acsami.9b07804
Yu, Y., Qian, L., Du, N., Liu, Y., Zhao, X., and Zhang, X. (2017). Ganoderma Lucidum Polysaccharide Enhances Radiosensitivity of Hepatocellular Carcinoma Cell Line HepG2 through Akt Signaling Pathway. Exp. Ther. Med. 14 (6), 5903–5907. doi:10.3892/etm.2017.5340
Zeidan, A. A., Poulsen, V. K., Janzen, T., Buldo, P., Derkx, P. M. F., Oregaard, G., et al. (2017). Polysaccharide Production by Lactic Acid Bacteria: from Genes to Industrial Applications. FEMS Microbiol. Rev. 41 (Suppl. p_1), S168–S200. doi:10.1093/femsre/fux017
Zeng, X., Li, P., Chen, X., Kang, Y., Xie, Y., Li, X., et al. (2019). Effects of Deproteinization Methods on Primary Structure and Antioxidant Activity of Ganoderma Lucidum Polysaccharides. Int. J. Biol. Macromol. 126, 867–876. doi:10.1016/j.ijbiomac.2018.12.222
Zhang, M., Kim, J. A., and Huang, A. Y. (2018). Optimizing Tumor Microenvironment for Cancer Immunotherapy: β-Glucan-Based Nanoparticles. Front. Immunol. 9, 341. doi:10.3389/fimmu.2018.00341
Zhang, S., Pang, G., Chen, C., Qin, J., Yu, H., Liu, Y., et al. (2019a). Effective Cancer Immunotherapy by Ganoderma Lucidum Polysaccharide-Gold Nanocomposites through Dendritic Cell Activation and Memory T Cell Response. Carbohydr. Polym. 205, 192–202. doi:10.1016/j.carbpol.2018.10.028
Zhang, Y., Ding, C., Zhu, W., Li, X., Chen, T., Liu, Q., et al. (2022). Chemotherapeutic Drugs Induce Oxidative Stress Associated with DNA Repair and Metabolism Modulation. Life Sci. 289, 120242. doi:10.1016/j.lfs.2021.120242
Zhang, Y., Jiang, Y., Zhang, M., and Zhang, L. (2019b). Ganoderma Sinense Polysaccharide: An Adjunctive Drug Used for Cancer Treatment. Prog. Mol. Biol. Transl. Sci. 163, 165–177. doi:10.1016/bs.pmbts.2019.02.008
Zhao, X., Zhou, D., Liu, Y., Li, C., Zhao, X., Li, Y., et al. (2018). Ganoderma Lucidum Polysaccharide Inhibits Prostate Cancer Cell Migration via the Protein Arginine Methyltransferase 6 Signaling Pathway. Mol. Med. Rep. 17 (1), 147–157. doi:10.3892/mmr.2017.7904
Zhong, J., Fang, L., Chen, R., Xu, J., Guo, D., Guo, C., et al. (2021). Polysaccharides from Sporoderm-Removed Spores of Ganoderma Lucidum Induce Apoptosis in Human Gastric Cancer Cells via Disruption of Autophagic Flux. Oncol. Lett. 21 (5), 425. doi:10.3892/ol.2021.12686
Keywords: cancer, Ganoderma, polysaccharides, anticancer properties, chemopreventive agents, therapeutic intervention
Citation: Wang M and Yu F (2022) Research Progress on the Anticancer Activities and Mechanisms of Polysaccharides From Ganoderma. Front. Pharmacol. 13:891171. doi: 10.3389/fphar.2022.891171
Received: 07 March 2022; Accepted: 17 June 2022;
Published: 05 July 2022.
Edited by:
Dejan S. Stojkovic, University of Belgrade, SerbiaReviewed by:
Jasmina Glamoclija, University of Belgrade, SerbiaCopyright © 2022 Wang and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Man Wang, d2FuZ21hbkBxZHUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.