- 1Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, University of Hong Kong, Hong Kong SAR, China
- 2Department of Medicine, School of Clinical Medicine, Li Ka Shing Faculty of Medicine, University of Hong Kong, Hong Kong SAR, China
- 3Research Department of Policy and Practice, School of Pharmacy, University College London, London, United Kingdom
- 4Laboratory of Data Discovery for Health (D24H), Hong Kong Science Park, Hong Kong SAR, China
- 5HKU-Shenzhen Hospital, Shenzhen, China
Background: PARP inhibitors have shown significant improvement in progression-free survival, but their costs cast a considerable financial burden. In line with value-based oncology, it is important to evaluate whether drug prices justify the outcomes.
Objectives: The aim of the study was to systematically evaluate PARP inhibitors on 1) cost-effectiveness against the standard care, 2) impact on cost-effectiveness upon stratification for genetic characteristics, and 3) identify factors determining their cost-effectiveness, in four cancer types.
Methods: We systematically searched PubMed, EMBASE, Web of Science, and Cochrane Library using designated search terms, updated to 31 August 2021. Trial-based or modeling cost-effectiveness analyses of four FDA-approved PARP inhibitors were eligible. Other studies known to authors were included. Reference lists of selected articles were screened. Eligible studies were assessed for methodological and reporting quality before review.
Results: A total of 20 original articles proceeded to final review. PARP inhibitors were not cost-effective as recurrence maintenance in advanced ovarian cancer despite improved performance upon genetic stratification. Cost-effectiveness was achieved when moved to upfront maintenance in a new diagnosis setting. Limited evidence indicated non–cost-effectiveness in metastatic breast cancer, mixed conclusions in metastatic pancreatic cancer, and cost-effectiveness in metastatic prostate cancer. Stratification by genetic testing displayed an effect on cost-effectiveness, given the plummeting ICER values when compared to the “treat-all” strategy. Drug cost was a strong determinant for cost-effectiveness in most models.
Conclusions: In advanced ovarian cancer, drug use should be prioritized for upfront maintenance and for patients with BRCA mutation or BRCAness at recurrence. Additional economic evaluations are anticipated for novel indications.
1 Introduction
The development of poly (ADP-ribose) polymerase (PARP) inhibitors represents a breakthrough in first harnessing the “synthetic lethality” concept in clinical use (Helleday, 2011; Sonnenblick et al., 2015) and kick-started the era of redefining a single tumor type for stratification into distinct diseases specific to genetic aberrations. Patients with tumor-harboring BRCA1/2 mutations or who show homologous recombination deficiency (HRD) are particularly sensitive to the effect of PARP inhibitors (O'Sullivan et al., 2014). At the time of writing, four PARP inhibitors have been approved by the U.S. Food and Drug Administration (FDA): olaparib, niraparib, rucaparib, and talazoparib (RUBRACA (rucaparib), 2020; LYNPARZA (olaparib), 2021; ZEJULA (niraparib) 2021; TELZENNA (talaparib), 2021).
Although efficacy as first-line monotherapy is as yet unproven, PARP inhibitors as maintenance therapy amplify the existing treatment effect. In advanced ovarian cancer, patients receive repeated courses of platinum-based chemotherapies with over 70% risk of recurrence until “platinum resistance” (Jiang et al., 2019; Ovarian Cancer Research Alliance, 2020). In metastatic pancreatic cancer, progression-free survival (PFS) following first-line chemotherapies last only 6 months with less than 10% of patients surviving after 5 years (Conroy et al., 2018; Rawla et al., 2019). PFS often diminishes with subsequent cycles; maintenance therapy between lines could prolong PFS and allow patient eligibility for subsequent strategies, thus enhancing survival likelihood (Evans and Matulonis, 2017). PARP inhibitors targeting ovarian cancer all demonstrated longer median PFS against placebo (olaparib, niraparib, and rucaparib: 16.6–21.0 vs. 5.4–5.5 months) in BRCAmut cohorts of recurrent platinum-sensitive cases, and in the first-line maintenance setting, olaparib and niraparib further extended PFS by 3 years and 1 year among BRCAmut and HRD-positive patients, respectively (Ledermann et al., 2012; Mirza et al., 2016; Coleman et al., 2017; Pujade-Lauraine et al., 2017; Moore et al., 2018; González-Martín et al., 2019). Patients with gBRCAmut metastatic pancreatic cancer also had longer PFS with maintenance olaparib against placebo (7.4 vs. 3.8 months) (Golan et al., 2019). Apart from maintenance, PARP inhibitors demonstrated efficacy in later lines as active treatment for gBRCAmut metastatic breast cancer (PFS extension with olaparib and talazoparib: 2.8–3 months) and gBRCAmut and/or HRD-positive metastatic castration-resistant prostate cancer (PFS with olaparib vs. placebo: 7.4 vs. 3.6 months; objective response rate with rucaparib: 43.5–50.8%) (Robson et al., 2017; Litton et al., 2018; Abida et al., 2020; Hussain et al., 2020).
Value-based oncology is thus warranted to address the cost-effectiveness of novel drugs, for which acceptable prices should be tied to justifiable patient outcomes by cost-effectiveness analyses (Neumann et al., 2021). Incremental cost-effectiveness ratio (ICER), a quotient of the cost difference between two therapeutic interventions divided by the outcome difference, denotes the incremental monetary value for an additional life-year or quality-adjusted life-year (QALY). When this falls below the willingness-to-pay (WTP) or when a strategy is both cost-saving and clinically superior (dominance), it is concluded as cost-effective. A previous literature review on the cost-effectiveness studies of PARP inhibitors focused, however, only on methodological quality and publications related to ovarian cancer (Gao et al., 2020).
Given the recently approved multiple indications in a variety of cancer types and the inconsistent genetic prerequisites for BRCA mutation and HRD status across indications, it is also questionable whether the full biomarker-guided use of PARP inhibitors would improve cost-effectiveness as they acted more profoundly on patient stratification. In this systematic review, we aimed to evaluate PARP inhibitors on 1) the cost-effectiveness against the standard of care, 2) impact on cost-effectiveness upon stratification for genetic characteristics, and 3) to elucidate the key factors that determine cost-effectiveness in the management of ovarian, breast, pancreatic, and prostate cancers.
2 Methods and Materials
This study was conducted according to the recommended checklist of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and benchmarked with the methodology of similar systematic reviews on medicinal cost-effectiveness (Moher et al., 2009; Verma et al., 2018; Yoshida et al., 2020).
2.1 Search Strategy and Eligibility
We systematically searched PubMed, EMBASE, Web of Science, and Cochrane Library, without language and date restriction, using the search terms: (“poly ADP-ribose polymerase inhibitors” OR “PARP inhibitors” OR “olaparib” OR “rucaparib” OR “niraparib” OR “talazoparib”) and (“cost” OR “cost-effectiveness” OR “cost-utility” OR “economics”) in any field, updated to 31 August 2021. Reference lists of eligible articles were checked for additional relevant articles, and other studies known to the authors were included. Eligibility criteria were trial-based, or modeling cost-effectiveness analyses published in English or Chinese language related to any of the four FDA-approved PARP inhibitors, regardless of cancer types, lines of treatment, and comparator interventions. Non-comparative studies, reviews, responses, editorials, protocols, and abstract-only articles were excluded.
2.2 Quality Assessment
Studies were assessed using the Quality of Health Economics Studies (QHES) instrument (for methodological quality) and the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist (for reporting quality) (Ofman et al., 2003; Husereau et al., 2013). Articles which obtained a QHES score above 74 out of 100 and CHEERS score above 20 out of 24 were qualified for final data extraction and synthesis.
2.3 Data Extraction and Synthesis
Data were extracted based on a pre-defined extraction framework for bibliography, methods, results, and conclusion, including ICER at base-case analysis and model impact at sensitivity analyses. All presented monetary values were converted to U.S. dollars in the year of publication. The primary outcome of interest was the ICER of PARP inhibitors compared with observation (no maintenance treatment after standard treatment), alternative PARP inhibitors, and the standard of care.
Two independent researchers (VKYC and RQY) performed literature screening and quality assessment. Data were extracted by one researcher (RQY) and cross-checked by another researcher (VKYC). All discrepancies were resolved in consensus meetings.
3 Results
3.1 Study Selection and Quality Assessment
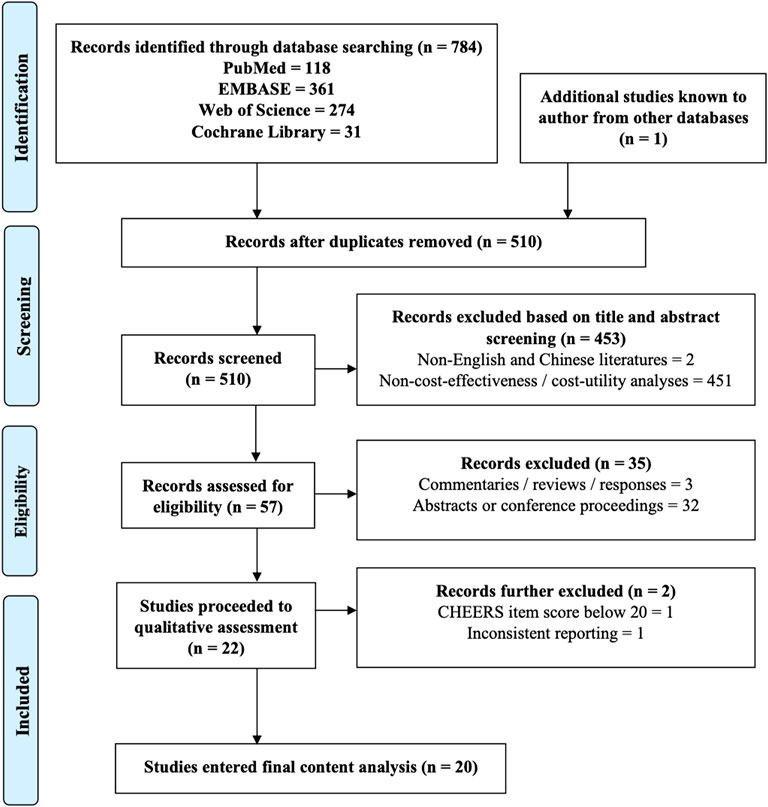
A total of 22 original full-text studies passed the initial screening for eligibility (Figure 1). Among them, 21 articles achieved good methodological and reporting quality (mean QHES score: 92.5/100 and a CHEERS score of 22.5/24) (Supplementary Table S1). One study was excluded further due to inconsistent reporting. Eventually, 20 articles proceeded into final review.
3.2 Study Characteristics
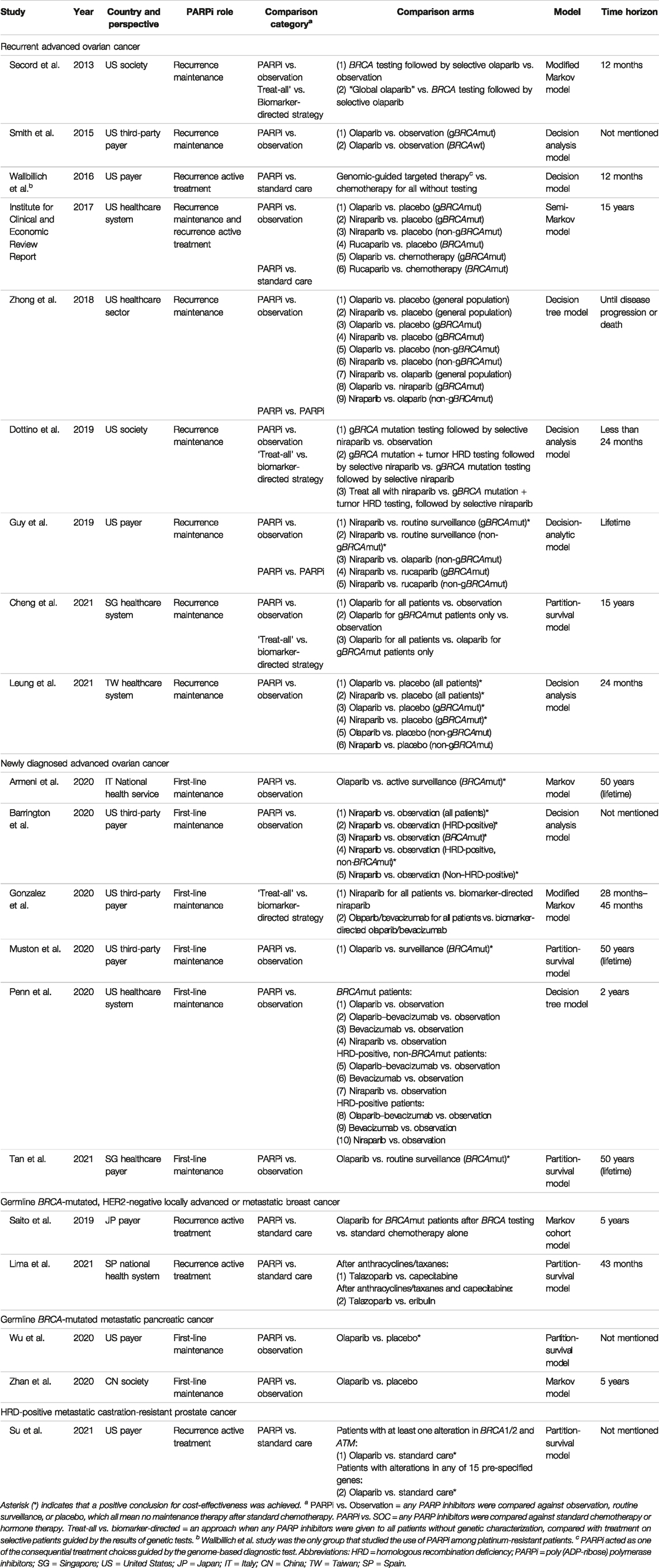
Table 1 illustrates the general characteristics of the included studies. The majority of studies were conducted in the United States (n = 13), five in Asia, and two in Europe. Most studies targeted patients with advanced ovarian cancer (n = 15), with nine focusing on recurrence and six covering new diagnosis setting. The remaining studied metastatic pancreatic (n = 2), breast (n = 2), and prostate (n = 1) cancers. Five studies investigated the role of PARP inhibitors as active treatment, 16 studies as maintenance treatment, and one covered both categories. All studies used decision modeling. The most frequently adopted time horizon was a short-term between 1 and 5 years or until disease progression (n = 10). Ten studies were set out from the payer’s perspective, seven from a healthcare system perspective, and three from a societal perspective. Major comparison arms were observation (n = 15), followed by standard care (n = 5), and alternative biomarker-directed strategies (n = 2). All studies stratified patients based on BRCA mutation, but only three studies examined the effect of HRD status. Figure 2 summarizes the economic outcomes by comparison arms, and Tables 2–4 provide detailed economic outcomes of each included study.
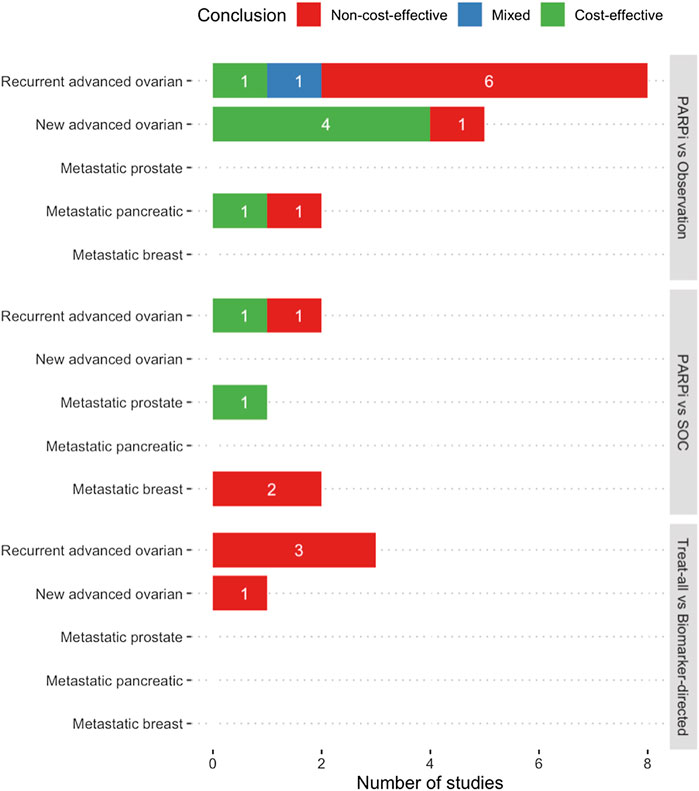
FIGURE 2. Summary of economic evaluation outcomes of included studies (a,b). (a) Mixed conclusion indicates the presence of both positive and negative conclusion for cost-effectiveness in different comparison arms in the same study. (b) PARPi vs Observation = Any PARP inhibitors were compared against observation, routine surveillance, or placebo, which all mean no maintenance therapy after standard chemotherapy. PARPi vs SOC = Any PARP inhibitors were compared against standard chemotherapy or hormone therapy. Treat-all vs Biomarker-directed = An approach when any PARP inhibitors were given to all patients without genetic characterization, compared with treatment on selective patients guided by the results of genetic tests. Abbreviations: PARPi − PARP inhibitors, SOC − Standard of care.
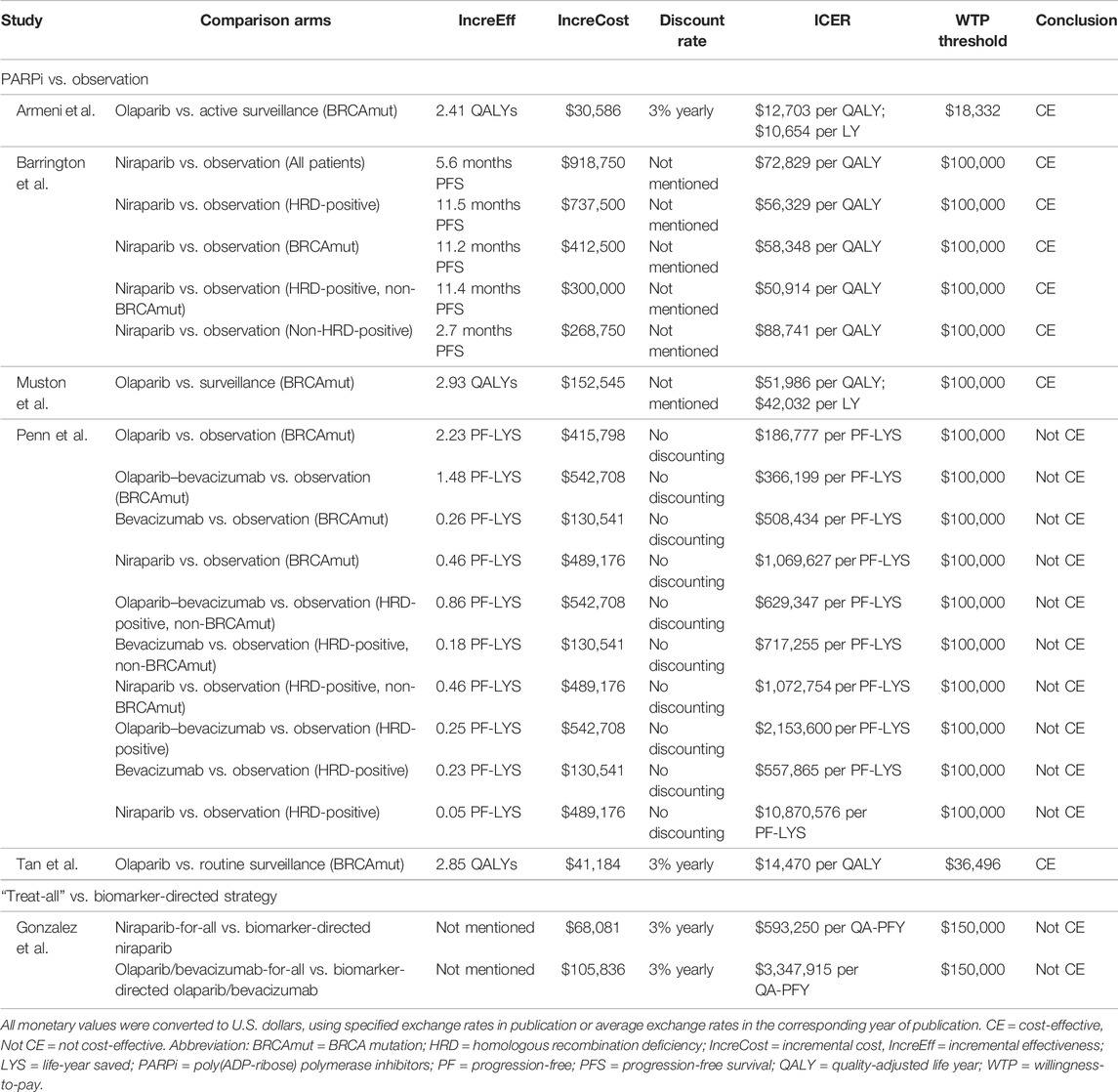
TABLE 3. Details of economic evaluation outcomes of studies in newly diagnosed advanced ovarian cancer.
3.3 Cost and Clinical Benefits of PARP Inhibitors
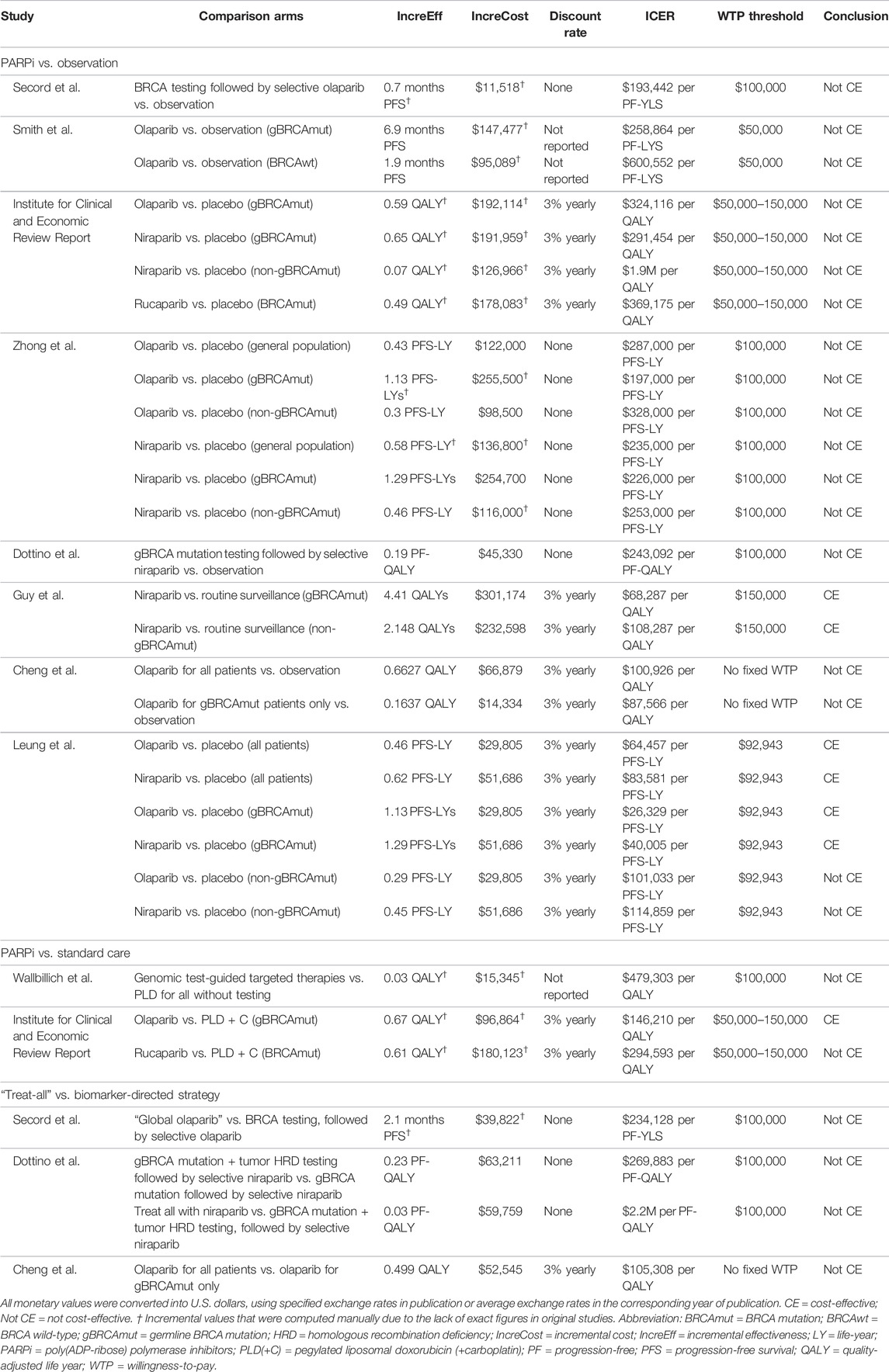
Overall, the maintenance strategies with PARP inhibitors generated additional 0.07–4.41 QALYs compared with observation. As active treatment, PARP inhibitors gave 0.03–0.67 QALY compared with the standard care. Clinical benefits varied across types and lines of PARP inhibitors, comparison arms, genetic characteristics, time horizon, and simulation methods of survival data. Costs varied greatly between health systems and country contexts.
3.3.1 Treating Recurrent Advanced Ovarian Cancer
PARP inhibitors were widely studied as maintenance treatment for patients with recurrent advanced ovarian cancer who were responsive to platinum-based chemotherapy. Eight studies compared PARP inhibitors against observation, with six of these concluding that they were not cost-effective. Specifically, Smith et al. (2015) determined that olaparib costs $600,552 per PF-YLS for BRCA wild-type patients, with improved ICER at $258,864 per PF-YLS when restricted to gBRCAmut patients, but this was still beyond the $50,000 WTP threshold. Zhong et al. (2018) later expanded the comparison to both olaparib and niraparib and identified their ICERs at $287,000 and $235,000 per PFS-LYS, which dropped only slightly to $197,000 and $226,000 per PFS-LY when restricted to gBRCAmut patients. Consistent findings were noted in the Institute for Clinical and Economic Review Report, two more studies in the United States (Secord et al., 2013; Institute for Clinical and Economic Review 2017; Dottino et al., 2019), and one study in the Singaporean context (Cheng et al., 2021). The Guy group in the United States was one of the two that concluded PARP inhibitors were cost-effective in comparison to observation, with niraparib in both gBRCAmut and wild-type patients giving ICERs at $68,287 and $108,287 per QALY, albeit a questionably high WTP threshold at $150,000 (Guy et al., 2019). Another similar conclusion was drawn in Taiwan by Leung et al., which later expanded the comparison to both niraparib and olaparib. Regardless of genetic features, the ICERs accounted for 69–90% of the WTP threshold. When restricted to gBRCAmut patients only, the ICERs even dropped to between 28 and 43% of the threshold (Leung et al., 2021).
In contrast to maintenance therapies, two studies evaluated the active role of PARP inhibitors in later lines in comparison with chemotherapies in the recurrence setting, with inconsistent results. In BRCAmut patients, the Institute for Clinical and Economic Review Report deemed olaparib cost-effective versus PLD + C (pegylated liposomal doxorubicin + carboplatin) at $146,210 per QALY, in contrast to rucaparib, which was not cost-effective at $294,593 per QALY against PLD + C (Institute for Clinical and Economic Review 2017). However, the genome-guided approach with next-generation sequencing (NGS) to test all concurrent targetable mutations was not cost-effective, although it was in the context of platinum-resistant cases (Wallbillich et al., 2016).
3.3.2 Treating Newly Diagnosed Advanced Ovarian Cancer
Since 2018, PARP inhibitors have been approved for earlier use as upfront maintenance rather than waiting for relapse occurrence following successful response to first-line chemotherapy, based on genetic characteristics. Six studies investigated their value as upfront maintenance. Five studies compared PARP inhibitors against observation with four concluding them to be cost-effective. In particular, Tan et al. (2021)compared first-line olaparib maintenance with routine surveillance in the Singaporean context and demonstrated $14,470 per QALY, an ICER far below their $36,496 WTP threshold. Olaparib was similarly found to be cost-effective in Italy and the United States, with ICERs accounting for 52–69% of their WTP thresholds (Armeni et al., 2020; Muston et al., 2020). Apart from olaparib, Barrington et al. (2020) comprehensively modeled five scenarios when offering niraparib to all patients, HRD-positive-only patients, BRCAmut-only patients, HRD-positive non-BRCAmut patients, and non–HRD-positive patients; all ICERs ranged from $50,914 to $88,741 per QALY, which remained lower than the $100,000 threshold. Without applying cost and benefit discounting, Penn et al. was the only group that showed negative findings for first-line maintenance with and without adding an antiangiogenic agent. Among the ten strategies composed of olaparib-only, niraparib-only, bevacizumab-only, and olaparib and bevacizumab with and without stratification by genetic characteristics, when compared against observation, the ICERs stayed high in the range from $366,100 to $10,870,576 per PF-LYS (Penn et al., 2020).
3.3.3 Treating Metastatic Breast, Pancreatic, and Prostate Cancers
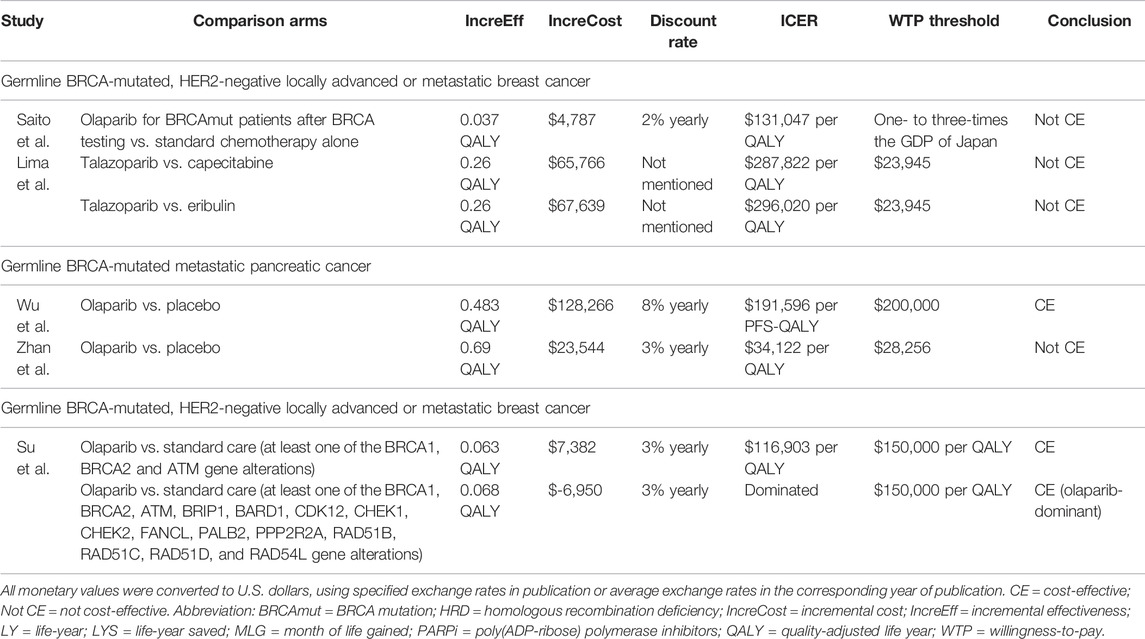
PARP inhibitors were approved for metastatic breast cancer with HER2-negative gBRCAmut patients who failed chemotherapy or for HR-positive gBRCAmut patients who failed or were ineligible for endocrine therapies. Two studies investigated the cost-effectiveness of PARP inhibitor versus standard chemotherapies in these patients, and both deemed the former not cost-effective. Saito’s group in Japan compared the strategy of olaparib monotherapy after positive BRCA mutation profiling to the use of capecitabine, eribulin, or vinorelbine without testing and discovered that the former costs $131,047 per QALY, which was hardly cost-effective at the WTP threshold of $89,286 (Saito et al., 2019). Regarding talazoparib, consistent findings were presented in a Spanish study by Lima and others, who obtained ICERs slightly above $280,000 per QALY in two scenarios compared with capecitabine or eribulin, which were ten-fold higher than the threshold of $23,945 (Olry de Labry Lima et al., 2021).
Olaparib, as maintenance after first-line platinum-based chemotherapy for gBRCAmut metastatic pancreatic cancer, a recent indication approved in 2019, was studied by two groups for its cost-effectiveness versus placebo, but the conclusions were mixed. In particular, Wu and Shi (2020) in the United States found that olaparib was cost-effective with an ICER at $191,596 per PFS-QALY, which was below the $200,000 threshold but not the case when modeling was based on overall survival data ($265,290 per QALY). In China, Zhan et al. (2020) identified an ICER at $34,122 per QALY which did not support cost-effectiveness at the WTP threshold of $28,255, although the ICER drastically dropped to $14,563 per QALY when the drug cost was calculated based on the donation plan for ovarian cancer.
Only one study evaluated the cost-effectiveness in metastatic castration-resistant prostate cancer, owing to the recent approval in 2020. In the two scenarios modeled by Su et al. in the United States, compared with the standard care, olaparib was cost-effective when used among patients with at least one gene alteration in BRCA1, BRCA2, and ATM, with an ICER yielded at $116,903 per QALY. In the case of expanding the treatment group to patients who had alterations in any of all 15 pre-specified genes (BRCA1/2, ATM, BRIP1, BARD1, CDK12, CHEK1/2, FANCL, PALB2, PPP2R2A, RAD51B/C/D, and RAD54L) after NGS testing, olaparib turned out to be a cost-effective option (Su et al., 2020).
3.4 Worthiness of the Biomarker-Directed Treatment Strategy
In the studies that separated their analyses based on genetic characteristics, the ICERs using PARP inhibitors among only BRCAmut and/or HRD-positive patients were always lower than those among all patients, implying that the biomarker-directed treatment strategy was potentially cost-effective. Four studies directly compared the biomarker-directed strategy against “global PARP inhibitors,” an approach that offers the drug to all patients, regardless of their genetic characteristics. In recurrent advanced ovarian cancer, Secord et al. (2013)concluded that “global olaparib” offered the greatest efficacy but was the costliest. In the United States and Singapore, “global olaparib” was associated with an incremental cost of $234,128 or $105,300 per progression-free life year compared with the BRCA1/2 testing stratification strategy (Secord et al., 2013; Cheng et al., 2021). However, compared with observation only, BRCA1/2 testing-directed treatment was still not cost-effective. The Dottino group demonstrated similar findings for niraparib, except that they also evaluated the addition of HRD testing alongside BRCA testing before treatment (Dottino et al., 2019). Consistently in newly diagnosed advanced ovarian cancer, Gonzalez et al. (2020) concluded that when compared with biomarker-directed treatment, adopting “global olaparib or niraparib” yielded ICERs as high as $3,347,915 per QA-PFY.
3.5 Key Cost-Effectiveness Determinants
All the articles performed sensitivity analyses to assess the factors which potentially impacted the cost-effectiveness of PARP inhibitors. Out of 20 studies, 17 highlighted that the drug price was a significant driver of the ICER (Secord et al., 2013; Smith et al., 2015; Wallbillich et al., 2016; Institute for Clinical and Economic Review, 2017; Zhong et al., 2018; Dottino et al., 2019; Armeni et al., 2020; Barrington et al., 2020; Gonzalez et al., 2020; Muston et al., 2020; Penn et al., 2020; Su et al., 2020; Wu and Shi, 2020; Zhan et al., 2020; Cheng et al., 2021; Leung et al., 2021; Olry de Labry Lima et al., 2021). In the United States system, to be cost-effective in treating recurrent ovarian cancer among BRCAmut patients, major cost reduction to $3,000–6,400 per cycle was warranted for olaparib, niraparib, and rucaparib, which was up to 76% reduction at the WTP of $100,000 (Secord et al., 2013; Smith et al., 2015; Wallbillich et al., 2016; Dottino et al., 2019). For newly diagnosed ovarian and metastatic pancreatic cancers, 47 and 50% reduction in olaparib cost for BRCAmut patients was highlighted, respectively (Penn et al., 2020; Wu and Shi, 2020). In the Spanish context, for metastatic breast cancer, an even more drastic price cut for talazoparib to $906 per cycle (85% reduction) was warranted to reach the $23,945 threshold (Olry de Labry Lima et al., 2021). ICERs were less sensitive to the costs of chemotherapies, hospital care, general adverse event management, and molecular testing. As for clinical estimates, models were more sensitive to the hazard ratios of PFS or the ratios used to project overall survival, time-receiving maintenance treatment, and utility values at progressive disease state (Smith et al., 2015; Zhong et al., 2018; Guy et al., 2019; Saito et al., 2019; Armeni et al., 2020; Barrington et al., 2020; Su et al., 2020; Wu and Shi 2020; Leung et al., 2021).
4 Discussion
PARP inhibitors marked a breakthrough in the burgeoning wave of precision oncology as they provide substantial progression-free survival benefit in a broad range of patients with actionable targets. The unbridled high costs may nonetheless hinder their presence in clinical routines; health economic studies are therefore warranted to assess priorities. This systematic review depicts several findings. First, the cost-effectiveness of PARP inhibitors varied with cancer types and lines of treatment. In most cases, they were not cost-effective as maintenance treatment for recurrent advanced ovarian cancer was compared with observation, but a stronger potential was attained when moved earlier to upfront maintenance in newly diagnosed advanced ovarian cancer. Limited evidence showed that PARP inhibitors were not cost-effective in metastatic breast cancer. The conclusions were mixed for metastatic pancreatic cancer, whilst olaparib in metastatic prostate cancer seemed to be cost-effective. Next, stratification by tumor genetic characteristics displayed an effect on ICERs, given the plummeting ICER values after confining treatment to BRCAmut- and/or HRD-only patients. Finally, drug cost was consistently highlighted in all models as a strong cost-effectiveness determinant, followed by the hazard ratio of PFS in some models. However, costs of comparator treatments, hospice care, general adverse event management, and molecular tests made minimal impact on all models.
This review serves to inform payers of the overall cost-effectiveness pattern of PARP inhibitors and key areas to intervene for resource prioritization. Although all included studies utilized registrational randomized data, the overall conclusions in the analyses are logical. In platinum-sensitive BRCAmut ovarian cancer, olaparib maintenance offered significant PFS improvement with 19.1 versus 5.5 months of placebo upon first recurrence in the landmark trial, but when the drug was moved to upfront maintenance in newly diagnosed advanced ovarian cancer, the estimated median PFS difference was numerically extended to 36 months (Pujade-Lauraine et al., 2017; Moore et al., 2018). Despite the higher cost, cost-effectiveness was reflected by the greater survival difference for earlier use along the patient’s journey. This finding echoes the recent guidance from the National Institute for Health and Care Excellence in 2020 and 2021 that olaparib alone or plus bevacizumab should be recommended for first-line maintenance in Cancer Drugs Fund with managed access agreement (National Institute for Health and Care Excellence 2019; National Institute for Health and Care Excellence 2021), heralding the importance of maximizing the value of the drug by re-adjusting the treatment position of PARP inhibitors and identifying BRCA mutation and HRD early at the time of diagnosis. In all relevant articles, biomarker-directed treatment was always more cost-effective than treating all patients with PARP inhibitors, regardless of genetic features. Comprehensive genome profiling was particularly valuable in metastatic prostate cancer as the targeted use of olaparib among patients with alterations in any of the 15 pre-specified genes yielded a cost-effective option, which was concluded as a more appropriate strategy than testing for only three gene alterations, owing to a lower number needed to screen for identifying eligible patients. When a funding decision has to be made, it is important to prioritize targeted use based on genetic stratification and to select the composition of test panels prudently to maximize the value of PARP inhibitors.
Undeniably, the poor cost-effectiveness of PARP inhibitors in recurrent ovarian cancer and metastatic breast cancer remains an issue. The standard comparator in platinum-sensitive ovarian cases was wait-and-watch, which explains the tremendous incremental cost, following the introduction of an extra treatment. In the case of metastatic breast cancer, however, it was more attributed by the relatively minuscule incremental QALY as olaparib and talazoparib were compared against an effective treatment. Another contributor for both was the steep drug price, a strong determining factor identified in 85% of the studies. Taking the United States system as an example, the per month wholesale acquisition costs of PARP inhibitors for ovarian cancer ranged from $13,679 to 18,070 between 2017 and 2018 (Institute for Clinical and Economic Review 2017; Gonzalez et al., 2020). In this review, PARP inhibitors could face a radical price cut to as low as $3,000 in order to fulfill the common WTP thresholds at $100,000, but the requirement seems unrealistic since other novel targeted therapies were commonly marketed at $5,000–10,000 per month or higher (Kaplan, 2017). Price negotiation could be an alternative measure as Zhan et al. (2020) found that olaparib turned out to be cost-effective in metastatic pancreatic cancer despite off-label use if the discounted price approved in ovarian cancer could similarly be applied to pancreatic cancer (Zhan et al., 2020).
There is a disproportionate distribution of economic evidence across different indications, countries, and health systems. The majority of studies were in the United States, and the remaining studies mostly originated from other developed countries, which signify an unmet need in developing countries where cost-effectiveness or even treatment access is questionable. Next, compared with advanced ovarian cancer, fewer studies evaluated the use of PARP inhibitors in metastatic breast, pancreatic, and prostate cancers, given that the latter indications were only approved recently. An assessment of cost-effectiveness consistency across systems was therefore less possible in these indications. Finally, owing to the lack of mature overall survival data, most studies either projected the overall survival impact based on the available PFS data or relied heavily on PF-QALY for interpretation. However, previous literature studies showed that a positive PFS correctly predicted a positive overall survival only 71% of the time (Lakdawalla et al., 2015). Since overall survival depicts the actual length of time until death, it is of greater clinical importance and accounts for any diminished effect in subsequent therapies after PARP inhibitor treatment. Therefore, further verification with mature data from trial or real-world evidence is highly encouraged as this will be critical for payers to confirm how PFS could be translated into overall survival benefit.
5 Conclusion
PARP inhibitors were not cost-effective as maintenance treatment for recurrent ovarian cancer but could be cost-effective if used for newly diagnosed patients. PARP inhibitor use should be prioritized for upfront maintenance and for patients with BRCA mutation or BRCAness at recurrence. Economic evidence in metastatic breast, pancreatic, and prostate cancers was less and with mixed conclusions. Drug cost is the most important determinant for cost-effectiveness. Additional economic evaluations across the globe with mature overall survival data and novel indications are anticipated.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author Contributions
VC and XL conceived the study idea and study design. VC and RY performed literature screening and data extraction and drafted the manuscript. XL supervised the study conduction. All authors interpreted the results and critically revised for intellectual content. All authors had full access to all the data in this study. All authors met the authorship criteria, approved manuscript submission, and agreed to be accountable for all aspects of the work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank our colleagues in the Department of Pharmacology and Pharmacy of the University of Hong Kong–Adrienne Chan, Kuan Peng, and Lauren Lau for assistance in data retrieval; and Lisa Lam for proof-reading the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.891149/full#supplementary-material
References
Abida, W., Patnaik, A., Campbell, D., Shapiro, J., Bryce, A. H., McDermott, R., et al. (2020). Rucaparib in Men with Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J. Clin. Oncol. 38 (32), 3763–3772. doi:10.1200/JCO.20.01035
Armeni, P., Borsoi, L., Fornaro, G., Jommi, C., Colombo, N., and Costa, F. (2020). Cost-Effectiveness and Net Monetary Benefit of Olaparib Maintenance Therapy versus No Maintenance Therapy after First-Line Platinum-Based Chemotherapy in Newly Diagnosed Advanced BRCA1/2-Mutated Ovarian Cancer in the Italian National Health Service. Clin. Ther. 42 (7), 1192–e12. e12. doi:10.1016/j.clinthera.2020.04.015
Barrington, D. A., Tubbs, C., Smith, H. J., Straughn, J. M., Senter, L., and Cohn, D. E. (2020). Niraparib Maintenance in Frontline Management of Ovarian Cancer: A Cost Effectiveness Analysis. Int. J. Gynecol. Cancer 30 (10), 1569–1575. doi:10.1136/ijgc-2020-001550
Cheng, L.-J., Wong, G., Chay, W.-Y., Ngeow, J., Tan, Y., Soon, S. S., et al. (2021). Cost-effectiveness of Olaparib Maintenance Therapy when Used with and without Restriction by BRCA1/2 Mutation Status for Platinum-Sensitive Relapsed Ovarian Cancer. Expert Rev. Pharmacoeconomics Outcomes Res. 21 (3), 441–448. doi:10.1080/14737167.2021.1890587
Coleman, R. L., Oza, A. M., Lorusso, D., Aghajanian, C., Oaknin, A., Dean, A., et al. (2017). Rucaparib Maintenance Treatment for Recurrent Ovarian Carcinoma after Response to Platinum Therapy (ARIEL3): a Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 390 (10106), 1949–1961. doi:10.1016/S0140-6736(17)32440-6
Conroy, T., Hammel, P., Hebbar, M., Ben Abdelghani, M., Wei, A. C., Raoul, J. L., et al. (2018). FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 379 (25), 2395–2406. doi:10.1056/NEJMoa1809775
Dottino, J. A., Moss, H. A., Lu, K. H., Secord, A. A., and Havrilesky, L. J. (2019). U.S. Food and Drug Administration-Approved Poly (ADP-Ribose) Polymerase Inhibitor Maintenance Therapy for Recurrent Ovarian Cancer: A Cost-Effectiveness Analysis. Obstet. Gynecol. 133 (4), 795–802. doi:10.1097/AOG.0000000000003171
Evans, T., and Matulonis, U. (2017). PARP Inhibitors in Ovarian Cancer: Evidence, Experience and Clinical Potential. Ther. Adv. Med. Oncol. 9 (4), 253–267. doi:10.1177/1758834016687254
Gao, W., Muston, D., Monberg, M., McLaurin, K., Hettle, R., Szamreta, E., et al. (2020). A Critical Appraisal and Recommendations for Cost-Effectiveness Studies of Poly(ADP-Ribose) Polymerase Inhibitors in Advanced Ovarian Cancer. PharmacoEconomics 38 (11), 1201–1218. doi:10.1007/s40273-020-00949-9
Golan, T., Hammel, P., Reni, M., Van Cutsem, E., Macarulla, T., Hall, M. J., et al. (2019). Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 381 (4), 317–327. doi:10.1056/NEJMoa1903387
Gonzalez, R., Havrilesky, L. J., Myers, E. R., Secord, A. A., Dottino, J. A., Berchuck, A., et al. (2020). Cost-effectiveness Analysis Comparing "PARP Inhibitors-For-All" to the Biomarker-Directed Use of PARP Inhibitor Maintenance Therapy for Newly Diagnosed Advanced Stage Ovarian Cancer. Gynecol. Oncol. 159 (2), 483–490. doi:10.1016/j.ygyno.2020.08.003
González-Martín, A., Pothuri, B., Vergote, I., DePont Christensen, R., Graybill, W., Mirza, M. R., et al. (2019). Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 381 (25), 2391–2402. doi:10.1056/NEJMoa1910962
Guy, H., Walder, L., and Fisher, M. (2019). Cost-Effectiveness of Niraparib versus Routine Surveillance, Olaparib and Rucaparib for the Maintenance Treatment of Patients with Ovarian Cancer in the United States. PharmacoEconomics 37 (3), 391–405. doi:10.1007/s40273-018-0745-z
Helleday, T. (2011). The Underlying Mechanism for the PARP and BRCA Synthetic Lethality: Clearing up the Misunderstandings. Mol. Oncol. 5 (4), 387–393. doi:10.1016/j.molonc.2011.07.001
Husereau, D., Drummond, M., Petrou, S., Carswell, C., Moher, D., Greenberg, D., et al. (2013). Consolidated Health Economic Evaluation Reporting Standards (CHEERS) Statement. BMC Med. 11, 80. doi:10.1186/1741-7015-11-80
Hussain, M., Mateo, J., Fizazi, K., Saad, F., Shore, N., Sandhu, S., et al. (2020). Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 383 (22), 2345–2357. doi:10.1056/NEJMoa2022485
Institute for Clinical and Economic Review (2017). Poly ADP-Ribose Polymerase (PARP) Inhibitors for Ovarian Cancer: Effectiveness and Value Final Evidence Report. Boston, MA: United States.
Jiang, X., Li, W., Li, X., Bai, H., and Zhang, Z. (2019). Current Status and Future Prospects of PARP Inhibitor Clinical Trials in Ovarian Cancer. Cancer Manag. Res. 11, 4371–4390. doi:10.2147/CMAR.S200524
Kaplan, B. (2017). Targeted Therapies: One Practice's Story. Am. J. Manag. Care 23 (10 Spec), SP409.
Lakdawalla, D. N., Chou, J. W., Linthicum, M. T., MacEwan, J. P., Zhang, J., and Goldman, D. P. (2015). Evaluating Expected Costs and Benefits of Granting Access to New Treatments on the Basis of Progression-free Survival in Non-small-cell Lung Cancer. JAMA Oncol. 1 (2), 196–202. doi:10.1001/jamaoncol.2015.0203
Ledermann, J., Harter, P., Gourley, C., Friedlander, M., Vergote, I., Rustin, G., et al. (2012). Olaparib Maintenance Therapy in Platinum-Sensitive Relapsed Ovarian Cancer. N. Engl. J. Med. 366 (15), 1382–1392. doi:10.1056/NEJMoa1105535
Leung, J. H., Lang, H. C., Wang, S. Y., Lo, H. F., and Chan, A. L. (2021). Cost-effectiveness Analysis of Olaparib and Niraparib as Maintenance Therapy for Women with Recurrent Platinum-Sensitive Ovarian Cancer. Expert Rev Pharmacoeconomics Outcomes Res.2021 doi:10.1080/14737167.2021.1954506
Litton, J. K., Rugo, H. S., Ettl, J., Hurvitz, S. A., Gonçalves, A., Lee, K. H., et al. (2018). Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N. Engl. J. Med. 379 (8), 753–763. doi:10.1056/NEJMoa1802905
Mirza, M. R., Monk, B. J., Herrstedt, J., Oza, A. M., Mahner, S., Redondo, A., et al. (2016). Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N. Engl. J. Med. 375 (22), 2154–2164. doi:10.1056/NEJMoa1611310
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. BMJ 339, b2535. doi:10.1136/bmj.b2535
Moore, K., Colombo, N., Scambia, G., Kim, B. G., Oaknin, A., Friedlander, M., et al. (2018). Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 379 (26), 2495–2505. doi:10.1056/NEJMoa1810858
Muston, D., Hettle, R., Monberg, M., McLaurin, K. K., Gao, W., Swallow, E., et al. (2020). Cost-effectiveness of Olaparib as a Maintenance Treatment for Women with Newly Diagnosed Advanced Ovarian Cancer and BRCA1/2 Mutations in the United States. Gynecol. Oncol. 159 (2), 491–497. doi:10.1016/j.ygyno.2020.08.013
National Institute for Health and Care Excellence (2019). Olaparib for Maintenance Treatment of BRCA Mutation-Positive Advanced Ovarian, Fallopian Tube or Peritoneal Cancer after Response to First-Line Platinum-Based Chemotherapy. London: Technology appraisal guidance.
National Institute for Health and Care Excellence (2021). Olaparib Plus Bevacizumab for Maintenance Treatment of Advanced Ovarian, Fallopian Tube or Primary Peritoneal Cancer. London: Technology appraisal guidance.
Neumann, P. J., Cohen, J. T., and Ollendorf, D. A. (2021). Drug-Pricing Debate Redux - Should Cost-Effectiveness Analysis Be Used Now to Price Pharmaceuticals? N. Engl. J. Med. 385 (21), 1923–1924. doi:10.1056/NEJMp2113323
O'Sullivan, C. C., Moon, D. H., Kohn, E. C., and Lee, J. M. (2014). Beyond Breast and Ovarian Cancers: PARP Inhibitors for BRCA Mutation-Associated and BRCA-like Solid Tumors. Front. Oncol. 4, 42. doi:10.3389/fonc.2014.00042
Ofman, J. J., Sullivan, S. D., Neumann, P. J., Chiou, C. F., Henning, J. M., Wade, S. W., et al. (2003). Examining the Value and Quality of Health Economic Analyses: Implications of Utilizing the QHES. J. Manag. Care Pharm. 9 (1), 53–61. doi:10.18553/jmcp.2003.9.1.53
LYNPARZA (olaparib) (2021). US Prescribing Information. Available from: http://www.azpicentral.com/pi.html?product=lynparza_tb.
Olry de Labry Lima, A., Špacírová, Z., Fénix-Caballero, S., Hoces, A. M., Vegas, A. S., Aranzana, M. C., et al. (2021). Cost-utility of Talazoparib Monotherapy Treatment for Locally Advanced or Metastatic Breast Cancer in Spain. Breast 58, 27–33. doi:10.1016/j.breast.2021.04.004
Ovarian Cancer Research Alliance (2020). Recurrence. Available from: https://ocrahope.org/patients/about-ovarian-cancer/recurrence/.
Penn, C. A., Wong, M. S., and Walsh, C. S. (2020). Cost-effectiveness of Maintenance Therapy Based on Molecular Classification Following Treatment of Primary Epithelial Ovarian Cancer in the United States. JAMA Netw. Open 3 (12), e2028620. doi:10.1001/jamanetworkopen.2020.28620
Pujade-Lauraine, E., Ledermann, J. A., Selle, F., Gebski, V., Penson, R. T., Oza, A. M., et al. (2017). Olaparib Tablets as Maintenance Therapy in Patients with Platinum-Sensitive, Relapsed Ovarian Cancer and a BRCA1/2 Mutation (SOLO2/ENGOT-Ov21): a Double-Blind, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 18 (9), 1274–1284. doi:10.1016/S1470-2045(17)30469-2
Rawla, P., Sunkara, T., and Gaduputi, V. (2019). Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 10 (1), 10–27. doi:10.14740/wjon1166
Robson, M., Im, S. A., Senkus, E., Xu, B., Domchek, S. M., Masuda, N., et al. (2017). Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 377 (6), 523–533. doi:10.1056/NEJMoa1706450
RUBRACA (rucaparib) (2020). US Prescribing Information. Available from: https://clovisoncology.com/pdfs/RubracaUSPI.pdf.
Saito, S., Nakazawa, K., Nagahashi, M., Ishikawa, T., and Akazawa, K. (2019). Cost-effectiveness of BRCA1/2 Mutation Profiling to Target Olaparib Use in Patients with Metastatic Breast Cancer. Per Med. 16 (6), 439–448. doi:10.2217/pme-2018-0141
Secord, A. A., Barnett, J. C., Ledermann, J. A., Peterson, B. L., Myers, E. R., and Havrilesky, L. J. (2013). Cost-Effectiveness of BRCA1 and BRCA2 Mutation Testing to Target PARP Inhibitor Use in Platinum-Sensitive Recurrent Ovarian Cancer. Int. J. Gynecol. Cancer 23 (5), 846–852. doi:10.1097/IGC.0b013e31829527bd
Smith, H. J., Walters Haygood, C. L., Arend, R. C., Leath, C. A., and Straughn, J. M. (2015). PARP Inhibitor Maintenance Therapy for Patients with Platinum-Sensitive Recurrent Ovarian Cancer: A Cost-Effectiveness Analysis. Gynecol. Oncol. 139 (1), 59–62. doi:10.1016/j.ygyno.2015.08.013
Sonnenblick, A., de Azambuja, E., Azim, H. A., and Piccart, M. (2015). An Update on PARP Inhibitors-Mmoving to the Adjuvant Setting. Nat. Rev. Clin. Oncol. 12 (1), 27–41. doi:10.1038/nrclinonc.2014.163
Su, D., Wu, B., and Shi, L. (2020). Cost-Effectiveness of Genomic Test-Directed Olaparib for Metastatic Castration-Resistant Prostate Cancer. Front. Pharmacol. 11, 610601. doi:10.3389/fphar.2020.610601
TELZENNA (talaparib) (2021). US Prescribing Information. Available from: http://labeling.pfizer.com/ShowLabeling.aspx?id=11046.
Tan, D. S., Chan, J. J., Hettle, R., Ghosh, W., Viswambaram, A., and Yu, C. C. (2021). Cost-effectiveness of Olaparib versus Routine Surveillance in the Maintenance Setting for Patients with BRCA-Mutated Advanced Ovarian Cancer after Response to First-Line Platinum-Based Chemotherapy in Singapore. J. Gynecol. Oncol. 32 (2), e27. doi:10.3802/jgo.2021.32.e27
Verma, V., Sprave, T., Haque, W., Simone, C. B., Chang, J. Y., Welsh, J. W., et al. (2018). A Systematic Review of the Cost and Cost-Effectiveness Studies of Immune Checkpoint Inhibitors. J. Immunother. Cancer 6 (1), 128. doi:10.1186/s40425-018-0442-7
Wallbillich, J. J., Forde, B., Havrilesky, L. J., and Cohn, D. E. (2016). A Personalized Paradigm in the Treatment of Platinum-Resistant Ovarian Cancer - A Cost Utility Analysis of Genomic-Based versus Cytotoxic Therapy. Gynecol. Oncol. 142 (1), 144–149. doi:10.1016/j.ygyno.2016.04.024
Wu, B., and Shi, L. (2020). Cost-effectiveness of Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. J. Natl. Compr. Cancer Netw. 18 (11), 1528–1536. doi:10.6004/jnccn.2020.7587
Yoshida, Y., Cheng, X., Shao, H., Fonseca, V. A., and Shi, L. (2020). A Systematic Review of Cost-Effectiveness of Sodium-Glucose Cotransporter Inhibitors for Type 2 Diabetes. Curr. Diab Rep. 20 (4), 12. doi:10.1007/s11892-020-1292-5
ZEJULA (niraparib) (2021). US Prescribing Information. Available from: https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Zejula_Capsules/pdf/ZEJULA-CAPSULES-PI-PIL.PDF.
Zhan, M., Zheng, H., Yang, Y., He, Z., Xu, T., and Li, Q. (2020). Cost-effectiveness Analysis of Maintenance Olaparib in Patients with Metastatic Pancreatic Cancer and a Germline BRCA1/2 Mutation Based on the Polo Trial. Cancer Manag. Res. 12, 12919–12926. doi:10.2147/CMAR.S283169
Keywords: cost-effectiveness, systematic review, PARP inhibitors, precision oncology, health economics, health policy
Citation: Chan VKY, Yang R, Wong ICK and Li X (2022) Cost-Effectiveness of Poly ADP-Ribose Polymerase Inhibitors in Cancer Treatment: A Systematic Review. Front. Pharmacol. 13:891149. doi: 10.3389/fphar.2022.891149
Received: 07 March 2022; Accepted: 19 May 2022;
Published: 11 July 2022.
Edited by:
Hye-Young Kwon, Mokwon University, South KoreaReviewed by:
Shawn D. Spencer, Philadelphia College of Osteopathic Medicine (PCOM), United StatesCiler Celik-Ozenci, Koç University, Turkey
Copyright © 2022 Chan, Yang, Wong and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xue Li, c3h1ZWxpQGhrdS5oaw==
†These authors have contributed equally to this work and share first authorship
 Vivien Kin Yi Chan
Vivien Kin Yi Chan Runqing Yang2†
Runqing Yang2† Ian Chi Kei Wong
Ian Chi Kei Wong Xue Li
Xue Li