- 1Research Center for Pharmacology and Toxicology, Institute of Medicinal Plant Development (IMPLAD), Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Sino-Pakistan Center on Traditional Chinese Medicine, Hunan University of Medicine, Huaihua, China
- 3Hunan University of Chinese Medicine, College of Traditional Chinese Medicine, Changsha, China
- 4School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 5Institute of Drug Discovery Technology, Ningbo University, Ningbo, China
Chronic restraint stress (CRS) is a classic animal model of stress that can lead to various physiological and psychological dysfunctions, including systemic neuroinflammation and memory deficits. Fresh Gastrodia elata Blume (FG), the unprocessed raw tuber of Gastrodia elata Blume, has been reported to alleviate the symptoms of headache, convulsions, and neurodegenerative diseases, while the protective effects of FG on CRS-induced cognitive deficits remain unclear. This work aimed to evaluate the effects of FG on CRS-induced cognitive deficits through multiplex animal behavior tests and to further explore the related mechanism by observing the expression of mitochondrial apoptosis-related proteins in the mouse hippocampus. In in vivo experiments, mice were subjected to the object location recognition test (OLRT), new object recognition test (NORT), Morris water maze test (MWMT), and passive avoidance test (PAT) to evaluate the learning and memory ability. In in vitro experiments, the expression of the AKT/CREB pathway, the fission- and apoptosis-related proteins (Drp1, Cyt C, and BAX), and the proinflammatory cytokines’ (TNF‐α and IL‐1β) level in the hippocampus was examined. Our results demonstrated that in spontaneous behavior experiments, FG significantly improved the cognitive performance of CRS model mice in OLRT (p < 0.05) and NORT (p < 0.05). In punitive behavior experiments, FG shortened the escape latency in long-term spatial memory test (MWMT, p < 0.01) and prolonged the latency into the dark chamber in non-spatial memory test (PAT, p < 0.01). Biochemical analysis showed that FG treatment significantly suppressed CRS‐induced Cyt C, Drp1, and BAX activation (p < 0.001, p < 0.01 and p < 0.05), promoted the CREB, p-CREB, AKT, and p-AKT level (p < 0.05, p < 0.01 and p < 0.001), and inhibited the CRS‐induced proinflammatory cytokines (TNF‐α and IL‐1β, p < 0.05 and p < 0.001) level in the hippocampus. Taken together, these results suggested that FG could attenuate cognitive deficits induced by CRS on multiple learning and memory behavioral tests.
1 Introduction
Stress is a common nonspecific response which results when individuals feel threatened, physically or mentally. Modern scientific research has demonstrated a high correlation between social and/or physical stress and memory impairment (Bowman et al., 2003). A chronic restraint stress (CRS) model simulates the physiological and psychological stress of humans after long-term exposure to confined spaces by placing animals in confined spaces and restricting their behavior and activities, which leads to depression, anxiety, learning, and memory deficits (Huang et al., 2015; Woo et al., 2018). Numerous research studies have shown that CRS diminished the learning and memory ability of animals (Kezhu et al., 2017; Dong et al., 2019). Studies have revealed that CRS activates glial cells to release proinflammatory factors, such as interleukin-1β (IL-1β) and tumor necrosis factor α (TNF- α) (Deak et al., 2015), which in turn promotes apoptosis, ultimately leading to apoptosis. BAX is a proapoptotic protein belonging to the Bcl-2 family, and it acts in conjunction with Drp1 to increase mitochondrial membrane permeability and division, which facilitates the release of cytochrome c (Cyt C) from the mitochondria into the cytoplasm (Reed, 1997; Sugioka et al., 2004; Hu et al., 2020). CREB serves an important role in neuronal regeneration, synaptic growth, learning, and memory. AKT also plays a major role in promoting neuronal survival and synaptic plasticity (Wang et al., 2020a). The activation of the AKT/CREB pathway provides direct links to neuroprotective mechanisms (Zhang et al., 2020).
The well-known herb plant fresh Gastrodia elata Blume (FG) has been commonly used as dietary supplement and medicine. The traditional function of FG is known for carminative, which is primarily used to treat headache, dizziness, and rheumatic ache (Zhan et al., 2016). Modern pharmacological studies have demonstrated that it is mainly effective on the central nervous system and cardiovascular system (Dai et al., 2017; Liu et al., 2018) and has a good effect on neuropsychiatric diseases such as insomnia, Alzheimer’s disease, depression, and anxiety disorder (Jung et al., 2006; Zhang et al., 2012; Huang et al., 2013; Huang et al., 2021a). These effects may be due to its anti-inflammatory, antiapoptotic, and promoting neurogenesis (Hsu et al., 2021; Wang et al., 2022). Our previous study has shown that FG possessed good function with improving learning and memory impairment caused by insufficient sleep (Huang et al., 2021b). However, the protective effects of FG on CRS-induced cognitive deficits are rarely investigated.
In this study, we researched the effects of FG on CRS-induced cognitive deficits through multiplex animal behavior tests. The possible neuroprotection mechanisms of FG were investigated through the modulation of inflammation and the AKT/CREB pathway in the hippocampus after CRS. Our research aims to address the effect of FG on the cognitive deficits induced by CRS and provides evidence for the application of FG in ameliorating stress-induced hypomnesia.
2 Methods
2.1 Animals
120 ICR mice weighing between 20 and 23 g were purchased from the Vital River Co., Ltd. (Qualified No. SCXK 2021–0006, Beijing, China). All experimental protocols adhered to the guidelines of the Animal Care and Use Committee of the Institute of Medical Plant Development, Chinese Academy of Medical Sciences, and Peking Union Medical College (Permit Number: 2020122411505). The animals were housed under standard laboratory conditions: room temperature was 24°C ± 2°C, relative humidity was 55°C ± 10°C, and maintained on a 12 h light/dark cycle (lights on at 8:00 a.m.). The animals arrived at least 3 days before the experiments and were handled carefully.
2.2 Chemicals and reagents
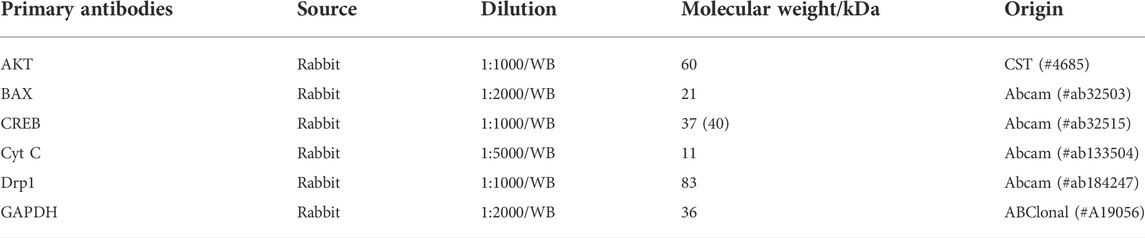
Donepezil hydrochloride (DNP [Aricept], Eisai Inc. [Ibaraki, Japan]) was used as a positive control. TNF-α (lot No. 040621210429) and IL-1β (lot No. 040921210429) commercial kits were obtained from Beyotime (Shanghai, China). A detailed description for primary antibodies used in the present research is displayed in Table 1, and secondary antibody is goat anti-rabbit IgG-HRP (Gene-Protein Link, lot No.07001). Reference substances: gastrodin (lot No. 110807–201507, purity ≥ 98%) was purchased from the China Institute for Food and Drug Control; p-hydroxybenzyl alcohol (lot No. H20806, purity ≥ 98%) was purchased from Sigma-Aldrich company; and parishin E (lot No. 1–1011606) was purchased from Chengdu Purifa Technology Development Co., Ltd. (Chengdu, China).
The equipment used included an open‐field computer‐aided controlling system (KSYY-OP- V4.0), OLRT and NORT system (KSYY-OR- V2.0), PAT system (KSYY-AD-V4.0), and MWMT apparatus (KSYY-MWM-V4.0), which were jointly developed by Beijing Kangsen High-technology Co., Ltd., China Astronaut Research and Training Center, the Institute of Medicinal Plant Development, and Chinese Academy of Medical Sciences. Ultra-high-performance liquid chromatography was performed (UPLC, Thermo Scientific Ultimate 3000, United States).
2.3 The preparation and standardization of fresh Gastrodia elata Blume
2.3.1 Sample prepared
Fresh Gastrodia elata Blume tuber was purchased from the local herbal grower in Jinkouhe, Sichuan Province, China. The specimen was identified as a tuber of Gastrodia elata Blume by Guang-hua Lu, professor at Chengdu University of Traditional Chinese Medicine, Chengdu, China.
To obtain FG, the fresh Gastrodia elata was pureed and distilled water was added 2–3 times in a liquidizer until completely smooth. Then, the puree was filtered with gauze, the filter residue was taken, and distilled water was added; the procedure was repeated once to ensure the complete extraction of the content. Then, the extract was filtered, freeze-dried, and stored at 4°C until utilization.
2.3.2 Fingerprint of fresh Gastrodia elata Blume
The FG (0.2 g) was dissolved in 60% ethanol (25 ml); ultrasound extraction was performed for 45 min followed by filtration through a 0.45 μm membrane filter, and the subsequent filtrate was gathered for testing. The sample was performed on an UPLC system with a Accucore Vanquish C18+ column (100 mm × 2.1 mm × 1.5 μm, Thermo Scientific). The column was operated at 35°C. The gradient elution consisted of a mobile phase of (A) 0.075% formic acid acetonitrile and (B) 0.1% formic acid water using a gradient elution of 2 %–5% A at 0–4 min, 5%–14% A at 4–10 min, 14%–53% A at 10–18 min, and 53%–95% A at 18–20 min. The flow rate was maintained at 0.4 ml/min, and aliquots of 1 μL were injected into the UPLC system, and the detection wavelength was set at 220 nm.
The content of gastrodin, p-hydroxybenzyl alcohol, and parishin E in the FG extract detected gastrodin, p-hydroxybenzyl alcohol, and parishin E was 0.264, 6.485, and 2.012 mg/g, respectively (shown in Figure 1).
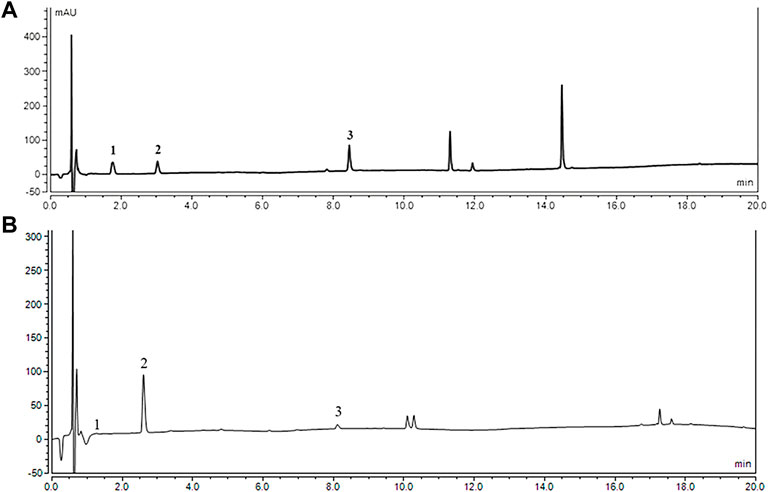
FIGURE 1. HPLC chromatograms of reference substance (A) and sample of FG. (B) (1) gastrodin; (2) p-hydroxybenzyl alcohol; and (3) Parishin E.
2.4 Groups and drug treatments
The mice were divided into five groups pseudorandomly (n = 12 in each group) as follows: the control group, CRS model group, CRS + DNP treatment group (i.g., 1.6 mg/kg), the dose is according to literature (Yang et al., 2020), and CRS + FG treatment groups (i.g., 0.5 and 1 g/kg, equivalent to the crude material), the dosage was based on our preliminary studies and literature (Lin et al., 2018; Huang et al., 2021b). The FG and DNP were dissolved in distilled water; mice in the control and CRS model group were administrated with dissolvent and treated with corresponding drugs from the 21st of CRS until sacrifice.
2.5 The chronic restraint stress procedure
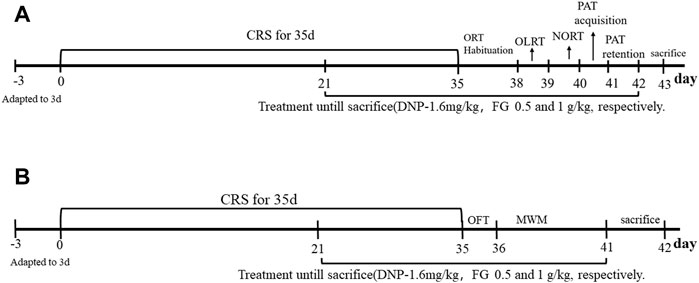
The animals were subjected to CRS as described in the prior study (Wang et al., 2020b). During the CRS period, the mice were kept in a transparent Plexiglas restrainer (L 13 × D 4 cm) with air holes and fixing plug for 10 h (from 22:00 p.m. to 8:00 a.m.) each day for 35 consecutive days. After stress, the mice returned to their cages under ad libitum access to food and water. The control mice were fasted and deprived of water during the CRS process. The experimental procedure is shown in Figure 2. Body weight was measured every 7 days during the experiment to monitor the effects of CRS and drug treatment on mice.
2.6 Behavioral tests
2.6.1 Open-field test
The open-field test (OFT) is used to evaluate the locomotor activity and sedative/excitatory states of animals. In neuropsychiatric behavioral experiments, when the locomotor activities of animals in each group have no significant difference, the next animal behavior tests have the significance of statistics (O’Connor et al., 2003).
The instrument consists of an infrared camera which is directly above the center of four circular test boxes (H 28 × D 28 cm). The drugs were administered 30 min before the experiment. Each animal was placed into the center of the tank in a proper sequence and allowed to explore freely for 3 min. The total distance and average speed in the next 10 min were recorded automatically by open-field computer-aided controlling system and as an index of locomotor activity.
2.6.2 Object recognition test
This sensitive cognitive behavior detection method allows to measure rodents’ instinct to approach and explore novel objects. The detection function of the object recognition test varies according to different experimental requirements, but the most commonly used are object location recognition test (OLRT) and new object recognition test (NORT).
2.6.3 Object location recognition test
The equipment and procedure of OLRT were consistent and described previously (Lu et al., 2018a). A 3-day habituation period of the OLRT was initiated. In this phase, the mice were exposed to an empty arena and allowed to explore for 10 min. The experiment test phase consisted of two parts: the familiar phase and the test phase. During the familiar phase, the mice were allowed to explore the arena for 5 min, containing two identical objects in a parallel position on the same side. After 30 min interval, the mice returned to the arena for 5 min, with one of the original objects stationed at the same position (Familiar, F) and another one placed diagonally referred as new location (Novelty, N). To avoid biases, locations for the objects were counterbalanced among the groups. In each step, the floor of the arena and objects were cleaned with 75% ethanol to avoid odor interference. The learning and memory abilities of animals in this test were evaluated by the discrimination index (DI).
where TN and TF indicate the time of exploring new and familiar location within 5 min, respectively.
2.6.4 New object recognition test
The familiar conduct of NORT is same as OLRT, whereas for the test phase, one object of the original objects was replaced by a new matching one (Novel, N) and another object was retained (Familiar, F). The learning and memory abilities of animals in this test were also evaluated by the discrimination index (DI).
where TN and TF indicate the time of exploring new and familiar object within 5 min, respectively.
2.6.5 Morris water maze test
The Morris water maze test (MWMT) is a classic experiment to evaluate the acquisition of animal spatial memory. In MWMT, the experimental animals are forced to swim to find the platform hidden in water to escape through repeated trials and reference distal cues (Tian et al., 2019). The animals’ spatial learning and memory ability can be effectively reflected by this method, which includes acquisition, maintenance, and reproduction (Lu et al., 2020). The MWMT device includes a circular pool (100 cm in diameter and 38 cm in height) filled with water (24–26°C) turbid by adding ink and divided into four quadrants. A cylindrical platform is placed in one quadrant (e.g., the first quadrant) and 1–1.5 cm below the water surface. There is a camera above for monitoring. The experiment was for five consecutive days and received training twice a day. Each time, the animals were introduced (facing the pool wall) into each of the four quadrants in turn (except for target quadrant), then they were given 90 s to find the platform, and the mice were placed on the platform for 10 s before and after entering the water. The escape latency (time of found the platform), escape success rate, and other indicators were recorded by the computerized tracking and image analyzer system.
2.6.6 Passive avoidance test
The passive avoidance test (PAT) was performed as described by previous the literature (Xu et al., 2017). The apparatus in trough-shape consists of a white illuminated chamber and a dark chamber (L 17 cm × W 13.5 cm × H 25 cm) connected by a small arch door. In acquisition trial, each mouse was placed into the light chamber for adapting for 180 s, followed by turning on the current for 300 s. When it entered the dark chamber, a 0.5 mA electric foot shock (5 s) was delivered. After 24 h, the retention trial was performed again as the acquisition trial without adaptation period. The mice were placed into the light chamber without adapting, and the latency (the first-time mice enter the dark chamber) was recorded in 300 s test session. If the mice did not enter the darkroom within 300 s, recorded as 300 s. Normal animals prefer to enter and stay in the dark room, but once entering or staying in the dark room, they receive electric shock. After several repetitions, they finally learn that the brighter room is the safety area to avoid electric injury (Xu et al., 2016a; Lu et al., 2018b).
2.7 Preparation of brain tissues
The animals were sacrificed after the behavioral tests, the hippocampus of which was dissected from the whole brain on ice immediately, snap-frozen, and stored at -80°C for further analysis.
2.8 Enzyme-linked immunosorbent assays
After the last behavioral test, the separated hippocampal from each hemisphere was weighed and homogenized in 0.9% saline (1:9, w: v). The levels of TNF-α and IL-1β were determined by commercial enzyme-linked immunosorbent assay (ELISA) kits according to the manufacture’s protocols from Beyotime (Shanghai, China).
2.9 Western blot assay
The hippocampus tissue samples were weighed and homogenized in lysis buffer (Solarbio, Beijing, China, lot: BC3710). After the homogenate was centrifuged at 12,000 rpm for 30 min at 4°C, the supernatant was collected and determined by a BCA protein assay kit (CWBIO, Beijing, China, lot: 32220). The equal amount of protein was separated by 10% SDS-PAGE gels (CWBIO, Beijing, China, lot: 01413), and then transferred onto polyvinylidene fluoride (PVDF) membrane (Millipore, MA, United States). The PVDF membrane was blocked by 5% skim milk in Tris-buffered saline and Tween 20 solution (TBST) at room temperature for 90 min. Subsequently, the membrane was incubated by appropriate primary antibodies overnight at 4°C. The membrane was washed in TBST three times for 10 min and incubated with corresponding HRP-conjugated secondary antibodies at room temperature for 90 min. After three washings for 10 min with TBST, the bands were detected by ECL kit (Absin, Shanghai, China, lot: OA16) and visualized by using a Molecular Imager ChemiDoc XRS + System (Bio-Rad, CA, United States).
2.10 Statistical analysis
Data were analyzed by SPSS software 21.0 (Chicago, IL, United States) and expressed as the mean ± standard error of the mean (Mean ± SEM). The exclusion of outliers follows a standard analytical procedure to avoid undue influence. Differences among the experimental groups were determined by one-way analysis of variance (ANOVA) followed by least significant difference post hoc test. The data recorded from the acquisition trails of the MWMT among the groups over a period of 5 days were analyzed with repeated measures and a multivariate analysis of variance (ANOVA) process of the general linear model. p < 0.05 was considered statistically significant and p < 0.01 indicates a very significant difference.
3 Results
3.1 Effects of fresh Gastrodia elata Blume on the body weight and locomotor activity in chronic restraint stress-treated mice
At the beginning of the experiment, there was no significant difference in body weight among the groups. Following 35 days of CRS, body weight of the CRS groups decreased significantly (p < 0.05) compared with the control group (Figure 3A). By the way, the CRS group showed grooming deficit after 5 days and this appearance defect remained until the end of the experiment.
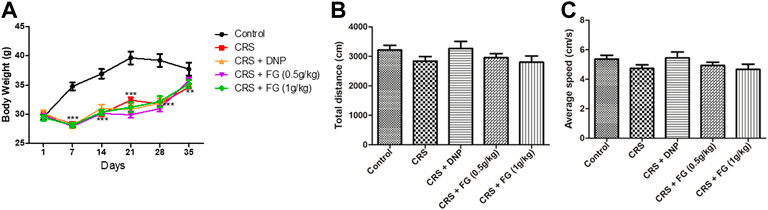
FIGURE 3. Effects of CRS, FG, and DNP on the locomotor activity of CRS mice in the open-field test and body weight. (A) Body weight; (B) total motion distance; and (C) average speed. Values represent the mean ± S.E.M. n = 10 ∼ 12 mice/group.
As shown in Figure 3B, there was no significant changes among all groups in the total distance (F4,52 = 1.351, p > 0.05) and the average speed (F4, 52 = 1.346, p > 0.05), which indicated that CRS and drugs treatment did not affect the locomotor activities of mice.
3.2 Effects of fresh Gastrodia elata Blume on the object recognition test in chronic restraint stress-treated mice
The effect of FG on spontaneity, spatial learning, and memory was evaluated by the OLRT. In a familiar phase, there was slight increase in drugs-treated groups in the total exploration time of the objects without significance differences among all the groups (F4, 43 = 0.772, p > 0.05, Figure 4A). The results guaranteed that there were no differences in the animals’ ability of exploration for the objects. In the test phase, as shown in Figure 4B, the DI of the CRS model group was decreased significantly (F4, 44 = 0.698, p < 0.01) compared with the control group, while the DNP and FG (1 g/kg) treatment groups elevated the discrimination index with significance (F4, 44 = 0.446, p < 0.05) compared with the CRS model group. It illustrated that these treatments could reverse the CRS-induced memory deficit.
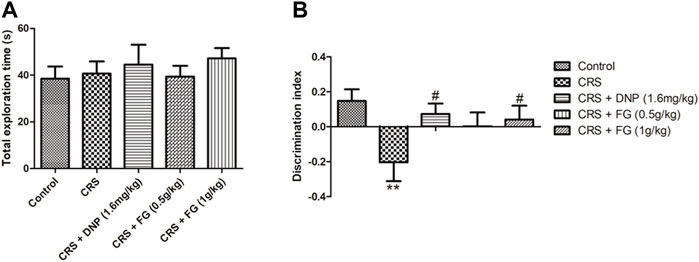
FIGURE 4. Effect of CRS, FG, and DNP on the object location recognition test of mice induced by CRS. (A) Total exploration time in familiar phase. (B) DI in test phase. Values represent the mean ± S.E.M. n = 8 ∼ 12 mice/group. **p < 0.01 compared with the control group; #p < 0.05 compared with the CRS group.
Moreover, NORT was used to evaluate the effects of FG on spontaneity non-spatial learning and memory in CRS-treated mice. Similarly, there was no significant difference among all groups in a familiar phase (F4, 46 = 0.704, p > 0.05, Figure 5A). In the test phase, the DI of the CRS model group was decreased significantly (p < 0.05) compared with the control group, while the FG (0.5 and 1 g/kg) treatment groups increased the discrimination index with significance (F4, 40 = 0.479, F4, 40 = 0.838, p < 0.05) compared with the CRS model group (Figure 5B). It illustrated that FG treatment could reverse the spontaneity, non-spatial learning, and memory deficit induced by CRS.
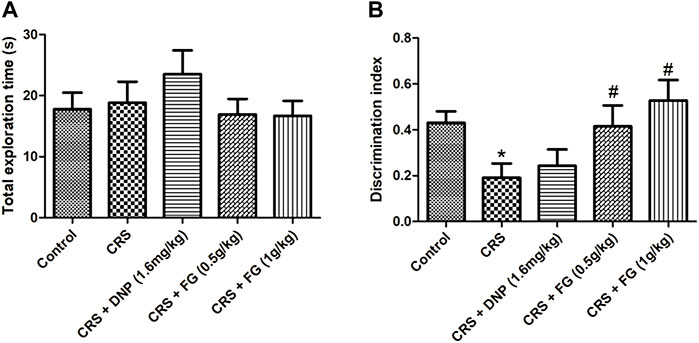
FIGURE 5. Effect of CRS, FG, and DNP on the new object recognition test of mice induced by CRS. (A) Total exploration time in familiar phase. (B) DI in test phase. Values represent the mean ± S.E.M. n = 8 ∼ 12 mice/group. *p < 0.05 compared with the control group; #p < 0.05 compared with the CRS group.
3.3 Effects of fresh Gastrodia elata Blume on the Morris water maze test in chronic restraint stress-treated mice
MWMT is used for detecting the effects of FG on the long‐term, punitive spatial memory performance of CRS-treated mice. We first used two-way repeated measures ANOVA to analyze the interaction effects between the groups and days of training. The statistical analysis results revealed that the group had a significant effect on the escape latency (F16, 416 = 1.18, p < 0.001) in the navigation phase. On day 5 (Figure 6), compared with the control group, the CRS model group displayed a significantly longer escape latency (p < 0.05) and compared with the CRS model group, FG (0.5 and 1 g/kg) and DNP group could significantly shorten the escape latency (p < 0.01, p < 0.05). It is worth mentioning that FG low does (0.5 g/kg) group significantly attenuated the prolong escape latency induced by CRS from day 3 to day 5 (p < 0.05, p < 0.01). These indicated that CRS could induce long-term spatial memory impairment, and FG treatment can reverse it.
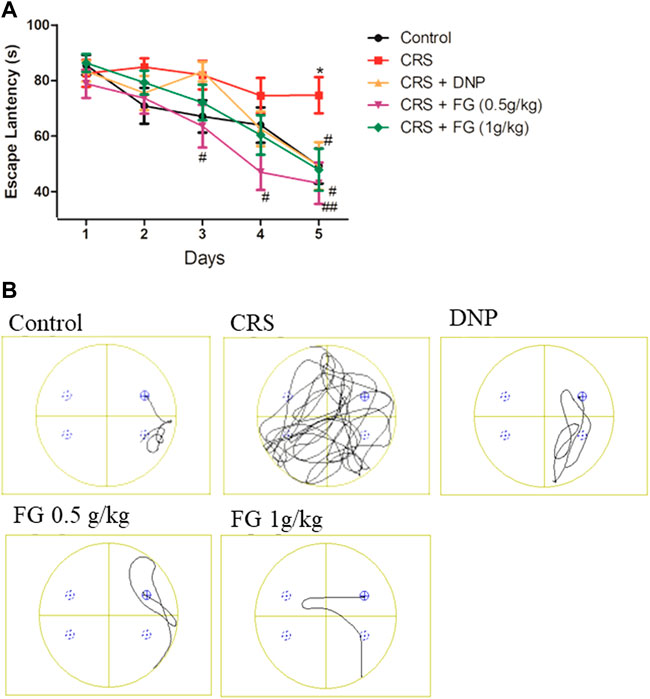
FIGURE 6. Effect of CRS, FG, and DNP on the MWMT of mice induced by CRS. Values represent the mean ± S.E.M. n = 9 ∼ 12 mice/group. (A) Escape latency; (B) Swimming track example (*p < 0.05 compared with the control group; #p < 0.05, ##p < 0.01 compared with the CRS group.
3.4 Effect of fresh Gastrodia elata Blume on the passive avoidance test
As shown in Figure 7, the latency into the dark chamber of the CRS model group mice was significantly shorter than the control group (F4,42 = 0.328, p < 0.05). While compared with the model group, FG (0.5 and 1 g/kg) treatment effectively extended the latency into the dark chamber (F4,42 = 3.024, F4,42 = 2.094, p < 0.01).
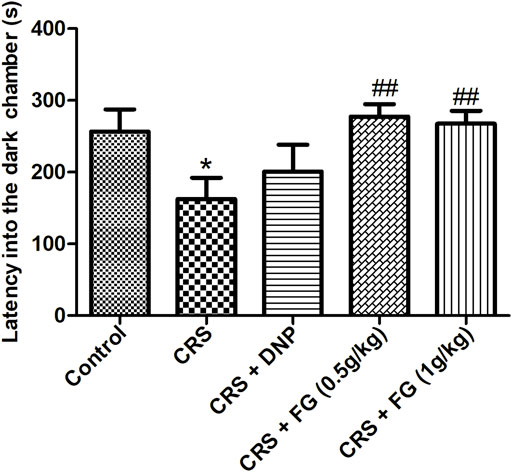
FIGURE 7. Effect of CRS, FG, and DNP on the PAT of mice induced by CRS. Values represent the mean ± S.E.M. n = 8 ∼ 10 mice/group. *p < 0.05 compared with the control group; ##p < 0.01 compared with the CRS group.
3.5 Effect of fresh Gastrodia elata Blume on inflammatory response
To observe the CRS-induced inflammatory response in the hippocampus, we measured the expression of TNF-α and IL-1β. As shown in Figure 8, the CRS model group increased the TNF‐α and IL-1β levels in the serum compared with the control group with significance (F4,34 = 3.847, F4,35 = 0.153, p < 0.01). However, compared with the CRS group, FG administrated at 1 g/kg significantly inhibited the increases of TNF-α (F4,34 = 0.488, p < 0.05), FG (0.5 and 1 g/kg) and DNP markedly diminished the level of IL-1β (F4,35 = 0.150, F4,35 = 2.986, F4,35 = 0.437, p < 0.01, p < 0.001 and p < 0.05).
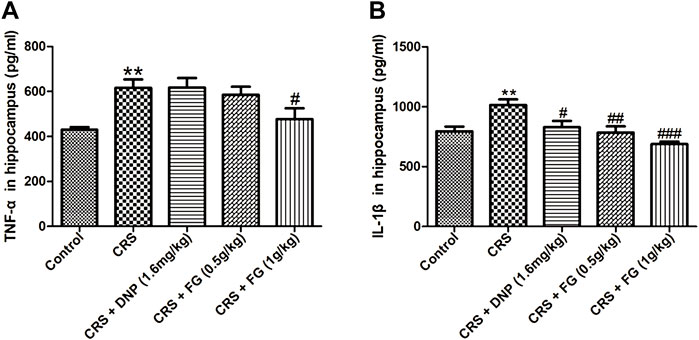
FIGURE 8. Effects of CRS, FG, and DNP on inflammation in serum of CRS-treated mice. (A) TNF-α and (B) IL-1β in serum. Values represent the mean ± S.E.M. n = 6 ∼ 8 mice/group. **p < 0.01 compared with the control group; #p < 0.05, ###p < 0.001 compared with the CRS group.
3.6 Effects of fresh Gastrodia elata Blume on the expression levels of molecules in the hippocampus
According to the results of the western blotting analysis (Figures 9, 10), the level of AKT, p-AKT, CREB, and p-CREB of the CRS group were lower than the control group (F4,10 = 4.721, F4,10 = 2.160, F4,10 = 3.832, F4,10 = 5.039, p < 0.05, p < 0.05, p < 0.001, p < 0.05), and the expression of Cyt C, DrP1, and BAX in the hippocampus of CRS-induced mice were significantly higher than the control group (F4,10 = 5.54, F4,10 = 4.164, F4,10 = 10.863, p < 0.01, p < 0.05 and p < 0.01, respectively). The administration of FG significantly reverse these changes (p < 0.05, p < 0.01, p < 0.001).
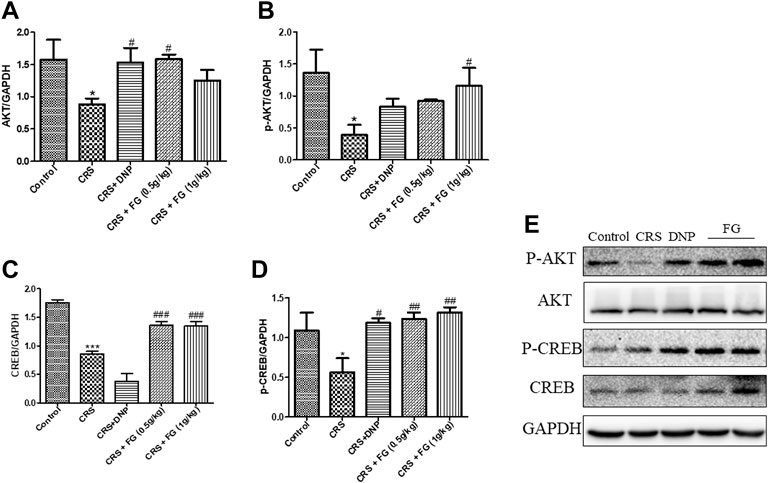
FIGURE 9. Effects of CRS, FG, and DNP on the AKT/CREB pathway expressed in the hippocampus of CRS-treated mice. (A–E) Expression levels of the protein were measured by western blot analysis and their respective histogram. n = 3 mice/group. Values represent the mean ± S.E.M. *p < 0.05, ***p < 0.01 compared with the control group; #p < 0.05, ##p < 0.01, ###p < 0.001 compared with the CRS group.
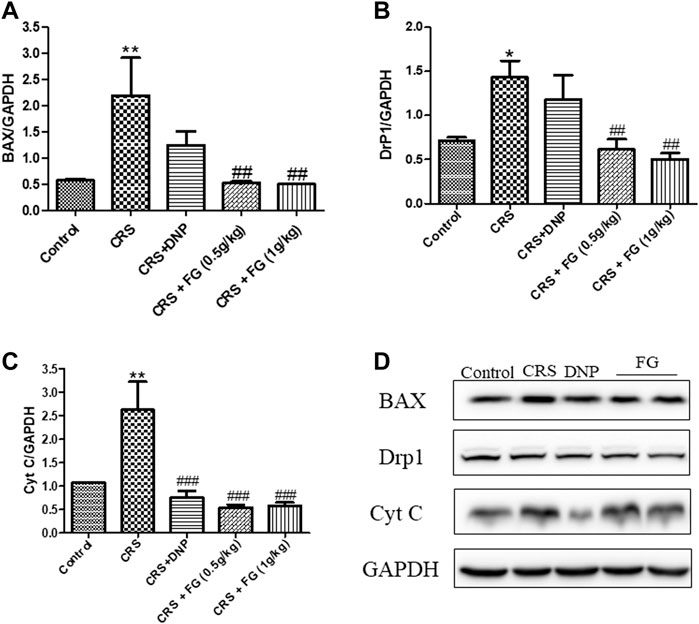
FIGURE 10. Effects of CRS, FG, and DNP on apoptosis-related proteins expressed in the hippocampus of CRS-treated mice. (A–D) Expression levels of the protein were measured by western blot analysis and their respective histogram. n = 3 mice/group. Values represent the mean ± S.E.M. *p < 0.05, **p < 0.01 compared with the control group; ##p < 0.01, ###p < 0.001 compared with the CRS group.
4 Discussion
The chronic restraint stress (CRS) model can simulate the physiological and psychological stress caused by long-term space-constrained environment and induce learning and memory impairment. In addition to studying the effects of general stress on human, CRS also plays an important role in investigating the effects of special environments such as aviation and sailing on human physical and mental health (Salehi et al., 2018; Salehpour et al., 2019; Peay et al., 2020). Also, CRS results in neuroinflammation, synaptic morphology alterations and mitochondrial apoptosis (Haack et al., 2008; Pietrogrande et al., 2018; Shen et al., 2021). Our research showed that the CRS model in mice could lead to cognitive impairment in the spontaneous and punishment behavioral test. FG was able to act against CRS‐induced memory impairment in mice and suggested that its action may be via anti-inflammatory and mediated mitochondrial apoptosis-related protein expression.
Animal behavior experiment is a subject that conducts observation and experiment in nature or in laboratory by collecting, observing, analyzing, and processing various behavior information of animals, which can be applied to study the physiological and pathological significance, as well as the generation mechanism of animal behavior expression (Sara, 2001; Xu et al., 2016b; Lu et al., 2018c; Lv et al., 2021). We applied a variety of animal behavior tests on learning and memory to assess the effect of FG. OLRT and NORT are sensitive, short-term, spontaneous activity behavioral methods to test the learning and memory ability of rodents through animals’ natural instinct of approaching and exploring novel object (Akkerman et al., 2012; Antunes and Biala, 2012). They were widely used in the study of memory neurobiology, brain injury mechanisms, and protective measures against cognitive impairment (Ennaceur, 2010; Xiong et al., 2020). The results showed that FG could reverse CRS-induced decrease in the DI, which implied that FG can ameliorate short-term spatial and non-spatial learning and memory. MWMT and PAT are punitive behavioral tests for learning and memory. Based on the rodents’ aversion of water, MWMT is widely used to test spatial memory (Vorhees and Williams, 2006). PAT is based on the dark preference habit of rodents, and mainly tests animals’ learning and memory ability to distinguish between light and dark (Zhang et al., 2018). In our results, FG significantly shortened the escape latency of animals in MWMT and prolonged the incubation period of mice to enter the dark room in PAT, indicating that the administration of FG can enhance the punitive long-term and short-term memory of mice. These animal learning and memory behavior results all together showed that FG could reverse various cognitive deficits induced by CRS.
Cellular activity in the brain depends on the high energetic support provided by the mitochondria (Hebert-Chatelain et al., 2016), enhancing the protein homeostasis of which can promote memory (Sorrentino et al., 2017). Inflammatory mediators regulate mitochondrial fission protein dynamin-related protein 1 (Drp1) (Yu et al., 2020), and the former can be induced to be released by CRS (24). Drp1 overexpression further accelerates the division of mitochondria and induces apoptosis (Shen et al., 2014), resulting in the release of Cyt c from the mitochondria into the cytoplasm (Tan et al., 2018), and meanwhile BAX plays a crucial role in the execution of apoptosis in the mitochondria (Rahmani et al., 2018). AKT directly protects the mitochondria from oxidants and its downstream substrate is cAMP response element binding (CREB) protein (Miyamoto et al., 2008), which is considered to increase the expression of synaptic plasticity protein, memory related protein, and new synapse formation. It is a key protein that affects memory storage and plays an important regulatory role in neuronal regeneration, synapse formation, learning, and memory (Villeda et al., 2014). Insulin and insulin growth factor can activate AKT through downstream signals, phosphorylate CREB, and increase the expression of synaptic plasticity genes and related proteins to promote synaptic neogenesis (Aguiar et al., 2011). It was previously reported that Gastrodia elata Blume can inhibit mitochondrial fragmentation and dysregulation of mitochondrial fusion and fission molecules (Huang et al., 2019). Therefore, we investigated whether the mechanism of FG reversing CRS-induced cognitive deficits is related to the control of mitochondrial-related protein. The results indicated that the FG can reduce the expression level of IL-1β and TNF-α significantly, which is consistent with the previous report (Xie et al., 2021). FG could significantly reverse CRS-induced mitochondrial fragmentation and dysregulation of mitochondrial fusion and fission molecule (Drp1 and Cyt C), and downregulate the level of BAX. We also observed that the levels of AKT, p-AKT, CREB, and p-CREB in the mice hippocampus were inhibited after CRS. Meanwhile, FG treatment dramatically reversed the decrease of AKT, p-AKT, CREB, and p-CREB protein expression in the hippocampus induced by CRS.
As mentioned previously, FG had a good effect on decreasing the level of TNF-α and IL-1β and promoting the expression of the AKT/CREB pathway and inhibition expression of Cyt C, DrP1, and BAX in the hippocampus. These may relate to a certain amount of gastrodin and p-hydroxybenzyl alcohol contained in FG (shown in Figure 1), which can reduce inflammation and inhibit division and apoptosis in the mitochondria (Jiao et al., 2019; Lei et al., 2020; Ma et al., 2020).
5 Conclusion
This study is the first to study the effects of FG on CRS-induced memory deficiency through both animal behavior tests and mitochondrial apoptosis-related protein detection. We found that the administration of FG exhibited an improvement effect in spontaneous short-term spatial and non-spatial memory (OLRT and NORT), as well as punitive non-spatial memory (PAT) and long-term spatial memory (MWMT). The enhancement of FG on learning and memory may be attributed to its mitochondrial apoptosis antagonism and anti-inflammatory effects. Thus, FG is considered to be a promising candidate supplement for ameliorating narrow space-induced hypomnesia, and this study provided a way for applying fresh Gastrodia elata Blume .
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by the Research Center for Pharmacology and Toxicology, Institute of Medicinal Plant Development (IMPLAD).
Author contributions
HH, NJ, and XL designed the research. HH, FC, and YZ conducted the experiments. HH, CY, HY, GL, and QH performed the data analysis. HH, YZ, and XL wrote and amended the manuscript. XL and NJ supervised the study and contributed to project administration. All authors approved the final version.
Funding
This work was supported by the International Cooperative Project of Traditional Chinese Medicine (GZYYG2020023); National Key R&D Program of China (No. 2018YFC1602105), and Key Laboratory of Agro-Products Processing, National Risk Assessment Laboratory of Agro-products Processing Quality and Safety, Ministry of Agriculture and Rural Affairs, China (S2021KFKT-02).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aguiar, A. S., Castro, A. A., Moreira, E. L., Glaser, V., Santos, A. R. S., Tasca, C. I., et al. (2011). Short bouts of mild-intensity physical exercise improve spatial learning and memory in aging rats: Involvement of hippocampal plasticity via AKT, CREB and BDNF signaling. Mech. Ageing Dev. 132, 560–567. doi:10.1016/j.mad.2011.09.005
Akkerman, S., Blokland, A., Reneerkens, O., van Goethem, N. P., Bollen, E., Gijselaers, H. J. M., et al. (2012). Object recognition testing: Methodological considerations on exploration and discrimination measures. Behav. Brain Res. 232, 335–347. doi:10.1016/j.bbr.2012.03.022
Antunes, M., and Biala, G. (2012). The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 13, 93–110. doi:10.1007/s10339-011-0430-z
Bowman, R. E., Beck, K. D., and Luine, V. N. (2003). Chronic stress effects on memory: Sex differences in performance and monoaminergic activity. Horm. Behav. 43, 48–59. doi:10.1016/s0018-506x(02)00022-3
Dai, R., Wang, T., Si, X., Jia, Y., Wang, L., Yuan, Y., et al. (2017). Vasodilatory effects and underlying mechanisms of the ethyl acetate extracts from Gastrodia elata. Can. J. Physiol. Pharmacol. 95, 564–571. doi:10.1139/cjpp-2016-0407
Deak, T., Quinn, M., Cidlowski, J. A., Victoria, N. C., Murphy, A. Z., and Sheridan, J. F. (2015). Neuroimmune mechanisms of stress: Sex differences, developmental plasticity, and implications for pharmacotherapy of stress-related disease. Stress 18, 367–380. doi:10.3109/10253890.2015.1053451
Dong, L., Wang, Y., Lv, J., Zhang, H., Jiang, N., Lu, C., et al. (2019). Memory enhancement of fresh ginseng on deficits induced by chronic restraint stress in mice. Nutr. Neurosci. 22, 235–242. doi:10.1080/1028415X.2017.1373928
Ennaceur, A. (2010). One-trial object recognition in rats and mice: Methodological and theoretical issues. Behav. Brain Res. 215, 244–254. doi:10.1016/j.bbr.2009.12.036
Haack, D., Luu, H., Cho, J., Chen, M. J., and Russo-Neustadt, A. (2008). Exercise reverses chronic stress-induced Bax oligomer formation in the cerebral cortex. Neurosci. Lett. 438, 290–294. doi:10.1016/j.neulet.2008.04.070
Hebert-Chatelain, E., Desprez, T., Serrat, R., Bellocchio, L., Soria-Gomez, E., Busquets-Garcia, A., et al. (2016). A cannabinoid link between mitochondria and memory. Nature 539, 555–559. doi:10.1038/nature20127
Hsu, W-H., Huang, N-K., Shiao, Y-J., Lu, C-K., Chao, Y-M., Huang, Y-J., et al. (2021). Gastrodiae rhizoma attenuates brain aging via promoting neuritogenesis and neurodifferentiation. Phytomedicine. 87, 153576. doi:10.1016/j.phymed.2021.153576
Hu, M., Zhao, Y., Cao, Y., Tang, Q., Feng, Z., Ni, J., et al. (2020). DRP1 promotes lactate utilization in KRAS-mutant non-small-cell lung cancer cells. Cancer Sci. 111, 3588–3599. doi:10.1111/cas.14603
Huang, G-B., Zhao, T., Muna, S. S., Jin, H-M., Park, J-I., Jo, K-S., et al. (2013). Therapeutic potential of Gastrodia elata Blume for the treatment of Alzheimer’s disease. Neural Regen. Res. 8, 1061–1070. doi:10.3969/j.issn.1673-5374.2013.12.001
Huang, H., Jiang, N., Zhang, Y. W., Lv, J. W., Wang, H. X., Lu, C., et al. (2021). Gastrodia elata blume ameliorates circadian rhythm disorder-induced mice memory impairment. Life Sci. Space Res. 31, 51–58. doi:10.1016/j.lssr.2021.07.004
Huang, N-K., Lin, C-C., Lin, Y-L., Huang, C-L., Chiou, C-T., Lee, Y-C., et al. (2019). Morphological control of mitochondria as the novel mechanism of Gastrodia elata in attenuating mutant huntingtin-induced protein aggregations. Phytomedicine. 59, 152756. doi:10.1016/j.phymed.2018.11.016
Huang, P., Li, C., Fu, T., Zhao, D., Yi, Z., Lu, Q., et al. (2015). Flupirtine attenuates chronic restraint stress-induced cognitive deficits and hippocampal apoptosis in male mice. Behav. Brain Res. 288, 1–10. doi:10.1016/j.bbr.2015.04.004
Huang, Y-J., Choong, L-X. C., Panyod, S., Lin, Y-E., Huang, H-S., Lu, K-H., et al. (2021). Gastrodia elata Blume water extract modulates neurotransmitters and alters the gut microbiota in a mild social defeat stress-induced depression mouse model. Phytother. Res. 35, 5133–5142. doi:10.1002/ptr.7091
Jiao, M., Yin, K., Zhang, T., Wu, C., Zhang, Y., Zhao, X., et al. (2019). Effect of the SSeCKS-TRAF6 interaction on gastrodin-mediated protection against 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin-induced astrocyte activation and neuronal death. Chemosphere 226, 678–686. doi:10.1016/j.chemosphere.2019.04.003
Jung, J. W., Yoon, B. H., Oh, H. R., Ahn, J-H., Kim, S. Y., Park, S-Y., et al. (2006). Anxiolytic-like effects of Gastrodia elata and its phenolic constituents in mice. Biol. Pharm. Bull. 29, 261–265. doi:10.1248/bpb.29.261
Kezhu, W., Pan, X., Cong, L., Liming, D., Beiyue, Z., Jingwei, L., et al. (2017). Effects of ginsenoside Rg1 on learning and memory in a reward-directed instrumental conditioning task in chronic restraint stressed rats. Phytother. Res. 31, 81–89. doi:10.1002/ptr.5733
Lei, X., Yuan, Y., and Zou, Q. (2020). The role and mechanism of gastrodin in the medial prefrontal cortex autophagy of PTSD rats. Int. J. Clin. Exp. Pathol. 13, 989–994.
Lin, Y-E., Chou, S-T., Lin, S-H., Lu, K-H., Panyod, S., Lai, Y-S., et al. (2018). Antidepressant-like effects of water extract of Gastrodia elata Blume on neurotrophic regulation in a chronic social defeat stress model. J. Ethnopharmacol. 215, 132–139. doi:10.1016/j.jep.2017.12.044
Liu, Y., Gao, J., Peng, M., Meng, H., Ma, H., Cai, P., et al. (2018). A review on central nervous system effects of gastrodin. Front. Pharmacol. 9, 24. doi:10.3389/fphar.2018.00024
Lu, C., Dong, L., Lv, J., Wang, Y., Fan, B., Wang, F., et al. (2018). 20(S)-protopanaxadiol (PPD) alleviates scopolamine-induced memory impairment via regulation of cholinergic and antioxidant systems, and expression of Egr-1, c-Fos and c-Jun in mice. Chem. Biol. Interact. 279, 64–72. doi:10.1016/j.cbi.2017.11.008
Lu, C., Lv, J., Dong, L., Jiang, N., Wang, Y., Wang, Q., et al. (2018). Neuroprotective effects of 20(S)-protopanaxatriol (PPT) on scopolamine-induced cognitive deficits in mice. Phytother. Res. 32, 1056–1063. doi:10.1002/ptr.6044
Lu, C., Wang, Y., Lv, J., Jiang, N., Fan, B., Qu, L., et al. (2018). Ginsenoside Rh2 reverses sleep deprivation-induced cognitive deficit in mice. Behav. Brain Res. 349, 109–115. doi:10.1016/j.bbr.2018.03.005
Lu, Z., Yang, T., Wang, L., Qiu, Q., Zhao, Y., Wu, A., et al. (2020). Comparison of different protocols of Morris water maze in cognitive impairment with heart failure. Brain Behav. 10, e01519. doi:10.1002/brb3.1519
Lv, J., Lu, C., Jiang, N., Wang, H., Huang, H., Chen, Y., et al. (2021). Protective effect of ginsenoside Rh2 on scopolamine-induced memory deficits through regulation of cholinergic transmission, oxidative stress and the ERK-CREB-BDNF signaling pathway. Phytother. Res. 35, 337–345. doi:10.1002/ptr.6804
Ma, C-L., Li, L., Yang, G-M., Zhang, Z-B., Zhao, Y-N., Zeng, X-F., et al. (2020). Neuroprotective effect of gastrodin in methamphetamine-induced apoptosis through regulating cAMP/PKA/CREB pathway in cortical neuron. Hum. Exp. Toxicol. 39, 1118–1129. doi:10.1177/0960327120911438
Miyamoto, S., Murphy, A. N., and Brown, J. H. (2008). Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-II. Cell Death Differ. 15, 521–529. doi:10.1038/sj.cdd.4402285
O’Connor, C., Heath, D. L., Cernak, I., Nimmo, A. J., and Vink, R. (2003). Effects of daily versus weekly testing and pre-training on the assessment of neurologic impairment following diffuse traumatic brain injury in rats. J. Neurotrauma 20, 985–993. doi:10.1089/089771503770195830
Peay, D. N., Saribekyan, H. M., Parada, P. A., Hanson, E. M., Badaruddin, B. S., Judd, J. M., et al. (2020). Chronic unpredictable intermittent restraint stress disrupts spatial memory in male, but not female rats. Behav. Brain Res. 383, 112519. doi:10.1016/j.bbr.2020.112519
Pietrogrande, G., Mabotuwana, N., Zhao, Z., Abdolhoseini, M., Johnson, S. J., Nilsson, M., et al. (2018). Chronic stress induced disturbances in laminin: A significant contributor to modulating microglial pro-inflammatory tone? Brain Behav. Immun. 68, 23–33. doi:10.1016/j.bbi.2017.09.012
Rahmani, M., Nkwocha, J., Hawkins, E., Pei, X., Parker, R. E., Kmieciak, M., et al. (2018). Cotargeting BCL-2 and PI3K induces BAX-dependent mitochondrial apoptosis in AML cells. Cancer Res. 78, 3075–3086. doi:10.1158/0008-5472.CAN-17-3024
Reed, J. C. (1997). Double identity for proteins of the Bcl-2 family. Nature 387, 773–776. doi:10.1038/42867
Salehi, A., Rabiei, Z., and Setorki, M. (2018). Effect of gallic acid on chronic restraint stress-induced anxiety and memory loss in male BALB/c mice. Iran. J. Basic Med. Sci. 21, 1232–1237. doi:10.22038/ijbms.2018.31230.7523
Salehpour, F., Farajdokht, F., Cassano, P., Sadigh-Eteghad, S., Erfani, M., Hamblin, M. R., et al. (2019). Near-infrared photobiomodulation combined with coenzyme Q10 for depression in a mouse model of restraint stress: Reduction in oxidative stress, neuroinflammation, and apoptosis. Brain Res. Bull. 144, 213–222. doi:10.1016/j.brainresbull.2018.10.010
Sara, J. (2001). Animal cognition and animal behaviour. Anim. Behav. 61, 277–286. doi:10.1006/anbe.2000.1606
Shen, J., Yang, L., and Wei, W. (2021). Role of Fto on CaMKII/CREB signaling pathway of hippocampus in depressive-like behaviors induced by chronic restraint stress mice. Behav. Brain Res. 406, 113227. doi:10.1016/j.bbr.2021.113227
Shen, Q., Yamano, K., Head, B. P., Kawajiri, S., Cheung, J. T. M., Wang, C., et al. (2014). Mutations in Fis1 disrupt orderly disposal of defective mitochondria. Mol. Biol. Cell 25, 145–159. doi:10.1091/mbc.E13-09-0525
Sorrentino, V., Romani, M., Mouchiroud, L., Beck, J. S., Zhang, H., D’Amico, D., et al. (2017). Enhancing mitochondrial proteostasis reduces amyloid-β proteotoxicity. Nature 552, 187–193. doi:10.1038/nature25143
Sugioka, R., Shimizu, S., and Tsujimoto, Y. (2004). Fzo1, a protein involved in mitochondrial fusion, inhibits apoptosis. J. Biol. Chem. 279, 52726–52734. doi:10.1074/jbc.M408910200
Tan, P-P., Zhou, B-H., Zhao, W-P., Jia, L-S., Liu, J., and Wang, H-W. (2018). Mitochondria-mediated pathway regulates C2C12 cell apoptosis induced by fluoride. Biol. Trace Elem. Res. 185, 440–447. doi:10.1007/s12011-018-1265-6
Tian, H., Ding, N., Guo, M., Wang, S., Wang, Z., Liu, H., et al. (2019). Analysis of learning and memory ability in an alzheimer’s disease mouse model using the Morris water maze. J. Vis. Exp. doi:10.3791/60055
Villeda, S. A., Plambeck, K. E., Middeldorp, J., Castellano, J. M., Mosher, K. I., Luo, J., et al. (2014). Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med. 20, 659–663. doi:10.1038/nm.3569
Vorhees, C. V., and Williams, M. T. (2006). Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 1, 848–858. doi:10.1038/nprot.2006.116
Wang, H., Jiang, N., Lv, J., Huang, H., and Liu, X. (2020). Ginsenoside Rd reverses cognitive deficits by modulating BDNF-dependent CREB pathway in chronic restraint stress mice. Life Sci. 258, 118107. doi:10.1016/j.lfs.2020.118107
Wang, M-Y., Meng, M., Yang, C-C., Zhang, L., Li, Y-L., Zhang, L., et al. (2020). Cornel iridoid glycoside improves cognitive impairment induced by chronic cerebral hypoperfusion via activating PI3K/Akt/GSK-3β/CREB pathway in rats. Behav. Brain Res. 379, 112319. doi:10.1016/j.bbr.2019.112319
Wang, R., Ren, Q., Gao, D., Paudel, Y. N., Li, X., Wang, L., et al. (2022). Ameliorative effect of Gastrodia elata Blume extracts on depression in zebrafish and cellular models through modulating reticulon 4 receptors and apoptosis. J. Ethnopharmacol. 289, 115018. doi:10.1016/j.jep.2022.115018
Woo, H., Hong, C. J., Jung, S., Choe, S., and Yu, S-W. (2018). Chronic restraint stress induces hippocampal memory deficits by impairing insulin signaling. Mol. Brain 11, 37. doi:10.1186/s13041-018-0381-8
Xie, H., Chen, Y., Wu, W., Feng, X., and Du, K. (2021). Gastrodia elata blume polysaccharides attenuate vincristine-evoked neuropathic pain through the inhibition of neuroinflammation. Mediat. Inflamm. 2021, 9965081. doi:10.1155/2021/9965081
Xiong, B., Zhang, W., Zhang, L., Huang, X., Zhou, W., Zou, Q., et al. (2020). Hippocampal glutamatergic synapses impairment mediated novel-object recognition dysfunction in rats with neuropathic pain. Pain 161, 1824–1836. doi:10.1097/j.pain.0000000000001878
Xu, P., Wang, K., Lu, C., Dong, L., Gao, L., Yan, M., et al. (2016). Protective effect of lavender oil on scopolamine induced cognitive deficits in mice and H2O2 induced cytotoxicity in PC12 cells. J. Ethnopharmacol. 193, 408–415. doi:10.1016/j.jep.2016.08.030
Xu, P., Wang, K., Lu, C., Dong, L., Gao, L., Yan, M., et al. (2017). The protective effect of lavender essential oil and its main component linalool against the cognitive deficits induced by D-galactose and aluminum trichloride in mice. Evid. Based. Complement. Altern. Med. 2017, 7426538. doi:10.1155/2017/7426538
Xu, P., Xu, S., Wang, K., Lu, C., Zhang, H., Pan, R., et al. (2016). Cognitive-enhancing effects of hydrolysate of polygalasaponin in SAMP8 mice. J. Zhejiang Univ. Sci. B 17, 503–514. doi:10.1631/jzus.B1500321
Yang, Y., Li, S., Huang, H., Lv, J., Chen, S., Pires Dias, A. C., et al. (2020). Comparison of the protective effects of ginsenosides Rb1 and Rg1 on improving cognitive deficits in SAMP8 mice based on anti-neuroinflammation mechanism. Front. Pharmacol. 11, 834. doi:10.3389/fphar.2020.00834
Yu, W., Wang, X., Zhao, J., Liu, R., Liu, J., Wang, Z., et al. (2020). Stat2-Drp1 mediated mitochondrial mass increase is necessary for pro-inflammatory differentiation of macrophages. Redox Biol. 37, 101761. doi:10.1016/j.redox.2020.101761
Zhan, H-D., Zhou, H-Y., Sui, Y-P., Du, X-L., Wang, W-H., Dai, L., et al. (2016). The rhizome of Gastrodia elata Blume - an ethnopharmacological review. J. Ethnopharmacol. 189, 361–385. doi:10.1016/j.jep.2016.06.057
Zhang, J., Yu, C., Zhang, X., Chen, H., Dong, J., Lu, W., et al. (2018). Porphyromonas gingivalis lipopolysaccharide induces cognitive dysfunction, mediated by neuronal inflammation via activation of the TLR4 signaling pathway in C57BL/6 mice. J. Neuroinflammation 15, 37. doi:10.1186/s12974-017-1052-x
Zhang, S., Xue, R., Geng, Y., Wang, H., and Li, W. (2020). Fisetin prevents HT22 cells from high glucose-induced neurotoxicity via PI3K/akt/CREB signaling pathway. Front. Neurosci. 14, 241. doi:10.3389/fnins.2020.00241
Keywords: chronic restraint stress, Gastrodia elata Blume, learning and memory, animal behavior, mitochondria
Citation: Huang H, Zhang Y, Yao C, He Q, Chen F, Yu H, Lu G, Jiang N and Liu X (2022) The effects of fresh Gastrodia elata Blume on the cognitive deficits induced by chronic restraint stress. Front. Pharmacol. 13:890330. doi: 10.3389/fphar.2022.890330
Received: 23 March 2022; Accepted: 03 August 2022;
Published: 29 August 2022.
Edited by:
Jacob Raber, Oregon Health and Science University, United StatesReviewed by:
Jong Hoon Ryu, Kyung Hee University, South KoreaIrena Maria Nalepa, Maj Institute of Pharmacology, Polish Academy of Sciences (IF PAS), Poland
Xi Rui He, Zunyi Medical University, China
Copyright © 2022 Huang, Zhang, Yao, He, Chen, Yu, Lu, Jiang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Jiang, amlhbmduaW5nMDYwM0AxNjMuY29t; Xinmin Liu, bGl1eGlubWluQGhvdG1haWwuY29t
 Hong Huang1
Hong Huang1 Yiwen Zhang
Yiwen Zhang Caihong Yao
Caihong Yao Qinghu He
Qinghu He Han Yu
Han Yu Guanghua Lu
Guanghua Lu Ning Jiang
Ning Jiang Xinmin Liu
Xinmin Liu