
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol., 11 May 2022
Sec. Pharmacology of Anti-Cancer Drugs
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.881493
This article is part of the Research TopicMulti-omics in Studying the Mechanisms of Anti-cancer Drugs Resistance and ToxicityView all 13 articles
 Kehai Lin1,2†
Kehai Lin1,2† Jie Lin2†
Jie Lin2† Zhong Huang2
Zhong Huang2 Jiding Fu3
Jiding Fu3 Qi Yi2
Qi Yi2 Jiazuo Cai2
Jiazuo Cai2 Muhammad Khan2
Muhammad Khan2 Yawei Yuan1,2*
Yawei Yuan1,2* Junguo Bu1*
Junguo Bu1*Background: The impact of smoking on the efficacy of anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC) treatment is controversial and has not been systematically explored in the first-line setting. We performed a systematic review based on a pairwise meta-analysis and a Bayesian network meta-analysis (NMA) to address this issue.
Methods: PubMed, Embase, Web of Science, Cochrane Library, Clinical-Trials.gov, and other resources were searched until 5 January 2022. Progression-free survival (PFS) was considered the main outcome of interest. Randomized controlled trials with smoking status analysis were included. Cochrane Risk of Bias Tool was performed to assess the risk of bias. Random effects models were adopted conservatively in meta-analysis. The NMA was performed in a Bayesian framework using the “gemtc” version 1.0–1 package of R-4.1.2 software.
Results: A total of 2,484 patients from nine studies were eligible for this study, with 1,547 never-smokers (62.3%) and 937 smokers (37.7%). In a pairwise meta-analysis, in the overall population, no significant difference was found between never-smokers and smokers. However, in the subgroup analyses based on crizotinib-controlled studies, anaplastic lymphoma kinase tyrosine kinase inhibitors (ALK-TKIs) derived better PFS in the smoking group over the never-smoking group in the Asian population (HR = 0.17, 95%CI = 0.09–0.31 in the smoking group, HR = 0.39, 95%CI = 0.24–0.65 in the never-smoking group, p = 0.04, low quality of evidence). In NMA, among never-smokers, lorlatinib ranked the highest for PFS (SUCRA = 96.2%), but no significant superiority was found among the new-generation ALK-TKIs except for ceritinib. In smokers, low-dose alectinib performed best (SUCRA = 95.5%) and also demonstrated a significant superiority over ensartinib (HR = 0.23, 95%CI = 0.08–0.68, very low quality of evidence), brigatinib (HR = 0.38, 95%CI = 0.14–0.99, low quality of evidence), ceritinib (HR = 0.24, 95%CI = 0.09–0.66, low quality of evidence), crizotinib (HR = 0.18, 95%CI = 0.08–0.41, moderate quality of evidence), and chemotherapy (HR = 0.11, 95%CI = 0.05–0.28, low quality of evidence).
Conclusion: In general, smoking may not affect the treatment efficacy of advanced ALK-positive NSCLC in the first-line setting. However, alectinib may perform better in the smoking Asian population. Moreover, lorlatinib in never-smokers and low-dose alectinib in smokers could be considered optimal first-line therapy for advanced ALK-positive NSCLC. Acceptable limitations of evidence, such as study risk of bias, inconsistency, and imprecision, were present in this NMA.
Lung cancer, one of the most malignant tumors in both sexes, ranked first in cancer-related deaths and second in newly diagnosed cancer cases worldwide, with percentages of 18.2% and 12.2%, respectively (Cancer today, 2022). Non-small cell lung cancer (NSCLC) accounts for approximately 85% of lung cancer cases (Thai et al., 2021). Oncogenic alterations gradually play an increasingly important role in the development. It is well-known that smoking rates are high, and the role of different smoking statuses varies significantly in carcinogenesis (Bossé and Amos, 2017; Li et al., 2017; Singal et al., 2019; Wang et al., 2021a; Thai et al., 2021), among which EGFR mutations are the most common oncogenic alterations, ranging from 15% in Europe to 62% in Asia in NSCLC of adenocarcinoma histology. EGFR tyrosine kinase inhibitors have performed well in the targeted treatments, extending patients’ median overall survival to more than 38 months by gefitinib or osimertinib alone, and even to more than 50 months when combined with chemotherapy. Interestingly, more and more oncogenic alterations have also been developed into useful treatment strategies in NSCLC, such as ALK, RET, NTRK, and ROS1 (Thai et al., 2021).
Anaplastic lymphoma kinase (ALK) gene translocation, leading to abnormal expression of constitutively active ALK fusion proteins, is a key mechanism for inducing lung tumorigenesis of ALK-positive NSCLC (3%–5% of NSCLCs). It is more common in never- or light-smokers and younger age and is associated with adenocarcinoma histology (Shaw and Engelman, 2013; Thai et al., 2021). During the past decade, ALK tyrosine kinase inhibitors (ALK-TKIs) have demonstrated remarkable efficacy in the treatment of advanced or metastatic NSCLC and now are the standard options in the first-line treatment of advanced ALK-positive NSCLCs instead of chemotherapy. Compared with cytotoxic chemotherapy, the first-generation ALK-TKI crizotinib significantly has extended median progression-free survival (PFS) of advanced NSCLC (around 11 vs. 7 months) (Solomon et al., 2014; Wu et al., 2018). Moreover, the PFS has been remarkably prolonged by the next-generation ALK-TKIs, such as ceritinib, alectinib, brigatinib, ensartinib, and lorlatinib (Soria et al., 2017; Zhou et al., 2019; Mok et al., 2020; Nakagawa et al., 2020; Shaw et al., 2020; Camidge et al., 2021; Horn et al., 2021). Undoubtedly, targeted therapy is the preferred treatment of oncogene-driven advanced NSCLC. Although never- or light-smokers account for a much higher proportion of ALK-positive NSCLC, it is of great interests and necessitous to figure out the effect of smoking on ALK-positive NSCLCs treatment efficacy in the first-line therapy.
To date, previous meta-analyses have investigated the correlation between smoking status and the efficacy of advanced NSCLC treatments (Breadner et al., 2020; Li et al., 2020). However, conflict occurs in the benefit of never-smoking, as one previous meta-analysis found that never-smokers tended to benefit from ALK-TKIs compared with cytotoxic chemotherapy, whereas another denoted that there were similar benefits regardless of the smoking status. Meanwhile, a network meta-analysis focusing on the relative efficacy of first-line targeted therapies in advanced ALK-positive NSCLCs has simply highlighted the role of smoking in the subgroup analysis (Wang et al., 2021b). These previous works could be systematically expanded to determine the correlation between smoking status and efficacy of ALK-targeted agents in the first-line treatment of advanced ALK-positive NSCLC.
Therefore, our study attempted to compare the impact of smoking on the efficacy of advanced ALK-positive NSCLC with high-quality first-line setting randomized controlled trials. Furthermore, a comprehensive NMA of the relative efficacy of first-line treatments according to different smoking statuses was also performed.
This study was carried out following the guidelines of the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) (Page et al., 2021) and the extension statement of NMA (Hutton et al., 2015). It was registered on the INPLASY website (registration number: INPLASY202180009, https://inplasy.com/inplasy-2021-8-0009/, accessed on 03 August 2021).
We searched PubMed, Embase, Web of Science, Cochrane Library, and Clinical-Trials.gov up to 19 August 2021 without language limitations for eligible studies, which was finally updated on 05 January 2022. In addition, abstracts were searched from the main oncology congresses databases, including the American Society of Clinical Oncology (ASCO), the European Society for Medical Oncology (ESMO), and the World Conference on Lung Cancer (WCLC). The search strategy is presented in Supplementary Table S2. The following search terms were used: non-small cell lung cancer (NSCLC), anaplastic lymphoma kinase (ALK), ALK tyrosine kinase inhibitors, crizotinib, ceritinib, alectinib, brigatinib, ensartinib, lorlatinib, entrectinib, and their medical subject headings (MeSH) terms. The inclusion criteria were as follows: 1) randomized controlled trials with clinical outcomes, such as PFS and OS; 2) patients with pathologically confirmed locally advanced or metastatic NSCLC; 3) studies with clear baseline characteristics of patients and ALK mutation status; and 4) studies including data of smoking status analysis required for meta-analysis. The relevant titles and abstracts were screened to remove duplicated and irrelevant publications. Then, the full texts and relevant reference lists of the other articles were browsed thoroughly for the final inclusion.
The following information was extracted: the trial name, publication year, design, interventions, sample size, race, patients age and gender, baseline brain metastases, adverse effects, previous treatments, number of smokers (defined as current and/or former smokers) and never-smokers, and hazard ratio (HR) with 95% confidence intervals (CIs) for PFS of whole group and subgroup. Quality assessment was performed using the Cochrane Risk of Bias Tool (Higgins et al., 2011). It includes seven domains (random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other bias), for which a judgment (low, high, or unclear risk) was assessed respectively. Study selection, data extraction, and risk of bias assessment were independently executed by two reviewers (KL, JL). For any unresolved discrepancies, a third reviewer (ZH) was concerned.
PFS was defined as the time from randomization to RECIST-defined disease progression or death from any cause. The HR was regarded as a measure of effect size for PFS. Because overall survival (OS) is immature for most trials and there is a lack of smoking subgroup analysis results, this part of the analysis was not performed.
PFS-HR of current smokers and former smokers was combined as the smoker group when smoking statuses were multiply categorized. The HR of current smokers in some studies was ignored because it was not applicable due to a small population. Heterogeneity across studies was assessed by I2 statistics, with I2 < 25%, 25% ≤ I2 ≤ 50%, and I2 > 50% being interpreted as signifying low-level, intermediate-level, and high-level heterogeneity, respectively. If necessary, subgroup analysis would be performed. Any heterogeneity between the smoker subgroup and never-smoker subgroup was detected by the Cochran Q test. A p-value (two-sided) of less than 0.05 was considered statistically significant. The analysis process was carried out by RevMan 5.4.1, applying the random-effect model conservatively.
With the model of the lower deviance information criterion (DIC), which is more feasible (Oravecz and Muth, 2017), a network meta-analysis of different therapeutic drugs in both never smokers and smokers based on a Bayesian framework was performed using the “gemtc 1.0–1” package of R software (version 4.1.2) (Neupane et al., 2014; Tonin et al., 2017). This method integrated both direct and indirect comparisons for any given pair of managements and certain endpoints. The function mtc.run was applied to generate samples, and we set 10000 simulations for each chain as the “burn-in” period, yielding 50,000 iterations to obtain the HR of model parameters when four Markov chains run simultaneously. The Brooks–Gelman–Rubin diagnosis plots method, trace plot, and density plot were used to access the model convergence (Wu et al., 2013). Rank probabilities were calculated to obtain the hierarchy of each treatment, and a plot of rank probabilities was created by the “gemtc” package (Gelman and Rubin, 1992). The probability of the competing treatments was ranked by the surface under the cumulative ranking curve (SUCRA), the highest and lowest values of which mean the highest probability of ranking the best and worst, respectively (Salanti et al., 2011; Tonin et al., 2017).
Stata/SE 15.1 and RevMan 5.4.1 were used to generate network and funnel plots for a visual illustration of relationships among each treatment and evaluation of the studies' publication bias. The mtc.anohe command of the “gemtc” package was used to evaluate global heterogeneity. A sensitivity analysis was performed by removing the trials deemed to be heterogeneous to ensure reliability.
The quality of evidence was assessed in accordance with the GRADE working group approach (Guyatt et al., 2008; Puhan et al., 2014). In this method, the quality of evidence was categorized into four levels (high, moderate, low, and very low), and the starting point of quality of direct evidence based on RCTs would be high, which could be downrated to moderate, low, or very low according to five domains (risk of bias, indirectness, imprecision, inconsistency, and publication bias). We used the GRADEpro Guideline Development Tool (www.gradepro.org) to rate the quality of evidence in a pairwise meta-analysis. In network meta-analysis (Puhan et al., 2014), the quality of indirect evidence was consistent with the lower confidence rating of two direct comparisons that contribute as first-order loops to the indirect estimates. As there are no closed loops in this NMA, the direct or indirect estimates constituted the final outcome.
Initially, 3,537 eligible studies were yielded from the searching strategies, including 322 studies from PubMed, 1,364 studies from Embase, 812 from Web of Science, 994 studies from Cochrane Library, and 8 studies from Clinical-Trials.gov. In addition, 37 studies were found from other sources. After removing 1,419 duplicates, additional 2,063 studies were excluded by screening the title and abstract. Eventually, nine studies were included in our analysis according to the inclusion criteria. The flowchart of the literature screening is presented in Figure 1.
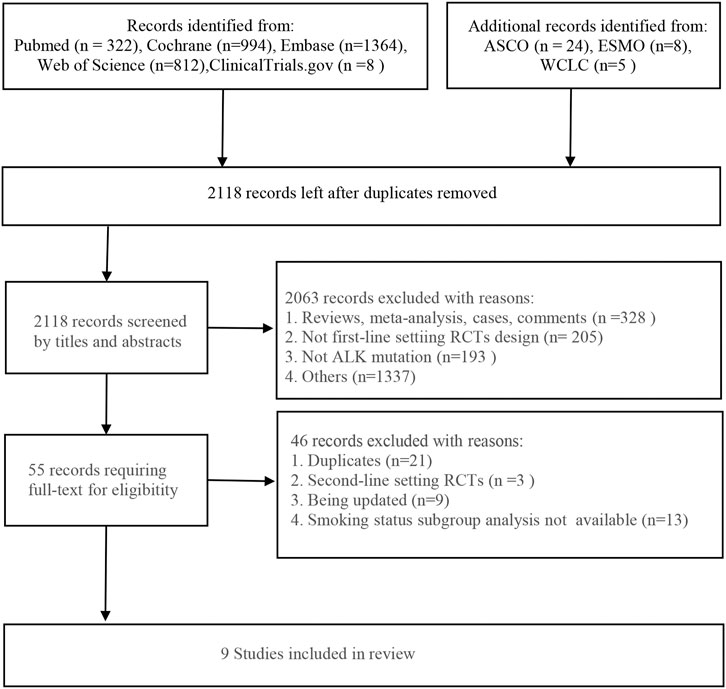
FIGURE 1. Flowchart of the literature screening. ASCO, the American Society of Clinical Oncology; ESMO, the European Society for Medical Oncology; WCLC, the World Conference on Lung Cancer; RCTs, randomized controlled trials; ALK, anaplastic lymphoma kinase.
Table 1 presents the detailed characteristics of included studies. All of the included nine studies were phase 3 randomized controlled trials, enrolling 2,484 participants totally, with 1,547 never-smokers (62.3%) and 937 smokers (37.7%). Among them, three were cytotoxic chemotherapy-controlled studies, with PROFILE1014 and PROFILE1029 investigating crizotinib and ASCEND-4 investigating ceritinib and six were crizotinib-controlled studies, with eXalt3 investigating ensartinib, ALTA-1L investigating brigatinib, CROWN investigating lorlatinib, and J-ALEX, ALEX, and ALESIA investigating alectinib). For race differences, three studies were Asian-only trials (PROFILE 1029, J-ALEX, and ALESIA), and six were multi-race trials (Crown, ALEX, ALTA-1L, PROFILE 1014, eXalt3, and ASCEND-4).
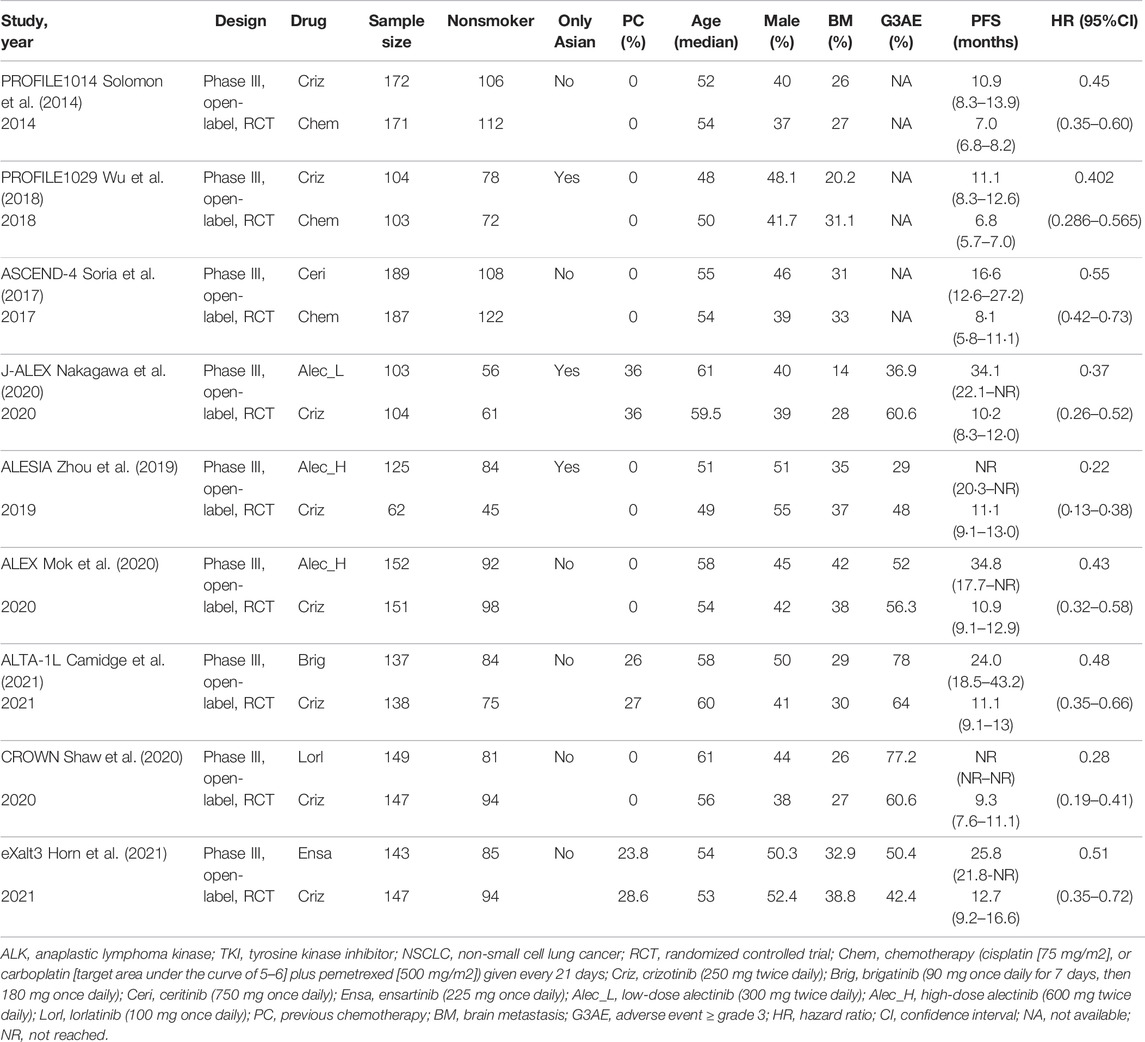
TABLE 1. Characteristics of the included studies of first-line ALK-TKI treatment for advanced ALK-positive NSCLC.
All nine studies were open-label studies prone to a high risk of performance bias. However, a low risk of detection bias was observed in all included studies due to the blinding of outcome assessment completed by a blinded independent review committee. Unclear risk occurred in selection bias, attrition bias, reporting bias, and other bias due to the lack of detailed information. Detailed quality assessment is illustrated in Supplementary Figure S1.
Both the trace and density plot (Supplementary Figure S2 for never-smoker, Supplementary Figure S3 for smoker) and Brooks–Gelman–Rubin diagnosis plot (Supplementary Figure S2 for never-smoker, Supplementary Figure S3 for smoker), showing no single chain fluctuation and normal distribution of density map, illustrated an excellent convergence of the models performed in NMA. As seen in Supplementary Figure S4, there was no significant publication bias in the pooled analyses. In terms of inconsistency analyses, the global analysis showed low heterogeneity in nonsmokers and moderate heterogeneity in the smokers (I2 = 4%, I2 = 26%, respectively). As no closed loop exits in this NMA, local inconsistency analysis was not performed. Thus, a satisfactory consistency among the studies was obtained. The transitivity across the included studies was well balanced by strictly including RCTs according to the selection criteria in this NMA.
We compared the pooled efficacy for never-smokers against smokers in the chemotherapy-controlled studies and crizotinib-controlled studies, respectively. In chemotherapy-controlled studies (Figure 2, Supplementary Table S3), the pooled PFS-HR for never-smokers was 0.42 (95%CI = 0.31–0.57, moderate quality of evidence), while 0.58 (95%CI = 0.44–0.76, moderate quality of evidence) for smokers. Compared with chemotherapy, treatment with ALK-TKIs exhibited no statistically significant difference between smokers and never-smokers (p = 0.14).
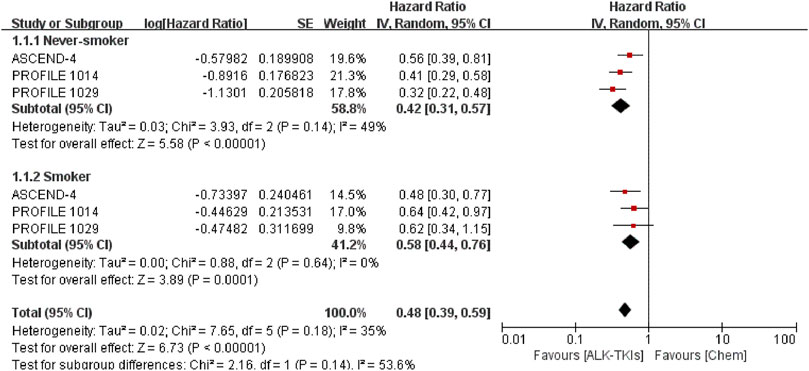
FIGURE 2. Comparison between smoking status subgroups in chemotherapy-controlled studies. ALK-TKIs, anaplastic lymphoma kinase tyrosine kinase inhibitors; Chem, chemotherapy.
In crizotinib-controlled studies (Figure 3, Supplementary Table S4), the pooled PFS-HR analysis yielded 0.37 (95%CI = 0.31–0.46, moderate quality of evidence) for never-smokers and 0.40 (95%CI = 0.26–0.60, very low quality of evidence) for smokers. Compared with crizotinib, treatment with both the second- and third-generation (2/3G) ALK-TKIs presented similar benefits between smokers and never-smokers (p = 0.80). The smoker subgroup exhibited significant heterogeneity (I2 = 59%). As such, we conducted subgroup analysis by race. In Asian-only studies, pooled PFS-HR was 0.39 (95%CI = 0.24–0.65, moderate quality of evidence) for never smokers and 0.17 (95%CI = 0.09–0.31, low quality of evidence) for smokers, with significant difference (p = 0.04). In others, pooled PFS-HR 0.37 (95%CI = 0.29–0.47, moderate quality of evidence) for never smokers and 0.50 (95%CI = 0.36–0.69, moderate quality of evidence) for smokers had no significant difference (p = 0.15). Moreover, no significant heterogeneity was found in both subgroup analysis (Asian-only subgroup: I2 = 29% in never-smoker subgroup, 0% in smoker subgroup; multirace subgroup: I2 = 9% in never-smoker subgroup, 25% in smoker subgroup).
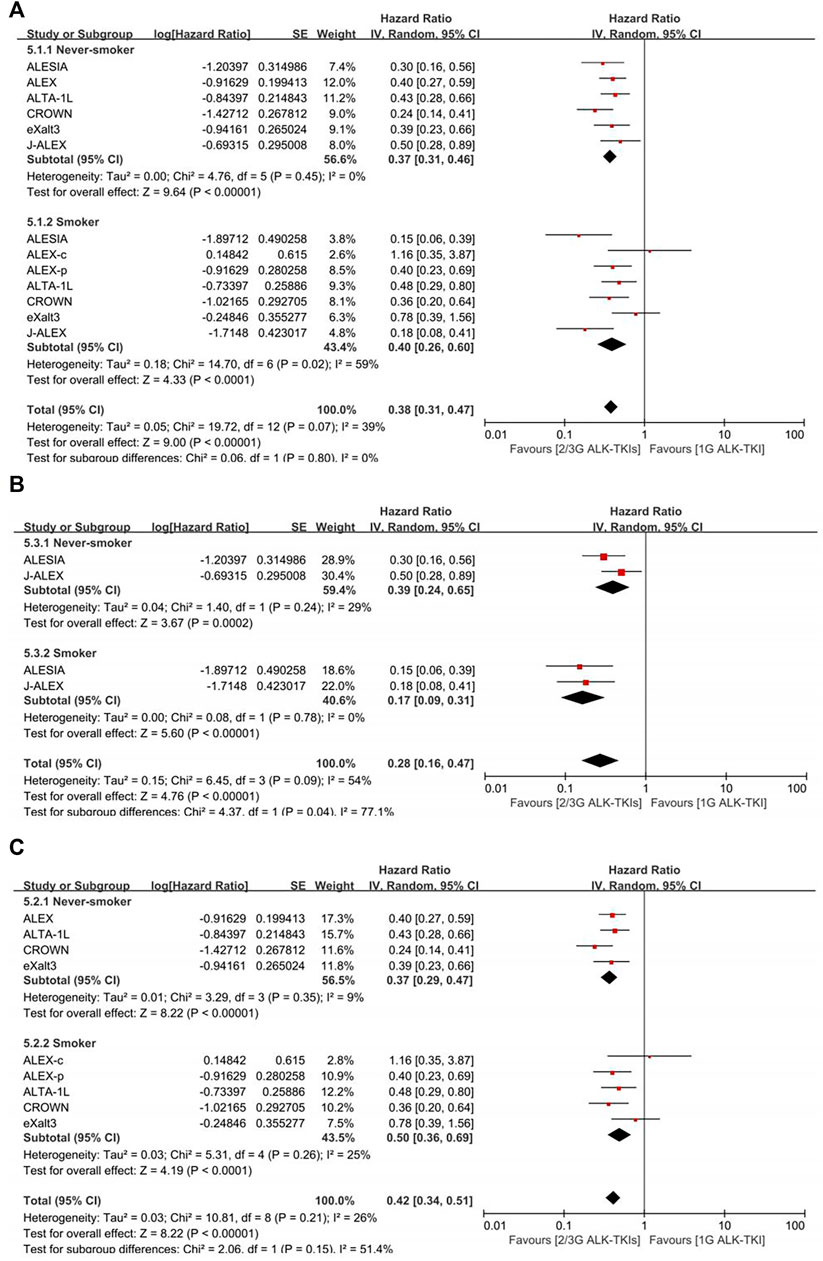
FIGURE 3. Comparison between smoking status subgroups in crizotinib-controlled studies. (A) Initial analysis. (B) Asian-only subgroup analysis. (C) Multiracial subgroup analysis. ALEX-c, current smoker subgroup of ALEX; ALEX-p, previous smoker subgroup of ALEX; 2/3G ALK-TKIs, both second- and third-generation anaplastic lymphoma kinase tyrosine kinase inhibitors; 1G ALK-TKI, first-generation anaplastic lymphoma kinase tyrosine kinase inhibitor.
Figure 4 presents the network plot of each treatment. Eight treatments were involved in this NMA, with low-dose alectinib (ld-alectinib, 300 mg twice daily) and high-dose alectinib (hd-alectinib, 600 mg twice daily) being regarded as separate treatments.
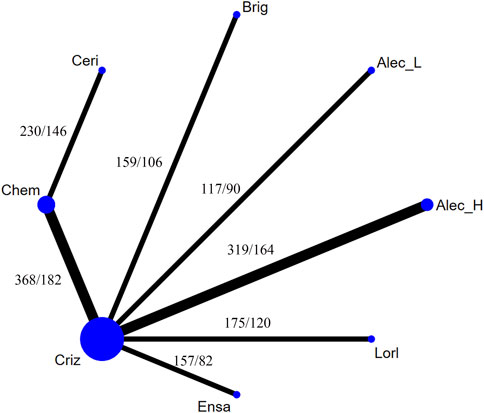
FIGURE 4. Network constructions for comparisons in progression-free survival of the never-smoker group or smoker group. Chem, chemotherapy; Criz, crizotinib; Brig, brigatinib; Ceri, ceritinib; Ensa, ensartinib; Alec_L, low-dose alectinib; Alec_H, high-dose alectinib; Lorl, lorlatinib. The “number 1/number 2” listed in the upper left of each comparison means that number 1 is the number of never-smokers and number 2 is the number of smokers in each comparison.
In the never-smoker group, as presented in Figure 5, all ALK-TKIs were significantly superior to chemotherapy; significant superiority was observed for all next-generation ALK-TKIs other than ceritinib when compared to crizotinib (HR = 0.24, 95%CI = 0.14–0.41, moderate quality of evidence for lorlatinib; HR = 0.37, 95%CI = 0.26–0.51, moderate quality of evidence for hd-alectinib; HR = 0.39, 95%CI = 0.23–0.66, moderate quality of evidence for ensartinib; HR = 0.43, 95%CI = 0.28–0.65, moderate quality of evidence for brigatinib; HR = 0.5, 95%CI = 0.28–0.89, low quality of evidence for ld-alectinib; HR = 1.51, 95%CI = 0.96–2.38, low quality of evidence for ceritinib); and no significant difference was noticed between hd- and ld-alectinib (HR = 0.74, 95%CI = 0.38–1.43, low quality of evidence), with the former showing relatively better efficacy. Additionally, lorlatinib, presenting highest SUCRA and Prbest values (SUCRA = 96.2%, Prbest = 82.2%) (Supplementary Tables S5, S6, Supplementary Figure S5), performed best, followed by hd-alectinib (SUCRA = 74.1%), ensartinib (SUCRA = 69.7%), brigatinib (SUCRA = 62.5%), ld-alectinib (SUCRA = 54.5%), crizotinib (SUCRA = 28.2%), ceritinib (SUCRA = 14.8%), and chemotherapy (SUCRA = 0.00%).
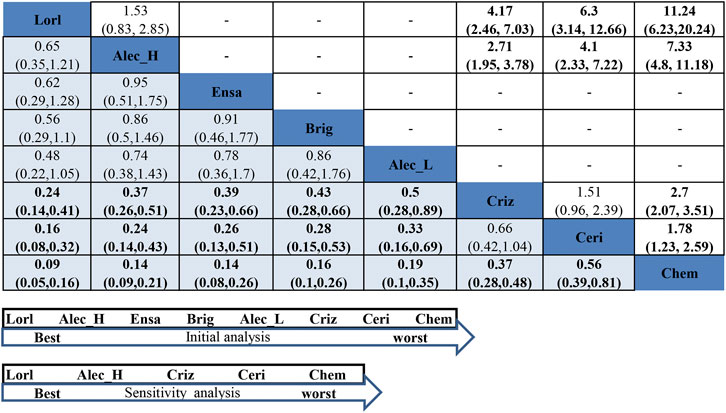
FIGURE 5. NMA results of never-smoker on progression-free survival (lower left) and sensitivity analysis (upper right), followed by the ranking distribution according to SUCRA values (lower arrow shape). NMA, network meta-analysis; SUCRA, the surface under the cumulative ranking curve; Chem, chemotherapy; Criz, crizotinib; Ceri, ceritinib; Alec_L, low-dose alectinib; Alec_H, high-dose alectinib; Brig, brigatinib; Lorl, lorlatinib; Ensa, ensartinib. Values in bold mean statistically significant.
For assessment of between-study heterogeneity (Supplementary Figure S6), we found no significant difference either in the crizotinib-chemotherapy comparison in both PROFILE1014 and PROFILE1029 or the comparison between crizotinib and hd-alectinib in both ALEX and ALESIA, with both showing no heterogeneity (I2 = 0.0%). Because three trials (J-ALEX, eXalt3, and ALTA-1L) had also enrolled patients with history of previous chemotherapy, a sensitivity analysis was performed by excluding these trials. Then, rank probabilities by SUCRA values were generated in the remaining studies. Figure 5 and Supplementary Table S6 reveal the same results for the relative ranking of the five remaining treatment groups. Based on these results, we may conclude that a history of previous treatment may not affect the outcomes of our NMA.
In the smoker group, as presented in Figure 6, ld-alectinib showed significantly better PFS than other ALK-TKIs except for hd-alectinib and lorlatinib (HR = 0.18, 95%CI = 0.08–0.41, moderate quality of evidence for crizotinib; HR = 0.23, 95%CI = 0.08–0.68, very low quality of evidence for ensartinib; HR = 0.24, 95%CI = 0.09–0.66, low quality of evidence for ceritinib; HR = 0.38, 95%CI = 0.14–0.99, low quality of evidence for brigatinib; HR = 0.5, 95%CI = 0.18–1.37, moderate quality of evidence for lorlatinib; HR = 0.62, 95%CI = 0.21–1.83, very low quality of evidence for hd-alectinib). Though lorlatinib has demonstrated a greater effect in the never-smoker group, it was not superior to both ld- and hd-alcetinib in PFS for the smoker group (HR = 0.5, 95%CI = 0.18–1.37, moderate quality of evidence for ld-alectinib; HR = 0.81, 95%CI = 0.33–1.97, very low quality of evidence for hd-alectinib). Additionally, ld-alectinib (SUCRA = 95.5%, Prbest = 76.9%) (Supplementary Tables S6, S7, Supplementary Figure S7) was considered to rank first with greatest probability, followed by hd-alectinib (SUCRA = 81.6%), lorlatinib (SUCRA = 72.8%), brigatinib (SUCRA = 58.8%), ceritinib (SUCRA = 36.2%), ensartinib (SUCRA = 34.3%), crizotinib (SUCRA = 20.2%), and chemotherapy (SUCRA = 0.01%).
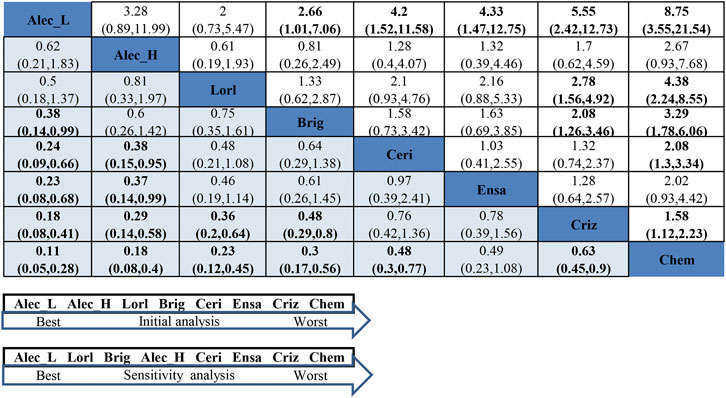
FIGURE 6. NMA results of smokers on progression-free survival (lower left) and sensitivity analysis (upper right), followed by the ranking distribution according to SUCRA values (lower arrow shape). NMA, network meta-analysis; SUCRA, the surface under the cumulative ranking curve; Chem, chemotherapy; Criz, crizotinib; Ceri, ceritinib; Alec_L, low-dose alectinib; Alec_H, high-dose alectinib; Brig, brigatinib; Lorl, lorlatinib; Ensa, ensartinib. Values in bold mean statistically significant.
For assessment of between-study heterogeneity (Supplementary Figure S8), a significant difference was found in the crizotinib-hd-alectinib comparison in both ALEX and ALESIA (I2 = 73.5%) but not in the crizotinib-chemotherapy group in both PROFILE1014 and PROFILE1029 (I2 = 0). High heterogeneity between ALEX and ALESIA was considered to result from a small number of smokers in the crizotinib arm (only 14) in the ALESIA study. Under such circumstances, we performed a sensitivity analysis by excluding ALESIA. The results (Figure 6 and Supplementary Table S6) showed that ld-alectinib still performed better, and the relative ranking of other treatments was consistent with the result from the initial NMA except for hd-alectinib, which was not better than lorlatinib and brigatinib. More sensitivity analyses by excluding studies enrolling patients with a history of previous chemotherapy are shown in Supplementary Table S6. It is concluded that the similar outcomes, to a certain extent, symbolized the inherent robustness of the NMA, which confirms the ultimate results.
On the one hand, it is well-known that smoking is of great importance in the morbidity and mortality of lung cancer (Loeb et al., 1984; Jung et al., 2016). On the other hand, the impact of smoking on treatment decisions is controversial and is recently being widely investigated (Lin et al., 2018; Li et al., 2020; Xiao et al., 2020; Chen et al., 2021; Zhao et al., 2021). To our knowledge, the comprehensive and systematic analysis of the relationship between smoking status and first-line treatment efficacy of advanced ALK-positive NSCLC has not been reported yet. Our present work may contribute to resolving this discrepancy and provide useful advice for clinical strategy.
As ALK-TKIs have replaced traditional chemotherapies as the upfront treatments with great advantages in efficacy and safety (Guidelines Detail, 2022), we classified the included studies into two groups: the chemotherapy-controlled and crizotinib-controlled groups. Although we found no difference between the smoking group and the never-smoking group in both chemotherapy- and crizotinib-controlled groups, the subgroup analysis by race in the crizotinib-controlled group indicated that ALK-TKIs in Asian-only derived better outcomes in the smoking group compared to the never-smoking group. In contrast to our outcomes, Breadner’s meta-analysis found a greater degree of benefit with ALK-TKIs in the never-smoker group (Breadner et al., 2020). Nonetheless, their study included second-line setting studies in the chemotherapy-controlled group. Moreover, it is possible that the advancement of lung cancer may have constrained the smoking factor from being detected. In real-world data, smoking history has been regarded as an independent negative prognostic factor for survival benefits (Jin et al., 2018; Britschgi et al., 2020). Tobacco use impairs the treatment efficacy of lung cancer and shortens patients’ survival as smoking tobacco directly influences response to anti-tumor drugs by affecting drugs metabolism (Gemine and Lewis, 2016). On the contrary, immunotherapy performs better in NSCLC patients with a smoking history (Li et al., 2020; Nie et al., 2020; Zhao et al., 2021). Notably, both studies in the Asian-only subgroup used alectinib, despite dosage differences, which may suggest the excellent efficacy of alectinib in smokers, while the experimental ALK-TKIs in another group dramatically varied in multiracial studies.
Recently, network meta-analyses have demonstrated the great advantage of both lorlatinib and alectinib on PFS, with the former being the best (Ando et al., 2021; Chuang et al., 2021). Our NMA of the never-smoking group also supports the advantageous effect of lorlatinib on PFS with significant superiority over crizotinib and ceritinib. As it was designed to easily penetrate the blood–brain barrier and against resistance to all known ALK mutants (Johnson et al., 2014; Zou et al., 2015; Solomon et al., 2018; El Darsa et al., 2020), lorlatinib had an extraordinarily good performance on PFS, not only in the frontline therapy but also in subsequent therapy owing to the failure of first- or second-generation ALK inhibitors (Shaw et al., 2020; Kuang and Leighl, 2021). Nevertheless, interestingly, consistent with Chuang’s finding of ld-alectinib ranking first in the patients with baseline brain metastasis (Chuang et al., 2021), the efficacy of ld-alectinib surpassed that of lorlatinib in the NMA of the smoking group, although there were no significant differences. Moreover, ld-alectinib administration resulted in significantly superior PFS to that of ensartinib, ceritinib, crizotinib, and chemotherapy. It is a feature of ld-alectinib, but not hd-alectinib, to show relatively high activity in the smoking subgroup of advanced ALK-positive NSCLC. However, there is little direct evidence to explain the potent mechanism of this discrepancy. To our knowledge, smokers suffer far more mutations than never-smokers, among which TP53 mutation deserves more attention in lung cancer (Le Calvez et al., 2005; Ding et al., 2008). It is noted that there is a high rate of TP53 co-mutation in ALK-positive NSCLC, which has shown a significantly worse prognosis (Aisner et al., 2017; Kron et al., 2018). In Yoda’s research, TP53 mutation coexisted in half of the lorlatinib-resistant samples (Yoda et al., 2018). In the ALTA-1L study (Camidge et al., 2021), patients with TP53 mutant derived apparently shorter PFS not only in the crizotinib treatment group but also in the brigatinib group. However, there was no more information concerning TP53 mutation and efficacy of other ALK-TKIs and relevant correlation with smoking. Probably, the abundant mutations in smokers complicate the drug efficacy. Therefore, additional studies are required to clarify the potential optimal treatment for smokers and never-smokers with elucidation on mechanistic details.
Importantly, adverse effects (AEs) play an indispensable role in clinical treatment decision-making. A lower dose of alectinib, 300 mg twice daily, is a legally experimental dose resulting from Japanese authority due to Japan’s historical maximum intake level of sodium lauryl sulfate, one of the capsule excipients for alectinib (Seto et al., 2013; Zhou et al., 2019). Inconsistent with theoretical assumptions, compared with hd-alectinib, 600 mg twice daily, dose reduction of alectinib did not obviously show a better safety profile in J-ALEX (≥ grade 3 AEs in J-ALEX = 36.9%; ALESIA = 29%; and ALEX = 52%). However, median follow-ups varied in these trials, which may have an impact on the safety profile. Median follow-up was 42.4 months for ld-alectinib in J-ALEX and 48.2 months for hd-alectinib in ALEX, which were much longer than 16.2 months for hd-alectinib in ALESIA (Zhou et al., 2019; Mok et al., 2020; Nakagawa et al., 2020). Also, 36% of participants had received previous chemotherapy in J-ALEX, which may have escalated their adverse effects. Accordingly, the safety profile of ld-alectinib is not worse than that of hd-alectinib, which means ld-alectinib is well tolerated. Lorlatinib (Shaw et al., 2020) is also well tolerated. Although the rate of adverse events (≥ grade 3) was as high as 77%, most of them were hyperlipidemia, weight gain, and hypertension. Moreover, cognitive effects and peripheral neuropathy were common but generally mild, all of which could be well managed.
Lastly, several limitations in our NMA are inevitable. First, in these RCTs, smoking history was not a stratification factor in randomization and small sample sizes existed, probably resulting in heterogeneity in patients selection for our meta-analysis and imprecision of evidence. Second, the direct comparisons were all based on chemotherapy- and crizotinib-controlled studies, which means a lack of direct comparison between next-generation ALK-TKIs and closed loops in the analysis, so the direct evidence among each comparison is insufficient. Third, there is only one trial for each of the next-generation ALK-TKI other than alectinib, and insufficient data may result in instability of the outcome. Fourth, the overall survival of most next-generation ALK-TKIs studies is immature, and subgroup outcome of smoking status is rarely presented. Consequently, extrapolation of long-term outcomes is prevented. Even with the inherent limitations of this NMA, we strictly follow the guidelines of PRISMA and the extension statement of NMA, which improves the quality of our analyses.
In summary, this systematic review compared the impact of smoking status on treatment efficacy and the relative efficacy of each frontline choice in the first-line setting of advanced ALK-positive NSCLC in terms of PFS. Although, in the overall population, there were no significant differences between smoking statuses, we found in the subgroup analyses that ALK-TKIs derived better PFS in the smoking group over the never-smoking group in the Asian population. Among never-smokers, lorlatinib ranks the highest for PFS, but no significant superiority was found among new-generation ALK-TKIs except for comparison with ceritinib. However, ld-alectinib performed better than lorlatinib among smokers, with ld-alectinib ranking first, followed by lorlatinib, brigatinib, hd-alectinib, ceritinib, ensartinib, crizotinib, and chemotherapy. Moreover, ld-alectinib was significantly superior to ensartinib, brigatinib, ceritinib, crizotinib, and chemotherapy. Given the limitations of this meta-analysis, further research focusing on the smoking status is needed to verify these conclusions.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
KL, JL, and ZH: study design. KL and JL: literature searching and extraction of data. ZH, QY, and JC: rechecking data. KL, JL, and JF: data analysis. KL and MK: writing-original draft. JB and YY: writing-review and editing, and study supervision. All authors contributed to the article and approved the submitted version.
This work was supported by the Key Clinical Technology of Guangzhou (Grant no. 2019ZD17).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We sincerely thank the support from all participants.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.881493/full#supplementary-material
Aisner, D. L., Sholl, L. M., Berry, L. D., Rossi, M. R., Chen, H., Fujimoto, J., et al. (2017). The Impact of Smoking and TP53 Mutations in Lung Adenocarcinoma Patients with Targetable Mutations-The Lung Cancer Mutation Consortium (LCMC2). Clin. Cancer Res. 24, 1038–1047. doi:10.1158/1078-0432.ccr-17-2289
Ando, K., Manabe, R., Kishino, Y., Kusumoto, S., Yamaoka, T., Tanaka, A., et al. (2021). Comparative Efficacy and Safety of Lorlatinib and Alectinib for ALK-Rearrangement Positive Advanced Non-small Cell Lung Cancer in Asian and Non-asian Patients: A Systematic Review and Network Meta-Analysis. Cancers (Basel) 13, 3704. doi:10.3390/cancers13153704
Bossé, Y., and Amos, C. I. (2017). A Decade of GWAS Results in Lung Cancer. Cancer Epidemiol. Biomarkers Prev. 27, 363–379. doi:10.1158/1055-9965.epi-16-0794
Breadner, D., Blanchette, P., Shanmuganathan, S., Boldt, R. G., and Raphael, J. (2020). Efficacy and Safety of ALK Inhibitors in ALK-Rearranged Non-small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Lung Cancer 144, 57–63. doi:10.1016/j.lungcan.2020.04.011
Britschgi, C., Addeo, A., Rechsteiner, M., Delaloye, R., Früh, M., Metro, G., et al. (2020). Real-World Treatment Patterns and Survival Outcome in Advanced Anaplastic Lymphoma Kinase (ALK) Rearranged Non-small-cell Lung Cancer Patients. Front. Oncol. 10, 1299. doi:10.3389/fonc.2020.01299
Camidge, D. R., Kim, H. R., Ahn, M. J., Yang, J. C. H., Han, J. Y., Hochmair, M. J., et al. (2021). Brigatinib versus Crizotinib in ALK Inhibitor-Naive Advanced ALK-Positive NSCLC: Final Results of Phase 3 ALTA-1L Trial. J. Thorac. Oncol. 16, 2091–2108. doi:10.1016/j.jtho.2021.07.035
Cancer today (2022). Global Cancer Observatory. Available at: https://gco.iarc.fr/today/home (Accessed February 17, 2022).
Chen, D. L., Li, Q. Y., and Tan, Q. Y. (2021). Smoking History and the Efficacy of Immune Checkpoint Inhibitors in Patients with Advanced Non-small Cell Lung Cancer: a Systematic Review and Meta-Analysis. J. Thorac. Dis. 13, 220–231. doi:10.21037/jtd-20-1953
Chuang, C. H., Chen, H. L., Chang, H. M., Tsai, Y. C., Wu, K. L., Chen, I. H., et al. (2021). Systematic Review and Network Meta-Analysis of Anaplastic Lymphoma Kinase (ALK) Inhibitors for Treatment-Naïve ALK-Positive Lung Cancer. Cancers (Basel) 13, 1966. doi:10.3390/cancers13081966
Ding, L., Getz, G., Wheeler, D. A., Mardis, E. R., McLellan, M. D., Cibulskis, K., et al. (2008). Somatic Mutations Affect Key Pathways in Lung Adenocarcinoma. Nature 455, 1069–1075. doi:10.1038/nature07423
El Darsa, H., Abdel-Rahman, O., and Sangha, R. (2020). Pharmacological and Clinical Properties of Lorlatinib in the Treatment of ALK-Rearranged Advanced Non-small Cell Lung Cancer. Expert Opin. Pharmacother. 21, 1547–1554. doi:10.1080/14656566.2020.1774552
Gelman, A., and Rubin, D. B. (1992). Inference from Iterative Simulation Using Multiple Sequences. Statist. Sci. 7. doi:10.1214/ss/1177011136
Gemine, R., and Lewis, K. (2016). Smoking Cessation With Lung Cancer: not Too Little, Never Too Late. EMJ Respir. 4, 86
Guidelines Detail (2022). National Comprehensive Cancer Network. Available at: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450 (Accessed February 17, 2022).
Guyatt, G. H., Oxman, A. D., Vist, G. E., Kunz, R., Falck-Ytter, Y., Alonso-Coello, P., et al. (2008). GRADE: an Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ 336, 924–926. doi:10.1136/bmj.39489.470347.ad
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised Trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Horn, L., Wang, Z., Wu, G., Poddubskaya, E., Mok, T., Reck, M., et al. (2021). Ensartinib vs Crizotinib for Patients with Anaplastic Lymphoma Kinase-Positive Non-small Cell Lung Cancer: A Randomized Clinical Trial. JAMA Oncol. 7, 1617–1625. doi:10.1001/jamaoncol.2021.3523
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 162, 777–784. doi:10.7326/m14-2385
Jin, Y., Chen, Y., Yu, X., and Shi, X. (2018). A Real-World Study of Treatment Patterns and Survival Outcome in Advanced Anaplastic Lymphoma Kinase-Positive Non-small-cell Lung Cancer. Oncol. Lett. 15, 8703–8710. doi:10.3892/ol.2018.8444
Johnson, T. W., Richardson, P. F., Bailey, S., Brooun, A., Burke, B. J., Collins, M. R., et al. (2014). Discovery of (10R)-7-Amino-12-Fluoro-2,10,16-Trimethyl-15-Oxo-10,15,16,17-Tetrahydro-2h-8,4-(metheno)pyrazolo[4,3-H][2,5,11]-Benzoxadiazacyclotetradecine-3-Carbonitrile (PF-06463922), a Macrocyclic Inhibitor of Anaplastic Lymphoma Kinase (ALK) and C-Ros Oncogene 1 (ROS1) with Preclinical Brain Exposure and Broad-Spectrum Potency against ALK-Resistant Mutations. J. Med. Chem. 57, 4720–4744. doi:10.1021/jm500261q
Jung, K. J., Jeon, C., and Jee, S. H. (2016). Smoking Effect on Lung Cancer: Ethnic Difference and Smoking Paradox. Epidemiol. Health 38, e2016060. doi:10.4178/epih.e2016060
Kron, A., Alidousty, C., Scheffler, M., Merkelbach-Bruse, S., Seidel, D., Riedel, R., et al. (2018). Impact of TP53 Mutation Status on Systemic Treatment Outcome in ALK-Rearranged Non-small-cell Lung Cancer. Ann. Oncol. 29, 2068–2075. doi:10.1093/annonc/mdy333
Kuang, S., and Leighl, N. B. (2021). Lorlatinib in ALK-Rearranged Lung Cancer. Cancer Cell 39, 25–27. doi:10.1016/j.ccell.2020.12.017
Le Calvez, F., Mukeria, A., Hunt, J. D., Kelm, O., Hung, R. J., Tanière, P., et al. (2005). TP53 and KRAS Mutation Load and Types in Lung Cancers in Relation to Tobacco Smoke: Distinct Patterns in Never, Former, and Current Smokers. Cancer Res. 65, 5076–5083. doi:10.1158/0008-5472.can-05-0551
Li, X., Huang, C., Xie, X., Wu, Z., Tian, X., Wu, Y., et al. (2020). The Impact of Smoking Status on the Progression-free Survival of Non-small Cell Lung Cancer Patients Receiving Molecularly Target Therapy or Immunotherapy versus Chemotherapy: A Meta-Analysis. J. Clin. Pharm. Ther. 46, 256–266. doi:10.1111/jcpt.13309
Li, Y., Xiao, X., Han, Y., Gorlova, O., Qian, D., Leighl, N., et al. (2017). Genome-wide Interaction Study of Smoking Behavior and Non-small Cell Lung Cancer Risk in Caucasian Population. Carcinogenesis 39, 336–346. doi:10.1093/carcin/bgx113
Lin, L., Zhao, J., Hu, J., Zou, G., Huang, F., Han, J., et al. (2018). Current Smoking Has a Detrimental Effect on Survival for Epidermal Growth Factor Receptor (EGFR) and Anaplastic Lymphoma Kinase (ALK) Negative Advanced Non-squamous Non-small Cell Lung Cancer (NSCLC) Patients Treated with Pemetrexed Continuation Maintenance. J. Cancer 9, 2140–2146. doi:10.7150/jca.24872
Loeb, L. A., Ernster, V. L., Warner, K. E., Abbotts, J., and Laszlo, J. (1984). Smoking and Lung Cancer: an Overview. Cancer Res. 44, 5940
Mok, T., Camidge, D. R., Gadgeel, S. M., Rosell, R., Dziadziuszko, R., Kim, D. W., et al. (2020). Updated Overall Survival and Final Progression-free Survival Data for Patients with Treatment-Naive Advanced ALK-Positive Non-small-cell Lung Cancer in the ALEX Study. Ann. Oncol. 31, 1056–1064. doi:10.1016/j.annonc.2020.04.478
Nakagawa, K., Hida, T., Nokihara, H., Morise, M., Azuma, K., Kim, Y. H., et al. (2020). Final Progression-free Survival Results from the J-ALEX Study of Alectinib versus Crizotinib in ALK-Positive Non-small-cell Lung Cancer. Lung Cancer 139, 195–199. doi:10.1016/j.lungcan.2019.11.025
Neupane, B., Richer, D., Bonner, A. J., Kibret, T., and Beyene, J. (2014). Network Meta-Analysis Using R: A Review of Currently Available Automated Packages. PLoS ONE 9, e115065. doi:10.1371/journal.pone.0115065
Nie, R. C., Duan, J. L., Liang, Y., Chen, X. J., Chen, Y. M., Luo, T. Q., et al. (2020). Smoking Status-Based Efficacy Difference in Anti-PD-1/pd-L1 Immunotherapy: a Systematic Review and Meta-Analysis. Immunotherapy 12, 1313–1324. doi:10.2217/imt-2020-0007
Oravecz, Z., and Muth, C. (2017). Fitting Growth Curve Models in the Bayesian Framework. Psychon. Bull. Rev. 25, 235–255. doi:10.3758/s13423-017-1281-0
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 Statement: an Updated Guideline for Reporting Systematic Reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Puhan, M. A., Schünemann, H. J., Murad, M. H., Li, T., Brignardello-Petersen, R., Singh, J. A., et al. (2014). A GRADE Working Group Approach for Rating the Quality of Treatment Effect Estimates from Network Meta-Analysis. BMJ 349, g5630. doi:10.1136/bmj.g5630
Salanti, G., Ades, A. E., and Ioannidis, J. P. (2011). Graphical Methods and Numerical Summaries for Presenting Results from Multiple-Treatment Meta-Analysis: an Overview and Tutorial. J. Clin. Epidemiol. 64, 163–171. doi:10.1016/j.jclinepi.2010.03.016
Seto, T., Kiura, K., Nishio, M., Nakagawa, K., Maemondo, M., Inoue, A., et al. (2013). CH5424802 (RO5424802) for Patients with ALK-Rearranged Advanced Non-small-cell Lung Cancer (AF-001JP Study): a Single-Arm, Open-Label, Phase 1-2 Study. Lancet Oncol. 14, 590–598. doi:10.1016/s1470-2045(13)70142-6
Shaw, A. T., Bauer, T. M., de Marinis, F., Felip, E., Goto, Y., Liu, G., et al. (2020). First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N. Engl. J. Med. 383, 2018–2029. doi:10.1056/nejmoa2027187
Shaw, A. T., and Engelman, J. A. (2013). ALK in Lung Cancer: Past, Present, and Future. J. Clin. Oncol. 31, 1105–1111. doi:10.1200/jco.2012.44.5353
Singal, G., Miller, P. G., Agarwala, V., Li, G., Kaushik, G., Backenroth, D., et al. (2019). Association of Patient Characteristics and Tumor Genomics with Clinical Outcomes Among Patients with Non-small Cell Lung Cancer Using a Clinicogenomic Database. JAMA 321, 1391–1399. doi:10.1001/jama.2019.3241
Solomon, B. J., Besse, B., Bauer, T. M., Felip, E., Soo, R. A., Camidge, D. R., et al. (2018). Lorlatinib in Patients with ALK-Positive Non-small-cell Lung Cancer: Results from a Global Phase 2 Study. Lancet Oncol. 19, 1654–1667. doi:10.1016/s1470-2045(18)30649-1
Solomon, B. J., Mok, T., Kim, D.-W., Wu, Y.-L., Nakagawa, K., Mekhail, T., et al. (2014). First-Line Crizotinib versus Chemotherapy in ALK-Positive Lung Cancer. N. Engl. J. Med. 371, 2167–2177. doi:10.1056/nejmoa1408440
Soria, J. C., Tan, D. S. W., Chiari, R., Wu, Y. L., Paz-Ares, L., Wolf, J., et al. (2017). First-line Ceritinib versus Platinum-Based Chemotherapy in Advanced ALK-Rearranged Non-small-cell Lung Cancer (ASCEND-4): a Randomised, Open-Label, Phase 3 Study. Lancet 389, 917–929. doi:10.1016/s0140-6736(17)30123-x
Thai, A. A., Solomon, B. J., Sequist, L. V., Gainor, J. F., and Heist, R. S. (2021). Lung Cancer. The Lancet 398, 535–554. doi:10.1016/s0140-6736(21)00312-3
Tonin, F. S., Rotta, I., Mendes, A. M., and Pontarolo, R. (2017). Network Meta-Analysis: a Technique to Gather Evidence from Direct and Indirect Comparisons. Pharm. Pract. (Granada) 15, 943. doi:10.18549/pharmpract.2017.01.943
Wang, L., Sheng, Z., Zhang, J., Song, J., Teng, L., Liu, L., et al. (2021). Comparison of Lorlatinib, Alectinib and Brigatinib in ALK Inhibitor-Naive/untreated ALK-Positive Advanced Non-small-cell Lung Cancer: a Systematic Review and Network Meta-Analysis. J. Chemother. 34, 1–10. doi:10.1080/1120009x.2021.1937782
Wang, Y., Ji, M., Zhu, M., Fan, J., Xie, J., Huang, Y., et al. (2021). Genome-wide Gene-Smoking Interaction Study Identified Novel Susceptibility Loci for Non-small Cell Lung Cancer in Chinese Populations. Carcinogenesis 42, 1154–1161. doi:10.1093/carcin/bgab064
Wu, H. Y., Huang, J. W., Lin, H. J., Liao, W. C., Peng, Y. S., Hung, K. Y., et al. (2013). Comparative Effectiveness of Renin-Angiotensin System Blockers and Other Antihypertensive Drugs in Patients with Diabetes: Systematic Review and Bayesian Network Meta-Analysis. BMJ 347, f6008. doi:10.1136/bmj.f6008
Wu, Y. L., Lu, S., Lu, Y., Zhou, J., Shi, Y. K., Sriuranpong, V., et al. (2018). Results of PROFILE 1029, a Phase III Comparison of First-Line Crizotinib versus Chemotherapy in East Asian Patients with ALK-Positive Advanced Non-small Cell Lung Cancer. J. Thorac. Oncol. 13, 1539–1548. doi:10.1016/j.jtho.2018.06.012
Xiao, J., Zhou, L., He, B., and Chen, Q. (2020). Impact of Sex and Smoking on the Efficacy of EGFR-TKIs in Terms of Overall Survival in Non-small-cell Lung Cancer: A Meta-Analysis. Front. Oncol. 10, 1531. doi:10.3389/fonc.2020.01531
Yoda, S., Lin, J. J., Lawrence, M. S., Burke, B. J., Friboulet, L., Langenbucher, A., et al. (2018). Sequential ALK Inhibitors Can Select for Lorlatinib-Resistant Compound ALK Mutations in ALK-Positive Lung Cancer. Cancer Discov. 8, 714–729. doi:10.1158/2159-8290.cd-17-1256
Zhao, W., Jiang, W., Wang, H., He, J., Su, C., and Yu, Q. (2021). Impact of Smoking History on Response to Immunotherapy in Non-small-cell Lung Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 11, 703143. doi:10.3389/fonc.2021.703143
Zhou, C., Kim, S. W., Reungwetwattana, T., Zhou, J., Zhang, Y., He, J., et al. (2019). Alectinib versus Crizotinib in Untreated Asian Patients with Anaplastic Lymphoma Kinase-Positive Non-small-cell Lung Cancer (ALESIA): a Randomised Phase 3 Study. Lancet Respir. Med. 7, 437–446. doi:10.1016/s2213-2600(19)30053-0
Keywords: non-small cell lung cancer, anaplastic lymphoma kinase, tyrosine kinase inhibitors, smoking, progression-free survival, network meta-analysis
Citation: Lin K, Lin J, Huang Z, Fu J, Yi Q, Cai J, Khan M, Yuan Y and Bu J (2022) Impact of Smoking on Response to the First-Line Treatment of Advanced ALK-Positive Non-Small Cell Lung Cancer: A Bayesian Network Meta-Analysis. Front. Pharmacol. 13:881493. doi: 10.3389/fphar.2022.881493
Received: 22 February 2022; Accepted: 08 April 2022;
Published: 11 May 2022.
Edited by:
Jian Zhang, Southern Medical University, ChinaReviewed by:
Umberto Malapelle, University of Naples Federico II, ItalyCopyright © 2022 Lin, Lin, Huang, Fu, Yi, Cai, Khan, Yuan and Bu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junguo Bu, YnVqZ0BnZDJoLm9yZy5jbg==; Yawei Yuan, eXVhbnlhd2VpQGd6aG11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.