- 1Obstetrics Gynecology Department, Faculty of Medicine, University of Indonesia, Cipto Mangunkusumo National General Hospital, Oncology Gynecology Division, Jakarta, Indonesia
- 2Department of Pharmacology and Therapeutics, Faculty of Medicine, University of Indonesia, Jakarta, Indonesia
- 3Department of Pathology and Anatomy, Faculty of Medicine, University of Indonesia, Cipto Mangunkusumo National General Hospital, Jakarta, Indonesia
- 4Community Medicine Department, Faculty of Medicine, University of Indonesia, Jakarta, Indonesia
Background: Early detection and treatment of cervical intraepithelial neoplasia (CIN) through a “see and treat” approach is a pillar of cervical cancer prevention programs in developing countries such as Indonesia. One of the major challenges faced is the limited N2O or CO2 gas supply for cryotherapy. Thus, an alternative therapeutic method such as trichloroacetic acid (TCA) topical application is needed as an alternative solution. The effectiveness of this therapy will depend on its destructive effect on eliminating the whole lesion in CIN.
Objective: To estimate the extent of damage in the normal cervical tissue after a single topical application of 85% TCA solution.
Design and Methods: This research was an intervention study carried out by applying ±5 ml of 85% TCA solution into the cervix of 40 patients scheduled for total hysterectomy for indications other than cervical pathology 24 h before surgery. The extent of tissue destruction was determined microscopically using histopathological specimens. The study protocol is registered at www.clinicaltrial.gov (ID NCT04911075).
Results: In the final analysis, 39 subjects were included. The necrotic area was detected at the superficial layer, accompanied by the full epithelial erosion thickness. In addition, there were also fibrotic areas resembling burned tissue in the stroma. The mean depth of destruction was 1.16 ± 0.01 mm in the anterior lip and 1.01 ± 0.06 mm in the posterior lip. There was no significant depth difference between the anterior and posterior lips (p ≥0.05). Moreover, the 85% TCA topical application was tolerable, as represented by the fact that the vast majority (82.1%) of participants experienced pain with a visual analog scale score of <4.
Conclusion: Single dose of TCA 85% in topical solution was able to destroy the normal cervical tissue with a deeper mean depth than the mean depth of CIN III in squamous epithelium.
Introduction
Cervical cancer is becoming the fourth most common cancer, with a high mortality rate for women globally (Bray et al., 2018). In developing countries, most of the cases are detected in the late stage of the disease (Anggraeni et al., 2006-2010; Ministry of Health, 2017; WHO. Cancer, 2018) even though the natural history of cancer development would take approximately 20 years (Woodman et al., 2007; Insinga et al., 2009; Chan et al., 2019). Thus, early detection and treatment of precancerous lesions will become crucial pillars for cervical cancer prevention (WHO, 2013; World Health Organization, 2013; Adhanom Ghebreyesus, 2019). Cervical intraepithelial neoplasia (CIN) is a cervical precancerous lesion that can histologically be found in one of three stages of development (I, II, or III). The higher the degree of CIN, the deeper the precancerous lesions will be found in the epithelial lining of the cervix (Campion and Canfell, 2015). Walker et al. (2003) had identified the mean depth of CIN III lesions, which was 0.32 ± 0.70 mm. Therefore, the effectiveness of the treatment of a precancerous lesion will be determined by its ability to create tissue damage beyond the depth of the CIN III lesion (Boonstra et al., 1990). Cryotherapy using the double freeze technique is effectively reaching the desired therapeutic effect for CIN lesions (Mariategui et al., 2008; Adepiti et al., 2016). Nevertheless, limited gas supply becomes a problem for the broader application of this method (Paul et al., 2013; Direktorat Pencegahan dan Pengendalian Penyakit Tidak Menular, 2018; Budiman et al., 2019). Hence, an alternative strategy is required to substitute the limited use of cryotherapy in developing countries (WHO, 2012; WHO, 2019).
Trichloroacetic acid (TCA) is an acetic acid analog that is characterized by a transparent red crystalline solid and soluble in water with excellent solubility and stability (PubChem. Trichloroacetic acid, 2020). TCA solution can be stored in amber glass bottles and be kept in the refrigerator for up to 23 weeks (Spinowitz and Rumsfield, 1989). Currently, the potential toxicity of topical TCA in humans is considered at low risk (Brody et al., 2000; Wiley et al., 2002; Yanofsky et al., 2012; Ayres, 1962; Resnik, 1984; Patel et al., 2017). Nonetheless, a minor burning sensation and ulceration were observed in the patient treated with 85% TCA solution (Taner et al., 2007). The principle of TCA therapy is the destruction of the epidermis and dermis due to necrosis accompanied by re-epithelialization and stimulation of new collagen formation. The intensity of the necrosis depends on the concentration used. The higher the concentration, the more rapid and profound the tissue destruction (Rakic et al., 2000; Yonei et al., 2007; Kimura et al., 2011; Brodland et al., 1989; Brodland et al., 1989; Rakic et al., 2000; Yonei et al., 2007; Kimura et al., 2011). Nevertheless, the evidence for the efficacy of 85% TCA in cases of cervical precancerous lesions has not been widely studied (Geisler et al., 2016; Suwartono, 2019). Furthermore, no studies have been found to analyze the depth of destruction levels of 85% TCA solution on normal cervical tissue.
Materials and Methods
Design, Location, and Time
The experimental research design had received approval from the Research Ethics Committee of the FKUI/RSCM Jakarta, Indonesia. The implementation time was from January to October 2021.
Population and Sample
The target population of the study was all patients who would undergo a total elective hysterectomy at the Obstetrics and Gynecology Department of the Faculty of Medicine/RSCM, which was not associated with a diagnosis of cervical pathology, whether benign, precancerous, or invasive carcinoma. The sample size is set at 40 people, which is obtained from the following formula:
Notes:
1) N: minimum sample size for each treatment group
2) Z: coefficient for confidence level α = 5% (1.96)
3) SD: standard deviation of examination results 0.64 (Adepiti et al. (2016), projections from single freeze cryotherapy
4) d: absolute error 5% of the average depth of 4.2 mm (0.21 mm)
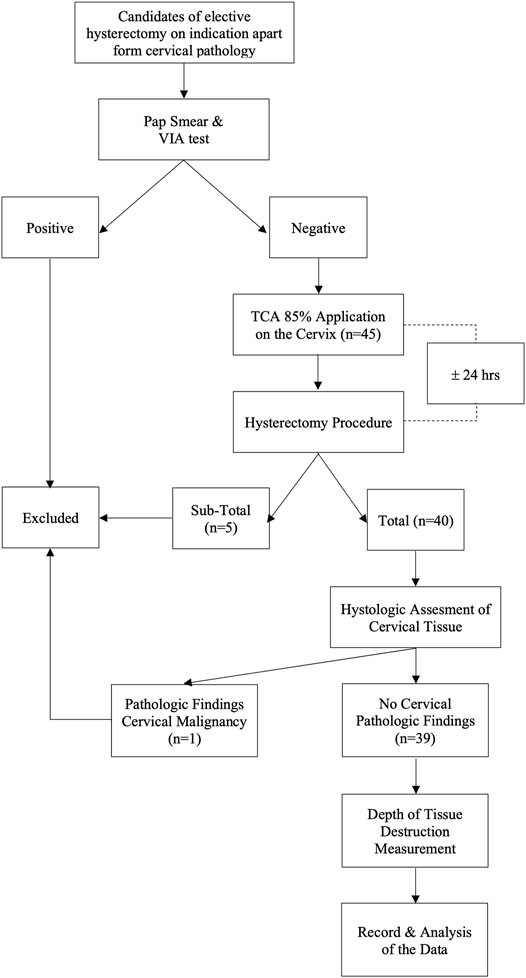
Inclusion criteria were normal cervix determined from clinical, radiological examinations, and negative visual inspection with acetic acid (VIA) test results. We consider the use of the VIA test to determine a normal cervix to be equivalent to the HPV test and pap-smear. According to a previous study, in subjects with a negative VIA test result, the chance of HPV infection was only 3.21 percent (Utami, 2016). VIA test has also been shown to be as effective as cytological examination for detecting cervical pre-cancerous lesions, with a sensitivity level of 90% and specificity of 92.2 percent. (Sankaranarayanan et al., 1998). Meanwhile, the participants who underwent a subtotal hysterectomy procedure or in whom cervical pathology was detected at the histological evaluation were excluded from the study. The flow chart of this study is depicted in Figure 1.
Intervention
The 85% TCA solution in this study was made according to the standard guideline from the Pharmacology Laboratory of the Faculty of Medicine/RSCM by using the weight-in-volume method (Harmon et al, 2006). Tools and materials utilized for making the solutions were previously sterilized using an autoclave. The 85% TCA solution was prepared by dissolving 85 g of pure crystal TCA (EMSURE® Merc®) in 100 ml of pure distilled water and stirring until homogenous. The solutions were freshly prepared before the subjects underwent the procedure. Solutions that were not used within seven days were discarded and replaced with new solutions. Twenty-four hours before the surgery, ± 5 ml of 85% TCA solution was applied to the cervix using a cotton swab by one investigator (DNL). The smear was made thinly into the ectocervical area, and the transformation zone appeared after a light pressing for 1–3 min until a white appearance occurred, indicating the precipitate of protein denaturation. For the endocervical canal, the tip of the stalk of a small cotton swab was dipped in the solution and inserted through the external uterine ostium (Figure 2).
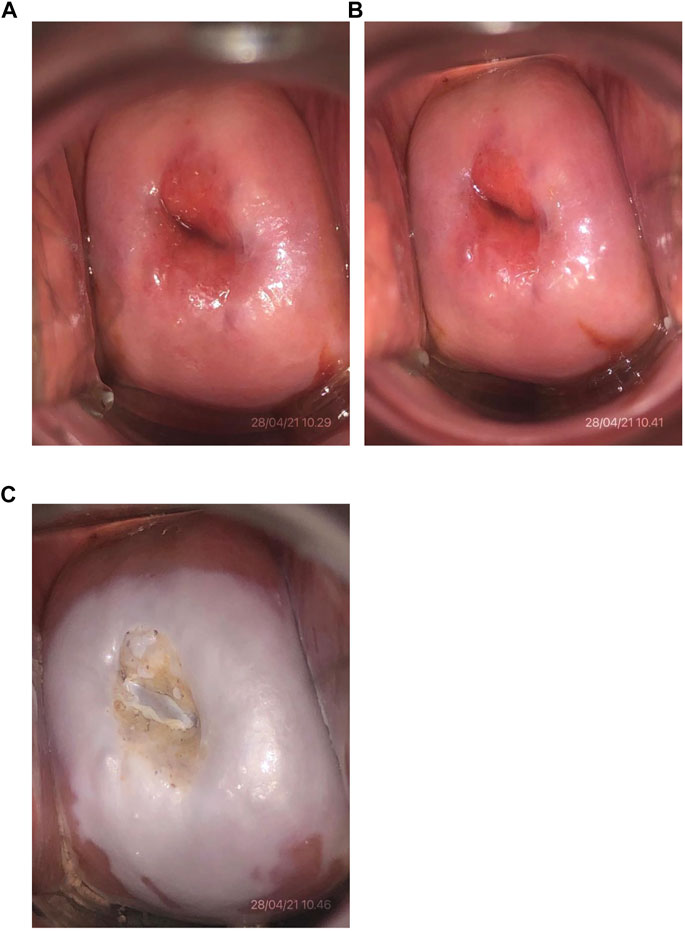
FIGURE 2. Cervical photo after 85% TCA application. (A) Initial cervical condition of one of the subjects (No. 4). (B) Condition after visual inspection with acetic acid test with a negative result. (C) Condition after application of 85% TCA solution.
Data Collection
Hysterectomy was performed 24 h after TCA application. A pathologist excised the cervix from the uterus and cut at the 12 and 6 o’clock positions to separate the left and right lips. Both pieces of tissue were fixed in formalin for 24 h. Multiple sections consisting of anterior and posterior division were obtained from each lip. The microscopic evaluation was focused on the measurement of the depth of necrosis with 40× magnification. The slides were then analyzed using ImageJ® (www.imagej.nih.gov) image processing software. The criterion to determine the deepest level of necrosis was based on the destruction of the glandular epithelium in the gland crypt in the stroma or the destruction of the endothelium of the stromal blood vessels (Mariategui et al., 2008; Adepiti et al., 2016). The final result of the measurement was corrected by adding 20% of the value to compensate for shrinkage from preanalytic tissue processing (Boonstra et al., 1990; Walker et al., 2003). This study protocol is registered at www.clinicaltrials.gov ID NCT04911075.
Data Analysis
The statistical analysis was performed using SPSS version 26. The data were presented as mean with a standard error of the margin (SEM). The difference between groups was analyzed using a two-tailed t-test. Correlation among the variables was assessed via the point biserial correlation test. Statistical significance was considered with p <0.05.
Results
Subject Characteristics
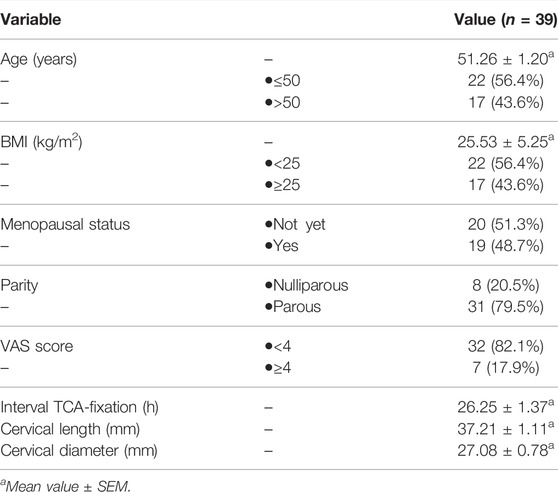
Thirty-nine subjects were included in the final analysis. One subject was excluded because a cancerous lesion was observed in the cervical stroma. Table 1 depicts the baseline characteristics. The mean age was 51.3 ± 1.2 years. Overall, 43.6% of the subjects had a body mass index (BMI) above the normal range. Over half (51.3%) of the subjects were still menstruating, and the majority (79.5%) had a history of childbirth. Macroscopically, the average cervical length was 37.2 ± 1.1 mm with a diameter of 27.1 ± 0.8 mm. Only 17.9% of the subjects complained of pain with a visual analog scale (VAS) score of ≥4 at the time of application. The time interval between TCA exposure and tissue fixation was 26.3 ± 1.4 h.
Microscopic Appearance of Tissue Destruction
Extensive areas of necrotizing lesions in the superficial layer were detected, accompanied by erosion of the full thickness of the epithelium in several places. Some histological samples found a fibrotic area of stroma resembling a burn wound under the superficial necrosis layer. Moreover, the burn-like tissue was bordered by the normal cervical stroma massively infiltrated with inflammatory cells (Figure 3).
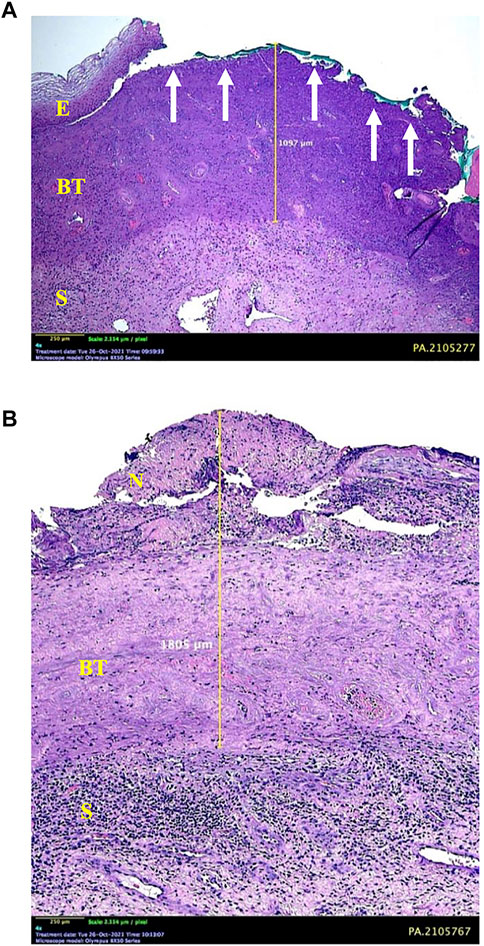
FIGURE 3. Microscopic appearance of cervical tissue destruction after 85% TCA application. (A) Some areas show erosion of the entire thickness of the epithelium (E) (arrows) with underlying burn tissue to a depth of 1.097 mm. (B) Superficial layer of necrosis (N), which continues with a burn-tissue–like layer into the stroma (S). A boundary between the layer of burn tissue and normal tissue accompanied by a massive infiltration of inflammatory cells is observed at the bottom. This is the limit for measuring depth, which is 1.805 mm.
Tissue Destruction Depth Analysis
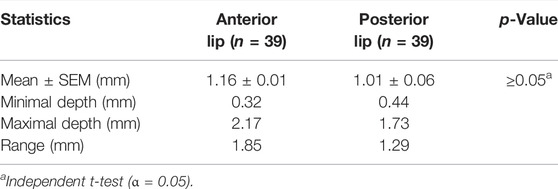
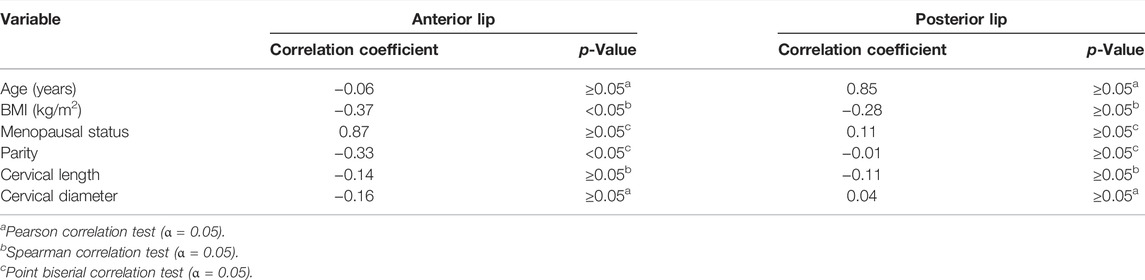
Table 2 and Figure 4 show the analyses of the depth of tissue destruction for all subjects and their description of data, respectively. There was no significant difference between the average depth of anterior and posterior lip tissue destruction (1.16 ± 0.01 vs. 1.01 ± 0.06 mm; p ≥0.05). The subgroup analysis also found a comparable average in age group, menopausal status, and parity status (p ≥0.05). Meanwhile, the difference was significant only in the anterior lip (1.30 ± 0.08 vs. 0.97 ± 0.08 mm; p <0.05), adjusted by the BMI (Table 3). Correlative analyses among the factors showed that age, menopausal status, length, and diameter of the cervix were not correlated to the depth of necrosis, both in the anterior and posterior lips (p ≥0.05). Conversely, BMI and parity status only showed a moderate negative correlation to the depth of the anterior cervical lip (p <0.05). (Table 4).
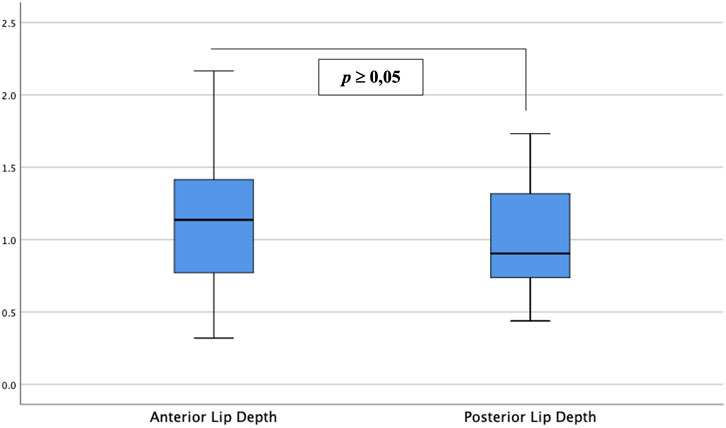
FIGURE 4. Depth of anterior and posterior cervical lip tissue destruction. Between the two groups, there was no significant difference in depth of tissue destruction between anterior and posterior cervical lips (p ≥0.05).
Discussion
To the best of our knowledge, this is the first study that analyzed the depth of necrosis in normal cervical tissue after a single topical application of 85% TCA solution. The pathophysiology of tissue destruction as the effect of TCA on epithelial tissue has been described in previous studies. This solution can cause protein denaturation, leading to cell necrosis accompanying an inflammatory reaction. A study by Rakic et al. (2000) showed that TCA was cytotoxic for keratinocytes and depressed protein–collagen synthesis and the expression of matrix metalloproteinases (MMPs) production of human dermal fibroblasts. In human skin, according to Yonei et al. (2007), platelet-derived growth factors subunit B (PDGF-B) mRNA expression became significantly upregulated after TCA application and then immediately downregulated. Immunoreactive PDGF-B in the cytoplasm of keratinocytes became detectable throughout the epidermis after TCA application, reached a maximum after the peak of mRNA expression, and then declined significantly over 24 h when the epidermis became completely necrotic. Kimura et al. (2011) stated that after TCA treatment, transient upregulation of proopiomelanocortin (POMC) and melanocortin receptor 1 (MC1R) mRNA expressions were observed in human skin. These results suggest that TCA activates the skin stress response system by inducing POMC and MC1R productions in keratinocytes. In this study, histologically, the necrotic area was detected in the superficial layer, accompanied by the full thickness of the epithelium erosion. In addition, there were areas of fibrotic stroma resembling burned tissue surrounding the normal stromal tissue with massive inflammatory cells. This appearance will undergo necrosis later (Sankaranarayanan et al., 1998; Harmon et al., 2006; Leopold et al., 2012).
The detailed measurement of the extent of necrotic tissue was analyzed. The depth of necrosis after application of 85% TCA was (1.16 ± 0.01 mm) in the anterior lip of the cervix and (1.01 ± 0.06 mm) in the posterior, respectively. Yonei et al. (2007) described that the cytotoxic effect of TCA was closely associated with the concentration of the solution. Another study further confirmed these data that showed the average depth destruction level of 80% TCA solution was 0.98 ± 0.17 mm (Brodland et al., 1989). In this study, the higher TCA concentration (85%) may achieve deeper necrosis in the normal tissue. Compared with the previous study (Walker et al., 2003), the deeper destruction induced by 85% TCA may reach beyond the average depth of CIN I (0.30 ± 0.93 mm), CIN II (0.36 ± 0.91), and even CIN III (0.32 ± 0.70 mm).
The comparable mean depth between anterior and posterior lip necrosis was also important. There was a discrepancy in the necrotic depth among anterior and posterior lips in the previous study. This fact could represent the noninferiority of TCA therapy compared with cryotherapy (Mariategui et al., 2008; Adepiti et al., 2016). In fact, several studies have also shown that the topographic positions in the cervix may influence the CIN lesions (Pastar et al., 2014). Of note, most severe lesions were found at the 8 and 7 o’clock positions, either in low- or high-grade lesions. In a similar manner, Zhao et al. (2015) identified that quadrants 2 and 3, specifically in the 4 and 7 o’clock positions, were susceptible to CIN on the cervix. CIN will be found more frequently on the posterior lip than on the anterior lip of the cervix. Thus, a therapeutic modality that can provide an equal depth effect between the anterior and posterior lips will be beneficial.
The potential of using an 85% TCA solution for therapy is also supported by the data that most of the subjects (82.1%) only experienced pain with a VAS score of less than four. Similar to the previous study, the main complaint of topical TCA application was mild pain or burning sensation. (Wiley et al., 2002; Yanofsky et al., 2012). However, no significant side effects were found in this study.
Several factors were thought to affect the depth of penetration level of TCA into cervical tissue, which was analyzed as an additional outcome in this study. The clinical characteristics of the subjects, particularly age or menopausal status, may influence the destruction level, although the results of previous studies were controversial. According to the study conducted by Mariategui et al. (2008), the cervical necrotic depth in the younger subjects was shallower due to the sufficient vascularity in the cervical area, thus making them resistant to hypoxic conditions. Conversely, the necrotic area may become shallower in the elderly or postmenopausal subjects due to increased tight junctional resistance. This factor becomes a major determinant for epithelial permeability that maintains the integrity and organization of cells through the action mechanism of various proteins, such as claudins (Blaskewicz et al., 2011; He et al., 2012; Zhao et al., 2015). Nevertheless, in this study, no association was found between age or menopausal status with the mean depth of tissue destruction or cervical physical dimensions, including length and diameter.
BMI and parity status may affect the extent of the destruction. Previous studies had shown that BMI and parity status might influence ovarian hormone levels, especially estrogen. Overweight and nulliparity are risk factors for higher estrogen levels (Barrett et al., 2014, Espetvedt Finstad et al., 2009, Lee et al., 2005). Gorodeski et al. (2000) stated that changing estrogen levels partly affected the transcervical paracellular permeability. This phenomenon, in turn, may have an influence on TCA’s penetration depth in cervical tissue. In this study, BMI and parity status were negatively correlated with the depth of necrosis. Nonetheless, the strength of the relationship that occurred is not so convinced and only occurred on the anterior lip. Based on these results, we conclude that the subject’s clinical and physical characteristics of their cervix did not significantly affect the depth of TCA’s tissue destruction in this study.
The strength of this study lies in the method of examining the depth of cervical tissue destruction, which is carried out microscopically through tissue histologic analysis. The data on the depth of tissue destruction from this study can be used as the evidence base literature for the application of 85% TCA in cervical precancer cases found through a “see and treat” approach as an alternative to cryotherapy. However, the limitation of this study was the insufficiency of samples number for performing subgroup analysis parametrically. A study with a larger number of samples is needed for achieving a better understanding of TCA’s depth penetration correlating factors.
Conclusion
The preliminary data on the destruction levels of a single topical application of 85% TCA in the normal cervical tissue can be obtained through this study, which is 1.16 ± 0.01 mm in the anterior lip and 1.01 ± 0.06 mm in the posterior, respectively. These results are deeper than the reference of CIN III mean depth in the cervical squamous epithelium. Based on the results of this pilot study, it is interesting to design a future study to determine the depth of tissue destruction through the application of a single dose of 85% TCA in cases of cervical pre-cancerous lesions (CIN).
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee Faculty of Medicine, Universitas Indonesia. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Conceptualization–LN, SP, and GP; investigation–DL; resources–WA and PR; formal analysis–AK; validation–AP and TU; writing–WA, DL, and AB.
Funding
Female Cancer Program Faculty of Medicine, University of Indonesia, Jakarta.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Andre Silalahi for helping in the data collection process, Lidya Kencana and colleagues (Pathology Anatomy Laboratorium/FKUI-RSCM) for helping in sample processing, and the Staff of Pharmacology and Therapeutic Laboratorium/FKUI-RSCM for providing the experimental solution.
References
Adepiti, A., Ajenifuja, O., Fadahunsi, O., Osasan, S., Pelemo, O., and Loto, M. (2016). Comparison of the Depth of Tissue Necrosis between Double-Freeze and Single-Freeze Nitrous Oxide-Based Cryotherapy. Niger. Med. J. 57(1), 1. doi:10.4103/0300-1652.180561
Adhanom Ghebreyesus, T. (2019). Draft: Global Strategy towards Eliminating Cervical Cancer as a Public Health Problem. World Health Organ. Available at: https://www.who.int/docs/default-source/cervical-cancer/cerv-cancer-elimn-strategy-16dec-12pm.pdf. (Accessed February 9, 2020).
Anggraeni, T. D., Nuranna, L., Sobur, C. S., Rahardja, F., Hia, C. W., Utami, T. W., et al. (2006-2010). Distribution of Age, Stage, and Histopathology of Cervical Cancer: A Retrospective Study on Patients at Dr. Cipto Mangunkusumo Hospital, Jakarta, Indonesia. Obstet. Gynecol. 35 (1), 4.
Ayres, S. (1962). Superficial Chemosurgery in Treating Aging Skin. Arch. Dermatol. 85 (3), 385. doi:10.1001/archderm.1962.01590030083011
Barrett, E. S., Parlett, L. E., Windham, G. C., and Swan, S. H. (2014). Differences in Ovarian Hormones in Relation to Parity and Time since Last Birth. Fertil. Steril 101, 1773–e1. doi:10.1016/j.fertnstert.2014.02.047
Blaskewicz, C. D, Pudney, J., and Anderson, D. J. (2011). Structure and Function of Intercellular Junctions in Human Cervical and Vaginal Mucosal Epithelia1. Biol. Reprod. 85(1), 97–104. doi:10.1095/biolreprod.110.090423
Boonstra, H., Aalders, J. G., Koudstaal, J., Oosterhuis, J. W., and Janssens, J. (1990). Minimum Extension and Appropriate Topographic Position of Tissue Destruction for Treatment of Cervical Intraepithelial Neoplasia. Obstet. Gynecol. 75 (2), 227–231.
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 68 (6), 394–424. doi:10.3322/caac.21492
Brodland, D. G., Cullimore, K. C., Roenigk, R. K., and Gibson, L. E. (1989). Depths of Chemexfoliation Induced by Various Concentrations and Application Techniques of Trichloroacetic Acid in a Porcine Model. J. Dermatol. Surg. Oncol. [Internet]. 15(9), 967–971. doi:10.1111/j.1524-4725.1989.tb03183.x
Brody, H. J, Monheit, G. D., Resnik, S. S., and Alt, T. H. (2000). A History of Chemical Peeling: Dermatol. Surg. 26(5), 405–409. Available at: http://Insights.ovid.com/crossref?an=00042728-200005000-00001. (Accessed February 9, 2020)
Budiman, B., Budi Harsono, A., and Mulyana Hidayat, Y. (2019). Evaluasi Program Deteksi Dini Kanker Serviks dengan Metode See and Treat di Kabupaten Karawang. Indones J. Obstet. Ginecol Sci. doi:10.24198/obgynia.v2n1.77
Campion, M. J., and Canfell, K. (2015). “Cervical Cancer Screening and Preinvasive Disease,” in Berek & Hacker’s Gynecologic Oncology. Sixth. (Philadelphia: Wolters Kluwer), 242–325.
Chan, C. K, Aimagambetova, G., Ukybassova, T., Kongrtay, K., and Azizan, A. (2019). Human Papillomavirus Infection and Cervical Cancer: Epidemiology, Screening, and Vaccination—Review of Current Perspectives. J. Oncol. 2019, 1–11. doi:10.1155/2019/3257939
Direktorat Pencegahan dan Pengendalian Penyakit Tidak Menular (2018). Laporan Kinerja 2018. Jakarta: Kementerian Kesehatan Republik Indonesia. Available at: http://p2ptm.kemkes.go.id/uploads/VHcrbkVobjRzUDN3UCs4eUJ0dVBndz09/2019/07/Laporan_Kinerja_2018.pdf.
Espetvedt Finstad, S., Emaus, A., Potischman, N., Barrett, E., Furberg, A-S., Ellison, P. T., et al. (2009). Influence of Birth Weight and Adult Body Composition on 17β-Estradiol Levels in Young Women. Cancer Causes Control. 20(2), 233–42. doi:10.1007/s10552-008-9238-2
Geisler, S., Speiser, S., Speiser, L., Heinze, G., Rosenthal, A., and Speiser, P. (2016). Short-Term Efficacy of Trichloroacetic Acid in the Treatment of Cervical Intraepithelial Neoplasia. Obstet. Gynecol. 127(2), 353–359. doi:10.1097/aog.0000000000001244
Gorodeski, G. I. (2000). Effects of Menopause and Estrogen on Cervical Epithelial Permeability. J. Clin. Endocrinol. Metab. 85 (7), 12. doi:10.1210/jc.85.7.2584
Harmon, C. B., Hadley, M., and Tristani, P. (2006). “Trichloroacetic Acid,” in Color Atlas of Chemical Peels (BerlinHeidelberg: Springer). doi:10.1007/3-540-30223-97
He, G., Li, H., Lin, H., Bian, M., Wang, Y., Sun, A., et al. (2012). Topographical Distribution Pattern of Cervical Intraepithelial Neoplasia across the Cervix. J. Int. Med. Res. 40(5):1897–1903. doi:10.1177/030006051204000530
Insinga, R. P., Dasbach, E. J., and Elbasha, E. H. (2009). Epidemiologic Natural History and Clinical Management of Human Papillomavirus (HPV) Disease: a Critical and Systematic Review of the Literature in the Development of an HPV Dynamic Transmission Model. BMC Infect. Dis. 9 (1), 119. doi:10.1186/1471-2334-9-119
Kimura, A., Kanazawa, N., Li, H-J., Yonei, N., Yamamoto, Y., and Furukawa, F. (2011). Influence of Trichloroacetic Acid Peeling on the Skin Stress Response System: Influence of TCA Peeling on the SSRS. J. Dermatol. 38(8), 740–747. doi:10.1111/j.1600-0625.2012.01495
Lee, J-W., Lee, S-J., Seo, J., Song, S. Y., Ahn, G, Park, C-S., et al. (2005). Increased Expressions of Claudin-1 and Claudin-7 during the Progression of Cervical Neoplasia. Gynecol. Oncol. 97(1), 53–59. doi:10.1016/j.ygyno.2004.11.058
Leopold, P. L., Vincent, J., and Wang, H. (2012). A Comparison of Epithelial-To-Mesenchymal Transition and Re-epithelialization. Semin. Cancer Biol. 22(5–6), 471–483. doi:10.1016/j.semcancer.2012.07.003
Mariategui, J., Santos, C., Taxa, L., Jeronimo, J., and Castle, P. E. (2008). Comparison of Depth of Necrosis Achieved by CO 2 - and N 2 O-Cryotherapy. Int. J. Gynecol. Obstet. 100(1), 24–26. doi:10.1016/j.ijgo.2007.07.009
Ministry of Health (2017). Republic of Indonesia. Indonesia Health Profile 2017 [Internet]. Jakarta; 2018. Available at: https://www.depkes.go.id/resources/download/pusdatin/profil-kesehatan-indonesia/indonesia-health-profile-2017.pdf. (Accessed August 2, 2020).
Pastar, I., Stojadinovic, O., Yin, N. C., Ramirez, H., Nusbaum, A. G., Sawaya, A., et al. (2014). Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care. 3(7), 445–464. doi:10.1089/wound.2013.0473
Patel, V. M., Schwartz, R. A., and Lambert, W. C. (2017). Topical Antiviral and Antifungal Medications in Pregnancy: a Review of Safety Profiles. J. Eur. Acad. Dermatol. Venereol. 31(9), 1440–1446. doi:10.1111/jdv.14297
Paul, P., Winkler, J. L., Bartolini, R. M., Penny, M. E, Huong, T. T., Nga, L. T., et al. (2013). Screen-and-Treat Approach to Cervical Cancer Prevention Using Visual Inspection with Acetic Acid and Cryotherapy: Experiences, Perceptions, and Beliefs from Demonstration Projects in Peru, Uganda, and Vietnam. The Oncologist. 18(12), 1278–1284. doi:10.1634/theoncologist.2013-0253
PubChem. Trichloroacetic acid (2020). [Internet]. National Library of Medicine National Center for Biotechnology Information. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Trichloroacetic-acid
Rakic, L., ChM, L., and Nusgens, B. V. (2000). Comparative Caustic and Biological Activity of Trichloroacetic and Glycolic Acids on Keratinocytes and Fibroblasts In Vitro. Skin Pharmacol. Physiol. 13(1), 52–59. doi:10.1159/000029908
Resnik, S. S. (1984). Chemical Peeling with Trichloroacetic Acid. J. Dermatol. Surg. Oncol. 10 (7), 549–550. doi:10.1111/j.1524-4725.1984.tb01250.x
Sankaranarayanan, R., Wesley, R., Somanathan, T., Dhakad, N., Shyamalakumary, B., Amma, N. S., et al. (1998). Visual Inspection of the Uterine Cervix after the Application of Acetic Acid in the Detection of Cervical Carcinoma and its Precursors, Cancer. 83.2150–6.
Spinowitz, A. L., and Rumsfield, J. (1989). Stability-Time Profile of Trichloroacetic Acid at Various Concentrations and Storage Conditions. J. Dermatol. Surg. Oncol. [Internet]. 15(9), 974–975. doi:10.1111/j.1524-4725.1989.tb03184.x
Suwartono, H. (2019). Efikasi Penggunaan Trichloroacetic Acid (TCA) Sebagai Terapi Pada IVA Positif Dibandingkan Dengan Cryotherapy [Quasi-Experimental]. [Jakarta]: Universitas Indonesia.
Taner, Z. M., Taskiran, C., Onan, A. M., Gursoy, R., and Himmetoglu, O. (2007). Therapeutic Value of Trichloroacetic Acid in the Treatment of Isolated Genital Warts on the External Female Genitalia. J. Reprod. Med. 52 (6), 521–525.
Utami, T. W. (2016). Prevalence of High-Risk Human Papillomavirus (HPV) Among Negative Visual Inspection of Acetic Acid (VIA). Indones J Obstet Gynecol [Internet]. Available at:http://inajog.com/index.php/journal/article/view/398. (Accessed March 29, 2022).
Walker, D. C., Brown, B. H., Blackett, A. D., Tidy, J., and Smallwood, R. H. (2003). A Study of the Morphological Parameters of Cervical Squamous Epithelium. Physiol Meas. 16, 121–35. doi:10.1088/0967-3334/24/1/309
Watts, A. M. I., Tyler, M. P. H., Perry, M. E., Roberts, A. H. N., and McGrouther, D. A. (2001). Burn Depth and its Histological Measurement. Burns [Internet]Mar27(2), 154–160. doi:10.1016/s0305-4179(00)00079-6
WHO. Cancer. (2018). Today - Cervical Cancer [Internet]. GLOBOCAN. Available at: http://gco.iarc.fr/today/data/factsheets/cancers/23-Cervix-uteri-fact-sheet.pdf.
WHO, (2019). WHO Guidelines for the Use of thermal Ablation for Cervical Pre-cancer Lesions. Jeneva: World Health Organization. Available at: https://apps.who.int/iris/rest/bitstreams/1256797/retrieve. (Accessed February 9, 2020).
WHO, (2013). Guidance Note: Comprehensive Cervical Cancer Prevention and Control: A Healthier Future for Girls and Women. Geneva, Switzerland: World Health Organization.
WHO, (2012). Technical Specifications Cryosurgical [Internet]. Jeneva: World Health Organization. Available from: https://apps.who.int/iris/bitstream/handle/10665/75853/9789241504560_eng.pdf?sequence=1&isAllowed=y.(Accessed, February 9, 2020).
Wiley, D. J., Douglas, J., Beutner, K., Cox, T., Fife, K., Moscicki, A., et al. (2002). External Genital Warts: Diagnosis, Treatment, and Prevention. Clin. Infect. Dis. 35(s2), S210–S224. doi:10.1086/342109
Woodman, C. B. J., Collins, S. I., and Young, L. S. (2007). The Natural History of Cervical HPV Infection: Unresolved Issues. Nat Rev Cancer. 12, 11–22. doi:10.1038/nrc2050
World Health Organization (Editor) (2013). WHO Guidelines for Screening and Treatment of Precancerous Lesions for Cervical Cancer Prevention (Geneva, Switzerland: World Health Organization), 40.
Yanofsky, V. R., Patel, R. V., and Goldenberg, G. (2012). Genital Warts: a Comprehensive Review. J. Clin. Aesthet. Dermatol. 5 (6), 25–36.
Yonei, N., Kanazawa, N., Ohtani, T., Furukawa, F., and Yamamoto, Y. (2007). Induction of PDGF-B in TCA-Treated Epidermal Keratinocytes. Arch. Dermatol. Res. 299 (9), 433–440. doi:10.1007/s00403-007-0781-6
Keywords: trichloroacetic acid, cervical intraepithelial neoplasia (CIN), topical application, tissue destruction, treatment
Citation: Nuranna L, Lubis DN, Arozal W, Purbadi S, Barinda AJ, Purwoto G, Rustamadji P, Putra AD, Utami TW and Kekalih A (2022) Pilot Study on the Effect of a Single Topical Application of Trichloroacetic Acid 85% on Normal Cervical Tissue. Front. Pharmacol. 13:880333. doi: 10.3389/fphar.2022.880333
Received: 21 February 2022; Accepted: 14 April 2022;
Published: 20 May 2022.
Edited by:
Catherine M T Sherwin, Wright State University, United StatesReviewed by:
Ahmed Mohamed Maged, Cairo University, EgyptErbil Karaman, Yüzüncü Yıl University, Turkey
Arnold Kruse, Isala Women and Children’s Hospital, Netherlands
Copyright © 2022 Nuranna, Lubis, Arozal, Purbadi, Barinda, Purwoto, Rustamadji, Putra, Utami and Kekalih. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wawaimuli Arozal, d2F3YWltdWxpQGdtYWlsLmNvbQ==
 Laila Nuranna1
Laila Nuranna1 Dolly N. Lubis
Dolly N. Lubis Wawaimuli Arozal
Wawaimuli Arozal Agian Jeffilano Barinda
Agian Jeffilano Barinda