- 1College of Pharmacy, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, MB, Canada
- 2Department of Psychiatry, Max Rady College of Medicine, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, MB, Canada
- 3Pharmaceutical, Laboratory and Blood Services Division, Ministry of Health, New Westminster, BC, Canada
Introduction: Second-generation long-acting injectable antipsychotics (SG-LAIAs) may improve outcomes compared to other antipsychotics. Real-world studies using linked administrative databases play an important role in assessing the comparative effectiveness of antipsychotic medications.
Methods: We used a prevalent new-user design in a population-based cohort of antipsychotic users with diagnosis of a psychotic disorder to compare the primary outcome of treatment failure, defined as psychiatric hospitalization, completed suicide, incarceration, or treatment discontinuation. Additional outcomes were all-cause mortality. SG-LAIA users were matched on a 1:1 basis with other antipsychotic users based on the time-conditional propensity score, calendar time, and prior antipsychotic exposure.
Results: The use of LAIAs was not associated with a lower risk of treatment failure than other antipsychotics (adjusted hazard ratio 1.07 and 95% confidence interval 0.98–1.15) but did reduce all-cause mortality (adjusted hazard ratio 0.69 and 95% confidence interval 0.48–0.99). Monotherapy with LAIAs was superior to other antipsychotic monotherapy (adjusted hazard ratio for treatment failure 0.83 and 95% confidence interval 0.78–0.89), and LAIAs were superior to other antipsychotics in antipsychotic-naïve users (adjusted hazard ratio for treatment failure 0.57 and 95% confidence interval 0.47–0.70).
Conclusion: In this population-based cohort, SG-LAIAs reduced the risk of treatment failure in incident new users but not in prevalent new users.
Introduction
Long-acting injectable antipsychotics (LAIAs) have an established role for patients who require long-term antipsychotic treatment and are at risk of poor adherence; LAIAs improve adherence and persistence to antipsychotic treatment, which subsequently reduces the risk of relapse (Correll et al., 2016; Pilon et al., 2017; Greene et al., 2018). Clinical practice guidelines for the treatment of schizophrenia recommend that all patients should be presented with LAIAs as a treatment option (Remington et al., 2017; American Psychiatric Association, 2021). The availability of long-acting injectable formulations of second-generation antipsychotics may improve patient acceptance of LAIAs, but their increased cost and formulary restrictions in some jurisdictions may be a barrier to the widespread use of second-generation long-acting injectable antipsychotics (Kane et al., 2019).
Observational study designs or pragmatic trials may be preferred over randomized controlled trials in studies of long-acting injectable antipsychotic effectiveness as they are more inclusive of patients with histories of non-adherence to treatment and multiple comorbidities (Alphs et al., 2014; Tiihonen et al., 2017). Randomized clinical trials have produced conflicting results, with some showing a reduced risk of relapse and treatment failure with LAIAs (Alphs et al., 2015; Subotnik et al., 2015), while others found no significant difference compared to oral antipsychotics (Kishimoto et al., 2014; Buckley et al., 2016). Improved clinical outcomes resulting from greater adherence to LAIAs may be obscured in controlled trials where adherence to assigned treatment is closely monitored (Correll et al., 2016). Observational studies also have limitations, most notably persistent confounding by unmeasured variables. Well-conducted observational studies can mitigate the risk of confounding with the incident user, active comparator designs, the use of propensity scores, and adjusting for measured covariates (Ray, 2003; Stürmer et al., 2014; Lund, Richardson and Sturmer, 2015). However, incident user designs limit sample size, particularly in the case of SG-LAIAs, where most new users have switched from an alternate antipsychotic. Prevalent new-user designs allow for the comparison of “switchers” to a newly marketed medication without restricting to treatment-naïve users (Suissa, Moodie and Dell’Aniello, 2017; Filion et al., 2020).
Many studies evaluate antipsychotic effectiveness in terms of treatment failure, treatment discontinuation, and hospitalization, but other outcomes may also be meaningful in this patient population. Population-based studies have established that patients with psychotic disorders are at increased risk of criminal justice system involvement (Khalifeh et al., 2015; Dean et al., 2018; Sariaslan et al., 2020). Antipsychotics may prevent reoffending in individuals with a history of incarceration (Fazel et al., 2014; Alphs et al., 2015; Chang et al., 2016; Rezansoff et al., 2017), but the literature on the role of antipsychotics in reducing crime in individuals without a history of justice system involvement is lacking. In the present study, we have used a prevalent new-user design in a population-based cohort of antipsychotic users to evaluate the risk of treatment failure, a composite endpoint of psychiatric hospitalization, completed suicide, incarceration, and treatment discontinuation, in SG-LAIA users versus oral antipsychotic users.
Materials and Methods
Data Source
We used the Manitoba Population Research Data Repository, a collection of administrative health, education, social, justice, and registry databases, housed at the Manitoba Centre for Health Policy in Manitoba, Canada, to form a cohort of second-generation long-acting injectable antipsychotic (SG-LAIA) users (Suissa, Moodi,e and Dell’Aniello, 2017; Smith et al., 2018). The repository captures all prescriptions dispensed in the province of Manitoba, Canada, excluding in-hospital pharmaceuticals, and has been validated for SG-LAIAs (Janzen et al., 2022). We linked prescription claims to hospital discharge abstracts, medical service claims, prosecutions, vital statistics, and insurance registry data by a scrambled personal identification number. This study received ethics approval from the University of Manitoba Health Research Ethics Board under the project number HS20380 (H2016:468), the Manitoba Centre for Health Policy, the Health Information Privacy Committee, and Manitoba Justice.
Cohort Selection and Exposure Definition
We formed a base cohort of all individuals who were dispensed antipsychotic medication on the first date. An SG-LAIA was dispensed in Manitoba between 14 February 2005 and 31 March 2020 and had
The cohort members were included in a monotherapy subgroup if they were dispensed only one antipsychotic medication on t0. Subjects in the monotherapy subgroup were censored upon the dispensation of an antipsychotic other than the incident antipsychotic.
Propensity Score Matching
Within each exposure set, we determined the propensity for initiation of SG-LAIAs at t0. For comparators, a time-conditional propensity score was calculated at each antipsychotic dispensation date in an exposure set. Covariates included in the propensity score were sex, age, income quintile, number of prior year medication classes dispensed, number of prior year hospitalizations, number of prior year physician visits, time since psychotic disorder diagnosis (defined as earliest of first antipsychotic dispensation or first hospitalization or medical claim with a diagnosis of psychotic disorder), prior year dispensation of psychotropic medication, psychiatric diagnoses in the previous 3 years, being accused of a crime in the previous 3 years, being a victim of a crime in the previous 3 years, and the calendar year of t0. Exposure sets were excluded if the propensity score of the SG-LAIA new user was outside the range of propensity scores of comparators in the exposure set. SG-LAIA new users were matched on the basis of 1:1 with replacement with the comparator in the exposure set with the nearest time-conditional propensity score. Matching was performed in the chronological order, starting with the subject with the earliest t0.
Outcome Definition
The cohort members were followed from t0 to the occurrence of the outcome, death, emigration from Manitoba, or 31 March 2020. In addition, comparators were censored if they received SG-LAIA dispensation. The primary outcome was treatment failure, defined as psychiatric hospitalization (including hospitalization for a mood/anxiety disorder, substance use disorder, psychotic disorder, schizophrenia, or attempted suicide), incarceration, suicide (the primary cause of death being self-inflicted injury or poisoning or poisoning of undetermined intent), or treatment discontinuation (defined as a gap in prescription dispensations greater than 90 days). Additional outcomes included all-cause mortality and individual components of the composite primary outcome. We also conducted a subgroup analysis of prevalent and incident new users and restricted to subjects exposed to antipsychotic monotherapy only during the follow-up. Detailed definitions of outcomes are provided in Supplementary Table S1.
Statistical Analysis
We used descriptive statistics to evaluate cohort characteristics. We determined standardized differences to assess the covariate balance between exposure groups before and after matching. Outcomes were analyzed using a Cox proportional hazards regression model stratified by matched pair, adjusting for age, sex, time since psychotic disorder diagnosis, decile of the time-conditional propensity score, prior year hospital admissions, history of being accused of a crime, and diagnosis of personality disorder, substance use disorder, or mood/anxiety disorder. A robust sandwich variance estimate was included in the Cox model to account for matching with replacement. In addition, we used a modified Cox model to perform adjustments for the time-varying use of antipsychotic polypharmacy during the follow-up. All analyses were conducted in SAS® 9.4 (SAS Institute; Cary, NC).
Sensitivity Analysis
We repeated analyses in cohort members who had received a diagnosis of schizophrenia (ICD-9-CM code 295 or ICD-10-CA code F20) in the 3 years prior to t0. We also conducted a post hoc sensitivity analysis including prior antipsychotics in the propensity score to evaluate the impact of baseline imbalance in prior antipsychotic medication.
Results
Description of the Cohort
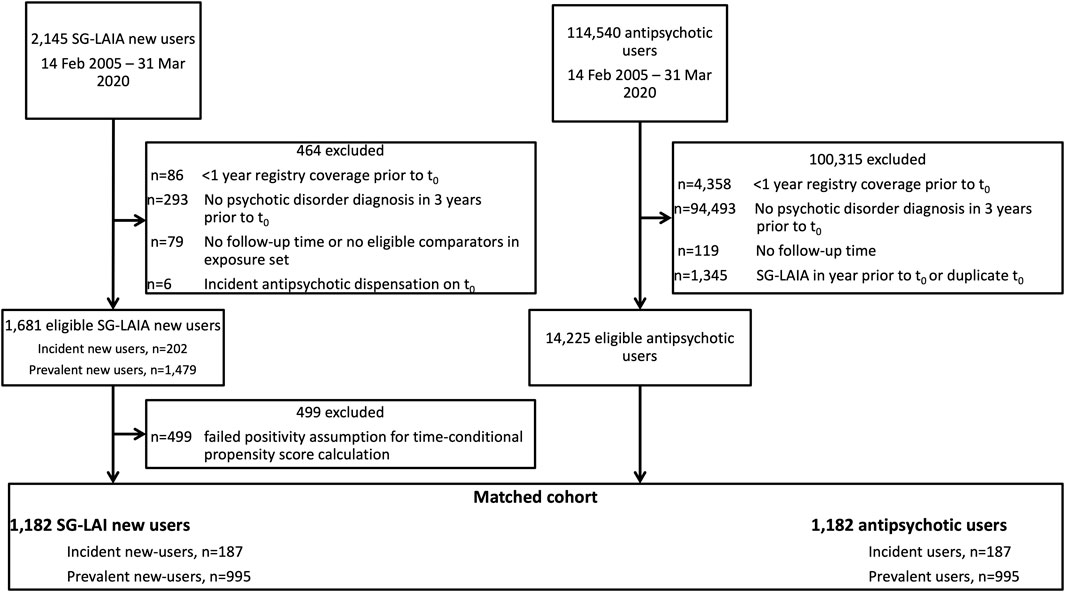
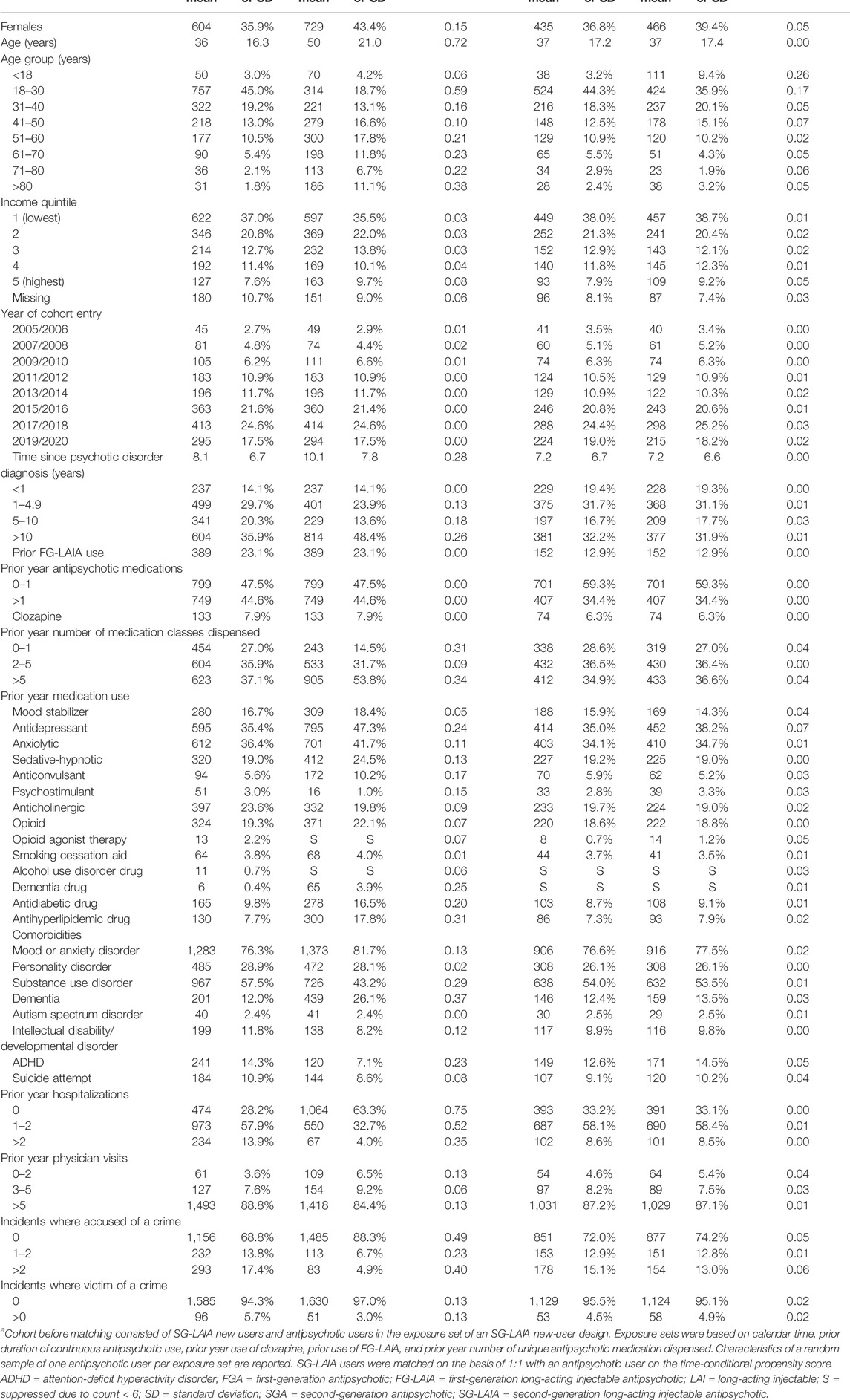
We identified 1,681 SG-LAIA new users and 14,225 antipsychotic user comparators eligible for matching. The final matched cohort included 1,182 matched pairs, with 187 in the incident new user cohort and 995 in the prevalent new-user cohort (Figure 1). The majority of SG-LAIA new users received risperidone-LAI (49.7%) on t0, followed by paliperidone-LAI (38.5%) and aripiprazole-LAI (11.8%). Among matched comparators, 86.9% received an oral SGA and 5.8% received an FG-LAIA on t0 (Supplementary Table S2). Baseline characteristics were well-balanced after matching, with standardized differences of less than 0.1 for all variables except for age groups less than 18 years and 18–30 years (Table 1, Supplementary Table S3). We adjusted Cox models for age to account for this imbalance.
The mean follow-up time for the primary outcome of treatment failure was 1.3 (SD 1.9) years for a total of 3,170 person years. In the SG-LAIA cohort, 913 experienced treatment failure in 1,512 person years of observation time for a crude incidence rate of 60.4 per 100 person years. Among matched comparators, there were 804 treatment failure events in 1,658 person years for a crude incidence rate of 48.5 per 100 person years. During 2,119 person years of observed monotherapy, there were 345 events in 653 person years of SG-LAIA monotherapy (crude incidence rate = 52.8 per 100 person years) and 704 events in 1,466 person years of monotherapy in the matched comparators (crude incidence rate = 48.0 per 100 person years). Baseline characteristics of the monotherapy subgroup are found in Supplementary Tables S4, S5.
Results of Cox Models
The SG-LAIA use was not associated with a reduced risk of treatment failure compared to the matched antipsychotic users (adjusted hazard ratio 1.07 and 95% confidence interval 0.98–1.15) (Table 2). However, the risk of treatment failure was reduced during SG-LAIA monotherapy compared to matched antipsychotic monotherapy (adjusted hazard ratio 0.83 and 95% confidence interval 0.78–0.89) (Table 3). The SG-LAIA use had no impact on the risk of incarceration (adjusted hazard ratio 0.97 and 95% confidence interval 0.76–1.25) or treatment discontinuation (adjusted hazard ratio 1.00 and 95% confidence interval 0.91–1.09) but increased the risk of psychiatric hospitalization (adjusted hazard ratio 1.38 and 95% confidence interval 1.23–1.54). A small number of suicides were observed during the follow-up, so results are not reported. In addition, the SG-LAIA use reduced the risk of all-cause mortality in the overall cohort (adjusted hazard ratio 0.69 and 95% confidence interval 0.48–0.99) and during monotherapy (adjusted hazard ratio 0.10 and 95% confidence interval 0.02–0.44).
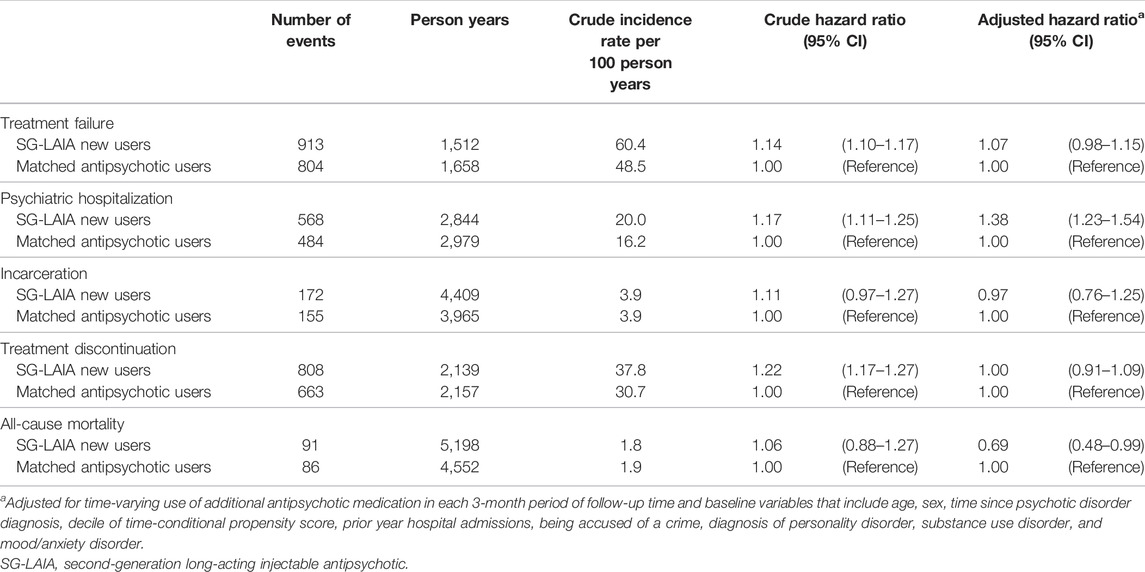
TABLE 2. Association between second-generation long-acting injectable antipsychotics versus oral antipsychotics and treatment failure, psychiatric hospitalization, incarceration, treatment discontinuation and all-cause mortality.
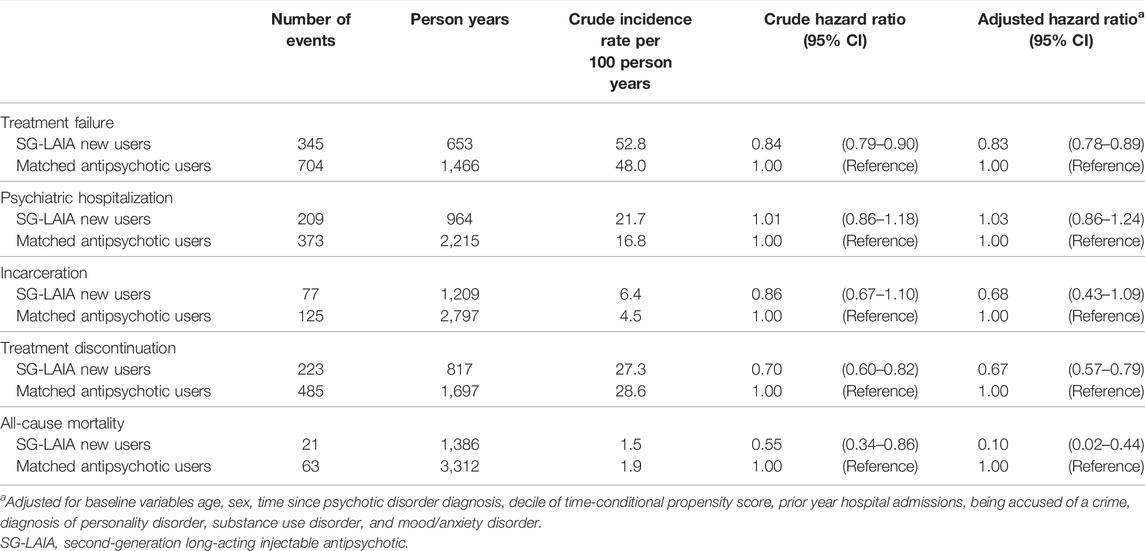
TABLE 3. Association between second-generation long-acting injectable antipsychotic monotherapy versus oral antipsychotic monotherapy and treatment failure, psychiatric hospitalization, incarceration, treatment discontinuation, and all-cause mortality.
Subgroups
Notable differences were observed in prevalent new users compared with incident new users (Table 4). Prevalent new users of SG-LAIAs had an increased risk of treatment failure (adjusted hazard ratio 1.20 and 95% confidence interval 1.01–1.32), psychiatric hospitalization (adjusted hazard ratio 1.50 and 95% confidence interval 1.31–1.71), and treatment discontinuation (adjusted hazard ratio 1.13 and 95% confidence interval 1.03–1.25). In contrast, a reduced risk of treatment failure (adjusted hazard ratio 0.57 and 95% confidence interval 0.47–0.70), incarceration (adjusted hazard ratio 0.32 and 95% confidence interval 0.11–0.99), and treatment discontinuation (adjusted hazard ratio 0.52 and 95% confidence interval 0.40–0.66) was observed in incident new users.
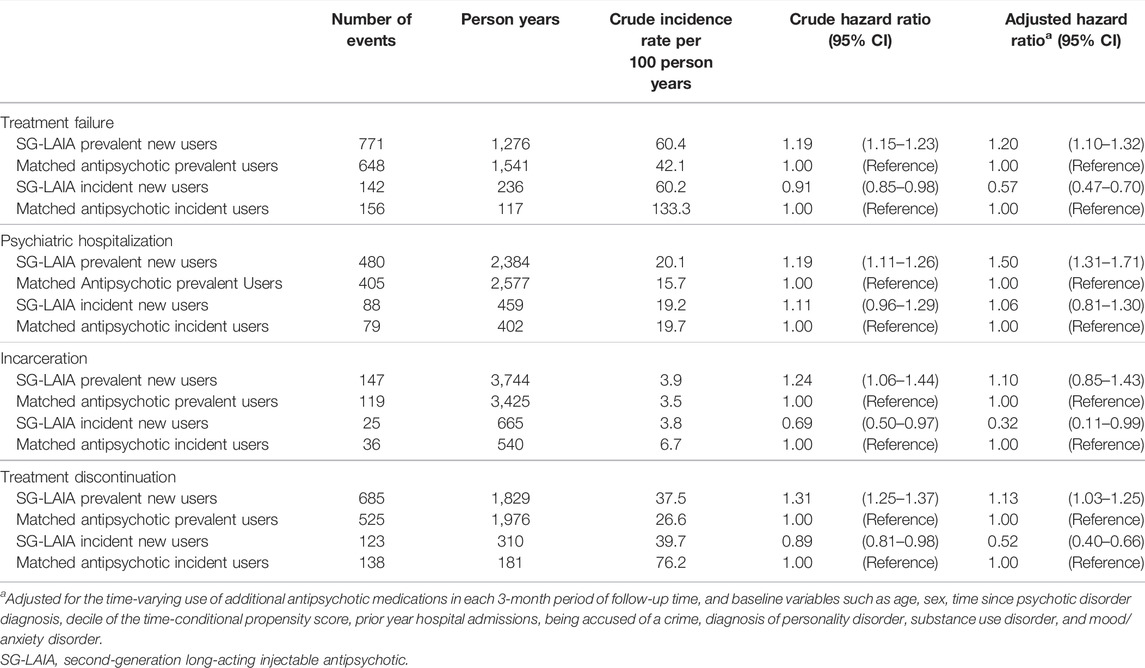
TABLE 4. Association between second-generation long-acting injectable antipsychotics versus oral antipsychotics and treatment failure, psychiatric hospitalization, incarceration, treatment discontinuation, and all-cause mortality in prevalent and incident new users.
Sensitivity Analysis
Results in the cohort of SG-LAIA new users who had a diagnosis of schizophrenia were similar, with a few notable exceptions (Supplementary Figures S1, S2). There was no observed reduction in all-cause mortality (adjusted hazard ratio 0.79 and 95% hazard ratio 0.55–1.12), and there was a reduced risk of psychiatric hospitalization during monotherapy (adjusted hazard ratio 0.81 and 95% confidence interval 0.72–0.92). Post hoc sensitivity analysis where a prior antipsychotic was included in the time-conditional propensity score improved the baseline balance in the number of subjects previously treated with quetiapine and aripiprazole, with a minimal change in hazard ratios (Supplementary Tables S6, S7).
Discussion
We used a prevalent new-user cohort design to evaluate the effectiveness of switching to an SG-LAIA compared with continuing an oral antipsychotic treatment regimen. In the overall cohort, we found the risk of treatment failure, incarceration, and treatment discontinuation was similar in SG-LAIA and oral antipsychotic users; the risk of psychiatric hospitalization was increased in SG-LAIA users, but the risk of all-cause mortality was decreased. Subsets of this population-based cohort benefitted from the SG-LAIA prescription, notably those receiving antipsychotic monotherapy and those who had no prior year of antipsychotic use, with the risk of treatment failure reduced by 17 and 43%, respectively. In contrast, prevalent antipsychotic users who switched to SG-LAIAs were found to have an increased risk of treatment failure, psychiatric hospitalization, and treatment discontinuation. Previous research has established that LAIAs have a greater benefit when used early in the course of the disease, but there is also evidence of effectiveness in prevalent antipsychotic users (Alphs et al., 2016; Tiihonen et al., 2017). Despite matching with the propensity score and a good balance of measured baseline variables including the duration of illness, prior antipsychotic exposure, and hospitalizations, we cannot rule out that this observation may be confounded. Patients switching to SG-LAIAs may not be comparable to those who were stabilized on a prior antipsychotic regimen. Crude hazard ratios shifted after adjusting for additional covariates, so the prevalent new-user design and propensity score matching were not sufficient to control confounding.
Other observational studies have found that the SG-LAIA use reduces the risk of treatment failure, treatment discontinuation, hospitalization, and mortality compared with oral antipsychotics (Tiihonen et al., 2011, 2017; Stip and Lachaine, 2018; Taipale et al., 2018; Song et al., 2019). Tiihonen et al. observed adjusted hazard ratios for the risk of treatment failure during monotherapy with paliperidone-LAI and risperidone-LAI of 0.80 and 0.72, respectively, compared with oral olanzapine monotherapy in a Swedish population-based cohort (Tiihonen et al., 2017). Taipale et al. also demonstrated an increased risk of mortality with oral antipsychotics and FG-LAIAs compared to SG-LAIAs in the same Swedish cohort (adjusted hazard ratio 1.51 for oral SGAs, 1.83 for oral FGAs, and 1.37 for FG-LAIAs) (Taipale et al., 2018). Despite the demonstrated benefits of LAIA treatment, event rates were considerable. Our study estimated crude incidence rates of approximately 60 treatment failure events, 25 psychiatric hospitalizations, and 38 treatment discontinuation events per 100 person years of SG-LAIA exposure. Similar or higher rates were observed in the Swedish cohort for treatment failure (IR 9.3 and 6.4 per 10 person years for paliperidone- and risperidone-LAI, respectively) and psychiatric hospitalization (IR 5.1 and 3.8 per 10 person years for paliperidone and risperidone LAI, respectively) (Tiihonen et al., 2017).
We observed a non-significant trend toward reduction in the risk of incarceration in the overall cohort and a remarkable 68% reduction in the risk of incarceration in incident new users of SG-LAIA. This finding is in line with previous research, including a pragmatic randomized trial that showed paliperidone-LAI reduced time to incarceration (Alphs et al., 2015) and a cohort study showing a 70% reduction in the risk of violent crimes during LAIA treatment (Fazel et al., 2014).
While observational designs of SG-LAIA effectiveness can be subject to unmeasured confounding, the direction of bias in this study is most likely in favor of an active comparator for a few reasons. First, LAIA users have been shown to have more severe diseases than patients who were not prescribed LAIAs (Kishimoto et al., 2018). Second, in Manitoba, SG-LAIA agents are reserved as second-line agents, for patients with evidence of non-adherence, treatment failure, or intolerance to another antipsychotic. Third, the increased frequency of contact between SG-LAIA users and healthcare providers introduces detection bias, as the need for hospitalization or treatment escalation is detected earlier in patients who are monitored more frequently. Thus, we are more confident in our results that show significant reductions in the risk of outcomes associated with SG-LAIA use than we are in those that show an increased risk.
This study has numerous strengths. By using a prevalent new-user design, we were able to increase our sample size by almost 1,000 patients. The data used from the Manitoba Population Research Data Repository have undergone a rigorous quality assessment, and we used established definitions to identify comorbidities and outcomes (Chartier et al., 2018; Smith et al., 2018). We had a 20-year study period with over 3,100 person years of observation time. We included incarceration as a reason for treatment failure and adjusted for having been accused of a crime. Finally, we have previously validated SG-LAIA exposure in prescription claim data (Janzen et al., 2022).
This study reinforces the evidence from previous work, suggesting LAIAs are superior to oral antipsychotics at the early stages of the disease and during monotherapy. We encourage clinicians to offer SG-LAIA treatment to all patients initiating antipsychotic therapy. However, it remains unclear whether there is a benefit to switching stable patients from oral antipsychotics to SG-LAIAs.
Conclusion
In this population-based cohort study, the SG-LAIA use was not associated with a reduced risk of treatment failure compared with other antipsychotics but did reduce mortality. Monotherapy with SG-LAIAs and the incident use of SG-LAIAs were associated with a reduced risk of treatment failure.
Data Availability Statement
The data analyzed in this study are subject to the following licenses/restrictions. Data used in this article were derived from administrative health and social data as a secondary use. The data were provided under specific data sharing agreements only for approved use at the Manitoba Centre for Health Policy (MCHP). The original source data are not owned by the researchers or MCHP and as such cannot be provided to a public repository. The original data source and approval for use have been noted in the acknowledgments of the article. If necessary, source data specific to this article or project may be reviewed at MCHP with the consent of the original data providers, along with the required privacy and ethical review bodies. Requests to access these datasets should be directed to the Manitoba Centre for Health Policy, https://umanitoba.ca/manitoba-centre-for-health-policy/data-repository.
Ethics Statement
The studies involving human participants were reviewed and approved by the University of Manitoba Health Research Ethics Board (project number HS20380/H2016:468). Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study, in accordance with the national legislation and the institutional requirements.
Author Contributions
DJ designed the study, conducted the literature review, analyzed the data, and wrote the first draft of the manuscript. CL and JB contributed to the study design and provided clinical expertise. IK and SA-S supervised all phases of the project. All authors contributed to and approved the final version of the manuscript.
Funding
This project was supported by the Evelyn Shapiro Award for Health Services Research to DJ, the Leslie F. Buggey Professorship in Pharmacy to SA-S and the University of Manitoba.
Acknowledgments
The authors acknowledge the Manitoba Centre for Health Policy for use of data contained in the Manitoba Population Research Data Repository under project #2017-016 (HIPC#2016/2017-64). The results and conclusions are those of the authors and no official endorsement by the Manitoba Centre for Health Policy, Manitoba Health, or other data providers is intended or should be inferred. Data used in this study are from the Manitoba Population Research Data Repository housed at the Manitoba Centre for Health Policy, University of Manitoba and were derived from data provided by Manitoba Health, Manitoba Vital Statistics and Manitoba Justice.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.879224/full#supplementary-material
References
Alphs, L., Benson, C., Cheshire-Kinney, K., Lindenmayer, J. P., Mao, L., Rodriguez, S. C., et al. (2015). Real-world Outcomes of Paliperidone Palmitate Compared to Daily Oral Antipsychotic Therapy in Schizophrenia: A Randomized, Open-Label, Review Board-Blinded 15-month Study. J. Clin. Psychiatry 76 (5), 554–561. doi:10.4088/JCP.14m09584
Alphs, L., Mao, L., Rodriguez, S. C., Hulihan, J., and Starr, H. L. (2014). Design and Rationale of the Paliperidone Palmitate Research in Demonstrating Effectiveness (PRIDE) Study: A Novel Comparative Trial of Once-Monthly Paliperidone Palmitate versus Daily Oral Antipsychotic Treatment for Delaying Time to Treatment Failure in Persons with Schizophrenia. J. Clin. Psychiatry 75 (12), 1388–1393. doi:10.4088/JCP.13m08965
Alphs, L., Nasrallah, H. A., Bossie, C. A., Fu, D. J., Gopal, S., Hough, D., et al. (2016). Factors Associated with Relapse in Schizophrenia Despite Adherence to Long-Acting Injectable Antipsychotic Therapy. Int. Clin. Psychopharmacol. 31 (4), 202–209. doi:10.1097/YIC.0000000000000125
American Psychiatric Association (2021). Practice Guideline for the Treatment of Patients with Schizophrenia. 3rd edn.. Washington, DC: American Psychiatric Association
Buckley, P. F., Schooler, N. R., Goff, D. C., Kopelowicz, A., Lauriello, J., Manschreck, T. C., et al. (2016). Comparison of Injectable and Oral Antipsychotics in Relapse Rates in a Pragmatic 30-month Schizophrenia Relapse Prevention Study. Psychiatr. Serv. 67 (12), 1370–1372. doi:10.1176/appi.ps.201500466
Chang, Z., Lichtenstein, P., Långström, N., Larsson, H., and Fazel, S. (2016). Association between Prescription of Major Psychotropic Medications and Violent Reoffending after Prison Release. JAMA 316 (17), 1798–1807. doi:10.1001/jama.2016.15380
Chartier, M., Bolton, J. M., Mota, N., MacWilliam, L., Ekuma, O., Nie, Y., et al. (2018). Mental Illness Among Adult Manitobans. Winnipeg, MB: Manitoba Centre for Health Policy
Correll, C. U., Citrome, L., Haddad, P. M., Lauriello, J., Olfson, M., Calloway, S. M., et al. (2016). The Use of Long-Acting Injectable Antipsychotics in Schizophrenia: Evaluating the Evidence. J. Clin. Psychiatry 77 (Suppl. 3), 1–24. doi:10.4088/jcp.15032su1
Dean, K., Laursen, T. M., Pedersen, C. B., Webb, R. T., Mortensen, P. B., and Agerbo, E. (2018). Risk of Being Subjected to Crime, Including Violent Crime, after Onset of Mental Illness: A Danish National Registry Study Using Police Data. JAMA Psychiatry 75 (7), 689–696. doi:10.1001/jamapsychiatry.2018.0534
Fazel, S., Zetterqvist, J., Larsson, H., Långström, N., and Lichtenstein, P. (2014). Antipsychotics, Mood Stabilisers, and Risk of Violent Crime. Lancet 384 (9949), 1206–1214. doi:10.1016/S0140-6736(14)60379-2
Filion, K. B., Lix, L. M., Yu, O. H., Dell'Aniello, S., Douros, A., Shah, B. R., et al. (2020). Sodium Glucose Cotransporter 2 Inhibitors and Risk of Major Adverse Cardiovascular Events: Multi-Database Retrospective Cohort Study. BMJ 370, m3342. doi:10.1136/bmj.m3342
Greene, M., Yan, T., Chang, E., Hartry, A., Touya, M., and Broder, M. S. (2018). Medication Adherence and Discontinuation of Long-Acting Injectable versus Oral Antipsychotics in Patients with Schizophrenia or Bipolar Disorder. J. Med. Econ. 21 (2), 127–134. doi:10.1080/13696998.2017.1379412
Janzen, D., Bolton, J. M., Leong, C., Kuo, I., Alessi-Severini, S., et al. (2022). Long-acting Injectable Antipsychotics in a Prescription Claims Data Source: a Validation Study. Drugs - Real World Outcomes.
Kane, J. M., Schooler, N. R., Marcy, P., Achtyes, E. D., Correll, C. U., Robinson, D. G., et al. (2019). Patients with Early-phase Schizophrenia Will Accept Treatment with Sustained-Release Medication (Long-acting Injectable Antipsychotics): Results from the Recruitment Phase of the PRELAPSE Trial. J. Clin. Psychiatry 80 (3), 22–18m12546. doi:10.4088/jcp.18m12546
Khalifeh, H., Johnson, S., Howard, L. M., Borschmann, R., Osborn, D., Dean, K., et al. (2015). Violent and Non-violent Crime against Adults with Severe Mental Illness. Br. J. Psychiatry 206 (4), 275–282. doi:10.1192/bjp.bp.114.147843
Kishimoto, T., Hagi, K., Nitta, M., Leucht, S., Olfson, M., Kane, J. M., et al. (2018). Effectiveness of Long-Acting Injectable vs Oral Antipsychotics in Patients with Schizophrenia: A Meta-Analysis of Prospective and Retrospective Cohort Studies. Schizophr Bull. 44 (3), 603–619. doi:10.1093/schbul/sbx090
Kishimoto, T., Robenzadeh, A., Leucht, C., Leucht, S., Watanabe, K., Mimura, M., et al. (2014). Long-acting Injectable vs Oral Antipsychotics for Relapse Prevention in Schizophrenia: a Meta-Analysis of Randomized Trials. Schizophr Bull. 40 (1), 192–213. doi:10.1093/schbul/sbs150
Lund, J. L., Richardson, D. B., and Stürmer, T. (2015). The Active Comparator, New User Study Design in Pharmacoepidemiology: Historical Foundations and Contemporary Application. Curr. Epidemiol. Rep. 2 (4), 221–228. doi:10.1007/s40471-015-0053-5
Pilon, D., Tandon, N., Lafeuille, M. H., Kamstra, R., Emond, B., Lefebvre, P., et al. (2017). Treatment Patterns, Health Care Resource Utilization, and Spending in Medicaid Beneficiaries Initiating Second-Generation Long-Acting Injectable Agents versus Oral Atypical Antipsychotics. Clin. Ther. 39 (10), 1972–e2. doi:10.1016/j.clinthera.2017.08.008
Ray, W. A. (2003). Evaluating Medication Effects outside of Clinical Trials: New-User Designs. Am. J. Epidemiol. 158 (9), 915–920. doi:10.1093/aje/kwg231
Remington, G., Addington, D., Honer, W., Ismail, Z., Raedler, T., and Teehan, M. (2017). Guidelines for the Pharmacotherapy of Schizophrenia in Adults. Can. J. Psychiatry 62 (9), 604–616. doi:10.1177/0706743717720448
Rezansoff, S. N., Moniruzzaman, A., Fazel, S., McCandless, L., and Somers, J. M. (2017). Adherence to Antipsychotic Medication and Criminal Recidivism in a Canadian Provincial Offender Population. Schizophr Bull. 43 (5), 1002–1010. doi:10.1093/schbul/sbx084
Sariaslan, A., Arseneault, L., Larsson, H., Lichtenstein, P., and Fazel, S. (2020). Risk of Subjection to Violence and Perpetration of Violence in Persons with Psychiatric Disorders in Sweden. JAMA Psychiatry 77 (4), 359–367. doi:10.1001/jamapsychiatry.2019.4275
Smith, M., Lix, L. M., Azimaee, M., Enns, J. E., Orr, J., Hong, S., et al. (2018). Assessing the Quality of Administrative Data for Research: A Framework from the Manitoba Centre for Health Policy. J. Am. Med. Inform. Assoc. 25 (3), 224–229. doi:10.1093/jamia/ocx078
Song, X., El Khoury, A. C., Brouillette, M., Smith, D., and Joshi, K. (2019). Treatment Discontinuation of Long-Acting Injectables or Oral Atypical Antipsychotics Among Medicaid Recipients with Schizophrenia. J. Med. Econ. 22 (11), 1105–1112. doi:10.1080/13696998.2019.1615927
Stip, E., and Lachaine, J. (2018). Real-world Effectiveness of Long-Acting Antipsychotic Treatments in a Nationwide Cohort of 3957 Patients with Schizophrenia, Schizoaffective Disorder and Other Diagnoses in Quebec. Ther. Adv. Psychopharmacol. 8 (11), 287–301. doi:10.1177/2045125318782694
Stürmer, T., Wyss, R., Glynn, R. J., and Brookhart, M. A. (2014). Propensity Scores for Confounder Adjustment when Assessing the Effects of Medical Interventions Using Nonexperimental Study Designs. J. Intern. Med. 275 (6), 570–580. doi:10.1111/joim.12197
Subotnik, K. L., Casaus, L. R., Ventura, J., Luo, J. S., Hellemann, G. S., Gretchen-Doorly, D., et al. (2015). Long-Acting Injectable Risperidone for Relapse Prevention and Control of Breakthrough Symptoms after a Recent First Episode of Schizophrenia. A Randomized Clinical Trial. JAMA Psychiatry 72 (8), 822–829. doi:10.1001/jamapsychiatry.2015.0270
Suissa, S., Moodie, E. E., and Dell'Aniello, S. (2017). Prevalent New-User Cohort Designs for Comparative Drug Effect Studies by Time-Conditional Propensity Scores. Pharmacoepidemiol. Drug Saf. 26 (4), 459–468. doi:10.1002/pds.4107
Taipale, H., Mittendorfer-Rutz, E., Alexanderson, K., Majak, M., Mehtälä, J., Hoti, F., et al. (2018). Antipsychotics and Mortality in a Nationwide Cohort of 29,823 Patients with Schizophrenia. Schizophrenia Res. 197, 274–280. doi:10.1016/j.schres.2017.12.010
Tiihonen, J., Haukka, J., Taylor, M., Haddad, P. M., Patel, M. X., and Korhonen, P. (2011). A Nationwide Cohort Study of Oral and Depot Antipsychotics after First Hospitalization for Schizophrenia. Am. J. Psychiatry 168 (6), 603–609. doi:10.1176/appi.ajp.2011.10081224
Keywords: antipsychotic treatment, long-acting injectable and oral antipsychotics, real-world data, comparative effectiveness, psychotic disorders
Citation: Janzen D, Bolton JM, Leong C, Kuo If and Alessi-Severini S (2022) Second-Generation Long-Acting Injectable Antipsychotics and the Risk of Treatment Failure in a Population-Based Cohort. Front. Pharmacol. 13:879224. doi: 10.3389/fphar.2022.879224
Received: 19 February 2022; Accepted: 11 April 2022;
Published: 19 May 2022.
Edited by:
Andrea Burden, ETH Zürich, SwitzerlandReviewed by:
Mary V. Seeman, University of Toronto, CanadaBo Ram Yang, Chungnam National University, South Korea
Copyright © 2022 Janzen, Bolton, Leong, Kuo and Alessi-Severini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Donica Janzen, amFuemVuZDZAbXl1bWFuaXRvYmEuY2E=
 Donica Janzen
Donica Janzen James M. Bolton2
James M. Bolton2 Christine Leong
Christine Leong Silvia Alessi-Severini
Silvia Alessi-Severini