- 1College of Pharmacy, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, MB, Canada
- 2Department of Epidemiology, University of Washington, Seattle, WA, United States
- 3Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Solna, Sweden
- 4College of Medicine, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, MB, Canada
- 5Department of Psychiatry, College of Medicine, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, MB, Canada
- 6Children’s Hospital Research Institute of Manitoba, Winnipeg, MB, Canada
Background: Evidence from developed countries demonstrates that the use of antiseizure medications (ASMs) has been increasing in the last decade. Pregnant women have a very challenging risk benefit trade-off in terms of ASM utilization, and it is crucial to know if increased utilization is seen among pregnant women.
Objective: To examine time-trends of utilization of ASM therapies among pregnant women in Manitoba, Canada.
Methods: We conducted a population-based cohort study using de-identified, linked administrative databases from Manitoba. Pregnancies between 1995 and 2018 were included. Four groups of pregnant people were created based on ASM exposure and epilepsy diagnosis.
Results: Of 273,492 pregnancies, 812 (3/1000) had epilepsy diagnosis and were exposed to ASMs, 963 (3.5/1000) had epilepsy diagnosis and were unexposed, and 2742 (10/1000) were exposed to ASMs and did not have epilepsy diagnosis. Overall, the number of pregnancies exposed to ASMs increased significantly from 0.56% in 1997 to 2.21% in 2018 (p < 0.0001). Subgroup analysis by epilepsy diagnosis showed no significant change in ASMs exposure among pregnant women with epilepsy [the proportion of women exposed to ASM from all pregnancies was 0.37% (in 1997) and 0.36% (in 2018), p = 0.24]. A drop in carbamazepine use was observed, while the number of lamotrigine prescriptions increased from 6.45% in 1997 to 52% by 2018. ASM use among pregnant women without epilepsy increased significantly from 0.19% in 1997 to 1.85% in 2018 (p < 0.0001). In the total cohort of pregnancies, 1439 (0.53%) were exposed during their entire pregnancy, and 1369 (0.5%) were exposed only in their first trimester. Clonazepam was the most used ASM during the study period (1953 users, 0.71%), followed by gabapentin (785 users, 0.29%) and carbamazepine (449 users, 0.16%).
Conclusion: No major shifts in the quantity of ASM use over the study period were observed among pregnant women with epilepsy. However, there was a significant increase in ASM use among pregnant women without epilepsy. The study results warrant further investigation into the implications of ASM use in pregnancy for indications other than epilepsy.
Introduction
The estimated prevalence of epilepsy among pregnant women ranges between 0.3 and 0.7% (Hauser et al., 1996; Whelehan and Delanty, 2019). Both epilepsy and antiseizure medications (ASMs) are associated with potential adverse effects to a pregnant person and their developing fetus (Pennell, 2016; Whelehan and Delanty, 2019). Pharmacological management with ASMs during pregnancy should be maintained at the lowest possible dose allowing for optimum seizure control and minimal fetal exposure (Patel and Pennell, 2016; Pennell, 2016). Worldwide, several studies have reported an increase in the use of ASMs for epilepsy and other indications such as neuropathic pain, other neurologic and psychiatric disorders, and movement disorders (restless leg syndrome) during pregnancy (Vajda et al., 2010; Kulaga et al., 2011; Bobo et al., 2012; Wen et al., 2015; Yeh et al., 2017; Kinney et al., 2018; Hurault-Delarue et al., 2019; Margulis et al., 2019; Cohen et al., 2020). In a recent study, the utilization trends of ASMs during pregnancy from five Nordic countries, the United States, and Australia were assessed between 2006 and 2016 (Cohen et al., 2020). A significant increase in the use of ASMs, particularly new generation ASMs such as lamotrigine, and a decrease in old generation ASMs such carbamazepine during pregnancy was found in all countries throughout the study period (Cohen et al., 2020). In Canada, a study from the province of Québec by Kulaga et al. (2011) found that the majority of pregnant women with epilepsy (79.6%) received ASM monotherapy, 5.8% received polytherapy, and 14.6% had no ASM exposure. Evidence shows that the adverse outcomes are dependent on the type of ASM used, the dose of fetal exposure at conception, and the trimester of exposure (Hill et al., 2010; Tomson and Battino, 2012; Pennell, 2016). Therefore, choosing the most appropriate ASM for women with epilepsy (WWE), with the lowest teratogenic risk is crucial (Hill et al., 2010; Tomson and Battino, 2012; Pennell, 2016).
In the Canadian province of Manitoba, evidence of an increase in ASM use among the general population exists (Leong et al., 2016). A study showed that ASM use increased significantly, from 8.3/1,000 to 23/1,000 between 1998 and 2013 (Leong et al., 2016). The study showed a 210% increase in ASM users with no epilepsy, and 55-fold increase in the use of gabapentin among users without epilepsy (Leong et al., 2016). The study, however, did not report subgroup analysis for the trends of utilization of ASMs in special populations, such as pregnant women (Leong et al., 2016). In the current study, we aim to examine the trends of utilization of ASMs during pregnancy and identify any changes in prescription patterns of ASM among pregnant people with epilepsy in Manitoba, Canada, between 1995 and 2019.
Materials and Methods
Data Sources and Design
A retrospective population-based cohort study was conducted using de-identified data from the province of Manitoba, Canada. We constructed a cohort of all pregnant women in Manitoba from 1 January 1997 to 31 March 2019, using the administrative databases for the provincial healthcare system from the Manitoba Research Data Repository at the Manitoba Centre for Health Policy (MCHP), University of Manitoba. The database repository is a secure data-rich environment containing person-level health information on the entire population of Manitoba. All records in the repository are de-identified; however, records are linkable at the individual and family levels using a scrambled health number attached to each record. For the current study, we used the following linked databases: (1) The Manitoba Health Insurance Registry (date of birth, sex, comorbidities); (2) Drug Program Information Network (DPIN), which includes drug names, brand names, and dispensation dates and captures the dispensation of all prescription drugs by community pharmacies in Manitoba regardless of the insurance coverage type (1995/96–2018/19); (3) Hospital Discharge Abstracts, which include records of all patients' hospital admissions with summaries for demographic data (1992/93–2018/19), (4) Medical Services Database, which includes physician claims used to identify diagnosis codes using the International Classification of Diseases (ICD-9 and ICD-10) (1992/93–2018/19), (5) The Hospital Newborn to Mother Link, which serves to match the baby's birth hospital record with the mother’s obstetrical delivery record, and (6) census data for income quintiles (IQ). All data sets were linked together using a scrambled personal health identification number that is unique for each mother (1995/96–2018/19). We conducted sensitivity analysis using diagnosis codes in 2, 5, and 10 years prior to pregnancy case. The 5 years' definition was used to minimize false-negative cases of epilepsy.
Study Population
We identified all pregnancies for women living in Manitoba between 1995 and 2018. A woman was considered to have epilepsy if she had ≥1 medical claims or ≥1 hospitalization for epilepsy during the 5 years prior to delivery (ICD-9: 345 or ICD-10: G40/G41) (Fisher et al., 2014; Tu et al., 2014; Leong et al., 2016). Four groups of pregnant women were created: (1) exposed pregnant women with epilepsy, (2) exposed pregnant women without epilepsy, (3) unexposed pregnant women with epilepsy, and (4) unexposed pregnant women without epilepsy. Women who did not have five-year coverage or whose children were born before 1 April 1997 were excluded due to <5 years of follow-up. The area of residence was defined as urban for women living in Winnipeg or Brandon or as rural for women living in all other areas of the province. Income quintiles were used to determine the socioeconomic status. Income quintile measures neighborhood socioeconomic status and divides the population into five income groups from the lowest to the highest income (approximately 20% of the population in each group) (Martens et al., 2015).
Exposure Definition
ASM utilization was identified using the Anatomical Therapeutic Chemical (ATC) codes. The exposure windows were first trimester (1st day of gestation–14th week), second trimester (15th week–25th week), third trimester (26th week–end of pregnancy), and anytime during pregnancy (1st day of gestation–end of pregnancy). Exposure to a prescribed ASM was defined as having ≥1 prescription filled during the exposure window of interest, or a prescription filled before the beginning of the exposure window but with a duration overlapping the exposure window. ASMs examined were identified using ATC codes within the prescription drug data, specifically all drugs coded as N03A for anti-epilepsy medication (Supplementary Table S1).
Statistical Analysis
The characteristics and comorbidities of women were evaluated using descriptive statistics. Patient comorbidities considered (including mood disorders, diabetes, and hypertension) are defined in Supplementary Table S2. The frequency and pattern of ASM use during the whole pregnancy and each trimester was estimated. The annual trend of use of ASMs was evaluated for the total study population and for women with epilepsy and women without epilepsy. Linear regression was used to model the annual trends of utilization of ASMs and specific ASMs in each group of pregnant women. Models were built using data from 1997 to 2018 as some medications were only available as of 1997. A p-value ≤0.05 was considered statistically significant. Statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC).
Results
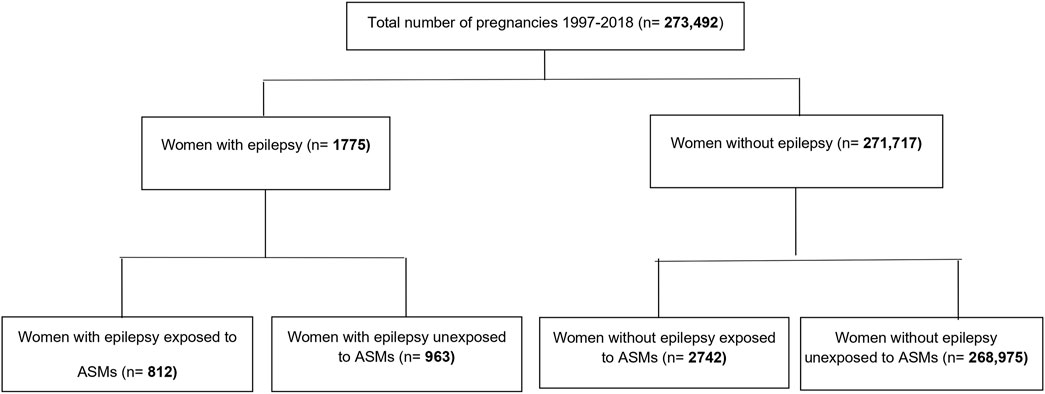
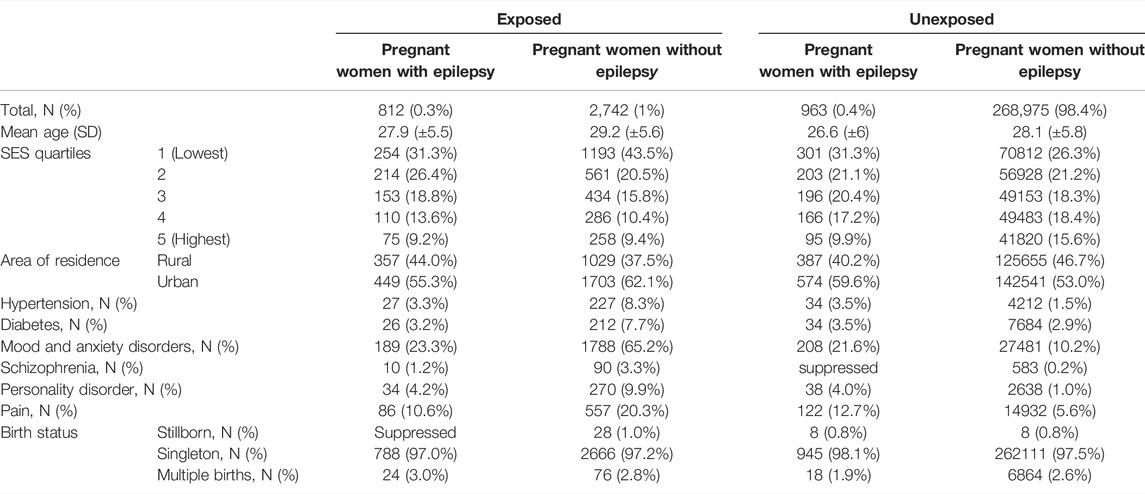
We identified 273,492 pregnancies, with a mean age of 28 years. Of these pregnancies, 0.3% (n = 812) were in women with epilepsy exposed to ASMs, 0.35% (n = 963) were pregnancies of women with epilepsy unexposed to ASM, and 1% (n = 2742) were women without epilepsy but exposed to ASMs (Figure 1). Among women with epilepsy, 31.3% (n = 254) of the exposed pregnancies and 31.3% (n = 301) of the unexposed pregnancies were in the lowest income quintile. Whereas, in women without epilepsy, 43.5% (n = 1193) of exposed pregnant women were in the lowest income quintile compared to 26.3% (n = 70812) in unexposed pregnant women (Table 1). Exposed pregnant women without epilepsy had higher rates of comorbidities compared to other groups. Among the exposed pregnant women without epilepsy, 65.21% were diagnosed with anxiety and 20.31% were diagnosed with pain when compared to 10.22 and 5.55%, respectively, in unexposed pregnant women without epilepsy (Table 1).
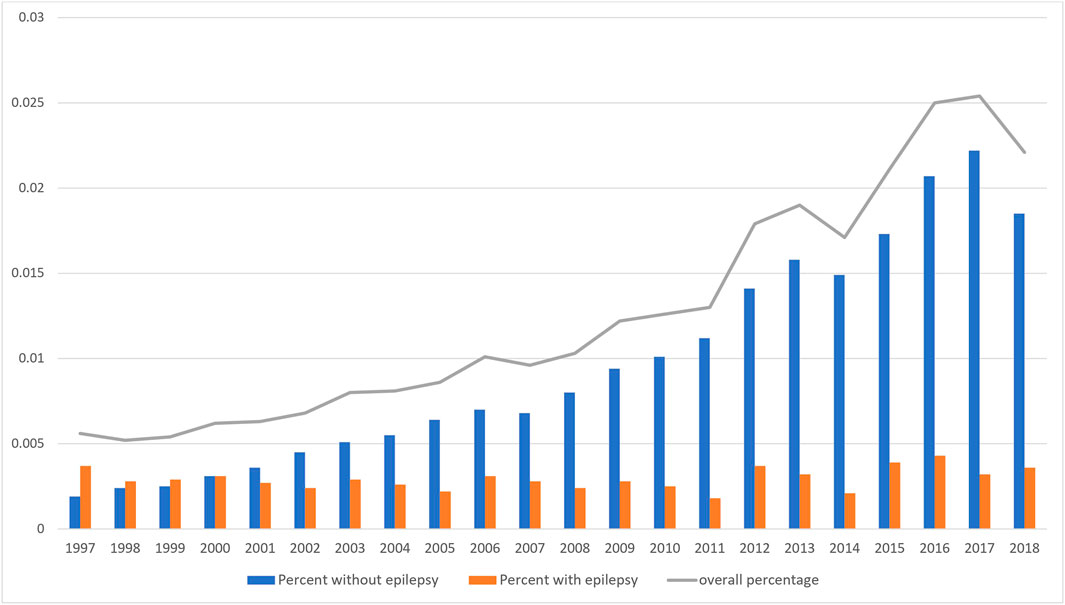
Linear regression analyses showed the number of pregnancies exposed to ASMs increased significantly from 0.56% in 1997 to 2.21% in 2018 (p < 0.0001) (Figure 2). There was no significant change in the percentage of pregnant women with epilepsy exposed to ASMs from 0.37% in 1997 to 0.36% in 2018 (p = 0.2354), while the percentage of ASM-exposures among pregnant women without epilepsy increased significantly (0.19% in 1997 to 1.85% in 2018, p < 0.0001) (Figure 2).
Trimester Analysis
Trimester analysis showed 0.53% (n = 1439) of women were exposed throughout their pregnancy, 0.5% (n = 1369) were exposed only in the first trimester, 0.02% (n = 63) were exposed only during the second trimester, and 0.07% (n = 184) were exposed only during the third trimester. Among women with epilepsy, 33.58% were exposed throughout the pregnancy. Detailed analysis of exposures by trimester is presented in Supplementary Figures S1–S3.
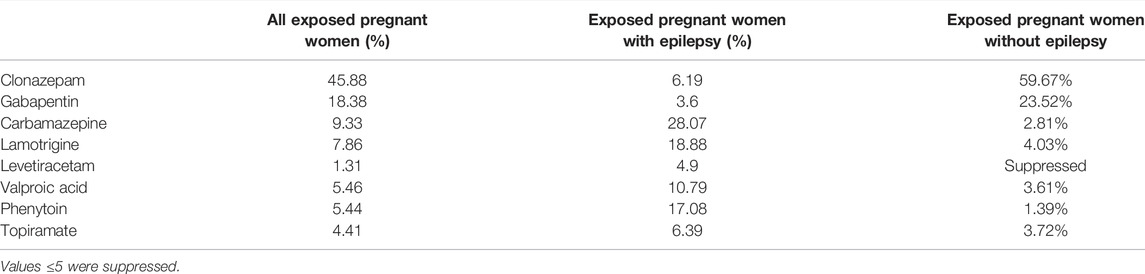
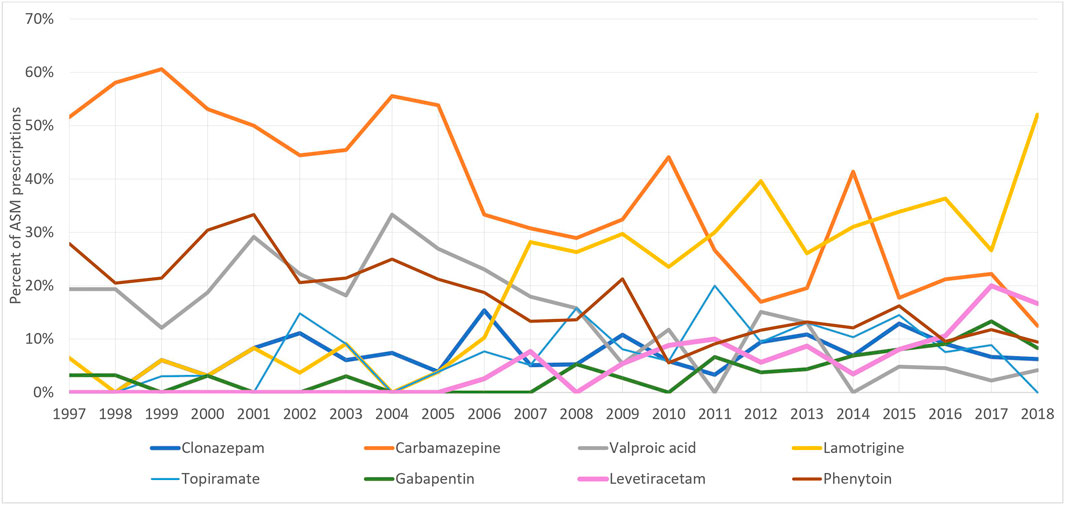
The most used ASM among pregnant women with and without epilepsy, throughout the study period was clonazepam (44.44% of all exposed pregnancies) followed by gabapentin (17.85%) and carbamazepine (10.22%) (Table 2). Whereas, among pregnant women with epilepsy, carbamazepine (33.86%), lamotrigine (22.77%), phenytoin (17.08%), and valproic acid (13%) were the most used (Figure 3).
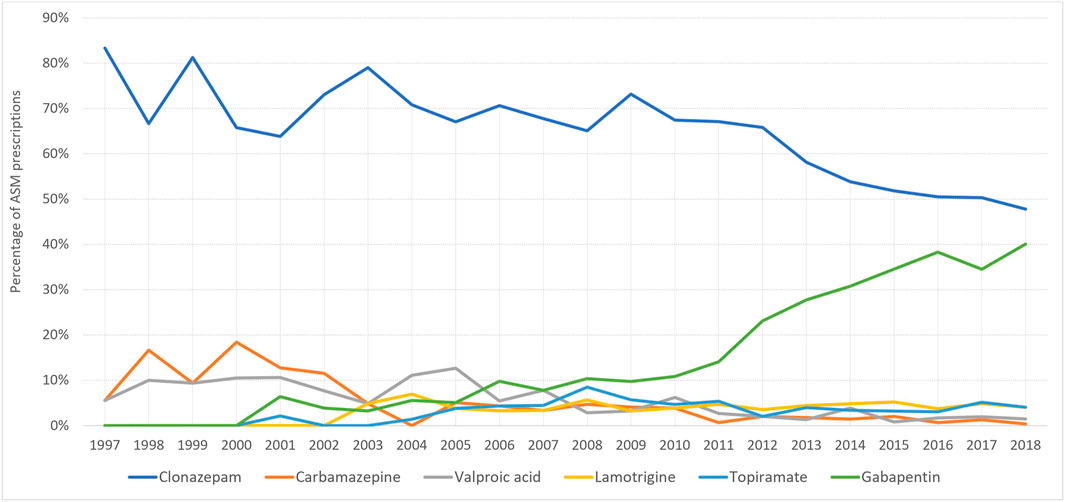
At the start of the study period, carbamazepine was the most prescribed ASM for pregnant women with epilepsy (51%), however, this decreased to 12.5% in 2018, whereas the number of lamotrigine prescriptions increased from 6.45% (1997) to 52% (2018) (Figure 4). On the other hand, among women without epilepsy, clonazepam remained the most used ASM throughout the study period. However, its utilization decreased from 88.2% in 1997 to 47.97% in 2018. Gabapentin first appeared among women without epilepsy in 2001 and its utilization increased to reach 40.2% of prescriptions in 2018 (Figure 5).
Discussion
In this population-based cohort study, we observed a significant increase in the utilization of ASMs among pregnant women in the Canadian province of Manitoba between 1997 and 2018. This increase was attributed mainly to the increased use of clonazepam and gabapentin among pregnant women without epilepsy. In general, there was no major shift in the utilization of ASMs among pregnant women with epilepsy over the study. By contrast, a significant increase in the utilization of ASMs among pregnant women without epilepsy was observed. Our study showed an increase in lamotrigine prescriptions among pregnant women with epilepsy and a decrease in valproic acid and carbamazepine use. Similar results were reported in the United Kingdom and Ireland, with an increase in lamotrigine and levetiracetam use and a decrease in valproic acid and carbamazepine between 1996 and 2016 (Kinney et al., 2018). Lamotrigine prescriptions increased from 15% of the total ASM prescriptions in the United Kingdom and Ireland in 2000 to 31% in 2016, while at the same time, valproic acid prescriptions decreased from 22% in 2000 to less than 5% in 2016 (Kinney et al., 2018). ASMs are frequently used for indications other than seizure control (LiverTox, 2012; Dokkedal-Silva et al., 2019). For example, valproic acid has been indicated for bipolar disorder and schizophrenia (LiverTox, 2012; Dokkedal-Silva et al., 2019). Lamotrigine has been indicated in bipolar depression in adults (Goldenberg, 2010). Gabapentin is primarily used to treat neuropathic pain, namely, trigeminal neuralgia, HIV-associated neuralgia, diabetic neuropathy, and neoplasia (Magnus, 1999; Goldenberg, 2010). It is also used in the treatment of psychiatric disorders, most notably bipolar disorder, and in movement disorders such as restless leg syndrome (Magnus, 1999; Goldenberg, 2010). Most exposed pregnant women with epilepsy (33.6%) were exposed throughout their pregnancy period, and while the main reasons are unknown, this could be a reflection to optimal management of seizures by practitioners. Among the pregnant women with epilepsy, >54% were unexposed to any ASM, this could be attributed to the presence of mild/non–medication-controlled epilepsy, or a potential misclassification of epilepsy definition used in our study (for example, isolated seizures not related to epilepsy).
Strengths and Limitations
The databases used in this study are a major strength in terms of size and coverage, and the validity and reliability of the MCHP Repository for epidemiological studies has been previously reported (Leong et al., 2016; Azimaee et al., 2018). The MCHP repository includes medical records for all Manitoba residents recorded in the process of routine care. Our study captured the prescription practices of prescribers in Manitoba during the past 20 years. Our study, however, has limitations. First, exposure was derived from dispensing records and not actual intake (Azimaee et al., 2018). Second, we did not have data on the severity of epilepsy cases. Finally, since many prescriptions started prior to pregnancy, a proportion of women may have stopped taking their medications as soon as they become pregnant, without a database record, thus overestimating some ASMs exposures.
Conclusion
Over the study period, no major shifts in the overall use of ASMs were observed among pregnant women with epilepsy. The reduction in carbamazepine and valproic acid use coupled with the increase in lamotrigine and levetiracetam use reflects Manitoba prescribers' adherence to updated guidelines (CCSO, 2015). Consistent with previous reports among the general population of Manitoba, gabapentin is increasingly used among pregnant women, mostly for non-epilepsy indications. Future studies on the utilization and safety outcomes of gabapentin and other new-generation ASMs in pregnancy, as well as studies focusing on pre-pregnancy counseling and management are warranted to inform prescribers and policymakers.
Data Availability Statement
The data sets presented in this article are not readily available because data used in this study are from the Manitoba Population Research Data Repository housed at the Manitoba Centre for Health Policy, University of Manitoba, and were derived from data provided by Manitoba Health. Requests to access the data sets should be directed to Manitoba Centre for Health Policy, https://umanitoba.ca/manitoba-centre-for-health-policy/.
Ethics Statement
The studies involving human participants were reviewed and approved by the Health Research Ethics Board (HREB) at the University of Manitoba and Manitoba Health Information Privacy Committee (HIPC). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
All authors contributed to the concept and design of the study. WS was responsible for the analysis and drafted the first draft of the manuscript. All authors contributed to drafting and revising of the full manuscript and have approved the manuscript as submitted. All authors have met the criteria of authorship and take public responsibility for the manuscript contents.
Funding
The study was funded through awards from the Manitoba Medical Services Foundation, Winnipeg Foundation, and Health Sciences Centre Foundation to cover data analysis, stipends, and publication fees.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors acknowledge the Manitoba Centre for Health Policy for use of data contained in the Manitoba Population Research Data Repository under project #H2020:088 (HS23673) (HIPC#2019/2020-60). The results and conclusions are those of the authors, and no official endorsement by the Manitoba Centre for Health Policy, Manitoba Health, or other data providers is intended or should be inferred. Data used in this study are from the Manitoba Population Research Data Repository housed at the Manitoba Centre for Health Policy, University of Manitoba, and were derived from data provided by Manitoba Health and Manitoba Health Insurance Registry, Drug Program Information Network, Hospital Discharge, and Medical Services database.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.871136/full#supplementary-material
References
Azimaee, M., Smith, M., Lix, L., Ostapyk, T., and Burchill, C. (2018). MCHP Data Quality Framework. Winnipeg, Manitoba, Canada: Manitoba Centre for Health Policy, University of Manitoba.
Bobo, W. V., Davis, R. L., Toh, S., Li, D. K., Andrade, S. E., Cheetham, T. C., et al. (2012). Trends in the Use of Antiepileptic Drugs Among Pregnant Women in the US, 2001-2007: a Medication Exposure in Pregnancy Risk Evaluation Program Study. Paediatr. Perinat. Epidemiol. 26, 578–588. doi:10.1111/ppe.12004
CCSO (2015). Provincial Guidelines for the Management of Epilepsy in Adults and Children. Available from: www.ontarioepilepsyguidelines.ca (Accessed December 11, 2021).
Cohen, J. M., Cesta, C. E., Furu, K., Einarsdóttir, K., Gissler, M., Havard, A., et al. (2020). Prevalence Trends and Individual Patterns of Antiepileptic Drug Use in Pregnancy 2006-2016: A Study in the Five Nordic Countries, United States, and Australia. Pharmacoepidemiol. Drug Saf. 29, 913–922. doi:10.1002/pds.5035
Dokkedal-Silva, V., Berro, L. F., Galduróz, J. C. F., Tufik, S., and Andersen, M. L. (2019). Clonazepam: Indications, Side Effects, and Potential for Nonmedical Use. Harv. Rev. Psychiatry 27, 279–289. doi:10.1097/HRP.0000000000000227
Fisher, R. S., Acevedo, C., Arzimanoglou, A., Bogacz, A., Cross, J. H., Elger, C. E., et al. (2014). ILAE Official Report: a Practical Clinical Definition of Epilepsy. Epilepsia 55, 475–482. doi:10.1111/epi.12550
Goldenberg, M. M. (2010). Overview of Drugs Used for Epilepsy and Seizures: Etiology, Diagnosis, and Treatment. P T 35, 392–415.
Hauser, W. A., Annegers, J. F., and Rocca, W. A. (1996). Descriptive Epidemiology of Epilepsy: Contributions of Population-Based Studies from Rochester, Minnesota. Mayo Clin. Proc. 71, 576–586. doi:10.4065/71.6.576
Hill, D. S., Wlodarczyk, B. J., Palacios, A. M., and Finnell, R. H. (2010). Teratogenic Effects of Antiepileptic Drugs. Expert Rev. Neurother. 10, 943–959. doi:10.1586/ern.10.57
Hurault-Delarue, C., Morris, J. K., Charlton, R., Gini, R., Loane, M., Pierini, A., et al. (2019). Prescription of Antiepileptic Medicines Including Valproate in Pregnant Women: A Study in Three European Countries. Pharmacoepidemiol. Drug Saf. 28, 1510–1518. doi:10.1002/pds.4897
Kinney, M. O., Morrow, J., Patterson, C. C., Campbell, E., Russell, A., Smithson, H. W., et al. (2018). Changing Antiepilepsy Drug-Prescribing Trends in Women with Epilepsy in the UK and Ireland and the Impact on Major Congenital Malformations. J. Neurol. Neurosurg. Psychiatry 89, 1320–1323. doi:10.1136/jnnp-2017-317368
Kulaga, S., Sheehy, O., Zargarzadeh, A. H., Moussally, K., and Bérard, A. (2011). Antiepileptic Drug Use during Pregnancy: Perinatal Outcomes. Seizure 20, 667–672. doi:10.1016/j.seizure.2011.06.012
Leong, C., Mamdani, M. M., Gomes, T., Juurlink, D. N., Macdonald, E. M., and Yogendran, M. (2016). Antiepileptic Use for Epilepsy and Nonepilepsy Disorders: A Population-Based Study (1998-2013). Neurology 86, 939–946. doi:10.1212/WNL.0000000000002446
LiverTox. Valproate - LiverTox - NCBI Bookshelf. 2012 Available from: https://www.ncbi.nlm.nih.gov/books/NBK548284/table/Valproate.Td/
Magnus, L. (1999). Nonepileptic Uses of Gabapentin. Epilepsia 40 (Suppl. 6), S66–S72. doi:10.1111/j.1528-1157.1999.tb00936.x
Margulis, A. V., Hernandez-Diaz, S., McElrath, T., Rothman, K. J., Plana, E., Almqvist, C., et al. (2019). Relation of In-Utero Exposure to Antiepileptic Drugs to Pregnancy Duration and Size at Birth. PLoS One 14, e0214180. doi:10.1371/journal.pone.0214180
Martens, P. J., Nickel, N., Forget, E., Lix, L., Turner, D., Prior, H., et al. (2015). The Cost of Smoking in Manitoba. Winnipeg MB: Manitoba Centre for Health Policy.
Patel, S. I., and Pennell, P. B. (2016). Management of Epilepsy during Pregnancy: an Update. Ther. Adv. Neurol. Disord. 9, 118–129. doi:10.1177/1756285615623934
Pennell, P. B. (2016). Use of Antiepileptic Drugs during Pregnancy: Evolving Concepts. Neurotherapeutics 13, 811–820. doi:10.1007/s13311-016-0464-0
Tomson, T., and Battino, D. (2012). Teratogenic Effects of Antiepileptic Drugs. Lancet Neurol. 11, 803–813. doi:10.1016/S1474-4422(12)70103-5
Tu, K., Wang, M., Jaakkimainen, R. L., Butt, D., Ivers, N. M., Young, J., et al. (2014). Assessing the Validity of Using Administrative Data to Identify Patients with Epilepsy. Epilepsia 55, 335–343. doi:10.1111/epi.12506
Vajda, F. J., Hollingworth, S., Graham, J., Hitchcock, A. A., O'Brien, T. J., Lander, C. M., et al. (2010). Changing Patterns of Antiepileptic Drug Use in Pregnant Australian Women. Acta Neurol. Scand. 121, 89–93. doi:10.1111/j.1600-0404.2009.01260.x
Wen, X., Meador, K. J., and Hartzema, A. (2015). Antiepileptic Drug Use by Pregnant Women Enrolled in Florida Medicaid. Neurology 84, 944–950. doi:10.1212/WNL.0000000000001304
Whelehan, A., and Delanty, N. (2019). Therapeutic Strategies for Treating Epilepsy during Pregnancy. Expert Opin. Pharmacother. 20, 323–332. doi:10.1080/14656566.2018.1550073
Keywords: utilization, pregnancy, antiepileptic, cohort, epilepsy
Citation: Shouman W, Delaney JA, Kowalec K, Ng M, Ruth C, Falk J, Leong C, Alessi-Severini S, Lavu A, Peymani P and Eltonsy S (2022) Trends of Utilization of Antiseizure Medications Among Pregnant Women in Manitoba, Canada: A 20-Year Population-Based Study. Front. Pharmacol. 13:871136. doi: 10.3389/fphar.2022.871136
Received: 07 February 2022; Accepted: 14 March 2022;
Published: 20 April 2022.
Edited by:
Andrea Burden, ETH Zürich, SwitzerlandReviewed by:
Barbara Mostacci, IRCCS Institute of Neurological Sciences of Bologna (ISNB), ItalyDick Lindhout, University Medical Center Utrecht, Netherlands
Copyright © 2022 Shouman, Delaney, Kowalec, Ng, Ruth, Falk, Leong, Alessi-Severini, Lavu, Peymani and Eltonsy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sherif Eltonsy, c2hlcmlmLmVsdG9uc3lAdW1hbml0b2JhLmNh
 Walid Shouman1
Walid Shouman1 Kaarina Kowalec
Kaarina Kowalec Marcus Ng
Marcus Ng Chelsea Ruth
Chelsea Ruth Christine Leong
Christine Leong Silvia Alessi-Severini
Silvia Alessi-Severini Alekhya Lavu
Alekhya Lavu Sherif Eltonsy
Sherif Eltonsy