- Department of Pharmaceutical Biology, Faculty of Pharmacy, University of Ljubljana, Ljubljana, Slovenia
While the chemical composition of vegetable butters and oils has been studied in detail, there is limited knowledge about their mechanisms of action after application on the skin. To understand their dermal effects better, 27 clinical studies evaluating 17 vegetable oils (almond, argan, avocado, borage, coconut, evening primrose, kukui, marula, mustard, neem, olive, rapeseed, sacha inchi, safflower, shea butter, soybean and sunflower oils) were reviewed in this research. The reviewed studies focused on non-affected skin, infant skin, psoriasis, xerosis, UVB-induced erythema, atopic dermatitis, molluscum contagiosum, tungiasis, scars, striae and striae gravidarum. We conclude that in inflammation-affected skin, vegetable oils with a high content of oleic acid, together with the lack of or a low linoleic acid content, may cause additional structural damage of the stratum corneum, while oils high in linoleic acid and saturated fatty acids may express positive effects. Non-affected skin, in contrast, may not react negatively to oils high in oleic acid. However, the frequency and duration of an oil’s use must be considered an important factor that may accelerate or enhance the negative effects on the skin’s structural integrity.
Introduction
Vegetable butters and oils have been used for centuries for their positive therapeutic and cosmetic effects on the skin’s health, and are also extensively used in the pharmaceutical and cosmetic industries today. They function, for example, as active ingredients, excipients and extraction solvents.
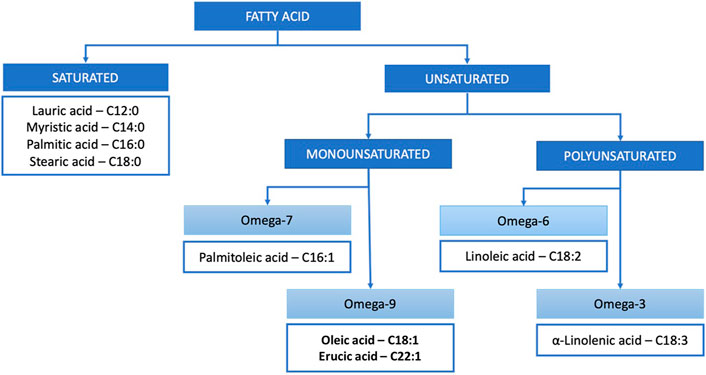
In terms of chemistry, vegetable butters and oils are composed of triglycerides (typically around 99%) and unsaponifiable matter (typically around 1%). Triglycerides are ester derivatives of glycerol and fatty acids. Depending on the number of double bonds, fatty acids are classified into saturated and mono- and polyunsaturated (Figure 1), which defines their susceptibility to light-, heat- or oxygen-induced changes. The main unsaponifiable compounds are phytosterols, phenols, squalene, carotenoids and vitamin E (Janeš and Kočevar Glavač, 2018). In terms of native, complex composition, vegetable butters and oils of the highest quality are obtained through cold pressing and CO2 extraction, without subsequent refining, as they are not exposed to temperature- or oxidation-dependant changes, and solvent residuals are not present (Sookwong and Mahatheeranont, 2017).
The dermal effects of vegetable butters and oils are based on triglycerides and fatty acids, and unsaponifiable matter. Scientific evidence regarding the exact mechanisms of action and the extent of dermal effects is still limited. However, important progress has been made in recent years in the area of clinical research, and a growing body of evidence indicates rationale for the science-based use of vegetable butters and oils in fields such as medicine, pharmacy and cosmetic science.
This article represents the most recent review of clinical studies evaluating the use of vegetable butters and oils in the treatment and care of different skin conditions and disorders after dermal application.
Methodology
A systematic search was performed on literature published until 2021 with PubMed, Science Direct and Google Scholar search engines. Key search words included “vegetable butter/oil”, “plant butter/oil”, “clinical study/trial”, “dermal” and “skin”. Only clinical studies evaluating the dermal effects of vegetable butters and oils were included, which resulted in 27 clinical studies. All other studies, such as studies with cosmetic or therapeutic products containing vegetable butters or oils, or studies with compounds isolated from vegetable butters or oils, were not included. Studies such as in vitro or in vivo studies not defined as clinical studies were also not included.
Composition
The general composition of vegetable butters and oils reviewed in this article is presented in Table 1. The content of fatty acids may vary in the range of 5–10%, mainly due to the different geographical origins of plant material (Janeš and Kočevar Glavač, 2018). An even higher variability is found for unsaponifiable matter, which is typically more affected by the method of production (Poljšak and Kočevar Glavač, 2021). Selected fatty acids and their dermal effects are summarized in Table 2.
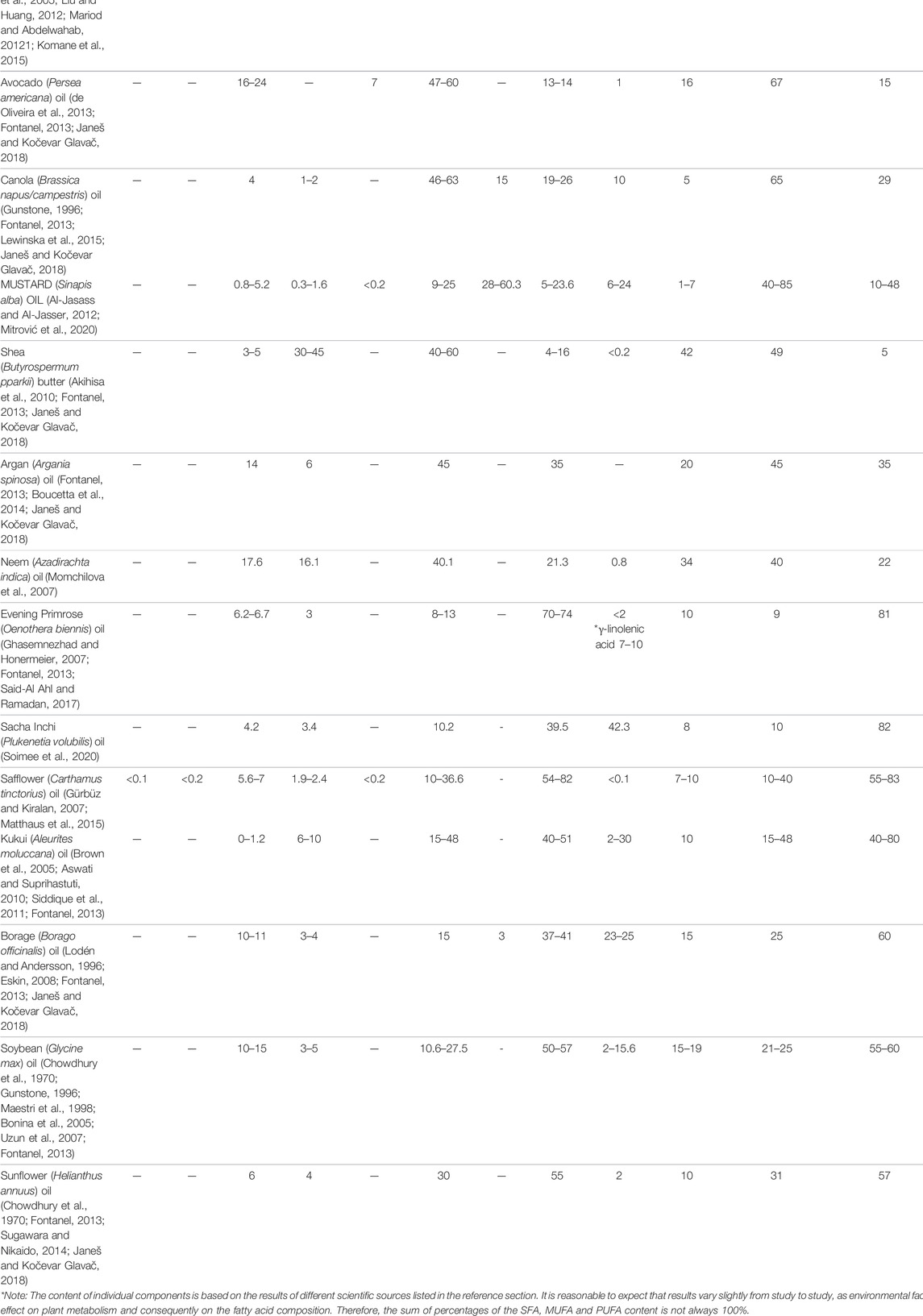
TABLE 1. Vegetable butters and oils, their fatty acid composition and unsaponifiable matter content; individual fatty acids were only included in the table when their content was at least 10% in at least one of the listed oils. Fatty acids of triglycerides and total unsaponifiable matter are given in percentages, “-” typically not present*.
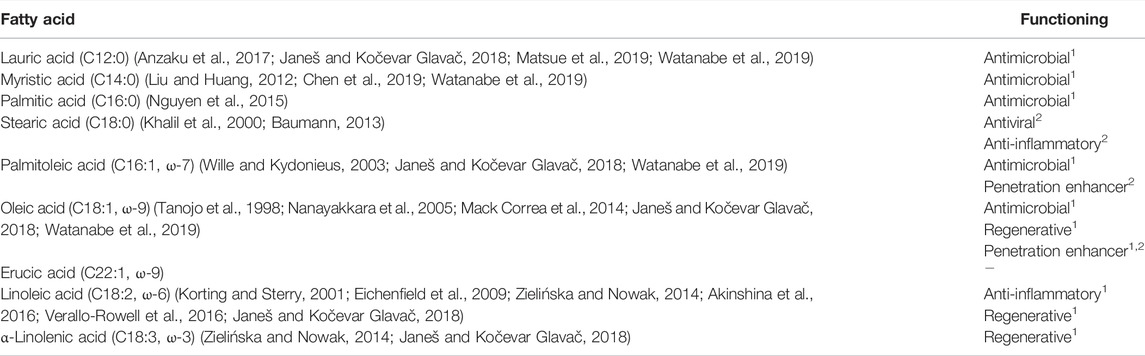
TABLE 2. Dermal activities of selected fatty acids; 1 = functioning of the isolated fatty acid, 2 = functioning of the isolated fatty acid in a dermal formulation, - not available.
In the context of dermal activity, the composition of vegetable butters and oils is intrinsically linked to the composition of skin lipids. Fatty acids in the skin are found in the stratum corneum (free and as structural units of ceramides) (Gray and Yardley, 1975; Hansen and Jensen, 1985; Wertz, 1992; Pappas, 2009), and in sebum (free and in diglycerides and triglycerides) (Pappas, 2009; Cunha et al., 2018). Free fatty acids in the stratum corneum are mostly saturated, with chain lengths of up to 36 carbon atoms, with tetracosanoic acid (lignoceric; C24) and hexacosanoic (ceric acid or ceratinic acid; C26) acids being the most abundant (39 M % and 23 M %, respectively) (Norlén et al., 1998). The proportion of total monounsaturated free fatty acids is approx. 20% (Van Smeden et al., 2014). Oleic (C18:1) and linoleic (C18:2) acids account for 6 and 2%, respectively, and are the only unsaturated fatty acids detected unbound in the stratum corneum (Menon et al., 2012). The human sebum is composed of triglycerides (41%), waxes (25%), free fatty acids (16%), squalene (12%), cholesterol and cholesterol esters (4%), and vitamin E (Cunha et al., 2018). Fatty acid chains range from C7 to C22 carbon atoms in length, with palmitic acid (C16) being the most abundant (Weitkamp et al. 1947; Wertz 2018). Monounsaturated fatty acids are of C14 to C18 atoms in length, the predominant acid being sapienic acid (C16:1Δ6) (Wertz 2018).
The main non-specific dermal activity of vegetable butters and oils is the emolliency of triglycerides, which results in improved skin hydration due to decreased transepidermal water loss (TEWL) (Danby et al., 2013). Specific effects include antimicrobial (Darmstadt et al., 2005; Verallo-Rowell et al., 2008), anti-inflammatory (Lucas et al., 2011) and antioxidative (Bardaa et al., 2016) action, expressed by free fatty acids and compounds of unsaponifiable matter (Poljšak et al., 2019). Dermally applied free fatty acids have also been shown to penetrate into the stratum corneum and enhance the penetration of other substances (Nanayakkara et al., 2005). Vegetable butters and oils can therefore be used to improve skin wound healing (Alves et al., 2019; Poljšak et al., 2019), ameliorate the severity of dermatitis (Desai, 2017; Hou et al., 2017), alleviate symptoms of inflammatory conditions (Styrczewska et al., 2019), etc.
The main fatty acids that express important dermal functions are briefly discussed below.
Oleic Acid
Oleic acid is a C18:1 unsaturated ω-9 fatty acid, generally present in the majority of vegetable butters and oils. It acts as a skin penetration enhancer, as it induces permeability defects in the stratum corneum structure (Jiang et al., 2000; Mack Correa et al., 2014). The disruption of the skin’s barrier function results in an increase in TEWL (Tanojo et al., 1998; Mack Correa et al., 2014) and irritation (Tanojo et al., 1998).
Linoleic Acid
Linoleic acid is an essential C18:2 unsaturated ω-6 fatty acid. It is a structural unit of phospholipid cell membranes, as well as ceramides in the stratum corneum, and is involved in the regulation of TEWL and lipid barrier homeostasis (Rabionet et al., 2013).
α-Linolenic Acid and γ-Linolenic Acid
The other essential fatty acid is α-linolenic acid, a C18:3 unsaturated ω-3 fatty acid, while γ-linolenic acid is an ω-6 fatty acid. α- and γ-linolenic acids are not structural components of the skin. However, together with linoleic acid they are involved in the skin’s metabolism of polyunsaturated fatty acids (Ziboh et al., 2000). A dietary deficiency of linoleic acid, but not of α-linolenic acid, has been shown to result in skin dysfunctions such as dryness and inflammation (Ziboh and Miller, 1990).
Unsaponifiable Compounds
While the triglyceride part of vegetable butters and oils has been researched extensively, significantly less studies have been performed on unsaponifiable compounds. This was the focus of a recently published review article from 2021 (Poljšak and Kočevar Glavač, 2021). Isolated unsaponifiable compounds were found to demonstrate wound healing, anti-acne and anti-dermatitis activities, as well as regenerative, hydrating, photoprotective and anti-wrinkle activities. However, dermal effects of unsaponifiable compounds as integral structural components of vegetable butters and oils remain largely unexplored in clinical studies. Selected unsaponifiable compounds and their dermal effects are summarized in Table 3.

TABLE 3. Dermal activities of selected unsaponifiable compounds; adopted from (Poljšak and Kočevar Glavač, 2021).
Clinical Studies
Essential progress in the understanding of the structure and functioning of the skin has been made since the first studies, which date back to about 1960 (Reinertson and Wheatley, 1959; Rawlings et al., 1994; Harding, 2004). However, in-depth investigations regarding physiological processes and the effects of individual components of the skin lipid matrix at the molecular level have only begun to emerge during the last decade (Akinshina et al., 2016; Badhe et al., 2019).
In terms of vegetable butters and oils, there is limited knowledge about their fate after dermal application, such as the extent of enzymatic hydrolysis or chemical degradation to glycerol and individual fatty acids and/or mono- or diglycerides, about penetration into the stratum corneum, inclusion in skin structures and processes, and the influence on the skin’s microbiota.
A number of in vitro, ex vivo, in silico and mathematical models have been developed for studying and predicting skin penetration and permeation (Moser et al., 2001; Netzlaff et al., 2007). However, none of these methodologies can thoroughly simulate real-life conditions in the human skin (Herkenne et al., 2008). Current research methods typically applied in vivo studies are suction blister fluid, microdialysis, skin biopsy and tape stripping. They exhibit disadvantages such as invasiveness and a lack of standardization (Herkenne et al., 2008). Among non-invasive in vivo methods, confocal Raman microspectroscopy is used most frequently (Darlenski et al., 2009). In general, the quantification of parameters, such as TEWL, stratum corneum hydration and skin surface acidity (pH), is essential for the integral evaluation of the lipid barrier status.
Table 4 represents a systematic review of clinical studies evaluating the effectiveness of vegetable butters and oils for dermal use. The studies were grouped according to skin condition (non-affected adult skin, infant skin, psoriasis, xerosis, UV-induced erythema, atopic dermatitis, molluscum contagiosum, tungiasis, and striae and scars), and listed chronologically together with the main characteristics and results.
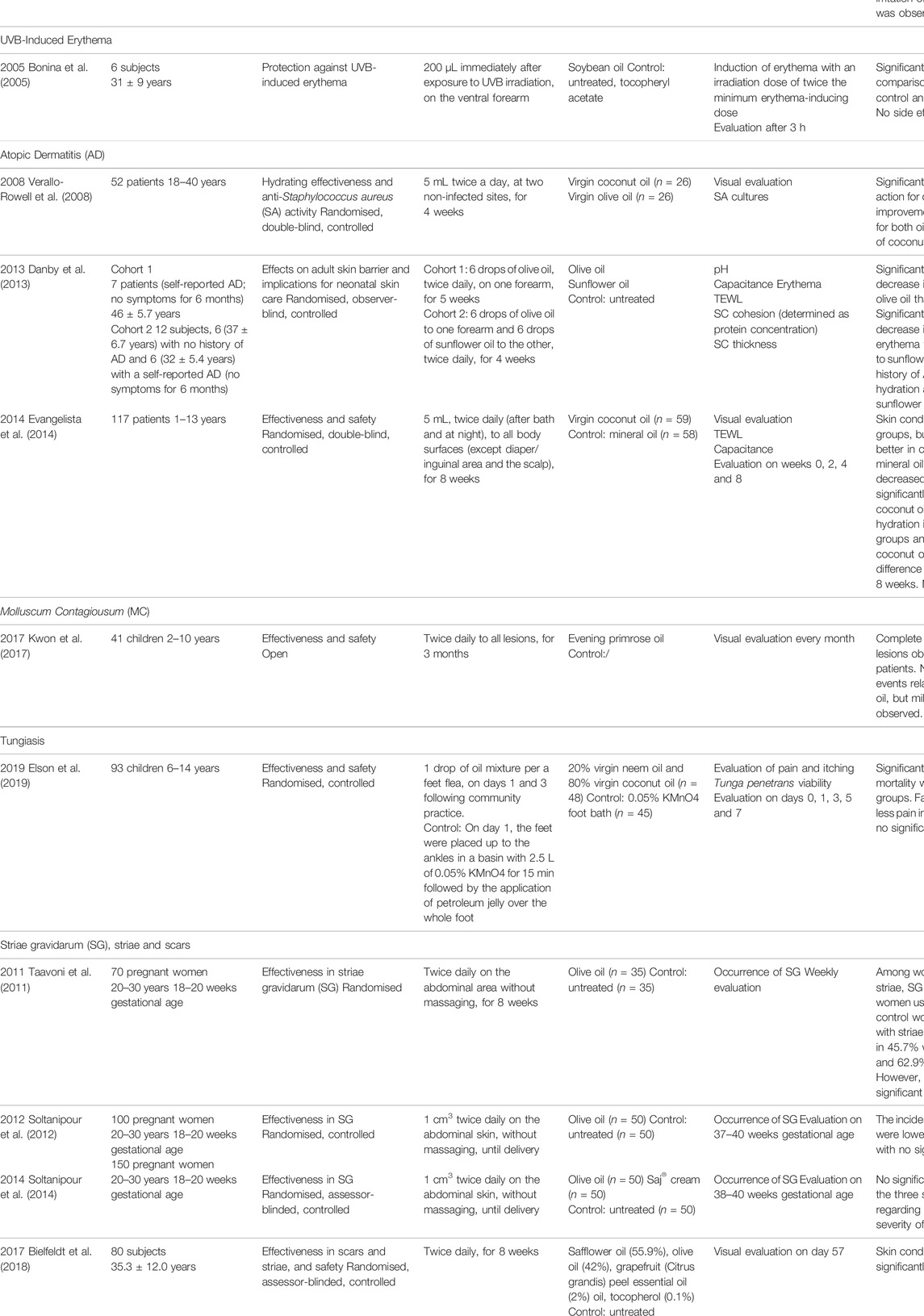
TABLE 4. Clinical studies evaluating the dermal effects of vegetable butters and oils. AD—atopic dermatitis, GC—gas chromatography, MC—molluscum contagiosum, SA—Staphylococcus aureus, SC—stratum corneum, SG—striae gravidarum, SLS—sodium lauryl sulphate.
Discussion
The dermal use of vegetable butters and oils probably dates back to the times of Ancient Egypt. Today, scores of different butters and oils are available for therapeutic and cosmetic purposes, and are researched in scientific studies. While their chemical composition has been studied in detail, significantly less research has been done to elucidate the mechanisms of action after application on the skin, particularly at the level of clinical effectiveness in the treatment of skin disorders (Janeš and Kočevar Glavač, 2018). Surprisingly, systematic studies were not available until the 1990s (Lodén and Andersson, 1996). Research then intensified after 2010 and, in the last few years, increased interest is reflected in comprehensive review articles (Lin et al., 2017; Vaughn et al., 2018; Poljšak, Kreft, and Kočevar Glavač, 2019; Moore, Wagner, and Komarnytsky, 2020). The reasons for the latter primarily derive from direct evidence that vegetable butters and oils function as effective active pharmaceutical ingredients in dermal treatments, and as cosmetically active ingredients in cosmetics, as evident from the clinical studies reviewed in Table 4. They are also generally linked to good skin compatibility, have fewer side effects, are affordable and easily accessible. Finally, in terms of today’s patients/consumers, we cannot neglect the fact that they are being increasingly used as alternatives for synthetic actives simply due to their natural origin.
The reviewed clinical studies on non-affected skin (Table 4) focused mainly on investigating penetration capacity, occlusive/hydrating effects and irritation potential, and included argan (Argania spinosa) oil, borage (Borago officinalis) oil, rapeseed (Brassica napus) oil, shea (Butyrospermum parkii) butter, soybean (Glycine max) oil, sunflower (Helianthus annuus) oil, olive (Olea europaea) oil, avocado (Persea americana) oil, sacha inchi (Plukenetia volubilis) oil, almond (Prunus dulcis) oil and marula (Sclerocarya birrea) oil (Lodén and Andersson, 1996; Stamatas et al., 2008; Patzelt et al., 2012; Boucetta et al., 2014; Komane et al., 2015; Soimee et al., 2020). The oils were proven to be semi-occlusive, which resulted in decreased levels of TEWL and/or increased stratum corneum hydration. Hydration was shown to improve very soon after application (30 min) and lasted for the duration of the studies (from 1 day to 60 days). The occlusive effects on non-affected skin were comparable to those of the controls (usually petrolatum or mineral oil), and were also directly confirmed in a clinical study with coconut (Cocos nucifera) oil on xerotic skin (Agero and Verallo-Rowell, 2004).
In this review, we placed special emphasis on the evaluation of possible connections between the fatty acid composition of triglycerides and the negative effects of the oils on the stratum corneum structural integrity. The skin’s lipid barrier disruption is assumed to be connected to vegetable oils with a content of predominantly oleic acid in triglycerides, and it was suggested that the ratio of oleic acid to linoleic acid may be crucial (Vaughn et al., 2018; Poljšak, Kreft, and Kočevar Glavač, 2019). However, in the case of non-affected skin, the reviewed vegetable oils were proven to be non-irritating, and this seems to be independent of the fatty acid composition. Sacha inchi and olive oils showed comparable effects and both were beneficial for dry skin (Soimee et al., 2020). Yet, their fatty acid composition is significantly different, with an approx. 1:4 ratio of oleic acid (10.2%) to linoleic acid (39.5%) for sacha inchi oil (Soimee et al., 2020), while oleic acid is typically predominant (>70%) over linoleic acid (10%; Table 1) in olive oil. Similar findings showing no irritation were reported for marula oil (69.0% oleic acid, <10% linoleic acid) (Komane et al., 2015). The resistance of the skin to the potentially damaging effects of vegetable oils with a high content of oleic acid in triglycerides may be explained by the physiological mechanisms of barrier repair in healthy skin not suffering from pathological conditions. Furthermore, almond, rapeseed and avocado oils represent vegetable oils with a 2–3:1 ratio of oleic acid to linoleic acid (Table 1), which corresponds closely to the physiological ratio of 3:1 (Menon et al., 2012), while argan oil has a ratio of approx. 1:1. However, no significant skin benefits were identified in connection with this ratio (Lodén and Andersson, 1996; Stamatas et al., 2008; Patzelt et al., 2012).
We conclude that studies have not yet proven whether the physiological ratio of oleic acid to linoleic acid could be considered a boundary between the positive and negative skin effects of dermally applied triglycerides. Moreover, non-affected skin is capable of resisting the damaging potential to disrupt the stratum corneum structure, resulting from the dermal use of vegetable oils with a high content of oleic acid in triglycerides.
Nine clinical studies (Darmstadt et al., 2004, 2005; Solanki et al., 2005; Kanti et al., 2014; Nangia et al., 2015; Cooke et al., 2016; Strunk et al., 2018; Summers et al., 2019; Konar et al., 2020) performed on infant skin explored the effects of safflower (Carthamus tinctorius) oil, coconut (Cocos nucifera) oil, sunflower (Helianthus annuus) oil, olive (Olea europaea) oil and mustard (Sinapis alba) oil. The results revealed a low cost, availability, simplicity, beneficial action and the effectiveness of treatments. Based on studies evaluating sunflower oil (Darmstadt et al., 2004; Darmstadt et al., 2005), vegetable oils were identified as an important intervention for treating infants in developing countries, especially for the reduction in the incidence of nosocomial infections. In addition, the oils significantly reduced TEWL and improved hydration, and generally no side effects were observed. In contrast to non-affected adult skin, the oil composition seems to be important for maintaining lipid barrier integrity in infants (Summers et al., 2019). Moreover, the skin’s structural integrity may be even more affected by the frequency and duration of the oil’s use. An increase in TEWL and a decrease in hydration was identified after the application of a refined sunflower oil every three to four hours. It is not clear, however, if low-oleic acid or mid-oleic acid sunflower oil was used in the study (Kanti et al., 2014). Oleic acid-rich triglycerides of olive oil were previously shown to damage the lipid barrier integrity in adult skin (Danby et al., 2013). Based on the aforementioned negative effect of the frequent use of refined sunflower oil every three to 4 hours (Kanti et al., 2014), the importance of unsaponifiable matter may also be taken into account. Finally, we must emphasize that in terms of long-term safety, it is advisable to use vegetable butters and oils on infants only when necessary, as the penetration of dermally applied oils through the non-mature skin of babies was found to be significant because the triglyceride profile in blood changed after an oil massage four times a day for five days (Solanki et al., 2005).
Coconut, sunflower and olive oils were used in three studies on skin affected by atopic dermatitis (Verallo-Rowell et al., 2008; Danby et al., 2013; Evangelista et al., 2014). Coconut oil, characterized by the predominant saturated fatty acids in triglycerides, was superior to mineral oil (Evangelista et al., 2014). In addition, treatment with coconut oil resulted in a significantly decreased Staphylococcus aureus colonization in comparison to olive oil (Verallo-Rowell et al., 2008). As expected, olive oil was proven not to be a good option for the treatment of atopic dermatitis. Oleic acid-rich triglycerides of olive oil (76.3% oleic acid, 4.6% linoleic acid) damaged the lipid barrier integrity, while sunflower oil (27.3% oleic acid, 60.9% linoleic acid) with an approx. 1:2 ratio of oleic acid to linoleic acid did not disturb the stratum corneum integrity, caused no erythema and improved skin hydration in adults with and without a history of atopic dermatitis (Danby et al., 2013).
Other skin conditions or diseases have been researched to a lesser extent. One study (Brown et al., 2005) investigated the effect of kukui (Aleurites moluccana) oil on psoriasis. Kukui oil was characterized by an approx. 1:2 ratio of oleic acid (21.21%) to linoleic acid (41.27%) in triglycerides. The oil had a positive effect but the reduction of symptoms was not significant compared to the effects of the control oil (mineral oil). In 2005, a study was conducted that evaluated the ability of soybean (Glycine max) oil to protect the skin from UVB-induced erythema (Bonina et al., 2005). Experiments showed that soybean oil (10.6% oleic acid, 56.5% linoleic acid) with an approx. 1:5 ratio of oleic acid to linoleic acid exhibited beneficial protective activity, which was stronger than that of tocopheryl acetate. A study evaluating the dermal use of evening primrose (Oenothera biennis) oil in children with molluscum contagiosum confirmed the potential for therapeutic treatment. The study, however, was not controlled (Kwon et al., 2017). For treating tungiasis, a mixture of 20% neem (Azadirachta indica) oil and 80% coconut oil was compared to 0.05% KMnO4 (Elson et al., 2019). The antiparasitic effectiveness of the oil mixture against Tunga penetrans was not superior to that of KMnO4. However, secondary outcomes were better. In terms of the composition of this oil mixture, coconut oil contributes mainly saturated fatty acids, with the predominant acid being lauric acid, while neem oil is characterized by an approx. 1:2 ratio of oleic acid to linoleic acid. Researchers stressed that the compounds of neem oil unsaponifiable matter (azadirachtin, azadirachtin derivatives and salanin) contribute significantly to the antiparasitic activity.
In the aforementioned studies, inflammation was the main process controlling/affecting skin conditions/disorders. We conclude that in inflammation-affected skin, vegetable oils with a highly dominant content of oleic acid, together with the lack of or a low linoleic acid content, may cause additional disruptive changes to the stratum corneum structure. This may result in an increase of TEWL and a decrease in hydration, and in erythema. In contrast, beneficial dermal effects may be expected in inflammation-affected skin from vegetable oils and their mixtures with a high content of linoleic acid in triglycerides.
Vegetable butters and oils are also frequently used in the prevention and treatment of striae. Three studies (Taavoni et al., 2011; Soltanipour et al., 2012; Soltanipour et al., 2014) investigating olive oil were conducted, but no significant effects were observed in reducing the incidence and severity of striae. However, positive results were reported for a body oil composed of 55.9% safflower oil, 42% olive oil, 2% grapefruit (Citrus grandis) essential oil and 0.1% tocopherol (Bielfeldt et al., 2018). The fatty acid composition of triglycerides supports a beneficial contribution of linoleic acid to the overall effect.
Finally, although the effectiveness of vegetable butters and oils for the improvement of skin conditions, or prevention and treatment of skin diseases is supported by clinical evidence, some of the conclusions that we have drawn must be further studied and backed up by new research of high quality. Namely, limitations of the reviewed clinical studies generally include a small number of patients, heterogeneity in terms of study design and duration, methods of evaluation, dosing regimen, and an unknown composition of the fatty acid profile and unsaponifiable compounds.
Conclusion
The reviewed studies focused on the effects of 17 vegetable oils on non-affected skin, infant skin, psoriasis, xerosis, UVB-induced erythema, atopic dermatitis, molluscum contagiosum, tungiasis, scars, striae and striae gravidarum. Coconut, olive and sunflower oils appeared most frequently, which demonstrates their availability and recognition in terms of a long history of dermal use. However, less-known and newly discovered oils are gaining attention.
The reviewed clinical studies show the importance of vegetable butters and oils as therapeutically and cosmetically active ingredients for dermal use. Chemical composition of both the triglyceride fraction and unsaponifiable matter is the basis for the comprehensive understanding of mechanisms of action and effects after their application on the skin, and enables a customized approach for the treatment of skin diseases and cosmetic care of the skin. However, a lack of knowledge of how vegetable butters and oils and their components are metabolized and/or incorporated in the skin following dermal application, and how they affect the structure and properties of the lipid matrix as well as the skin’s microbiota call for further research.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was funded by the Slovenian Research Agency, grant number P1-0208.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agero, A. L., and Verallo-Rowell, V. M. (2004). A Randomized Double-Blind Controlled Trial Comparing Extra Virgin Coconut Oil with Mineral Oil as a Moisturizer for Mild to Moderate Xerosis. Dermatitis 15, 109–116. doi:10.2310/6620.2004.04006
Akihisa, T., Kojima, N., Katoh, N., Ichimura, Y., Suzuki, H., Fukatsu, M., et al. (2010). Triterpene Alcohol and Fatty Acid Composition of Shea Nuts from Seven African Countries. J. Oleo Sci. 59, 351–360. doi:10.5650/jos.59.351
Akinshina, A., Das, C., and Noro, M. G. (2016). Effect of Monoglycerides and Fatty Acids on a Ceramide Bilayer. Phys. Chem. Chem. Phys. 18, 17446–17460. doi:10.1039/C6CP01238H
Al-Jasass, F. M., and Al-Jasser, M. S. (2012). Chemical Composition and Fatty Acid Content of Some Spices and Herbs under Saudi Arabia Conditions. Scientific World J. 2012, 1–5. doi:10.1100/2012/859892
Alves, A. Q., Da Silva, V. A., Góes, A. J. S., Silva, M. S., De Oliveira, G. G., Bastos, I. V. G. A., et al. (2019). The Fatty Acid Composition of Vegetable Oils and Their Potential Use in Wound Care. Adv. Skin Wound Care 32, 1–8. doi:10.1097/01.ASW.0000557832.86268.64
Anzaku, A. A., Akyala, J. I., Juliet, A., and Obianuju, E. C. (2017). Antibacterial Activity of Lauric Acid on Some Selected Clinical Isolates. Ann. Clin. Lab. Res. 05, 2. doi:10.21767/2386-5180.1000170
Aswati, M., and Suprihastuti, S. R. (2010). “Epoxidation of Candlenut Oil,” in 2010 International Conference on Chemistry and Chemical Engineering (ICCCE) (Yogyakarta: IEEE). Available at: https://ieeexplore.ieee.org/stamp/stamp.jsp?tp=&arnumber=5560371 (Accessed February 3, 2020).
Badhe, Y., Gupta, R., and Rai, B. (2019). Structural and Barrier Properties of the Skin Ceramide Lipid Bilayer: a Molecular Dynamics Simulation Study. J. Mol. Model. 25, 140. doi:10.1007/s00894-019-4008-5
Bardaa, S., Ben Halima, N., Aloui, F., Ben Mansour, R., Jabeur, H., Bouaziz, M., et al. (2016). Oil from Pumpkin (Cucurbita Pepo L.) Seeds: Evaluation of its Functional Properties on Wound Healing in Rats. Lipids Health Dis. 15, 73. doi:10.1186/s12944-016-0237-0
Baumann, L. S. (2013). Stearic Acid. MDedge Dermatology. Available at: https://www.mdedge.com/dermatology/article/78248/aesthetic-dermatology/stearic-acid (Accessed January 28, 2020).
Berasategi, I., Barriuso, B., Ansorena, D., and Astiasarán, I. (2012). Stability of Avocado Oil during Heating: Comparative Study to Olive Oil. Food Chem. 132, 439–446. doi:10.1016/j.foodchem.2011.11.018
Bielfeldt, S., Blaak, J., Staib, P., Simon, I., Wohlfart, R., Manger, C., et al. (2018). Observer-blind Randomized Controlled Study of a Cosmetic Blend of Safflower, Olive and Other Plant Oils in the Improvement of Scar and Striae Appearance. Int. J. Cosmet. Sci. 40, 81–86. doi:10.1111/ics.12438
Bonina, F., Puglia, C., Avogadro, M., Baranelli, E., and Cravotto, G. (2005). The Topical Protective Effect of Soybean-Germ Oil against UVB-Induced Cutaneous Erythema: An In Vivo Evaluation. Arch. Pharm. (Weinheim) 338, 598–601. doi:10.1002/ardp.200500159
Boucetta, K. Q., Charrouf, Z., Derouiche, A., Rahali, Y., and Bensouda, Y. (2014). Skin Hydration in Postmenopausal Women: Argan Oil Benefit with Oral And/or Topical Use. Prz Menopauzalny 13, 280–288. doi:10.5114/pm.2014.46470
Brown, A. C., Koett, J., Johnson, D. W., Semaskvich, N. M., Holck, P., Lally, D., et al. (2005). Effectiveness of Kukui Nut Oil as a Topical Treatment for Psoriasis. Int. J. Dermatol. 44, 684–687. doi:10.1111/j.1365-4632.2005.02634.x
Chen, X., Zhao, X., Deng, Y., Bu, X., Ye, H., and Guo, N. (2019). Antimicrobial Potential of Myristic Acid against Listeria Monocytogenes in Milk. J. Antibiot. (Tokyo) 72, 298–305. doi:10.1038/s41429-019-0152-5
Chowdhury, K., Banu, L., Khan, S., and Latif, A. (1970). Studies on the Fatty Acid Composition of Edible Oil. Bangladesh J. Sci. Ind. Res. 42, 311–316. doi:10.3329/bjsir.v42i3.669
Čolić, S. D., Fotirić Akšić, M. M., Lazarević, K. B., Zec, G. N., Gašić, U. M., Dabić Zagorac, D. Č., et al. (2017). Fatty Acid and Phenolic Profiles of almond Grown in Serbia. Food Chem. 234, 455–463. doi:10.1016/J.FOODCHEM.2017.05.006
Cooke, A., Cork, M. J., Victor, S., Campbell, M., Danby, S., Chittock, J., et al. (2016). Olive Oil, sunflower Oil or No Oil for Baby Dry Skin or Massage: A Pilot, Assessor-Blinded, Randomized Controlled Trial (The Oil in Baby Skincare [observe] Study). Acta Derm. Venereol. 96, 323–330. doi:10.2340/00015555-2279
Cunha, M., Daza, F., Machado Filho, C. D. A., da Veiga, G. L., and Fonseca, F. L. A. (2018). The Relevance of Sebum Composition in the Etiopathogeny of Acne. Eur. J. Biol. Res. 8, 21–25. doi:10.5281/zenodo.1184139
Danby, S. G., AlEnezi, T., Sultan, A., Lavender, T., Chittock, J., Brown, K., et al. (2013). Effect of Olive and sunflower Seed Oil on the Adult Skin Barrier: Implications for Neonatal Skin Care. Pediatr. Dermatol. 30, 42–50. doi:10.1111/j.1525-1470.2012.01865.x
Darlenski, R., Sassning, S., Tsankov, N., and Fluhr, J. W. (2009). Non-invasive In Vivo Methods for Investigation of the Skin Barrier Physical Properties. Eur. J. Pharm. Biopharm. 72, 295–303. doi:10.1016/j.ejpb.2008.11.013
Darmstadt, G. L., Badrawi, N., Law, P. A., Ahmed, S., Bashir, M., Iskander, I., et al. (2004). Topically Applied sunflower Seed Oil Prevents Invasive Bacterial Infections in Preterm Infants in Egypt: a Randomized, Controlled Clinical Trial. Pediatr. Infect. Dis. J. 23, 719–725. doi:10.1097/01.inf.0000133047.50836.6f
Darmstadt, G. L., Saha, S. K., Ahmed, A. S., Chowdhury, M. A., Law, P. A., Ahmed, S., et al. (2005). Effect of Topical Treatment with Skin Barrier-Enhancing Emollients on Nosocomial Infections in Preterm Infants in Bangladesh: a Randomised Controlled Trial. Lancet 365, 1039–1045. doi:10.1016/S0140-6736(05)71140-5
de Oliveira, A. P., Franco, Ede. S., Rodrigues Barreto, R., Cordeiro, D. P., de Melo, R. G., de Aquino, C. M., et al. (2013). Effect of Semisolid Formulation of Persea Americana Mill (Avocado) Oil on Wound Healing in Rats. Evid. Based. Complement. Alternat. Med. 2013, 472382. doi:10.1155/2013/472382
Desai, A. S. (2017). Coconut Oil: The Future of Atopic Dermatitis Treatment? Dermatol. Ther. 30, e12472. doi:10.1111/dth.12472
D. Janeš, and N. Kočevar Glavač (Editors) (2018). Modern Cosmetics, Ingredients of Natural Origin, a Scientific View (Velenje, Slovenia: Širimo dobro besedo d.o.o.), Vol. 1. Available at: https://moderncosmethics.com/product/modern-cosmetics/.
Eichenfield, L. F., McCollum, A., and Msika, P. (2009). The Benefits of sunflower Oleodistillate (SOD) in Pediatric Dermatology. Pediatr. Dermatol. 26, 669–675. doi:10.1111/j.1525-1470.2009.01042.x
Elson, L., Randu, K., Feldmeier, H., and Fillinger, U. (2019). Efficacy of a Mixture of Neem Seed Oil (Azadirachta indica) and Coconut Oil (Cocos Nucifera) for Topical Treatment of Tungiasis. A Randomized Controlled, Proof-Of-Principle Study. Plos Negl. Trop. Dis. 13, e0007822. doi:10.1371/journal.pntd.0007822
Eskin, N. A. M. (2008). Borage and Evening Primrose Oil. Eur. J. Lipid Sci. Technol. 110, 651–654. doi:10.1002/ejlt.200700259
Evangelista, M. T., Abad-Casintahan, F., and Lopez-Villafuerte, L. (2014). The Effect of Topical virgin Coconut Oil on SCORAD index, Transepidermal Water Loss, and Skin Capacitance in Mild to Moderate Pediatric Atopic Dermatitis: a Randomized, Double-Blind, Clinical Trial. Int. J. Dermatol. 53, 100–108. doi:10.1111/ijd.12339
Fontanel, D. (2013). Unsaponifiable Matter in Plant Seed Oils. Berlin, Heidelberg: Springer. doi:10.1007/978-3-642-35710-7
Ghasemnezhad, A., and Honermeier, B. (2007). Seed Yield, Oil Content and Fatty Acid Composition of Oenothera Biennis L. Affected by Harvest Date and Harvest Method. Ind. Crops Prod. 25, 274–281. doi:10.1016/j.indcrop.2006.12.005
Gray, G. M., and Yardley, H. J. (1975). Lipid Compositions of Cells Isolated from Pig, Human, and Rat Epidermis. J. Lipid Res. 16, 434–440. doi:10.1016/s0022-2275(20)34493-x
Gunstone, F. D. (1996). Fatty Acid and Lipid Chemistry. Boston, MA: Springer US. doi:10.1007/978-1-4615-4131-8
Gürbüz, B., and Kiralan, M. (2007). Oil Content and Fatty Acid Composition of Some Safflower (Carthamus tinctorius L.) Varieties Sown in Spring and Winter. Available at: https://www.researchgate.net/publication/242606175 (Accessed March 29, 2021).
Hansen, H. S., and Jensen, B. (1985). Essential Function of Linoleic Acid Esterified in Acylglucosylceramide and Acylceramide in Maintaining the Epidermal Water Permeability Barrier. Evidence from Feeding Studies with Oleate, Linoleate, Arachidonate, Columbinate and Alpha-Linolenate. Biochim. Biophys. Acta 834, 357–363. doi:10.1016/0005-2760(85)90009-8
Harding, C. R. (2004). The Stratum Corneum: Structure and Function in Health and Disease. Dermatol. Ther. 17 (Suppl. 1), 6–15. [pii]. doi:10.1111/j.1396-0296.2004.04s1001.x
Herkenne, C., Alberti, I., Naik, A., Kalia, Y. N., Mathy, F. X., Préat, V., et al. (2008). In Vivo methods for the Assessment of Topical Drug Bioavailability. Pharm. Res. 25, 87–103. doi:10.1007/s11095-007-9429-7
Hou, D. D., Di, Z. H., Qi, R. Q., Wang, H. X., Zheng, S., Hong, Y. X., et al. (2017). Sea Buckthorn (Hippophaë Rhamnoides L.) Oil Improves Atopic Dermatitis-like Skin Lesions via Inhibition of NF-Κb and STAT1 Activation. Skin Pharmacol. Physiol. 30, 268–276. doi:10.1159/000479528
Jiang, S. J., Hwang, S. M., Choi, E. H., Elias, P. M., Ahn, S. K., and Lee, S. H. (2000). Structural and Functional Effects of Oleic Acid and Iontophoresis on Hairless Mouse Stratum Corneum. J. Invest. Dermatol. 114, 64–70. doi:10.1046/j.1523-1747.2000.00834.x
Kanti, V., Grande, C., Stroux, A., Bührer, C., Blume-Peytavi, U., and Garcia Bartels, N. (2014). Influence of Sunflower Seed Oil on the Skin Barrier Function of Preterm Infants: A Randomized Controlled Trial. Dermatology 229, 230–239. doi:10.1159/000363380
Khalil, M. H., Marcelletti, J. F., Katz, L. R., Katz, D. H., and Pope, L. E. (2000). Topical Application of Docosanol- or Stearic Acid-Containing Creams Reduces Severity of Phenol Burn Wounds in Mice. Contact Dermatitis 43, 79–81. doi:10.1034/j.1600-0536.2000.043002079.x
Komane, B., Vermaak, I., Summers, B., Viljoen, A., Liu, C.-H., Huang, H.-Y., et al. (2015). Safety and Efficacy of Sclerocarya Birrea (A.Rich.) Hochst (Marula) Oil: A Clinical Perspective. J. Ethnopharmacol. 176, 327–335. doi:10.1016/j.jep.2015.10.037
Konar, M. C., Islam, K., Roy, A., and Ghosh, T. (2020). Effect of virgin Coconut Oil Application on the Skin of Preterm Newborns: A Randomized Controlled Trial. J. Trop. Pediatr. 66, 129–135. doi:10.1093/tropej/fmz041
Korting, H. C., and Sterry, W. (2001). Therapeutische Verfahren in der Dermatologie: Dermatika und Kosmetika. Berlin: Blackwell Berlin.
Kwon, H. S., Lee, J. H., Kim, G. M., Choi, E. H., and Bae, J. M. (2017). Topical Evening Primrose Oil as a Possible Therapeutic Alternative in Children with Molluscum Contagiosum. Clin. Exp. Dermatol. 42, 923–925. doi:10.1111/ced.13226
Lewinska, A., Zebrowski, J., Duda, M., Gorka, A., and Wnuk, M. (2015). Fatty Acid Profile and Biological Activities of Linseed and Rapeseed Oils. Molecules 20, 22872–22880. doi:10.3390/molecules201219887
Lin, T. K., Zhong, L., Santiago, J. L., Zhong, L., and Santiago, J. L. (2017). Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 19, 70. doi:10.3390/ijms19010070
Liu, C. H., and Huang, H. Y. (2012). Antimicrobial Activity of Curcumin-Loaded Myristic Acid Microemulsions against Staphylococcus Epidermidis. Chem. Pharm. Bull. (Tokyo) 60, 1118–1124. doi:10.1248/cpb.c12-00220
Lodén, M., and Andersson, A. C. (1996). Effect of Topically Applied Lipids on Surfactant-Irritated Skin. Br. J. Dermatol. 134, 215–220. doi:10.1111/j.1365-2133.1996.tb07604.x10.1046/j.1365-2133.1996.978714.x
Lucas, L., Russell, A., and Keast, R. (2011). Molecular Mechanisms of Inflammation. Anti-inflammatory Benefits of virgin Olive Oil and the Phenolic Compound Oleocanthal. Curr. Pharm. Des. 17, 754–768. doi:10.2174/138161211795428911
Mack Correa, M. C., Mao, G., Saad, P., Flach, C. R., Mendelsohn, R., and Walters, R. M. (2014). Molecular Interactions of Plant Oil Components with Stratum Corneum Lipids Correlate with Clinical Measures of Skin Barrier Function. Exp. Dermatol. 23, 39–44. doi:10.1111/exd.12296
Maestri, D. M., Guzmán, G. A., and Giorda, L. M. (1998). Correlation between Seed Size, Protein and Oil Contents, and Fatty Acid Composition in Soybean Genotypes. Grasas y Aceites 49, 450–453. doi:10.3989/gya.1998.v49.i5-6.757
Mariod, A. A., and Abdelwahab, S. I. (2012). Sclerocarya birrea(Marula), an African Tree of Nutritional and Medicinal Uses: A Review. Food Rev. Int. 28, 375–388. doi:10.1080/87559129.2012.660716
Mariod, A. A., Ali, A. O., Elhussein, S. A., and Hussien, I. H. (2005). A Re-investigation of Physiochemical Characteristics and Fatty Acid Composition of Sclerocarya Birrea (Homeid) Kernel Oil. J. Sci. Technol. 6, 1–4. Available at: http://www.sustech.edu/staff_publications/20101216051603385.pdf (Accessed June 17, 2019).
Matsue, M., Mori, Y., Nagase, S., Sugiyama, Y., Hirano, R., Ogai, K., et al. (2019). Measuring the Antimicrobial Activity of Lauric Acid against Various Bacteria in Human Gut Microbiota Using a New Method. Cel Transpl. 28, 1528–1541. doi:10.1177/0963689719881366
Matthaus, B., Özcan, M. M., and Al Juhaimi, F. Y. (2015). Fatty Acid Composition and Tocopherol Profiles of Safflower (Carthamus tinctorius L.) Seed Oils. Nat. Prod. Res. 29, 193–196. doi:10.1080/14786419.2014.971316
Menon, G. K., Cleary, G. W., and Lane, M. E. (2012). The Structure and Function of the Stratum Corneum. Int. J. Pharm. 435, 3–9. doi:10.1016/j.ijpharm.2012.06.005
Mericli, F., Becer, E., Kabadayı, H., Hanoglu, A., Yigit Hanoglu, D., Ozkum Yavuz, D., et al. (2017). Fatty Acid Composition and Anticancer Activity in colon Carcinoma Cell Lines of Prunus Dulcis Seed Oil. Pharm. Biol. 55, 1239–1248. doi:10.1080/13880209.2017.1296003
Mitrović, P. M., Stamenković, O. S., Banković-Ilić, I., Djalović, I. G., Nježić, Z. B., Farooq, M., et al. (2020). White Mustard (Sinapis alba L.) Oil in Biodiesel Production: A Review. Front. Plant Sci. 11, 299. doi:10.3389/fpls.2020.00299
Momchilova, S., Antonova, D., Marekov, I., Kuleva, L., Nikolova‐Damyanova, B., and Jham, G. (2007). Fatty Acids, Triacylglycerols, and Sterols in Neem Oil (Azadirachta Indica A. Juss) as Determined by a Combination of Chromatographic and Spectral Techniques. J. Liquid Chromatogr. Relat. Tech. 30, 11–25. doi:10.1080/10826070601034188
Moore, E. M., Wagner, C., and Komarnytsky, S. (2020). The Enigma of Bioactivity and Toxicity of Botanical Oils for Skin Care. Front. Pharmacol. 11, 785. doi:10.3389/fphar.2020.00785
Moser, K., Kriwet, K., Naik, A., Kalia, Y. N., and Guy, R. H. (2001). Passive Skin Penetration Enhancement and its Quantification In Vitro. Eur. J. Pharm. Biopharm. 52, 103–112. doi:10.1016/S0939-6411(01)00166-7
Nanayakkara, G. R., Bartlett, A., Forbes, B., Marriott, C., Whitfield, P. J., and Brown, M. B. (2005). The Effect of Unsaturated Fatty Acids in Benzyl Alcohol on the Percutaneous Permeation of Three Model Penetrants. Int. J. Pharm. 301, 129–139. doi:10.1016/j.ijpharm.2005.05.024
Nangia, S., Paul, V. K., Deorari, A. K., Sreenivas, V., Agarwal, R., and Chawla, D. (2015). Topical Oil Application and Trans-epidermal Water Loss in Preterm Very Low Birth Weight Infants-A Randomized Trial. J. Trop. Pediatr. 61, 414–420. doi:10.1093/tropej/fmv049
Netzlaff, F., Kaca, M., Bock, U., Haltner-Ukomadu, E., Meiers, P., Lehr, C. M., et al. (2007). Permeability of the Reconstructed Human Epidermis Model Episkin in Comparison to Various Human Skin Preparations. Eur. J. Pharm. Biopharm. 66, 127–134. doi:10.1016/j.ejpb.2006.08.012
Nguyen, M. T., Hanzelmann, D., Härtner, T., Peschel, A., and Götz, F. (2016). Skin-Specific Unsaturated Fatty Acids Boost the Staphylococcus aureus Innate Immune Response. Infect. Immun. 84, 205–215. doi:10.1128/IAI.00822-15
Norlén, L., Nicander, I., Lundsjö, A., Cronholm, T., and Forslind, B. (1998). A New HPLC-Based Method for the Quantitative Analysis of Inner Stratum Corneum Lipids with Special Reference to the Free Fatty Acid Fraction. Arch. Dermatol. Res. 290, 508–516. doi:10.1007/s004030050344
Patzelt, A., Lademann, J., Richter, H., Darvin, M. E., Schanzer, S., Thiede, G., et al. (2012). In Vivo investigations on the Penetration of Various Oils and Their Influence on the Skin Barrier. Skin Res. Technol. 18, 364–369. doi:10.1111/j.1600-0846.2011.00578.x
Poljšak, N., and Kočevar Glavač, N. (2021). Tilia Sp. Seed Oil-Composition, Antioxidant Activity and Potential Use. Appl. Sci. 11, 4932. doi:10.3390/app11114932
Poljšak, N., Kreft, S., and Kočevar Glavač, N. (2019). Vegetable Butters and Oils in Skin Wound Healing: Scientific Evidence for New Opportunities in Dermatology. Phytotherapy Res. 34, 254–269. doi:10.1002/ptr.6524
Rabionet, M., Bayerle, A., Marsching, C., Jennemann, R., Gröne, H. J., Yildiz, Y., et al. (2013). 1-O-acylceramides Are Natural Components of Human and Mouse Epidermis. J. Lipid Res. 54, 3312–3321. doi:10.1194/jlr.M040097
Rawlings, A. V., Scott, I. R., Harding, C. R., and Bowser, P. A. (1994). Stratum Corneum Moisturization at the Molecular Level. J. Invest. Dermatol. 103, 731–741. doi:10.1111/1523-1747.ep12398620
Reinertson, R. P., and Wheatley, V. R. (1959). Studies on the Chemical Composition of Human Epidermal Lipids. J. Invest. Dermatol. 32, 49–59. Available at: http://www.ncbi.nlm.nih.gov/pubmed/13620967 (Accessed November 25, 2019). doi:10.1038/jid.1959.11
Said-Al Ahl, H. A. H., and Ramadan, M. F. (2017). Oil Yield and Fatty Acid Profile of Oenothera Biennis as Affected by Different Levels of Nitrogen and Zinc Fertilization. La Riv. Ital. Delle Sostanze Grasse XCIV. Available at: https://www.innovhub.com//c/document_library/get_file?uuid=5958f2d4-5d7d-4d18-9341-7e1111a77e94&groupId=11654 (Accessed June 12, 2019).
Siddique, B. M., Ahmad, A., Alkarkhi, A. F., Ibrahim, M. H., and K, M. O. (2011). Chemical Composition and Antioxidant Properties of Candlenut Oil Extracted by Supercritical CO2. J. Food Sci. 76, C535–C542. doi:10.1111/j.1750-3841.2011.02146.x
Soimee, W., Nakyai, W., Charoensit, P., Grandmottet, F., Worasakwutiphong, S., Phimnuan, P., et al. (2020). Evaluation of Moisturizing and Irritation Potential of Sacha Inchi Oil. J. Cosmet. Dermatol. 19, 915–924. doi:10.1111/jocd.13099
Solanki, K., Matnani, M., Kale, M., Joshi, K., Bavdekar, A., Bhave, S., et al. (2005). Transcutaneous Absorption of Topically Massaged Oil in Neonates. Indian Pediatr. 42 (10), 998–1005.
Soltanipoor, F., Delaram, M., Taavoni, S., and Haghani, H. (2012). The Effect of Olive Oil on Prevention of Striae Gravidarum: A Randomized Controlled Clinical Trial. Complement. Ther. Med. 20, 263–266. doi:10.1016/j.ctim.2012.05.001
Soltanipour, F., Delaram, M., Taavoni, S., and Haghani, H. (2014). The Effect of Olive Oil and the Saj® Cream in Prevention of Striae Gravidarum: A Randomized Controlled Clinical Trial. Complement. Ther. Med. 22, 220–225. doi:10.1016/j.ctim.2013.11.011
Sookwong, P., and Mahatheeranont, S. (2017). Supercritical CO2 Extraction of Rice Bran Oil -the Technology, Manufacture, and Applications. J. Oleo Sci. 66, 557–564. doi:10.5650/jos.ess17019
Stamatas, G. N., de Sterke, J., Hauser, M., von Stetten, O., and van der Pol, A. (2008). Lipid Uptake and Skin Occlusion Following Topical Application of Oils on Adult and Infant Skin. J. Dermatol. Sci. 50, 135–142. doi:10.1016/j.jdermsci.2007.11.006
Strunk, T., Pupala, S., Hibbert, J., Doherty, D., and Patole, S. (2018). Topical Coconut Oil in Very Preterm Infants: An Open-Label Randomised Controlled Trial. Neonatology 113, 146–151. doi:10.1159/000480538
Styrczewska, M., Zuk, M., Boba, A., Zalewski, I., and Kulma, A. (2019). Use of Natural Components Derived from Oil Seed Plants for Treatment of Inflammatory Skin Diseases. Curr. Pharm. Des. 25, 2241–2263. doi:10.2174/1381612825666190716111700
Sugawara, E., and Nikaido, H. (2014). Properties of AdeABC and AdeIJK Efflux Systems of Acinetobacter Baumannii Compared with Those of the AcrAB-TolC System of Escherichia coli. Antimicrob. Agents Chemother. 58, 7250–7257. doi:10.1128/AAC.03728-14
Summers, A., Visscher, M. O., Khatry, S. K., Sherchand, J. B., Leclerq, S. C., Katz, J., et al. (2019). Impact of sunflower Seed Oil versus Mustard Seed Oil on Skin Barrier Function in Newborns: A Community-Based, Cluster-Randomized Trial. BMC Pediatr. 19, 512. doi:10.1186/s12887-019-1871-2
Taavoni, S., Soltanipour, F., Haghani, H., Ansarian, H., and Kheirkhah, M. (2011). Effects of Olive Oil on Striae Gravidarum in the Second Trimester of Pregnancy. Complement. Ther. Clin. Pract. 17, 167–169. doi:10.1016/j.ctcp.2010.10.003
Tanojo, H., Boelsma, E., Junginger, H. E., Ponec, M., and Boddé, H. E. (1998). In Vivo human Skin Barrier Modulation by Topical Application of Fatty Acids. Skin Pharmacol. Appl. Skin Physiol. 11, 87–97. sph11087 [pii]. doi:10.1159/000029813
Uzun, B., Arslan, C., Karhan, M., and Toker, C. (2007). Fat and Fatty Acids of white Lupin (Lupinus Albus L.) in Comparison to Sesame (Sesamum indicum L.). Food Chem. 102, 45–49. doi:10.1016/j.foodchem.2006.03.059
Van Smeden, J., Boiten, W. A., Hankemeier, T., Rissmann, R., Bouwstra, J. A., and Vreeken, R. J. (2014). Combined LC/MS-platform for Analysis of All Major Stratum Corneum Lipids, and the Profiling of Skin Substitutes. Biochim. Biophys. Acta 1841, 70–79. doi:10.1016/j.bbalip.2013.10.002
Vaughn, A. R., Clark, A. K., Sivamani, R. K., and Shi, V. Y. (2018). Natural Oils for Skin-Barrier Repair: Ancient Compounds Now Backed by Modern Science. Am. J. Clin. Dermatol. 19, 103–117. doi:10.1007/s40257-017-0301-1
Verallo-Rowell, V. M., Dillague, K. M., and Syah-Tjundawan, B. S. (2008). Novel Antibacterial and Emollient Effects of Coconut and virgin Olive Oils in Adult Atopic Dermatitis. Dermatitis 19, 308–315. doi:10.2310/6620.2008.08052
Verallo-Rowell, V. M., Katalbas, S. S., and Pangasinan, J. P. (2016). Natural (Mineral, Vegetable, Coconut, Essential) Oils and Contact Dermatitis. Curr. Allergy Asthma Rep. 16, 51. doi:10.1007/s11882-016-0630-9
Watanabe, T., Yamamoto, Y., Miura, M., Konno, H., Yano, S., and Nonomura, Y. (2019). Systematic Analysis of Selective Bactericidal Activity of Fatty Acids against Staphylococcus aureus with Minimum Inhibitory Concentration and Minimum Bactericidal Concentration. J. Oleo Sci. 68, 291–296. doi:10.5650/jos.ess18220
Weitkamp, A. W., Smiljanic, A. M., and Rothman, S. (1947). The Free Fatty Acids of Human Hair Fat. J. Am. Chem. Soc. 69, 1936–1939. doi:10.1021/ja01200a027
Wertz, P. W. (1992). Epidermal Lipids. Semin. Dermatol. 11, 106–113. doi:10.1007/978-3-642-97234-8_3
Wertz, P. W. (2018). Lipids and the Permeability and Antimicrobial Barriers of the Skin. J. Lipids 2018, 5954034. doi:10.1155/2018/5954034
Wille, J. J., and Kydonieus, A. (2003). Palmitoleic Acid Isomer (C16:1delta6) in Human Skin Sebum Is Effective against Gram-Positive Bacteria. Skin Pharmacol. Appl. Skin Physiolphysiol 16, 176–187. doi:10.1159/000069757
Ziboh, V. A., Miller, C. C., and Cho, Y. (2000). Metabolism of Polyunsaturated Fatty Acids by Skin Epidermal Enzymes: Generation of Antiinflammatory and Antiproliferative Metabolites. Am. J. Clin. Nutr. 71, 361s–6S. doi:10.1093/ajcn/71.1.361s
Ziboh, V. A., and Miller, C. C. (1990). Essential Fatty Acids and Polyunsaturated Fatty Acids: Significance in Cutaneous Biology. Annu. Rev. Nutr. 10, 433–450. doi:10.1146/annurev.nu.10.070190.002245
Keywords: vegetable butters and oils, skin barrier, fatty acids, infant skin, xerosis, atopic dermatitis, psoriasis
Citation: Poljšak N and Kočevar Glavač N (2022) Vegetable Butters and Oils as Therapeutically and Cosmetically Active Ingredients for Dermal Use: A Review of Clinical Studies. Front. Pharmacol. 13:868461. doi: 10.3389/fphar.2022.868461
Received: 02 February 2022; Accepted: 21 March 2022;
Published: 25 April 2022.
Edited by:
Alessandra Durazzo, Council for Agricultural Research and Economics, ItalyReviewed by:
Guevara Nonviho, Université Nationale des Sciences, BeninAshraf Hamed, Minia University, Egypt
Copyright © 2022 Poljšak and Kočevar Glavač. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nina Kočevar Glavač, bmluYS5rb2NldmFyLmdsYXZhY0BmZmEudW5pLWxqLnNp
†These authors have contributed equally to this work and share first authorship
 Nina Poljšak
Nina Poljšak Nina Kočevar Glavač
Nina Kočevar Glavač