- 1Department of Obstetrics and Gynecology, University Hospital Hradec Kralove, Faculty of Medicine in Hradec Kralove, Charles University, Hradec Kralove, Czech Republic
- 2Biomedical Research Center, University Hospital Hradec Kralove, Hradec Kralove, Czech Republic
- 3Institute of Clinical Biochemistry and Diagnostics, Faculty of Medicine in Hradec Kralove, University Hospital Hradec Kralove, Charles University, Hradec Kralove, Czech Republic
- 4Institute of Clinical Microbiology, University Hospital Hradec Kralove, Charles University, Faculty of Medicine in Hradec Kralove, Hradec Kralove, Czech Republic
- 5Department of Social Medicine, Charles University, Faculty of Medicine in Hradec Kralove, Hradec Kralove, Czech Republic
- 6Department of Obstetrics and Gynecology, Institute of Clinical Science, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
- 7Region Västra Götaland, Sahlgrenska University Hospital, Department of Obstetrics and Gynecology, Gothenburg, Sweden
- 8Department of Genetics and Bioinformatics, Domain of Health Data and Digitalization, Institute of Public Health, Oslo, Norway
Objectives: To determine the prevalence and load of Ureaplasma spp. DNA in the cervical fluid of women with singleton pregnancies complicated by preterm prelabor rupture of membranes (PPROM) with respect to intra-amniotic infection, sterile intra-amniotic inflammation, and colonization of the amniotic fluid.
Methods: A total of 217 women with PPROM between gestational ages 24 + 0 and 33 + 6 weeks were included in this study. Paired amniotic and cervical fluid samples were collected at the time of admission via transabdominal amniocentesis and using a Dacron polyester swab, respectively. Microbial invasion of the amniotic cavity was diagnosed using a combination of culture and molecular biology methods. Intra-amniotic inflammation was determined based on the concentration of interleukin-6 in the amniotic fluid. Based on the presence or absence of these conditions, the women were stratified into the following subgroups: intra-amniotic infection (with both), sterile intra-amniotic inflammation (with inflammation only), colonization (with microorganisms only), and negative amniotic fluid (without either). The Ureaplasma spp. DNA load in the cervical fluid was assessed using PCR.
Results: Ureaplasma spp. DNA in the cervical fluid was found in 61% (133/217) of the women. Women with negative amniotic had similar prevalence of Ureaplasma spp. DNA in cervical fluid (55%) to those with sterile intra-amniotic inflammation (54%) but lower than those with intra-amniotic infection (73%) and colonization (86%; p < 0.0001). Women with negative amniotic fluid had a lower load of Ureaplasma spp. DNA in their cervical fluid (median: 4.7 × 103 copies of DNA/ml) than those with intra-amniotic infection (median: 2.8 × 105 copies DNA/ml), sterile intra-amniotic inflammation (median: 5.3 × 104 copies DNA/ml), and colonization (median: 1.2 × 105 copies DNA/mL; p < 0.0001).
Conclusion: In conclusion, in PPROM at <34 weeks, the presence of intra-amniotic infection, sterile intra-amniotic inflammation, or colonization of the amniotic fluid was associated with a higher prevalence and/or load of Ureaplasma spp. DNA in the cervical fluid than the absence of intra-amniotic complications.
Introduction
Preterm prelabor rupture of the membranes (PPROM) is defined as rupture of the fetal membranes with leakage of amniotic fluid before the onset of regular uterine activity before 37 weeks of gestation (Mercer, 2003; Mercer, 2005). PPROM is one of the “great obstetrical syndromes,” with considerable medical and socio-economic impacts (Romero, 1996; Romero et al., 2006; Di Renzo, 2009; Brosens et al., 2011). PPROM remains under intensive debate among scientists, researchers, and clinicians given the necessity: 1) to fully unravel the underlying pathophysiological mechanisms to make the prevention of PPROM possible (Moore et al., 2020); 2) to understand the underlying mechanisms affecting the interval between PPROM and delivery to optimize the timing of induction of lung maturity (Battarbee et al., 2020); 3) to characterize the causality and consequences of microbial invasion of the amniotic cavity (the presence of microorganisms and/or their DNA in the amniotic fluid) and intra-amniotic inflammation (elevation of inflammatory mediators in the amniotic fluid) (Menon and Richardson, 2017); and 4) to identify risk factors and reliable biomarkers of microbial invasion of the amniotic cavity and intra-amniotic inflammation to enable a individualized therapeutic approach (Stranik et al., 2021).
The presence of microbial invasion of the amniotic cavity and intra-amniotic inflammation complicates approximately 23–41% and 17–58%, respectively, of pregnancies with PPROM (Romero et al., 2015; Kacerovsky et al., 2020a; Kacerovsky et al., 2021b). Based on these two intra-amniotic complications, the following scenarios can occur in PPROM pregnancies: 1) intra-amniotic infection (presence of both), 2) sterile intra-amniotic inflammation (intra-amniotic inflammation only), 3) colonization of the amniotic fluid (microbial invasion of the amniotic cavity only), and 4) negative amniotic fluid (absence of both) (Musilova et al., 2015; Romero et al., 2015).
There is plethora of evidence that Ureaplasma spp. are the most common microorganisms recovered from the amniotic fluid obtained from PPROM pregnancies. Therefore, these low-virulence bacteria with sizes comparable to those of viruses represent the most common cause of intra-amniotic infection or colonization of the amniotic cavity in PPROM (Kacerovsky et al., 2021b). Because they are commonly found in the cervical/vaginal niche of women with PPROM, the lower genital tract is considered the main source of amniotic fluid Ureaplasma spp.
The presence of Ureaplasma spp. in the cervical/vaginal niche in PPROM pregnancies is related to a higher risk of ascension and their subsequent presence in amniotic fluid (Musilova et al., 2016). Nevertheless, there is a shortage of information on whether their prevalence and loads in the cervical niche might be prone to a specific subtype of intra-amniotic complications, such as intra-amniotic infection, sterile intra-amniotic inflammation, and colonization of the amniotic cavity.
To fill this knowledge gap, this study aimed to determine the prevalence of Ureaplasma spp. DNA in the cervical fluid of the subgroups of women with PPROM with intra-amniotic infection, sterile intra-amniotic inflammation, colonization of the amniotic cavity, and negative amniotic fluid. The secondary aim was to compare the microbial load of Ureaplasma spp. DNA in the cervical fluid among subgroups of women with PPROM. The final aim was to compare the relative abundance of Ureaplasma spp. DNA in the cervical fluid among subgroups of women with PPROM.
Materials and Methods
In this retrospective study, we included pregnant women admitted to the Department of Obstetrics and Gynecology of the University Hospital Hradec Kralove, Czech Republic, between May 2015 and May 2021, who met the following criteria: 1) age ≥18 years; 2) singleton pregnancy; 3) gestational ages between 24 + 0 and 33 + 6 weeks; 4) PPROM; and 5) amniocentesis to assess the intra-amniotic environment. The exclusion criteria were as follows: 1) pregnancy-related complications (e.g., fetal growth restriction, gestational, gestational hypertension, and preeclampsia); 2) chronic medical complications (e.g., pregestational diabetes mellitus and chronic hypertension); 3) congenital or chromosomal fetal abnormalities; 4) signs of fetal hypoxia; 4) significant vaginal bleeding; and 5) preterm labor with intact membranes.
PPROM was diagnosed based on visual confirmation of amniotic fluid pooling in the posterior vaginal fornix by sterile speculum examination. If uncertainty about amniotic fluid leakage remained after the clinical examination, the presence of insulin-like growth factor-binding protein in the vaginal fluid was assessed (Actim PROM test; Medix Biochemica, Kauniainen, and Finland).
Body fluid samples (amniotic fluid first, cervical fluid second) were collected at the time of admission before the administration of corticosteroids, antibiotics, or tocolytics. Transabdominal amniocentesis to obtain an amniotic fluid sample is a standard part of the department’s clinical management of women with PPROM to assess the intra-amniotic environment. Women with PPROM received corticosteroids to accelerate lung maturation and intravenous antibiotics. Women with intra-amniotic inflammation received clarithromycin for 7 days unless delivery occurred. Those without intra-amniotic inflammation were treated with benzylpenicillin for 7 days unless delivery occurred. In the case of penicillin allergy, women were treated with clindamycin for 7 days unless delivery occurred. Once the final results regarding microbial invasion of the amniotic cavity from cultivation or PCR were known, the attending clinician modified the women’s antibiotic therapies accordingly. Tocolysis was used only in women who developed regular uterine activity during the course of corticosteroids or within 24 h after their administration. The women were managed expectantly, except those with intra-amniotic infection (the presence of both microbial invasion of the amniotic cavity and intra-amniotic inflammation) beyond the 28th gestational week. This subset of women with PPROM was managed actively (labor was induced or an elective cesarean section was performed after finalizing corticosteroid treatment within 72 h of membrane rupture).
This study was approved by the institutional review board of the University Hospital Hradec Kralove (June 2014, No. 201408 S07P). All study participants were Caucasian, and informed consent was obtained from all participants.
Amniotic fluid samples, cervical fluid samples, and the clinical and demographic data of some women from this cohort were used in our previous studies (Musilova et al., 2017a; Musilova et al., 2017b; Hornychova et al., 2018; Kacerovsky et al., 2018; Musilova et al., 2018; Janku et al., 2019; Kacerovsky et al., 2020a; Kacerovsky et al., 2020b; Musilova et al., 2020; Soucek et al., 2020; Spacek et al., 2020; Kacerovsky et al., 2021a; Kacerovsky et al., 2021b; Matulova et al., 2021; Musilova et al., 2021; Stranik et al., 2021)
Amniotic Fluid Sampling
Ultrasonography-guided transabdominal amniocentesis was performed before administration of corticosteroids, antibiotics, or tocolytics. The details of amniotic fluid sampling have been previously described (Kacerovsky et al., 2020a; Stranik et al., 2021).
Cervical Fluid Sampling
Cervical fluid samples were collected using Dacron polyester swabs. The details of the procedure have been described previously. Pellets were used to assess the bacterial DNA and Ureaplasma spp. DNA (Musilova et al., 2016; Kacerovsky et al., 2020a; Kacerovsky et al., 2021c).
Assessment of IL-6
Amniotic fluid samples obtained from May 2015 to November 2018 were assessed using a Milenia QuickLine IL-6 lateral flow immunoassay and Milenia POC-Scan Reader (Milenia Biotec, GmbH, Giessen, Germany) (Kacerovsky et al., 2014). The measurement range was 50–10,000 pg/ml. Samples obtained between December 2018 and May 2021 were evaluated using an automated electrochemiluminescence immunoassay method with a Cobas e602 immunoanalyzer, which is part of the Cobas 8,000 platform (Roche Diagnostics, Basel, Switzerland) (Musilova et al., 2020). The measurement range was 1.5–5,000 pg/ml, which could be extended to 50,000 pg/ml with a 10-fold dilution of the sample.
Detection of Ureaplasma spp., Mycoplasma hominis, and Chlamydia trachomatis in the amniotic fluid.
To assess the amniotic fluid, a commercial AmpliSens® C. trachomatis/Ureaplasma/M. hominis-FRT kit (Federal State Institution of Science, Central Research Institute of Epidemiology, Moscow, Russia) was used to detect DNA from Ureaplasma spp., M. hominis, and Ch. trachomatis in a single PCR tube for each fluid. The details of this procedure have been described previously (Kacerovsky et al., 2020a; Kacerovsky et al., 2020b; Stranik et al., 2021).
Detection of bacteria other than Ureaplasma spp., Mycoplasma hominis, or Chlamydia trachomatis in the amniotic fluid.
The detection of bacteria other than Ureaplasma spp., M. hominis, and Ch. trachomatis in the women’s amniotic fluid using aerobic/anaerobic cultivation and non-cultivation methods has been described previously (Kacerovsky et al., 2020a; Kacerovsky et al., 2020b; Stranik et al., 2021).
Detection of Ureaplasma spp. and Mycoplasma hominis in the Cervical Fluid
To assess the cervical fluid, a commercial AmpliSens® C. trachomatis/Ureaplasma/M. hominis-FRT kit (Federal State Institution of Science, Central Research Institute of Epidemiology, Moscow, Russia) was used to detect DNA from Ureaplasma spp., M. hominis, and Ch. trachomatis in a single PCR tube for each fluid. The details of this procedure have been described previously. The load of Ureaplasma spp. (copies/ml) was determined using an absolute quantification technique with an external calibration curve. Plasmid DNA (pCR3, Invitrogen, Carlsbad, CA, United States) was used to prepare a calibration curve.
The relative abundance of Ureaplasma spp. DNA in the cervical fluid and total bacterial DNA detection were performed using quantitative RT-PCR–BactQuant (Liu et al., 2012). To quantify the bacterial load, we used the forward primer CCTACGGGDGGCWGCA, reverse primer GGA CTACHVGGGTMTCTAATC, and hydrolysis probe FAM-BHQ1 CAGCCGCGGTA. A calibration curve was generated using 10-fold dilutions of linearized and normalized plasmids containing the cloned target sequence of the 466-bp region in the V3-V4 domain of 16S rRNA at a concentration of 107 copies/µl (Generi Biotech, Hradec Kralove, Czech Republic) (Kacerovsky et al., 2019). The relative abundance of Ureaplasma spp. in the cervical microbiota was calculated [(Ureaplasma spp. DNA load/total bacterial DNA load) × 100] and expressed as percentages. The PCR conditions used in the BactQuant assay were the same as those used for Ureaplasma spp.
Clinical Definitions
Microbial invasion of the amniotic cavity was determined based on a positive PCR analysis for Ureaplasma spp., M. hominis, or Ch. trachomatis in the amniotic fluid, their combination, positivity for the 16S rRNA gene in the amniotic fluid, aerobic/anaerobic cultivation of the amniotic fluid, or a combination of these parameters. Intra-amniotic inflammation was defined as a concentration of IL-6 in the amniotic fluid that was ≥745 pg/ml when measured using a lateral flow immunoassay point-of-care test (Chaemsaithong et al., 2016a; Chaemsaithong et al., 2016b) or ≥3,000 pg/ml when measured using an automated electrochemiluminescence immunoassay method (Musilova et al., 2020). Intra-amniotic infection was defined as the presence of microbial invasion of the amniotic cavity and intra-amniotic inflammation. Women with intra-amniotic infection were further subdivided into those with and without Ureaplasma spp. in the amniotic fluid. Sterile intra-amniotic inflammation was defined as the presence of intra-amniotic inflammation without microbial invasion into the amniotic cavity. Colonization of the amniotic cavity was defined as the presence of microbial invasion in the amniotic cavity in the absence of intra-amniotic inflammation. Women with colonization were further subdivided into those with and without Ureaplasma spp. in the amniotic fluid. Negative amniotic fluid was defined as amniotic fluid without microbial invasion of the amniotic cavity or intra-amniotic inflammation.
Statistical Analyses
The demographic and clinical characteristics of the patients were compared using the non-parametric Kruskal–Wallis test for continuous variables and chi-square test for categorical variables, and the results are presented as medians (interquartile range [IQR]) and numbers (%), respectively. The normality of the data was tested using the Anderson–Darling test. The non-parametric Kruskal–Wallis or Mann-Whitney U tests were used, as appropriate, to compare the loads of bacteria and Ureaplasma spp. DNA in the cervical fluid and the relative abundance of Ureaplasma spp. DNA in the cervical fluid. Chi-squared or Fisher’s exact tests were used, as appropriate, to compare the prevalence of Ureaplasma spp. DNA in the cervical fluid. Differences were considered statistically significant at p < 0.05. All p-values were determined using two-tailed tests, and all statistical analyses were performed using GraphPad Prism 8.4.3 for Mac OS X (GraphPad Software, San Diego, CA, United States).
Results
Overall, 217 women with singleton pregnancies and PPROM between gestational ages 24 + 0 and 33 + 6 weeks were included in the study. Intra-amniotic infection, sterile intra-amniotic inflammation, colonization of the amniotic cavity, and negative amniotic fluid were observed in 20% (44/217), 11% (24/217), 10% (21/217), and 59% (128/217) of women, respectively. The demographic and clinical data of the women with PPROM, as well as short-term neonatal outcomes are summarized in Table 1.

TABLE 1. Demographical and clinical characteristics and short-term neonatal outcomes of pregnancies with preterm prelabor rupture of membranes prior to 34 weeks of gestation according to the presence of intra-amniotic infection, sterile intra-amniotic inflammation, colonization of the amniotic cavity, and negative amniotic fluid.
The most common microorganisms in the amniotic fluid were Ureaplasma spp., which were found in 59% (26/44) and 71% (15/21) of women with intra-amniotic infection and colonization of the amniotic cavity, respectively. All microbial findings in the amniotic fluid that were identified in the women with intra-amniotic infection are shown in Table 2.
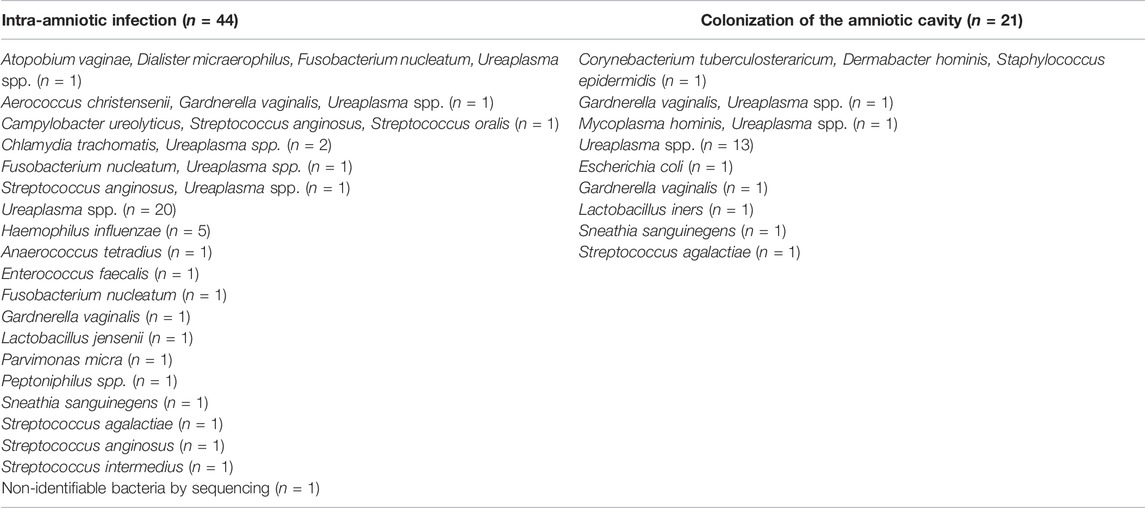
TABLE 2. The microbial species identified in the amniotic fluid from pregnancies with preterm prelabor rupture of membranes prior to 34 weeks of gestation complicated with intra-amniotic infection and colonization of the amniotic cavity.
Ureaplasma spp. DNA in the cervical fluid was identified in 61% (133/217) of the women. All of the women with Ureaplasma spp. DNA in their amniotic fluid (n = 41) also had Ureaplasma spp. DNA in their cervical fluid.
Prevalence of Ureaplasma spp. DNA in the Cervical Fluid
The prevalence of Ureaplasma spp. DNA in the cervical fluid varied among the subgroups of women with intra-amniotic infection (73% [32/44]), sterile intra-amniotic inflammation (54% [13/24]), colonization (86% [18/21]), and negative amniotic fluid (55% [70/128]; p = 0.01; Figure 1).
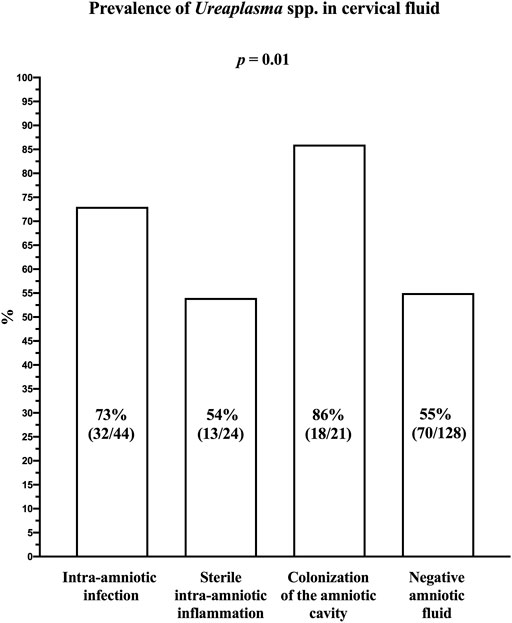
FIGURE 1. Prevalence of Ureaplasma spp. DNA in the cervical fluid of women with preterm prelabor rupture of membranes before 34 weeks of gestation according to the presence of intra-amniotic infection, sterile intra-amniotic inflammation, colonization of the amniotic cavity, and negative amniotic fluid.
Women with negative amniotic fluid had a lower prevalence of Ureaplasma spp. DNA in their cervical fluid compared to the women with intra-amniotic infections (p = 0.05) and colonization (p = 0.008); however, they had a similar prevalence to those with sterile intra-amniotic inflammation (p = 1.00).
Microbial Load of Ureaplasma spp. DNA in the Cervical Fluid
The load of Ureaplasma spp. DNA in the cervical fluid varied among the subgroups (median [IQR]; intra-amniotic infection: 2.8 × 105 copies DNA/mL [8.9 × 104–1.1 × 106]; sterile intraamniotic inflammation: 5.3 × 104 copies DNA/mL [7.8 × 103–9.2 × 105]; colonization 1.2 × 105 copies DNA/ml [5.2 × 104–1.1 × 106]; and negative amniotic fluid: 4.7 × 103 copies DNA/mL [9.3 × 102–4.6 × 104]; p < 0.0001; Figure 2A), as well as when the women with Ureaplasma spp. in the amniotic fluid were excluded (median [IQR]; intra-amniotic infection: 1.1 × 105 copies DNA/mL [7.3 × 103–6.7 × 105]; colonization 1.3 × 105 copies DNA/mL [1.1 × 105–1.6 × 105]; p = 0.003; Figure 2B).
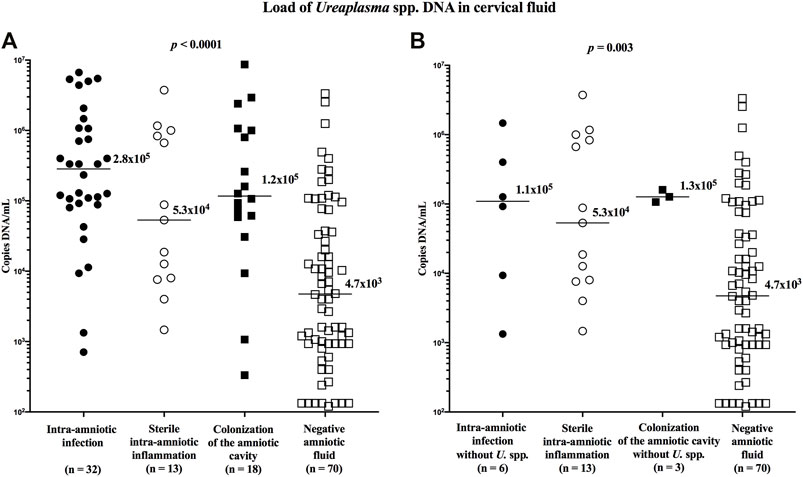
FIGURE 2. Comparison of the loads of Ureaplasma spp. DNA in the cervical fluid among groups of the women with intra-amniotic infection, sterile intra-amniotic inflammation, colonization of the amniotic fluid, and negative amniotic fluid (A) and among the same groups when women with the presence of Ureaplasma spp. in amniotic fluid were excluded (B). The median values are marked.
Women with negative amniotic fluid had a lower load of Ureaplasma spp. DNA in the cervical fluid compared to those with intra-amniotic infection (p < 0.0001; without Ureaplasma spp. in amniotic fluid: p = 0.04), sterile intra-amniotic inflammation (p = 0.004), and colonization (p = 0.0001; without Ureaplasma spp. in amniotic fluid: p = 0.04).
Relative Abundance of Ureaplasma spp. in the Cervical Fluid
To assess the relative abundance of Ureaplasma spp. in the cervical fluid between the subgroups of women with PPROM, the amount of bacterial DNA in the cervical fluid was first evaluated. No difference in the concentration of bacterial DNA was identified between the subgroups (median [IQR]; intra-amniotic infection: 5.7 × 106 copies DNA/ml [2.9 × 105–3.1 × 107]; sterile intra-amniotic inflammation: 5.8 × 106 copies DNA/ml [4.4 × 105–5.4 × 107]; colonization: 5.8 × 106 copies DNA/ml [9.0 × 105–6.1 × 107]; and negative amniotic fluid: 4.3 × 106 copies DNA/ml [5.9 × 105–2.0 × 107]; p = 0.89; Figure 3).
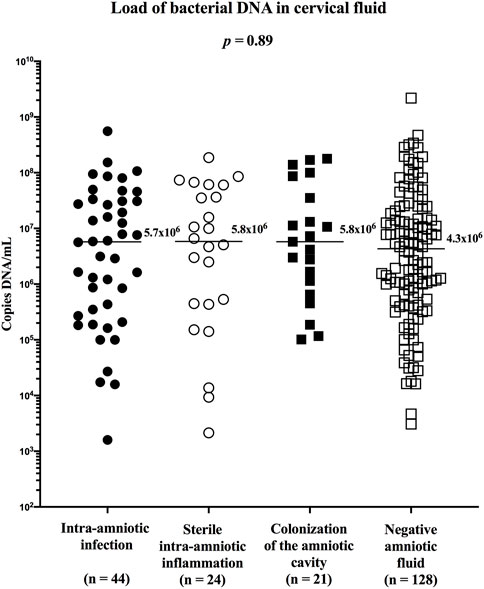
FIGURE 3. Comparison of the load of bacterial DNA in the cervical fluid among groups of women with intra-amniotic infection, sterile intra-amniotic inflammation, colonization of the amniotic fluid, and negative amniotic fluid.
The relative abundance of Ureaplasma spp. in the cervical fluid differed among the subgroups (median [IQR]; intra-amniotic infection: 14.2% [2.6–100.0]; sterile intra-amniotic inflammation: 0.8% [0.4–5.9]; colonization: 4.4% [0.5–9.4]; and negative amniotic fluid: 0.3% [0.1–0.9]; p < 0.0001; Figure 4A). However, after excluding the women with Ureaplasma spp. in the amniotic fluid, the difference reached borderline statistical significance (median [IQR]; intra-amniotic infection: 0.3% [0.2–7.7]; colonization: 9.5% [0.4–23.7]; p = 0.06; Figure 4B).
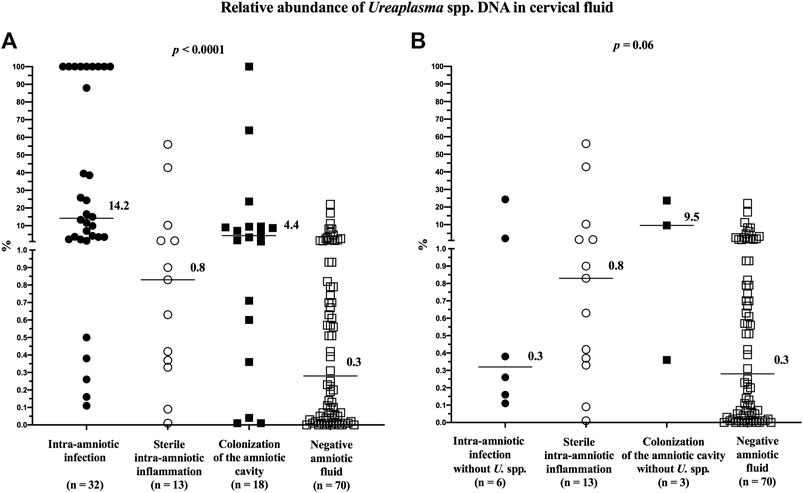
FIGURE 4. Comparison of the relative abundance of Ureaplasma spp. DNA in the cervical fluid among groups of women with intra-amniotic infection, sterile intra-amniotic inflammation, colonization of the amniotic fluid, and negative amniotic fluid (A) and among the same groups when women with the presence of Ureaplasma spp. in their amniotic fluid were excluded (B). The median values are marked.
Women with negative amniotic fluid had a lower relative abundance than those with intra-amniotic infection (p < 0.0001), sterile intra-amniotic inflammation (p = 0.05), or colonization (p = 0.008). After excluding the women with Ureaplasma spp. in the amniotic fluid, there was no difference (intra-amniotic infection: p = 0.36; colonization: p = 0.06).
Discussion
The principal findings of this study in women with PPROM before 34 weeks were as follows: 1) the total prevalence of Ureaplasma spp. DNA in the cervical fluid was 61%; 2) the presence of intra-amniotic infection and colonization was related to higher rates of Ureaplasma spp. DNA in the cervical fluid compared to those with negative amniotic fluid results; and 3) the presence of intra-amniotic infection, sterile intra-amniotic inflammation, or colonization of the amniotic fluid was associated with higher microbial loads of Ureaplasma spp. DNA in the cervical fluid compared to those with negative amniotic fluid; and 4) the presence of sterile intra-amniotic inflammation was related to a higher relative abundance of Ureaplasma spp. DNA in the cervical fluid compared to that in negative amniotic fluid.
Ureaplasma spp. are considered commensal microorganisms of the cervical/vaginal niche owing to: 1) their high prevalence in pregnant or non-pregnant women, and 2) the absence of a difference in the prevalence between women of reproductive ages with and without symptoms of infection of the urogenital tract (Marovt et al., 2015; Sweeney et al., 2017). In pregnant women, the presence of Ureaplasma spp. in the cervical/vaginal niche has been shown to be associated with adverse pregnancy outcomes (Abele-Horn et al., 2000; Kafetzis et al., 2004; Kataoka et al., 2006). Accordingly, Ureaplasma spp. in the cervical/vaginal niche is more frequently observed in pregnancies complicated by PPROM than in uncomplicated pregnancies (Larsen and Hwang, 2010; Donders et al., 2017; Sprong et al., 2020). Its prevalence in PPROM pregnancies varies between 53 and 73% (Kwak et al., 2014; Kwak et al., 2015; Musilova et al., 2016). The observations from this study (61%) are in agreement with previously published findings.
Interestingly, in this study, women with intra-amniotic infection and colonization of the amniotic cavity had higher rates of Ureaplasma spp. DNA in their cervical fluid than did women with sterile intra-amniotic inflammation and negative amniotic fluid. In other words, a higher proportion of Ureaplasma spp. DNA in the cervical fluid was found in the subgroups of women with microbial invasion of the amniotic cavity. This observation is consistent with the findings of our previous study, in which women with microbial invasion of amniotic inflammation had a higher rate of Ureaplasma spp. DNA in the cervical fluid than did those without this complication (Musilova et al., 2016). Notably, in this study, no difference in the frequency of Ureaplasma spp. in the cervical fluid between women with sterile inflammation and negative amniotic fluid was identified. This observation differs from that recently reported in women with preterm labor and intact membranes. In that study, the prevalence of Ureaplasma spp. DNA in the group of women with sterile intra-amniotic inflammation was comparable to that in women with intra-amniotic infection; however, it was 2-fold higher than that in women with negative amniotic fluid (Kacerovsky et al., 2021c). The diversity in observations between the studies on different phenotypes of spontaneous preterm delivery supports the hypothesis that pathophysiological pathways leading to the development of sterile intra-amniotic inflammation might differ between PPROM and preterm labor with intact membranes.
Abnormal microbiota in the cervical/vaginal niche have been shown to be associated not only with a higher frequency of Ureaplasma spp. but also with higher loads of these bacteria in that niche (Donders et al., 2017). Kwak et al. reported that a higher load of Ureaplasma spp. in the vaginal niche is associated with a higher frequency of histological chorioamnionitis in women with PPROM (Kwak et al., 2014). In line with their report, a difference in the microbial load of Ureaplasma spp. DNA in the cervical fluid among the subgroups with intra-amniotic infection, sterile intra-amniotic inflammation, colonization, and negative amniotic fluid (with the lowest levels found in those with negative amniotic fluid) was found in this study. However, this observation was not in concordance with our previous study, where no difference in the load of Ureaplasma spp. DNA in the cervical fluid was identified in women with and without microbial invasion of the amniotic cavity (Musilova et al., 2016). The reason for this difference is not fully clear; however, it can be explained by the differences between the studies, including: 1) the sample sizes of women with Ureaplasma spp. DNA (previous: n = 40; current: n = 133) that were studied; and 2) the gestational ages considered during sampling (previous: 24 + 0 to 36 + 6; current: 24 + 0 to 33 + 6).
The relative abundance of Ureaplasma spp. DNA in the cervical fluid might represent a more precise tool with which to characterize the association between the composition of the cervical microbiome and intra-amniotic complications, as opposed to the assessment of the absolute number of copies of Ureaplasma spp. DNA that are present. In this study, women with PPROM and sterile intra-amniotic inflammation had an approximately 2.5-fold higher relative abundance of Ureaplasma spp. DNA compared to women with negative amniotic fluid results. This observation should be considered in terms of the pathophysiology of the development of sterile intra-amniotic inflammation in PPROM.
Taken together, the results from this study suggest that 1) the subgroup of women with PPROM and negative amniotic fluid differs from those with intra-amniotic complications in terms of frequency, loads, and relative abundance of Ureaplasma spp. DNA in the cervical fluid; and 2) differences exist between PPROM and preterm labor with intact membranes with regard to the association between Ureaplasma spp. DNA in the cervical fluid and intra-amniotic complications. Collectively, these observations further support that pathophysiological pathways leading to the development of intra-amniotic complications might differ between PPROM and preterm labor with intact membranes.
This study has several strengths. First, a relatively large cohort of women with a well-defined clinical phenotype for spontaneous preterm delivery (PPROM) was assessed. Secondly, the presence of Ureaplasma spp. DNA in the cervical fluid was evaluated with a specific PCR procedure, which allowed us to identify even very low loads of Ureaplasma spp. DNA. Third, thorough assessment of microbial invasion of the amniotic cavity, with the use of a combination of non-specific PCR (16S rRNA) followed by sequencing, specific PCR for Ureaplasma spp., Mycoplasma hominis, and Chlamydia trachomatis, and aerobic/anaerobic cultivation allowed us to precisely dissect the subgroups of women with sterile intra-amniotic inflammation.
This study has limitations that are worth mentioning. First, in women with PPROM, amniotic fluid leaks from the amniotic cavity through the cervix into the posterior vaginal fornix. This means that the leaking amniotic fluid can contaminate the cervical fluid. Therefore, the fluid obtained with a Dacron swab from the endocervical canal of women with PPROM should be considered not just as cervical fluid but as a compound fluid, composed of both cervical fluid and amniotic fluid components. Its composition, as well as the ratio between amniotic and cervical fluids, might vary among women with PPROM and depend on various clinical parameters and scenarios (e.g., amount of residual amniotic fluid, location of the membrane rupture site, position of the fetus, and interval between PPROM and sampling) that occur at the time of sampling. Therefore, if the leaked amniotic fluid contains Ureaplasma spp. DNA (in cases of intra-amniotic infection and colonization of the amniotic cavity caused by Ureaplasma spp.), it can affect the total microbial load of the Ureaplasma spp. DNA and its relative abundance in the fluid obtained from the endocervical canal. Unfortunately, the presence of this phenomenon is inevitable in PPROM and could affect the assessment of microbial loads and the relative abundance of Ureaplasma spp. DNA in the cervical fluid among subgroups of women with PPROM. On the other hand, it is highly unlikely that the presence of Ureaplasma spp. DNA in leaked amniotic fluid could affect the assessment of the prevalence of Ureaplasma spp. in the cervical fluid, since the cervical/vaginal niche is considered to be a primary source of Ureaplasma spp.
Second, biovars, serovars, and other types of Ureaplasma spp. were not assessed and considered in this study. This fact should be taken as a shortcoming of this study. However, there is evidence of the possibility of horizontal gene transfer in Ureaplasma spp., allowing them to carry markers of multiple serovars (Xiao et al., 2011; Sweeney et al., 2017). Thus, the assessment of serovars could have a limited diagnostic value. Third, during the study period, the methods to assess the concentrations of IL-6 in amniotic fluid were modified. Therefore, the concentrations of IL-6 in this study were determined using two different approaches (lateral flow immunoassay point-of-care test and automated electrochemiluminescence). This fact prevents us from assessing the association between the intensity of the intra-amniotic inflammatory response, measured by IL-6 concentrations in amniotic fluid, and the loads of Ureaplasma spp. DNA in the cervical fluid.
In conclusion, in PPROM at < 34 weeks, the presence of intra-amniotic infection, sterile intra-amniotic inflammation, or colonization of the amniotic fluid was associated with a higher prevalence and/or load of Ureaplasma spp. DNA in cervical fluid than in the absence of intra-amniotic complications.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional review board of the University Hospital Hradec Kralove. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MK, IM—writing of the manuscript MK, JM, JS, JM, IM, RK, RB, and PB -data acquistion MK, IM, BJ, and JM - data analysis MK, IM, BJ, PB, RK, RB, and JM—interpretation of data RK, RB, PB, JS, JMa, JMl, and BJ—critical revising of the manuscript MK, RK, RP, PB, JS, JMa, JMl, BJ, and IM—approval for publication.
Funding
This work was supported by the Faculty Hospital in Hradec Kralove (a long-term organization development plan).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abele-Horn, M., Scholz, M., Wolff, C., and Kolben, M. (2000). High-density Vaginal Ureaplasma Urealyticum Colonization as a Risk Factor for Chorioamnionitis and Preterm Delivery. Acta Obstet. Gynecol. Scand. 79, 973–978. doi:10.3109/00016340009169245
Battarbee, A. N., Ros, S. T., Esplin, M. S., Biggio, J., Bukowski, R., Parry, S., et al. (2020). Optimal Timing of Antenatal Corticosteroid Administration and Preterm Neonatal and Early Childhood Outcomes. Am. J. Obstet. Gynecol. MFM 2, 100077. doi:10.1016/j.ajogmf.2019.100077
Brosens, I., Pijnenborg, R., Vercruysse, L., and Romero, R. (2011). The "Great Obstetrical Syndromes" Are Associated with Disorders of Deep Placentation. Am. J. Obstet. Gynecol. 204, 193–201. doi:10.1016/j.ajog.2010.08.009
Chaemsaithong, P., Romero, R., Korzeniewski, S. J., Martinez-Varea, A., Dong, Z., Yoon, B. H., et al. (2016a). A point of Care Test for Interleukin-6 in Amniotic Fluid in Preterm Prelabor Rupture of Membranes: a Step toward the Early Treatment of Acute Intra-amniotic Inflammation/infection. J. Matern. Fetal Neonatal. Med. 29, 360–367. doi:10.3109/14767058.2015.1006621
Chaemsaithong, P., Romero, R., Korzeniewski, S. J., Martinez-Varea, A., Dong, Z., Yoon, B. H., et al. (2016b). A Rapid Interleukin-6 Bedside Test for the Identification of Intra-amniotic Inflammation in Preterm Labor with Intact Membranes. J. Matern. Fetal Neonatal. Med. 29, 349–359. doi:10.3109/14767058.2015.1006620
Di Renzo, G. C. (2009). The Great Obstetrical Syndromes. J. Matern. Fetal Neonatal. Med. 22, 633–635. doi:10.1080/14767050902866804
Donders, G. G. G., Ruban, K., Bellen, G., and Petricevic, L. (2017). Mycoplasma/Ureaplasma Infection in Pregnancy: to Screen or Not to Screen. J. Perinat Med. 45, 505–515. doi:10.1515/jpm-2016-0111
Hornychova, H., Kacerovsky, M., Musilova, I., Pliskova, L., Zemlickova, H., Matejkova, A., et al. (2018). Cervical Human Papillomavirus Infection in Women with Preterm Prelabor Rupture of Membranes. PLoS One 13, e0207896. doi:10.1371/journal.pone.0207896
Janku, P., Kacerovsky, M., Zednikova, B., Andrys, C., Kolackova, M., Drahosova, M., et al. (2019). Pentraxin 3 in Noninvasively Obtained Cervical Fluid Samples from Pregnancies Complicated by Preterm Prelabor Rupture of Membranes. Fetal Diagn. Ther. 46, 402–410. doi:10.1159/000499482
Kacerovsky, M., Matulova, J., Andrys, C., Mls, J., Hornychova, H., Kukla, R., et al. (2021a). Preterm Prelabor Rupture of Membranes without Microbial Invasion of the Amniotic Cavity and Intra-amniotic Inflammation: a Heterogeneous Group with Differences in Adverse Outcomes. J. Matern. Fetal Neonatal. Med., 1–12. doi:10.1080/14767058.2021.2017875
Kacerovsky, M., Musilova, I., Hornychova, H., Kutova, R., Pliskova, L., Kostal, M., et al. (2014). Bedside Assessment of Amniotic Fluid Interleukin-6 in Preterm Prelabor Rupture of Membranes. Am. J. Obstet. Gynecol. 211, 385–389. doi:10.1016/j.ajog.2014.03.069
Kacerovsky, M., Pliskova, L., Bolehovska, R., Gerychova, R., Janku, P., Matlak, P., et al. (2020a). Lactobacilli-dominated Cervical Microbiota in Women with Preterm Prelabor Rupture of Membranes. Pediatr. Res. 87, 952–960. doi:10.1038/s41390-019-0692-1
Kacerovsky, M., Pliskova, L., Bolehovska, R., Lesko, D., Gerychova, R., Janku, P., et al. (2021b). Cervical Gardnerella Vaginalis in Women with Preterm Prelabor Rupture of Membranes. PLoS One 16, e0245937. doi:10.1371/journal.pone.0245937
Kacerovsky, M., Romero, R., Stepan, M., Stranik, J., Maly, J., Pliskova, L., et al. (2020b). Antibiotic Administration Reduces the Rate of Intraamniotic Inflammation in Preterm Prelabor Rupture of the Membranes. Am. J. Obstet. Gynecol. 223, 114–e20. doi:10.1016/j.ajog.2020.01.043
Kacerovsky, M., Vlkova, B., Musilova, I., Andrys, C., Pliskova, L., Zemlickova, H., et al. (2018). Amniotic Fluid Cell-free DNA in Preterm Prelabor Rupture of Membranes. Prenat Diagn. 38, 1086–1095. doi:10.1002/pd.5366
Kacerovsky, M., Pliskova, L., Bolehovska, R., Gerychova, R., Janku, P., Matlak, P., et al. (2019). Lactobacilli-dominated Cervical Microbiota in Women with Preterm Prelabor Rupture of Membranes. Pediatr. Res. 87 (5), 952–960. doi:10.1038/s41390-019-0692-1
Kacerovsky, M., Stranik, J., Kukla, R., Bolehovska, R., Bostik, P., Matulova, J., et al. (2021c). Intra-amniotic Infection and Sterile Intra-amniotic Inflammation in Women with Preterm Labor with Intact Membranes Are Associated with a Higher Rate of Ureaplasma Species DNA Presence in the Cervical Fluid. J. Maternal-Fetal Neonatal Med., 1–9. doi:10.1080/14767058.2021.1947231
Kafetzis, D. A., Skevaki, C. L., Skouteri, V., Gavrili, S., Peppa, K., Kostalos, C., et al. (2004). Maternal Genital Colonization with Ureaplasma Urealyticum Promotes Preterm Delivery: Association of the Respiratory Colonization of Premature Infants with Chronic Lung Disease and Increased Mortality. Clin. Infect. Dis. 39, 1113–1122. doi:10.1086/424505
Kataoka, S., Yamada, T., Chou, K., Nishida, R., Morikawa, M., Minami, M., et al. (2006). Association between Preterm Birth and Vaginal Colonization by Mycoplasmas in Early Pregnancy. J. Clin. Microbiol. 44, 51–55. doi:10.1128/JCM.44.1.51-55.2006
Kwak, D. W., Cho, H. Y., Kwon, J. Y., Park, Y. W., and Kim, Y. H. (2015). Usefulness of Maternal Serum C-Reactive Protein with Vaginal Ureaplasma Urealyticum as a Marker for Prediction of Imminent Preterm Delivery and Chorioamnionitis in Patients with Preterm Labor or Preterm Premature Rupture of Membranes. J. Perinat Med. 43, 409–415. doi:10.1515/jpm-2014-0142
Kwak, D. W., Hwang, H. S., Kwon, J. Y., Park, Y. W., and Kim, Y. H. (2014). Co-infection with Vaginal Ureaplasma Urealyticum and Mycoplasma Hominis Increases Adverse Pregnancy Outcomes in Patients with Preterm Labor or Preterm Premature Rupture of Membranes. J. Matern. Fetal Neonatal. Med. 27, 333–337. doi:10.3109/14767058.2013.818124
Larsen, B., and Hwang, J. (2010). Mycoplasma, Ureaplasma, and Adverse Pregnancy Outcomes: a Fresh Look. Infect. Dis. Obstet. Gynecol. 2010. doi:10.1155/2010/521921
Liu, C. M., Aziz, M., Kachur, S., Hsueh, P. R., Huang, Y. T., Keim, P., et al. (2012). BactQuant: an Enhanced Broad-Coverage Bacterial Quantitative Real-Time PCR Assay. BMC Microbiol. 12, 56. doi:10.1186/1471-2180-12-56
Marovt, M., Keše, D., Kotar, T., Kmet, N., Miljković, J., Šoba, B., et al. (2015). Ureaplasma Parvum and Ureaplasma Urealyticum Detected with the Same Frequency Among Women with and without Symptoms of Urogenital Tract Infection. Eur. J. Clin. Microbiol. Infect. Dis. 34, 1237–1245. doi:10.1007/s10096-015-2351-8
Matulova, J., Kacerovsky, M., Bolehovska, R., Stranik, J., Spacek, R., Burckova, H., et al. (2021). Birth Weight and Intra-amniotic Inflammatory and Infection-Related Complications in Pregnancies with Preterm Prelabor Rupture of Membranes: a Retrospective Cohort Study. J. Maternal-Fetal Neonatal Med., 1–11. doi:10.1080/14767058.2021.1956458
Menon, R., and Richardson, L. S. (2017). Preterm Prelabor Rupture of the Membranes: A Disease of the Fetal Membranes. Semin. Perinatol 41, 409–419. doi:10.1053/j.semperi.2017.07.012
Mercer, B. M. (2003). Preterm Premature Rupture of the Membranes. Obstet. Gynecol. 101, 178–193. doi:10.1016/s0029-7844(02)02366-9
Mercer, B. M. (2005). Preterm Premature Rupture of the Membranes: Current Approaches to Evaluation and Management. Obstet. Gynecol. Clin. North. Am. 32, 411–428. doi:10.1016/j.ogc.2005.03.003
Moore, Rm., Katri, R., Kumar, D., Mansour, J. M., Mercer, B., and Moore, J. J. (2020). α-Lipoic Acid Blocks the GMCSF Induced Protease/protease Inhibitor Spectrum Associated with Fetal Membrane Weakening In-Vitro. Placenta 97, 79–88. doi:10.1016/j.placenta.2020.06.021
Musilova, I., Andrys, C., Holeckova, M., Kolarova, V., Pliskova, L., Drahosova, M., et al. (2020). Interleukin-6 Measured Using the Automated Electrochemiluminescence Immunoassay Method for the Identification of Intra-amniotic Inflammation in Preterm Prelabor Rupture of Membranes. J. Matern. Fetal Neonatal. Med. 33, 1919–1926. doi:10.1080/14767058.2018.1533947
Musilova, I., Andrys, C., Krejsek, J., Drahosova, M., Zednikova, B., Pliskova, L., et al. (2018). Amniotic Fluid Pentraxins: Potential Early Markers for Identifying Intra-amniotic Inflammatory Complications in Preterm Pre-labor Rupture of Membranes. Am. J. Reprod. Immunol. 79, e12789. doi:10.1111/aji.12789
Musilova, I., Kacerovsky, M., Stepan, M., Bestvina, T., Pliskova, L., Zednikova, B., et al. (2017a). Maternal Serum C-Reactive Protein Concentration and Intra-amniotic Inflammation in Women with Preterm Prelabor Rupture of Membranes. PLoS One 12, e0182731. doi:10.1371/journal.pone.0182731
Musilova, I., Kolackova, M., Andrys, C., Drahosova, M., Baranová, I., Chmelarova, M., et al. (2021). Nicotinamide Phosphoribosyltransferase and Intra-amniotic Inflammation in Preterm Prelabor Rupture of Membranes. J. Matern. Fetal Neonatal. Med. 34, 736–746. doi:10.1080/14767058.2019.1615049
Musilova, I., Kutová, R., Pliskova, L., Stepan, M., Menon, R., Jacobsson, B., et al. (2015). Intraamniotic Inflammation in Women with Preterm Prelabor Rupture of Membranes. PLoS One 10, e0133929. doi:10.1371/journal.pone.0133929
Musilova, I., Pliskova, L., Gerychova, R., Janku, P., Simetka, O., Matlak, P., et al. (2017b). Maternal white Blood Cell Count Cannot Identify the Presence of Microbial Invasion of the Amniotic Cavity or Intra-amniotic Inflammation in Women with Preterm Prelabor Rupture of Membranes. PLoS One 12, e0189394. doi:10.1371/journal.pone.0189394
Musilova, I., Pliskova, L., Kutova, R., Hornychova, H., Jacobsson, B., and Kacerovsky, M. (2016). Ureaplasma Species and Mycoplasma Hominis in Cervical Fluid of Pregnancies Complicated by Preterm Prelabor Rupture of Membranes. J. Matern. Fetal Neonatal. Med. 29, 1–7. doi:10.3109/14767058.2014.984606
Romero, R., Espinoza, J., Kusanovic, J. P., Gotsch, F., Hassan, S., Erez, O., et al. (2006). The Preterm Parturition Syndrome. BJOG 113 Suppl 3 (Suppl. 3), 17–42. doi:10.1111/j.1471-0528.2006.01120.x
Romero, R., Miranda, J., Chaemsaithong, P., Chaiworapongsa, T., Kusanovic, J. P., Dong, Z., et al. (2015). Sterile and Microbial-Associated Intra-amniotic Inflammation in Preterm Prelabor Rupture of Membranes. J. Matern. Fetal Neonatal. Med. 28, 1394–1409. doi:10.3109/14767058.2014.958463
Soucek, O., Kacerovsky, M., Musilova, I., Pliskova, L., Bolehovska, R., and Andrys, C. (2020). Amniotic Fluid CD11b Levels in Pregnancies Complicated by Preterm Prelabor Rupture of Membranes. J. Matern. Fetal Neonatal. Med., 1–9. doi:10.1080/14767058.2020.1767578
Spacek, R., Musilova, I., Andrys, C., Soucek, O., Burckova, H., Pavlicek, J., et al. (2020). Extracellular Granzyme A in Amniotic Fluid Is Elevated in the Presence of Sterile Intra-amniotic Inflammation in Preterm Prelabor Rupture of Membranes. J. Matern. Fetal Neonatal. Med., 1–10. doi:10.1080/14767058.2020.1817895
Sprong, K. E., Mabenge, M., Wright, C. A., and Govender, S. (2020). Ureaplasma Species and Preterm Birth: Current Perspectives. Crit. Rev. Microbiol. 46, 169–181. doi:10.1080/1040841X.2020.1736986
Stranik, J., Kacerovsky, M., Soucek, O., Kolackova, M., Musilova, I., Pliskova, L., et al. (2021). IgGFc-binding Protein in Pregnancies Complicated by Spontaneous Preterm Delivery: a Retrospective Cohort Study. Sci. Rep. 11, 6107. doi:10.1038/s41598-021-85473-2
Sweeney, E. L., Dando, S. J., Kallapur, S. G., and Knox, C. L. (2017). The Human Ureaplasma Species as Causative Agents of Chorioamnionitis. Clin. Microbiol. Rev. 30, 349–379. doi:10.1128/CMR.00091-16
Keywords: microbial invasion of the amniotic cavity, genital mycoplasma, intra-amniotic inflammation, non-invasive sample, preterm delivery
Citation: Kacerovsky M, Kukla R, Bolehovska R, Bostik P, Matulova J, Mls J, Stranik J, Jacobsson B and Musilova I (2022) Prevalence and Load of Cervical Ureaplasma Species With Respect to Intra-amniotic Complications in Women With Preterm Prelabor Rupture of Membranes Before 34 weeks. Front. Pharmacol. 13:860498. doi: 10.3389/fphar.2022.860498
Received: 23 January 2022; Accepted: 02 March 2022;
Published: 31 March 2022.
Edited by:
Nanbert Zhong, New York State Institute for Basic Research in Developmental Disabilities, United StatesReviewed by:
Kirsten Glaser, University Hospital Leipzig, GermanyMartin Mueller, University Hospital Bern, Switzerland
Copyright © 2022 Kacerovsky, Kukla, Bolehovska, Bostik, Matulova, Mls, Stranik, Jacobsson and Musilova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marian Kacerovsky, a2FjZXJtYXJAZm5oay5jz
 Marian Kacerovsky
Marian Kacerovsky Rudolf Kukla
Rudolf Kukla Radka Bolehovska
Radka Bolehovska Pavel Bostik4
Pavel Bostik4 Jana Matulova
Jana Matulova Bo Jacobsson
Bo Jacobsson Ivana Musilova
Ivana Musilova