
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 04 April 2022
Sec. Gastrointestinal and Hepatic Pharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.857956
This article is part of the Research Topic Advances in Non-Alcoholic Fatty Liver Disease Therapeutics: Pathogenic Mechanisms and Targets View all 15 articles
The liver plays an important role in glucose and lipid homeostasis, drug metabolism, and bile synthesis. Metabolic disorder and inflammation synergistically contribute to the pathogenesis of numerous liver diseases, such as metabolic-associated fatty liver disease (MAFLD), liver injury, and liver cancer. Celastrol, a triterpene derived from Tripterygium wilfordii Hook.f., has been extensively studied in metabolic and inflammatory diseases during the last several decades. Here we comprehensively review the pharmacological activities and the underlying mechanisms of celastrol in the prevention and treatment of liver diseases including MAFLD, liver injury, and liver cancer. In addition, we also discuss the importance of novel methodologies and perspectives for the drug development of celastrol. Although celastrol has been claimed as a promising agent against several metabolic diseases, both preclinical and clinical studies are highly required to accelerate the clinical transformation of celastrol in treating different liver illness. It is foreseeable that celastrol-derived therapeutics is evolving in the field of liver ailments.
The liver is a metabolic organ that is responsible for glucose and lipid homeostasis, drug metabolism, and bile synthesis (Diehl-Jones and Askin, 2002; Almazroo et al., 2017; Tappy, 2021). Liver diseases contain non-alcoholic fatty liver disease (NAFLD) and its related diseases, drugs or infections induced liver injury, liver cancer, and among others. With the aging of the population and the prevalence of obesity, NAFLD, known as metabolic-associated fatty liver disease (MAFLD), is becoming a threat to human health in the world, (Eslam et al., 2020). MAFLD begins with the accumulation of hepatic lipid, metabolic syndrome (central adiposity, hyperglycemia, dyslipidemia, and hypertension) (Mundi et al., 2020). Environment factors like dietary habits and the genetic factors such as PNPLA3 (encoding patatin-like phospholipase domain-containing protein3), TM6SF2 (encoding transmembrane 6 superfamily member 2), and epigenetic factors are the risk factors of MAFLD (Younossi et al., 2018). The current global prevalence of MAFLD is approximately 25%, which may develop into non-alcoholic steatohepatitis, eventually leading to liver fibrosis, cirrhosis, and even liver cancer if not treated in a timely fashion (Ye et al., 2020). Except for the liver damage, MAFLD and non-alcoholic steatohepatitis can also aggravate or induce insulin resistance, which are closely related to the high incidence of type 2 diabetes and cardiovascular disease (Liang et al., 2022). Liver injury as a loss of liver function is mainly caused by drug, infections, and intrahepatic cholestasis (Thawley, 2017). The drug-induced liver injury (DILI) is one of the most common and serious adverse drug reactions, which can lead to acute liver failure and even death in severe cases (Licata, 2016). With 831,000 death cases and 905,000 new cases in 2020, liver cancer with high mortality and incidence remains a global menace (Sung et al., 2021). Although many patients are diagnosed and treated at an early stage of the disease, the recurrence rate is still high. For middle and late-stage hepatic carcinoma, the prognosis is even less optimistic. Therefore, new therapy or drugs with better efficacy and lower toxicity are of great importance.
Traditional Chinese Medicine (TCM) was used to treat various diseases such as metabolic diseases, liver diseases, inflammation, and cancers for many years. In light of their numerous pharmacological activities, low toxicity, and low side effects, nature compounds from TCM are widely used to protect against liver diseases, such as berberine, curcumin, ginsenoside, celastrol, to name a few (Xu et al., 2018a). In Europe and America, up to 65% of patients choose herbal medicines to treat liver diseases (Zhang et al., 2013; Madrigal-Santillán et al., 2014; Bagherniya et al., 2018). Celastrol, a pharmacologically active compound from Tripterygium wilfordii Hook.f., possesses therapeutic properties for multiple diseases, such as rheumatoid arthritis, inflammatory bowel diseases, hepatitis, as well as anti-cancer, anti-obesity, and metabolic modulating (Feng et al., 2013; Lin et al., 2015; Liu et al., 2015; Tseng et al., 2017; Saito et al., 2019; Song et al., 2019). In recent years, celastrol has attracted the attention of researchers and the pharmaceutical industry due to its benefits on human health. Here, we summarized the studies exploring the pharmacological properties of celastrol in liver protection during 15 years from 2007 to 2021, and made a general statement about its mechanisms in liver-related diseases.
The PubMed and Web of Science databases were used to search related articles from 2007 to 2021. Cellular, animal, or human studies were searched with the keywords (celastrol) and (liver). Papers were extracted using the following inclusion criteria: (1) these studies should be research articles to estimate the hepatoprotective effects of celastrol; (2) only articles written in English were included. All titles and abstracts of publications retrieved were reviewed to select potentially eligible studies. Full texts of literatures potentially eligible were reviewed by two independent investigators. From a total of 181 results, 76 unique studies were identified, 88 duplication, 5 reviews, and 12 other article styles were excluded. After carefully reviewing the title and/or abstract, 18 were excluded 5 publications are irrelevant. A total of 53 articles were included in this review. A flowchart of this is illustrated in Figure 1.
MAFLD, characterized by the hepatic fat accumulation, is usually correlated with the metabolic disorder related obesity (Samuel and Shulman, 2018; Jarvis et al., 2020), hepatic inflammation (Kazankov et al., 2019), oxidative stress (Ore and Akinloye, 2019; Świderska et al., 2019), and intestinal microbiome dysbiosis (Caussy and Loomba, 2018). Lipotoxicity induced by abnormal hepatic fat deposition activated Kuepfer cell (KC) to promote inflammation in MAFLD patients (Lanthier, 2015). Oxidative stress and endoplasmic reticulum (ER) stress resulted in mitochondrial reactive oxygen species (ROS) accumulation in hepatocytes, which in turn impaired glycolipid metabolism, accelerated inflammatory response, and introduced cell death in MAFLD (Ore and Akinloye, 2019; Świderska et al., 2019; Chen et al., 2020a). The reasonable management of body weight and dietary habits should be given a high priority in MAFLD patients. Although the antidiabetic and lipid-lowering drugs may help to reduce hepatic lipid accumulation (Mundi et al., 2020), there are no specific drugs for MAFLD approved in Europe and the United States. Recent research mainly focused on the metabolic targets, immune targets, fibrosis, cell stress, and apoptosis (Friedman et al., 2018). Celastrol as a promising compound exhibits good anti-obesity and anti-inflammatory effect on treating MAFLD.
The liver is vital for lipid and glucose metabolism and homeostasis, protein, and amino acid metabolism, where bile acids produce and flux to the digestive system. Numerous evidences supported that obesity is strongly associated with MAFLD development (Fabbrini et al., 2010). The liver protective effect and molecular mechanism of celastrol on high fat diet (HFD) induced MAFLD animal models have been well-investigated (Wang et al., 2014; Ma et al., 2015; Zhang et al., 2017a; Hu et al., 2017; Luo et al., 2017; Kyriakou et al., 2018; Pfuhlmann et al., 2018; Zhang et al., 2018; Feng et al., 2019a; Zhao et al., 2019a; Feng et al., 2019b; Chellappa et al., 2019; Fang et al., 2019; Abu Bakar et al., 2020; Hu et al., 2020; Zhu C. et al., 2021; Zhu Y. et al., 2021; Hua et al., 2021; Ouyang et al., 2021; Yang et al., 2021).
As a typical feature of obesity, leptin resistance was involved in the hepatic steatosis during the development of MAFLD (Myers et al., 2010; Boutari and Mantzoros, 2020). Leptin, a hormone secreted by adipose tissue, directly interacted with the leptin receptor in the brain and mediated food intake, energy metabolism, body weight, and other physiological processes (Xu et al., 2018b; Caron et al., 2018; Pan and Myers, 2018). Brain leptin promoted hepatic lipid flux and reduced lipogenesis in the liver (Hackl et al., 2019). Celastrol as a leptin sensitizer exhibited significant weight loss and food consumption reduction in HFD-obesity animals (Wang et al., 2014; Liu et al., 2015; Ma et al., 2015; Zhang et al., 2017a; Hu et al., 2017; Luo et al., 2017; Kyriakou et al., 2018; Pfuhlmann et al., 2018; Zhang et al., 2018; Feng et al., 2019a; Zhao et al., 2019a; Feng et al., 2019b; Chellappa et al., 2019; Fang et al., 2019; Saito et al., 2019; Abu Bakar et al., 2020; Hu et al., 2020; Zhu C. et al., 2021; Zhu Y. et al., 2021; Hua et al., 2021; Ouyang et al., 2021; Yang et al., 2021) (Table 1).
Celastrol (100 μg/kg by intraperitoneally injection [i.p], or 10 mg/kg by oral administration [OA]) increased the leptin sensitivity to suppress food intake and reduce body weight in HFD induced C57BL/6J mice and was firstly reported in 2015 by Ozcan’s group (Liu et al., 2015), while the benefits of celastrol on obesity were abrogated in lean mice, leptin receptor-deficient (db/db), or leptin-deficient (ob/ob) mice/rats (Liu et al., 2015; Pfuhlmann et al., 2018; Feng et al., 2019a; Feng et al., 2019b; Saito et al., 2019; Hu et al., 2020). Compared with leptin or celastrol alone, the combined use of leptin and celastrol could significantly inhibit appetite and weight gain of different mice, even for ob/ob mice (Liu et al., 2015). Numerous studies verified the effect of leptin sensitizer celastrol on appetite as showing in Table 1.
The underlying molecular mechanism of celastrol anti-obesity and increase the leptin sensitivity can be attributed to Sirtuin 1(Sirt1) (Zhang et al., 2017a; Abu Bakar et al., 2020), interleukin-1 receptor 1(IL1R1) (Feng et al., 2019b), protein tyrosine phosphatase (PTP) 1B (PTP1B), and T-cell PTP (TCPTP) (Kyriakou et al., 2018). SIRT1 as a deacetylase activated by energy deprivation was involved in glucose, lipid, and bile acid metabolism regulation (Purushotham et al., 2009; García-Rodríguez et al., 2014). It has been reported that celastrol exacerbated liver metabolic disorder by suppressing the phosphorylation of AMP-activated protein kinase α (AMPKα) in liver specific Sirt1 knock out mice fed with HFD (Zhang et al., 2017a). In line with that, celastrol (3 mg/kg/day for 8 weeks) significantly increased insulin sensitivity and weight loss through enhancing AMPK/SIRT1 signaling in HFD-induced rats (Abu Bakar et al., 2020). IL1R1, a cytokine receptor, was associated with cell death and inflammation. IL1R1 deficient mice showed the phenotype of mature-onset obesity and leptin resistance (García et al., 2006). Feng and his colleagues demonstrated that the protective property of celastrol on obesity reversed by IL1R1 deficiency, which indicated that IL1R1 is essential for celastrol to reduce food consumption and body weight, alleviate glucose intolerance and insulin tolerance, as well as hepatic steatosis in HFD mice (Feng et al., 2019). However, these studies were only performed in genetic deficient animals, and whether the hepatoprotective effects of celastrol depended on its direct interaction with Sirt1 or IL1R1 need further studies. The surface plasmon resonance (SPR) assay, circular dichroism (CD) spectroscopy, and other methods might be effective methods to verify the above hypothesis. PTP1B and TCPTP negatively regulate leptin signaling in the hypothalamus (Dodd et al., 2019). Eleni et al. demonstrated that celastrol-induced weight loss was attributed to the supression of PTP1B and TCPTP, and inhibition of PTP1B and TCPTP is mediated by reversible noncompetitive binding to an allosteric pocket close to the active site (Kyriakou et al., 2018). In addition, different studies demonstrated the anti-obesity effect of celastrol was independent on melanocortin 4 receptor (MC4R), and lipocalin 2 (Lcn2) (Feng et al., 2019a; Saito et al., 2019), because the deletion of these genes did not weaken the weight-loss and liver-protecting effect of celastrol in mice.
Obesity related MAFLD was triggered by energy imbalance, with features of excessive lipid deposition. Peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α), a nuclear transcriptional coactivator factor, increased energy expenditure by regulating mitochondrial function and biogenesis (Vega et al., 2000; Rodgers et al., 2005; Minsky and Roeder, 2015). Liver-specific deficient PGC-1α mice exhibit hepatic steatosis (Rodgers et al., 2005). Accumulating evidences uncovered that celastrol promotes the white adipose tissue (iWAT) browning and brown adipose tissue (BAT) activation by upregulating PGC-1α and uncoupling protein 1(UCP1, a downstream effector of PGC-1α, is responsible for electron transport of mitochondrial oxidative phosphorylation) expression (Table 2). A total of 3 mg/kg/day celastrol (OA) significantly increased the HSF1 (Heat Shock Transcription Factor 1)/PGC-1α axis activity and protected against obesity in HFD mice by increasing energy expenditure, including iWAT browning, BAT activation, and mitochondrial gene transcription, while such benefit was abrogated by HSF1 depletion (Ma et al., 2015). In agreement with this result, several reports also clarified that celastrol increased the energy expenditure by activating iWAT browning through upregulation of PGC-1α and UCP1 (Fang et al., 2019; Yang et al., 2021). However, these studies were challenged by Katrin’s group, and they showed that celastrol intervention only upregulated the UCP1, while the expression of PGC-1α in BAT of HFD mice was not altered, and UCP1 deletion in HFD mice did not abolish food consumption and body weight reduction effects of celastrol (Pfuhlmann et al., 2018). This indicated that weight loss effect of celastrol was not dependent on UCP1-mediated thermogenesis under nutrient stress. In accordance with Katrin’s study, other researchers did not observe that the upregulation of UCP1 and PGC-1α expression in iWAT and BAT of HFD induced mice and rats with celastrol administration (Saito et al., 2019; Hu et al., 2020; Hua et al., 2021). The disparity of above observations of celastrol’s hepatoprotective effects might be caused by different celastrol-delivery way (OA vs. i.p), dosage, and intervention time period (Table 2). All above results indicated that iWAT browning happened at a specific time after celastrol treatment, and celastrol improved energy expenditure through other pathways not only depending on browning of iWAT.
Numerous studies proved that gut microbiota dysbiosis can also be attributed to fatty liver disease development (Le Roy et al., 2013; Canfora et al., 2019; Frazier and Chang, 2020). Oral administration of 500 μg/kg/day celastrol markedly enhanced energy expenditure and enhanced liver lipid metabolism in HFD rats by improving the gut microbiota homeostasis and activating the hypothalamic leptin signaling pathway rather than affecting food intake (Hu et al., 2020). Firmicutes promoted lipid absorption not fatty acid oxidation (Murphy et al., 2013). Increased ratio of Bacteroidetes to Firmicutes played a critical role in improvement of lipid consumption in celastrol-treated HFD rats (Murphy et al., 2013). Consistent with this, oral administration of celastrol (3 mg/kg/d) inhibited intestinal lipid absorption to ameliorate metabolic disturbance by reconstructing the gut microbiota profile in HFD mice (Hua et al., 2021). Although it has been reported that celastrol improved lipid metabolism by modulating gut microbiota, its specific mechanism of gut microbiota involved in MAFLD still needs to be explored. In addition, recent study demonstrated that age-associated obesity mice injected with celastrol (100–200 μg/kg) exhibited reduction body weight and fasting glucose level owing to fat loss and circadian rhythms restored without impact on lean mass (Chellappa et al., 2019), and whether gut microbiota act as an actuator of host circadian rhythms involved in the procedure remains to be studied (Frazier and Chang, 2020; Saran et al., 2020).
In summary, celastrol reduced body weight and hepatic fat accumulation mainly by increasing leptin sensitivity, enhancing energy metabolism, and modulating gut microbiota. Above all, Sirt1, IL1R1, PTP1B, and TCPTP might be the potential targets of celastrol, but whether celastrol directly interacted with these targets should be deeply explored. Moreover, the effect of celastrol on PGC-1α and UCP1 is controversial, and further studies should be performed to verify this effect. Gut microbiota have been extensively studied in recent years, but the molecular mechanism of celastrol on anti-obesity through gut microbiota is still unclear.
Macrophage infiltration and inflammation caused by dyslipidemia is associated with progression of MAFLD (Kazankov et al., 2019; Katsiki et al., 2016; Luo et al., 2018). Different animal experiments have elucidated that the inflammation in fatty liver mice treated with celastrol are remarkably attenuated (Table 1). Macrophage infiltration and inflammatory factors (IL-1β, IL-18, MCP-1α, and TNF-α) were reduced in liver of HFD induced C57BL/6J or C57BL/6N mice by celastrol treated for 3 weeks (Hu et al., 2017; Luo et al., 2017; Zhang et al., 2018; Ouyang et al., 2021; Zhu Y. et al., 2021). In addition, adipose tissue inflammation and mitochondrial dysfunction in skeletal muscle ameliorated in HFD-induced SD rats treated with 3 mg/kg/day celastrol for 8 weeks (Abu Bakar et al., 2020). The mechanism exhibited that celastrol can activate AMPK/SIRT1 signaling pathways for mitochondrial function improvement and attenuate inflammatory responses via decreasing nuclear factor kappa-B (NF-κB) activity (Abu Bakar et al., 2020). Celastrol-mediated hepatoprotective effects in C57BL/6J mice fed with HFD abolished in liver specific Sirt1-deficient mice fed HFD (Zhang et al., 2017a). Celastrol directly interacted with adenylyl cyclase associated protein 1 (CAP1) in macrophages contributed to improvement of metabolism and attenuation of inflammatory response through NF-κB signaling pathway in HFD-induced mice (Zhu Y. et al., 2021). Apart from that, LPS induced acute liver inflammation and HFD induced chronic inflammation are both inhibited by celatrol through binding Nur77 and promoting Nur77 interact with TRAF2 inducing autophagy (Hu et al., 2017). Deletion of Nur77 or Sirt1 impaired the anti-inflammation ability of celastrol, but LCN2 or ApoE deficiency did not influence the anti-hepatosteatosis of celastrol (Hu et al., 2017; Zhang et al., 2017a; Feng et al., 2019b; Ouyang et al., 2021). In general, the protective effects of celastrol against inflammation might be attributed to suppression of NF-κB involved signaling pathway.
In summary, different animal experiments confirmed that celastrol had metabolic improvement effect on HFD induced SD rats/mice or aged-obesity mice, but has no effect on gene deficiency mice such as HSF1 deletion mice, IL1R1 null mice, Sirt1 deficient mice, etc. (Ma et al., 2015; Zhang et al., 2017a; Feng et al., 2019b). This indicated that HSF1, IL1R1, and Sirt1 might act as the target of celastrol. In addition, oral administration of celastrol hardly affects food intake but has the same benefit on metabolic compared to intraperitoneal injection. Therefore, it is better to use oral administration to explore the pharmacological mechanism of celastrol. The effects of celastrol on MAFLD are summarized in Figure 2.
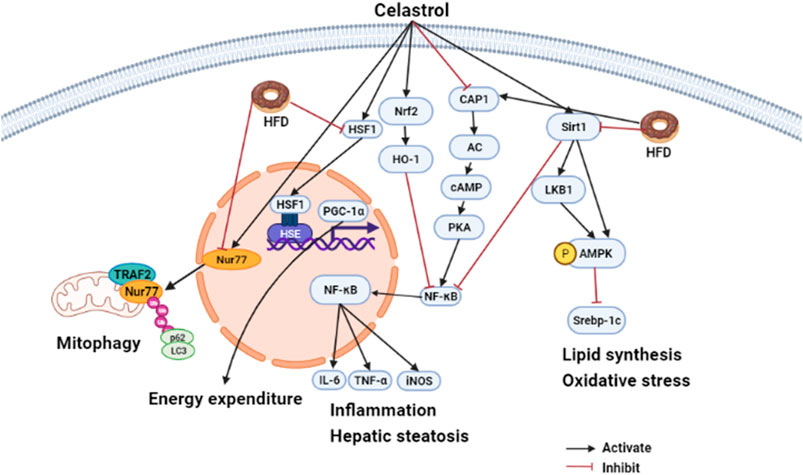
FIGURE 2. Hepatoprotective effects of celastrol in HFD induced animal model (edited by Biorender software). Diagram of the mechanism of celastrol in regulating hepatic energy expenditure, inflammation, and lipid metabolism. Under HFD stress, celastrol attenuated inflammation by inducing Nur77 interaction with TRAF2 to promote mitochondrial autophagy. Macrophages mediated inflammation was also ameliorated by celastrol targeted CAP1 via inhibition of cAMP-PKA-NF-κB signaling pathway, and macrophage M1 polarization was suppressed by celastrol via regulating Nrf2-HO-1-NF-κB pathways. In addition, celastrol can also regulate metabolism disorder. On the one hand, it can promote energy metabolism by activating HSF1-PGC1α; on the other hand, it can target SIRT1 to promote AMPK α phosphorylation and inhibit Srebp-1c-mediated lipid synthesis against oxidative stress and inflammation TRAF2, TNFR-associated factor2; CAP1, adenylyl cyclase-associated; protein 1 cAMP, cyclic adenylate monophosphate; PKA, protein kinase A; NF-κB, nuclear factor kappa-B; Srebp-1c, sterol regulatory element binding protein 1c; AMPKα, AMP-activated protein kinase α.
Liver injury characterized by a loss of hepatocyte function in patients resulted from mitochondrial oxidative stress, endoplasmic reticulum stress, inflammation, and autophagy in hepatocyte (Vento and Cainelli, 2020). Drugs, infections, and intrahepatic cholestasis can all lead to liver injury (Fontana et al., 2014). Celastrol, an anti-inflammatory drug, ameliorated the damage in hepatocyte or liver injury (Abdelaziz et al., 2017; El-Tanbouly et al., 2017; Jannuzzi et al., 2018; Wu et al., 2018; Zhao et al., 2019b; Guo et al., 2019; Wang et al., 2020; Zhao et al., 2020; Yan et al., 2021) (Table 3).
As the number and variety of drug applications grow rapidly, drug-induced liver injury (DILI), especially acetaminophen (APAP) overdose, is becoming the most common cause of acute liver failure (ALF) in America and Europe (Shehu et al., 2017). A series of studies demonstrated that pretreatment with celastrol attenuated the APAP-induced oxidative stress and inflammation by raising antioxidant enzyme activities such as glutathione peroxidase (GPx), glutathione reductase (GR), catalase, and superoxide dismutase (SOD) activities both in vitro and in vivo (Abdelaziz et al., 2017; Jannuzzi et al., 2018). CCl4 as another hepatotoxic chemical could induce liver injury by inflammatory activation and antioxidant enzymes inhibition (Zhang et al., 2017b). Celastrol might also suppress inflammation in CCl4 induced liver injury rat by activating AMPK-SIRT3 signaling (Wang et al., 2020). In the latest research, Li and his colleagues found that celastrol could eliminate oxidative stress and reduce the release of proinflammatory cytokines by activating PPARα signaling pathway in CCl4 intoxicant mice (Zhao et al., 2020). However, celastrol itself as a drug also has toxicity, the balance between the liver protection and liver injury needs to be further studied, and new structure modification and formulation should be developed to reduce toxicity and enhance efficacy.
Cholestasis is a pathophysiological process caused by bile secretion and excretion disorders. It is manifested as excessive accumulation of bile components such as bile acid, cholesterol, and bilirubin in the liver and systemic circulation, causing damage to liver cells and whole body. Long-term continuous cholestasis will progress to liver fibrosis and even cirrhosis (Zollner and Trauner, 2008; Padda et al., 2011). Li and his colleagues demonstrated that celastrol ameliorated cholestatic liver injury induced by α-naphthyl isothiocyanate (ANIT) and thioacetamide (TAA) through activation of SIRT1-FXR signaling pathway, while the hepatoprotective effects of celastrol were abrogated in FXR deficiency or SIRT1 inhibition mice (Zhao et al., 2019b). In addition to that, 5 mg/kg/day celastrol remarkably alleviated intrahepatic cholestasis of pregnancy via downregulating matrix metalloproteinase (MMP-2 and MMP-9) and total bile acid in pregnant rats (Guo et al., 2019).
Sepsis is a serious clinical syndrome caused by many organisms including bacteria, viruses, and fungi, which might lead to liver injury (Strnad et al., 2017). Cecal ligation and puncture (CLP) model and lipopolysaccharide (LPS) inducement is often used to mimics sepsis (Zhang et al., 2014; Xiong et al., 2017; Li et al., 2020). Celastrol attenuated CLP induced hepatic dysfunction in rats by inhibiting toll-like receptor-4 (TLR-4)/NF-κB (El-Tanbouly et al., 2017). In addition, celastrol pretreatment was found to attenuate propionibacterium acnes/lipopolysaccharides induced liver damage via NLRP3 inflammasome suppression and inflammatory inhibition (Yan et al., 2021). On the contrary, celastrol aggravated liver injury by exacerbation of inflammatory and elevation of oxidative stress in LPS intoxicant mice (Wu et al., 2018). The contradictory results were attributed to the different dosage of LPS and celastrol, and it is hard for high dosage of celastrol to reverse the severe liver failure that resulted from high dose of LPS, and the toxicity of celastrol should be taken into consideration in treating sepsis.
In short, these data suggested that celastrol is a potential candidate for treatment of hepatic injury, mainly owing to its ability to inhibit inflammation and oxidative stress and recover bile acid homeostasis. While protecting the liver injury, the toxicity of celastrol should be also taken into consideration to avoid side effects.
Hepatocellular carcinoma (HCC) as one highly malignant cancer remains a global menace. Effective systemic treatment of HCC is impeded by its complicated molecular pathogenesis, high rate of metastasis, recurrence, and chemo-resistance. It is imperative to explore effective therapy strategies for liver cancer. Natural medicine with long history of clinical use exhibited great potential in anti-cancer due to its high efficiency and availability, as well as low side effects (Hu et al., 2013). Numerous studies have shown that celastrol exerts anticancer effects by inhibiting their proliferation, metastasis, and inflammatory properties depending on modulating a variety of signaling pathways (Kashyap et al., 2018).
Celastrol arrested cell cycle and promoted apoptosis through suppression of STAT3/JAK2 signaling, PI3 K/Akt signaling, and ER-stress/UPR signaling in different hepatocellular cell lines (Table 4) (Chen et al., 2011; Rajendran et al., 2012; Li et al., 2013; Wei et al., 2014; Li et al., 2015; Ma et al., 2017; Ren et al., 2017; Du et al., 2020; Kun-Ming et al., 2020). In addition, celastrol inhibited hepatoma cell line growth and proliferation via arresting cell cycle in sub-G1 (Rajendran et al., 2012) and G2/M phase (Chen et al., 2011; Ren et al., 2017). Further studies showed that celastrol reduced the expression of c-myc and cyclin D1 which was closely related to cell cycle arrest of tumor cells in vivo on a time- and dose-dependent manner (Rajendran et al., 2012; Ma et al., 2017). Mechanistic studies revealed that celastrol could impede liver cancer through downregulation of HSP90 proteins, activation of c-Jun NH2 terminal kinase (JNK), and weakened MRC complex I activity (Chen et al., 2011). Apart from that, celastrol could also inhibit the migration, invasion, and metastasis of liver cancer by inhibiting the ROCK2-mediated phosphorylation of ezrin at Thr567 (Du et al., 2020), suppressing NF-κB and Akt activity, and downregulating miR-224, MMP-2, and MMP-9 (Li et al., 2013).
As show in Table 5 (Chen et al., 2011; Rajendran et al., 2012; Wei et al., 2014; Chang et al., 2016; Kun-Ming et al., 2020; Saber et al., 2020; Si et al., 2021), studies have manifested that celastrol exhibits anticancer activity against a variety of liver cancer animal models such as HCC patient-derived xenografts BALB/cJ mice (Wei et al., 2014), different hepatocellular carcinoma cells derived xenografts mouse models (Rajendran et al., 2012; Ma et al., 2014; Ren et al., 2017; Si et al., 2021), and DEN induced HCC rats/mice (Chang et al., 2016; Saber et al., 2020) with dosage of 1–10 mg/kg for 3–10 weeks. Celastrol (4 mg/kg) prevented tumor proliferation and increased apoptosis via inhibition of STAT3/JAK2 signaling pathway in PLC/PRF5 cells derived xenografts HCC mice (Rajendran et al., 2012). Recently, different studies validated that inhibition of circ_SLIT3/miR-223-3p/CXCR4 signaling is involved in the anti-HCC activity of celastrol both in vitro and in vivo (Kun-Ming et al., 2020; Si et al., 2021). Meanwhile, celastrol has been demonstrated to decrease the hepatic lesions and elevation of serum alanine aminotransferase (ALT), glutamic oxalacetic transaminase (AST), alkaline phosphatase (ALP), and alpha fetoprotein (AFP) in diethylnitrosamine (DEN)-induced hepatocellular carcinoma (HCC) rats, due to activation of mitochondrial apoptosis induced by p53 (Chang et al., 2016; Saber et al., 2020). Consistent with this, combination therapy with celastrol (2 mg/kg) and metformin (200 mg/kg) not only attenuated hepatic injury with elevated liver enzymes but also decreased NLRP3 mediated NF-κB signaling to suppress anti-apoptotic processes in mice with DEN-induced HCC (Saber et al., 2020). Meanwhile, Jiang and his colleagues found that synergistic use of celastrol and PHA665752 (a c-Met inhibitor) significantly inhibited cell growth, migration in c-met deficient Huh7 cells, and Huh7 xenografts nude mice (Jiang et al., 2013). Besides that, celastrol could enhance the activity of anti-liver cancer drugs including sorafenib, lapatinib, and ABT-737 by cell growth inhibition and apoptosis induction (Zhu et al., 2012; Yan et al., 2014; Zhang et al., 2019). These results indicated that celastrol not only has good efficiency on killing cancer cells but also can improve the anti-cancer ability of first-line drugs for tumor therapy.
Hypoxia was one of most important regulators in liver cancer progression. Hypoxia-inducible factor (HIF-1), a nuclear transcription factor, might be activated under hypoxia and specifically regulated oxygen and metabolic homeostasis (Balamurugan, 2016). HIF-1/2α protein is highly expressed in human HCC tissues, and correlates with tumor invasion and metastasis in HCC patients (Bangoura et al., 2004; Wong et al., 2014). In addition to antiproliferative and proapoptotic effects, celastrol repressed tumor growth, angiogenesis, invasion, and metastasis through reduced hypoxia-induced accumulation of HIF-1α protein (Ma et al., 2014). Ma et al. demonstrated that celastrol inhibited the hypoxia-induced accumulation of HIF-1α protein due to the downregulation of mTOR/p70S6K/EIF4E and ERK1/2 phosphorylation in Hep3B cells derived xenografts nude female mice, and the expression of angiogenesis related factors-vascular endothelial growth factor (VEGF) and erythropoietin (EPO) are both prevented (Ma et al., 2014). However, this result was challenged by Han and his co-workers, who found that celastrol promoted HIF-1α protein accumulation by inducing ROS and activating Akt/p70S6K signaling in HepG2 cells under hypoxia, and celastrol suppressed cancer cell growth by promoting mitochondrial autophagy and apoptosis in vitro (Han et al., 2014). The difference in hypoxia exposure time (16 h in Han et al.’s work vs. 6 h in Ma et al.’s study) of HepG2 cells might contribute to the discrepant results on HIF-1a expression for celastrol in the above studies.
Summarily, celastrol might be a potential therapeutic candidate for the treatment of liver cancer due to reduction of the oxidative stress, inhibition of inflammatory response, and activation of mitochondrial autophagy. Although the studies were performed both in vitro and in vivo, the clinical research still should be performed to evaluate whether celastrol could apply as effective drug or adjuvant drug to further improve clinical outcomes. The mechanisms of celastrol on anti-cancer are summarized in Figure 3.
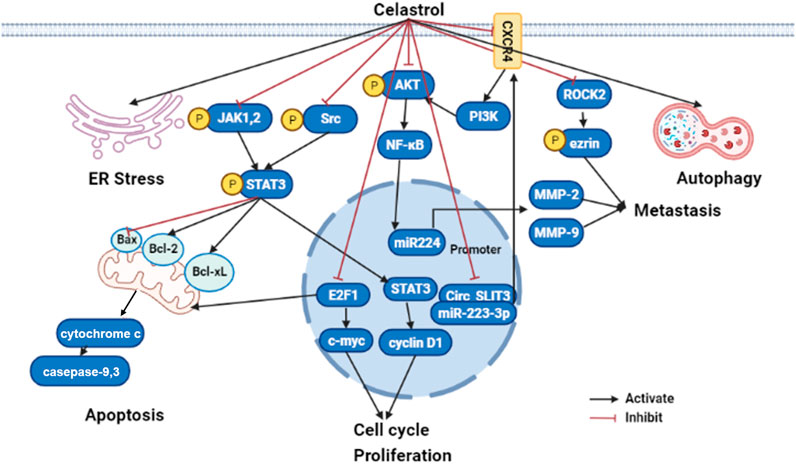
FIGURE 3. The molecular mechanisms of celastrol in liver cancer (edited by Biorender software). Celastrol exert anticancer effect by restraining their cell growth, metastasis, and inflammatory response, while boosting ER stress, apoptosis, and autophagy depending on modulated different signaling pathways. Firstly, celastrol can activate cancer cell apoptosis through inhibiting JAK2 and STAT3 phosphorylation, then downregulated BCL-2 family proteins (Bcl-2 and Bcl-xl) and upregulated caspase family proteins (caspase 3 and caspase 9). Secondly, celastrol repressed tumor cell proliferation by suppressing cyclin D1 and c-myc through E2F1 and STAT3; Lastly, celastrol prohibited tumor metastasis by NF-κB signaling pathway modulated MMP-2 and MMP-9 through the CXCR4-related signaling pathway, as well as the ROCK2-mediated phosphorylation of ezrin at Thr567. ER, endoplasmic reticulum; JAK1, Janus kinase1; JAK2, Janus kinase2; STAT3, signal transducer and activator of transcription 3; Bcl-2, B-cell lymphoma-2; PI3K, phosphatidylinositol-3-kinase; AKT, protein kinase B; NF-kB, nuclear factor kappa B; MMP2, Matrix metallopeptidase 2; MMP9, Matrix metallopeptidase 9; circ_SLIT3, circRNA slit guidance ligand 3; ROCK2, Rho Associated Coiled-Coil Containing Protein kinase 2.
Although celastrol exhibited many benefits on various liver diseases, the shortcomings of celastrol limited its clinical transformation. On the one hand, the poor water solubility of celastrol (13.25 ± 0.83 μg/ml at 37°C) results in low oral bioavailability (17.06% in rat) (Qi et al., 2014), and more researches should be conducted on improving its water solubility and oral bioavailability. On the other hand, the side effects of celastrol and narrow therapeutic window of dose also could be a big problem for its clinical use. While 0.25 mg or 0.5 mg/kg/d, celastrol (i.p.) attenuated the LPS induced inflammation (Yan et al., 2021), 1.5 mg/kg/d, celastrol (i.p.) (Wu et al., 2018) aggravated the liver injury induced by celastrol. Beyond that, it was insufficient for CYP450s to metabolize celastrol, which led to the hepatotoxicity. In addition to damaging the liver, celastrol also has side effects on kidney (Wu et al., 2018), cardiovascular system, and hematopoietic system (Zhang et al., 2012). New modification and formulation are urgently needed to overcome the shortcomings and improve its efficacy.
Hopefully, new approaches have been reported to improve the water solubility and bioavailability of celastrol. Lipid-based nanocarriers (including the self-microemulsifying drug delivery system [SMEDDS] [Chen et al., 2012], nanostructured lipid carrier [NLC], and phytosomes) (Freag et al., 2018), polymer-based nanocarriers like PEG-PCL (poly(ethylene glycol)-poly(ɛ-caprolactone) copolymers) nanoparticles (Zhao et al., 2019a), and galactosylated liposomes (Chen et al., 2020b) markedly enhanced the water solubility and oral bioavailability of celastrol. Combination with other drugs can be an effective approach to reduce the toxicity and enhance the efficacy of celastrol. Currently, celastrol combined with chemotherapeutic agents, tumor necrosis factor superfamily, active ingredients of TCM, ionizing radiation, nucleic acid, and other therapies have been applied to the treatment of various cancers in vitro (Shi et al., 2020). The combined therapy reduced the dosage of celastrol and the related adverse effect, while the efficacy of celastrol improved. In addition, structural modifications will be a promising way to reduce the toxicity and improve the water bioavailability of celastrol, and the correlation between structure and toxicity have been well studied (Hou et al., 2020). Chemical modifications of celastrol mainly focused on the A/B ring and C-20 carboxyl group. In addition to chemical modifications, Chang et al. demonstrated that the glycosylation of celastrol formed by biotransformation showed over 53-fold higher water solubility and exhibited 50-fold less toxicity than celastrol in vivo (Chang et al., 2021), which indicated that biotransformation or biosynthesis might be a useful strategy for the clinical use of celastrol.
A large number of studies have shown that celastrol might be a potential drug for different liver diseases treatment. In this review, we summarized the recent studies about the protective effects and mechanisms of celastrol in several liver diseases, including MAFLD, and it caused liver injury, or liver cancer. The liver protection effect could be attributed to modulating metabolic balance, inhibiting inflammatory response, and activating autophagy by altering different cellular pathways (Tables 1–5). Although many researchers elucidated the molecular mechanisms of celastrol in liver diseases, the direct targets of celastrol are unknown. The SPR assay, affinity chromatographic methods, and other methods might be used to explore the direct interaction between celastrol and the potential targets. At the same time, with the development of omics, celastrol regulates gut microbes and liver metabolism to exert hepatoprotective effects required further investigated.
Moreover, although accumulating evidence demonstrated that celastrol grants tremendous potential in liver diseases at cellular or animal levels, the clinical studies of celastrol in liver diseases are very rare. Comprehensive studies on efficacy, safety, and toxicity in humans urgently need to be carried out. Besides, the poor water solubility, low bioavailability, and narrow therapeutic window of dose also limited the clinical application of celastrol. New modifications and formulations are needed to overcome the shortcomings and improve its efficacy. It is foreseeable that celastrol and its derivatives will be a promising drug for the treatment of liver diseases.
SJ, ML, and L-WQ conceived the study, SJ and ML worte the manuscript, FX and LW drew the figure, ML, SJ, and GZ performed tables analysis. All authors contributed to the article and approved the submitted version.
This research funded by the National Natural Science Foundation of China (Grant No. 81900780 and Grant No. 82003933).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The author acknowledges and thanks Dr. Haijian Sun and Dr. Raphael N. Alolga for editing our article.
Abdelaziz, H. A., Shaker, M. E., Hamed, M. F., and Gameil, N. M. (2017). Repression of Acetaminophen-Induced Hepatotoxicity by a Combination of Celastrol and Brilliant Blue G. Toxicol. Lett. 275, 6–18. doi:10.1016/j.toxlet.2017.04.012
Abu Bakar, M. H., Shariff, K. A., Tan, J. S., and Lee, L. K. (2020). Celastrol Attenuates Inflammatory Responses in Adipose Tissues and Improves Skeletal Muscle Mitochondrial Functions in High Fat Diet-Induced Obese Rats via Upregulation of AMPK/SIRT1 Signaling Pathways. Eur. J. Pharmacol. 883, 173371. doi:10.1016/j.ejphar.2020.173371
Almazroo, O. A., Miah, M. K., and Venkataramanan, R. (2017). Drug Metabolism in the Liver. Clin. Liver Dis. 21, 1–20. doi:10.1016/j.cld.2016.08.001
Bagherniya, M., Nobili, V., Blesso, C. N., and Sahebkar, A. (2018). Medicinal Plants and Bioactive Natural Compounds in the Treatment of Non-alcoholic Fatty Liver Disease: A Clinical Review. Pharmacol. Res. 130, 213–240. doi:10.1016/j.phrs.2017.12.020
Balamurugan, K. (2016). HIF-1 at the Crossroads of Hypoxia, Inflammation, and Cancer. Int. J. Cancer 138, 1058–1066. doi:10.1002/ijc.29519
Bangoura, G., Yang, L. Y., Huang, G. W., and Wang, W. (2004). Expression of HIF-2alpha/EPAS1 in Hepatocellular Carcinoma. World J. Gastroenterol. 10, 525–530. doi:10.3748/wjg.v10.i4.525
Boutari, C., and Mantzoros, C. S. (2020). Adiponectin and Leptin in the Diagnosis and Therapy of NAFLD. Metabolism 103, 154028. doi:10.1016/j.metabol.2019.154028
Canfora, E. E., Meex, R. C. R., Venema, K., and Blaak, E. E. (2019). Gut Microbial Metabolites in Obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 15 (5), 261–273. doi:10.1038/s41574-019-0156-z
Caron, A., Lee, S., Elmquist, J. K., and Gautron, L. (2018). Leptin and Brain-Adipose Crosstalks. Nat. Rev. Neurosci. 19, 153–165. doi:10.1038/nrn.2018.7
Caussy, C., and Loomba, R. (2018). Gut Microbiome, Microbial Metabolites and the Development of NAFLD. Nat. Rev. Gastroenterol. Hepatol. 15, 719–720. doi:10.1038/s41575-018-0058-x
Chang, T. S., Wang, T. Y., Chiang, C. M., Lin, Y. J., Chen, H. L., Wu, Y. W., et al. (2021). Biotransformation of Celastrol to a Novel, Well-Soluble, Low-Toxic and Anti-oxidative Celastrol-29-O-β-Glucoside by Bacillus Glycosyltransferases. J. Biosci. Bioeng. 131, 176–182. doi:10.1016/j.jbiosc.2020.09.017
Chang, W., He, W., Li, P. P., Song, S. S., Yuan, P. F., Lu, J. T., et al. (2016). Protective Effects of Celastrol on Diethylnitrosamine-Induced Hepatocellular Carcinoma in Rats and its Mechanisms. Eur. J. Pharmacol. 784, 173–180. doi:10.1016/j.ejphar.2016.04.045
Chellappa, K., Perron, I. J., Naidoo, N., and Baur, J. A. (2019). The Leptin Sensitizer Celastrol Reduces Age-Associated Obesity and Modulates Behavioral Rhythms. Aging cell 18, e12874. doi:10.1111/acel.12874
Chen, G., Zhang, X., Zhao, M., Wang, Y., Cheng, X., Wang, D., et al. (2011). Celastrol Targets Mitochondrial Respiratory Chain Complex I to Induce Reactive Oxygen Species-dependent Cytotoxicity in Tumor Cells. BMC cancer 11, 170. doi:10.1186/1471-2407-11-170
Chen, X., Hu, X., Hu, J., Qiu, Z., Yuan, M., and Zheng, G. (2020). Celastrol-Loaded Galactosylated Liposomes Effectively Inhibit AKT/c-Met-Triggered Rapid Hepatocarcinogenesis in Mice. Mol. Pharm. 17, 738–747. doi:10.1021/acs.molpharmaceut.9b00428
Chen, Y., Yuan, L., Zhou, L., Zhang, Z. H., Cao, W., and Wu, Q. (2012). Effect of Cell-Penetrating Peptide-Coated Nanostructured Lipid Carriers on the Oral Absorption of Tripterine. Int. J. Nanomedicine 7, 4581–4591. doi:10.2147/IJN.S34991
Chen, Z., Tian, R., She, Z., Cai, J., and Li, H. (2020). Role of Oxidative Stress in the Pathogenesis of Nonalcoholic Fatty Liver Disease. Free Radic. Biol. Med. 152, 116–141. doi:10.1016/j.freeradbiomed.2020.02.025
Diehl-Jones, W. L., and Askin, D. F. (2002). The Neonatal Liver, Part 1: Embryology, Anatomy, and Physiology. Neonatal. Netw. 21, 5–12. doi:10.1891/0730-0832.21.2.5
Dodd, G. T., Xirouchaki, C. E., Eramo, M., Mitchell, C. A., Andrews, Z. B., Henry, B. A., et al. (2019). Intranasal Targeting of Hypothalamic PTP1B and TCPTP Reinstates Leptin and Insulin Sensitivity and Promotes Weight Loss in Obesity. Cell Rep 28, 2905–e5. doi:10.1016/j.celrep.2019.08.019
Du, S., Song, X., Li, Y., Cao, Y., Chu, F., Durojaye, O. A., et al. (2020). Celastrol Inhibits Ezrin-Mediated Migration of Hepatocellular Carcinoma Cells. Sci. Rep. 10, 11273. doi:10.1038/s41598-020-68238-1
El-Tanbouly, G. S., El-Awady, M. S., Megahed, N. A., Salem, H. A., and El-Kashef, H. A. (2017). The NF-Κb Inhibitor Celastrol Attenuates Acute Hepatic Dysfunction Induced by Cecal Ligation and Puncture in Rats. Environ. Toxicol. Pharmacol. 50, 175–182. doi:10.1016/j.etap.2017.02.002
Eslam, M., Sanyal, A. J., George, J., and International Consensus, P. (2020). MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 158, 1999–2014. doi:10.1053/j.gastro.2019.11.312
Fabbrini, E., Sullivan, S., and Klein, S. (2010). Obesity and Nonalcoholic Fatty Liver Disease: Biochemical, Metabolic, and Clinical Implications. Hepatology 51, 679–689. doi:10.1002/hep.23280
Fang, P., He, B., Yu, M., Shi, M., Zhu, Y., Zhang, Z., et al. (2019). Treatment with Celastrol Protects against Obesity through Suppression of Galanin-Induced Fat Intake and Activation of PGC-1α/GLUT4 axis-mediated Glucose Consumption. Biochim. Biophys. Acta Mol. Basis Dis. 1865, 1341–1350. doi:10.1016/j.bbadis.2019.02.002
Feng, L., Zhang, D., Fan, C., Ma, C., Yang, W., Meng, Y., et al. (2013). ER Stress-Mediated Apoptosis Induced by Celastrol in Cancer Cells and Important Role of Glycogen Synthase Kinase-3β in the Signal Network. Cell Death Dis 4, e715. doi:10.1038/cddis.2013.222
Feng, X., Guan, D., Auen, T., Choi, J. W., Salazar Hernández, M. A., Lee, J., et al. (2019). IL1R1 Is Required for Celastrol's Leptin-Sensitization and Antiobesity Effects. Nat. Med. 25, 575–582. doi:10.1038/s41591-019-0358-x
Feng, X., Guan, D., Auen, T., Choi, J. W., Salazar-Hernandez, M. A., Faruk, F., et al. (2019). Lipocalin 2 Does Not Play A Role in Celastrol-Mediated Reduction in Food Intake and Body Weight. Sci. Rep. 9, 12809. doi:10.1038/s41598-019-49151-8
Fontana, R. J., Hayashi, P. H., Gu, J., Reddy, K. R., Barnhart, H., Watkins, P. B., et al. (2014). Idiosyncratic Drug-Induced Liver Injury Is Associated with Substantial Morbidity and Mortality within 6 Months from Onset. Gastroenterology 147, 96–e4. doi:10.1053/j.gastro.2014.03.045
Frazier, K., and Chang, E. B. (2020). Intersection of the Gut Microbiome and Circadian Rhythms in Metabolism. Trends Endocrinol. Metab. 31, 25–36. doi:10.1016/j.tem.2019.08.013
Freag, M. S., Saleh, W. M., and Abdallah, O. Y. (2018). Self-assembled Phospholipid-Based Phytosomal Nanocarriers as Promising Platforms for Improving Oral Bioavailability of the Anticancer Celastrol. Int. J. Pharm. 535, 18–26. doi:10.1016/j.ijpharm.2017.10.053
Friedman, S. L., Neuschwander-Tetri, B. A., Rinella, M., and Sanyal, A. J. (2018). Mechanisms of NAFLD Development and Therapeutic Strategies. Nat. Med. 24, 908–922. doi:10.1038/s41591-018-0104-9
García, M. C., Wernstedt, I., Berndtsson, A., Enge, M., Bell, M., Hultgren, O., et al. (2006). Mature-onset Obesity in Interleukin-1 Receptor I Knockout Mice. Diabetes 55, 1205–1213. doi:10.2337/db05-1304
García-Rodríguez, J. L., Barbier-Torres, L., Fernández-Álvarez, S., Gutiérrez-de Juan, V., Monte, M. J., Halilbasic, E., et al. (2014). SIRT1 Controls Liver Regeneration by Regulating Bile Acid Metabolism through Farnesoid X Receptor and Mammalian Target of Rapamycin Signaling. Hepatology 59, 1972–1983. doi:10.1002/hep.26971
Guo, J., Wang, Y., Wang, N., Bai, Y., and Shi, D. (2019). Celastrol Attenuates Intrahepatic Cholestasis of Pregnancy by Inhibiting Matrix Metalloproteinases-2 and 9. Ann. Hepatol. 18, 40–47. doi:10.5604/01.3001.0012.7860
Hackl, M. T., Fürnsinn, C., Schuh, C. M., Krssak, M., Carli, F., Guerra, S., et al. (2019). Brain Leptin Reduces Liver Lipids by Increasing Hepatic Triglyceride Secretion and Lowering Lipogenesis. Nat. Commun. 10, 2717. doi:10.1038/s41467-019-10684-1
Han, X., Sun, S., Zhao, M., Cheng, X., Chen, G., Lin, S., et al. (2014). Celastrol Stimulates Hypoxia-Inducible Factor-1 Activity in Tumor Cells by Initiating the ROS/Akt/p70S6K Signaling Pathway and Enhancing Hypoxia-Inducible Factor-1α Protein Synthesis. PloS one 9, e112470. doi:10.1371/journal.pone.0112470
Hou, W., Liu, B., and Xu, H. (2020). Celastrol: Progresses in Structure-Modifications, Structure-Activity Relationships, Pharmacology and Toxicology. Eur. J. Med. Chem. 189, 112081. doi:10.1016/j.ejmech.2020.112081
Hu, M., Luo, Q., Alitongbieke, G., Chong, S., Xu, C., Xie, L., et al. (2017). Celastrol-Induced Nur77 Interaction with TRAF2 Alleviates Inflammation by Promoting Mitochondrial Ubiquitination and Autophagy. Mol. Cell 66, 141–e6. doi:10.1016/j.molcel.2017.03.008
Hu, W., Wang, L., Du, G., Guan, Q., Dong, T., Song, L., et al. (2020). Effects of Microbiota on the Treatment of Obesity with the Natural Product Celastrol in Rats. Diabetes Metab. J. 44, 747–763. doi:10.4093/dmj.2019.0124
Hu, Y., Wang, S., Wu, X., Zhang, J., Chen, R., Chen, M., et al. (2013). Chinese Herbal Medicine-Derived Compounds for Cancer Therapy: a Focus on Hepatocellular Carcinoma. J. Ethnopharmacol 149, 601–612. doi:10.1016/j.jep.2013.07.030
Hua, H., Zhang, Y., Zhao, F., Chen, K., Wu, T., Liu, Q., et al. (2021). Celastrol Inhibits Intestinal Lipid Absorption by Reprofiling the Gut Microbiota to Attenuate High-Fat Diet-Induced Obesity. iScience 24, 102077. doi:10.1016/j.isci.2021.102077
Jannuzzi, A. T., Kara, M., and Alpertunga, B. (2018). Celastrol Ameliorates Acetaminophen-Induced Oxidative Stress and Cytotoxicity in HepG2 Cells. Hum. Exp. Toxicol. 37, 742–751. doi:10.1177/0960327117734622
Jarvis, H., Craig, D., Barker, R., Spiers, G., Stow, D., Anstee, Q. M., et al. (2020). Metabolic Risk Factors and Incident Advanced Liver Disease in Non-alcoholic Fatty Liver Disease (NAFLD): A Systematic Review and Meta-Analysis of Population-Based Observational Studies. Plos Med. 17, e1003100. doi:10.1371/journal.pmed.1003100
Jiang, H. L., Jin, J. Z., Wu, D., Xu, D., Lin, G. F., Yu, H., et al. (2013). Celastrol Exerts Synergistic Effects with PHA-665752 and Inhibits Tumor Growth of C-Met-Deficient Hepatocellular Carcinoma In Vivo. Mol. Biol. Rep. 40, 4203–4209. doi:10.1007/s11033-013-2501-y
Kashyap, D., Sharma, A., Tuli, H. S., Sak, K., Mukherjee, T., and Bishayee, A. (2018). Molecular Targets of Celastrol in Cancer: Recent Trends and Advancements. Crit. Rev. Oncol. Hematol. 128, 70–81. doi:10.1016/j.critrevonc.2018.05.019
Katsiki, N., Mikhailidis, D. P., and Mantzoros, C. S. (2016). Non-alcoholic Fatty Liver Disease and Dyslipidemia: An Update. Metabolism 65, 1109–1123. doi:10.1016/j.metabol.2016.05.003
Kazankov, K., Jørgensen, S. M. D., Thomsen, K. L., Møller, H. J., Vilstrup, H., George, J., et al. (2019). The Role of Macrophages in Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 16, 145–159. doi:10.1038/s41575-018-0082-x
Kun-Ming, C., Chih-Hsien, C., Chen-Fang, L., Ting-Jung, W., Hong-Shiue, C., and Wei-Chen, L. (2020). Potential Anticancer Effect of Celastrol on Hepatocellular Carcinoma by Suppressing CXCR4-Related Signal and Impeding Tumor Growth In Vivo. Arch. Med. Res. 51, 297–302. doi:10.1016/j.arcmed.2020.03.001
Kyriakou, E., Schmidt, S., Dodd, G. T., Pfuhlmann, K., Simonds, S. E., Lenhart, D., et al. (2018). Celastrol Promotes Weight Loss in Diet-Induced Obesity by Inhibiting the Protein Tyrosine Phosphatases PTP1B and TCPTP in the Hypothalamus. J. Med. Chem. 61, 11144–11157. doi:10.1021/acs.jmedchem.8b01224
Lanthier, N. (2015). Targeting Kupffer Cells in Non-alcoholic Fatty Liver Disease/non-Alcoholic Steatohepatitis: Why and How? World J. Hepatol. 7, 2184–2188. doi:10.4254/wjh.v7.i19.2184
Le Roy, T., Llopis, M., Lepage, P., Bruneau, A., Rabot, S., Bevilacqua, C., et al. (2013). Intestinal Microbiota Determines Development of Non-Alcoholic Fatty Liver Disease in Mice. Gut. 62 (12), 1787–1794. doi:10.1136/gutjnl-2012-303816
Li, H., Li, Y., Liu, D., Sun, H., and Liu, J. (2013). miR-224 Is Critical for Celastrol-Induced Inhibition of Migration and Invasion of Hepatocellular Carcinoma Cells. Cell Physiol Biochem 32, 448–458. doi:10.1159/000354450
Li, P. P., He, W., Yuan, P. F., Song, S. S., Lu, J. T., and Wei, W. (2015). Celastrol Induces Mitochondria-Mediated Apoptosis in Hepatocellular Carcinoma Bel-7402 Cells. Am. J. Chin. Med. 43, 137–148. doi:10.1142/S0192415X15500093
Li, Z., Feng, H., Han, L., Ding, L., Shen, B., Tian, Y., et al. (2020). Chicoric Acid Ameliorate Inflammation and Oxidative Stress in Lipopolysaccharide and D-Galactosamine Induced Acute Liver Injury. J. Cell Mol Med 24, 3022–3033. doi:10.1111/jcmm.14935
Liang, Y., Chen, H., Liu, Y., Hou, X., Wei, L., Bao, Y., et al. (2022). Association of MAFLD with Diabetes, Chronic Kidney Disease, and Cardiovascular Disease: A 4.6-Year Cohort Study in China. J. Clin. Endocrinol. Metab. 107, 88–97. doi:10.1210/clinem/dgab641
Licata, A. (2016). Adverse Drug Reactions and Organ Damage: The Liver. Eur. J. Intern. Med. 28, 9–16. doi:10.1016/j.ejim.2015.12.017
Lin, L., Sun, Y., Wang, D., Zheng, S., Zhang, J., and Zheng, C. (2015). Celastrol Ameliorates Ulcerative Colitis-Related Colorectal Cancer in Mice via Suppressing Inflammatory Responses and Epithelial-Mesenchymal Transition. Front. Pharmacol. 6, 320. doi:10.3389/fphar.2015.00320
Liu, J., Lee, J., Salazar Hernandez, M. A., Mazitschek, R., and Ozcan, U. (2015). Treatment of Obesity with Celastrol. Cell 161, 999–1011. doi:10.1016/j.cell.2015.05.011
Luo, D., Guo, Y., Cheng, Y., Zhao, J., Wang, Y., and Rong, J. (2017). Natural Product Celastrol Suppressed Macrophage M1 Polarization against Inflammation in Diet-Induced Obese Mice via Regulating Nrf2/HO-1, MAP Kinase and NF-Κb Pathways. Aging (Albany NY) 9, 2069–2082. doi:10.18632/aging.101302
Luo, X., Li, H., Ma, L., Zhou, J., Guo, X., Woo, S. L., et al. (2018). Expression of STING Is Increased in Liver Tissues from Patients with NAFLD and Promotes Macrophage-Mediated Hepatic Inflammation and Fibrosis in Mice. Gastroenterology 155, 1971–e4. doi:10.1053/j.gastro.2018.09.010
Ma, J., Han, L. Z., Liang, H., Mi, C., Shi, H., Lee, J. J., et al. (2014). Celastrol Inhibits the HIF-1α Pathway by Inhibition of mTOR/p70S6K/eIF4E and ERK1/2 Phosphorylation in Human Hepatoma Cells. Oncol. Rep. 32, 235–242. doi:10.3892/or.2014.3211
Ma, L., Peng, L., Fang, S., He, B., and Liu, Z. (2017). Celastrol Downregulates E2F1 to Induce Growth Inhibitory Effects in Hepatocellular Carcinoma HepG2 Cells. Oncol. Rep. 38, 2951–2958. doi:10.3892/or.2017.5971
Ma, X., Xu, L., Alberobello, A. T., Gavrilova, O., Bagattin, A., Skarulis, M., et al. (2015). Celastrol Protects against Obesity and Metabolic Dysfunction through Activation of a HSF1-Pgc1α Transcriptional Axis. Cell Metab 22, 695–708. doi:10.1016/j.cmet.2015.08.005
Madrigal-Santillán, E., Madrigal-Bujaidar, E., Álvarez-González, I., Sumaya-Martínez, M. T., Gutiérrez-Salinas, J., Bautista, M., et al. (2014). Review of Natural Products with Hepatoprotective Effects. World J. Gastroenterol. 20, 14787–14804. doi:10.3748/wjg.v20.i40.14787
Minsky, N., and Roeder, R. G. (2015). Direct Link between Metabolic Regulation and the Heat-Shock Response through the Transcriptional Regulator PGC-1α. Proc. Natl. Acad. Sci. U S A. 112, E5669–E5678. doi:10.1073/pnas.1516219112
Mundi, M. S., Velapati, S., Patel, J., Kellogg, T. A., Abu Dayyeh, B. K., and Hurt, R. T. (2020). Evolution of NAFLD and its Management. Nutr. Clin. Pract. 35, 72–84. doi:10.1002/ncp.10449
Murphy, E. F., Cotter, P. D., Hogan, A., O'Sullivan, O., Joyce, A., Fouhy, F., et al. (2013). Divergent Metabolic Outcomes Arising from Targeted Manipulation of the Gut Microbiota in Diet-Induced Obesity. Gut 62, 220–226. doi:10.1136/gutjnl-2011-300705
Myers, M. G., Leibel, R. L., Seeley, R. J., and Schwartz, M. W. (2010). Obesity and Leptin Resistance: Distinguishing Cause from Effect. Trends Endocrinol. Metab. 21, 643–651. doi:10.1016/j.tem.2010.08.002
Ore, A., and Akinloye, O. A. (2019). Oxidative Stress and Antioxidant Biomarkers in Clinical and Experimental Models of Non-alcoholic Fatty Liver Disease. Medicina (Kaunas) 55. doi:10.3390/medicina55020026
Ouyang, M., Qin, T., Liu, H., Lu, J., Peng, C., and Guo, Q. (2021). Enhanced Inflammatory Reaction and Thrombosis in High-Fat Diet-Fed ApoE-/- Mice Are Attenuated by Celastrol. Exp. Clin. Endocrinol. Diabetes 129, 339–348. doi:10.1055/a-1010-5543
Padda, M. S., Sanchez, M., Akhtar, A. J., and Boyer, J. L. (2011). Drug-induced Cholestasis. Hepatology 53, 1377–1387. doi:10.1002/hep.24229
Pan, W. W., and Myers, M. G. (2018). Leptin and the Maintenance of Elevated Body Weight. Nat. Rev. Neurosci. 19, 95–105. doi:10.1038/nrn.2017.168
Pfuhlmann, K., Schriever, S. C., Baumann, P., Kabra, D. G., Harrison, L., Mazibuko-Mbeje, S. E., et al. (2018). Celastrol-Induced Weight Loss Is Driven by Hypophagia and Independent from UCP1. Diabetes 67, 2456–2465. doi:10.2337/db18-0146
Purushotham, A., Schug, T. T., Xu, Q., Surapureddi, S., Guo, X., and Li, X. (2009). Hepatocyte-specific Deletion of SIRT1 Alters Fatty Acid Metabolism and Results in Hepatic Steatosis and Inflammation. Cell Metab 9, 327–338. doi:10.1016/j.cmet.2009.02.006
Qi, X., Qin, J., Ma, N., Chou, X., and Wu, Z. (2014). Solid Self-Microemulsifying Dispersible Tablets of Celastrol: Formulation Development, Charaterization and Bioavailability Evaluation. Int. J. Pharm. 472, 40–47. doi:10.1016/j.ijpharm.2014.06.019
Rajendran, P., Li, F., Shanmugam, M. K., Kannaiyan, R., Goh, J. N., Wong, K. F., et al. (2012). Celastrol Suppresses Growth and Induces Apoptosis of Human Hepatocellular Carcinoma through the Modulation of STAT3/JAK2 Signaling cascade In Vitro and In Vivo. Cancer Prev. Res. (Phila) 5, 631–643. doi:10.1158/1940-6207.CAPR-11-0420
Ren, B., Liu, H., Gao, H., Liu, S., Zhang, Z., Fribley, A. M., et al. (2017). Celastrol Induces Apoptosis in Hepatocellular Carcinoma Cells via Targeting ER-Stress/UPR. Oncotarget 8, 93039–93050. doi:10.18632/oncotarget.21750
Rodgers, J. T., Lerin, C., Haas, W., Gygi, S. P., Spiegelman, B. M., and Puigserver, P. (2005). Nutrient Control of Glucose Homeostasis through a Complex of PGC-1alpha and SIRT1. Nature 434, 113–118. doi:10.1038/nature03354
Saber, S., Ghanim, A. M. H., El-Ahwany, E., and El-Kader, E. M. A. (2020). Novel Complementary Antitumour Effects of Celastrol and Metformin by Targeting IκBκB, Apoptosis and NLRP3 Inflammasome Activation in Diethylnitrosamine-Induced Murine Hepatocarcinogenesis. Cancer Chemother. Pharmacol. 85, 331–343. doi:10.1007/s00280-020-04033-z
Saito, K., Davis, K. C., Morgan, D. A., Toth, B. A., Jiang, J., Singh, U., et al. (2019). Celastrol Reduces Obesity in MC4R Deficiency and Stimulates Sympathetic Nerve Activity Affecting Metabolic and Cardiovascular Functions. Diabetes 68, 1210–1220. doi:10.2337/db18-1167
Samuel, V. T., and Shulman, G. I. (2018). Nonalcoholic Fatty Liver Disease as a Nexus of Metabolic and Hepatic Diseases. Cell Metab 27, 22–41. doi:10.1016/j.cmet.2017.08.002
Saran, A. R., Dave, S., and Zarrinpar, A. (2020). Circadian Rhythms in the Pathogenesis and Treatment of Fatty Liver Disease. Gastroenterology 158, 1948–1966. doi:10.1053/j.gastro.2020.01.050
Shehu, A. I., Ma, X., and Venkataramanan, R. (2017). Mechanisms of Drug-Induced Hepatotoxicity. Clin. Liver Dis. 21, 35–54. doi:10.1016/j.cld.2016.08.002
Shi, J., Li, J., Xu, Z., Chen, L., Luo, R., Zhang, C., et al. (2020). Celastrol: A Review of Useful Strategies Overcoming its Limitation in Anticancer Application. Front. Pharmacol. 11, 558741. doi:10.3389/fphar.2020.558741
Si, H., Wang, H., Xiao, H., Fang, Y., and Wu, Z. (2021). Anti-Tumor Effect of Celastrol on Hepatocellular Carcinoma by the circ_SLIT3/miR-223-3p/CXCR4 Axis. Cancer Manag. Res. 13, 1099–1111. doi:10.2147/CMAR.S278023
Song, X., Zhang, Y., Dai, E., Du, H., and Wang, L. (2019). Mechanism of Action of Celastrol against Rheumatoid Arthritis: A Network Pharmacology Analysis. Int. Immunopharmacol 74, 105725. doi:10.1016/j.intimp.2019.105725
Strnad, P., Tacke, F., Koch, A., and Trautwein, C. (2017). Liver - Guardian, Modifier and Target of Sepsis. Nat. Rev. Gastroenterol. Hepatol. 14, 55–66. doi:10.1038/nrgastro.2016.168
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A. Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
Świderska, M., Maciejczyk, M., Zalewska, A., Pogorzelska, J., Flisiak, R., and Chabowski, A. (2019). Oxidative Stress Biomarkers in the Serum and Plasma of Patients with Non-alcoholic Fatty Liver Disease (NAFLD). Can Plasma AGE Be a Marker of NAFLD? Oxidative Stress Biomarkers in NAFLD Patients. Free Radic. Res. 53, 841–850. doi:10.1080/10715762.2019.1635691
Tappy, L. (2021). Metabolism of Sugars: A Window to the Regulation of Glucose and Lipid Homeostasis by Splanchnic Organs. Clin. Nutr. 40, 1691–1698. doi:10.1016/j.clnu.2020.12.022
Thawley, V. (2017). Acute Liver Injury and Failure. Vet. Clin. North America: Small Anim. Pract. 47, 617–630. doi:10.1016/j.cvsm.2016.11.010
Tseng, C. K., Hsu, S. P., Lin, C. K., Wu, Y. H., Lee, J. C., and Young, K. C. (2017). Celastrol Inhibits Hepatitis C Virus Replication by Upregulating Heme Oxygenase-1 via the JNK MAPK/Nrf2 Pathway in Human Hepatoma Cells. Antivir. Res 146, 191–200. doi:10.1016/j.antiviral.2017.09.010
Vega, R. B., Huss, J. M., and Kelly, D. P. (2000). The Coactivator PGC-1 Cooperates with Peroxisome Proliferator-Activated Receptor Alpha in Transcriptional Control of Nuclear Genes Encoding Mitochondrial Fatty Acid Oxidation Enzymes. Mol. Cell Biol 20, 1868–1876. doi:10.1128/mcb.20.5.1868-1876.2000
Vento, S., and Cainelli, F. (2020). Acute Liver Failure. Lancet 395, 1833. doi:10.1016/S0140-6736(20)30046-5
Wang, C., Shi, C., Yang, X., Yang, M., Sun, H., and Wang, C. (2014). Celastrol Suppresses Obesity Process via Increasing Antioxidant Capacity and Improving Lipid Metabolism. Eur. J. Pharmacol. 744, 52–58. doi:10.1016/j.ejphar.2014.09.043
Wang, Y., Li, C., Gu, J., Chen, C., Duanmu, J., Miao, J., et al. (2020). Celastrol Exerts Anti-inflammatory Effect in Liver Fibrosis via Activation of AMPK-SIRT3 Signalling. J. Cell Mol Med 24, 941–953. doi:10.1111/jcmm.14805
Wei, W., Wu, S., Wang, X., Sun, C. K., Yang, X., Yan, X., et al. (2014). Novel Celastrol Derivatives Inhibit the Growth of Hepatocellular Carcinoma Patient-Derived Xenografts. Oncotarget 5, 5819–5831. doi:10.18632/oncotarget.2171
Wong, C. C., Kai, A. K., and Ng, I. O. (2014). The Impact of Hypoxia in Hepatocellular Carcinoma Metastasis. Front. Med. 8, 33–41. doi:10.1007/s11684-013-0301-3
Wu, M., Chen, W., Yu, X., Ding, D., Zhang, W., Hua, H., et al. (2018). Celastrol Aggravates LPS-Induced Inflammation and Injuries of Liver and Kidney in Mice. Am. J. Transl Res. 10, 2078–2086.
Xiong, X., Ren, Y., Cui, Y., Li, R., Wang, C., and Zhang, Y. (2017). Obeticholic Acid Protects Mice against Lipopolysaccharide-Induced Liver Injury and Inflammation. Biomed. Pharmacother. 96, 1292–1298. doi:10.1016/j.biopha.2017.11.083
Xu, J., Bartolome, C. L., Low, C. S., Yi, X., Chien, C. H., Wang, P., et al. (2018). Genetic Identification of Leptin Neural Circuits in Energy and Glucose Homeostases. Nature 556, 505–509. doi:10.1038/s41586-018-0049-7
Xu, L., Zhao, W., Wang, D., and Ma, X. (2018). Chinese Medicine in the Battle against Obesity and Metabolic Diseases. Front. Physiol. 9, 850. doi:10.3389/fphys.2018.00850
Yan, C. Y., Ouyang, S. H., Wang, X., Wu, Y. P., Sun, W. Y., Duan, W. J., et al. (2021). Celastrol Ameliorates Propionibacterium acnes/LPS-Induced Liver Damage and MSU-Induced Gouty Arthritis via Inhibiting K63 Deubiquitination of NLRP3. Phytomedicine 80, 153398. doi:10.1016/j.phymed.2020.153398
Yan, Y. Y., Guo, Y., Zhang, W., Ma, C. G., Zhang, Y. X., Wang, C., et al. (2014). Celastrol Enhanced the Anticancer Effect of Lapatinib in Human Hepatocellular Carcinoma Cells In Vitro. J. BUON 19, 412–418.
Yang, X., Wu, F., Li, L., Lynch, E. C., Xie, L., Zhao, Y., et al. (2021). Celastrol Alleviates Metabolic Disturbance in High-Fat Diet-Induced Obese Mice through Increasing Energy Expenditure by Ameliorating Metabolic Inflammation. Phytother Res. 35, 297–310. doi:10.1002/ptr.6800
Ye, Q., Zou, B., Yeo, Y. H., Li, J., Huang, D. Q., Wu, Y., et al. (2020). Global Prevalence, Incidence, and Outcomes of Non-obese or Lean Non-alcoholic Fatty Liver Disease: a Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 5, 739–752. doi:10.1016/S2468-1253(20)30077-7
Younossi, Z., Anstee, Q. M., Marietti, M., Hardy, T., Henry, L., Eslam, M., et al. (2018). Global burden of NAFLD and NASH: Trends, Predictions, Risk Factors and Prevention. Nat. Rev. Gastroenterol. Hepatol. 15, 11–20. doi:10.1038/nrgastro.2017.109
Zhang, A., Sun, H., and Wang, X. (2013). Recent Advances in Natural Products from Plants for Treatment of Liver Diseases. Eur. J. Med. Chem. 63, 570–577. doi:10.1016/j.ejmech.2012.12.062
Zhang, D. G., Zhang, C., Wang, J. X., Wang, B. W., Wang, H., Zhang, Z. H., et al. (2017). Obeticholic Acid Protects against Carbon Tetrachloride-Induced Acute Liver Injury and Inflammation. Toxicol. Appl. Pharmacol. 314, 39–47. doi:10.1016/j.taap.2016.11.006
Zhang, J., Li, C. Y., Xu, M. J., Wu, T., Chu, J. H., Liu, S. J., et al. (2012). Oral Bioavailability and Gender-Related Pharmacokinetics of Celastrol Following Administration of Pure Celastrol and its Related Tablets in Rats. J. Ethnopharmacol 144, 195–200. doi:10.1016/j.jep.2012.09.005
Zhang, R., Chen, Z., Wu, S. S., Xu, J., Kong, L. C., and Wei, P. (2019). Celastrol Enhances the Anti-liver Cancer Activity of Sorafenib. Med. Sci. Monit. 25, 4068–4075. doi:10.12659/MSM.914060
Zhang, S., Yang, N., Ni, S., Li, W., Xu, L., Dong, P., et al. (2014). Pretreatment of Lipopolysaccharide (LPS) Ameliorates D-GalN/LPS Induced Acute Liver Failure through TLR4 Signaling Pathway. Int. J. Clin. Exp. Pathol. 7, 6626–6634.
Zhang, X., Wang, Y., Ge, H. Y., Gu, Y. J., Cao, F. F., Yang, C. X., et al. (2018). Celastrol Reverses Palmitic Acid (PA)-caused TLR4-MD2 Activation-dependent Insulin Resistance via Disrupting MD2-related Cellular Binding to PA. J. Cell Physiol 233, 6814–6824. doi:10.1002/jcp.26547
Zhang, Y., Geng, C., Liu, X., Li, M., Gao, M., Liu, X., et al. (2017). Celastrol Ameliorates Liver Metabolic Damage Caused by a High-Fat Diet through Sirt1. Mol. Metab. 6, 138–147. doi:10.1016/j.molmet.2016.11.002
Zhao, J., Luo, D., Zhang, Z., Fan, N., Wang, Y., Nie, H., et al. (2019). Celastrol-loaded PEG-PCL Nanomicelles Ameliorate Inflammation, Lipid Accumulation, Insulin Resistance and Gastrointestinal Injury in Diet-Induced Obese Mice. J. Control. Release 310, 188–197. doi:10.1016/j.jconrel.2019.08.026
Zhao, Q., Liu, F., Cheng, Y., Xiao, X. R., Hu, D. D., Tang, Y. M., et al. (2019). Celastrol Protects from Cholestatic Liver Injury through Modulation of SIRT1-FXR Signaling. Mol. Cell Proteomics 18, 520–533. doi:10.1074/mcp.RA118.000817
Zhao, Q., Tang, P., Zhang, T., Huang, J. F., Xiao, X. R., Zhu, W. F., et al. (2020). Celastrol Ameliorates Acute Liver Injury through Modulation of PPARα. Biochem. Pharmacol. 178, 114058. doi:10.1016/j.bcp.2020.114058
Zhu, C., Yang, J., Zhu, Y., Li, J., Chi, H., Tian, C., et al. (2021). Celastrol Alleviates Comorbid Obesity and Depression by Directly Binding Amygdala HnRNPA1 in a Mouse Model. Clin. Transl Med. 11, e394. doi:10.1002/ctm2.394
Zhu, H., Yang, W., He, L. J., Ding, W. J., Zheng, L., Liao, S. D., et al. (2012). Upregulating Noxa by ER Stress, Celastrol Exerts Synergistic Anti-cancer Activity in Combination with ABT-737 in Human Hepatocellular Carcinoma Cells. PloS one 7, e52333. doi:10.1371/journal.pone.0052333
Zhu, Y., Wan, N., Shan, X., Deng, G., Xu, Q., Ye, H., et al. (2021). Celastrol Targets Adenylyl Cyclase-Associated Protein 1 to Reduce Macrophages-Mediated Inflammation and Ameliorates High Fat Diet-Induced Metabolic Syndrome in Mice. Acta Pharm. Sin B 11, 1200–1212. doi:10.1016/j.apsb.2020.12.008
Keywords: celastrol, liver diseases, liver injury, liver cancer, MAFLD
Citation: Li M, Xie F, Wang L, Zhu G, Qi L- and Jiang S (2022) Celastrol: An Update on Its Hepatoprotective Properties and the Linked Molecular Mechanisms. Front. Pharmacol. 13:857956. doi: 10.3389/fphar.2022.857956
Received: 19 January 2022; Accepted: 21 February 2022;
Published: 04 April 2022.
Edited by:
Francisco Javier Cubero, Complutense University of Madrid, SpainReviewed by:
Lili Ding, Shanghai University of Traditional Chinese Medicine, ChinaCopyright © 2022 Li, Xie, Wang, Zhu, Qi and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lian-Wen Qi, UWlsd0BjcHUuZWR1LmNu; Shujun Jiang, ZmFpcnlqc2pAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.